Abstract
Background
American Indian (AI) and Alaska Native (AN) communities experience disproportionately high rates of tobacco use when compared to the overall U.S. population, especially among rural populations.
Methods
We implemented a single-blind, randomized clinical trial of a text messaging-based smoking cessation intervention through the tobacco quitlines of five states (Alaska, Minnesota, New Mexico, Oklahoma, and Wisconsin) with high percentages of AI residents. We partnered with state quitlines and Optum, a multi-state entity that manages quitlines. Participants who called the quitlines and identified as AI/AN were given the option to enroll in this trial. Upon consent, they were randomly assigned to either the standard quitline program (control) or a program culturally tailored for AI/ANs (intervention), which used a text messaging intervention to encourage smoking cessation. We adapted the text messages based on key informant and focus group input. Baseline data was analyzed for differences across age, sex, and the Fagerström Test for Nicotine Dependence.
Results
We recruited n = 487 AIs into the trial. Participants had an average age of 41.9 years (SD = 11.7) and 66% were female. The average Fagerström Test for Nicotine Dependence score was 5.38 (SD = 2.37). The intervention and control arms did not significantly differ across any of the baseline characteristics.
Conclusion
Implementation of this trial illustrated important lessons in adapting, implementing, and evaluating trials in collaboration with AI communities and local and national organizations. This work will inform future efforts to implement culturally-tailored interventions with AI/ANs and advance our knowledge about adapting and implementing smoking cessation interventions.
Keywords: Culturally-tailored intervention, American indians, Alaska Native and American Indian adults, Tobacco treatment, Text-messaging smoking cessation, Quitlines
1. Introduction
Lifetime cigarette smoking prevalence in American Indian (AI) and Alaska Native (AN) communities are among the highest in the United States (U.S.) [[1], [2], [3], [4], [5], [6]]. Prevalence is as high as 58% in some communities, and for virtually all AI communities it exceeds that of the general population [3,[7], [8], [9]]. Although traditional tobacco use is common among AI adults of certain tribes, in many communities ceremonial tobacco has been supplemented or largely replaced by commercial tobacco [10,11].
Few effective culturally-tailored smoking-cessation programs are available for AI adult smokers [12,13]. A randomized controlled trial that combined culturally-tailored components and varenicline did not result in differences in cessation between treatment and control groups [14]. In another recent randomized controlled trial, AI adults receiving the culturally tailored cessation intervention were twice as likely to be abstinent at 3 and 6 months than individuals in the control group receiving a best practice program [13].
Tobacco cessation treatment guidelines recommend that brief counseling and pharmacotherapy be offered to smokers interested in quitting [15]. Pharmacotherapy alone doubles smoking quit rates when compared to placebo [16], but is especially potent when combined with brief counseling [17]. Reaching rural-dwelling smokers remains a public health challenge, and interventions delivered in environments in which people smoke are needed to achieve optimal impact [18].
Smoking cessation interventions are well suited for delivery through mobile phones, as people appreciate the confidentiality and ease of use [19,20]. Phone-based cessation interventions have shown to be effective [20]. According to a 2009 Cochrane review and meta-analysis of 12 randomized clinical trials, using text messaging as a component in combination with face to face or telephone counseling was associated with smoking abstinence at 6 months [21]. We previously reported that among AIs living in rural communities, similar to the regions included in the current study, 86% owned a cellphone. Of these, 93% had unlimited texting and 80% had internet access [22].
This study is an adaptation of the STop SmOking with Mobile Phones (STOMP) protocol, developed in New Zealand [23]. In the original STOMP study, the intervention group received individually-tailored texts, including culturally-tailored texts using Maori language and the control group received texts that thanked participants for their participation or provided cessation resources. Participants in the intervention group were more likely to decrease smoking at six weeks than their peers in the control group. A trial of 5800 people in the United Kingdom, using a version of the STOMP protocol, found that biochemically-validated continuous abstinence at 6 months was higher in the intervention group compared to the control group (10.7% vs. 4.9%) [24]. The primary aim of the present trial is to determine if a culturally adapted version of STOMP is an effective smoking cessation intervention for AI/AN adults.
2. Methods
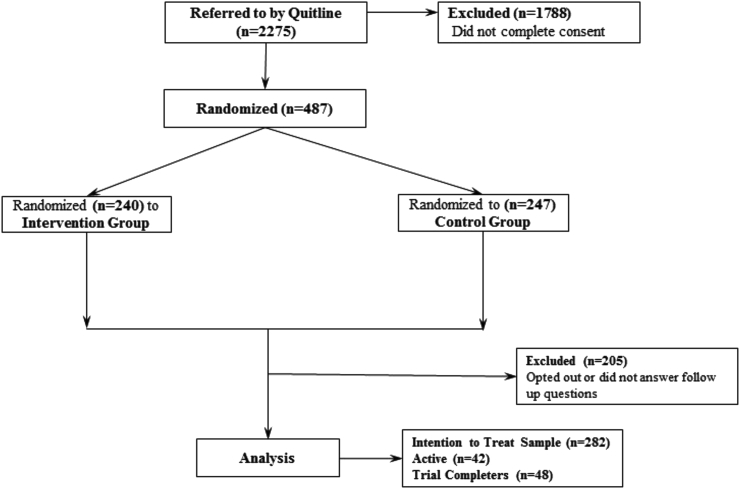
This project is a single blind, randomized trial of a culturally-tailored smoking-cessation intervention for AI/AN adults delivered through text-messaging. Baseline data was collected through a secure online form that participants can access on a smart phone and follow up data is collected exclusively through text messages. The trial's primary outcome is self-reported continuous smoking abstinence at 6-month follow-up. In this communication, we report on the study methods; including how messages were adapted for use in the intervention, initial and revised recruitment strategies, and the baseline characteristics of the recruited sample. Recruitment is ongoing and thus the CONSORT diagram (see Fig. 1) does not yet report the loss to follow-up numbers.
Fig. 1.
Sample intervention text messages.
2.1. Message adaptation
We obtained the bank of messages used for Maori young adults in the original STOMP investigation from the original investigators [23]. These messages were reviewed and edited by two AI research team members (Dr. Nelson and Ms. Young). Messages that could be adapted to be consistent with AI/AN cultures were retained and those that were specific to Maori culture were removed from the bank.
To further adapt the text messages, we conducted eight focus groups at four tribal colleges in Montana. Focus groups were comprised of 55 AI/AN tribal college students who were current or former smokers. We displayed each of the 74 text messages on a single Power Point® slide. Using the Turning Point® audience response system, students rated text message as evoking a positive, neutral, or negative emotional response. Messages receiving less than 70% positive responses were reviewed further by focus group members. Either the wording was improved or the message was removed from the message bank. Thirty new messages were also generated through this process, resulting in a total of 104 culturally adapted messages for use in the intervention arm of this trial.
In general, focus group members strongly disliked “text speak” (e.g., using “B4” for the word “before”), metaphors, and poetic language; they expressed a strong preference for positive, encouraging messages that supported self-efficacy, and concrete suggestions for handling cravings. Some examples of the resulting messages are: “Health is priceless. Cigarettes aren't”; “What is something you can make with your hands that will be a treasure to your family?”; “Respect yourself before everyone else – don't smoke,” and “It is never too late to make a change to better your health and life. It's just a decision away.”
2.2. Original design and rationale for methodology change
Originally, this study focused on AI college students at eight rural tribal colleges and state universities in five states with a high percentage of AIs. A research coordinator and faculty sponsor at each of the sites worked to recruit AI students during university rallies, pow-wows, and other cultural events affiliated with the colleges and universities. Although we convened annual community action board meetings to modify our project, of the 247 students contacted, only nine were enrolled in the study at the end of the first year of recruitment [19]. Meetings held at each site revealed a variety of challenges to recruitment, with the most common reason for non-participation being that students were not ready to quit. This repeated pattern of response from students who were approached prompted the local faculty and staff working on the study to advise us to seek other recruitment venues. This recommendation resulted in the decision to approach AIs/ANs using the state quitlines. We anticipated that smokers already interested in quitting would call the quitline, which in turn, would greatly increase recruitment. Using this approach, we enrolled n = 487 participants into the trial.
2.3. Change to state quitline partnership
State quitlines are evidence-based tobacco cessation interventions that help tobacco users quit through various supportive services, including counseling, practical information on how to quit, referral to other cessation resources, nicotine replacement therapy, and self-help materials. Our new approach evaluated a potential enhancement to services received through the quitline. We worked closely with Optum Health, the largest state quitline provider in the U.S. and the program directors for the individual state helplines. We approached states served by Optum, with a high proportion of AIs in their populations; namely Alaska, Minnesota, New Mexico, Oklahoma and Wisconsin. These states included much of our original catchment area, but greatly expanded the pool of potential participants, resulting in a more heterogeneous and generalizable sample. We retained the original STOMP study follow-up period of 26 weeks and adapted the informed consent process to an online form [23,24]. With these changes, the entire intervention was a self-contained, online experience where participants could complete the study from a smart phone. This resulted in an investigation that is more cost-efficient and reaches AI smokers who are rural dwelling and interested in quitting smoking.
2.4. Eligibility
Callers to the state's quitlines were required to self-identify as AI or AN, speak English and be over the age of 18. Potential participants were given the option to be contacted by our research team after confirming this information. Following their consent, Optum forwarded information on potential participants to our team through a secure online system. Our team sent an introductory text message with a link to the online consent form. After electronically signing the consent form, participants were directed to the online baseline questionnaires.
2.5. Randomization
Participants were randomly assigned to either receive the culturally-tailored text-messages intervention or a usual care control group who only completed the online consent form and questionnaires at baseline and follow-up time points. Both groups received standard quitline services (phone counseling and nicotine replacement therapy). The randomization process was stratified across Fagerström Test for Nicotine Dependence (FTND) scores, gender and age [25]. After randomization, a member of the research team added the participant's mobile phone number to the online texting service, Mozeo, which automatically delivered the appropriate text messages based on programmed schedules for each arm of the trial.
2.6. Study intervention
The culturally-tailored text-message intervention group received 200 texts (140 texts in the first 4–6 weeks, 60 in texts in weeks 7–26). Texts began one week prior to the participant's quit date, with a text such as “Thank you for participating in AI STOMP! Your quit date is in one week” followed by the intervention texts, such as “Be determined – you can be smoke free”. At the end of the intervention, control group participants were offered the same intervention as what the intervention group received during the clinical trial.
2.7. Measures
We collected information on demographics, smoking history, as well as the FTND and the Hooked On Nicotine Checklist (HONC) [26,27]. Participants completed these measures through a secure online survey system which can be accessed on smartphones or computers, depending on their preference. Participants were also asked to identify a quit date within 30 days of signing the consent form, similar to earlier reported STOMP protocols [22]. All subsequent data collection was through their personal mobile phones or computers.
Participants in both arms of the intervention were sent six follow-up questions by text-message at six, 12, and 18 weeks. Participants were asked the following: “Have you smoked a cigarette in the last 7 days?; How many cigarettes have you smoked in the last 7 days?; Since your quit date, how many quit tries longer than 24 h did you have?; How many car accidents have you been in since your quit date?; Have you gained weight since your quit date?; Have you experienced withdrawal symptoms since your quit date? Participant were asked all of the above questions, in addition to the following three questions at week 26: Have you smoked a cigarette in the last 6 months?; Have you shown any of your text messages to other friends or family?; Have you seen texts sent to someone else who's in this study?”
2.8. Ethical oversight
All study procedures were reviewed and approved by the Washington State University Institutional Review Board (IRB). As appropriate, the study was also reviewed and approved by other Tribal and college/university IRBs.
3. Analytic plan
For this report, we calculated percentages for categorical variables and means with standard deviations for continuous variables separately by study group. Upon completion of all follow-up data collection, we will perform an intention-to-treat analysis. Seven-day point prevalence of smoking abstinence reflects the immediate impact of the intervention on complete smoking cessation during (six weeks) and after (26 weeks) the active intervention phase as our primary outcome. We will also evaluate the intervention effects on the secondary outcomes of craving and withdrawal. We will use chi-square tests and t-tests to compare these outcomes between the two study groups. We will then perform unadjusted logistic regression analyses using the robust variance estimator (i.e., sandwich estimator) to appropriately account for clustering of multiple participants within each state and generate confidence intervals and p values that accurately model the effect of participant clustering on the precision of our point estimates [28]. This analysis will assess whether the intervention is associated with any smoking cessation, regardless of whether abstinence is maintained to the end of the study. Our a priori hypothesis is that the group receiving culturally-tailored text messages will have significantly higher prevalence of smoking cessation.
Missing data will be handled in a manner consistent with current expert recommendations for randomized trials, some of which have been established by our own team [29]. This approach emphasizes either maximum likelihood or multiple imputation. Maximum likelihood and multiple imputation have both shown exceptional performance compared to other, more common methods of handling missing data when the assumption of “missing at random” can be safely satisfied. Our threshold for statistical significance will be set at 0.05. For this baseline and design manuscript, we describe our sample's baseline characteristics.
4. Results
Demographic and clinical characteristics of the n = 487 participants and n = 9 tribal college participants are shown in Table 1, Table 2. Importantly, Table 2 is not referenced further in our characterization of the sample below.
Table 1.
Baseline characteristics of quitline participants by experimental randomization group.
| Outcome | Intervention (n = 240) |
Control (n = 247) |
Total (n = 487) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 41.63 | 11.11 | 42.15 | 12.21 | 41.89 | 11.67 |
| Age of first Cigarette (years) | 15.33 | 4.08 | 14.97 | 4.70 | 15.15 | 4.40 |
| Sex (Female) %, n | 65.00 | 156 | 66.80 | 165 | 65.91 | 321 |
| FTND | 5.31 | 2.26 | 5.44 | 2.49 | 5.38 | 2.37 |
| HONC | 8.40 | 1.95 | 8.03 | 2.65 | 8.21 | 2.35 |
| Education %, n | ||||||
| 1-8th Grade | 2.58 | 6 | 2.53 | 6 | 2.55 | 12 |
| Some HS | 10.73 | 25 | 9.28 | 22 | 10.00 | 47 |
| HS or GED | 28.33 | 66 | 33.33 | 79 | 30.85 | 145 |
| Some College | 45.06 | 105 | 43.04 | 102 | 44.04 | 207 |
| College Graduate | 13.30 | 31 | 11.81 | 28 | 12.55 | 59 |
Table 2.
Baseline characteristics of student participants by experimental randomization group.
| Outcome | Intervention (n = 4) |
Control (n = 5) |
Total (n = 9) |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 33.50 | 18.43 | 30.80 | 14.34 | 32.00 | 15.24 |
| Age of first Cigarette (years) | 13.50 | 3.70 | 15.40 | 2.79 | 14.56 | 3.17 |
| Sex (Female) %, n | 25 | 1 | 40 | 2 | 33.33 | 3 |
| FTND | 5.25 | 2.63 | 3.20 | 2.68 | 4.11 | 2.71 |
| HONC | 8.25 | 1.50 | 7.80 | 1.64 | 8.00 | 1.50 |
Our sample (n = 487) has an average age of 41.9 years (SD = 11.7) and is 66% female. The average FTND score was 5.4 (SD = 2.4) and the mean age of smoking initiation was 15.1 years (SD = 4.4). The average FTND score translates to a population of moderately dependent smokers, not unlike averages among smoking cessation populations our team and others have reported [[30], [31], [32]]. The randomized sample did not significantly differ across group assignment on any of the baseline characteristics (all ps > 0.05). Fig. 2 is our up-to-date CONSORT diagram illustrating participant flow through the clinical trial thus far. Upon completion of this clinical trial, we will finalize the CONSORT diagram. Notably, most of our participants were recruited from Oklahoma (66.3%). There were no statistically significant differences, however, when comparing those recruited from Oklahoma to those in other states on age, sex, age of first cigarette, education, FTND or HONC scores (p > 0.05).
Fig. 2.
CONSORT diagram for study participant flow.
5. Discussion
We designed a study to evaluate whether receiving culturally appropriate text messages could improve cessation among AI smokers. Text messaging has the advantage of anonymity and convenience and is well-suited for a smoking cessation intervention by allowing for cost-effective delivery and scalability to large numbers of smokers regardless of their location. Text messaging is an invaluable tool for increasing smoking cessation rates because it affords the ability to 1) tailor messages to key user characteristics such as age, sex, and race; 2) send time-sensitive messages; 3) devise content that can distract the smoker from cravings; and 4) link the smoker with others for social support [20].
Our study design is innovative and has the potential to improve public health by reducing the risk of cancer and other chronic diseases in AI communities. It is the first study to examine a culturally-tailored text-message smoking cessation intervention in AI populations. In addition, by partnering with state quitlines to adapt, implement, and evaluate this intervention, we increased our enrollment and knowledge of evidence-based smoking cessation treatments. While the focus group data was collected amongst college students who were interested in quitting, given the low amount of needed adaptation, we are confident that the intervention will be relevant to those seeking smoking cessation services through the quitline. The result is that this intervention is likely generalizable to many AI community members who are interested in quitting smoking. We do not have reason to suspect that this intervention would differ significantly in potential efficacy among those who called the quitline and were eligible but opted not to participate.
5.1. Lessons learned
We experienced substantial challenges with recruitment and implementation of this trial which required us to modify our recruitment strategy. However, as a result, we learned several lessons relevant to future clinical trial design considerations for this population of AI smokers who are rural dwelling. This trial was originally designed for students in tribal colleges and universities with a high enrollment of AIs. Our team worked closely with these communities prior to the implementation of the first version of this study holding Community Action Board meetings and focus groups. Although our original partners were tribal colleges whose representatives were interested in smoking cessation interventions for their student bodies, the students were not responsive to recruitment efforts. A critical lesson from this experience is to involve future participants when preparing an intervention such as this, and not just administrators and clinicians who are involved in the treatment being constructed and delivered. Research staff at each site heavily engaged throughout, but the primary hurdle for recruitment was identifying potential participants who were actively pursuing help to quit smoking. After nearly four years of work on the part of the tribal university partners and our research staff, we agreed to develop changes to our recruitment approach. We expanded our inclusion criteria from AI students to AI smokers of all ages and occupations, and chose to partner with quitlines to identify potential subjects. The increase in participation after making those changes is likely a direct result of being able to offer the intervention to AIs interested in quitting tobacco. In both the college and quitline settings, we found that participants were starting to smoke later than previously reported by others [33].
We also made a couple of simple, but critical protocol changes when engaging the quitline approach that had a significant impact. The switch to recruiting from callers to quitlines decreased the need for research staff labor. In addition, we removed the in-person consent process and implemented an entirely online intervention format, which further decreased the demand on research staff time. One aspect of the design that may be valuable to incorporate into a future approach would be to collect basic data from individuals who opt not to enroll into the study after being deemed eligible. This could help recruitment strategies or other aspects of our approach. Lastly, we also found that Oklahoma state produced the highest number of participants, which we plan to investigate further in order to identify how our approaches in other states could be modified to emulate our success in Oklahoma.
6. Conclusion
In conclusion, our intervention design will assist future research teams in designing interventions to better fit the needs of diverse and hard-to-reach populations. As in many community-based investigations such as this, changes in design are common and often unanticipated by pilot data or early community communications. Research with and for communities is a continuous process that requires flexibility, true partnership, and continuous examination of assumptions. These three issues are highlighted further when working with diverse and hard-to-reach populations, such as those that are rural dwelling. Fortunately, we have continued our strong research relationship with these communities and have current, active collaborations in many of them in an ongoing attempt to improve the health of AIs. Through the many lessons learned, this work can serve as a case example of how a failure of one approach should not be perceived to be an unsurmountable barrier. Rather, it is a call to lean on community feedback and modify one's approach that fits the needs of that specific community more closely to identify a design that can overcome the previously identified barriers.
Acknowledgments
Funding for this research is provided by the National Institute on Minority Health and Health Disparities, research grant P20MD006871, Principal Investigators McPherson and Buchwald. Funding support was also provided by the Oklahoma Tobacco Settlement Endowment Trust. We thank our community partners for their continuing support and partnership throughout all stages of this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100363.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nez Henderson P., Jacobsen C., Beals J. Correlates of cigarette smoking among selected Southwest and Northern plains tribal groups: the AI-SUPERPFP Study. Am. J. Public Health. 2005;95(5):867–872. doi: 10.2105/AJPH.2004.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welty T.K., Rhoades D.A., Yeh F., Lee E.T., Cowan L.D., Fabsitz R.R., Robbins D.C., Devereux R.B., Henderson J.A., Howard B.V. Changes in cardiovascular disease risk factors among American Indians: the Strong Heart Study. Ann. Epidemiol. 2002;12(97–106) doi: 10.1016/s1047-2797(01)00270-8. [DOI] [PubMed] [Google Scholar]
- 3.Kropp F., Somoza E., Lilleskov M., Moccasin M.G.-B., Moore M., Lewis D., Boetel B., Smith C., Winhusen T. Characteristics of Northern Plains American Indians seeking substance abuse treatment in an urban, non-tribal clinic: a descriptive study. Community Ment. Health J. 2013;49(6):714–721. doi: 10.1007/s10597-012-9537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nez Henderson P., Kanekar S., Wen Y., Buchwald D., Goldberg J., Choi W., Okuyemi K.S., Ahluwalia J., Henderson J.A. Patterns of cigarette smoking initiation in two culturally distinct American Indian tribes. Am. J. Public Health. 2009;99(11):2020–2025. doi: 10.2105/AJPH.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess D., Fu S.S., Joseph A.M., Hatsukami D.K., Solomon J., van Ryn M. Beliefs and experiences regarding smoking cessation among American Indians. Nicotine Tob. Res. 2007;9(Suppl_1):S19–S28. doi: 10.1080/14622200601083426. [DOI] [PubMed] [Google Scholar]
- 6.Gryczynski J., Feldman R., Carter-Pokras O., Kanamori M., Chen L., Roth S. Contexts of tobacco use and perspectives on smoking cessation among a sample of urban American Indians. J. Health Care Poor Underserved. 2010;21(2):544–558. doi: 10.1353/hpu.0.0276. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control (CDC) Cigarette smoking among American Indians, Alaskan Natives--behavioral risk factor surveillance system, 1987-1991. J. Am. Med. Assoc. 1992;268(21):3052–3055. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 1998. Tobacco Use Among U.S. Racial/ethnic Minority Groups – African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: A Report of the Surgeon General. [Google Scholar]
- 9.Centers for Disease Control and Prevention Cigarette smoking among adults--United States, 2001. MMWR (Morb. Mortal. Wkly. Rep.) 2003;52(40):953–956. [PubMed] [Google Scholar]
- 10.Kunitz S.J. Historical influences on contemporary tobacco use by Northern Plains and Southwestern American Indians. Am. J. Public Health. 2016;106(2):246–255. doi: 10.2105/AJPH.2015.302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redwood D., Lanier A.P., Renner C., Smith J., Tom-Orme L., Slattery M.L. Differences in cigarette and smokeless tobacco use among American Indian and Alaska Native people living in Alaska and the Southwest United States. Nicotine Tob. Res. 2010;12(7):791–796. doi: 10.1093/ntr/ntq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Silva J., BASW R.L.H. The Wiidookowishin program: results from a qualitative process evaluation of a culturally tailored commercial tobacco cessation program. Am. Indian Alsk Native Ment. Health Res. 2014;21(1):18. doi: 10.5820/aian.2101.2014.18. [DOI] [PubMed] [Google Scholar]
- 13.Choi W.S., Beebe L.A., Nazir N., Kaur B., Hopkins M., Talawyma M., Shireman T.I., Yeh H.-W., Greiner K.A., Daley C.M. All nations breath of life: a randomized trial of smoking cessation for American Indians. Am. J. Prev. Med. 2016;51(5):743–751. doi: 10.1016/j.amepre.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith S.S., Rouse L.M., Caskey M., Fossum J., Strickland R., Culhane J.K., Waukau J. Culturally-tailored smoking cessation for adult American Indian smokers: a clinical trial. Counsel. Psychol. 2014;42(6):852–886. doi: 10.1177/0011000014542601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiore M.C., Smith S.S., Jorenby D.E., Baker T.B. The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. Jama. 1994;271(24):1940–1947. [PubMed] [Google Scholar]
- 16.Hughes J.R., Goldstein M.G., Hurt R.D., Shiffman S. Recent advances in the pharmacotherapy of smoking. Jama. 1999;281(1):72–76. doi: 10.1001/jama.281.1.72. [DOI] [PubMed] [Google Scholar]
- 17.Stitzer M.L. Combined behavioral and pharmacological treatments for smoking cessation. Nicotine Tob. Res. 1999;1(Suppl 2):S181–S187. doi: 10.1080/14622299050012041. discussion S207-10. [DOI] [PubMed] [Google Scholar]
- 18.Burhansstipanov L., Christopher S., Schumacher S.A. Lessons learned from community-based participatory research in Indian country. Cancer Control. 2005;12(Suppl 2):70–76. doi: 10.1177/1073274805012004s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuckovic N., Polen M.R., Hollis J.F. The problem is getting us to stop. What teens say about smoking cessation. Prev. Med. 2003;37(3):209–218. doi: 10.1016/s0091-7435(03)00115-4. [DOI] [PubMed] [Google Scholar]
- 20.Balch G.I., Tworek C., Barker D.C., Sasso B., Mermelstein R., Giovino G.A. Opportunities for youth smoking cessation: findings from a national focus group study. Nicotine Tob. Res. 2004;6(1):9–17. doi: 10.1080/1462200310001650812. [DOI] [PubMed] [Google Scholar]
- 21.Whittaker R., Borland R., Bullen C., Lin R.B., McRobbie H., Rodgers A. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2009;(4):CD006611. doi: 10.1002/14651858.CD006611.pub4. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dotson J., Nelson L., Young S., Buchwald D., Roll J. Use of cell phones and computers for health promotion and tobacco cessation by American Indian college students in Montana. Rural Rem. Health. 2017;17:4014. doi: 10.22605/rrh4014. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers A., Corbett T., Bramley D., Riddell T., Wills M., Lin R.B., Jones M. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tobac. Contr. 2005;14(4):255–261. doi: 10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Free C., Knight R., Robertson S., Whittaker R., Edwards P., Zhou W., Rodgers A., Cairns J., Kenward M.G., Roberts I. Society for Research on Nicotine and Tobacco 17th Annual Meeting. Society for Research on Nicotine and Tobacco; Toronto, Ontario: 2011. A randomised controlled trial of mobile (cell) phone text messaging smoking cessation support: TXT2STOP. [Google Scholar]
- 25.Korte K.J., Capron D.W., Zvolensky M., Schmidt N.B. The Fagerstrom test for nicotine dependence: do revisions in the item scoring enhance the psychometric properties? Addict. Behav. 2013;38(3):1757–1763. doi: 10.1016/j.addbeh.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellman R.J., Savageau J.A., Godiwala S., Savageau N., Friedman K., Hazelton J., DiFranza J.R. A comparison of the hooked on nicotine checklist and the Fagerström test for nicotine dependence in adult smokers. Nicotine Tob. Res. 2006;8(4):575–580. doi: 10.1080/14622200600789965. [DOI] [PubMed] [Google Scholar]
- 27.DiFranza J.R., Savageau J.A., Fletcher K., Ockene J.K., Rigotti N.A., McNeill A.D., Coleman M., Wood C. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch. Pediatr. Adolesc. Med. 2002;156(4):397–403. doi: 10.1001/archpedi.156.4.397. [DOI] [PubMed] [Google Scholar]
- 28.Diggle P., Diggle P.J., Heagerty P., Heagerty P.J., Liang K.-Y., Zeger S. Oxford University Press; 2002. Analysis of Longitudinal Data. [Google Scholar]
- 29.McPherson S., Barbosa-Leiker C., Burns G.L., Howell D., Roll J. Missing data in substance abuse treatment research: current methods and modern approaches. Exp. Clin. Psychopharmacol. 2012;20(3):243. doi: 10.1037/a0027146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherson S., Howell D., Lewis J., Barbosa-Leiker C., Bertotti Metoyer P., Roll J. Self-reported smoking effects and comparative value between cigarettes and high dose e-cigarettes in nicotine-dependent cigarette smokers. Behav. Pharmacol. 2016;27(2–3):301–307. doi: 10.1097/FBP.0000000000000226. Spec Issue. [DOI] [PubMed] [Google Scholar]
- 31.Packer R.R., Howell D.N., McPherson S., Roll J.M. Investigating reinforcer magnitude and reinforcer delay: a contingency management analog study. Exp. Clin. Psychopharmacol. 2012;20(4):287–292. doi: 10.1037/a0027802. [DOI] [PubMed] [Google Scholar]
- 32.McClure E.A., Baker N.L., Sonne S.C., Ghitza U.E., Tomko R.L., Montgomery L., Babalonis S., Terry G.E., Gray K.M. Tobacco use during cannabis cessation: use patterns and impact on abstinence in a national drug abuse treatment clinical trials network study. Drug Alcohol Depend. 2018;192:59–66. doi: 10.1016/j.drugalcdep.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark T.T., Doyle O., Clincy A. Age of first cigarette, alcohol, and marijuana use among US biracial/ethnic youth: a population-based study. Addict. Behav. 2013;38(9):2450–2454. doi: 10.1016/j.addbeh.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.