Abstract
Epilepsy is the most common serious childhood neurological disorder. In the low- and middle-income countries (LMICs) of Africa, children with epilepsy suffer increased morbidity and mortality compared to their counterparts in high-income countries, and the majority do not receive treatment - the childhood epilepsy treatment gap. Reports of the childhood epilepsy treatment gap in Africa are likely underestimates; most surveys do not include several common childhood seizure types, including most types of non-convulsive epilepsy. Efforts to scale up childhood epilepsy care services in the LMICs of Africa must contend with a shortage of physicians and diagnostic technology [e.g., electroencephalograms (EEGs)]. One pragmatic solution is to integrate epilepsy care into primary care by task-shifting to community health extension workers. The aims of this project (BRIDGE) are to: 1) train, develop, and pilot task-shifted epilepsy care teams; 2) develop and pilot innovative childhood epilepsy screening and diagnostic paradigms adapted to the local Hausa language/culture in Kano, northern Nigeria; and, 3) quantify and map the childhood epilepsy treatment gap, using geographic information systems (GIS), to target limited resources to areas of greatest need. Task-shifted teams will diagnose and manage childhood epilepsy using an innovative epilepsy screening tools and diagnostic and management paradigms in environments with limited EEG access. If validated and demonstrated efficacious in clinical trials, this project can be taken to scale across broader areas of west Africa's LMICs that share language and culture. BRIDGE has the potential to enhance access to basic childhood epilepsy care and establish the foundation for childhood epilepsy clinical trials in west Africa.
Keywords: Childhood epilepsy, Epilepsy treatment gap, Task shifting, Nigeria, Hausa
1. Introduction
Epilepsy, the most common serious childhood neurological disorder, affects over 65 million people, about 80% of whom live in low- and middle-income countries (LMICs) [1,2]. In LMICs of Africa, where half or more of the population is under 18 years of age [3,4], children with epilepsy suffer especially poor health outcomes and have high risk of serious comorbidities [5] and injuries [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. Epilepsy-associated deaths are a major cause of overall childhood accidental mortality in Africa [16,17]. Childhood epilepsy imposes hidden burdens associated with stigma, discrimination, and human rights violations [18].
The childhood epilepsy treatment gap, or the percentage of children with epilepsy who do not receive adequate treatment in Africa is 75% or higher in Africa's low-income countries [[19], [20], [21], [22]], with the exception of Senegal (23%) [23] and Madagascar (32%) [24], probably underestimates because of under-ascertainment of non-convulsive seizures [25]. The epilepsy treatment gap may not be evenly distributed geographically. Spatial epidemiology methods utilizing Geographic Information Systems (GIS) techniques with population-based maps of epilepsy treatment gaps [[26], [27], [28], [29]], may help target limited resources to improve epilepsy care access.
LMICs in Africa that need to scale up epilepsy care must contend with physician shortages [30], and lack of electroencephalogram (EEG) access [11,19]. Integrating epilepsy care into primary care by task-shifting epilepsy care to community health extension workers (CHEWs) is recommended by the World Health Organization (WHO) [31] for LMICs in Africa [[32], [33], [34]]. Task-shifting care has been piloted for depression [35], and demonstrated to be effective in HIV care [36]. Further, preliminary studies in LMICs suggest that task-shifting interventions for brain disorders may be feasible if increased numbers of CHEWs are available, medications are reasonably available, primary-care level supervision of task-shifted care is maintained, and CHEWs are adequately trained and compensated [19]. However, few studies and no clinical trials have evaluated task-shifted epilepsy care in Africa [37,38] or elsewhere [39]. The WHO recommendations for reducing the childhood epilepsy treatment gap have been accepted by various African governments [40]. However, the WHO-recommended strategies for task-shifted epilepsy care have not been widely implemented in Africa probably because: (a) task-shifted childhood epilepsy care has potential, but is inadequately grounded in evidence; (b) published methods for implementing task-shifted epilepsy care are insufficient, without demonstrated methods for epilepsy education, training, and supervision of CHEWs; (c) barriers to implementing task-shifted epilepsy care, and strategies to overcome these barriers, have not been well-established [[41], [42], [43], [44]], and d) protocols for task-shifted (to CHEWs) epilepsy diagnosis and management, which address all major epilepsy and seizure types in settings with very limited EEG access, have not been developed, piloted, and tested. There is equipoise with regard to task-shifted epilepsy care versus usual physician epilepsy care in the LMICs of Africa.
The long-term aim of BRIDGE is to develop a scalable toolkit to reduce the childhood epilepsy treatment gap in Africa, with methods that include task-shifted care. This innovative project lays a foundation for a large cluster-randomized clinical trial of task-shifted childhood epilepsy care, and for clinical and spatial epidemiology studies of childhood epilepsy in Hausa-speaking Africa.
2. Methods
2.1. Setting
Since 2008, Vanderbilt University Medical Center (VUMC), Aminu Kano Teaching Hospital (AKTH), and key partners in northern Nigeria have supported clinical care and research targeting the needs of people in northern Nigeria with HIV, sickle cell disease (e.g., stroke prevention), renal disease and epilepsy with funding from the U.S. National Institutes of Health (NIH), the U.S. Centers for Disease Control and Prevention/U.S. President's Emergency Plan for AIDS Relief (CDC/PEPFAR), and other organizations [36,[45], [46], [47], [48], [49], [50], [51], [52]].
Kano is the state capital of Kano State in Nigeria and the second largest metropolis in Nigeria. Kano metropolis is comprised of eight local government areas (LGAs) —Dala, Fagge, Gwale, Kano Municipal, Kumbotso, Nasarawa, Tarauni, and Ungogo. The total area of metropolitan Kano urban area is 251 square kilometers, with an estimated population of 3,680,000. Kano state has an estimated population of 13,076,892. Although Nigeria has made economic progress over the past few decades, about half of people in northern Nigeria live in extreme poverty. Almost half of people in Kano are under the age of 18 years. These studies will be conducted in eight primary healthcare centers (PHCs) randomly selected from a pool of 167 PHCs in metropolitan Kano that offer primary maternal and child health care services.
Kano is the largest population center of the Hausa people, who are heavily concentrated in northern Nigeria and southern Niger, with significant Hausa populations in Chad, Ghana, Cameroon, Togo, and Sudan. Spoken by up to 120 million people, Hausa is the third most commonly spoken language in Africa, behind only Arabic and Swahili, and is the most commonly spoken language in west Africa [53,54]. Hausa-translated educational materials and instruments demonstrated as effective in this study can be used across other Hausa-speaking populations. Other minority ethnic groups in Kano include the Fulani (most speak Hausa), Yoruba, Igbo and Kanuri.
2.2. Rationale
BRIDGE seeks to close the epilepsy treatment gap by developing and testing sustainable models of childhood epilepsy care that have the potential to be taken to scale in northern Nigeria, and throughout the LMICs of Africa, with an initial focus on childhood epilepsy among the Hausa people. Novel systems of epilepsy care (e.g., task-shifting epilepsy care to specially-trained community health extension workers [CHEWs]) and innovative diagnostic paradigms that most efficiently and effectively utilize scarce diagnostic resources (e.g. EEGs) have not been well-established or tested in clinical trials in African LMICs. BRIDGE will emphasize work performed at a network of primary healthcare centers (PHCs) in Kano, and a major tertiary care referral center (Aminu Kano Teaching Hospital, AKTH), to enhance access to epilepsy care and establish the foundation for childhood epilepsy clinical trials.
2.3. Specific aims
BRIDGE will build upon the clinical and research platforms in Kano to enhance clinical capacity and increase access to epilepsy care, and establish the foundation for childhood epilepsy clinical trials through the following Specific aims:
-
1)
Train, develop, and pilot epilepsy care teams of CHEWs and physicians under the direction of epilepsy-trained physicians and nurses to facilitate task-shifting and improve access to epilepsy care. We hypothesize that graduates of the training program in childhood epilepsy will demonstrate good knowledge of epilepsy diagnosis and treatment on post-course examinations administered by epilepsy experts (≥80% correct answers on examinations).
-
2)
Develop and pilot innovative task-shifted epilepsy diagnostic paradigms for low-resource environments with limited diagnostic EEG access. We will extend and modify existing diagnostic paradigms used by the WHO and other organizations to include diagnosis of all major childhood epilepsy syndromes and seizure types, and will adapt these diagnostic paradigms for use in Kano to be piloted by epilepsy care teams. We hypothesize that epilepsy diagnostic paradigm education will allow the epilepsy care teams to implement care and optimally utilize scarce EEG resources.
-
3)
Quantify and map the childhood epilepsy treatment gap in Kano, Nigeria, and compare the urban versus rural childhood epilepsy treatment gaps to (a) help target the implementation of childhood epilepsy care teams, and (b) establish data collection systems to monitor the impact of task-shifted epilepsy care teams and innovative epilepsy diagnostic paradigms on reducing the treatment gap. Based upon previous estimates in other African LMICs, we hypothesize that the childhood epilepsy treatment gap varies across areas of Kano, and by urban and rural areas within Kano.
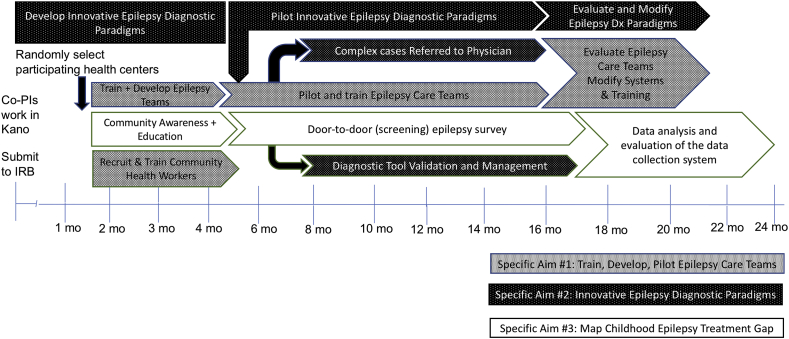
In addition, three qualitative evaluations will be conducted to: 1) assess patients’ family satisfaction with health services delivered by epilepsy care teams and CHEWs; 2) evaluate health care worker satisfaction with the new epilepsy care team and task-shifted care approach; and 3) perform an audit of diagnostic EEG services in Kano, including physicians and technologists with EEG expertise, and available EEG machines. An overview of the BRIDGE timeline is shown in Fig. 1.
Fig. 1.
BRIDGE timeline.
2.4. Study design overview
The three specific aims of this study are tightly integrated and coordinated during the two-year timeline (Fig. 1). Following approval by the internal review boards (IRB), and approval by the Kano State Ministry of Health, eight participating PHCs will be randomly selected – one from each of the eight local government areas of metropolitan Kano. One of the local government areas (Kumbotso) is primarily a rural area, with a small urban component. An epilepsy education program, developed for community health workers, will be administered to a cohort of CHEWs. From this cohort, CHEWs who successfully complete the training program will be selected to conduct community cross-sectional surveys to quantify and map the epilepsy treatment gap in Kano, and participate as members of epilepsy care teams working at the participating PHCs.
Parallel to the epilepsy education program the epilepsy diagnostic screening instruments and paradigms were developed. The childhood epilepsy screening instrument, modified for use in Kano by adding questions to enhance seizure type classification and by translating to Hausa, will undergo validation in two populations – pediatric clinic populations in Kano, and door-to-door surveys in neighborhoods served by the participating PHCs. In addition, qualitative evaluations will be performed to assess satisfaction of patients, family members, and healthcare workers. An audit of the diagnostic EEG services available in Kano will be performed (Fig. 1).
2.4.1. Institutional Review Board (IRB) and ethics committee approval
Ethical approval for this study was obtained from the Aminu Kano Teaching Hospital (AKTH) Research Ethics Committee, and the Institutional Review Board at Vanderbilt University Medical Center. The Kano State Ministry of Health also approved the study.
2.4.2. Random selection of participating primary healthcare centers (PHCs)
Following approvals from the IRBs and the Kano State Ministry of Health, a computer-based random number generator will be used to randomly select eight PHCs from a pool of a total of 167 PHCs in the eight local governmental areas of metropolitan Kano. The site chosen in one of the eight local governmental areas, Kumbotso, will be located in a rural area. The managers of each of the randomly selected eight PHCs will give permission for their PHC to participate in the BRIDGE project. If any of the eight randomly selected PHCs declines to participate, the reason(s) will be documented, and another PHC from the same local government area will be randomly selected for participation. By randomly selecting PHCs from each of the governmental areas of metropolitan Kano, we will seek to achieve a representative sample of PHCs, avoid a clustering of the study sites within a single region of the city, and reduce the likelihood of movement of patients between the participating PHCs.
2.4.3. Selection of community health extension workers (CHEWs)
One CHEW currently working in each of the eight randomly selected PHCs will be selected for training in childhood epilepsy. Following successful completion of training, these eight CHEWs will work half-time on the project. An additional 15 CHEWs who are recent graduates of the CHEW training program at the Kano State School of Health Technology or similar schools who show an interest in the project and the training will also be enrolled in the epilepsy training program. From these additional CHEWs, 10 who academically excel in the training program will also be hired to work on the project, each assigned to one of the eight participating PHCs. In addition, we will train 2 additional Community Health Officers (CHOs) in the epilepsy training program, each of whom will be hired at 50% effort to work on the project and supervise the CHEWs.
2.5. Epilepsy education program development and pilot (aim 1)
The training program was modified from nurse training programs in pediatric epilepsy designed for new-onset seizure clinics in the U.S., adapted to the needs of Kano. This program was then refined by investigators at Vanderbilt University Medical Center and Aminu Kano Teaching Hospital (AKTH) using a flipped classroom pedagogical model, including a series of 33 brief recorded lectures (10–18 min each), with each lecture paired with a case-based group activity that reinforces the new material taught in the lecture. Each CHEW in the training program will be provided an android tablet, loaded with the recorded lectures, case-based group activities, and other learning materials to facilitate review and study.
The greater than 60-hour epilepsy training program will be free of charge. The initial period of training will include one to two weeks of full-time lectures and group activities, followed by weekly to bi-weekly follow-up lectures and meetings with other CHEWs and community health officers (CHOs). After the initial week of intensive epilepsy training, CHEWs will see patients under the supervision of physicians with expertise in epilepsy, as well as have follow-up lectures and group activities. CHEWs and CHOs will take daily quizzes during the initial week of training, and then a comprehensive quiz after the first two months of the epilepsy education program. Focus groups will be conducted, before and after completion of the epilepsy course as part of the evaluation of the epilepsy education program. All CHEWs who complete the epilepsy education program will receive a certificate from Vanderbilt Institute for Global Health and Aminu Kano Teaching Hospital.
The material covered during the intensive epilepsy education program will include: 1) clinical neuroanatomy and neurophysiology, with an emphasis on the cerebral cortex; 2) the basics of childhood stroke (common among children with sickle cell anemia in Kano); 3) basics of the neurological examination; 4) seizure definition, classification, and semiology (with video examples of seizures); 5) multiple lectures on major childhood epilepsy syndromes; 6) common co-morbid conditions associated with childhood epilepsy; 7) febrile seizures; 8) status epilepticus; 9) single seizures and post-traumatic seizures; 10) symptomatic seizures associated with infections (e.g., meningitis, malaria); 11) non-epileptic events; and, 12) an introduction to epilepsy treatment, with a review of the major anti-epileptic drugs (AEDs) available for use in Kano - phenobarbital, carbamazepine, and valproate. Other important topics covered include stigma, challenges of sustaining AED therapy, and seizure precautions and safety measures (e.g., keeping child aware from fires). Lectures will also be provided on newer AEDs that are rarely available (e.g., levetiracetam), and older medications still in use in northern Nigeria (e.g., paraldehyde, used for status epilepticus).
The pharmacy technicians working at the participating PHCs will receive basic additional education in epilepsy and the typically available AEDs. In addition, primary care physicians working in Kano who receive referrals from the participating PHCs will also be provided access to the epilepsy education program materials and consultations with the physician epilepsy specialists on the BRIDGE project.
Following the initial month of training, the CHEWs and CHOs will continue to evaluate patients under the supervision of physicians with expertise in epilepsy for approximately five months. Supplemental education sessions will continue on a regular basis, and supplemental epilepsy education sessions will be scheduled for the group. The CHEWs will learn and practice the details of the new childhood epilepsy screening instruments and diagnostic paradigms as they are developed by the study team and will begin functioning in teams of 2–4 CHEWs, with CHEWs supervised by a CHO and by physicians with expertise in epilepsy. The CHEWs and CHOs who complete the epilepsy training program will undergo a comprehensive evaluation/examination that will include a written examination as well as an evaluation by one or more of the physician epilepsy specialists regarding clinical evaluation of epilepsy patients.
2.6. Development and feasibility of task-shifted epilepsy diagnosis and treatment protocol (aim 2)
The childhood epilepsy diagnostic guidelines from WHO for use in low-resource settings of Africa will inform our task-shifted care protocol, with a focus on addressing the needs of children with epilepsy who are not sufficiently addressed by the WHO guidelines [41] - those with absence epilepsy, myoclonic seizures, atonic seizures, and less common epilepsy syndromes (e.g., epileptic spasms), including those with epilepsy who do not achieve seizure freedom after the first AED is administered in appropriate doses. These diagnostic guidelines will reflect the reality of limited access to EEGs for children in Kano. The diagnostic and treatment guidelines and systems utilized in the Paediatric Epilepsy Training (PET) courses and modules (developed by the British Pediatric Neurology Association, https://www.bpna.org.uk/pet/), previously piloted throughout Africa, will also inform this further development of the childhood epilepsy paradigms.
A recently validated epilepsy screening, diagnosis and seizure type classification tool [55] has been modified and expanded with the intent of improving ascertainment of specific non-convulsive seizures thought to possibly be under-ascertained with the initial instrument. The modified epilepsy screening, diagnosis and seizure type classification tool will be validated in both clinics and in community screening (e.g., door-to-door surveys) after translation to Hausa, and will be a useful tool for gathering preliminary information on patients with suspected epilepsy, and assist the CHEW in obtaining additional information to clarify the history of possible seizures (Fig. 2). The proposed childhood epilepsy diagnostic and management guidelines will be reviewed by an advisory panel of experts in pediatric neurology.
Fig. 2.
Childhood Epilepsy Screening & Seizure Classification Tool∗.
∗Modified and expanded from Patel AA et al. Epilepsy & Behavior 2016; 59:57–61, with permission.
#Question 2 added to screen for absence seizures & focal non-motor seizures
%Questions 11 and 12 were separated into two questions.
2.6.1. Community epilepsy education and awareness campaign (aims 2 and 3)
A community epilepsy education and awareness campaign will begin with meetings with key community leaders and stakeholders in areas served by the participating PHCs, followed by educational programs at local schools and/or local community centers, and radio programs in Hausa language. The radio broadcasts will feature AKTH physicians with special expertise in epilepsy and child neurologists who will present basic information about epilepsy, the BRIDGE studies and the community surveys, and answer questions from the audience. As per prior agreement, these public service radio broadcasts will be recorded, and the radio shows will be re-played weekly for four weeks, and then be made available for ongoing community information sessions. The primary educational messages will be: 1) seizures and epilepsy are disorders of the brain that can be effectively treated; 2) a general description of types of seizures; 3) some, perhaps many, children with seizures who can be treated effectively with medication are not receiving treatment; and 4) AKTH is conducting research to develop new ways to deliver medical care to more children with epilepsy. Focus group discussions (FGDs) and key informant interviews (KIIs) will be conducted with community leaders and community representatives.
2.6.2. Validation of epilepsy diagnosis and seizure type classification (aims 2 and 3)
A validated Hausa version of an epilepsy screening and seizure type classification tool is key to both the pilot of task-shifted childhood epilepsy care (Aim 2) and mapping the childhood epilepsy treatment gap in Kano (Aim 3). An epilepsy screening tool initially validated among children in Zambia and Tanzania [55] was modified to increase the predictive values for types of focal-onset seizures (e.g., frontal and temporal lobe), generalized seizures (e.g., absence, atonic, myoclonic), and specific epilepsy syndromes (e.g., juvenile myoclonic epilepsy [56], nodding syndrome [57], Lennox-Gastaut syndrome [17,58,59], and infantile spasms [60,61]). The epilepsy screening tool consists of three initial screening questions, with a “yes” answer to any screening question prompting an additional 15 questions to refine descriptions for seizure type classification and recognition of childhood epilepsy syndromes (Fig. 2).
With active collaborations between Hausa-speaking epilepsy specialists at AKTH and linguists at the Bayero University Center for Nigerian Languages and Folklore, the tool was translated to Hausa, back-translated to English and piloted by physician epilepsy specialists in Hausa. Epilepsy-trained CHEWs participated in the final editing of the Hausa translation of the tool and trained in its administration. Both Hausa and English versions of the epilepsy screening and seizure type classification tool will be put into REDCap®, a secure web application developed by Vanderbilt University for managing surveys and databases [62], and loaded onto android tablet computers used by the epilepsy-trained CHEWs and physicians. The childhood epilepsy screening questionnaire (Fig. 2) will be validated in two separate populations – (1) a pediatric clinic-based population at Murtala Muhammad Hospital (Kano) and at AKTH, and (2) a community-based population of children ascertained using the questionnaire. We will not exclude non-Hausa speakers from the study. Such participants will be administered the English version of the epilepsy screening and seizure type classification tool. Children (≥6 months, < 18 years) who screen positive in the survey, or in the pediatric clinics, will obtain an evaluation, including a history and physical examination, by a CHEW and by one of the physician epilepsy specialists at AKTH at no charge to the family. The diagnosis (epilepsy versus not epilepsy) and seizure type classification by a physician epilepsy specialist in Kano who speaks the local languages and knows the local culture will be used as the gold standard in the validation studies. A senior pediatric neurologist and epilepsy expert based at the Vanderbilt data coordinating center (ET) will review the screening tool data from all positive screens and the random sample of negative screens; blinded to the results of the physician and CHEW exams, and use a previously developed paradigm to determine “tool epilepsy diagnosis and classification of seizure types (generalized or focal onset)” based on the tool data alone. The sensitivity and specificity of the tool for epilepsy diagnosis, and the predictive values for epilepsy diagnosis will be determined. Among those children who screen positive for epilepsy, the predictive value of a positive screen for generalized seizures and for focal-onset seizures, as well as the predictive value of a negative screen for generalized seizures and for focal-onset seizures, will be determined.
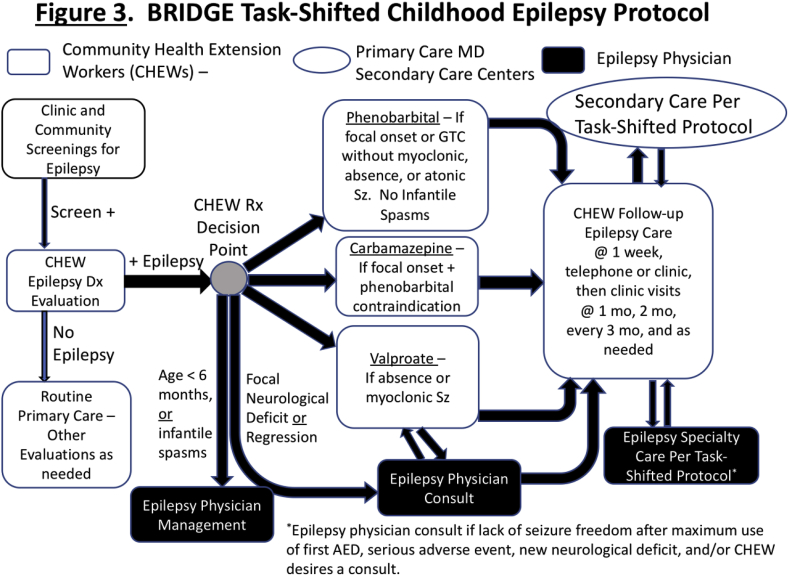
Parental consent and childhood assent will be obtained prior to the examinations. If the patient identified with epilepsy is not receiving treatment, and if medical treatment is deemed necessary after the CHEW and physician exams, subsequent treatment will be provided by a CHEW in the pilot of task-shifted childhood epilepsy care, after informed consent. Parents/guardians of children with untreated epilepsy who do not consent for task-shifted epilepsy care (Fig. 3), will be referred to available local healthcare resources for epilepsy care, including community physicians. We will not exclude non-Hausa speakers from the study. Such participants will be administered the English version of the epilepsy screening and seizure type classification tool.
Fig. 3.
BRIDGE Task-shifted Childhood epilepsy Protocol.
2.6.3. Feasibility assessment of task-shifted childhood epilepsy care system (aim 2)
Screening for childhood epilepsy, diagnostic evaluations of children who screen positive, and classification of childhood seizure types by clinical criteria will be piloted by epilepsy-trained CHEWs under the supervision of BRIDGE Kano-based physicians with expertise in epilepsy, and with adherence to the task-shifted protocol monitored by Kano-based investigators, and via the case report forms (CRFs) uploaded to the Vanderbilt data coordinating center. Parents of children (ages ≥ 6 months, < 18 years) who screen positive for epilepsy during the door-to-door surveys or the clinic surveys and are found to have epilepsy after their initial evaluation by a CHEW and a physician epilepsy specialist will be given the option for their child to receive epilepsy care by an epilepsy-trained CHEW at a local randomly selected PHC. After informed consent, the patient will be followed for epilepsy care by an epilepsy-trained CHEW, who will in turn be supervised by a CHO with specialized training in epilepsy and a physician epilepsy specialist – together, these form the epilepsy care team. Parents who do not wish for their child to receive task-shifted care via CHEWs at their local PHCs will be able to seek epilepsy care via the usual standard of care in Kano. The overview of the task-shifted childhood epilepsy care protocol is outlined in Fig. 3.
Children who are clinically diagnosed with epilepsy by a study physician and receive care from an epilepsy care team will receive standard of care AEDs available in northern Nigeria. Ours is not a study of a new drug(s), and only available and long-approved AEDs typically used in epilepsy care will be utilized. Nevertheless, for families who are unable to pay for their child's medication, efforts will be made to subsidize the costs of AEDs using private donor funds, based upon the financial needs of the child's family. Children diagnosed as having focal-onset seizures will be prescribed carbamazepine or phenobarbital. Children with absence seizures and/or myoclonic seizures, with or without generalized tonic-clonic seizures, will be prescribed valproate. Phenobarbital may be used in selected situations for patients with myoclonic seizures. If ethosuximide is available for absence seizures, this may also be prescribed. Children with generalized tonic-clonic seizures, without absence seizures or myoclonic seizures, will be prescribed phenobarbital or valproate. Children with focal epilepsy with secondary generalized tonic-clonic seizures may also be treated with carbamazepine. Levetiracetam, typically not available and expensive, may also be used for children with seizures associated with focal-onset epilepsy or primary generalized epilepsy, if available and affordable. Typical contraindications for specific anti-epileptic drugs used in clinical practice (e.g., previous allergy to the drug) will be followed (Fig. 3) [[63], [64], [65]]. All females of childbearing age enrolled in task-shifted care will be referred by CHEWs to a family planning clinic (CHEW-operated, and typically in the PHC) as per routine practice, where oral contraceptives are available, and all patients will be prescribed multivitamins with folic acid. Any patient who becomes pregnant while enrolled in task-shifted epilepsy care will be referred to a physician for consultation [66]. Standard of care for managing epilepsy during pregnancy will be followed, consistent with medications and treatments available in northern Nigeria.
Medication dosing guidelines will be according to standard of care and will be followed by the CHEW as outlined in the epilepsy training, and by the physician epilepsy specialist who prescribes the medication during the initial diagnostic visit [63]. Children who continue to have seizures, despite appropriate doses of the first AED, will be referred by the CHEW to the physician epilepsy specialist for further evaluation (Fig. 3). Due to limited EEG availability, EEGs will only be performed on children for whom the diagnosis of epilepsy is not clear at the initial evaluation and/or children who continue to have seizures while on anti-epileptic medication(s), and will be ordered by the physician epilepsy specialist upon referral by the CHEW (Fig. 3).
Following the initial diagnostic evaluation by the physician epilepsy specialist, routine clinic visits with epilepsy-trained CHEWs will occur at months 1 and 2, every three months, and as needed. At each clinic visit, seizure frequency and the presence or absence of AED adverse events and any other potential adverse events will be noted in the RedCap CRFs for each child, with CRFs uploaded to the Vanderbilt coordinating center, with review by both the Vanderbilt-based data coordinating center and the physician epilepsy specialists/investigators at AKTH in Kano, Nigeria. Outcome measures from this feasibility study will include; (a) feasibility of randomization of PHCs in Kano, (b) feasibility of recruitment from clinic settings and community (e.g., door-to-door surveys), (c) estimating resources (including personnel) needed for a cluster randomized clinical trial of task-shifted care, (d) piloting the data collection for primary and secondary outcome measures, and (e) determining the acceptability of the task-shifted intervention.
2.6.4. Audit of diagnostic EEG services in kano
The PIs will meet at least twice per year in Kano, and weekly via web-based conferencing, to evaluate the effectiveness of the epilepsy diagnostic guidelines and the functioning of the task-shifted epilepsy teams. The PIs will also evaluate the impact that the task-shifted care teams using the epilepsy diagnostic paradigms have on EEG laboratory referrals. The BRIDGE team will also conduct a detailed audit of the EEG laboratory capacity, including the number of physicians able to read pediatric EEGs, the number of healthcare professionals able to operate EEG machines in Kano, and the number and functioning of EEG machines in Kano. A report regarding the EEG capacity in Kano relative to the current demand for EEG diagnostic services will be developed by the BRIDGE Epilepsy investigators, reviewed by the BRIDGE Advisory Panel, and a final report will be submitted to the Kano State Ministry of Health and the senior management at AKTH.
2.7. Mapping the childhood epilepsy treatment gap (aim 3)
Specific Aim 3 will have three components (Fig. 1): 1) community epilepsy awareness and community education, during which time information about epilepsy and the upcoming survey will be provided to communities who will participate in the survey; 2) validation of epilepsy screening and seizure-type classification tool; and 3) the door-to-door childhood epilepsy survey with collection of GIS data to map the childhood epilepsy treatment gap.
2.7.1. Door-to-door survey (aim 3)
The door-to-door survey to ascertain children (≥6 months, < 18 years) with epilepsy will utilize the childhood epilepsy screening and seizure type classification tool translated to Hausa by the Bayero University Center for Nigerian Languages, Translation, and Folklore, and administered by epilepsy-trained CHEWs. This tool has been validated in Zambia and Tanzania [55], and modified by the BRIDGE investigators to better ascertain focal-onset seizures, myoclonic seizures, infantile spasms, and nodding seizures for use in Kano (Fig. 2). As emphasized by linguists at the Center for Nigerian Languages, Translation, and Folklore the Hausa word typically used for the English term “epilepsy” literally refers only to convulsions that are associated with falls. Therefore, given the known under-ascertainment of non-convulsive epilepsy in prior door-to-door surveys and other epidemiology studies, and to avoid confusion among the Hausa people participating in the surveys, we are using the term “seizures” and referring to “seizures that benefit from treatment” (2 or more unprovoked afebrile seizures) instead of using the typical Hausa term for “epilepsy” in consent and assent documents. Additional questions designed to ascertain generalized absence seizures, atonic seizures, and myoclonic seizures, and infantile spasms have been added to the tool. The questionnaire will be uploaded into REDCap, and additional demographic data will be collected.
The door-to-door questionnaire will be administered by epilepsy-trained CHEWs with additional training in administering questionnaires, and using REDCap for survey tools and CRFs. These CHEWs will be supervised by two CHOs, who will also receive additional training in epilepsy and questionnaire administration, and study physicians with training in epilepsy. After informed consent, the questionnaire will be administered by the CHEW via an interview with the parent at the home (likely the mother). Data collection will be performed using tablet computers. GPS coordinates of the residences of the participants will be recorded using the built-in GPS on the tablet computers used by the CHEWs to administer the epilepsy diagnostic questionnaire, and when needed, with separate hand-held GPS devices.
2.7.2. Methods of selecting homes/households for the community survey
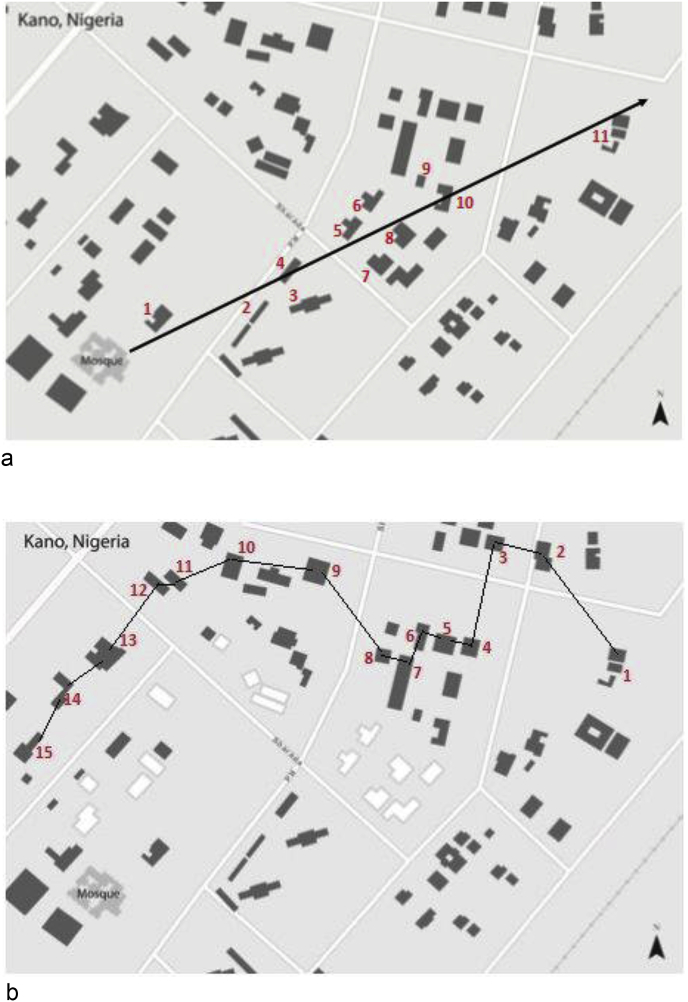
An estimated 2,418 households will be surveyed in communities served by the eight participating PHCs in the metropolitan Kano area. To select the households, the survey team will use the ‘random walk’ method previously validated in WHO community surveys and utilized in other surveys in Kano [[67], [68], [69], [70]]. The steps used in selecting households for the survey is outlined in Fig. 4. In each sampled house, a structured questionnaire will be administered to the parent/guardian (typically mother) of children in the eligible age group after obtaining parental informed consent and assent for older children. All eligible respondents found in a household who are parents/guardians of children will be interviewed. After completing interviews in the first house, the next house whose door is nearest to the one just completed will be approached for the survey (Fig. 4). This process will be completed until the houses in the referral regions of each PHC have been surveyed, and/or when the total number of households surveyed exceeds 2,418 and an adequate number of households have been surveyed to adequately validate and pilot the survey questionnaire.
Fig. 4.
Selecting Houses within communities for the childhood epilepsy door-to-door survey.
The epilepsy-trained community health extension workers (CHEWs) conducting the community survey in Kano, Nigeria, often without standardized street signs or addresses, will take the following steps, as documented in this map derived from satellite images of Kano (a) Start at a central location (e.g., a mosque as in Fig. 4a). determined by the survey team with local health workers. (b) Spin a pen or pencil in the air and let it to fall on the ground. Investigators will begin walking in the direction indicated by the point of the pen/pencil. (c) The number of houses between the starting point and the boundary of the community will be counted (In Fig. 4a example, there are 11 houses.). (d) A random number (between 1 and 11) will be selected. To select the random number, the numbers will be written on pieces of paper, the pieces of papers will be mixed together, then one will be pulled out of the pile. (In the example, the number 11 was randomly selected.) (3) The random walk will begin at the house that matched the random number (house no. 11 in Fig. 4b). After completing the study in the first selected house, the interviewer will walk to the next closest house. Whenever it seemed that two houses are the same distance away, a coin will be flipped to decide between the two.
2.7.3. Estimation and mapping of the childhood epilepsy treatment gap
Utilizing the Hausa version of the epilepsy screening and seizure type classification tool administered by epilepsy-trained CHEWs, children (≥6 months, < 18 years) who screen positive will undergo exams by CHEWs and physicians. Children who receive a physician's diagnosis of epilepsy and are determined to require treatment serve as the denominator; children who require medical treatment and who are not receiving treatment comprise the numerator for the calculation of the childhood epilepsy treatment gap.
2.8. Data collection, management, and analysis
Study data will be collected and managed using REDCap, hosted at Vanderbilt University Medical Center [62]. REDCap is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
2.8.1. Record retention and privacy/confidentiality
Study subject-level information will be collected as part this study. For all study subject-level data, only the program coordinator in Kano and the clinicians in Kano at each clinic site will have access to identifiable patient information that is also required for routine patient care. AKTH will store research data, including medical records and questionnaires, in locked cabinets, and all e-files will be password protected. This security of the data includes the mapping of the location of the children who are not receiving epilepsy care. These data will not have personal identifiers and will be separate from the data on children found to have epilepsy, and it should not be possible to identify an individual child by simply seeing a dot on a map from the region of Kano where the child resides. Raw coordinate data will be used only by necessary members of the study team, and published data mapping will be at the “ward” level.
No data will be released with any information that may directly or indirectly identify participants, their survey responses, or laboratory results to outside agencies. CHEWs will use portable tablet computers to collect data in the field; these computers will be data-encrypted and password-protected. Additional measures to enhance security for data housed at Vanderbilt Institute for Global Health (VIGH) will be utilized. Access to all data will be ID and password-protected, including data warehouse software and computers managing and analyzing survey data. Enhanced security measures exist for VIGH, including 24-hour building security guards, after-hours elevator and stair lockouts, and key pad locks on all doors leading into offices.
CRF data will contain personal identifying information for the duration of the BRIDGE project. After completion of the project, the electronic CRFs with personal identifying information will be transmitted to the patient's primary care patient record. The electronic data with only unique study numbers (and not names or other personal identifying information) will be used for identifying study subject records for data analysis at the completion of the BRIDGE project.
2.8.2. Power and sample size considerations for aim 3
A power analysis was performed to determine the urban and rural sample sizes needed to detect the difference between the urban childhood epilepsy treatment gap and the rural childhood epilepsy treatment gap in Kano. Two-sided tests were performed using the formula below, where n1 was rural sample size, n2 was urban sample size, α was the Type I error (significant level at 0.05), β was Type II error of 0.20 (power = 1–0.20 = 0.80), z1-α/2 was the critical value of the standard normal distribution at 1- α/2, z1- β was the critical value of the standard normal distribution at 1- β, p1 was the rural childhood epilepsy treatment gap, p2 was the urban childhood epilepsy treatment gap, and k was the ratio of n2 to n1.
We calculated a range of sample sizes needed using a range of prevalence of active childhood epilepsy of 5–7 per 1000 in urban areas, a range of prevalence of active childhood epilepsy of 7–15 per 1000 in rural areas of Kano, and a range of epilepsy treatment gaps of 45%–65% in urban areas and of 75%–90% in rural areas, based upon prior published prevalence and treatment gap estimates in sub-Saharan Africa [[19], [20], [21], [22],[41], [42], [43], [44],[71], [72], [73], [74], [75], [76], [77]]. We estimated an average of 5.5 children per household in Kano. Based upon these assumptions, we estimated a likely necessary sample of households for the survey at 1768, with the maximum estimated 2418 households required in the sample. Our 18 epilepsy-trained CHEWs, who will also be trained in the use of the door-to-door questionnaire, will administer the questionnaire to mothers at approximately 1768 households to 2418 households over a period of 12–18 months, or fewer than 15 households per CHEW per month, which is about three households per CHEW per week, assuming 48 working weeks per year.
2.8.3. Statistical considerations for aim 3
The childhood epilepsy treatment gap will be a dichotomous variable. We will use SatScan to identify clusters of children with treatment gaps using the Bernoulli model of purely spatial clusters. A ‘case’ will be a child with a treatment gap (child with epilepsy but without treatment), whereas a ‘control’ will be a child without such gap (child with epilepsy who at the time of ascertainment was receiving appropriate medical treatment). Appropriate medical treatment will be defined as treatment with an AED appropriate for the child's seizure type, regardless of whether the treatment has rendered the child seizure free at the time of the door-to-door survey. Separate locations of households are specified for each case and each control. We will vary the size and shape of the window that moves across Kano to identify these areas. The kml output file from the SatScan software will be overlaid with Google Earth to visually represent the clusters. The childhood epilepsy treatment gap within areas served by the PHCs, and the childhood epilepsy prevalence estimate using the door-to-door survey data will be determined.
2.8.4. Focus groups to assess patient and provider satisfaction (aim 2)
Four focus group discussions with patients and their family members will be conducted under the supervision of the Kano-based investigator to determine satisfaction with the epilepsy care team system. Similarly, four focus groups with CHOs, CHEWs, and of PHC leadership will be conducted near the end of the grant period to determine satisfaction with the epilepsy care team system. Each focus group will have about 8 participants. A consent form will be signed prior to the focus group sessions involving CHEWs, CHOs, and PHC Leadership.
For qualitative data analysis, audio files will be transcribed, coded and analyzed using Atlas Ti© software. The data will be analyzed using mixed methods: Framework Analysis will be used to identify responses to our questions about the drivers, core facilitators, and barriers to the CHEWs successfully providing epilepsy care for children in the community with sub-analysis of each category done using Grounded Theory [78,79]. Two code maps will be developed to categorize data: (1) to understand the social, structural, and informational drivers, facilitators, barriers to CHEWs providing epilepsy care, and (2) to understand perceptions patients and family members have of CHEWs involvement in improving uptake of epilepsy care services. The data will be coded by our qualitative data analyst; analysis will be facilitated by NVivo [80,81].
2.9. Study outcomes and dissemination of results
We will track process outcomes related to Aim 1 activities, including the numbers of CHEWs who successfully complete the epilepsy education program and their performance in the program (as denoted by their scores on module quizzes). Outcomes for Specific Aim 2 will include CHEW and CHO adherence to the protocol, the level of accuracy and consistency in documentation of clinical activities by CHEWs and CHOs, and acceptance of the task-shifted epilepsy care by the physicians, CHEWs, CHOs, clinic administrators, and patient families. Aim 3 will be monitored with selected metrics, such as the number of completed meetings with key community leaders and stakeholders, in addition to quantitative data obtained from door-to-door surveys (e.g., number of children who screen positive for epilepsy, confirmed by epilepsy specialists and linked to care).
2.9.1. Plans for dissemination of study results
All publications and reports as products of this research will be produced with leadership and/or involvement of all key personnel. Because the results of this research may have an impact on the Nigerian Federal Ministry of Health's plan for task-shifted care for mental health, and for the local healthcare officials in Kano, the final report of this study will be provided to local healthcare officials in Kano, as well as the Nigerian Federal Ministry of Health. The study PIs will meet with Nigerian Federal Ministry of Health officials, state and local government healthcare authorities in Kano, who have been engaged with the BRIDGE project from the beginning of the planning, to discuss the results of the research. The results of this research will be submitted for publication in the peer-reviewed literature. We anticipate several peer-reviewed publications on topics including, but not limited to the following: (a) an epilepsy education program for CHEWs and CHOs; (b) validation of a Hausa version of the childhood epilepsy and seizure type classification tool; (c) development of diagnostic paradigms supplementing the WHO guidelines for epilepsy diagnosis and management in LMICs of Africa; (d) mapping of the childhood epilepsy treatment gap in Kano; and, (e) developing task-shifted epilepsy care teams to reduce the childhood epilepsy treatment gap.
The materials used in the training of the CHEWs and the data regarding the outcomes of the CHEW training will be published on a BRIDGE study website, and made available free to the public. In addition to the publication of articles in the peer-reviewed literature, PHCs and communities participating in this research will also receive presentations of the results of the research via community educational sessions in PHCs and other appropriate venues (e.g., radio). A final report submitted to NIH will also be provided to the AKTH Research Ethics Committee, the Kano State Ministry of Health, and the Vanderbilt University Medical Center IRB.
3. Discussion
The long-term goal of the BRIDGE project is to bring basic epilepsy care to the majority of children with epilepsy living in LMICs who remain untreated [1,2,[19], [20], [21]]. Our approach to closing the childhood epilepsy treatment gap is based upon the framework proposed by the WHO, which is thus far unimplemented. We are not pursuing other alternative methods unless this task-shifted epilepsy care framework is demonstrated ineffective or not feasible.
Our overall approach to reducing the childhood epilepsy treatment gap in sub-Saharan Africa is characterized by a few strengths: (1) Epilepsy training and education designed specifically for CHEWs to facilitate task-shifted epilepsy care in regions with shortages of physicians; (2) epilepsy education, screening, diagnosis and seizure type classification tools in the local language (Hausa), and developed for use in resource-limited settings with few physicians and limited EEG access; (3) integrated into the primary health care system, in partnership with the local ministry of health, permitting randomization of primary healthcare centers and enhancing generalizability of results within Hausa-speaking west Africa (4) a mixture of qualitative methods (e.g., focus groups); and novel quantitative methods (e.g., spatial epidemiology) to allow mapping of the childhood epilepsy treatment gap, and implementation of task-shifted childhood epilepsy care; and, (5) a strategic location in the largest population of children and families speaking the most commonly spoken local language in west Africa (Hausa), potentially facilitating generalizability to a much larger population.
3.1. Epilepsy training and education designed specifically for CHEWs
Our approach to bridging the childhood epilepsy treatment gap, consistent with WHO recommendations [41], is dependent upon the use of CHEWs in clinical roles that until recently have been deemed only suitable for physicians. Over the past fifteen years in both Europe and the US, task-shifting to nurse practitioners for initial epilepsy care has become commonplace [82,83], and has been integrated into national models of epilepsy care [84]. Although task shifting to CHEWs for HIV care has been demonstrated effective in HIV/AIDS care in sub-Saharan Africa [36,85], and preliminary evidence suggests that task-shifted depression care may be effective [86], the diagnosis and management of the many types of childhood epilepsy may be a more challenging role for CHEWs, who have significantly less educational background in neurology than do nurse practitioners. Because half of the CHEWs in our epilepsy education program will be randomly selected among CHEWs practicing in primary care settings, and half will be selected among new graduates of CHEW training programs, our group of CHEWs may be representative of the larger group of CHEWs working in northern Nigeria primary care. Even if our epilepsy education program for CHEWs is highly successful in these pre-clinical trial studies, we may not know whether our approach to training CHEWs can be taken to scale until a much larger group of CHEWs are trained, and likely until after the completion of a cluster randomized clinical trial of task-shifted childhood epilepsy care, which will require training of a much larger group of CHEWs.
3.2. Emphasis on epilepsy education and diagnostic tools in a common local language (Hausa)
In rich countries the clinical diagnosis of epilepsy is traditionally accomplished by physicians combining the art of history taking with the science of EEGs, with EEGs typically serve a supporting role in epilepsy diagnosis [87,88]. EEGs, although prioritized by many clinical neuroscience professionals when building new epilepsy care programs in rich countries, under optimal conditions are often normal or non-diagnostic between events among people with epilepsy; the cost of acquiring, maintaining and utilizing standard EEG technology is prohibitive in many LMICs [89]. The well-prepared clinician listening in the patient's native language, and sensitive to the patient's culture, is the most effective diagnostic tool for epilepsy [87,88,90]; therefore, we have prioritized epilepsy education and diagnostic tools [55] in the local language that facilitate the clinicians' ability to diagnose epilepsy from a history of events, rather than expensive, and difficult to maintain EEG technology [19].
Our emphasis on the diagnosis of epilepsy using tools to enhance history-taking by CHEWs should not suggest that there is no role for strategic use of EEG, such as when an epilepsy diagnosis is difficult, or in doubt (as when AEDs are ineffective). Even if EEG is utilized only when it will alter clinical management, as is the case in our proposed task-shifted system of childhood epilepsy care (Fig. 3), increasing EEG access will also be essential to eliminate the childhood epilepsy treatment gap. For example, Kano, Nigeria probably has the greatest access to routine EEG of any area in Hausa-speaking west Africa - about 2–3 functioning EEG machines, and about 4–5 physicians trained to interpret EEGs, for a population of about 12 million people, approximately half of whom are children, with a childhood epilepsy treatment gap of 67%–90%. Even by very conservative estimates, we know that a few thousand additional children per year would benefit from EEG in Kano state alone - a number that far exceeds the capacity of the current system of care. Methods for scaling-up EEG services, and how to staff and pay for these services, are not addressed by our initial research on the childhood epilepsy treatment gap, and will need to be emphasized in future investigations.
3.3. Task-shifted childhood epilepsy care integrated into the primary health care system
Our integration of task-shifted childhood epilepsy care into the local primary healthcare system is consistent with the WHO [41] and with the Nigerian Federal Ministry of Health recommendations [40] for closing the epilepsy treatment gap. We have included the Kano State Ministry of Health into our planning, and have obtained Kano State Ministry of Health's approval to randomly select primary healthcare centers for our research, increasing the representativeness of our sample, and minimizing the odds of bias from both known and unknown confounding variables [91]. Furthermore, our inclusion of the Kano State Ministry of Health in our early pre-clinical trials studies increases the odds of successful completion of our studies, and eventual scaling up task-shifted epilepsy care if it is proven efficacious and cost-effective.
We have not yet included detailed cost-effectiveness studies and policy studies as part of our research on closing the childhood epilepsy treatment gap. Eventually robust cost-effectiveness data will be essential for a large-scale implementation of task-shifted epilepsy care, as ministries of health have multiple competing health agendas and limited resources. Kano, the largest and probably one of the more prosperous states in Hausa-speaking west Africa, may be better capable of integrating task-shifted epilepsy care into its primary healthcare system than some less resourced areas where there are fewer CHEWs available, and where local budgets will not be able to expand the number of CHEWs, or even make them available for epilepsy training. Long-term, it may be necessary to document that the cost-effectiveness of task-shifted epilepsy care is substantial, and is actually a net positive for the health system - not unthinkable given the very high rate of morbidity from untreated epilepsy [8].
3.4. Mixed qualitative and novel quantitative methods to inform future implementation
If task-shifted epilepsy care is proven efficacious in cluster randomized clinical trials, then implementation will be a complex undertaking. We are using qualitative methods, such as qualitative analysis of focus groups of CHEWs, parents/guardians of enrolled children, and of other health professionals to identify potential barriers to implementation of the components task-shifted epilepsy care. We are also developing methods for mapping the epilepsy treatment gap, or identifying the areas of Kano with the highest epilepsy treatment gap to help target scarce resources. Our initial studies will not likely have adequate sample size to provide a comprehensive map of the epilepsy treatment gap in Kano state, but we will be able to estimate the distribution of the treatment gap in Kano, and refine the methods for a larger more comprehensive study. Likewise, our initial focus group data will probably identify areas of future study that will be essential to address potential barriers to identifying children with untreated epilepsy and to task-shifted epilepsy care.
3.5. Located within the largest population in Hausa-speaking africa
Addressing childhood epilepsy treatment gap in resource-limited areas of Africa requires diagnostic and management tools that can be adapted for use by CHEWs, and also understood by the local people. We have chosen Kano for our research because of the long-standing Vanderbilt-AKTH collaborative relationships, and Kano has the largest population and the highest population density of anywhere in Hausa-speaking Africa. If our studies of task-shifted epilepsy care in Kano demonstrate efficacy, then we will be able to pursue extending the methods and materials to other Hausa speaking areas of west Africa. Further, the methods for adapting our epilepsy care system to the Hausa language and culture should be useful in developing a toolkit for adapting task-shifted childhood epilepsy care to other local languages and cultures in sub-Saharan Africa. Kano is probably one of the most prosperous of areas in Hausa-speaking west Africa, and it is possible that strategies that are successful in Kano may be more difficult to implement in a somewhat poorer and more resource limited area of Africa.
4. Conclusion
Developing and piloting methods for task-shifted childhood epilepsy care in Africa's LMICs are required to prepare for a definitive cluster randomized clinical trial of task-shifted childhood epilepsy care. These studies represent only the initial steps necessary to bring epilepsy care to the approximately fifty percent of the world's children with epilepsy who do not receive treatment. Scaled-up epilepsy training programs, cost-effective strategies for AED distribution, policy interventions, and technology for improving epilepsy diagnosis, patient/family self-care, and physician-supervision of CHEWs will all be required to eliminate the childhood epilepsy treatment gap.
Acknowledgment
This study is made possible by support from the National Institutes of Health/Fogarty International Center award number 1R21TW010899, and the Amos Christie Chair in Global Health. The findings and conclusions are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100362.
Contributor Information
Edwin Trevathan, Email: edwin.trevathan@vanderbilt.edu, edwin.trevathan@vumc.org.
the BRIDGE Advisory Panel:
Gretchen Birbeck, Paul Carney, Tracy Glauser, Angelina Kakooza, Phillip Pearl, Angela Wabulya, James Wheless, and Jo Wilmshurst
Financial support
This study was made possible by support from the National Institutes of Health/Fogarty International Center award number,1R21TW010899and the Amos Christie Chair in Global Health. The findings and conclusions are those of the authors and do not necessarily represent the official position of the National Institutes of Health.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.de Boer H.M. ILAE/IBE/WHO global campaign against epilepsy: a partnership that works. Curr. Opin. Neurol. 2013;26(2):219–225. doi: 10.1097/WCO.0b013e32835f2037. [DOI] [PubMed] [Google Scholar]
- 2.de Boer H.M., Mula M., Sander J.W. The global burden and stigma of epilepsy. Epilepsy Behav. 2008;12(4):540–546. doi: 10.1016/j.yebeh.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 3.CIA . 2016. The World Factbook - Africa - Nigeria.https://www.cia.gov/library/publications/the-world-factbook/geos/ni.html 2016 [cited 2016 December 15, 2016]; Available from: [Google Scholar]
- 4.Canning D., Sangeeta R., Yazbeck A.S., editors. Africa Development Forum. World Bank; Washington, DC: 2015. Africa's demographic transition: dividend or disaster? p. 182. [Google Scholar]
- 5.Keezer M.R., Sisodiya S.M., Sander J.W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 6.Carpio A. Mortality of epilepsy in developing countries. Epilepsia. 2005;46(Suppl 11):28–32. doi: 10.1111/j.1528-1167.2005.00404.x. [DOI] [PubMed] [Google Scholar]
- 7.Lagunju I.A., Oyinlade A.O., Babatunde O.D. Seizure-related injuries in children and adolescents with epilepsy. Epilepsy Behav. 2016;54:131–134. doi: 10.1016/j.yebeh.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Charlson F.J. Excess mortality from mental, neurological, and substance use disorders in the global burden of disease study. In: Patel V., editor. third ed. vol 4. 2010. (Mental, Neurological, and Substance Use Disorders: Disease Control Priorities). 2016: Washington (DC) [Google Scholar]
- 9.Diop A.G. Epilepsy and mortality in Africa: a review of the literature. Epilepsia. 2005;46(Suppl 11):33–35. doi: 10.1111/j.1528-1167.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilmshurst J.M., Birbeck G.L., Newton C.R. Epilepsy is ubiquitous, but more devastating in the poorer regions of the world... or is it? Epilepsia. 2014;55(9):1322–1325. doi: 10.1111/epi.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton C.R., Garcia H.H. Epilepsy in poor regions of the world. Lancet. 2012;380(9848):1193–1201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- 12.Levira F. Epilepsia; 2016. Premature Mortality of Epilepsy in Low- and Middle-Income Countries: A Systematic Review from the Mortality Task Force of the International League against Epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner R.G. Incidence, remission and mortality of convulsive epilepsy in rural northeast South Africa. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibinda F. Burden of epilepsy in rural Kenya measured in disability-adjusted life years. Epilepsia. 2014;55(10):1626–1633. doi: 10.1111/epi.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boschini L.P. The role of seizure disorders in burn injury and outcome in Sub-Saharan Africa. J. Burn Care Res. 2014;35(6):e406–e412. doi: 10.1097/BCR.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chasimpha S. Patterns and risk factors for deaths from external causes in rural Malawi over 10 years: a prospective population-based study. BMC Public Health. 2015;15:1036. doi: 10.1186/s12889-015-2323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autry A.R. Increased risk of death among children with Lennox-Gastaut syndrome and infantile spasms. J. Child Neurol. 2010;25(4):441–447. doi: 10.1177/0883073809348355. [DOI] [PubMed] [Google Scholar]
- 18.Baskind R., Birbeck G.L. Epilepsy-associated stigma in sub-Saharan Africa: the social landscape of a disease. Epilepsy Behav. 2005;7(1):68–73. doi: 10.1016/j.yebeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Chin J.H. Epilepsy treatment in sub-Saharan Africa: closing the gap. Afr. Health Sci. 2012;12(2):186–192. doi: 10.4314/ahs.v12i2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilmshurst J.M. Children with epilepsy in Africa: recommendations from the international child neurology association/african child neurology association workshop. J. Child Neurol. 2013;28(5):633–644. doi: 10.1177/0883073813482974. [DOI] [PubMed] [Google Scholar]
- 21.Nwani P.O. Epilepsy treatment gap: prevalence and associated factors in Southeast Nigeria. Acta Neurol. Scand. 2013;128(2):83–90. doi: 10.1111/ane.12096. [DOI] [PubMed] [Google Scholar]
- 22.Meyer A.C. Global disparities in the epilepsy treatment gap: a systematic review. Bull. World Health Organ. 2010;88(4):260–266. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndoye N.F. Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal an ILAE/IBE/WHO study. Seizure. 2005;14(2):106–111. doi: 10.1016/j.seizure.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Andriantseheno L.M., Andrianasy T.E., Andriambao D.S. [Psychiatric disorders in Madagascar: clinical study of 376 cases registered in Mahajanga] Bull. Soc. Pathol. Exot. 2004;97(2):122–126. [PubMed] [Google Scholar]
- 25.Ngugi A.K. Incidence of convulsive epilepsy in a rural area in Kenya. Epilepsia. 2013;54(8):1352–1359. doi: 10.1111/epi.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schootman M. Emerging technologies to measure neighborhood conditions in public health: implications for interventions and next steps. Int. J. Health Geogr. 2016;15(1):20. doi: 10.1186/s12942-016-0050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schootman M. Geographical spatial analysis. In: Boslaugh S., editor. Encyclopedia of Epidemiology. SAGE Publications; Thousand Oaks, CA: 2008. pp. 433–435. [Google Scholar]
- 28.Schootman M. Geographic clustering of adequate diagnostic follow-up after abnormal screening results for breast cancer among low-income women in Missouri. Ann. Epidemiol. 2007;17(9):704–712. doi: 10.1016/j.annepidem.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Schootman M. Positional accuracy and geographic bias of four methods of geocoding in epidemiologic research. Ann. Epidemiol. 2007;17(6):464–470. doi: 10.1016/j.annepidem.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Tsolekile L.P., Abrahams-Gessel S., Puoane T. Healthcare professional shortage and task-shifting to prevent cardiovascular disease: implications for low- and middle-income countries. Curr. Cardiol. Rep. 2015;17(12):115. doi: 10.1007/s11886-015-0672-y. [DOI] [PubMed] [Google Scholar]
- 31.Abdulmalik J. Country contextualization of the mental health gap action programme intervention guide: a case study from Nigeria. PLoS Med. 2013;10(8) doi: 10.1371/journal.pmed.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birbeck G.L. The health care workforce for epilepsy in resource-poor settings: what will work? What is realistic? Epilepsia. 2008;49(9):1642–1643. doi: 10.1111/j.1528-1167.2008.01580_3.x. [DOI] [PubMed] [Google Scholar]
- 33.Petersen I. Lessons from case studies of integrating mental health into primary health care in South Africa and Uganda. Int. J. Ment. Health Syst. 2011;5:8. doi: 10.1186/1752-4458-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman R., Gill G., Wilkinson D. Noncommunicable disease management in resource-poor settings: a primary care model from rural South Africa. Bull. World Health Organ. 1998;76(6):633–640. [PMC free article] [PubMed] [Google Scholar]
- 35.Odejide A.O. Integrating mental health into primary health care in Nigeria: management of depression in a local government (district) area as a paradigm. Sishin shinkeigaku Zasshi. 2002;104(10):802–809. [PubMed] [Google Scholar]
- 36.Aliyu M.H. Integrated prevention of mother-to-child HIV transmission services, antiretroviral therapy initiation, and maternal and infant retention in care in rural north-central Nigeria: a cluster-randomised controlled trial. Lancet HIV. 2016;3(5):e202–e211. doi: 10.1016/S2352-3018(16)00018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kengne A.P. Nurse-led care for epilepsy at primary level in a rural health district in Cameroon. Epilepsia. 2008;49(9):1639–1642. doi: 10.1111/j.1528-1167.2008.01580_2.x. [DOI] [PubMed] [Google Scholar]
- 38.Some D. Task shifting the management of non-communicable diseases to nurses in kibera, Kenya: does it work? PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0145634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleeman N., Bradley P.M., Lindsay B. Care delivery and self management strategies for children with epilepsy. Cochrane Database Syst. Rev. 2015;(12):CD006245. doi: 10.1002/14651858.CD006245.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Federal Ministry of Health . Federal Ministry of Health; Abuja, Nigeria: 2015. MhGAP Intervention Guide for Nigeria: for Management of Mental, Neurological, and Substance Abuse Disoders in Non-specialized Health Settings. [Google Scholar]
- 41.WHO . Mental Health Gap Action Programme, Ed. WHO. 2.0. WHO; Geneva, Switzerland: 2016. mhGAP Intervention Guide for mental, neurological, and substance abuse disorders in non-specialized health settings. [PubMed] [Google Scholar]
- 42.WHO . World Health Organization; Geneva, Switzerland: 2014. Mental Health Atlas 2014. [Google Scholar]
- 43.WHO . WHO; Geneva, Switzerland: 2013. Mental Health Action Plan 2013-2020; p. 50. [Google Scholar]
- 44.WHO, Mental Health . World Health Organization; Geneva, Switzerland: 2008. Gap Action Programme: Scaling up for Mental, Neurological and Substance Use Disorders. [PubMed] [Google Scholar]
- 45.Boehmer A. Patient and provider satisfaction with a comprehensive strategy to improve prevention of mother-to-child HIV transmission services in rural Nigeria. J. Acquir. Immune Defic. Syndr. 2016;72(Suppl 2):S117–S123. doi: 10.1097/QAI.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Debaun M.R. Primary stroke prevention in children with sickle cell anemia living in Africa: the false choice between patient-oriented research and humanitarian service. Trans. Am. Clin. Climatol. Assoc. 2016;127:17–33. [PMC free article] [PubMed] [Google Scholar]
- 47.Boafor T.K. Pregnancy outcomes in women with sickle-cell disease in low and high income countries: a systematic review and meta-analysis. BJOG. 2016;123(5):691–698. doi: 10.1111/1471-0528.13786. [DOI] [PubMed] [Google Scholar]
- 48.Musa B.M. The global burden of pulmonary hypertension in sickle cell disease: a systematic review and meta-analysis. Ann. Hematol. 2016;95(11):1757–1764. doi: 10.1007/s00277-016-2693-z. [DOI] [PubMed] [Google Scholar]
- 49.Galadanci N.A. Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): challenges of conducting a feasibility trial. Pediatr. Blood Canc. 2015;62(3):395–401. doi: 10.1002/pbc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galadanci N.A. Wheezing is common in children with sickle cell disease when compared with controls. J. Pediatr. Hematol. Oncol. 2015;37(1):16–19. doi: 10.1097/MPH.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iliyasu Z. Prevalence and risk factors for domestic violence among pregnant women in northern Nigeria. J. Interpers Violence. 2013;28(4):868–883. doi: 10.1177/0886260512455872. [DOI] [PubMed] [Google Scholar]
- 52.Iliyasu Z. Adherence to intermittent preventive treatment for malaria in pregnancy in urban Kano, northern Nigeria. Pathog. Glob. Health. 2012;106(6):323–329. doi: 10.1179/2047773212Y.0000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danladi B. Department of African Languages and Cultures, Ahmadu Bello University (ABU) Zaria Kaduna State; Nigeria: 2016. Hausa Speakers in Nigeria Now 120m– Communiqué. [Google Scholar]
- 54.Eugene G. 2016 September 26, 2016 October 15. Top 10 Most Widely Spoken Languages In Africa. I Think, Therefore I Am; p. 2018.https://geneeugene.wordpress.com/2016/09/26/top-10-most-widely-spoken-languages-in-african/ Available from. [Google Scholar]
- 55.Patel A.A. A pediatric epilepsy diagnostic tool for use in resource-limited settings: a pilot study. Epilepsy Behav. 2016;59:57–61. doi: 10.1016/j.yebeh.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Camfield C.S., Striano P., Camfield P.R. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S15–S17. doi: 10.1016/j.yebeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 57.Sejvar J.J. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol. 2013;12(2):166–174. doi: 10.1016/S1474-4422(12)70321-6. [DOI] [PubMed] [Google Scholar]
- 58.Trevathan E. Infantile spasms and Lennox-Gastaut syndrome. J. Child Neurol. 2002;17(Suppl 2):2S9–2S22. doi: 10.1177/08830738020170021201. [DOI] [PubMed] [Google Scholar]
- 59.Trevathan E., Murphy C.C., Yeargin-Allsopp M. Prevalence and descriptive epidemiology of Lennox-Gastaut syndrome among Atlanta children. Epilepsia. 1997;38(12):1283–1288. doi: 10.1111/j.1528-1157.1997.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 60.Wong M., Trevathan E. Infantile spasms. Pediatr. Neurol. 2001;24(2):89–98. doi: 10.1016/s0887-8994(00)00238-1. [DOI] [PubMed] [Google Scholar]
- 61.Trevathan E., Murphy C.C., Yeargin-Allsopp M. The descriptive epidemiology of infantile spasms among Atlanta children. Epilepsia. 1999;40(6):748–751. doi: 10.1111/j.1528-1157.1999.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 62.Harris P.A. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerrini R. Safety and tolerability of antiepileptic drug treatment in children with epilepsy. Drug Saf. 2012;35(7):519–533. doi: 10.2165/11630700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Guerrini R., Belmonte A., Genton P. Antiepileptic drug-induced worsening of seizures in children. Epilepsia. 1998;39(Suppl 3):S2–S10. doi: 10.1111/j.1528-1157.1998.tb05118.x. [DOI] [PubMed] [Google Scholar]
- 65.Glauser T. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- 66.Pennell P.B. Use of antiepileptic drugs during pregnancy: evolving concepts. Neurotherapeutics. 2016;13(4):811–820. doi: 10.1007/s13311-016-0464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett S. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat. Q. 1991;44(3):98–106. [PubMed] [Google Scholar]
- 68.Milligan P., Njie A., Bennett S. Comparison of two cluster sampling methods for health surveys in developing countries. Int. J. Epidemiol. 2004;33(3):469–476. doi: 10.1093/ije/dyh096. [DOI] [PubMed] [Google Scholar]
- 69.WHO, World Health Organization . World Health Organization; Geneva, Switzerland: 2015. Vaccination Coverage Cluster Surveys: Reference Manual; p. 259. [Google Scholar]
- 70.WHO . World Health Organization, Regional Office for the Eastern Mediterranean; Alexandria, Egypt: 1995. Field Guide on Rapid Nutritional Assessment in Emergencies. [Google Scholar]
- 71.Wilmshurst J.M., Kakooza-Mwesige A., Newton C.R. The challenges of managing children with epilepsy in Africa. Semin. Pediatr. Neurol. 2014;21(1):36–41. doi: 10.1016/j.spen.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koba Bora B. Living with epilepsy in Lubumbashi (Democratic Republic of Congo): epidemiology, risk factors and treatment gap. Pan. Afr. Med. J. 2015;21:303. doi: 10.11604/pamj.2015.21.303.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kessler R.C. Lifetime prevalence and age-of-onset distributions of mental disorders in the world health organization's world mental health survey initiative. World Psychiatr. 2007;6(3):168–176. [PMC free article] [PubMed] [Google Scholar]
- 74.Carter J.A. The reasons for the epilepsy treatment gap in Kilifi, Kenya: using formative research to identify interventions to improve adherence to antiepileptic drugs. Epilepsy Behav. 2012;25(4):614–621. doi: 10.1016/j.yebeh.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mbuba C.K. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol. 2012;11(8):688–696. doi: 10.1016/S1474-4422(12)70155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mbuba C.K. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49(9):1491–1503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nigeria W.H.O. 2014. Mental Health Atlas Country Profile.http://www.who.int/mental_health/evidence/atlas/profiles-2014/nga.pdf?ua=1 2015 2015; Available from: [Google Scholar]
- 78.Mays N., Pope C. Qualitative research in health care. Asses. Qual. Qual. Res. BMJ. 2000;320(7226):50–52. doi: 10.1136/bmj.320.7226.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pope C., Ziebland S., Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320(7227):114–116. doi: 10.1136/bmj.320.7227.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bazeley P., Jackson K. SAGE Publications; Los Angeles, CA: 2013. Qualitative Data Analysis With NVivo. [Google Scholar]
- 81.Hutchison A.J., Johnston L.H., Breckon J.D. Using QSR-NVivo to facilitate the development of a grounded theory project: an account of a worked example. Int. J. Soc. Res. Methodol. 2010;13(4):283–302. [Google Scholar]
- 82.Hill C.E. Improved availability and quality of care with epilepsy nurse practitioners. Neurol. Clin. Pract. 2017;7(2):109–117. doi: 10.1212/CPJ.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel A.D. Reduction of emergency department visits using an urgent clinic for children with established epilepsy. Neurol. Clin. Pract. 2016;6(6):480–486. doi: 10.1212/CPJ.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Clinical Programme for Epilepsy . National Clinical and Integrated Care Programmes; Dublin, Ireland: 2016. Model of Care: Epilepsy; p. 72. [Google Scholar]
- 85.Seidman G., Atun R. Does task shifting yield cost savings and improve efficiency for health systems? A systematic review of evidence from low-income and middle-income countries. Hum. Resour. Health. 2017;15(1):29. doi: 10.1186/s12960-017-0200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gureje O. Integrating mental health into primary care in Nigeria: report of a demonstration project using the mental health gap action programme intervention guide. BMC Health Serv. Res. 2015;15:242. doi: 10.1186/s12913-015-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trevathan E. The diagnosis of epilepsy and the art of listening. Neurology. 2003;61(12):E13–E14. doi: 10.1212/wnl.61.12.e13. [DOI] [PubMed] [Google Scholar]
- 88.Pillai J., Sperling M.R. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47(Suppl 1):14–22. doi: 10.1111/j.1528-1167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 89.WHO. Role of EEG in the Management of Convulsive Epilepsy. mhGAP. 2012. http://www.who.int/mental_health/mhgap/evidence/epilepsy/q5/en/ [cited 2016 December 15, 2016]; Available from: [Google Scholar]
- 90.Smith S.J. EEG in the diagnosis, classification, and management of patients with epilepsy. J. Neurol. Neurosurg. Psychiatry. 2005;76(Suppl 2):ii2–7. doi: 10.1136/jnnp.2005.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Campbell M.K. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328(7441):702–708. doi: 10.1136/bmj.328.7441.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.