Abstract
Bone resorption in the joints is the characteristic finding in patients with rheumatoid arthritis (RA). Osteoclast-like cells are present in the synovial tissues and invade the bone of patients with RA. The characteristics of these cells are not completely known. In the work reported here, we generated these cells from peripheral-blood monocytes from healthy individuals. The monocytes were co-cultured with nurse-like cells from synovial tissues of patients with RA (RA-NLCs). Within 5 weeks of culture, the monocytes were activated and differentiated into mononuclear cells positive for CD14 and tartrate-resistant acid phosphatase (TRAP). These mononuclear cells then differentiated into multinucleated giant bone-resorbing cells after stimulation with IL-3, IL-5, IL-7, and/or granulocyte-macrophage-colony-stimulating factor. TRAP-positive cells with similar characteristics were found in synovial fluid from patients with RA. These results indicate that multinucleated giant bone-resorbing cells are generated from monocytes in two steps: first, RA-NLCs induce monocytes to differentiate into TRAP-positive mononuclear cells, which are then induced by cytokines to differentiate into multinucleated giant bone-resorbing cells.
Keywords: monocytes, nurse cells, osteoclasts, rheumatoid arthritis, stromal cells
Our laboratory has established that nurse-like cells (NLCs) are present in the synovial tissues and bone marrow of patients with rheumatoid arthritis (RA) [1,2,3]. Such cells, which were first discovered in thymus, play an important role in thymocyte maturation and differentiation [4,5,6]. In vitro, they form unique complexes with thymocytes, which initially adhere to them and then crawl beneath them [7,8,9]. This phenomenon, which is unique to NLCs at various tissue sites, has been called 'pseudoemperipolesis'. NLCs from RA synovial tissue (RA-NLCs) promote survival of B cells [2,3] and maintain the growth of myeloid cells of patients with RA [1], suggesting that they contribute profoundly to pathogenesis in RA.
Multinucleated cells in synovial tissues have been reported to invade the bone of patients with RA [10]. The cells' expression of tartrate-resistant acid phosphatase (TRAP) and calcitonin receptor suggested that they are osteoclasts [11,12]. Although the presence of osteoclast-like cells in rheumatoid synovium is well understood, the mechanism by which they differentiate is not. In order to examine the effect of RA-NLCs on monocyte functions, we co-cultured peripheral-blood monocytes with RA-NLCs and looked for morphological and functional alterations of CD14- and TRAP-positive cells. We also found such cells in synovial fluid from patients with RA. These cells differentiated into multinucleated giant bone-resorbing cells in the presence of IL-3, IL-5, IL-7, and/or granulocyte/-macrophage-colonystimulating factor (GM-CSF). In this way we defined the process by which bone-resorbing cells are generated from monocytic cells.
Materials and methods
Isolation of NLCs from RA synovial tissues
RA-NLCs were established from RA synovial tissues as previously described [1]. Briefly, synovial tissues were obtained from knee joints of five patients with RA who fulfilled American College of Rheumatology criteria for RA [13], after informed consent had been obtained. The cells were cultured in DMEM (Dulbecco's modified Eagle's medium [DMEM; Gibco BRL, Gaithersburg, MD, USA] supplemented with 10% fetal calf serum [FCS; Hyclone, Logan, UT, USA], 100 units/ml of penicillin [Gibco BRL], and 100 μg/ml of streptomycin [Gibco BRL] at 37ºC in 7.5% CO2. RA-NLCs were identified by their ability to support pseudoemperipolesis, seen in vitro in the migration of a T-cell lymphoma line, MOLT-17, beneath the NLCs, as previously described [3].
Isolation of mononuclear cells from RA synovial fluid
Synovial fluid was obtained from patients with RA who fulfilled the American College of Rheumatology criteria for RA [13]. The infiltrating cells were collected from the fluid by centrifugation at 1900 g and were cultured in supplemented DMEM. After 3 to 5 weeks of culture, most of the lymphocytes and granulocytes disappeared and monocyte-like cells became dominant. CD14-positive monocyte-like cells were purified from this population with a magnetic-activated cell sorter (MACS; Myltenyi Biotec GmbH, Germany) using anti-CD14 antibody conjugated to magnetic beads in accordance with the manufacturer's instructions. The purity of CD14-positive cells was analyzed using a fluorescence-activated cell sorter (FACScan™; see Supplementary material).
Isolation and culture of monocytes from peripheral blood
Peripheral-blood monocytes were collected as plastic-adherent cells, as described previously [14]. Mononuclear cells were isolated from heparinized peripheral blood from five healthy volunteers [15]. Over 97% of the adherent cells were determined to be monocytes by morphology and CD14 expression.
Monocytes (1 x 106) were co-cultured with RA-NLCs. After 3 to 5 weeks, TRAP-positive mononuclear cells with abundant cytoplasm became dominant. They were collected by gently washing the culture with warm supplemented DMEM and their purity was confirmed cytochemically.
Formation of multinucleated giant cells by TRAP-positive mononuclear cells
The CD14-positive and TRAP-positive mononuclear cells from the synovial fluid of patients with RA were examined for expression of surface antigen and for phagocytic activity and were stimulated with various cytokines (see Supplementary material).
TRAP-positive mononuclear cells (5 x 104) were cultured in supplemented DMEM in the presence or absence of various cytokines or in conditioned medium ([15]; and see Supplementary material) for 96–120 h. In the presence of receptor activator nuclear factor κB ligand (RANKL), cultures were maintained for 14 days. At the end of the culture period, May–Grunwald–Giemsa (Wako Pure Chemical Co., Osaka, Japan) and TRAP staining (TRAP-staining kit; Sigma, St Louis, MO, USA) were conducted. The frequency of osteoclasts was evaluated from the fusion index, as previously described [16]. More than 1000 nuclei within TRAP-positive multinucleated cells (>4 nuclei/cell) were counted. The fusion index (%) was calculated according to the formula:
(total no. of nuclei within multinucleated cells' 100)/total no. of nuclei counted
where 'multinucleated cells' are cells with >4 nuclei.
Examination of bone resorption
TRAP-positive mononuclear cells (5 x 104) were stimulated with various cytokines on a dentin slice for 7 days. In order to examine resorption areas with a scanning electron microscope, the differentiated cells were washed off the slices with distilled water. Then the slices were dehydrated, air-dried, and sputtered with gold.
Results
Morphological changes of peripheral-blood monocytes after co-culture with RA-NLCs
After peripheral-blood monocytes had been cultured with RA-NLCs for 3 to 4 weeks, we recovered TRAP-positive mononuclear cells (Fig. 1a,1b,1c) with abundant cytoplasm and an off-center nucleus (Fig. 1a and 1b). These cells strongly expressed CD11b, CD11c, CD14, CD45, and human major histocompatibility antigen (HLA)-DR, suggesting that they were of monocyte lineage (Table 1). However, they did not express CD11a, CD35, or CD68, which are expressed on freshly isolated monocytes (see Supplementary material).
Figure 1.
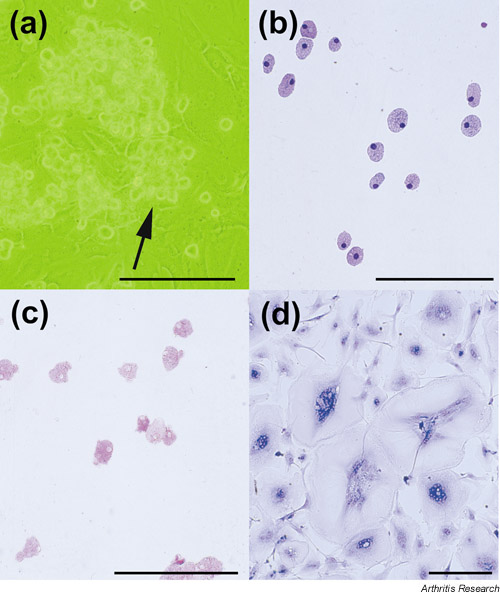
Morphology of TRAP-positive mononuclear cells induced from peripheral-blood monocytes with RA-NLCs. (a) Phase-contrast micrograph of monocytes co-cultured with RA-NLCs. Mononuclear cells (arrow) are growing on the RA-NLCs. (b) Mononuclear cells collected from the culture shown in (a). May–Grunwald–Giemsa staining. (c) Detection of TRAP expressed by the mononuclear cells (TRAP-positive cells were stained red with their cytoplasm). (d) Differentiated mononuclear cells. The cells shown here are multinucleated giant cells. May–Grunwald–Giemsa staining. Scale lines = 100 –m.
Table 1.
Differentiation of human TRAP-positive mononuclear cells derived from peripheral-blood monocytes into multinucleated cells after stimulation with IL-3, IL-5, IL-7, and GM-CSF
| Stimulator | Concentration | Fusion index (%) a |
| None | 1.8 | |
| Conditioned mediumb | (10% v/v) | 86.1 |
| IL-1α | (1 ng/ml) | 1.1 |
| IL-1β | (1 ng/ml) | 1.3 |
| IL-2 | (250 U/ml) | 7.7 |
| IL-3 | (5 ng/ml) | 64.8 |
| IL-4 | (100 U/ml) | 1.3 |
| IL-5 | (1 ng/ml) | 66.1 |
| IL-6 | (20 ng/ml) | 5.6 |
| IL-6 + sIL-6R | (sIL-6R: 200 ng/ml) | 5.3 |
| IL-7 | (20 ng/ml) | 72.4 |
| IL-8 | (20 ng/ml) | 2.1 |
| IFN-γ | (100 U/ml) | 7.1 |
| GM-CSF | (1 ng/ml) | 73.8 |
| M-CSF | (25 ng/ml) | 5.8 |
| TNF-a | (1 ng/ml) | 6.9 |
| VD3 | (10-7 mol/l) | 3.4 |
| Dexamethasone | (10-8 mol/l) | 0.8 |
| M-CSF + IL-4 | (M-CSF, 25 ng/ml; IL-4,100 U/ml) | 0.1 |
| IL-3 + IL-7 | 75.4 | |
| IL-7 + GM-CSF | 77.3 | |
| IL-3 + IL-7 + GM-CSF | 78.7 | |
| Phytohemagglutinin | (1% v/v) | 2.0 |
| RANKLc | (100 ng/ml) | 2.6 |
| RANKL + M-CSF c | (RANKL/ODF, 100 ng/ml; | 38.1 |
| M-CSF, 25 ng/ml) |
aTRAP-positive mononuclear cells were stimulated with various cytokines for 96–120 h. Fusion indices were calculated using the formula given in the text and previously [16]. Data are representative of three independent experiments using TRAP-positive cells induced from monocytes. bSee [15], and Supplementary material. cThe culture was maintained for 14 days. GM-CSF = granulocyte macrophage-colony-stimulating factor; IFN = interferon; IL = interleukin; M-CSF = macrophage-colony-stimulating factor; ODF = osteoclast differentiation factor; RANKL= receptor activator of nuclear-factor-κB ligand; sIL-6R = soluble interleukin-6 receptor; TNF = tumor necrosis factor; VD3 = 1,25-dihydroxyvitamin D3.
Presence of TRAP-positive mononuclear cells in synovial fluid from patients with RA
We detected monocytic cells positive for CD14 and TRAP in synovial fluids of patients with RA. These cells also strongly expressed CD11b, CD11c, CD14, CD45, and HLA-DR (see Supplementary meterial) but not CD1a, CD1b, CD2, CD5, or CD86, which are expressed on den-dritic cells derived from monocytes [17]. These results indicate that TRAP-positive monocytic cells present in synovial fluid and those induced in vitro in cultures with RA-NLCs are morphplogically and phenotypically the same. These cells were obtained from the synovial fluid of all patients with RA, regardless of age and sex. CD14-positive monocyte-like cells accounted for 20–91% of the mononuclear cells freshly isolated from the synovial fluid of such patients (data not presented).
Differentiation of TRAP-positive mononuclear cells into multinucleated giant bone-resorbing cells in the absence of RA-NLCs
The TRAP-positive cells induced in vitro and those isolated from synovial fluid both differentiated into multinucleated cells after being cultured for 72 to 96 h with the conditioned medium (Fig. 1d). These multinucleated cells still possessed TRAP activity (data not shown) and formed resorption areas on dentin slices (Fig. 2), suggesting that they had bone-resorbing activity as osteoclasts.
Figure 2.
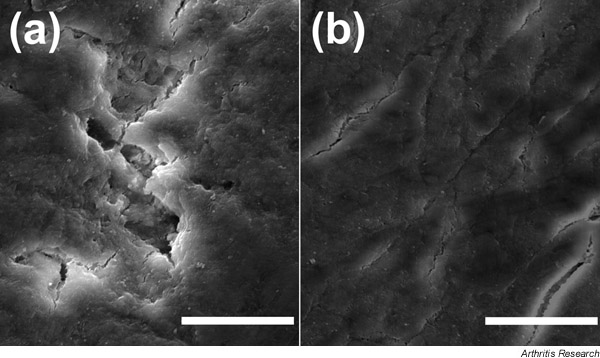
Scanning electron micrographs of dentin slices, showing (a) resorption areas formed on dentin by TRAP-positive multinucleated giant cells derived from monocytes and stimulated with granulocyte-macrophage-colony-stimulating factor for 96 h and (b) a control slice incubated with the TRAP-positive mononuclear cells in the absence of cytokines. Scale lines = 50 μm.
Induction of multinucleated cells by IL-3, IL-5, IL-7 or GM-CSF
The cytokines IL-3, IL-5, IL-7, and GM-CSF induced differentiation of TRAP-positive cells induced in vitro or those isolated from synovial fluid into osteoclasts (Table 1). Regardless of which cytokine was used to stimulate differentiation of the osteoclasts, they were all positive for TRAP and formed resorption pits on dentin slices, suggesting that they were all identical to the cells induced by conditioned medium (data not shown). The fusion index of osteoclasts induced by a mixture of cytokines was higher than those stimulated with a single cytokine. The cytokines IL-6 and IL-8, which are produced by RA-NLCs [2], did not induce osteoclast formation. RANKL was recently reported to induce osteoclasts from human peripheral blood [18,19]; however, a mixture of macrophage-colony-stimulating factor and RANKL exhibited only weak activity for induction of osteoclasts from the TRAP-positive mononuclear cells (Table 1). Phytohemagglutinin, which was contained in the conditioned medium, did not induce differentiation. TRAP-positive cells from the synovial fluid of patients with RA have a fusion index similar to that found for the TRAP-positive cells obtained experimentally from monocytes (data not shown). Induction of the osteoclasts was completely neutralized by the antibody to each cytokine (see Supplementary material).
Discussion
We have shown that the novel ability of RA-NLCs may contribute to the pathogenesis of RA by encouraging the generation of TRAP-positive mononuclear cells, which are osteoclast precursors. The TRAP-positive precursor cells have phagocytic activity and are negative for CD83, suggesting that they are different from peripheral-blood monocytes and dendritic cells [14,17].
Fujikawa et al reported that synovial macrophages differentiated into osteoclasts after incubation in the presence of a rat osteoblast-like cell line [20]. The fibroblasts isolated from RA synovia induced differentiation of monocytes into multinucleated cells in the presence of 1,25-dihydroxyvitamin D3 and macrophage-colony-stimulating factor [21]. Further study will be required to determine the identity of those monocytic cells and our cells. Fibroblastic cells in synovial fluid from patients with RA have been reported to support pseudoemperipolesis, which was considered to be the unique feature of the nurse cells, in the presence of IL-4 [22]. Shigeyama et al recently reported that RA synovial fluid may promote osteoclastogenesis from monocytes by expressing osteoclast differentiation factor [23]. It is likely that RA fibroblasts and RA-NLCs share several roles in the pathogenesis of RA, including activation of monocytes. However, the molecules required in our study for osteoclastogenesis from monocytes were different from those in the study of Shigeyama et al [23]. These findings suggest that multiple pathways for osteoclastogenesis in RA synovia may cause severe joint destruction.
There may be two steps for generation of the osteoclasts in the joints of patients with RA: first, differentiation of monocytes into TRAP-positive mononuclear cells induced and maintained by RA-NLCs, followed by cytokine-induced differentiation of these mononuclear cells into osteoclasts. The interaction between monocytes and RA-NLCs required adhesion molecules, but RANK (receptor activator of nuclear-factor-κB) and RANKL were not necessary to induce the TRAP-positive cells in preliminary studies in our laboratory (unpublished observation). The molecules required in the interaction are under investigation. In addition, we found the presence of TRAP-positive mononuclear cells which differentiated into osteoclasts in synovial fluids of patients with RA in vitro. Monocytes may infiltrate the affected joints and differentiate into TRAP-positive mononuclear cells under the influence of RA-NLCs. This conclusion is consistent with previous findings of TRAP-positive multinucleated giant cells in the synovial tissue of patients with RA [10,11,12]. Further studies are required to characterize these osteoclasts derived from the TRAP-positive mononuclear cells and to delineate the unique course of differentiation into bone-resorbing cells promoted by RA-NLCs.
Conclusion
In order to elucidate the role of RA-NLCs, monocytes were co-cultured with RA-NLCs. Monocytes differentiated into TRAP-positive mononuclear cells, the precursor cells of osteoclasts. Osteoclasts were generated from TRAP-positive mononuclear cells in the presence of IL-3, IL-5, IL-7, and GM-CSF. TRAP-positive cells were also present in synovial fluids of patients with RA. RA-NLCs may play a significant role in the activation of monocytes and long-term maintenance of differentiated monocytes (osteoclast precursors). The present study suggests that monocytes may differentiate into osteoclast precursor cells in the affected joints of patients with RA.
Supplementary materials and methods
Cell lines
Human lung fibroblasts CCD-19Lu were obtained from American Type Culture Collection (Rockville, MD, USA). Human T cell line MOLT-17 was a generous gift from Dr J Minowada (Fujisaki Cell Center, Okayama, Japan). These cell lines were cultured as recommended by the providers.
Examination of pseudoemperipolesis
Pseudoemperipolesis was measured as previously described [3]. RA synovial stromal cells (3 x 104) were incubated in supplemented DMEM in 24-well culture plates overnight. The next day, MOLT-17 cells (1 x 106) were added to the RA-NLC culture. Stromal cells with more than three MOLT-17 cells beneath them after 6 h of incubation were considered NLCs.
Long-term maintenance of monocytes by RA-NLCs
Monocytes (2.5 x 105) were co-cultured with RA-NLCs or CCD-19Lu with or without culture inserts (Becton Dickinson, Franklin Lakes, NJ, USA) in supplemented DMEM in for up to 48-well culture plates at 37ºC in 7.5% CO2 70 days. Half of the medium was changed once a week. The TRAP-positive mononuclear cells were collected from the culture, stained with trypan blue, and counted for viability under a microscope.
Antibodies and staining of cells
The cell-surface antigens on freshly isolated monocytes and TRAP-positive mononuclear cells were examined by staining with monoclonal antibodies specific for CD1a, CD4, CD5, CD11a, CD11b, CD11c, CD13, CD14, CD15, CD16, CD19, CD20, CD34, CD45, CD45RA, CD45RO, CD54, HLA-DR (Becton Dickinson), CD1b (Nichirei, Tokyo, Japan), CD2, CD3 (Ortho Diagnostics, Raritan, NJ, USA), CD35, CD68 (DAKO Japan, Kyoto, Japan), CD51/61, CD83, HLA-A, B and C (Pharmingen, San Diego, CA, USA), CD80, and CD86 (Ancell, Bayport, MN, USA). Antigen-expression was analyzed with a FACScan flow cytometer (Becton Dickinson). Multinucleated giant bone-resorbing cells differentiated from TRAP-positive mononuclear cells were fixed with cold acetone and stained immunohistochemically with rabbit polyclonal antibodies specific for actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), carbonic anhydrase II (Rockland, Gilbertsville, PA, USA), or vitronectin receptor (Chemicon International, Inc., Temecula, CA, USA). TRAP activity in TRAP-positive mononuclear cells and multinucleated giant bone-resorbing cells was examined using a TRAP-staining kit (Sigma, St Louis, MO, USA). Neutralizing antibodies to human IL-3, IL-5, IL-7, and GM-CSF were purchased from Genzyme (Cambridge, MA, USA).
Cytokines and reagents
Conditioned media were prepared as previously reported [15]. Briefly, a mixture of peripheral-blood mononuclear cells from 10 healthy donors was stimulated with phytohemagglutinin at 37ºC for 72 h. Culture supernatant fluids were collected, filtered, and used as conditioned media. IL-1α was purchased from Immugenex (Los Angeles, CA). IL-1α, IL-2, IL-3, IL-4, IL-5, IL-6, soluble IL-6 receptor (sIL-6R), interferon gamma (IFN-γ), granulocyte/macrophage-colony stimulating factor (GM-CSF), macrophage-colony stimulating factor, and tumor necrosis factor (TNF)-α were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). IL-7 and IL-8 were purchased from Genzyme. 1,25-dihydroxyvitamin D3 and dexamethasone were purchased from Wako Pure Chemical Co. (Osaka, Japan). Receptor activator of nuclear-factor-κB ligand (RANKL) was obtained from Peprotech (London, UK).
Assessment of phagocytic activity
Phagocytic activity of TRAP-positive mononuclear cells was assessed from their ingestion of heat-killed yeast. TRAP-positive mononuclear cells (1 x 106) were incubated with 2 x 107 yeast cells in phosphate-buffered saline supplemented with 10% fresh human serum, type AB, at 37ºC for 45 min. The cells were washed and stained with fuchsin (Wako Pure Chemical Co.), and the cells with ingested yeast were counted under a microscope.
Inhibition of the generation of multinucleated giant cells from TRAP-positive mononuclear cells by neutralizing antibodies
Neutralizing antibodies specific for IL-3, IL-5, IL-7, and GM-CSF were used for inhibition of the generation of multinucleated giant bone-resorbing cells. Irrelevant polyclonal mouse IgG from Jackson ImmunoResearch (West Grove, PA, USA) was used as a control. The TRAP-positive mononuclear cells (5 x 104) were pre-incubated with each antibody in DMEM containing 10% FCS in microtubes at 37ºC for 1 h. The cells were cultured in 4-well chamber slides, and stimulated with a cytokine for 96–120 h at 37ºC in 7.5% CO2. At the end of the culture period, the cells were stained for TRAP and the fusion index was calculated as described in the main paper.
Detection of calcitonin receptors
Calcitonin receptors on the multinucleated giant bone-resorbing cells were detected in situ using 125I-human cal-citonin were performed as described elsewhere [23]. TRAP-positive mononuclear cells (5 x 104) were stimulated with IL-3, IL-5, IL-7, or GM-CSF at the optimal concentrations in 4-well chamber slides (Nalge Nunc International, Rochester, NY, USA) for 96–120 h at 37ºC in 7.5% CO2. After formation of multinucleated giant bone-resorbing cells had been confirmed microscopically, the cells were incubated in 0.4 ml αMEM (Gibco BRL) with 0.1% bovine serum albumin and 0.2 mCi/ml of 125I-labeled human calcitonin (Amersham Pharmacia Biotech, Buckinghamshire, UK) for 1 h at 22ºC. Nonspecific binding was assessed on each slide in the presence of an excess amount of unlabeled human calcitonin. Then the cells were washed three times with phosphate-buffered saline solution and fixed with 2.5% formaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer. The slides were washed and dried as previously described [S1]. Air-dried slides were dipped in photographic emulsion (Kodak NTB3; Eastman Kodak, Rochester, NY, USA), drained, and dried for 2 h and were kept in a light-proof container with desiccant at 4ºC for 10 days. The slides were developed in accordance with the manufacturer's instructions.
Supplementary results
Phenotypic characterization of the TRAP-positive mononuclear cells induced by NLCs
The TRAP-positive mononuclear cells, whether induced from monocytes or collected from the synovial fluid of patients with RA, strongly expressed CD11b, CD11c, CD14, CD45, HLA-A, HLA-B, HLA-C, and HLA-DR but did not express CD1a, CD1b, CD2, CD4, CD5, CD16, CD19, CD20, or CD83. These observations suggest that these cells belong to the monocyte/macrophage lineage (Supplementary Table 1). However, the cells did not express CD11a, CD35, or CD68, which are expressed on freshly isolated monocytes from peripheral blood (Supplementary Table 2). The TRAP-positive mononuclear cells had strong phagocytic activity against heat-killed yeast (data not presented). In addition, the cells were positive for carbonic anhydrase II, actin, and vitronectin receptor (Supplementary Fig. 1), and calcitonin receptors were also detected (Supplementary Fig. 1); these four receptors are considered characteristic of osteoclasts [10,23].
Supplementary Table 1.
Expression of surface antigen by TRAP-positive mononuclear cells generated by co-culture with RA-NLC
| % Positivea | ||||
| Monocytes from | TRAP-positive cells induced | TRAP-positive cells | ||
| Antigen | peripheral blood | from monocytes | from RA-SF | |
| CD1a | 1.24 | 0.11 | 0.24 | |
| CD1b | 0.22 | 0.13 | 0.12 | |
| CD2 | 2.20 | 0.18 | 1.01 | |
| CD3 | 0.82 | 0.85 | 0.82 | |
| CD4 | 0.28 | 0.61 | 0.35 | |
| CD5 | 0.23 | 0.44 | 0.29 | |
| CD11a | LFA-1 | 94.72 | 1.16 | 1.18 |
| CD11b | CR3 a chain | 95.46 | 99.42 | 99.56 |
| CD11c | CR4 a chain | 99.47 | 99.22 | 99.01 |
| CD13 | 99.44 | 96.15 | 93.87 | |
| CD14 | 95.70 | 99.28 | 99.63 | |
| CD15 | Lex | 22.81 | 0.49 | 15.97 |
| CD16 | FcgR III | 0.72 | 0.24 | 0.30 |
| CD19 | 0.33 | 0.13 | 0.13 | |
| CD20 | 0.36 | 0.11 | 0.10 | |
| CD34 | 0.54 | 0.18 | 0.18 | |
| CD35 | CR1 | 93.49 | 0.50 | 0.60 |
| CD45 | 98.73 | 94.28 | 92.90 | |
| CD45RA | 0.37 | 0.15 | 0.46 | |
| RA45RO | 0.65 | 4.18 | 3.30 | |
| CD51/61 | VNRb | 0.22 | 0.10 | 0.17 |
| CD54 | ICAM-1 | 0.21 | 0.30 | 0.33 |
| CD68 | 94.59 | 1.06 | 1.10 | |
| CD80 | B7/BB1 | 8.19 | 7.41 | 6.52 |
| CD83 | 0.37 | 0.10 | 1.42 | |
| CD86 | B70/FUN-1 | 90.01 | 0.55 | 1.21 |
| HLA-A, -B, -C | 97.44 | 99.68 | 99.53 | |
| HLA-DR | 96.65 | 98.45 | 98.16 | |
aCells were stained with monoclonal antibody specific for various antigens, and analyzed by FACScan. Data are representative of four independent analyses. bVitronectin receptor. RA-SF = synovial fluid from patients with rheumatoid arthritis.
Supplementary Table 2.
Inhibition of formation of multinucleated giant cells by neutralizing antibodies specific for IL-3, IL-5, IL-7, and GM-CSF
| Fusion index (%) a | ||||
| Antibodyb | IL-3 (5 ng/ml) | IL-5 (1 ng/ml) | IL-7 (20 ng/ml) | GM-CSF (1 ng/ml) |
| Polyclonal mouse IgG | 63.8 ± 1.2 | 62.3 ± 3.6 | 66.2 ± 5.4 | 67.7 ± 6.0 |
| Anti-IL-3 | 7.3 ± 1.6 | - | - | - |
| Anti-IL-5 | - | 3.8 ± 1.8 | - | - |
| Anti-IL-7 | - | - | 5.5 ± 5.8 | - |
| Anti-GM-CSF | - | - | - | 4.0 ± 1.3 |
aFusion indices were calculated using the formula given in the Methods section. Each value is the mean ± SD of three independent experiments using TRAP-positive cells induced from monocytes of three individuals. bConcentrations of cytokines: polyclonal mouse IgG, 10 mg/ml; anti-IL-3, 10 mg/ml; anti-IL-5, 5 mg/ml; anti-IL-7, 10 mg/ml, anti-GM-CSF, 2 mg/ml. - = not examined; GM-CSF = granulocyte/macrophage-colony-stimulating factor; IL = interleukin.
Supplementary Figure 1.
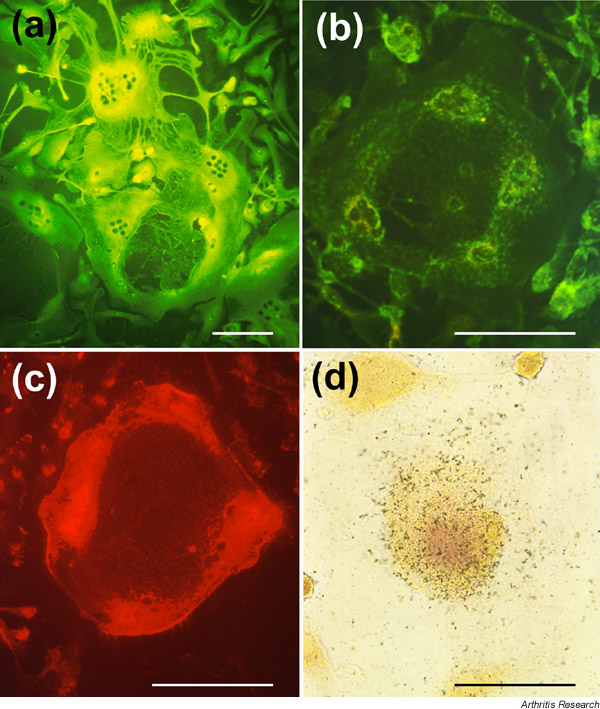
Immunohistochemical staining of multinucleated giant bone-resorbing cells. The cells were stained green for (a) carbonic anhydrase II and (b) vitronectin receptor. The cells were also positive for (c) actin, which showed red, ring-form staining. (d)In situ detection of calcitonin receptor using 125I-human calcitonin. Black grains mark the cells expressing calcitonin receptor. The multinucleated giant bone-resorbing cells were incubated with 125I-human calcitonin for 1 h. The cells were washed, fixed, and dried as described in the Supplementary materials and methods section. Air-dried slides were exposed at 4ºC for 10 days. Scale lines = 100 μm.
Long-term maintenance of monocytes by RA-NLCs
Monocytes obtained from all five healthy individuals and co-cultured with RA-NLCs grew and differentiated into TRAP-positive mononuclear cells (Supplementary Fig. 2). We also used five RA-NLC lines, each from a different patient with RA. All five cell lines induced the generation of TRAP-positive mononuclear cells from human peripheral blood monocytes (data not presented). The generation of these TRAP-positive cells required direct contact with RA-NLCs.
Supplementary Figure 2.
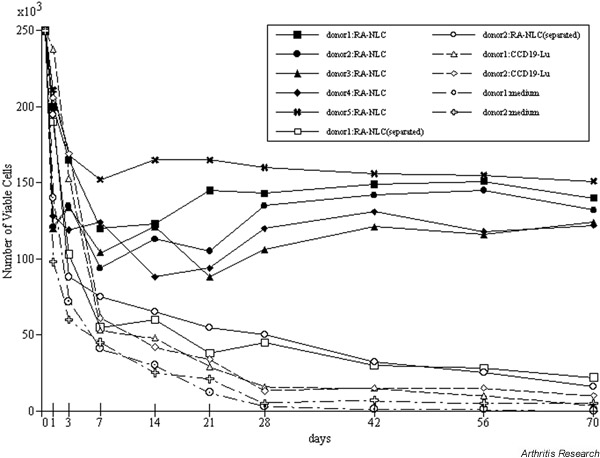
Effects of culture conditions on survival of monocytes from five donors with RA-NLCs or CCD-19Lu, in the presence (separated) or absence (no indication) of culture inserts. Each culture was maintained for up to 70 days. The resulting mononuclear cells were collected, stained with trypan blue, and counted under a microscope. Each experiment was conducted in duplicate, and each point represents the mean number of viable cells in two cultures.
Induction of osteoclasts by IL-3, IL-5, IL-7, or GM-CSF
The conditioned medium contains several cytokines. In order to determine which cytokines induce TRAP-positive mononuclear cells to differentiate into multinucleated giant bone-resorbing cells, in vitro induced TRAP-positive cells and those isolated from synovial fluid of patients with RA were collected and stimulated with various cytokines in the absence of RA-NLCs. In a preliminary study, a high-performance liquid chromatography fraction of the conditioned medium, which was used to induce TRAP-positive mononuclear cells to differentiate into multinucleated giant bone-resorbing cells, contained proteins with a molecular weight of approximately 20 kDa (data not shown). Therefore, we mainly examined cytokines with approximately that molecular weight. The activities of IL-3, IL-5, IL-7, and GM-CSF for inducing multinucleated giant bone-resorbing cells were completely neutralized by the antibody to the respective cytokine (Supplementary Table 2).
Abbreviations
DMEM = Dulbecco's modified Eagle's medium; FCS = fetal calf serum; GM-CSF = granulocyte/macrophage-colony-stimulating factor; HLA = human major histocompatibility antigen; IL = interleukin; NLC = nurse-like cell; RA = rheumatoid arthritis; RANKL = receptor activator of nuclear-factor-κB ligand; RA-NLC = nurse-like cell derived from rheumatoid arthritis synovial tissue; TRAP= tartrate-resistant acid phosphatase.
Acknowledgments
Acknowledgements
This work was supported by the Program for Promotion of Fundamental Studies in Health Science of the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan. The authors are grateful to Dr Ichiro Kurane (Department of Virology 1, National Institute of Infectious Diseases, Tokyo, Japan) for helpful discussions and critical reading of the manuscript.
References
- Tomita T, Takeuchi E, Toyosaki-Maeda T, Oku H, Kaneko M, Takano , Sugamoto H, Ohzono K, Suzuki R, Ochi T. Establishment of nurse-like stromal cells from bone marrow patients with rheumatoid arthritis: indication of characteristic bone marrow microenvironment in patients with rheumatoid arthritis. Rheumatology (Oxford) 1999;38:854–863. doi: 10.1093/rheumatology/38.9.854. [DOI] [PubMed] [Google Scholar]
- Takeuchi E, Tomita T, Toyosaki-Maeda T, Hashimoto H, Kaneko M, Takano H, Sugano K, Suzuki R, Ochi T. Establishment and characterization of nurse-cell like stromal cell lines from synovial tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:221–228. doi: 10.1002/1529-0131(199902)42:2<221::AID-ANR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Shimaoka Y, Attrep JF, Hirano T, Ishihara K, Suzuki R, Toyosaki T, Ochi T, Lipsky PE. Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest. 1998;102:606–618. doi: 10.1172/JCI3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzano M, Li Y, Phlip D, Omene C, Cantey M, Saunders G, Guyden JC. Thymic nurse cell rescue of early CD4+ CD8+ thymocytes from apoptosis. Cell Mol Biol. 1995;41:1099–1111. [PubMed] [Google Scholar]
- Pezzano M, Phlip D, Stephenson S, Li Y, Reid V, Maitta R, Guyden JC. Positive selection by thymic nurse cells requires IL-1 beta and is associated with an increased Bcl-2 expression. Cell Immunol. 1996;169:174–184. doi: 10.1006/cimm.1996.0108. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Nakayama K, McCoy RL, Wang F, Nishimura T, Habu S, Murphy KM, Loh DY. Cellular and peptide requirements for in vitro clonal deletion of immature thymocytes. Proc Natl Acad Sci U S A. 1992;89:9000–9004. doi: 10.1073/pnas.89.19.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Doi H, Chin S, Nishimura T, Kasahara S. Establishment of mouse thymic nurse cell clones from a spontaneous BALB/c thymic tumor. Eur J Immunol. 1988;18:821–824. doi: 10.1002/eji.1830180525. [DOI] [PubMed] [Google Scholar]
- Miyake K, Hasunuma Y, Yagita H, Kimoto M. Requirement for VLA-4 and VLA-5 integrins in lymphoma cells binding to and migration beneath stromal cells in culture. J Cell Biol. 1992;119:653–662. doi: 10.1083/jcb.119.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiai H, Nishi Y, Miyazawa T, Matsudaira Y, Nishizuka Y. Mouse lymophoid leukemias: symbiotic complexes of neoplastic lymphocytes and their microenvironments. J Natl Cancer Inst. 1981;66:713–722. [PubMed] [Google Scholar]
- Gravallese EM, Harada Y, Wang JT, Gom AH, Thomhil TS, Goldring SR. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol. 1998;152:943–951. [PMC free article] [PubMed] [Google Scholar]
- Chang JS, Quinn JM, Demaziere A, Bulstrode CJ, Francis MJ, Duthie RB, Athanasou NA. Bone resorption by cells isolated from rheumatoid synovium. Ann Rheum Dis. 1992;51:1223–1229. doi: 10.1136/ard.51.11.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa Y, Shingu M, Torisu T, Itonaga I, Masumi S. Bone resorption by tartrate-resistant acid phosphatase-positive multinuclear cells isolated from rheumatoid synovium. Br J Rheumatol. 1996;35:213–217. doi: 10.1093/rheumatology/35.3.213. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HA, Medsger TA, Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JG, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Akagawa KS, Takatsuka N, Nozaki Y, Komuro I, Azuma M, Ueda M, Naito M, Takahashi K. Generation of CD1+RelB+ dendritic cells and tartrate-resistant acid phosphatase-positive osteoclast-like multinucleated giant cells from human monocytes. Blood. 1996;88:4029–4039. [PubMed] [Google Scholar]
- Toyosaki T, Tsuruta Y, Yoshioka T, Takemoto H, Suzuki R, Tomita T, Ochi T. Recognition of rheumatoid arthritis synovial antigen by CD4+, CD8-T cell clones established from rheumatoid arthritis joints. Arthritis Rheum. 1998;41:92–100. doi: 10.1002/1529-0131(199801)41:1<92::AID-ART12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Tabata N, Tajima M, Ito M, Tsurudome M, Sudo A, Uchida A, Ito Y. Induction of human osteoclast-like cells by treatment of blood monocytes with anti-fusion regulatory protein-1/CD98 monoclonal antibodies. J Bone Miner Res. 1998;13:44–49. doi: 10.1359/jbmr.1998.13.1.44. [DOI] [PubMed] [Google Scholar]
- Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci U S A. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashino K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Ostreoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–178. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y, Sabokbar A, Neale S, Athanasou NA. Human osteoclast formation and bone resorption by monocytes and synovial macrophages in rheumatoid arthritis. Ann Rheum Dis. 1996;55:816–822. doi: 10.1136/ard.55.11.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H, Oda H, Yamamoto S, Kawaguchi H, Tanaka S, Nishikawa T, Koshihara Y. A new mechanism of bone destruction in rheumatoid arthritis: synovial fibroblasts induce osteoclastogenesis. Biochem Biophys Res Commun. 1997;240:279–286. doi: 10.1006/bbrc.1997.7404. [DOI] [PubMed] [Google Scholar]
- Burger JA, Zvaifler NJ, Tsukada N, Firestein GS, Kipps TJ. Fibroblast-like synoviocytes support pseudoemperipolesis via a stromal cell-derived B-cell factor-1- and CD106 (VCAM-1)-dependent mechanism. J Clin Invest. 2001;107:305–315. doi: 10.1172/JCI11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43:2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
Supplementary references
- Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FAO, Martin TJ. Abundant calcitonin receptors in isolated rat osteoclasts. Biochemical and autoradiographic characterization. J Clin Invest. 1986;78:355–360. doi: 10.1172/JCI112584. [DOI] [PMC free article] [PubMed] [Google Scholar]


