Abstract
There are no studies to date,describing changes in the diffusion tensor imaging (DTI) metrics of the white matter (WM) regions of the entire cervical and thoracic spinal cord (SC) remote from the lesion in pediatric spinal cord injury (SCI) subjects. The purpose of this study was to determine whether DTI at sites cephalad and caudal to a lesion provides measures of cord abnormalities in children with chronic SCI. A retrospective study included 10 typically developing subjects (TD) and 10 subjects with chronic SCI who underwent SC imaging in 2014–2017. Axial diffusion tensor images using an inner field of view DTI sequence were acquired to cover the entire cervical and thoracic SC. Regions of interest were drawn on the SC WM: right and left lateral (motor), ventral (motor), and dorsal (sensory) tracts. To detect differences in DTI metrics between TD and SCI of the cord, a one way analysis of variance with pooled t test was performed. A stepwise regression analysis was performed to assess the correlation between DTI metrics and clinical scores. In motor and sensory tracts, fractional anisotropy (FA) and axial diffusivity (AD) were significantly decreased in the proximal segments of the caudal cord. In motor tracts cephalad to the lesion, FA was significantly decreased whereas AD was significantly increased in the proximal segment; however, AD was decreased in the distal and middle segments. International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) total score was significantly correlated with FA and AD of the motor and sensory tracts cephalad to the lesion. This study demonstrates that FA and AD have the potential to be sensitive biomarkers of the full extent of cord injury and might be useful in detecting remote injuries to the SC and in guiding new treatments.
Keywords: DTI, ISNCSCI score, motor and sensory tracts, SC, SCI, WM
Introduction
Spinal cord injury (SCI) can result in extensive neurological consequences leading to axonal damage, degeneration, and neuronal loss. SCI studies on cell transplantation in the chronic phase have shown no significant recovery in locomotor and sensory function because glial scar and cystic cavities at the injury site create a greater impediment to axonal regeneration compared with acute SCI.1,2 Examining the perilesional areas distant from the injury site may provide additional information to help guide therapies and to promote axonal regeneration and functional recovery in these patients.
A non-invasive functional evaluation of axonal injury in SCI is important for understanding its mechanism and developing new therapeutic treatments. Diffusion tensor imaging (DTI) has the potential to be an invaluable tool for evaluating the structural changes in the entire SC after an SCI; however, most of the DTI studies have only shown diffusion measurements at the lesion site or within a few segments of the lesion.3–5 Examining perilesional SC and regions distant from the lesion may provide valuable insights into the full extent of injury. In addition, this information may help better explain the so called “zone of partial preservation” as described in the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI). It is well known that beyond the level of injury, changes occur in the remaining cord tissues.6,7 As time passes, the axons degenerate distal to the injury with a breakdown of myelin sheath and activation of microglia with subsequent gliosis.8,9 In chronic SCI, the lesion is more or less static except for the continuously advancing degeneration of descending and ascending spinal tracts and the effects of plasticity on the neuronal synaptic network.6 Previous adult DTI studies have shown changes in diffusivity values in white matter (WM) regions distant from the injury site in the cervical cord alone.10–13 Therefore, DTI might be sensitive to changes in the structure of the cord tissue in regions distant from the lesion in chronic injury.
To the best of our knowledge, there are no studies to date describing changes in the DTI metrics of the WM areas in the segments of the entire cervical and thoracic SC remote from the lesion in pediatric subjects with chronic SCI. We hypothesized that diffusion properties of the SC WM areas would be altered distant from the lesion, which may reflect changes in tissue microstructure. The purpose of this study was to determine whether DTI at sites cephalad (above) and caudal (below) the lesion provides measures of cord abnormalities in children with chronic SCI.
Methods
Study design
Written informed assent (child) and consent (parent) were obtained under the protocol approved by the institutional review board. A retrospective study included 10 typically developing (TD) subjects and 10 subjects with chronic SCI who underwent SC imaging in 2014–2017. A sample of consecutive subjects were studied consisting of 10 TD (age range, 6–16 years; mean age, 12.38 ± 2.81; 3 male and 7 female) and 10 subjects with chronic SCI (age range, 6–16 years; mean age, 12.33 ± 3.03; 6 male and 4 female). Table 1 shows the demographic information of the SCI subjects and the extent of the lesion as defined by magnetic resonance imaging (MRI). All MRI lesions represented contiguous abnormality. There were three patients who had non-traumatic SCI as follows: subject 205 had a focal non-traumatic spinal cord hemorrhage, subject 203 had a spinal cord tumor and a post-resection SCI, and subject 115 had focal transverse myelitis with chronic residual neurological symptoms. Two SCI subjects, 205 and 115, had no focal abnormality on MRI and, therefore, represented Spinal Cord Injury without Radiographic Abnormality (SCIWORA). The cephalad and caudal margins of the lesion present on MRI in these subjects were based on the neurological level determined by the ISNCSCI examination (Table 1).
Table 1.
Demographic Information of the SCI Subjects
| Subject ID | Age at enrollment (years) | Age at the time of injury(years) | Time interval from injury to DTI studies (years) | Gender | Cephalad and caudal margins of the lesion present on MRI | NL | AIS grade | ISNCSCI motor scores | ISNCSCI sensory scores | Cause |
|---|---|---|---|---|---|---|---|---|---|---|
| 201 | 11.12 | 4.73 | 6.38 | M | C5-C6 to mid T5 | C6 | A | 42 | 23 | MVA |
| 204 | 12.65 | 5 | 7.65 | F | C6-C7 to conus | T3 | A | 50 | 44 | MVA |
| 215 | 14.96 | 13.66 | 1.30 | M | Mid C6 to T1-T2 | C8 | A | 50 | 31 | MVA |
| 208 | 8.08 | 2.09 | 5.98 | M | Mid C6 to C7-T1 | C8 | A | 50 | 36 | MVA |
| 205 | 9.41 | 8.29 | 1.13 | F | aMid T8 to mid T9 | T9 | A | 50 | 69 | Bleed |
| 209 | 15.71 | 12 | 3.71 | M | T8-T9 to conus | T9 | A | 50 | 64 | MVA |
| 203 | 11.04 | 1.5 | 9.54 | F | Mid T5 to mid T8 | T4 | A | 50 | 48 | Tumor resection |
| 217 | 16.99 | 15.82 | 1.16 | M | Mid C3 to mid C4 | C4 | B | 24 | 38 | Diving |
| 115 | 9.24 | 1.82 | 7.41 | M | aMid C5 to mid C6 | C6 | B | 45 | 108 | TM |
| 221 | 14.12 | 0.7 | 13.43 | F | C6-C7 to mid T6 | T3 | B | 50 | 44 | MVA |
Based on NL.
SCI, spinal cord injury; MRI, magnetic resonance imaging; NL, neurological level; AIS, American Spinal Injury Association Impairment Scale; ISNCSCI, International Standard of Neurological Classification for Spinal Cord Injury; TM, transverse myelitis; MVA, motor vehicle accident.
Inclusion criteria for the TD subjects were: no evidence of SCI or pathology, as assessed by performing a neurological screen and a brief assessment of motor and sensory function and reflexes. Inclusion criteria for SCI subjects were: stable SCI in the past 3 months and being at least 6 months post-SCI. Exclusion criteria were: being unable to tolerate MRI without sedation, scoliosis, any abnormality (unrelated to the SCI) of the nervous and/or musculoskeletal system, or orthodontic hardware or other internal metal material.
ISNCSCI
All the subjects with SCI underwent a full neurological evaluation based on ISNCSCI.14 The evaluation consisted of manual muscle strength testing of five upper and lower extremity muscles bilaterally, testing of light touch and sharp–dull discrimination at 28 dermatomes bilaterally, and a rectal examination of sensory and motor function.14 The upper extremity and lower extremity total motor possible scores ranged from 0 to 25 for each limb (maximum score of 50 for each side). The sensory score ranged from 0 to 56 for each modality on each side (maximum score of 112 on each side). The ISNCSCI total motor score refers to the sum of the bilateral upper extremity and lower extremity motor scores, and the ISNCSCI total light touch sensory score is the sum of right and left light touch scores.
Imaging acquisition
A 3T MR scanner (Siemens, Germany) with a four channel neck matrix and an eight channel spine matrix coil was used. Conventional T1- and T2-weighted MRI scans were obtained for all the subjects. The T2-weighted image of the cervical and thoracic SC in the sagittal plane was initially obtained using a gradient echo (GRE) sequence and was used to facilitate slice prescription for the subsequent scans perpendicular to the cord. Next, an axial GRE T2-weighted, sagittal T2-weighted 3D SPACE; sagittal turbo spin-echo (TSE), T1-weighted; sagittal TSE T2-weighted; and axial DTI scans using an inner field of view (iFOV) sequence were obtained for the subjects. The iFOV sequence used in this study has been described in detail elsewhere15 and was based on a diffusion sensitized single-shot echo planar imaging sequence using 2D radiofrequency excitations, which allows for higher in-plane resolution with fewer geometric distortions and improved signal-to-noise ratio. Manual shim volume adjustments were also performed prior to data acquisition in order to restrict the adjustment volume to the anatomy of interest in an effort to further improve signal.
The iFOV sequence was optimized for both signal and scan duration to image the entire cervical and thoracic SC. DTI scans were acquired axially in the same anatomical location prescribed for the T2-weighted GRE images to cover the cervical (C1-upper thoracic region) and thoracic (upper thoracic-L1) SC using two overlapping slabs.16 The imaging parameters for each slab of DTI acquisition included: field of view, 164 mm; phase field of view, 47 mm; number of excitations, 3; number of diffusion directions, 20; b0 images, 6; b-value, 800 s/mm2; voxel, 0.8 × 0.8 × 6 mm3; axial slices, 40; repetition time, 7900 ms; echo time, 110 ms; and acquisition time, 8:49 min. Anesthesia and cardiac and respiratory gating were not used, to shorten scan times.
Data pre-processing
A central mask was applied to the raw DTI images to eliminate the anatomy outside the SC. Motion correction was performed first on the 6 b0 images to create a mean b0 image, and the diffusion weighted images were subsequently co-registered to the mean b0 image.17 The diffusion data sets were corrected for motion-induced artifacts based on 3D registration technique using an in-house software developed in Matlab (MathWorks, Natick, MA).17 The technique uses rigid body transformation with 6 degrees of freedom (3 translational, 3 axis rotations) paired with normalized mutual information as cost function to align target images (20 diffusion directional images) with the reference image (b0). A robust, iterative diffusion tensor estimation scheme, RESTORE, was implemented to ensure removal of data outliers from the final tensor fit.18 All the processing was performed using in-house software developed in Matlab.6
Region of interest (ROI) analysis
ROIs were drawn manually on axial fractional anisotropy (FA) maps to extract information from the SC WM areas: right and left lateral, ventral, and dorsal (Fig. 1). ROI's included: right and left posterolateral (lateral corticospinal and rubrospinal motor tracts), ventral (medial reticulospinal, ventral corticospinal, and tectospinal motor tracts); and dorsal (fasciculus gracilis and fasciculus cuneatus sensory tracts). The right and left posterolateral ROIs were specifically placed dorsal to a line that bisected the anterior and posterior cord to attempt to not include the lateral spinothalamic tract (Fig. 2). The ROI was drawn at each intervertebral disk and mid-vertebral body level of the cervical and thoracic SC to compute DTI metrics FA, mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). There was a consistent sparring of the outer margin of the SC of approximately one voxel width, to minimize volume averaging with cerebrospinal fluid. These ROIs were anatomically localized by a board-certified pediatric neuroradiologist using axial T2-weighted GRE images as a reference.
FIG. 1.
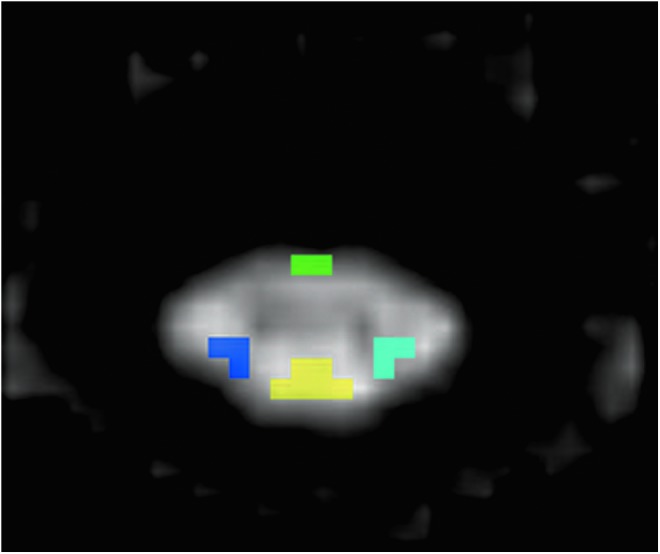
Regions of interest (ROIs) are anatomically defined on the gray scale axial fractional anisotropy (FA) map (for diffusion tensor imaging [DTI] metrics) to extract information from the spinal cord white matter (WM) areas: Right and left lateral (dark and light blue), ventral (green) and dorsal (yellow) regions respectively. Color image is available online at www.liebertpub.com/neu
FIG. 2.
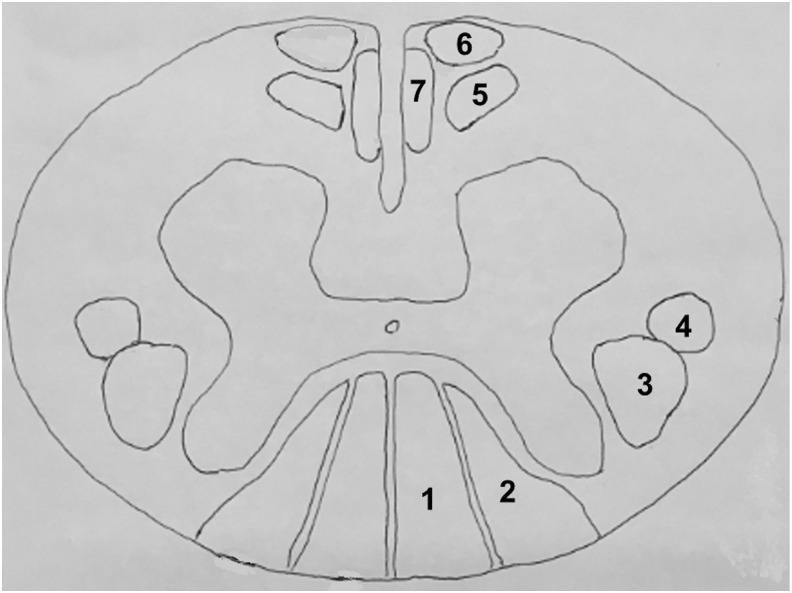
Spinal cord showing the location of the ascending (sensory labelled as 1, 2) and descending (motor labelled as 3–7) tracts. Regions of interest (ROIs) for diffusion tensor imaging (DTI) are drawn on the dorsal region to include fasciculus gracilis (1) and fasciculus cuneatus (2), right and left lateral regions to include the lateral corticospinal tract (3) and rubrospinal tract (4), and ventral region to include the medial reticulospinal tract (5) tectospinal tract (6), and ventral corticospinal tract (7).
For each SCI subject, we chose to separate the normal-appearing cord on the MRI cephalad and caudal to the lesion into three equal segments: proximal third, middle third, and distal third in relation to the lesion present on MRI, or the neurological level of injury in patients with SCIWORA (Table 1). For choosing the TD cord for comparison, we took the epicenter as the average of the SC lesion in all the 10 SCI subjects, which was mid T3, C1 to T2-T3 was defined as the cephalad SC, and T3-T4 to T12-L1 was defined as the caudal SC. In the group analysis of the six segments (three cephalad, three caudal) of the entire cord, the TD cord was separated into proximal third, middle third, and distal third and compared with the respective segments in the SCI subjects.
Additionally, we analyzed five vertebral body levels cephalad and caudal to the lesion for all subjects, to examine the more detailed DTI changes in the immediate perilesional SC regions. For the TD group, the cephalad levels included C5 to mid T2, and the caudal levels included mid T4 to mid T8. The mean of each of the five vertebral body levels both cephalad and caudal to the lesion was determined for all subjects (Figs. 3 and 4).
FIG. 3.
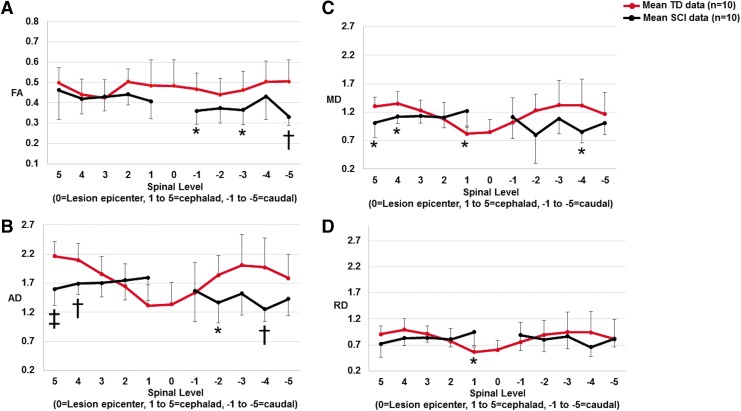
Scatter plots showing mean fractional anisotropy (FA) (A), axial diffusivity (AD) (B), mean diffusivity (MD) (C), and radial diffusivity (RD) (D) measurements for the motor tracts (right and left lateral and ventral white matter) for five vertebral body levels cephalad and caudal to the lesion for typically developing (TD) subjects and subjects with chronic spinal cord injury (SCI). The dark red solid line represents the mean TD data and the black line represents the mean SCI data. In the TD population, “0” level (mid T3) represents the average epicenter of the SCI subjects. In the SCI subjects, the full extent of the lesion (which may include multiple levels) is represented as the “0” level. In our cohort of SCI subjects, there was minimal diffusion tensor imaging (DTI) signal at the lesion levels, and, therefore, we did not perform DTI measurements at these levels. *p < 0.05, †p < 0.01, ‡p < 0.001 indicates significant difference from TD. Color image is available online.
FIG. 4.
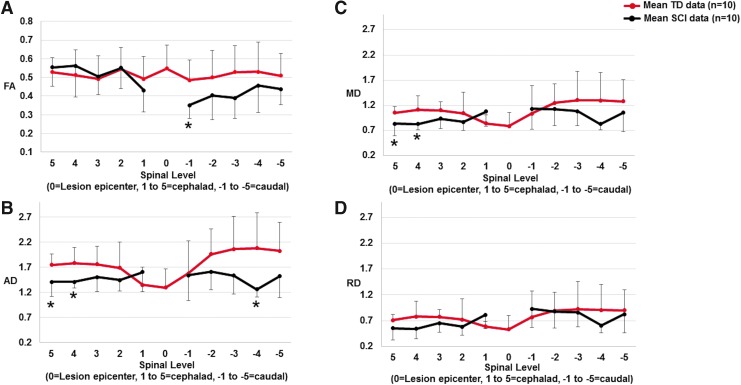
Scatter plots showing mean fractional anisotropy (FA) (A), axial diffusivity (AD) (B), mean diffusivity (MD) (C), and radial diffusivity (RD) (D) measurements for the sensory tracts (dorsal white matter) for five vertebral body levels cephalad and caudal to the lesion for typically developing (TD) subjects and subjects with chronic spinal cord injury (SCI). The dark red solid line represents the mean TD data and the black line represents the mean SCI data. In the TD population, “0” level (mid T3) represents the average epicenter of the SCI subjects. In the SCI subjects, the full extent of the lesion (which may include multiple levels) is represented as the “0” level. In our cohort of SCI subjects, there was minimal diffusion tensor imaging (DTI) signal at the lesion levels and, therefore, we did not perform DTI measurements at these levels. *p < 0.05 indicates significant difference from TD. Color image is available online.
Statistical analysis
Statistical analysis was performed using JMP pro 13.0 software. The data were checked for normality using the Shapiro–Wilk test, and the data were normally distributed. There was no statistical correction applied to the data. A p < 0.05 was used for statistical significance.
Group differences
To detect differences in DTI metrics between TD and SCI in the segments of the cord, a one way analysis of variance with pooled t test was performed.
Correlation between DTI metrics and clinical scores
A stepwise regression analysis was performed to assess the correlation between DTI metrics obtained from the averaged WM ROIs, motor tracts, and sensory tracts to the respective ISNCSCI total motor score and ISNCSCI total light touch sensory score in the cephalad and caudal regions. The ISNCSCI scores were defined as separate independent variables. DTI metrics obtained from all levels of motor and sensory tracts were considered as separate dependent variables.
Results
Group analysis of segments of the cord cephalad and caudal to the lesion comparing SCI to TD
Motor tracts
As shown in Table 2, FA was significantly decreased in the proximal segment of the cord both cephalad (p < 0.0001) and caudal (p < 0.0001) to the lesion and in the middle segment (p = 0.0002) of the caudal cord.
Table 2.
Summary of Changes of FA in the Motor and Sensory Tracts of the Segments of the SC Cephalad and Caudal to the Level of MRI Lesion in TD and SCI Subjects
| FA cephalad | FA caudal | |||||
|---|---|---|---|---|---|---|
| Distal | Middle | Proximal | Proximal | Middle | Distal | |
| Motor tracts | ||||||
| TD | 0.49 ± 0.06 | 0.46 ± 0.08 | 0.48 ± 0.08 | 0.45 ± 0.07 | 0.48 ± 0.09 | 0.43 ± 0.05 |
| SCI | 0.50 ± 0.12 | 0.43 ± 0.07 | 0.40 ± 0.07↓‡ | 0.35 ± 0.06↓‡ | 0.40 ± 0.08↓‡ | 0.41 ± 0.09 |
| Sensory tracts | ||||||
| TD | 0.57 ± 0.10 | 0.51 ± 0.09 | 0.51 ± 0.11 | 0.50 ± 0.12 | 0.50 ± 0.13 | 0.40 ± 0.10 |
| SCI | 0.57 ± 0.14 | 0.55 ± 0.12 | 0.47 ± 0.12 | 0.38 ± 0.09↓‡ | 0.44 ± 0.10↓* | 0.43 ± 0.10 |
Bold indicates significantly different DTI value in SCI subjects than in TD.
↓ indicates decreased DTI value; ↑ indicates increased DTI value in SCI compared with TD.
p < 0.001, *p < 0.05 indicates significant difference from TD.
FA, fractional anisotropy; SC, spinal cord; MRI, magnetic resonance imaging; TD, typically developing; SCI, spinal cord injury.
Cephalad to the lesion, AD was significantly decreased in the distal (p = 0.0017) and middle (p < 0.0001) segments; however, significantly increased AD was found in the proximal segment (p = 0.0327). Caudal to the lesion, AD showed a significant decrease in both the proximal (p = 0.0017) and middle (p = 0.0104) segments (Table 3).
Table 3.
Summary of Changes of AD in the Motor and Sensory Tracts of the Segments of the SC Cephalad and Caudal to the Level of MRI Lesion in TD and SCI Subjects
| AD cephalad | AD caudal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distal | Middle | Proximal | Proximal | Middle | Distal | |||||
| Motor tracts | ||||||||||
| TD | 2.15 ± 0.31 | 2.11 ± 0.28 | 1.59 ± 0.38 | 1.80 ± 0.51 | 1.84 ± 0.41 | 1.49 ± 0.32 | ||||
| SCI | 1.96 ± 0.30↓† | 1.87 ± 0.25↓‡ | 1.76 ± 0.31↑* | 1.47 ± 0.42↓† | 1.61 ± 0.35↓* | 1.44 ± 0.35 | ||||
| Sensory tracts | ||||||||||
| TD | 1.81 ± 0.33 | 1.78 ± 0.30 | 1.59 ± 0.40 | 1.87 ± 0.62 | 2.02 ± 0.61 | 1.48 ± 0.36 | ||||
| SCI | 1.81 ± 0.37 | 1.62 ± 0.26↓† | 1.48 ± 0.29 | 1.45 ± 0.38↓‡ | 1.70 ± 0.42↓† | 1.41 ± 0.47 | ||||
Unit for AD is given in 10−3 mm2/s.
Bold indicates significantly different DTI value in SCI subjects than in TD.
↓ indicates decreased DTI value; ↑ indicates increased DTI value in SCI compared to TD.
p < 0.01, ‡p < 0.001, *p < 0.05 indicates significant difference from TD.
AD, axial diffusivity; SC, spinal cord; MRI, magnetic resonance imaging; TD, typically developing; SCI, spinal cord injury.
Cephalad to the lesion, MD was significantly decreased in the distal (p = 0.0041) and middle (p = 0.0008) segments of the cord; however, a significant increase in MD was found in the proximal segment (p = 0.0006), whereas the caudal cord did not show any significant changes (Table 4).
Table 4.
Summary of Changes of MD in the Motor and Sensory Tracts of the Segments of the SC Cephalad and Caudal to the Level of MRI Lesion in TD and SCI Subjects
| MD cephalad | MD caudal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distal | Middle | Proximal | Proximal | Middle | Distal | |||||
| Motor tracts | ||||||||||
| TD | 1.39 ± 0.25 | 1.38 ± 0.21 | 1.02 ± 0.24 | 1.18 ± 0.36 | 1.20 ± 0.33 | 1.01 ± 0.23 | ||||
| SCI | 1.24 ± 0.29↓† | 1.24 ± 0.18↓‡ | 1.22 ± 0.24↑‡ | 1.06 ± 0.31 | 1.12 ± 0.26 | 1.00 ± 0.24 | ||||
| Sensory tracts | ||||||||||
| TD | 1.07 ± 0.26 | 1.09 ± 0.21 | 0.99 ± 0.28 | 1.19 ± 0.48 | 1.29 ± 0.50 | 1.03 ± 0.28 | ||||
| SCI | 1.08 ± 0.35 | 0.96 ± 0.19↓‡ | 0.95 ± 0.20 | 1.04 ± 0.30 | 1.13 ± 0.34 | 0.95 ± 0.30 | ||||
Unit for MD is given in 10−3 mm2/s
Bold indicates significantly different DTI value in SCI subjects than in TD.
↓ indicates decreased DTI value; ↑ indicates increased DTI value in SCI compared with TD.
p < 0.01, ‡p < 0.001 indicates significant difference from TD.
MD, mean diffusivity; SC, spinal cord; MRI, magnetic resonance imaging; TD, typically developing; SCI, spinal cord injury.
Cephalad to the lesion, RD was significantly decreased in the distal (p = 0.0150) and middle (p = 0.0432) segments of the cord; however, a significant increase in RD was found in the proximal segment (p < 0.0001), whereas the caudal cord did not show any significant changes (Table 5).
Table 5.
Summary of Changes of RD in the Motor and Sensory Tracts of the Segments of the SC Cephalad and Caudal to the Level of MRI Lesion in TD and SCI Subjects
| RD cephalad | RD caudal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distal | Middle | Proximal | Proximal | Middle | Distal | |||||
| Motor tracts | ||||||||||
| TD | 1.01 ± 0.23 | 1.01 ± 0.21 | 0.74 ± 0.20 | 0.87 ± 0.30 | 0.87 ± 0.31 | 0.76 ± 0.20 | ||||
| SCI | 0.88 ± 0.31↓* | 0.93 ± 0.17↓* | 0.95 ± 0.22↑‡ | 0.86 ± 0.26 | 0.87 ± 0.23 | 0.77 ± 0.21 | ||||
| Sensory tracts | ||||||||||
| TD | 0.69 ± 0.25 | 0.75 ± 0.21 | 0.69 ± 0.26 | 0.85 ± 0.44 | 0.93 ± 0.47 | 0.81 ± 0.26 | ||||
| SCI | 0.72 ± 0.38 | 0.62 ± 0.20↓† | 0.68 ± 0.19 | 0.83 ± 0.28 | 0.85 ± 0.32 | 0.71 ± 0.24 | ||||
Unit for RD is given in 10−3 mm2/s.
Bold indicates significantly different DTI value in SCI subjects than in TD.
↓ indicates decreased DTI value; ↑ indicates increased DTI value in SCI compared with TD.
p < 0.05, ‡p < 0.001, †p < 0.01 indicates significant difference from TD.
RD, radial diffusivity; SC, spinal cord; MRI, magnetic resonance imaging; TD, typically developing; SCI, spinal cord injury.
Sensory tracts
As shown in Table 2, no significant changes in FA were seen cephalad to the lesion, whereas FA was significantly decreased in the proximal (p < 0.0001) and middle (p = 0.0383) segments caudally.
AD was significantly decreased in the middle segment (p = 0.0038) of the cephalad cord and in both the proximal (p = 0.0005) and middle (p = 0.0092) segments of the caudal cord (Table 3).
MD showed a significant decrease only in the middle segment (p = 0.0008) of the cephalad cord, whereas the caudal cord did not show any significant changes (Table 4).
RD showed a significant decrease only in the middle segment (p = 0.0024) of the cephalad cord while the caudal cord did not show any significant changes (Table 5).
Group analysis of five vertebral body levels of the cord cephalad and caudal to the lesion comparing SCI to TD
Motor tracts
As shown in Figure 3A, a decrease in FA (not statistically significant) was observed in the cephalad cord at levels 1 and 2. Caudal to the lesion, FA was significantly decreased at levels −1 (p = 0.0231), −3 (p = 0.0490), and −5 (p = 0.0045).
AD was significantly decreased at levels 4 (p = 0.0055) and 5 (p = 0.0005) cephalad to the lesion. AD showed an increased trend at levels 1 and 2. In the caudal cord, AD was increased at level −1. However, AD was significantly decreased at levels −2 (p = 0.0434) and −4 (p = 0.0057) (Fig. 3B).
MD was significantly decreased at levels 4 (p = 0.0206) and 5 (p = 0.0107) in the cephalad cord. At level 1, MD (p = 0.0193) showed a significant increase. Caudally, MD was increased at level −1, whereas at levels −2, −3, −4 and −5, it was decreased. MD was statistically different at level −4 (p = 0.0348) (Fig. 3C).
RD was decreased at levels 4 and 5 in the cephalad cord. At level 1, RD (p = 0.0136) showed a significant increase. Caudally, RD was increased at level −1, whereas at levels −2, −3, −4 and −5, it was decreased (Fig. 3D).
Sensory tracts
As shown in Figure 4A, FA was increased at levels 2, 3, 4 and 5; however it was decreased at level 1 cephalad to the lesion. Caudally, FA decreased at levels −2, −3, −4 and −5, with level −1 (p = 0.0253) being significantly decreased.
Cephalad to the lesion, AD significantly decreased at levels 4 (p = 0.0101) and 5 (p = 0.0147); at level 1 it increased. In the caudal cord, AD was decreased at all levels: −1, −2, −3, −4, −5, with level −4 (p = 0.0143) being significantly decreased (Fig. 4B).
MD was decreased at levels 2, 3, 4, and 5 with MD being significantly decreased at levels 4 (p = 0.0236) and 5 (p = 0.0238) in the cephalad cord. At level 1, MD was increased cephalad to the lesion. Caudally, MD was increased at level −1; however, it decreased at levels −2, −3, −4, and −5 (Fig. 4C).
RD was decreased at levels 2, 3, 4, and 5 in the cephalad cord except for level 1, where RD was increased. Caudally, RD was increased at level −1; however, it decreased at levels −2, −3, −4, and −5 (Fig. 4D).
Correlation between DTI metrics and clinical scores
The ISNCSCI total motor score showed significant correlation for FAcephalad (p = 0.0258), ADcephalad (p = 0.0383), and ADcaudal (p = 0.0231) (Table 6). The ISNCSCI total light touch sensory score had significant correlation for ADcephalad (p = 0.0046) and ADcaudal (p = 0.0447) (Table 6). Table 7 shows the stepwise regression model with the dependent variables used for analysis with their likely ratios and confidence intervals for motor tracts and sensory tracts cephalad and caudal to the lesion.
Table 6.
Effect of ISNCSCI Scores (Total Motor and Total Light Touch Sensory Scores) on DTI Metrics Obtained from All Levels of Motor Tracts Only and Sensory Tracts Only Cephalad and Caudal to the Lesion
| DTI metrics (motor tracts) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FAcephalad | FAcaudal | MDcephalad | MDcaudal | ADcephalad | ADcaudal | RDcephalad | RDcaudal | |
| Coefficients | 0.00380 | 0.00151 | 0.00285 | 0.00492 | 0.01022 | 0.00944 | −0.00082 | 0.00266 |
| Std. Error | 0.00168 | 0.00096 | 0.00396 | 0.00301 | 0.00488 | 0.00409 | 0.00410 | 0.00264 |
| p value | 0.0258 | 0.1184 | 0.4723 | 0.1058 | 0.0383 | 0.0231 | 0.8413 | 0.3161 |
| DTI metrics (sensory tracts) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FAcephalad | FAcaudal | MDcephalad | MDcaudal | ADcephalad | ADcaudal | RDcephalad | RDcaudal | |
| Coefficients | 0.00069 | −0.00034 | 0.00183 | 0.00169 | 0.00409 | 0.00271 | 0.00070 | 0.00118 |
| Std. Error | 0.00059 | 0.00031 | 0.00116 | 0.00098 | 0.00142 | 0.00133 | 0.00121 | 0.0008 |
| P value | 0.2456 | 0.2843 | 0.1170 | 0.0887 | 0.0046 | 0.0447 | 0.5612 | 0.1802 |
Bold indicates significantly different DTI value in SCI subjects.
ISNCSCI, International Standard of Neurological Classification for Spinal Cord Injury; DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity.
Table 7.
Explanation of the Stepwise Regression Model with the Dependent Variables Used for Analysis with Their Likely Ratios and Confidence Intervals for Motor Tracts Only and Sensory Tracts Only Cephalad and Caudal to the Lesion
| DTI metrics (motor tracts) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FAcephalad | FAcaudal | MDcephalad | MDcaudal | ADcephalad | ADcaudal | RDcephalad | RDcaudal | |
| t-Ratio | 2.26 | 1.58 | 0.72 | 1.63 | 2.09 | 2.31 | −0.20 | 1.01 |
| Lower 95% CI | 0.000466 | −0.000394 | −0.00499 | −0.001062 | 0.000558 | 0.001321 | −0.008953 | −0.002585 |
| Upper 95% CI | 0.007144 | 0.003432 | 0.010709 | 0.010914 | 0.019894 | 0.017567 | 0.007304 | 0.007918 |
| DTI metrics (sensory tracts) | ||||||||
|---|---|---|---|---|---|---|---|---|
| FAcephalad | FAcaudal | MDcephalad | MDcaudal | ADcephalad | ADcaudal | RDcephalad | RDcaudal | |
| t-Ratio | 1.17 | −1.08 | 1.58 | 1.72 | 2.88 | 2.03 | 0.58 | 1.35 |
| Lower 95% CI | −0.000481 | −0.000973 | −0.000467 | −0.000261 | 0.001284 | 6.574e-5 | −0.001699 | −0.000558 |
| Upper 95% CI | 0.001864 | 0.000288 | 0.004142 | 0.003654 | 0.006907 | 0.005364 | 0.003117 | 0.002933 |
DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity; RD, radial diffusivity, CI, confidence interval.
Discussion
This study demonstrates for the first time changes in diffusion characteristics of the motor and sensory WM tracts at the segments of the cord cephalad and caudal to an SC lesion in children with chronic SCI compared with TD. An a priori theory was that there is more abnormality in the adjacent perilesional (proximal cord cephalad and caudal to the lesion) WM. Our data demonstrated abnormal DTI values cephalad and caudal to the SCI lesion that may correlate to pathological changes in the motor and sensory tracts.
Previous DTI studies in animal models of SCI have shown changes within the SC cephalad and caudal to the injury site.3,4,19,20 Previous human adult DTI studies have shown that diffusion characteristics in the SCI subjects and healthy subjects differed in the cervical SC WM regions that appeared normal on conventional MRI.10–13 In an adult cervical DTI study, Kim and coworkers showed that FA and MD of the WM areas were significantly lower and higher respectively at only one level cephalad and caudal to the site of injury in SCI patients compared with controls.13 Cohen-Adad and coworkers studied the diffusion parameters in the normal-appearing WM areas of adult cervical SC on MRI at vertebral levels remote from the lesion, and showed a significant decrease in FA and AD and increase in RD in SCI patients compared with controls.10 In our study, of pediatric TD and SCI subjects, DTI changes were examined in the entire cervical and thoracic SC.
Decreasing FA values reflects anterograde and retrograde degeneration of the WM tracts.21 Histologically, axonal loss, demyelination, gliosis, astrocytic scarring, and an increase in the extracellular matrix have been shown in the secondary degeneration of the WM tracts.22 The DTI findings that we observed may represent a component of the late consequences of secondary axonal injury, which include Wallerian degeneration. The precise mechanisms of Wallerian degeneration and associated post-injury-mediated cellular and membrane receptors cascades are poorly understood. There are metabolic receptor processes that likely contribute to the degenerative changes in the SC. In SCI, in ex vivo experiments, upregulation of sulfonylurea receptor 1 (Sur1)-regulated non-selective cation (NC)Ca-ATP channels, which leads to edema, hemorrhage, and cell death has been described.23 It has also been suggested in an SCI animal model that intra-axonal Ca2+ store channels, ryanodine receptor (RyR), or inositol 1,4,5-trisphosphate receptors (IP3R) contribute to secondary axonal degeneration following SCI.24 From a DTI perspective, the lower FA in SCI subjects might be the result of the reduced number of fibers and associated increased diffusivity in the extracellular space.25 In our study, the decreased FA and AD values seen in the segments of the cord, cephalad and caudal to the lesion for both motor and sensory tracts, may be associated with the anterograde and retrograde degeneration of the axonal tracts in the cord.
Cohen-Adad and coworkers observed changes in DTI metrics cephalad to the lesion on the dorsal aspect of the cord and caudal to the lesion on the ventral and lateral aspect, suggesting anterograde degeneration of ascending tracts cephalad to the injury and anterograde degeneration of descending tracts caudal to injury in cats.19 Cohen-Adad and coworkers also observed significant changes in the dorsal aspect caudal to the injury and in the ventral and lateral aspect cephalad to the injury indicating that retrograde degeneration also occurred consistent with disruption of several axonal transport mechanisms after spinal lesion.19,26 Similarly in our study, when comparing FA and AD changes in the motor tracts, we found that both FA and AD were significantly decreased in the proximal and middle segments of the caudal cord, suggesting anterograde degeneration of the tracts caudal to the lesion. However, in the cephalad cord, we found more significant AD abnormality as compared with FA, consistent with a greater degree of retrograde degeneration. In the sensory tracts, we found a similar pattern of lower FA and AD in the proximal and middle caudal cord, suggesting retrograde degeneration. In the cephalad cord, FA did not show any significant changes, whereas AD showed significant decrease in the middle segment consistent with the anterograde degeneration.
We found increased AD value in the proximal cephalad cord in the motor tracts above the SC injury. This was different than the typical pattern of lower AD in regions with cord injury. Tang and coworkers described that within an individual imaging voxel in an atrophied cord, there may be a decrease in axonal caliber, which may increase relative neuronal density that could cause an increase in AD.27
In the current study, MD and RD showed significantly increased values in the proximal segment of the cephalad cord and statistically decreased DTI values in the middle and distal segments of the cephalad cord in the motor tracts. Additionally, in the sensory tracts, MD and RD showed statistically decreased values only in the middle cephalad cord. The increased MD and RD values in our study in the proximal cephalad cord is in agreement with the previous studies showing a significant increase in the upper cervical cord levels.12,13
In the chronic stages of SCI, progressive demyelination even in areas remote to the injury site, and subsequent remyelination, results a preferential loss of large diameter axons as well as a permanent decrease in axon diameter in surviving axons.28–30 As the mean diameter of axons decreases, the spacing between axons also decreases and axons become more densely packed.31 As the axons are densely packed, the water molecules cannot diffuse larger distances, as more barriers are encountered contributing to a decrease in extracellular volume fraction. Our results of decreased MD and RD diffusivity values in the motor and sensory tracts cephalad to the lesion may be the result of structural changes in the SC caused by demyelination and chronic atrophy of the cord, causing the axons to be more densely packed.
Additionally, we performed analysis at five vertebral body levels cephalad and caudal to the lesion to examine the more detailed DTI changes in the immediate perilesional SC regions. Overall, these DTI values followed a similar pattern compared with the group analysis that examined the proximal third, middle third, and distal third SC segments cephalad and caudal to the lesion. To our knowledge, this is the first human study to analyze the perilesional changes in DTI at multiple vertebral body levels.
We tested the correlation of the ISNCSCI total motor score to the right and left lateral and ventral WM regions as well as the correlation with the ISNCSCI total light touch sensory score to the dorsal WM regions. We observed that the ISNCSCI total motor score significantly correlated with AD values of all levels of the motor tracts both cephalad and caudal to the lesion; however, with FA values it was correlated cephalad to the lesion only. Additionally, ISNCSCI total light touch sensory score significantly correlated with AD values of all levels of the sensory tracts both cephalad and caudal to the lesion. Overall, FA and AD had a better correlation than MD and RD with the ISNCSCI total motor score and the ISNCSCI light touch sensory score.
It is important to note that in regions of the cord that had a normal MRI and clinical ISNCSCI examination, statistical differences in DTI metrics were seen, primarily FA and AD, which illustrates that these DTI metrics are potentially more sensitive than ISNCSCI scores at detecting damaged SC tissue. In the clinical examination, the cord cephalad to the lesion can generally be assessed; however, the caudal boundary of abnormality is more difficult to discern.32 Hence, DTI may provide additional information on the viability of SC tissue above and below an SC lesion level, which is particularly important when considering newly developing interventions.
One of the limitations of the study was the use of manual ROI selection, which may have introduced partial volume averaging of the surrounding cerebrospinal fluid and adjacent gray matter. Currently, no automatic ROI methods exist, and manual segmentation is operator dependent and time consuming. Therefore, for future analysis, an automated or semi-automated segmentation method is required for accurately delineating WM. Another limitation is the resolution and signal-to-noise ratio of the DTI images, which can be improved with higher field strengths and improved coils with shorter imaging times. Cardiac gating was not used in this study. The lower cervical, thoracic, and thoracolumbar levels are the most sensitive to cardiac motion. Therefore, some cardiac-related artifacts may have biased the DTI values. Although this may be considered a limitation in this study, it is important to note that gating increases the acquisition time by a factor of 3–6, which would be hard for pediatric subjects to tolerate, and would increase the probability of voluntary subject movements. The age range of the TD and SCI subjects was fairly broad, which could have impacted the values observed in these subjects. The low number of subjects, both males and females, might have impacted the results. Future work with a larger number of subjects is needed to validate DTI data for the WM tracts. Further research with a larger number of SCI subjects including all American Spinal Injury Association Impairment Scale (AIS) grades would be important to further validate DTI. Specifically, there was a wide range of time since injury and the first DTI examination in our study, and future studies could look at grouping patients with similar time delays between injury and DTI scans.
Conclusion
This study showed significant alteration in FA and AD values of the motor and sensory tracts at different segments of the SC both cephalad and caudal to the lesion in pediatric subjects with chronic SCI compared with TD. In contrast, conventional MRI showed no cord signal abnormalities in these regions. This suggests that FA and AD have the potential to be sensitive biomarkers of the full extent of cord injury, and might be useful in detecting remote injuries to the SC and be complementary to the clinical examination. This may be helpful to determine the regions of the cord that can functionally be improved with evolving treatment therapies such as stem cell therapy for neuronal replacement and axon remyelination.
Acknowledgments
This work was supported by National Institute of Neurological Disorders of the National Institutes of Health under award number R01NS079635 to Drs. Mohamed and Mulcahey.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Karimi-Abdolrezaee S., Eftekharpour E., Wang J., Schut D., and Fehlings M.G. (2010) Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J. Neurosci. 30, 1657–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishimura S., Yasuda A., Iwai H., Takano M., Kobayashi Y., Nori S., Tsuji O., Fujiyoshi K., Ebise H., Toyama Y., Okano H., and Nakamura M. (2013) Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol. Brain 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deo A.A., Grill R.J., Hasan K.M., and Narayana P.A. (2006) In vivo serial diffusion tensor imaging of experimental spinal cord injury. J. Neurosci. Res. 83, 801–810 [DOI] [PubMed] [Google Scholar]
- 4. Sundberg L.M., Herrera J.J., and Narayana P.A. (2010) In vivo longitudinal MRI and behavioral studies in experimental spinal cord injury. J. Neurotrauma 27, 1753–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim J., Song S., Burke D., Magnuson D.S. (2012) Comprehensive locomotor outcomes correlate to hyperacute diffusion tensor measures after spinal cord injury in the adult rat. Exp. Neurol. 235, 188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kakulas B.A. (2004) Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord 42, 549–563 [DOI] [PubMed] [Google Scholar]
- 7. Norenberg M.D., Smith J., and Marcillo A. (2004) The pathology of human spinal cord injury: defining the problems. J. Neurotrauma 21, 429–440 [DOI] [PubMed] [Google Scholar]
- 8. Buss A., and Schwab M.E. (2003) Sequential loss of myelin proteins during Wallerian degeneration in the rat spinal cord. Glia 42, 424–432 [DOI] [PubMed] [Google Scholar]
- 9. George R., Griffin J.W. (1994) Delayed macrophage responses and myelin clearance during Wallerian degeneration in the central nervous system: the dorsal radiculotomy model. Exp. Neurol. 129, 225–236 [DOI] [PubMed] [Google Scholar]
- 10. Cohen-Adad J., El Mendili M.M., Lehericy S., Pradat P.F., Blancho S., Rossignol S., and Benali H. (2011) Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 55, 1024–1033 [DOI] [PubMed] [Google Scholar]
- 11. Petersen J.A., Wilm B.J., von Meyenburg J., Schubert M., Seifert B., Najafi Y., Dietz V., and Kollias S. (2012) Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J. Neurotrauma 29, 1556–1566 [DOI] [PubMed] [Google Scholar]
- 12. Koskinen E., Brander A., Hakulinen U., Luoto T., Helminen M., Ylinen A., and Ohman J. (2013) Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J. Neurotrauma 30, 1587–1595 [DOI] [PubMed] [Google Scholar]
- 13. Kim S.Y., Shin M.J., Chang J.H., Lee C.H., Shin Y.I., Shin Y.B., and Ko H.Y. (2015) Correlation of diffusion tensor imaging and phase-contrast MR with clinical parameters of cervical spinal cord injuries. Spinal Cord 53, 608–614 [DOI] [PubMed] [Google Scholar]
- 14. Kirshblum S.C., Waring W., Biering-Sorensen F., Burns S.P., Johansen M., Schmidt-Read M., Donovan W., Graves D., Jha A., Jones L., Mulcahey M.J., and Krassioukov A. (2011) Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J. Spinal Cord Med. 34, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finsterbusch J. (2012) Improving the performance of diffusion-weighted inner field-of-view echo-planar imaging based on 2D-selective radiofrequency excitations by tilting the excitation plane. J. Magn. Reson. Imaging 35, 984–992 [DOI] [PubMed] [Google Scholar]
- 16. Saksena S., Middleton D.M., Krisa L., Shah P., Faro S.H., Sinko R., Gaughan J., Finsterbusch J., Mulcahey M.J., and Mohamed F.B. (2016) Diffusion tensor imaging of the normal cervical and thoracic pediatric spinal cord. AJNR Am. J. Neuroradiol. 37, 2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Middleton D.M., Mohamed F.B., Barakat N., Hunter L.N., Shellikeri S., Finsterbusch J., Faro S.H., Shah P., Samdani A.F., and Mulcahey M.J. (2014) An investigation of motion correction algorithms for pediatric spinal cord DTI in healthy subjects and patients with spinal cord injury. Magn. Reson. Imaging 32, 433–439 [DOI] [PubMed] [Google Scholar]
- 18. Chang L.C., Jones D.K., and Pierpaoli C. (2005) RESTORE: Robust estimation of tensors by outlier rejection. Magn. Reson. Med. 53, 1088–1095 [DOI] [PubMed] [Google Scholar]
- 19. Cohen-Adad J., Leblond H., Delivet-Mongrain H., Martinez M., Benali H., Rossignol S. (2011) Wallerian degeneration after spinal cord lesions in cats detected with diffusion tensor imaging. Neuroimage 57, 1068–1076 [DOI] [PubMed] [Google Scholar]
- 20. Brennan F.H., Cowin G.J., Kurniawan N.D., and Ruitenberg M.J. (2013) Longitudinal assessment of white matter pathology in the injured mouse spinal cord through ultra-high field (16.4 T) in vivo diffusion tensor imaging. Neuroimage 82, 574–585 [DOI] [PubMed] [Google Scholar]
- 21. Beirowski B., Adalbert R., Wagner D., Grumme D.S., Addicks K., Ribchester R.R., and Coleman M.P. (2005) The progressive nature of Wallerian degeneration in wildtype and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buss A., Brook G.A., Kakulas B., Martin D., Franzen R., Schoenen J., Noth J., and Schmitt A.B. (2004) Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127, 34–44 [DOI] [PubMed] [Google Scholar]
- 23. Simard J.M., Woo S.K., Schwartzbauer G.T., and Gerzanich V. (2012) Sulfonylurea receptor 1 in central nervous system injury: a focused review. J. Cereb. Blood Flow Metab. 32, 1699–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orem B.C., Pelisch N., Williams J., Nally J.M., and Stirling D.P. (2017) Intracellular calcium release through IP3R or RyR contributes to secondary axonal degeneration. Neurobiol. Dis. 106, 235–243 [DOI] [PubMed] [Google Scholar]
- 25. Facon D., Ozanne A., Fillard P., Lepeintre J.F., Tournoux-Facon C., and Ducreux D. (2005) MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am. J. Neuroradiol. 26, 1587–1594 [PMC free article] [PubMed] [Google Scholar]
- 26. Perlson E., Maday S., Fu M.M., Moughamian A.J., and Holzbaur E.L. (2010) Retrograde axonal transport: pathways to cell death? Trends Neurosci. 33, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang Y.Y., Lu Q., Fan M., Yang Y., and Posner M.I. (2012) Mechanisms of white matter changes induced by meditation. Proc. Natl. Acad. Sci. 109, 10,570–10,574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Totoiu M.O., and Keirstead H.S. (2005) Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 486, 373–383 [DOI] [PubMed] [Google Scholar]
- 29. Potter K., and Saifuddin A. (2003) MRI of chronic spinal cord injury. Br. J. Radiol. 76, 347–352 [DOI] [PubMed] [Google Scholar]
- 30. Blight A.R., and Decrescito V. (1986) Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience 19, 321–341 [DOI] [PubMed] [Google Scholar]
- 31. Nashmi R., and Fehlings M. G. (2001) Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience 104, 235–251 [DOI] [PubMed] [Google Scholar]
- 32. Marino R.J., Ditunno J.F., Jr., Donovan W.H., and Maynard F., Jr. (1999) Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil. 80, 1391–1396 [DOI] [PubMed] [Google Scholar]