Abstract
Objectives: Acupuncture has been used for treating gastrointestinal (GI) disorders such as postoperative nausea and vomiting. Electroacupuncture (EA) accelerates GI transit following surgery and ameliorates postoperative ileus (POI) to restore colonic transit (CT); however, the mechanisms of this EA-induced restoration remain unclear. The aims of this study were to show CT following surgery and the effects of EA at ST 36 on POI induced by surgical stress (SS) in 45 conscious, male Sprague–Dawley rats.
Materials and Methods: An operation was performed in each rat, setting a cannula into the cecum to connect the proximal colon to inject markers. On the day after surgery, 20 metal radiopaque markers were administered to the proximal colon of each rat. These markers were visible throughout the GI tract on soft X-ray immediately after administration and up to 240 minutes afterward. The rats were divided into 5 groups with 9 rats in each group: (1) SS; (2) 5 days post surgery (POST-5D); (3) SS + phentolamine; (4) EA alone; and (5) EA + atropine. The EA was performed at ST 36 for 20 minutes at a frequency of 10 Hz and agents were administered in the appropriate groups before markers were administered and measurements were taken. Measurements were performed the day after surgery except in the POST 5-D group. CT was calculated by the geometric center on the images showing the CT for each rat.
Results: CT after surgery was delayed significantly and phentolamine accelerated CT. EA restored CT following surgery and atropine abolished the effect of EA on CT.
Conclusions: The current study demonstrated that surgery induced a delay in CT through the sympathetic pathway via α-adrenoreceptors; CT was restored by EA. These results suggest that EA can be used to treat POI through mediation of the autonomic nervous system.
Keywords: colonic transit, postoperative ileus, electroacupuncture, autonomic nervous system
Introduction
The primary symptoms of postoperative ileus (POI) include nausea and vomiting, inability to tolerate oral intake, abdominal distension, and delayed passage of flatus and/or stools.1 POI is a common occurrence following gastrointestinal (GI) and other types of surgery,2–5 and leads to increased morbidity and hospital costs.6–8 The pathogenesis of POI is multifactorial and involves inflammatory and humoral factors, which mediate the first phase of a reaction to surgery.1 Furthermore, colonic transit (CT) following surgery in rats is delayed via α2-adrenoceptors.9
Acupuncture is used to treat GI disorders such as postoperative nausea and vomiting.10 In a clinical study, electroacupuncture (EA) was found to be effective for reducing the time to defecation after surgery.11 It has been reported that EA stimulates distal colonic motility and accelerates CT via the sacral parasympathetic efferent pathway in normal rats.12 Enhancement of the parasympathetic pathway, which includes the vagus and pelvic nerves, accelerates colonic motility.13–15 In addition, the enteric nervous system consists of cholinergic nerves that function as excitatory muscle motor neurons.16 EA ameliorates colonic disorders, such as constipation and/or diarrhea, in rats.17,18 It was also reported that EA accelerates GI transit following surgery. However, the mechanism of EA-induced restoration of CT is still unclear.
General techniques for measuring colonic motility include the use of strain-gauge transducers and administration of nonabsorbable radioactive markers. Although the strain-gauge transducer method can record gut contractions over an extended period in conscious animals, it cannot be used to measure transit in the gut. CT can be measured using the nonabsorbable radioactive marker method, which is currently widely used. However, the sacrifice of many animals is necessary to obtain data for time-series experiments. To circumvent these problems, the current authors developed a new method that uses radiopaque markers under X-ray visualization to measure CT over a time-series in rats.
It is important to measure total CT in a time-course analysis to understand problems with colonic regulation due to complications in innervation. With the new method, the effects of surgery and EA on rat CT can be measured over a time-series. The aims of this study were to demonstrate CT following surgery on rats, to determine the effects of EA administered at the ST 36 acupoint on POI induced by surgical stress (SS), and to investigate the mechanisms of EA-induced CT restoration by using pharmacologic techniques in conscious rats.
Materials and Methods
Animals
Forty-five male Sprague–Dawley rats (weight, 200–340 g) were obtained from a commercial supplier (CLEA Japan, Inc., Tokyo, Japan). The animals were individually housed under controlled temperature (22°C–24°C), humidity, and light (a 12:12-hour light/dark cycle with the light cycle starting at 7:00 am), with ad libitum access to laboratory food and water. To ensure proper acclimatization, all rats were housed under standard conditions for at least 7 days before any experimentation. All experiments were started at 9:00 am. All procedures were performed according to the United Kingdom Home Office Guidelines on Animals (Scientific Procedures) Act of 1986 and the protocols were approved by the Animal Research Committee of the Meiji University of Integrative Medicine, Kyoto, Japan.
Operative Procedure Including SS to Induce POI
The rats were fasted overnight and anesthetized using 2% isoflurane. After a midline abdominal incision was made, an indwelling silastic cannula (inner diameter, 2 mm; outer diameter, 4 mm) was inserted into each rat's cecum and positioned to enter the proximal colon. The proximal part of the tube was moved through the left abdominal wall and tunneled beneath the skin to the posterior neck, where it was fixed to the skin.
Measurement of CT
The rats were randomly divided into 5 groups as follows: (1) an SS group (n = 9), designated as the POI group, with measurement performed on the day after surgery; (2) a POST-5D group (n = 9), designated as the recovery group (normal state), with measurement performed 5 days after surgery; (3) an SS + PA group (n = 9), with measurement performed following the administration of phentolamine on the day after surgery; (4) an EA alone group (n = 9), with EA performed before measurement on the day after surgery; and (5) an EA + A group (n = 9), with EA and administration of atropine performed before measurement on the day after surgery.
The animals were fasted for 24 hours before measurement. Twenty metal radiopaque markers (diameter, 1.5 mm) were administered to each rat's proximal colon with 1.0 mL of saline on the first or fifth day after surgery. The markers could be observed through the entire GI tract with soft X-ray visualization under light anesthesia with 2% isoflurane for ∼2 minutes from immediately after the administration of markers to 240 minutes thereafter. Nine images were captured every 30 minutes. After the measurement of CT, 1 mL of barium was administered to the proximal colon and was visible on the imaging of the entire colon. The rats were free to move in their cages under no anesthetic influence and with ad libitum access to water between the 9 periods of X-ray imaging.
Calculation of the Geometric Center
After processing the images using GIMP, version 2.8.22 software, the distance covered by the marker was calculated, and the colon was divided into 10 equal segments (ImageJ 1.50i, National Institutes of Health, Bethesda, MD). The geometric center (GC) of distribution of the radiopaque markers within the colon was calculated using the following formula for each X-ray image:
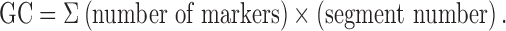 |
GC was expressed in arbitrary units.
Pharmacologic Treatment
To investigate the role of α-adrenergic receptors in mediating the effects of SS, 1 mg/kg of phentolamine (Sigma-Aldrich, St Louis, MO) with 0.5 mL of saline were administered intraperitoneally (i.p.) just before the administration of the markers.
To investigate the role of the cholinergic pathway in mediating the effects of EA on SS-induced changes in CT, 0.05 mg/kg of atropine (Mitsubishi Tanabe Pharma Corporation, Osaka, Japan) with 0.5 mL of saline were administered i.p. just before the EA.
EA Protocol
Hook-shaped needles were used to avoid spontaneous removal of inserted acupuncture needles from the rats' bodies.12,19 Before administration of the markers, stainless-steel acupuncture needles (diameter, 0.34 mm; length, 30 mm; Asahi Co., Saitama, Japan) were inserted bilaterally into the skin and underlying muscles at the ST 36 acupoint to a depth of 5 mm. The ST 36 acupoint is located ∼5 mm lateral and inferior to the anterior tubercle of the tibia. The inserted needles were connected to an electrical pulse generator (Ohm Pulser LFP-2000e, Zeniryoki Co., Fukuoka, Japan) and stimulated by an electric current (alternating current with a frequency of 10 Hz, duration of 50 ms, and intensity of 0.01 mA) for 20 minutes with each rat under anesthesia maintained with 1% isoflurane.
Statistical Analysis
Data were expressed as mean ± standard error of the mean. Statistical comparisons between groups for each period were first made using a one-way analysis of variance, and post hoc analysis was performed if differences were found. A Dunnett test was used to determine changes in GC in each group compared with those in the SS group; P < 0.05 was considered statistically significant. Data were analyzed using JMP version 12.2.0 (SAS Institute Japan).
Results
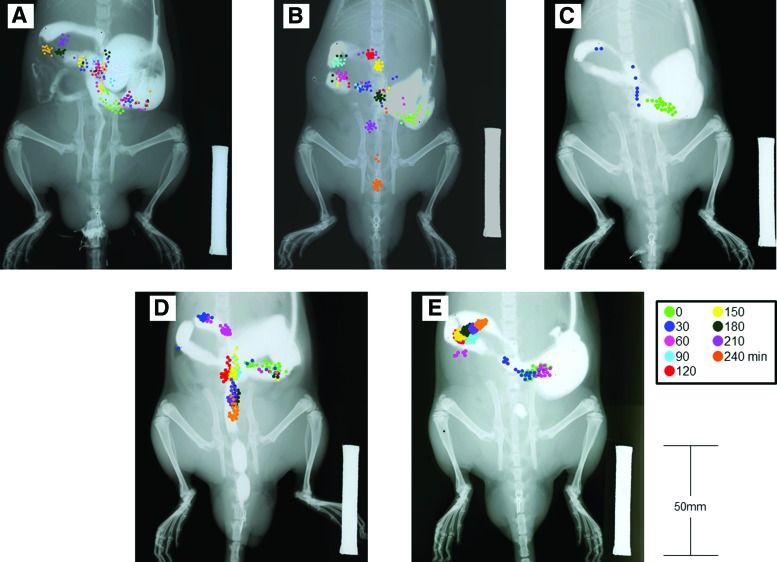
Representative images of colonic transit from all procedures in each group are shown in Figure 1. The markers were in the proximal colon from 0 to 240 minutes in the SS group (1 day after the operation; Fig. 1A). The markers reached the distal colon at 180 minutes in the POST-5D group when the rats had recovered following the operation (Fig. 1B). In the SS + PA group, some markers were excreted via the anus at 30 minutes as CT was accelerated by the action of phentolamine (Fig. 1C). Furthermore, markers were found in the distal colon at 90 minutes in the EA group, which means that CT was accelerated increasingly in the EA group more than in the SS group, and the transportation of markers was similar to that in the POST-5D group (Fig. 1D). However, markers were detected at the end of the proximal colon at 240 minutes in the EA + A group (Fig. 1E). This means that atropine neutralized the effect of EA on CT.
FIG. 1.
Imaging of each groups' colonic transit of radiopaque markers. Scale bar = 50 mm. The markers were colored differently for each time point. (A) Surgical stress (SS) group. (B) Five days after surgery [POST-5D] group. (C) SS + PA [phentolamine] group. (D) EA [electroacupuncture] alone group. (E) EA + A [atropine] group. Color images are available online.
Although no differences were found between the groups with respect to GC immediately after marker injection, significant changes from 30 to 240 minutes were noted (Table 1). At 30 and 60 minutes, GC increased significantly more in the SS + PA group than in the SS group. However, there were no differences in GC between the SS group and the other groups.
Table 1.
The Geometric Centers of All Groups at Each Time Period
| Minutes | SS | POST-5Da | SS+PA | EA | EA + A | p | |||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 21 ± 1 | 21 ± 1 | 25 ± 2 | 22 ± 1 | 25 ± 2 | 0.1681 | |||
| 30 | 40 ± 7 | 50 ± 7 | 158 ± 13 | ** | 52 ± 4 | 36 ± 6 | <0.01 | ||
| 60 | 43 ± 7 | 69 ± 13 | 166 ± 12 | ** | 78 ± 13 | 47 ± 6 | <0.01 | ||
| 90 | 42 ± 6 | 86 ± 16 | * | 169 ± 11 | ** | 89 ± 13 | * | 58 ± 6 | <0.01 |
| 120 | 47 ± 7 | 109 ± 19 | ** | 179 ± 7 | ** | 98 ± 17 | * | 70 ± 8 | <0.01 |
| 150 | 51 ± 8 | 116 ± 20 | ** | 183 ± 7 | ** | 101 ± 18 | * | 77 ± 9 | <0.01 |
| 180 | 55 ± 9 | 123 ± 19 | ** | 181 ± 9 | ** | 112 ± 15 | * | 90 ± 10 | <0.01 |
| 210 | 62 ± 9 | 131 ± 19 | ** | 184 ± 8 | ** | 111 ± 16 | * | 101 ± 12 | <0.01 |
| 240 | 72 ± 12 | 143 ± 21 | ** | 185 ± 8 | ** | 115 ± 16 | 106 ± 13 | <0.01 |
Data are expressed as mean ± standard error of the mean.
Five days after surgery.
p < 0.05; **p < 0.01; both significantly different from SS group.
SS, surgical stress; PA phentolamine; EA, electroacupuncture; A, atropine.
At 90 minutes, the GCs in the POST-5D, SS + PA, and EA groups, unlike the GC in the EA + A group, were significantly greater than the GC in the SS group. Compared to the SS group, significant changes in GC were found in the POST-5D, SS + PA, and EA groups from 120 to 210 minutes. However, no differences in GC were found between the EA + A and SS group from 120 to 210 minutes. Compared to the SS group, significant changes were found in the POST-5D and SS + PA groups at 240 minutes, while the GCs in the EA and EA + A groups did not differ from the GC in the SS group.
Discussion
Utility of the Method for Measuring CT
The advantage of the new measurement method, which used radiopaque markers under X-ray visualization, was the ability to make repeated evaluations of CT in the same rat. It was therefore possible to perform time-series experiments with smaller sample sizes. The current authors previously did measurements for 2 consecutive days to assess reproducibility.* For comparison with earlier studies,12,17 male Sprague–Dawley rats were used in the current study. Female rats was not chosen because the estrous cycle affects the autonomic nervous system (ANS) of female rats.20 Many studies on GI function reported recovery after ∼5 days.9,21 CT in the POST-5D (recovery) group was regarded as the normal state in this study.
Pathophysiology of POI
POI is characterized by cessation of transit and coordinated bowel motility after surgical intervention, which prevents effective transit of intestinal contents or tolerance to oral intake.22,23 A complex interplay among neurogenic, inflammatory, humoral, and pharmacologic factors is implicated in the development of POI.1,24–26 The physiologic stress response seems to allow a short period of intestinal paralysis after surgery with return of GI function occurring in the following stages: (1) the small bowel recovers between 0 and 24 hours; (2) the stomach recovers between 24 and 48 hours; and (3) the colon recovers between 48 and 72 hours.27 In the current study, the operation to fix the indwelling cannula was considered the SS that induced POI in the rats. Thus, although handling and incision primarily involved the cecum, the current authors reckoned that damage to the stomach and colon as well as the resulting bowel-wall inflammation pervaded the GI tract.28,29
Impairment of nerve activity occurs during and immediately after surgery, which, in turn, induces delay of GI motility.1 Pain sensations from somatic wounds to the abdominal wall and from visceral wounds caused by surgery are carried via the somatic neurons of the lower intercostal and upper lumbar nerves and the vagus nerve, respectively.30 The open abdominal surgery and cannulation of the cecum were integral aspects of the operative procedure in this study. Therefore, the afferent pathway perceived that the abovementioned nerves carried information to the central nervous system, resulting in the delayed CT observed on the first day after surgery. Neurogenically mediated GI dysmotility following surgery favors sympathetic over parasympathetic outflow as the efferent pathway.24
Catecholamines, released by sympathetic nerves, act on presynaptic parasympathetic nerves to inhibit the release of acetylcholine, which increases smooth-muscle excitability,31–33 and act directly on myocytes to stimulate production of nitric monoxide.34 After abdominal surgery, the activated sympathetic pathway inhibits upper GI motility via α2-adrenoreceptors, but not via β-adrenoreceptors.9 In this time-series study, it was investigated if PA could eliminate CT delay following surgery. PA reduced CT delay caused by SS 30 minutes after administration, with CT in the SS + PA group being faster than in the POST-5D (recovery) group. The α2-receptor agonist idazoxan was reported as the most likely candidate for controlling fecal excretion via inhibition of acetylcholine release.35 The antagonization of α-adrenoreceptors induces enhancement of the parasympathetic pathway in the colon.31,32,35 Surgically induced delay of CT is regarded as being caused by enhancement of sympathetic-nerve activity via α-adrenoreceptors.
Effect of EA on Delayed CT Induced by SS
It has been reported that EA improves CT by restraint stress via corticotropin-releasing factor (CRF) type-1 receptors and mitigates delayed gastric emptying via CRF type-2 receptors, which mediate outflow of the parasympathetic efferent pathway.36–38 Furthermore, impaired GI transit following abdominal surgery was ameliorated by EA at the ST 36 acupoint.17 EA at the ST 36 acupoint stimulated colonic motility significantly, but did not do so at BL 21, which is located 5 mm lateral to the spinous process of the twelfth thoracic vertebrae in rats.12 The current study demonstrated that EA at the ST 36 acupoint improved CT following surgery. Moreover, it has been reported that EA stimulated the distal colon, but not the proximal colon, via the pelvic nerves, and that the effect persisted for 1–3 hours.12 In the current study, EA was performed before measurement and the effect was observed from 90 to 210 minutes in the EA group. Almost all of the markers were in the proximal colon at 90 minutes in the SS group (Fig. 1A). This result suggests that EA affects the proximal colon as well as the distal colon.
Additionally, EA + A was administered to 9 rats to test if the effect of EA on the colon involved the parasympathetic pathway. No changes were found in CT following EA + A administration. A previous study reported no changes in CT following administration of 0.1 mg/kg of atropine; however, administration of a higher concentration of atropine (0.3 mg/kg) was found to delay CT significantly compared to controls.39 It was therefore unlikely that 0.05 mg/kg of atropine would delay CT directly in the current study. Given that the effect of EA on CT was neutralized by atropine, these results suggest that EA enhances the parasympathetic pathway. It is also possible that EA inhibits sympathetic nerves, as catecholamines act on presynaptic parasympathetic nerves to inhibit release of acetylcholine.31–33 Thus, the enhancement of parasympathetic nerves observed in the current study might have resulted from inhibition of sympathetic nerves by EA. Although it is unclear if EA affects parasympathetic nerves directly or through the sympathetic pathway, as the effect of EA was neutralized by atropine, this study at least revealed that EA improved CT following surgery through the parasympathetic pathway.
Opioid release after surgery inhibits GI motility peripherally via μ-opioid receptors.40 The μ-opioid receptors inhibit release of acetylcholine from the myenteric plexus in the gut.41,42 There is a possibility that peripheral opioids are involved in CT delay following surgery. Additionally, EA induces release of peripheral opioids to relieve pain.43 However, the peripheral opioids induced by EA would not lead to a disorder of colonic motility, as EA was shown in the current study to improve CT following surgery. Although pain was not evaluated in the current study as in previous reports,43 peripheral opioids might have had an analgesic effect against surgical pain. It is well-known that pain upregulates the sympathetic pathway,44 which induces CT delay. The analgesic effect of EA might play a role in improving impaired CT.
Furthermore, it has been shown that endogenous CRF inhibits gastric motor function following SS.45–47 It was also reported that the most significant effects of EA included prevention of postoperative stress and hyperglycemia in anesthetized patients.48 Intracerebroventricular injection of CRF was reported to cause delayed gastric emptying and inhibition of accelerated CT.37 EA was also shown to evoke upregulation of hypothalamic oxytocin (OXT) and inhibition of parasympathetic nerve activity, and to improve CT associated with acute and chronic heterotypic stress.18,37
This study showed that EA improved CT after surgery through the enhancement of the parasympathetic pathway. However, the involvement of EA and its effect via the sympathetic pathway, opioids, OXT, and anti-inflammatory responses are yet unclear. This study enabled description of rat CT in greater detail than has been shown in earlier studies. With the new measurement method, CT can be studied using fewer experiments and animals, which, in turn, can accelerate data accumulation.
Conclusions
This study showed that EA reduces CT delay caused by SS through the sympathetic pathway via α-adrenoreceptors. This suggests that EA can be used to treat POI through mediation of the ANS.
Acknowledgments
The authors would like to thank Sazu Taniguchi, PhD, and Hiroshi Taniguchi, PhD, for useful discussions.
Footnotes
Article on this previous research is in submission.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Bragg D, El-Sharkawy AM, Psaltis E, Maxwell-Armstrong CA, Lobo DN. Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr. 2015;34(3):367–376 [DOI] [PubMed] [Google Scholar]
- 2. Berend KR, Lombardi AV, Jr, Mallory TH, Dodds KL, Adams JB. Ileus following total hip or knee arthroplasty is associated with increased risk of deep venous thrombosis and pulmonary embolism. J Arthroplasty. 2004;19(7[suppl2]):82–86 [DOI] [PubMed] [Google Scholar]
- 3. Althausen PL, Gupta MC, Benson DR, Jones DA. The use of neostigmine to treat postoperative ileus in orthopedic spinal patients. J Spinal Disord. 2001;14(6):541–545 [DOI] [PubMed] [Google Scholar]
- 4. Finan MA, Barton DP, Fiorica JV, Hoffman MS, Roberts WS, Gleeson N, Cavanagh D. Ileus following gynecologic surgery: Management with water-soluble hyperosmolar radiocontrast material. South Med J. 1995;88(5):539–542 [PubMed] [Google Scholar]
- 5. Stanley BK, Noble MJ, Gilliland C, Weigel JW, Mebust WK, Austenfeld MS. Comparison of patient-controlled analgesia versus intramuscular narcotics in resolution of postoperative ileus after radical retropubic prostatectomy. J Urol. 1993;150(5[pt1]):1434–1436 [DOI] [PubMed] [Google Scholar]
- 6. Goldstein JL, Matuszewski KA, Delaney CP, et al. Inpatient economic burden of postoperative ileus associated with abdominal surgery in the United States. P&T. 2007;32(2):82–90 [Google Scholar]
- 7. Senagore AJ. Pathogenesis and clinical and economic consequences of postoperative ileus. Am J Health Syst Pharm. 2007;64(20[suppl13]):S3–S7 [DOI] [PubMed] [Google Scholar]
- 8. Salvador CG, Sikirica M, Evans A, Pizzi L, Goldfarb N. Clinical and economic outcomes of prolonged postoperative ileus in patients undergoing hysterectomy and hemicolectomy. P&T. 2005;30(10):590–595 [Google Scholar]
- 9. Fukuda H, Tsuchida D, Koda K, Miyazaki M, Pappas TN, Takahashi T. Inhibition of sympathetic pathways restores postoperative ileus in the upper and lower gastrointestinal tract. J Gastroenterol Hepatol. 2007;22(8):1293–1299 [DOI] [PubMed] [Google Scholar]
- 10. Cheong KB, Zhang JP, Huang Y, Zhang ZJ. The effectiveness of acupuncture in prevention and treatment of postoperative nausea and vomiting—a systematic review and meta-analysis. PLoS One. 2013;8(12):e82474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng SS, Leung WW, Mak TW, et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. 2013;144(2):307.e1–313.e1 [DOI] [PubMed] [Google Scholar]
- 12. Iwa M, Matsushima M, Nakade Y, Pappas TN, Fujimiya M, Takahashi T. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G285–G292 [DOI] [PubMed] [Google Scholar]
- 13. Langley JN, Anderson HK. On the innervation of the pelvic and adjoining viscera: Part I. The lower portion of the intestine. J Physiol. 1895;18(1–2):67–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Langley JN, Anderson HK. The innervation of the pelvic and adjoining viscera: Part VI. Histological and physiological observations upon the effects of section of the sacral nerves. J Physiol. 1896;19(4):372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil. 2010;22(6):688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv Exp Med Biol. 2014;817:39–71 [DOI] [PubMed] [Google Scholar]
- 17. Balestrini JL, Tsuchida D, Fukuda H, Pappas TN, Takahashi T. Acupuncture accelerates delayed gastrointestinal transit after abdominal surgery in conscious rats. Scand J Gastroenterol. 2005;40(6):734–735 [DOI] [PubMed] [Google Scholar]
- 18. Yoshimoto S, Babygirija R, Dobner A, Ludwig K, Takahashi T. Anti-stress effects of transcutaneous electrical nerve stimulation (TENS) on colonic motility in rats. Dig Dis Sci. 2012;57(5):1213–1221 [DOI] [PubMed] [Google Scholar]
- 19. Imai K, Ariga H, Chen C, Pappas TN, Takahashi T. Effects of electroacupuncture on gastric motility and heart rate variability in conscious rats. Auton Neurosci. 2008;138(1–2):91–98 [DOI] [PubMed] [Google Scholar]
- 20. Pinkham MI, Barrett CJ. Estradiol alters the chemosensitive cardiac afferent reflex in female rats by augmenting sympathoinhibition and attenuating sympathoexcitation. Clin Exp Pharmacol Physiol. 2015;42(6):622–631 [DOI] [PubMed] [Google Scholar]
- 21. Fukuda H, Tsuchida D, Koda K, Miyazaki M, Pappas TN, Takahashi T. Impaired gastric motor activity after abdominal surgery in rats. Neurogastroenterol Motil. 2005;17(2):245–250 [DOI] [PubMed] [Google Scholar]
- 22. Vather R, Trivedi S, Bissett I. Defining postoperative ileus: Results of a systematic review and global survey. J Gastrointest Surg. 2013;17(5):962–972 [DOI] [PubMed] [Google Scholar]
- 23. Delaney CP, Leslie JB, Marks J, et al. Postoperative Ileus: Profiles, Risk Factors, and Definitions—a Framework for Optimizing Surgical Outcomes in Patients Undergoing Major Abdominal and Colorectal Surgery. Findings, Definitions, and Analysis of The Postoperative Ileus Management Council (PIMC) National Experts' Clinical Consensus Panel—Applying Landmark Evidence to Surgical Principles and Practice: Focus on the Natural History of Postoperative Ileus. Clinical Consensus Update® in General Surgery. May 1, 2006. Online document at: www.clinicalwebcasts.com/pdfs/GenSurg_WEB.pdf Accessed November12, 2018
- 24. Vather R, O'Grady G, Bissett IP, Dinning PG. Postoperative ileus: Mechanisms and future directions for research. Clin Exp Pharmacol Physiol. 2014;41(5):358–370 [DOI] [PubMed] [Google Scholar]
- 25. Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004;16(suppl2):54–60 [DOI] [PubMed] [Google Scholar]
- 26. Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003;138(2):206–214 [DOI] [PubMed] [Google Scholar]
- 27. Holte K, Kehlet H. Postoperative ileus: A preventable event. Br J Surg. 2000;87(11):1480–1493 [DOI] [PubMed] [Google Scholar]
- 28. Koscielny A, Engel D, Maurer J, Hirner A, Kurts C, Kalff JC. Impact of CCR7 on the gastrointestinal field effect. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G665–G675 [DOI] [PubMed] [Google Scholar]
- 29. Schwarz NT, Kalff JC, Türler A, Speidel N, Grandis JR, Billiar TR, Bauer AJ. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology. 2004;126(1):159–169 [DOI] [PubMed] [Google Scholar]
- 30. Kahokehr A, Sammour T, Srinivasa S, Hill AG. Metabolic response to abdominal surgery: The 2-wound model. Surgery. 2011;149(3):301–304 [DOI] [PubMed] [Google Scholar]
- 31. Yokotani K, Okuma Y, Nakamura K, Osumi Y. Release of endogenous acetylcholine from a vascularly perfused rat stomach in vitro; inhibition by M3 muscarinic autoreceptors and alpha-2 adrenoceptors. J Pharmacol Exp Ther. 1993;266(3):1190–1195 [PubMed] [Google Scholar]
- 32. Fuder H, Muscholl E. Heteroreceptor-mediated modulation of noradrenaline and acetylcholine release from peripheral nerves. Rev Physiol Biochem Pharmacol. 1995;126:265–412 [DOI] [PubMed] [Google Scholar]
- 33. Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9(11):633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kreiss C, Toegel S, Bauer AJ. Alpha2-adrenergic regulation of NO production alters postoperative intestinal smooth muscle dysfunction in rodents. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G658–G666 [DOI] [PubMed] [Google Scholar]
- 35. Croci T, Bianchetti A. Stimulation of faecal excretion in rats by alpha 2-adrenergic antagonists. J Pharm Pharmacol. 1992;44(4):358–360 [DOI] [PubMed] [Google Scholar]
- 36. Tatewaki M, Harris M, Uemura K, et al. Dual effects of acupuncture on gastric motility in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2003;285(4):R862–R872 [DOI] [PubMed] [Google Scholar]
- 37. Iwa M, Nakade Y, Pappas TN, Takahashi T. Electroacupuncture elicits dual effects: Stimulation of delayed gastric emptying and inhibition of accelerated colonic transit induced by restraint stress in rats. Dig Dis Sci. 2006;51(8):1493–1500 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi T. Mechanism of acupuncture on neuromodulation in the gut—a review. Neuromodulation. 2011;14(1):8–12 [DOI] [PubMed] [Google Scholar]
- 39. Haga K, Asano K, Fukuda T, Kobayakawa T. The function of 5-HT3 receptors on colonic transit in rats. Obes Res. 1995;3(suppl5):801S–810S [DOI] [PubMed] [Google Scholar]
- 40. Fukuda H, Suenaga K, Tsuchida D, et al. The selective mu opioid receptor antagonist, alvimopan, improves delayed GI transit of postoperative ileus in rats. Brain Res. 2006;1102(1):63–70 [DOI] [PubMed] [Google Scholar]
- 41. Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988;40(2):121–162 [PubMed] [Google Scholar]
- 42. Yokotani K, Osumi Y. Involvement of mu-receptor in endogenous opioid peptide-mediated inhibition of acetylcholine release from the rat stomach. Jpn J Pharmacol. 1998;78(1):93–95 [DOI] [PubMed] [Google Scholar]
- 43. Taguchi R, Taguchi T, Kitakoji H. Involvement of peripheral opioid receptors in electroacupuncture analgesia for carrageenan-induced hyperalgesia. Brain Res. 2010;1355:97–103 [DOI] [PubMed] [Google Scholar]
- 44. Nathan PW. Pain and the sympathetic system. J Auton Nerv Syst. 1983;7(3–4):363–370 [DOI] [PubMed] [Google Scholar]
- 45. Barquist E, Bonaz B, Martinez V, Rivier J, Zinner MJ, Taché Y. Neuronal pathways involved in abdominal surgery–induced gastric ileus in rats. Am J Physiol. 1996;270(4[pt2]):R888–R894 [DOI] [PubMed] [Google Scholar]
- 46. Luckey A, Wang L, Jamieson PM, et al. Corticotropin-releasing factor receptor 1–deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125(3):654–659 [DOI] [PubMed] [Google Scholar]
- 47. Taché Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain–gut motor response to stress. Can J Gastroenterol. 1999;13(supplA):18A–25A [DOI] [PubMed] [Google Scholar]
- 48. Grech D, Li Z, Morcillo P, Kalyoussef E, Kim DD, Bekker A, Ulloa L. Intraoperative low-frequency electroacupuncture under general anesthesia improves postoperative recovery in a randomized trial. J Acupunct Meridian Stud. 2016;9(5):234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]