Abstract
A hallmark of the progressive cascade of damage referred to as secondary spinal cord injury (SCI) is vascular disruption resulting in decreased oxygen delivery and loss of mitochondria homeostasis. While therapeutics targeting restoration of single facets of mitochondrial function have proven largely ineffective clinically post-SCI, comprehensively addressing mitochondrial function via pharmacological stimulation of mitochondrial biogenesis (MB) is an underexplored strategy. This study examined the effects of formoterol, a mitochondrial biogenic Food and Drug Administration-approved selective and potent β2-adrenoreceptor (ADRB2) agonist, on recovery from SCI in mice. Female C57BL/6 mice underwent moderate SCI using a force-controlled impactor-induced contusion model, followed by daily formoterol intraperitoneal administration (0.1 mg/kg) beginning 1 h post-SCI. The SCI resulted in decreased mitochondrial protein expression, including PGC-1α, in the injury and peri-injury sites as early as 3 days post-injury. Formoterol treatment attenuated this decrease in PGC-1α, indicating enhanced MB, and restored downstream mitochondrial protein expression to that of controls by 15 days. Formoterol-treated mice also exhibited less histological damage than vehicle-treated mice 3 days after injury—namely, decreased lesion volume and increased white and gray matter sparing in regions rostral and caudal to the injury epicenter. Importantly, locomotor capability of formoterol-treated mice was greater than vehicle-treated mice by 7 days, reaching a Basso Mouse Scale score two points greater than that of vehicle-treated SCI mice by 15 days. Interestingly, similar locomotor restoration was observed when initiation of treatment was delayed until 8 h post-injury. These data provide evidence of ADRB2-mediated MB as a therapeutic approach for the management of SCI.
Keywords: β2-adrenoreceptor, formoterol, mitochondrial biogenesis, recovery, spinal cord injury
Introduction
There are estimated to be 180,000 new cases of spinal cord injury (SCI) yearly worldwide, and while individuals at any age can fall victim, the majority of injuries occur in males younger than age 30.1,2 The only pharmacological intervention for the management of SCI is the anti-inflammatory methylprednisolone, which has marginal effects in only a small subpopulation of patients and, as of 2013, was no longer recommended for the management of acute SCI by the “Guidelines for the management of acute cervical spine and spinal cord injuries.”3,4 Given the lack of therapies capable of restoring function to affected regions, this relatively young patient population is dependent on healthcare support for most of their lifetime, emphasizing the need for more effective therapeutics for the management of SCI.
Spinal cord injury occurs in two phases: primary injury, or the immediate mechanical trauma to the spinal cord, and secondary injury, which is initiated shortly after injury and can continue for months to years.5 The initial injury disrupts the vasculature around the spinal cord, leading to vasoconstriction, hemorrhage, edema, hypoperfusion, and ischemia, directly contributing to secondary injury.6,7 Interestingly, the degree of functional loss has been shown to be proportional to the degree of ischemia post-SCI, indicating the significant impact of ischemia during secondary injury.8 The subsequent local decrease in oxygen delivery reduces the ability of mitochondria to maintain cellular energetics.7,9 Neurons are highly susceptible to ischemic injury and mitochondrial dysfunction because of their strict reliance on adenosine triphosphate (ATP)-dependent processes and limited capacity to buffer oxidative stress.10,11 Loss of mitochondrial homeostasis ultimately leads to excitotoxicity, calcium overload, and neuronal cell death, all of which are characteristic of SCI and propagate secondary injury.5,12,13
Evidence suggests that pharmacological therapeutics that interrupt this secondary injury could improve neuron survival and encourage functional recovery. Furthermore, temporal data indicate that restoration of mitochondrial function shortly after injury may be a beneficial approach for the management of SCI.3,14Thus far, the majority of studies targeting mitochondria after SCI have focused on specific aspects of mitochondrial function, such as increasing antioxidant defenses or altering mitochondrial dynamics (fission/fusion).14–18 While several of such strategies have shown promise in vivo, none has yet been clinically successful. Pharmacological activation of mitochondrial biogenesis (MB), however, is a heretofore unexploited strategy for the management of SCI.
Mitochondrial biogenesis, or the repair, growth, and/or division of existing mitochondria, involves an intricate network of multiple pathways regulating various nuclear- and mitochondrial deoxyribonucleic acid (DNA)-encoded genes and is governed by peroxisomal proliferator γ coactivator-1 α (PGC-1α), the “master regulator of MB.”19,20 Hu and associates21 recently reported decreased PGC-1α expression in the spinal cord after contusion SCI in rats. In addition, both maintaining and overexpressing PGC-1α expression post-SCI reduced neuronal death and improved functional recovery.21,22 These data are suggestive of the potential therapeutic efficacy of increased PGC-1α and MB after SCI.
Our laboratory recently developed a drug discovery program to identify drugs that induce MB.23–25 Through this program, the Food and Drug Administration (FDA)-approved highly specific β2-adrenergic receptor (ADRB2) agonist formoterol was found to be a powerful inducer of MB in mice.26,27 Previously, we subjected mice to renal ischemia-reperfusion (IR) injury followed by treatment with formoterol beginning 24 h after injury, when renal dysfunction was maximal. After five days of treatment, formoterol completely restored mitochondrial and renal function, supporting the idea that pharmacological stimulation of MB can promote recovery of mitochondrial and organ function after ischemic injury.28
Interestingly, studies have shown that treatment with the ADRB2 agonist clenbuterol enhanced recovery in rats post-SCI.29–31 Recent work from our laboratory determined that formoterol and clenbuterol possess unique structural features resulting in divergent signaling pathways. Specifically, formoterol contains a longer methoxyphenyl group, which clenbuterol lacks, that extends across the ADRB2 binding pocket, and leads to activation of the Gβγ-Akt-eNOS-sGC pathway and the subsequent induction of MB.32 Therefore, formoterol is not only a more potent, selective, and long-acting ADRB2 agonist than clenbuterol, but also induces MB,26,33 giving this pharmacological agent the potential to alleviate multiple aspects of injury progression and promote recovery post-SCI. Given the importance of ischemia during secondary injury and the detrimental mitochondrial dysfunction that accompanies SCI, this study sought to determine the therapeutic potential of formoterol-mediated MB on functional recovery using an impactor-induced contusion mouse model of SCI.
Methods
Animals and SCI model
Female wild-type C57Bl/6 mice between eight and nine weeks of age were obtained from The Jackson Laboratories (Bar Harbor, ME), housed in groups of 3–5, and allowed to habituate for seven days before use. To determine the mitochondrially biogenic effect of formoterol in the spinal cord under physiological conditions, naïve mice were treated with intraperitoneally with 0.1 mg/kg formoterol once daily for 48 h, followed by euthanasia via anesthesia overdose and isolation of the T9-13 region of the spinal cord.
For SCI studies, mice were randomized into sham and SCI groups. Animals were anesthetized with ketamine 10 mg/kg and xylazine 6 mg/kg via intraperitoneal injection and continuously monitored for spontaneous breathing. Mice underwent a complete single-level laminectomy at the 10th–12th thoracic vertebrae (T10–12). The vertebral column was clamped and stabilized at the upper thoracic and lumbar levels, and a controlled contusion with a force of 80 kilodynes was administered using the Infinite Horizon IH-0400 impactor (Lexington, KY) with the dura intact. Sham mice received laminectomy only. Manual bladder expression was performed twice daily until functional recovery. Injured mice were divided further into formoterol- or vehicle-treated groups. Groups were euthanized three days, seven days, or 15 days post-SCI via anesthesia overdose, and spinal cords were isolated for analysis.
All studies were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and the University of Arizona in accordance with the guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The schematic representation of the experimental design is seen in Figure 1.
FIG. 1.
Schematic representation of experimental design.
Drug treatment
Formoterol fumarate dehydrate was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in saline/0.1% dimethyl sulfoxide to 0.01 mg/mL. Given that we have observed mitochondrially biogenic effects in mice with doses as low as 0.1 mg/kg,27 mice were treated intraperitoneally daily with either formoterol 0.1 mg/kg or vehicle control beginning 1 h or 8 h after injury.
Locomotor capability assessment
Locomotor capability was assessed using the 10-point (0–9) Basso Mouse Scale (BMS)34 by an observer blinded to the experimental groups. Each mouse was observed for 3 min, with bladder expression taking place before assessment. Animals were observed 24 h after the surgical procedure and every other day thereafter until euthanasia. Sham animals maintained a BMS score of 9 throughout the experiment.
Ribonucleic acid (RNA) and mitochondrial DNA (mtDNA) expression
The injury site and spinal cord regions approximately 5 mm rostral and caudal to the injury site (peri-injury sites) were collected and total RNA extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) based on the manufacturer's protocol. The complementary DNA (cDNA) was synthesized using the iScript cDNA Synthesis Kit and quantitative polymerase chain reaction (qPCR) performed using 500 ng of cDNA template and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA). Fold changes were calculated using the ΔΔCt method. The primers used can be found in Table 1.
Table 1.
Primers
| Target | Sense | Antisense |
|---|---|---|
| PGC-1α | 5′-AGGAAATCCGAGCGGACTGA-3′ | 5′-GCAAGAAGGCGACACATCGAA-3′ |
| ND1 | 5′-TGAATCCGAGCATCCTACC-3′ | 5′-ATTCCTGCTAGGAAAATTGG-3′ |
| β-Actin | 5′-GGGATGTTTGCTCCAACCAA-3′ | 5′-GCGCTTTTGACTCAGGATTTAA-3′ |
DNA was isolated from the injury and peri-injury spinal cord sites using the Qiagen DNeasy Blood and Tissue Kit (Valencia, CA) and 5 ng used for qPCR quantification of relative mtDNA content. ND1, a mitochondrial gene, was measured and normalized to the nuclear encoded gene β-actin.
Immunoblot
Protein was extracted from the injury and peri-injury sites of the spinal cord using radioimmunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1% Triton X-100, pH 7.4) with protease inhibitor cocktail (1:100), 1 mM sodium fluoride, and 1 mM sodium orthovanadate (Sigma-Aldrich, St. Louis, MO). Samples were agitated for 2 h at 4°C and then centrifuged at 14,000 × g for 15 min and the supernatant collected. Protein was quantified using a bicinchoninic acid assay, and 10–12 μg of protein was separated via electrophoresis using 4–15% SDS-polyacrylamide gels, then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked in 5% milk in TBST and incubated overnight with primary antibodies with constant agitation at 4°C. Membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody and visualized using chemiluminescence (Thermo Scientific, Waltham, MA) on a GE ImageQuant LAS4000 (GE Life Sciences, Pittsburgh, PA). Optical density was determined using ImageJ software. Primary antibodies used were as follows: Nrf2 (1:1000, Santa Cruz Biotechnology, Dallas, TX), PGC-1α (1:1000), TFAM (1:1000), NDUFS1 (1:1000), ATP Syn β (1:1000), α-tubulin (1:1000, Abcam, Cambridge, UK).
Histopathological analysis
Spinal cord tissues were processed as described previously.35 Briefly, mice were transcardially perfused with 0.1 M phosphate buffered saline followed by 4% paraformaldehyde (PFA). A 1 cm segment of the spinal cord centered on the injury epicenter was removed and post-fixed in PFA for 2 h, then washed in 0.2 M phosphate buffer overnight at 4°C. Tissues were then cryoprotected in 20% sucrose with 0.1% sodium azide at 4°C until the spinal cords sank (>3 days). Spinal cords were trimmed to 6 mm segments centered on the injury sight and frozen in optimal cutting temperature compound at −80°C. The entire 6 mm was cryosectioned into 10 μm coronal sections and every section collected.
Eriochrome cyanine (EC) staining for myelin was used to distinguish damaged and spared tissue.35 Slides were warmed for 60 min, then hydrated in dH2O, submerged in acetone for 2 min, and rehydrated in dH2O. Slides were exposed to serial dilutions of decreasing concentrations of ethanol and incubated in EC solution for 30 min. Selective myelin staining was obtained by differentiation in 0.3% ammonium hydroxide for 30 sec. Slides were then exposed to serial dilutions of increasing ethanol concentrations. Analyses were performed in a blinded fashion, with respect to treatment group, using an Olympus BX41 microscope (Tokyo, Japan) and ImageJ software. Lesion and spared tissue areas were quantified across 2 mm of spinal cord centered on the epicenter at 100 μm intervals using the Cavalieri method,35 totaling 21 sections per animal.
Statistical analysis
Tissue isolated from a single animal or a single animal's behavior represented n = 1. Behavioral assays were on a total of n = 8 sham mice and n = 12 injured mice per group, and data were analyzed using two-way analysis of variance (ANOVA) with repeated measures followed by the Tukey post hoc test. All data sets underwent and passed the Shapiro-Wilk normality test. Differences in mtDNA and messenger ribonucleic acid (mRNA) expression between two groups were analyzed using the two-tailed Student t test, while that of three or more groups was analyzed using a one-way ANOVA followed by the Tukey post hoc test. Differences in individual protein expression between all three groups (Sham, SCI + Vehicle, SCI + Formoterol) were analyzed using one-way ANOVA followed by the Tukey post hoc test. Two-way ANOVA was used to analyze tissue histopathology (lesion, gray and white matter area) across the spinal cord. Total lesion, gray matter and white matter volumes were analyzed using the two-tailed Student t test.
For expression analyses, different superscripts are indicative of statistically significant differences, while bars with the same superscript are not significantly different (Fig. 2–5, 6E). For body weight and BMS scores (Fig. 4, 7), “a” denotes a statistically significant difference compared with SCI + Vehicle, while “b” denotes a statistically significant difference compared with day 1. For lesions, gray matter and white matter area (Fig. 6B–D), “a” denotes a main effect of treatment. In all cases, GraphPad Prism software (La Jolla, CA) was used and a p < 0.05 was considered indicative of a statistically significant difference between mean values.
FIG. 2.
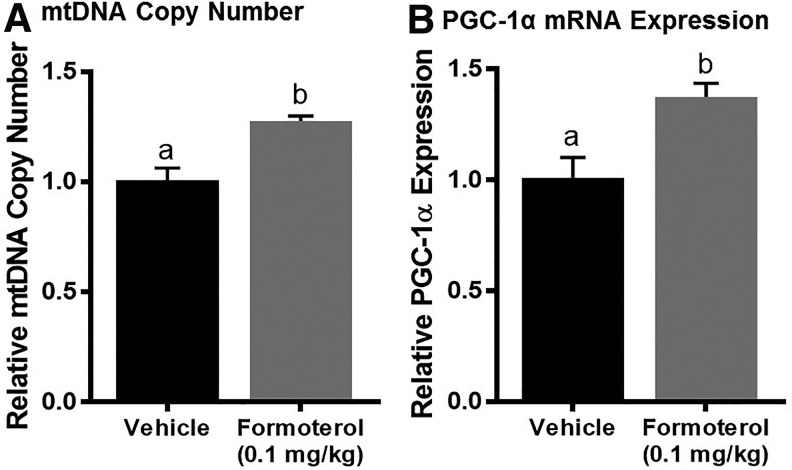
Effect of formoterol on mitochondrial biogenesis in naïve mice. Mice were treated with vehicle or formoterol (0.1 mg/kg) daily for 48 h. The spinal cord was extracted and analyzed for mitochondrial DNA (mtDNA) content (A) and peroxisomal proliferator γ coactivator-1α (PGC-1α) messenger ribonucleic acid (mRNA) expression (B). Data represent 4–6 mice per group and are expressed as mean ± standard error of the mean. Different superscripts are indicative of statistically significant differences (p < 0.05 by Student t test).
FIG. 3.
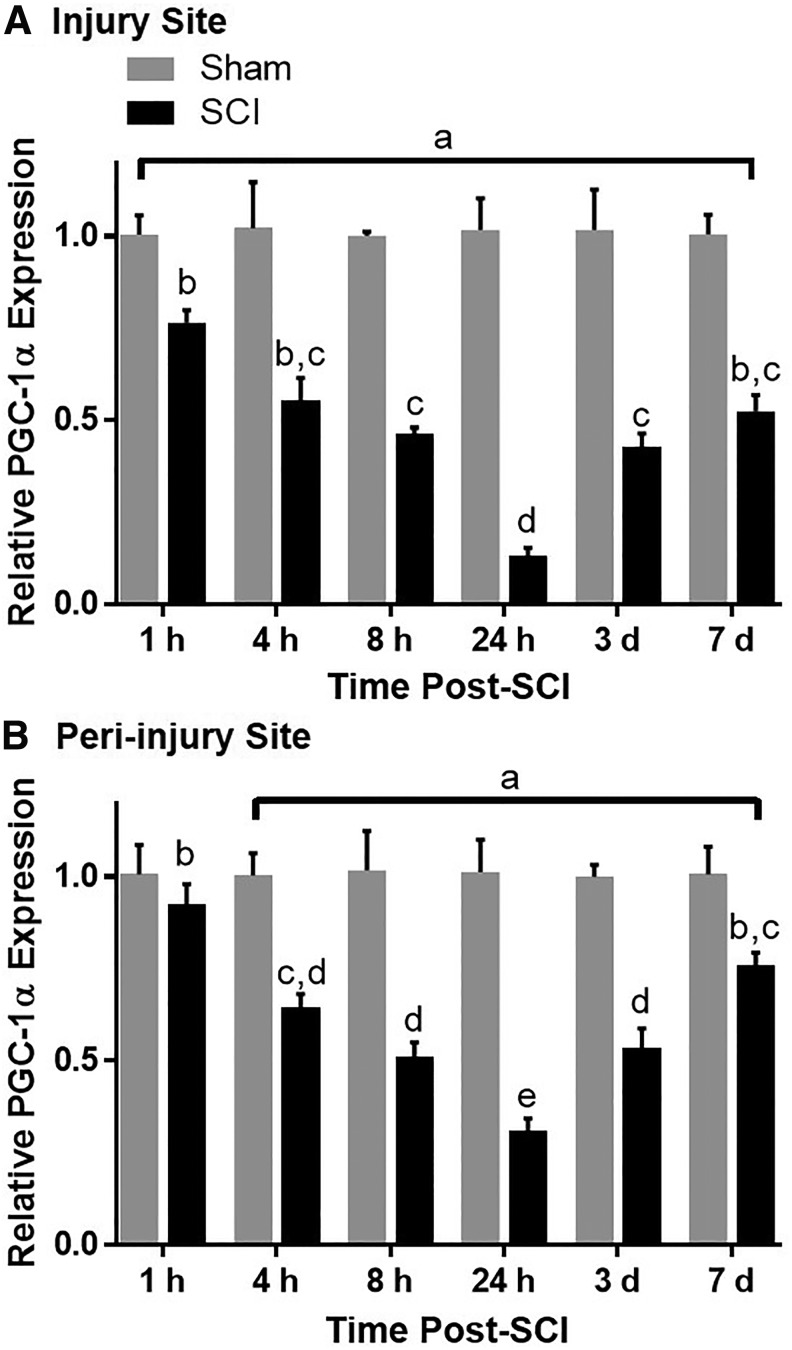
Time-dependent effects of spinal cord injury (SCI) on PGC-1α messenger ribonucleic acid (mRNA) in mice. Mice underwent moderate SCI using an 80 Kdyn force-controlled impactor induced contusion model. The injury (A) and peri-injury (B) sites were extracted and analyzed for PGC-1α mRNA expression. Data represent four mice per group and are expressed as mean ± standard error of the mean. Different superscripts are indicative of statistically significant differences in expression between time points. As denoted by “a”, all groups in A and groups 4 h–7 days in B are significantly different from their respective sham controls (p < 0.05 by two-way analysis of variance followed by Tukey post hoc test).
FIG. 4.
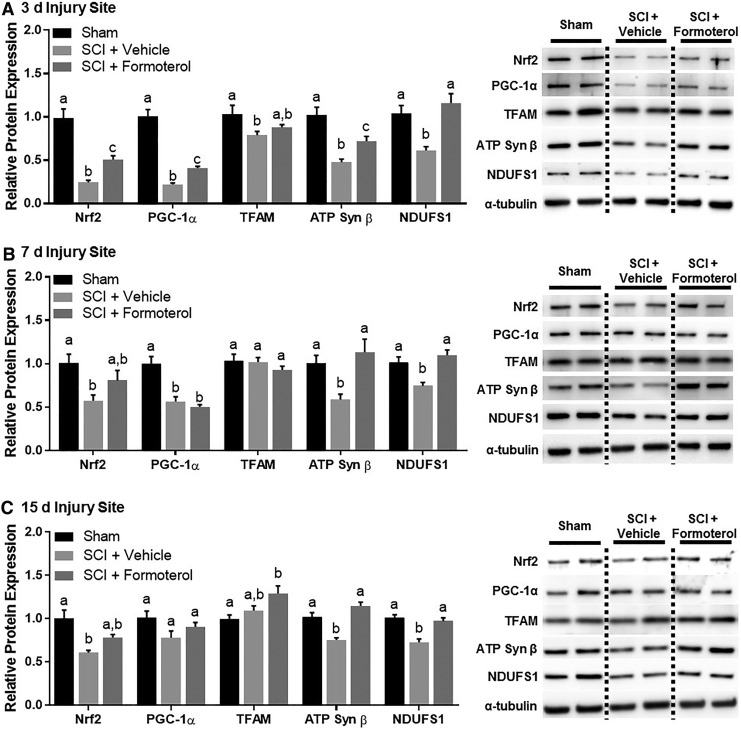
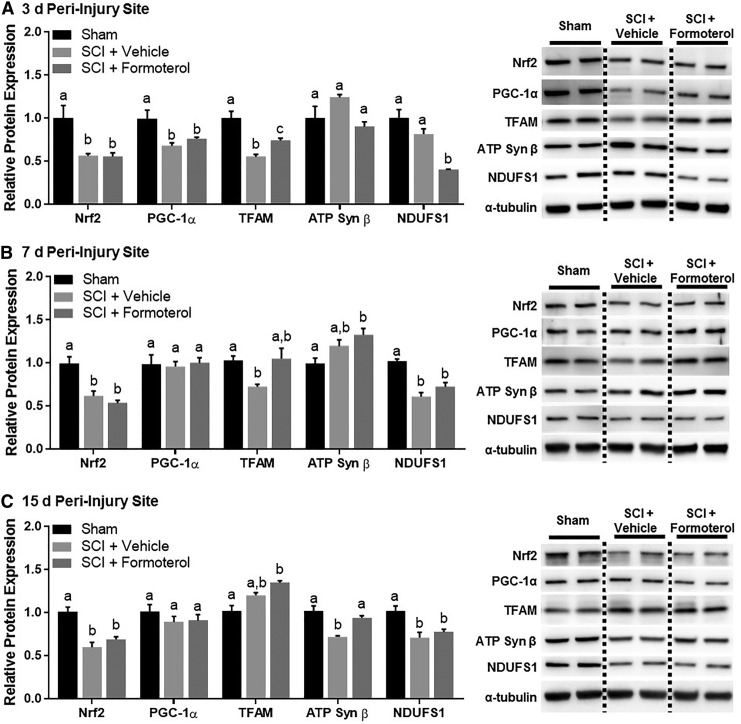
Effect of formoterol on mitochondrial proteins in the injury site of mice subjected to spinal cord injury (SCI). Mice underwent moderate SCI using an 80 Kdyn force-controlled impactor induced contusion model followed by daily intraperitoneal administration of vehicle or formoterol (0.1 mg/kg) beginning 1 h post-injury. Subsets of mice were euthanized three days (A), seven days (B), and 15 days (C) post-SCI and the injury site extracted and analyzed for protein. Data represent 5–6 mice per group and are expressed as mean ± standard error of the mean. For each protein, different superscripts are indicative of statistically significant differences in expression (p< 0.05 by one-way analysis of variance followed by Tukey post hoc test). Bars with the same superscripts are not significantly different. Nrf2, nuclear respiratory factor 2; PGC-1α, peroxisomal proliferator γ coactivator-1α; TFAM, mitochondrial transcription factor A; ATP, adenosine triphosphate; NDUFS1, nicotinamide adenine dinucleotide:ubiquinone oxidoreductase core subunit S1.
FIG. 5.
Effect of formoterol on mitochondrial proteins in the peri-injury site of mice subjected to spinal cord injury (SCI). Mice underwent moderate SCI using an 80 Kdyn force-controlled impactor induced contusion model followed by daily intraperitoneal administration of vehicle or formoterol (0.1 mg/kg) beginning 1 h post-injury. Subsets of mice were euthanized three days (A), seven days (B), and 15 days (C) post-SCI and the peri-injury site extracted and analyzed for protein. Data represent 5–6 mice per group and are expressed as mean ± standard error of the mean. For each protein, different superscripts are indicative of statistically significant differences in expression (p < 0.05 by one-way analysis of variance followed by Tukey post hoc test). Bars with the same superscripts are not significantly different. Nrf2, nuclear respiratory factor 2; PGC-1α, peroxisomal proliferator γ coactivator-1α; TFAM, mitochondrial transcription factor A; ATP, adenosine triphosphate; NDUFS1, nicotinamide adenine dinucleotide:ubiquinone oxidoreductase core subunit S1.
FIG. 6.
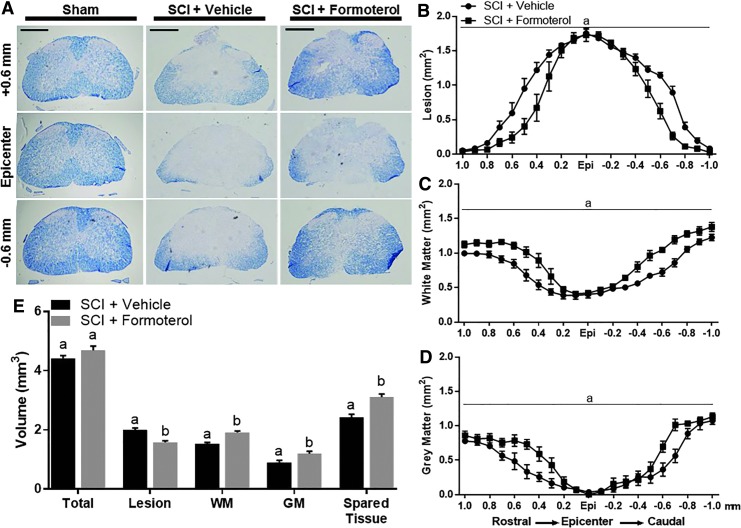
Effect of formoterol on spinal cord histopathology in injured mice. Mice underwent moderate spinal cord injury (SCI) using an 80 Kdyn force-controlled impactor induced contusion model followed by daily intraperitoneal administration of vehicle or formoterol (0.1 mg/kg) beginning 1 h post-injury and continuing for three days. Spinal cords were extracted and evenly spaced tissue sections stained with Eriochrome cyanine (A) and analyzed for lesion (B), white matter (WM) (C), and gray matter (GM) (D) volume, with “a” being indicative of a main effect of treatment (p < 0.05 by two-way analysis of variance followed by Tukey post hoc test). Panel E depicts total quantification across 2 mm of injured spinal cord and for each outcome, different superscripts are indicative of statistically significant differences (p < 0.05 by Student t test). Bars with the same superscripts are not significantly different. Data represent 5–6 mice per group and are expressed as mean ± standard error of the mean. Scale bar = 0.5 mm. Color image is available online.
FIG. 7.
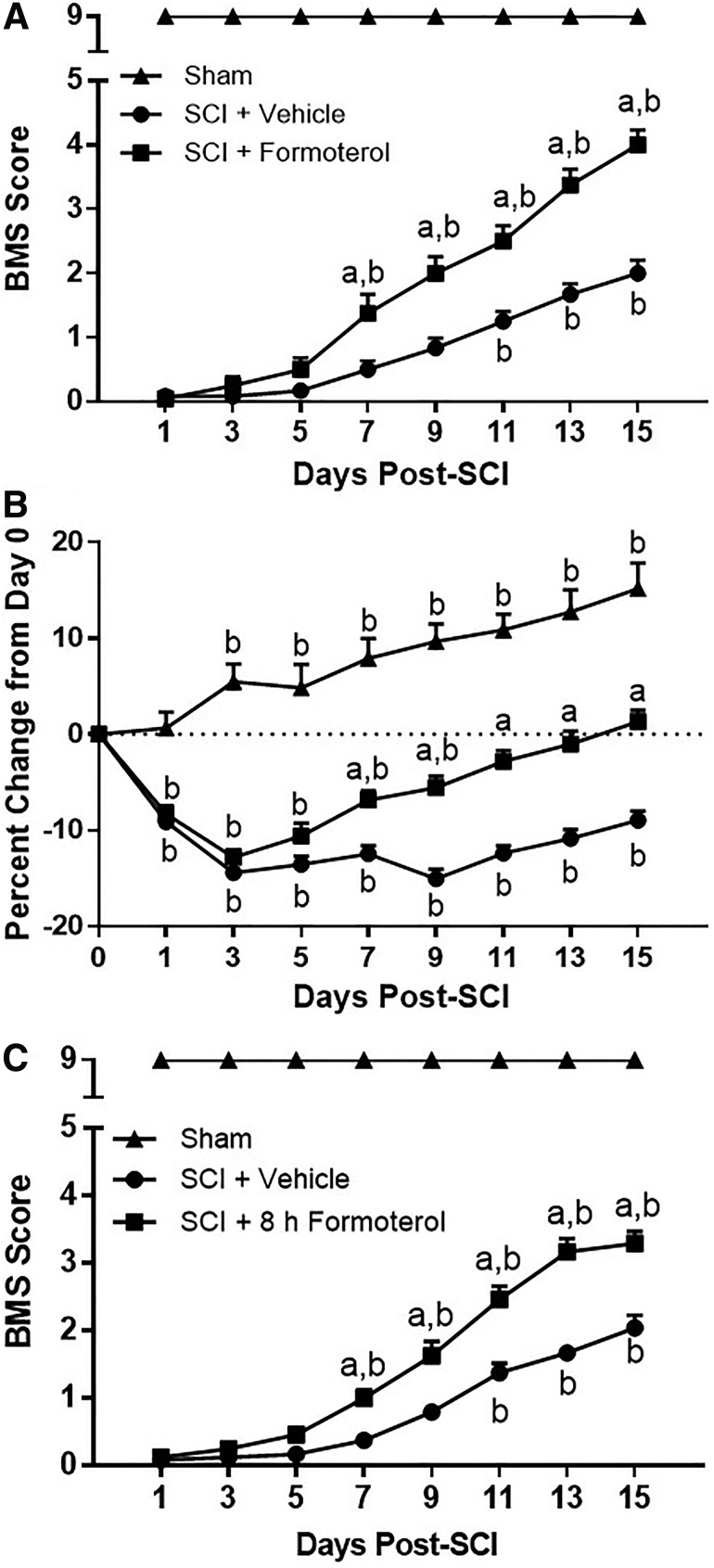
Effect of formoterol treatment on functional recovery and body weight after spinal cord injury (SCI) in mice. Mice underwent moderate SCI using an 80 Kdyn force-controlled impactor induced contusion model followed by daily intraperitoneal administration of vehicle or formoterol (0.1 mg/kg) beginning 1 h (A,B) or 8 h (C) post-injury and continuing for 15 days. Locomotor function was assessed using the Basso-Mouse Scale beginning 24 h after injury and continuing every alternate day (A,C). Body weight was assessed before injury (day 0), and every day locomotor activity was assessed (B). Data are representative of eight sham and 12 injured mice per treatment group and are expressed as mean ± standard error of the mean. Statistically significant difference compared with SCI + Vehicle is denoted by “a,” and statistically significant difference compared with the initial assessment (day 1 for A and C, day 0 for B) is denoted by “b” (p < 0.05 by two-way analysis of variance with repeated measures followed by Tukey post hoc test).
Results
Effect of formoterol on MB in the naïve spinal cord
Naïve mice were treated with formoterol 0.1 mg/kg or vehicle intraperitoneally daily for 48 h. After treatment, the T9-13 portion of the thoracic spinal cord was extracted and analyzed for mtDNA copy number (Fig. 2A) and PGC-1α mRNA expression (Fig. 2B). Both mitochondrial end-points were increased more than 25% in the spinal cord of formoterol compared with vehicle-treated mice. The 1.3–1.4-fold increases in these end-points are both significant and physiologically relevant.36–38
Time course of loss of PGC-1α expression post-SCI
Mice underwent a force-controlled impactor-induced contusion model of SCI using a force of 80 Kdyn. To determine the time-course of mitochondrial disruption after moderate SCI, PGC-1α mRNA expression was assessed in injury and peri-injury sites. Using this model, PGC-1α decreased 25% in the injury site within the first hour post-SCI, ultimately decreasing 80% by 24 h (Fig. 3A). In the peri-injury site, while unaltered at 1 h post-SCI, PGC-1α decreased 30% by 4 h, reaching 40% of sham levels by 24 h (Fig. 3B). There was a significant effect of both injury and time post-injury on PGC-1α expression in both the injury (F[1, 36] = 111, p < 0.0001; F[5,36] = 5.169, p = 0.0011) and peri-injury (F[5,36] = 4.938, p = 0.0015; F[1,36] = 106, p < 0.0001) sites.
While diminished PGC-1α expression continued to be observed in the injury and peri-injury sites three and seven days post-injury, expression began to recover in both sites at these time points, indicating that the injury was not so severe as to leave no opportunity for pharmacological intervention. PGC-1α expression was decreased compared with respective sham levels in the injury site at all time points assessed and in the peri-injury site beginning 4 h after SCI and continuing to seven days.
Effect of formoterol on mitochondrial protein expression post-SCI in the injury site
Mice underwent SCI followed by daily administration of intraperitoneal formoterol 0.1 mg/kg beginning 1 h post-injury and continuing until euthanasia. Protein expression of PGC-1α, nuclear respiratory factor 2 (Nrf2), a transcription factor induced by PGC-1α that has transcriptional control of multiple mitochondrial genes,39 mitochondrial transcription factor A (TFAM), and the mitochondrial proteins ATP synthase β (ATP Syn β), a subunit of ATP synthase of the electron transport chain (ETC), and nicotinamide adenine dinucleotide:ubiquinone oxidoreductase core subunit S1 (NDUFS1), a subunit of ETC complex I, were investigated. Analysis of the injury site three days post-SCI revealed a significant effect of injury and treatment on the expression of Nrf2 (F[2,15] = 31.77, p < 0.0001), PGC-1α (F[2,15] = 79.14, p < 0.0001), TFAM (F[2,15] = 4.64, p = 0.270), ATP Syn β (F[2,15] = 17.9, p = 0.0001) and NDUFS1 (F[2,15] = 11.15, p = 0.0011), all of which were decreased markedly in the injury site of vehicle-treated SCI mice compared with sham (Fig. 4A). Treatment with formoterol partially restored PGC-1a, Nrf2, TFAM, and ATP Syn b, and fully restored NDUFS1.
Additional analyses revealed persistent effects of injury and treatment on expression of Nrf2 (F[2,14] = 4.718, p = 0.0271), PGC-1α (F[2,14] = 23.45, p < 0.001), ATP Syn β (F[2,15] = 6.723, p = 0.0082) and NDUFS1 ([2,14] = 10.85, p = 0.0014), but not TFAM (F[2,14] = 0.7771, p = 0.4786). PGC-1α, Nrf2, ATP Syn b and NDUFS1 expression remained decreased in the injury site of SCI mice at 7 d (Fig. 4B). Formoterol treatment completely restored NDUFS1 and ATP Syn b, partially restored Nrf2, and had no effect on PGC-1a. By 15 days, only Nrf2 (F[2,15] = 11.69, p = 0.0009), ATP Syn b (F[2,15] = 21.26, p < 0.0001), and NDUFS1 (F[2,15] = 18.21, p < 0.0001) remained decreased after SCI, while PGC-1a (F[2,12] = 2.915, p = 0.0929) was equivalent to sham controls (Fig. 4C). Formoterol treatment maintained ATP Syn b and NDUFS1 and increased TFAM (F[2,14] = 5.766, p = 0.0149) beyond sham controls.
Effect of formoterol on mitochondrial protein expression post-SCI in the peri-injury site
Altered mitochondrial protein expression was also present in the peri-injury site three days post-SCI (Fig. 5A). Nrf2 (F[2,15] = 8.445, p = 0.0035), PGC-1α (F[2,15] = 6.728, p = 0.0082) and TFAM (F[2,15] = 20.75, p < 0.0001) were decreased in the peri-injury site of SCI mice compared with shams, while ATP Syn β (F[2,13] = 3.103, p = 0.0791) and NDUFS1 were not. Formoterol treatment again partially restored TFAM, but had no effect on PGC-1α or Nrf2. Interestingly, NDUFS1 (F[2,14] = 22.78, p < 0.0001) was decreased 50% in SCI mice treated with formoterol.
The Nrf2 (F[2,15] = 17.97, p = 0.0001), TFAM (F[2,14] = 4.648, p = 0.0283) and NDUFS1 (F[2,15] = 27.46, p < 0.0001) were decreased in the peri-injury site of injured mice at seven days, while there was no difference in PGC-1α (F[2,15] = 0.06426, p = 0.9380) or ATP Syn β (F[2,15] = 5.974, p = 0.0123, Fig. 5B). Formoterol treatment had no effect on Nrf2 or NDUFS1, restored TFAM expression, and increased ATP Syn β to levels greater than sham controls. At 15 days, Nrf2 (F[2,15] = 20.26, p < 0.0001), ATP Syn β (F[2,13] = 15.37, p = 0.0004) and NDUFS1 (F[2,15] = 9.267, p = 0.0024) were decreased in SCI mice, while PGC-1α (F[2,15] = 0.8784, p = 0.4358) and TFAM were not (Fig. 5C). Formoterol had no effect on Nrf2 or NDUFS1, yet maintained ATP Syn β, and again increased TFAM (F[2,14] = 10.64, p = 0.0016) to greater than sham levels.
Effect of formoterol on spinal cord histopathology three days post-SCI
Given that increases in mitochondrial protein expression were observed with formoterol as early as three days post-injury, we assessed spinal cord histopathology at this time point using Eriochrome cyanine staining for myelin (Fig. 6). Cross-sectional analysis revealed decreased lesioned tissue in formoterol- compared with vehicle-treated injured mice, particularly in the sections rostral and caudal to the epicenter (Fig. 6B). Similarly, quantification of both white (Fig. 6C) and gray matter (Fig. 6D) showed increased sparing with formoterol treatment, again in areas adjacent to the epicenter. Main effects of treatment (F[1,189] = 58.88, p < 0.0001; F[1,189] = 133.5, p < 0.0001; F[1,189] = 40.92, p < 0.0001] and spinal level (F[20,189] = 114.8, p < 0.0001; F[20,189] = 62.42, p < 0.0001; F[20,189] = 47.31, p < 0.0001) were observed with all three outcomes. Analysis of 2 mm of spinal cord centered on the injury epicenter indicated that formoterol-treated mice also had decreased lesion volume, and increased white matter, gray matter, and spared tissue volume (Fig. 6E).
Effect of formoterol on functional recovery and body weight post-SCI
Functional capabilities were assessed beginning 24 h after injury and continuing every alternate day thereafter up to 15 days post-SCI using the BMS, a 10-point scale ranging from 0 (complete hindlimb paralysis) to 9 (normal function).34 As expected, all injured mice depicted complete paralysis by 24 h post-injury, and sham controls maintained a score of 9 throughout assessment. Analysis revealed a significant effect of both formoterol treatment (F[2, 27] = 686.7, p < 0.0001) and days after injury (F[7,189] = 113.9, p < 0.0001), as well as a significant interaction (F[14,189] = 35.33, p < 0.0001). Mice treated with daily formoterol beginning 1 h after SCI displayed an increased BMS score compared with vehicle treated mice by seven days post-injury (1.2 vs. 0.5). (Fig. 7A). By 15 days post-SCI, formoterol-treated mice exhibited a BMS score of 4, indicative of occasional plantar stepping and two points higher than that of vehicle-treated injured mice, which displayed a score of 2, indicating ankle movement. In addition, SCI mice treated with formoterol presented an increased BMS score by day 7 compared with day 1, while this did not occur until day 11 in vehicle-treated mice.
Animals were weighed daily beginning before SCI (day 0) until euthanasia. The data are presented based on the weights on the day of surgery and days when locomotor activity was assessed (Fig. 7B). Analysis of all data revealed a significant effect of both formoterol treatment (F[2,32] = 98.05, p < 0.0001) and days post-injury (F[15,480] = 37.76, p < 0.0001), as well as a significant interaction (F[30,480] = 16.52, p < 0.0001). While all injured mice lost a comparable percentage of body weight post-injury, formoterol-treated mice gained body weight compared with vehicle-treated mice beginning on day 7, and reached a weight no different than their initial starting weight by day 11. Injured mice treated with vehicle did not return to pre-surgery weight at any point during the 15 day experiment.
Effect of delayed initiation of formoterol treatment on functional recovery and post-SCI
In a separate cohort of SCI mice, daily administration with formoterol 0.1 mg/kg or vehicle was delayed until 8 h post-injury and functional recovery assessed. Analysis again revealed a significant effect of formoterol treatment (F[2,21] = 1445, p < 0.0001) and days post-injury (F[7,147] = 92.27, p < 0.0001), and a significant interaction (F[14,147] = 32.36, p < 0.0001). Mice treated with vehicle alone again reached a maximum BMS score of approximately 2 by 15 days post-injury, and those treated with formoterol had a score of 3.5, slightly less than that observed when treatment was initiated 1 h after injury (Fig. 7C). Similar to what was observed when treatment began 1 h post-SCI, formoterol-treated mice displayed an increased BMS score by seven days after injury compared with both day 1 and vehicle-treated mice.
Discussion
Nervous system mitochondria have been implicated in not only energy supply, but also neuronal homeostasis and degeneration, with even minor mitochondrial defects leading to pathological conditions.40 Loss of mitochondrial function results in excitotoxicity, Ca2+ overload, and the initiation of cell death cascades, all of which are hallmarks of SCI and worsen injury.5,12,13 After accumulating above a certain threshold, Ca2+ triggers the opening of the mitochondrial permeability transition pore (mPTP), which has been linked to neuronal cell death after brain injury, neurodegenerative disorders, and SCI.14,41–48 Sullivan and colleagues49 demonstrated that spinal cord mitochondria have a reduced Ca2+ threshold for opening of the mPTP than mitochondria isolated from the brain, indicating the importance of restoring mitochondrial homeostasis after SCI.
Given the complex nature of secondary injury, targeting specific downstream occurrences is likely to be ineffective50; data reveal that rapidly restoring mitochondrial homeostasis, however, may be a more inclusive strategy.3,14,51 Mitochondrial dysfunction post-SCI has been suggested to be critical to the progression of secondary injury and concurrent cell death51 and, as such, mitochondria have become an appealing therapeutic target. Many studies targeting mitochondria post-SCI, however, have focused on singular aspects of dysfunction, including opening of the mPTP or reactive oxygen species (ROS) production.14–18,52 Here, we assessed the therapeutic efficacy of pharmacological enhancement of MB, an alternate approach for comprehensively pursuing restoration of mitochondrial function that has not yet been fully explored for the management of SCI.
Rapid and persistent mitochondrial dysfunction after moderate SCI was observed in both the injury and peri-injury sites, with peak injury in both regions occurring 24 h after impact, corresponding with studies reporting decreased spinal cord PGC-1α after contusive SCI in rats.21 Decreased expression of mitochondrial proteins was observed in both sites of injured mice at all time points examined, and formoterol-induced rescue of said proteins began as early as three days post-SCI. Importantly, formoterol increased PGC-1α expression in both regions by three days after injury, suggestive of MB, yet by seven and 15 days, PGC-1α was comparable among injured mice regardless of treatment.
The PGC-1α activity can be altered via post-translational modifications including, but not limited to, phosphorylation, methylation, and acetylation,53 which have not yet been investigated in this model. Nonetheless, these early formoterol-induced increases in spinal cord PGC-1α expression likely contributed to the additional mitochondrial protein restoration observed throughout treatment. Formoterol treatment ultimately returned all proteins assessed in the injury site to sham levels, with TFAM expression surpassing that of controls. Similarly, in the peri-injury site, formoterol-treated mice exhibited rescued expression of ATP Syn β, and TFAM again exceeded that of sham controls. These data indicate that formoterol-induced MB improved mitochondrial homeostasis after SCI.
Interestingly, formoterol-treated mice had decreased NDUFS1, a subunit of complex I of the ETC, in the peri-injury site three days post-SCI, which could be indicative of decreased complex I activity. Complex I is a major site of ROS production, and inhibition of its activity has been shown to decrease oxidative mitochondrial damage during ischemia.54,55 Furthermore, administration of the complex I inhibitor rotenone decreased lipid peroxidation after SCI in rats,56 a possible result of decreased ROS production. An opposite effect was observed in the injury site, with formoterol treatment increasing NDUFS1. While the reason for this difference remains to be discerned, such dissimilar effects may be indicative of formoterol-induced neuroprotection at the peri-injury site, as opposed to the injury epicenter, inhibiting the diffusion of injury at this early time point.
Histological analysis revealed formoterol-induced tissue sparing three days after injury, with total white and gray matter volume increased with formoterol treatment. Interestingly, the majority of this sparing was evident in the areas rostral and caudal to the epicenter. These data indicate that formoterol may be acting as a neuroprotectant, preventing the spread of secondary injury.
Formoterol treatment enhanced locomotor capability in injured mice as early as seven days after SCI, aligning with increases in body weight, with these mice ultimately displaying a BMS score of 4 by 15 days. Clenbuterol, a less potent and selective ADRB2 than formoterol, has been examined for the management of SCI with studies showing improved functional recovery after SCI in rats beginning three weeks post-injury,29,30 a full two weeks after our initial observed effects with formoterol. Furthermore, our data were obtained using 0.1 mg/kg formoterol, a dose nearly 20-fold less than the 1.6–2.0 mg/kg used for clenbuterol. This accelerated functional improvement at a substantially lower dose is likely partially because of the increased potency, selectivity, and half-life of formoterol.54 Remarkably, similar locomotor effects were observed when formoterol treatment initiation was delayed until 8 h after injury, which speaks to the potential clinical applicability of this therapeutic strategy.
Based on increases in mtDNA and mitochondrial gene and protein expression, our studies have shown that formoterol induces MB in the kidney, heart, skeletal muscle, and spinal cord of mice, while clenbuterol has not been found to induce MB.26,27,32,55 Recent work from our laboratory has attributed this difference to unique structural features of the two agonists resulting in distinct receptor-ligand interactions and divergent signaling pathways. Specifically, formoterol is able to extend across the ADRB2 binding pocket, leading to activation of the Gβγ-Akt-eNOS-sGC pathway, which is necessary for the induction of MB; clenbuterol and other non-mitochondrially biogenic ADRB2 agonists do not activate this pathway.32 Therefore, despite both formoterol and clenbuterol being ADRB2 agonists, dissimilar structures and signaling leading to MB with formoterol has a greater therapeutic potential for the management of SCI.
Mitochondrial dysfunction after SCI is a well-characterized consequence of secondary injury and, as such, therapeutically targeting mitochondria is not a novel approach. While multiple pharmacological agents targeting mitochondria have proven beneficial for the management of SCI in vivo,50,56–58 there remains no approved therapeutic strategy. This could be, in part, because these compounds generally target singular aspects of mitochondrial dysfunction (i.e., mPTP opening, oxidative stress), which may not be sufficient to effectively improve overall mitochondrial health and ultimately patient outcome. It has been suggested that enhancing multiple facets of mitochondrial function could address this inadequacy.38,50,59 While this could be accomplished potentially via combinatorial treatment with multiple agents that target specific mitochondrial functions, pharmacological induction of MB has the potential to more efficiently combat this deficit.
The data presented here are the first to suggest pharmacological activation of MB via agonism of the ADRB2 receptor for the management of SCI. Further, these studies were performed using an FDA-approved compound with the ability to be repurposed, reinforcing the potential clinical applicability of these findings and demonstrating the value of continued investigation into this approach.
Acknowledgments
This study was supported by the South Carolina Spinal Cord Injury Research Fund: SCIRF #2015 I-03 (R.G.S), the National Institutes of Health National Institute of General Medical Sciences: GM084147 (R.G.S), and the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs: BX: 000851 (R.G.S.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Devivo M.J. (2012). Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 [DOI] [PubMed] [Google Scholar]
- 2. Fitzharris M., Cripps R.A., and Lee B.B. (2014). Estimating the global incidence of traumatic spinal cord injury. Spinal Cord 52, 117–122 [DOI] [PubMed] [Google Scholar]
- 3. Rabchevsky A.G., Patel S.P., and Springer J.E. (2011). Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol. Ther. 132, 15–29 [DOI] [PubMed] [Google Scholar]
- 4. Walters B.C., Hadley M.N., Hurlbert R.J., Aarabi B., Dhall S.S., Gelb D.E., Harrigan M.R., Rozelle C.J., Ryken T.C., and Theodore N; American Association of Neurological Surgeons; Congress of Neurological Surgeons. (2013). Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery 60, Suppl 1, 82–91 [DOI] [PubMed] [Google Scholar]
- 5. Oyinbo C.A. (2011). Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol. Exp. (Wars) 71, 281–299 [DOI] [PubMed] [Google Scholar]
- 6. Baptiste D.C. and Fehlings M.G. (2006). Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma 23, 318–334 [DOI] [PubMed] [Google Scholar]
- 7. Graumann U., Ritz M.F., and Hausmann O. (2011). Necessity for re-vascularization after spinal cord injury and the search for potential therapeutic options. Curr. Neurovasc. Res. 8, 334–341 [DOI] [PubMed] [Google Scholar]
- 8. Tator C.H. and Fehlings M.G. (1991). Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 [DOI] [PubMed] [Google Scholar]
- 9. Kundi S., Bicknell R., and Ahmed Z. (2013). The role of angiogenic and wound-healing factors after spinal cord injury in mammals. Neurosci. Res. 76, 1–9 [DOI] [PubMed] [Google Scholar]
- 10. Moskowitz M.A., Lo E.H., and Iadecola C. (2010). The science of stroke: mechanisms in search of treatments. Neuron 68, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adibhatla R.M. and Hatcher J.F. (2010). Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 12, 125–169 [DOI] [PubMed] [Google Scholar]
- 12. Rowland J.W., Hawryluk G.W., Kwon B., and Fehlings M.G. (2008). Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg. Focus 25, E2. [DOI] [PubMed] [Google Scholar]
- 13. Choi D.W. and Rothman S.M. (1990). The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13, 171–182 [DOI] [PubMed] [Google Scholar]
- 14. McEwen M.L., Sullivan P.G., Rabchevsky A.G., and Springer J.E. (2011). Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics 8, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel S.P., Sullivan P.G., Lyttle T.S., and Rabchevsky A.G. (2010). Acetyl-L-carnitine ameliorates mitochondrial dysfunction following contusion spinal cord injury. J. Neurochem. 114, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall E.D. (2011). Antioxidant therapies for acute spinal cord injury. Neurotherapeutics 8, 152–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monaco E.A., 3rd, Weiner G.M., and Friedlander R.M. (2013). Randomized-controlled trial of minocycline for spinal cord injury shows promise. Neurosurgery 72, N17–N19 [DOI] [PubMed] [Google Scholar]
- 18. Teng Y.D., Choi H., Onario R.C., Zhu S., Desilets F.C., Lan S., Woodard E.J., Snyder E.Y., Eichler M.E., and Friedlander R.M. (2004). Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A. 101, 3071–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly D.P. and Scarpulla R.C. (2004). Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 [DOI] [PubMed] [Google Scholar]
- 20. Ventura-Clapier R., Garnier A., and Veksler V. (2008). Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc. Res. 79, 208–217 [DOI] [PubMed] [Google Scholar]
- 21. Hu J., Lang Y., Cao Y., Zhang T., and Lu H. (2015). The neuroprotective effect of tetramethylpyrazine against contusive spinal cord injury by activating PGC-1α in rats. Neurochem. Res. 40, 1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu J., Lang Y., Zhang T., Ni S., and Lu H. (2016). Lentivirus-mediated PGC-1α overexpression protects against traumatic spinal cord injury in rats. Neuroscience 328, 40–49 [DOI] [PubMed] [Google Scholar]
- 23. Beeson C.C., Beeson G.C. and Schnellmann R.G. (2010). A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal. Biochem. 404, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rasbach K.A. and Schnellmann R.G. (2008). Isoflavones promote mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 325, 536–543 [DOI] [PubMed] [Google Scholar]
- 25. Rasbach K.A., Funk J.A., Jayavelu T., Green P.T. and Schnellmann R.G. (2010). 5-hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 332, 632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peterson Y.K., Cameron R.B., Wills L.P., Trager R.E., Lindsey C.C., Beeson C.C. and Schnellmann R.G. (2013). beta2-Adrenoceptor agonists in the regulation of mitochondrial biogenesis. Bioorg. Med. Chem. Lett. 23, 5376–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wills L.P., Trager R.E., Beeson G.C., Lindsey C.C., Peterson Y.K., Beeson C.C. and Schnellmann R.G. (2012). The beta2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 342, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jesinkey S.R., Funk J.A., Stallons L.J., Wills L.P., Megyesi J.K., Beeson C.C., and Schnellmann R.G. (2014). Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 25, 1157–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeman R.J., Feng Y., Peng H., and Etlinger J.D. (1999). Clenbuterol, a beta(2)-adrenoceptor agonist, improves locomotor and histological outcomes after spinal cord contusion in rats. Exp. Neurol. 159, 267–273 [DOI] [PubMed] [Google Scholar]
- 30. Zeman R.J., Peng H., Feng Y., Song H., Liu X., and Etlinger J.D. (2006). Beta2-adrenoreceptor agonist-enhanced recovery of locomotor function after spinal cord injury is glutathione dependent. J. Neurotrauma 23, 170–180 [DOI] [PubMed] [Google Scholar]
- 31. Brown A., Nabel A., Oh W., Etlinger J.D., and Zeman R.J. (2014). Perfusion imaging of spinal cord contusion: injury-induced blockade and partial reversal by beta2-agonist treatment in rats. J. Neurosurg. Spine 20, 164–171 [DOI] [PubMed] [Google Scholar]
- 32. Cameron R.B., Beeson C.C., and Schnellmann R.G. (2017). Structural and pharmacological basis for the induction of mitochondrial biogenesis by formoterol but not clenbuterol. Sci. Rep. 7, 10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker J.G. (2010). The selectivity of β-adrenoceptor agonists at human β(1)-, β(2)- and β(3)-adrenoceptors. Br. J. Pharmacol. 160, 1048–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Basso D.M., Fisher L.C., Anderson A.J., Jakeman L.B., McTigue D.M., and Popovich P.G. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 [DOI] [PubMed] [Google Scholar]
- 35. Patel S.P., Cox D.H., Gollihue J.L., Bailey W.M., Geldenhuys W.J., Gensel J.C., Sullivan P.G., and Rabchevsky A.G. (2017). Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery. Exp. Neurol. 293, 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Funk J.A. and Schnellmann R.G. (2013). Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol. Appl. Pharmacol. 273, 345–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garrett S.M., Whitaker R.M., Beeson C.C., and Schnellmann R.G. (2014). Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J. Pharmacol Exp. Ther. 350, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitaker R.M., Corum D., Beeson C.C., and Schnellmann R.G. (2016). Mitochondrial biogenesis as a pharmacological target: a new approach to acute and chronic diseases. Annu. Rev. Pharmacol. Toxicol. 56, 229–249 [DOI] [PubMed] [Google Scholar]
- 39. Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R.C., and Spiegelman B.M. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 [DOI] [PubMed] [Google Scholar]
- 40. Dubinsky J.M. (2005). CNS mitochondria in neurodegenerative disorders. Antioxid. Redox Signal. 7, 1089–1091 [DOI] [PubMed] [Google Scholar]
- 41. Pivovarova N.B. and Andrews S.B. (2010). Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 277, 3622–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Norenberg M.D. and Rao K.V. (2007). The mitochondrial permeability transition in neurologic disease. Neurochem. Int. 50, 983–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friberg H. and Wieloch T. (2002). Mitochondrial permeability transition in acute neurodegeneration. Biochimie 84, 241–250 [DOI] [PubMed] [Google Scholar]
- 44. Bezprozvanny I. (2009). Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 15, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kinnally K.W., Peixoto P.M., Ryu S.Y., and Dejean L.M. (2011). Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta 1813, 616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hirsch T., Susin S.A., Marzo I., Marchetti P., Zamzami N., and Kroemer G. (1998). Mitochondrial permeability transition in apoptosis and necrosis. Cell Biol. Toxicol. 14, 141–145 [DOI] [PubMed] [Google Scholar]
- 47. Lemasters J.J., Qian T., Elmore S.P., Trost L.C., Nishimura Y., Herman B., Bradham C.A., Brenner D.A., and Nieminen A.L. (1998). Confocal microscopy of the mitochondrial permeability transition in necrotic cell killing, apoptosis and autophagy. BioFactors 8, 283–285 [DOI] [PubMed] [Google Scholar]
- 48. Lemasters J.J., Nieminen A.L., Qian T., Trost L.C., Elmore S.P., Nishimura Y., Crowe R.A., Cascio W.E., Bradham C.A., Brenner D.A., and Herman B. (1998). The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366, 177–196 [DOI] [PubMed] [Google Scholar]
- 49. Sullivan P.G., Rabchevsky A.G., Keller J.N., Lovell M., Sodhi A., Hart R.P., and Scheff S.W. (2004). Intrinsic differences in brain and spinal cord mitochondria: Implication for therapeutic interventions. J. Comp. Neurol. 474, 524–534 [DOI] [PubMed] [Google Scholar]
- 50. Scholpa N.E. and Schnellmann R.G. (2017). Mitochondrial-based therapeutics for the treatment of spinal cord injury: mitochondrial biogenesis as a potential pharmacological target. J. Pharmacol. Exp. Ther. 363, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sullivan P.G., Krishnamurthy S., Patel S.P., Pandya J.D., and Rabchevsky A.G. (2007). Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J. Neurotrauma 24, 991–999 [DOI] [PubMed] [Google Scholar]
- 52. Casha S., Zygun D., McGowan M.D., Bains I., Yong V.W., and Hurlbert R.J. (2012). Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 135, 1224–1236 [DOI] [PubMed] [Google Scholar]
- 53. Fernandez-Marcos P.J. and Auwerx J. (2011). Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884s–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baker J.G. (2010). The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br. J. Pharmacol. 160, 1048–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jesinkey S.R., Korrapati M.C., Rasbach K.A., Beeson C.C., and Schnellmann R.G. (2014). Atomoxetine prevents dexamethasone-induced skeletal muscle atrophy in mice. J. Pharmacol. Exp. Ther. 351, 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Springer J.E., Visavadiya N.P., Sullivan P.G., and Hall E.D. (2018). Post-injury treatment with NIM811 promotes recovery of function in adult female rats after spinal cord contusion: a dose-response study. J. Neurotrauma 35, 492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Z.Y., Fan Z.K., Cao Y., Jia Z.Q., Li G., Zhi X.D., Yu D.S., and Lv G. (2015). Acetyl-L-carnitine ameliorates mitochondrial damage and apoptosis following spinal cord injury in rats. Neurosci. Lett. 604, 18–23 [DOI] [PubMed] [Google Scholar]
- 58. Patel S.P., Sullivan P.G., Pandya J.D., Goldstein G.A., VanRooyen J.L., Yonutas H.M., Eldahan K.C., Morehouse J., Magnuson D.S., and Rabchevsky A.G. (2014). N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol. 257, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hall E.D., Wang J.A., Bosken J.M., and Singh I.N. (2016). Lipid peroxidation in brain or spinal cord mitochondria after injury. J. Bioenerg. Biomemb. 48, 169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]