Abstract
C-terminal telopeptide of type I collagen (CTX) and procollagen type 1 N-terminal propeptide (P1NP) are bone turnover markers (BTMs) that are promising surrogate measures of fracture healing; however, it is unknown if their response is affected by other bone healing metabolites. Since 70% of fracture patients are reported to have insufficient serum vitamin D, we sought to determine if serum 25(OH)D levels are associated with differential changes in CTX and P1NP concentrations after hip fracture. This prospective cohort included hip fracture patients 65 years of age or older admitted to one of eight Baltimore-area hospitals. Serum samples were collected at baseline, 2-, 6-, and 12-month post-fracture. A mixed-effects repeated-measures analysis was used to determine the longitudinal association between vitamin D deficiency (25(OH)D < 20ng/ml) and CTX and P1NP. Baseline lab values were obtained for 296 participants (mean age, 80.8 years; 51% male; 55% 25(OH)D < 20ng/ml). During the acute fracture healing period P1NP concentrations increased by 14% (95%CI: 7–21%, p < 0.01) while CTX levels did not change (p = 0.07). Both CTX and P1NP decreased below baseline at 6 and 12 months. CTX levels were higher in participants with baseline 25(OH)D < 20ng/ml (p = 0.01). There was no association between 25(OH)D < 20ng/ml and P1NP levels over the study duration (p = 0.33). Data from this large, longitudinal cohort support claims that CTX and P1NP concentrations change during fracture healing; however, the differential response of CTX among vitamin D deficient patients highlights important questions for its utility as a reliable surrogate marker of fracture healing.
Keywords: hip fracture, bone turnover markers, vitamin D, CTX, P1NP
Bone turnover markers show distinct patterns after long bone fractures, making them an appealing non-invasive method to predict fracture healing.1 C-terminal telopeptide of type I collagen (CTX) and procollagen type I N-terminal propeptide (P1NP) have been recommended as the reference bone turnover markers (BTMs) for predicting fracture risk and monitoring of osteoporosis.2,3 However, current understanding of CTX and P1NP after fractures is insufficient to monitor the course of fracture healing4 and is based on heterogeneous long bone fractures5–9 with limited data on trends after hip fractures.7,10
Vitamin D is involved in each stage of the fracture healing process, and the overall effect on fracture healing has yet to be elucidated.11 A recent systematic review showed that more than 70% of fracture patients are reported to have serum 25-hydroxy-vitamin D (25(OH)D) levels <30ng/ml during the acute fracture healing period.12 The clinical significance of these findings is unclear,13 but many surgeons believe low levels of vitamin D may be associated with impaired fracture healing.14
Literature assessing the effect of vitamin D deficiency on CTX and P1NP is limited,3 and to our knowledge, no study has assessed the relationship of baseline serum 25(OH)D on CTX and P1NP during the fracture healing period. Our objective was to assess the trajectory of CTX and P1NP after hip fracture in the elderly, and to determine if serum 25(OH)D levels are associated with differential changes in CTX and P1NP concentrations after hip fracture.
METHODS
This is a secondary analysis of a larger prospective cohort, the Baltimore Hip Study-7 (BHS-7) (level of evidence: II). The purpose of this parent study was to assess the trajectories of physiologic composition, physical activity and function, and psychosocial function after hip fractures in males in comparison to females. Participants were enrolled from May 2006 to June 2011 at eight hospitals in Maryland. The University of Maryland Baltimore Institutional Review Board (IRB) and the IRBs at each of the study hospitals approved the study.
Study Participants
Participants were 65 or older who had an operative hip fracture and were enrolled within 15 days of their index hospitalization. Exclusion criteria included patients who were non-community dwelling at the time of fracture, resided greater than 70 miles from the study hospital, were non-English speaking, had a pathologic fracture, weighed >300 pounds, were bedridden for 6 months before the fracture, or had hardware in the contralateral hip. Participants or their proxies provided written informed consent within 15 days of admission. Males were enrolled on an ongoing basis, and females were enrolled on a one-to-one basis with males at each hospital, in order to keep the number of males and females in the study approximately equal. Participants were followed for 12 months, and in-person assessments and blood draws were collected at baseline (within 22 days of admission), and at 2, 6, and 12 months after admission.
Over the study period, 362 patients with hip fractures were enrolled; 23 participants were withdrawn (5 participants failed to provide data at baseline, and another 18 participants were removed as a result of an IRB-requested post-procedure audit), leaving a final sample of 339 (168 men, 171 women). Of these, 43 did not have baseline levels of CTX, P1NP, or 25(OH)D. The sample for this analysis included 296 participants.
Outcomes and Measures
Our primary outcomes were serum CTX and P1NP, measured within 22 days of hospitalization for fracture, 2-, 6-, and 12-month post-admission. Due to the high variability of CTX and P1NP levels among patients, we expressed values as a percent of baseline measurement.15 The association between serum 25(OH)D and CTX and P1NP was our primary interest. We used a cutoff of 20ng/ml to determine deficiency and 30ng/ml to determine insufficiency.16 Several covariates that have been demonstrated to affect CTX and P1NP were tested as possible confounders in the analysis.3 Age and sex were obtained from the medical records upon patient enrollment. Body mass index (BMI), pre-injury activity level, and Charlson Comorbidity Index data were collected by the study staff.
Blood Samples
Fasting blood specimens at each study visit were collected between the hours of 7:00 AM and 10:00 AM and processed no later than 1:00 pm, so that samples were processed within 6h of collection and serum was stored at −80°C until assayed.
Serum CTX was measured by enzyme-linked immunosorbent assay (ELISA) from IDX Cat # AC02F1. The sensitivity was 0.020 ng/ml, intra-assay coefficient of variability (CV%) was 8.15, and inter-assay CV% was 10.31. P1NP was measured by radioimmunoassay (RIA) from IDS inc. Cat # OD 67034. The sensitivity was 2.0 μg/L, intra-assay CV% was 4.14, and inter-assay CV% was 2.74. The CTX and P1NP assay measurements were performed at the Johns Hopkins Bayview Medical Center General Clinical Research Center (GCRC).
Serum 25(OH)D was extracted by solid phase extraction (SPE), separated and eluted by high performance of liquid chromatography (HPLC), and determined by mass spectrometry (MS) in atmospheric-pressure chemical ionization (APCI) source at positive ionization mode and multiple reaction monitoring (MRM) of transition. Deuterated stable isotope 25(OH)D3-d6 or 25(OH)D2-d6 was utilized as an internal standard for the calibrations of 25(OH)D3 and 25 (OH)D2, respectively. The intra-assay CV% for 25(OH)D3 was 6% at 10.0 ng/ml and 5% at 50.0 ng/ml, and the inter-assay CV% was 7% at 10.0 ng/ml and at 50.0 ng/ml. The intra-assay CV% for 25(OH)D2 was 8% at 10.0 ng/ml and 4% at 50.0 ng/ml, and the inter-assay CV% was 9% at 10.0 ng/ml and 4% at 50.0 ng/ml. The serum 25(OH)D assay measurement was performed at the Brigham and Women’s Hospital Research Assay Core. Samples for all assays were measured in duplicate and samples from all time points for a single subject were assayed together.
Statistical Methods
Patient characteristics were described as means with standard deviations for continuous variables and counts with percentages for categorical variables. The longitudinal CTX and P1NP data were analyzed using mixed-effects repeated-measures regression with each patient uniquely coded as a random effect. Time was coded as a categorical variable (baseline, 2-, 6-, and 12-months) in all models. The initial models tested the association between time and serum CTX and P1NP levels. Secondary models were then developed to compare the association between 25(OH)D insufficiency and deficiency with serum CTX and P1NP levels. The 25(OH)D insufficiency models included time, serum 25(OH)D ≥ or <30 ng/ml, and the interaction between time and serum 25(OH)D as fixed effects with serum CTX and P1NP levels as dependent variables in the models. The 25(OH)D deficiency models included time, serum 25(OH)D ≥ or <20 ng/ml, and the interaction between time and serum 25(OH)D as fixed effects with serum CTX and P1NP levels as dependent variables in the models. One benefit of a mixed-model repeated-measures analysis is the ability to model all participant data, regardless if a particular subject missed a study visit. In all models, data were assumed to be missing at random. Age, sex, BMI, pre-injury activity level, and Charlson Comorbidity Index data were tested but not determined to be confounders in any of the analyses, and therefore not included as variables in the models. A baseline diagnosis of osteoporosis was tested as a possible mediator or effect measure modifier in the models but did not satisfy the assumptions for inclusion in final models. All statistical analysis was conducted using JMP Pro Version 13 (SAS Institute, Cary, NC).
RESULTS
Sample Characteristics and Follow-Up
Of the 296 patients included in the final sample, serum samples analyzed for CTX and P1NP were obtained for 190 (65%) participants at 2 months, 147 (50%) at 6 months, and 132 (45%) at 12 months (Fig. 1). Baseline blood draws were performed on average at 12 days after hospitalization, 68 days at 2 months, 188 days at 6 months, and 374 days at 12 months.
Figure 1.
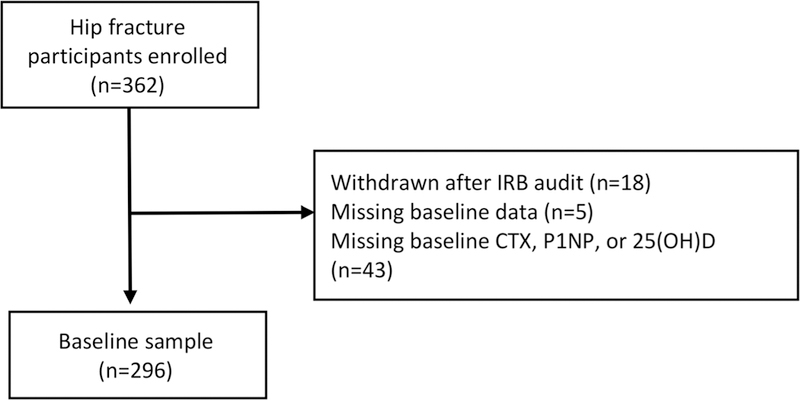
Flow diagram.
The study population included 152 males and 144 females. The mean age was 80.8 (±7.7), BMI was 25.2 (±5.0), and 93% were Caucasian (Table 1). Serum 25 (OH)D levels ranged from 2.3 to 48.0 ng/ml, with a mean of 19.8 ng/ml at baseline. Prior to the injury, the mean weekly activity level of the study participants was estimated at 15.8 ± 12.8 h.
Table 1.
Baseline Characteristics of Study Participants
| 25(OH)D ≥ 20 ng/ml (n = 132) |
25(OH)D < 20 ng/ml (n = 164) |
Total Cohort (n = 296) |
p-Value | |
|---|---|---|---|---|
| Age, mean ± SD | 80.5 ± 7.6 | 81.0 ± 7.8 | 80.8 ± 7.7 | 0.54 |
| Sex, female, n (%) | 89 (67) | 55 (34) | 144 (49) | <0.01 |
| Race, n (%) | ||||
| Caucasian | 120 (91) | 151 (92) | 271 (93) | 0.89 |
| African American | 7 (5) | 11 (7) | 18 (6) | |
| Other/missing | 5 (4) | 2 (1) | 7 (2) | |
| Non-Hispanic ethnicity, n (%) | 127 (96) | 161 (98) | 288 (97) | 0.81 |
| Body mass index, kg/m2, mean ± SD | 24.8 ± 4.8 | 25.5 ± 5.1 | 25.2 ± 5.0 | 0.19 |
| Pre-injury activity time, h/wk, | 17.8 ± 13.1 | 15.0 ± 13.0 | 15.8 ± 12.8 | 0.08 |
| mean ± SD | ||||
| Charlson comorbidity index, | 1.8 ± 1.7 | 2.2 ± 1.9 | 2.0 ± 1.8 | 0.05 |
| mean ± SD | ||||
| CTX, ng/ml, mean ± SD | 0.85 ± 0.43 | 0.97 ± 0.65 | 0.91 ± 0.56 | 0.07 |
| P1NP, μg/L, mean ± SD | 127.2 ± 100.9 | 110.5 ± 61.8 | 117.9 ± 81.9 | 0.08 |
| 25(OH)D, ng/ml, mean ± SD | 28.5 ± 6.4 | 12.8 ± 4.4 | 19.8 ± 9.5 | <0.01 |
CTX
The mean baseline serum CTX level was 0.91 (ng/ml) and ranged from 0.19 (ng/ml) to 4.8 (ng/ml). After relatively stable serum CTX levels from baseline to 2-month post-injury (mean difference −4.6%, 95%CI: −9.6% to 4.4%, p = 0.07), we observed a significant decline in serum CTX for 6 months (−14.2%, 95%CI: −19.7% to −8.7%, p < 0.01) and 12 months (−26.5%, 95%CI: −32.2% to −20.7%, p < 0.01), relative to baseline levels (Fig. 2A). 25(OH)D deficiency (<20 ng/ml) at baseline was associated with a 3.6% longitudinal increase in serum CTX levels (95%CI: −0.08 to 6.4%, p = 0.01) (Fig. 3A). However, there was no association between 25(OH)D insufficiency (<30ng/ml) and serum CTX levels (−0.5%, 95%CI: −4.5 to 3.5%, p = 0.81) (Fig. 4A).
Figure 2.
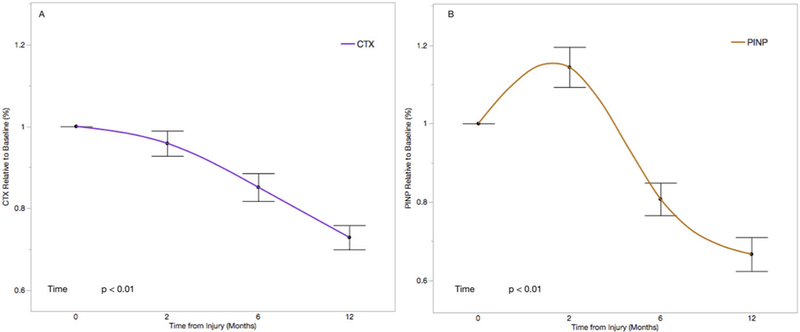
Change in CTX (A) and P1NP (B) relative to baseline in the full cohort. Each time point is represented as the mean serum level relative to baseline with standard error. Time is coded as a continuous variable based on months from injury.
Figure 3.
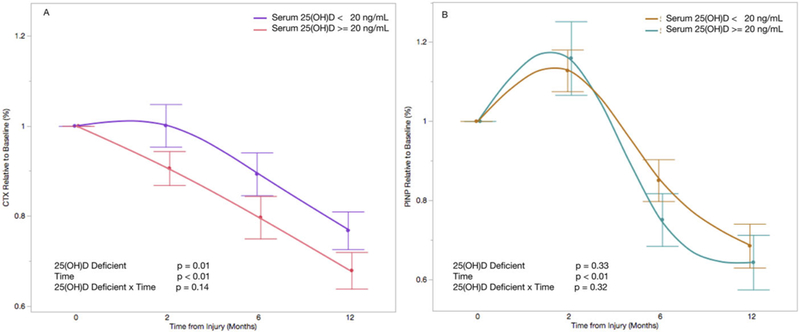
Change in CTX (A) and P1NP (B) relative to baseline and stratified by baseline serum 25(OH)D level of 20ng/ml. Each time point is represented as the mean serum level relative to baseline with standard error. Time is coded as a continuous variable based on months from injury. Time, serum 25(OH)D, and the interaction between time and serum 25(OH)D are included as fixed effects with serum CTX (A) and P1NP (B) levels as dependent variables.
Figure 4.
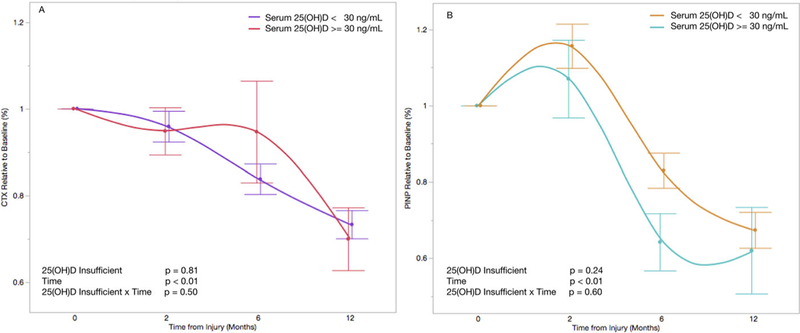
Change in CTX (A) and P1NP (B) relative to baseline and stratified by baseline serum 25(OH)D level of 30ng/ml. Each time point is represented as the mean serum level relative to baseline with standard error. Time is coded as a continuous variable based on months from injury. Time, serum 25(OH)D, and the interaction between time and serum 25(OH)D are included as fixed effects with serum CTX (A) and P1NP (B) levels as dependent variables.
P1NP
The mean baseline serum P1NP level was 117.9 (μg/L) and ranged from 16.1 (μg/L) to 750.2 (μg/L). Serum P1NP levels increased by 14.3% (95%CI: 7.3–21.4%, p < 0.01) from baseline to 2 months, and then declined to 18.5% below baseline at 6 months (95%CI: −26.3% to −10.8%, p < 0.01) and 32.9% below baseline at 12-month post-injury (95%CI: −41.0% to 24.9%, p < 0.01) (Fig. 2B). There was no association between serum 25(OH)D deficiency (< 20ng/ml) and serum P1NP levels (2.1%, 95%CI: −2.2% to 6.4%, p = 0.33) (Fig. 3B). Similarly, there was no association between serum 25(OH)D insufficiency (<30ng/ml) and serum P1NP levels (4.0%, 95%CI: −2.4% to 9.7%, p = 0.24) (Fig. 4B).
DISCUSSION
The findings of this study describe consistent trends in serum CTX (resorption marker) and P1NP (formation marker) in the 1 year following hip fracture in the elderly. During the first 2 months post-injury when the majority of fracture healing is expected, serum CTX levels remained relatively stable from baseline, whereas serum P1NP increased substantially during that same time period. Both BTMs progressively decreased at 6 and 12 months relative to baseline levels. A serum 25(OH)D level <20ng/ml was associated with elevated serum CTX levels following the fracture. There was no association between serum 25 (OH)D and P1NP.
Previous studies have shown an initial peak in serum CTX or P1NP shortly after tibial,5,6 femur,6 forearm,6–8 and ankle9 fractures. These levels were observed to be slightly elevated through 1-year post-fracture.6–9 The time at which CTX and P1NP peaked varied among the studies, from 2 weeks7 to 3 months5,8,9 post-fracture. These results were obtained from younger samples,5,6 consisted of heterogeneous fractures,6,7 and had lower sample sizes,5,8,9 reducing their generalizability to hip fractures in the elderly. Further, these BTMs have been observed to be elevated at 3 months following fragility fractures.17,18 Ivaska et al. observed greater changes in CTX and P1NP in hip fractures compared to other fractures.7 Since hip fractures have healing trajectories distinct from long bone fractures, such as metaphyseal bone involvement and lower weight-bearing status during recovery, it is likely that these differences could cause distinct changes in BTMs after fracture. The relationship between serum 25(OH)D and CTX or P1NP has provided mixed results,19–22 and none have evaluated this trajectory in a fracture population. Trials investigating the relationship between vitamin D supplementation and CTX or P1NP have also shown mixed results, and to our knowledge, only a single small study has explored this relationship in hip fracture patients.23–26
Our results demonstrate the relationship between baseline serum 25(OH)D and CTX and P1NP after hip fracture and confirm that observed trends in these BTMs after long bone fractures are similar in elderly hip fractures. We demonstrated that peak CTX and P1NP levels occur between 2- and 6-month post-fracture, and that mean CTX and P1NP remained slightly elevated at 12 months (0.59ng/ml and 63.1 mg/L, respectively) when compared to older adults without fractures.27 These data further show that baseline vitamin D levels have a significant relationship to CTX in the post-fracture period. The lack of a significant association between 25(OH)D and P1NP is unexpected. It is possible that the high variability in P1NP levels15 made our sample size insufficient to detect a difference. In previous studies that assessed vitamin D and P1NP, only the larger studies found a significant relationship.19–22 Szulc et al. found an inverse, but not a significant relationship between CTX/P1NP and 25(OH)D based on a sample size of 582, while Bhattoa et al. found no differences between groups with a cutoff of 30 ng/ml and a sample size of 206. Lu et al. found a significant inverse relationship in 2,588 individuals, as did Zhao et al. in their study of 1,724 individuals. These studies were not conducted in fracture populations, however, so additional underlying biochemical mechanisms during fracture healing may play a role in these observed differences. It is possible that CTX is a more sensitive marker to metabolites and fractures. A previous study that assessed the relationship between fracture healing BTMs found a significant relationship with CTX but not P1NP,6 however, the effect of vitamin D was not assessed in that study. These issues highlight that the utility of P1NP in fracture healing has yet to be determined.
This study has several limitations. First, our base-line measurement occurred at approximately 2-week post-fracture. Previous studies have presented findings that BTMs are elevated by this time compared with pre-fracture levels7 and immediately post-fracture.5 While all samples for each subject were measured in batch and duplicate, not all subjects were assayed together, potentially introducing measurement error. However, our results are presented as intra-individual differences,15 mitigating this variability and making the results comparable to much of the available literature,5,7–9 but not all.6 Additionally, blood draws were performed at varying times of day, however, they were all performed between 7 and 10 am. Circadian variation has been demonstrated to affect BTM levels,3,15 peaking in the morning and decreasing throughout the day. Attrition in the sample increased over the duration of the study and exceeded 50% at 12-month post-injury despite monthly phone calls to try to contact study participants. However, the analyses used mixed-effects repeated-measures models and data were assumed to be missing at random, limiting the bias of missing data on the estimates. Finally, this prospective cohort did not obtain fracture outcome imaging, so we were not able to test the association between BTMs and fracture union.
Despite these limitations, our study has several strengths. In addition to the prospective design, this study was conducted at both academic and community-based institutions in Maryland, increasing the generalizability of the results. The study sample was equally split between males and females, allowing us to account for known sex effects on BTMs. Our study population included a homogenous group of hip fractures and only included patients over 65 years of age, limiting the potential bias of age and fracture location on CTX and P1NP. Finally, this is the largest study to prospectively measure serum CTX and P1NP after hip fractures, and our sample size provided the power to detect several associations of interest.
Serologic biomarkers have the potential to be useful for early markers of bone union, but there is currently not enough available evidence in human trials.4,28 Few studies have assessed this relationship between BTMs and fracture healing6,29,30 with only two assessing CTX or P1NP.6,29 Interestingly, Moghaddam et al. found a significantly lower CTX during the first week in the nonunion group. However, the differences were not statistically significant at subsequent time points. This may have been due to insufficient power rather than a true lack of association. Our results that 25(OH)D is inversely related to CTX during the fracture healing period, combined with Moghaddam’s findings present the complex relationship of these BTMs during fracture healing. Further, one recent systematic review on serum 25(OH)D and fracture healing showed there is currently a dearth of research on the efficacy of vitamin D supplementation and fracture healing,14 while another concluded that vitamin D supplementation does not reduce fractures.31 Additionally, a recent study has questioned the utility of CTX and P1NP in predicting hip fracture risk.32 These findings highlight the limited knowledge of the effect of vitamin D on fractures, and BTMs as predictors of clinical outcomes.
Our results add significant evidence to the current body of literature on the normal course of CTX and P1NP following hip fractures, showing a peak near 2-month post-fracture followed by a decline through 12 months. We demonstrated that serum 25(OH)D is inversely correlated with CTX during hip fracture healing in elderly patients. The overall effect of vitamin D on BTMs and bone healing currently is unknown, and future prospective studies should investigate this complex relationship.
ACKNOWLEDGMENTS
This work was supported by grant from the NIH National Institute on Aging (R37 AG09901, R01 AG029315, and P30 AG208747). GPS is a consultant for Smith & Nephew and Zimmer and receives funding from Patient-Centered Outcomes Research Institute and the US Department of Defense. JM is a consultant for Ammonett, Novartis, Pluristem, and Viking. DO is a consultant for Kinexum and Viking.
Grant sponsor: National Institute on Aging; Grant numbers: P30 AG208747, R01 AG029315, R37 AG09901.
REFERENCES
- 1.Cunningham BP, Brazina S, Morshed S, et al. 2017. Fracture healing: a review of clinical, imaging and laboratory diagnostic options. Injury 48:S69–S75. [DOI] [PubMed] [Google Scholar]
- 2.Vasikaran S, Eastell R, Bruyere O, et al. 2011. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22:391–420. [DOI] [PubMed] [Google Scholar]
- 3.Szulc P, Naylor K, Hoyle NR, et al. 2017. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28:2541–2556. [DOI] [PubMed] [Google Scholar]
- 4.Pountos I, Georgouli T, Pneumaticos S, et al. 2013. Fracture non-union: can biomarkers predict outcome? Injury 44:1725–1732. [DOI] [PubMed] [Google Scholar]
- 5.Veitch SW, Findlay SC, Hamer AJ, et al. 2006. Changes in bone mass and bone turnover following tibial shaft fracture. Osteoporos Int 17:364–372. [DOI] [PubMed] [Google Scholar]
- 6.Moghaddam A, Muller U, Roth HJ, et al. 2011. TRACP 5b and CTX as osteological markers of delayed fracture healing. Injury 42:758–764. [DOI] [PubMed] [Google Scholar]
- 7.Ivaska KK, Gerdhem P, Akesson K, et al. 2007. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res 22:1155–1164. [DOI] [PubMed] [Google Scholar]
- 8.Ingle BM, Hay SM, Bottjer HM, et al. 1999. Changes in bone mass and bone turnover following distal forearm fracture. Osteoporos Int 10:399–407. [DOI] [PubMed] [Google Scholar]
- 9.Ingle BM, Hay SM, Bottjer HM, et al. 1999. Changes in bone mass and bone turnover following ankle fracture. Osteoporos Int 10:408–415. [DOI] [PubMed] [Google Scholar]
- 10.Yu-Yahiro JA, Michael RH, Dubin NH, et al. 2001. Serum and urine markers of bone metabolism during the year after hip fracture. J Am Geriatr Soc 49:877–883. [DOI] [PubMed] [Google Scholar]
- 11.Gorter EA, Hamdy NA, Appelman-Dijkstra NM, et al. 2014. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone 64:288–297. [DOI] [PubMed] [Google Scholar]
- 12.Sprague S, Petrisor B, Scott T, et al. 2016. What is the role of vitamin d supplementation in acute fracture patients? A systematic review and meta-Analysis of the prevalence of hypovitaminosis D and supplementation efficacy. J Orthop Trauma 30:53–63. [DOI] [PubMed] [Google Scholar]
- 13.Sprague S, Slobogean GP, Bogoch E, et al. 2017. Vitamin D use and health outcomes after surgery for hip fracture. Orthopedics 40:e868–e875. [DOI] [PubMed] [Google Scholar]
- 14.Sprague S, Bhandari M, Devji T, et al. 2016. Prescription of vitamin D to fracture patients: a lack of consensus and evidence. J Orthop Trauma 30:e64–e69. [DOI] [PubMed] [Google Scholar]
- 15.Cox G, Einhorn TA, Tzioupis C, et al. 2010. Bone-turnover markers in fracture healing. J Bone Joint Surg Br 92:329–334. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 17.Rozental TD, Herder LM, Walley KC, et al. 2015. 25-Hydroxyvitamin-D and bone turnover marker levels in patients with distal radial fracture. J Bone Joint Surg Am 97:1685–1693. [DOI] [PubMed] [Google Scholar]
- 18.Ting BL, Walley KC, Travison TG, et al. 2017. Elevated bone turnover markers are associated with distal radius fractures in premenopausal women. J Hand Surg Am 42:71–77. [DOI] [PubMed] [Google Scholar]
- 19.Bhattoa HP, Nagy E, More C, et al. 2013. Prevalence and seasonal variation of hypovitaminosis D and its relationship to bone metabolism in healthy Hungarian men over 50 years of age: the HunMen Study. Osteoporos Int 24:179–186. [DOI] [PubMed] [Google Scholar]
- 20.Lu HK, Zhang Z, Ke YH, et al. 2012. High prevalence of vitamin D insufficiency in China: relationship with the levels of parathyroid hormone and markers of bone turnover. PLoS ONE 7:e47264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, Xia W, Nie M, et al. 2011. The levels of bone turnover markers in Chinese postmenopausal women: Peking Vertebral Fracture study. Menopause 18:1237–1243. [DOI] [PubMed] [Google Scholar]
- 22.Szulc P, Munoz F, Marchand F, et al. 2003. Role of vitamin D and parathyroid hormone in the regulation of bone turnover and bone mass in men: the MINOS study. Calcif Tissue Int 73:520–530. [DOI] [PubMed] [Google Scholar]
- 23.Madar AA, Knutsen KV, Stene LC, et al. 2015. Effect of vitamin D3-supplementation on bone markers (serum P1NP and CTX): a randomized, double blinded, placebo controlled trial among healthy immigrants living in Norway. Bone Rep 2:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimnes G, Joakimsen R, Figenschau Y, et al. 2012. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass-a randomized controlled 1-year trial. Osteoporos Int 23:201–211. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald HM, Wood AD, Aucott LS, et al. 2013. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: a 1-year double-blind RCT in postmenopausal women. J Bone Miner Res 28:2202–2213. [DOI] [PubMed] [Google Scholar]
- 26.Torbergsen AC, Watne LO, Frihagen F, et al. Effects of nutritional intervention upon bone turnover in elderly hip fracture patients. Randomized controlled trial. Clin Nutr ESPEN. In press. [DOI] [PubMed] [Google Scholar]
- 27.Michelsen J, Wallaschofski H, Friedrich N, et al. 2013. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone 57:399–404. [DOI] [PubMed] [Google Scholar]
- 28.Morshed S 2014. Current options for determining fracture union. Adv Med 2014:708574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrmann M, Klitscher D, Georg T, et al. 2002. Different kinetics of bone markers in normal and delayed fracture healing of long bones. Clin Chem 48:2263–2266. [PubMed] [Google Scholar]
- 30.Emami A, Larsson A, Petren-Mallmin M, et al. 1999. Serum bone markers after intramedullary fixed tibial fractures. Clin Orthop Relat Res 368:220–229. [PubMed] [Google Scholar]
- 31.Bolland MJ, Grey A, Avenell A. 2018. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol 6:847–858. [DOI] [PubMed] [Google Scholar]
- 32.Crandall CJ, Vasan S, LaCroix A, et al. 2018. Bone turnover markers are not associated with hip fracture risk: a case-control study in the women’s health initiative. J Bone Miner Res 33:1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]


