INTRODUCTION
Malnutrition affects 52 million children under age 5 years, contributing to 45% of child mortality. Chronic malnutrition results in stunting (low height for age), present in 155 million children—87 million in Asia and 59 million in Africa. The 16.9 million children with severe wasting (low weight for height), including 12.6 million in Asia and 4.1 million in Africa,1 have a 9.4-fold greater chance of dying compared with healthy-weight children.2 The World Health Organization (WHO) defines severe acute malnutrition (SAM) as mid-upper arm circumference (MUAC) less than 115 mm or weight-for-height z score (WHZ) less than −3 for ages 6 months to 59 months. Both acute and chronic malnutrition can cause long-term cognitive deficits. Low birthweight, stunting, and wasting correlate with lower scores on intelligence tests, developmental delays, and decreased lifetime earnings, perpetuating the poverty-malnutrition cycle.3
The WHO recommends that all children with SAM are treated with therapeutic foods. Children with minimal appetite or medical complications should receive inpatient treatment with therapeutic milk (F-75 and later F-100) and an antibiotic with gram-negative coverage and then be transitioned to community-based treatment with ready-to-use therapeutic food (RUTF). Children should be monitored until recovery, defined as WHZ greater than or equal to −2 or MUAC greater than or equal to 125 mm and greater than or equal to 2 weeks without edema.4 Despite these guidelines, SAM mortality rates in the hospital setting remain as high as 10% to 40%, and meta-analyses examining long-term outcomes reveal mixed results.5,6 Among the key barriers to improving care is an incomplete understanding of mechanisms underlying the metabolic and physiologic abnormalities of SAM.
Recent insights into the pathogenesis of malnutrition instill hope that better outcomes might soon be possible. This review highlights new evidence relevant to 5 topics, including early-life determinants of malnutrition, the role of protein deficiency in the development and perpetuation of malnutrition, the drivers of malnutrition-associated immune deficiencies, impaired gut barrier function and resulting inflammation, and potential roles of the intestinal microbiota in the pathogenesis and treatment of malnutrition.
PRENATAL AND PERINATAL FACTORS
Intrauterine growth restriction (IUGR), being small for gestational age, and preterm birth all contribute to child mortality7 and malnutrition.8 Maternal micronutrient status is 1 determinant of low birthweight and IUGR. Iron supplementation during pregnancy reduces the risk of low birthweight and child mortality within the first 5 years of life,9 and multiple micronutrient supplementation during pregnancy increases birthweight and decreases infant mortality.10 Low vitamin D receptor expression has been observed in placentas of IUGR pregnancies,11 and single-nucleotide polymorphisms in placental genes governing vitamin D metabolism are associated with low birthweight.12 IUGR can be driven by many other factors, including low insulin-like growth factor 1 (IGF-1)13 (discussed later), highlighting the complex, systemic nature of metabolic derangements in malnutrition.
Low birthweight could promote malnutrition via fetal epigenetic alterations.14 Differential DNA methylation in infants with IUGR was observed in genes involved in lipid metabolism, transcriptional regulation, metabolic disease, and T-cell development.15 Although a “thrifty phenotype” may be protective during early-life nutrient deprivation, its persistence into adulthood can have detrimental effects. Adult survivors of infant or prenatal famines have increased rates of obesity, diabetes, and cardiovascular diseases.16 Epigenetic changes caused by episodes of prenatal or childhood malnutrition can persist for generations17; however, some changes can be rescued by early nutrient supplementation in preclinical models.18
Maternal genotype also influences the risk of child malnutrition. For example, vitamin D status and fetal growth are impacted by maternal variants of vitamin D metabolizing genes.19 IUGR might be avoided in certain cases by individualizing prenatal supplementation regimens. Similarly, mothers lacking a functional FUT2 gene secrete lower concentrations of fucosylated human milk oligosaccharides in breast milk and are more likely to have stunted children.20 When nutritional quality of breast milk is inadequate, complementary feeding might be required to reduce an infant’s risk of malnutrition.
THE ROLE OF PROTEIN DEFICIENCY
Significant food insecurity arising seasonally or with political or natural crises increases the incidence of malnutrition and child mortality. SAM incidence is highest in the rainy season, or preharvest hungry period, and declines just after harvest.21 One recent study in India found that children with SAM had increased odds of relapse if they completed treatment during seasons of moderate or severe food insecurity.22 Just as important as macronutrient quantity are protein quality and digestibility.23 Protein inadequacy, which correlates with stunting,24 is highest in Africa and southern Asia.25 Up to 70% of protein consumption in these regions is in the form of cereals and roots, which lack many of the essential amino acids found in animal meat and dairy proteins.
Although stunted children have lower circulating levels of all essential amino acids, it is uncertain whether this results from inadequate intake, increased catabolism, or both. Malnourished children are particularly deficient in arginine, glycine, glutamine, asparagine, glutamate, and serine.26 These amino acids serve in a variety of biological roles, including protein synthesis, enterocyte growth, bile acid conjugation, intestinal barrier function, and neurotransmitter biosynthesis. Serum amino acids are sensed by, and influence the activity of, the mTORC1 pathway, a master regulator of growth.27 Protein synthesis, proteolysis, and bone growth are inhibited during SAM, because lipolysis and fatty acid oxidation meet a greater proportion of energy needs.28–30 Decreased circulating polyunsaturated fatty acid levels further suggest compensatory fat catabolism in SAM,31 whereas elevated cortisol and growth hormone (GH) and decreased leptin and insulin may reflect hormonal regulation of these processes.28,32 Decreased leptin is a strong independent predictor of mortality in children with SAM.28,30
Protein deficiency also results in liver dysfunction (Fig. 1). The most dramatic manifestation is steatosis,33 although mechanisms by which this occurs are poorly understood. A murine model of protein deficiency linked mitochondrial dysfunction and loss of peroxisomes to impaired fatty acid oxidation and steatosis. By activating the nutrient-sensing nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα), peroxisome numbers and fatty acid oxidation and steatosis were normalized. Peroxisome loss was associated with decreased markers of bile acid synthesis,34 suggesting that peroxisomal dysfunction may contribute to the altered bile acid profiles observed in SAM. Specifically, children with SAM have increased total bile acids in serum, whereas their intestine contains decreased conjugated and increased secondary bile acids.35 Secondary bile acids, deoxycholic acid and lithocholic acid, products of metabolism by gut microbes, can be toxic to intestinal epithelial cells, increasing permeability and apoptosis.36 Thus, although liver dysfunction and microbiome alterations influence bile acid metabolism, the resulting bile acid changes may in turn cause liver and intestinal dysfunction. In a neonatal mouse model of protein deficiency, primary and secondary bile acid content within liver was decreased greater than 80%; mice exhibited evidence of oxidative stress, inflammation, autophagy, and liver dysfunction.37 Decreased intestinal conjugated bile acids likely also contribute to the impaired fat digestion, fat-soluble vitamin deficiencies, and small bowel bacterial overgrowth that contribute to the clinical picture of SAM.
Fig. 1.
Effects of malnutrition on liver structure and function.
COMPROMISED GUT BARRIER FUNCTION
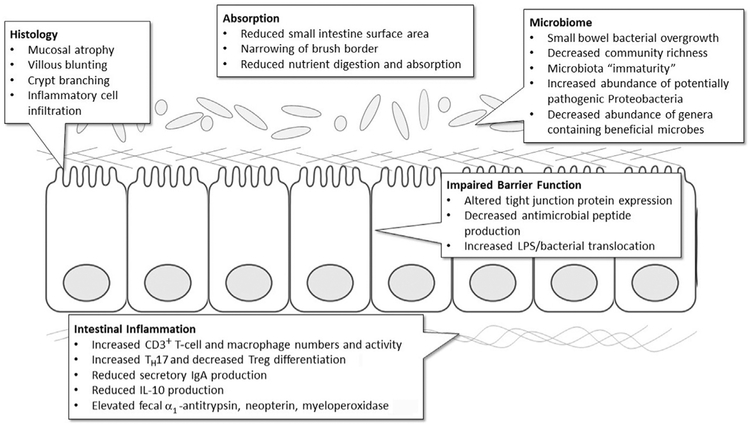
Malnutrition affects all organ systems, including the intestinal mucosa (Fig. 2). Hallmark histologic changes include mucosal and villous atrophy, crypt branching, and narrowing of the brush border.38 Malnourished children also have inflammatory cells infiltrating the lamina propria, increased numbers and activity of CD3 cells, increased macrophage number and activity, and reduced interleukin (IL)-10 production.39 Animal models of protein malnutrition reveal an inverse relationship between dietary protein quantity and the severity of intestinal histopathology.40,41
Fig. 2.
Effects of malnutrition on intestinal function. Observations predominantly from clinical studies, although some mechanistic data are from preclinical models of malnutrition.
This intestinal damage impairs digestion and absorption of macronutrients and micronutrients, resulting in increased nutritional requirements.42 In this context, proteins from breast milk and animal sources are more bioavailable than those derived from plants, which could explain why dairy proteins improve growth in children with SAM.43,44 Compared with other carbohydrates, lactose is often more easily digested by children with SAM.45
Animal models of malnutrition exhibit minimal intestinal histopathology unless an infectious insult is provided. Nonetheless, human studies and animal models suggest that malnutrition (with or without infection) impairs intestinal barrier function41,46 by altering the expression of antimicrobial peptides41 and tight junction proteins.47,48 Historically, intestinal absorption and permeability has been assessed with the lactulose:mannitol test, which has high variability in children due to inaccurate carbohydrate dosing, incorrect urine collection, variable rates of gastric emptying or renal excretion, and concurrent diarrhea.49 Recent studies have sought to identify biomarkers that correlate with intestinal damage, inflammation, and barrier function. Promising candidates include serum endotoxin core antibody; circulating bacterial products, such as lipopolysaccharide (LPS) and flagellin; and fecal markers, including α1-antitrypsin, myeloperoxidase, and neopterin.50 Serum LPS and bacterial 16S DNA are elevated in children with SAM, and correlate with decreased expression of mucosal repair peptides and IGF-1, which suggests GH resistance.47 Thus, decreased barrier function and bacterial translocation may contribute to chronic inflammation and growth failure by modulating the GH/IGF-1 axis.29,32 Not surprisingly, intestinal and systemic inflammatory markers and elevated GH predict mortality in malnourished children.32,51
IMMUNE DEFICIENCIES ATTRIBUTED TO MALNUTRITION
Malnutrition causes deficits in both adaptive and innate immune function, leading to increased childhood mortality from infectious disease.52 These deficits are multifactorial, driven in part by impaired immune cell production and function. Animal models of protein malnutrition demonstrate bone marrow atrophy and decreased numbers of hematopoietic stem cells and hematopoietic progenitor cells. Cell cycle arrest occurs in the latter due to reduced expression of cell cycle proteins and increased expression of inhibitory proteins.53 Bone marrow mesenchymal stem cells are more likely to differentiate into adipocytes in protein-malnourished mice, further limiting their ability to produce cytokines.54 Bone marrow polymorphonuclear cells from protein-malnourished mice also exhibit reduced migration and IL-1β production in response to LPS challenge.55 Lymphoid organs, including thymus, spleen, and lymph nodes, also show atrophy, reduced cellularity, arrested cell cycle, and impaired cellular function in acute malnutrition.56
In addition to the reduced cellularity of hematopoietic and lymphoid tissues, a shortened lifespan from increased apoptosis also contributes to reduced numbers of circulating monocytes, macrophages, dendritic cells, and natural killer cells.56 Circulating innate immune cells from malnourished mice also exhibit impaired cytokine expression in response to LPS as a result of nuclear factor κB dysregulation.56 In addition to protein deficiency, multiple micronutrient deficiencies can contribute to immune dysfunction.56 In preclinical models, natural killer cell and neutrophil functions are restored by reversal of vitamin A deficiency and vitamin C deficiency, respectively.57,58
Reduced numbers and function of innate immune cells contribute to deficits in adaptive immunity. Dendritic cells from severely malnourished children have reduced HLA-DR expression and consequently are unable to stimulate T cells.59 Peripheral blood mononuclear cells from malnourished children underexpress helper T-cell (TH)1 differentiation cytokines and overexpress TH2 cytokines, contributing to their inability to clear certain infections.60,61 CD3+ T cells from cord blood of children with IUGR revealed hypermethylation of genes that participate in T-cell regulation and activation and metabolic diseases.62 T cells from malnourished children also overexpress the apoptotic marker CD95.60 CD8+ T cells from malnourished mice recover their functional deficits when transferred to a healthy mouse,63 suggesting that environmental cues contribute to impaired function. T cells require glucose uptake and metabolism—both leptin-dependent processes. Low leptin levels in malnutrition inhibit T-cell activation and skew differentiation of T cells from TH1 to TH2.64,65 Leptin also protects against thymic atrophy, prevents apoptosis of innate immune cells, and improves cytokine production in macrophages and T cells in models of malnutrition.65,66 Changes to the gut microbiota drive differentiation of TH17 cells over regulatory T cells, because transcription factors for each cell type are sensitive to different commensal species.67 Finally, deficits in mucosal immunity in malnourished children can result in poor response to mucosal vaccines.68 In a mouse model of malnutrition, poor secretory IgA production mediated decreased response to Salmonella and cholera vaccines.69
Malnutrition also impairs hepatic synthesis of complement proteins, especially in children with edematous malnutrition, among whom low circulating C3 correlates with low serum albumin.52 Increased consumption of complement, however, measured via elevated circulating levels of the C3 degradation product C3d, might also contribute to the low levels reported in numerous studies of malnourished children.35
INTESTINAL MICROBIOTA AS A CAUSE AND THERAPEUTIC TARGET FOR MALNUTRITION
Enteropathogens induce malnutrition, mediating growth impairment by reducing nutrient absorption and increasing nutrient and energy needs.70 Most malnourished children in low-income countries harbor multiple pathogens.71 As the number of pathogens isolated from stool increases, weight-for-age z scores and height-for-age z scores decrease.72 A single episode of diarrhea can have an impact on mortality and linear growth for 2 months to 3 months after infection.51,71 Even in the absence of diarrhea, however, the malnourished gut microbiota is abnormal.73–75 Decades ago, culture-dependent studies revealed bacterial overgrowth in the proximal gastrointestinal tract, and microbial DNA sequencing technologies have facilitated a more detailed characterization of this malnutrition-associated dysbiosis75 (Box 1). Numerous factors may drive these microbiome alterations. For example, a monotonous diet containing specific nondigestible dietary carbohydrates20,76 provides a selective advantage to microbes that metabolize these substrates. Likewise, inflammation can alter the microbiome by triggering an immune response in which subsets of commensal microbes may be eliminated by host-secreted antimicrobial peptides,77 by disruption of the oxygen gradient at the mucosal surface78–80 and by generation of reactive oxygen and nitrogen species.81,82
Box 1. Summary of features that characterize the gut microbiota from malnourished children.
The fecal microbiome derived from malnourished versus healthy children is characterized by
-
Increased relative abundance of pathogenic genera within the phylum Proteobacteria
Enterobacter, Escherichia, Klebsiella, and Shigella
-
Decreased relative abundance of genera containing beneficial bacteria
Bifidobacterium, Butyrivibrio, Faecalibacterium, Lactobacillus, and Roseburia
Decreased microbial community richness (fewer unique organisms)
Microbiome “immaturity,” with delayed acquisition of age-specific microbes and microbial genes
Data from Velly H, Britton RA, Preidis GA. Mechanisms of cross-talk between the diet, the intestinal microbiome, and the undernourished host. Gut Microbes 2017;8(2):98–112.
Recent preclinical studies demonstrate a causal link between the malnourished microbiome and growth impairment. Fecal microbes isolated from malnourished children can induce weight loss in gnotobiotic mice under specific conditions.83–85 Similarly, mice iteratively challenged with a combination of nonpathogenic commensal microbes demonstrate impaired growth.86 Intriguingly, the microbiota’s effect on growth in each of these mouse models is dependent on administration of a low-protein, low-fat diet—if the animals consume standard chow, growth impairment is not observed. Furthermore, the presence of a malnourished microbiota can exacerbate weight loss due to pathogenic infection.87 On the other hand, specific beneficial microbes have been positively linked to growth. For example, Lactobacillus plantarum increases IGF-1 expression and linear growth in a model of chronic malnutrition.88
Several large double-blind, randomized controlled trials have examined the role of microbiome-targeting therapies for SAM but have demonstrated conflicting results (Table 1).89–94 Current WHO guidelines recommend treating all cases of complicated or uncomplicated SAM with broad-spectrum antibiotics because their use results in decreased mortality, and 1 study associated early-life antibiotic exposure with an increase in ponderal growth among children in low-income settings.95 These guidelines are warranted, but vigilance must be kept for adverse events that may emerge as well.96,97 Although trials of probiotics and prebiotics have not revealed growth benefits for malnourished children,93,94 these microbiome-targeting therapies were not tailored to microbial species or functional deficiencies within the target populations; thus, the full potential of microbiome-targeting therapies for child malnutrition has not yet been realized.
Table 1.
Randomized, double-blind,placebo-controlled trials examining microbiome-targeting therapies in the treatment of malnourished children
| Microbiome-Targeting Therapy | Study Population | Primary Outcome | Secondary Outcomes | Reference |
|---|---|---|---|---|
| (Super Synbiotics AB, Stockholm, Sweden)a in RUTF vs RUTF alone for treatment duration (mean 33 d) | N = 795, 5 mo–168 mo with WFH <70%, nutritional edema, or MUAC <11 cm, receiving inpatient treatment in Blantyre, Malawi | No effect on nutritional cure (WFH >80% on 2 consecutive outpatient visits): 53.9% treatment vs 51.3% placebo, P = .40 | No effect on mortality, outpatient defaulting, treatment failures, hospital readmissions, loss to followup, rate of weight gain, or days to nutritional cure | Kerac et al,93 2009 |
| Amoxicillin (80–90 mg/kg/d) vs cefdinir (14 mg/kg/d) vs placebo in 2 daily doses during the initial 7 d of RUTF | N = 2767, 6 mo–59 mo with nutritional edema or WHZ < −3 and able to consume RUTF as outpatient therapy at 18 feeding clinics in rural Malawi | Placebo increased risk of treatment failure vs amoxicillin (RR 1.32; 95% CI, 1.04–1.68) and vs cefdinir (RR 1.64; 95% CI, 1.27–2.11) and risk of death vs amoxicillin (RR 1.55; 95% CI, 1.07–2.24) and vs cefdinir (RR 1.80; 95% CI, 1.22–2.64) | Placebo decreased rate of weight gain vs cefdinir (3.1 ± 4.1 vs 3.9 ± 6.3 g/kg/d, P = .002); placebo decreased rate of MUAC gain (0.22±0.41 mm/d) vs amoxicillin (0.27 ± 0.42 mm/d, P = .01) and vs cefdinir (0.28 ± 0.42 mm/d, P = .002); no effect on time to recovery or rate of gain of length/height | Trehan et al,89 2013 |
| Amoxicillin (80 mg/kg/d) vs placebo twice daily for 7 d | N = 2412, 6 mo–59 mo with WHZ < −3 or MUAC <115 mm (but no nutritional edema), in outpatient therapy at 4 health centers in rural Niger | No effect on nutritional recovery (WHZ ≥ −2 on 2 consecutive visits and MUAC ≥115 and no nutritional edema ≥7 d and all treatments completed) by 8 wk of followup, risk ratio for amoxicillin vs placebo 1.05; 95% CI, 0.99–1.12 | Amoxicillin decreased risk of hospital admission by 14%; no effect on nonresponse rate at 8 wk, death from any cause, or default | Isanaka et al,91 2016 |
| Cotrimoxazole (120 mg/d for age <6 mo or 240 mg/d for age 6 mo to 5 y) vs placebo daily for 6 mo | N = 1778, 60 d–59 mo with MUAC <115 mm (≥6 mo) or <110 mm (2–5 mo) or nutritional edema, and HIV-antibody rapid test negative and completed initial stabilization phase of treatment at 2 rural and 2 urban hospitals in Kenya | No effect on mortality during the 365-d study period, hazard ratio for cotrimoxazole vs placebo 0.90; 95% CI, 0.71–1.16 | Cotrimoxazole increased incidence of diarrhea and decreased incidence of confirmed malaria, skin or soft tissue infections, and positive urine culture; no effect on frequency of other nonfatal illness episodes, pathogens detected from blood culture, suspected toxic effects, or changes in anthropometric or hematological indices | Berkley et al,92 2016 |
| Bifidobacterium animalis subsp lactis and Lactobacillus rhamnosus (109 CFUs/d) vs placebo from admission to completion of outpatient treatment (range 8–12 wk) | N = 400, 6 mo–59 mo with MUAC <115 mm or WHZ/WLZ < −3 or nutritional edema, in inpatient therapy in Kampala, Uganda | No effect on mean duration of diarrhea, adjusted effect size for probiotics vs placebo during inpatient treatment +0.2; 95% CI, −0.8 d-1.2 d | Probiotics decreased duration of diarrhea vs placebo (−2.2 d; 95% CI −3.5–0.3); no effect on incidence or severity of diarrhea, pneumonia incidence or duration or severity, nutritional recovery, weight gain, fever, vomiting, duration of hospitalization, or mortality | Grenov et al,94 2017 |
Abbreviations: CFUs, colony-forming units; RR, relative risk; WFH, weight-for-height as mean of National Center for Health Statistics data.
Freeze-dried 1011 CFUs Pediococcus pentosaceus, Leuconostoc mesenteroides, Lactobacillus paracasei, and Lactobacillus plantarum; 2.5 g each of oat bran, inulin, pectin, and resistant starch.
PROSPECTUS
Although recovery rates in SAM treatment programs routinely exceed 85%, hundreds of thousands of children still die every year. The next generation of treatment modalities must reflect the growing knowledge of the pathogenesis of malnutrition and the comorbidities that affect multiple organ systems. There also exists, however, an enormous coverage gap, with less than 15%98 of affected children globally, including less than 2% in East Asia and the Pacific,99 having access to malnutrition treatment. Among the barriers to access are caretaker awareness of malnutrition and local treatment programs, high opportunity costs of seeking treatment, and proximity.98 Interventions that may reduce the coverage gap include educating and engaging mothers, integrating community-based management of SAM with existing health programs, and strengthening government involvement to increase coverage and data collection.99 There is now a unique and extraordinary opportunity to work together—basic and clinical scientists, policymakers, and mothers of afflicted children—to ease the suffering from the most dire health problem plaguing children today.
KEY POINTS.
Prenatal and perinatal influences can have lifelong and intergenerational effects on nutritional status, primarily via epigenetic modifications.
Protein deficiency results in hepatic dysfunction driven by loss of peroxisomes and mitochondrial impairment.
Compromised gut barrier function increases nutrient needs and predisposes to infection and systemic inflammation.
Malnutrition-associated immune deficiencies are driven by lymphoid atrophy, cell cycle arrest of progenitor cells, and impaired effector function.
The intestinal microbiota can contribute to malnutrition and represents a promising therapeutic target.
Abbreviations
- GH
Growth hormone
- HMO
Human milk oligosaccharides
- IUGR
Intrauterine growth restriction
- IL
Interleukin
- LPS
Lipopolysaccharide
- MUAC
Mid-upper arm circumference
- PPARα
Peroxisome proliferator-activated receptor alpha
- RUTF
Ready-to-use therapeutic food
- SAM
Severe acute malnutrition
- WHO
World Health Organization
- WHZ
Weight-for-height z score
Footnotes
Disclosure Statement: The authors have no commercial or financial conflicts to disclose.
REFERENCES
- 1.UNICEF/WHO/World Bank Group. Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key findings of the 2017 edition. Geneva (Switzerland): UNICEF/WHO/World Bank; 2017. [Google Scholar]
- 2.Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243–60. [DOI] [PubMed] [Google Scholar]
- 3.Guerrant RL, DeBoer MD, Moore SR, et al. The impoverished gut–a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 2013; 10(4):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Guideline: updates on the management of severe acute malnutrition in infants and children. Geneva (Switzerland): World Health Organization; 2013. [PubMed] [Google Scholar]
- 5.Lenters LM, Wazny K, Webb P, et al. Treatment of severe and moderate acute malnutrition in low- and middle-income settings: a systematic review, meta-analysis and Delphi process. BMC Public Health 2013;13(Suppl 3):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoonees A, Lombard M, Musekiwa A, et al. Ready-to-use therapeutic food for home-based treatment of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst Rev 2013;6:CD009000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz J, Lee AC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013;382(9890):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian P, Lee SE, Donahue Angel M, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol 2013;42(5):1340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013; 382(9890):427–51. [DOI] [PubMed] [Google Scholar]
- 10.Persson LA, Arifeen S, Ekstrom EC, et al. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. JAMA 2012; 307(19):2050–9. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen TP, Yong HE, Chollangi T, et al. Placental vitamin D receptor expression is decreased in human idiopathic fetal growth restriction. J Mol Med (Berl) 2015; 93(7):795–805. [DOI] [PubMed] [Google Scholar]
- 12.Workalemahu T, Badon SE, Dishi-Galitzky M, et al. Placental genetic variations in vitamin D metabolism and birthweight. Placenta 2017;50:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Estal I, de la Garza RG, Castilla-Cortazar I. Intrauterine growth retardation (IUGR) as a novel condition of insulin-like growth factor-1 (IGF-1) deficiency. Rev Physiol Biochem Pharmacol 2016;170:1–35. [DOI] [PubMed] [Google Scholar]
- 14.Tobi EW, Goeman JJ, Monajemi R, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun 2014;5:5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding YX, Cui H. Integrated analysis of genome-wide DNA methylation and gene expression data provide a regulatory network in intrauterine growth restriction. Life Sci 2017;179:60–5. [DOI] [PubMed] [Google Scholar]
- 16.Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care 2006;9(4):388–94. [DOI] [PubMed] [Google Scholar]
- 17.Martorell R, Zongrone A. Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol 2012;26(Suppl 1):302–14. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Rodriguez P, Cantu J, O’Neil D, et al. Alterations in expression of imprinted genes from the H19/IGF2 loci in a multigenerational model of intrauterine growth restriction (IUGR). Am J Obstet Gynecol 2016;214(5):625.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dror DK. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Curr Opin Obstet Gynecol 2011;23(6):422–6. [DOI] [PubMed] [Google Scholar]
- 20.Charbonneau MR, O’Donnell D, Blanton LV, et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016;164(5):859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaitla B, Devereux S, Swan SH. Seasonal hunger: a neglected problem with proven solutions. PLoS Med 2009;6(6):e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burza S, Mahajan R, Marino E, et al. Seasonal effect and long-term nutritional status following exit from a Community-Based Management of Severe Acute Malnutrition program in Bihar, India. Eur J Clin Nutr 2016;70(4):437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh S Protein quality in the first thousand days of life. Food Nutr Bull 2016; 37(Suppl 1):S14–21. [DOI] [PubMed] [Google Scholar]
- 24.Mal-Ed Network Investigators. Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED longitudinal birth cohort study. PLoS Med 2017;14(10):e1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh S Assessment of protein adequacy in developing countries: quality matters. Food Nutr Bull 2013;34(2):244–6. [DOI] [PubMed] [Google Scholar]
- 26.Semba RD, Shardell M, Sakr Ashour FA, et al. Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016;6:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149(2):274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freemark M Metabolomics in nutrition research: biomarkers predicting mortality in children with severe acute malnutrition. Food Nutr Bull 2015;36(1 Suppl):S88–92. [DOI] [PubMed] [Google Scholar]
- 29.Wong SC, Dobie R, Altowati MA, et al. Growth and the growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directions. Endocr Rev 2016;37(1):62–110. [DOI] [PubMed] [Google Scholar]
- 30.Bartz S, Mody A, Hornik C, et al. Severe acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J Clin Endocrinol Metab 2014;99(6):2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semba RD, Trehan I, Li X, et al. Low serum omega-3 and omega-6 polyunsaturated fatty acids and other metabolites are associated with poor linear growth in young children from rural Malawi. Am J Clin Nutr 2017;106(6):1490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeBoer MD, Scharf RJ, Leite AM, et al. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition 2017;33: 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doherty JF, Adam EJ, Griffin GE, et al. Ultrasonographic assessment of the extent of hepatic steatosis in severe malnutrition. Arch Dis Child 1992;67(11):1348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Zutphen T, Ciapaite J, Bloks VW, et al. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J Hepatol 2016;65(6):1198–208. [DOI] [PubMed] [Google Scholar]
- 35.Preidis GA, Kim KH, Moore DD. Nutrient-sensing nuclear receptors PPARalpha and FXR control liver energy balance. J Clin Invest 2017;127(4):1193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ajouz H, Mukherji D, Shamseddine A. Secondary bile acids: an underrecognized cause of colon cancer. World J Surg Oncol 2014;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preidis GA, Keaton MA, Campeau PM, et al. The undernourished neonatal mouse metabolome reveals evidence of liver and biliary dysfunction, inflammation, and oxidative stress. J Nutr 2014;144(3):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanfield JP, Hutt MS, Tunnicliffe R. Intestinal biopsy in kwashiorkor. Lancet 1965; 2(7411):519–23. [DOI] [PubMed] [Google Scholar]
- 39.Welsh FK, Farmery SM, MacLennan K, et al. Gut barrier function in malnourished patients. Gut 1998;42(3):396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sampaio IC, Medeiros PH, Rodrigues FA, et al. Impact of acute undernutrition on growth, ileal morphology and nutrient transport in a murine model. Braz J Med Biol Res 2016;49(10):e5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attia S, Feenstra M, Swain N, et al. Starved guts: morphologic and functional intestinal changes in malnutrition. J Pediatr Gastroenterol Nutr 2017;65(5):491–5. [DOI] [PubMed] [Google Scholar]
- 42.Kvissberg MA, Dalvi PS, Kerac M, et al. Carbohydrate malabsorption in acutely malnourished children and infants: a systematic review. Nutr Rev 2016;74(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arsenault JE, Brown KH. Effects of protein or amino-acid supplementation on the physical growth of young children in low-income countries. Nutr Rev 2017;75(9):699–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manary M, Callaghan M, Singh L, et al. Protein quality and growth in malnourished children. Food Nutr Bull 2016;37(Suppl 1):S29–36. [DOI] [PubMed] [Google Scholar]
- 45.Grenov B, Briend A, Sangild PT, et al. Undernourished children and milk lactose. Food Nutr Bull 2016;37(1):85–99. [DOI] [PubMed] [Google Scholar]
- 46.Campbell DI, Elia M, Lunn PG. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr 2003;133(5):1332–8. [DOI] [PubMed] [Google Scholar]
- 47.Amadi B, Besa E, Zyambo K, et al. Impaired barrier function and autoantibody generation in malnutrition enteropathy in Zambia. EBioMedicine 2017;22:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wada M, Tamura A, Takahashi N, et al. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013;144(2):369–80. [DOI] [PubMed] [Google Scholar]
- 49.Denno DM, VanBuskirk K, Nelson ZC, et al. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis 2014;59(Suppl 4):S213–9. [DOI] [PubMed] [Google Scholar]
- 50.Harper KM, Mutasa M, Prendergast AJ, et al. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl Trop Dis 2018;12(1):e0006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attia S, Versloot CJ, Voskuijl W, et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: an observational cohort study. Am J Clin Nutr 2016;104(5):1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rytter MJ, Kolte L, Briend A, et al. The immune system in children with malnutrition–a systematic review. PLoS One 2014;9(8):e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakajima K, Crisma AR, Silva GB, et al. Malnutrition suppresses cell cycle progression of hematopoietic progenitor cells in mice via cyclin D1 down-regulation. Nutrition 2014;30(1):82–9. [DOI] [PubMed] [Google Scholar]
- 54.Cunha MC, Lima Fda S, Vinolo MA, et al. Protein malnutrition induces bone marrow mesenchymal stem cells commitment to adipogenic differentiation leading to hematopoietic failure. PLoS One 2013;8(3):e58872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fock RA, Vinolo MA, Blatt SL, et al. Impairment of the hematological response and interleukin-1beta production in protein-energy malnourished mice after endo-toxemia with lipopolysaccharide. Braz J Med Biol Res 2012;45(12):1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim MK, Zambruni M, Melby CL, et al. Impact of Childhood Malnutrition on Host Defense and Infection. Clin Microbiol Rev 2017;30(4):919–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowman TA, Goonewardene IM, Pasatiempo AM, et al. Vitamin A deficiency decreases natural killer cell activity and interferon production in rats. J Nutr 1990; 120(10):1264–73. [DOI] [PubMed] [Google Scholar]
- 58.Vissers MC, Wilkie RP. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha. J Leukoc Biol 2007;81(5):1236–44. [DOI] [PubMed] [Google Scholar]
- 59.Hughes SM, Amadi B, Mwiya M, et al. Dendritic cell anergy results from endotoxemia in severe malnutrition. J Immunol 2009;183(4):2818–26. [DOI] [PubMed] [Google Scholar]
- 60.Badr G, Sayed D, Alhazza IM, et al. T lymphocytes from malnourished infants are short-lived and dysfunctional cells. Immunobiology 2011;216(3):309–15. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Torres C, Gonzalez-Martinez H, Miliar A, et al. Effect of malnutrition on the expression of cytokines involved in Th1 cell differentiation. Nutrients 2013; 5(2):579–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams L, Seki Y, Delahaye F, et al. DNA hypermethylation of CD3(+) T cells from cord blood of infants exposed to intrauterine growth restriction. Diabetologia 2016;59(8):1714–23. [DOI] [PubMed] [Google Scholar]
- 63.Chatraw JH, Wherry EJ, Ahmed R, et al. Diminished primary CD8 T cell response to viral infection during protein energy malnutrition in mice is due to changes in microenvironment and low numbers of viral-specific CD8 T cell precursors. J Nutr 2008;138(4):806–12. [DOI] [PubMed] [Google Scholar]
- 64.Saucillo DC, Gerriets VA, Sheng J, et al. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol 2014;192(1):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodriguez L, Graniel J, Ortiz R. Effect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected children. Clin Exp Immunol 2007;148(3):478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruno A, Conus S, Schmid I, et al. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol 2005;174(12):8090–6. [DOI] [PubMed] [Google Scholar]
- 67.Sefik E, Geva-Zatorsky N, Oh S, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science 2015;349(6251):993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Czerkinsky C, Holmgren J. Vaccines against enteric infections for the developing world. Philos Trans R Soc Lond B Biol Sci 2015;370(1671). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rho S, Kim H, Shim SH, et al. Protein energy malnutrition alters mucosal IgA responses and reduces mucosal vaccine efficacy in mice. Immunol Lett 2017;190: 247–56. [DOI] [PubMed] [Google Scholar]
- 70.Guerrant RL, Oria RB, Moore SR, et al. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 2008;66(9):487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kotloff KL. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am 2017;64(4):799–814. [DOI] [PubMed] [Google Scholar]
- 72.Platts-Mills JA, Taniuchi M, Uddin MJ, et al. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr 2017;105(5):1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blanton LV, Barratt MJ, Charbonneau MR, et al. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016;352(6293):1533. [DOI] [PubMed] [Google Scholar]
- 74.Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res 2015;77(1–2):256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velly H, Britton RA, Preidis GA. Mechanisms of cross-talk between the diet, the intestinal microbiome, and the undernourished host. Gut Microbes 2017;8(2):98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanchez de Medina F, Romero-Calvo I, Mascaraque C, et al. Intestinal inflammation and mucosal barrier function. Inflamm Bowel Dis 2014;20(12):2394–404. [DOI] [PubMed] [Google Scholar]
- 78.Marteyn B, West NP, Browning DF, et al. Modulation of Shigella virulence in response to available oxygen in vivo. Nature 2010;465(7296):355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albenberg L, Esipova TV, Judge CP, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014;147(5):1055–63.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morris RL, Schmidt TM. Shallow breathing: bacterial life at low O(2). Nat Rev Microbiol 2013;11(3):205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010;467(7314):426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winter SE, Winter MG, Xavier MN, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013;339(6120):708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013;339(6119):548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blanton LV, Charbonneau MR, Salih T, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016; 351(6275):854–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kau AL, Planer JD, Liu J, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med 2015;7(276):276ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown EM, Wlodarska M, Willing BP, et al. Diet and specific microbial exposure trigger features of environmental enteropathy in a novel murine model. Nat Commun 2015;6:7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wagner VE, Dey N, Guruge J, et al. Effects of a gut pathobiont in a gnotobiotic mouse model of childhood undernutrition. Sci Transl Med 2016;8(366):366ra164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwarzer M, Makki K, Storelli G, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016;351(6275):854–7. [DOI] [PubMed] [Google Scholar]
- 89.Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013;368(5):425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dubray C, Ibrahim SA, Abdelmutalib M, et al. Treatment of severe malnutrition with 2-day intramuscular ceftriaxone vs 5-day amoxicillin. Ann Trop Paediatr 2008;28(1):13–22. [DOI] [PubMed] [Google Scholar]
- 91.Isanaka S, Langendorf C, Berthe F, et al. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med 2016;374(5):444–53. [DOI] [PubMed] [Google Scholar]
- 92.Berkley JA, Ngari M, Thitiri J, et al. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial. Lancet Glob Health 2016; 4(7):e464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kerac M, Bunn J, Seal A, et al. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. Lancet 2009;374(9684):136–44. [DOI] [PubMed] [Google Scholar]
- 94.Grenov B, Namusoke H, Lanyero B, et al. Effect of probiotics on diarrhea in children with severe acute malnutrition: a randomized controlled study in uganda. J Pediatr Gastroenterol Nutr 2017;64(3):396–403. [DOI] [PubMed] [Google Scholar]
- 95.Rogawski ET, Platts-Mills JA, Seidman JC, et al. Early antibiotic exposure in low-resource settings is associated with increased weight in the first two years of life. J Pediatr Gastroenterol Nutr 2017;65(3):350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488(7413):621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.UNICEF/Coverage Monitoring Network/ACF International. The State of Global SAM Management Coverage 2012. New York and London: (UNICE/CMN/ACF; ); 2012. [Google Scholar]
- 99.UNICEF. Annual Results Report 2016 Nutrition. New York: United Nations Children’s Fund (UNICEF); 2017. [Google Scholar]