Abstract
Ethnopharmacological relevance
Paullinia pinnata L. (Sapindaceae) is an African woody vine, traditionally used for the treatment of itch and pain-related conditions such as rheumatoid arthritis.
Aim
This work evaluates, in vitro and in vivo, the anti-inflammatory and analgesic effects of aqueous (AEPP) and methanol (MEPP) extracts from Paullinia pinnata leaves.
Methods
AEPP and MEPP (100, 200 and 300 mg/kg/day) were administered orally in monoarthritic rats induced by a unilateral injection of 50 μl of Complete Freund’s Adjuvant (CFA) in the ankle joint. During the 14 days of treatment, pain and inflammation were evaluated alternatively in both ankle and paw of the CFA-injected leg. Malondialdehyde (MDA) and glutathione (GSH) levels were assessed in serum and spinal cord. Histology of smooth tissue of the paw was also analyzed. For in vitro studies, AEPP and MEPP (10, 30 and 100 μg/ml) were evaluated against nitric oxide (NO) production by macrophages that were either non-stimulated or stimulated with LPS, 8-Br-AMPc and the mixture of both substances after 8h exposure. These extracts were also evaluated on TNF-α and IL-1β production in cells stimulated with LPS for 8 hours. Finally, the ability of the extracts to bind to neuroactive receptors was evaluated in vitro using competitive binding assays with >45 molecular targets.
Results
AEPP and MEPP significantly reduced by 20 to 98% (p<0.001) the inflammation and pain sensation in both the ankle and paw. AEPP significantly increased glutathione levels (p<0.05) in serum. Both extracts reduced MDA production in serum and spinal cord (p<0.001), and significantly improved tissue reorganization in treated arthritic rats. P. pinnata extracts did not affect NO production in non-stimulated macrophages but significantly reduced it by 47 to 88% in stimulated macrophages. AEPP and MEPP also significantly inhibited TNF-α (35 to 68%) and IL-1β (31 to 36%) production in LPS stimulated macrophages. No cytotoxic effect of plant extracts was observed. MEPP showed concentration-dependent affinity for Sigma 2 receptors with an IC50 of 50 μg/ml.
Conclusion
These results demonstrate the analgesic and anti-inflammatory effects of P. pinnata extracts on monoarthritis and further support its traditional use for pain and inflammation. These activities are at least partly due to the ability of these extracts to inhibit the production of NO, TNF-α, IL-1β and to likely modulate Sigma 2 receptors.
Keywords: Paullinia pinnata, rheumatoid arthritis, analgesic, anti-inflammatory, cytokines, Sigma 2 receptor
Graphical Abstract
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by warm, swollen, and painful joints that can be extended to surrounding tissues and bone loss. It is a worldwide health problem with a global lifetime prevalence of about 1% (Wasserman, 2018). It is often associated with significant disability, suffering, anxiety and emotional distress (Wong et al., 2010). In Cameroon, RA has a prevalence of 5.4% in the population over 60 years of age (Atabonkeng et al., 2015). The bone loss and joint destruction in this pathology are mediated by immunological insults, proinflammatory cytokines, and various immune cells (Jung et al., 2014). The intense activity of immune cells in RA leads to over production of reactive oxygen species (ROS), resulting in oxidative stress; in fact, significant oxidative stress has been observed in RA patients (Veselinovic et al., 2014; García-González et al., 2015). The produced ROS significantly potentiate the inflammatory processes, activate the immune system, and likely maintain or worsen the pathology.
Therapeutic options in the treatment of RA have improved in the last decade, moving from the use of simple non-steroidal anti-inflammatory drugs to strategies that target the harmful effects of up-regulated cytokines or other inflammatory mediators and inhibit their associated signaling events. The utility of cytokines as therapeutic targets in RA has been unequivocally demonstrated by the success of tumor necrosis factor (TNF)-α blockade in clinical practice (Alghasham and Rasheed, 2014). However, some patients only respond partially or do not respond at all to TNF-α blocking agents. The availability/cost of this new class of drug is also a serious matter of concern. Further therapeutic strategies need to be identified. One intriguing direction is a polypharmacological approach to target multiple signaling pathways at once. In this manuscript, we utilize medicinal plant extracts to achieve this aim, given their potential pleotropic effects related to the large chemical content of the extracts.
Paullinia pinnata (P. pinnata) is an African woody vine traditionally used in the west region of Cameroon for the treatment of pruritis (Mozouloua et al., 2011), rheumatoid arthritis, and bacterial infection (Lunga et al., 2014). In eastern Africa, a decoction of the leaves is used in the treatment of snake bites (Chabra et al., 1991). Phytochemical studies of this plant have revealed the presence of phenols (Zamble et al., 2006), flavonoids, triterpene, saponins and tannins (Abourashed et al., 1999), steroids, steroidal glycosides as well as cerebroside and a ceramide (Dongo et al., 2009). Compounds such as methylinositol, β-sitosterol and friedelin have been isolated from P. pinnata (Lunga et al., 2014).
Based on the traditional use of this plant, we conducted the present study with an aim to (1) test the efficacy of aqueous and methanol extracts of the leaves of P. pinnata on CFA-induced monoarthritis in Wistar rats and (2) identify the potential mechanisms of the analgesic and anti-inflammatory effects of the extracts. The novelty of the present work is two-fold. First, by rigorously testing the efficacy of these extracts in laboratory models of pain, we provide evidence that human use of these plants is likely driven by real anti-inflammatory and analgesic action rather than placebo effects. Second, our in vitro experiments have begun the process of determining the mechanism of action of the P. pinnata plant extracts, especially the relationship to cytokines and Sigma 2 receptor binding.
2. Material and methods
2.1. Plant material and extracts’ preparation
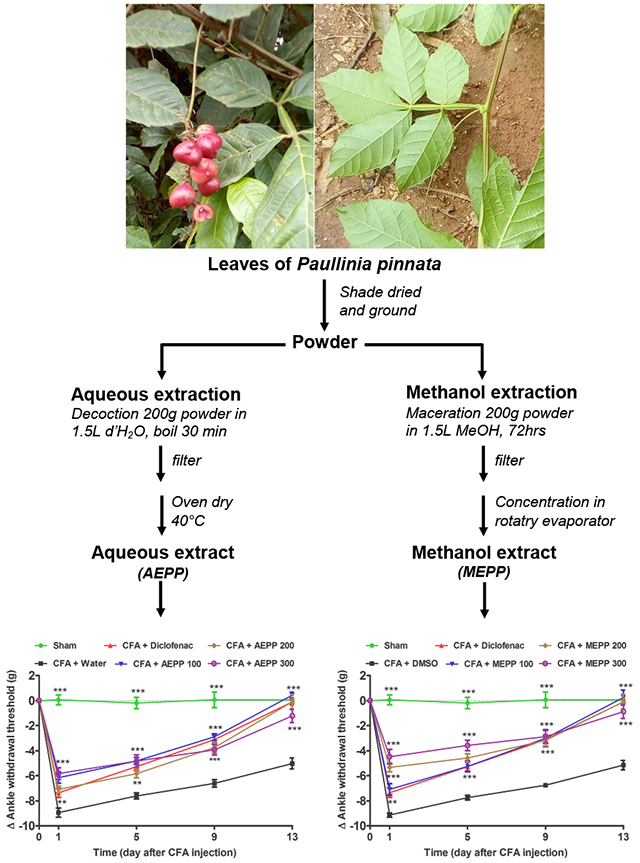
P. pinnata leaves were collected in Koung-khi Division (West Region, Cameroon) in April 2016. The identification of plant specimens was done at the Cameroon National Herbarium in Yaoundé by Mr. Tadjouteu Fulbert in comparison with a voucher specimen under the reference number 10701/SRF.Cam.
The plant material was shade dried and ground into a fine powder. The aqueous extract (AEPP) was prepared as a decoction, by boiling 200 g of the powder in 1.5 L of distilled water for 15 minutes. The mixture was filtered on Whatman paper N° 1. The residue was re-extracted and filtered using the same procedure in 1 L of distilled water. The two filtrates obtained were mixed and evaporated in a ventilated oven for 72 hours at 40°C to yield 12.23 g.
The methanol extract (MEPP) was prepared by macerating 200 g of the powder in 1.5 L of methanol for 72 hours. The mixture was filtered on Whatman paper N° 1. The residue was re-extracted in 1 L of methanol for 24 hours and filtered. The two filtrates were mixed and concentrated at 40°C and at low pressure in a rotary evaporator to obtain 21.19 g of the methanol extract.
The aqueous extract was diluted in distilled water while the methanol extract was prepared in 4% DMSO. For in vitro experiments, extract solutions were prepared and filtered using a 0.2 μM mesh. Samples (1 ml) of the filtered solutions were completely evaporated in an oven and the residue weighed to determine the concentration of each solution. Thereafter, solutions were diluted in culture medium (RPMI) prior to experiments.
2.2. Chemicals and drugs
Complete Freund’s adjuvant (CFA), lipopolysaccharide, 8-Bromo-cAMP, sodium chloride, H-89 Dihydrochloride, Roswell Park Memorial Institute (RPMI) Fetal Bovine Serum (FBS), NG-nitro-L-Arginine methyl ester (L-NAME), 8-Bromo-cyclic monophosphate Adenosine (8-Br-AMPc), and (H89) were purchased from Sigma-Aldrich Chemical Co. (Taufkirchen, Germany). 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl-tetrazolium bromide (MTT), Dimethylsufoxide (DMSO), Penicillin and Streptomycin were purchased from Carl-Roth (Kalshur, Germany). Sodium Diclofenac was obtained from Denk-Pharma (Germany). Dexamethasone was obtained from Enzo-life Science (Sweden). Ether was purchased from a local pharmacy (Dschang, Cameroon). Griess reagent was prepared in the laboratory with ingredients from Sigma-Aldrich Chemical Co. (Taufkirchen, Germany). Mouse TNF-α and IL-1β ELISA kit were purchased from R&D Systems.
2.3. Animals
For all in vivo components of this study, 60 Wistar rats (10 to 12 weeks old), weighing between 150-200 g, were divided into 10 groups of 6 animals each (3 males and 3 females). Animals were bred on-site in the Laboratory of Physiology and Phytopharmacology of the University of Dschang, Cameroon. For in vitro primary macrophage analysis, Swiss mice were utilized. Rats and mice were maintained under standard laboratory conditions and had ad libitum access to water and chow. All procedures used in the present study were approved by the local Ethical Committee and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain (protocol number PP00032). Rats were habituated to pain threshold recording and paw volume measurement before the experiment. Rats with baseline pain threshold below 7 g were discarded to ensure a more precise measurement of mechanical withdrawal thresholds following CFA-induced arthritis.
2.4. CFA-Induced Painful Inflammatory Arthritis
The experiment was conducted following the protocol previously described by Mannelli et al. (2013) with some modifications. Rats were briefly anesthetized with ether, the right leg skin was sterilized with 75% ethanol and the lateral malleolus located by palpation. Then, an insulin syringe needle was inserted vertically to penetrate the skin and turned distally for insertion into the articular cavity at the gap between the tibiofibular and tarsal bone until a distinct loss of resistance was felt. Arthritis was induced by injection of 50 μl of CFA into the articulation (day 0). In the sham group, which served as naive controls, an empty needle was introduced in the articulation without injection. The CFA-injected rats (54) were subdivided in 9 groups: aqueous extract negative control treated orally with distilled water, methanol extract negative control treated orally with DMSO (4% in distilled water), positive control treated with diclofenac (5 mg/kg/day; dissolved in distilled water) and the 6 remaining groups were treated with AEPP or MEPP at the doses of 100, 200 and 300 mg/kg/day. All CFA-injected groups were treated daily for 14 consecutive days. During the experimental period, pain threshold at the ankle and paw was recorded with the aid of an analgesiometer (UgoBasile, type 37215) on the 1st, 5th, 9th and 13th days. Briefly, awake rats were scruffed and placed in the meter. Two trials were completed on the CFA-injected (or sham-treated) ankle or ipsilateral paw per rat per day and the values averaged.
Paw volume was measured using an electronic caliper (Fine Science, Heidelberg, Germany) on the 2nd, 6th, 10th and 14th days after CFA administration. Briefly, rats were slightly restrained and the diameter of the paw was measured at the space between all footpads from the back (dorsal surface) to the front (plantar surface) of the paw. At the 15th day, animals were anesthetized by intraperitoneal injection of 15% ethyl carbamate solution (1.5 g/kg). Blood and spinal cord (lumbar portion L3 - L5) were collected for antioxidant assays.
Blood collected in heparinized tubes was centrifuged at 3000 rpm and the plasma was kept at −20°C until use. The lumbar portion of spinal cord was homogenized in 5% solution of ice-cold 0.1 M Tris buffer (pH 7.4) at 4°C and centrifuged twice for 15 min at 3000 and 10,000 rpm at 4°C and the supernatant was collected. Both plasma sera and spinal cord supernatant were used for Glutathione (GSH) and malondialdehyde (MDA) estimation. The CFA-injected and sham rats’ ankle joints were collected and kept in 4% buffered formalin for histopathological examination.
2.5. Biochemical and histopathological examinations
2.5.1. GSH Measurement
A modified method of Sehirli et al., (2008) was used. Briefly, 250 μl of sample was added to 1 ml of Na2HPO4. 2H2O (0.3 M) and 0.1 ml of dithiobisnitrobenzoate (0.4 mg/ml in 1% of trisodic acid). The optical density was read with a spectrophotometer (Helios Epsilon) at 412 nm 1 hour after mixture of reagents. The rate of GSH was calculated according to the following formula: D.O. = ε .C.L (where D.O. = optical density, ε = Coefficient of molar extinction of the GSH (1.36 ×104 M−1.Cm−1), C = Concentration in GSH, L = Length of the optical journey (1 cm)).
2.5.2. MDA Measurement
The MDA assay was conducted as previously described by Chen et al., (2008). 500 μl of orthophosphoric acid (1%) and 500 μl of thiobarbituric acid was added to 100 μl of plasma or supernatant. The mixture was homogenized and placed in boiling water bath for 15 minutes. The tubes were cooled with an ice bath and centrifuged to 3000 rpm for 10 minutes. The supernatant was collected and the absorbance was read at 532 nm using a spectrophotometer. The concentration of MDA was calculated based on the absorbance coefficient according to the following formula: D.O. = ε .C.L (where D.O. = optical density, ε = Coefficient of molar extinction of the MDA (1.56 × 105 M−1. Cm−1), C = Concentration in MDA, L = Length of the optic journey (1 cm)).
2.5.3. Histopathological Analysis of Ankle Joint
The fixed soft tissue of collected joint was removed and put into graded ethanol 80%, 95%, and 100% for dehydration, follow by impregnation in 2 trays of xylene (2 hours per tray of ethanol and xylene). Paraffin inclusion was done and 5 μm thick sections were cut and stained with hematoxylen-eosin for histological examination with a light microscope at 100× magnification. The histological pictures of all samples were blindly analyzed by an anatomo-pathologist.
2.6. In vitro macrophage isolation and culture
Mice aged 8 to 10 weeks were sacrificed by cervical dislocation. Their abdomens were sterilized with ethanol 75%. The skin was incised to expose the peritoneal wall and 5 ml of PBS-EDTA were injected into peritoneal cavity. The abdomen was massaged, the fluid from peritoneum collected and dispensed into a centrifuge tube on ice. After centrifugation at 1500 rpm for 5 min at 4°C, the pellet was collected and washed in PBS by second similar centrifugation. The cell pellet was resuspended in 5 ml of RPMI medium (supplemented with 10% heat-inactivated FBS, 100U/mL penicillin and 100 μg/mL streptomycin) at 37 °C with 5% CO2 for 3 hours. During this time, peritoneal macrophages adhered to the plastic surface and the floating non-macrophages and dead cells were washed away by adding and aspirating PBS. The adherent macrophages were collected, suspended in supplemented RPMI and incubated in a 96-well plate, 1×105 cells/well in 200μl RPMI medium for 3 hours. After this period, cells were exposed to plant extracts, L-NAME, or Dexamethaxone at the concentrations of 10, 30 and 100 μg/ml for 8 hours. In another set of experiments, cells were additionally stimulated with LPS (1 μg/ml), 8-Br-cAMP (0.5 μM) or both. When stimulated, cells were incubated with tested substances for an hour before adjunction of stimulating substances. In the experiments using 8-Br-cAMP alone or in combination with LPS, cells were plated at a density of 1×7.54 cells/well to avoid cytotoxicity of over produced inflammatory mediators. In all cases, the final volume of each well was adjusted to 300 μl with RPMI. At the end of the experimental period, the supernatant was collected for the assay of NO, TNF-α or IL-1β. Attached cells were washed twice with PBS and incubated with MTT for cell viability. NO was assayed as previously described (Nguelefack-Mbuyo et al., 2012), while TNF-α and IL-1β were quantified using commercial kits as indicated by the manufacturer (R&D System).
2.7. In vitro screening of extract targets
To evaluate the potential of P. pinnata extracts for lead compounds to treat pain and inflammation, AEPP and MEPP were screened using a radioligand competition-binding assay. Extracts were screened for their ability to bind to a panel of 43 central nervous system targets including G protein couple receptors (GPCRs), monoamine transporters (MATs), and ion channels. All binding data for AEPP and MEPP extracts were performed and generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP) Besnard et al. (2012). Experimental details are available online at http://pdsp.med.unc.edu/. In brief, extracts that passed primary binding criterion of greater than 50% inhibition at 10 mg/ml, were subjected to secondary screening to determine IC50 at the specific target of interest.
2.8. Statistical analysis
All the data were expressed as mean ± SEM. Statistical analysis was done using Graph Pad Prism software version 5.0. One-way ANOVA followed by the Tukey’s post-test was used when analyzing one independent variable data. Two-way ANOVA-repeated measure followed by Bonferroni post-test was used to analyze two independent variable data. P values less than 0.05 were considered to be statistically significant.
3. RESULTS
3.1. Effect of Paullinia pinnata extracts on CFA-induced inflammation
Oral administration of P. pinnata extracts significantly inhibited ankle and paw edema induced by CFA injection (two way ANOVA main effects time, treatment and interaction p<0.0001). Bonferroni post hoc analysis demonstrated analgesic-effects of P. pinnata at all doses (100, 200 and 300 mg/kg) used (Figure 1A-D). The methanol extract was the most effective with maximal inhibitory percentages of 79 and 98% at the ankle and paw, respectively. The positive control diclofenac (5 mg/kg) induced significant anti-inflammatory activity with inhibitory percentage ranging from 46 to 91%, but was in general less efficient than P. pinnata extracts (Figure 1).
Figure 1:
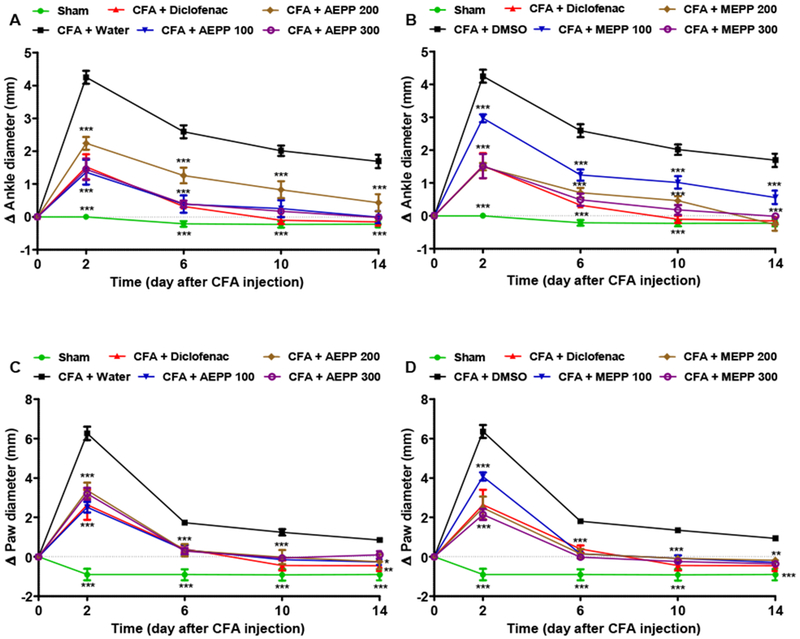
Effects of the aqueous (AEPP 100, 200, 300mg/kg/day; A, C) and methanol (MEPP 100, 200, 300mg/kg/day; B, D) extracts of Paullinia pinnata on the primary (Ankle; A-B) and secondary (Paw; C-D) inflammatory arthritis induced by ankle injection of CFA in rat. (A) AEPP induces significant decreases in ankle swelling compared to rats treated with CFA and vehicle (water). (B) MEPP induces significant decreases in ankle swelling compared to rats treated with CFA and vehicle. (C) AEPP induces significant decreases in paw swelling compared to rats treated with CFA and vehicle. (D) MEPP induces significant decreases in paw swelling compared to rats treated with CFA and vehicle. NSAID diclofenac (5 mg/kg/day) is included as a positive control for anti-inflammatory effects. Each point represents the mean ± SEM of 6 individual rats. **p<0.01, ***p<0.001 significant difference compared to negative control group (CFA+Water) using 2 way ANOVA with Bonferroni post-hoc test.
3.2. Effect of Paullinia pinnata extracts on CFA-induced hyperalgesia
P. pinnata extracts administered orally showed significant antihyperalgesic effects (two way ANOVA effects time, treatment and interaction p<0.0001). As demonstrated by a Bonferroni post hoc test, aqueous and methanol extracts at all the doses used significantly inhibited (p<0.05 to p<0.001) mechanical hyperalgesia induced by CFA, both at the level of ankle and on the paw. The maximal inhibitory effects of 89 and 80% respectively for ankle and paw were observed with aqueous extract at the dose of 100 mg/kg (Figure 2).
Figure 2:
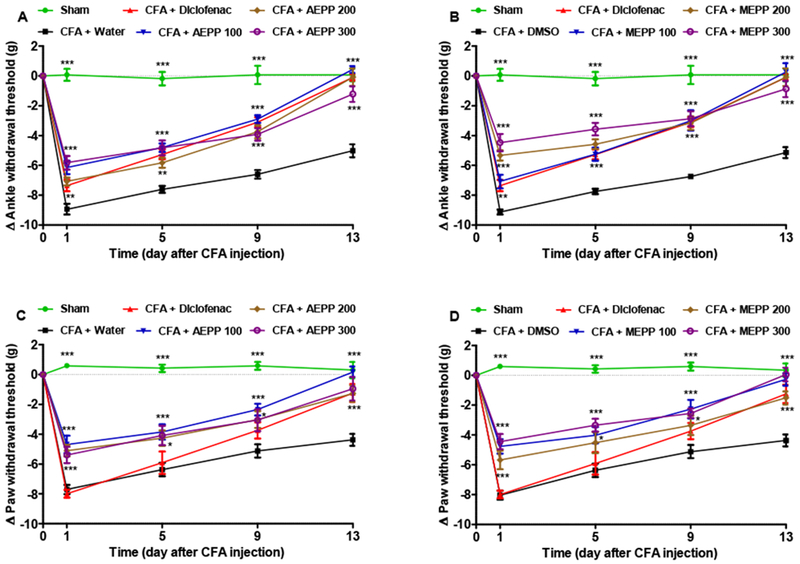
Effects of the aqueous (AEPP; 100, 200, 300 mg/kg/day) and methanol (MEPP; 100, 200, 300 mg/kg/day) extracts of Paullinia pinnata on the primary (Ankle) and secondary (Paw) mechanical hypersensitivity induced by ankle injection of CFA in rat. (A) AEPP induces significant decreases in mechanical hypersensitivity (i.e. decreased change in threshold from baseline) compared to rats treated with CFA and vehicle (water). (B) MEPP induces significant decreases in mechanical hypersensitivity compared to rats treated with CFA and vehicle. (C) AEPP induces significant decreases in mechanical hypersensitivity compared to rats treated with CFA and vehicle. (D) MEPP induces significant decreases in mechanical hypersensitivity compared to rats treated with CFA and vehicle. NSAID diclofenac (5 mg/kg/day) is included as a positive control for anti-inflammatory effects. Each point represents the mean ± SEM of 6 individual rats. **p<0.01, ***p<0.001 significant difference compared to negative control group (CFA+Water) using 2 way ANOVA with Bonferroni post-hoc test.
3.3. Effects of Paullinia pinnata on GSH content in plasma and spinal cord of arthritic rat
To evaluate the impact of P. pinnata extracts on oxidative stress, we measured glutathione (GSH) production in the plasma and spinal cord on day 15 after CFA administration (Figure 3). As expected, CFA paw injection led to a significant reduction of GSH both in the spinal cord and in the plasma compared to control injected rats. AEPP and MEPP significantly (p<0.05) increased the GSH level in the plasma respectively at the doses of 100 and 200 mg/kg (Figure 3C and 3D). The positive control diclofenac-treated group (5 mg/kg) also significantly (p<0.05) increased GSH levels in plasma as compare to untreated CFA control. In contrast to effects in plasma, P. pinnata extracts did not show any significant increase of GSH level in the spinal cord as compared to untreated CFA control.
Figure 3:
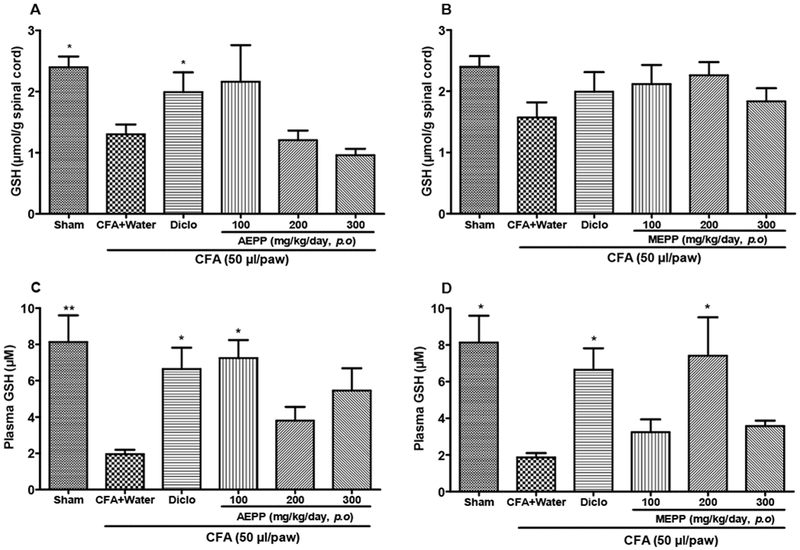
Effects of the aqueous (AEPP) and methanol (MEPP) extracts of Paullinia pinnata on the glutathione (GSH) content in the spinal cord and plasma of CFA-injected animals. NSAID diclofenac (5 mg/kg/day) is included as a positive control for anti-inflammatory effects. (A) GSH levels in the spinal cord decreased significantly after treatment with diclofenac. There was a trend for decreased GSH with AEPP 100 treatment that went away with increasing concentration. (B) MEPP treatment fails to alter GSH levels in the spinal cord. (C) AEPP 100 and diclofenac significantly reverse CFA induced GSH depletion in the plasma. (D) MEPP 200 and diclofenac MEPP significantly reverse CFA induced GSH depletion in the plasma. Each bar represents the mean ± SEM of 6 individual rats, *p<0.05, **p< 0.01 significant difference compared to negative control group (CFA+Water) using 1-Way ANOVA with Tukey as post hoc test.
3.4. Effects of Paullinia pinnata on MDA content in plasma and spinal cord of arthritic rat
To complement the data showing that P. pinnata extracts can increase protective GSH, we also evaluated the impact of extracts on malondialdehyde (MDA). MDA is the final product of lipid peroxidation and typically increases in the context of increased oxidative stress. As expected, we found a significant increase of MDA content in CFA paw injected rats as compared to the sham injected group. This effect was observed in the spinal cord (Figure 4) but not in the plasma (data not show). AEPP and MEPP significantly (p<0.001) reduced the MDA content in the spinal cord, when compared to the CFA control group. MEPP at the dose of 200 mg/kg exhibited the highest effect with an inhibition percentage of 86% (Figure 4B). The positive control diclofenac was less effective (76%) than MEPP.
Figure 4:
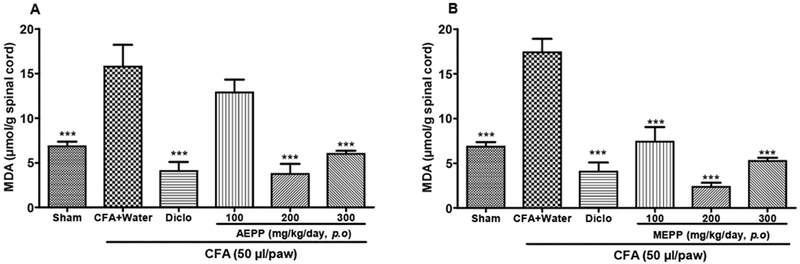
Effects of the aqueous (AEPP) and methanol (MEPP) extracts of Paullinia pinnata on the malondialdehyde (MDA) content in the spinal cord of CFA injected animals. NSAID diclofenac (5 mg/kg/day) is included as a positive control for anti-inflammatory effects. (A) AEPP treatment at 200 mg/kg and 300 mg/kg reversed CFA-induced increases in MDA spinal cord content (1-Way ANOVA significant main effect p<0.001). (B) MEPP treatment at 100, 200 and 300 mg/kg reversed CFA-induced increases in MDA spinal cord content (1-Way ANOVA significant main effect p<0.001). Each bar represents the mean ± SEM of 6 individual rats. ***p< 0.001 significant difference compared to negative control group (CFA+Water) using 1-Way ANOVA with Tukey as post hoc test.
3.5. Histopathological analysis of ankle soft tissue
To further characterize this inflammation and potential anti-inflammatory effects of P. pinnata we evaluated the histopathology of CFA injection. We found that CFA injection into the ankle induced a marked inflammation of the soft surrounding tissue. This inflammation was materialized by intensive infiltration of leukocytes and formation of giant cells (Figure 5B). Diclofenac, used as reference drug, significantly reduced cell infiltration and inhibited the formation of giant cells (Figure 5C). AEPP and MEPP dose-dependently reduced the inflammatory characteristics such as the tissue hyperplasia (Figure 5G), giant cells (Figure 5D-E), and cell infiltration. MEPP at 300 mg/kg exhibited the best anti-inflammatory effect, with almost complete inhibition of cell infiltration (Figure 5I).
Figure 5:
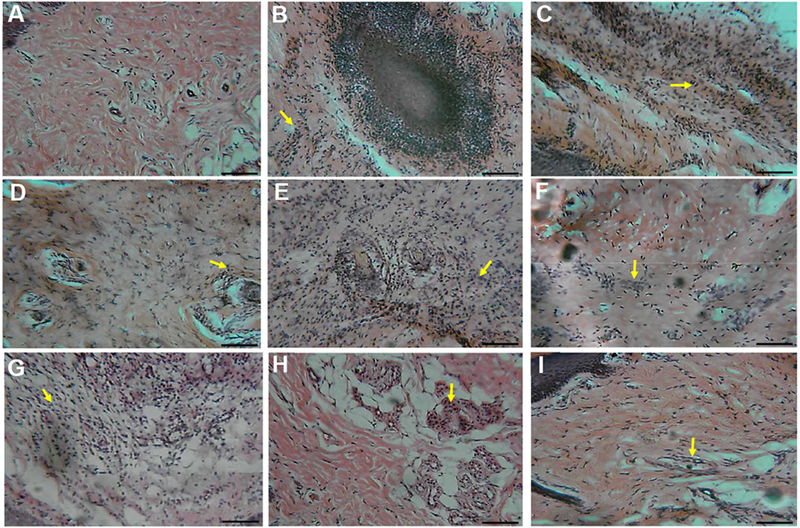
Histopathological examination of ankle soft tissues in sham (A), arthritic control (CFA+water) (B) and treated (CFA+diclofenac or extract) rats (C-I). 100× magnification. A: normal deep tissue in sham rats. B: (arthritic control; CFA+water) granulomatous inflammation comprising giant cells (white arrow) at the center of a plaque of infiltrating cells, C: (CFA+Diclofenac) deep tissue with infiltrating cells reduced compared to B. D: (AEPP 100 mg/kg/day) and E: (AEPP 200 mg/kg/day) deep tissue with scattered giant cells (white arrow) and cell infiltration (yellow arrow). F: (AEPP 300 mg/kg/day) moderate inflammation with low cell infiltration. G: (MEPP 100 mg/kg/day) dense inflamed and infiltrated cells in deep tissue. H: (MEPP 200 mg/kg/day) and I: (MEPP 300 mg/kg/day) dispersed infiltrated cells. Scale bar = 10 μm.
3.6. Effects of Paullinia pinnata extracts on NO production
In the inflammatory process, macrophages play a pivotal role by producing pro-inflammatory substances such as cytokines and nitrogen monoxide (NO). We evaluated the effects AEPP and MEPP on the NO production to further consolidate the anti-inflammatory effects of P. pinnata. Extracts as well as dexamethasone and L-NAME did not show any significant effect on NO production in non-stimulated macrophages. However, MEPP at 100 μg/ml reduced the viability of non-stimulated macrophages (Figure 6). When macrophages were stimulated with LPS, nearly all the tested substances significantly reduced the NO production. In contrast, MEPP (10 μg/ml) induced a statistically significant increase in NO production. After LPS stimulation, AEPP increased cell viability while MEPP had no effect (Figure 7).
Figure 6:
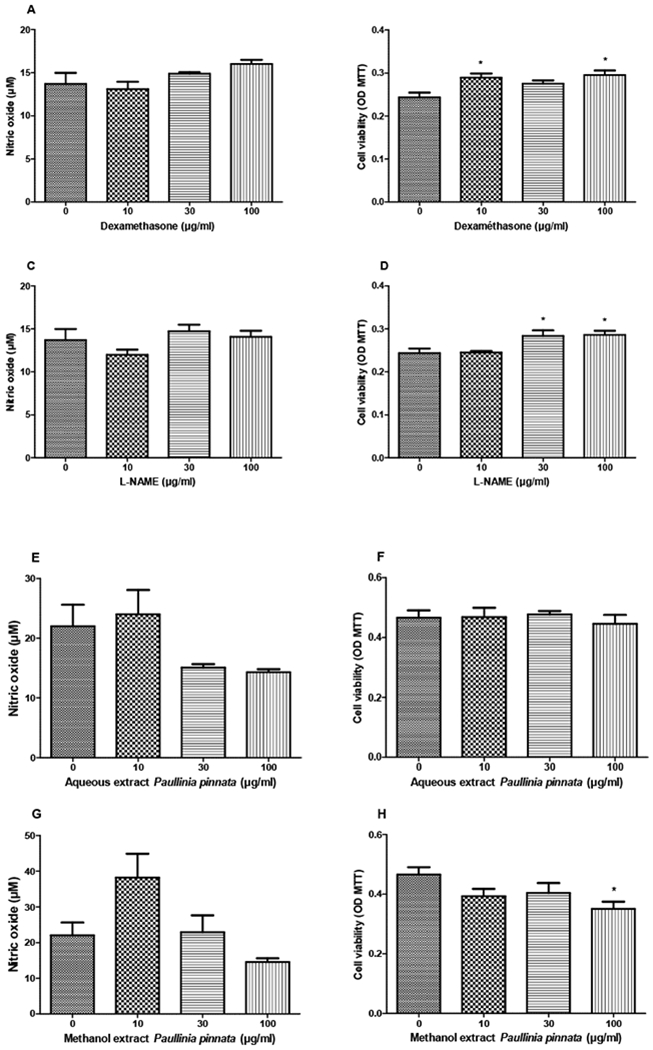
Effects of Dexamethasone (A, B), L-NAME (C, D), aqueous (E, F) and methanol (G, H) extracts of Paullinia pinnata on cell viability and nitric oxide production by non-stimulated macrophages. None of the treatments significantly affected the NO production although extracts at 30 and 100 μg/ml (E, G) tend to reduce it. The methanol extract at 100 μg/ml significantly reduced the cell viability. Each bar represents the mean ± SEM of five repetitions. *p<0.05 significant difference compared to control (0 μg/ml) using 1-Way ANOVA with Tukey as post hoc test.
Figure 7:
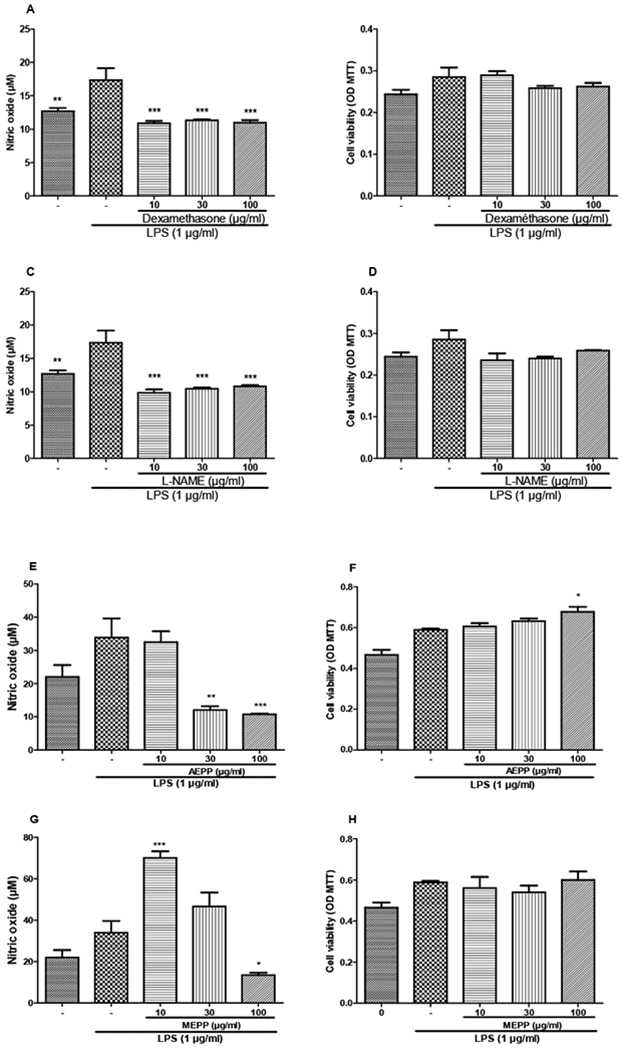
Preventive effects of dexamethasone (A, B), L-NAME (C, D), aqueous (E, F) and methanol (G, H) extracts of Paullinia pinnata on cell viability and nitric oxide production by LPS-stimulated macrophages. Reference substances significantly inhibited the NO production at all the concentrations used (A, C). AEPP concentration dependently inhibited the NO production (E) but showed concentration dependent increase in cell viability. The concentration 10 μg/ml of MEPP significantly increased the NO production while 100 μg/ml reduced it (G). Each bar represents the mean ± SEM of 5 repetitions. *p<0.05, **p<0.01, ***p<0.001 significant differences compared to control (0 μg/ml) using 1-Way ANOVA with Tukey as post hoc test.
Stimulation of macrophages with 8-Br-cAMP increased the NO production by 133%. This overproduction was completely inhibited by both extracts at the concentrations of 10 and 30 μg/ml. Surprisingly, P. pinnata extracts at 100 μg/ml had no effect. In this set of experiment, none of the treatments affected the cell viability (Figure 8). As shown in Figure 9, the combination of LPS and 8-Br-cAMP significantly reduced the NO production inhibitory effects of AEPP and MEPP.
Figure 8:
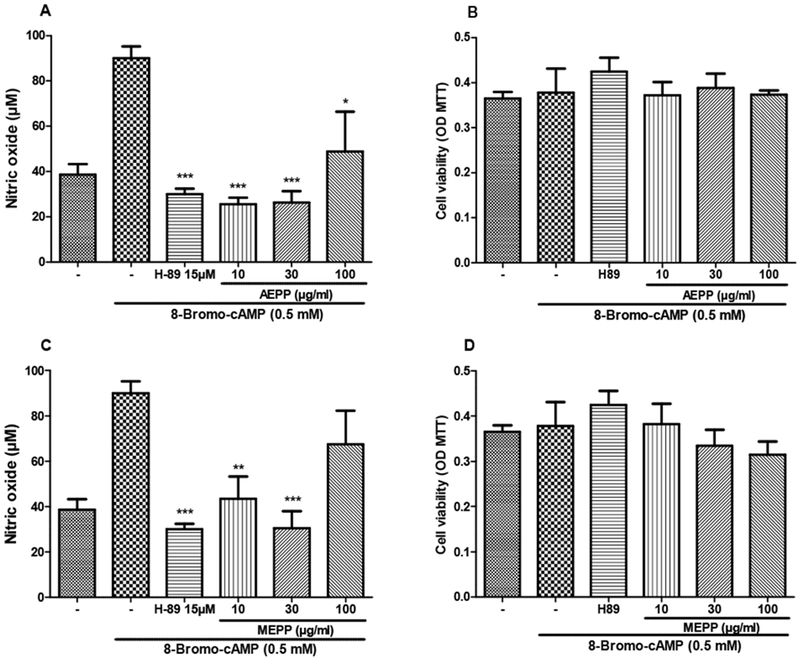
Preventive effects of the aqueous (AEPP 10, 30, 100 ug/mL; A, B) and methanol (MEPP 10, 30, 100 ug/ml; C, D) extracts of Paullinia pinnata on cell viability (B, D) and nitric oxide production (A, C) by 8-Bromo-AMPc-stimulated macrophages. H-89 (15uM) is included as a positive control for pharmacologic PKA inhibition. (A) AEPP extracts significantly decrease 8-Bromo-AMPc induced nitric oxide production. (B) Cell viability is not affected by 8-Bromo-AMPc treatment nor with AEPP extract treatment. (C) MEPP extracts (10 and 30 ug/mL) significantly decrease 8-Bromo-AMPc induced nitric oxide production. (D) Cell viability is not affected by 8-Bromo-AMPc treatment nor with MEPP extract treatment. Each bar represents the mean ± SEM of 5 repetitions. *p<0.05, **p<0.01, ***p<0.001 significant differences compared to untreated 8-Bromo-AMPc-stimulated macrophages. Analysis were performed using 1-Way ANOVA with Tukey as post hoc test.
Figure 9:
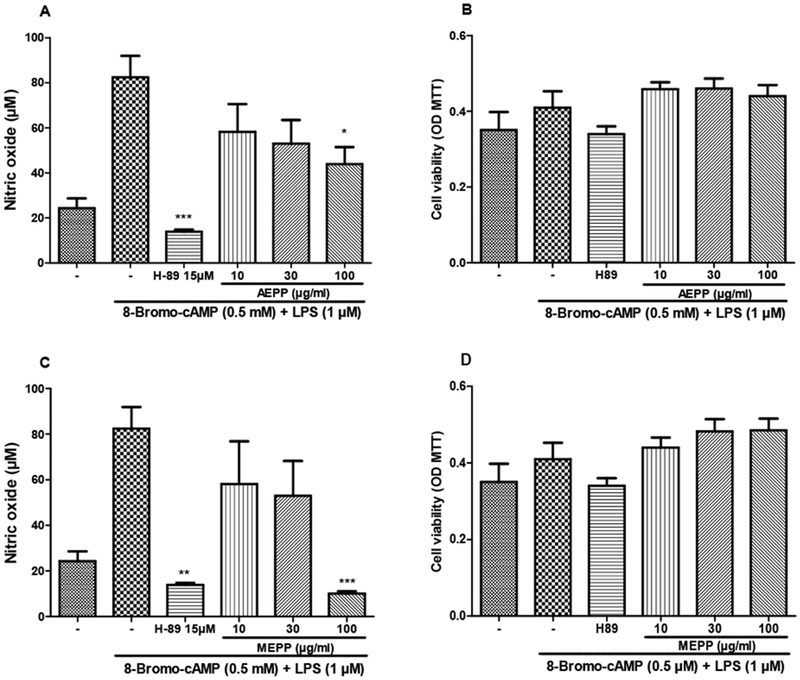
preventive effects of the aqueous (AEPP 10, 30, 100 ug/mL; A, B) and methanol (MEPP 10, 30, 100 ug/mL C, D) extracts of Paullinia pinnata on cell viability (B, D) and nitric oxide production (A, C) by LPS + 8-Bromo-AMPc-stimulated macrophages. Only the concentration of 100 μg/ml of both extracts significantly reduced the NO production (A, C). None of the treatment affected the cell viability. Each bar represents the mean ± SEM of 5 repetitions. *p<0.05, **p<0.01, ***p<0.001 significant difference compared to untreated LPS + 8-Bromo-AMPc-stimulated macrophages. Analysis were performed using 1 way ANOVA and Turkey post hoc test.
3.7. Effects of Paullinia pinnata extracts on TNF-α and IL-1β production by LPS stimulated macrophages
LPS stimulation induced a potent and significant (p<0.001) increase in cytokines production. TNF-α and IL-1β in stimulated cells increased respectively by 225 and 195% as compared to non-stimulated cells. AEPP and MEPP both significantly reduced the production of these pro-inflammatory mediators. MEPP was the most efficient on IL-1β, with a maximal inhibition of 47%, while AEPP showed the best activity on TNF-α, completely inhibiting the LPS-induced production (Figure 10).
Figure 10:
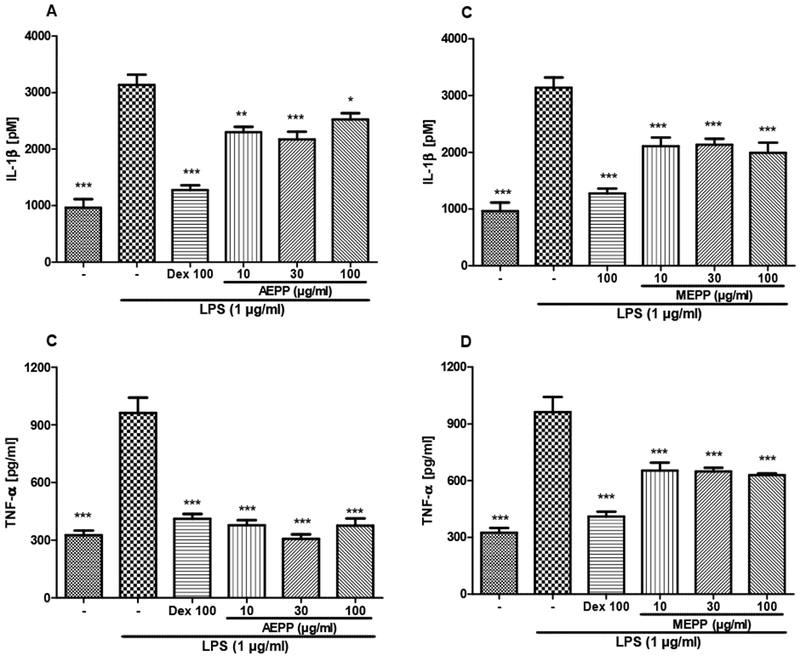
Preventive effects of the aqueous (AEPP 10, 30, 100 ug/ml; A, C) and methanol (MEPP 10, 30, 100 ug/ml; B, D) extracts of Paullinia pinnata on IL-1β (A, B) and TNF-α production (C, D) by EPS-stimulated (1ug/ml) macrophages. Both extracts at all the concentrations used and dexamethasone introduced as positive control significantly prevented the IL-1β and TNF-α production. AEPP induced the best inhibitory effect on TNF-α production (C). Each bar represents the mean ± SEM of 5 repetitions. *p<0.05, **p<0.01, ***p<0.001 significant difference compared to untreated LPS-stimulated macrophages. Analysis was performed using 1 way ANOVA followed by Tukey post hoc test.
3.8. Radioligand competition-binding to identify potential molecular targets of P. pinnata extracts
To begin the analysis of the target(s) of P. pinnata extracts, we utilized in vitro competitive binding assays for over 40 GPCR and ion channel targets. The results obtained from the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP) for the two extracts screened are summarized in Tables 1 focusing on opioid receptors and strong “hits.” Only MEPP showed potential binding on Sigma2 and peripheral benzodiazepine receptors (PBR) with respective inhibition of 68% and 77.4%. In the secondary binding assays, this extract displayed 30-fold selectivity for Sigma2 (IC50 = 50 μg/ml) over PBR (IC50 = 1661 μg/ml).
Table 1.
Psychoactive Drug Screening Program primary binding assay of aqueous (AEPP) and methanol (MEPP) extracts of Paullinia pinnata.
| Sigma 1 | Sigma 2 | PBR | MOR | KOR | DOR | GABAA | |
|---|---|---|---|---|---|---|---|
| AEPP | 31.1 | 14.6 | 0.7 | 6.8 | −18.0 | −9.4 | 38.8 |
| MEPP | n.d | 68.6* | 77.4** | −1.3 | −14.0 | 10.5 | 38.0 |
Data are presented as percent inhibition of standards. Data represented as mean % inhibition (N = 4 separate determinations). Negative inhibition represents stimulation of binding by extract. Percent inhibition > 50% is retested in secondary binding assay to determine IC50. PBR: peripheral benzodiazepine receptor, MOR: mu opioid receptor, KOR: kappa opioid receptor, DOR: delta opioid receptor.
Secondary binding assay IC50 = 50 μg/ml;
Secondary binding assay IC50 = 1661 μg/ml
4. Discussion
Rheumatoid arthritis (RA) in a multifactorial disease, combining inflammatory pain, oxidative stress and articular destruction. CFA-induced RA is a well-studied, reliable and reproducible experimental model of RA (Pan et al., 2017). More, it reflects clinical experience (Mannelli et al., 2013). Time course of hyperalgesia and oedema development in this model, generally run for at least a month. In the present study, CFA-injection induced a marked inflammation and hyperalagesia that were sustained for more than two weeks. These ailments were accompanied with a substantial oxidative stress. AEPP and MEPP significantly and time dependently reduced inflammation and hyperalgesia. They also exhibited significant in vivo antioxidant and in vitro inhibitory effects on NO and cytokine production by stimulated macrophages. In addition, MEPP showed binding affinity with Sigma2 receptors, which have recently been shown to be involved in neuropathic pain (Sahn et al, 2017).
To assess the effects of P. pinnata extracts on mechanical hyperalgesia, we performed the Randall Sellito method both on the ankle and the paw of injected ankle. AEPP and MEPP showed a time dependent analgesic effects at the range of the reference drug. As the model used herein undergoes sustained inflammation, the analgesic effect of P. pinnata extracts may result from the inhibition of inflammatory mediators. It was noticed that plant extracts were efficient both at the injected ankle and at the paw, indicating analgesic effect both on primary and secondary hyperalgesia. A differential modulation of the two types of hyperalgesia are now well documented (Treede and Magerl, 2000; Vanegas, 2004). Cumulative evidence shows that secondary hyperalgesia especially in inflammatory arthritis, is due to central sensitization and involves myelinated A-fibers (Walker et al., 2007; Hsieh et al., 2015; Van der Breoke et al., 2017). The fact that P. pinnata extracts were able to significantly reduce secondary hyperalgesia in this animal model suggest that, in addition to probable inhibitory effects on pro-inflammatory mediators, they may also act the central nervous system, stimulating receptors involved in analgesic pathways and/or inhibiting those participating in pain signalization.
In order to identify the potential targets of P. pinnata extracts and further understand the mechanism of the analgesic effect of these extracts, a cell based competitive binding assays for over 40 central nervous known GPCRs and ion channels was performed. MEPP but not AEPP exhibited high binding affinity to PBR and Sigma 2 receptors. Two conclusions can be drawn from these results: (1) the analgesic effects of the two extracts likely results from different active principles; (2) PBR and/or Sigma 2 receptors pathways may participate at least partially in the analgesic effect of MEPP. Secondary binding assays were further performed to better characterize the effects of MEPP on these receptors. MEPP showed a high binding affinity to Sigma2 receptor (IC50: 50 μg/ml). This is an exciting result, given that Sigma 2 receptor is one of the most recently characterized non-opioid receptors with an analgesic effect (Sahn et al., 2017) and the molecular identification of this receptor was only discovered in 2017 (Alon et al, 2017). An overall non-opioid mechanism of action is also suggested by the failure of P pinnata extracts to bind to opioid receptors in competitive binding assays. These results give the hope for the development of a non-opioid analgesic for the management of chronic pain.
Inflammation in a CFA-induced RA is a complex process and various mediators such as bradykinin, serotonin, proinflammatory cytokines, prostaglandins, ROS and nitric oxide have been reported to be involved in its development (Kshirsagar et al., 2014). P. pinnata extracts time dependently inhibited oedema induced by the intrarticular injection of CFA. These findings were further supported by the histological analysis that shows reduced inflammatory features, including formation of giant cells and infiltration of inflammatory cells in soft tissues from the injected ankle. These findings support the previous hypothesis on the inhibition of pro-inflammatory mediators. Indeed, CFA-induced oedema in rats is known to induce the sensitization of immune cells (macrophages and T-cells) which will be attracted to the inflammatory site, inducing swelling. These immune cells also release proinflammatory cytokines such as TNF, IL-1B, IL-6. The activity of these mediators will lead to the increased level of various inducible enzymes including inducible nitric oxide synthase (iNOS) implicated in high production of nitric oxide (NO) (Zhen et al., 2017). To evaluate whether P. pinnata extracts could interfered with these process, we conducted in vitro tests using non-stimulated and stimulated mature macrophages isolated from rat peritoneum. AEPP and MEPP did not show any significant effect on NO production by non-stimulated macrophages. These data suggest little inhibitory effect on quiescent macrophages, but when macrophages were stimulated with LPS, AEPP and MEPP significantly reduced NO production. It is well known that the LPS activation of macrophages through Toll-like receptor (TLR) induces, in macrophages, the up regulation of iNOS (An et al., 2015) leading to increase NO release. The results obtained with P. pinnata extracts demonstrate that these extracts modulate the NO production by macrophages in inflammatory conditions probably by inhibiting the up regulation of iNOS or the related pathways.
Chen et al. (1999) demonstrated that the PKA inhibitors, KT-5720 and H8, reduced LPS-induced NO release and iNOS expression. Concordantly, the direct PKA activator, Bt2cAMP, caused concentration-dependent NO release and iNOS expression. Similar results were obtained in the present study where 8-Br-cAMP, a direct PKA activator, increased by more than two-fold, the NO release in macrophages. We used this to verify the effect of P. pinnata extracts on PKA. AEPP and MEPP significantly reduced NO release in 8-Br-cAMP stimulated macrophages, suggesting a potential inhibitory effect of P. pinnata extracts on PKA. CD14, a glycoprotein that binds bacterial LPS, plays a critical role in the inflammatory response to infection by gram negative bacteria. It was also demonstrated that 8-Br-cAMP significantly increase the expression of CD14 (Liu et al., 2000). We hypothesized that combining the stimulation with 8-Br-cAMP and LPS will potentiate the NO release and help to differentiate the effect of plant extract on the overexpression of CD14 and PKA. Surprisingly, the combination of the two stimuli increased the NO production in the same range as 8-Br-cAMP alone. But, this combination significantly reduced the effects of low concentrations of AEPP and MEPP. The explanation might be simply that the stimulation is so high that low concentration of extracts are not able to overcome the resulting production. However, the alternative explanation that the plant extracts do not have any effect on CD14 expression cannot be ruled out from the present data. It was also noticed that some effects of plant extracts were not concentration-dependent especially in the in vitro test using 8-Br-cAMP. This may imply interactions between stimulating constituents in the plant extracts that expressed their activity at high concentration. In fact, the pleiotropic effects of plant extracts is a well-known phenomenon (Lorenc-Kukula et al, 2005) due to the large variety of metabolite content in many plants.
The LPS stimulation of macrophages also induced significant increase in TNF-α and IL-1β release. These effects were significantly reduced by EAPP and MEPP, indicating that the inhibition of TNF-α and IL-1β release counts for the anti-inflammatory effect of P. pinnata extracts. Oxidative stress, induced by free radical production, is believed to be a primary factor in various degenerative diseases such as arthritis (Afonso et al., 2007). The free radicals produced during the development of arthritis in the joint cartilage leads to the reductions of enzymes such as, superoxide dismutase (SOD), catalase (CAT) and GSH (Djordjevic et al., 2004; Anuradha and Krishnamoorthy, 2011). GSH is synthesized in the liver and known as the first line of defense against peroxidation. It was also reported that the increase levels of ROS in the arthritic joint leads to lipid peroxidation, marked by increased malonaldehyde (MDA) production (Ajay et al., 2014). The results obtained show that, both aqueous and methanol extracts of P. pinnata significantly increased the GSH level in the plasma and decreased MDA content in the spinal cord. These effects suggest the ability of the extracts to increase GSH activity, leading to the reduction of hydrogen peroxide (H2O2) and therefore, reduction of lipid peroxidation. Besides, Lunga and al (2014), working on the methanol extract of the leaves of P. pinnata, isolated methyl inositol and proved its in vitro antioxidant properties. This suggest that methyl inositol may contribute to the antioxidant effects of P. pinnata extracts observed in this study.
5. Conclusion
The present study showed that aqueous and methanol extracts of P. pinnata leaves possess anti-hyperalgesic and anti-inflammatory effect on experimental monoarthritis. These effects may result from the inhibition of inflammatory processes and activation of neuronal analgesic pathways. The anti-inflammatory effect is at least partially due to the inhibition of inflammatory cells infiltration, the reduction of NO, TNF-α and IL-1β release. The activation of Sigma 2 receptors may also contribute to the analgesic effect of MEPP. The in vivo antioxidant activity of these extracts is an additional benefit effect in the anti-inflammatory process of P. pinnata extracts. These results support the use of the leaves of P. pinnata extracts in Cameroonian folk medicine for the management of arthritic pain.
Acknowledgment
The authors express their gratitude to the Alexander von Humboldt Foundation, Duquesne University (Loogman Faculty Research Grant for Research in Africa BJK, KJT, TBN), the International Association for the Study of Pain (IASP Collaborative Research Grant BJK, KJT, TBN), and the National Institutes of Health (NCCIH R15AT008060-02 BJK, KJT) for supporting this work. IC50 determinations, and receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2018-00023-C (NIMH PDSP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
All the authors declare no conflict of interest
References
- 1.Abourashed A, Toyang NJ, Chinski J Jr., Khan JA, 1999. Two new flavone glycosides from Paullinia pinnata. Journal of Natural Products, 62: 1179–81. [DOI] [PubMed] [Google Scholar]
- 2.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A, 2007. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint. Bone Spine, 74: 324–329. [DOI] [PubMed] [Google Scholar]
- 3.Ajay DK, Prashant VP, Uday NH, Rabindra KN, Haidarali MS 2014. Anti-Inflammatory and Antiarthritic Activity of Anthraquinone Derivatives in Rodents. International Journal of Inflammation, 12 p. 10.1155/2014/690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alghasham A, Rasheed Z, 2014. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity, 47(2):77–94. [DOI] [PubMed] [Google Scholar]
- 5.Alon A, Schmidt HR, Wood MD, Sahn JJ, Martin SF, Kruse AC, 2017. Identification of the gene that codes for the sigma2 receptor. Proceedings of the National Academy of Sciences of the United States of America, 114 (27), 7160–7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An H-J, Nugroho A, Song B-M, Park H-J 2015. Isoeugenin, a Novel Nitric Oxide Synthase Inhibitor Isolated from the Rhizomes of Imperata cylindrical. Molecules, 20: 21336–21345; doi: 10.3390/molecules201219767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anuradha R, Krishnamoorthy P (2011). Antioxidant activity of methanolic extract of Pongamia pinnata on lead acetate induced hepatic damage in rats. African Journal of Biochemistry Research, 5(12): 348–351. [Google Scholar]
- 8.Atabonkeng EP, Adiogo D, Emmanuel ME, Hervé FO, Owono MA, Assoumou OMC, 2015. Evaluation of the Prevalence of Rheumatoid Factor in Five Regions of Cameroon. Archive Rheumatology, 30(3): 226–230. [Google Scholar]
- 9.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL, 2012. Automated design of ligands to polypharmacological profiles. Nature. 492(7428):215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabra S, Makuna R, Mshiu E, 1991. Plant used in traditional medicine in Eastern Tanzania. Journal of Ethnopharmacology , 33: 143–157. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-C, Chiu K-T, Sun Y-T, Chen W-C, 1999. Role of the Cyclic AMP-Protein Kinase A Pathway in Lipopolysaccharide-induced Nitric Oxide Synthase Expression in RAW 264.7 Macrophages the journal of biological chemistry, 274(44): 31559–31564. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y, Jia L, Yin H, Chen C, 2008. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides (ELLIS) and Crocus sativus (L): A relationship investigation between antioxidant activity and crocin contents. Food Chemistry, 109: 484–492. [Google Scholar]
- 13.Djordjevic A, Spasic S, Jovanovic-Galovic A, Djordjevic R Grubor-Lajsic G, 2004. Oxidative stress in diabetic pregnancy: SOD, CAT and GSH-Px activity and lipid peroxidation products. The Journal of Fetal-Maternal & Neonatal Medicine, 16(6): 367–372.. [DOI] [PubMed] [Google Scholar]
- 14.Dongo E, Hussain H, Miemanang SR, Tazoo D, Schulz B Krohn K, 2009. Chemical Constituents of Klainedoxagabonenses and Paullinia pinnata. Records of Natural Products. 3(3): 165–169. [Google Scholar]
- 15.García-González A, Gaxiola-Robles R, Zenteno-Savín T, 2015. Oxidative stress in patients with rheumatoid arthritis. Rev Invest Clin. 67(1):46–53. [PubMed] [Google Scholar]
- 16.Hsieh MT, Donaldson LF, Lumb BM, 2015. Differential contributions of A- and C-nociceptors to primary and secondary inflammatory hypersensitivity in the rat. Pain, 156(6):1074–83. doi: 10.1097/j.pain.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SM, Kim KW, Yang C-W, Park S-H, Ju J-H, 2014. Cytokine-Mediated Bone Destruction in Rheumatoid Arthritis. J Immunol Res, 263625. doi: 10.1155/2014/263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kshirsagar AD, Panchal PV, Harle UN, Nanda RK, Shaikh HM, 2014. Anti-Inflammatory and Antiarthritic Activity of Anthraquinone Derivatives in Rodents. International Journal of Inflammation. 12 p. 10.1155/2014/690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Morris M, Nie S, Shapiro RA, Billiar TR, 2000. cAMP induces CD14 expression in murine macrophages via increased transcription. J. Leukocyte Biol, 67: 894–901.. [DOI] [PubMed] [Google Scholar]
- 20.Lorenc-Kukula K, Amarowicz R, Oszmiański J, Doermann P, Starzycki M, Skała J, Żuk M, Kulma A, Szopa J, 2005. Pleiotropic effect of phenolic compounds content increases in transgenic Flax plant. J Agric Food Chem, 53 (9): 3685–3692. DOI: 10.1021/jf047987z. [DOI] [PubMed] [Google Scholar]
- 21.Lunga PK, Tamokou JJ, Fodouop SPC, Kuiate JR, Tchoumboue J, Gatsing D, 2014. Antityphoid and radical scavenging properties of the methanol extracts and compounds from the aerial part of Paullinia pinnata. Springer Plus. 3:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lunga KP, Qin X-J, Yang XW, Kuiate J-R, Du ZZ, Gatsing D, 2014. Antimicrobial steroidal saponin and oleanane-type triterpenoidsaponins from Paullinia pinnata. Biomed Central, Complementary and Alternative Medicine, 14:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannelli LDC, Bani D, Bencini A, Brandi ML, Calosi L, Cantore M, Carossino AM, Ghelardini C, Valtancoli B, Failli P, 2013. Therapeutic Effects of the Superoxide Dismutase Mimetic Compound MnII Me2DO2A on Experimental Articular Pain in Rats. Mediators of Inflammation. 11 pages, 10.1155/2013/905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozouloua D, Apema AKR, Nguengue JP, 2011. Etude préliminaire des plantes médicinales à effets antidermatosiques utilisées en pharmacopée à Bangui. Unité de Recherche en Sciences Appliqées au Développement (URSAD). [Google Scholar]
- 25.Nguelefack-Mbuyo PE, Dongmo BA, Nguelefack TB, Kamanyi A, Kamtchouing P, Dimo T, 2012. Endothelium/Nitric Oxide Mediate Vasorelaxant and Antihypertensive Effects of the Aqueous Extract from the Stem Bark of Mammea Africana Sabine (Guttiferae). Evidence-based Complementary and Alternative Medicine. DOI: 10.1155/2012/961741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan T, Cheng T-F, Jia Y-R, Li P, Li F, 2017. Anti-rheumatoid arthritis effects of traditional Chinese herb couple in adjuvant-induced arthritis in rats. Journal of Ethnopharmacology, 205: 1–7. [DOI] [PubMed] [Google Scholar]
- 27.Sahn JJ, Mejia GL, Ray PR, Martin SF, Price. TJ, 2017. Sigma 2 Receptor/Tmem97 Agonists Produce Long Lasting Antineuropathic Pain Effects in Mice. ACS Chem Neurosci., 8(8): 1801–1811. doi: 10.1021/acschemneuro.7b00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehirli O, Tozan A, Omurtag GZ, Cetine S, Contuk G, Gedik N (2008). Protective effects of resveratol against naphtylene-induiced oxidative stress in mice. Ecotoxicology and environmental safety, 71: 301–308. [DOI] [PubMed] [Google Scholar]
- 29.Treede RD, Magerl W, 2000. Multiple mechanisms of secondary hyperalgesia. Prog Brain Res, 129: 331–341. [DOI] [PubMed] [Google Scholar]
- 30.Van den Broeke EN, Lenoir C, Mouraux A, 2016. Secondary hyperalgesia is mediated by heat-insensitive A-fibre nociceptors. J. Physiol, 594(22): 6767–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanegas H, 2004. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett, 361(1-3): 225–228. [DOI] [PubMed] [Google Scholar]
- 32.Veselinovic M, Barudzic N, Vuletic M, Zivkovic V, Tomic-Lucic A, Djuric D, Jakovljevic V, 2014. Oxidative stress in rheumatoid arthritis patients: relationship to diseases activity. Molecular Cell Biochemistry, 391(1–2):225–32. [DOI] [PubMed] [Google Scholar]
- 33.Walker SM, Meredith-Middleton J, Lickiss T, Moss A, Fitzgerald M, 2007. Primary and secondary hyperalgesia can be differentiated by postnatal age and ERK activation in the spinal dorsal horn of the rat pup. Pain, 128(1):157–168, DOI: 10.1016/j.pain.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Wasserman A, 2018. Rheumatoid Arthritis: Common questions about diagnosis and management. American Family Physician, 97(7): 455–462. [PubMed] [Google Scholar]
- 35.Wong R, Davis AM, Badley E, Grewal R, Mohammed M, 2010. Prevalence of Arthritis and Rheumatic diseases around the World-A Growing Burden and Implications for Health Care Needs. Models of Care in Arthritis, Bone and Joint Disease, 002. [Google Scholar]
- 36.Zamble A, Carpentier M, Kandoussi A, 2006. Paullinia pinnata extracts rich in polyphenols promote vascular relaxation via endothelium-dependent mechanism. Journal of Cardiovascular Pharmacology, 47(4): 599–608. [DOI] [PubMed] [Google Scholar]
- 37.Zheng K, Zhao Z, Lin N, Wu Y, Xu Y, Zhang W, 2017. Protective effect of pinitol against inflammatory mediators of rheumatoid arthritis via inhibition of protein tyrosine phosphatase non-receptor type 22 (PTPN22). Med Sci Monit. 20(23): 1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]


