Abstract
Background
Since the mid‐2000s, the field of metastatic renal cell carcinoma (mRCC) has experienced a paradigm shift from non‐specific therapy with broad‐acting cytokines to specific regimens, which directly target the cancer, the tumour microenvironment, or both.
Current guidelines recommend targeted therapies with agents such as sunitinib, pazopanib or temsirolimus (for people with poor prognosis) as the standard of care for first‐line treatment of people with mRCC and mention non‐specific cytokines as an alternative option for selected patients.
In November 2015, nivolumab, a checkpoint inhibitor directed against programmed death‐1 (PD‐1), was approved as the first specific immunotherapeutic agent as second‐line therapy in previously treated mRCC patients.
Objectives
To assess the effects of immunotherapies either alone or in combination with standard targeted therapies for the treatment of metastatic renal cell carcinoma and their efficacy to maximize patient benefit.
Search methods
We searched the Cochrane Library, MEDLINE (Ovid), Embase (Ovid), ISI Web of Science and registers of ongoing clinical trials in November 2016 without language restrictions. We scanned reference lists and contacted experts in the field to obtain further information.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐RCTs with or without blinding involving people with mRCC.
Data collection and analysis
We collected and analyzed studies according to the published protocol. Summary statistics for the primary endpoints were risk ratios (RRs) and mean differences (MD) with their 95% confidence intervals (CIs). We rated the quality of evidence using GRADE methodology and summarized the quality and magnitude of relative and absolute effects for each primary outcome in our 'Summary of findings' tables.
Main results
We identified eight studies with 4732 eligible participants and an additional 13 ongoing studies. We categorized studies into comparisons, all against standard therapy accordingly as first‐line (five comparisons) or second‐line therapy (one comparison) for mRCC.
Interferon (IFN)‐α monotherapy probably increases one‐year overall mortality compared to standard targeted therapies with temsirolimus or sunitinib (RR 1.30, 95% CI 1.13 to 1.51; 2 studies; 1166 participants; moderate‐quality evidence), may lead to similar quality of life (QoL) (e.g. MD ‐5.58 points, 95% CI ‐7.25 to ‐3.91 for Functional Assessment of Cancer ‐ General (FACT‐G); 1 study; 730 participants; low‐quality evidence) and may slightly increase the incidence of adverse events (AEs) grade 3 or greater (RR 1.17, 95% CI 1.03 to 1.32; 1 study; 408 participants; low‐quality evidence).
There is probably no difference between IFN‐α plus temsirolimus and temsirolimus alone for one‐year overall mortality (RR 1.13, 95% CI 0.95 to 1.34; 1 study; 419 participants; moderate‐quality evidence), but the incidence of AEs of 3 or greater may be increased (RR 1.30, 95% CI 1.17 to 1.45; 1 study; 416 participants; low‐quality evidence). There was no information on QoL.
IFN‐α alone may slightly increase one‐year overall mortality compared to IFN‐α plus bevacizumab (RR 1.17, 95% CI 1.00 to 1.36; 2 studies; 1381 participants; low‐quality evidence). This effect is probably accompanied by a lower incidence of AEs of grade 3 or greater (RR 0.77, 95% CI 0.71 to 0.84; 2 studies; 1350 participants; moderate‐quality evidence). QoL could not be evaluated due to insufficient data.
Treatment with IFN‐α plus bevacizumab or standard targeted therapy (sunitinib) may lead to similar one‐year overall mortality (RR 0.37, 95% CI 0.13 to 1.08; 1 study; 83 participants; low‐quality evidence) and AEs of grade 3 or greater (RR 1.18, 95% CI 0.85 to 1.62; 1 study; 82 participants; low‐quality evidence). QoL could not be evaluated due to insufficient data.
Treatment with vaccines (e.g. MVA‐5T4 or IMA901) or standard therapy may lead to similar one‐year overall mortality (RR 1.10, 95% CI 0.91 to 1.32; low‐quality evidence) and AEs of grade 3 or greater (RR 1.16, 95% CI 0.97 to 1.39; 2 studies; 1065 participants; low‐quality evidence). QoL could not be evaluated due to insufficient data.
In previously treated patients, targeted immunotherapy (nivolumab) probably reduces one‐year overall mortality compared to standard targeted therapy with everolimus (RR 0.70, 95% CI 0.56 to 0.87; 1 study; 821 participants; moderate‐quality evidence), probably improves QoL (e.g. RR 1.51, 95% CI 1.28 to 1.78 for clinically relevant improvement of the FACT‐Kidney Symptom Index Disease Related Symptoms (FKSI‐DRS); 1 study, 704 participants; moderate‐quality evidence) and probably reduces the incidence of AEs grade 3 or greater (RR 0.51, 95% CI 0.40 to 0.65; 1 study; 803 participants; moderate‐quality evidence).
Authors' conclusions
Evidence of moderate quality demonstrates that IFN‐α monotherapy increases mortality compared to standard targeted therapies alone, whereas there is no difference if IFN is combined with standard targeted therapies. Evidence of low quality demonstrates that QoL is worse with IFN alone and that severe AEs are increased with IFN alone or in combination. There is low‐quality evidence that IFN‐α alone increases mortality but moderate‐quality evidence on decreased AEs compared to IFN‐α plus bevacizumab. Low‐quality evidence shows no difference for IFN‐α plus bevacizumab compared to sunitinib with respect to mortality and severe AEs. Low‐quality evidence demonstrates no difference of vaccine treatment compared to standard targeted therapies in mortality and AEs, whereas there is moderate‐quality evidence that targeted immunotherapies reduce mortality and AEs and improve QoL.
Immunotherapy for advanced kidney cancer
Review question
Kidney cancer is rarely curable once it has spread to other organs at the time of diagnosis. Targeted agents are currently considered as the standard treatment for advanced kidney cancer that has spread to other organs. This review examines clinical studies that have directly compared immunotherapies or combination therapies to current standard therapy.
Background
Prior to the use of the new targeted agents, drugs that boosted the immune response against the cancer in a non‐specific way (immunotherapies) were the most widely used treatment form for people with kidney cancer that had spread to other organs. Newer immunotherapeutic agents, including vaccines and so called 'checkpoint inhibitors,' have been developed to specifically target the body's immune system and enable it to recognize and attack cancer cells more specifically. In this review, we evaluated all types of immunotherapy or combination therapies by comparing it to the current standard therapy.
Study characteristics
A systematic search up to the end of October 2016 identified eight studies that looked at four different types of immunotherapy in 4732 people. Studies were only included if patients were randomized to a form of immunotherapy included in this review or a standard form of targeted therapy. One study was funded by a public institution whereas all the others were supported by drug companies.
The study participants were generally representative of people with advanced kidney cancer. The majority of people had their kidney cancer removed before starting treatment. We compared studies of people who had previously received standard medicine (821 participants) to those of people who had not (3911 participants). All studies reported our main outcome of interest; the chance of longer survival including the survival for one year. We also focused on the frequency of severe treatment side effects, quality of life and the delay in disease worsening.
Key results
Interferon‐α was the most commonly used therapy option prior to the era of targeted therapies. Two studies with 1166 participants compared interferon‐α alone (monotherapy) to targeted standard therapy. Interferon‐α is probably inferior to tested targeted therapies called sunitinib and temsirolimus. Patients with interferon‐α monotherapy probably have a shorter time to worsening of cancer. They may have similar quality of life and a slightly more severe treatment side effects.
Adding temsirolimus to interferon‐α probably does not improve survival compared to temsirolimus alone, but may result in more major side effects (one study).
Two studies compared interferon‐α to a combination of interferon‐α and bevacizumab in 1381 previously untreated participants. There was a slightly increased death rate with probably fewer major side effects for people treated with interferon‐α alone.
Two studies evaluated vaccines. Vaccines may lead to similar death rates and side effects in people with advanced kidney cancer.
For patients who had already undergone systemic treatment, one study with nivolumab, a novel checkpoint inhibitor, improved average survival by more than five months when compared to the targeted standard therapy, everolimus. The effects are probably accompanied by better quality of life and fewer major side effects.
Quality of the evidence
We had reduced confidence in the results of the studies we analyzed (moderate‐ or low‐quality evidence) because patients and treating physicians were often not blinded to the treatment and involved relatively few patients.
Summary of findings
Summary of findings for the main comparison.
Interferon‐α alone versus standard targeted therapies (sunitinib or temsirolimus) in first‐line therapy of metastatic renal cell carcinoma
| IFN‐α alone versus standard targeted therapy for mRCC | ||||||
|
Patient population: previously untreated patients with mRCC Settings: phase III, international, multicentre, open‐label Intervention: IFN‐α alone Comparison: standard targeted therapy (sunitinib or temsirolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapy | Risk difference with IFN‐α alone (95% CI) | |||||
| 1‐year mortality Follow‐up: 1 to 36 months | Lowa | RR 1.3 (1.13 to 1.51) | 1166 (2 studies) | ⊕⊕⊕⊝ Moderate1 | ‐ | |
| 150 per 1000 | 45 more per 1000 (from 20 more to 76 more) | |||||
| Moderatea | ||||||
| 280 per 1000 | 84 more per 1000 (from 36 more to 143 more) | |||||
| Higha | ||||||
| 550 per 1000 | 165 more per 1000 (from 71 more to 280 more) | |||||
| QoL FACT‐G Follow‐up: median 17 weeks | The mean QoL in the control group was 82.3 pointsb | MD 5.58 lower (7.25 to 3.91 lower) | ‐ | 730 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| QoL FKSI‐15 Follow‐up: median 17 weeks | The mean QoL in the control group was 45.3 pointsb |
MD 3.27 lower (4.18 to 2.36 lower) |
‐ | 730 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| QoL FKSI‐DRS Follow‐up: median 17 weeks | The mean QoL in the control group was 29.4 pointsb |
MD 1.98 lower (2.51 to 1.46 lower) |
‐ | 730 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| QoL EQ‐5D Follow‐up: range 12 to 17 weeks | The mean QoL in the control group was 0.711pointsb |
MD 0.06 lower (0.12 lower to 0 higher) |
‐ | 1002 (2 studies) |
⊕⊕⊝⊝ Low2,3 | ‐ |
| QoL EQ‐VAS Follow‐up: range 12 to 17 weeks | The mean QoL in the control groups was 70.4pointsb |
MD 4.68 lower (6.53 to 2.83 lower) |
‐ | 1002 (2 studies) |
⊕⊕⊝⊝ Low2,3 | ‐ |
| Adverse events (grade ≥ 3) Follow‐up: 14 to 36 months | 668 per 1000 | 114 more per 1000 (from 20 more to 214 more) | RR 1.17 (1.03 to 1.32) | 408 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D: EuroQol 5‐Dimension; EQ‐VAS: EuroQol Visual Analogue Scale; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; FKSI‐15: FACT‐Kidney Symptom Index; FKSI‐DRS: FACT‐Kidney Symptom Index Disease Related Symptoms; IFN‐α: interferon‐α; MD: mean difference; mRCC: metastatic renal cell carcinoma; QoL: quality of life; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for selection bias and performance bias due to cross‐over. 2 Downgraded for performance and detection bias. 3 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. a Moderate risk of 1‐year mortality from the SEER (Surveillance, Epidemiology, and End Results) Cancer Statistics Review (Howlader 2015), low risk from participants with favourable risk in Rini 2015, high risk from Hudes 2007. b Mean postbaseline value on treatment.
Summary of findings 2.
Interferon‐α combined with targeted therapies versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma
| IFN‐α alone or combined with targeted therapy compared to standard targeted therapy in first‐line therapy of mRCC | ||||||
|
Patient population: previously untreated patients with mRCC Setting: phase III, international, multicentre, open‐label Intervention: IFN‐α combined with targeted therapy Comparison: standard targeted therapy (temsirolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapy | Risk difference with IFN‐α combined with targeted therapy (95% CI) | |||||
| 1‐year mortality Follow‐up: 14‐36 months | Lowa | RR 1.13 (0.95 to 1.34) | 419 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ | |
| 150 per 1000 | 20 more per 1000 (from 8 fewer to 51 more) | |||||
| Moderatea | ||||||
| 280 per 1000 | 36 more per 1000 (from 14 fewer to 95 more) | |||||
| Higha | ||||||
| 550 per 1000 | 71 more per 1000 (from 28 fewer to 187 more) | |||||
| Quality of life | No evidence available | |||||
| Adverse events (grade ≥ 3) Follow‐up: 14 to 36 months | 668 per 1000 | 200 more per 1000 (from 114 more to 301 more) | RR 1.30 (1.17 to 1.45) | 416 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IFN‐α: interferon‐α; mRCC: metastatic renal cell carcinoma; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. 2 Downgraded for performance and detection bias. a Moderate risk of 1‐year mortality from the SEER Cancer Statistics Review (Howlader 2015), low risk from participants with favourable risk in Rini 2015, high risk from Hudes 2007.
Summary of findings 3.
Interferon‐α alone versus interferon‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma
| IFN‐α alone versus IFN‐α + bevacizumab in first‐line therapy of mRCC | ||||||
|
Patient population: previously untreated patient with mRCC Setting: phase III, international, multicentre, Escudier 2007: double‐blind, placebo‐controlled; Rini 2010: open‐label Intervention: IFN‐α alone Comparison: IFN‐α alone + bevacizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | ||||||
| Risk with standard therapy (IFN‐α + bevacizumab) | Risk difference with IFN‐α alone (95% CI) | |||||
| 1‐year mortality Follow‐up: 13.3 to 22 months | Low | RR 1.17 (1.00 to 1.36) | 1381 (2 studies) | ⊕⊕⊝⊝ Low1,2 | ‐ | |
| 150 per 1000 | 25 more per 1000 (from 0 more to 54 more) | |||||
| Moderate | ||||||
| 280 per 1000 | 48 more per 1000 (from 0 more to 101 more) | |||||
| High | ||||||
| 550 per 1000 | 93 more per 1000 (from 0 more to 198 more) | |||||
| Quality of life | No evidence available | |||||
|
Adverse events (grade ≥ 3) Follow‐up: up to 28 days after last dose to 65 months |
705 per 1000 | 162 fewer per 1000 (from 113 fewer to 205 fewer) | RR 0.77 (0.71 to 0.84) | 1350 (2 studies) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IFN‐α: interferon‐α; mRCC: metastatic renal cell carcinoma; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for selection bias and performance bias due to substantial cross‐over. 2 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. 3 Downgraded for performance and detection bias.
Summary of findings 4.
Interferon‐α plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
| IFN‐α + bevacizumab versus targeted therapies in first‐line therapy of mRCC | ||||||
|
Patient population: previously untreated patients with mRCC Setting: phase II, national (France), multicentre, open‐label Intervention: IFN‐α + bevacizumab Comparison: standard targeted therapies (sunitinib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapies | Risk difference with IFN‐α + bevacizumab (95% CI) | |||||
|
1‐year mortality median Follow‐up: 23.2 months |
Lowa | RR 0.37 (0.13 to 1.08) | 83 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ | |
| 150 per 1000 | 95 fewer per 1000 (131 fewer to 12 more) | |||||
| Moderatea | ||||||
| 280 per 1000 | 176 fewer per 1000 (from 244 fewer to 22 more) | |||||
| Higha | ||||||
| 550 per 1000 | 347 fewer per 1000 (from 479 fewer to 44 more) | |||||
| Quality of life | No evidence available | |||||
|
Adverse events (grade ≥ 3) Follow‐up: 48 weeks |
595 per 1000 | 107 more per 1000 (from 89 fewer to 369 more) | RR 1.18 (0.85 to 1.62) | 82 (1 study) | ⊕⊕⊝⊝ Low2,3 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; mRCC: metastatic renal cell carcinoma; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for reporting and performance bias due to differences in second‐line treatment. 2 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. 3 Downgraded for performance and detection bias. a Moderate risk of 1‐year mortality from the SEER Cancer Statistics Review (Howlader 2015), low risk from participants with favourable risk in Rini 2015, high risk from Hudes 2007.
Summary of findings 5.
Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma
| Vaccine treatment versus standard therapies in first‐line therapy of mRCC | ||||||
|
Patient population: previously untreated patients with mRCC Setting: phase III, international, multicentre, double‐blind, placebo‐controlled (Amato 2010), open‐label (Rini 2015) Intervention: vaccine treatment (MVA‐5T4 or IMA0901) Comparison: placebo and standard therapies (IL‐2, IFN‐α and sunitinib) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | ||||||
| Risk with standard therapies | Risk difference with vaccine treatment (95% CI) | |||||
| 1‐year mortality Follow‐up: 12 to 48 months | Lowa | RR 1.10 (0.91 to 1.32) | 1034 (2 studies) | ⊕⊕⊝⊝ Low1,2,3 | ‐ | |
| 150 per 1000 | 15 more per 1000 (from 13 fewer to 48 more) | |||||
| Moderatea | ||||||
| 280 per 1000 | 28 more per 1000 (from 25 fewer to 90 more) | |||||
| Higha | ||||||
| 550 per 1000 | 55 more per 1000 (from 49 fewer to 176 more) | |||||
| Quality of life | No evidence available | |||||
|
Adverse events (grade ≥ 3) Follow‐up: not reported |
241 per 1000 | 39 more per 1000 (from 7 fewer to 94 more) | RR 1.16 (0.97 to 1.39) | 1065 (2 studies) | ⊕⊕⊝⊝ Low3,4,5 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; mRCC: metastatic renal cell carcinoma; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Not downgraded for performance bias, borderline decision due to second‐line therapies in one study. 2 Downgraded for indirectness due to non‐standard therapies (low‐dose interleukin‐2, IFN‐α) in 75% participants of both treatment arms. 3 Downgraded for imprecision due to wide confidence intervals; clinical action would differ between lower and upper boundary of the confidence interval. 4 Downgraded for performance and detection bias. 5 Not downgraded for indirectness, borderline decision due to non‐standard therapies in both treatment arms. a Moderate risk of 1‐year mortality from the SEER Cancer Statistics Review (Howlader 2015), low risk from participants with favourable risk in Rini 2015, high risk from Hudes 2007.
Summary of findings 6.
Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma
| Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with mRCC | ||||||
|
Patient population: previously treated patients with mRCC Setting: phase III, international, multicentre, open‐label Intervention: targeted immunotherapy (nivolumab) alone Comparison: standard targeted therapies (everolimus) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | ||||||
| Risk with standard targeted therapies | Risk difference with targeted immunotherapy alone (95% CI) | |||||
| 1‐year mortality Follow‐up: > 14 months | 341 per 1000 | 102 fewer per 1000 (from 44 fewer to 150 fewer) | RR 0.70 (0.56 to 0.87) | 821 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Quality of life: Clinically relevant improvement in FKSI‐DRS Follow‐up: 1 to 104 weeks | 367 per 1000 |
187 more per 1000 (from 103 more to 287 more) |
RR 1.51 (1.28 to 1.78) | 704 (1 study) | ⊕⊕⊕⊝ Moderate2 | ‐ |
| Quality of life: clinically relevant improvement in EQ‐5D VAS Follow‐up: 1 to 104 weeks | 391 per 1000 |
145 more per 1000 (from 63 more to 238 more) |
RR 1.37 (1.16‐1.61) | 703 (1 study) |
⊕⊕⊕⊝ Moderate2 | ‐ |
| Adverse events (grade ≥ 3) | 365 per 1000 | 179 fewer per 1000 (from 128 fewer to 219 fewer) | RR 0.51 (0.40 to 0.65) | 803 (1 study) | ⊕⊕⊕⊝ Moderate2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D VAS: EuroQol 5‐Dimension Visual Analogue Scale; FKSI‐DRS: FACT‐Kidney Symptom Index Disease Related Symptoms; mRCC: metastatic renal cell carcinoma; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded for performance bias due to cross‐over. 2 Downgraded for performance and detection bias.
Background
Description of the condition
Kidney cancer is classified into renal cell carcinoma (RCC) and urothelial carcinoma of the renal pelvis. Kidney cancer is the 14th most common malignancy worldwide with approximately 337,800 new cases diagnosed in 2012 (Ferlay 2013). In 2012, 375,925 people had kidney and pelvis cancer in the US with an estimated 63,920 newly diagnosed cancer cases and 13,860 deaths in 2014 (Howlader 2015; Siegel 2014). Incidence is highest in Europe, North America and Australia and lowest in India, Japan, Africa and China (Ljungberg 2011). In the European Union, 85,215 new cases of kidney cancer occurred in 2012 with 35,134 patient deaths (Ferlay 2013).
RCC is the most common tumour of the kidney comprising 90% of cases. It is a heterogeneous cancer and is classified into three major histological RCC types; clear‐cell RCC (70% to 85% of cases), papillary RCC (7% to 15%) and chromophobe RCC (5% to 10%) (Escudier 2014).
In the Western world, RCC shows an age‐standardized incidence rate of 5.8 per 100,000 people and a mortality rate of 1.4 per 100,000 people. Despite advances in diagnosis, about 30% of people with RCC have already developed metastatic RCC (mRCC) at presentation (Gupta 2008), and another 20% of people with clinically localized RCC eventually develop metastases during the course of the disease despite treatment (Athar 2008; Motzer 1996; Zisman 2002). The annual incidence of mRCC was estimated at 8567 cases in the US and 3026 cases in Germany (2002 figures). These numbers correspond to incidence rates of 3.9 (US males), 2.1 (US females), 4.7 (German males) and 2.7 (German females) per 100,000 inhabitants in these countries (Gupta 2008). Prognosis of patients is directly related to the dissemination stage of the tumour. Therefore, the five‐year survival for people with localized RCC in the US is 92.1% decreasing to 65.4% for people with regional disease and down to 11.8% for people with mRCC (Howlader 2015). The estimated economic burden of mRCC has not been adequately studied and can only be estimated from incidences and costs for all types of RCC and kidney cancer. Annual healthcare costs and lost productivity accounted for between USD 107 million to USD 556 million spent in the US whereas the worldwide mRCC cost was estimated to lie between USD 1.1 billion and USD 1.6 billion (2006 figures) (Gupta 2008). In the case of metastatic disease, the central aim of treatment is to optimize improvement in quality and quantity of life. Therefore, the development of new agents with more effective antitumour activity is urgently required for the enhancement of quality of life (QoL) in people with mRCC.
Description of the intervention
RCC has been reported to be a highly immunogenic tumour, an observation that explains the rationale behind the application of immunotherapy to promote an antitumour effect (Michael 2003; Rayman 2004). Due to high levels of intratumoural immune cell infiltration and spontaneous remission rates, various immunotherapeutic approaches have been developed for the treatment of this disease. Most studies have focused on the implementation of non‐specific cytokines, such as interferon (IFN)‐α and interleukin (IL)‐2 and their combinations. Studies have so far produced inconsistent results and failed to define a globally recognized, standardized immunotherapy regimen for metastatic disease (Johannsen 2007). Since the mid‐2000s, the transformation of mRCC treatment following advances that led to improved understanding of RCC biology and the approval of targeted agents inhibiting the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) signalling pathways. This has led to a switch from cytokine‐based therapies to targeted therapies in the treatment of mRCC, a change that is further highlighted by the omission of cytokine monotherapy from current evidence‐based guidelines of the European Association of Urology (Ljungberg 2015). For most people with metastatic disease, cytoreductive nephrectomy alone is merely palliative and thus, additional systemic treatments are necessary. Current guidelines recommend systemic treatment with targeted therapies (sunitinib, bevacizumab plus IFN‐α, pazopanib, temsirolimus, sorafenib, everolimus and axitinib) according to histology, patient risk stratification and treatment line (Ljungberg 2015). Despite remarkable improvements in progression‐free survival (PFS) and objective response rates (ORR) with targeted therapies, an increase in complete remission of mRCC has not been achieved perhaps due to intrinsic or acquired drug resistance of patients (Abe 2013).
The novel immune‐mediated therapeutic options block the immunosuppressive cancer mechanisms culminating in the stimulation of the host antitumour immune response leading to long‐term, persistent tumour destruction (Draube 2011; Postow 2015).
This review summarizes pivotal studies reported since the last version of this review that demonstrate the superiority of targeted agents over IFN‐α as first‐line treatment (Coppin 2007; Hudes 2007; Motzer 2007), including studies focusing on the possible synergy between therapeutic vaccines and antiangiogenic agents (Amato 2010; Rini 2015), personalized immunotherapy (Figlin 2014), or immune checkpoint inhibitors against current standard therapy options (Motzer 2015a).
How the intervention might work
The better understanding of the tumour microenvironment and of T‐cell responses has led to the development of specific immunotherapeutic strategies and as such, a new class of cancer immunotherapy agents, known as immune checkpoint inhibitors, is at the focus for clinical application (Bedke 2014; Postow 2015). These agents mainly comprise of antibodies that target inhibitory molecules such as the cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4), or the programmed death protein 1 pathway (PD‐1 and its ligand PD‐L1). The expression of these inhibitory co‐receptors on T lymphocytes can cause complete suppression or weakening of the antitumour T‐cell responses. It is now recognized that people with mRCC characterized by PD‐1‐positive cancer‐infiltrating lymphocytes often have larger and more aggressive tumours (Thompson 2007). Furthermore, PD‐L1 expression by RCC cancer cells is often associated with a worse clinical outcome of these patients.
Vaccines are another alternative in immunotherapy for the treatment of mRCC. The aim of vaccines is the activation of immune cells to recognize and destroy tumour cells. Vaccination of RCC patients with synthetic peptides representing epitopes derived from tumour‐associated antigens (TAA) and recognized by T‐cell receptors has been shown to induce a well‐defined T‐cell response (Brookman‐May 2011; Dutcher 2013). Different vaccination strategies are under development including cell‐based vaccines that utilize either tumour cells or dendritic cells and cell‐free vaccines that are based on the application of TAA. Furthermore, studies have proposed that the addition of immune‐modulator drugs such as cyclophosphamide can enhance the infiltration of vaccine‐induced effector T cells into tumours (Walter 2012).
Why it is important to do this review
This Cochrane Review serves as the latest update of the Cochrane Review first published in 2000 and previously updated in 2005 and 2007 (Coppin 2000; Coppin 2005; Coppin 2007). In summary, the old review indicates that no cytokine‐based immunotherapy is significantly effective for advanced RCC. IFN‐α and high‐dose interleukin‐2 (HD‐IL‐2) are of unknown survival benefit prior to current first‐line therapy of mRCC with targeted agents. HD‐IL‐2 has been associated with durable complete responses in a small number of patients, but it is of limited use due to its severe toxicity. Furthermore, no clinical factors or biomarkers exist to accurately predict a durable response in patients treated with HD‐IL‐2 (McDermott 2015a). The update will focus on the current role of non‐specific cytokines and implementation of new, specific immunotherapeutic approaches for treatment of people with mRCC. Comparisons were made against the current standard of care options (Ljungberg 2015).
Despite the availability of targeted therapies inhibiting angiogenesis or signal transduction pathways, progress with these agents has reached a plateau and therapy remains non‐curative. Stadler 2014 refers to the 'maturing' of RCC therapy suggesting little progress has been made beyond fine‐tuning the choice and order of the targeted agents.
As a result of this as well as an enhanced understanding of the complex interaction between cancer and host cells (e.g. of the ability of cancer cells to evade immune surveillance or the efficacy of checkpoint inhibitors, such as ipilimumab and nivolumab), interest in immunotherapy has rekindled. Novel therapeutic options are focusing on the possible synergy between standard targeted therapy and immunotherapeutic agents or vaccine approaches (Combe 2015).
The key aims of this review were: 1. to determine the role of non‐specific and new immunotherapies in the development of standard of care guidelines and their place in the current management of mRCC and 2. determine which immunotherapeutic approach, either alone or in combination with standard targeted therapies, is the most efficient to maximize patient benefit. We focused on the entire body of evidence for our clinical question, as well as on patient‐important outcomes and used the GRADE approach to rate quality of evidence (Guyatt 2011a).
Objectives
To assess the effects of immunotherapies either alone or in combination with standard targeted therapies for the treatment of metastatic renal cell carcinoma and their efficacy to maximize patient benefit.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs with or without blinding. Trials were included regardless of their publication status and language of publication. We excluded cross‐over trials and cluster‐randomized trials.
Types of participants
Participants diagnosed with all types of histologically confirmed mRCC including stage IV (T4 any N M0, any T any N M1) (Sobin 2009; Wittekind 2012).
On one occasion, we permitted inclusion of locally advanced cancer patients in a single study that mostly included people with metastatic disease but we excluded studies that focused on locally advanced disease. Studies of mixed solid tumours were eligible only if participants with RCC were stratified and reported separately from other tumour types. In studies where participants had received prior systemic therapy or prior nephrectomy, such prior interventions were documented in the Characteristics of included studies table. We expected that most studies would have been performed in participants with clear‐cell histology (Escudier 2014), but we also included studies investigating histology other than clear‐cell RCC.
Types of interventions
We investigated comparisons of experimental intervention versus comparator interventions utilizing at least one immunotherapeutic agent. We included only studies that compared protocol‐defined immunotherapeutic, experimental interventions to standard treatment options (comparator interventions) as defined in current, evidence‐based guidelines for systemic therapy in people with mRCC (e.g. Escudier 2014; German Guideline Programme in Oncology 2015; Ljungberg 2015). We included trials independently of who, when or by whom the intervention was delivered.
Experimental interventions
ILs alone or combined with other immunotherapy or targeted therapies.
IFN‐α alone or combined with other immunotherapy or targeted therapies.
Vaccine treatment (dentritic cell (DC)‐mediated, Bacillus Calmette‐Guérin (BCG) with tumour antigen, tumour‐associated peptides) alone or in combination with other immunotherapy or targeted therapies.
Adoptive T‐cell therapies.
Targeted immunotherapy (checkpoint inhibitors) either alone or in combination with other immunotherapy or targeted therapies.
Other immunotherapies identified from the searches.
Comparator interventions
Current standard therapy in the form of:
targeted therapies in first‐, second‐ or third‐line therapies;
immunotherapies and targeted therapies (IFN‐α plus bevacizumab) in first‐line therapy.
Comparisons
IFN‐α alone versus standard targeted therapy in first‐line therapy of mRCC.
IFN‐α combined with targeted therapies versus standard targeted therapy in first‐line therapy of mRCC.
IFN‐α alone versus IFN‐α plus bevacizumab in first‐line therapy of mRCC.
IFN‐α plus bevacizumab versus standard targeted therapies in first‐line therapy of mRCC.
Vaccine treatment versus standard therapies in first‐line therapy of mRCC.
Targeted immunotherapies versus standard targeted therapy in previously treated patients with mRCC.
We identified no studies comparing current standard therapies against adoptive T‐cell therapies (experimental intervention 4) and other immunotherapies (experimental intervention 6).
Types of outcome measures
We did not use measurement of outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Overall survival (OS) including one‐year mortality.
Quality of life (QoL).
Adverse events (AEs) (grade 3 or greater).
Secondary outcomes
Progression‐free survival (PFS) (progression may have been measured using clinical or radiological indices).
Tumour remission (both partial and complete remission).
Method and timing of outcome measurement
QoL: measured by cancer‐specific instruments such as the Functional Assessment of Cancer ‐ General (FACT‐G) and FACT‐Kidney Symptom Index (FKSI) questionnaires.
AEs (e.g. vascular leak syndrome, severe infections, severe influenza‐like symptoms): measured by the US National Cancer Institute's (NCI) Common Terminology Criteria for Adverse Events (CTCAE) criteria as worst grade per patient during treatment and follow‐up.
PFS: measured by Response Evaluation Criteria In Solid Tumours (RECIST) criteria during treatment until disease progression (Eisenhauer 2009).
Tumour remission: measured by RECIST criteria during treatment (Eisenhauer 2009).
If we were unable to retrieve the necessary information to analyse time‐to‐event outcomes, we attempted to assess the number of events per total for dichotomized outcomes at 12 months after randomization.
'Summary of findings' tables
We presented 'Summary of findings' tables reporting the following outcomes listed according to priority:
OS (one‐year mortality);
QoL;
AEs (grade 3 or 4);
tumour remission (both partial and complete remission).
We could not perform analyses to estimate absolute effects on the basis of time‐to‐event outcomes and, therefore, we used a predefined approach and describe relative and absolute effects based on one‐year mortality rates.
Search methods for identification of studies
We performed a comprehensive search with no restrictions on the language of publication or publication status.
Electronic searches
We searched the following sources from inception of each database and re‐ran the database search three months prior to the date of review submission.
-
Cochrane Library (via wiley.com; for search strategy, see Appendix 1, 15 April 2015, 16 February 2016, 27 October 2016):
Cochrane Central Register of Controlled Trials (CENTRAL);
Cochrane Database of Systematic Reviews (CDSR);
Database of Abstracts of Reviews of Effects (DARE);
Health Technology Assessment Database (HTA);
MEDLINE (via Ovid; see Appendix 2, 14 April 2015, 7 March 2016, 27 October 2016);
Embase (via Ovid; see Appendix 3, 14 April 2015, 3 March 2016, 16 November 2016).
We applied the Cochrane sensitivity‐maximizing RCT filter (Lefebvre 2011) to the MEDLINE (Ovid) search strategy (Appendix 2), and adaptations of it to remaining databases, except the Cochrane Library (16 April 2015).
We also searched:
ClinicalTrials.gov (www.clinicaltrials.gov/; see Appendix 4); 27 September 2015, 21 March 2016 and 5 November 2016;
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/; see Appendix 5), a meta‐register of studies with links to numerous other trials registers; 27 September 2015, 21 March 2016 and 5 November 2016;
EORTC database of clinical trials and trials in which EORTC has been/is participating (www.eortc.be/protoc/listprot.asp?kind=sites&site=24; see Appendix 6); 27 September 2015, 21 March 2016 and 5 November 2016;
Web of Science Core Collection ‐ Meeting Abstracts (apps.webofknowledge.com/; from 2011; see Appendix 7); 16 April 2015 and 27 October 2016.
No additional relevant key words were detected during any of the electronic or other searches, so there was no need to modify our search strategies or incorporate any changes.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses and health technology assessment reports. In addition, we contacted authors of included trials to identify any further published or unpublished studies (including grey literature) that we may have missed. We contacted drug manufacturers for ongoing or unpublished trials in February and March 2016.
Data collection and analysis
Selection of studies
We used reference management software (EndNote and Citavi) to identify and remove potential duplicate records. Two review authors (IM, DR) independently scanned the abstract or title, or both, of remaining records retrieved, to determine which studies should be assessed further. Two review authors (IM, DR) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification or ongoing studies in accordance with the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any discrepancies through consensus or recourse to a third review author (SU or BS). If resolution of a disagreement was not possible, we designated the study as 'awaiting classification' and we contacted trial authors for clarification. We documented reasons for exclusion of studies that may have reasonably been expected to be included in the Cochrane Review in the Characteristics of excluded studies table. We presented an adapted PRISMA flow diagram showing the process of study selection (Liberati 2009; Figure 1).
Figure 1.
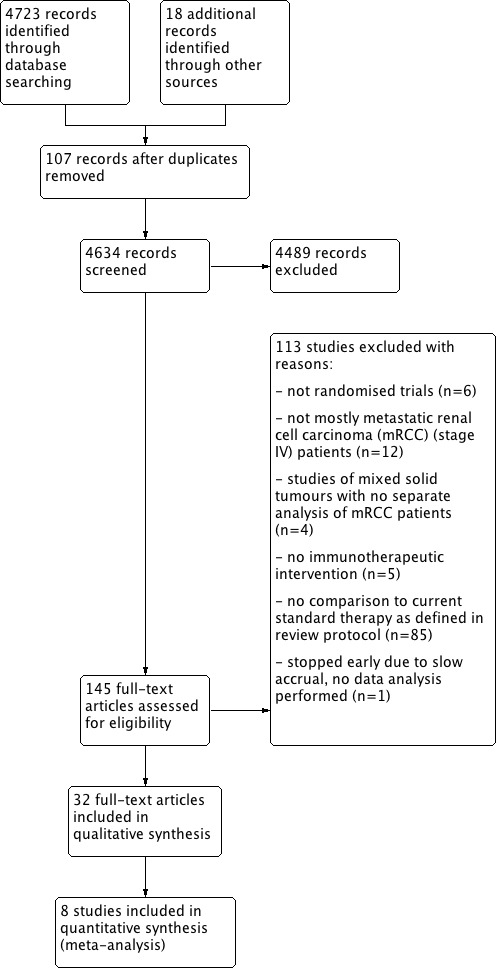
Study flow diagram.
Data extraction and management
We developed a dedicated data abstraction form that was pilot tested ahead of time.
For trials that fulfilled inclusion criteria, two review authors (two of SU, IM, DR, AVH and FP) independently abstracted the following information from individual studies, which were provided in the Characteristics of included studies tables:
study design;
study dates (if dates were not available then this was reported as such);
study settings and country;
participant inclusion and exclusion criteria;
participant details, baseline demographics;
number of participants by study and by study arm;
details of relevant experimental and comparator interventions such as dose, route, frequency and duration;
definitions of relevant outcomes, and method and timing of outcome measurement as well as any relevant subgroups;
study funding sources;
declarations of interest by primary investigators.
We extracted outcome data relevant to this Cochrane Review as needed for calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events and totals for population of a 2 × 2 table, and summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or data necessary to calculate this information. For time‐to‐event outcomes, we attempted to obtain hazard ratios (HRs) with corresponding measures of variance or data necessary to calculate this information; HRs and their confidence intervals (CIs) were estimated directly or indirectly from the published data as from reported log rank Chi2, log rank P values, from observed and expected event ratios, or survival curves (Parmar 1998; Tierney 2007; Williamson 2002).
We resolved any disagreements by discussion, or, if required, by consultation with a third review author (SU or IM).
We provided information including trial identifier, about potentially relevant ongoing studies in the Characteristics of ongoing studies table. We added references of the most recent abstracts or notifications in ClinicalTrials.gov.
We attempted to contact authors of included trials to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we tried to maximize yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data‐set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (two of SU, IM, DR, AVH and FP) assessed the risk of bias of each included study independently. We resolved disagreements by consensus or by consultation with a third review author (SU or IM).
We assessed risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2011b). We assessed the following domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other sources of bias.
We judged risk of bias domains as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We presented a 'Risk of bias' summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome. We grouped outcomes according to whether measured subjectively or objectively when reporting our findings in the 'Risk of bias' tables.
We also assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with like judgements when reporting our findings in the 'Risk of bias' tables.
We further summarized the risk of bias across domains for each outcome in each included study as well as across studies and domains for each outcome.
We defined the following endpoints as subjective outcomes:
QoL;
AEs (grade 3 and 4) (e.g. vascular leak syndrome, severe infections, severe influenza‐like symptoms);
PFS;
tumour remission.
We defined OS as an objective outcome.
Measures of treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% CIs and continuous data as mean differences (MDs) with 95% CIs unless different studies use different measures to assess the same outcome, in which case we used standardized mean differences (SMDs) with 95% CIs. We expressed time‐to‐event data as HRs with 95% CIs. We used results from analyses, stratified by randomization strata or unstratified analyses and most objective results of central or independent reviews.
We used the following threshold for minimal important differences (MIDs):
EuroQol 5‐Dimension Index (EQ‐5D): 0.06‐0.08 (Pickard 2007);
EuroQol Visual Analogue Scale (EQ‐VAS): 7 (Pickard 2007);
FACT‐G: 4 points for better rating and 8 points for worse rating (Ringash 2007);
Functional Assessment of Cancer Therapy ‐ Biologic Response Modifier (FACT‐BRM): 2 points (Cella 1997);
FACT‐Kidney Symptom Index FKSI‐15: 3 points (Cella 1997);
FACT‐Kidney Symptom Index Disease Related Symptoms (FKSI‐DRS): 2 points (Cella 1997).
We qualified MDs above these MIDs as clinically important.
Unit of analysis issues
The unit of analysis was the individual participant.
We included two studies with more than two intervention groups and included pairs of interventions into different comparisons (Hudes 2007), and selected one pair of interventions to create single‐wise comparisons (Negrier 2011), according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We tried to obtain missing data from trial authors, if feasible, and performed intention‐to‐treat (ITT) analyses if data were available; we otherwise performed available‐case analyses. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
In the event of excessive heterogeneity unexplained by subgroup analyses, we did not report outcome results as the pooled effect estimate in a meta‐analysis but we provided a narrative description of the results of each study.
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); we interpreted the I2 statistic as follows:
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When there was heterogeneity, the possible reasons for it were determined by examining individual study and subgroup characteristics.
Assessment of reporting biases
We attempted to obtain study protocols to assess selective outcome reporting.
We did not include 10 trials or more investigating a particular outcome therefore no funnel plots to assess small‐study effects and explanations for their asymmetry were presented.
Data synthesis
Due to the diversity of studies, differing in participant selection, treatment regimens and comparison groups, we did not expect a single study effect and thus, summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects. In addition, statistical analysis was performed according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes, we used the Mantel‐Haenszel method; for continuous outcomes, we used the inverse variance method and for time‐to‐event outcomes, we used the generic inverse variance method. We performed analyses with Review Manager 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
Since following consistently reported and used characteristics to introduce clinical heterogeneity was expected, we carried out subgroup analyses for all primary outcomes with investigation of interactions:
prior nephrectomy (yes versus no);
prior systemic therapies (yes versus no);
performance status (Eastern Cooperative Oncology Group (ECOG) Performance Status 0 or 1 versus greater than 1) or Karnofsky Performance Status (KPS) (90% to 100%; 70% to 80%; less than 70%);
risk prognosis (good/favourable (0) versus intermediate (1 to 2) versus poor prognosis (3 or greater) from the Motzer criteria (good (0) versus intermediate (1 to 2) versus poor prognosis (greater than 3)) (Motzer 2002) or the International Metastatic RCC Database Consortium (IMDC) criteria (Heng 2009; Heng 2013).
We did not use the test for subgroup differences in Review Manager 5 to compare subgroup analyses due to the insufficient number of available studies with prospectively planned subgroup analyses (RevMan 2014).
Sensitivity analysis
We calculated HRs as sensitivity analyses. We additionally planned to perform sensitivity analyses to explore the influence of the following factor (when applicable) on effect sizes of all primary outcomes, but we did not perform these analyses due to the small number of studies.
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high' risk of bias and 'unclear' risk of bias.
'Summary of findings' tables
We present the overall quality of the evidence for the primary outcomes and tumour remission according to the GRADE approach, having taken into account five criteria not only related to internal validity such as risk of bias (Guyatt 2011b), publication bias (Guyatt 2011c), imprecision (Guyatt 2011d), and inconsistency (Guyatt 2011e), but also to external validity, such as directness of results (Guyatt 2011f). For each comparison, two review authors (SU, MN) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low' or 'very low' using GRADEpro GDT. Any discrepancies were resolved by consensus, or, if needed, by arbitration by a third review author (IM). For each comparison, we presented a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing these outcomes and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011a; Schünemann 2011). If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table.
Results
Description of studies
Results of the search
The literature search identified 4723 records, 18 additional records were identified through other sources including handsearching of reference lists of included studies, congress abstracts and articles identified by contacted experts. After removal of 107 duplicate records, we screened 4634 records, and assessed 145 full‐text articles for eligibility. We excluded 113 studies. Detailed reasons for exclusion are summarized in the Figure 1.
We included 32 publications. Out of these 32 publications, eight were primary publications and included in the meta‐analysis (Amato 2010; Escudier 2007; Hudes 2007; Motzer 2007; Motzer 2015a; Negrier 2011; Rini 2010; Rini 2015). We identified 18 secondary (Bellmunt 2008; Bracarda 2013; Castellano 2009; Cella 2008; Cella 2010; Cella 2016; Dutcher 2009; Escudier 2010; Figlin 2009; Kim 2015; Kwitkowski 2010; Melichar 2008; Motzer 2009; Oudard 2011; Patil 2012; Pickering 2009; Summers 2010; Yang 2010) and six preliminary publications or published study protocols (Escudier 2011; Motzer 2015b; Reddy 2006; Rini 2004; Rini 2008; Sharma 2015).
We included eight studies into six comparisons to current evidence‐based standard therapy with targeted therapies or IFN‐α plus bevacizumab. They compared IFN‐α monotherapy or IFN‐α combined with targeted therapy versus targeted standard therapy (Hudes 2007; Motzer 2007), IFN‐α alone with IFN‐α combined with bevacizumab (Escudier 2007; Rini 2010), or IFN‐α combined with bevacizumab with standard targeted therapy (Negrier 2011). In addition, Amato 2010 and Rini 2015 compared vaccine treatment with standard targeted therapies and one study compared targeted immunotherapy with a standard targeted therapy (Motzer 2015a). According to the review protocol, we included only comparisons with standard of care options and most studies from the last versions of this review were excluded (Coppin 2005; Coppin 2007). We identified 13 ongoing studies (EudraCT2016‐002170‐13; Figlin 2014; Hammers 2015; NCT00930033; NCT01984242; NCT02014636; NCT02089685; NCT02210117; NCT02420821; NCT02432846; NCT02684006; NCT02781506; NCT02853331).
Included studies
Since the introduction of targeted agents in the mid‐2000s, clear‐cell and non‐clear‐cell renal cancers have been recognized as distinct entities, and studies have been stratified for or have exclusively recruited these types separately. Similarly, it is now recognized that the magnitude or even the direction of benefit (Armstrong 2015) may depend on prognostic/predictive variables as in the low‐/intermediate‐/high‐risk categories as defined by Motzer 2002 for cytokines, and modified by Heng 2013 for targeted agents. We have examined studies by histological type and prognostic category where possible, that is where these are exclusive or stratified patient inclusion criteria. Line of therapy is discussed for phase III trials recruiting since the introduction of targeted agents.
In this review, we presented the results in groups related to the type of immunotherapy: IFN‐α alone or combined with targeted therapy as comparator to current standard of care treatment (two included studies), IFN‐α plus bevacizumab as standard of care option with IFN‐α or targeted therapies (three included studies), vaccines (two included studies) and checkpoint inhibitor therapy (one included study).
We included studies based on six different comparisons. All major information of these studies may be found in the Characteristics of included studies table.
Comparison 1. Interferon‐α alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
We included two phase III studies (Hudes 2007; Motzer 2007). Both studies were conducted worldwide as multicentre international parallel‐group RCTs with two (Motzer 2007) or three (Hudes 2007) treatment arms. Altogether, 1166 mRCC patients with no previous systemic therapies were randomized. All participants in Motzer 2007 and 80% of participants in Hudes 2007 had mRCC with a clear‐cell component. Participants had a comparable median age of 62 years, two‐thirds of the participants were male. Motzer 2007 restricted inclusion to participants with ECOG Performance Status 1 or less, Hudes 2007 included 82% of participants with KPS 70% or less. Memorial Sloan‐Kettering Cancer Center (MSKCC) risk score was poor in 72% of participants included in Hudes 2007, but only in 6% of participants in Motzer 2007. Neither study allowed prior systemic therapies. Most RCC patients had prior nephrectomy (67% in Hudes 2007 and 90% in Motzer 2007). In both studies, altogether 582 participants were randomized to IFN‐α monotherapy. Participants in the control groups were treated with targeted drugs such as temsirolimus (209 participants in Hudes 2007) or sunitinib (375 participants in Motzer 2007). Participants in Motzer 2007 received IFN‐α at a dose of 9 milli‐International Units (MIU) given subcutaneously three times weekly. In Hudes 2007, temsirolimus was administered as a weekly intravenous infusion of 25 mg and IFN‐α 3 MIU (with an increase to 18 MIU) subcutaneously three times weekly.
Comparison 2. Interferon‐α combined with targeted therapies versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
We included one phase III study (Hudes 2007). The study was conducted worldwide as a multicentre international parallel‐group RCT with three treatment arms (Hudes 2007). One comparison was included into comparison 1, the second into comparison 2. The control group from Hudes 2007 was included in both comparisons. Altogether, 419 mRCC patients with no previous systemic therapies were randomized. See 'Comparison 1. IFN‐α alone versus standard targeted therapies in first‐line therapy of mRCC' for baseline characteristics of this study. A total of 210 participants were randomized to therapy with IFN‐α plus temsirolimus and 209 participants in the control group were treated with temsirolimus alone. The dose of temsirolimus for combination with IFN‐α (6 MIU) was 15 mg and therefore more than 40% lower than the dose of the monotherapy arm.
Comparison 3. Interferon‐α alone versus interferon‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma
We included two studies (Escudier 2007; Rini 2010). Both studies were conducted as two‐arm parallel‐group international and multicentre RCTs in Europe, Asia and Australia (Escudier 2007), or North America (Rini 2010). The studies randomized 1381 mRCC patients with no previous systemic therapy. All participants had clear‐cell mRCC with a comparable median age between 60 and 62 years and two‐thirds were male. Nearly all participants had good performance status (ECOG Performance Status 0 or 1) with favourable and intermediate‐risk score. In total, 690 participants were treated with IFN‐α alone and 691 participants were treated with IFN‐α plus bevacizumab. The studies were slightly different in design. The Escudier 2007 trial (AVOREN; Avastin and Roferon in Renal Cell Carcinoma) was designed as a double‐blind study and included a placebo control. Furthermore, all participants in AVOREN were post nephrectomy, whereas in the CALGB (Cancer and Leukemia Group B) trial, only 85% were nephrectomized.
Comparison 4. Interferon‐α plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
We included one study (Negrier 2011). This study was conducted as a parallel‐group, multicentre national RCT in France (Negrier 2011), mainly to evaluate the combination of temsirolimus plus bevacizumab to improve treatment efficacy. They randomized participants into three treatment arms (Negrier 2011), but only the comparisons of two of them met the inclusion criteria of our review and were included. In total, 83 mRCC patients with no previous systemic treatment for mRCC were randomized. Nearly all participants had clear‐cell mRCC. Participants had a comparable median age of about 62 years and 71% of them were male. Most participants (88%) had an ECOG Performance Status 0 or 1, had undergone nephrectomy (91%) and intermediate (47%) or favourable (31%) risk prognosis. Forty‐one participants received IFN‐α plus bevacizumab and 42 participants received targeted monotherapy with sunitinib (Negrier 2011).
Comparison 5. Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma
Two studies investigated the efficacy of two different vaccines in comparison to targeted therapy with standard‐of‐care in first‐line therapy of mRCC according to local practice including sunitinib (Amato 2010; Rini 2015). Both studies were conducted in Europe and the US as multicentre, international, two‐arm, parallel‐group RCTs and randomized 1071 mRCC patients with no previous systemic therapies. All participants had clear‐cell mRCC. Participants had a median age of 60 years, two‐thirds of the participants were male and most of them had undergone nephrectomy. Nearly all participants in Rini 2015 had favourable or intermediate‐risk prognosis and only 13% had a KPS of 80 or lower. However, 26% of participants had a poor‐risk prognosis in Amato 2010 and 30% had a KPS performance status of 80. In total, 365 participants were treated with MVA‐5T4 (Amato 2010), 204 participants with IMA901 (Rini 2015), and 502 participants with placebo or no additional treatment to standard‐of‐care according to local practice.
Comparison 6. Targeted immunotherapy alone versus targeted standard therapy in previously treated patients with metastatic renal cell carcinoma
We included one study (Motzer 2015a). This study was conducted worldwide as a parallel‐group, international, multicentre RCT. A total of 821 participants with advanced or metastatic RCC with a clear‐cell component and a median age of 62 years were included and 75% of them were male. Nearly all participants had a KPS of 70 or more and prior nephrectomy. All participants had prior systemic treatment for mRCC with sunitinib, pazopanib or axitinib. A total of 410 participants were treated with nivolumab and 411 with everolimus. Two additional studies assessed the dose‐response relationship, activity and safety of this targeted immunotherapy in phase I and phase II RCTs (Choueiri 2014; Motzer 2015c).
Excluded studies
We assessed 210 full‐text articles for eligibility and excluded 178 of them. Exclusion criteria are summarized in the Characteristics of excluded studies table and include:
not randomized trials (Amato 2009; Amin 2015; Bromwich 2002; Harlin 2004; Wang 2015; Yang 2007);
mostly no mRCC (stage IV) patients (including adjuvant studies) (Atzpodien 2005; Clark 2003; Fenton 1996; Galligioni 1996; Jocham 2004; Majhail 2006; Passalacqua 2014; Pizzocaro 2001; Messing 2003; Soret 1996; Wood 2008; Zhan 2012);
studies of mixed solid tumours with no separate analysis of mRCC patients (Dillman 2003; Du Bois 1997; Margolin 1997; Smith 2003);
no immunotherapeutic intervention (Keefe 2015; Negrier 2010; Powles 2015; Rini 2012; Sternberg 2013);
no comparison to current standard therapy as defined in review protocol (Aass 2005; Adler 1987; Atkins 1993; Atzpodien 2001; Atzpodien 2004; Atzpodien 2006; Boccardo 1998; Borden 1990; Bracarda 2013; Brinkmann 2004; Buzogany 2001; Choueiri 2014; Creagan 1991; De Mulder 1995; Dexeus 1989; Donskov 2006; Dudek 2008; Dutcher 2003; Edsmyr 1985; Elkord 2013; Escudier 2009; Figlin 1999; Flanigan 2001; Foon 1988; Fosså 1992; Fosså 2004; Fujita 1992; Gleave 1998; Gore 2010; Henriksson 1998; Jayson 1998; Jonasch 2010; Kempf 1986; Kinouchi 2004; Kirkwood 1985; Koretz 1991; Kriegmair 1995; Law 1995; Lissoni 1993; Lissoni 2000;Lissoni 2003; Liu 2012; Lummen 1996; McCabe 1991; McDermott 2005; Mickisch 2001; Motzer 2000; Motzer 2001; Motzer 2015c; MRCRCC 1999; Muss 1987; Naglieri 1998; NCT00352859; Negrier 1998; Negrier 2000; Negrier 2007; Negrier 2008; Neidhart 1991; Osband 1990; Otto 1988; Passalacqua 2010; Patel 2008; Pedersen 1980; Porzsolt 1988; Procopio 2011; Pyrhönen 1999; Quesada 1985; Radosavljevic 2000; Ravaud 2015; Rini 2014; Rosenberg 1993; Rossi 2010; Sagaster 1995; Scardino 1997; Schwaab 2000; Simons 1997; Steineck 1990; Tannir 2006; Tsavaris 2000; Walter 2012; Weiss 1992; Witte 1995; Yang 1995; Yang 2003; Zhao 2015);
stopped early due to slow accrual, no data analysis performed (NCT00678288).
Risk of bias in included studies
Risk of bias of all included studies is summarized in Figure 2 and Figure 3.
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
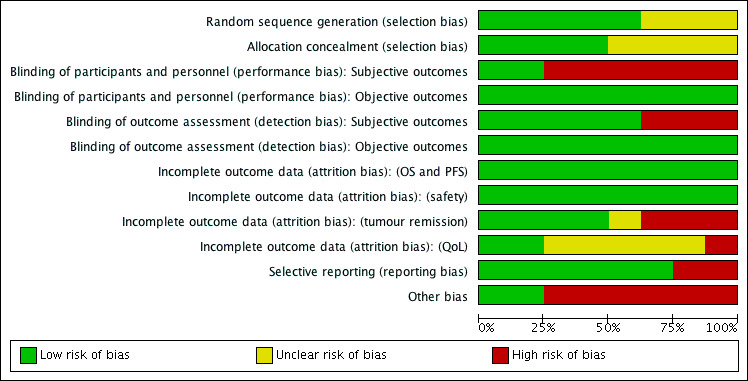
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Sequence generation was at low risk of bias in five studies (Escudier 2007; Motzer 2007; Motzer 2015a; Negrier 2011; Rini 2010), and unclear risk of bias in three studies. Six studies described blocked randomization. All studies used a stratified randomization procedure stratified by region, prognostic risk group, prior therapy (nephrectomy or antiangiogenic therapies) or performance score.
Allocation concealment
Allocation concealment was at low risk of bias in four studies (Escudier 2007; Motzer 2015a; Negrier 2011;Rini 2015), and information was missing in four studies. These studies used central allocation based on an interactive voice recognition system (Hudes 2007), fax or email (Rini 2015), or reported no more detailed information.
Blinding
Blinding of participants and personnel
Two studies were double‐blind, placebo‐controlled trials with the same route and timing of administration of placebo and the intervention and blinding of participants and study personnel (Amato 2010; Escudier 2007). We judged these studies at low risk of performance and detection bias for subjective outcomes (QoL, AEs, PFS and tumour remission) and judged all remaining studies at high risk of bias. Of the three studies in this review that reported formal QoL assessment, none were double‐blind, making this outcomes of questionable reliability (Hudes 2007; Motzer 2007; Motzer 2015a).
We judged all studies at low risk of bias for objective outcomes (OS).
Blinding of outcome assessment
Four studies described blinded or independent outcome assessment of subjective outcomes (Amato 2010; Escudier 2007; Motzer 2007; Negrier 2011). These studies were at low risk of bias in respect to subjective outcomes. High risk of bias was judged if treatment effects of blinded assessment were not shown (Hudes 2007), and in non‐blinded studies without independent assessment of tumour remission and PFS (Motzer 2015a; Rini 2010; Rini 2015).
We considered a double‐blind design for subjective outcomes such as QoL. Of the three studies included in this review that reported formal QoL assessment, none were double‐blind making this outcome of questionable reliability; therefore, we discussed QoL outcomes separately.
We downgraded the quality of evidence for our subjective outcomes (e.g. QoL, AEs) for risk of bias due to no double blinding or blinded assessment of these outcomes in all comparisons (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
We judged the assessment of objective outcomes (OS) in all studies at low risk of bias.
Incomplete outcome data
Treatment effects for OS and PFS were based on the analysis of all randomized participants according to randomization with high completeness and no differences in censoring between treatment groups and were judged as low risk of bias in all included studies.
Safety analysis was based in all studies on all treated participants.
Tumour remission was completely reported for all participants with measurable disease and postbaseline tumour assessment in four studies with no differences between groups (Amato 2010, Escudier 2007, Negrier 2011; Rini 2015). These studies were at low risk of bias. Three studies reported differences in the number of tumour assessments between treatment groups (Hudes 2007; Motzer 2007; Motzer 2015a). These studies were at high risk of bias. One study reported no numbers of participants with tumour assessment and we judged it at unclear risk of bias (Rini 2010).
Four studies of two trials reported QoL with low risk of bias due to high completion rates (greater than 90%) and small differences between treatment groups (Motzer 2007; Motzer 2015a). Risk of bias was unclear due to missing completion rates and differences in completion between groups in one study (Hudes 2007).
Selective reporting
Five studies stated no differences between outcomes planned in the protocol and those reported (Hudes 2007; Motzer 2007; Motzer 2015a; Rini 2010; Rini 2015). Therefore, these studies were at low risk of bias. We could not rule out high risk of bias due to selective reporting in two studies due to missing reports of preplanned outcomes as PFS (Amato 2010), long‐term OS (Negrier 2011), and QoL (Negrier 2011).
Other potential sources of bias
Four studies identified other potential sources of bias. These sources included the frequent use of second‐line therapies after progression (Amato 2010; Escudier 2007; Negrier 2011; Rini 2010), permitted cross‐over to the intervention group (Motzer 2007; Motzer 2015a), and the use of a blocked randomization in centres in an unblinded trial (Negrier 2011).
We downgraded the quality of evidence for one‐year mortality for risk of bias in all comparisons where participants with progressive disease frequently crossed over to the comparison arm, where a relevant amount of participants received second‐line systemic anticancer therapies subsequent to progression (Table 1; Table 3; Table 5), or where studies were stopped for early benefit and participants were permitted to cross over from the control to the intervention groups during follow‐up for OS (Table 6).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Comparison 1. Interferon‐α alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
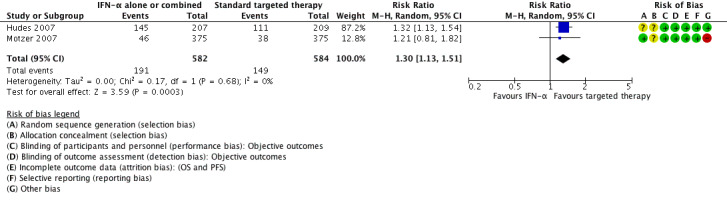
Two studies with 1376 participants compared the efficacy and safety of IFN‐α monotherapy with targeted therapies (Hudes 2007; Motzer 2007). In this comparison, we evaluated 1166 participants. The intervention group received IFN‐α monotherapy and had 582 participants (Hudes 2007, 207 participants; Motzer 2007, 375 participants), and the control group received standard targeted therapies as sunitinib (Motzer 2007, 375 participants) and temsirolimus (Hudes 2007, 209 participants).
1.1. Overall survival
In total, 191/582 (33%) participants treated with IFN‐α alone died within one year after randomization compared to 149/584 (26%) participants with targeted therapies (RR 1.30, 95% CI 1.13 to 1.51; Figure 4). We applied this RR to participants with mRCC and low‐risk score (favourable) one‐year mortality rates of 150/1000 participants, moderate‐risk score of 280/1000 participants and high‐risk score of 550/1000 participants with standard targeted therapies. These numbers are probably increased by 45 deaths/1000 (from 20 more to 76 more) in low‐risk participants, 84 deaths/1000 (from 36 more to 143 more) in moderate‐risk participants and 165 deaths/1000 (from 71 more to 280 more) in high‐risk participants if they were treated with IFN‐α as monotherapy (Table 1).
Figure 4.
Forest plot of comparison: 1 Interferon‐α (IFN‐α) alone versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, outcome: 1.1 1‐year mortality.
Treatment effects of both studies were comparable with no important statistical heterogeneity (I2 = 0%), but the two trials varied in median survival and one‐year mortality rates. In Motzer 2007, median OS was 21.8 months with IFN‐α alone versus 26.6 months with targeted therapies and one‐year mortality was 12% with IFN‐α alone versus 10% with targeted therapies. In contrast, in Hudes 2007 who included participants with a worse risk prognosis, median OS was only 7.3 months with IFN‐α alone versus 10.9 months with temsirolimus with one‐year mortality of approximately 70% with IFN‐α alone versus 53% with temsirolimus (Table 13; Figure 4). Subgroup analyses in Hudes 2007 suggested a higher benefit from targeted therapies for participants with a low performance status (KPS 70 or less) or no prior nephrectomy (Table 14).
Table 1.
Overall survival
| Study ID | Comparison (group 1 vs group 0) | Median OS (95% CI) (months) | 1‐year mortality | Comments | ||
| Group 1 | Group 0 | Group 1 | Group 0 | |||
| Hudes 2007 | 1 (IFN‐α alone vs standard targeted therapies) | 7.3 (6.1 to 8.8) | 10.9 (8.6 to 12.7) | 70% | 53% | From curves. |
| Motzer 2007 | 21.8 (17.9 to 26.9) | 26.4 (23.0 to 32.9) | 12.3% | 10.1% | Numbers reported. | |
| Hudes 2007 | 2 (IFN‐α + targeted therapies vs standard targeted therapies) | 8.4 (6.6 to 10.3) | 10.9 (8.6 to 12.7) | 60% | 53% | From curves. |
| Escudier 2007 | 3 (IFN‐α alone vs IFN‐α + bevacizumab) | 21.3 | 23.3 | 32% | 26% | From curves. |
| Rini 2010 | 17.4 (14.4 to 20.0) | 18.3 (16.5 to 22.5) | 39% | 34% | From curves with numbers and censoring marks. | |
| Negrier 2011 | 4 (IFN‐α + bevacizumab vs standard targeted therapies) | Not reported | Not reported | 10% | 26% | Reported. |
| Amato 2010 | 5 (Vaccine treatment vs standard therapies) | 19.2 | 20.1 | 35% | 33% | From curves with censoring. |
| Rini 2015 | 33.1 | Not reached | 17% | 22% | Numbers reported. | |
| Motzer 2015a | 6 (Targeted immunotherapy alone vs targeted standard therapy) | 25.0 (21.8 to NE) | 19.6 (17.6 to 23.1) | 24% | 34% | From curves with numbers with censoring marks. |
CI: confidence interval; IFN‐α: interferon‐α; NE: not estimable; OS: overall survival.
Table 2.
Overall survival subgroups
| Comparison (group 1 vs group 0) | Study | Subgroup | Sample size | Treatment effects (95% CI) or P values |
| 1 (IFN‐α alone vs standard targeted therapies) | Hudes 2007 | Prior nephrectomy | 278 | HR 1.2 (0.9 to 1.6) |
| Motzer 2007 | Prior nephrectomy | 674 | HR 1.2 (0.95 to 1.5) | |
| Pooled | Prior nephrectomy | 952 | HR 1.2 (1.0 to 1.43), I2 = 0% | |
| Hudes 2007 | No prior nephrectomy | 138 | HR 1.7 (1.1 to 2.5) | |
| Motzer 2007 | No prior nephrectomy | 76 | HR 1.23 (0.8 to 2.1) | |
| Pooled | No prior nephrectomy | 214 | HR 1.48 (1.1 to 2.0), I2 = 1% | |
| Hudes 2007 | KPS ≤ 70 | 340 | HR 1.39 (1.11 to 1.75) | |
| Hudes 2007 | KPS > 70 | 75 | HR 0.93 (0.53 to 1.67) | |
| Motzer 2007 | With poor risk | 48 | HR 1.15 (0.83 to 2.78) | |
| Motzer 2007 | Intermediate risk | 421 | HR 1.27 (1.00 to 1.62) | |
| Motzer 2007 | ECOG Performance Status 0 | 460 | HR 1.1 (0.87 to 1.5) | |
| Motzer 2007 | ECOG Performance Status 1 | 290 | HR 1.4 (1.05 to 1.7) | |
| 3 (IFN‐α alone vs IFN‐α + bevacizumab) | Escudier 2007 | Favourable risk | 180 | HR 1.09 (0.73 to 1.61), P = 0.6798 |
| Rini 2010 | Favourable risk | 192 | HR 1.11 (0.8 to 1.56), P = 0.5189 | |
| Pooled | Favourable risk | 372 | HR 1.10 (0.85 to 1.43), I2 = 0% | |
| Escudier 2007 | Intermediate risk | 392 | HR 1.20 (0.95 to 1.54), P = 0.1230 | |
| Rini 2010 | Intermediate risk | 465 | HR 1.15 (0.94 to 1.41), P = 0.1688 | |
| Pooled | Intermediate risk | 857 | HR 1.18 (1.01 to 1.37), I2 = 0% | |
| Escudier 2007 | Poor risk | 59 | HR 1.18 (0.68 to 2.04), P = 0.5594 | |
| Rini 2010 | Poor risk | 75 | HR 1.33 (0.82 to 2.17), P = 0.2439 | |
| Pooled | Poor risk | 124 | HR 1.27 (0.88 to 1.82), I2 = 0% | |
| Rini 2010 | Prior nephrectomy | 620 | HR 1.10 (0.93 to 1.32), P = 0.2871 | |
| Rini 2010 | No prior nephrectomy | 112 | HR 1.54 (1.02 to 2.27), P = 0.0381 | |
| 5 (Vaccine treatment vs standard therapies) | Amato 2010 | Favourable risk, treated with IL‐2 (SOC) | 100 | HR 0.54 (0.30 to 0.98), P = 0.046 |
| Amato 2010 | Favourable risk, treated with IFN‐α (SOC) | 206 | P > 0.05 | |
| Amato 2010 | Good prognosis, treated with sunitinib (SOC) | 119 | P > 0.05 | |
| Amato 2010 | Intermediate prognosis, treated with IL‐2 (SOC) | 70 | P > 0.05 | |
| Amato 2010 | Intermediate prognosis, treated with IFN‐α (SOC) | 169 | P > 0.05 | |
| Amato 2010 | Intermediate prognosis, treated with sunitinib (SOC) | 65 | P > 0.05 | |
| Rini 2015 | Favourable risk (n = 92) | 92 | HR 0.82, P = 0.59 | |
| Rini 2015 | Intermediate risk (n = 240) | 240 | HR 1.52, P < 0.05 | |
| 6 (Targeted immunotherapy alone vs standard targeted therapies) | Motzer 2015a | Favourable risk group (MSKCC risk group) | 293 | HR 0.89 (0.59 to 1.32) |
| Motzer 2015a | Intermediate risk group (MSKCC risk group) | 404 | HR 0.76 (0.58 to 0.99) | |
| Motzer 2015a | Poor risk group (MSKCC risk group) | 124 | HR 0.47 (0.30 to 0.73) | |
| Motzer 2015a | 1 previous antiangiogenic regimen | 591 | HR 0.71 (0.56 to 0.90) | |
| Motzer 2015a | 2 previous antiangiogenic regimens | 230 | HR 0.89 (0.61 to 1.29) |
CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; HR: hazard ratio; IFN‐α: interferon‐α; IL: interleukin; KPS: Karnovsky Performance Score; MSKCC: Memorial Sloan‐Kettering Cancer Center; n: number of participants; SOC: standard of care.
The pooled HRs for OS of 1.28 (95% CI 1.10 to 1.49; Analysis 1.2) stated a better OS in participants treated with standard targeted therapies compared to IFN‐α alone.
Analysis 1.2.
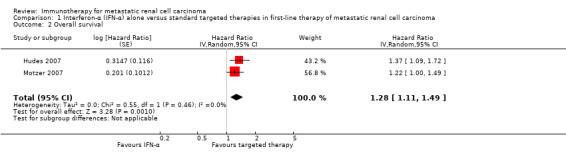
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.
1.2. Quality of life
Motzer 2007 reported QoL based on approximately 95% of 750 included participants. Participants in the IFN‐α arm reported similar QoL with no clinically important differences in all QoL assessments compared to standard targeted therapies during treatment.
FACT‐G assessment stated lower mean postbaseline scores by ‐5.58 points (95% CI ‐7.24 to ‐3.91). Across all treatment cycles, the overall MD in scores of FKSI assessments were slightly lower with IFN‐α compared to sunitinib by an MD of ‐3.27 points (95% CI ‐4.18 to ‐2.36) for FKSI‐15 and by an MD of ‐1.98 points (95% CI ‐2.51 to ‐1.46) for FKSI‐DRS (Motzer 2007). These differences were stated by EuroQol assessment with an overall MD of ‐0.0364 points (95% CI ‐0.0620 to ‐0.0109) in EQ‐5D score and ‐4.74 points (95% CI ‐6.87 to ‐2.60) for EQ‐VAS with lower values with IFN‐α alone.
In addition, treatment effects on the mean last score from Hudes 2007 stated a slightly lower (least favourable) score in all EQ‐5D assessments in the IFN‐α group compared to the temsirolimus group: the mean EQ‐5D index score at the last measure was lower in the IFN‐α arm compared to the temsirolimus arm by ‐0.099 points (95% CI ‐0.162 to ‐0.036) and the mean EQ‐VAS was lower by ‐4.50 points (95% CI ‐8.184 to ‐0.819).
Pooled treatment effects of EQ‐5D and EQ‐VAS stated similar QoL with IFN‐α and standard therapies (Table 15; Analysis 1.3).
Table 3.
Quality of life
| Comparison (group 1 vs group 0) | Study (reported in) | Measurement instrument | Group 1 | Group 0 | Favours | Difference (95% CI) or P values |
| 1 (IFN‐α alone vs standard targeted therapies) | Motzer 2007 (reported in Cella 2008) | FACT‐G total score, mean postbaseline score over 17 weeks | 76.8 (n = 357) | 82.3 (n = 373) | Group 0 | ‐5.58 (‐7.25 to ‐3.91) |
| Motzer 2007 (reported in Cella 2008) | FKSI‐15, mean postbaseline score over 17 weeks | 42.1 (n = 357) | 45.3 (n = 373) | Group 0 | ‐3.27 (‐4.18 to ‐2.36) | |
| Motzer 2007 (reported in Cella 2008) | FKSI‐DRS, mean postbaseline score over 17 weeks | 27.4 (n = 357) | 29.4 (n = 373) | Group 0 | ‐1.98 (‐2.51 to ‐1.46) | |
| Hudes 2007, (reported in Yang 2010) | EQ‐5D Index, mean score on treatment | 0.492 (n = 115) | 0.590 (n = 157) | Group 0 | ‐0.099 (95% CI ‐0.162 to ‐0.036) | |
| Motzer 2007 (reported in Cella 2008) | EQ‐5D Index, mean postbaseline score over 17 weeks | 0.725 (n = 357) | 0.762 (n = 373) | Group 0 | ‐0.0364 (‐0.0620 to ‐0.0109) | |
| Pooled EQ‐5D | 472 | 530 | Group 0 | ‐0.06 (‐0.12 to 0), I2 = 69% | ||
| Hudes 2007, (reported in Yang 2010) | EQ‐VAS, mean score on treatment | 58.83 (n = 115) | 63.33 (n = 157) | Group 0 | ‐4.50 (‐8.184 to ‐0.819) | |
| Motzer 2007, (reported in Cella 2008) | EQ‐VAS, mean postbaseline score over 17 weeks | 68.7 (n = 357) | 73.4 (n = 373) | Group 0 | ‐4.74 (‐6.87 to ‐2.60) | |
| Pooled EQ‐VAS | 472 | 530 | Group 0 | ‐4.68 (‐6.53 to ‐2.83), I2 = 0% | ||
| 6 (Targeted immunotherapy alone vs standard targeted therapies) | Motzer 2015a (reported in Cella 2016) | FKSI‐DRS, mean score at baseline | 30.2 ± 4.4 (n = 362) | 30.1 ± 4.8 (n = 344) | ‐ | Difference in mean change 1.6 (1.4 to 1.9), P < 0.0001 |
| FKSI‐DRS, mean change from baseline to week 28 | 0.4 ± 5 (n = 164) | ‐1.2 ± 4 (n = 122) | Group 1 | |||
| FKSI‐DRS, mean change from baseline to week 52 | 1.6 ± 4 (n = 97) | ‐1.0 ± 6 (n = 63) | Group 1 | |||
| FKSI‐DRS, mean change from baseline to week 104 | 3.5 ± 4.1 (n = 20) | 0.2 ± 6 (n = 9) | Group 1 | |||
| Clinically important improvement from baseline by ≥ 2 FKSI‐DRS points | 200 (55%)/361 | 126 (37%)/343 | Group 1 | RR 1.51 (1.28 to 1.78); P < 0.0001 | ||
| Time to clinically important improvement ≥ 2 FKSI‐DRS points | Median: 4.7 months (3.7 to 7.5) | Median not reached | Group 1 | HR 1.66 (1.33 to 2.08); P < 0.0001 | ||
| Clinically important improvement from baseline by ≥ 3 FKSI‐DRS points | 148 (41%)/361 | 95 (28%)/343 | Group 1 | RR 1.48 (1.20 to 1.83); P = 0.0002 | ||
| Time to clinically important improvement ≥ 3 FKSI‐DRS points | Median not estimable | Median not estimable | Group 1 | HR 1.61 (1.24 to 2.09); P < 0.0003 | ||
| EQ‐5D utility index, mean score at baseline | 0.78 ± 0.24 (n = 362) | 0.78 ± 0.21 (n = 344) | ‐ | No significant differences in proportion of participants with clinical important improvements (P = 0.070) or time to improvement (P = 0.86). | ||
| EQ‐5D utility index, mean change from baseline to week 28 | 0.052 ± 0.22 (n = 164) | ‐0.03 ± 0.2 (n = 122) | Group 1 | |||
| EQ‐5D utility index, mean change from baseline to week 52 | 0.06 ± 0.1 (n = 98) | ‐0.01 ± 0.2 (n = 63) | Group 1 | |||
| EQ‐5D utility index, mean change from baseline to week 104 | 0.13 ± 0.7 (n = 20) | ‐0.02 ± 0.15 (n = 9) | Group 1 | |||
| EQ‐5D VAS, mean score at baseline | 73.3 ± 18.5 (n = 362) | 72.5 ± 18.7 (n = 344) | ‐ | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 28 | 5 ± 13 (n = 164) | ‐3 ± 11 (n = 122) | Group 1 | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 52 | 7 ± 15 (n = 98) | ‐2 ± 16 (n = 63) | Group 1 | ‐ | ||
| EQ‐5D VAS, mean change from baseline to week 104 | 9 ± 9 (n = 20) | 1 ± 18 (n = 9) | Group 1 | ‐ | ||
| Clinically important improvement from baseline by ≥ 7 EQ‐5D VAS points | 192 (53%)/360 | 134(39%)/343 | ‐ | RR 1.37 (1.16 to 1.61); P = 0.0001 | ||
| Time to clinically important improvement | Median: 6.5 months (3.9 to 12.2) | Median: 23.1 months (15.4 to not estimated) | ‐ | HR 1.37 (1.10 to 1.71) |
CI: confidence interval; EQ‐5D Index: EuroQol 5‐Dimension (MID 0.06 to 0.08, Pickard 2007); EQ‐VAS: EuroQol Visual Analog Scale (MID 7, Pickard 2007); FACT‐G: Functional Assessment of Cancer Therapy ‐ General (MID 4 points for better rating and 8 points for worse rating, Ringash 2007); FKSI‐15: FACT‐Kidney Symptom Index (MID 3 points, Cella 1997); FKSI‐DRS: FACT‐Kidney Symptom Index Disease Related Symptoms (MID 2 points, Cella 1997); HR: hazard ratio; MID: minimal important difference; n: number of participants.
Analysis 1.3.
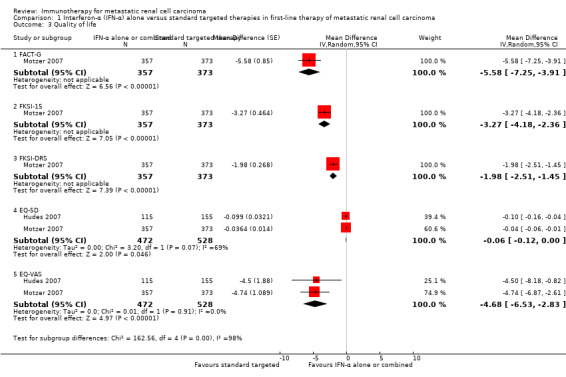
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Quality of life.
Summarizing these results, the treatment options may lead to similar QoL.
1.3. Adverse events (grade 3 or greater)
One study reported the total number of participants with AEs (grade 3 or greater) based on 408 participants (Hudes 2007). AEs occurred in 78% of participants with IFN‐α alone compared to 67% of participants with temsirolimus alone (RR 1.17, 95% CI 1.03 to 1.32; Analysis 1.4). We applied these RRs to participants with mRCC with a mean risk of AEs (grade 3 or greater) in 668/1000 participants in the temsirolimus group. These number may be slightly increased by 114/1000 participants (from 20 more to 214 more), if they are treated with IFN‐α alone instead of standard targeted therapies (Table 1).
Analysis 1.4.
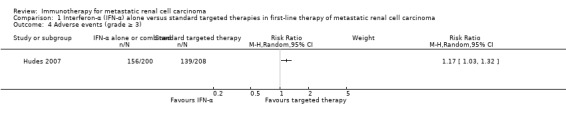
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3).
Asthenia was the most common AE grade 3 or greater. Similar numbers of participants reported dyspnoea, diarrhoea, nausea or vomiting, but more participants with temsirolimus had mild‐to‐moderate rash (0% with IFN‐α alone versus 4% with temsirolimus alone), peripheral oedema, hyperglycaemia and hyperlipidaemia (Hudes 2007).
Motzer 2007 reported higher proportions of participants with treatment‐related fatigue (grade 3 or greater) in the IFN‐α group than in the sunitinib group (12% with IFN‐α versus 7% with sunitinib). In contrast, participants in the sunitinib group had higher rates of grade 3 diarrhoea (0% with IFN‐α versus 5% with sunitinib), vomiting (1% with IFN‐α versus 4% with sunitinib), hypertension (1% with IFN‐α versus 8% with sunitinib), hand‐foot syndrome (0% with IFN‐α versus 5% with sunitinib), leukopenia (7% with IFN‐α versus 12% with sunitinib), neutropenia (2% with IFN‐α versus 5% with sunitinib) and thrombocytopenia (0% with IFN‐α versus 8% with sunitinib).
1.4. Progression‐free survival
PFS was inferior with IFN‐α compared to targeted therapies with no important heterogeneity between studies (HR 2.23, 95% CI 1.79 to 2.77; Analysis 1.5). Hudes 2007 also compared IFN‐α plus temsirolimus versus temsirolimus alone and there were no differences between groups (HR 1.09, 95% CI 0.90 to 1.31).
Analysis 1.5.
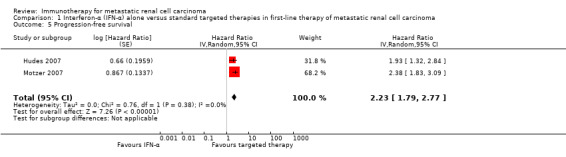
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Progression‐free survival.
1.5. Tumour remission
Both studies reported tumour remission based on 1007 participants. Fewer participants showed treatment response with IFN‐α compared to targeted therapies in both studies with a pooled RR of 0.30 (95% CI 0.12 to 0.75); there was substantial heterogeneity between studies (I2 = 73%). In Motzer 2007, 6% of RCC participants showed response with IFN‐α versus 31% with sunitinib whereas the overall response rates were much lower in Hudes 2007 with 5% of participants treated with IFN‐α versus 9% with temsirolimus.
Comparison 2. Interferon‐α combined with targeted therapies versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
One study with 419 participants (210 in the intervention group and 209 in the control group) compared the efficacy of IFN‐α plus temsirolimus with temsirolimus alone (Hudes 2007).
2.1. Overall survival
A total of 126/210 participants treated with IFN‐α plus temsirolimus died compared to 111/209 participants treated with temsirolimus alone (RR 1.13, 95% CI 0.95 to 1.34; Analysis 2.1). We applied this RR to participants with mRCC and low‐risk score (favourable) one‐year mortality rates of 150/1000 participants, moderate‐risk score of 280/1000 participants and high‐risk score of 550/1000 participants with standard targeted therapies, respectively. These numbers are probably not changed (Table 2).
Analysis 2.1.
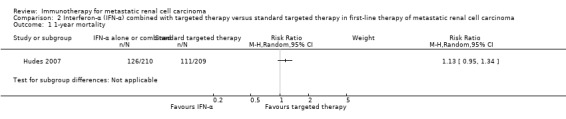
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
The median OS was 8.4 months with IFN‐α plus temsirolimus and 10.9 months with temsirolimus alone with one‐year mortality of approximately 60% for IFN‐α plus temsirolimus and 53% for temsirolimus alone in Hudes 2007, who included participants with a worse risk prognosis (Table 13; Figure 4).
These results corresponded to no difference of a combined therapy with IFN‐α as combined therapy compared to standard targeted therapy on mortality (HR 1.20, 95% CI 0.97 to 1.49; Analysis 2.1).
2.2 Quality of life
QoL was not reported for participants treated with IFN‐α plus temsirolimus, because no survival advantage was observed.
2.3. Adverse events (grade 3 or greater)
The total number of participants with AEs (grade 3 or greater) was reported based on 416 participants. AEs occurred in 87% of participants in the IFN‐α plus temsirolimus group compared to 67% of participants in the temsirolimus group (RR 1.30, 95% CI 1.17 to 1.45; Analysis 2.3). We applied these RRs to participants with mRCC with a mean risk of AEs (grade 3 or greater) in 668/1000 participants in the temsirolimus alone group. These numbers may be increased by 200 (from 114 more to 301 more) with AEs (grade 3 or greater) if they are treated with combination therapy instead of temsirolimus alone (Table 2).
Analysis 2.3.
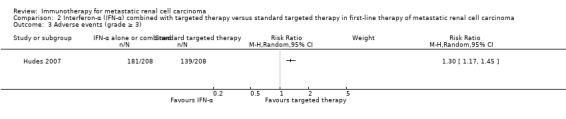
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).
2.4. Progression‐free survival
There were no differences in PFS between IFN‐α plus temsirolimus and temsirolimus alone (HR 1.09, 95% CI 0.90 to 1.31; Analysis 2.4).
Analysis 2.4.
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.
2.5. Tumour remission
Tumour remission was reported based on 357 participants. There were no differences between IFN‐α plus temsirolimus and temsirolimus (RR 0.92, 95% CI 0.46 to 1.83; Analysis 2.5). The overall response rates were low in Hudes 2007 with 8% of participants treated with IFN‐α plus temsirolimus versus 9% with temsirolimus alone.
Analysis 2.5.
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.
Comparison 3. Interferon‐α alone versus interferon‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma
Two studies with 1381 participants compared the efficacy and safety of IFN‐α alone versus IFN‐α plus bevacizumab (Escudier 2007; Rini 2010). OS and PFS were reported in both included studies on all participants, the frequency of AEs (grade 3 or greater) was reported based on all treated participants and tumour remission was assessed in both studies for 1205 participants.
3.1. Overall survival
In total, 244/685 (36%) participants with IFN‐α alone died within one year after randomization compared to 212/696 (30%) participants treated with IFN‐α plus bevacizumab (RR 1.17, 95% CI 1.00 to 1.36) with no important statistical heterogeneity (I2 = 0%) (Figure 5). We applied this RR to participants with mRCC and low‐risk score (favourable) with one‐year mortality rates of 150/1000 participants, moderate‐risk score of 280/1000 participants and high‐risk score of 550/1000 participants with standard therapies. These numbers may be slightly increased by 25 deaths/1000 (from 0 to 54 more) in low‐risk participants, 48 deaths/1000 (from 0 to 101 more) in moderate‐risk participants and 93 deaths/1000 (from 0 more to 198 more) in high‐risk participants if they are treated with IFN‐α as monotherapy (Table 3). Small differences in median OS of 21.3 months with IFN‐α alone versus 23.3 months with IFN‐α plus bevacizumab (Escudier 2007) and 17.4 months with IFN‐α alone versus 18.3 months with IFN‐α plus bevacizumab (Rini 2010) were consistent with these results. Furthermore, their efficacy might depend on prognostic scores with highest benefit in participants with intermediate‐risk score and no prior nephrectomy (Table 14).
Figure 5.
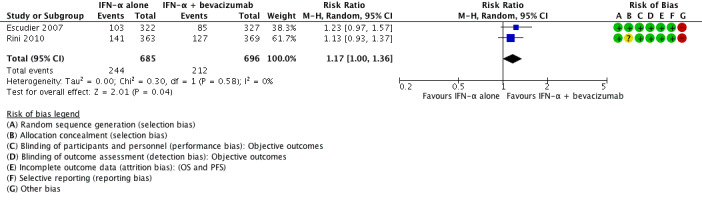
Forest plot of comparison: 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, outcome: 3.1 1‐year mortality.
The primary endpoint for both studies was OS, which was not different in either study alone, but the pooled HR of 1.13 (95% CI 1.00 to 1.28) demonstrated a disadvantage of IFN‐α alone compared to IFN‐α plus bevacizumab with respect to survival with no important heterogeneity (I2 = 0%) (Analysis 3.2).
Analysis 3.2.
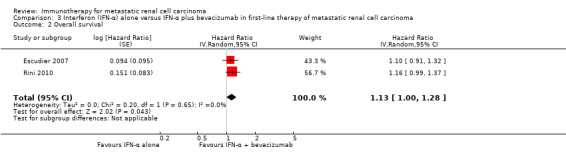
Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.
3.2. Quality of life
Neither study evaluated QoL.
3.3. Adverse events (grade 3 or greater)
AEs (grade 3 or greater) occurred in both studies less frequently in participants treated with IFN‐α alone compared to IFN‐α plus bevacizumab (RR 0.77, 95% CI 0.71 to 0.84) with no important heterogeneity (I2 = 0%) (Analysis 3.3). We applied this RR to participants with mRCC with a mean risk of AEs (grade 3 or greater) in 705/1000 participants in the IFN‐α alone group. This number is probably decreased by 162/1000 participants (from 113 to 205 fewer) with AEs grade 3 or 4 if they are treated with IFN‐α plus bevacizumab (Table 3).
Analysis 3.3.
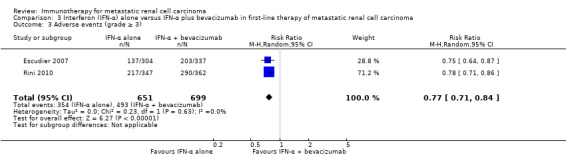
Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).
The number of participants with AEs grade 3 or greater was higher in Rini 2010 where 63% of participants receiving IFN‐α lone experienced grade 3 toxicity compared to 80% of participants receiving IFN‐α plus bevacizumab.
However, Escudier 2007 reported 203 AEs grade 3 or greater in participants who received one or more doses of bevacizumab compared to 137 AEs in participants who did not receive bevacizumab. Therefore, a maximum 137/304 (45%) participants with IFN‐α alone and 203/337 (60%) participants with IFN‐α plus bevacizumab had an AE with grade 3 or greater. In both studies, participants treated with IFN‐α plus bevacizumab reported AEs grade 3 or greater in established IFN‐α‐related toxicities (e.g. fatigue and neutropenia) and an increase in bevacizumab‐related toxicities (most common were bleeding/haemorrhage, hypertension, proteinuria and thromboembolic events).
3.4. Progression‐free survival
The pooled HR of 1.53 (95% CI 1.36 to 1.73) demonstrated a clear disadvantage for participants of IFN‐α monotherapy compared to IFN‐α plus bevacizumab with respect to PFS with no important heterogeneity (I2 = 0%) (Analysis 3.4).
Analysis 3.4.
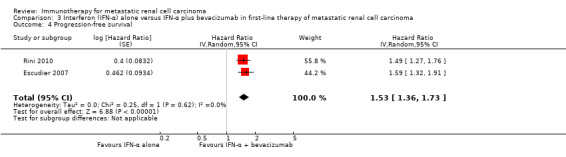
Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.
3.5. Tumour remission
In total, 85/639 (13%) participants with IFN‐α alone compared to 190/566 (34%) participants with IFN‐α plus bevacizumab showed a response. Tumour remission showed a high disadvantage for participants treated with IFN‐α monotherapy compared to IFN‐α plus bevacizumab (RR 0.39, 95% CI 0.31 to 0.50) with no important heterogeneity (I2 = 0%) (Analysis 3.5).
Analysis 3.5.
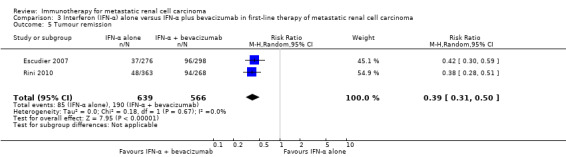
Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.
Comparison 4. Interferon‐α plus bevacizumab versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma
One study compared the efficacy and safety of the combination standard first‐line therapy with IFN‐α plus bevacizumab with standard targeted therapy (sunitinib) (Negrier 2011). One‐year mortality and PFS were reported based on 83 participants and AEs and tumour remission based on 82 participants who received treatment.
4.1. Overall survival
In total, 4/41 participants (10%) treated with IFN‐α plus bevacizumab died within one year after randomization compared to 11/42 (26%) participants with standard targeted therapy. This result demonstrated similar mortality with both treatment options (RR 0.37, 95% CI 0.13 to 1.08; Analysis 4.1).
Analysis 4.1.
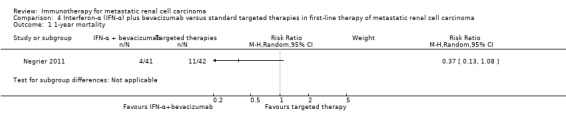
Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
We applied this RR to participants with mRCC with low‐risk score (favourable) with one‐year mortality rates of 150/1000 participants, moderate‐risk score of 280/1000 participants and high‐risk scores of 550/1000 participants with standard targeted therapy. The numbers of deaths may be similar with IFN‐α plus bevacizumab and standard targeted therapy (Table 4).
4.2. Quality of life
We found no data.
4.3. Adverse events (grade 3 or greater)
There was uncertainty if AEs (grade 3 or greater) occur more frequently in participants treated with IFN‐α plus bevacizumab compared to participants with targeted therapy (70% with IFN‐α plus bevacizumab versus 60% with targeted therapy; RR 1.18, 95% CI 0.85 to 1.62; Analysis 4.2). We applied this RR to participants with mRCC with a mean risk that AEs (grade 3 or greater) occur in 595/1000 participants in the control group. The treatment options may lead to similar numbers of participants with AEs (grade 3 or greater) (Table 4).
Analysis 4.2.

Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Adverse events (grade ≥ 3).
4.4. Progression‐free survival
There was uncertainty surrounding the effects of IFN‐α plus bevacizumab compared to targeted therapy on PFS (HR 0.66, 95% CI 0.38 to 1.14; Analysis 4.3). This is the only head‐to‐head comparison for both established first‐line therapies.
Analysis 4.3.
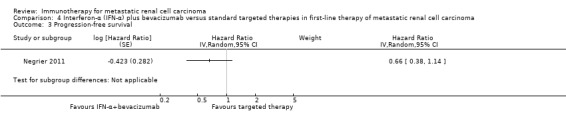
Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Progression‐free survival.
4.5. Tumour remission
There was uncertainty surrounding the effects of IFN‐α and bevacizumab compared to standard targeted therapy on tumour remission (RR 1.49, 95% CI 0.82 to 2.71; Analysis 4.4). In total, 17/40 (43%) participants with IFN‐α plus bevacizumab compared to 12/42 (29%) participants with targeted therapy showed remission. Negrier 2011 reported a very high response rate of 43% for the IFN‐α plus bevacizumab group compared to response rates of 29% with the standard targeted therapy group.
Analysis 4.4.
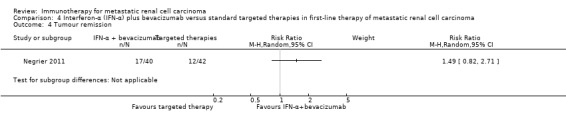
Comparison 4 Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Tumour remission.
Comparison 5. Vaccine treatment versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
Two studies compared the efficacy and safety of vaccine treatment (MVA‐5T4 or IMA901) with standard targeted therapies based on 1071 participants. A total of 365 participants received treatment with a modified vaccinia Ankara encoding the tumour antigen 5T4 (MVA‐5T4) in combination with sunitinib, IL‐2 or IFN‐α. Participants in the control groups received sunitinib, IL‐2 or IFN‐α alone (Amato 2010). Another 204 participants received treatment with IMA901 in addition to standard therapy and 135 participants with a targeted monotherapy with sunitinib (Rini 2015).
OS and tumour remission were reported in both studies on 1071 participants and AEs were reported based on all 1065 treated participants. PFS was evaluated in one included study on 339 participants (Rini 2015).
5.1. Overall survival
A total of 165/546 (30.2%) participants treated with the vaccine in addition to standard therapy died within one year after randomization compared to 140/488 (28.7%) participants with standard therapy. This result demonstrated similar mortality (RR 1.10, 95% CI 0.91 to 1.32) with no important heterogeneity (I2 = 0%) (Figure 6). We applied this RR to participants with mRCC and low‐risk score (favourable) with one‐year mortality rates of 150/1000 participants, moderate‐risk score of 280/1000 participants and high‐risk scores of 550/1000 participants with standard targeted therapies. The numbers of deaths may be similar between treatments (Table 5).
Figure 6.
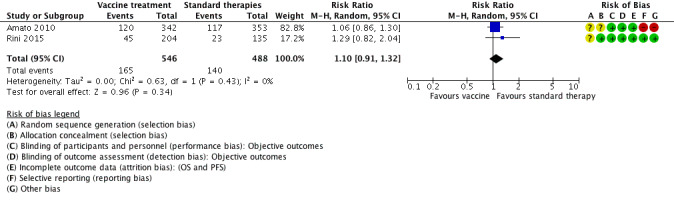
Forest plot of comparison: 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, outcome: 5.1 1‐year mortality.
Different inclusion criteria on performance status and prognosis in both included studies resulted in different median OS. Amato 2010 reported median OS of 19.2 with vaccine versus 20.1 months with standard targeted therapy and one‐year mortality rates of 35% with vaccine versus 33% with standard targeted therapy, whereas Rini 2015 reported median OS of more than 33 months for vaccine versus 'not reached' for standard targeted therapy and one‐year mortality rates of 17% with vaccine and 22% with standard targeted therapy. Both studies reported a higher benefit of vaccine treatment for participants with favourable risk (Table 14).
These results corresponded to similar OS (HR of 1.14, 95% CI 0.96 to 1.37) with no important heterogeneity (I2 = 22%) (Analysis 5.2).
Analysis 5.2.
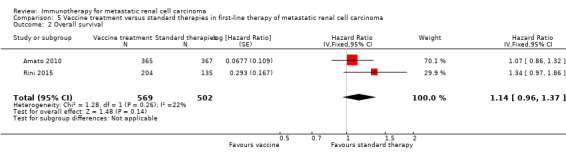
Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.
5.2. Quality of life
Neither study evaluated QoL.
5.3. Adverse events (grade 3 or greater)
There was uncertainty if AEs (grade 3 or greater) were increased in participants treated with vaccine compared to standard targeted therapy (RR 1.16, 95% CI 0.97 to 1.39) with no important heterogeneity (I2 = 0%) (Analysis 5.3). We applied this RR to participants with mRCC with a risk of AEs (grade 3 or greater) in 241/1000 participants in the control group with standard targeted therapies. The treatment options may lead to a similar number of AEs (Table 5).
Analysis 5.3.
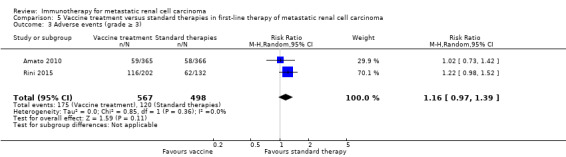
Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 3 Adverse events (grade ≥ 3).
Amato 2010 reported treatment‐emergent AEs (grade 3 or greater) in 59/365 (16%) participants with SOC/MVA‐5T4 compared to 58/367 (16%) participants with SOC/placebo. MVA‐5T4 was well tolerated with each regimen with no significant difference in the incidence of AEs. There were no clinically relevant MVA‐5T4‐specific AEs observed. Participants with an intermediate prognosis experienced thrombocytosis more frequently compared with participants of favourable prognosis (39% with intermediate versus 8% with favourable). Rini 2015 reported AEs grade 3 or greater in 116/220 (53%) participants who were treated with IMA901 compared to 62/132 (47%) participants with no IMA901. IMA901 was generally well tolerated with no differences between treatment groups and transient injection‐site reactions related to IMA901.
5.4. Progression‐free survival
The HR of 1.05 (95% CI 0.87 to 1.27) demonstrated no influence of vaccines (Analysis 5.4).
Analysis 5.4.
Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 4 Progression‐free survival.
5.5. Tumour remission
Tumour remission showed no benefit from vaccine treatment (RR 0.93, 95% CI 0.76 to 1.13) with no important heterogeneity (I2 = 0%) of treatment effects, but with high differences in tumour remission of around 13% in Amato 2010 and 45% in Rini 2015 but no differences depending on vaccine treatment in both studies (Analysis 5.5).
Analysis 5.5.
Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 5 Tumour remission.
Comparison 6. Targeted immunotherapies versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma
One study compared efficacy and safety of targeted immunotherapy with nivolumab with standard targeted therapy with everolimus based on 821 participants (Motzer 2015a). Of them, 410 were randomized to treatment with the PD‐1 inhibitor nivolumab and 411 were randomized to treatment with everolimus. OS and PFS were evaluated for all randomized participants, safety was evaluated for all treated 803 participants and tumour remission in 750 participants.
6.1. Overall survival
A total of 98/410 (24%) participants randomly assigned to receive nivolumab and 140/411 (34%) participants randomly assigned to receive everolimus died within one year of randomization (RR 0.70, 95% CI 0.56 to 0.87; Analysis 6.1). We applied this RR to participants with mRCC with a mean risk to die within one year after the start of second‐line therapy in the control group, in which 341/1000 participants died. This number is probably reduced by 102 deaths out of 1000 participants (from 44 to 150 fewer) if they were treated with targeted immunotherapy (Table 6). The median OS were 25.0 months with nivolumab and 19.6 months with everolimus after a minimum follow‐up of 14 months.
Analysis 6.1.
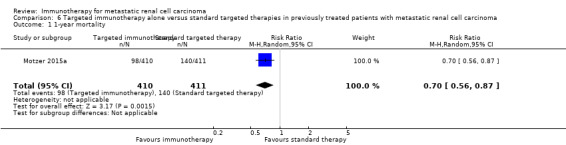
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
This benefit in OS was observed across prespecified subgroups with slightly better results in participants with poor prognostic score and no differences depending on the number of previous antiangiogenic regimens (Table 14). A posthoc subgroup analysis demonstrated the prognostic influence of PD‐L1 expression. Participants with PD‐L1 expression of 1% or greater demonstrated a median OS of 21.8 months for nivolumab versus 18.8 months for everolimus. In participants with PD‐L1 expression of 1% or less, median OS was 27.4 months for nivolumab versus 21.2 months for everolimus.
OS showed the superiority of nivolumab over everolimus (HR 0.73, 95% CI 0.60 to 0.89; Analysis 6.2).
Analysis 6.2.
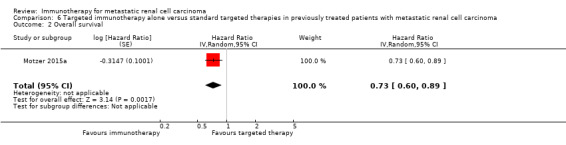
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 2 Overall survival.
6.2. Quality of life
Mean health‐related QoL scores (FKSI‐DRS and EQ‐5D) were comparable at baseline. Clinically relevant differences in improvements FKSI‐DRS by at least 2 FKSI‐DRS points were more frequently observed in participants treated with nivolumab. They were reported in 200/361 (55%) participants with nivolumab compared to 126/343 (37%) participants with everolimus (RR 1.51, 95% CI 1.28 to 1.78). Similar proportions were reported in clinically relevant improvements in EQ‐VAS by at least 7 EQ‐VAS points. A total of 192/360 (53%) participants treated with nivolumab and 134/343 (39%) participants treated with everolimus showed relevant QoL improvements (RR 1.37, 95% CI 1.16 to 1.61), but there were no important or significant differences reported for EQ utility score (Table 15; Table 6).
We applied these RRs to participants with mRCC with a mean chance of a clinically relevant improvement in FKSI‐DRS in 367/1000 participants who received standard targeted therapies. This number is probably increased by 187/1000 participants (from 103 more to 287 more) for FKSI‐DRS if they are treated with IFN‐α monotherapy.
Furthermore, there was clinically relevant improvement in EQ‐5D VAS in 391/1000 participants treated with standard targeted therapies. This number is probably increased by 145/1000 participants (from 63 more to 238 more) for EQ‐5D VAS if they are treated with IFN‐α monotherapy.
6.3. Adverse events (grade 3 or greater)
AEs (grade 3 or greater) were less frequent for nivolumab and occurred in 76/406 (19%) participants compared to 145/397 (37%) participants treated with everolimus (RR 0.51, 95% CI 0.40 to 0.65; Analysis 6.4).
Analysis 6.4.
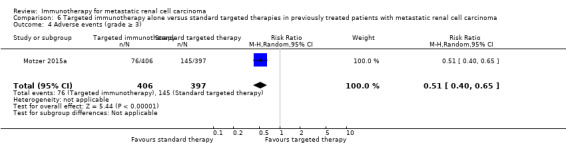
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 4 Adverse events (grade ≥ 3).
We applied this RR to participants with mRCC in second‐line treatment in the control group with a risk of AEs (grade 3 or greater) in 365/1000 participants. This number is probably decreased by 179/1000 participants who might experience AEs grade 3 or 4 (from 128 to 219 fewer) if they are treated with targeted immunotherapy (Table 6).
The most common grade 3 or 4 AEs were anaemia (2% with nivolumab versus 8% with everolimus), fatigue (2% with nivolumab versus 3% with everolimus), hyperglycaemia (1% with nivolumab versus 4% with everolimus), hypertriglyceridaemia (0% with nivolumab versus 5% with everolimus) and stomatitis (0% with nivolumab versus 4% with everolimus). Clinical manifestations of a potentially fatal pneumonitis described in 4% (nivolumab) versus 15% (everolimus).
6.4. Progression‐free survival
The HR of 0.88 (95% CI 0.75 to 1.03) demonstrated a slight benefit for participants treated with nivolumab compared to everolimus with respect to PFS (Analysis 6.5).
Analysis 6.5.
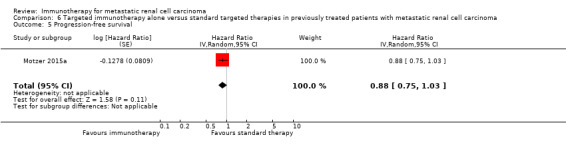
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 5 Progression‐free survival.
6.5. Tumour remission
Partial or complete response occurred in 103/387 (25%) participants treated with nivolumab and 22/363 (5%) participants treated with everolimus. Tumour remission was higher with nivolumab compared to everolimus (RR 4.39, 95% CI 2.84 to 6.80; Analysis 6.6).
Analysis 6.6.
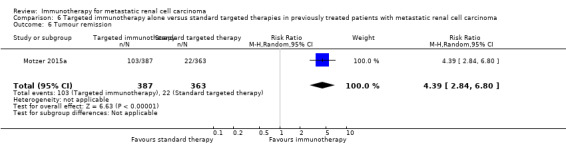
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 6 Tumour remission.
Discussion
Summary of main results
Cytokines in the era of targeted therapies
Several pivotal phase III trials have been reported since the previous version of this review (Coppin 2007), demonstrating superiority in first‐line therapy for targeted agents as sunitinib, bevacizumab plus IFN‐α or temsirolimus over IFN‐α.
Summarizing the evidence, IFN‐α monotherapy is probably inferior to standard targeted therapies with temsirolimus or sunitinib with respect to OS (HR 1.28, 95% CI 1.11 to 1.49) with a higher one‐year mortality (RR 1.30, 95% CI 1.13 to 1.51). Patients with IFN‐α monotherapy may have similar QoL and may experience slightly more AEs grade 3 or greater (RR 1.17, 95% CI 1.03 to 1.32). These results support the recommendations in the treatment guidelines of sunitinib as the first‐line treatment option for patients regardless of prognostic factors and temsirolimus in poor‐risk mRCC patients (Escudier 2014; German Guideline Programme in Oncology 2015; Ljungberg 2015).
The combined treatment of temsirolimus plus IFN‐α may result in greater toxicity with a higher frequency of patients with AEs grade 3 or 4 (RR 1.30, 95% CI 1.17 to 1.45), but there is probably no difference on the effects on OS when compared with temsirolimus alone (HR 1.20, 95% CI 0.97 to 1.49) or IFN monotherapy (HR 0.96, 95% CI 0.76 to 1.20) (Hudes 2007), perhaps, at least partially, due to lower administered doses of temsirolimus and IFN‐α.
Treatment with IFN‐α alone may slightly increase mortality in comparison to IFN‐α plus intravenous bevacizumab, a humanized monoclonal antibody directed against VEGF in treatment‐naive, low‐ and intermediate‐risk patients (Escudier 2007; Rini 2010). This effect is probably accompanied by a lower incidence of AEs grade 3 or greater.
All trials that evaluated sunitinib and IFN‐α plus bevacizumab enrolled a small proportion of participants (less than 10%) with poor risk classification. In the case of sunitinib, subgroup analysis of the pivotal phase III trial demonstrated efficacy and feasibility of treatment in people with high‐risk disease and thus, sunitinib remains a therapeutic option for this group of patients. Treatment with IFN‐α plus bevacizumab or targeted monotherapy sunitinib may lead to similar one‐year mortality (RR 0.37, 95% CI 0.13 to 1.08) and incidence of AEs grade 3 or greater (Negrier 2011).
New immunotherapy approaches ‐ vaccines
Amato 2010 conducted a large placebo‐controlled study of the addition of MVA‐5T4 vaccine to three different standard‐of‐care first‐line options (rd‐IL‐2, or IFN‐α, or sunitinib) in use at the participating centres in the US and Europe. Despite the induction of a strong immune response to MVA‐5T4 in some participants, survival outcomes were comparable to standard of care alone (HR 1.07, 95% CI 0.86 to 1.32).
Results of a phase III trial with IMA901 showed no beneficial effect on OS when added to sunitinib in first‐line mRCC patients (HR 1.34, 95% CI 0.97 to 1.86) (IMPRINT study; Rini 2015). There was no clear association between T‐cell responses and clinical outcomes.
Summarizing these effects, the outcomes may be similar in mRCC. The uncertainty includes the effect of vaccine treatment with MVA‐5T4 or IMA901 in addition to sunitinib compared to standard therapies on overall mortality (HR 1.14, 95% CI 0.96 to 1.37), one‐year mortality (RR 1.10, 95% CI 0.91 to 1.32) and AEs grade 3 or greater (RR 1.16, 95% CI 0.97 to 1.39).
New immunotherapy ‐ targeted immunotherapy (immune checkpoint inhibitors)
Immune checkpoint inhibitors exploit new understanding of the molecular mechanisms of interaction between tumour cells and host immune cells such as antigen‐presenting cells and immune effector cells (Postow 2015). Nivolumab is the first of these therapies to be validated for mRCC, specifically the clear‐cell histological subtype, and the first therapy to demonstrate improved OS (HR 0.73, 95% CI 0.60 to 0.89) as part of the second‐line treatment for patients in this group (Motzer 2015a). Poor‐ and intermediate‐risk patients treated with nivolumab experienced the greatest OS benefit compared with patients of favourable risk. Nivolumab reduced mortality by 27% (95% CI ‐43% to ‐7%) compared to everolimus, a previously validated second‐line standard of care. The incidence of AEs grade 3 or 4 was lower with nivolumab (RR 0.51, 95% CI 0.40 to 0.65), but the types of AEs observed were also different. In particular, nivolumab may result in severe autoimmune illness such as pneumonitis or colitis that can be rapidly progressive and potentially fatal if not quickly recognized and treated (Weber 2015). No treatment‐related deaths occurred in the large pivotal study of 821 participants (Motzer 2015a). Patients treated with nivolumab experienced an improvement in their health‐related QoL in this unblinded study and had significantly lower symptom burden throughout treatment compared to patients receiving everolimus.
Overall completeness and applicability of evidence
We identified no studies comparing current standard therapies against adoptive T‐cell therapies (comparison 4) during the literature search. Furthermore, we found no studies comparing other immunotherapies (comparison 6) than defined in comparisons 1 to 5 against standard therapies. Given that we identified no studies devoted to or stratified for participants with non‐clear‐cell RCC, our conclusions can only be applied to clear‐cell RCC. There are multiple uncommon subtypes of non‐clear‐cell RCC, and only international efforts can identify the most efficient and preferred therapy for these patients.
Quality of the evidence
We identified eight eligible studies with 4732 participants. We categorized these included studies into six comparisons against current evidence‐based standard therapy with either targeted therapies or IFN‐α plus bevacizumab: four as first‐line and one as second‐line therapy of mRCC. Effect estimates for our primary outcomes (OS, QoL, AEs grade 3 or 4) in these six comparisons were based on the results obtained from one to three RCTs. All available RCTs were of moderate size (between 171 and 821 participants). Seven out of eight studies were funded by the drug manufacturers and one by a government agency (Rini 2010). This may raise the possibility of publication bias, but the number of studies was insufficient to meet rigorous criteria to create funnel plots.
Quality of evidence from RCTs is generally considered to be high but was downgraded in cases of relevant risk of bias, inconsistency, imprecision or publication bias. We downgraded quality of evidence for the following outcomes for study limitations (risk of bias) as recommended in Guyatt 2011b:
OS: downgraded when patients were allowed to cross‐over to targeted therapy or when a relevant number of participants received second‐line systemic anticancer therapies subsequent to progression of disease;
QoL: downgraded because of no blinding of participants, physicians or blinded assessment of this subjective outcome;
adverse events, grade 3 or 4: downgraded because of no double blinding or blinded assessment of this subjective outcome if participant‐reported outcomes were included.
We generally downgraded the quality of evidence for all outcomes for imprecision if the CI included a possible benefit from both therapeutic approaches (Guyatt 2011d). We strongly suspected reporting bias and downgraded quality of evidence when assessment of outcomes were planned in the protocol but not reported (Guyatt 2011c).
Comparison 1: IFN‐α alone versus standard targeted therapy with temsirolimus or sunitinib in first‐line therapy of mRCC: we judged the quality of evidence for one‐year mortality as moderate, downgrading for study limitations because participants with progressive disease and randomized to IFN‐α monotherapy in Motzer 2007 were allowed to cross‐over to targeted therapy with sunitinib. We judged the quality of evidence on QoL as low, downgrading for imprecision and study limitations with a high risk of bias due to performance and detection bias given lack of blinding of participants, physicians and outcome assessors. We judged the quality of evidence on AEs as low and downgraded the evidence for imprecision and lack of blinding of participants, physicians and outcome assessors of AEs in both studies (Table 1).
Comparison 2: IFN‐α combined with temsirolimus versus standard targeted therapies with temsirolimus in first‐line therapy of mRCC: the evidence for one‐year mortality was downgraded to moderate by one point for imprecision. QoL could not be evaluated due to insufficient data. We judged the evidence on AEs to be low quality and downgraded for imprecision and lack of blinding in both studies (Table 2).
Comparison 3: IFN‐α alone versus IFN‐α plus bevacizumab in first‐line therapy of mRCC: we judged the quality of evidence for one‐year mortality to be low and downgraded for risk of bias and imprecision. We found study limitations due to a high risk of selection bias and performance bias due to substantial cross‐over due to the use of second‐line systemic anticancer therapies subsequent to progression in more than 50% of participants in Rini 2010 and approximately 20% in Escudier 2007. QoL could not be evaluated due to insufficient data. We judged the evidence on AEs to be of moderate quality and downgraded due to high risk of performance and detection bias on subjective outcomes in Rini 2010 with no blinding of participants, physicians and outcome assessors (Table 3).
Comparison 4: IFN‐α plus bevacizumab versus targeted therapies in first‐line therapy of mRCC: we judged the quality of the evidence to be low for one‐year mortality, downgrading for non‐reporting of information on long‐term OS and imprecision. QoL could not be evaluated due to insufficient data. We judged the evidence on AEs to be of low quality and downgraded for lack of blinding and imprecision (Table 4).
Comparison 5: vaccine treatment versus standard therapies in first‐line therapy of mRCC: we downgraded the quality of evidence for one‐year mortality to low and downgraded by one point for indirectness due to the use of present‐day non‐standard therapies (low‐dose IL‐2, IFN‐α) in 75% of participants in both treatment groups (Amato 2010), and by additional point for imprecision. We decided not to downgrade for risk of bias due to the use of additional second‐line therapies in one‐third of participants in this study. QoL could not be evaluated due to insufficient data. We judged the evidence on AEs to be of low quality and downgraded it by one point for imprecision and one point for risk of bias due to the absence of double blinding or blinded assessment of AEs (Table 5), and decided not to downgrade due to the use of present‐day non‐standard therapies in Amato 2010.
Comparison 6: targeted immunotherapies versus standard targeted therapy in previously treated patients with mRCC: we judged the evidence to be of moderate quality for one‐year mortality and downgraded by one point for risk of bias due to permitted cross‐over during follow‐up for OS. The evidence for AEs and QoL was of moderate quality and was downgraded for lack of blinding (Table 6).
Potential biases in the review process
We have searched all available English‐language databases for published studies of immunotherapy but recognize there is a potential for publication bias that would have excluded any unpublished studies or studies published in unlisted journals. Missed studies can be expected to be negative, and consequently the magnitude of observed effects might be overestimated. In addition, unlike previous editions of this review, we have not attempted to systematically conduct a manual search for meeting abstracts but instead, searched in the Web of Science Core Collection for meeting abstracts.
The number of studies per comparisons was insufficient to generate funnel plots and the risk of reporting bias was based on comparisons between protocol and reported results thus, might be underestimated. We included one study where participants were assigned by their physician to one standard‐of‐care regimens consistent with local practice (low‐dose IL‐2, IFN‐α or sunitinib) (Amato 2010). Only one of these arms with 185/732 randomized participants was consistent with standard therapies in this review.
Agreements and disagreements with other studies or reviews
Since the mid‐2000s, we have experienced a transition from non‐specific therapy with cytokines to rather specific immunotherapy regimens, which directly target the RCC cancer cell and the tumour microenvironment. To the best of our knowledge this is the first systematic review that focuses on the potential of immunotherapy in mRCC following the dismissal of non‐specific cytokines as potential therapeutic agents by current guidelines.
Reviews have reflected on the role of targeted therapies in the current management of mRCC after their approval at the beginning of 2005. Their conclusions are in line with our results. In general, these targeted agents have replaced non‐specific immunotherapy in the majority of patients (Abe 2013; Albiges 2015; Coppin 2008; Dutcher 2013; Jonasch 2014; Takyar 2016; Xu 2015). The combination of bevacizumab plus IFN‐α is the only remaining therapy that involves a non‐specific cytokine and is still currently recommended as first‐line treatment for people with RCC with good or intermediate prognosis, despite this, most experts do not routinely use this regimen (Rothermundt 2015).
Other immunotherapy reviews and overview articles have mainly focused on the future potential of immunotherapy (Bedke 2015; Grünwald 2015; Yang 2016), and highlighted possible further investigations to enhance the effectiveness of new immunotherapeutic agents exploring the use of combination therapy with PD‐1 or PD‐L1 blockade (Lee 2016; Massari 2015), or combination of vaccines with targeted therapies (Combe 2015; Figlin 2015), as key areas of research. These research topics are also reflected in the many ongoing studies that our review identified.
Data from Weber 2015 and Ciccarese 2016 focused on the toxicity profile of novel immunotherapeutic agents, including nivolumab and are consistent with our present findings on the safety profile of nivolumab. In general, AEs of immune checkpoint inhibitors are mostly mild to moderate in severity with fewer AEs compared with currently used targeted therapies.
Authors' conclusions
The use of cytokines in the era of targeted therapies
Interferon (IFN)‐α is inferior to most targeted therapies with respect to progression‐free survival (PFS) and overall survival (OS) and was, in addition, poorly tolerated in comparison to the tested agents. The toxicity of the combination is greater than that of IFN‐α alone with most participants able to tolerate it.
New immunotherapies
Second‐line nivolumab is currently the only new agent demonstrated to improve OS in metastatic renal cell carcinoma (mRCC). In addition, nivolumab may have a role as third‐line therapy, since 28% of participants had received two prior antiangiogenic agents and subset analyses could neither confirm nor exclude a survival benefit to this group (Motzer 2015a). At the time of interim analysis, no participant or tumour factors such as risk score or absence of programmed death (PD)‐1 staining of tumour cells have been able to identify people unable to benefit from nivolumab.
It seems reasonable to conclude that we are entering a third era of systemic therapy for mRCC, that of targeted immune therapy with changing management guidelines for mRCC (e.g. Powles 2016).
With rare exceptions, mRCC remains incurable with limited survival. Therefore, the main implication of this review was the importance of continuing the search for treatment that can reliably induce durable remissions of this disease. Immunotherapy continues to be the modality that holds hope of achieving this objective, since some therapies are specific to the individual tumour's neoantigen profile and the development of immune memory cells have the potential to maintain long‐lasting tumour suppression even without cure (Desrichard 2016; McDermott 2015b). Furthermore, there remains many immunotherapy avenues to be explored (Ghasemzadeh 2016). In addition, it is mandatory to identify people who will benefit from the respective (immuno)therapy. Therefore, biomarkers are urgently required for treatment decision and to monitor therapy outcome, which also allows the early detection of therapy resistances. High‐throughput analysis should be further used for the identification of neoantigens, which represent patient‐specific, novel therapeutic targets for T‐cell‐based immunotherapies. This further leads to the development of personalized medicine.
OS has been improved by current best first‐ and second‐line options for mRCC and is, therefore, the required outcome for future phase III studies, compared to the appropriate control arm. For third‐line therapy, improved PFS remains a reasonable objective. With immune checkpoint inhibitors, remission seems even less reliable as an indicator of benefit than for other immunotherapies since response is not necessary for prolongation of survival. Tumour decrease with cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) inhibitors in particular (ipilimumab, tremelimumab) may be delayed many months, and may be preceded by initial pseudo‐progression possibly because the tumours are swelled by infiltrating inflammatory cells; a revised set of clinical/radiological criteria to evaluate response has been recommended and should be introduced for these agents (Postow 2015). Comparisons of adverse effects (AEs) and preferably blinded quality of life (QoL) assessment are especially required for non‐inferiority trials of adequate size.
PD‐L1 expression was not found to significantly impact the efficacy of nivolumab, so that reliable biomarkers of response to these agents are needed to identify people who will benefit most (Motzer 2016). Next‐generation DNA sequencing may provide a way forward and has already shown that higher overall mutational burden may predict responsiveness to nivolumab and may identify a potential genetic signature for benefit of CTLA‐4 inhibition (Le 2015). Genetic studies specific to mRCC are required. The efficacy and safety of PD‐1/PD‐L1‐targeting antibodies in specific RCC subpopulations, such as older people, people with poor risk status or non‐clear‐cell histologies should be further investigated. The question, if patients continue to have tumour control after cessation of blockade of the pathway between PD‐1 and PD‐L1, reflecting the development of an effective immunological memory to control tumour growth, needs to be addressed.
The most promising immunotherapeutic approaches hinge on individualization of the immune attack. This can be achieved by use of autologous reagents such as individually prepared vaccines or cellular reinfusion, but the logic of harnessing the individual patient's immune system is very appealing and underlies the blockade of immune checkpoints and other immune receptor‐ligand pathways including B7‐H3 and B7‐H4. Several PD‐1/PD‐L1 and CTLA‐4 inhibitors are reaching the clinic or are in development. Durable remissions have been seen in melanoma treated with the CTLA‐4 inhibitor ipilimumab with or without nivolumab (Postow 2015), changing the standard of care for that condition. In mRCC, the results of the ongoing phase III studies with more than 2000 randomized participants are eagerly awaited (Hammers 2015; NCT02420821; NCT02684006).
Vaccines continue as work in progress. The IMPRINT phase III trial of sunitinib with or without IMA901 vaccine has had preliminary reporting (Rini 2015). The ongoing ADAPT (Autologous Dendritic Cell Immunotherapy (AGS‐003) Plus Standard Treatment of Advanced Renal Cell Carcinoma) phase III trial is comparing standard vascular endothelial growth factor receptor (VEGFR) inhibitor therapy with or without autologous AGS‐003 vaccine (Figlin 2014; Figlin 2015).
Based on previous randomized studies with IFN‐α, upfront nephrectomy is advised in appropriately selected patients (Flanigan 2001; Mickisch 2001). The most effective setting of new immunotherapeutic and targeted therapies in locally advanced or metastatic RCC patients who undergo cytoreductive nephrectomy are currently under investigation and results should be discussed in the update of this review (NCT00930033; NCT02210117; NCT02432846; Zibelman 2015).
Clarification of the role of immunotherapy for non‐clear‐cell renal cancers is also urgently needed.
Acknowledgements
We appreciate the excellent support from the Cochrane Urology Group, its trials search co‐ordinator team and Chris Coppin.
Appendices
Appendix 1. Cochrane Library (Wiley.com) search strategy
1. MeSH descriptor: [Carcinoma, Renal Cell] explode all trees and with qualifier(s): [Drug therapy ‐ DT, Immunology ‐ IM, Pathology ‐ PA, Prevention & control ‐ PC, Therapy ‐ TH]
2. MeSH descriptor: [von Hippel‐Lindau Disease] explode all trees and with qualifier(s): [Drug therapy ‐ DT, Immunology ‐ IM, Pathology ‐ PA, Prevention & control ‐ PC, Therapy ‐ TH]
3. advance* NEAR (renal OR kidney OR nephron*) NEAR (cancer* OR neoplasm* OR carcinoma* OR tumour* OR tumour*)
4. advance* NEAR (renal or kidney or nephron*) NEXT cell NEAR cancer*
5. metasta* NEAR (renal or kidney or nephron*) NEAR (cancer* or neoplasms* or carcinoma* or tumour* or tumour*)
6. metasta* NEAR (renal or kidney or nephron*) NEXT cell NEAR cancer*
7. local* NEAR advance* NEAR (renal or kidney or nephro*) NEAR (cancer* or neoplasms* or carcinoma* or tumour* or tumour*)
8. local* NEAR advance* NEAR (renal or kidney or nephro*) NEXT cell NEAR cancer*
9. "clear cell type" NEXT/3 carcinom*
10. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
11. MeSH descriptor: [Immunotherapy, Active] explode all trees
12. MeSH descriptor: [Immunization, Passive] explode all trees
13. MeSH descriptor: [Adoptive Transfer] explode all trees
14. MeSH descriptor: [Cancer Vaccines] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
15. MeSH descriptor: [Antigens, Neoplasm] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
16. MeSH descriptor: [Antibodies, Monoclonal] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
17. MeSH descriptor: [Hematopoietic Stem Cell Transplantation] explode all trees
18. MeSH descriptor: [Immunomodulation] explode all trees and with qualifier(s): [Drug effects ‐ DE]
19. MeSH descriptor: [Immunologic Factors] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
20. MeSH descriptor: [Immunosuppressive Agents] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
21. MeSH descriptor: [Superantigens] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
22. MeSH descriptor: [Antigens, Neoplasm] explode all trees and with qualifier(s): [Adverse effects ‐ AE, Therapeutic use ‐ TU]
23. vaccin*:ti,ab
24. (immune?ation OR immunotherap* OR immunosuppress* OR immunotox*):ti,ab
25. tum*r NEXT antigen*:ti,ab
26. adjuvants:ti,ab OR adoptive T cell therap*:ti,ab
27. (car or "containment and autonomic regulation") NEXT therapy
28. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29. MeSH descriptor: [Interferon‐alpha] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
30. MeSH descriptor: [Interleukin‐2] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
31. MeSH descriptor: [Interleukin‐7] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
32. MeSH descriptor: [Interleukin‐12] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
33. MeSH descriptor: [Interleukin‐15] explode all trees and with qualifier(s): [Therapeutic use ‐ TU]
34. MeSH descriptor: [Dendritic Cells] explode all trees
35. MeSH descriptor: [BCG Vaccine] explode all trees
36. MeSH descriptor: [Cytokine‐Induced Killer Cells] explode all trees
37. MeSH descriptor: [Recombinant Proteins] explode all trees
38. vaccin* NEXT/2 (BCG or "Bacillus Calmette Guerin" or Calmette*):ti,ab
39. (DNA or mRNA or cellular or peptid* or protein or genetic) NEXT/2 vaccin*:ti,ab
40. Dendritic cell NEXT/2 bas* NEXT/3 vaccin*:ti,ab
41. immun* NEXT/2 checkpoint:ti,ab
42. Cytotoxic T lymphocyt* or CTL:ti,ab
43. inhibit* NEXT/2 (receptor* or antibod*):ti,ab
44. (humanized or agonistic) NEXT antibod*.ab
45. Anti NEXT/2 (CTLA or "PD‐L1" or "PD‐1" or "sLAG‐3"):ti,ab
46. ("cytotoxic T lymphocytes antigen" or "soluble human LAG‐3 protein"):ti,ab
47. (ipilimumab or tremelimumab or nivolumab or naptumomab or estafenox):ti,ab
48. (IMP321 or IMA901 or AGS‐003 or CMDSC or ABR‐217620 or ANYARA):ti,ab
49. activating receptor*:ti,ab
50. ("denileukin difitox" or reniale or "recombinant human interleukin" or lymphocine):ti,ab
51. "autologous tumour lysate" NEXT/2 "loaded dendritic cells":ti,ab
52. HSPPC NEXT/3 vaccin*:ti,ab
53. mRNA NEXT transfected NEXT dendritic NEXT cell*:ti,ab
54. (co NEXT stimulation) or (co NEXT inhibitation):ti,ab
55. activat* NEXT killer NEXT cell*:ti,ab
56. ("CD4+" or regulat*) NEXT/3 "T" NEXT cell*:ti,ab
57. myeloid NEXT deriv* NEXT/3 suppressor* NEXT cell*:ti,ab
58. (tum*r NEXT associat* NEXT macrophag*) or TAM:ti,ab
59. "CpG" NEXT oligonucleotid*:ti,ab
60. 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59
61. 28 or 60
62. 10 and 61
Appendix 2. MEDLINE (Ovid) search strategy
1. exp Carcinoma, Renal Cell/dt, pa, th, im, pc [Drug Therapy, Pathology, Therapy, Immunology, Prevention & Control]
2. von Hippel‐Lindau Disease/dt, pa, th, im, pc [Drug Therapy, Pathology, Therapy, Immunology, Prevention & Control]
3. (advance* adj6 (renal OR kidney OR nephron$) adj6 (cancer$ OR neoplasms$ OR carcinoma$ OR tumour$ OR tumour$)).mp
4. (advance* adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp.
5. (metasta* adj6 (renal or kidney or nephron$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp
6. (metasta* adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp
7. (local* adj6 advance* adj6 (renal or kidney or nephro*) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp
8. (local* adj6 advance* adj6 (renal or kidney or nephro*) adj cell adj6 cancer$).mp
9. ("clear cell type" adj3 carcinom$).mp
10. or/1‐9
11. exp Immunotherapy, Active /ae, tu [therapeutic use]
12. Immunization, Passive/ae, tu [Adverse Effects, Therapeutic Use]
13. Adoptive Transfer/ or adoptive T cell therap$.ti,ab
14. Cancer Vaccines /ae, tu
15. exp Antigens, Neoplasm /ae, tu
16. exp Antibodies, Monoclonal /ae, tu
17. Hematopoietic Stem Cell Transplantation
18. exp Immunomodulation/de [Drug Effects]
19. Immunologic Factors/ ae, tu
20. Immunosuppressive Agents/ae, tu
21. exp Superantigens/ae, tu
22. exp Antigens, Neoplasm/ae, tu
23. vaccin$.ti,ab
24. (immuni#ation OR immunotherap$ OR immunosuppress$ OR immunotox$).ti,ab
25. ((tumour or tumour) adj antigen$).ti,ab
26. Adjuvants.ti,ab
27. ((car or "containment and autonomic regulation") adj therapy).mp
28. or/11‐27
29. Interferon‐alpha/tu OR Interleukin‐2/tu OR Interleukin‐7/tu OR Exp Interleukin‐12/tu OR Interleukin‐15/tu
30. Exp Dendritic Cells/ OR BCG vaccine/
31. exp Cytokine‐Induced Killer Cells/de
32. exp Recombinant Proteins/tu
33. (vaccin$ adj2 (BCG or "Bacillus Calmette Guerin" or Calmette$)).ti,ab
34. ((DNA or mRNA or cellular or peptid$ or protein or genetic) adj2 vaccin$).ti,ab
35. (Dendritic cell adj2 bas$ adj3 vaccin$).ti,ab
36. (immun$ adj2 checkpoint).ti,ab
37. (Cytotoxic T lymphocyt$ or CTL).ti,ab
38. (inhibit$ adj2 (receptor$ or antibod$)).ti,ab
39. (humanized or agonistic) adj antibod$.ab
40. (Anti adj2 (CTLA or "PD‐L1" or "PD‐1" or "sLAG‐3")).ti,ab
41. ("cytotoxic T lymphocytes antigen" or "soluble human LAG‐3 protein").ti,ab
42. (ipilimumab or tremelimumab or nivolumab or naptumomab or estafenox).ti,ab
43. (IMP321 or IMA901 or AGS‐003 or CMDSC or ABR‐217620 or ANYARA).ti,ab
44. (activating receptor$).ti,ab
45. ("denileukin difitox" or reniale or "recombinant human interleukin" or lymphocine).ti,ab
46. ("autologous tumour lysate" adj2 "loaded dendritic cells").ti,ab
47. (HSPPC adj3 vaccin$).ti,ab
48. (mRNA transfected dendritic cell$).ti,ab
49. ((co adj stimulation) or (co adj inhibitation)).ti,ab
50. (activat$ adj killer adj cell$).ti,ab
51. ((CD4+ or regulat$) adj3 T adj cell$).ti,ab
52. (myeloid adj deriv$ adj3 suppressor$ adj cell$).ti,ab
53. ((tumour adj associat$ macrophag$) or (tumour adj associat$ macrophag$) or TAM).ti,ab
54. (CpG adj oligonucleotid$).ti,ab
55. or/29‐54
56. 28 or 55
57. randomised controlled trial.pt.
58. controlled clinical trial.pt.
59. randomised.ab.
60. placebo.ab.
61. drug therapy.fs.
62. randomly.ab.
63. trial.ab.
64. groups.ab.
65. or/57‐64
66. exp animals/ not humans/
67. 65 not 66
68. 10 and 56 and 67
Appendix 3. Embase (Ovid) search strategy
1 kidney carcinoma/
2 von Hippel Lindau disease/
3 (advance$ adj6 (renal or kidney or nephron$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp.
4 (advance$ adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp.
5 (metasta$ adj6 (renal or kidney or nephron$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp.
6 (metasta$ adj6 (renal or kidney or nephron$) adj cell adj6 cancer$).mp.
7 (local$ adj6 advance$ adj6 (renal or kidney or nephro$) adj6 (cancer$ or neoplasms$ or carcinoma$ or tumour$ or tumour$)).mp.
8 (local$ adj6 advance$ adj6 (renal or kidney or nephro$) adj cell adj6 cancer$).mp.
9 ("clear cell type" adj3 carcinom$).mp.
10 or/1‐9
11 exp immunotherapy/
12 exp passive immunization/
13 adoptive transfer.ti,ab.
14 adoptive t cell therap*.ti,ab.
15 cancer vaccine/
16 exp tumour antigen/
17 exp monoclonal antibody/
18 exp hematopoietic stem cell transplantation/
19 immunomodulation/
20 immunologic factor/
21 exp immunosuppressive agent/
22 superantigen/
23 alpha interferon/
24 interleukin 2/
25 interleukin 7/
26 interleukin 12/
27 interleukin 15/
28 exp dendritic cell/
29 BCG vaccine/
30 vaccin$.ti,ab.
31 (immuni#ation or immunotherap$ or immunosuppress$ or immunotox$).ti,ab.
32 ((tumour or tumour) adj antigen$).ti,ab.
33 Adjuvants.ti,ab.
34 ((car or "containment and autonomic regulation") adj therapy).mp.
35 (vaccin$ adj2 (BCG or "Bacillus Calmette Guerin" or Calmette$)).ti,ab.
36 ((DNA or mRNA or cellular or peptid$ or protein or genetic) adj2 vaccin$).ti,ab.
37 (Dendritic cell adj2 bas$ adj3 vaccin$).ti,ab.
38 (immun$ adj2 checkpoint).ti,ab.
39 (Cytotoxic T lymphocyt$ or CTL).ti,ab.
40 (inhibit$ adj2 (receptor$ or antibod$)).ti,ab.
41 ((humanized or agonistic) adj antibod$).ab.
42 (Anti adj2 (CTLA or "PD‐L1" or "PD‐1" or "sLAG‐3")).ti,ab.
43 ("cytotoxic T lymphocytes antigen" or "soluble human LAG‐3 protein").ti,ab.
44 (ipilimumab or tremelimumab or nivolumab or naptumomab or estafenox).ti,ab.
45 (IMP321 or IMA901 or AGS‐003 or CMDSC or ABR‐217620 or ANYARA).ti,ab.
46 activating receptor$.ti,ab.
47 ("denileukin difitox" or reniale or "recombinant human interleukin" or lymphocine).ti,ab.
48 ("autologous tumour lysate" adj2 "loaded dendritic cells").ti,ab.
49 (HSPPC adj3 vaccin$).ti,ab.
50 mRNA transfected dendritic cell$.ti,ab.
51 ((co adj stimulation) or (co adj inhibitation)).ti,ab.
52 (activat$ adj killer adj cell$).ti,ab.
53 ((CD4+ or regulat$) adj3 T adj cell$).ti,ab.
54 (myeloid adj deriv$ adj3 suppressor$ adj cell$).ti,ab.
55 ((tumour adj associat$ macrophag$) or (tumour adj associat$ macrophag$) or TAM).ti,ab.
56 (CpG adj oligonucleotid$).ti,ab.
57 or/11‐56
58 10 and 57
59 Crossover Procedure/
60 double‐blind procedure/
61 randomised controlled trial/
62 single‐blind procedure/
63 (random$ or factorial$ or crossover$ or cross over$ or placebo$ or assign$ or allocat$ or volunteer$).mp.
64 ((doubl$ or singl$) adj blind$).mp.
65 or/59‐64
66 exp animal/ not human.sh.
67 65 not 66
68 58 and 67
Appendix 4. ClinicalTrials.gov search strategy
Search strategies in Advanced Search
Search 1:
Interventions: Immunotherapy OR Immunization OR Cancer Vaccines OR tumour antigen OR monoclonal antibody OR hematopoietic stem cell transplantation OR immunosuppressive agent OR superantigen OR adoptive transfer
Conditions: metastatic renal cell carcinoma
Search 2:
Interventions: tumour‐associated peptides OR interferon alpha OR interleukin 2 OR interleukin 7 OR interleukin 12 OR interleukin 15 OR dendritic cell OR BCG vaccine OR DNA vaccine OR mRNA vaccine OR cellular vaccine
Conditions: metastatic renal cell carcinoma
Search 3:
Interventions: peptide vaccine OR peptid vaccine OR protein vaccine OR genetic vaccine OR IMP321 OR IMA901 OR AGS‐003 OR CMDSC OR ABR‐217620 OR ANYARA
Conditions: metastatic renal cell carcinoma
Search 4:
Interventions: immune checkpoint inhibitor OR CTLA OR PD‐L1 OR PD‐1 OR sLAG‐3 OR cytotoxic T lymphocytes antigen OR soluble human LAG‐3 protein OR ipilimumab OR tremelimumab OR nivolumab OR atezolizumab OR pembrolizumab OR pidilizumab OR durvalumab
Conditions: metastatic renal cell carcinoma
Appendix 5. WHO ICTRP search strategy
In the Condition:
metastatic renal cell carcinoma
In the Intervention:
Immunotherapy OR Immunization OR Cancer Vaccines OR tumour antigen OR monoclonal antibody OR hematopoietic stem cell transplantation OR immunosuppressive agent OR superantigen OR tumour‐associated peptides OR interferon alpha OR interleukin 2 OR interleukin 7 OR interleukin 12 OR interleukin 15 OR dendritic cell OR BCG vaccine OR DNA vaccine OR mRNA vaccine OR cellular vaccine OR peptide vaccine OR peptid vaccine OR protein vaccine OR genetic vaccine OR IMP321 OR IMA901 OR AGS‐003 OR CMDSC OR ABR‐217620 OR ANYARA OR adoptive transfer OR immune checkpoint inhibitor OR CTLA OR PD‐L1 OR PD‐1 OR sLAG‐3 OR cytotoxic T lymphocytes antigen OR soluble human LAG‐3 protein OR ipilimumab OR tremelimumab OR nivolumab OR atezolizumab OR pembrolizumab OR pidilizumab OR durvalumab
Appendix 6. EORTC search strategy
We browsed the website www.eortc.be/protoc/listprot.asp?kind=sites&site=24 and screened all trials by tumour site (kidney cancer).
Appendix 7. Web of Science Core Collection ‐ Meeting Abstracts search strategy
Search in "TOPIC" field:
"metastatic renal cell carcinoma" OR (metastatic NEAR/2 (renal or kidney or nephron*) NEAR/2 (cancer* or neoplasms* or carcinoma* or tumour* or tumour*))
AND
Immunotherapy OR Immunization OR Cancer Vaccines OR adoptive transfer OR tumour antigen OR monoclonal antibody OR hematopoietic stem cell transplantation OR immunosuppressive agent OR superantigen OR tumour‐associated peptides OR interferon alpha OR interleukin 2 OR interleukin 7 OR interleukin 12 OR interleukin 15 OR dendritic cell OR BCG vaccine OR DNA vaccine OR mRNA vaccine OR cellular vaccine OR peptide vaccine OR peptid vaccine OR protein vaccine OR genetic vaccine OR IMP321 OR IMA901 OR AGS‐003 OR CMDSC OR ABR‐217620 OR ANYARA
Refine to Document Type: Meeting Abstract
Data and analyses
Comparison 1.
Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year mortality | 2 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.13, 1.51] |
| 2 Overall survival | 2 | Hazard Ratio (Random, 95% CI) | 1.28 [1.11, 1.49] | |
| 3 Quality of life | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 FACT‐G | 1 | 730 | Mean Difference (Random, 95% CI) | ‐5.58 [‐7.25, ‐3.91] |
| 3.2 FKSI‐15 | 1 | 730 | Mean Difference (Random, 95% CI) | ‐3.27 [‐4.18, ‐2.36] |
| 3.3 FKSI‐DRS | 1 | 730 | Mean Difference (Random, 95% CI) | ‐1.98 [‐2.51, ‐1.45] |
| 3.4 EQ‐5D | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.12, ‐0.00] |
| 3.5 EQ‐VAS | 2 | 1000 | Mean Difference (Random, 95% CI) | ‐4.68 [‐6.53, ‐2.83] |
| 4 Adverse events (grade ≥ 3) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | 2.23 [1.79, 2.77] | |
| 6 Tumour remission | 2 | 1007 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.75] |
Analysis 1.1.
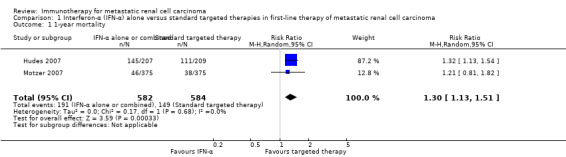
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
Analysis 1.6.
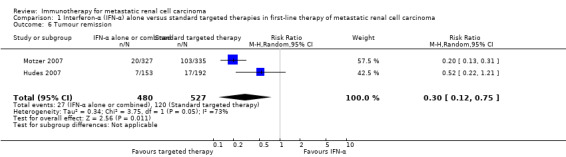
Comparison 1 Interferon‐α (IFN‐α) alone versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 6 Tumour remission.
Comparison 2.
Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 Adverse events (grade ≥ 3) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 5 Tumour remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Analysis 2.2.
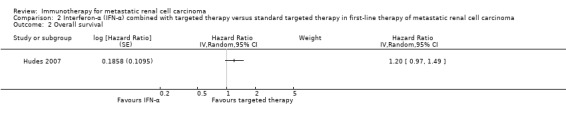
Comparison 2 Interferon‐α (IFN‐α) combined with targeted therapy versus standard targeted therapy in first‐line therapy of metastatic renal cell carcinoma, Outcome 2 Overall survival.
Comparison 3.
Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year mortality | 2 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.00, 1.36] |
| 2 Overall survival | 2 | Hazard Ratio (Random, 95% CI) | 1.13 [1.00, 1.28] | |
| 3 Adverse events (grade ≥ 3) | 2 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.71, 0.84] |
| 4 Progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | 1.53 [1.36, 1.73] | |
| 5 Tumour remission | 2 | 1205 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.31, 0.50] |
Analysis 3.1.
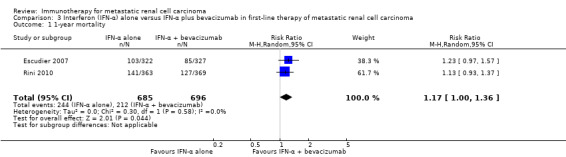
Comparison 3 Interferon (IFN‐α) alone versus IFN‐α plus bevacizumab in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
Comparison 4.
Interferon‐α (IFN‐α) plus bevacizumab versus standard targeted therapies in first‐line therapy of metastatic renal cell carcinoma
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Adverse events (grade ≥ 3) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Tumour remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 5.
Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year mortality | 2 | 1034 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
| 2 Overall survival | 2 | 1071 | Hazard Ratio (Fixed, 95% CI) | 1.14 [0.96, 1.37] |
| 3 Adverse events (grade ≥ 3) | 2 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.97, 1.39] |
| 4 Progression‐free survival | 1 | 339 | Hazard Ratio (Random, 95% CI) | 1.05 [0.87, 1.27] |
| 5 Tumour remission | 2 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
Analysis 5.1.
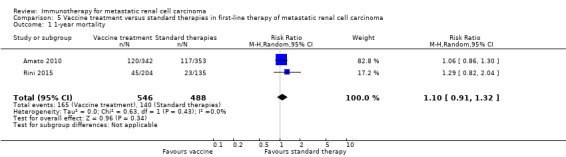
Comparison 5 Vaccine treatment versus standard therapies in first‐line therapy of metastatic renal cell carcinoma, Outcome 1 1‐year mortality.
Comparison 6.
Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 1‐year mortality | 1 | 821 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.87] |
| 2 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.60, 0.89] | |
| 3 Quality of life | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Clinical important MID in FKSI‐DRS | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.28, 1.78] |
| 3.2 Clinical important MID in EQ‐5D‐VAS | 1 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.16, 1.61] |
| 4 Adverse events (grade ≥ 3) | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.40, 0.65] |
| 5 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | 0.88 [0.75, 1.03] | |
| 6 Tumour remission | 1 | 750 | Risk Ratio (M‐H, Random, 95% CI) | 4.39 [2.84, 6.80] |
Analysis 6.3.
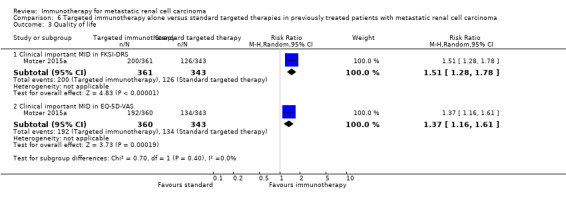
Comparison 6 Targeted immunotherapy alone versus standard targeted therapies in previously treated patients with metastatic renal cell carcinoma, Outcome 3 Quality of life.
Differences between protocol and review
This review was based on a published protocol (Unverzagt 2015), with differences as described here.
We included only studies that compared protocol‐defined immunotherapeutic interventions (experimental interventions) to standard treatment options as defined in current guidelines for systemic therapy in people with mRCC (e.g. Escudier 2014; German Guideline Programme in Oncology 2015; Ljungberg 2015). While working on the review, the comparator interventions were adapted accordingly.
We omitted tumour remission from our 'Summary of Findings' tables due to determining its low patient‐importance during the editorial process.
We corrected the outcome serious adverse effects (grade 3 or greater) to adverse events (grade 3 or greater).
We adapted the definition of stage IV RCC patients according to updated TNM classification to T4 any N M0 and any T, any N M1 (Sobin 2009; Wittekind 2012).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
Study design: 2‐arm, parallel‐group,
double‐blind, placebo‐controlled RCT. Study dates: recruitment from October 2006 to March 2008, follow‐up to March 2009, range of follow‐up 12 to 29 months. Setting: multicentre, international, phase III. Countries: France, Germany, Israel, Poland, Romania, Russia, Spain, UK, Ukraine, US. |
|
| Participants |
Inclusion criteria: people with histologically
confirmed advanced or metastatic clear‐cell RCC with prior
nephrectomy who required first‐line treatment, aged ≥ 18
years, measurable disease, KPS ≥ 80%, MSKCC 0‐2, life
expectancy > 12 weeks. Exclusion criteria: cerebral metastases, prior exposure to MVA‐5T4, known allergy to vaccinia vaccinations or egg proteins, pregnancy. Sample size:732. Age (years, median with range): group 1: 58 (18 to 86); group 0: 58 (24 to 85). Sex (M/F, %): group 1: 69.6/30.4; group 0: 65.1/34.9. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 365): MVA‐5T4 (modified vaccinia
Ankara encoding the tumour antigen 5T4). Group 0 (n = 367): placebo. Both as IM injection into the deltoid muscle at weeks 1, 3, 6, 9, 13, 17, 21, 25, 33, 41, 49, 57, 65. Cointerventions (standard‐of‐care according to local practice):
|
|
| Outcomes |
OS (primary outcome) How measured: active follow‐up. Time points measured: censored to March 2009. Time points reported: Kaplan‐Meier survival curves over up to 30 months, HR (with 95% CI), median. Subgroups: risk prognosis and standard of care (no comparison group 1 vs group 0 reported). AEs, grade ≥ 3 (primary safety outcome) How measured: NCI‐CTC (treatment‐emergent SAE all, grade 3, 4, 5). Time points measured and reported: not reported. Subgroups: not reported. QoL not evaluated. PFS (secondary outcome) How measured: not reported. Time points measured: week 26. Time points reported: not reported. Subgroups: not reported. Tumour remission (secondary outcome) How measured: complete response, partial response, stable disease. Time points measured and reported: week 26. Subgroups: not reported. |
|
| Funding sources | Sponsored by Oxford BioMedica. | |
| Declarations of interest | RH, WHS, SN: named inventors on several Oxford BioMedica patents. REH: minor consultancy role for Oxford BioMedica. | |
| Notes | Trial registration: NCT00397345, at the recommendation of the data safety monitoring board, the sponsor terminated the administration of MVA‐5T4/placebo to participants in July 2008 due to little or no prospect of demonstrating a significant survival benefit. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratification for standard of care, severity of disease and geographic location. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | No performance bias assumed due to blinding of participants and study personnel. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias assumed due to blinding of participants and study personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No detection bias on OS assumed due to blinding of participants, study personnel and outcome assessors. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed due to blinding of participants, study personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | No attrition bias on OS and PFS assumed, analysis with active follow‐up on survival data after termination of drug administration with no further description, no differences in censoring detected. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | No attrition bias on safety outcomes assumed, assessment on the basis of all participants as randomized. |
| Incomplete outcome data (attrition bias) (tumour remission) | Low risk | No attrition bias on tumour remission assumed, assessment on the basis of all participants as randomized. |
| Incomplete outcome data (attrition bias) (QoL) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | High risk | PFS was mentioned as secondary outcome, but no information provided. |
| Other bias | High risk | Safety monitoring board recommended stopping in July 2008 because there was little prospect of demonstrating a significant benefit in OS, second‐line therapies in 32% of placebo and 29% of MVA‐5T4 patients. |
| Methods |
Study design: 2‐arm, parallel‐group,
double‐blind, placebo‐controlled RCT. Study dates: randomization from June 2004 to October 2005, clinical cutoff: September 2006, median follow‐up of 13.3 months (0 to 25.6) (clinical cutoff), median follow‐up for OS: group 1: 21, group 0: 23 months to September 2008. Setting: multicentre, international, phase III. Countries: Europe (Belgium, Czech Republic, Finland, France, Germany, Hungary, Israel, Italy, Netherlands, Norway, Poland, Russia, Spain, Switzerland, UK), Asia (Russia, Singapore, Taiwan), Australia. |
|
| Participants |
Inclusion criteria: people with measurable or
non‐measurable, predominantly clear‐cell mRCC with no prior
systemic therapy, prior total or partial nephrectomy, KPS ≥
70%; aged ≥ 18 years; normal hepatic, haematopoietic and renal
function, and only minimal proteinuria. Exclusion criteria: prior systemic treatment for mRCC, recent major surgical procedures, evidence of brain metastases, ongoing full‐dose oral or parenteral anticoagulant or antiplatelet aggregation treatment, uncontrolled hypertension on medication, clinically significant cardiovascular disease, chronic corticosteroid treatment. Sample size:649. Age (years, median with range): group 1: 61 (30 to 82); group 0: 60 (18 to 81). Sex (M/F, %): group 1: 68/32; group 0: 73/27. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 322): IFN‐α + placebo IFN α‐2a (Hoffmann‐La Roche Ltd, Basel, Switzerland) 9 MIU 3 times/week SC for maximum 52 weeks or until disease progression, unacceptable toxicity or withdrawal of consent, an initial dose < 9 MIU permitted if the recommended dose was reached within the first 2 weeks of treatment, dose reduction; to 6 MIU or 3 MIU to manage AE attributable to IFN‐α. Placebo: every 2 weeks until disease progression, unacceptable toxicity or withdrawal of consent. Group 0 (n = 327): IFN‐α + bevacizumab IFN α‐2a (Hoffmann‐La Roche Ltd, Basel, Switzerland) 9 MIU 3 times/week SC for maximum 52 weeks or until disease progression, unacceptable toxicity or withdrawal of consent, an initial dose < 9 MIU permitted if the recommended dose was reached within the first 2 weeks of treatment, dose reduction; to 6 MIU or 3 MIU to manage AE attributable to IFN‐α. Bevacizumab (Hoffmann‐La Roche Ltd, Basel, Switzerland) 10 mg/kg IV every 2 weeks until disease progression, unacceptable toxicity or withdrawal of consent, no dose reduction permitted. Cointerventions (standard‐of‐care according to local practice): not reported. |
|
| Outcomes |
OS (primary outcome) How measured: investigator‐assessed, time between the date of randomization and death due to any cause, censoring on the day of last follow‐up or the last day of study administration if no follow‐up was done. Time points measured: during treatment and follow‐up before unblinding with cross‐over and second‐line therapies. Time points reported: Kaplan‐Meier curves over up to 24 months, median OS, number of deaths at data cutoff before cross‐over and second‐line therapies. Subgroups: MSKCC score. AEs, grade ≥ 3 (secondary outcome) How measured: investigator‐assessed, ongoing documentation of AEs (CTCAE v.3.0), physical examination, electrocardiography, urinalysis, measurement of blood pressure. Time points measured: at each visit, weekly monitoring in participants who developed ≥ grade 3 hypertension, 24‐hour urine collection if protein was observed with a dipstick analysis. Time points reported: frequency of participants with AEs, SAEs (safety population), AEs with grade ≥ 3, most commonly reported AEs with grade ≥ 3 up to 28 days after the last dose, deaths due to AEs. Subgroups: not reported. QoL not evaluated PFS (secondary outcome) How measured: time between randomization and first documented disease progression or death due to any cause, investigator‐assessment and independent review committee. Time points measured: every 8 weeks up to week 32 and every 12 weeks thereafter until disease progression. Time points reported: Kaplan‐Meier survival curves for up to 24 months, median PFS. Subgroups: age, sex, MSKCC score, baseline VEGF, number of metastatic sites. Tumour remission (secondary outcome) How measured: assessment by the investigator with RECIST, non‐measurable lesions were used to define complete response and disease progression only. Time points measured: every 8 weeks up to week 32, every 12 weeks thereafter until disease progression, responses had to be confirmed by a second assessment ≥ 4 weeks after the first response was recorded. Time points reported: best tumour response for participants with measurable disease. Subgroups: not reported. |
|
| Funding sources | Hoffmann‐La Roche Ltd. | |
| Declarations of interest | BE: consulted for and received honoraria from Roche, Bayer, Wyeth, Pfizer, Inate and Antigenics. SB: consulted for Roche, Pfizer, Wyeth and Bayer. AR: acted as an adviser for Bayer, Pfizer, GSK, Novartis and Wyeth. NM: employee of and owns stock of Roche. BM: received honoraria and research funding from Roche. | |
| Notes | BO17705E, NCT00738530 (registered October 2008), preplanned interim analysis with significant benefit in OS, unblinding, data and safety monitoring board recommended cross‐over of participants from the placebo to the bevacizumab group, differences in availability of new second‐line therapies in countries might confound OS results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No selection bias assumed, the randomization list bases on block design procedure, stratified by country and MSKCC risk group. |
| Allocation concealment (selection bias) | Low risk | No selection bias assumed, central allocation by an interactive voice recognition system, list kept in a secure location, not available to any person directly involved in the study. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | No performance bias on subjective outcomes assumed, blinding of participants and study personnel with same route and timing as bevacizumab until preplanned interim analysis and data cutoff. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on objective outcomes assumed, blinding of participants and study personnel with same route and timing as bevacizumab until preplanned interim analysis and data cutoff. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No performance bias on subjective outcomes assumed, blinding of participants and study personnel with same route and timing as bevacizumab until preplanned interim analysis and data cutoff. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed due to blinding of participants, study personnel and outcome assessors. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | No attrition bias on OS and PFS assumed due similar censoring between treatment groups, 251 deaths and 505 progression events from 649 participants had occurred at the time of data cutoff. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | No attrition bias on safety outcomes assumed, all participants who included at least 1 dose of bevacizumab were compared to participants who received no bevacizumab. |
| Incomplete outcome data (attrition bias) (tumour remission) | Low risk | No attrition bias on tumour remission assumed, all participants with measurable disease at baseline were included. |
| Incomplete outcome data (attrition bias) (QoL) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | Low risk | No reporting bias on tumour remission assumed, no reporting bias assumed, all preplanned outcomes reported. |
| Other bias | High risk | Cross‐over of 13 (4%) participants from group 0 to group 1, 49 (15%) participants in group 1 and 64 (20%) participants in group 0 received second‐line therapy with tyrosine kinase inhibitors. |
| Methods |
Study design: 3‐arm, parallel‐group,
open‐label RCT. Study dates: recruitment from July 2003 to April 2005, follow‐up until second interim analysis after 446 deaths (June 2006), range of follow‐up: 14 to 36 months. Setting: multicentre, international, phase III. Countries: Global Advanced Renal Cell Carcinoma (ARCC) Trial including countries of all continents, specifically: Argentina, Australia, Canada, Czech Republic, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Netherlands, Poland, Russia, Serbia, Montenegro, Slovakia, South Africa, Spain, Sweden, Taiwan, Turkey, Ukraine, UK, US. |
|
| Participants |
Inclusion criteria: histologically confirmed advanced
RCC (stage IV or recurrent disease), KPS ≥ 60, no previous
systemic therapy, measurable disease, adequate bone marrow, renal and
hepatic functions (neutrophil count > 1500 cells/mm3, platelet
count > 100,000 cells/mm3, haemoglobin count > 8 g/dL.
People with a history of brain metastases if their condition was
neurologically stable and they did not require corticosteroids after
surgical resection or radiotherapy. Exclusion criteria: serum creatinine level ≤ 1.5 times ULN; aspartate aminotransferase level ≤ 3 times ULN (≤ 5 times if liver metastases present); total bilirubin level ≤ 1.5 times ULN; fasting level of total cholesterol ≤ 350 mg/dL, triglyceride level ≤ 400 mg/dL. Sample size:626. Age (years, median with range): group 1: 59 (32 to 82); group 1a: 60 (23 to 86); group 0: 58 (32 to 81). Sex (M/F, %): group 1: 69/31; group 1a: 71/28; group 0: 66/33. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 210): IFN‐α +
temsirolimus Temsirolimus (Wyeth Research, 15 mg IV weekly, 30‐minute infusion) + IFN‐α (Roferon‐A, Roche, starting dose 3 MU 3 times/week for week 1 and 6 MU SC 3 times/week thereafter). Group 1a (n = 207): IFN‐α IFN‐α starting dose of 3 MU SC 3 times/week for the first week, dose was raised to 9 MU 3 times/week for the second week and to 18 MU 3 times/week for week 3, if tolerated. Participants who were unable to tolerate 9 MU or 18 MU received the highest tolerable dose (3 MU, 4.5 MU or 6 MU). Group 0 (n = 209): temsirolimus Temsirolimus 25 mg IV weekly 30‐minute infusion. Cointerventions for participants treated with temsirolimus (standard‐of‐care according to local practice): premedication with diphenhydramine 25 mg to 50 mg IV or a similar histamine H1 blocker given approximately 30 minutes before each weekly temsirolimus infusion as prophylaxis against an allergic reaction. |
|
| Outcomes |
OS (primary outcome) How measured: investigator assessed, time between date of randomization and date of death. Time points measured: not reported. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median with 95% CI, HR with 95% CI. Subgroups: prior nephrectomy, KPS (≤ 70, > 70). AEs, grade ≥3 How measured: AEs (NCI‐CTC 3.0) occurring in ≥ 20% of participants in any group (all grades and grade 3 or 4), number of AEs, grade 3 or 4 per treatment group, any visit at which the participant reported a symptomatic NCI‐CTC (v.3) grade 3 or 4. Time points measured: weekly or biweekly. Time points reported: treatment period. Subgroups: not evaluated. QOL How measured: EQ‐5D and EQ‐VAS questionnaire (self‐report). Time points measured: at screening, week 12, week 32. Time points reported: week 12, withdrawal or last recorded visit (only IFN‐α vs temsirolimus) (Yang 2010). Subgroups: prior nephrectomy. PFS (secondary outcome) How measured: determined by the site investigators' assessment and a blinded assessment of imaging studies (performed by Bio‐Imaging Technologies, not shown), time between date of randomization and date of disease progression or death, whichever occurred first. Time points measured: not reported. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median with 95% CI, HR with P value. Subgroups: not reported. Tumour remission (secondary outcome) How measured: CT scans of the chest, abdomen and pelvis; radionuclide bone scan MRI or CT scan of the brain; classification into participants with stable disease or objective response (RECIST); % participants who had a confirmed objective response (complete or partial) as their best response to treatment. Time points measured: before treatment, repeated at 8‐week intervals. Time points reported: 24 weeks. Subgroups: not reported. |
|
| Funding sources | Wyeth Research, Cambridge, MA, US. | |
| Declarations of interest | GH: financial support from Pfizer and Wyeth; MC, RF, IGHS‐W, RJM: financial support from Wyeth; JD: financial support from Novartis, Chiron, Bayer, Onyx, Pfizer and Wyeth; AK: financial support from Bayer and Wyeth; DMcD: financial support from Bayer, Onyx, Genentech and Novartis. TO'T, SL and LM: full‐time employee of Wyeth Research. | |
| Notes | Trial registration: NCT00065468, study was stopped as a result of the second predefined interim analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified block randomization. |
| Allocation concealment (selection bias) | Unclear risk | No information reported. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Different delivery of the interventions, no placebo‐controlled trial, participants and physicians not blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Kaplan‐Meier estimates of blinded assessment for PFS not shown, outcome assessors not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | No attrition bias on PFS or OS assumed due to small censoring rates. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | No attrition bias on safety outcomes assumed, Inclusion of all participants as treated. |
| Incomplete outcome data (attrition bias) (tumour remission) | High risk | Differences in postbaseline tumour assessment (group 1: 74% vs group 1a: 80% vs group 0: 92%). |
| Incomplete outcome data (attrition bias) (QoL) | High risk | High risk of attrition on QoL due to high differences in completion rates between treatment groups. |
| Selective reporting (reporting bias) | Low risk | No reporting bias assumed, nearly all outcomes reported (besides quality‐adjusted time without symptoms or toxicity). |
| Other bias | Low risk | Early stop for benefit, no other risk of bias assumed. |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Study dates: enrolment between August 2004 and October 2005, median duration of treatment: 6 months, range 1 month to 15 months. Setting: inpatients, multicentre (101 centres), international, phase III. Countries: Australia, Brazil, Canada, Europe, US. |
|
| Participants |
Inclusion criteria: people with histologically
confirmed clear‐cell mRCC; aged ≥ 18 years; no previous
treatment with systemic therapy for RCC; measurable disease; ECOG
Performance Status 0 to 1; adequate haematological, coagulation, hepatic,
renal and cardiac function. Exclusion criteria: brain metastases, uncontrolled hypertension or clinically significant cardiovascular events or disease during the preceding 12 months. Sample size:750. Age (years, median with range): group 1: 59 (34 to 85); group 0: 62 (27 to 87). Sex (M/F, %): group 1: 72/28; group 0: 71/29. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 375): IFN‐α IFN‐α‐2a (Roche) SC injection 3 times/week on non‐consecutive days at 3 MU per dose during first week, 6 MU per dose during second week and 9 MU per dose thereafter) until the occurrence of disease progression, unacceptable AEs or withdrawal of consent. Group 0 (n = 375): sunitinib Sunitinib orally 50 mg once daily in 6‐week cycles consisting of 4 weeks of treatment followed by 2 weeks without treatment. Cointerventions (standard‐of‐care according to local practice): not specified. |
|
| Outcomes |
OS (secondary endpoint) How measured: not specified. Time points measured: during follow‐up; off‐study every 2 months. Time points reported: Kaplan‐Meier survival curves over up to 36 months (data from Motzer 2009), median with 95% CI, unstratified HR and stratified HR with 95% CI. Subgroups: MSKCC criteria; previous nephrectomy; ECOG Performance Status; lactate dehydrogenase level; time since diagnosis; haemoglobin level; corrected serum calcium level; number of metastatic sites; bone, lung and liver metastases. AEs, grade ≥ 3 (not specified) How measured: CTCAE v.3.0. Time points measured and reported: not reported, no summarized frequencies reported. Subgroups: not reported. QoL (secondary outcome) How measured: FKSI‐15, FACT‐G, EQ‐5D, EQ‐VAS ‐ completion, if > 80% of items in FACT‐G and > 50% of items in FKSI completed. Time points measured and reported: before randomization, on days 1 and 28 of each cycle, overall mean score after 17 weeks (Cella 2008). Subgroups: not reported. PFS (primary endpoint) How measured: time from randomization to the first documentation of objective disease progression or to death from any cause, whichever occurred first with a blinded central review of radiological images. Time points measured: during follow‐up (central review to September 2007). Time points reported: Kaplan‐Meier survival curves over up to 14 months (Motzer 2007), median PFS with 95% CI; HR with 95% CI (central review and investigator‐assessed). Subgroups: MSKCC criteria, previous nephrectomy, age, sex, ECOG Performance Status, lactate dehydrogenase level, time since diagnosis, haemoglobin level, corrected serum calcium level. Tumour remission (secondary outcome) How measured: RECIST with blinded central review of radiological images, complete and partial response, stable disease. Time points measured: day 28 of cycles 1 through 4, and every two cycles thereafter until the end of treatment. Time points reported: independent central review to September 2007 (interim analysis), investigator‐assessed update results (not included). Subgroups: not reported. |
|
| Funding sources | IFN‐α‐2a (Roferon‐A, Roche) and sunitinib were provided by Pfizer. | |
| Declarations of interest | RJM: research grants from Pfizer and Genentech, consulting fees from Wyeth and lecture fees from Bayer Pharmaceuticals; TEH: consulting and lecture fees from Pfizer, Bayer Pharmaceuticals and Onyx Pharmaceuticals; DM: consulting fees from Pfizer and Wyeth Pharmaceuticals and lecture fees from Pfizer; RMB: research grants from Pfizer, Bayer Pharmaceuticals, Genentech, Genzyme and Bristol‐Myers Squibb and consulting and lecture fees from Pfizer, Bayer Pharmaceuticals, Onyx Pharmaceuticals and Genentech; OR: consulting and lecture fees from Pfizer; SO: consulting and lecture fees from Pfizer; SN: consulting fees from Pfizer and Bayer Pharmaceuticals; RAF: research grants from Pfizer, consulting fees from Pfizer and Onyx Pharmaceuticals, and lecture fees from Pfizer and Bayer Pharmaceuticals, STK, IC, PWB, CMB: full‐time employees of Pfizer and equity owners in the company. | |
| Notes | Registration: NCT00098657 and NCT00083889, protocol amendment (February 2006), cross‐over of 7% (n = 25) participants from IFN‐α to sunitinib. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk of selection bias due to stratified randomization according to baseline levels of lactate dehydrogenase (> 1.5 vs ≤ 1.5 ULN), ECOG Performance Status (0 vs 1), and previous nephrectomy (yes vs no) with random permuted blocks of 4. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Blinding not possible due to different routes of interventions, participants and physicians were not blinded. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinded central review of radiological images used to assess primary endpoint and ORR, no blinded assessment of AEs and QoL reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | All participants were included, similar censoring. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | All treated participants included. |
| Incomplete outcome data (attrition bias) (tumour remission) | High risk | Images of 88 (12%) participants had not been assessed by a central review at the time of interim analysis and were assessed by investigators (results not included). |
| Incomplete outcome data (attrition bias) (QoL) | Low risk | Completion rates for FKSI, FACT‐G and EQ‐5D questionnaires: 95% with at least 1 postbaseline assessment and slightly lower rates in the group with IFN‐α. |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes from the protocol reported. |
| Other bias | High risk | After the interim analysis, participants in the IFN‐α group with progressive disease were allowed to cross over to the sunitinib group and 25 participants crossed over which may influence OS. |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Study dates: randomization October 2012 to March 2014, data cutoff: June 2015, minimal follow‐up of 14 months. Setting: multicentre (146 centres), international, phase III. Countries: North America (US, Canada), Western Europe (Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Norway, Poland, Romania, Spain, Sweden, UK), South America (Argentina, Brazil) and Asia (Israel, Japan). |
|
| Participants |
Inclusion criteria: people with histologically
confirmed advanced or mRCC with a clear‐cell component, aged
≥ 18 years, measurable disease, prior treatment with 1 or 2
antiangiogenic therapies, ≤ 3 previous systemic therapies with cytokines
and cytotoxic drugs and disease progression during or > 1 treatment
regimen within 6 months before study enrolment, KPS ≥ 70. Exclusion criteria: central nervous system metastasis, previous treatment with an mTOR inhibitor, condition requiring treatment with glucocorticoids (equivalent to > 10 mg of prednisone > 10 mg daily). Sample size:821. Age (years, median with range): group 1: 62 (23‐88); group 0: 62 (18‐86). Sex (M/F, %): group 1: 77/23; group 0: 74/26. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 410): nivolumab Nivolumab 3 mg/kg IV every 2 weeks, no dose modifications permitted). Group 0 (n = 411): everolimus Everolimus 10 mg orally, every day, dose modifications permitted. Cointerventions (standard‐of‐care according to local practice): not specified. |
|
| Outcomes |
OS (primary endpoint) How measured: time from randomization to the date of death. Time points measured: every 8 weeks for the first year, and then every 12 weeks until disease progression or discontinuation of treatment, after discontinuation of treatment, participants followed every 3 months for assessment of survival and subsequent anticancer therapy. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median with 95% CI, unstratified HR with 98.5% CI. Subgroups: MSKCC prognostic score, previous antiangiogenic regimens, region, age, gender. AEs, grade ≥ 3 (secondary endpoint) How measured: NCI‐CTC AE V4.0. Time points measured: at each clinic visit. Time points reported: overall frequency of all and most common treatment‐related AEs (fatigue, pruritus, stomatitis, anaemia) and AEs grade 3/4 including treatment‐related deaths. Subgroups: not reported. QoL (secondary endpoint) How measured: health‐related QoL assessments with FACT FKSI‐DRS and a resulting summary score and EQ‐5D. Time points measured: baseline, after randomization but before cycle 1 of therapy, on day 1 of each cycle, at the first 2 follow‐up visits (each assessment before physician contact, treatment doses and any procedures), about 30 and 100 days after last dose, EQ‐5D: additional at each of the 10 survival follow‐ups visits (every 3 months). Time points reported: FKSI‐DRS and EQ‐5D (utility index and VAS) with completion rates at baseline, mean change from baseline to weeks 4 to 104, clinically important improvements, time to improvement (Cella 2016). Subgroups: not reported. PFS (secondary endpoint) How measured: time from randomization to first documented RECIST‐defined tumour progression or death from any cause. Time points measured: CT and MRI at baseline, every 8 weeks for the first year and then every 12 weeks until disease progression or discontinuation of treatment. Time points reported: Kaplan‐Meier survival curves over up to 30 months, median. Subgroups: not reported. Tumour remission (secondary endpoint) How measured: evaluated by the investigator (RECIST 1.1), number of randomized participants with a complete or partial response. Time points measured: CT and MRI at baseline, every 8 weeks for the first year and then every 12 weeks until disease progression or discontinuation of treatment. Time points reported: overall response. Subgroups: not reported. |
|
| Funding sources | Bristol Myers Squibb. | |
| Declarations of interest | RJM: honoraria from Bayer, Pfizer, Novartis and GlaxoSmithKline. SG: fees for consulting and serving on advisory boards from Bristol‐Myers Squibb, Novartis, Bayer, Sanofi‐Aventis, Astellas, Xcenda and Onclive; grant support from Bristol‐Myers Squibb, Novartis, Bayer, Pfizer, Acceleron, Merck and Agensys. HJH: grant support from Pfizer, Newlink Genetics, GlaxoSmithKline and SFJ Pharmaceuticals. SST: fees for serving on advisory boards from Prometheus; consulting fees from Amgen; grant support through his institution from Prometheus, Argos Therapeutics, Immatics Biotechnologies, Novartis and Exelixis. GP: fees for serving on advisory boards from Janssen and Novartis; lecture fees from Astellas and Pfizer; grant support from Bayer. ERP: fees for serving on advisory boards from Merck, Dendreon, GlaxoSmithKline, Pfizer, Astellas, Novartis and Genentech; grant support from AstraZeneca, Eli Lilly, Merck, Dendreon, GlaxoSmithKline, Acceleron and Pfizer. TKC: fees for consulting and for serving on advisory boards from GlaxoSmithKline, Novartis, Pfizer, Merck, AstraZeneca, Bayer and Prometheus; grant support through his institution from Bristol‐Myers Squibb, GlaxoSmithKline, Novartis, Exelixis, Pfizer, Merck, Roche, AstraZeneca, TRACON Pharmaceuticals and Peloton. HG: fees for serving on advisory boards from Novartis, Bayer, Sanofi‐Aventis, Astellas and Pfizer. FD: grant support from Novartis, Pfizer and GlaxoSmithKline. PB: honoraria from GlaxoSmithKline, Pfizer and Orion. JW: fees for serving on advisory boards, paid to his institution, from Bristol‐Myers Squibb, Novartis, GlaxoSmithKline, Roche and Amgen. YT: fees for serving on advisory boards from ONO Pharmaceuticals and Pfizer; honoraria and grant support from ONO Pharmaceuticals, Novartis and Pfizer. TCG: fees for consulting and serving on advisory boards from Boehringer Ingelheim, Merck Serono, Novartis, Pfizer, GlaxoSmithKline, Merck Sharp & Dohme, Bayer HealthCare, Roche, Bristol‐Myers Squibb, Eli Lilly and Janssen‐Cilag; honoraria from Boehringer Ingelheim, Merck Serono, Novartis, Pfizer, GlaxoSmithKline, Bayer, Roche, Eli Lilly, Janssen‐Cilag, Sanofi‐Aventis; travel support from Boehringer Ingelheim, Merck Serono, Pfizer, Roche and Eli Lilly; owning stock in Bayer. FAS: fees for serving on advisory boards from Pfizer, GlaxoSmithKline and Novartis; lecture fees from GlaxoSmithKline. CK: fees for serving on advisory boards from Pfizer, Novartis, Sanofi‐Aventis, Bayer and Seattle Genetics; lecture fees from Pfizer and Novartis. AR: lecture fees from Merck Sharp & Dohme. JSS, LAX, IMW: employees of and hold stock in Bristol‐Myers Squibb. PS: reports receiving consulting fees from Jounce Therapeutics, Amgen, Bristol‐Myers Squibb, GlaxoSmithKline and AstraZeneca/MedImmune; also founder of and holds stock in Jounce Therapeutics. | |
| Notes | Registration: NCT01668784 (CheckMate025). Study was stopped early due to the results of a planned interim analysis by the independent data monitoring committee showing significant benefit for OS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk of selection bias assumed due to block randomization, stratified by region, MSKCC prognostic risk group and the number of previous antiangiogenic therapy regimens (1 or 2) for advanced RCC. |
| Allocation concealment (selection bias) | Low risk | Central randomization. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Open‐label study. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | Low risk of attrition bias assumed, high completeness of follow‐up with similar censoring in between treatment groups. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | Low risk of attrition bias assumed, safety analysis bases on all treated participants. |
| Incomplete outcome data (attrition bias) (tumour remission) | High risk | High risk of bias on attrition bias for tumour remission assumed due to different numbers of non‐evaluated participants (6% with nivolumab vs 12% with everolimus). |
| Incomplete outcome data (attrition bias) (QoL) | Low risk | High completion rates (≥ 80% in the first year) with no differences between treatment groups. |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes from the protocol reported. |
| Other bias | High risk | Stopped early for benefit in OS, subsequent systematic therapies (group 1: 55%; group 0: 63%) with cross‐over (25% from group 1 to group 0; 1.7% from group 0 to group 1). |
| Methods |
Study design: 3‐arm, parallel‐group,
open‐label RCT. Study dates: randomization from May 2008 to May 2009, median follow‐up 23.2 months, study completion date February 2012. Setting: multicentre (24 centres), national, phase II. Country: France. |
|
| Participants |
Inclusion criteria: histologically confirmed mRCC of
all histological subtypes except papillary carcinomas; aged ≥
18 years; ECOG Performance Status 0 to 2; measurable metastases; liver,
renal and haematological functions in the range of 1.5 to 2 times above or
below normal values; normal lipid and glycaemic concentrations; normal
cardiac function within 6 weeks before randomization. Exclusion criteria: brain metastases, hypertension, systemic treatment for the disease, history of arterial or venous thrombosis in the past 6 months. Sample size: 171. Age (years, median with range): group 1: 62 (40 to 79); group 0a: 62 (33 to 83); group 0b: 61 (33 to 83). Sex (M/F, %): group 1: 66/34; group 0a: 74/26; group 0b: 76/24. Prognostic factors (all randomized participants):
|
|
| Interventions |
Group 1 (n = 41): IFN‐α +
bevacizumab IFN‐α 9 mIU SC 3 times/week + bevacizumab 10 mg/kg IV every 2 weeks. Group 0 (n = 42): sunitinib Sunitinib 50 mg/day for 4 weeks, followed by 2 weeks off. Group 0a (n = 88): temsirolimus + bevacizumab (excluded, not standard treatment). Temsirolimus 25 mg IV weekly + bevacizumab 10 mg/kg IV every 2 weeks. Treatments continued until disease progression, unacceptable toxicity or protocol violation. Cointerventions (standard‐of‐care according to local practice): not specified. |
|
| Outcomes |
OS (secondary endpoint) How measured: time from randomization to death from any cause. Time points measured: during follow‐up. Time points reported: 12‐months OS (study is ongoing for long‐term OS). Subgroups: not reported. AEs, grade ≥ 3 (secondary endpoint) How measured:participants on study medication were assessed (NCI‐CTCAE v.3.0), data safety monitoring committee. Time points measured: at day 15 and then at least every 6 weeks over 48 weeks. Time points reported: main types of AEs (all grades and grade ≥ 3), frequency of AEs and SAEs ≥ 3. Subgroups: not reported. QoL not evaluated PFS (primary endpoint) How measured: time from randomization to disease progression or death from any cause (central reviewed data), 4 follow‐up CT scans according to RECIST 1.0. Time points measured: baseline and then every 12 weeks over 48 weeks with 4 follow‐up CT scans. Time points reported: Kaplan‐Meier survival curves over up to 30 weeks, median PFS with 95% CI. Subgroups: not reported. Tumour remission (secondary endpoint) How measured: thoracic, abdominal and pelvic CT scan, brain MRI or CT and bone scan. Time points measured: baseline and then every 12 weeks. Time points reported: best response. Subgroups: not reported. |
|
| Funding sources | French Ministry of Health and Wyeth Pharmaceuticals. | |
| Declarations of interest | SN: honoraria from Novartis, Wyeth, Pfizer, GlaxoSmithKline and Roche; research funding from Wyeth, Roche and Novartis. DP: honoraria from Bayer, Eli Lilly and Roche. JOB: honoraria from Amgen; consultant with Novartis. LG, BL: honoraria from Novartis. BE: honoraria from Bayer, Roche, Pfizer, Genentech, Novartis, GlaxoSmithKline and Aveo; consultant with Bayer, Pfizer and Roche. All other authors declared no conflicts of interest. | |
| Notes | Registration: NCT00619268 (TORAVA). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Low risk of selection bias assumed, computer‐generated list, permutated blocks, stratification by participating centre and performance status. |
| Allocation concealment (selection bias) | Low risk | Low risk of selection bias assumed due to central allocation. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and investigators were unmasked. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Masked central review of CT scans done in 89% of all randomized participants. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | No attrition bias assumed due to high completeness of, and similar censoring in, different treatment groups during follow‐up. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | No attrition bias assumed, all participants who received at least 1 dose of the study drug were included. |
| Incomplete outcome data (attrition bias) (tumour remission) | Low risk | No attrition bias assumed, response was reported for all participants. |
| Incomplete outcome data (attrition bias) (QoL) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | High risk | Information on long‐term OS and QoL not published despite planning in protocol. |
| Other bias | High risk | Blocked randomization in centres in an unblinded trial, differences in second‐line treatment after study treatment failure because of toxicity or progression with lower rates of second‐line therapies with sunitinib (48%) compared to 68% to 69% in other groups. |
| Methods |
Study design: 2‐arm, parallel‐group
RCT. Study dates: randomization from October 2003 to July 2005, data cutoff March 2009, median follow‐up among surviving participants 46.2 months. Setting: multicentre, international. Countries: Canada, US. |
|
| Participants |
Inclusion criteria: people with mRCC;
clear‐cell histological component confirmed by local pathology
review; no prior systemic therapy for RCC; KPS ≥ 70%; aged
≥ 18 years; adequate bone marrow, hepatic and renal function;
serum creatinine ≤ 1.5 times ULN. Exclusion criteria: central nervous system metastases; NYHA class II to IV heart failure; bleeding within 6 months; blood pressure that could not be controlled < 160/90 mmHg with medication; history of venous thrombosis within 1 year or arterial thrombosis within 6 months or who required ongoing therapeutic anticoagulation; uncontrolled thyroid function; pregnancy; requirement for systemic corticosteroids greater than physiological replacement doses or delayed healing wounds, ulcers or bone fractures. Sample size:732. Age (years, median with IQR): group 1: 61 (56 to 70); group 0: 62 (55 to 70). Sex (M/F, %): group 1: 73/27; group 0: 66/34. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 363): IFN‐α IFN‐α‐2a (Intron; Schering‐Plough, Kenilworth, NJ), provided by the NCI Cancer Therapy Evaluation Program, 9 MU SC 3 times/week (non‐consecutive days). Dose reduction to 6 MU and 3 MU if IFN‐related toxicity present. Group 0 (n = 369): IFN‐α + bevacizumab IFN‐α‐2a (Intron; Schering‐Plough, Kenilworth, NJ), provided by the NCI Cancer Therapy Evaluation Program, 9 MU SC 3 times/week (non‐consecutive days). Dose reduction to 6 MU and 3 MU if IFN‐related toxicity present. Bevacizumab (provided by the NCI Cancer Therapy Evaluation Program) 10 mg/kg IV every 2 weeks. Cointerventions (standard‐of‐care according to local practice): not specified. |
|
| Outcomes |
OS (primary outcome) How measured: time from registration to death from any cause. Time points measured: during treatment and follow‐up. Time points reported: Kaplan‐Meier curves over up to 60 months, median OS. Subgroups: nephrectomy, MSKCC, liver metastases, age, gender. AEs, grade ≥ 3 (secondary outcome) How measured: ongoing documentation of AEs (CTCAE v.3.0). Time points measured: baseline, every 12 weeks. Time points reported: frequency of participants with AEs grade ≥ 3, deaths due to AEs, treatment‐related AEs to March 2009. Subgroups: not reported. QoL not evaluated PFS (secondary outcome) How measured: time between randomization and date of progression or death, investigator assessment of x‐rays. Time points measured: baseline, every 12 weeks. Time points reported: Kaplan‐Meier survival curves for up to 60 months, median PFS. Subgroups: number of adverse risk factors. Tumour remission (secondary outcome) How measured: investigator assessment of x‐rays, RECIST criteria. Time points measured: baseline, every 12 weeks. Time points reported: overall response rate. Subgroups: not reported. |
|
| Funding sources | Supported in part by National Cancer Institute to the Cancer and Leukemia Group B (CALGB) (Grant No. CA31946, CA33601) and by National Cancer Institute (Grants No. CA60138, CA41287, CA47642, CA45808, CA77440, CA14985, CA77202). | |
| Declarations of interest | BIR: consultant or advisory role: Genentech. WMS: consultant or advisory role: Genentech; research funding: Genentech. JP: consultant or advisory role: Genentech; honoraria: Genentech. JD: consultant or advisory role: Genentech and Novartis; honoraria: Pfizer, Novartis; research funding: Novartis, Genentech, Pfizer. DAV: research funding: Genentech. | |
| Notes | Registration: NCT00072046 (CALGB 90206). Results on PFS and overall response rate published in Rini 2008, no cross‐over was permitted for participants randomly assigned to IFN‐α monotherapy, a substantial percentage of participants in both arms received systemic anticancer therapy subsequent to progression (62% of participants on IFN‐ monotherapy and 54% of participants on bevacizumab + IFN‐α) (mostly with sunitinib or sorafenib). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random block design, stratified by nephrectomy status and number of adverse prognostic factors. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Non‐blinded trial and no independent review of x‐rays could potentially have contributed to the improved PFS and overall response rate. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | No attrition bias assumed due to similar censoring, 657/732 (90%) participants experienced progression or death. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | Based on all participants who were eligible for evaluation for toxicity (362/363 from the intervention and 347/369 from the control group, reasons not reported). |
| Incomplete outcome data (attrition bias) (tumour remission) | Unclear risk | No data reported. |
| Incomplete outcome data (attrition bias) (QoL) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | Low risk | No differences in outcomes to the protocol. |
| Other bias | High risk | Treatment with second‐line systemic anticancer therapy subsequent to progression (62% of participants on IFN‐α monotherapy and 54% of participants on bevacizumab + IFN‐α) might bias OS. |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Study dates: December 2010 to July 2015 (study start to study completion date according to the trial registration). Setting: multicentre, international, phase III. Countries: Europe (France, Germany, Hungary, Italy, Poland, Russia, UK, Netherlands, Romania, Norway), US. |
|
| Participants |
Inclusion criteria: metastatic or locally advanced
(or both) RCC with clear‐cell histology (histological confirmation by
local pathologist required), aged > 18 years,
HLA‐A*02‐positive type, candidates for a first‐line
therapy with sunitinib, favourable or intermediate‐risk (favourable
risk: none, intermediate risk: 1 or 2 of the following criteria applied:
haemoglobin < LLN, serum corrected calcium > ULN, KPS < 80%, time
from initial diagnosis to initiation of therapy < 1 year, absolute
neutrophil count > ULN, platelets > ULN), women who were
postmenopausal or surgically sterile or practiced medically acceptable
method of contraception, men willing to use contraception or had undergone
vasectomy. Exclusion criteria: prior systemic therapy for metastatic disease; history of or current brain metastases; abnormal ≥ CTC grade 3 laboratory values for haematology, liver and renal function; metastatic second malignancy; localized second malignancy expected to influence the person's lifespan; history or evidence of systemic autoimmune disease; known HIV infection; active infections requiring oral or IV antibiotics; any other known infection with a biological agent that can cause a severe disease and posed a severe danger to laboratory personnel working on participants' blood or tissue; received study drug within any clinical study within 4 weeks before sunitinib start; serious intercurrent illness, which according to the investigator, posed an undue risk for the person when participating in the trial; < 12 months since myocardial infarction, severe or unstable angina, coronary or peripheral artery bypass graft or cerebrovascular event. Sample size:339. Age (years, mean): group 1: 62.2; group 0: 59.8. Sex (M/F, %): group 1: 70/30; group 0: 65/35. Prognostic factors:
|
|
| Interventions |
Group 1 (n = 204): IMA901 + sunitinib Single infusion of cyclophos 300 mg/m2 3 days prior to first vaccination, 10 intradermal vaccination IMA901 + GM‐CSF 75 µg. 1 cycle sunitinib prior to randomization, sunitinib 50 mg orally (4 weeks/2 weeks off). Group 0 (n = 135): sunitinib alone 1 cycle sunitinib prior to randomization, sunitinib 50 mg orally (4 weeks/2 weeks off). Cointerventions (standard‐of‐care according to local practice): not specified. |
|
| Outcomes |
OS (primary endpoint) How measured: investigator‐assessed. Time points measured: not reported. Time points reported: Kaplan‐Meier‐curves over 42 months, median OS, log rank P value. Subgroups: favourable and intermediate risk. AEs, grade ≥ 3 (secondary endpoint) How measured: investigator‐assessed, AEs, physical examinations, vital signs, haematology, clinical chemistry, urinalysis and electrocardiographic changes. Time points measured: not reported. Time points reported: most frequent (≥ 10% of participants) AEs. Subgroups: not reported. QoL: not measured. PFS (secondary endpoint) How measured: RECIST 1.1, central review and investigator analysis. Time points measured: not reported. Time points reported: Kaplan‐Meier‐curves over 24 months, median PFS, log rank P value. Subgroups: not reported. Tumour remission (secondary endpoint) How measured: RECIST 1.1, images collected centrally and interpreted by independent radiologists and oncologists who assessed the tumour images without being informed about participant's treatment and the local assessment of site investigators. Time points measured: not reported. Time points reported: best objective response. Subgroups: not reported. |
|
| Funding sources | Immatics Biotechnologies GmbH, Pfizer. | |
| Declarations of interest | CR, HS, TW: shareholders of Immatics biotechnologies GmbH. JL, DM, RM, AM, JF, AK: employees of Immatics biotechnologies GmbH. | |
| Notes | Registration: NCT01265901. Published as abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Missing information on generation of randomization, 3:2 block randomization, stratified by factors included risk group, nephrectomy and region (Western EU, US, Central Eastern EU). |
| Allocation concealment (selection bias) | Low risk | Low risk of selection bias assumed due to central allocation via fax or email (or both). |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Open‐label trial. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No performance bias on OS assumed. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No performance bias, assessment of response by blinded assessors. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No detection bias on OS assumed. |
| Incomplete outcome data (attrition bias) (OS and PFS) | Low risk | No attrition bias assessed due to similar censoring in the treatment groups. |
| Incomplete outcome data (attrition bias) (safety) | Low risk | No attrition bias assessed due to reporting in the safety population of all treated participants. |
| Incomplete outcome data (attrition bias) (tumour remission) | Low risk | No attrition bias assessed due to reporting in all randomized participants. |
| Incomplete outcome data (attrition bias) (QoL) | Unclear risk | Not evaluated. |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes reported. |
| Other bias | Low risk | No other bias identified. |
AE: adverse event; CI: confidence interval; CT: computer tomography; CTC: Common Terminology Criteria; ECOG: Eastern Cooperative Oncology Group; EQ‐5D: EuroQol 5‐Dimension; EQ‐VAS: EuroQol Visual Analogue Scale; FACT‐G: Functional Assessment of Cancer Therapy ‐ General; FKSI‐15: 15‐item Kidney Symptom Index; FKSI‐DRS: Kidney Symptom Index ‐ Disease‐Related Symptoms; HR: hazard ratio; IL: interleukin; IM: intramuscular; IFN‐α: interferon‐α; IV: intravenous; KPS: Karnovsky Performance Status; LLN: lower limit of normal; mIU: milli‐international unit; mRCC: metastatic renal cell carcinoma; MRI: magnetic resonance imaging; MSKCC: Memorial Sloan‐Kettering Cancer Center; MU: million units; n: number of participants; NCI‐CTC: National Cancer Institute Common Terminology Criteria; NYHA: New York Heart Association; OS: overall survival; PFS: progression‐free survival; QoL: quality of life; RCC: renal cell carcinoma; RCT: randomized controlled trial; RECIST: Response Evaluation Criteria In Solid tumours; SAE: serious adverse event; SC: subcutaneous; ULN: upper limit of normal; VAS: visual analogue score; VEGF: vascular endothelial growth factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aass 2005 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± 13 cRA). |
| Adler 1987 | No comparison to current standard therapy as defined in review protocol (autologous tumour cells + BCG vs norprogesterone). |
| Amato 2009 | Not an RCT. |
| Amin 2015 | Not an RCT. |
| Atkins 1993 | No comparison to current standard therapy as defined per review protocol (IFN‐α + IL‐2 vs IL‐2). |
| Atzpodien 2001 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α + 5‐FU vs tamoxifen). |
| Atzpodien 2004 | No comparison to current standard therapy as defined per review protocol (SC IL‐2/SC IFN‐α + IL‐2/IV 5‐FU vs SC IL‐2/SC IFN‐α + IL‐2/IV 5‐FU/OP 13 cRA vs SC IFN‐α/IV vinblastine). |
| Atzpodien 2005 | Not mostly mRCC (stage IV) patients. |
| Atzpodien 2006 | No comparison to current standard therapy as defined per review protocol (IL‐2 + IFN‐α + PO 13cRA/inhaled + inhaled IL‐2 vs IL‐2 + IFN‐α + PO 13cRA/inhaled). |
| Bellmunt 2008 | Secondary publication to Hudes 2007. |
| Boccardo 1998 | No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 vs IFN‐α vs IL‐2). |
| Borden 1990 | No comparison to current standard therapy as defined in review protocol. |
| Bracarda 2010 | Secondary publication to Escudier 2007. |
| Bracarda 2013 | No comparison to current standard therapy as defined in review protocol (sorafenib + high‐dose IFN‐α vs sorafenib + low‐dose IFN‐α). |
| Brinkmann 2004 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α + 5‐FU vs misletoe lectin). |
| Bromwich 2002 | Not an RCT. |
| Buzogany 2001 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs vinblastine). |
| Castellano 2009 | Secondary publication to Motzer 2007. |
| Cella 2008 | Secondary publication to Motzer 2007. |
| Cella 2010 | Secondary publication to Motzer 2007. |
| Cella 2016 | Secondary publication to Motzer 2015a. |
| Choueiri 2014 | No comparison to current standard therapy as defined in review protocol (pharmacodynamic study with nivolumab). |
| Clark 2003 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Creagan 1991 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs aspirin). |
| De Mulder 1995 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± IFN‐γ). |
| Dexeus 1989 | No comparison to current standard therapy as defined in review protocol (combined chemotherapy ± IFN‐α). |
| Dillman 2003 | Study of mixed solid tumours with no separate analysis of mRCC patients. |
| Donskov 2006 | No comparison to current standard therapy as defined in review protocol (reduced dose IL‐2 ± histamine). |
| Du Bois 1997 | Study of mixed solid tumours with no separate analysis of mRCC patients. |
| Dudek 2008 | No comparison to current standard therapy as defined in review protocol (LMI vaccination + cyclophosphamide + IL‐2 vs LMI + cyclophosphamide vs LMI). |
| Dutcher 2003 | No comparison to current standard therapy as defined in review protocol (IFN‐γ + IFN‐α vs IFN‐γ). |
| Dutcher 2009 | Secondary publication to Hudes 2007. |
| Edsmyr 1985 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs pulmonal irradiation + vincristine + bleomycin). |
| Elkord 2013 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± naptumomab estafenox). |
| Escudier 2009 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs first‐line sorafenib). |
| Escudier 2010 | Secondary publication to Escudier 2007. |
| Escudier 2011 | Preliminary publication to Negrier 2011. |
| Fenton 1996 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Figlin 1999 | No comparison to current standard therapy as defined in review protocol (TIL vaccination + IL‐2 vs IL‐2). |
| Figlin 2009 | Secondary publication to Hudes 2007. |
| Flanigan 2001 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± initial nephrectomy). |
| Foon 1988 | No comparison to current standard therapy as defined in review protocol (IFN‐α + IFN‐γ vs IFN‐γ). |
| Fosså 1992 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± vinblastine). |
| Fosså 2004 | No comparison to current standard therapy as defined in study protocol (IFN‐α ± 13 cRA). |
| Fujita 1992 | No comparison to current standard therapy as defined in review protocol (IFN‐α different doses). |
| Galligioni 1996 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Gleave 1998 | No comparison to current standard therapy as defined in review protocol (IFN‐γ vs placebo). |
| Gore 2010 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs IFN‐α + IL‐2 + 5‐FU). |
| Harlin 2004 | Not an RCT. |
| Henriksson 1998 | No comparison to current standard therapy as defined in review protocol (IFN‐α + tamoxifen vs tamoxifen). |
| Jayson 1998 | No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 vs IFN‐α). |
| Jocham 2004 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Jonasch 2010 | No comparison to current standard therapy as defined in review protocol (first‐line sorafenib + IFN‐α vs sorafenib). |
| Keefe 2015 | No immunotherapeutic intervention (CRLX101 + bevacizumab). |
| Kempf 1986 | No comparison to current standard therapy as defined in review protocol (high IFN‐α vs low‐dose IFN‐α). |
| Kim 2015 | Secondary publication to Rini 2015. |
| Kinouchi 2004 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± cimetidine). |
| Kirkwood 1985 | No comparison to current standard therapy as defined in review protocol (high‐dose vs low‐dose IFN‐α). |
| Koretz 1991 | No comparison to current standard therapy as defined in review protocol (LAK vaccination + IL‐2 vs IL‐2). |
| Kriegmair 1995 | No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine vs medroxyprogesterone acetate). |
| Kwitkowski 2010 | Secondary publication to Hudes 2007. |
| Law 1995 | No comparison to current standard therapy as defined in review protocol (LAK vaccination + IL‐2 vs IL‐2). |
| Lissoni 1993 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2). |
| Lissoni 2000 | No comparison to current standard therapy as defined in review protocol (reduced‐dose IL‐2 ± melatonin). |
| Lissoni 2003 | No comparison to current standard therapy as defined in review protocol (IL‐2 + GM‐CSF vs IL‐2). |
| Liu 2012 | No comparison to current standard therapy as defined in review protocol (CIK vaccination vs IL‐2 + IFN‐α). |
| Lummen 1996 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IFN‐γ). |
| Majhail 2006 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Margolin 1997 | Study of mixed solid tumours with no separate analysis of mRCC patients. |
| McCabe 1991 | No comparison to current standard therapy as defined in review protocol (reduced‐dose IL‐2 vs LAK). |
| McDermott 2005 | No comparison to current standard therapy as defined in review protocol (high‐dose IL‐2 vs reduced‐dose IL‐2 + IFN‐α). |
| Melichar 2008 | Secondary publication to Escudier 2007. |
| Messing 2003 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Mickisch 2001 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± initial nephrectomy). |
| Motzer 2000 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± 13 cRA). |
| Motzer 2001 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2). |
| Motzer 2009 | Secondary publication to Motzer 2007. |
| Motzer 2015b | Preliminary study to Motzer 2015a. |
| Motzer 2015c | No comparison to current standard therapy as defined in review protocol (dose‐response study of nivolumab). |
| MRCRCC 1999 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs medroxyprogesterone acetate). |
| Muss 1987 | No comparison to current standard therapy as defined in review protocol (IV IFN‐α vs SC IFN‐α). |
| Naglieri 1998 | No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 vs IL‐2 + epidoxorubicin). |
| NCT00352859 | No comparison to current standard therapy as defined in review protocol (sorafenib + IFN‐α vs sorafenib + gemcitabine ‐ early termination of study because of slow accrual with no data analysis). |
| NCT00678288 | Stopped early due to slow accrual, no data analysis performed (sorafenib + IFN‐α vs sorafenib). |
| Negrier 1998 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2). |
| Negrier 2000 | No comparison to current standard therapy as defined in review protocol (IFN‐α + IL‐2 ± 5‐FU). |
| Negrier 2007 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐α vs IL‐2 vs IFN‐α vs medroxyprogesterone acetate). |
| Negrier 2008 | No comparison to current standard therapy as defined in review protocol (IV IL‐2 + IFN‐α vs SC IL‐2 + IFN‐α ). |
| Negrier 2010 | No immunotherapeutic intervention (sorafenib vs placebo). |
| Neidhart 1991 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± vinblastine). |
| Osband 1990 | No comparison to current standard therapy as defined in review protocol (cimetidine + ALT vaccination vs cimetidine). |
| Otto 1988 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± vinblastine). |
| Oudard 2011 | Secondary publication to Motzer 2007. |
| Passalacqua 2010 | No comparison to current standard therapy as defined in review protocol (maintenance therapy after disease progression). |
| Passalacqua 2014 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Patel 2008 | No comparison to current standard therapy as defined in review protocol (study A. no RCT; study B: IL‐2 + SRL172 vaccination vs IL‐2). |
| Patil 2012 | Secondary publication to Motzer 2007. |
| Pedersen 1980 | No comparison to current standard therapy as defined in review protocol (nephrectomy ± plasma). |
| Pickering 2009 | Secondary publication to Motzer 2007. |
| Pizzocaro 2001 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Porzsolt 1988 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± medroxyprogesterone acetate). |
| Powles 2015 | No immunotherapeutic intervention. |
| Procopio 2011 | No comparison to current standard therapy as defined in review protocol (first‐line sorafenib + IL‐2 vs sorafenib). |
| Pyrhönen 1999 | No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine vs vinblastine). |
| Quesada 1985 | No comparison to current standard therapy as defined in review protocol (high‐dose vs low‐dose IFN‐α). |
| Radosavljevic 2000 | No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine ± medroxyprogesterone acetate). |
| Ravaud 2015 | No comparison to current standard therapy (IFN + bevacizumab vs everolimus + bevacizumab). |
| Reddy 2006 | Preliminary publication to Motzer 2007. |
| Rini 2004 | Protocol to Rini 2010. |
| Rini 2008 | Preliminary publication without OS to Rini 2010. |
| Rini 2012 | No immunotherapeutic intervention (sorafenib ± AMG 386). |
| Rini 2014 | No comparison to current standard therapy (IFN + bevacizumab vs temsirolimus + bevacizumab). |
| Rosenberg 1993 | No comparison to current standard therapy as defined in review protocol (LAK vaccination + IL‐2 vs IL‐2). |
| Rossi 2010 | No comparison to current standard therapy as defined in review protocol (dose‐response study of siltuximab). |
| Sagaster 1995 | No comparison to current standard therapy as defined in review protocol (IFN‐α ± coumarin + cimetidine). |
| Scardino 1997 | No comparison to current standard therapy as defined in review protocol (postoperative + preoperative IL‐2 vs postoperative IL‐2). |
| Schwaab 2000 | No comparison to current standard therapy as defined in review protocol (AV vaccination + IFN‐α + IFN‐γ (together with AV) vs AV + IFN‐α + IFN‐γ (after initiation of AV)). |
| Sharma 2015 | Preliminary study to Motzer 2015a. |
| Simons 1997 | No comparison to current standard therapy as defined in review protocol (autologous vaccine ± GM‐CSF). |
| Smith 2003 | Study of mixed solid tumours with no separate analysis of mRCC patients. |
| Soret 1996 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Steineck 1990 | No comparison to current standard therapy as defined in review protocol (IFN‐α vs medroxyprogesterone acetate). |
| Sternberg 2013 | No immunotherapeutic intervention (pazopanib vs placebo). |
| Summers 2010 | Secondary publication to Escudier 2007. |
| Tannir 2006 | No comparison to current standard therapy as defined in review protocol (intermediate‐dose vs low‐dose IFN‐α). |
| Tsavaris 2000 | No comparison to current standard therapy as defined in review protocol (IFN‐α + vinblastine vs IFN‐α). |
| Voss 2015 | No immunotherapeutic intervention (CRLX101 + bevacizumab vs SOC). |
| Walter 2012 | No comparison to current standard therapy as defined in review protocol (cyclophosphamide + IMA 901 vaccination vs IMA 901 vaccination). |
| Wang 2015 | Not an RCT. |
| Weiss 1992 | No comparison to current standard therapy as defined in review protocol (continuous IL‐2 vs bolus IL‐2 + LAK vaccination). |
| Witte 1995 | No comparison to current standard therapy as defined in review protocol (IL‐2 + IFN‐β vs IL‐2). |
| Wood 2008 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Yang 1995 | No comparison to current standard therapy as defined in review protocol (IL‐2 + PEG‐IL‐2 vs IL‐2). |
| Yang 2003 | No comparison to current standard therapy as defined in review protocol (IL‐2 different doses). |
| Yang 2007 | Not an RCT. |
| Yang 2010 | Secondary publication to Hudes 2007. |
| Zhan 2012 | Not mostly mRCC (stage IV) patients (adjuvant study). |
| Zhao 2015 | No comparison to current standard therapy as defined in review protocol (DC‐CIK vaccination vs IL‐2 + IFN‐α). |
5‐FU: 5‐fluorouracil; ALT: alanine transaminase; CIK: cytokine‐induced killer; DC‐CIK: dendritic cell cytokine‐induced killer; GM‐CSF: granulocyte‐macrophage colony‐stimulating factor; IL: interleukin; IV: intravenous; LAK: lymphokine‐activated killer; mRCC: metastatic renal cell carcinoma; PEG‐IL‐2: pegylated interferon‐2; PO: per os (orally); RCT: randomized controlled trial; SC: subcutaneous; SOC: standard of care.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | NIVOSWITCH: a Randomized Phase II Study with NIVOlumab or Continuation of Therapy as an Early SWITCH Approach in Patients with Advanced or Metastatic Renal Cell Carcinoma (RCC) and Disease Control after 3 Months of Treatment with a Tyrosine Kinase Inhibitor. |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Setting: multicentre, international, phase II. Countries: Europe. |
| Participants |
Main inclusion criteria: aged ≥ 18
years, either gender, histological confirmation of RCC with a
clear‐cell component, ECOG Performance Status 0 to 2, metastatic or
locally advanced RCC with clear‐cell component, not amenable to
surgery with curative intention, first‐line treatment with a TKI for
10 to 12 weeks (limited to sunitinib or pazopanib), people with measurable
disease (RECIST 1.1), adequate blood count, liver‐enzymes, and renal
function. Main exclusion criteria: prior systemic therapy other than 10 to 12 weeks SOC TKI treatment, complete remission or progression during SOC TKI first‐line treatment, termination of first‐line treatment with TKI due to intolerance, prior therapy with antitumour vaccines, anti‐PD‐L1, anti‐PD1, anti‐CTLA‐4, or other immunomodulatory antitumour agents, known chronic infection and intercurrent illness. Sample size planned: 244. |
| Interventions |
Group 1: nivolumab after TKI (sunitinib or pazopanib)
and disease control. Group 0: pazopanib after TKI (sunitinib or pazopanib) and disease control. |
| Outcomes |
Primary outcome: 24 months OS. Secondary outcomes: best overall response, PFS, QoL, safety, other. |
| Starting date | September 2016. |
| Contact information | AIO‐Studien‐gGmbH, Dr Aysun Karatas, info@aio‐studien‐ggmbh.de. |
| Notes | Sponsor protocol no: AIO‐NZK‐0116. |
| Trial name or title | ADAPT: an International Phase 3 Randomised Trial of Autologous Dendritic Cell Immunotherapy (AGS‐003) Plus Standard Treatment of Advanced Renal Cell Carcinoma (NCT01582672). |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Setting: multicentre, international, phase III. Countries: Canada, Czech Republic, Hungary, Israel, Italy, Spain, UK, US. |
| Participants |
Main inclusion criteria: aged ≥ 18
years; either gender; histological confirmation of advanced RCC with
predominantly clear‐cell histology; advanced disease; metastatic
disease (measurable or non‐measurable per RECIST 1.1); people who
were candidates for standard first‐line therapy initiating with
sunitinib; time from diagnosis to treatment < 1 year; KPS ≥
70%; life expectancy ≥ 6 months; resolution of all acute toxic
effects of prior radiotherapy or surgical procedures to grade ≤ 1
(NCI‐CTC 4.0); adequate haematological, renal, hepatic and
coagulation function; negative serum pregnancy test for women with
reproductive potential and agreement of both men and women of reproductive
potential to use a reliable form of contraception during the study and for
12 weeks after the last dose of study drug. Main exclusion criteria: prior systemic therapy of any type for RCC, including immunotherapy, chemotherapy, hormonal or investigational therapy; prior history of malignancy within the preceding 3 years, except for adequately treated in situ carcinomas or non‐melanoma skin cancer; adequately treated early‐stage breast cancer, superficial bladder cancer and non‐metastatic prostate cancer with a normal PSA; history of, or known, brain metastases; spinal cord compression, carcinomatous meningitis or evidence of brain or leptomeningeal disease; people with ≥ 4 of the following risk factors: haemoglobin < LLN, corrected calcium > 10 mg/dL, KPS < 80%, neutrophils > ULN, platelets > ULN, planned or elective surgical treatment postnephrectomy for the direct management of RCC, within 28 days before visit 1, NCI CTCAE grade 3 haemorrhage < 28 days before day 0, clinically significant comorbidities. Sample size planned:450. |
| Interventions |
Group 1: AGS‐003 + standard treatment
(sunitinib). Group 0: standard treatment (sunitinib). |
| Outcomes |
Primary outcome: OS, duration from randomization to
death. Secondary outcomes: PFS, tumour response, AEs. |
| Starting date | November 2012. |
| Contact information | Robert Figlin, MD, principal Investigator. |
| Notes | Final data collection date for primary outcome measure: April 2017. |
| Trial name or title | (CheckMate 214): A Phase 3, Randomised, Open‐Label Study of Nivolumab Combined with Ipilimumab versus Sunitinib Monotherapy in Subjects with Previously Untreated, Advanced or Metastatic Renal Cell Carcinoma (NCT02231749). |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Setting: multicentre, international, phase III. Countries: Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, Colombia, Czech Republic, Denmark, Finland, France, Germany, Hungary, Ireland, Israel, Italy, Japan, Korea, Republic of, Mexico, Netherlands, Poland, Spain, Sweden, Taiwan, Turkey, UK, US. |
| Participants |
Main inclusion criteria: aged ≥ 18
years, either gender, histological confirmation of RCC with a
clear‐cell component, advanced or metastatic (AJCC Stage IV) RCC, no
prior systemic therapy for RCC with predefined exceptions (regular adjuvant
or neoadjuvant therapy), KPS ≥ 70%, measurable disease (RECIST
1.1), archival or recent tumour tissue. Exclusion criteria: cerebral metastases; prior systemic treatment with VEGF or VEGF receptor targeted therapy; prior treatment with an anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibody or any other antibody or drug specifically targeting T‐cell costimulation or checkpoint pathways; any active or recent history of a known or suspected autoimmune disease or recent history of a syndrome that required systemic corticosteroids or immunosuppressive medications except for syndromes which would not be expected to recur in the absence of an external trigger; vitiligo or type 1 diabetes mellitus or residual hypothyroidism due to autoimmune thyroiditis only requiring hormone replacement are permitted to enrol, any condition requiring systemic treatment with corticosteroids or other immunosuppressive medications within 14 days prior to first dose of study drug; inhaled steroids and adrenal replacement steroid doses > 10 mg daily; prednisone equivalents are permitted in the absence of active autoimmune disease. Sample size planned:1070. |
| Interventions |
Group 1: nivolumab 3 mg/kg + ipilimumab 1 mg/kg. Group 0: sunitinib 50 mg. |
| Outcomes |
Primary outcome: OS, PFS (coprimary). Secondary outcomes: ORR, safety. |
| Starting date | October 2014. |
| Contact information | Sponsor: Bristol‐Myers Squibb. |
| Notes | Estimated primary completion date: May 2019. |
| Trial name or title | CARMENA: Randomised Phase III Trial Evaluating the Importance of Nephrectomy in Patients Presenting with Metastatic Renal Cell Carcinoma Treated with Sunitinib. |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Setting: phase III, multicentre, national. Countries: France. |
| Participants |
Main inclusion criteria: aged ≥ 18
years, either gender, ECOG Performance Status 0 or 1, biopsy (primary tumour
or metastases) confirming the diagnosis of clear‐cell carcinoma,
documented metastatic disease, absence of prior systemic treatment for
kidney cancer including antiangiogenic, tumour amenable to nephrectomy in
the opinion of the patient's urologist, patients for whom the
indication of sunitinib is considered according to the recommendation rules
given by national health authorities of participating countries,
prescription of sunitinib in the circumstances of the study is considered as
a standard treatment, people with predefined adequate organ function. Main exclusion criteria: prior systemic treatment for kidney cancer, bilateral kidney cancer, pregnant or breastfeeding women, specified comorbidities, symptomatic brain metastases. Sample size planned: 576. |
| Interventions |
Group 1: nephrectomy + sunitinib. Group 0: sunitinib. |
| Outcomes |
Primary outcome: OS. Secondary outcome: ORR, PFS. |
| Starting date | September 2009. |
| Contact information | Principal Investigator: Arnaud Mejean, MD PhD, arnaud.mejean@nck.aphp.fr. |
| Notes | Estimated primary completion date: September 2019. |
| Trial name or title | IMmotion 150: a Phase II, Randomised Study of Atezolizumab Administered as Monotherapy or In Combination with Bevacizumab versus Sunitinib In Patients with Untreated Advanced Renal Cell Carcinoma. |
| Methods |
Study design: 3‐arm, parallel‐group,
open‐label RCT. Setting: phase II, multicentre, international. Countries: Czech Republic, France, Germany, Italy, Poland, Romania, Spain, UK, US. |
| Participants |
Main inclusion criteria: aged ≥18 years,
either gender, unresectable advanced or metastatic renal cell carcinoma with
component of clear‐cell histology or component of sarcomatoid
histology that has not been previously treated with any systemic agents (or
both), including treatment in the adjuvant setting, measurable disease, as
defined by RECIST v1.1, KPS ≥ 70, adequate haematological and
end‐organ function as defined by protocol. Main exclusion criteria: cerebral metastases; radiotherapy for RCC within 28 days prior to cycle 1; uncontrolled pleural effusion, pericardial effusion or ascites; pregnancy and lactating women; life expectancy < 12 weeks. Sample size planned: 305. |
| Interventions |
Group 1: atezolizumab + bevacizumab. Group 1.1: atezolizumab (following PD ‐ atezolizumab + bevacizumab). Group 0: sunitinib (following PD ‐ atezolizumab + bevacizumab). |
| Outcomes |
Primary outcome: PFS central reading. Secondary outcomes: PFS investigator assessed, ORR, OS. |
| Starting date | January 2014. |
| Contact information | Sponsor: Hoffmann La Roche. |
| Notes | Estimated primary completion date: September 2018. |
| Trial name or title | A Phase I/II Study to Assess the Safety and Efficacy of Pazopanib and MK 3475 in Subjects with Advanced Renal Cell Carcinoma. |
| Methods |
Study design: part 1. non‐randomized dose
escalation, part 2: 3‐arm, parallel‐group, open‐label
RCT. Setting: multicentre, international. Countries: UK, US. |
| Participants |
Inclusion criteria: aged ≥ 18 years,
either gender, people with histologically confirmed advanced or mRCC,
measurable disease, no prior systemic therapy, ECOG Performance Status 0 or
1, adequate organ function. Exclusion criteria: cerebral metastases, active autoimmune disease, pregnancy, history of a malignancy (other than the disease under treatment in the study) within 5 years. Sample size planned:228. |
| Interventions |
Group 1: MK 3475 (pembrolizumab) + pazopanib. Group 1.1: MK 3475 (pembrolizumab). Group 0: pazopanib. |
| Outcomes |
Primary outcome: part 2: PFS. Secondary outcomes: part 2: ORR, OS, safety. |
| Starting date | December 2013. |
| Contact information | Sponsor: Novartis Pharmaceuticals. |
| Notes | Estimated primary completion date: May 2021. |
| Trial name or title | KEYNOTE 029 ‐ a Phase I/II Clinical Trial to Study the Safety and Tolerability of MK‐3475 + Pegylated Interferon Alfa‐2b (PEG‐IFN) and MK‐3475 + Ipilimumab (IPI) in Subjects with Advanced Melanoma (MEL) and Renal Cell Carcinoma (RCC). |
| Methods |
Study design: 3‐arm, parallel‐group,
open‐label RCT. Setting: multicentre, international. Countries: Australia, New Zealand, US. |
| Participants |
Inclusion criteria: aged ≥ 18 years,
either gender, people with histologically confirmed advanced or metastatic
melanoma or RCC, RCC participants must have received ≥ 1 prior
line of therapy for metastatic disease, ECOG Performance Status of 0 or 1,
adequate organ function. Exclusion criteria: cerebral metastases, diagnosis of immunodeficiency or receiving systemic steroid therapy, additional malignancy, active infection requiring systemic therapy, pregnancy, breastfeeding women. Sample size planned:343. |
| Interventions |
Group 1: pembrolizumab + PegIFN‐2b. Group 1.1: pembrolizumab + ipilimumab. Group 0: pembrolizumab. |
| Outcomes |
Primary outcome: dose‐limiting toxicities, AE,
PFS. Secondary outcomes: ORR, OS. |
| Starting date | March 2014. |
| Contact information | Sponsor: Merck Sharp & Dohme Corp. |
| Notes | Estimated primary completion date: April 2017. |
| Trial name or title | A Pilot Randomised Tissue‐Based Study Evaluating Anti‐PD1 Antibody or Anti‐PD1 + Bevacizumab or Anti‐PD1 + Anti‐CTLA‐4 in Patients with Metastatic Renal Cell Carcinoma who are Eligible for Cytoreductive Nephrectomy, Metastasectomy or Post‐Treatment Biopsy. |
| Methods |
Study design: 3‐arm, parallel‐group,
double‐blind RCT. Setting: multicentre. Countries: US. |
| Participants |
Main inclusion criteria: informed consent;
histologically or cytologically confirmed clear‐cell mRCC who are
eligible for cytoreductive nephrectomy; metastasectomy or
post‐treatment biopsy; diagnosis must be confirmed by pathologist
review of screening biopsy; measurable disease defined as a lesion that can
be accurately measured in at least 1 dimension and measures ≥
15 mm with conventional techniques or ≥ 10 mm with more
sensitive techniques such as MRI or spiral CT scan; prior treatment for RCC
including prior surgery, radiotherapy, immunotherapy with IL‐2 or
interferon (but not anti‐PD1 or anti‐CTLA‐4); target
therapy with RTK inhibitors/mTOR inhibitors, such as sunitinib, sorafenib,
pazopanib, axitinib, everolimus and temsirolimus (but not bevacizumab) or
chemotherapy allowed; ECOG Performance Status 0 or 1. Main exclusion criteria: any other malignancy from which the person has been disease‐free for < 2 years, except for non‐melanoma skin cancer, in situ carcinoma of any site, organ allografts, major surgical procedure, open biopsy or significant traumatic injury with poorly healed wound within 6 weeks prior to first dose of study drug; or anticipation of need for major surgical procedure during the course of the study (other than in protocol); autoimmune disease; known history of testing positive for HIV or known AIDS; positive test for hepatitis B virus or positive test for hepatitis C virus. Sample size planned: 60. |
| Interventions |
Group 1: nivolumab. Group 1.1: nivolumab + bevacizumab. Group 1.2: nivolumab + ipilimumab. |
| Outcomes |
Primary outcome: safety. Secondary outcomes: immunological changes in tumour tissue, ORR. |
| Starting date | November 2014. |
| Contact information | Padmanee Sharma, MD, PhD. |
| Notes | Estimated primary data: November 2018. |
| Trial name or title | A Phase III, Open‐Label, Randomised Study of Atezolizumab (Anti‐PD‐L1 Antibody) in Combination with Bevacizumab versus Sunitinib in Patients with Untreated Advanced Renal Cell Carcinoma. |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT. Setting: multicentre, international, phase III. Countries: Australia, Bosnia and Herzegovina, Brazil, Canada, Czech Republic, Denmark, France, Germany, Italy, Japan, Korea, Republic of, Mexico, Poland, Russian Federation, Serbia, Singapore, Spain, Taiwan, Thailand, Turkey, Ukraine, UK, US. |
| Participants |
Inclusion criteria: aged ≥ 18 years,
definitive diagnosis of unresectable locally advanced or mRCC with
clear‐cell histology or a component of sarcomatoid carcinoma (or
both), with no prior treatment in the metastatic setting, evaluable MSKCC
risk score, measurable disease (RECIST 1.1), KPS ≥ 70%,
adequate haematological and end‐organ function. Exclusion criteria: radiotherapy for RCC within 14 days prior to treatment; central nervous system disease; uncontrolled pleural effusion, pericardial effusion or ascites; uncontrolled hypercalcaemia; any other malignancies within 5 years except for low‐risk prostate cancer or those with negligible risk of metastasis or death; life expectancy < 12 weeks. Sample size planned:830. |
| Interventions |
Group 1: atezolizumab (MPDL3280A ‐
PD‐L1 AB) + bevacizumab. Group 0: sunitinib. |
| Outcomes |
Primary outcome: PFS, OS. Secondary outcomes: ORR, safety, pharmacokinetics. |
| Starting date | May 2015. |
| Contact information | global.rochegenentechtrials@roche.com. |
| Notes | Primary results expected: June 2020. |
| Trial name or title | An Open‐Label, Randomised, Controlled, Multicenter, Phase II Study Evaluating Safety and Efficacy of Intratumourally Administered Intuvax Pre‐nephrectomy Followed by Sunitinib Post‐Nephrectomy, Compared to Sunitinib Post‐Nephrectomy in Metastatic Renal Cell Carcinoma Patients (MERECA). |
| Methods |
Study design: 2‐arm, parallel‐group,
open‐label RCT, 2:1 randomization. Setting: multicentre, phase II. Countries: Sweden. |
| Participants |
Main inclusion criteria: aged ≥ 18
years, either gender, informed consent, recent (< 6 months) diagnosed RCC
with at least 1 CT‐verified metastasis, planned resection of primary
tumour, primary tumour diameter ≥ 4 cm, candidate for standard
first‐line therapy with sunitinib, adequate haematological parameters
and liver function. Main exclusion criteria: life expectancy < 4 months; active autoimmune disease requiring treatment with systemic immunosuppressive agents, e.g. inflammatory bowel disease, multiple sclerosis, sarcoidosis, psoriasis, autoimmune haemolytic anaemia, rheumatoid arthritis, systemic lupus erythematosus, vasculitis, Sjögren's syndrome, scleroderma and autoimmune hepatitis; treatment with systemic corticosteroids within 7 days before screening, known cardiomyopathy or clinical significant finding in electrocardiography at screening (or both); KPS < 70%. Sample size planned:90. |
| Interventions |
Group 1: intuvax + nephrectomy + sunitinib. Group 0: nephrectomy + sunitinib. |
| Outcomes |
Primary outcome: median OS from randomization for
high‐risk patients, 18‐month OS in the
intermediate‐risk mRCC patients. Secondary outcomes: safety, PFS. |
| Starting date | April 2015. |
| Contact information | henrik.elofsson@immunicum.com. |
| Notes | Estimated primary completion date: February 2018. |
| Trial name or title | JAVELIN RENAL 101 ‐ A Phase 3, Multinational, Randomised, Open‐Label, Parallel‐Arm Study of Avelumab (MSB0010718C) in Combination with Axitinib (Inlyta(Registered)) versus Sunitinib (Sutent(Registered)) Monotherapy in the First‐Line Treatment of Patients with Advanced Renal Cell Carcinoma. |
| Methods |
Study design: parallel‐arm, open‐label
RCT. Setting: multicentre, international phase III. Countries: Japan, US. |
| Participants |
Main inclusion criteria: aged ≥ 18
years; either gender; histologically or cytologically confirmed advanced or
mRCC with clear‐cell component; availability of a recent
formalin‐fixed, paraffin‐embedded tumour tissue block;
≥ 1 measurable lesion (RECIST 1.1) not previously irradiated;
ECOG Performance Status 0 or 1; adequate bone marrow function, renal and
liver functions. Main exclusion criteria: prior systemic therapy directed at advanced or mRCC; prior adjuvant or neoadjuvant therapy for RCC if disease progression or relapse has occurred during or within 12 months after the last dose of treatment; prior immunotherapy with IL‐2, IFN‐α or anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibody (including ipilimumab) or any other antibody or drug specifically targeting T cell costimulation or immune checkpoint pathways; prior therapy with axitinib or sunitinib (or both) and any prior therapies with other VEGF pathway inhibitors; known severe hypersensitivity reactions to monoclonal antibodies (grade ≥ 3), any history of anaphylaxis or uncontrolled asthma; any of the following in the previous 6 months: myocardial infarction, severe/unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure, cerebrovascular accident, transient ischaemic attack, deep vein thrombosis or symptomatic pulmonary embolism; vaccination within 4 weeks of the first dose of avelumab and while on trial is prohibited except for administration of inactivated vaccines. Sample size planned: 583. |
| Interventions |
Group 1: avelumab in combination with axitinib. Group 0: sunitinib. |
| Outcomes |
Primary outcome: PFS. Secondary outcomes: OS, ORR, QoL, safety. |
| Starting date | March 2016. |
| Contact information | Pfizer CT.gov call centre. |
| Notes | Estimated primary completion date: June 2018. |
| Trial name or title | Nivolumab and Stereotactic Ablative Radiation Therapy versus Nivolumab Alone for Metastatic Renal Cancer. |
| Methods |
Study design: parallel‐arm, open‐label
RCT. Setting: national, phase II. Countries: US. |
| Participants |
Main inclusion criteria: aged 18 to 100 years, either
gender, pathological diagnosis of metastatic RCC with clear‐cell
component, measurable disease in at least 2 non‐radiated sites,
progression or intolerance to ≥ 1 prior systemic antiangiogenic
therapy, ECOG Performance Status 0, 1, 2 or 3, adequate organ and marrow
function. Main exclusion criteria: major surgery within 2 weeks prior to first dose; prior treatment with any anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2, anti‐CD137 or anti‐CTLA‐4 antibody or any other antibody or drug specifically targeting T‐cell costimulation or checkpoint pathways; active known or suspected autoimmune disease; history of hypersensitivity to monoclonal antibodies. Sample size planned: 87. |
| Interventions |
Group 1: nivolumab. Group 0: nivolumab with radiation. |
| Outcomes |
Primary outcome: ORR. Secondary outcomes: OS, PFS, safety, other. |
| Starting date | June 2016. |
| Contact information | Raquibu Hannan, MD, PhD, tel: +1 214‐645‐8525. |
| Notes | Contact: Jean Wu, RN, MSN, OCN. |
| Trial name or title | KEYNOTE‐426 ‐ Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK‐3475) in Combination with Axitinib versus Sunitinib Monotherapy in Participants with Renal Cell Carcinoma. |
| Methods |
Study design: parallel‐arm, open‐label
RCT. Setting: multicentre, international phase III. Countries: Japan, Hungary, Spain, Korea, Russia, US. |
| Participants |
Main inclusion criteria: aged ≥ 18
years, either gender, histologically or cytologically confirmed advanced or
mRCC (stage IV) with clear‐cell component with or without sarcomatoid
features, availability of an archival tumour tissue sample or fresh biopsy,
measurable disease (RECIST 1.1), no previously systemic therapy, KPS
≥ 70%. Main exclusion criteria: prior treatment with VEGF receptor or mTOR targeting agents; prior treatment with anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2 or other immunotherapy; known severe hypersensitivity reactions; active autoimmune disease; active infection; major surgery within 4 weeks prior to randomization; heart failure NYHA III or IV. Sample size planned: 840. |
| Interventions |
Group 1: pembrolizumab and axitinib. Group 0: sunitinib. |
| Outcomes |
Primary outcome: PFS, OS. Secondary outcomes: ORR, safety. |
| Starting date | September 2016. |
| Contact information | Merck Sharp & Dohme Corp. |
| Notes | Estimated primary completion date: December 2019; EudraCT: 2016‐000588‐17. |
AE: adverse event; AJCC: American Joint Committee on Cancer; CT: computer tomography; CTLA‐4: cytotoxic T‐lymphocyte antigen 4; ECOG: Eastern Cooperative Oncology Group; IL: interleukin; IFN‐α: interferon‐α; KPS: Karnovsky Performance Status; LLN: lower limit of normal; mRCC: metastatic renal cell carcinoma; MRI: magnetic resonance imaging; MSKCC: Memorial Sloan‐Kettering Cancer Center; mTOR: mammalian target of rapamycin; NCI‐CTC: National Cancer Institute Common Terminology Criteria; NCI CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NYHA: New York Heart Association; ORR: objective response rate; PD: programmed death; PD‐1: programmed death‐1; PFS: progression‐free survival; PSA: prostate‐specific antigen; QoL: quality of life; RCC: renal cell carcinoma; RCT: randomized controlled trial; RECIST: Response Evaluation Criteria In Solid tumours; SOC: standard of care; ULN: upper limit of normal; VEGF: vascular endothelial growth factor.
Contributions of authors
SU: wrote the protocol, contributed to search strategy development, performed the systematic search, extracted data, judged risk of bias and quality of evidence, summarized treatment effects and wrote the review.
IM: wrote the protocol, contributed to search strategy development, scanned the abstract or title, read all potentially relevant records as full text, screened ongoing trials, extracted data, judged risk of bias and wrote the review.
MN: graded the evidence and critically commented the review.
DR: scanned the abstract or title, read all potentially relevant records as full text, extracted data and judged risk of bias.
AVH: extracted data, judged risk of bias and critically commented the review.
FP: extracted data, judged risk of bias and critically commented the review.
FG: wrote the protocol and screened ongoing trials.
BS: wrote the protocol, contributed to search strategy development and critically commented the review.
Sources of support
Internal sources
-
Wilhelm‐Roux‐Programme, Martin Luther University Halle‐Wittenberg, Germany.
Financial support (grant number 26/18)
External sources
-
Federal Ministry of Education and Research, Germany.
Financial support (grant number: 01KG1402)
Declarations of interest
SU: reported the following financial activities related to the submitted work: institution received support from the Federal Ministry of Education and Research, Germany (grant number: 01KG1024) and from the Wilhelm‐Roux‐Program, Martin Luther University Halle‐Wittenberg, Germany, to write this review.
IM: reported the following financial activities related to the submitted work: institution received support from the Wilhelm‐Roux‐Program, Martin Luther University Halle‐Wittenberg, Germany, for travel to meetings for this review.
MN: none known.
DR: reported the following financial activities related to the submitted work: institution received support from the Wilhelm‐Roux‐Program, Martin Luther University Halle‐Wittenberg, Germany, for travel to meetings for this review.
AVH: none known.
FP: none known.
FG: none known.
BS: none known.
Notes
We have based parts of the Methods section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by the Cochrane Urology Group.
New
References
References to studies included in this review
- Amato RJ, Hawkins RE, Kaufman HL, Thompson JA, Tomczak P, Szczylik C, et al. Vaccination of metastatic renal cancer patients with MVA‐5T4: a randomized, double‐blind, placebo‐controlled phase III study. Clinical Cancer Research 2010;16(22):5539‐47. [DOI: 10.1158/1078-0432.CCR-10-2082] [DOI] [PubMed] [Google Scholar]
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa‐2a for treatment of metastatic renal cell carcinoma: a randomised, double‐blind phase III trial. Lancet 2007;370(9605):2103‐11. [DOI: 10.1200/JCO.2009.26.7849] [DOI] [PubMed] [Google Scholar]
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. New England Journal of Medicine2007; Vol. 356, issue 22:2271‐81. [DOI] [PubMed]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson D, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. New England Journal of Medicine 2007;356(2):115‐24. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. New England Journal of Medicine 2015;373(19):1803‐13. [DOI: 10.1056/NEJMoa1510665] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrier S, Gravis G, Perol D, Chevreau C, Delva R, Bay JO, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncology 2011;12(7):673‐80. [DOI] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. Journal of Clinical Oncology 2010;28(13):2137‐43. [DOI: 10.1200/JCO.2009.26.5561] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B, Stenzl A, Zdrojowy R, Kogan M, Shkolnik M, Oudard S, et al. Results from an open‐label, randomized, controlled Phase 3 study investigating IMA901 multipeptide cancer vaccine in patients receiving sunitinib as first‐line therapy for advanced/metastatic RCC. European Cancer Congress. 2015:17LBA. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aass N, Mulder PH, Mickisch GH, Mulders P, Oosterom AT, Poppel H, et al. Randomized phase II/III trial of interferon Alfa‐2a with and without 13‐cis‐retinoic acid in patients with progressive metastatic renal cell Carcinoma: the European Organisation for Research and Treatment of Cancer Genito‐Urinary Tract Cancer Group (EORTC 30951). Journal of Clinical Oncology2005; Vol. 23, issue 18:4172‐8. [DOI] [PubMed]
- Adler A, Gillon G, Lurie H, Shaham J, Loven D, Shachter Y, et al. Active specific immunotherapy of renal cell carcinoma patients: a prospective randomized study of hormono‐immuno versus hormonotherapy. Preliminary report of immunological and clinical aspects. Journal of Biological Response Modifiers 1987;6(6):610‐24. [PubMed] [Google Scholar]
- Amato RJ, Shingler W, Goonewardena M, Belin J, Naylor S, Jac J, et al. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon‐alpha (IFN‐alpha): a phase 2 trial. Journal of Immunotherapy2009; Vol. 32, issue 7:765‐72. [DOI] [PubMed]
- Amin A, Dudek AZ, Logan TF, Lance RS, Holzbeierlein JM, Knox JJ, et al. Survival with AGS‐003, an autologous dendritic cell‐based immunotherapy, in combination with sunitinib in unfavourable risk patients with advanced renal cell carcinoma (RCC): phase 2 study results. Journal for Immunotherapy of Cancer 2015;3:14. [DOI: 10.1186/s40425-015-0055-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins MB, Sparano J, Fisher RI, Weiss GR, Margolin KA, Fink KI, et al. Randomized phase II trial of high‐dose interleukin‐2 either alone or in combination with interferon alfa‐2b in advanced renal cell carcinoma. Journal of Clinical Oncology1993; Vol. 11, issue 4:661‐70. [DOI] [PubMed]
- Atzpodien J, Kirchner H, Illiger HJ, Metzner B, Ukena D, Schott H, et al. IL‐2 in combination with IFN‐alpha and 5‐FU versus tamoxifen in metastatic renal cell carcinoma: long‐term results of a controlled randomized clinical trial. British Journal of Cancer 2001;85(8):1130‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, et al. Interleukin‐2‐ and interferon alfa‐2a‐based immunochemotherapy in advanced renal cell carcinoma: a prospectively randomized trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). Journal of Clinical Oncology2004; Vol. 22, issue 7:1188‐94. [DOI] [PubMed]
- Atzpodien J, Schmitt E, Gertenbach U, Fornara P, Heynemann H, Maskow A, et al. Adjuvant treatment with interleukin‐2‐ and interferon‐alpha2a‐based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). British Journal of Cancer 2005;92(5):843‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzpodien J, Kirchner H, Rebmann U, Soder M, Gertenbach U, Siebels M, et al. Interleukin‐2/interferon‐alpha2a/13‐retinoic acid‐based chemoimmunotherapy in advanced renal cell carcinoma: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). British Journal of Cancer 2006;95(4):463‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J, Szczylik C, Feingold J, Strahs A, Berkenblit A. Temsirolimus safety profile and management of toxic effects in patients with advanced renal cell carcinoma and poor prognostic features. Annals of Oncology 2008;19(8):1387‐92. [DOI] [PubMed] [Google Scholar]
- Boccardo F, Rubagotti A, Canobbio L, Galligioni E, Sorio R, Lucenti A, et al. Interleukin‐2, interferon‐alpha and interleukin‐2 plus interferon‐alpha in renal cell carcinoma. A randomized phase II trial. Tumori 1998;84(5):534‐9. [DOI] [PubMed] [Google Scholar]
- Borden EC, Rinehart JJ, Storer BE, Trump DL, Paulnock DM, Teitelbaum AP. Biological and clinical effects of interferon‐beta ser at two doses. Journal of Interferon Research1990; Vol. 10, issue 6:559‐70. [DOI] [PubMed]
- Bracarda S, Bellmunt J, Melichar B, Negrier S, Bajetta E, Ravaud A, et al. Overall survival in patients with metastatic renal cell carcinoma initially treated with bevacizumab plus interferon‐α2a and subsequent therapy with tyrosine kinase inhibitors: a retrospective analysis of the phase III AVOREN trial. BJU International 2010;107:214‐9. [DOI: 10.1111/j.1464-410X.2010.09707.x] [DOI] [PubMed] [Google Scholar]
- Bracarda S, Porta C, Boni C, Santoro A, Mucciarini C, Pazzola A, et al. Could interferon still play a role in metastatic renal cell carcinoma? A randomized study of two schedules of sorafenib plus interferon‐alpha 2a (RAPSODY). European Urology2013; Vol. 63, issue 2:254‐61. [DOI] [PubMed]
- Brinkmann OA, Hertle L. Combined cytokine therapy vs mistletoe treatment in metastatic renal cell cancer. Clinical comparison of therapy success with combined administration of interferon‐alpha2b, interleukin‐2, and 5‐fluorouracil compared to treatment with mistletoe lectin [in German]. Onkologe 2004;10(9):978‐85. [Google Scholar]
- Bromwich E, Hendry D, Aitchison M. Cytoreductive nephrectomy: is it a realistic option in patients with renal cancer?. BJU International 2002;89(6):523‐5. [DOI] [PubMed] [Google Scholar]
- Buzogany I, Feher G, Kocsis J, Molnar I, Nagy G, Laszlo K, et al. Interferon alfa‐2b versus interferon alfa 2b + vinblastin treatment in advanced renal cell carcinoma; randomized multicenter trial [Intron‐A vs Intron‐A‐vinbalstin‐kezeles hatekonysaganak es bistonsagossaganak vizsgalata ketkaru, randomizalt multicentrikus vizsgalatban]. Magyar Urologia 2001;13(1):19‐31. [Google Scholar]
- Castellano D, Muro XG, Perez‐Gracia JL, Gonzalez‐Larriba JL, Abrio MV, Ruiz MA, et al. Patient‐reported outcomes in a phase III, randomized study of sunitinib versus interferon‐{alpha} as first‐line systemic therapy for patients with metastatic renal cell carcinoma in a European population. Annals of Oncology 2009;20(11):1803‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Li JZ, Cappelleri JC, Bushmakin A, Charbonneau C, Kim ST, et al. Quality of life in patients with metastatic renal cell carcinoma treated with sunitinib or interferon alfa: results from a phase III randomized trial. Journal of Clinical Oncology 2008;26(22):3763‐9. [DOI] [PubMed] [Google Scholar]
- Cella D, Michaelson MD, Bushmakin AG, Cappelleri JC, Charbonneau C, Kim ST, et al. Health‐related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon‐alpha in a phase III trial: final results and geographical analysis. British Journal of Cancer 2010;102(4):658‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Grünwald V, Nathan P, Doan J, Dastani H, Taylor F, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open‐label, phase 3 trial. Lancet Oncology 2016;17:994‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri TK, Fishman MN, Escudier B, McDermott DF, Kluger H, Stadler WM, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma (mRCC): association of biomarkers with clinical outcomes. Asia‐Pacific Journal of Clinical Oncology 2015;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JI, Atkins MB, Urba WJ, Creech S, Figlin RA, Dutcher JP, et al. Adjuvant high‐dose bolus interleukin‐2 for patients with high‐risk renal cell carcinoma: a cytokine working group randomized trial. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 2003;21(16):3133‐40. [DOI] [PubMed] [Google Scholar]
- Creagan ET, Twito DI, Johansson SL, Schaid DJ, Johnson PS, Flaum MA, et al. A randomized prospective assessment of recombinant leukocyte a human interferon with or without aspirin in advanced renal adenocarcinoma. Journal of Clinical Oncology1991; Vol. 9, issue 12:2104‐9. [DOI] [PubMed]
- Mulder PH, Oosterhof G, Bouffioux C, Oosterom AT, Vermeylen K, Sylvester R. EORTC (30885) randomised phase III study with recombinant interferon alpha and recombinant interferon alpha and gamma in patients with advanced renal cell carcinoma. The EORTC Genitourinary Group. British Journal of Cancer 1995;71(2):371‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexeus FH, Logothetis CJ, Sella A, Finn L. Interferon alternating with chemotherapy for patients with metastatic renal cell carcinoma. American Journal of Clinical Oncology1989; Vol. 12, issue 4:350‐4. [DOI] [PubMed]
- Dillman RO, Wiemann M, Nayak SK, DeLeon C, Hood K, DePriest C. Interferon‐gamma or granulocyte‐macrophage colony‐stimulating factor administered as adjuvants with a vaccine of irradiated autologous tumor cells from short‐term cell line cultures: a randomized phase 2 trial of the cancer biotherapy research group. Journal of Immunotherapy 2003;26(4):367‐73. [DOI] [PubMed] [Google Scholar]
- Donskov F, Hokland M, Marcussen N, Torp Madsen HH, Maase H. Monocytes and neutrophils as 'bad guys' for the outcome of interleukin‐2 with and without histamine in metastatic renal cell carcinoma ‐ results from a randomised phase II trial. British Journal of Cancer 2006;94(2):218‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Bois JS, Trehu EG, Mier JW, Shapiro L, Epstein M, Klempner M, et al. Randomized placebo‐controlled clinical trial of high‐dose interleukin‐2 in combination with a soluble p75 tumor necrosis factor receptor immunoglobulin G chimera in patients with advanced melanoma and renal cell carcinoma. Journal of Clinical Oncology 1997;15(3):1052‐62. [DOI] [PubMed] [Google Scholar]
- Dudek AZ, Mescher MF, Okazaki I, Math VT, Luo X, Curtsinger JM, et al. Autologous large multivalent immunogen vaccine in patients with metastatic melanoma and renal cell carcinoma. American Journal of Clinical Oncology 2008;31(2):173‐81. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Fine JP, Krigel RL, Murphy BA, Schaefer PL, Ernstoff MS, et al. Stratification by risk factors predicts survival on the active treatment arm in a randomized phase II study of interferon‐gamma plus/minus interferon‐alpha in advanced renal cell carcinoma (E6890). Medical Oncology 2003;20(3):271‐81. [DOI] [PubMed] [Google Scholar]
- Dutcher JP, Souza P, McDermott D, Figlin RA, Berkenblit A, Thiele A, et al. Effect of temsirolimus versus interferon‐alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Medical Oncology 2009;26(2):202‐9. [DOI] [PubMed] [Google Scholar]
- Edsmyr F, Esposti PL, Andersson L. Interferon therapy in disseminated renal cell carcinoma. Radiotherapy and Oncology 1985;4(1):21‐6. [DOI] [PubMed] [Google Scholar]
- Elkord E, Burt DJ, Sundstedt A, Nordle Ö, Hedlund G, Hawkins RE. Immunological response and overall survival in a subset of advanced renal cell carcinoma patients from a randomized phase 2/3 study of naptumomab estafenox plus IFN‐a versus IFN‐a. Oncotarget 2015;6(6):4428‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first‐line treatment with sorafenib versus interferon alfa‐2a in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(8):1280‐9. [DOI] [PubMed] [Google Scholar]
- Escudier B, Bellmunt J, Negrier S, Bajetta E, Melichar B, Bracarda S, et al. Phase III trial of bevacizumab plus interferon alfa‐2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. Journal of Clinical Oncology 2010;28(13):2144‐50. [DOI: 10.1200/JCO.2009.26.7849] [DOI] [PubMed] [Google Scholar]
- Escudier BJ, Negrier S, Perol D, Gravis G, Delva R, Bay J, et al. Prognostic factors for progression‐free survival (PFS) in patients with metastatic renal cell carcinoma (mRCC): results from the French randomized phase II study TORAVA. Journal of Clinical Oncology 2011;29(Suppl):15. [Google Scholar]
- Fenton RG, Steis RG, Madara K, Zea AH, Ochoa AC, Janik JE, et al. A phase I randomized study of subcutaneous adjuvant IL‐2 in combination with an autologous tumor vaccine in patients with advanced renal cell carcinoma. Journal of Immunotherapy 1996;19(5):364‐74. [DOI] [PubMed] [Google Scholar]
- Figlin RA, Thompson JA, Bukowski RM, Vogelzang NJ, Novick AC, Lange P, et al. Multicenter, randomized, phase III trial of CD8(+) tumor‐infiltrating lymphocytes in combination with recombinant interleukin‐2 in metastatic renal cell carcinoma. Journal of Clinical Oncology1999; Vol. 17, issue 8:2521‐9. [DOI] [PubMed]
- Figlin RA, Souza P, McDermott D, Dutcher JP, Berkenblit A, Thiele A, et al. Analysis of PTEN and HIF‐1alpha and correlation with efficacy in patients with advanced renal cell carcinoma treated with temsirolimus versus interferon‐alpha. Cancer 2009;115(16):3651‐60. [DOI] [PubMed] [Google Scholar]
- Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa‐2b compared with interferon alfa‐2b alone for metastatic renal‐cell cancer. New England Journal of Medicine 2001;345(23):1655‐9. [DOI] [PubMed] [Google Scholar]
- Foon K, Doroshow J, Bonnem E, Fefer A, Graham S, Grosh B, et al. A prospective randomized trial of alpha 2B‐interferon/gamma‐interferon or the combination in advanced metastatic renal cell carcinoma. Journal of Biological Response Modifiers1988; Vol. 7, issue 6:540‐5. [PubMed]
- Fosså SD, Martinelli G, Otto U, Schneider G, Wander H, Oberling F, et al. Recombinant interferon alfa‐2a with or without vinblastine in metastatic renal cell carcinoma: results of a European multi‐center phase III study. Annals of Oncology1992; Vol. 3, issue 4:301‐5. [DOI] [PubMed]
- Fosså SD, Mickisch GH, Mulder PH, Horenblas S, Oosterom AT, Poppel H, et al. Interferon‐alpha‐2a with or without 13‐cis retinoic acid in patients with progressive, measurable metastatic renal cell carcinoma. Cancer 2004;101(3):533‐40. [DOI] [PubMed] [Google Scholar]
- Fujita T, Fukushima M. Interferon therapy for advanced renal cell carcinoma [in Japanese]. Hinyokika Kiyo ‐ Acta Urologica Japonica 1992;38(11):1293‐8. [MEDLINE: ] [PubMed] [Google Scholar]; Fujita T, Inagaki J, Asano H, Naide Y, Ono Y, Ohshima S, et al. Effects of low‐dose interferon‐alpha (Hlbi) following nephrectomy in metastatic renal cell carcinoma. 28th Annual Meeting of the American Society of Clinical Oncology; 1992 May 17‐19; San Diego (CA); A685. [MEDLINE: ]
- Galligioni E, Quaia M, Merlo A, Carbone A, Spada A, Favaro D, et al. Adjuvant immunotherapy treatment of renal carcinoma patients with autologous tumor cells and bacillus Calmette‐Guerin: five‐year results of a prospective randomized study. Cancer 1996;77(12):2560‐6. [DOI] [PubMed] [Google Scholar]
- Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, et al. Interferon gamma‐1b compared with placebo in metastatic renal‐cell carcinoma. Canadian Urologic Oncology Group. New England Journal of Medicine1998; Vol. 338, issue 18:1265‐71. [DOI] [PubMed]
- Gore ME, Griffin CL, Hancock B, Patel PM, Pyle L, Aitchison M, et al. Interferon alfa‐2a versus combination therapy with interferon alfa‐2a, interleukin‐2, and fluorouracil in patients with untreated metastatic renal cell carcinoma (MRC RE04/EORTC GU 30012): an open‐label randomised trial. Lancet 2010;375(9715):641‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Artz AS, Mahowald M, Rini BI, Zimmerman T, Vogelzang NJ, et al. Clinical responses following nonmyeloablative allogeneic stem cell transplantation for renal cell carcinoma are associated with expansion of CD8+ IFN‐gamma‐producing T cells. Bone Marrow Transplantation 2004;33(5):491‐7. [DOI] [PubMed] [Google Scholar]
- Henriksson R, Nilsson S, Colleen S, Wersall P, Helsing M, Zimmerman R, et al. Survival in renal cell carcinoma‐a randomized evaluation of tamoxifen vs interleukin 2, alpha‐interferon (leucocyte) and tamoxifen. British Journal of Cancer 1998;77(8):1311‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayson GC, Middleton M, Lee SM, Ashcroft L, Thatcher N. A randomized phase II trial of interleukin 2 and interleukin 2‐interferon alpha in advanced renal cancer. British Journal of Cancer 1998;78(3):366‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham D, Richter A, Hoffmann L, Iwig K, Fahlenkamp D, Zakrzewski G, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal‐cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 2004;363(9409):594‐9. [DOI] [PubMed] [Google Scholar]
- Jonasch E, Corn P, Pagliaro LC, Warneke CL, Johnson MM, Tamboli P, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low‐dose interferon alfa in patients with advanced renal cell carcinoma ‐ clinical and biomarker analysis. Cancer 2010;116(1):57‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe SM, Hoffmann‐Censits JH, Mamtani R, Walicki M, Robinson J, Smith A, et al. HIF inhibition in metastatic renal cell carcinoma (mRCC): final results of a phase Ib/IIa clinical trial evaluating the nanoparticle drug conjugate (NDC), CRLX101, in combination with bevacizumab (bev). Journal of Clinical Oncology 2015;33(Suppl 1):15. [Google Scholar]
- Kempf RA, Grunberg SM, Daniels JR, Skinner DG, Venturi CL, Spiegel R, et al. Recombinant interferon alpha‐2 (INTRON A) in a phase II study of renal cell carcinoma. Journal of Biological Response Modifiers 1986;5(1):27‐35. [PubMed] [Google Scholar]
- Kim H, Halabi S, Li P, Mayhew G, Simko J, Nixon A, et al. A prognostic model for overall survival in patients with metastatic clear cell renal carcinoma: results from CALGB 90206 (alliance). Journal of Urology 2015;193(Suppl):e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouchi T, Sakamoto J, Tsukamoto T, et al. Prospective randomized trial of natural interferon‐alpha (IFN) versus IFN + cimetidine in advanced renal cell carcinoma with pulmonary metastasis. 40th Annual Meeting of the American Society of Clinical Oncology; 2004 June 5‐8; New Orleans (LA); A4676. [DOI] [PMC free article] [PubMed]
- Kirkwood JM, Harris JE, Vera R. A randomized study of low and high doses of leukocyte alpha‐interferon in metastatic renal cell carcinoma: the American Cancer Society Collaborative Trial. Cancer Research 1985;45(2):863‐71. [PubMed] [Google Scholar]
- Koretz MJ, Lawson DH, York RM, Graham SD, Murray DR, Gillespie TM, et al. Randomized study of interleukin 2 (IL‐2) alone vs IL‐2 plus lymphokine‐activated killer cells for treatment of melanoma and renal cell cancer. Archives of Surgery 1991;126(7):898‐903. [DOI] [PubMed] [Google Scholar]
- Kriegmair M, Oberneder R, Hofstetter A. Interferon alfa and vinblastine versus medroxyprogesterone acetate in the treatment of metastatic renal cell carcinoma. Urology 1995;45(5):758‐62. [DOI] [PubMed] [Google Scholar]
- Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist 2010;15(4):428‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law TM, Motzer RJ, Mazumdar M, Sell KW, Walther PJ, O'Connell M, et al. Phase III randomized trial of interleukin‐2 with or without lymphokine‐activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995;76(5):824‐32. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Barni S, Ardizzoia A, Andres M, Scardino E, Cardellini P, et al. A randomized study of low‐dose interleukin‐2 subcutaneous immunotherapy versus interleukin‐2 plus interferon‐alpha as first line therapy for metastatic renal cell carcinoma. Tumori 1993;79(6):397‐400. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Mandala, Brivio F. Abrogation of the negative influence of opioids on IL‐2 immunotherapy of renal cell cancer by melatonin. European Urology 2000;38:115‐8. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Mengo S, Bucovec R, Brivio F, Fumagalli L, Tancini G, et al. Clinical and biological effects of interleukin‐2 with or without a concomitant administration of granulocyte‐macrophage colony‐stimulating factor in metastatic cancer patients. In Vivo 2003;17(1):73‐5. [PubMed] [Google Scholar]
- Liu L, Zhang W, Qi X, Li H, Yu J, Wei S, et al. Randomized study of autologous cytokine‐induced killer cell immunotherapy in metastatic renal carcinoma. Clinical Cancer Research 2012;18(6):1751‐9. [DOI] [PubMed] [Google Scholar]
- Lummen G, Goepel M, Mollhoff S, Hinke A, Otto T, Rubben H. Phase II study of interferon‐gamma versus interleukin‐2 and interferon‐alpha 2b in metastatic renal cell carcinoma. Journal of Urology 1996;155(2):455‐8. [PubMed] [Google Scholar]
- Majhail NS, Wood L, Elson P, Finke J, Olencki T, Bukowski RM. Adjuvant subcutaneous interleukin‐2 in patients with resected renal cell carcinoma: a pilot study. Clinical Genitourinary Cancer 2006;5(1):50‐6. [DOI] [PubMed] [Google Scholar]
- Margolin K, Atkins M, Sparano J, Sosman J, Weiss G, Lotze M, et al. Prospective randomized trial of lisofylline for the prevention of toxicities of high‐dose interleukin 2 therapy in advanced renal cancer and malignant melanoma. Clinical Cancer Research 1997;3(4):565‐72. [PubMed] [Google Scholar]
- McCabe MS, Stablein D, Hawkins MJ. The Modified Group C experience ‐ phase III randomized trials of IL‐2 vs IL‐2+LAK in advanced renal cell carcinoma and advanced melanoma. 35th Annual Meeting of the American Society of Clinical Oncology; 1999 May 15‐18; Atlanta, GA; A714.
- McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high‐dose interleukin‐2 versus subcutaneous interleukin‐2 and interferon in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology2005; Vol. 23, issue 1:133‐41. [DOI] [PubMed]
- Melichar B, Koralewski P, Ravaud A, Pluzanska A, Bracarda S, Szczylik C, et al. First‐line bevacizumab combined with reduced dose interferon‐alpha2a is active in patients with metastatic renal cell carcinoma. Annals of Oncology 2008;19(8):1470‐6. [DOI] [PubMed] [Google Scholar]
- Messing EM, Manola J, Wilding G, Propert K, Fleischmann J, Crawford ED, et al. Phase III study of interferon alfa‐NL as adjuvant treatment for resectable renal cell carcinoma: an Eastern Cooperative Oncology Group/Intergroup trial. Journal of Clinical Oncology 2003;21(7):1214‐22. [DOI] [PubMed] [Google Scholar]
- Mickisch GHJ, Garin A, Poopel H, Prijck L, Sylvester R, European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon‐alfa‐based immunotherapy compared with interferon alfa alone in metastatic renal‐cell carcinoma: a randomized trial. Lancet 2001;358:966‐70. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Murphy BA, Bacik J, Schwartz LH, Nanus DM, Mariani T, et al. Phase III trial of interferon alfa‐2a with or without 13‐cis‐retinoic acid for patients with advanced renal cell carcinoma. Journal of Clinical Oncology 2000;18(16):2972‐80. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin‐12 versus interferon‐alpha 2a for patients with advanced renal cell carcinoma. Journal of Interferon & Cytokine Research 2001;21(4):257‐63. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 2009;27(22):3584‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Bono P, Hudes GR, Tomita Y, Ravaud A, Waxman I, et al. A phase III comparative study of nivolumab (anti‐PD‐1; BMS‐936558; ONO‐4538) versus everolimus in patients (pts) with advanced or metastatic renal cell carcinoma (mRCC) previously treated with antiangiogenic therapy. Journal of Clinical Oncology 2015;31(15 (suppl)):TP4592. [Google Scholar]
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel T, Harrison MR, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. Journal of Clinical Oncology 2015;33(13):1430‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Research Council Renal Cancer Collaborators. Interferon‐alpha and survival in metastatic renal cell carcinoma: early results of a randomized controlled trial. Lancet 1999;353:14‐7. [PubMed] [Google Scholar]
- Muss HB, Costanzi JJ, Leavitt R. Recombinant alfa interferon in renal cell carcinoma: a randomized trial of two routes of administration. Journal of Clinical Oncology 1987;5(2):286‐91. [DOI] [PubMed] [Google Scholar]
- Naglieri E, Gebbia V, Durini E, Lelli G, Abbate I, Selvaggi FP, et al. Standard interleukin‐2 (IL‐2) and interferon‐alpha immunotherapy versus an IL‐2 and 4‐epirubicin immuno‐chemotherapeutic association in metastatic renal cell carcinoma. Anticancer Research 1998;18(38):2021‐6. [PubMed] [Google Scholar]
- NCT00352859. A randomized discontinuation trial to determine the clinical benefit of continuation of sorafenib following disease progression in patients with advanced renal cell carcinoma, 2006. clinicaltrials.gov/ct2/show/NCT00352859 (assessed 30 May 2016).
- NCT00678288. A phase II, randomized, open‐label, multicenter, study evaluating the efficacy of sorafenib alone and sorafenib in combination with low dose interferon alpha‐2a as second‐line treatment of sunitinib failure in patients with metastatic renal cell carcinoma, 2008. clinicaltrials.gov/ct2/show/NCT00678288 (assessed 30 May 2016).
- Escudier B, Chevreau C, Lasset C, Douillard JY, Ravaud A, Fabbro M, et al. Cytokines in metastatic renal cell carcinoma: is it useful to switch to interleukin‐2 or interferon after failure of a first treatment? Groupe Francais d'Immunotherape. Journal of Clinical Oncology 1999;17(7):2039‐43. [DOI] [PubMed] [Google Scholar]; Negrier S, Escudier B, Lasset C, Douillard J Y, Savary J, Chevreau C, et al. Recombinant human interleukin‐2, recombinant human interferon alfa‐2a, or both in metastatic renal‐cell carcinoma. Groupe Français d'Immunothérapie. New England Journal of Medicine 1998;338(18):1272‐8. [DOI] [PubMed] [Google Scholar]
- Negrier S, Caty A, Lesimple T, Douillard JY, Escudier B, Rossi JF, et al. Treatment of patients with metastatic renal carcinoma with a combination of subcutaneous interleukin‐2 and interferon alfa with or without fluorouracil. Groupe Français d'Immunothérapie, Fédération Nationale des Centres de Lutte Contre le Cancer. Journal of Clinical Oncology2000; Vol. 18, issue 24:4009‐15. [DOI] [PubMed]
- Negrier S, Perol D, Ravaud A, Chevreau C, Bay JO, Delva R, et al. Medroxyprogesterone, interferon alfa‐2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer 2007;110(11):2468‐77. [DOI] [PubMed] [Google Scholar]
- Negrier S, Perol D, Ravaud A, Bay JO, Oudard S, Chabaud S, et al. Randomized study of intravenous versus subcutaneous interleukin‐2, and IFNalpha in patients with good prognosis metastatic renal cancer. Clinical Cancer Research 2008;14(18):5907‐12. [DOI] [PubMed] [Google Scholar]
- Negrier S, Jager E, Porta C, McDermott D, Moore M, Bellmunt J, et al. Efficacy and safety of sorafenib in patients with advanced renal cell carcinoma with and without prior cytokine therapy, a subanalysis of TARGET. Medical Oncology 2010;27(3):899‐906. [DOI] [PubMed] [Google Scholar]
- Neidhart JA, Anderson SA, Harris JE, Rinehart JJ, Laszlo J, Dexeus FH, et al. Vinblastine fails to improve response of renal cancer to interferon alfa‐n1: high response rate in patients with pulmonary metastases. Journal of Clinical Oncology 1991;9(5):832‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Osband ME, Lavin PT, Babayan RK, Graham S, Lamm W, Parker B, et al. Effect of autolymphocyte therapy on survival and quality of life in patients with metastatic renal‐cell carcinoma. Lancet 1990;335(8696):994‐8. [DOI] [PubMed] [Google Scholar]
- Otto U, Schneider A, Denkhaus H, Conrad S. Treatment of metastatic kidney cancer with recombinant alpha‐2 or gamma interferon. Results of 2 clinical phase II and III studies [Die Behandlung des metastasierenden Nierenkarzinoms mit rekombinantem alpha‐2‐ oder gamma‐Interferon. Ergebnisse zweier klinischer Phase‐II‐ bzw. Phase III‐Studien]. Onkologie 1988;11(4):185‐91. [DOI] [PubMed] [Google Scholar]
- Oudard S, Beuselinck B, Decoene J, Albers P. Sunitinib for the treatment of metastatic renal cell carcinoma. Cancer Treatment Reviews 2011;37(3):178‐84. [DOI] [PubMed] [Google Scholar]
- Passalacqua R, Buzio C, Buti S, Porta C, Labianca R, Pezzuolo D, et al. Phase III, randomised, multicentre trial of maintenance immunotherapy with low‐dose interleukin‐2 and interferon‐alpha for metastatic renal cell cancer. Cancer Immunology, Immunotherapy 2010;59(4):553‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passalacqua R, Caminiti C, Buti S, Porta C, Camisa R, Braglia L, et al. Adjuvant low‐dose interleukin‐2 (IL‐2) plus interferon‐alpha (IFN‐alpha) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian oncology group for clinical research (GOIRC). Journal of Immunotherapy 2014;37(9):440‐7. [DOI] [PubMed] [Google Scholar]
- Patel PM, Sim S, O'Donnell DO, Protheroe A, Beirne D, Stanley A, et al. An evaluation of a preparation of Mycobacterium vaccae (SRL172) as an immunotherapeutic agent in renal cancer. European Journal of Cancer 2008;44(2):216‐23. [DOI] [PubMed] [Google Scholar]
- Patil S, Figlin RA, Hutson TE, Michaelson MD, Negrier S, Kim ST, et al. Q‐TWiST analysis to estimate overall benefit for patients with metastatic renal cell carcinoma treated in a phase III trial of sunitinib vs interferon‐alpha. British Journal of Cancer 2012;106(10):1587‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F, Hippe E, Hvidt V, Francis D, Jensen HS, Dybkjaer E, et al. Treatment of metastasizing renal adenocarcinoma with specific plasma transfusion. A controlled trial of the effects on metastases and survival time [in Danish] [Behandling af metastaserende adenocarcinoma renis med specifik plasmatransfusion]. Ugeskrift for Laeger 1980;142(48):3167‐70. [MEDLINE: ] [PubMed] [Google Scholar]
- Pickering LM, Pyle L, Larkin JMG. Sunitinib is superior to interferon alfa with respect to quality of life for patients with renal cell carcinoma. Nature Review Clinical Oncology 2009;6(1):6‐7. [DOI] [PubMed] [Google Scholar]
- Pizzocaro G, Piva L, Colavita M, Ferri S, Artusi R, Boracchi P, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. Journal of Clinical Oncology 2001;19(2):425‐31. [DOI] [PubMed] [Google Scholar]
- Porzsolt F, Messerer D, Hautmann R, Gottwald A, Sparwasser H, Stockamp K, et al. Treatment of advanced renal cell cancer with recombinant interferon alpha as a single agent and in combination with medroxyprogesterone acetate. A randomized multicenter trial. Journal of Cancer Research & Clinical Oncology 1988;114(1):95‐100. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Wheater MJ, Din O, Geldart TR, Boleti E, Stockdale A, et al. A randomized phase II study of AZ2014 versus everolimus in patients with VEGF refractory metastatic clear cell renal cancer (mRCC). Journal of Clinical Oncology 2015;33(Suppl 1):7. [DOI] [PubMed] [Google Scholar]
- Procopio G, Verzoni E, Bracarda S, Ricci S, Sacco C, Ridolfi L, et al. Sorafenib with interleukin‐2 vs sorafenib alone in metastatic renal cell carcinoma: the ROSORC trial. British Journal of Cancer 2011;104(8):1256‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrhönen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, et al. Prospective randomized trial of interferon alfa‐2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. Journal of Clinical Oncology1999; Vol. 17, issue 9:2859‐67. [DOI] [PubMed]
- Quesada JR, Rios A, Swanson D. Antitumor activity of recombinant‐derived interferon alpha in metastatic renal cell carcinoma. Journal of Clinical Oncology 1985;3(11):1522‐8. [DOI] [PubMed] [Google Scholar]
- Radosavljevic D, Jelic S, Babovic N, Popov I, Kreacic M, Stamatovic L, et al. Addition of medroxyprogesterone‐acetate to interferon‐a ‐ vinblastine combination in advanced renal‐cell carcinoma ‐ is there any impact on quality of life?. Annals of Oncology 2000;11 Suppl 4:332P. [Google Scholar]
- Ravaud A, Barrios C, Alekseev B, Tay MH, Agarwala SS, Yalcin S, et al. RECORD‐2: phase II randomized study of everolimus and bevacizumab versus interferon α‐2a and bevacizumab as first‐line therapy in patients with metastatic renal cell carcinoma. Annals of Oncology 2015;26(7):1378‐84. [DOI: 110.1093/annonc/mdv170] [DOI] [PubMed] [Google Scholar]
- Reddy K. Phase III study of sunitinib malate (SU11248) versus interferon‐alpha as first‐line treatment in patients with metastatic renal cell carcinoma. Clinical Genitourinary Cancer 2006;5(1):23‐5. [DOI] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Taylor J, Small EJ, Schilsky RL. Cancer and Leukemia Group B 90206: a randomized phase III trial of interferon‐alpha or interferon‐alpha plus anti‐vascular endothelial growth factor antibody (bevacizumab) in metastatic renal cell carcinoma. Clinical Cancer Research 2004;10(8):2584‐6. [DOI] [PubMed] [Google Scholar]
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou S‐S, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. Journal of Clinical Oncology 2008;26(33):5422‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B, Szczylik C, Tannir NM, Koralewski P, Tomczak P, Deptala A, et al. AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney: a randomized, double‐blind, placebo‐controlled, phase 2 study. Cancer 2012;118(24):6152‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. Journal of Clinical Oncology 2014;32(8):752‐9. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, et al. Prospective randomized trial of high‐dose interleukin‐2 alone or in conjunction with lymphokine‐activated killer cells for the treatment of patients with advanced cancer. Journal of the National Cancer Institute 1993;85(8):622‐32. [DOI] [PubMed] [Google Scholar]
- Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, et al. A phase I/II study of siltuximab (CNTO 328), an anti‐interleukin‐6 monoclonal antibody, in metastatic renal cell cancer. British Journal of Cancer 2010;103(8):1154‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagaster P, Micksche M, Flamm J, Ludwig H. Randomised study using IFN‐alpha versus IFN‐alpha plus coumarin and cimetidine for treatment of advanced renal cell cancer. Annals of Oncology 1995;6(10):999‐1003. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scardino E, Lissoni P, Andres M, Frea B, Favini P, Kocjancic E, et al. Preoperative subcutaneous immunotherapy with interleukin‐2 in renal cell carcinoma with synchronous metastases: a clinicobiological randomized study [in Italian]. Archivio Italiano di Urologia e Andrologia 1997;69(1):49‐54. [PubMed] [Google Scholar]
- Schwaab T, Heaney JA, Schned AR, Harris RD, Cole BF, Noelle RJ, et al. A randomized phase II trial comparing two different sequence combinations of autologous vaccine and human recombinant interferon gamma and human recombinant interferon alpha2B therapy in patients with metastatic renal cell carcinoma: clinical outcome and analysis of immunological parameters. Journal of Urology 2000;163(4):1322‐7. [DOI] [PubMed] [Google Scholar]
- Sharma P, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025: a randomized, open‐label phase III study of nivolumab (NIVO) versus everolimus (EVE) in advanced renal cell carcinoma (RCC). European Journal Cancer 2015;51:S708. [Google Scholar]
- Simons JW, Jaffee EM, Weber CE, Levitsky HI, Nelson WG, Carducci MA, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte‐macrophage colony‐stimulating factor gene transfer. Cancer Research 1997;57(8):1537‐46. [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Kurt RA, Baher AG, Denman S, Justice L, Doran T, et al. Immune effects of escalating doses of granulocyte‐macrophage colony‐stimulating factor added to a fixed, low‐dose, inpatient interleukin‐2 regimen: a randomized phase I trial in patients with metastatic melanoma and renal cell carcinoma. Journal of Immunotherapy 2003;26(2):130‐8. [DOI] [PubMed] [Google Scholar]
- Soret JY, Escudier B. Adjuvant treatment with IL‐2 or interferon‐alpha in renal cell carcinoma: a French multicentric study. Cancer Biotherapy & Radiopharmaceuticals 1996;11(5):301‐2. [DOI] [PubMed] [Google Scholar]
- Steineck G, Strander H, Carbin BE, Borgström E, Wallin L, Achtnich U, et al. Recombinant leukocyte interferon alpha‐2a and medroxyprogesterone in advanced renal cell carcinoma. A randomized trial. Acta Oncology1990; Vol. 29, issue 2:155‐62. [DOI] [PubMed]
- Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double‐blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. European Journal of Cancer 2013;49(6):1287‐96. [DOI] [PubMed] [Google Scholar]
- Summers J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus interferon for advanced renal cell carcinoma. Oncologist 2010;15(1):104‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannir NM, Cohen L, Wang X, Thall P, Mathew PF, Jonasch E, et al. Improved tolerability and quality of life with maintained efficacy using twice‐daily low‐dose interferon‐alpha‐2b: results of a randomized phase II trial of low‐dose versus intermediate‐dose interferon‐alpha‐2b in patients with metastatic renal cell carcinoma. Cancer 2006;107(9):2254‐61. [DOI] [PubMed] [Google Scholar]
- Tsavaris N, Skarlos D, Bacoyiannis C, Aravantinos G, Kosmas C, Retalis G, et al. Combined treatment with low‐dose interferon plus vinblastine is associated with less toxicity than conventional interferon monotherapy in patients with metastatic renal cell carcinoma. Journal of Interferon & Cytokine Research2000; Vol. 20, issue 8:685‐90. [DOI] [PubMed]
- Voss MH, Plimack ER, Rini BI, Atkins MB, Alter R, Bhatt RS, et al. The DART Study: part 1 results from the dalantercept plus axitinib dose escalation and expansion cohorts in patients with advanced renal cell carcinoma (RCC). Journal of Clinical Oncology 2015;33(Suppl 1):15. [Google Scholar]
- Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single‐dose cyclophosphamide associates with longer patient survival. Nature Medicine 2012;18(8):1254‐61. [DOI] [PubMed] [Google Scholar]
- Wang H, Feng F, Zhu M, Wang R, Wang X, Wu Y, et al. Therapeutic efficacy of dendritic cells pulsed by autologous tumor cell lysate in combination with CIK cells on advanced renal cell carcinoma. Xibao Yu Fenzi Mianyixue Zazh 2015;31(1):61‐71. [PubMed] [Google Scholar]
- Weiss GR, Margolin KA, Aronson FR, Sznol M, Atkins MB, Dutcher JP, et al. A randomized phase II trial of continuous infusion interleukin‐2 or bolus injection interleukin‐2 plus lymphokine‐activated killer cells for advanced renal cell carcinoma. Journal of Clinical Oncology1992; Vol. 10, issue 2:275‐81. [DOI] [PubMed]
- Witte RS, Leong T, Emstoff MS, Krigel RL, Oken MM, Harris J, et al. A phase II study of interleukin‐2 with and without beta‐interferon in the treatment of advanced renal cell carcinoma. Investigational New Drugs 1995;13(3):241‐7. [DOI] [PubMed] [Google Scholar]
- Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An adjuvant autologous therapeutic vaccine (HSPPC‐96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open‐label, randomised phase III trial. Lancet 2008;372(9633):145‐54. [DOI] [PubMed] [Google Scholar]
- Yang JC, Topalian SL, Schwartzentruber DJ, Parkinson DR, Marincola FM, Weber JS, et al. The use of polyethylene glycol‐modified interleukin‐2 (PEG‐IL‐2) in the treatment of patients with metastatic renal cell carcinoma and melanoma. A phase I study and a randomized prospective study comparing IL‐2 alone versus IL‐2 combined with PEG‐IL‐2. Cancer 1995;76(4):687‐94. [DOI] [PubMed] [Google Scholar]
- Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high‐dose and low‐dose interleukin‐2 in patients with metastatic renal cancer. Journal of Clinical Oncology 2003;21(4):3127‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti‐CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. Journal of Immunotherapy 2007;30(8):825‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Souza P, Alemao E, Purvis J. Quality of life in patients with advanced renal cell carcinoma treated with temsirolimus or interferon‐alpha. British Journal of Cancer 2010;102(10):1456‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan HL, Gao X, Pu XY, Li W, Li ZJ, Zhou XF, et al. A randomized controlled trial of postoperative tumor lysate‐pulsed dendritic cells and cytokine‐induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma. Chinese Medical Journal 2012;125(21):3771‐7. [PubMed] [Google Scholar]
- Zhao X, Zhang Z, Huang J, Yang S, Xie T, Yue D, et al. Clinical study of cytokine‐induced killer cells based immunotherapies in different stages of renal cell carcinoma. Cytotherapy 2015;17:S20‐21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- AIO‐Studien‐gGmbH. NIVOSWITCH ‐ a randomized phase II study with NIVOlumab or continuation of therapy as an early SWITCH approach in patients with advanced or metastatic renal cell carcinoma (RCC) and disease control after 3 months of treatment with a tyrosine kinase inhibitor, 2016. www.clinicaltrialsregister.eu/ctr‐search/trial/2016‐002170‐13/DE (assessed 5 November 2016).
- Figlin RA, Wood CG. ADAPT: an ongoing international phase III randomized trial of autologous dendritic cell immunotherapy (AGS‐003) plus standard treatment in advanced renal cell carcinoma (RCC). Journal of Clinical Oncology 2014;32(Suppl 4):Abstract 449. [Google Scholar]
- Hammers HJ, Plimack E, Sternberg C, McDermott DF, Larkin J, Ravaud A, Rini BI, et al. CheckMate 214: a phase III, randomized, open‐label study of nivolumab combined with ipilimumab versus sunitinib monotherapy in patients with previously untreated metastatic renal cell carcinoma. Journal of Clinical Oncology 2015;33(Suppl):Abstract TPS4578. [Google Scholar]
- NCT0930033. CARMENA: randomized phase III trial evaluating the importance of nephrectomy in patients presenting with metastatic renal cell carcinoma treated with sunitinib, 2009. clinicaltrials.gov/ct2/show/NCT0930033 (assessed 24 March 2016).
- NCT01984242. Phase 2 study of atezolizumab (an engineered anti‐PDL1 antibody) as monotherapy or in combination with avastin (bevacizumab) compared to sunitinib in patients with untreated advanced renal cell carcinoma [IMmotion150], 2013. clinicaltrials.gov/ct2/show/NCT01984242 (assessed 24 March 2016).
- NCT02014636. Safety and efficacy study of pazopanib and MK 3475 in advanced renal cell carcinoma (RCC), 2013. clinicaltrials.gov/ct2/show/NCT02014636 (assessed 24 March 2016).
- NCT02089685. Safety and tolerability of pembrolizumab (MK‐3475) + pegylated interferon alfa‐2b and pembrolizumab + ipilimumab in participants with advanced melanoma or renal cell carcinoma (MK‐3475‐029/KEYNOTE‐29), 2014. clinicaltrials.gov/ct2/show/NCT02089685 (assessed 24 March 2016).
- NCT02210117. Nivolumab vs nivolumab + bevacizumab vs nivolumab + ipilimumab in metastatic renal cell carcinoma (mRCC), 2014. clinicaltrials.gov/ct2/show/NCT02210117 (assessed 24 March 2016).
- NCT02420821. A study of atezolizumab in combination with bevacizumab versus sunitinib in participants with untreated advanced renal cell carcinoma, 2015. clinicaltrials.gov/ct2/show/NCT02420821 (assessed 24 March 2016).
- NCT02432846. Intratumoral vaccination with intuvax pre‐nephrectomy followed by sunitinib post‐nephrectomy vs sunitinib post‐nephrectomy in newly diagnosed metastatic renal cell carcinoma (mRCC) (MERECA), 2015. clinicaltrials.gov/ct2/show/NCT02432846 (assessed 24 March 2016).
- NCT02684006. JAVELIN RENAL 101 ‐ a phase 3, multinational, randomized, open‐label, parallel‐arm study of avelumab (MSB0010718C) in combination with axitinib (Inlyta(Registered)) versus sunitinib (Sutent(Registered)) monotherapy in the first‐line treatment of patients with advanced renal cell carcinoma, 2016. clinicaltrials.gov/ct2/show/NCT02684006 (assessed 01 May 2016).
- NCT02781506. Nivolumab and stereotactic ablative radiation therapy versus nivolumab alone for metastatic renal cancer, 2016. clinicaltrials.gov/ct2/show/NCT02781506 (assessed 05 November 2016).
- NCT02853331. KEYNOTE‐426 ‐ a study to evaluate the efficacy and safety of pembrolizumab (MK‐3475) in combination with axitinib versus sunitinib monotherapy in participants with renal cell carcinoma, 2016. clinicaltrials.gov/ct2/show/NCT02853331 (assessed 05 November 2016).
Additional references
- Abe H, Kamai T. Recent advances in the treatment of metastatic renal cell carcinoma. International Journal of Urology 2013;20(10):944‐55. [DOI: 10.1111/iju.12187] [DOI] [PubMed] [Google Scholar]
- Albiges L, Choueiri T, Escudier B, Galsky M, George D, Hofmann F, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. European Urology 2015;67(1):100‐10. [DOI: 10.1016/j.eururo.2014.04.006] [DOI] [PubMed] [Google Scholar]
- Armstrong AJ, Broderick S, Eisen T, Stadler WM, Jones RJ, Garcia JA, et al. Final clinical results of a randomized phase II international trial of everolimus vs. sunitinib in patients with metastatic non‐clear cell renal cell carcinoma (ASPEN). Journal of Clinical Oncology 2015;15(Suppl 1):Abstract 4507. [Google Scholar]
- Athar U, Gentile TC. Treatment options for metastatic renal cell carcinoma: a review. Canadian Journal of Urology 2008;15(2):3954‐66. [PubMed] [Google Scholar]
- Bedke J, Gouttefangeas C, Singh‐Jasuja H, Stevanović S, Behnes C‐L, Stenzl A. Targeted therapy in renal cell carcinoma: moving from molecular agents to specific immunotherapy. World Journal of Urology 2014;32(1):31‐8. [DOI: 10.1007/s00345-013-1033-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedke J, Kruck S, Gakis G, Stenzl A, Goebell PJ. Checkpoint modulation ‐ a new way to direct the immune system against renal cell carcinoma. Human Vaccines and Immunotherapeutics 2015;11(5):1201‐8. [DOI: 10.1080/21645515.2015.1016657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman‐May S, Burger M, Wieland WF, Rössler W, May M, Denzinger S. Vaccination therapy in renal cell carcinoma: current position and future options in metastatic and localized disease. Expert Reviews of Vaccines 2011;10(6):837‐52. [DOI: 10.1586/erv.11.64] [DOI] [PubMed] [Google Scholar]
- Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT Scales), Version 4. Evanston (IL): Northwestern Healthcare & Northwestern University, Center on Outcomes, Research and Education, 1997. [Google Scholar]
- Ciccarese C, Alfieri S, Santoni M, Santini D, Brunelli M, Bergamini C, et al. New toxicity profile for novel immunotherapy agents: focus on immune‐checkpoint inhibitors. Expert Opinion on Drug Metabolism and Toxicology 2016;12(1):57‐75. [DOI: 10.1517/17425255.2016.1120287] [DOI] [PubMed] [Google Scholar]
- Combe P, Guillebon E, Thibault C, Granier C, Tartour E, Oudard S. Trial Watch: therapeutic vaccines in metastatic renal cell carcinoma. Oncoimmunology 2015;4(5):e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin C. Immunotherapy for renal cell cancer in the era of targeted therapy. Expert Review of Anticancer Therapy 2008;8(6):907‐19. [DOI: 10.1586/14737140.8.6.907] [DOI] [PubMed] [Google Scholar]
- Desrichard A, Snyder A, Chan TA. Cancer Neoantigens and Applications for Immunotherapy. Clinical Cancer Research 2016;22(4):807‐12. [DOI: 10.1158/1078-0432.CCR-14-3175] [DOI] [PubMed] [Google Scholar]
- Draube A, Klein‐González N, Mattheus S, Brillant C, Hellmich M, Engert A, et al. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta‐analysis. PLoS One 2011;6(4):e18801. [PUBMED: 21533099] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher JP. Recent developments in the treatment of renal cell carcinoma. Therapeutic Advances in Urology 2013;5(6):338‐53. [PUBMED: 24294292] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228‐47. [DOI] [PubMed] [Google Scholar]
- Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2014;25(Suppl 3):iii49‐56. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. globocan.iarc.fr (accessed 12 February 2015).
- Figlin RA. Personalized immunotherapy (AGS‐003) when combined with sunitinib for the treatment of metastatic renal cell carcinoma. Expert Opinion on Biological Therapy 2015;15(8):1241‐8. [DOI: 10.1517/14712598.2015.1063610] [DOI] [PubMed] [Google Scholar]
- The German Guideline Programme in Oncology (German Cancer Society, German Cancer Aid, AWMF). S3‐Guideline on Diagnostics, Therapy and Follow‐up of renal cell carcinoma, long version 1.0, AWMF registration 043/017OL, 2015 [Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 1.0, AWMF Registernummer: 043/017OL]. leitlinienprogramm‐onkologie.de/Leitlinien.7.0.html (assessed 31 May 2016).
- Ghasemzadeh A, Bivalacqua TJ, Hahn NM, Drake CG. New strategies in bladder cancer: a second coming for immunotherapy. Clinical Cancer Research 2016;22(4):793‐801. [DOI: 10.1158/1078-0432.CCR-15-1135] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster University. GRADEpro GDT. Version accessed 17 April 2015. McMaster University, 2014.
- Grünwald V. T‐cell checkpoint inhibitors in metastatic renal cell carcinoma. Current Opinion in Urology 2015;25(5):411‐5. [DOI: 10.1097/MOU.0000000000000199] [DOI] [PubMed] [Google Scholar]
- Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treatment Reviews 2008;34(3):193‐205. [DOI: 10.1016/j.ctrv.2007.12.001] [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl E, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [DOI: 10.1016/j.jclinepi.2010.07.017] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI: 10.1016/j.jclinepi.2010.01.011] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI: 10.1016.j.jclinepi.2011.01.012] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI: 10.1016/j.jclinepi.2011.03.017] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [DOI: 10.1016.j.jclinepi.2011.04.014] [DOI] [PubMed] [Google Scholar]
- Heng DYC, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor‐targeted agents: results from a large, multicenter study. Journal of Clinical Oncology 2009;27(34):5794‐9. [DOI] [PubMed] [Google Scholar]
- Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal‐Cell Carcinoma Database Consortium prognostic model: a population‐based study. Lancet Oncology 2013;14(2):141‐8. [DOI: 10.1016/S1470-2045(12)70559-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al (editors). SEER cancer statistics review, 1975‐2012, National Cancer Institute. Bethesda, MD. seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER website on April 2015 (accessed 30 May 2016).
- Johannsen M, Brinkmann OA, Bergmann L, Heinzer H, Steiner T, Ringsdorf M, et al. The role of cytokine therapy in metastatic renal cell cancer. European Urology Supplements 2007;6:658‐64. [Google Scholar]
- Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ 2014;349:g4797. [DOI: 10.1136/bmj.g4797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD‐1 Blockade in tumors with mismatch‐repair deficiency. New England Journal Medicine 2015;372(26):2509‐20. [DOI: 10.1056/NEJMoa1500596] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Motzer RJ. Immune checkpoint therapy in renal cell carcinoma. Cancer Journal 2016;22(2):92‐5. [DOI: 10.1097/PPO.0000000000000177] [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg B, Campbell SC, Cho HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. European Urology 2011;60:615‐21. [DOI: 10.1016/j.eururo.2011.06.049] [DOI] [PubMed] [Google Scholar]
- Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. European Urology 2015;67(5):913‐24. [DOI] [PubMed] [Google Scholar]
- Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Martignoni G, et al. PD‐1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treatment Reviews 2015;41(2):114‐21. [DOI: 10.1016/j.ctrv.2014.12.013] [DOI] [PubMed] [Google Scholar]
- McDermott DF, Cheng SC, Signoretti S, Margolin KA, Clark JI, Sosman JA, et al. The high‐dose aldesleukin "select" trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clinical Cancer Research 2015;21(3):561‐8. [DOI: 10.1158/1078-0432.CCR-14-1520] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DF, Choueiri TK, Puzanov I, Hodi S, Drake CG, Brahmer JR, et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. Journal of Clinical Oncology 2015;33(18):2013‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Pandha HS. Renal‐cell carcinoma: tumour markers, T‐cell epitopes, and potential for new therapies. Lancet Oncology 2003;4(4):215‐23. [PUBMED: 12681265] [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bander NH, Nanus DM. Renal‐cell carcinoma. New England Journal of Medicine 1996;335(12):865‐75. [DOI: 10.1056/NEJM199609193351207] [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon‐alfa as a comparative treatment for clinical trials of new therapies against renal cell carcinoma. Journal of Clinical Oncology 2002;20(1):289‐96. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Sharma P, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 phase III trial: Outcomes by key baseline factors and prior therapy for nivolumab (NIVO) versus everolimus (EVE) in advanced renal cell carcinoma (RCC). Journal Clinical Oncology 2016;34(Suppl 2S):Abstract 498. [Google Scholar]
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
- Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ‐5D utility and VAS scores in cancer. Health and Quality of Life Outcomes 2007;5:70. [DOI: 10.1186/1477-7525-5-70] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. Journal of Clinical Oncology 2015;33(17):1974‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Staehler M, Ljungberg B, Bensalah K, Canfield SE, Dabestani S, et al. Updated EAU guidelines for clear cell renal cancer patients who fail VEGF targeted therapy. European Urology 2016;69(1):4‐6. [DOI: 10.1016/j.eururo.2015.10.017] [DOI] [PubMed] [Google Scholar]
- Rayman P, Wesa AK, Richmond AL, Das T, Biswas K, Raval G, et al. Effect of renal cell carcinomas on the development of type 1 T‐cell responses. Clinical Cancer Research 2004;10(18 Pt 2):6360S‐6S. [PUBMED: 15448031] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Ringash J, O'Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient‐reported outcomes. Cancer 2007;110(1):196‐202. [DOI] [PubMed] [Google Scholar]
- Rothermundt C, Bailey A, Cerbone L, Eisen T, Escudier B, Gillessen S, et al. Algorithms in the first‐line treatment of metastatic clear cell renal cell carcinoma ‐ analysis using diagnostic nodes. Oncologist 2015;20(9):1028‐35. [DOI: 10.1634/theoncologist.2015-0145] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a Cancer Journal for Clinicians 2014;64(1):9‐29 (erratum in: CA: a Cancer Journal for Clinicians 2014;64(5):364). [DOI: 10.3322/caac.21208] [DOI] [PubMed] [Google Scholar]
- Sobin LH, Gospodariwicz MK, Wittekind C. TNM Classification of Malignant Tumours. UICC International Union Against Cancer. 7th Edition. Hoboken (NJ): Wiley‐Blackwell, 2009:255‐257. [Google Scholar]
- Stadler WM. Maturing of renal cancer therapeutics. Journal of Clinical Oncology 2014;32(8):722‐4. [DOI] [PubMed] [Google Scholar]
- Takyar S, Diaz J, Sehgal M, Sapunar F, Pandha H. First‐line therapy for treatment‐naive patients with advanced/metastatic renal cell carcinoma: a systematic review of published randomized controlled trials. Anticancer Drugs 2016;27(5):383‐97. [DOI: 10.1097/CAD.0000000000000335] [DOI] [PubMed] [Google Scholar]
- Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD‐1 is expressed by tumor‐infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clinical Cancer Research 2007;13(6):1757‐61. [PUBMED: 17363529] [DOI] [PubMed] [Google Scholar]
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt S, Moldenhauer I, Coppin C, Greco F, Seliger B. Immunotherapy for metastatic renal cell carcinoma. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011673] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. Journal of Clinical Oncology 2015;33(18):2092‐9. [DOI: 10.1200/JCO.2014.60.0379] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [PUBMED: 15685717] [DOI] [PubMed] [Google Scholar]
- Wittekind BJ, Compton CC, Sobin LH, editor(s). A commentary on uniform use. UICC International Union Against Cancer. 4th Edition. New York (NY): Wiley‐Liss, 2001:106. [Google Scholar]
- Xu KY, Wu S. Update on the treatment of metastatic clear cell and non‐clear cell renal cell carcinoma. Biomarker Research 2015;3:5. [DOI: 10.1186/s40364-015-0030-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC. The immunotherapy roadmap. Clinical Cancer Research 2016;22(2):275‐6. [DOI: 10.1158/1078-0432.CCR-15-2144] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibelman M, Barth P, Handorf E, Smaldone MC, Kutikov A, Uzzo RG, et al. A review of international clinical trials in renal cell carcinoma: a status report from the ClinicalTrials.gov website. Clinical Genitourinary Cancer 2015;13(2):142‐9. [PUBMED: 25450029] [DOI] [PubMed] [Google Scholar]
- Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. Journal of Clinical Oncology 2002;20(23):4559‐66. [PUBMED: 12454113] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Coppin C, Porzsolt F, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD001425] [DOI] [PubMed] [Google Scholar]
- Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001425.pub2] [DOI] [PubMed] [Google Scholar]
- Coppin C, Porzsolt F, Autenrieth M, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD001425.pub2] [DOI] [PubMed] [Google Scholar]


