ABSTRACT
Background: Oral candidiasis (OC) has a profound effect on the life quality of immunocompromised patients, such as those undergoing chemotherapy. Objective: Systematic investigation of clinical outcome and microbiological features of yeast isolates recovered from the oral cavity of 150 Iranian patients with hematological malignancies. Design: MALDI-TOF MS, 21-plex PCR, and rDNA sequencing were used for identification. Antifungal susceptibility testing (broth microdilution, CLSI M27-A3/S4) and genotypic diversity of yeast isolates (amplified fragment length polymorphism) were assessed. Results: Nystatin treatment resulted in 70% therapeutic failure and administration of 150 mg fluconazole (FLZ) + nystatin for patients with OC relapse showed 70% clinical failure. Previous history of OC was significantly correlated with FLZ treatment requirement and nystatin failure (P = 0.005, α < 0.05). Candida albicans (80.3%) and Kluyveromyces marxianus (C. kefyr) (12.7%) were the two most prevalent yeast species isolated. FLZ and AMB exhibited the highest geometric mean values. 21-PCR showed 98.9% agreement with MALDI-TOF MS. K. marxianus isolates had the same genotype, while C. albicans isolates grouped in 15 genotypes. Conclusions: Marked rate of therapeutic failure of nystatin necessitated OC treatment with systemic antifungals. K. marxianus was the second most prevalent yeast and 21-plex PCR could be considered as an inexpensive identification tool.
KEYWORDS: Oral candidiasis, hematological malignancies, Nystatin, Fluconazole, 21-plex PCR, and MALDI-TOF MS
In recent decades, we have witnessed an increase in the number of patients prone to develop candidiasis [1,2]. According to epidemiological studies, more than 100 million people develop mucosal-surface fungal infections annually [3] and among these candidiasis is the most prevalent [4]. High-risk patients suffering from oral candidiasis (OC) not only experience a lower life quality [5], but importantly they may develop life-threatening invasive candidiasis, where the causative agents enter the bloodstream and cause candidemia [6,7]. It is well-known that various immunocompromised patient populations, such as those infected with HIV [8], with inherited genetic disorders, and cancer patients undergoing chemotherapy are among the most susceptible individuals to develop OC [5,9–11]. Indeed, meta-analysis studies proved that chemotherapy and radiotherapy treatments are independent risk factors for the development of OC [5,9,10].
Previous studies indicated that C. albicans is still the most common agent of OC, whereas non-albicans Candida (NAC) species, although with a lower frequency, are increasingly encountered in clinical settings [12,13]. Unfortunately, the majority of these species either intrinsically are less responsive to azole drugs [14] or they acquire resistance to antifungal agents [15]. As a result, accurate identification at the species level is of paramount importance to initiate a timely and appropriate therapeutic regimen. Nowadays, Sanger sequencing and matrix-assisted laser-desorption time-of-flight mass spectrometry (MALDI-TOF MS), owing to comprehensivity and accuracy, are being widely used in clinical settings of developed countries (European countries) [16]. However, high costs required for the purchase and maintenance of these devices hamper their use in developing countries [17,18]. In contrast, not only commercial PCR devices cost significantly less ($2 K–$4 K), but also the development of affordable and reproducible PCR devices with initial costs of $130 contributed to the popularity and extensive use of such a device in developing countries [19]. Therefore, PCR has been recommended as a reliable identification platform by the World Health Organization (WHO) [20]. Currently, Arastehfar et al., developed a comprehensive multiplex PCR that identifies the most prevalent yeast species causing infections in human [21].
Despite extensive studies on various immunocompromised populations [22–24], patients with hematological disorders were less studied in Iran. Moreover, none of the previous studies from Iran, extensively and systematically investigated the clinical outcomes that might lead to raising awareness among of clinicians. As a result, the purpose of this study was to investigate the clinical outcome, distribution, antifungal susceptibility, and genotypic diversity of yeast species isolated from the oral cavity of patients suffering from hematological disorders with oral candidiasis hospitalized in a referral hospital (Firoozgar, Tehran, Iran). Moreover, due to the lack of MALDI-TOF MS equipment in Iran, an alternative comprehensive multiplex PCR assay, 21-plex PCR, was evaluated for its identification accuracy.
Material and methods
Study design
One-hundred and 50 patients suffering from hematological malignancies undergoing chemotherapeutic regimens (1 December 2017 to 31 May 2018) retrospectively were enrolled in this study. All patients were admitted in the teaching hospital of Firoozgar, Tehran, Iran, which is affiliated with the Iran University of Medical Sciences (Tehran, Iran). Adults (>18 years old) without limitations in sex and the hematologic malignancy types (acute lymphocytic leukemia, chronic lymphocytic leukemia, acute myeloid leukemia, and chronic myeloid leukemia) were recruited to this study.
Patients’ characteristics
All patients were examined by specialist clinicians for signs and symptoms of OC. Patients that met the following criteria were considered as OC positive, (a) presence of thrush (pseudomembranous) in the oral cavity, and (b) obtaining positive yeast growth from swab samples [25]. Oral mucositis was defined by observing erythematous and ulcerative lesions and all stages of mucositis (mild, moderate and severe) were included [26]. This study was reviewed by ethical committee members of the Iran University of Medical Sciences (IUMS) and ethical approval was granted (IR.IUMS.FMD.REC.1397.098). Informed consent was obtained from all patients whose identity was anonymized to researchers through the use of a numerical code identifier (1–150). Various demographic information, such as age, gender, type of cancer, sampling season (to observe the possible seasonality pattern) [27], clinical symptoms of OC, and previous history of OC were recorded for OC positive patients.
Treatment options
In the neutropenic phase (absolute neutrophil count <500 cells/mcl), patients were prophylactically treated with Ciprofloxacin (500 mg BD/orally and Acyclovir (400 mg/TDS/orally)) [28]. All patients initially underwent treatment with topical nystatin and those with relapse were treated with the combination therapy of 150 mg fluconazole (FLZ) and topical nystatin for one week, which is not recommended by National Comprehensive Cancer Network (NCCN) clinical practice guideline [28]. Off note, owing to the research nature of this study, authors did not have any authority for the choice of antifungal agents used to treat patients recruited to this study. The respective decisions were made solely by in-charge clinicians.
Clinical specimens processing and culture conditions
Symptomatic patients were examined and positive OC cases were confirmed by specialist resident clinicians. Sterile cotton swabs, samples were taken from the tongue and buccal mucosal lesions of patients and immediately transferred into falcon tubes containing sterile phosphate buffer saline (PBS 1×).
Initially, samples were subjected to direct microscopic examination to observe the yeast structures, followed by streaking on Sabouraud dextrose agar medium (SDA, Sigma–Aldrich, USA) and incubated at 35°C for 48 h. As some oral samples might harbor more than a single species, and in order to obtain pure colonies for each species, they were streaked on CHROMagar (CHROMagar Candida, France) and incubated for 48 h at 35°C. As results obtained from the aforementioned conventional assays (direct microscopy and CHROM agar) were not definitive, hence, their respective phenotypic were not further analyzed and the identity of each isolate was determined by LSU rDNA sequencing, MALDI-TOF MS, and 21-plex PCR.
Identification of candida species and DNA extraction
Yeast isolates were identified by Bruker MALDI-TOF MS (MicroFlex-LT, Bremen, Germany) using a previously described full extraction method [29]. Scores > 2 indicated successful identification to the species level, and scores < 2 were regarded as correct identification at the genus level. Prior to proceeding with PCR identification, DNA samples were extracted by a CTAB method [30]. Using yeast species obtained from this study, the efficacy of a previously described 21-plex PCR [21] was evaluated. Species with failed identification in the first multiplex PCR were further tested by the second multiplex PCR and in case of obtaining negative results, isolates were checked by a third multiplex PCR [21]. PCR products were run on 2% agarose gel, stained with GelRed (BioTium, USA) and visualized with a gel documentation device (Gel Doc XR+, BioRad, USA). As the gold standard technique, emerging Candida isolates were further checked by sequencing of the D1/D2 domain of a large subunit of the rDNA gene using LROR and LR5 primers [31]. Mixed samples identified as C. albicans and C. dubliniensis by 21-plex were further tested using previously described hyphal-wall protein 1 primers [32].
Genotyping by AFLP
In order to assess the genotypic diversity of all isolates, they were subjected to a previously published AFLP protocol [33]. AFLP data were analyzed by Bionumerics software V7.6 (Applied Math Inc., Austin, Texas).
Antifungal susceptibility testing
The broth microdilution method of the Clinical and Laboratory Standards Institute (CLSI, M27-A3/S4) was used for antifungal susceptibility testing [34,35]. Eight routinely used antifungal drugs, including amphotericin B (AMB) (Sigma Chemical Corporation, St. Louis, MO), fluconazole (FLZ) (Pfizer, New York), itraconazole (ITZ) (Santa Cruz Biotech, Dallas), voriconazole (VOR) (Pfizer, New York), posaconazole (PSZ) (MSD, Kenilworth, USA), 5-fluorocytosine (5-FC) (Sigma Aldrich, Steinheim, Germany), caspofungin (CAS) (Merck & Co., Inc.), and anidulafungin (AND) (Pfizer, New York), were used. Plates were incubated at 35°C for 24 h and visual data were recorded. C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used for quality control purposes. Depending on species, clinical breakpoints and epidemiological cut-off values were determined [35]. For isolates of C. albicans, the MIC values of FLZ, ITZ, VRZ, CAS, and AND were interpreted based on clinical breakpoints (CBP), while PSZ, AMB and 5-FC MIC values were evaluated based on epidemiological cut-off values (ECV) [35]. As for C. tropicalis, the MIC values of FLZ, VRZ, AND, and CAS were evaluated based on CBP and the rest of the antifungals (PSZ, ITZ, AMB, and 5-FC) based on ECV [35]. The MIC values of all antifungal agents obtained for the isolates of K. marxianus and C. dubliniensis, were evaluated based on ECV [35]. MIC50 for all antifungals were defined as the minimum concentration of drugs to inhibit 50% of fungal growth, whereas for amphotericin B the MIC endpoint was considered as the lowest concentration that inhibited 100% of fungal growth compared to the drug-free control.
Statistical analyses
All the data presented in this study were analyzed by SPSS software v.24 (Windows, Chicago, IL). Statistical analysis included Pearson correlation Chi-square T-test, Eta correlation coefficient (for nominal and continuous variables), and Fei and Kramarz (for two nominal variables).
Results
Patients and isolates
The present study included 150 patients with hematological disorders, among them 85 patients among them 85 patients developed OC. The majority of OC patients were men (n= 53; 62.4%). Women represented almost one-third of the patients (n= 32; 37.6%). The median age of the OC patients was 53 years. Acute myeloid leukemia (AML) with the prevalence of 53% constituted the most dominant hematological malignancy followed by chronic lymphocytic leukemia (CLL) (n= 17; 20%), acute lymphocytic leukemia (ALL) (n= 15; 18%), and chronic myeloid leukemia (CML) (n= 7; 8%). The vast majority of the patients (n= 51; 60%) were prescribed with chemotherapeutic combination therapy of arsenic trioxide and cytarabine and the rest of the patients with ritoximab/cyclophosphamide (n= 15; 17.7%), cyclophosphamide (n= 13; 15.3), and fluorouracil (n= 6; 7.1). Erythema, inflammation and burning sensation of the buccal mucosa and tongue were the most common complaints that were detected in 59 patients (69.4%). C. albicans (n= 69; 80.3%) was the most prevalent causative agent of OC, followed by K. marxianus (= Candida kefyr) (n= 11; 12.7%), C. tropicalis (n= 3; 3.5%), C. dubliniensis (n= 2; 2.3%), and Hanseniaspora opuntiae (n= 1; 1.2%). One of the patients concurrently was colonized with both C. albicans and H. opuntiae.
Lack of efficacy of nystatin for clearance of OC
Primarily all patients underwent treatment with topical nystatin, which resulted in OC clearance in approximately 30% (n= 28; 32.9%) of the cases. Subsequently, the rest of the patients (n= 57; 67.1%) were treated with a combination of 150 mg of FLZ and topical nystatin, among them 63% (n= 36) experienced at least one episode of OC relapse. A significant correlation was noticed between the previous experience of OC and treatment with FLZ (α < 0.05, P = 0.005) and therapeutic failure with nystatin (P < 0.05).
Identification
MALDI-TOF MS consistent with LSU rDNA sequencing successfully identified all isolates to the species level (green score; >2), while the 21-plex PCR could not identify the only isolate of H. opuntiae. Interestingly, in agreement with primers targeting hyphal wall protein-1 [32], two isolates showed double bands representing both C. albicans and C. dubliniensis, while both MALDI-TOF MS and sequencing identified both as C. albicans (Figure 1). Those mixed samples on CHROMagar yielded the same color as C. albicans (green-colored colonies). As for the mixed sample containing both isolates of C. albicans and H. opuntiae, green and cream-colored colonies were observed on CHROMagar, respectively.
Figure 1.
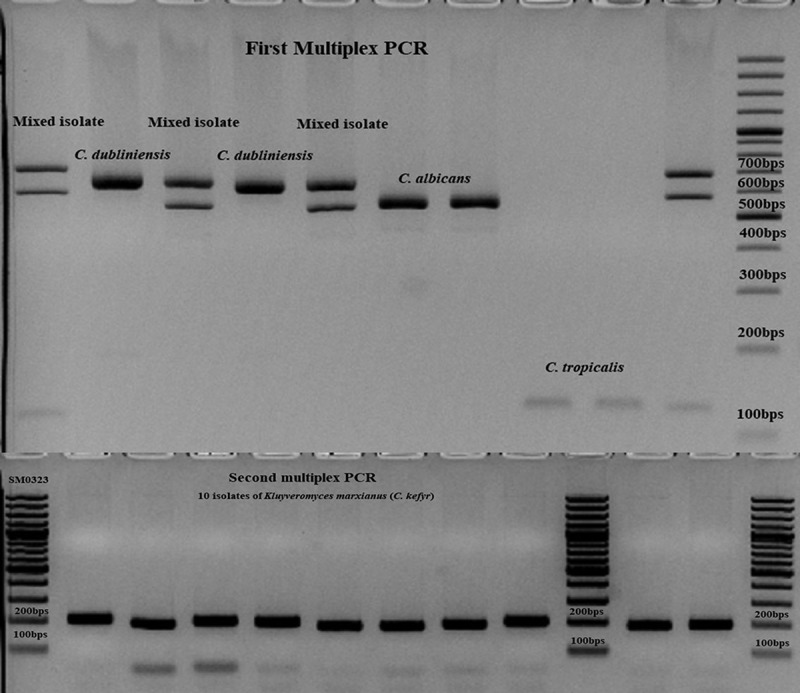
Application of 21-plex PCR for identification of clinical isolates. The majority of the strains were identified in the first multiplex PCR (C. albicans, C. dubliniensis, and C. tropicalis), the isolates of K. marxianus (C. kefyr) by the second multiplex PCR, and the only isolate of Hanseniaspora opuntiae was not identified by any of the PCR reactions.
AFLP genotyping
Although, infections due to C. albicans are mainly endogenous, some studies have shown cases of hospital-acquired infections for this species [36]. Accordingly, the high prevalence of C. albicans and K. marxianus in the oral cavities of patients included in this study, convinced us, to perform the AFLP for all isolates (except for H. opuntiae, n= 1) to observe the genotypic diversity. Moreover, all of the patients enrolled in this study were from the same hospital and the same unit (blood unit). One of the most important findings of this study was encountering a high number of K. marxianus isolates (n= 11; 12.7%) and through AFLP those isolates belonged to the same genotype (Supplementary Figure 1). As for the isolates of C. albicans, we found three major clusters (C), including C1 (n= 16), C2 (n= 17) and C3 (n= 22) and 12 minor and unique genotypes each containing a single isolate. No significant relationships were found between genotype, the season of isolation, and hospitalized ward. Two DNA samples of C. albicans isolates did not yield any banding patterns (probably due to the presence of inhibitors), therefore they were excluded from the final AFLP figure.
Antifungal susceptibility testing
Antifungal susceptibility results and MIC values are represented in Table 1. Among isolates of C. albicans, only 2.8% (n= 2) were resistant to FLZ (≥ 8 µg/ml) and 4 isolates (5.7%) were intermediate for ITZ (0.25–0.5 µg/ml), while they were all either susceptible to VRZ (≤ 0.12 µg/ml), CAS (≤ 0.25 µg/ml), and AND (≤ 0.25 µg/ml) or wild-type (WT) for AMB (≤ 2 µg/ml), 5-FC (≤ 0.5 µg/ml), and PSZ (≤ 0.06 µg/ml). All of the K. marxianus isolates showed WT phenotype for all antifungals used [5-FC (≤ 0.5 µg/ml), AMB (≤ 2 µg/ml), FLZ (≤ 1 µg/ml), ITZ (≤ 0.12 µg/ml), PSZ (≤ 0.25 µg/ml), VRZ (≤ 0.015 µg/ml), AND (≤ 0.25 µg/ml), and CAS (≤ 0.0.3 µg/ml)]. All isolates of C. tropicalis, except for two isolates that were intermediate for ITZ (0.25–0.5 µg/ml), were susceptible to FLZ (≤ 2 µg/ml), VRZ (≤ 0.12 µg/ml), AND (≤ 0.25 µg/ml), and CAS (≤ 0.25 µg/ml) and WT for PSZ (≤ 0.12 µg/ml), AMB (≤ 2 µg/ml), and 5-FC (≤ 0.5 µg/ml). WT phenotype and low MIC values against all antifungal agents were noticed for the two C. dubliniensis isolates and the only isolate of H. opuntiae, respectively. Two isolates of C. dubliniensis were WT. For all of the tested antifungal agents these isolates and the only isolate of H. opuntiae exhibited low MIC values.CAS, VRZ, and AND, and showed the lowest geometric mean values for both C. albicans and K. marxianus, while FLZ and AMB showed the highest GM mean values for C. albicans and K. marxianus isolates, respectively.
Table 1.
Antifungal susceptibility testing against yeast species isolated from oral lesions of patients with hematological malignancies.
| ECVa (µG/ml) |
CBPb (µG/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Organism | Antifungal | <ECV | >ECV | S | SDD | I | R | GM | Range |
| C. albicans (n= 69) | AMB | 69 | 0 | NA | NA | NA | NA | 0.439 | 0.12–1 |
| FLZ | 67 | 2 | 67 | 0 | NA | 2 | 0.461 | 0.125–8 | |
| ITZ | 65 | 4 | 65 | 4 | NA | 0 | 0.046 | 0.016–0.5 | |
| VRZ | 53 | 16 | 69 | NA | 0 | 0 | 0.022 | ≤0.015–0.06 | |
| PSZ | 69 | 0 | NA | NA | NA | NA | 0.026 | ≤0.015–0.06 | |
| CAS | 69 | 0 | 100% | NA | 0 | 0 | 0.019 | ≤0.008–0.12 | |
| AND | 68 | 1 | 100% | NA | 0 | 0 | 0.026 | 0.015–0.12 | |
| 5-FC | 69 | 0 | NA | NA | NA | NA | 0.060 | <0.06–0.06 | |
| K. marxianus (n= 11) | AMB | 10 | 0 | NA | NA | NA | NA | 1.149 | 0.012–2C |
| FLZ | 10 | 0 | NA | NA | NA | NA | 0.326 | 0.12–0.5 | |
| ITZ | 10 | 0 | NA | NA | NA | NA | 0.053 | 0.06–0.25d | |
| VRZ | 9 | 1 | NA | NA | NA | NA | 0.016 | 0.015–0.03 | |
| PSZ | 10 | 0 | NA | NA | NA | NA | 0.028 | 0.015–0.03 | |
| CAS | 10 | 0 | NA | NA | NA | NA | 0.014 | ≤0.008–0.03 | |
| AND | 10 | 0 | NA | NA | NA | NA | 0.024 | 0.015–0.12 | |
| 5-FC | 10 | 0 | NA | NA | NA | NA | 0.056 | <0.06–0.12e | |
| C. tropicalis (n= 3) | AMB | 3 | 0 | NA | NA | NA | NA | NA | 0.12–0.5 |
| FLZ | 3 | 0 | 3 | 0 | NA | 0 | NA | 0.25–1 | |
| ITZ | 3 | 0 | NA | NA | NA | NA | NA | 0.03–0.25 | |
| VRZ | 1 | 2 | 1 | NA | 2 | 0 | NA | ≤0.008–0.25 | |
| PSZ | 3 | 0 | NA | NA | NA | NA | NA | ≤0.015–0.06 | |
| CAS | 3 | 0 | 3 | NA | 0 | 0 | NA | ≤0.008–0.06 | |
| AND | 3 | 0 | 3 | NA | 0 | 0 | NA | 0.015–0.06 | |
| 5-FC | 3 | 0 | NA | NA | NA | NA | NA | ≤0.12–0.25 | |
| C. dubliniensis (n= 2) | AMB | 2 | 0 | NA | NA | NA | NA | NA | 0.03–0.25 |
| FLZ | 2 | 0 | NA | NA | NA | NA | NA | 0.06–0.25 | |
| ITZ | 2 | 0 | NA | NA | NA | NA | NA | 0.015–0.03 | |
| VRZ | 2 | 0 | NA | NA | NA | NA | NA | <0.008–0.03 | |
| PSZ | 2 | 0 | NA | NA | NA | NA | NA | 0.06–0.25 | |
| CAS | 2 | 0 | NA | NA | NA | NA | NA | 0.008–0.06 | |
| AND | 2 | 0 | NA | NA | NA | NA | NA | 0.015–0.06 | |
| 5-FC | 2 | 0 | NA | NA | NA | NA | NA | 0.06–0.12 | |
| H. opuntiae (n= 1) | AMB (0.5) | NA | NA | NA | NA | NA | NA | NA | 0.5 |
| FLZ (1) | NA | NA | NA | NA | NA | NA | NA | 1 | |
| ITZ (0.03) | NA | NA | NA | NA | NA | NA | NA | 0.03 | |
| VRZ (0.015) | NA | NA | NA | NA | NA | NA | NA | 0.015 | |
| PSZ (0.015) | NA | NA | NA | NA | NA | NA | NA | 0.015 | |
| CAS (0.03) | NA | NA | NA | NA | NA | NA | NA | 0.03 | |
| AND (0.015) | NA | NA | NA | NA | NA | NA | NA | 0.015 | |
| 5-FC (0.06) | NA | NA | NA | NA | NA | NA | NA | 0.06 | |
a. Epidemiological cut-off value
b. Clinical breakpoint
c. For the isolates of K. marxianus, the epidemiological cut-off value of >2 were considered resistant to AMB
d and e. For the isolates of K. marxianus, the epidemiological cut-off value of ≥0.5 were considered resistant to ITZ and 5-FC
S; Susceptible, SDD; Susceptible dose-dependent, I; Intermediate, R; Resistant, NA; Not applicable
Geometric mean value was not calculated for species containing less than 10 isolates.
The only isolate of H. opuntiae showed low MIC values for all antifungal agents, which have are mentioned next to each antifungal used.
Discussion
The worrisome escalation of cancer incidence may facilitate the emergence of OC in this population of patients. Unfortunately, despite a high annual burden of OC and the possible link with the development of candidemia [10,37] and even cancer [38], many clinical and microbiological aspects of this complication remained to be elucidated. Conducting local, national, and worldwide studies might result in a better clinical outcome, ease of management of OC cases, establishing appropriate empiric therapy, and finally lower hospital costs. Consequently, in order to bridge the gap between the lack of knowledge on clinical outcome and microbiological features of OC among patients suffering from hematological disorders we conducted this study in Iran.
In our study, more than half (56%) of the enrolled patients were positive for OC, from whom 86 yeast isolates were recovered. Encountering such a high number of OC positive patients is likely due to the fact that all our patients underwent chemotherapy [5], which is known to be the main risk factor for the manifestation of OC [5]. C. albicans (81%) was the most encountered Candida species, followed by K. marxianus (12%), C. tropicalis (3.5%), C. dubliniensis (2.3%) and H. opuntiae (1.2%). It was not surprising that C. albicans was the most prevalent Candida species, as this fungus is the most abundant yeast agent inhabiting mucosal surfaces of the gastrointestinal (GI), pulmonary, and urinary tract [27,39]. Predominance of C. albicans is in agreement with other studies [40–42], while unlike those studies we found K. marxianus as the second most frequently encountered yeast species. Previous studies have shown that patients with hematological disorders are prone to develop candidiasis due to K. marxianus [27,43], which corroborates the high rate of this yeast species in our study. Together with Jamel et al., we reported the second clinical case of H. opuntiae [44]. Studies of English abstracts published from 2009 to 2018 revealed an increasing trend in the spectrum and/or prevalence of emerging Candida and yeast species isolated from the oral cavity of Iranian HIV positive and cancer patients [22,45–48]. The noted differences in the distribution of diverse yeast species found in various studies might reflect the variations in patient subgroups, diet, sample types, or even the administered antifungal regimen. In line with those facts, application of informative and useful next-generation sequencing platforms have revealed that smoking [49], hygiene, and even the composition of the tap water [50] may cause microbial diversity in the oral microbiome.
Approximately 30% of our patients were successfully treated with nystatin and the remaining required combination of 150 mg of FLZ + topical nystatin, which indicates insufficient efficiency of this drug for clearance of OC. Interestingly, we observed a significant correlation between previous occurrence of OC and the requirement of more than one period of FLZ + nystatin treatment and nystatin therapeutic failure. Some studies showed a superiority of nystatin pastilles or pastille and suspension over nystatin alone, whilst the adverse side effects on the gastrointestinal tract, unpleasant taste, use of high doses and extended time of prescriptions are the drawbacks of nystatin formulations [51]. On the contrary, other studies have shown a similar efficacy of nystatin to that of placebo and an inferiority to FLZ when used in severe immunodeficient patients [52]. Although, we did not test nystatin in our antifungal susceptibility panel, other studies have shown high MIC values for topical agents [48] and recommended the use of systemic antifungal drugs, such as FLZ [5,53]. Moreover, randomized, double-blind clinical trials have shown a higher efficacy of FLZ and MCF when they were used in HIV-positive patients suffering from OC [8,54]. In agreement with other studies that showed a superiority of a single dose of 750 mg of FLZ rather than two-week-long 150 mg FLZ therapy in HIV-positive individuals [8], we found that almost 70% of patients treated with 150 mg FLZ and topical nystatin therapy showed OC relapse on more than one occasion. Adopting such a single high dose of FLZ regimen instead of a standard two-week long FLZ therapy of 150 mg, might result in lower risk of development of FLZ-resistant isolates stemming from a more extensive exposure time [55]. In case of FLZ-refractory cases, utilization of other azole agents, including PSZ, VRZ, ITZ [5], and micafungin [19] have been recommended [5]. This is in line with our findings that ITZ, VRZ, and PSZ showed the lowest geometric mean values and FLZ the highest. Nowadays, due to the expanding spectrum of yeast species in clinical settings that are possibly less responsive to azoles, it might be useful to implement such a therapeutic regimen of systemic antifungals for patients suffering from OC.
Approximately, 3% of the isolates of C. albicans were resistant to FLZ and infected patients underwent treatment with FLZ + nystatin, which is in concordance with other studies that previous exposure with FLZ results in the emergence of FLZ resistant isolates in patients suffering from candidemia [56]. Although a newer generation of antifungals targeting CYP51A have shown promising results when tested in animal models infected with C. auris [57], Cryptococcus neoformans [44], Cocciodiodes posadasii and Coccidioides immitis [58], their efficacy remains to be evaluated for the treatment of OC patients. Moreover, if proved to be efficient when tested in human OC positive cases, utilization of these drugs in clinical settings might contribute in preserving the limited systemic antifungal armamentarium and reducing the burden of systemic drug-resistant yeast species.
With regards to the identification of our clinical yeast isolates, MALDI-TOF MS and 21-plex showed 100% and 99% consistency with LSU rDNA sequencing, respectively. Although rare, MALDI-TOF MS successfully identified H. opuntiae, while the 21-plex PCR did not. Previous studies have shown the superiority of MALDI-TOF MS for identification of clinically important yeast species [59,60], but unfortunately, this device is not available in Iran as a developing country. However, PCR as an affordable and reproducible device increasingly proves to be a popular identification platform in developing countries [19,20]. Moreover, samples with mixed species could be differentiated via 21-plex PCR, while neither MALDI-TOF MS nor Sanger sequencing were able to differentiate the respective yeast species.
Subjecting our isolates to AFLP revealed that all 11 isolates of K. marxianus were in the same cluster. That may suggest that these isolates originated from the same source. Unfortunately, as a limitation of our study, we could not find the source of the K. marxianus isolates. However, the lack of dairy products in the hospital diet of our patients, precludes the fact that they have acquired K. marxianus during their hospitalization stay. Moreover, it is even likely that outside the hospitalization period those patients might have consumed dairy products containing K. marxianus and subsequently were infected with this yeast.
Unexpectedly, we found 15 genotypes of C. albicans, including three major clones constituting approximately 82% of C. albicans isolates and 12 minor genotypes each represented by a single isolate. As the three major genotypes encompassed more than 80% of the C. albicans isolates, the phenomenon of intrahospital transmission might be likely. Despite some studies using AFLP have ruled out the intrahospital transmission phenomenon for C. albicans isolates recovered from blood samples [61], others using pulse-field gel electrophoresis (PFGE) proved otherwise [36]. Encountering with such a contradictory finding might stem from the variation in the technology used, but it is noteworthy that both AFLP and PFGE possess a high discriminatory power. Unfortunately, similar to the isolates of K. marxianus, we did not carry out any screening protocols to prove the concept of intrahospital transmission for the C. albicans isolates, which is the main limitation of our study. Although, AFLP proved its superiority and higher resolution over multi-locus sequence typing (MLST) [61], application of platforms with a higher resolution, such as next-generation sequencing, might provide a better explanation for the intrahospital transmission phenomenon of C. albicans isolates.
Conclusion
Clinical outcome data derived from our study was compelling enough to persuade the clinicians to abandon the usage of nystatin as a choice of antifungal therapy for the treatment of OC positive patients with hematological malignancies. Interestingly, K. marxianus after C. albicans represented the second most prevalent yeast species isolated from the oral cavity of our patients, and 21-plex proved its efficiency for identification of clinically encountered yeast species. Moreover, through performing AFLP, we found that the majority of C. albicans and 100% of K. marxianus isolates showed the same genotypic background that might be an indication of intrahospital transmission. However, proving this fact requires environmental screening to find the same clinical source, which is one of the shortages of our study.
Funding Statement
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 642095, National Health Department of China [2018ZX10101003], National Natural Science Foundation of China [31770161], Second Military Medical University [2017JZ47], Shanghai Science and Technology Committee [14DZ2272900 and 14495800500], and the Iran University of Medical Sciences (33725).
Acknowledgments
We would like to express our sincere appreciation to Bart Theelen, who facilitated the experimental studies in the Westerdijk Institute.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
References
- [1].Spampinato C, Leonardi D.. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int. 2013;2013:204237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today. 2009;14(3–4):214–9. [DOI] [PubMed] [Google Scholar]
- [3].Gow NA, Netea MG. Medical mycology and fungal immunology: new research perspectives addressing a major world health challenge. Philos Trans R Soc Lond B Biol Sci. 2016;371(1709):20150462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13. [DOI] [PubMed] [Google Scholar]
- [5].Lalla RV, Latortue MC, Hong CH, et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer. 2010;18(8):985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Soysa NS, Ellepola AN. The impact of cigarette/tobacco smoking on oral candidosis: an overview. Oral Dis. 2005;11(5):268–273. [DOI] [PubMed] [Google Scholar]
- [7].Raber-Durlacher JE, Barasch A, Peterson DE, et al. Oral complications and management considerations in patients treated with high-dose chemotherapy. Support Cancer Ther. 2004;1(4):219–229. [DOI] [PubMed] [Google Scholar]
- [8].Hamza OJ, Matee MI, Brüggemann RJ, et al. Single-dose fluconazole versus standard 2-week therapy for oropharyngeal candidiasis in HIV-infected patients: a randomized, double-blind, double-dummy trial. Clin Infect Dis. 2008;47(10):1270–1276. [DOI] [PubMed] [Google Scholar]
- [9].Dunic I, Vesic S, Jevtovic DJ. Oral candidiasis and seborrheic dermatitis in HIV‐infected patients on highly active antiretroviral therapy. HIV Med. 2004;5(1):50–54. [DOI] [PubMed] [Google Scholar]
- [10].Ramirez-Garcia A, Rementeria A, Aguirre-Urizar JM, et al. Candida albicans and cancer: can this yeast induce cancer development or progression?. Crit Rev Microbiol. 2016;42(2):181–193. [DOI] [PubMed] [Google Scholar]
- [11].Vaezi A, Fakhim H, Khodavaisy S, et al. Epidemiological and mycological characteristics of candidemia in Iran: a systematic review and meta-analysis. J Mycol Med. 2017;27(2):146–152. [DOI] [PubMed] [Google Scholar]
- [12].Jan A, Bashir G, Fomda BA, et al. Molecular identification of Candida dubliniensis among Candida albicans isolated from oral cavity of cancer patients using PCR-RFLP, in a tertiary care hospital in Kashmir, India. Br Microbiol Res J. 2016;14:2. [Google Scholar]
- [13].Lamoth F, Lockhart SR, Berkow EL, et al. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73(suppl_1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Whaley SG, Berkow EL, Rybak JM, et al. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. 2017;7:2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014;27(6):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murray PR. What is new in clinical microbiology—microbial identification by MALDI-TOF mass spectrometry: a paper from the 2011 William Beaumont Hospital Symposium on molecular pathology. J Mol Diagn. 2012;14(5):419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahmed A, Azim A, Baronia AK, et al. Invasive candidiasis in non neutropenic critically ill-need for region-specific management guidelines. Indian J Crit Care Med. 2015;19(6):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rajkumari N, Mathur P, Xess I, et al. Distribution of different yeasts isolates among trauma patients and comparison of accuracy in identification of yeasts by automated method versus conventional methods for better use in low resource countries. Indian J Med Microbiol. 2014;32(4):391–397. [DOI] [PubMed] [Google Scholar]
- [19].Wong G, Wong I, Chan K, et al. A rapid and low-cost PCR thermal cycler for low resource settings. PLoS One. 2015;10(7):e0131701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ragheb SM, Jimenez L. Polymerase chain reaction/rapid methods are gaining a foothold in developing countries. PDA J Pharm Sci Technol. 2014May6;68(3):239–255. [DOI] [PubMed] [Google Scholar]
- [21].Arastehfar A, Fang W, Pan W, et al. YEAST PANEL Multiplex PCR for identification of clinically important yeast species: stepwise diagnostic strategy, useful for developing countries. Diagn Microbiol Infect Dis. 2019;93(2):112–119. [DOI] [PubMed] [Google Scholar]
- [22].Khedri S, Santos ALS, Roudbary M, et al. Iranian HIV/AIDS patients with oropharyngeal candidiasis: identification, prevalence and antifungal susceptibility of Candida species. Lett Appl Microbiol. 2018;67(4):392–399. [DOI] [PubMed] [Google Scholar]
- [23].Dagi HT, Findik D, Senkeles C, et al. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Ann Clin Microbiol Antimicrob. 2016;15(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Badiee P, Badali H, Boekhout T, et al. Antifungal susceptibility testing of Candida species isolated from the immunocompromised patients admitted to ten university hospitals in Iran: comparison of colonizing and infecting isolates. BMC Infect Dis. 2017;17(1):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78(922):455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52(1):61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dufresne SF, Marr KA, Sydnor E, et al. Epidemiology of Candida kefyr in patients with hematologic malignancies. J Clin Microbiol. 2014;52(6):1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(7):882–913. [DOI] [PubMed] [Google Scholar]
- [29].Cassagne C, Cella AL, Suchon P, et al. Evaluation of four pretreatment procedures for MALDI-TOF MS yeast identification in the routine clinical laboratory. Med Mycol. 2013;51(4):371–377. [DOI] [PubMed] [Google Scholar]
- [30].Theelen B, Silvestri M, Guého E, et al. Identification and typing of Malassezia yeasts using amplified fragment length polymorphism (AFLPTm), random amplified polymorphic DNA (RAPD) and denaturing gradient gel electrophoresis (DGGE). FEMS Yeast Res. 2001;1(2):79–86. [DOI] [PubMed] [Google Scholar]
- [31].Stielow JB, Lévesque CA, Seifert KA, et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 2015;35:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Romeo O, Racco C, Criseo G. Amplification of the hyphal wall protein 1 gene to distinguish Candida albicans from Candida dubliniensis. J Clin Microbiol. 2006;44(7):2590–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marchetta A, Gerrits van Den Ende B, Al-Hatmi AMS, et al. Global molecular diversity of the halotolerant fungus Hortaea werneckii. Life (Basel). 201823;8(3):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alexander BD, Procop GW, Dufresne P, et al. Reference method for broth dilution antifungal susceptibility testing of teasts (M27-S4). Clin Lab Stand Inst. 2017;37:1–33. [Google Scholar]
- [35].Pfaller M, Diekema D. Progress in antifungal susceptibility testing of Candida spp. using clinical and laboratory standards institute broth microdilution methods, 2010–2012. J Clin Microbiol. 2012;50(9):2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fanello S, Bouchara JP, Jousset N, et al. Nosocomial Candida albicans acquisition in a geriatric unit: epidemiology and evidence for person-to-person transmission. J Hosp Infect. 2001;47(1):46–52. [DOI] [PubMed] [Google Scholar]
- [37].Sroussi HY, Epstein JB, Bensadoun RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6(12):2918–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- [39].Richardson JP, Moyes DL. Adaptive immune responses to Candida albicans infection. Virulence. 2015;6(4):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Aslani N, Janbabaei G, Abastabar M, et al. Identification of uncommon oral yeasts from cancer patients by MALDI-TOF mass spectrometry. BMC Infect Dis. 2018;18(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Glažar I, Prpić J, Muhvić-Urek M, et al. Identification of Candida spp. in the oral cavity in patients with malignant diseases. Vsp. 2017;74(11):1066–1070. [Google Scholar]
- [42].Jayachandran AL, Katragadda R, Thyagarajan R, et al. Oral candidiasis among cancer patients attending a tertiary Care Hospital in Chennai, South India: an evaluation of clinicomycological association and antifungal susceptibility pattern. Can J Infect Dis Med Microbiol. 2016;2016:8758461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sendid B, Lacroix C, Bougnoux ME. Is Candida kefyr an emerging pathogen in patients with oncohematological diseases? Clin Infect Dis. 20061;43(5):666–667. [DOI] [PubMed] [Google Scholar]
- [44].Wiederhold NP, Najvar LK, Garvey EP, et al. The fungal Cyp51 inhibitor VT-1129 is efficacious in an experimental model of cryptococcal meningitis. Antimicrob Agents Chemother. 2018;62(9):e01071–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Katiraee F, Khosravi A, Khalaj V, et al. Oral candidiasis in Human Immunodeficiency Virus (HIV) infected individuals in Iran. Tum J. 2010;68(1):37–44. [Google Scholar]
- [46].Azizi A, Rezaei M. Prevalence of Candida species in the oral cavity of patients undergoing head and neck radiotherapy. J Dent Res Dent Clin Dent Prospects. 2009;3(3):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Katiraee F, Teifoori F, Soltani M. Emergence of azole-resistant Candida species in AIDS patients with oropharyngeal candidiasis in Iran. Curr Med Mycol. 2015;1(3):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jabalameli Z, Sabzghabaee AM, Mohaghegh MA, et al. Antifungal susceptibility of Candida species isolated from cancer patients with oral lesions undergoing chemotherapy. Int J Infect. 2017;4(4):e14178. [Google Scholar]
- [49].Vallès Y, Inman CK, Peters BA, et al. Types of tobacco consumption and the oral microbiome in the United Arab Emirates Healthy Future (UAEHFS) Pilot Study. Sci Rep. 201827;8(1):11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Willis JR, González-Torres P, Pittis AA, et al. Citizen science charts two major “stomatotypes” in the oral microbiome of adolescents and reveals links with habits and drinking water composition. Microbiome. 2018;6(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lyu X, Zhao C, Yan ZM, et al. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gøtzsche PC, Johansen HK. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database Syst Rev. 2002;4:CD002033. [DOI] [PubMed] [Google Scholar]
- [53].Garcia-Cuesta C, Sarrion-Pérez MG, Bagán JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. 2014;6(5):e576–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].de Wet N, Llanos-Cuentas A, Suleiman J. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis. 2004;39(6):842–849. [DOI] [PubMed] [Google Scholar]
- [55].Zhang L, Xiao M, Watts MR. Development of fluconazole resistance in a series of Candida parapsilosis isolates from a persistent candidemia patient with prolonged antifungal therapy. BMC Infect Dis. 2015;15:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Garnacho-Montero J, Díaz-Martín A, García-Cabrera E, et al. Risk factors for fluconazole-resistant candidemia. Antimicrob Agents Chemother. 2010;54(8):3149–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wiederhold NP, Lockhart SR, Najvar LK, et al. The fungal Cyp51 specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother. 2018;63(3):02233–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shubitz LF, Roy ME, Trinh HT, et al. Efficacy of the investigational antifungal VT-1161 in treating naturally occurring coccidioidomycosis in dogs. Antimicrob Agents Chemother. 2017;61(5):e00111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Broyer P, Perrot N, Rostaing H, et al. An automated sample preparation instrument to accelerate positive blood cultures microbial identification by MALDI-TOF mass spectrometry (Vitek® MS). Front Microbiol. 2018;9:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].. [Google Scholar]
- [61].Asadzadeh M, Ahmad S, Al-Sweih N, et al. Molecular fingerprinting studies do not support intrahospital transmission of Candida albicans among candidemia patients in Kuwait. Front Microbiol. 2017;8:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


