Key Points
Question
Is bariatric surgery cost-effective in patients with nonalcoholic steatohepatitis and compensated cirrhosis?
Findings
In this simulation model study, laparoscopic sleeve gastrectomy had an incremental cost-effectiveness ratio of $66 119 per quality-adjusted life-year in overweight patients, $18 716 per quality-adjusted life-year in patients with mild obesity, $10 274 per quality-adjusted life-year in patients with moderate obesity, and $6563 per quality-adjusted life-year in patients with severe obesity.
Meaning
Bariatric surgery could be highly cost-effective in patients with nonalcoholic steatohepatitis and compensated cirrhosis, even in those with a lower baseline body mass index.
This economic evaluation study uses a simulation model to assess the cost-effectiveness of bariatric surgery in patients with nonalcoholic steatohepatitis (NASH) and compensated cirrhosis who have varying baseline weight.
Abstract
Importance
Obesity is the most common risk factor for nonalcoholic steatohepatitis (NASH), the progressive form of nonalcoholic fatty liver disease that can lead to cirrhosis and hepatocellular carcinoma. Weight loss can be an effective treatment for obesity and may slow the progression of advanced liver disease.
Objective
To assess the cost-effectiveness of bariatric surgery in patients with NASH and compensated cirrhosis.
Design, Setting, and Participants
This economic evaluation study used a Markov-based state-transition model to simulate the benefits and risks of laparoscopic sleeve gastrectomy (SG), laparoscopic Roux-en-Y gastric bypass (GB), and intensive lifestyle intervention (ILI) compared with usual care in patients with NASH and compensated cirrhosis and varying baseline weight (overweight, mild obesity, moderate obesity, and severe obesity). Patients faced varied risks of perioperative mortality and complications depending on the type of surgery they underwent. Data were collected on March 22, 2017.
Main Outcomes and Measures
Life-years, quality-adjusted life-years (QALYs), costs (in 2017 $US), and incremental cost-effectiveness ratios (ICERs) were calculated.
Results
Demographic characteristics of the patient population were based on a previously published prospective study (n = 161). Patients in the model were 41.0% female, and the base case age was 54 years. Compared with usual care, SG was associated with an increase in QALYs of 0.263 to 1.180 (bounds of ranges represent overweight to severe obesity); GB, 0.263 to 1.207; and ILI, 0.004 to 0.216. Sleeve gastrectomy was also associated with an increase in life-years of 0.693 to 1.930; GB, 0.694 to 1.947; and ILI, 0.012 to 0.114. With usual care, expected life-years in overweight, mild obesity, moderate obesity, and severe obesity were 12.939, 11.949, 10.976, and 10.095, respectively. With usual care, QALY in overweight was 6.418; mild obesity, 5.790; moderate obesity, 5.186; and severe obesity, 4.577. Sleeve gastrectomy was the most cost-effective option for patients across all weight classes assessed: ICER for SG in patients with overweight was $66 119 per QALY; mild obesity, $18 716 per QALY; moderate obesity, $10 274 per QALY; and severe obesity, $6563 per QALY. A threshold analysis on the procedure cost of GB found that for GB to be cost-effective, the cost of the surgery must be decreased from its baseline value of $28 734 by $4889 for mild obesity, by $3189 for moderate obesity, and by $2289 for severe obesity. In overweight patients, GB involved fewer QALYs than SG, and thus decreasing the cost of surgery would not result in cost-effectiveness.
Conclusions and Relevance
Bariatric surgery could be highly cost-effective in patients with NASH compensated cirrhosis and obesity or overweight. The findings from this analysis suggest that it can inform clinical trials evaluating the effect of bariatric procedures in patients with NASH cirrhosis, including those with a lower body mass index.
Introduction
The prevalence of adult obesity in the United States is expected to reach approximately 50% by 2030.1 Obesity is the most common risk factor for nonalcoholic fatty liver disease and the progressive subtype of the disease, nonalcoholic steatohepatitis (NASH), which can give rise to cirrhosis and hepatocellular carcinoma (HCC). In recent prospective studies, more than 70% of patients with compensated cirrhosis were overweight or obese.2 In addition to contributing to the development of advanced liver disease, obesity also leads to worse outcomes among patients with compensated cirrhosis. Compared with their normal-weight counterparts, obese patients with compensated cirrhosis face a nearly 3-fold increased risk of decompensation.2
Weight loss can be an effective treatment for NASH.3 In a recent 12-month trial of lifestyle interventions, fibrosis improved or stabilized in patients with NASH who lost at least 5% of total body weight.4 Weight loss through diet and exercise also decreases portal pressure in patients with NASH and compensated cirrhosis, which likely reduces the likelihood of decompensation.5 In addition, weight loss may also improve the likelihood of receiving a liver transplant for patients on the wait list.6 Although diet and exercise may improve outcomes in patients with NASH, long-term maintenance of weight loss is often inadequate. In a trial of lifestyle intervention in patients with NASH, only 50% achieved 7% total body weight loss after 1 year.4
In contrast with lifestyle interventions, bariatric surgery often induces excellent long-term weight loss outcomes and may also have the potential to halt or reverse liver damage in cirrhosis.7,8 However, bariatric surgery carries heightened perioperative risks in patients with cirrhosis. An analysis of patients undergoing bariatric surgery in the Nationwide Inpatient Sample showed that mortality was higher in those with compensated (0.9%) and decompensated cirrhosis (16.3%) than in those without cirrhosis (0.3%).9 In addition, the cost of bariatric surgery may pose a potential barrier for some patients; payers typically deny coverage of these procedures to individuals with body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of less than 35.0, making surgery inaccessible to many who might benefit from it.
Despite the benefits of weight loss in NASH cirrhosis and the increasing burden of this condition, no randomized clinical trial has been performed, to date, to assess the effect of bariatric surgery and nonsurgical weight loss interventions on the progression of advanced liver disease. Under these circumstances, mathematical modeling provides a platform to integrate the best available data to help guide medical decision making. The aim of our study was to perform a comparative cost-effectiveness analysis of bariatric surgery in patients with NASH cirrhosis who were overweight or obese.
Methods
Model Structure
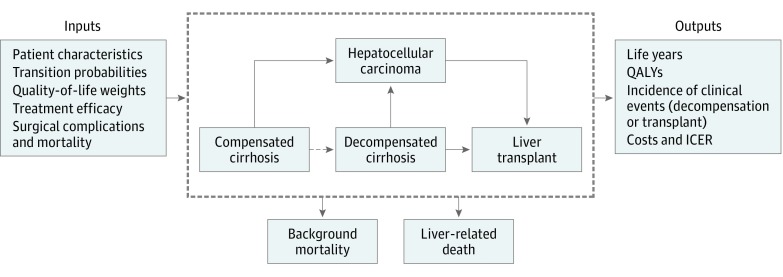
This report follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline for economic evaluations. We developed a Markov-based state-transition model using TreeAge Pro software (version 2017; TreeAge) to assess cost-effectiveness of the following 4 different weight loss strategies: usual care, intensive lifestyle intervention (ILI), laparoscopic sleeve gastrectomy (SG), and laparoscopic Roux-en-Y gastric bypass (GB) (Figure). Institutional review board approval was not required for this study that did not use human participants.
Figure. Simplified Model Schematic.
The dashed line between the compensated cirrhosis and decompensated cirrhosis states indicates that the probability of decompensating varies according to body mass index and thus decreases with weight loss. ICER indicates incremental cost-effectiveness ratio; QALYs, quality-adjusted life-years.
The baseline patient characteristics of the simulated population were based on a previously published prospective study2 (n = 161). Patients in the model were 41.0% female, and the base case age was 54 years. Note that this population represents patients with cirrhosis and thus includes a higher proportion of older and male individuals than the overall population of patients undergoing bariatric surgery. Our analysis was conducted for patients with overweight, mild obesity, moderate obesity, and severe obesity. The model cycle length, or time between state transitions, was 1 year. Each year, patients could remain in the same health state, progress to another, or die. Patients with compensated cirrhosis could progress to the decompensated cirrhosis state. Based on an analysis that associated obesity with clinical decompensation,2 we adjusted the probability of decompensation according to patients’ BMI. Patients with compensated or decompensated cirrhosis could progress to HCC.10,11,12,13,14 In the model, liver transplant was a possibility only for individuals with decompensated cirrhosis or HCC (Figure).15,16,17,18 After transplant, these patients faced a risk of death based on mortality rates among transplant recipients.19 Possible causes of death included surgical mortality, liver-related mortality, and background mortality, which was based on patients’ age, sex, and BMI, using data from the US Third National Health and Nutritional Examination Survey.9,20,21,22,23 Patients with compensated and decompensated cirrhosis had excess liver-related mortality of 2.1% and 13.0%, respectively.11,12,22 Simulated patients were followed up until death, to capture the long-term benefits of surgery.
Competing Strategies
In the usual-care strategy, patients did not undergo surgery or other weight loss interventions, and their liver disease progressed according to probabilities derived from the literature. We assumed that, without intervention, patients’ weight remained constant during their lifetime.
In SG and GB strategies, patients faced a risk of 30-day surgical mortality after the operation, which varied depending on the type of procedure, based on the American College of Surgeons National Quality Improvement Survey.23 They also encountered the possibility of major and minor complications after surgery.23 Major complications required a second operation; minor complications required readmission but no additional operation. The estimates from the American College of Surgeons National Quality Improvement Survey were adjusted for our population using data from a study that compared mortality in patients with compensated cirrhosis and patients without cirrhosis.9
After surgery, patients underwent weight loss, which could slow the progression of liver disease in our model by decreasing the probability of decompensation. We incorporated the association of weight loss with survival and quality of life for patients in the following 4 weight classes: overweight (BMI, 25.0-29.9), mild obesity (BMI, 30.0-34.9), moderate obesity (BMI, 35.0-39.9), and severe obesity (BMI, ≥40.0). Postsurgical weight loss was derived from a recent meta-analysis.24 Weight loss in the ILI strategy was derived from the Look AHEAD (Action for Health in Diabetes) trial.25 Long-term trends in weight were extrapolated using data from the Swedish Obese Subjects study.26 We applied our estimates of percentage of excess weight loss (eTable 1 in the Supplement) to all of the initial weight categories in our analysis.
Costs and Quality-of-Life Adjustments
Costs associated with treatment strategies, surgical complications, and health states were derived from the literature.27,28,29,30,31,32 The initial cost of GB was based on a prior analysis,27 and the cost of SG was calculated using cost data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample.28 All costs from prior years were converted to 2017 $US using the Consumer Price Index.33 Costs were evaluated from the perspective of a third-party payer.
Utility estimates were derived from studies on cirrhosis due to other causes because no quality-of-life studies for NASH cirrhosis were available to inform our model.34 This approach has been used in a previous cost-effectiveness analysis studying NASH.10 All patients who underwent surgery had a decrement in their quality of life, which was applied for 6 weeks. An additional quality-of-life decrement was applied for 6 weeks to patients who experienced major complications, and a 4-week decrement was applied after mild complications. Complications also incurred additional costs.27 We adjusted quality of life based on patients’ age, sex, and weight class (eTable 2 in the Supplement). Costs and utilities were discounted at an annual rate of 3%.35
Outcomes
Compared with usual care, we estimated the gain in life-years, quality-adjusted life-years (QALYs), and total costs for patients undergoing ILI, SG, and GB. We assessed cost-effectiveness for each treatment strategy by estimating incremental cost-effectiveness ratios (ICERs) for patients with overweight, mild obesity, moderate obesity, and severe obesity. The ICERs were calculated on an efficiency frontier. The willingness-to-pay threshold to determine cost-effectiveness was $100 000 per QALY.
Sensitivity Analysis
Data were collected on March 22, 2017. We conducted 1-way sensitivity analyses to examine the association of model parameter uncertainty on the cost-effectiveness results for patients in each weight class. One-way sensitivity analyses were performed for the cost-effective strategy in each weight class (ie, SG). We also performed probabilistic sensitivity analysis (PSA) to assess how model parameter uncertainty could affect our results. In PSA, all parameter values were varied simultaneously, using statistical distributions derived from the literature (Table 1). We conducted PSA using second-order sampling for 10 000 iterations for each weight class.
Table 1. Model Inputs: Baseline Values, Ranges, and Parameters for Distributions Used in Deterministic and Probabilistic Sensitivity Analyses.
| Parameter | Source | Base Case (Range or Variation) | Distribution | Parameter 1a | Parameter 2b |
|---|---|---|---|---|---|
| Age, y | Berzigotti et al,2 2011 | 54 (34.4 to 73.6) | NA | NA | NA |
| Female, % | Berzigotti et al,2 2011 | 41 (35 to 49) | β | 59.122 | 85.078 |
| Annual discount rate, % | Weinstein et al,35 1996 | 3 (0 to 5) | β | 8.354 | 270.122 |
| Transition probabilities | |||||
| HR per 1-U increase in BMI | Berzigotti et al,2 2011 | 1.06 (1.01 to 1.12) | Log normal | 4.907 | 86.682 |
| CC to DC (BMI, 27.2), % | NA | 7.21 (3.21 to 10.77) | β | 13.0128 | 167.469 |
| CC/DC to HCC, %10,11,12,13,14 | Mahady et al,10 2012; Sanyal et al,11 2006; Ratziu et al,12 2002; Ascha et al,13 2010; Yatsuji et al,14 2009 | 0.69 (0.50 to 16.8) | β | 1.03 | 147.97 |
| CC to liver-related death, % | Sanyal et al,11 2006; Hui et al,22 2003 | 2.1 (2.0 to 4.0) | β | 4.483 | 210.003 |
| DC to liver-related death, % | Ratziu et al,12 2002 | 13.0 (10.0 to 38.0) | β | 0.774 | 5.178 |
| HCC to liver-related death, % | Fattovich et al,21 2002 | 42.7 (33.0 to 86.0) | β | 2.14 | 2.87 |
| DC to transplantation, % | Thuluvath et al,15 2010; Davis et al,16 2011 | 2.3 (1.0 to 6.2) | β | 1.282 | 54.473 |
| HCC to transplantation, % | Lang et al,17 2009; Saab et al,18 2010 | 4.0 (0.0 to 14.0) | β | 0.59 | 14.16 |
| Posttransplant to liver-related death in year 1, % | Wolfe et al,19 2010 | 11.6 (6.0 to 42.0) | β | 0.38 | 2.88 |
| Posttransplant to liver-related death in year 2 or later, %19 | Wolfe et al,19 2010 | 4.4 (2.4 to 11.0) | β | 1.59 | 34.51 |
| 30-Day mortality for GB, % | Mosko and Nguyen,9 2011; Young et al,23 2015 | 0.3255 (0.10 to 1.00) | β | 2.000 | 612.491 |
| 30-Day mortality for SG, % | Mosko and Nguyen,9 2011; Young et al,23 2015 | 0.217 (0.041 to 1.14) | β | 0.596 | 273.886 |
| Minor complications for GB, % | Mosko and Nguyen,9 2011; Young et al,23 2015 | 7.86 (3.73 to 16.47) | β | 5.311 | 62.255 |
| Major complications for GB, % | Mosko and Nguyen,9 2011; Young et al,23 2015 | 5.34 (2.53 to 11.19) | β | 5.477 | 97.095 |
| Minor complications for SG, % | Mosko and Nguyen,9 2011; Young et al,23 2015 | 5.32 (2.52 to 11.15) | β | 5.476 | 97.449 |
| Major complications for SG, % | Mosko and Nguyen,9 2011; Young et al,23 2015 | 3.47 (1.65 to 7.28) | β | 5.600 | 155.785 |
| Health-related quality-of-life weights | |||||
| CC | Chhatwal et al,34 2015 | 0.90 (0.81 to 0.99) | Β | 37.52 | 4.17 |
| DC | Chhatwal et al,34 2015 | 0.80 (0.57 to 0.99) | β | 8.50 | 2.12 |
| HCC | Chhatwal et al,34 2015 | 0.79 (0.54 to 0.99) | β | 7.27 | 1.93 |
| Liver transplant at year 1 | Chhatwal et al,34 2015 | 0.84 (0.77 to 0.93) | β | 52.70 | 10.04 |
| Liver transplant at year 2 or later | Chhatwal et al,34 2015 | 0.93 (0.84 to 0.99) | β | 27.78 | 2.09 |
| Treatment-related quality-of-life weights | |||||
| Initial surgery | Campbell et al,27 2010 | –0.22 (–0.24 to –0.20) | β | 300.96 | 1067.03 |
| Minor complications | Campbell et al,27 2010 | –0.11 (–0.12 to –0.10) | β | 369.31 | 2988.03 |
| Major complications | Campbell et al,27 2010 | –0.36 (–0.40 to –0.32) | β | 258.87 | 460.21 |
| Weight-related quality-of-life weights | |||||
| BMI <30.0 | Campbell et al,27 2010 | 0.88 (0.79 to 0.97) | β | 48.22 | 6.58 |
| BMI 30.0-34.9 | Campbell et al,27 2010 | 0.85 (0.77 to 0.93) | β | 60.27 | 10.64 |
| BMI 35.0-39.9 | Campbell et al,27 2010 | 0.82 (0.74 to 0.90) | β | 72.33 | 15.88 |
| BMI ≥40.0 | Campbell et al,27 2010 | 0.78 (0.70 to 0.86) | β | 88.40 | 24.93 |
| Health state costs, 2017 $US | |||||
| CC | Saab et al,30 2014; Gordon et al,31 2012; McAdam-Marx et al,32 2011 | $5886 (±25%) | γ | 61.466 | 0.010 |
| DC | Saab et al,30 2014; Gordon et al,31 2012; McAdam-Marx et al,32 2011 | $41 082 (±25%) | γ | 61.466 | 0.001 |
| HCC | Saab et al,30 2014; Gordon et al,31 2012; McAdam-Marx et al,32 2011 | $90 344 (±25%) | γ | 61.466 | 6.804 |
| Liver transplant year 1 | Saab et al,30 2014; Gordon et al,31 2012; McAdam-Marx et al,32 2011 | $183 279 (±25%) | γ | 61.466 | 3.354 |
| Liver transplant year 2 or later | Saab et al,30 2014; Gordon et al,31 2012; McAdam-Marx et al,32 2011 | $45 107 (±25%) | γ | 61.466 | 0.001 |
| Treatment costs, 2017 $US | |||||
| GB | Campbell et al,27 2010; AHRQ,28 2014 | $28 734 (±25%) | γ | 61.466 | 0.002 |
| SG | Campbell et al,27 2010; AHRQ,28 2014 | $23 660 (±25%) | γ | 61.466 | 0.003 |
| Minor complications | Campbell et al,27 2010 | $1414 (±25%) | γ | 61.466 | 0.043 |
| Major complications | Campbell et al,27 2010 | $46 091 (±25%) | γ | 61.466 | 0.001 |
| ILI in year 1 | Rushing et al,29 2017 | $1410 (±25%) | γ | 61.466 | 0.044 |
| ILI in year 2 | Rushing et al,29 2017 | $1087 (±25%) | γ | 61.466 | 0.057 |
| ILI in year 3 | Rushing et al,29 2017 | $932 (±25%) | γ | 61.466 | 0.067 |
| ILI in year 4 | Rushing et al,29 2017 | $750 (±25%) | γ | 61.466 | 0.083 |
| ILI in years 5-8 | Rushing et al,29 2017 | $564 (±25%) | γ | 61.466 | 0.111 |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CC, compensated cirrhosis; DC, decompensated cirrhosis; GB, laparoscopic Roux-en-Y gastric bypass; HCC, hepatocellular carcinoma; HR, hazard ratio; ILI, intensive lifestyle intervention; NA, not applicable; SG, laparoscopic sleeve gastrectomy.
Corresponds to the α parameter for β distribution, the k (shape) parameter for γ distribution, and μ for log normal distribution.
Corresponds to the β parameter for β distribution, θ (scale) parameter for γ distribution, and α for log normal distribution.
Results
Base-Case Results
All weight loss strategies involved a gain in life-years and QALYs in patients with compensated cirrhosis (41% female and 59% male; base case age, 54 years). Compared with usual care, SG was associated with an increase in QALYs of 0.263 to 1.180 (bounds of ranges represent overweight to severe obesity); GB, 0.263 to 1.207; and ILI, 0.004 to 0.216. Sleeve gastrectomy was also associated with an increase in life-years of 0.693 to 1.930; GB, 0.694 to 1.947; and ILI, 0.012 to 0.114 (Table 2). With usual care, expected life-years in overweight, mild obesity, moderate obesity, and severe obesity were 12.939, 11.949, 10.976, and 10.095, respectively. These results are consistent with prospective studies that found median survival of greater than 12 years in patients with compensated cirrhosis.36 With usual care, QALY in overweight was 6.418; mild obesity, 5.790; moderate obesity, 5.186; and severe obesity, 4.577. For patients with obesity (mild, moderate, and severe), GB involved the greatest increase in life-years and QALYs, followed by SG and then ILI. In contrast, for overweight patients, GB had the largest increase in life-years; however, SG had the largest increase in QALYs, followed very closely by GB and then by ILI. Note that these differences between GB and SG for overweight patients were very small. When rounded to the nearest thousandth, SG and GB both involved an equal number of QALYs, and GB involved 0.001 additional life-years compared with SG.
Table 2. Results of Cost-effectiveness Analysesa.
| Strategy | Cost, 2017 $US | Incremental Cost, 2017 $US | QALYs | Incremental QALYs | Life-Years | Incremental Life-Years | ICER, $/QALY |
|---|---|---|---|---|---|---|---|
| Severe obesity (BMI ≥40.0) | |||||||
| Usual care | 214 412 | NA | 4.577 | NA | 10.095 | NA | NA |
| ILI | 223 087 | 934 | 4.793 | −0.964 | 10.209 | −1.815 | Absolutely dominated |
| SG | 222 153 | 7741 | 5.757 | 1.179 | 12.025 | 1.930 | 6563 |
| GB | 228 369 | 6216 | 5.784 | 0.027 | 12.042 | 0.017 | 229 919 |
| Moderate obesity (BMI 35.0-39.9) | |||||||
| Usual care | 206 809 | NA | 5.186 | NA | 10.976 | NA | NA |
| ILI | 214 953 | 8145 | 5.259 | 0.073 | 11.063 | 0.087 | Extendedly dominated |
| SG | 216 075 | 1122 | 6.088 | 0.829 | 12.593 | 1.530 | 10 274 |
| GB | 222 174 | 6099 | 6.106 | 0.019 | 12.604 | 0.011 | 329 002 |
| Mild obesity (BMI 30.0-34.9) | |||||||
| Usual care | 197 486 | NA | 5.790 | NA | 11.949 | NA | NA |
| ILI | 205 128 | 7642 | 5.809 | 0.020 | 12.001 | 0.052 | Extendedly dominated |
| SG | 209 976 | 4848 | 6.457 | 0.648 | 13.131 | 1.130 | 18 716 |
| GB | 215 990 | 6014 | 6.458 | 0.002 | 13.137 | 0.006 | 3 667 701 |
| Overweight (BMI 25.0-29.9) | |||||||
| Usual care | 186 264 | NA | 6.418 | NA | 12.939 | NA | NA |
| ILI | 193 506 | 7242 | 6.422 | 0.004 | 12.951 | 0.012 | Extendedly dominated |
| SG | 203 660 | 10 153 | 6.681 | 0.258 | 13.632 | 0.681 | 66 119 |
| GB | 209 606 | 5946 | 6.681 | 0 | 13.633 | 0.001 | Absolutely dominated |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GB, laparoscopic Roux-en-Y gastric bypass; ICER, incremental cost-effectiveness ratio; ILI, intensive lifestyle intervention; NA, not applicable; QALYs, quality-adjusted life-years; SG, laparoscopic sleeve gastrectomy.
An extendedly dominated strategy has an ICER that is higher than that of the next most effective strategy. An absolutely dominated strategy is more expensive and less effective than other strategies. Note that the ILI strategy has negative incremental QALYs and life-years for the severe obesity category; as the ILI strategy was absolutely dominated, the incremental QALYs, life-years, and costs for this group are calculated against the SG strategy.
Cost-effectiveness analysis found that SG was the optimal strategy in patients with NASH cirrhosis in all weight categories. Compared with the next nondominated strategy, the ICER for SG was $66 119 per QALY in overweight, $18 716 per QALY in mild obesity, $10 274 per QALY in moderate obesity, and $6563 per QALY in severe obesity (Table 2). Although GB involved a greater increase in QALYs than SG in obese patients, GB was also more expensive, and the ICER for GB ($229 919 per QALY for severe, $329 002 per QALY for moderate, and $3 667 701 per QALY for mild obesity) exceeded the commonly accepted willingness-to-pay threshold of $100 000 per QALY.
A threshold analysis on the procedure cost of GB found that for GB to be cost-effective, the cost of the surgery must be decreased from its baseline value of $28 734 by $4889 for mild obesity, by $3189 for moderate obesity, and by $2289 for severe obesity (Table 3). In overweight patients, GB involved fewer QALYs than SG, and thus decreasing the cost of surgery would not result in cost-effectiveness.
Table 3. Threshold Analysis: Procedure Cost That Could Make GB Cost-effectivea.
| Patient Profile | Reduced Cost of GB at Which GB Is the Most Cost-effective Intervention $ | Cost Reduction Relative to Baseline Cost of GB, 2017 $US |
|---|---|---|
| Severe obesity (BMI ≥40.0) | 25 965 | 2289 |
| Moderate obesity (BMI 35.0-39.9) | 25 065 | 3189 |
| Mild obesity (BMI 30.0-34.9) | 23 365 | 4889 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GB, laparoscopic Roux-en-Y gastric bypass.
Baseline cost of GB was $28 734. Overweight is not included because GB was absolutely dominated for this BMI class (ie, GB resulted in fewer quality-adjusted life-years than sleeve gastrectomy).
Sensitivity Analysis
We conducted 1-way sensitivity analysis only on the cost-effective interventions identified in the base case analyses (ie, SG). eFigures 1 to 4 in the Supplement show the 10 model parameters for each weight class that had the largest association with the ICER, using tornado diagrams. We found that in patients with mild, moderate, or severe obesity, SG remained cost-effective despite variations in all model inputs. In overweight patients, the ICER for SG remained below the willingness-to-pay threshold ($100 000/QALY), despite modifications to all model inputs except for age and the hazard ratio for decompensation associated with a 1-U increase in BMI. The ICER rose above $100 000 per QALY (vs usual care) when age was varied to its high extreme (73.6 years) and when the hazard ratio for decompensation associated with a 1-U increase in BMI was varied to its low extreme (1.01).
In the PSA, model parameters were varied simultaneously 10 000 times, and cost-effectiveness was calculated. According to our PSA results, SG was the most cost-effective option for patients with overweight or obesity in most iterations. In patients with severe obesity, SG was the optimal strategy in 79% of iterations, using a willingness-to-pay threshold of $100 000 per QALY (eFigure 5 in the Supplement). In those with moderate obesity, SG was the most cost-effective option in 64% of iterations (eFigure 6 in the Supplement). In patients with mild obesity, it was the most cost-effective option in 76% of iterations (eFigure 7 in the Supplement). In the overweight population, SG was the optimal strategy in 53% of model iterations (eFigure 8 in the Supplement).
Discussion
In this modeling-based study, we examined whether the benefits of bariatric surgery outweighed the heightened risks of mortality and complications in surgical candidates with NASH cirrhosis. Our results projected that surgery would outperform usual care and ILI across all weight classes assessed, including overweight, in terms of life expectancy and QALY. Furthermore, we found that bariatric surgery was cost-effective for patients with obesity (mild, moderate, and severe) and those in the overweight range.
Although studies demonstrate that decompensation is more likely at higher BMIs2 and that weight loss leads to decreased portal hypertension in patients with cirrhosis and obesity,5 less is known regarding the effect of bariatric surgery on clinical outcomes in NASH cirrhosis.7,8 In a previous analysis, Klebanoff et al37 examined the long-term cost-effectiveness of bariatric surgery in patients with NASH and varying degrees of fibrosis, but that study did not include patients with NASH and cirrhosis. However, given the continued rise in the prevalence of NASH cirrhosis,38 we should explore the potential role of weight loss in treating this disease. Therefore, our analysis underscores a real and urgent need for clinical studies of bariatric surgery and other weight loss therapies in NASH cirrhosis.
For patients with obesity, bariatric surgery remained cost-effective in our 1-way sensitivity analyses, which included varying the probability of surgical mortality and complications. These findings are important because the weighing of risks and benefits of bariatric surgery in the setting of cirrhosis may not appear obvious to patients or physicians. Surgery may involve a 3-fold increased risk of death in patients with compensated cirrhosis, compared with those without cirrhosis.9 In addition, individuals with cirrhosis have a diminished life expectancy, and thus may not derive long-term health benefits from surgery, including improvements in comorbidities such as hypertension, dyslipidemia, and diabetes.39,40 Despite these considerations, our analysis suggests that the benefits of surgery may outweigh the risks. In sensitivity analyses, surgery remained cost-effective in patients with obesity, including those with mild obesity (BMI, 30.0-35.0), even when we assumed a small benefit of weight loss on liver disease progression and an increase in the the risk of surgical mortality. In addition, a recent analysis found no association between compensated cirrhosis and increased mortality in patients undergoing bariatric surgery, suggesting that our analysis may even overstate the dangers of bariatric surgery.41 Our findings are consistent with the available evidence, suggesting that bariatric surgery offers an acceptably low risk in patients with compensated cirrhosis, potentially even in those with portal hypertension.42,43
To date, no clinical studies have compared outcomes of SG and GB in patients with NASH cirrhosis. We chose to include both procedures in our model, because they are the 2 most common bariatric procedures performed in the United States.44 Of the treatment options in this analysis, GB involved the largest increase in life expectancy and quality of life for patients with obesity, but SG was the most cost-effective. Although GB involved a slightly greater increase in QALYs, it was also marginally more expensive than SG. Our findings suggest that the slightly superior weight loss attributable to GB might not be worth the higher cost of the procedure. In addition to the extra cost of GB, it also entails a higher risk of complications, which may limit its cost-effectiveness. Data from patients without cirrhosis show that GB induces more weight loss and more successful resolution of comorbidities compared with SG, but also involves a higher rate of deep wound infections, serious morbidity, and 30-day reoperation.23 Despite the overall lower rate of complications for SG, it is associated with an increased risk of portal vein thrombosis, a rare but serious complication with mortality exceeding 40% among affected patients.45 Although this risk poses an important clinical consideration, patients and physicians are increasingly opting for SG, owing to various potential advantages. For example, SG preserves endoscopic access to the gastric tube in the event of variceal bleeding, as well as access to the biliary system.46 While our model is informed by limited data and should not be used to guide selection of a particular bariatric procedure, our findings support the idea that SG may provide a more favorable combination of risk, benefit, and cost compared with GB. Given the rapidly increasing popularity of SG at the expense of GB, our results may reflect a growing conventional wisdom concerning these procedures among bariatric surgeons.
Strengths and Limitations
Our study has several strengths. This model is, to our knowledge, the first cost-effectiveness analysis of bariatric surgery and lifestyle intervention among patients with NASH cirrhosis. In addition, although 1 previous modeling study explored weight loss in patients with cirrhosis,47 it did not assess cost-effectiveness and did not include SG, which is now the most popular bariatric procedure in the United States.48 Compared with prior analyses, ours also incorporated not only individuals with severe obesity, but also those with lower baseline BMI.
As with any simulation model, our analysis must be viewed in the context of some limitations. First, we used weight loss data from studies of patients without cirrhosis; weight loss after bariatric surgery may differ between patients with and without cirrhosis. This limitation is not major, because the available data suggest excellent weight loss outcomes for patients with and without cirrhosis.7 We also extrapolated long-term trends in BMI using data from the Swedish Obese Subjects study,26 given a lack of long-term data surrounding trends in weight after SG and GB. In addition, we assumed that weight loss would slow the progression of compensated cirrhosis to decompensated cirrhosis. This decision gains support from an analysis that described an increased risk of decompensation associated with high BMI,2 as well as a trial that found decreased portal hypertension after weight loss.5 Evidence also suggests that bariatric surgery induces resolution of noncirrhotic NASH,49 and some limited data show that surgery may even cause regression of fibrosis in patients with cirrhosis.8,50 Future studies that explore the effects of bariatric surgery in this population will confirm or refute our modeling assumptions. We also assumed that weight loss would benefit patients in our model by leading to a decrease in background mortality, a decrease in the probability of decompensation, and an increase in weight-related quality of life. Thus, we may have overestimated the benefit of surgery on survival and quality of life. However, we also did not explicitly model other comorbidities that resolve or improve with weight loss, such as diabetes, hypertension, and dyslipidemia, which may counterbalance any potential overestimation of clinical benefit. In addition, to inform the effect of weight loss on the probability of decompensation, our analysis used data from a study that included a large portion of patients with cirrhosis secondary to viral hepatitis or alcohol use.2 Given the association between NASH and obesity, we suspect that weight loss might have an even greater benefit in individuals with NASH. Accordingly, our estimates of the benefits of bariatric surgery in this population may be conservative.
Our results for patients with a BMI of less than 35.0 should also be viewed with some caution. The available evidence suggests that bariatric surgery is safe and does not lead to excessive weight loss in patients with mild obesity. Based on the current literature, the American Society for Metabolic and Bariatric Surgery51 and the International Federation for the Surgery of Obesity and Metabolic Disorders52 recommend bariatric surgery as an option for suitable patients with a BMI of 30.0 to 35.0. For patients with a BMI of less than 30.0, scant data are available to guide clinical decision making. The lack of data to inform modeling for patients with a BMI of less than 30.0 makes our analysis of the overweight patients an exploratory one, although we can reasonably expect similar beneficial results of surgery in the lower BMI group.
Conclusions
Our results underscore the promise that bariatric procedures may hold for patients with NASH cirrhosis, including those with lower BMI. Our analysis affirms that the benefits of surgery likely outweigh the risks in otherwise eligible individuals, and surgery could be a cost-effective intervention. Given the increasing burden of NASH cirrhosis and the lack of effective treatments, it would appear that future studies must explore the effects of bariatric surgery on disease progression in individuals with NASH cirrhosis.
eTable 1. Percentage of Excess Weight Loss After Bariatric Surgery and Lifestyle Intervention
eTable 2. Health-Related Quality-of-Life Utilities of the United States Population
eFigure 1. One-Way Sensitivity Analysis for SG in Severe Obesity
eFigure 2. One-Way Sensitivity Analysis for SG in Moderate Obesity
eFigure 3. One-Way Sensitivity Analysis for SG in Mild Obesity
eFigure 4. One-Way Sensitivity Analysis for SG in Overweight
eFigure 5. Probabilistic Sensitivity Analysis for Severe Obesity
eFigure 6. Probabilistic Sensitivity Analysis for Moderate Obesity
eFigure 7. Probabilistic Sensitivity Analysis for Mild Obesity
eFigure 8. Probabilistic Sensitivity Analysis for Overweight
eReferences.
References
- 1.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):-. doi: 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 2.Berzigotti A, Garcia-Tsao G, Bosch J, et al. ; Portal Hypertension Collaborative Group . Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54(2):555-561. doi: 10.1002/hep.24418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55(4):885-904. doi: 10.1007/s00125-011-2446-4 [DOI] [PubMed] [Google Scholar]
- 4.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367-78.e5. doi: 10.1053/j.gastro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 5.Berzigotti A, Albillos A, Villanueva C, et al. ; Ciberehd SportDiet Collaborative Group . Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet Study. Hepatology. 2017;65(4):1293-1305. doi: 10.1002/hep.28992 [DOI] [PubMed] [Google Scholar]
- 6.Segev DL, Thompson RE, Locke JE, et al. Prolonged waiting times for liver transplantation in obese patients. Ann Surg. 2008;248(5):863-870. doi: 10.1097/SLA.0b013e31818a01ef [DOI] [PubMed] [Google Scholar]
- 7.Jan A, Narwaria M, Mahawar KK. A systematic review of bariatric surgery in patients with liver cirrhosis. Obes Surg. 2015;25(8):1518-1526. doi: 10.1007/s11695-015-1727-2 [DOI] [PubMed] [Google Scholar]
- 8.Kral JG, Thung SN, Biron S, et al. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135(1):48-58. doi: 10.1016/j.surg.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(10):897-901. doi: 10.1016/j.cgh.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 10.Mahady SE, Wong G, Craig JC, George J. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: a cost utility analysis. Hepatology. 2012;56(6):2172-2179. doi: 10.1002/hep.25887 [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682-689. doi: 10.1002/hep.21103 [DOI] [PubMed] [Google Scholar]
- 12.Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35(6):1485-1493. doi: 10.1053/jhep.2002.33324 [DOI] [PubMed] [Google Scholar]
- 13.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972-1978. doi: 10.1002/hep.23527 [DOI] [PubMed] [Google Scholar]
- 14.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24(2):248-254. doi: 10.1111/j.1440-1746.2008.05640.x [DOI] [PubMed] [Google Scholar]
- 15.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4, pt 2):1003-1019. doi: 10.1111/j.1600-6143.2010.03037.x [DOI] [PubMed] [Google Scholar]
- 16.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45(2):e17-e24. doi: 10.1097/MCG.0b013e3181e12c09 [DOI] [PubMed] [Google Scholar]
- 17.Lang K, Danchenko N, Gondek K, Shah S, Thompson D. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50(1):89-99. doi: 10.1016/j.jhep.2008.07.029 [DOI] [PubMed] [Google Scholar]
- 18.Saab S, Hunt DR, Stone MA, McClune A, Tong MJ. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver Transpl. 2010;16(6):748-759. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4, pt 2):961-972. doi: 10.1111/j.1600-6143.2010.03021.x [DOI] [PubMed] [Google Scholar]
- 20.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187-193. doi: 10.1001/jama.289.2.187 [DOI] [PubMed] [Google Scholar]
- 21.Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E; European Concerted Action on Viral Hepatitis (EUROHEP) . Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97(11):2886-2895. doi: 10.1111/j.1572-0241.2002.07057.x [DOI] [PubMed] [Google Scholar]
- 22.Hui JM, Kench JG, Chitturi S, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38(2):420-427. doi: 10.1053/jhep.2003.50320 [DOI] [PubMed] [Google Scholar]
- 23.Young MT, Gebhart A, Phelan MJ, Nguyen NT. Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American College of Surgeons NSQIP. J Am Coll Surg. 2015;220(5):880-885. doi: 10.1016/j.jamcollsurg.2015.01.059 [DOI] [PubMed] [Google Scholar]
- 24.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149(3):275-287. doi: 10.1001/jamasurg.2013.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Look AHEAD Research Group Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2014;22(1):5-13. doi: 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56-65. doi: 10.1001/jama.2011.1914 [DOI] [PubMed] [Google Scholar]
- 27.Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC. Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care. 2010;16(7):e174-e187. [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project National Inpatient Sample (NIS). https://hcupnet.ahrq.gov/. Modified August 13, 2018. Accessed March 22, 2017.
- 29.Rushing J, Wing R, Wadden TA, et al. ; Look AHEAD Research Group . Cost of intervention delivery in a lifestyle weight loss trial in type 2 diabetes: results from the Look AHEAD clinical trial. Obes Sci Pract. 2017;3(1):15-24. doi: 10.1002/osp4.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675. doi: 10.1111/apt.12871 [DOI] [PubMed] [Google Scholar]
- 31.Gordon SC, Pockros PJ, Terrault NA, et al. Impact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology. 2012;56(5):1651-1660. doi: 10.1002/hep.25842 [DOI] [PubMed] [Google Scholar]
- 32.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17(7):531-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Bureau of Labor Statistics Consumer Price Index Inflation Calculator. 2015. https://data.bls.gov/cgi-bin/cpicalc.pl. Accessed July 25, 2017.
- 34.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406. doi: 10.7326/M14-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253-1258. doi: 10.1001/jama.1996.03540150055031 [DOI] [PubMed] [Google Scholar]
- 36.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217-231. doi: 10.1016/j.jhep.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff MJ, Corey KE, Chhatwal J, Kaplan LM, Chung RT, Hur C. Bariatric surgery for nonalcoholic steatohepatitis: a clinical and cost-effectiveness analysis. Hepatology. 2017;65(4):1156-1164. doi: 10.1002/hep.28958 [DOI] [PubMed] [Google Scholar]
- 38.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Lopez-Jimenez F. Effect of bariatric surgery on the metabolic syndrome: a population-based, long-term controlled study. Mayo Clin Proc. 2008;83(8):897-907. doi: 10.1016/S0025-6196(11)60766-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schauer PR, Bhatt DL, Kirwan JP, et al. ; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017;376(7):641-651. doi: 10.1056/NEJMoa1600869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipshultz H, Issak A, Porter A, et al. 3–Bariatric surgery is safe in patients with compensated cirrhosis: an update on outcomes from 2008-2013. Gastroenterology. 2018;154(6)(suppl 1):S1. doi: 10.1016/S0016-5085(18)30492-X [DOI] [Google Scholar]
- 42.Pestana L, Swain J, Dierkhising R, Kendrick ML, Kamath PS, Watt KD. Bariatric surgery in patients with cirrhosis with and without portal hypertension: a single-center experience. Mayo Clin Proc. 2015;90(2):209-215. doi: 10.1016/j.mayocp.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 43.Hanipah ZN, Punchai S, McCullough A, et al. Bariatric surgery in patients with cirrhosis and portal hypertension. Obes Surg. 2018;28(11):3431-3438. doi: 10.1007/s11695-018-3372-z [DOI] [PubMed] [Google Scholar]
- 44.Chhatwal J, Samur S, Kues B, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017;65(3):777-788. doi: 10.1002/hep.28926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belnap L, Rodgers GM, Cottam D, Zaveri H, Drury C, Surve A. Portal vein thrombosis after laparoscopic sleeve gastrectomy: presentation and management. Surg Obes Relat Dis. 2016;12(10):1787-1794. doi: 10.1016/j.soard.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 46.Takata MC, Campos GM, Ciovica R, et al. Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis. 2008;4(2):159-164. doi: 10.1016/j.soard.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 47.Bromberger B, Porrett P, Choudhury R, Dumon K, Murayama KM. Weight loss interventions for morbidly obese patients with compensated cirrhosis: a Markov decision analysis model. J Gastrointest Surg. 2014;18(2):321-327. doi: 10.1007/s11605-013-2298-y [DOI] [PubMed] [Google Scholar]
- 48.American Society for Metabolic and Bariatric Surgery Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed December 8, 2017.
- 49.Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379-388. doi: 10.1053/j.gastro.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 50.Woodford RM, Burton PR, O’Brien PE, Laurie C, Brown WA. Laparoscopic adjustable gastric banding in patients with unexpected cirrhosis: safety and outcomes. Obes Surg. 2015;25(10):1858-1862. doi: 10.1007/s11695-015-1623-9 [DOI] [PubMed] [Google Scholar]
- 51.ASMBS Clinical Issues Committee Bariatric surgery in class I obesity (body mass index 30-35 kg/m2). Surg Obes Relat Dis. 2013;9(1):e1-e10. doi: 10.1016/j.soard.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 52.Busetto L, Dixon J, De Luca M, Shikora S, Pories W, Angrisani L. Bariatric surgery in class I obesity: a Position Statement from the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO). Obes Surg. 2014;24(4):487-519. doi: 10.1007/s11695-014-1214-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Percentage of Excess Weight Loss After Bariatric Surgery and Lifestyle Intervention
eTable 2. Health-Related Quality-of-Life Utilities of the United States Population
eFigure 1. One-Way Sensitivity Analysis for SG in Severe Obesity
eFigure 2. One-Way Sensitivity Analysis for SG in Moderate Obesity
eFigure 3. One-Way Sensitivity Analysis for SG in Mild Obesity
eFigure 4. One-Way Sensitivity Analysis for SG in Overweight
eFigure 5. Probabilistic Sensitivity Analysis for Severe Obesity
eFigure 6. Probabilistic Sensitivity Analysis for Moderate Obesity
eFigure 7. Probabilistic Sensitivity Analysis for Mild Obesity
eFigure 8. Probabilistic Sensitivity Analysis for Overweight
eReferences.