Key Points
Question
Does the outcome of subthalamic deep brain stimulation vary among common monogenic forms of Parkinson disease?
Findings
In this systematic review and meta-analysis involving 518 patients from 17 published studies, treatment with subthalamic deep brain stimulation for patients with Parkinson disease with LRRK2, GBA, or PRKN gene mutation yielded similar motor outcomes but different changes in dopaminergic doses, activities of daily living, motor complications, and cognitive functions.
Meaning
Genetic screening for LRRK2, GBA, and PRKN mutations in patients with Parkinson disease who are candidates for subthalamic deep brain stimulation may serve to inform outcomes.
Abstract
Importance
Comparative outcomes among different monogenic forms of Parkinson disease after subthalamic nucleus deep brain stimulation (STN DBS) remain unclear.
Objective
To compare clinical outcomes in patients with the most common monogenic forms of Parkinson disease treated with STN DBS.
Design, Setting, and Participants
Systematic review and meta-analysis in which a PubMed search of interventional and noninterventional studies of Parkinson disease with LRRK2, GBA, or PRKN gene mutations published between January 1, 1990, and May 1, 2018, was conducted. Among the inclusion criteria were articles that reported the Motor subscale of the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) before and after STN DBS treatment, that involved human participants, and that were published in the English language. Studies that used aggregated data from patients with different genetic mutations were excluded, and so were studies with assumed but not confirmed genetic data or incomplete follow-up data.
Main Outcomes and Measures
Changes in UPDRS-III scores and levodopa equivalent daily dose (LEDD) were analyzed for each monogenic form of Parkinson disease. Additional end points included activities of daily living (UPDRS-II), motor complications (UPDRS-IV), and cognitive function.
Results
Of the 611 eligible studies, 17 (2.8%) met the full inclusion criteria; these 17 studies consisted of 8 cohort studies (47.1%), 3 case series (17.6%), and 6 case reports (35.3%), and they involved a total of 518 patients. The UPDRS-III score improved by 46% in LRRK2 (mean change, 23.0 points; 95% CI, 15.2-30.8; P < .001), 49% in GBA (20.0 points; 95% CI, 4.5-35.5; P = .01), 43% in PRKN (24.1 points; 95% CI, 12.4-35.9; P < .001), and 53% in idiopathic Parkinson disease (25.2 points; 95% CI, 21.3-29.2; P < .001). The LEDD was reduced by 61% in LRRK2 (mean change, 711.9 mg/d; 95% CI, 491.8-932.0; P < .001), 22% in GBA (269.2 mg/d; 95% CI, 226.8-311.5; P < .001), 61% in PRKN (494.8 mg/d; 95% CI, –18.1 to –1007.8; P = .06), and 55% in idiopathic Parkinson disease (681.8 mg/d; 95% CI, 544.4-819.1; P < .001). Carriers of the PRKN mutations showed sustained improvements in UPDRS-II and UPDRS-IV, whereas LRRK2 mutation carriers sustained improvements only in UPDRS-IV. Carriers of the GBA mutation showed worse postsurgical cognitive and functional performance.
Conclusions and Relevance
Treatment with STN DBS for patients with Parkinson disease with LRRK2, GBA, or PRKN mutations appears to be associated with similar motor outcomes but different changes in dopaminergic dose, activities of daily living, motor complications, and cognitive functions.
This systematic review and meta-analysis examines research articles indexed in PubMed on the genetic mutations in Parkinson disease and their response to subthalamic nucleus deep brain stimulation treatment.
Introduction
The traditional view of Parkinson disease as a single idiopathic disorder has been useful for the development of symptomatic treatments, such as dopaminergic oral medications. However, the selection of optimal candidates for subthalamic nucleus deep brain stimulation (STN DBS) demands a more nuanced characterization of the distinctive and heterogeneous pathogenic mechanisms involved in the different subtypes of Parkinson disease.
A range of genetic mutations has been associated with variable clinical phenotypes of Parkinson disease. Carriers of glucosylceramidase β (GBA [OMIM *606463]) gene mutations, for instance, have a greater probability of developing cognitive impairment, postural instability, and falls.1 Carriers of PRKN (OMIM *602544), PINK1 (OMIM *608309), and DJ-1 (OMIM *602533) gene mutations, on the contrary, exhibit milder progression of motor and nonmotor features.2
Treatment with STN DBS can yield greater than 50% of motor improvement,3,4,5 60% amelioration of levodopa-related motor complications,4,5 40% to 60% improvement in quality of life,6,7 and 50% reduction in the levodopa equivalent daily dose (LEDD).8 Nonetheless, the clinical outcomes after STN DBS have remained variable,9 with nearly half of patients with Parkinson disease developing stimulation-resistant symptoms such as gait impairment, postural instability, falls, cognitive impairment, and other nonmotor deficits within 5 years from the procedure.9 This variability in outcomes warrants an examination of clinical and biologic factors. To this end, we sought to examine whether different monogenic forms of Parkinson disease are associated with different responses to STN DBS in motor, functional, and pharmacologic end points.
Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline.10,11
Search Methods
We searched PubMed for interventional and noninterventional studies published between January 1, 1990, and May 1, 2018, that reported data on patients treated with STN DBS and screened for monogenic forms of Parkinson disease. We used the following search terms: deep brain stimulation, mutation, gene, genetics, inherited, familial, Parkinson's disease, and parkinsonism.
Three of us (C.A.A., A.R., and D.P.) independently reviewed abstracts and full-text articles for eligibility criteria. Duplicated studies were identified and excluded. Only studies that referred to human participants and were published in the English language were considered. No restrictions were applied to participant sex, age, ethnicity, follow-up duration, disease duration, or disease severity. The reference list of each article was screened for additional pertinent studies not captured by the original search strategy.
Inclusion and Exclusion Criteria
We included studies in which patients with genetically confirmed monogenic forms of Parkinson disease were treated with STN DBS, that included a minimum postsurgical follow-up of 3 months, and that reported the Motor subscale of the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) in the presurgical medication-off and postsurgical medication-off/stimulation-on conditions.12 Studies of aggregated data from patients with different genetic mutations (eg, genetic data were pooled rather than reported separately) were excluded. We excluded studies with assumed but not confirmed genetic data or incomplete follow-up data. For recessive mutations such as PRKN, only data from homozygous or compound heterozygous were included. Data from heterozygous carriers were extracted but only evaluated in ancillary analyses.
Study End Points
We used a data collection form to extract the following variables of interest: (1) UPDRS-III in the presurgical medication-off and postsurgical medication-off/stimulation-on conditions; (2) LEDD, according to a previously published conversion table13; (3) UPDRS Part II (activities of daily living); (4) UPDRS Part IV (motor complications); and (5) cognitive evaluation by Montreal Cognitive Assessment,14 Mini–Mental State Examination,15 or Mattis Dementia Rating Scale,16 according to availability. Additional data included in the data collection were study population, sample size, genetic mutations evaluated, year of publication, study design, age at Parkinson disease onset, disease duration at STN DBS, and follow-up duration in months.
Data were expressed as mean with SD or mean percentage change, as appropriate. If multiple data points were available from the same cohort, we included the most recent publication with the longest follow-up. The control group was formed by patients with Parkinson disease from the same study data sets with confirmed negative genetic screening.
Assessment of Risk of Bias
Two of us (C.A.A. and D.P.) independently performed the quality appraisal of qualifying studies. Given the heterogeneity of study designs, the risk of bias of individual studies was evaluated using the National Heart, Lung, and Blood Institute quality appraisal tools, per the Cochrane handbook recommendations.17 Visual inspection of funnel plots was conducted to assess for publication bias.18
Statistical Analysis
Two sets of analyses were performed: (1) meta-analyses for quantitative outcomes (UPDRS-III and LEDD) from different studies with varying sample sizes after assigning appropriate weights by specific gene mutations and controls, and (2) descriptive data analyses without weights for case report studies (intraindividual analyses) and outcomes of variable definitions and measurements, such as activities of daily living, motor complications, and cognitive outcomes. In the descriptive data analyses, we used summary statistics (mean, SD, and range) for continuous data and frequency for categorical data. To estimate the proportion of specific genetic mutations, we conducted for each gene a separate meta-analysis for proportions, using a random-effects model with the DerSimonian and Laird method.19
The 95% CI for proportion was computed according to the score (Wilson) method. Mean percentage change in study outcomes were converted to mean and SD wherever feasible. The effect size for each end point was computed using the mean change between the presurgical and postsurgical periods along with the pooled SD. After estimating the correlation coefficients between presurgical and postsurgical values for a specific gene mutation, pooled SD was computed for each data set using a previously published formula.20 The heterogeneity in the studies was measured with the I2 statistic, which provides the proportion of observed variance likely to remain even after eliminating sampling error.21 An I2 statistic greater than 50% was considered as substantial heterogeneity.17 Given the sample size, inclusion of observational studies, and heterogeneity across the studies, the pooled effect size was computed using a random-effects model by means of the DerSimonian and Laird method.19 We further confirmed the findings of the study by performing Hartung-Knapp-Sidik-Jonkman method for a random-effects meta-analysis.22
Given the small number of studies included, we performed validation analyses with 2 different methods to estimate the pooled SD and confirm the robustness of the meta-analysis–estimated associations between the presurgical and postsurgical treatment for each specific gene. In the validation analysis, meta-analyses using a random-effects model with the DerSimonian and Laird method were conducted for each outcome using the pooled SD, computed after estimating the correlation coefficient between presurgical and postsurgical values for all data sets (irrespective of gene mutations) and ignoring the correlation between presurgical and postsurgical values. Publication bias was assessed using the Egger test and a funnel plot. Key findings were displayed using forest plots.
Two-sided P < .05 was considered statistically significant. Data sets for meta-analyses and statistical codes were included (eTables 1 to 5 and eAppendix 1 in the Supplement). Our biostatistician (A.D.) carried out the analyses using Stata, version 13.1 (StataCorp LLC) (eAppendix 2 in the Supplement).
Results
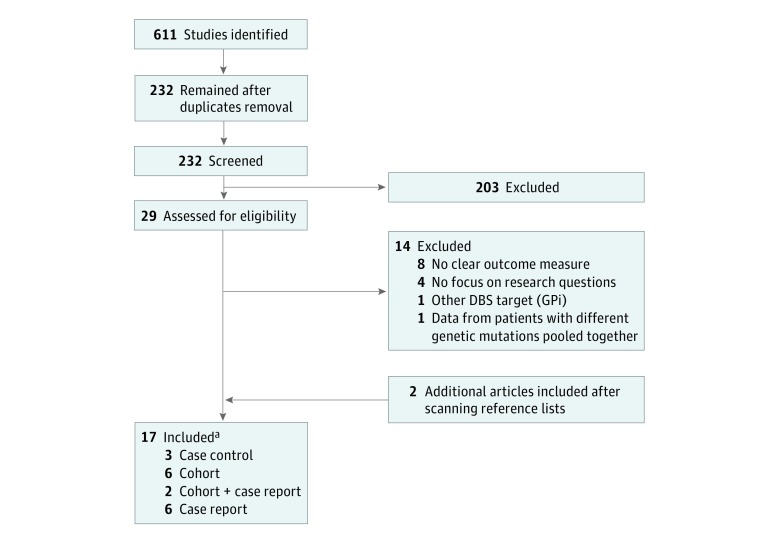
Of the 611 eligible studies, 17 (2.8%) met the full inclusion criteria (8 cohort studies [47.1%], 3 case series [17.6%], and 6 case reports [35.3%])23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39 and underwent data extraction, individual quality assessment, and risk-of-bias evaluation (Figure 1). Of the 17 studies, 9 (53.0%) yielded data sets for meta-analysis, 6 (35.3%) for intraindividual analysis, and 2 (11.8%) for both. No signs of publication bias were detected through visual inspection of funnel plots and publication bias tests.
Figure 1. Study Flowchart.
DBS indicates deep brain stimulation; GPi, globus pallidus pars interna.
aTwenty-four patients were carriers of heterozygous PRKN mutation and were analyzed separately.23,24,25,26,28,29,30
Clinical and Demographic Data
We found a total of 518 patients (135 with monogenic forms of Parkinson disease and 383 controls) from 17 studies of Parkinson disease–associated genetic mutations (Table 1). Twelve carriers (8.9%) of monogenic Parkinson disease were excluded because of non-STN targeting (n = 9)24 or incomplete follow-up data (n = 3).26,33 Five controls (1.3%) were excluded because of incomplete follow-up data.25,26
Table 1. Reviewed Studies.
| Source | Study Design | Patients Screened, No. | Selection Criteria | Patients Genetically Assessed, No. | Gene | Patients With Genetic Mutation, No. | Patients for Meta-analysis (Motor), No. | Patients for Meta-analysis (Therapy), No. | Control Patients, No. | Mean Follow-up, mo | Mean (SD), y | Quality Assessment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at PD Onset in Carriers | PD Duration at DBS in Carriers | Age at PD Onset in Controls | PD Duration at DBS in Controls | ||||||||||||
| Studies Included in Meta-analysis | |||||||||||||||
| Romito et al,28 2005 | Cohort | 36 | NA | 36 | PRKN | 1 PRKN
a; 4 heterozygous PRKN |
4 Heterozygous PRKN | 4 Heterozygous PRKN | 31 | 18 | PRKN heterozygous: 33.0 (5.8) | PRKN heterozygous: 15.7 (4.9) | 43.5 (6.2) | 13.9 (5.5) | Fair |
| Schüpbach et al,31 2007 | Cohort | 69 | NA | 69 | LRRK2 | 9 Heterozygous LRRK2 | 9 Heterozygous LRRK2 | 9 heterozygous LRRK2 | 60 | 12 | 41.1 (6.1) | 13.4 (2.7) | 43.1 (7.8) | 13.0 (8.2) | Fair |
| Gómez-Esteban et al,33 2008 | Cohort | 48 | Family history | 8 | LRRK2 | 5 Heterozygous LRRK2b | 4 Heterozygous LRRK2 | NA | 43 | 6 | 43.2 (10.8) | 12.8 (3.6) | 58.0 (1.2) | 14.2 (6.9) | Fair |
| Moro et al,23 2008 | Cohort | 312 | Age at PD onset <45 y | 80 |
PRKN PINK1 LRRK2 |
6 PRKN; 5 heterozygous PRKN; 1 homozygous PINK1a |
6 PRKN; 5 heterozygous PRKN |
NA | 68 | 12 |
PRKN: 26.5 (10.1); PRKN heterozygous: 34.4 (6.4) |
PRKN: 22.2 (8.1); PRKN heterozygous: 15.6 (3.6) |
36.3 (7.0) | 17.3 (7.4) | Fair |
| Lohmann et al,25 2008 | Cohort | 134 | Young onset and/or family history | 54 |
PRKN LRRK2 |
7 PRKN; 7 heterozygous PRKN |
7 PRKN; 7 heterozygous PRKN |
7 PRKN; 7 heterozygous PRKN |
40 (1 excluded from meta-analysis) | 18 |
PRKN: 26.4 (9.6); PRKN heterozygous: 38.4 (9.9) |
PRKN: 19.9 (7.9); PRKN heterozygous: 13.4 (2.2) |
38.0 (9.2) | 15.0 (4.6) | Fair |
| Weiss et al,36 2012 | Case-control | 98 | GBA mutation | 98 | GBA | 3 Heterozygous GBA | 3 Heterozygous GBA | NA | 6c | 24-48 | 49.7 (3.8) | 17.3 (5.5) | 49.3 (5.1) | 17.8 (6.8) | Fair |
| Angeli et al,24 2013 | Cohort | 94 | NA | 94 |
PRKN PINK1 LRRK2 DJ1 SNCA GBA |
5 PRKN (2 STN DBS) 3 heterozygous PRKN (DBS target NR); 5 heterozygous LRRK2 (5 STN DBS); 16 heterozygous GBA (13 STN DBS)d |
2 PRKN; 5 heterozygous LRRK2 13 heterozygous GBA |
2 PRKN; 5 heterozygous LRRK2; 13 heterozygous GBA |
67 | 12 |
PRKN: 39.7 (1.2) PRKN heterozygous: NR; LRRK2: 43.0 (8.7); GBA heterozygous: 42.9 (6.2) |
PRKN: 25.2 (12.8); PRKN heterozygous: NR LRRK2: 12.1 (1.8); GBA heterozygous: 11.2 (5.0) |
40.8 (7.2) | 15.1 (5.5) | Fair |
| Greenbaum et al,32 2013 | Case-control | NR | LRRK2 mutation | NR |
PRKN PINK1 LRRK2 |
13 Heterozygous LRRK2 | 13 Heterozygous LRRK2 | 13 Heterozygous LRRK2 | 26e | 12 | 49.5 (6.8) | 11.7 (4.9) | 49.2 (6.6) | 13.2 (5.8) | Good |
| Kim et al,26 2014 | Cohort | 122 | Age at PD onset <40 y | 18 |
PRKN PINK1 LRRK2 DJ1 SNCA |
3 PRKN; 2 heterozygous PRKNf |
3 PRKN | 3 PRKN | 13 (4 Excluded from meta-analysis) | 45 |
PRKN: 21.7 (8.5); PRKN heterozygous: NR |
PRKN: 28.3 (7.6); PRKN heterozygous: NR |
34.6 (3.9) | 15.4 (3.4) | Good |
| Sayad et al,29 2016 | Cohort | 27 | NA | 27 |
PRKN PINK1 LRRK2 DJ1 |
2 Heterozygous PRKN; 15 heterozygous LRRK2 |
2 Heterozygous PRKN; 15 heterozygous LRRK2 |
NA | 12 (Authors included 2 hetorzygous PRKN patients as controls) | 24 |
PRKN heterozygous: 48.0 (0.0); LRRK2: 40.1 (9.4) |
PRKN heterozygous: 11.5 (2.1); LRRK2: 16.1 (3.0) |
40.3 (8.2) | 14.3 (2.7) | Good |
| Lythe et al,37 2017 | Case-control | NR | GBA mutation | NR |
PRKN PINK1 LRRK2 DJ1 SNCA GBA |
15 Heterozygous GBA; 2 homozygous GBA |
15 Heterozygous GBA; 2 homozygous GBA |
15 Heterozygous GBA; 2 homozygous GBA |
17g | 90 | 41.4 (5.8) | 12.1 (1.3) | 43.0 (5.3) | 14.7 (5.0) | Good |
| Total patients included in meta-analysis, No. | NA | NA | NA | NA | NA | NA | 115 | 80 | 378 | NA | NA | NA | |||
| Case Reports (Intraindividual Patient Analysis) | |||||||||||||||
| Capecci et al,27 2004 | Case report | NA | NA | 1 | PRKN | 1 PRKN | NA | NA | NA | 12 | 22 | 19 | NA | NA | NA |
| Romito et al,28 2005 | Case report | 36 | NA | 36 | PRKN | 1 PRKN | NA | NA | NA | 18 | 45 | 8 | NA | NA | NA |
| Moro et al,23 2008 | Case report | 312 | Age at PD onset <45 y | 80 |
PRKN PINK1 LRRK2 |
1 Homozygous PINK1 | NA | NA | NA | 12 | 31 | 30 | NA | NA | NA |
| Breit et al,34 2010 | Case report | NA | NA | 1 | LRRK2 | 1 Heterozygous LRRK2 | NA | NA | NA | 12 | 42 | 18 | NA | NA | NA |
| Stefani et al,35 2013 | Case report | NA | NA | 1 | LRRK2 | 1 Heterozygous LRRK2 | NA | NA | NA | 3 | 49 | 7 | NA | NA | NA |
| Antonini et al,38 2012 | Case report | NA | NA | 1 | SNCA | 1 Heterozygous SNCA | NA | NA | NA | 12 | 41 | 5 | NA | NA | NA |
| Nakahara et al,39 2014 | Case report | NA | NA | 1 |
PRKN PINK1 |
1 PRKN + heterozygous PINK1 | NA | NA | NA | 8 | 15 | 45 | NA | NA | NA |
| Genç et al,30 2016 | Case report | NA | NA | 1 | PRKN | 1 Heterozygous PRKN | NA | NA | NA | NR | 10 | 4 | NA | NA | NA |
Abbreviations: DBS, deep brain stimulation; NA, not applicable; NR, not reported; PD, Parkinson disease; PRKN, homozygous or compound heterozygous PRKN mutations; STN, subthalamic nucleus.
Data reported in the Case Reports section.
One patient was excluded from meta-analysis because of incomplete data.
Control group matched 1:2 with patients with GBA mutation (sex, age, PD duration at STN DBS).
Nine patients were excluded from meta-analysis because of DBS target other than STN or unknown.
Control group matched 1:2 with patients with LRRK2 mutation (sex, age at onset, PD duration at STN DBS).
Two patients were excluded from meta-analysis because of incomplete data.
Control group matched 1:1 with patients with GBA mutation (sex, PD duration at STN DBS).
In the population tested for specific mutations, the proportion of LRRK2 carriers was 29% (95% CI, 10%-47%)24,29,31,32,33; GBA carriers, 5.0% (95% CI, 2%-8%)24,36,37; and PRKN carriers, 6.0% (95% CI, 2%-10%) (Table 2).23,24,25,26,28 Our search yielded only 1 case of PINK1,23 1 case of SNCA (OMIM *163890),38 and 1 case of combined PRKN and PINK1 mutations (Table 3).39 The proportion of heterozygous PRKN carriers was 9.0% (95% CI, 5%-12%) (eTable 6 in the Supplement).23,24,25,26,28,29,30
Table 2. Proportion and Type of Mutations.
| Gene | Patients With Mutation, % (95% CI) | Source | Gene Assessment | Type of Mutation Found |
|---|---|---|---|---|
| PRKN | 6 (2-10) | Capecci et al,27 2004 | PRKN | ex3del |
| Romito et al,28 2005 | PRKN | G828A-duplEx1 | ||
| Moro et al,23 2008 | PRKN; PINK1; LRRK2 (only G2019S) | Q34fsX43 (2 patients); N58_Q178del; V2445fsX318; Q57fsX96-Q347fsX368; I2fsX7-Q311fsX318 | ||
| Lohmann et al,25 2008 | PRKN; LRRK2 (only G2019S) | C289G; ex5del–255delA; ex3del–prom-ex1del; ex2-4dupl–ex3del; ex5del–C441R; ex2del–ex3del; ex4-7del–IVS7-1G>C | ||
| Angeli et al,24 2013 | PRKN; PINK1; LRRK2 (exons 1, 2, 10, 15, 27, 41, 49); DJ-1 (exons 3, 5, 6, 7); SNCA; GBA | c.101_102delAG; ex3-4del; c.1289G>A-c.823C>T; c.337_376del-c.465–466del; c.823C>T-ex6dupl | ||
| Kim et al,26 2014 | PRKN; PINK1; LRRK2 (only G2019S); DJ-1; SNCA | NR (3 patients) | ||
| LRRK2 | 29 (10-47) | Schüpbach et al,31 2007 | LRRK2 (exon 41) | G2019S (8 patients); T2031S |
| Gómez-Esteban et al,33 2008 | LRRK2 | R1441G (5 patients) | ||
| Breit et al,34 2010 | LRRK2 | R793M | ||
| Stefani et al,35 2013 | LRRK2 (exon 41) | G2019S | ||
| Angeli et al,24 2013 | PRKN; PINK1; LRRK2 (exons 1, 2, 10, 15, 27, 41, 49); DJ-1 (exons 3, 5, 6, 7); SNCA; GBA; PRKN; PINK1; LRRK2 (only G2019S); PRKN | G2019S (5 patients) | ||
| Greenbaum et al,32 2013 | PINK1; LRRK2 (exon 41); DJ-1; GBA (N370S and L444P) | G2019S (13 patients) | ||
| Sayad et al,29 2016 | PRKN | G2019S (15 patients) | ||
| GBA | 5 (2-8) | Weiss et al,36 2012 | PINK1; LRRK2 (exons 1, 2, 10, 15, 27, 41, 49); DJ-1 (exons 3, 5, 6, 7); SNCA; GBA; PRKN | L444P (2 patients); N370S |
| Angeli et al,24 2013 | PINK1; LRRK2; DJ1; SNCA; GBA; PRKN | 13 Patients heterozygous: E326K (4 patients); N370S; D409H; recNcil; R463C; N188S; R275Q; IVS211 G>A; L444P; T369M 3 patients homozygous/compound heterozygous: E326K; R463C; L444P-E326K |
||
| Lythe et al,37 2017 | PINK1; LRRK2 (only G2019S); SNCA | 15 Patients heterozygous: NR; 2 patients homozygous: NR |
||
| PINK1 | NA | Moro et al,23 2008 | PRKN | V170G |
| SNCA | NA | Antonini et al,38 2012 | PINK1 | dupl 4q22.1 |
| PRKN + PINK1 | NA | Nakahara et al,39 2014 | NA | T175PfsX2 (PRKN) + R58-V59insGR (heterozygous PINK1) |
Abbreviations: NA, not applicable; NR, not reported.
Table 3. Intraindividual Patient Analyses.
| Genes | No. of Studies | Mean (Range) | Improvement (Range), % | |
|---|---|---|---|---|
| Baseline Values | Improvement | |||
| Motor improvement (UPDRS-III score)a | ||||
| PRKN | 2 | 53 (45-61) | 32 (26-38) | 64 (43-94) |
| LRRK2 | 2 | 48.5 (27-70) | 32.5 (19-46) | 68 (66-70) |
| PINK1 | 1 | 35.5 | 16.5 | 47 |
| SNCA | 1 | 22 | 9.5 | 43 |
| PRKN + PINK1 | 1 | 86 | 53 | 62 |
| LEDD reduction, mg | ||||
| PRKN | 2 | 700 (500-900) | 406 (220-592) | 55 (44-66) |
| LRRK2 | 2 | 875 (850-900) | 445 (400-490) | 51 (44-58) |
| PINK1 | 0 | NA | NA | NA |
| SNCA | 1 | 1250 | 790 | 63 |
| PRKN + PINK1 | 1 | 1181 | 691 | 59 |
Abbreviations: LEDD, levodopa equivalent daily dose; NA, not applicable; UPDRS-III, Unified Parkinson’s Disease Rating Scale Part III.
Motor improvement was defined as the change in the UPDRS-III score between the presurgical medication-off condition and the postsurgical medication-off/stimulation-on condition. The presurgical motor outcome (UPDRS-III score) associated with levodopa is reported in eTable 9 in the Supplement.
The mean (SD) age at Parkinson disease onset was 43.4 (3.7) years in LRRK2, 44.7 (4.4) years in GBA, and 28.6 (7.7) years in PRKN carriers. For the single cases, the age at onset was 31 years in PINK1, 41 years in SNCA, and 15 years in the combined PRKN and PINK1 carriers. The mean (SD) age at onset in the control population was 44.6 (6.7) years. The mean (SD) disease duration at the time of STN DBS was 13.4 (1.6) years in LRRK2, 13.5 (3.3) years in GBA, and 23.9 (3.6) years in PRKN carriers. For the single cases, it was 30 years in the PINK1, 5 years in SNCA, and 45 years in the combined PRKN and PINK1 carriers. The mean (SD) disease duration at the time of STN DBS among the controls was 14.6 (1.4) years.
Meta-analysis
Motor End Points
Of the 123 patients with Parkinson disease–associated genetic mutations, 115 (93.5%; 46 LRRK2, 33 GBA, 18 homozygous PRKN, and 18 heterozygous PRKN) were included in the meta-analysis for motor end points, and 8 single cases (6.5%) underwent intraindividual patient analyses (Tables 1 and 3).
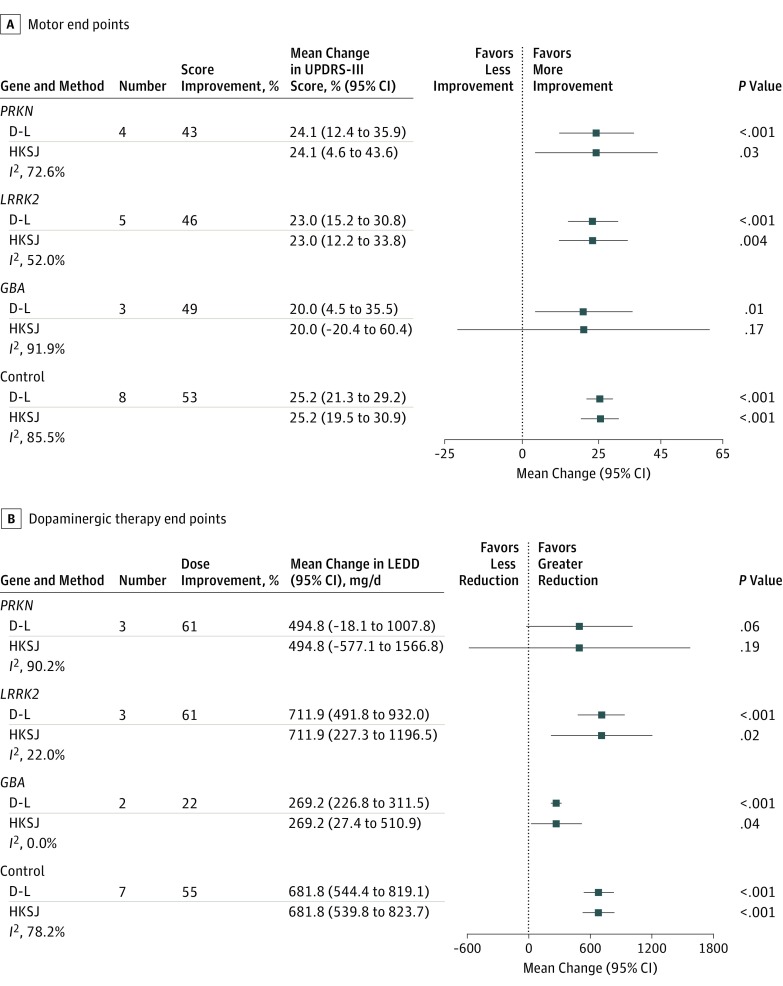
The UPDRS-III score improved by 46% in LRRK2 (mean change, 23.0 points; 95% CI, 15.2-30.8; P < .001), 49% in GBA (20.0 points; 95% CI, 4.5-35.5; P = .01), 43% in PRKN (24.1 points; 95% CI, 12.4-35.9; P < .001), and 53% in control (25.2 points; 95% CI, 21.3-29.2; P < .001) patients (Figure 2A and eFigure in the Supplement). Data from the heterozygous PRKN carriers are reported in eTable 6 in the Supplement. The validation analyses confirmed the robustness of the findings (eTable 7 in the Supplement).
Figure 2. Meta-analysis of Motor Improvement and Levodopa Equivalent Daily Dose (LEDD) Reduction After Subthalamic Nucleus Deep Brain Stimulation.
A, The DerSimonian and Laird (D-L) meta-analysis method produced slightly less precise estimates compared with the Hartung-Knapp-Sidik-Jonkman (HKSJ) method. Both methods produced similar findings, except for the GBA gene owing to the extremely high heterogeneity and small number of studies. The presurgical motor outcome (Unified Parkinson’s Disease Rating Scale Part III [UPDRS-III] score) associated with levodopa is reported in eTable 8 in the Supplement. B, Both D-L and HKSJ meta-analysis methods produced similar findings for all genes. Error bars represent the 95% CI of the mean changes reported.
Dopaminergic Therapy
Among the 123 patients with Parkinson disease–associated genetic mutations, 80 (65.0%) (27 LRRK2, 30 GBA, 12 homozygous PRKN, and 11 heterozygous PRKN) were included in the meta-analysis for therapy end points, and 7 single cases (5.7%) underwent intraindividual patient analyses (Tables 1 and 3).
The LEDD was reduced by 61% (mean change, 711.9 mg/d; 95% CI, 491.8-932.0 mg/d; P < .001) in LRRK2, 22% (269.2 mg/d; 95% CI, 226.8-311.5 mg/d; P < .001) in GBA, 61% (494.8 mg/d; 95% CI, −18.1 to 1007.8 mg/d; P = .06) in PRKN, and 55% (681.8 mg/d; 95% CI, 544.4-819.1 mg/d; P < .001) in control patients (Figure 2B and eFigure in the Supplement). Data from the heterozygous PRKN carriers are reported in eTable 6 in the Supplement. The validation analyses confirmed the robustness of the findings (eTable 7 in the Supplement). The presurgical motor outcome (UPDRS-III score) associated with levodopa is reported in eTables 8 and 9 in the Supplement.
Systematic Review of Individual-Level Data
Activities of Daily Living
Three studies and 5 case reports analyzed changes in the UPDRS-II in patients with a monogenic form of Parkinson disease treated with STN DBS.26,27,31,33,34,35,38,39 The LRRK2 carriers (n = 15) (4 studies with a mean [SD] follow-up of 14.4 [22.5] months) showed variable results, varying from 45.2% to 66.7% improvement in the G2019S variant (n = 10) (2 studies with a mean [SD] follow-up of 11.1 [2.8] months)31,35 and 21.4% in the R793M variant (n = 1) (1 study with a 12-month follow-up)34 to 10.0% deterioration in the R1441G variant (n = 4) (1 study with a 6-month follow-up).33 The PRKN carriers (n = 4) (2 studies with a mean [SD] follow-up of 36.8 [16.5] months) showed improvement by 62.0% to 81.8%.26,27 The single case of SNCA showed a 37.2% improvement at 12-months of follow-up, and the single case of combined PRKN and PINK1 showed a 12.5% worsening at 8 months of follow-up.38,39
Motor Complications
Five studies and 4 case reports involved motor complications (UPDRS-IV) in patients with a monogenic form of Parkinson disease treated with STN DBS.24,25,26,27,31,34,36,38,39 Improvements were observed in LRRK2 carriers (n = 15) (3 studies with a mean [SD] follow-up of 12.0 [0.0] months) by 50% to 75%,24,31,34 GBA carriers (n = 16) (2 studies with a mean [SD] follow-up of 14.3 [4.8] months) by 37% to 80%,24,36 and PRKN carriers (n = 13) (4 studies with a mean [SD] follow-up of 22.8 [12.9] months) by 20% to 100%.24,25,26,27 The single case of SNCA improved by 87.5% at 12 months, and the combined PRKN and PINK1 case improved by 80% at 8 months.38,39
Cognitive Outcomes
Six studies and 1 case report analyzed cognitive data in patients with a monogenic form of Parkinson disease treated with STN DBS.24,25,28,31,36,37,38 The LRRK2 carriers (n = 9) (1 study with 12-month follow-up) had stable postsurgical Mattis Dementia Rating Scale scores.31 The GBA carriers (n = 26) (3 studies with a mean [SD] follow-up of 72.2 [21.1] months) developed progressive cognitive decline after STN DBS.24,36,37 A 7-year follow-up study found worse performance in all of the 5 cognitive domains in 17 GBA carriers, compared with 17 controls.37 A 5-year prospective study found a steeper decline in Mattis Dementia Rating Scale scores in 13 GBA carriers, compared with 67 controls,24 and a case series of 3 GBA carriers and 6 controls found a higher prevalence of dementia in the GBA group after 24 to 48 months of STN DBS.36 The PRKN carriers (n = 8) (2 studies with a mean [SD] follow-up of 18.0 [0.0] months) showed no or minimal postsurgical cognitive decline in the Mattis Dementia Rating Scale score25 and at full neuropsychological testing.28 Finally, the single case of SNCA (1 study with a follow-up of 12 months) showed a 1-point loss in the Mini–Mental State Examination (from 30 to 29 points).38
Discussion
The results of this systematic review and meta-analysis confirmed that STN DBS is consistently associated with improved motor outcomes in monogenic forms of Parkinson disease. However, STN DBS showed differences in LEDD reduction, motor complications, and cognitive outcomes.
The overall proportion of monogenic Parkinson disease carriers in this meta-analysis (2%-8% for GBA, 2%-10% for PRKN, and 10%-47% for LRRK2) was in keeping with findings in previous studies, suggesting a relatively high prevalence of LRRK2, GBA, and PRKN mutations in Parkinson disease cohorts selected for surgical treatments.40 Although this observation highlights the importance of clarifying the contribution of genetic factors to functional outcomes after STN DBS, the variability associated with monogenic forms of Parkinson disease (eg, the age at onset ranged from 15 years in combined PRKN and PINK1 carriers to 44 years in GBA carriers; the duration of disease at STN DBS varied from 5 years in SNCA carriers to 45 years in combined PRKN and PINK1 carriers) and the associated but unmeasured epigenetic factors should be considered when interpreting these results.
The LRRK2 mutation in Parkinson disease showed an excellent motor response to STN DBS, with 46% reduction in the UPDRS-III score and more than 60% reduction in dopaminergic therapy. Carriers of the G2019S, the most frequent variant in the LRRK2 gene, had activities-of-daily-living outcomes similar to those of carriers of idiopathic Parkinson disease, whereas carriers of the R1441G variant rapidly deteriorated after STN DBS.33 These findings are in agreement with the notion that G2019S-associated Parkinson disease exhibits a milder motor decline and slower progression of medication- and stimulation-resistant symptoms compared with idiopathic Parkinson disease.41,42 So far, 7 missense mutations have been identified within the LRRK2 gene, accounting for 1% to 2% of all cases of Parkinson disease.43 The G2019S variant is by far the most prevalent, whereas 6 other variants are infrequently observed, with the exception of the R1441G variant in patients of Basque descent.44 No clear differences have been identified in the phenotypes associated with these mutations, but rarer mutations seem to have higher clinical penetrance.45 Still, current data remain insufficient to definitively conclude that G2019S variant carriers receive a more favorable outcome after STN DBS.
The GBA mutation in Parkinson disease exhibited a substantial improvement of motor symptoms but a considerably higher rate of cognitive complications compared with other monogenic forms of Parkinson disease, and lower LEDD reduction after STN DBS (22% vs 55% of patients with sporadic Parkinson disease).24,36,37
We cannot exclude that the knowledge that GBA mutation leads to a more aggressive clinical phenotype46 could affect the therapeutic decision of maintaining higher post-DBS doses of dopaminergic medications. Carriers of the GBA mutation may present with a spectrum of clinical phenotypes, from akinetic-rigid Parkinson disease to dementia with Lewy bodies, with variable motor complications in the form of dyskinesia and wearing-off. In a cohort of 20 patients with the GBA mutation in Parkinson disease, compared with 27 patients with sporadic Parkinson disease, the mutation was found to be associated with a relatively younger age at onset and more rapid progression of cognitive symptoms, postural instability, and gait abnormalities.46 Depression, anxiety, social dysfunction, and hallucinations may also be more frequently observed in these patients.47,48,49,50 Up to 5% of patients with Parkinson disease undergoing STN DBS might be carriers of the GBA mutation, but we could not clarify which of the numerous GBA variants is the most represented in this specific population.51 Overall, this meta-analysis confirms the motor advantages of STN DBS for GBA mutation carriers but suggests a higher rate of cognitive complications. A thorough neuropsychological assessment and a careful discussion of the risk/reward profile of STN DBS are, therefore, important in this particular population. Future studies will need to examine whether DBS of the globus pallidus pars interna should be preferred by GBA mutation carriers, given the possible lower rate of cognitive complications.52
The PRKN mutation in Parkinson disease showed a good response to STN DBS, with substantial improvement of motor complications and a relatively low prevalence of dementia up to 4 years after surgical treatment. These data suggest that this population might be particularly suitable for STN DBS, particularly because of the early development of dyskinesia and other levodopa-related motor fluctuations.53 On the other hand, the high prevalence of behavioral and psychiatric symptoms among PRKN mutation carriers warrants a careful neuropsychological evaluation before these carriers’ eligibility for STN DBS is considered.53,54 The PRKN mutation is the most common known cause of early-onset Parkinson disease, accounting for up to 77% of familial Parkinson disease with an age at onset younger than 30 years55 and 10% to 20% of early-onset Parkinson disease in general.56 Approximately 30% of PRKN mutations result from single-nucleotide polymorphism changes, 10% from small deletions, and more than 50% from deletions or duplications of 1 or several exons.57
Heterozygous PRKN mutations are not deemed pathogenic, but the possibility exists that cases of homozygous or compound heterozygous PRKN mutation have been erroneously diagnosed in older studies as heterozygous, given that not all exons were tested or gene doses analyses performed. Results from this selected subgroup showed a 41% motor improvement after STN DBS, compared with 53% in the control group, and a 76% LEDD reduction, compared with 55% controls (eTable 6 in the Supplement). In sum, these data suggest that STN DBS in carriers of PRKN mutations (both homozygous and heterozygous) might yield motor improvements at least comparable to what has been observed in patients with sporadic or idiopathic Parkinson disease.
Only single cases reported the outcomes of STN DBS in rarer forms of monogenic Parkinson disease. Single case reports showed moderate motor improvements in SNCA and PINK1 mutations, as well as in a case with combined PRKN and PINK1 mutation. Still, the variability in the pattern of progression associated with these rare genetic variants renders these data of uncertain value at this time. Carriers of the SNCA mutation are prone to developing cognitive decline, autonomic dysfunction, speech problems, and behavioral changes, which may affect the overall outcome of STN DBS.58 Carriers of the PINK1 mutation, on the other hand, usually manifest a slow progression of nonmotor symptoms,59 which suggests that this particular subtype of Parkinson disease may be a good candidate for STN DBS to address motor complications. However, the high prevalence of psychiatric symptoms has to be considered in the presurgical screening.60
No data have been reported for carriers of the DJ-1 mutation, a rare autosomal recessive monogenic form of early-onset Parkinson disease. Mutations in the DJ-1, PRKN, and PINK1 genes might present with a similar phenotype, characterized by an age at onset of 25 to 30 years, mild nonmotor symptoms, and a tendency to develop dyskinesia and dystonia in response to even minimal doses of levodopa, which may be optimally treated with STN DBS.42
This study suggests that LRRK2, GBA, and PRKN mutations in Parkinson disease are associated with motor improvements after STN DBS, comparable to idiopathic Parkinson disease. Carriers of the G2019 variant in LRRK2 and PRKN mutations showed sustained advantages in motor complications and activities of daily living, whereas GBA mutation carriers frequently developed cognitive impairment and stimulation-resistant symptoms within 2 to 7 years after surgical treatment. Whether this latter finding resulted from an incomplete response to STN DBS or to a faster accrual of disability intrinsic to the GBA phenotype remains unclear. The limited data available for SNCA and PINK1 mutations highlight the critical unmet need for large, multicenter studies aimed at characterizing the natural pattern of disease progression associated with rare genetic variants of Parkinson disease. Emerging possibilities for a more common genetic analysis arise from a substantial drop in the cost (and therefore wider availability) of gene panels that are designed to detect the presence of pathogenic variants in SNCA, LRRK2, PRKN, GBA, PINK1, DJ-1, and VPS35 (OMIM *601501) genes. Considering the pathogenic role of copy number variations in the pathogenesis of SNCA and PRNK mutations in Parkinson disease, these gene panel assays should combine sequencing and gene doses. Exome sequencing might be a more comprehensive alternative to predesigned gene panels, but difficulties remain in thoroughly assessing PRKN and GBA gene variability.61
Limitations
Several limitations should temper the strength of these results. First, the vast heterogeneity of the sample size, demographic features, and outcome measures of the source studies prevented the possibility of conducting a meta-analysis of the association between STN DBS and motor complications, activities of daily living, and cognitive outcomes. The analysis of these data was, therefore, limited to a systematic review of the few cases reported in the literature. Second, the sample size of the monogenic variants examined is low, consistent with their low prevalence but also with inconsistent genotyping across clinics. Third, the prevalence of the genetic mutations in Parkinson disease may have been overestimated because of a selection bias toward genetically screening patients with early-onset Parkinson disease or with a family history of neurodegenerative disorders. Variability in disease duration and length of observation period may also have accounted for some of the differences observed.
Fourth, the differences in study designs and length of follow-up, among other variables, generated large heterogeneity across the eligible studies. The unknown frequency of monogenic Parkinson disease variants precluded sensitivity analyses, but validation analyses confirmed the consistency of our results. Fifth, the cosegregation of factors known to be associated with clinical outcomes after STN DBS, such as young age at onset and at surgical treatment as well as the usually younger age of patients with monogenic forms of Parkinson disease, might have played a role in the observed results.8,62,63 Because the predictive interval for the estimated pooled effect could not be reported owing to the small number of studies, the extent to which the differences in clinical outcomes might be associated with a heterogeneous genetic background or with factors that cosegregate with the genetic background needs to be clarified in large multicenter prospective clinical trials.
Conclusions
This meta-analysis and systematic review suggests that patients with Parkinson disease who are carriers of LRRK2, GBA, and PRKN gene mutations show good motor advantages after STN DBS, comparable to patients with idiopathic Parkinson disease. Patients with the G2019 variant of LRRK2 and PRKN mutations showed sustained advantages on motor complications and activities of daily living, whereas patients with GBA mutations frequently developed cognitive impairment and stimulation-resistant symptoms within 2 to 7 years after surgical treatment. However, the current level of evidence remains insufficient to recommend genetic screening in patients with Parkinson disease who are considered candidates for STN DBS. Larger, ideally prospective, studies may establish the areas in which genetic information serves to inform the process of selecting the optimal candidates for advanced therapy for Parkinson disease.
eFigure. Percent Motor Improvement and Dopaminergic Dose Reduction Following STN DBS
eTable 1. Meta-analysis Data for Proportion
eTable 2. Meta-analysis Data for Motor and Therapy Endpoints
eTable 3. Case Report Data
eTable 4. Meta-analysis Output
eTable 5. Motor Levodopa Response
eTable 6. Heterozygous PRKN Analysis
eTable 7. Validation analyses for UPDRS-III improvement and LEDD reduction
eTable 8. Pre STN DBS Motor Outcome (UPDRS-III Score) Associated With Levodopa - Meta-analysis
eTable 9. Pre STN DBS Motor Outcome (UPDRS-III Score) Associated With Levodopa - Intra-Individual Patient Analysis
eAppendix 1. Statistical Codes
eAppendix 2. Methods of Data Analysis
References
- 1.Scholz SW, Jeon BS. GBA mutations and Parkinson disease: when genotype meets phenotype. Neurology. 2015;84(9):-. doi: 10.1212/WNL.0000000000001321 [DOI] [PubMed] [Google Scholar]
- 2.Puschmann A. Monogenic Parkinson’s disease and parkinsonism: clinical phenotypes and frequencies of known mutations. Parkinsonism Relat Disord. 2013;19(4):407-415. doi: 10.1016/j.parkreldis.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 3.Obeso JA, Olanow CW, Rodriguez-Oroz MC, Krack P, Kumar R, Lang AE; Deep-Brain Stimulation for Parkinson’s Disease Study Group . Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345(13):956-963. doi: 10.1056/NEJMoa000827 [DOI] [PubMed] [Google Scholar]
- 4.Zibetti M, Merola A, Rizzi L, et al. Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson’s disease. Mov Disord. 2011;26(13):2327-2334. doi: 10.1002/mds.23903 [DOI] [PubMed] [Google Scholar]
- 5.Rizzone MG, Fasano A, Daniele A, et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord. 2014;20(4):376-381. doi: 10.1016/j.parkreldis.2014.01.012 [DOI] [PubMed] [Google Scholar]
- 6.Diamond A, Jankovic J. The effect of deep brain stimulation on quality of life in movement disorders. J Neurol Neurosurg Psychiatry. 2005;76(9):1188-1193. doi: 10.1136/jnnp.2005.065334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dafsari HS, Reker P, Stalinski L, et al. ; EUROPAR; IPMDS (International Parkinson’s and Movement Disorders Society) Non-Motor Parkinson’s Disease Study Group . Quality of life outcome after subthalamic stimulation in Parkinson’s disease depends on age. Mov Disord. 2018;33(1):99-107. doi: 10.1002/mds.27222 [DOI] [PubMed] [Google Scholar]
- 8.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(suppl 14):S290-S304. doi: 10.1002/mds.20962 [DOI] [PubMed] [Google Scholar]
- 9.Buhmann C, Huckhagel T, Engel K, et al. Adverse events in deep brain stimulation: a retrospective long-term analysis of neurological, psychiatric and other occurrences. PLoS One. 2017;12(7):e0178984. doi: 10.1371/journal.pone.0178984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 12.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738-750. doi: 10.1002/mds.10473 [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649-2653. doi: 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 16.Mattis S. Mental status examination for organic mental syndrome in the elderly patient In: Bellack L, Karusu TB, eds. Geriatric Psychiatry. New York, NY: Grune & Stratton; 1976:77-121. [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(pt A):139-145. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-analysis. Chichester, UK: John Wiley & Sons; 2009. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- 21.Borenstein M, Higgins JPT, Hedges LV, Rothstein HR. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5-18. doi: 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
- 22.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moro E, Volkmann J, König IR, et al. Bilateral subthalamic stimulation in Parkin and PINK1 parkinsonism. Neurology. 2008;70(14):1186-1191. doi: 10.1212/01.wnl.0000307748.11216.03 [DOI] [PubMed] [Google Scholar]
- 24.Angeli A, Mencacci NE, Duran R, et al. Genotype and phenotype in Parkinson’s disease: lessons in heterogeneity from deep brain stimulation. Mov Disord. 2013;28(10):1370-1375. doi: 10.1002/mds.25535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohmann E, Welter ML, Fraix V, et al. ; French Parkinson’s Disease Genetics Study Group . Are parkin patients particularly suited for deep-brain stimulation? Mov Disord. 2008;23(5):740-743. doi: 10.1002/mds.21903 [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Yun JY, Kim YE, et al. Parkin mutation and deep brain stimulation outcome. J Clin Neurosci. 2014;21(1):107-110. doi: 10.1016/j.jocn.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 27.Capecci M, Passamonti L, Annesi F, et al. Chronic bilateral subthalamic deep brain stimulation in a patient with homozygous deletion in the parkin gene. Mov Disord. 2004;19(12):1450-1452. doi: 10.1002/mds.20250 [DOI] [PubMed] [Google Scholar]
- 28.Romito LM, Contarino MF, Ghezzi D, Franzini A, Garavaglia B, Albanese A. High frequency stimulation of the subthalamic nucleus is efficacious in Parkin disease. J Neurol. 2005;252(2):208-211. doi: 10.1007/s00415-005-0638-x [DOI] [PubMed] [Google Scholar]
- 29.Sayad M, Zouambia M, Chaouch M, et al. Greater improvement in LRRK2 G2019S patients undergoing subthalamic nucleus deep brain stimulation compared to non-mutation carriers. BMC Neurosci. 2016;17:6. doi: 10.1186/s12868-016-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genç G, Apaydın H, Gündüz A, et al. Successful treatment of Juvenile parkinsonism with bilateral subthalamic deep brain stimulation in a 14-year-old patient with parkin gene mutation. Parkinsonism Relat Disord. 2016;24:137-138. doi: 10.1016/j.parkreldis.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 31.Schüpbach M, Lohmann E, Anheim M, et al. Subthalamic nucleus stimulation is efficacious in patients with Parkinsonism and LRRK2 mutations. Mov Disord. 2007;22(1):119-122. doi: 10.1002/mds.21178 [DOI] [PubMed] [Google Scholar]
- 32.Greenbaum L, Israeli-Korn SD, Cohen OS, et al. The LRRK2 G2019S mutation status does not affect the outcome of subthalamic stimulation in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(11):1053-1056. doi: 10.1016/j.parkreldis.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 33.Gómez-Esteban JC, Lezcano E, Zarranz JJ, et al. Outcome of bilateral deep brain subthalamic stimulation in patients carrying the R1441G mutation in the LRRK2 dardarin gene. Neurosurgery. 2008;62(4):857-862. doi: 10.1227/01.neu.0000318171.82719.35 [DOI] [PubMed] [Google Scholar]
- 34.Breit S, Wächter T, Schmid-Bielenberg D, et al. Effective long-term subthalamic stimulation in PARK8 positive Parkinson’s disease. J Neurol. 2010;257(7):1205-1207. doi: 10.1007/s00415-010-5493-8 [DOI] [PubMed] [Google Scholar]
- 35.Stefani A, Marzetti F, Pierantozzi M, et al. Successful subthalamic stimulation, but levodopa-induced dystonia, in a genetic Parkinson’s disease. Neurol Sci. 2013;34(3):383-386. doi: 10.1007/s10072-012-1014-0 [DOI] [PubMed] [Google Scholar]
- 36.Weiss D, Brockmann K, Srulijes K, et al. Long-term follow-up of subthalamic nucleus stimulation in glucocerebrosidase-associated Parkinson’s disease. J Neurol. 2012;259(9):1970-1972. doi: 10.1007/s00415-012-6469-7 [DOI] [PubMed] [Google Scholar]
- 37.Lythe V, Athauda D, Foley J, et al. GBA-associated Parkinson’s disease: progression in a deep brain stimulation cohort. J Parkinsons Dis. 2017;7(4):635-644. doi: 10.3233/JPD-171172 [DOI] [PubMed] [Google Scholar]
- 38.Antonini A, Pilleri M, Padoan A, et al. Successful subthalamic stimulation in genetic Parkinson’s disease caused by duplication of the α-synuclein gene. J Neurol. 2012;259(1):165-167. doi: 10.1007/s00415-011-6162-2 [DOI] [PubMed] [Google Scholar]
- 39.Nakahara K, Ueda M, Yamada K, et al. Juvenile-onset parkinsonism with digenic parkin and PINK1 mutations treated with subthalamic nucleus stimulation at 45 years after disease onset. J Neurol Sci. 2014;345(1-2):276-277. doi: 10.1016/j.jns.2014.07.053 [DOI] [PubMed] [Google Scholar]
- 40.Pal GD, Hall D, Ouyang B, et al. ; Consortium on Risk for Early Onset Parkinson’s Disease (CORE-PD) Investigators . Genetic and clinical predictors of deep brain stimulation in young-onset Parkinson’s disease. Mov Disord Clin Pract. 2016;3(5):465-471. doi: 10.1002/mdc3.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders-Pullman R, Mirelman A, Alcalay RN, et al. ; LRRK2 Ashkenazi Jewish Consortium . Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol. 2018;75(3):312-319. doi: 10.1001/jamaneurol.2017.4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Healy DG, Falchi M, O’Sullivan SS, et al. ; International LRRK2 Consortium . Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583-590. doi: 10.1016/S1474-4422(08)70117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domingo A, Klein C. Genetics of Parkinson disease. Handb Clin Neurol. 2018;147:211-227. doi: 10.1016/B978-0-444-63233-3.00014-2 [DOI] [PubMed] [Google Scholar]
- 44.Mata IF, Hutter CM, González-Fernández MC, et al. Lrrk2 R1441G-related Parkinson’s disease: evidence of a common founding event in the seventh century in Northern Spain. Neurogenetics. 2009;10(4):347-353. doi: 10.1007/s10048-009-0187-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee AJ, Wang Y, Alcalay RN, et al. ; Michael J. Fox LRRK2 Cohort Consortium . Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord. 2017;32(10):1432-1438. doi: 10.1002/mds.27059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brockmann K, Srulijes K, Pflederer S, et al. GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407-411. doi: 10.1002/mds.26071 [DOI] [PubMed] [Google Scholar]
- 47.Barrett MJ, Shanker VL, Severt WL, et al. Cognitive and antipsychotic medication use in monoallelic GBA-related Parkinson disease. JIMD Rep. 2014;16:31-38. doi: 10.1007/8904_2014_315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol. 2015;72(2):201-208. doi: 10.1001/jamaneurol.2014.2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goker-Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol. 2008;65(10):1353-1357. doi: 10.1001/archneur.65.10.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Cai Y, Gu Z, et al. ; Chinese Parkinson Study Group . Clinical profiles of Parkinson’s disease associated with common leucine-rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging. 2014;35(3):725.e1-725.e6. doi: 10.1016/j.neurobiolaging.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 51.Thaler A, Bregman N, Gurevich T, et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 2018;55:45-49. doi: 10.1016/j.parkreldis.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 52.Rughani A, Schwalb JM, Sidiropoulos C, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation for the Treatment of Patients With Parkinson’s Disease: Executive Summary. Neurosurgery. 2018;82(6):753-756. doi: 10.1093/neuros/nyy037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahlskog JE. Parkin and PINK1 parkinsonism may represent nigral mitochondrial cytopathies distinct from Lewy body Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(10):721-727. doi: 10.1016/j.parkreldis.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kilarski LL, Pearson JP, Newsway V, et al. Systematic review and UK-based study of PARK2 (parkin), PINK1, PARK7 (DJ-1) and LRRK2 in early-onset Parkinson’s disease. Mov Disord. 2012;27(12):1522-1529. doi: 10.1002/mds.25132 [DOI] [PubMed] [Google Scholar]
- 55.Lücking CB, Dürr A, Bonifati V, et al. ; French Parkinson’s Disease Genetics Study Group; European Consortium on Genetic Susceptibility in Parkinson’s Disease . Association between early-onset Parkinson’s disease and mutations in the parkin gene. N Engl J Med. 2000;342(21):1560-1567. doi: 10.1056/NEJM200005253422103 [DOI] [PubMed] [Google Scholar]
- 56.Klein C, Lohmann-Hedrich K. Impact of recent genetic findings in Parkinson’s disease. Curr Opin Neurol. 2007;20(4):453-464. doi: 10.1097/WCO.0b013e3281e6692b [DOI] [PubMed] [Google Scholar]
- 57.Klein C, Westenberger A. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(1):a008888. doi: 10.1101/cshperspect.a008888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson’s disease. Mov Disord. 2012;27(7):831-842. doi: 10.1002/mds.24962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158-1160. doi: 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- 60.Samaranch L, Lorenzo-Betancor O, Arbelo JM, et al. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain. 2010;133(pt 4):1128-1142. doi: 10.1093/brain/awq051 [DOI] [PubMed] [Google Scholar]
- 61.Martínez-Arias R, Comas D, Mateu E, Bertranpetit J. Glucocerebrosidase pseudogene variation and Gaucher disease: recognizing pseudogene tracts in GBA alleles. Hum Mutat. 2001;17(3):191-198. doi: 10.1002/humu.4 [DOI] [PubMed] [Google Scholar]
- 62.Merola A, Zibetti M, Artusi CA, et al. Subthalamic nucleus deep brain stimulation outcome in young onset Parkinson’s disease: a role for age at disease onset? J Neurol Neurosurg Psychiatry. 2012;83(3):251-257. doi: 10.1136/jnnp-2011-300470 [DOI] [PubMed] [Google Scholar]
- 63.Charles PD, Van Blercom N, Krack P, et al. Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology. 2002;59(6):932-934. doi: 10.1212/WNL.59.6.932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Percent Motor Improvement and Dopaminergic Dose Reduction Following STN DBS
eTable 1. Meta-analysis Data for Proportion
eTable 2. Meta-analysis Data for Motor and Therapy Endpoints
eTable 3. Case Report Data
eTable 4. Meta-analysis Output
eTable 5. Motor Levodopa Response
eTable 6. Heterozygous PRKN Analysis
eTable 7. Validation analyses for UPDRS-III improvement and LEDD reduction
eTable 8. Pre STN DBS Motor Outcome (UPDRS-III Score) Associated With Levodopa - Meta-analysis
eTable 9. Pre STN DBS Motor Outcome (UPDRS-III Score) Associated With Levodopa - Intra-Individual Patient Analysis
eAppendix 1. Statistical Codes
eAppendix 2. Methods of Data Analysis