This prognostic study investigates the significance of missense polymorphism in the HSD3B1 gene among men treated with primary androgen-deprivation therapy or abiraterone for prostate cancer.
Key Points
Question
What is the clinical impact of genetic variant in HSD3B1 when treated with androgen-deprivation therapy (ADT) and abiraterone for prostate cancer?
Findings
In this prognostic study of 203 Japanese men, the prognosis in variant carriers of HSD3B1 was worse in the ADT group among 104 men with metastatic hormone-sensitive prostate cancer. However, variant carriers of HSD3B1 among 99 men with castration-resistant prostate cancer showed distinctly better response to abiraterone therapy.
Meaning
Genotype in HSD3B1 may serve as a promising biomarker for ADT and abiraterone, suggesting an application for upfront abiraterone with ADT for individuals with hormone-sensitive prostate cancer.
Abstract
Importance
Recently, genetic polymorphism in HSD3B1 encoding 3β-hydroxysteroid dehydrogenase-1 has been shown to be associated with oncological outcome when treated with androgen-deprivation therapy (ADT) for prostate cancer. Upfront abiraterone combined with ADT has proved survival benefit. However, its effect on oncological outcome among different ethnicities and in abiraterone treatment remain unclear.
Objective
To investigate the significance of missense polymorphism in HSD3B1 gene among men treated with primary ADT or abiraterone.
Design, Setting, and Participants
This prognostic study included Japanese patients with metastatic hormone-sensitive prostate cancer between June 1993 and July 2005 and with castration-resistant prostate cancer between September 2014 and February 2018. Genome DNA was obtained from patient whole blood samples, and genotyping on HSD3B1 (rs1047303, 1245C) was performed by Sanger sequencing.
Exposures
Primary ADT for metastatic hormone-sensitive prostate cancer and abiraterone for castration-resistant prostate cancer.
Main Outcomes and Measures
The association of genotype in HSD3B1 with clinicopathological parameters and oncological outcome, including prostate-specific antigen response, progression-free survival, treatment failure–free survival, and overall survival was examined.
Results
Of 203 men, 104 were in the primary ADT cohort (median [interquartile range] age, 72 [67-76] years) and 99 men were in the abiraterone group (median [interquartile range] age, 74 [67-80] years). Most patients carried metastatic lesions in each cohort. Among the cohort of primary ADT, men carrying heterozygous and homozygous variant types in HSD3B1 gene showed higher progression risk (hazard ratio [HR], 2.34; 95% CI, 1.08-4.49; P = .03) but not any-caused death risk (HR, 1.36; 95% CI, 0.52-2.92; P = .50), compared with men carrying homozygous wild type. In contrast, among the abiraterone cohort, men carrying variant type in HSD3B1 gene showed lower progression risk (HR, 0.32; 95% CI, 0.12-0.69; P = .006) and lower all-cause mortality risk (HR, 0.40; 95% CI, 0.13-0.94; P = .04) compared with men carrying homozygous wild type.
Conclusions and Relevance
This study showed that HSD3B1 genetic variant is distinctly associated with oncological outcome between primary ADT and abiraterone in Japanese men, suggesting universal significance among different ethnicities in primary ADT, as well as promise as a predictive biomarker of ADT and abiraterone.
Introduction
Androgens play critical roles in prostate carcinogenesis as well as prostate cancer progression. Since 1941, androgen-deprivation therapy (ADT), which reduces testosterone production and inhibits androgen action in prostate cancer cells, has been the criterion standard therapy for metastatic prostate cancer.1 Although initially most prostate cancers respond well to ADT, most patients eventually progress to castration-resistant prostate cancer (CRPC), which is mainly thought to be because androgen receptor reactivation is induced by several mechanisms.2 One of those mechanisms has been identified to be intratumoral androgen synthesis mostly from adrenal precursor steroids and at least in part due to de novo synthesis from cholesterol,3,4 which is supported by increased expression of several genes encoding steroidogenic enzymes including HSD3B, HSD17B, and SRD5A in CRPC.5 Among them, HSD3B1 encodes 3β-hydroxysteroid dehydrogenase-1, which is mainly expressed in peripheral tissues including the prostate, breast, skin, and placenta (another isoform, 3β-hydroxysteroid dehydrogenase-2 was mainly expressed in adrenal gland and gonad in human) and is a rate-limiting enzyme required for all pathways of dihydrotestosterone synthesis.6 Recently, a mutation (1245A→C) in HSD3B1 was shown to provide a novel mechanism of resistance to ADT,7 where amino acid 367 Asn→Thr is changed and 3β-hydroxysteroid dehydrogenase-1 is rendered to be resistant to proteasomal degradation, causing substantial accumulation of this enzyme and gain of function. Although the HSD3B1 (1245C) allele can be acquired by mutation, germ-line single-nucleotide polymorphism (rs1047303) is also known to exist.
Recently, upfront abiraterone in combination with primary ADT has been shown to improve survival for metastatic hormone-sensitive prostate cancer (HSPC).8,9 However, it remains unclear who is suitable for upfront abiraterone therapy to metastatic HSPC. Intriguingly, it has been reported that abiraterone is converted by 3β-hydroxysteroid dehydrogenase to Δ4-abiraterone (D4A), which blocks multiple steroidogenic enzymes and antagonizes the androgen receptor, providing an additional explanation for clinical activity by abiraterone.10 Therefore, tumors in men carrying variant genotype in HSD3B1 showing higher enzymatic activity of 3β-hydroxysteroid dehydrogenase-1 may be vulnerable to abiraterone owing to higher concentration of D4A.
Recent studies have demonstrated that genetic polymorphism in HSD3B1 is associated with oncological outcome among residents in the United States treated with ADT, where men carrying variant alleles showed worse prognosis.11,12,13 Thus, genetic variation in HSD3B1 (1245C) genotype is a promising predictive biomarker of positive ADT response among men with prostate cancer. However, its impact on prognosis among people of different ethnicities remains unclear, where the frequency of the variant allele would differ among ethnicities. In addition, the significance of HSD3B1 genotype in abiraterone treatment is rarely investigated. Accordingly, in this study, we aimed to investigate the association between genetic variants in HSD3B1 (1245C) and oncological outcome in Japanese men treated with primary ADT for metastatic HSPC as well as abiraterone for CRPC.
Methods
Patients
Japanese patients who had undergone primary ADT for metastatic HSPC to regional lymph nodes or distant sites at the University of Occupational and Environmental Health (Kitakyushu, Japan) and Kyushu University Hospital (Fukuoka, Japan) between June 1993 and July 2005 were consecutively included in the cohort of primary ADT.14,15,16 All patients were histopathologically diagnosed as having adenocarcinoma of the prostate. Clinical TNM staging was determined in accordance with the unified TNM criteria based on the results of digital rectal examination, transrectal ultrasound, magnetic resonance imaging, computed tomography, and bone scan.17 All patients were primarily treated with surgical castration or medical castration using a gonadotropin-releasing hormone agonist (goserelin acetate or leuprorelin acetate) with or without an antiandrogen agent (bicalutamide, flutamide, or chlormadinone acetate). Progressive disease was defined as an increase in serum prostate-specific antigen (PSA) levels of more than 2 ng/mL and a 25% increase over the nadir, the appearance of a new lesion, or the progression of 1 or more known lesions classified according to the Response Evaluation Criteria in Solid Tumors.18
Japanese patients who were treated with abiraterone for CRPC at the Akita University Hospital (Akita, Japan), Kyoyo University Hospital (Kyoto, Japan), and Kyushu University Hospital (Fukuoka, Japan) between September 2014 and February 2018 were included in the abiraterone cohort. Clinical staging was determined based on the results of computed tomography and bone scan.17 Patients were treated with 1000 mg of abiraterone plus 10 mg of prednisolone daily with surgical castration or medical castration using a gonadotropin-releasing hormone agonist/antagonist (goserelin acetate, leuprorelin acetate, or degarelix acetate). Prostate-specific antigen response was defined as maximum decline of PSA after abiraterone administration. Treatment failure was defined as radiological progression including the appearance of a new metastatic lesion, the progression of 1 or more known lesions classified according to the Response Evaluation Criteria in Solid Tumors,17 or discontinuation of abiraterone owing to no clinical benefit judged by physicians.
Written informed consent was obtained from all patients. The patients who declined to be included in this study were excluded. Informed consent and blood samples were obtained before the initiation of therapy. This study was performed in accordance with the principles described in the Declaration of Helsinki19 and the Ethical Guidelines for Epidemiological Research enacted by the Japanese government and approved by each institutional review board. Reporting followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.20
Genotyping
Genomic DNA was extracted from patient whole blood samples. HSD3B1 (rs1047303) genotyping was performed by sequencing as described previously.21 Briefly, pathologic complete response amplification was performed using TaKaRa EmeraldAmp PCR Master Mix (TaKaRa). The primers, annealing temperature, and cycle numbers were as follows: 5′-GTCAAATAGCGTATTCACCTTCTCTTAT-3′ and 5′-GAGGGTGGAGCTTGATGACATCT-3′, annealing temperature: 65°C, 35 cycles, respectively. The pathologic complete response products were purified using the TaKaRa NucleoSpin Gel and PCR clean-up (TaKaRa) and sequenced using the BigDye Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems) on a Genetic Analyzer 3130XL (Applied Biosystems). Sequence data were visualized using Sequence Scanner Software version 1.0 (Applied Biosystems).
Statistical Analysis
All statistical analyses were performed using JMP software, version 13 (SAS Institute). Categorical and continuous data were analyzed by Pearson χ2 and Wilcoxon rank sum tests, respectively. Survival analyses were conducted using the Kaplan-Meier method and the log-rank test. Univariate analyses were performed using the Cox hazard proportional model to estimate hazard ratios (HRs and 95% CIs). All P values were 2-sided. P values less than .05 were considered significant.
Results
Significance of Genetic Variation in HSD3B1 in Primary ADT
Clinical and pathological characteristics of 104 Japanese patients in the primary ADT cohort are displayed in eTable 1 in the Supplement. The median patient age was 72 years (interquartile range [IQR], 67-76 years; range, 44-87 years), and the median PSA at diagnosis was 244.0 ng/mL (IQR, 85.5-744.3 ng/mL). The Gleason scores of biopsy specimens from 31 patients (32.6%) and 64 patients (67.4%) were less than 8 and 8 or higher, respectively. The clinical T-stages were cT2/3 and cT4 in 65 patients (72.2%) and 25 patients (27.8%), respectively. Metastasis to the regional lymph nodes (N1) and distant sites (M1) were detected in 51 patients (56.0%) and 94 patients (90.4%), respectively. Twelve patients (11.5%) were primarily treated with castration alone and 92 patients (88.5%), with combined androgen blockade. During a median follow-up of 3.7 years (IQR, 1.6-7.6 years), disease progression and any caused death occurred in 85 cases (81.7%) and 60 cases (57.7%), respectively. The median progression-free survival (PFS) and overall survival (OS) were 1.2 years and 6.0 years, respectively.
First, associations between genetic polymorphisms in HSD3B1 and clinicopathological parameters were analyzed. Homozygous wild type, heterozygous variant type, and homozygous variant type were detected in 95 men (91.3%), 7 men (6.7%), and 2 men (1.9%), respectively. Among them, no differences in clinicopathological parameters including PSA at diagnosis, Gleason score, and clinical stage were observed (eTable 1 in the Supplement).
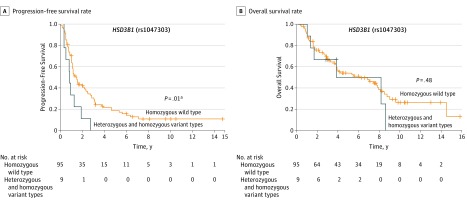
Next, we analyzed the prognostic impact of HSD3B1 genotype status on PFS and OS. Using univariate analyses, heterozygous and homozygous variant types were significantly associated with higher risk of progression (HR, 2.16; 95% CI, 1.14-3.85; P = .02) compared with homozygous wild type (Table 1). Kaplan-Meier analyses demonstrated an advantage in PFS of men with HSD3B1 homozygous wild type status compared with men carrying heterozygous and homozygous variant types (Figure 1A). However, OS was comparable between men with homozygous wild type and men with heterozygous and homozygous variant types (HR, 1.3; 95% CI, 0.52-2.92; P = .50) as shown with Kaplan-Meier curve (Figure 1B).
Table 1. Associations Between Clinicopathological Parameters and Prognosis in Primary ADT.
| Variable | Progression-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, HR (range), y | 1.56 (0.49-5.26) | .46 | 3.99 (0.98-17.53) | .05 |
| PSA at diagnosis (range) | 2.94 (0.69-9.33) | .13 | 1.00 (0.11-5.78) | >.99 |
| Biopsy Gleason score | ||||
| <8 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥8 | 1.67 (1.03-2.77) | .04a | 1.25 (0.72-2.28) | .44 |
| Clinical T-stage | ||||
| cT2/3 | 1 [Reference] | NA | 1 [Reference] | NA |
| cT4 | 1.20 (0.70-1.97) | .50 | 1.49 (0.79-2.67) | .21 |
| Clinical N-stage | ||||
| N0 | 1 [Reference] | NA | 1 [Reference] | NA |
| N1 | 1.30 (0.82-2.07) | .26 | 1.08 (0.62-1.89) | .77 |
| Clinical M-stage | ||||
| M0 | 1 [Reference] | NA | 1 [Reference] | NA |
| M1 | 1.67 (0.79-4.31) | .19 | 1.83 (0.74-6.10) | .21 |
| Hormonal therapy | ||||
| Combined androgen blockade | 1 [Reference] | NA | 1 [Reference] | NA |
| Castration | 0.74 (0.36-1.37) | .35 | 1.07 (0.44-2.21) | .86 |
| HSD3B1 (rs1047303) | ||||
| Homozygous wild type | 1 [Reference] | NA | 1 [Reference] | NA |
| Heterozygous and homozygous variant types | 2.34 (1.08-4.49) | .03a | 1.36 (0.52-2.92) | .50 |
Abbreviations: ADT, androgen-deprivation therapy; HR, hazard ratio; NA, not applicable; PSA, prostate-specific antigen.
Statistically significant.
Figure 1. Association of Gene Polymorphism in HSD3B1 rs1047303 With Prognosis in Cases With Metastatic Hormone-Sensitive Prostate Cancer Treated With Primary Androgen-Deprivation Therapy .
Progression-free survival rate (A) and overall survival rate (B) stratified by gene polymorphism in HSD3B1 rs1047303 are shown.
aStatistically significant.
Significance of Genetic Variation in HSD3B1 in Abiraterone
Clinical and pathological characteristics of 99 Japanese patients in the abiraterone cohort are displayed in eTable 2 in the Supplement. The median patient age was 74 years (IQR, 67-80 years). The Gleason scores of biopsy specimens from 14 patients (15.4%) and 77 patients (84.6%) were less than 8 and 8 or higher, respectively. The median PSA at pretreatment of abiraterone was 14.7 ng/mL (IQR, 4.7-87.1 ng/mL). The clinical M-stages were M0, M1a, M1b, and M1c in 8 patients (8.1%), 10 patients (10.1%), 68 patients (68.7%), and 13 patients (13.1%), respectively. Prior enzalutamide and prior docetaxel were administered in 46 patients (46.5%) and 41 patients (41.4%), respectively. During a median follow-up of 1.2 years (IQR, 0.8-1.9 years), treatment failure occurred in 75 patients (75.8%), and all-cause mortality occurred in 50 patients (50.5%). The median time to treatment failure was 0.5 years, and median OS was 1.8 years.
First, associations between genetic polymorphisms in HSD3B1 and clinicopathological parameters were analyzed. Homozygous wild type and heterozygous variant type were detected in 85 men (85.9%) and 14 men (14.1%), respectively, while homozygous variant type was not detected in this cohort. Among them, prior enzalutamide was more frequent in homozygous wild type although no differences in other clinicopathological parameters were observed (eTable 2 in the Supplement).
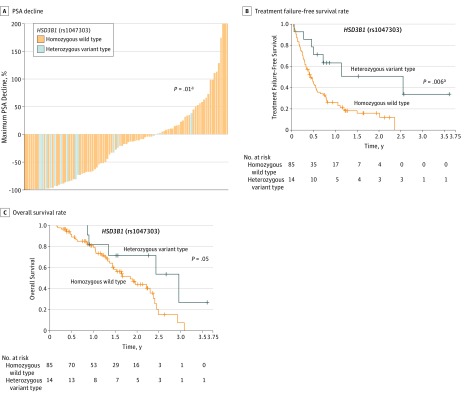
Next, we analyzed clinical significance of genetic polymorphism in HSD3B1 on PSA response, treatment failure–free survival, and OS. Maximum PSA declines during abiraterone treatment in heterozygous variant type (median, −83.4%; IQR, −97.4% to −18.7%) were significantly superior to those in homozygous wild type (median, −12.2%; IQR, −67.9% to 25.3%; P = .01) (Figure 2A). Using univariate analyses, heterozygous variant type was significantly associated with lower risk of treatment failure (HR, 0.32; 95% CI, 0.12-0.69; P = .002) compared with homozygous wild type (Table 2). Kaplan-Meier curve distinguished treatment failure–free survival by genetic polymorphism in HSD3B1 between men carrying homozygous wild type and men carrying heterozygous variant type (Figure 2B). When adjusted with PSA at pretreatment, clinical M-stage, prior enzalutamide, and prior docetaxel using univariate analyses, heterozygous variant type was significantly associated with lower risk of treatment failure (HR, 0.35; 95% CI, 0.13-0.80; P = .01) compared with homozygous wild type. Similarly, heterozygous variant type was significantly associated with lower risk of all-cause mortality (HR, 0.40; 95% CI, 0.13-0.94; P = .04) compared with homozygous wild type (Table 2), as shown with Kaplan-Meier curve (Figure 2C). When adjusted with PSA at pretreatment, clinical M-stage, prior enzalutamide, and prior docetaxel using multivariate analyses, the significance of genetic polymorphism in HSD3B1 was not relevant with all-cause mortality (HR, 0.48; 95% CI, 0.16-1.25; P = .14).
Figure 2. Association of Gene Polymorphism in HSD3B1 rs1047303 With Sensitivity and Prognosis in Cases With Castration-Resistant Prostate Cancer Treated With Abiraterone.
A, Waterfall plots showing the greatest decline in prostate-specific antigen (PSA) values from baseline during abiraterone treatment in 97 men whose PSA response were available. B and C, Treatment failure–free survival rate (B) and overall survival rate (C) stratified by gene polymorphism in HSD3B1 rs1047303 are shown.
aStatistically significant.
Table 2. Associations Between Clinicopathological Parameters and Prognosis in Abiraterone.
| Variable | Treatment Failure–Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at pretreatment, HR (range), y | 0.80 (0.24-2.76) | .72 | 2.09 (0.40-10.87) | .38 |
| PSA at diagnosis, HR (range) | 0.52 (0.047-2.54) | .47 | 0.33 (0.0017-4.57) | .50 |
| Biopsy Gleason score | ||||
| <8 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥8 | 1.14 (0.45-1.58) | .67 | 0.89 (0.45-1.99) | .77 |
| PSA at pretreatment, HR (range) | 9.88 (2.85-27.11) | .001a | 50.20 (12.26-191.36) | <.001a |
| ECOG PS at pretreatment | ||||
| 0 | 1 [Reference] | NA | 1 [Reference] | NA |
| 1 | 1.48 (0.88-2.43) | .14 | 1.15 (0.60-2.14) | .67 |
| ≥2 | 1.89 (0.77-3.99) | .15 | 2.00 (0.78-4.48) | .14 |
| Clinical M-stage | ||||
| M0 | 0.87 (0.30-1.99) | .86 | 1.18 (0.19-3.97) | .83 |
| M1a | 0.79 (0.32-1.63) | .54 | 0.46 (0.075-1.55) | .24 |
| M1b | 1 [Reference] | NA | 1 [Reference] | NA |
| M1c | 2.23 (1.09-4.21) | .03a | 2.96 (1.24-6.29) | .02a |
| Prior enzalutamide | ||||
| Absence | 1 [Reference] | NA | 1 [Reference] | NA |
| Presence | 3.51 (2.15-5.81) | <.001a | 2.23 (1.25-4.05) | .007a |
| Prior docetaxel | ||||
| Absence | 1 [Reference] | NA | 1 [Reference] | NA |
| Presence | 1.87 (1.18-2.97) | .008a | 1.34 (0.75-2.40) | .32 |
| HSD3B1 (rs1047303) | ||||
| Homozygous wild type | 1 [Reference] | NA | 1 [Reference] | NA |
| Heterozygous variant type | 0.32 (0.12-0.69) | .002a | 0.40 (0.13-0.94) | .04a |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NA, not applicable; PS, performance status; PSA, prostate-specific antigen.
Statistically significant.
Discussion
Previous studies investigating the effect of HSD3B1 on oncological outcome have demonstrated higher risk of progression and all-cause mortality in men carrying the variant allele.11,12,13 Although ethnic distribution in the study by Agarwal et al12 is not presented, the study by Hearn et al11 included mainly white men. In contrast to these US reports, the Chinese study by Wu et al22 showed significant risk of CRPC in men carrying variant allele but failed to show significant differences in PFS and OS. Thus, to our knowledge, for the first time in Asian individuals, the current study clearly shows significant detrimental PFS associated with variant allele. Superior quality of the sample origin might contribute to the successful demonstration of the significant results in this study, contrary to the results of the Chinese study by Wu et al22 that used samples obtained from formalin-fixed archival tissues, which may cause errors in sequencing and may contain tumor-derived DNA. In addition, an imbalance in patients with nonmetastasis and metastasis between genotypes may explain the reason of failure to show prognostic significance in the study by Wu et al.22 Higher frequency in the current study of variant allele in CRPC (14.1%) compared with that in HSPC (8.7%) also supports the hypothesis that men carrying variant allele tend to become resistant to ADT. However, the current study failed to show significant results in OS, contrary to the findings of the US study by Hearn et al,11 which may be due to the rare frequency of variant type in Japanese men or subsequent therapy such as abiraterone for CRPC.
Intriguingly, this study showed opposite oncological outcomes in abiraterone treatment among men carrying variant type, in contrast to those in primary ADT. Abiraterone was shown to be converted into more active D4A by 3β-hydroxysteroid dehydrogenase-110 and further into paradoxically androgen receptor agonist 3-keto-5α-abiraterone by 5α-reductase as 1 of 6 metabolites from D4A.23 Then, it may theoretically be reasonable that men with variant type in HSD3B1 with higher activity of 3β-hydroxysteroid dehydrogenase-1 showed favorable response and prognosis in abiraterone treatment. Thus, increased metabolism of abiraterone into more active D4A leading to better therapeutic effect of abiraterone may be achieved in men with higher activity of 3β-hydroxysteroid dehydrogenase-1, while extragonadal androgen synthesis leading to progression to CRPC may be augmented. Moreover, opposite prognostic impact of HSD3B1 genotype indicated promise as a predictive biomarker for ADT and abiraterone treatment. Recently, upfront abiraterone treatment was shown to improve survival in metastatic hormone-sensitive prostate cancer by the LATITUDE8 and STAMPEDE9 trials although a predictive biomarker was not identified so far. According to the result in the current study, men carrying variant type in HSD3B1 gene may be suitable candidates for upfront abiraterone treatment even in a low-risk group because their tumors would be resistant to ADT but vulnerable to abiraterone. Meanwhile, some men with homozygous wild type in HSD3B1 gene may be treated with ADT monotherapy even in a high-risk group. However, controversial pharmacological and prognostic significance in therapies using CYP17 inhibitor of genetic polymorphism in HSD3B1 has recently been reported.24,25,26 Almassi et al24 have reported improved PFS in variant carrier of HSD3B1 gene when treated with nonsteroidal CYP17A1 inhibitor ketoconazole. Meanwhile, Hahn et al25 failed to show significance in abiraterone treatment, which may be due to statistical underpower. Also, increased serum 3-keto-5α-abiraterone levels were recently observed in variant carriers of HSD3B1 gene among 30 patients,26 where differential enzymatic activity of 5α-reductase among ethnic groups might influence. Then, the value of HSD3B1 genotype as a predictive marker for ADT and abiraterone for HSPC should be investigated by prospective randomized clinical trials in the future.
In the present study including only Japanese men, the frequency of variant allele (8.7% in metastatic hormone-sensitive prostate cancer and 14.1% in CRPC) appeared to be less than that of the studies from the United States (62.7%, 47.9%,11 and 52.9%12) but comparable with the Chinese study by Wu et al22 (17.5%). This finding suggests less variant frequency in Asian individuals compared with white individuals. Previously, several studies reported the survival of Asian individuals to be better than that of white or black individuals.27,28,29 This ethnic difference of HSD3B1 genetic polymorphism frequency may explain better outcome of ADT in Asian individuals. Meanwhile, according to the results in the current study, the differential frequency of genetic variation in HSD3B1 may result in distinct oncological outcome among Asian men when treated with abiraterone, which should be investigated in the future.
Limitations
This study had several limitations, including its retrospective design, relatively small sample size, and lack of abiraterone metabolites measurement. In addition, this study was conducted using the data from multiple institutions, which may have resulted in diagnostic and therapeutic variations among the institutions. Included ethnicity was only Japanese, and abiraterone was used for only CRPC in this study.
Conclusions
This study confirmed the finding that HSD3B1 genetic variation may be associated with detrimental outcomes of ADT on prognosis in Japanese men with prostate cancer, augmenting the robustness of previous findings and suggesting universal significance among different ethnicities. Furthermore, opposite prognostic significance in abiraterone treatment was indicated, suggesting promise as a predictive biomarker in ADT and abiraterone including upfront abiraterone therapy for HSPC. However, the present study was limited as described above. Thus, prospective validation studies would be warranted.
eTable 1. Clinicopathological characteristics according to genetic polymorphism in patients treated with primary ADT
eTable 2. Clinicopathological characteristics according to genetic polymorphism in patients treated with abiraterone
References
- 1.Shiota M, Eto M. Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int J Urol. 2016;23(5):-. doi: 10.1111/iju.13091 [DOI] [PubMed] [Google Scholar]
- 2.Shiota M, Yokomizo A, Naito S. Pro-survival and anti-apoptotic properties of androgen receptor signaling by oxidative stress promote treatment resistance in prostate cancer. Endocr Relat Cancer. 2012;19(6):R243-R253. doi: 10.1530/ERC-12-0232 [DOI] [PubMed] [Google Scholar]
- 3.Montgomery RB, Mostaghel EA, Vessella R, et al. . Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447-4454. doi: 10.1158/0008-5472.CAN-08-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Locke JA, Guns ES, Lubik AA, et al. . Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407-6415. doi: 10.1158/0008-5472.CAN-07-5997 [DOI] [PubMed] [Google Scholar]
- 5.Stanbrough M, Bubley GJ, Ross K, et al. . Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815-2825. doi: 10.1158/0008-5472.CAN-05-4000 [DOI] [PubMed] [Google Scholar]
- 6.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005;26(4):525-582. doi: 10.1210/er.2002-0050 [DOI] [PubMed] [Google Scholar]
- 7.Chang KH, Li R, Kuri B, et al. . A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154(5):1074-1084. doi: 10.1016/j.cell.2013.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fizazi K, Tran N, Fein L, et al. ; LATITUDE Investigators . Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360. doi: 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 9.James ND, de Bono JS, Spears MR, et al. ; STAMPEDE Investigators . Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338-351. doi: 10.1056/NEJMoa1702900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Bishop AC, Alyamani M, et al. . Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523(7560):347-351. doi: 10.1038/nature14406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hearn JWD, AbuAli G, Reichard CA, et al. . HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 2016;17(10):1435-1444. doi: 10.1016/S1470-2045(16)30227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal N, Hahn AW, Gill DM, Farnham JM, Poole AI, Cannon-Albright L. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 2017;3(6):856-857. doi: 10.1001/jamaoncol.2017.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hearn JWD, Xie W, Nakabayashi M, et al. . Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 2018;4(4):558-562. doi: 10.1001/jamaoncol.2017.3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiota M, Fujimoto N, Yokomizo A, et al. . SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur J Cancer. 2015;51(14):1962-1969. doi: 10.1016/j.ejca.2015.06.122 [DOI] [PubMed] [Google Scholar]
- 15.Shiota M, Fujimoto N, Imada K, et al. . Potential role for YB-1 in castration-resistant prostate cancer and resistance to enzalutamide through the androgen receptor V7. J Natl Cancer Inst. 2016;108(7):djw005. doi: 10.1093/jnci/djw005 [DOI] [PubMed] [Google Scholar]
- 16.Shiota M, Fujimoto N, Itsumi M, et al. . Gene polymorphisms in antioxidant enzymes correlate with the efficacy of androgen-deprivation therapy for prostate cancer with implications of oxidative stress. Ann Oncol. 2017;28(3):569-575. [DOI] [PubMed] [Google Scholar]
- 17.International Union Against Cancer Urologic Tumors. Prostate In: Sobin LH, Wittekind CH, eds. TNM Classification of Malignant Tumors. 5th ed New York, NY: John Wiley & Sons; 1997:170-173. [Google Scholar]
- 18.Scher HI, Halabi S, Tannock I, et al. ; Prostate Cancer Clinical Trials Working Group . Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148-1159. doi: 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 20.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. doi: 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto N, Kubo T, Inatomi H, et al. . Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 2013;16(4):336-340. doi: 10.1038/pcan.2013.23 [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Huang S, Nastiuk KL, et al. . Variant allele of HSD3B1 increases progression to castration-resistant prostate cancer. Prostate. 2015;75(7):777-782. doi: 10.1002/pros.22967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Alyamani M, Li J, et al. . Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547-551. doi: 10.1038/nature17954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almassi N, Reichard C, Li J, et al. . HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 2018;4(4):554-557. doi: 10.1001/jamaoncol.2017.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn AW, Gill DM, Nussenzveig RH, et al. . Germline variant in HSD3B1 (1245 A > C) and response to abiraterone acetate plus prednisone in men with new-onset metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2018;16(4):288-292. doi: 10.1016/j.clgc.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 26.Alyamani M, Emamekhoo H, Park S, et al. . HSD3B1(1245A>C) variant regulates dueling abiraterone metabolite effects in prostate cancer. J Clin Invest. 2018;128(8):3333-3340. doi: 10.1172/JCI98319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukagai T, Namiki TS, Carlile RG, Yoshida H, Namiki M. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97(6):1190-1193. doi: 10.1111/j.1464-410X.2006.06201.x [DOI] [PubMed] [Google Scholar]
- 28.Holmes L Jr, Chan W, Jiang Z, Ward D, Essien EJ, Du XL. Impact of androgen deprivation therapy on racial/ethnic disparities in the survival of older men treated for locoregional prostate cancer. Cancer Control. 2009;16(2):176-185. doi: 10.1177/107327480901600210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooperberg MR, Hinotsu S, Namiki M, Carroll PR, Akaza H. Trans-Pacific variation in outcomes for men treated with primary androgen-deprivation therapy (ADT) for prostate cancer. BJU Int. 2016;117(1):102-109. doi: 10.1111/bju.12937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Clinicopathological characteristics according to genetic polymorphism in patients treated with primary ADT
eTable 2. Clinicopathological characteristics according to genetic polymorphism in patients treated with abiraterone