Key Points
Question
Does a model considering the features of individuals predict the glycemic responses to food better than models based on the calorie or carbohydrate content?
Findings
In this cohort study using a personalized predictive model among 327 participants without diabetes, glycemic responses to food varied significantly across the cohort. Observed postprandial glycemic responses showed higher correlation with values predicted by the model taking into consideration the features of individuals than with the calorie and carbohydrate content of the meals.
Meaning
A personalized predictive model that takes into consideration unique features of an individual in addition to food characteristics may allow individuals to better manage their glycemic responses to food consumed.
This cohort study using a personalized predictive model describes and predicts the glycemic responses of individuals without diabetes to a diverse array of foods using a model that considers the physiology and microbiome of the individual in addition to the characteristics of the foods consumed.
Abstract
Importance
Emerging evidence suggests that postprandial glycemic responses (PPGRs) to food may be influenced by and predicted according to characteristics unique to each individual, including anthropometric and microbiome variables. Interindividual diversity in PPGRs to food requires a personalized approach for the maintenance of healthy glycemic levels.
Objectives
To describe and predict the glycemic responses of individuals to a diverse array of foods using a model that considers the physiology and microbiome of the individual in addition to the characteristics of the foods consumed.
Design, Setting, and Participants
This cohort study using a personalized predictive model enrolled 327 individuals without diabetes from October 11, 2016, to December 13, 2017, in Minnesota and Florida to be part of a study lasting 6 days. The study measured anthropometric variables, described the gut microbial composition, and assessed blood glucose levels every 5 minutes using a continuous glucose monitor. Participants logged their food and activity information for the duration of the study. A predictive model of individualized PPGRs to a diverse array of foods was trained and applied.
Main Outcomes and Measures
Glycemic responses to food consumed over 6 days for each participant. The predictive model of personalized PPGRs considered individual features, including the microbiome, in addition to the features of the foods consumed.
Results
Postprandial response to the same foods varied across 327 individuals (mean [SD] age, 45 [12] years; 78.0% female). A model predicting each individual’s responses to food that considers several individual factors in addition to food features had better overall performance (R = 0.62) than current standard-of-care approaches using nutritional content alone (R = 0.34 for calories and R = 0.40 for carbohydrates) to control postprandial glycemic levels.
Conclusions and Relevance
Across the cohort of adults without diabetes who were examined, a personalized predictive model that considers unique features of the individual, such as clinical characteristics, physiological variables, and the microbiome, in addition to nutrient content was more predictive than current dietary approaches that focus only on the calorie or carbohydrate content of foods. Providing individuals with tools to manage their glycemic responses to food based on personalized predictions of their PPGRs may allow them to maintain their blood glucose levels within limits associated with good health.
Introduction
Hyperglycemia, prediabetes, and type 2 diabetes have become common conditions in the US population, with approximately 40% of individuals at risk for diabetes and up to 1 in 14 diagnosed as having type 2 diabetes.1,2 Elevated blood glucose levels are associated with a higher risk of developing serious health conditions, such as type 2 diabetes3 and cardiovascular disease.4 Given this forecast, the pursuit of preventive interventions makes sense as a broad strategy to curb and reverse the epidemic rise of these conditions.5,6
Dietary interventions for normalizing glycemic levels are associated with favorable changes in a variety of health markers, such as fasting blood glucose level and glycated hemoglobin level, among others, especially in persons who are overweight, obese, diabetic, or at risk for cardiovascular disease.7,8 However, these observations have not been evaluated in many populations, including in the United States. Interventions often focus on nutritional modifications, with recommendations suggesting the consumption of foods with low calories or carbohydrate content.7,8 The effect of dietary caloric restriction is most pronounced with respect to weight loss.9,10 In contrast, consumption of foods with low carbohydrate content or diets with a high protein content have been shown to be effective in improving glycated hemoglobin levels in patients with diabetes.7,11 However, prescription of these diets focuses specifically on the characteristics of the foods and does not take into consideration the features of individual patients.
Growing evidence suggests that glycemic responses to the same foods differ significantly among individuals. In addition to the characteristics of the foods consumed, the glycemic responses of individuals vary with physiological12 and genetic13 variables and their microbiome.14 In fact, the microbial composition is different in individuals with and without diabetes, with species influencing other members and potentially driving the dynamics of the community they are part of and the health status of the individual differently.15 The gut microbiome may also have a role in energy metabolism and the regulation of insulin response.16,17,18,19
Consideration of diverse individual and nutritional variables is often challenging and may hinder the adoption of improved strategies for the control of glycemic responses. However, current commercialization of cutting-edge and easy-to-use biological assays to measure genomic variation and microbiome contents, as well as improvements and developments in food labeling20,21 and personal self-tracking devices and applications (eg, continuous glucose monitors [CGMs]22,23 and food logging apps), allows better quantification of personal traits that could assist in defining personalized approaches for glycemic control.6 However, the various inputs from these devices, loggers, and assays still need integration into actionable tools for facilitating the maintenance of normoglycemic levels. Doing so would be an important step to curtail the epidemic rise of hyperglycemia.
In 2015, a tool for predicting personalized postprandial glycemic responses (PPGRs) of individuals in an Israeli cohort using many of the above-mentioned personal features was described.14 That research study demonstrated significantly better performance of this tool in predicting glycemic responses to food compared with models based only on food characteristics and showed considerable success in normalizing blood glucose levels of participants in short-term dietary interventions using model-based, personally tailored diets. However, this model has not been studied or evaluated in other populations, including the United States, to our knowledge. The objective of the present study was to test the hypothesis that individual diversity observed in PPGRs to food among a cohort of 327 free-living individuals without diabetes mostly based in the Midwestern region of the United States would be captured by a personalized approach that considers a variety of characteristics of the individual for the management of glycemic levels.
Methods
Study Participants
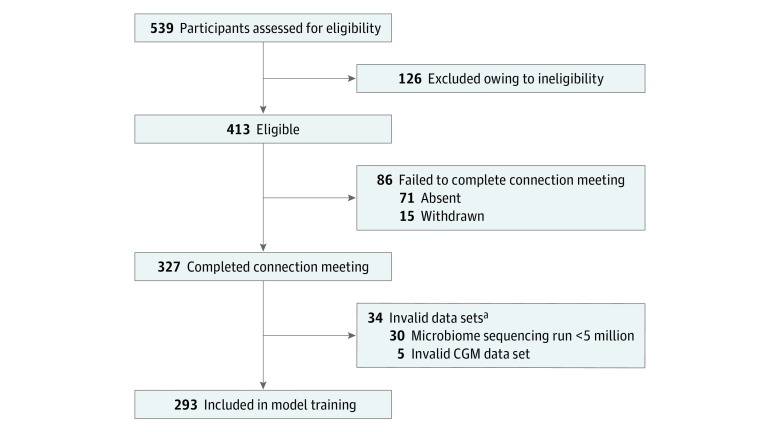
A total of 327 participants without diabetes recruited from October 11, 2016, to December 13, 2017, in Olmsted County and Hennepin County in Minnesota and in Duval County in Florida completed this 6-day study (Figure 1), which was approved by the Mayo Clinic Institutional Review Board. Only a small fraction of the total participants were from Florida (9 of 327); therefore, this cohort will henceforth be referred to as Midwestern. Because the focus of the study is on a particular geographic population, it followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Written informed consent was obtained from all participants. This study is registered with ClinicalTrials.gov (NCT02945514). Inclusion criteria were men and women 18 years or older with access to a mobile device and web browser. Individuals were excluded if, among other criteria, they were younger than 18 years, were initially seen with a chronic gastrointestinal or metabolic disorder, or had undergone certain medical or pharmacological interventions in the past 2 years (a full list of exclusion criteria is available in the eAppendix in the Supplement).
Figure 1. Flow Diagram of Study Participants.
CGM indicates continuous glucose monitor.
aOne participant experienced a microbiome sequencing run less than 5 million and an invalid CGM data set.
Intervention
Two days before the beginning of the study week, participants were asked to provide a stool sample that was shipped to the DayTwo facility in Adanim, Israel, processed, and analyzed for microbiome composition. Details are available in the eAppendix in the Supplement.
Participants attended a connection meeting at the beginning of the study week, during which staff (H.M.-S., K.E., and other nonauthors) provided a review of the study objective and requirements, participants were offered an opportunity to ask questions, and personal variables were recorded. They were instructed on the use of the food and activity–logging mobile application to be used throughout the week and the use of the manual blood glucometer (Contour Next Link; Bayer AG) to measure blood glucose levels every 5 minutes. During the study week, participants were asked to wear the CGM, complete manual glucose monitoring at least 4 times a day for its calibration, and log their food intake. They were asked to maintain their normal eating habits except for 4 standardized meals provided by the research team, which were to be consumed as the first meal of the day. These represented 2 different meals composed of defined food items (eAppendix in the Supplement) to allow for comparison of the glycemic responses across all individuals and to assess the reproducibility of the individual measures of glycemic levels.
Outcomes
Glucose was measured using a CGM (iPro2; Medtronic). Postprandial glycemic responses were computed following the method by Zeevi et al14: logged meal times and continuous glucose measurements were used to calculate the incremental area under the curve in the 2 hours after a meal as previously described,24 and values were truncated to the range of 0 to 80 mg/dL*h (details are available in the eAppendix in the Supplement). Five participants had corrupted CGM files that could not be processed and were excluded from the data set used to train the predictive model as well as from the validation of the accuracy step (Figure 1).
Participants logged meals using a mobile device application (DayTwo Food & Activity Logger on Google Play and the iTunes App Store) containing the full MyNetDiary food catalog.25 Participants were asked to choose the food items consumed from this extensive, curated food database and to log the amount consumed and the time and duration of the meal. The nutritional values of each reported meal, including the calorie and carbohydrate content, were computed based on the nutritional values reported in the MyNetDiary database. To avoid inaccuracies in PPGR measurements and to better inform training of the predictive model, some of the logged meal information suspected to be misreported was excluded from the data set, as detailed in the eAppendix in the Supplement.
Predictive Model Creation and Validation
To predict personal glycemic responses to food in this cohort, we applied the modeling framework described by Zeevi et al.14 Postprandial glycemic responses were predicted based on stochastic gradient boosting regression (XGBoost, version 0.6)26 using the XGBRegressor class. Postprandial glycemic responses are predicted as the sum of predictions from thousands of decision trees. Trees are inferred sequentially, with each trained on the residual of all previous trees and making a small contribution to the overall prediction. The features incorporated in each tree are selected by an inference procedure from a pool of 72 features, representing meal content and personal, CGM, and microbiome features (a full list is available in the eAppendix in the Supplement).
To increase robustness and generalizability, the model was trained on data from 293 American individuals described herein and a subset of 397 individuals from the previously published Israeli cohort study14 using the same methods and framework. Similar to the present study, the Israeli cohort was representative of the Israeli adult population without diabetes, with 54% overweight individuals and 22% obese individuals (complete details are given by Zeevi et al14). To reduce noise, a subset of 397 individuals from the Israeli cohort whose fecal samples were collected using the same sample collection methods as applied herein was used (eAppendix in the Supplement). Performance was assessed by 10-fold cross-validation in which participants are divided into 10 groups, the model is trained on 9 parts (containing both American and Israeli participants), and performance is measured by the ability to accurately predict meals reported by the left-out participants (out-of-bag predictions). Prediction results on Midwestern participants from all left folds were aggregated, and Pearson product moment correlation with the measured PPGRs was reported.
The standard error for the calculated performance was assessed using 50 iterations of bootstrapping. Random data sets of the same size as the original were sampled with replacement from the original data set, and the entire training and validation process was repeated.
Statistical Analysis
Pearson product moment correlation was used to quantify the accuracy of the predicted PPGRs from the model relative to those obtained from the CGM. It was also used to quantify the correlation between the calorie and carbohydrate content in a meal and PPGRs estimated from the CGM measurements.
Receiver operating characteristic curve analyses were created for model comparison at 3 threshold values. These analyses represented the 50th, 75th, and 90th percentiles of all measured PPGRs in the Midwestern cohort.
For additional model performance statistics, we binned the predicted values into 10 quantiles and computed Pearson product moment correlation between the mean predicted PPGRs and the mean measured PPGRs in each quantile. To assess the frequency of gross errors, we computed the percentage of cases in which the measured PPGRs were low (in the bottom 15% quantile) and the predicted PPGRs were high (in the top 15% quantile) and vice versa.
Results
Baseline characteristics of our cohort are listed in the Table. In summary, the 327 individuals who were enrolled in the study had a mean (SD) age of 45 (12) years, and 255 (78.0%) were female. Glycated hemoglobin levels indicative of diabetes (>6.5%) served as an exclusion criterion for the study participants (to convert glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01), and thus none of the participants had frank type 2 diabetes based on this measurement. Therefore, the mean clinical variable values measured were associated with good health (Table).27,28,29,30,31
Table. Characteristics of the Study Participants.
| Characteristic | Value |
|---|---|
| Demographic Variables | |
| No. of participants | 327 |
| Female, No. (%) | 255 (78.0) |
| Age, mean (95% CI), y | 45 (43-46) |
| Ethnicity, No. (%) | |
| Non-Hispanic/non-Latino | 261 (79.8) |
| Hispanic/Latino | 8 (2.4) |
| Unknown | 58 (17.7) |
| Race, No. (%) | |
| White | 259 (79.2) |
| Asian | 12 (3.7) |
| Black or African American | 5 (1.5) |
| American Indian/Alaskan Native | 1 (0.3) |
| Other | 4 (1.2) |
| Undisclosed | 46 (14.1) |
| Physical Variables | |
| BMI, mean (95% CI)a | 27.32 (26.69-27.95) |
| <25, No. (%) | 137 (39.8) |
| ≥25, No. (%) | 190 (58.1) |
| ≥30, No. (%) | 88 (26.9) |
| Waist to hip ratio, mean (95% CI) | |
| Overallb | 0.84 (0.83-0.85) |
| Male | 0.93 (0.92-0.95) |
| Female | 0.81 (0.80-0.82) |
| Clinical Laboratory Variables | |
| Glycated hemoglobin, mean (95% CI), %c | 5.2 (5.2-5.2) |
| Glycated hemoglobin ≥5.7%, No. (%) | 29 (8.9) |
| Cholesterol, mean (95% CI), mg/dL | |
| Totald | 202.3 (198.2-106.3) |
| High-density lipoproteine | 63.3 (61.2-65.4) |
| Thyrotropin level, mean (95% CI), mIU/Lf | 2.4 (2.3-2.6) |
| Nutrient Content of Foods Consumed, % | |
| Beef, poultry, and pork | 12.5 |
| Dairy products | 10.7 |
| Breads | 9.1 |
| Sweets | 8.1 |
| Vegetables | 7.9 |
| Cakes and cookies | 4.9 |
| Fruit | 4.5 |
| Nuts | 4.3 |
| Pizza, pies, and other pastries | 4.0 |
| Alcoholic drinks | 3.0 |
| Other | 31.0 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
SI conversion factors: To convert glycated hemoglobin level to a proportion of total hemoglobin, multiply by 0.01; to convert cholesterol level to millimoles per liter, multiply by 0.0259.
For BMI, less than 18.5 is underweight, 18.5 to 24.9 is normal, 25.0 to 29.9 is overweight, and 30.0 or greater is obese.27
For waist to hip ratio, 0.90 or greater in men and 0.85 or greater in women indicate substantially increased risk of a metabolic condition.28
For glycated hemoglobin, less than 5.7% is healthy, 5.7% to 6.4% is prediabetic, and 6.5% or greater is diabetic.29
For total cholesterol, less than 200 mg/dL is desirable, 200 to 239 mg/dL is borderline high, and 240 mg/dL or greater is high.30
For high-density lipoprotein cholesterol, 60 mg/dL or greater is desirable.31
For thyrotropin, less than 0.1 mIU/L indicates hyperthyroidism and greater than 10.0 mIU/L indicates hypothyroidism.31
Participants logged a total of approximately 3.2 million kcal during the study. The meals logged that were later used for training of the predictive model (data processing information is available in the eAppendix in the Supplement), representing about 1.8 million kcal, provide an overview of our cohort’s dietary habits in terms of their contribution to the overall energy intake (Table). For the meal information that was used for training of the predictive model (information regarding exclusion of meal information is available in the eAppendix in the Supplement), the mean number of calories logged per meal was 400.1 kcal (0.3 kcal minimum and 3136.1 kcal maximum), and the mean carbohydrate content was 37.9 g (0 g minimum and 443.3 g maximum). For the meals that were consumed and used to train the predictive model, carbohydrates composed 43% of the nutrients, proteins composed 18%, and lipids composed 39%.
The microbiome composition was consistent with prior observations among healthy individuals in this geographic region.32 The most prevalent phyla among our cohort were Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria. The most prevalent genera were Bacteroides, Subdoligranulum, Blautia, Roseburia, Eubacterium, Faecalibacterium, Oscillobacter, Alistipes, and Dorea, as well as an unnamed member of the Lachnospiraceae family. All of these phyla and genera occurred in 95% to 100% of participants, albeit at varying abundances (H.M.-S., unpublished data, 2018).
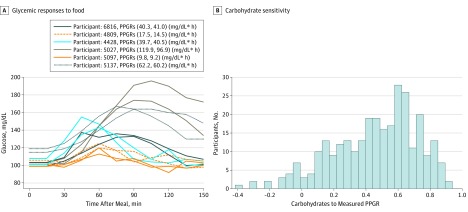
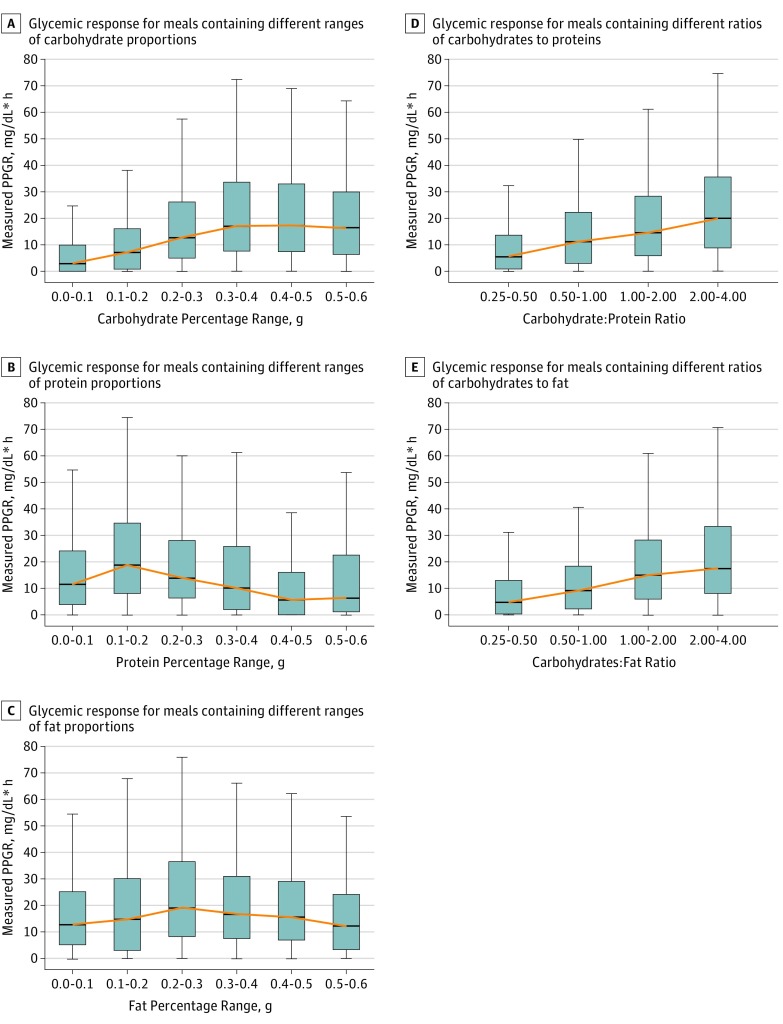
The response to the standardized meal of bagel and cream cheese consumed by our cohort varied substantially across participants, with glycemic excursions (eg, Figure 2A) (ie, the maximum glycemic elevation from baseline over time after eating a meal33) ranging from 6 to 94 mg/dL (mean, 30.7 mg/dL). Glycemic excursion values can be translated to a single variable often considered to be a good predictor of overall blood glucose sensitivity (namely, PPGRs), measured as the incremental area under the curve. The carbohydrate sensitivity of an individual is given as the correlation between the carbohydrate content of a meal and PPGRs. Figure 2B shows that the carbohydrate sensitivity of the participants in our study to the foods consumed varied significantly. However, there was significant intraindividual reproducibility of the glycemic responses to the bagel and cream cheese meal (Pearson product moment correlation R = 0.66). For the responses associated with the different proportions of carbohydrates in foods, it would be expected that amounts of carbohydrates would be positively associated with the levels of response; on average, this was the trend observed (yellow line in Figure 3A). However, extensive personal variation is observed for any percentage of carbohydrates in the meal, and glycemic response levels in individuals will range from low to high values (Figure 3A). Similar variations in response levels to protein (Figure 3B) and fat (Figure 3C) were also observed in our participants. For the responses observed in terms of the ratios of the nutrients, our results are consistent with the expected increase in PPGRs with amount of carbohydrates relative to protein (Figure 3D) or fat (Figure 3E) in the meal, while we still observed a wide range of values, reflecting the individuality of these responses.
Figure 2. Variability in Glycemic Responses to Food Among the Individuals in the Cohort.
A, Shown is an example of intraindividual consistency and interindividual variability in response to the bagel and cream cheese meal. The key shows the participant number and the computed postprandial glycemic response (PPGR) for meals eaten by the same participant on different days. Postprandial glycemic response was computed as the incremental area under the curve of blood glucose levels in the 2 hours after a meal (to convert glucose to mmol/L, multiply by 0.0555). B, Carbohydrate sensitivity is measured as the correlation between carbohydrates (in grams) in the meal consumed and the PPGR.
Figure 3. Distribution of the Glycemic Responses to the Different Proportions and Ratios of Nutrients.
Postprandial glycemic response (PPGR) is defined as the incremental area under the curve of blood glucose levels in the 2 hours after a meal. Boxes show the 25th percentile (bottom of box), median (box midline), and 75th percentile (top of box). The error bars show the distribution, excluding outliers (Q1 − 1.5*IQR and Q3 + 1.5*IQR, where IQR = Q3 − Q1, Q3 is the 75th percentile, and Q1 is the 25th percentile), and the yellow line shows how the median changes across different ranges of nutrients. IQR indicates interquartile range; Q, quantile.
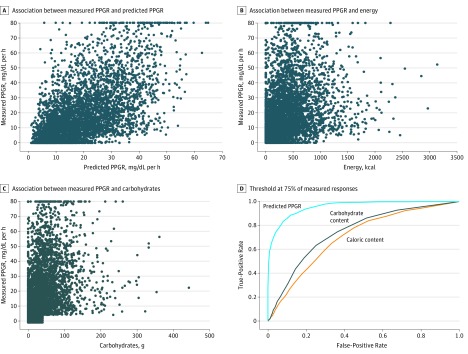
From the information collected from the study participants, we retrained a model for personalized predictive PPGRs using the same framework as previously applied to an Israeli population.14 The model combining data from the Israeli and American cohorts was used to predict the response for different foods for each individual who participated in our study (eAppendix in the Supplement). We then tested these predictions against our data set by using 10-fold cross-validation (R = 0.62 ± 0.01). The correlation between PPGRs predicted by our model and those observed in our participants was substantially higher than that between observed PPGRs and the calorie or carbohydrate content of the meals (R = 0.62 vs R = 0.34 for calories and R = 0.40 for carbohydrates) (Figure 4A-C). Given the R = 0.66 intraindividual correlation observed for standardized meals, our model appears to explain a large percentage of the explainable variance. Receiver operating characteristic curves across 3 threshold values, representing the 50th, 75th, and 90th percentiles of all measured PPGRs in the Midwestern cohort, further show that our predictive model consistently and substantially outperformed the models based on the calorie and carbohydrate content of the meals consumed (Figure 4D and eFigure in the Supplement).
Figure 4. Performance of the Model Developed in This Study, as Well as Calorie-Only or Carbohydrate-Only Models, in Predicting the Postprandial Glycemic Response (PPGR) to Food.
A-C, Correlation between measured and various PPGR predictors. For definition of the measured PPGRs, see the Outcomes subsection in the Methods section. C, Note that rare cases in which meals were reported to have more than 40 g of carbohydrates showed a remarkably low glycemic response (<5 mg/dL*h), and were excluded to reduce potential influences of meal misreporting. D, Receiver operating characteristic curve analysis for comparison of the model presented herein, as well as models based on the calorie or carbohydrate content alone for classifying high PPGR, where high PPGR was defined as the 75th percentile of all measured PPGRs in the US cohort. Additional receiver operating characteristic curve analyses at other PPGR quantiles are available in the eFigure in the Supplement.
To further assess the accuracy of predictions, we computed both the root-mean-square error (14.82 mg/dL*h) and the median absolute error (8.23 mg/dL*h) for PPGR predictions. In addition, to estimate how prediction accuracy varied across the range of PPGRs and to eliminate the possibility that the correlations reported were driven by extreme values only, we computed the correlation between the mean predicted PPGRs and the mean measured PPGRs when data were binned to 10 quantile bins and observed a high correlation (R = 0.99). Finally, we observed a low percentage of cases with gross prediction errors in which the measured PPGR was low and the predicted one was high (0.004 of cases) or vice versa (0.009 of cases). Taken together, these results and statistics indicate that the model constructed provides more accurate predictions relative to more commonly used models considering solely the calorie or carbohydrate content of the foods consumed and demonstrate that prediction accuracy is consistent across large ranges of measured PPGRs.
Discussion
In this study, we demonstrated that a tool generating personalized predictions considering a variety of an individual’s features can predict his or her personal glycemic responses to diverse foods. The tool empowers each person to accurately predict his or her glycemic response to specific foods, exceeding the accuracy of the information on the calorie or carbohydrate content alone.
Our cohort was homogeneous and representative of a Midwestern population consuming a Western diet, and the baseline clinical characteristics, dietary habits, and microbiome composition of our participants show that they were generally healthy (Table).27,28,29,30,31,32 Despite this, our results demonstrate a wide variation in individual glycemic responses to the foods consumed. To rule out the possible influence of the diverse food items consumed on this variability, a test meal of bagel and cream cheese was provided to all participants, allowing the comparison of responses with a standardized meal. The type and amplitude of the response were fairly consistent for each individual; however, the interindividual variability was significantly higher and consistent with previous observations.34 The specific factors contributing to this variability were not further explored in this study but have been previously suggested to include both the unique characteristics of foods7,10,35,36 and the unique features of individuals.13,14,37,38,39 Therefore, it is important to take an individual’s characteristics into consideration when determining and recommending the best dietary approach for managing glycemic levels.
The glycemic responses to the meals recorded by the participants of our study were overall consistent with trends suggesting a dampening influence on the glycemic response induced by carbohydrates in meals by addition of proteins or fats.9,10 However, we still show substantial variability around these trends, reflecting the individuality of a participant’s responses and the need for personalized approaches for the control of these responses.
The tool used in our study to predict PPGRs of individuals to food represents a modification and extension of the model created by Zeevi et al14 for the individuals in the Israeli population. We added the information gathered from our study participants to the original data set and retrained the model. Similar to the model by Zeevi et al,14 the predicted and observed PPGRs were highly correlated with each other. Furthermore, this correlation was substantially higher than that observed for current standard-of-care approaches used to inform management of postprandial glycemic levels, such as those based on the calorie or carbohydrate content, demonstrating that our model is more accurate at predicting individual glycemic responses to food.
As in the model presented by Zeevi et al,14 a large number of the features that make up the predictive model in the present study are related to the fecal microbiome of the individual. While the mechanisms underlying this relationship remain elusive and are not the focus of the present work, results of previous studies16,18,19 suggested that individual microbes may influence blood glucose homeostasis. The fact that the approach presented herein has been shown to predict personalized responses in 2 distinct populations (Israel and the US Midwest) suggests that such an individually based approach to the development of dietary interventions may also be useful in normalizing the glycemic response in groups at higher risk of developing diabetes, such as Asian,38 Pima Indian,40 or Nauruan populations.41
Limitations
Our study has several limitations. We found that the correlation of the intraindividual PPGRs was lower than that in the report by Zeevi et al14 for the Israeli population. This result may have been due to the choice of bagel and cream cheese as the standardized meal provided to the participants. Zeevi et al14 observed that, for standardized meals consisting of bread, the intraindividual response also varied more than for meals consisting solely of glucose. However, the intraindividual variability for this meal was still significantly lower than the interindividual variability that was captured by our model.
The accuracy of the predictions in our cohort was also slightly lower than that observed in the Israeli cohort.14 However, our results demonstrate that the overall accuracy of the individual predictions of the model was still substantially higher compared with current dietary approaches. Follow-up studies should assess the long-term beneficial health consequences of the personalized dietary approach while considering adherence and overall effectiveness.
The model presented herein is composed of a wide array of features. While CGM measurements and fecal sample analyses are not easily obtained by all patients, they are becoming increasingly affordable and accessible. Nevertheless, exploring the performance costs of simplified versions of our model containing only easily measurable clinical variables could help in making personalized nutrition accessible to larger populations.
Our model is trained based on self-reported meals. While self-reporting may be subjective and various biases may be present,42 the use of a mobile application for meal logs, allowing for privacy, instant logging, and the use of standardized food databases substantially mitigated these factors. We suggest that future studies could focus on quantitatively estimating and characterizing misreporting in different populations of interest so that any biases found can be taken into account when applying our model to specific populations.
Conclusions
Our tool generating personalized predictions provides individuals with access to actionable, person-specific information for managing their blood glucose levels and maintaining a normoglycemic status. Personalized PPGR predictions are accessible in the form of a mobile or web-based application, enabling real-time assessment of influences of foods and combinations of foods on blood glucose levels of the individual at the time of consumption. This capability adds to strategies already in place that are aimed at decreasing the incidence of diabetes while enabling individuals to better control their nutritional behaviors.10,43 Follow-up clinical studies assessing the changes induced by this approach compared with current practices on cardiometabolic markers of diabetes risk will be required to assess the long-term health benefits of using this tool. Nonetheless, results presented herein point toward the potentially significant contribution of measurement-based personalized approaches across different populations in harnessing nutrition as a means of improving PPGRs, with subsequent reduction of the consequences of prolonged and repetitive exposure to hyperglycemia.
eAppendix. Supplemental Methods
eFigure. Receiver Operating Characteristic (ROC) Curve Analysis for Comparison of the Model Presented Herein and Models Based on Caloric or Carbohydrate Content Alone for Classifying High PPGR Responses
References
- 1.Dodds RF. Understanding Diabetes: A Biochemical Perspective. Hoboken, NJ: John Wiley & Sons; 2013. doi: 10.1002/9781118530665 [DOI] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):-. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Hanefeld M, Leiter L, et al. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164(19):2090-2095. doi: 10.1001/archinte.164.19.2090 [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899-904. doi: 10.1016/j.amjcard.2007.03.107 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuomilehto J, Lindström J, Eriksson JG, et al. ; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343-1350. doi: 10.1056/NEJM200105033441801 [DOI] [PubMed] [Google Scholar]
- 7.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505-516. doi: 10.3945/ajcn.112.042457 [DOI] [PubMed] [Google Scholar]
- 8.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health: a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87(1)(suppl):258S-268S. doi: 10.1093/ajcn/87.1.258S [DOI] [PubMed] [Google Scholar]
- 9.Bantle JP, Wylie-Rosett J, Albright AL, et al. ; American Diabetes Association . Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(suppl 1):S61-S78. doi: 10.2337/dc08-S061 [DOI] [PubMed] [Google Scholar]
- 10.Evert AB, Boucher JL, Cypress M, et al. ; American Diabetes Association . Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821-3842. doi: 10.2337/dc13-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand-Miller JC. Glycemic load and chronic disease. Nutr Rev. 2003;61(5, pt 2):S49-S55. doi: 10.1301/nr.2003.may.S49-S55 [DOI] [PubMed] [Google Scholar]
- 12.Robert JJ, Cummins JC, Wolfe RR, et al. Quantitative aspects of glucose production and metabolism in healthy elderly subjects. Diabetes. 1982;31(3):203-211. doi: 10.2337/diab.31.3.203 [DOI] [PubMed] [Google Scholar]
- 13.Ortlepp JR, Metrikat J, Albrecht M, von Korff A, Hanrath P, Hoffmann R. The vitamin D receptor gene variant and physical activity predicts fasting glucose levels in healthy young men. Diabet Med. 2003;20(6):451-454. doi: 10.1046/j.1464-5491.2003.00971.x [DOI] [PubMed] [Google Scholar]
- 14.Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079-1094. doi: 10.1016/j.cell.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Sung J, Kim S, Cabatbat JJT, et al. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat Commun. 2017;8:15393. doi: 10.1038/ncomms15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MT, Nieuwdorp M, Bäckhed F. Microbial modulation of insulin sensitivity. Cell Metab. 2014;20(5):753-760. doi: 10.1016/j.cmet.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Kreznar JH, Keller MP, Traeger LL, et al. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep. 2017;18(7):1739-1750. doi: 10.1016/j.celrep.2017.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin SC, Kim SH, You H, et al. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science. 2011;334(6056):670-674. doi: 10.1126/science.1212782 [DOI] [PubMed] [Google Scholar]
- 19.Suez J, Shapiro H, Elinav E. Role of the microbiome in the normal and aberrant glycemic response. Clin Nutr Exp. 2016;6:59-73. doi: 10.1016/j.yclnex.2016.01.001 [DOI] [Google Scholar]
- 20.Campos S, Doxey J, Hammond D. Nutrition labels on pre-packaged foods: a systematic review. Public Health Nutr. 2011;14(8):1496-1506. doi: 10.1017/S1368980010003290 [DOI] [PubMed] [Google Scholar]
- 21.Rothman RL, Housam R, Weiss H, et al. Patient understanding of food labels: the role of literacy and numeracy. Am J Prev Med. 2006;31(5):391-398. doi: 10.1016/j.amepre.2006.07.025 [DOI] [PubMed] [Google Scholar]
- 22.Mazze RS, Strock E, Wesley D, et al. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10(3):149-159. doi: 10.1089/dia.2007.0293 [DOI] [PubMed] [Google Scholar]
- 23.Tsujino D, Nishimura R, Taki K, Miyashita Y, Morimoto A, Tajima N. Daily glucose profiles in Japanese people with normal glucose tolerance as assessed by continuous glucose monitoring. Diabetes Technol Ther. 2009;11(7):457-460. doi: 10.1089/dia.2008.0083 [DOI] [PubMed] [Google Scholar]
- 24.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43(1):167-172. doi: 10.1093/ajcn/43.1.167 [DOI] [PubMed] [Google Scholar]
- 25.MyNetDiary Food Catalog. https://www.mynetdiary.com/food-catalog/. Accessed January 14, 2019.
- 26.Chen T, Guestrin C XGBoost: a scalable tree boosting system. https://www.kdd.org/kdd2016/papers/files/rfp0697-chenAemb.pdf. Published August 2016. Accessed October 2017.
- 27.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. WMJ. 1998;97(9):20-21, 24-25, 27-37. [PubMed] [Google Scholar]
- 28.World Health Organization Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation: Geneva, 8-11 December 2008 https://www.who.int/nutrition/publications/obesity/WHO_report_waistcircumference_and_waisthip_ratio/en/. Published 2011.
- 29.American Diabetes Association Standards of medical care in diabetes: 2013. Diabetes Care. 2013;36(suppl 1):S11-S66. doi: 10.2337/dc13-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 31.Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573-1575. doi: 10.1001/archinte.160.11.1573 [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Ryu E, Hathcock M, et al. Impact of demographics on human gut microbial diversity in a US Midwest population. PeerJ. 2016;4:e1514. doi: 10.7717/peerj.1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644-655. doi: 10.2337/diab.19.9.644 [DOI] [PubMed] [Google Scholar]
- 34.Matthan NR, Ausman LM, Meng H, Tighiouart H, Lichtenstein AH. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104(4):1004-1013. doi: 10.3945/ajcn.116.137208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanzerstorfer P, Rechenmacher E, Lugmayr O, et al. Effects of various commercial whole-grain breads on postprandial blood glucose response and glycemic index in healthy subjects. Austin J Clin Med. 2018;5(1):1031. [Google Scholar]
- 36.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78(4):734-741. doi: 10.1093/ajcn/78.4.734 [DOI] [PubMed] [Google Scholar]
- 37.Parker HL, Tucker E, Hoad CL, et al. Development and validation of a large, modular test meal with liquid and solid components for assessment of gastric motor and sensory function by non-invasive imaging. Neurogastroenterol Motil. 2016;28(4):554-568. doi: 10.1111/nmo.12752 [DOI] [PubMed] [Google Scholar]
- 38.Dickinson S, Colagiuri S, Faramus E, Petocz P, Brand-Miller JC. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr. 2002;132(9):2574-2579. doi: 10.1093/jn/132.9.2574 [DOI] [PubMed] [Google Scholar]
- 39.Wolever TM, Jenkins DJ, Ocana AM, Rao VA, Collier GR. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988;48(4):1041-1047. doi: 10.1093/ajcn/48.4.1041 [DOI] [PubMed] [Google Scholar]
- 40.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319(23):1500-1506. doi: 10.1056/NEJM198812083192302 [DOI] [PubMed] [Google Scholar]
- 41.Sicree RA, Zimmet PZ, King HO, Coventry JS. Plasma insulin response among Nauruans: prediction of deterioration in glucose tolerance over 6 yr. Diabetes. 1987;36(2):179-186. doi: 10.2337/diab.36.2.179 [DOI] [PubMed] [Google Scholar]
- 42.Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327(27):1893-1898. doi: 10.1056/NEJM199212313272701 [DOI] [PubMed] [Google Scholar]
- 43.Franz MJ, Boucher JL, Evert AB. Evidence-based diabetes nutrition therapy recommendations are effective: the key is individualization. Diabetes Metab Syndr Obes. 2014;7:65-72. doi: 10.2147/DMSO.S45140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Methods
eFigure. Receiver Operating Characteristic (ROC) Curve Analysis for Comparison of the Model Presented Herein and Models Based on Caloric or Carbohydrate Content Alone for Classifying High PPGR Responses