Abstract
The feasibility of 3D printing in clinical practice depends not only on the usability but also on the reliability of the method. The aims of this study were to demonstrate the feasibility of a 3D printing method for pediatric patients planned for pelvic triple osteotomy and to present a reliable quality assessment strategy for these printed models. A 10-year-old boy with Legg-Calvé-Perthes disease underwent a triple pelvic osteotomy. Preoperative and postoperative CT scans were printed as 3D models. An image-based quality assessment strategy was proposed: The printed 3D models were imaged with CT. The model images were systematically compared with the corresponding ground truth images, ie, patient images, to determine the reliability using distance measurements in the model and ground truth images. The 3D printed models were found useful in both the preoperative and postoperative stages. The models were found reliable: Strong linear correlation between the model and ground truth images both preoperatively (R = 0.99; P < 0.001) and postoperatively (R = 1.00; P < 0.001) was found. The study demonstrates the usefulness of 3D printed models in clinical practice. We also present a robust and simple strategy, using common clinical tools, to assess the reliability of 3D printed models.
Over the past decades, significant developments have been made in the field of 3D printing with benefits, especially for medical professions facing complex surgery. Patient-specific 3D models have enabled the surgeons to take preoperative planning away from screens and into the physical space and perform mock surgery on the models for evaluation of expected result before cutting the skin. Research studies have shown that such strategies can reduce the operation time and give the surgeon a subjective and substantial boost in understanding of the unique anatomic situation before surgery.1-3 Fields ranging from cardiology to facial reconstructive surgery have adopted custom-made 3D printed models as a method to improve planning and outcome.4,5,6,7,8,9,10
Orthopaedic surgery, and pediatric orthopaedic surgery in particular, is well suited to benefit from 3D printed models because bone is easy to model from CT scans, and deformities can clearly be visualized by plastic models. The pediatric skeleton is under constant development and can be affected by a large number of rare deformities and dysplasias. Hence, the pediatric skeleton is well suited for individual models.
Common pediatric orthopaedic conditions such as Legg-Calvé-Perthes disease (LCPD), developmental dysplasia of the hip (DDH), and slipped capital femoral epiphysis present complex three-dimensional shapes that are unique for each patient and can pose a challenge for the surgeon. A number of articles have been published presenting case studies in which 3D printed bone models have provided benefit to the orthopaedic surgeon in pediatric orthopaedic surgery.3,11 To our knowledge, however, no reports have been published describing 3D printed models as a preoperative tool for a triple osteotomy in children; a procedure that is used to treat selected cases of both LCPD and DDH. Multiple surgical methods for triple osteotomies in children have been described, and they all serve to realign the acetabulum to provide better coverage of the femoral head.12-14 This realignment is achieved by osteotomies of the ilium, pubis, and ischium bones to enable rotation and better anterior coverage by the acetabulum. Triple osteotomy is generally a preferred surgical method for children approximately older than 10 years when the symphysis has lost some of its elasticity and a Salter osteotomy (only one osteotomy of the ilium bone) is no longer sufficient.
The feasibility of 3D printing in clinical practice depends not only on the usability but also on the reliability of the method. Most people assume that 3D printed models are replicas of the true anatomy, but this assumption is seldom verified. As for all diagnostic methods within the medical field, quality assessments of the 3D printed model should be performed to ensure high performance of the method. The models might lack in reliability when low-end printers are used, and this issue might be even more pronounced when the printer is managed by staff who is not properly acquainted with the equipment—a situation likely to be more common as 3D printers become more widely available. Hence, the need for suitable quality assessment strategies is prominent. George et al15 describe different methods to measure the accuracy and reproducibility of 3D printed models, including calipers but also methods for redigitalization of the model using optical scanners, CT, or MRI. Such image-based strategies should be suitable for validation of the geometry in 3D models of complex structures, such as the pediatric pelvis. With such methods, quality assessments can be performed in-house by radiologists using the workstations implemented in the hospitals' system architecture. The need for a simple and accessible assessment strategy is even more relevant if hospitals acquire their own 3D printers for local production of custom-made models. Implementation of an assessment method in the workflow for pediatric patients with DDH or LCD would ensure accurate and reproducible modeling and enable recognition of change patterns for efficient monitoring of abnormalities and diseases. Moreover, access to a 3D printed model with an assured high accuracy would enable the surgeon to trust the anatomy of the model and better perform preoperative measurements of angles and dysplasia and evaluate postoperative results. To our knowledge, an image-based strategy for validation of 3D printed models of pediatric patients with DDH or LCDP has not previously been described.
The aims of this study were (1) to present a case and a proof-of-concept study, involving production and evaluation, of 3D printed models pre- and postoperatively for a patient treated with a triple osteotomy of the pelvis and (2) to present a promising image-based strategy for quality assessment of 3D printed pelvic models using locally available diagnostic tools.
Methods
Patient Characteristics
The patient was a 10.5-year-old boy with LCPD with an onset earlier the same year. The preoperative radiographs revealed a fragmented caput femori with incomplete acetabular coverage (Reimer index 8%) (Figure 1). Clinically, the patient had a limp, a positive Trendelenburg, severely impaired abduction of the hip, and moderate ambulating pain. The patient was considered too old for a Salter osteotomy, and the team decided on a triple pelvic osteotomy ad modum Carlioz as the treatment of choice. The patient underwent a successful surgery in 2017, and the postoperative rehabilitation went according to plan. Six months after surgery, a new CT scan was performed to evaluate the late postoperative results. Clinical postoperative results were satisfactory with reduced pain, no limp, and greatly improved abduction. Postoperative radiographs (Figures 2 and 3) revealed a Reimer index of 0% and satisfying acetabular coverage.
Figure 1.
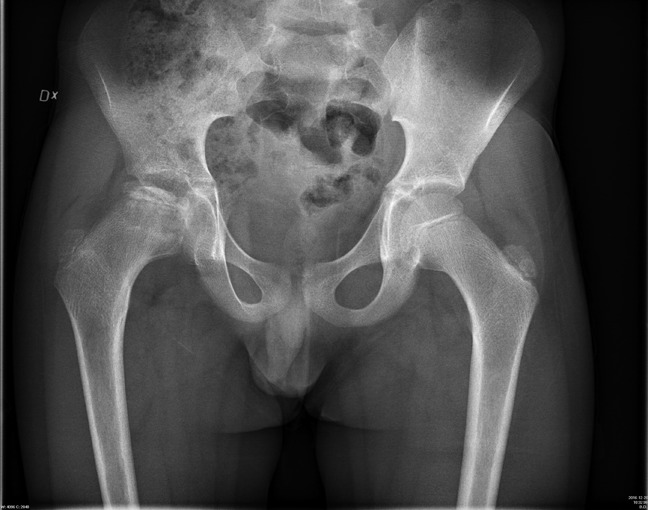
Preoperative radiograph of the patient with Legg-Calvé-Perthes disease. Note the right hip joint with typical deformity of the femoral head and incomplete coverage by a steep acetabulum.
Figure 2.
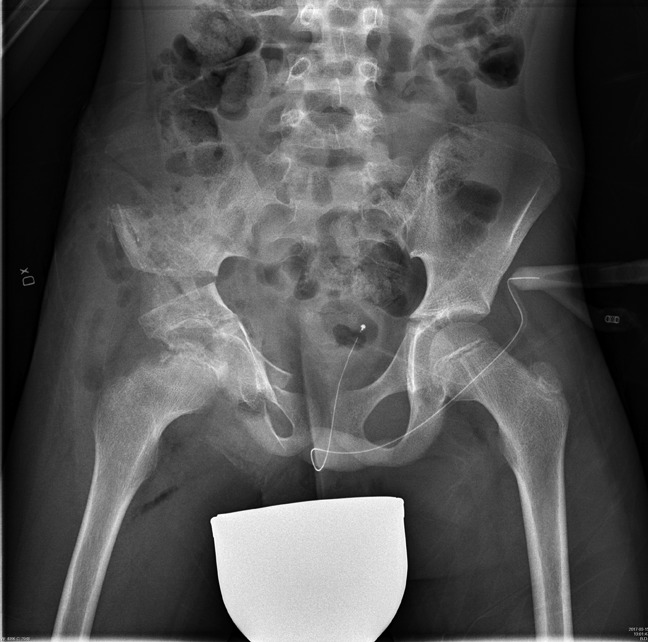
Perioperative radiograph after a triple osteotomy with improved coverage of the femoral head. Bioresorbable screws were used for fixation.
Figure 3.
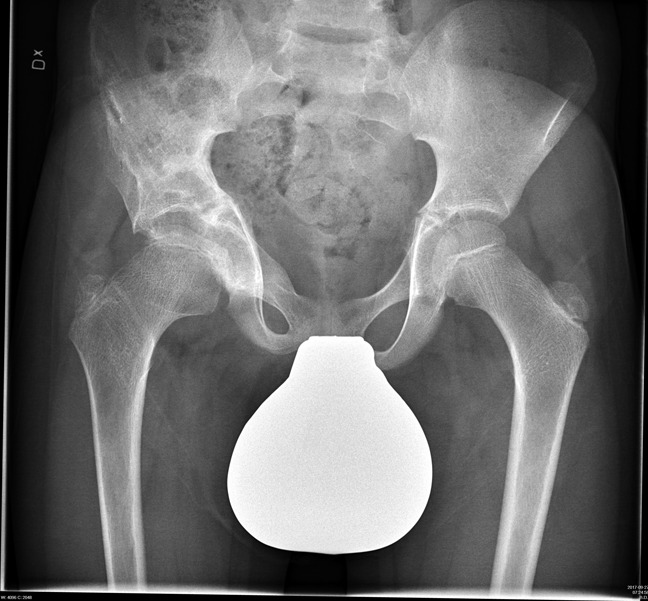
Postoperative radiograph 6 months after surgery with a healed osteotomy.
Production of 3D Printed Models
As part of the clinical routine, a preoperative CT scan of the patient was performed, using a CT scanner (Discovery CT750 HD; GE Healthcare) with an image slice thickness of 0.625 mm (The Project Was Approved by the Head of Department, Responsible for Ethical Review of Such Projects. The Project Did Not Affect the Treatment of the Patient). The DICOM images were transferred to a multimodality postprocessing workstation (software Volume Viewer 12.3 ext. 8; GE Healthcare), where 3D volume rendering and image segmentation was performed to create a digital 3D model. To only visualize bone, the threshold limit for the image segmentation was adjusted, and objects without relevance, eg, CT table top, were removed. The digital model was then exported to an STL file, which is a triangulated representation of the digital 3D model. The STL file was further prepared for printing in the 3D software Meshmixer (Autodesk). Preparations involved removing vertices and peripheral image artifacts from the digital model. In addition, to make the printed model able to stand on its own, a foundation and a support pin were added for stability in the software. The STL file was finally scaled down to fit the printers printing table (scale: 1:2.1) using the selected 3D printers software Cura (Ultimaker).
Printing was performed in cooperation with the innovative research institute RISE Interactive (Gothenburg, Sweden). Both preoperative and postoperative 3D printed models of the pelvis were produced using a 3D printer model Ultimaker 3 (Ultimaker), which uses Fused Deposition Modeling with an XYZ accuracy of 12.5, 12.5, 2.5 μm. Because of the access of dual-extrusion print heads, both the model and its crucial support material could be printed simultaneously. The model was printed in acrylonitrile butadiene styrene material, and the water-soluble support material was polyvinyl alcohol. The support material was removed by soaking the finalized printed model in water.
On the basis of previous experience and expertise from similar cases, an experienced radiologist (K.M.) decided on the 10 most pertinent anatomic landmarks that were both sufficiently identifiable on a CT scan and representative of the relevant anatomy. Distance measurements of the predefined anatomic structures were performed for each of the four different data sets, ie, the CT images of the patient's pelvis both preoperatively and postoperatively and the CT images of the model preoperatively and postoperatively.
All distance measurements were performed by an experienced radiologist (K.M.) using the AW 3.2 (GE Healthcare). The radiologist performed the measurements consecutively according to the index number of the anatomic structure (Table 1). For all data sets, each anatomic structure was measured twice during the same session. In total, 80 measurements were performed.
Table 1.
Anatomic Structures Used in the Quality Assessment

Before the distance measurements were performed, the pelvis was aligned in the axial, coronal, and sagittal views using specific anatomic landmarks, eg, the symphysis, the promontorium, and the anterior aspect of the sacrum with the purpose to improve the reproducibility of the reformations.
On the basis of the distance measurements, the feasibility of the 3D print reproduction method and the feasibility of the present quality assurance strategy were assessed. The reliability of the presented 3D print reproduction was determined by bias and limits of agreement between distance measurements in images of the model and the ground truth, both visualized by Bland-Altman plots. Agreement between the model and ground truth was also determined by means of the Pearson regression coefficient.
The reproducibility of the distance measurement was determined as the variation between repeated measurements, calculated as 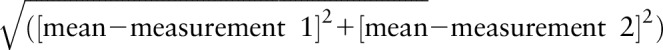 . The measurement reproducibility was used not only as an indicator of how accurate the measurements can technically be performed but also to study the reliability of the proposed quality assurance strategy.
. The measurement reproducibility was used not only as an indicator of how accurate the measurements can technically be performed but also to study the reliability of the proposed quality assurance strategy.
Results
Subjective Benefit of the 3D Model to the Surgeon (T.L.)
The experienced orthopaedic surgeon forms a 3D model based on radiographs in their mind, but to be able to hold and turn the printed model, visualized in Figures 4 and 5, during the planning of surgery does add a new dimension and benefit to the planning of complex surgery. This is of particular interest for demanding surgeries, where the bone anatomy of the patient is far beyond the normal variation. The procedure could also first be tried out on the 3D model to ensure that all cuts and angles are properly optimized, but this process was not deemed necessary in this case.
Figure 4.
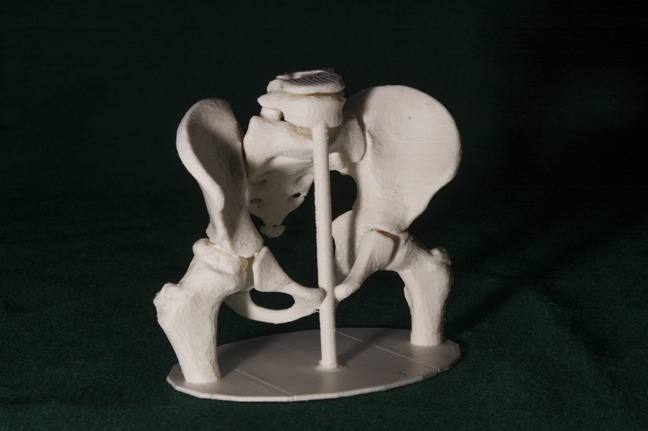
Preoperative 3D printed model visualizing the deformed femoral head due to LCPD and poor coverage by the acetabulum. LCPD = Legg-Calvé-Perthes disease
Figure 5.
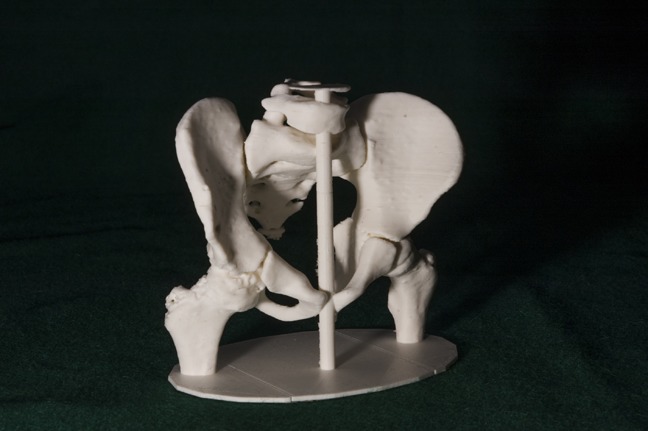
Postoperative 3D printed model 6 months after surgery showing a healed osteotomy and improved coverage of the femoral head.
Quality Assessments of the 3D Printed Models
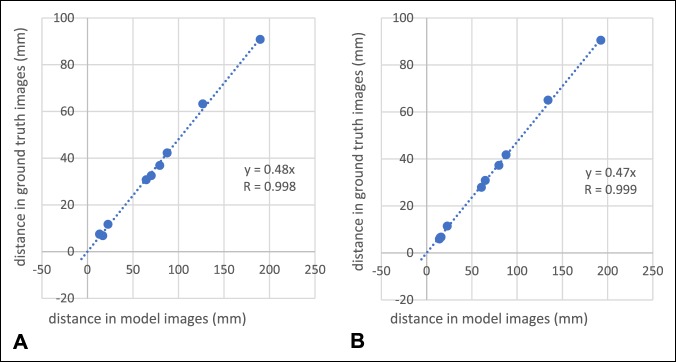
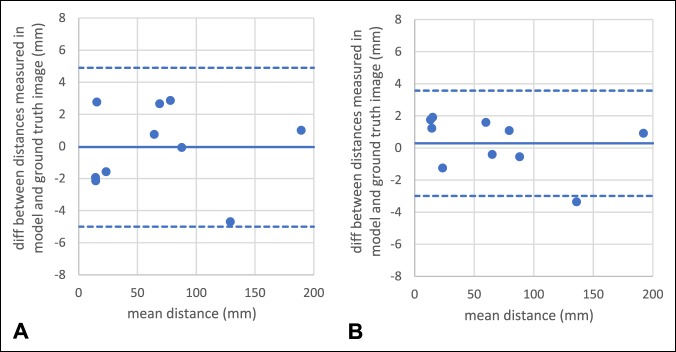
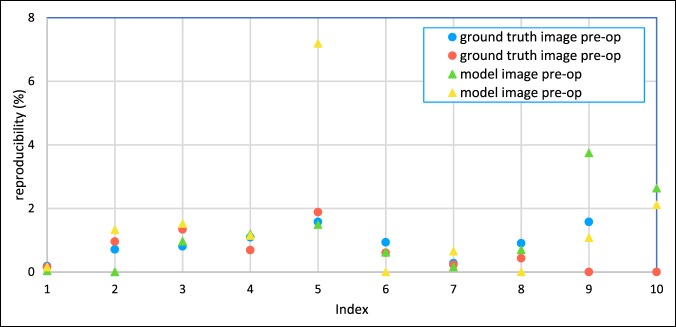
Results demonstrated high reliability of the present 3D printed method as strong linear correlation between the model and ground truth images for both preoperative (R = 0.99; P < 0.001) and postoperative measurements (R = 1.00; P < 0.001; Figure 6). Moreover, low bias, 0 and 0 mm, and small limits of agreement, 5 and 4 mm, were found for the preoperative and postoperative measurements, respectively (Figure 7). The proposed quality assessment strategy displayed high reproducibility (Figure 8), with mean and maximal percentage variation in repeated measurements of 1.5% and 7%, respectively.
Figure 6.
Strong and significant linear correlations were found between distances measured in the ground truth image and the corresponding model image for the (A) preoperative and (B) postoperative measurements.
Figure 7.
Bland-Altman plots displaying small bias and small limits of agreement between ground truth and the corresponding model image for (A) the preoperative and (B) postoperative measurements.
Figure 8.
Graph showing the reproducibility of the preoperative and postoperative measurements for the model and ground truth images measured in percentage variation between repeated measurements. See Table 1 for definition of index.
Discussion
We present the production of a 3D printed model of a pediatric pelvis as a preoperative tool using the CT scans that are part of our normal clinical practice. Second, and of more general interest, we present a method for a radiologist to evaluate the anatomic accuracy of a 3D printed model of a pediatric pelvis using equipment available at most modern hospitals. Our results suggest that this method is easy, reliable, and reproducible. We also found the use of a 3D printed model to be useful in preoperative planning and of particular benefit when going over the procedure preoperatively with younger colleagues. However, these results are subjective because it is a case study. Nevertheless, the postoperative model provided good visualization of the 3D result, and the model was found greatly appreciated by the parents as a tool to explain the indication for surgery.
Previous articles have presented 3D printed models to aid in advanced pelvic and hip surgery, and our results are in line with their general conclusions, suggesting the feasibility and benefit of 3D printed models as an adjunct tool in planning and evaluation of surgery in selected cases. We do not present a detailed algorithm of how to produce a 3D model. This analysis has been done in detail in numerous other articles, and because the field is in rapid evolution, any specific method of printing is likely to have a limited life span.2,4,16,17 The cost of a 3D printed model today is highly variable because of the myriad of materials and printers available. The model used for this proof of concept costs approximately 150€ to produce. Once again, the rapid expansion of the field and the increasing availability of 3D printers make a gradual decrease in production cost likely.
However, as 3D printed models are likely to become increasingly common in clinical practice, the need to be able to standardize quality assessment is evident. Calipers are easy to use, but which parts of the 3D model to measure remains subjective. Moreover, calipers are often limited regarding the accessibility, especially for complex structures. Optic scanners do not face the same limitations, but are not widely available, and require expensive equipment and complex interpretation of the results. Our proposed image-based strategy, based on equipment that is common in the clinic, is simple and seems to be reproducible. When 3D printers become more widely available at hospitals, simplicity and making use of available equipment are probably of value.
Producing a digital model by converting CT images in DICOM to STL file format using the method described here proved to involve few steps and required little technical know-how to reproduce. Once a representative STL file is obtained, there are many solutions for the physical printing of the model, which can be done using a local 3D printer at the hospital or an external commercial company. Regardless of how and where the printing is performed, the need to verify the quality and reliability of the model remains. Using the present quality assessment method, it was shown that the 3D printing method reproduced the complex structure of the pelvis accurately; the model and ground truth images showed high agreement for all geometric measurements.
With the expansion of 3D printing model technologies, the question arises regarding their effect and benefit on patient care. Development and design of these technologies should also take into account the concept of quality as it might affect the ability of practicians to use the diagnostic information. Toward that goal, one should consider several human factors involved in the analysis and interpretation, eg, perception issues and decision process. That is, the validation of the 3D printing model should include not only measurements of reliability but also attributes for usability. Empirical validation of the present 3D printed method demonstrated high functionality regarding usability for the diagnostic task and chosen skeletal dysplasia. In addition, the method satisfied the predetermined needs of the clinic, and the understandability of the method was considered as high. Moreover, we believe that the method has high extendibility, reusability, and flexibility and, as such, can be easily reapplied and adapted for other orthopaedic diagnosis.
The same could also be said about the quality assessment strategy, which was found to have high usability. In addition, the quality assessment strategy was found to be robust even for complex structures, such as a pelvic triple osteotomy in children. For repeated measurements, including the whole procedure of image angulation and distance measurements, we found that the percentage variation in repeated measurements was below 7%. The highest variation was detected for measurements of more complex structures, where the landmarks used for the image angulation and distance measurement were less easily identified. For less complex structures, the reproducibility of the quality assessment method was even higher (<4%).
There are obvious limitations in our study investigating the feasibility of a 3D printed model for a pediatric pelvis using only one case. As a proof of concept, however, we find the results relevant for surgeons in the field. Furthermore, the distance measurements were performed by only one radiologist, who had performed the reproducibility measurements consecutively on the same day. The anatomic landmarks were also chosen by the radiologist to optimize relevance and reproducibility. These landmarks can, of course, be subject to further improvement. Additional studies on the subject will likely yield additional aspects to this rapidly expanding field.
Conclusion
A 3D printed model of a pediatric pelvis, pre- and postoperative a triple osteotomy, was a useful tool for the surgeon both to plan the surgery and to evaluate the results. We also present a simple method, using common clinical tools, to evaluate the anatomic accuracy of a 3D printed model of a pelvis, and this method seems reliable and reproducible.
Footnotes
None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Hedelin, Dr. Swinkels, Dr. Laine, Dr. Mack, and Dr. Lagerstrand.
References
- 1.Hurson C, Tansey A, O'Donnchadha B, Nicholson P, Rice J, McElwain J: Rapid prototyping in the assessment, classification and preoperative planning of acetabular fractures. Injury 2007;38:1158-1162. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio F, Marconi S: 3D printing: Clinical applications in orthopaedics and traumatology. EFORT Open Rev 2016;1:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherkasskiy L, Caffrey JP, Szewczyk AF, et al. : Patient-specific 3D models aid planning for triplane proximal femoral osteotomy in slipped capital femoral epiphysis. J Child Orthop 2017;11:147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. : 3D printing based on imaging data: Review of medical applications. Int J Comput Assist Radiol Surg 2010;5:335-341. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto JS, Morris JM, Foley TA, et al. : Three-dimensional physical modeling: Applications and experience at Mayo clinic. Radiographics 2015;35:1989-2006. [DOI] [PubMed] [Google Scholar]
- 6.Rose AS, Webster CE, Harrysson OL, Formeister EJ, Rawal RB, Iseli CE: Pre-operative simulation of pediatric mastoid surgery with 3D-printed temporal bone models. Int J Pediatr Otorhinolaryngol 2015;79:740-7444. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock P, Prabhu SP, Flynn K, Orbach DB, Smith E: Optimizing cerebrovascular surgical and endovascular procedures in children via personalized 3D printing. J Neurosurg Pediatr 2015;16:584-589. [DOI] [PubMed] [Google Scholar]
- 8.Deferm S, Meyns B, Vlasselaers D, Budts W: 3D-Printing in congenital cardiology: From flatland to spaceland. J Clin Imaging Sci 2016;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannopoulos AA, Steigner ML, George E, et al. : Cardiothoracic applications of 3-dimensional printing. J Thorac Imaging 2016;31:253-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kappanayil M, Koneti NR, Kannan RR, Kottayil BP, Kumar K: Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: Early experience and proof of concept in a resource-limited environment. Ann Pediatr Cardiol 2017;10:117-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guarino J, Tennyson S, McCain G, Bond L, Shea K, King H: Rapid prototyping technology for surgeries of the pediatric spine and pelvis: Benefits analysis. J Pediatr Orthop 2007;27:955-960. [DOI] [PubMed] [Google Scholar]
- 12.Steel HH: Triple osteotomy of the innominate bone. A procedure to accomplish coverage of the dislocated or subluxated femoral head in the older patient. Clin Orthop Relat Res 1977;116-127. [PubMed] [Google Scholar]
- 13.Tönnis D, Behrens K, Tscharani F: A modified technique of the triple pelvic osteotomy: Early results. J Pediatr Orthop 1981;1:241-249. [DOI] [PubMed] [Google Scholar]
- 14.Carlioz H: Pelvic osteotomies in children and adolescents [in French]. Acta Orthop Belg 2000;66:321-28. [PubMed] [Google Scholar]
- 15.George E, Liacouras P, Rybicki FJ, Mitsouras D: Measuring and establishing the accuracy and reproducibility of 3D printed medical models. Radiographics 2017;37:1424-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu XB, Wang JQ, Zhao CP, et al. : Printed three-dimensional anatomic templates for virtual preoperative planning before reconstruction of old pelvic injuries: Initial results. Chin Med J 2015;128:477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bücking TM, Hill ER, Robertson JL, Maneas E, Plumb AA, Nikitichev DI: From medical imaging data to 3D printed anatomical models. PLoS One 2017;12:e0178540. [DOI] [PMC free article] [PubMed] [Google Scholar]