To the Editor:
Isocitrate dehydrogenase (IDH1/2) genes encode for ubiquitinously expressed enzymes that catalyze a redox reaction that converts isocitrate to α-ketoglutarate while reducing NADP to NADPH and liberating CO2 [1]. IDH1 exerts his function in the cytoplasm and peroxisomes whilst IDH2 is localized in the mitochondrial matrix [1]. When mutated, the IDH1 and IDH2 enzymes acquire a neomorphic activity leading to the conversion of α-ketoglutarate to D-2-hydroxyglutarate [2–4]. The latter compound acts as an oncometabolite by inhibiting the α-ketoglutarate-dependent enzymes that regulates epigenetic modeling, collagen synthesis and cell signaling [1]. IDH1 and IDH2 mutations are mutually exclusive with TET2 mutations that are known to promote leukemia with a similar mechanism [5].
IDH1 gene mutations have been detected in 6.6–7.6% [6, 7] of AML patients, most frequently carrying a normal karyotype, and their presence has not been associated with prognostic relevance. They are heterozygous missense mutations confined to a single arginine residue, R132, in the enzyme active site [1]. Five R132 mutations leading to different amino acid exchanges have been described [6, 7]: p.R132H, p.R132C, p.R132G, p.R132S, and p.R132L, with R132H being the most frequent [7]. As a whole group, the IDH1-R132 mutations are more frequent in cases carrying NPM1 mutations [6, 7] but it is yet unknown how the amino acid substitution of arginine at position 132 correlates with the mutational status of NPM1 and other mutations in AML. Here, combining molecular analyses and immunohistochemistry we demonstrate that the R132H and R132C substitutions show a different distribution pattern among AML genotypes.
We first investigated 140 AML patients with normal cytogenetics enrolled in Northern Italy Leukemia Group (NILG) multicenter clinical trial (NCT00495287), for which both molecular and immunohistochemical data were available (Supplementary Information). In all 140 patients, the results of next generation sequencing (NGS) for IDH1 and NPM1 mutations were blindly compared with those of immunohistochemistry on bone marrow (BM) biopsies using monoclonal antibodies against IDH1-R132H and NPM1, respectively. The antibody against the IDH1-R132H mutant was previously produced by Capper et al. [8] and extensively investigated in various kind of tumors. The antibody directed against the nucleophosmin (NPM1) [9] was generated in BF laboratory. Cytoplasmic nucleophosmin-1 expression was regarded as predictive of NPM1 mutations [9, 10] (Supplementary Information). For all studies described below, written informed consent to examine leukemic samples was obtained in accordance with the Declaration of Helsinki and approval was obtained from Local Ethic Committee.
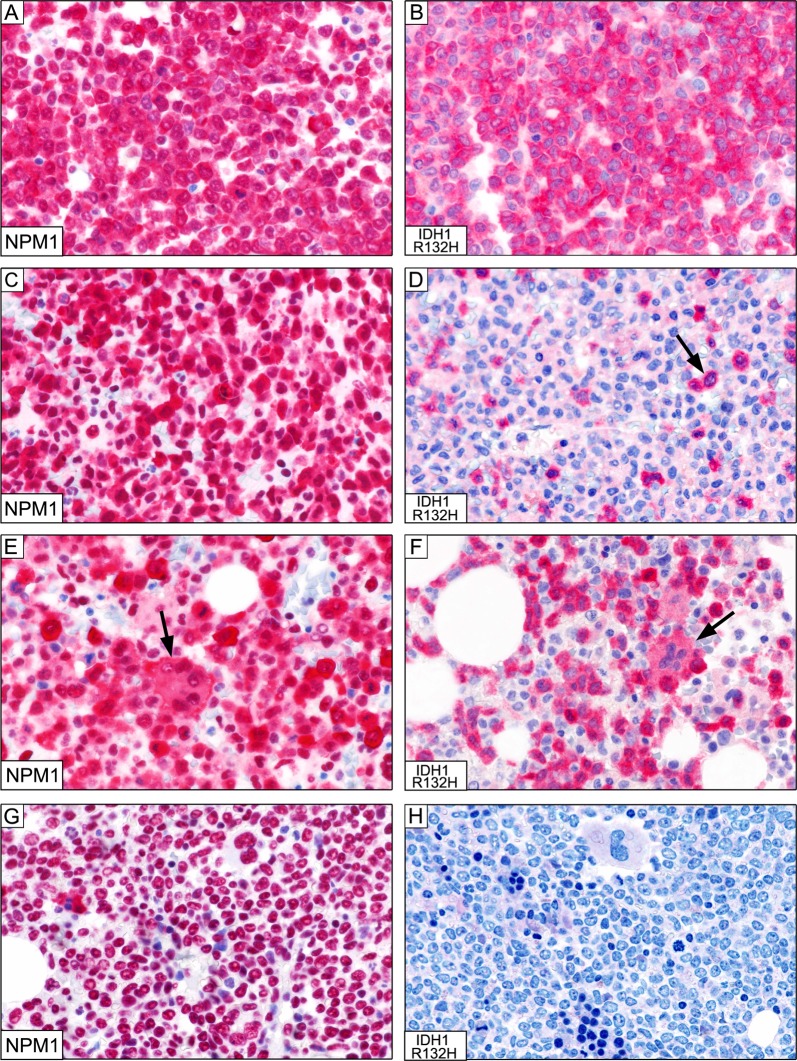
Molecular analyses revealed NPM1 mutations in 71/140 (51%) cases. These findings were fully confirmed by immunohistochemistry that showed cytoplasmic NPM1 (predictive of NPM1 mutations) (Fig. 1a, c, e) in the same 71 cases. In the remaining 69 cases, NPM1 expression was nucleus-restricted, as expected in cases with NPM1 wild-type status [9].
Fig. 1.
a Massive infiltration of BM by NPM1-mutated AML cells showing the expected nuclear plus aberrant cytoplasmic positivity for nucleophosmin-1 (×400). b The same case as (a), showing a comparable number of leukemic cells expressing the IDH1-R132H mutant; positivity is mostly restricted to the cytoplasm of blast cells (×400). c Marked infiltration of BM by NPM1-mutated AML cells showing the expected nuclear plus aberrant cytoplasmic positivity for nucleophosmin-1. The rare elements with nucleus-restricted positivity for NPM1 represent residual normal hematopoietic cells (×400). d The same case as (c), showing that leukemic cells expressing the IDH1-R132H mutant represent only a small subclone of the total population of NPM1-mutated cells (×400). e Marked infiltration of BM by NPM1-mutated AML cells showing the expected nuclear plus aberrant cytoplasmic positivity for nucleophosmin-1 (×400). The arrow points to a positive megakaryocyte. Elements with nucleus-restricted positivity for NPM1 represent normal residual hematopoietic cells (×400). f The same case as (c), showing that the percentage of leukemic cells expressing the IDH1-R132H is slightly inferior to that of NPM1 cytoplasmic-positive cells. As in (e), the IDH1-R132H mutant is present both in mononuclear blast cells and in a megakaryocyte (arrow). The IDH1-R132H negative cells represent normal residual hematopoietic cells (×400). g Massive bone marrow infiltration by leukemic cells with nucleus-restricted positivity for nucleophosmin-1 (predictive of absence of NPM1 mutations, confirmed molecularly) (×400). h Specificity of the antibody against IDH1-R132H is demonstrated by the negativity of leukemic cells molecularly carrying the IDH1-R132C mutation (×400). (a–h) Dako REAL Detection System Alkaline Phosphatase/RED rabbit/mouse
Molecular analyses revealed IDH1-R132H mutations in 10/140 (7%) cases. Notably, these 10 cases were all NPM1-mutated and showed cytoplasmic NPM1 at immunohistochemistry (10/71:14%). The same 10 cases, revealed R132H mutant expression at cytoplasmic level (Fig. 1b, d, f), as expected for the cytosolic function of the enzyme [1]. At diagnosis, the percentage of IDH1-R132H-positive leukemic cells and with aberrant cytoplasmic NPM1 were comparable in 6/10 cases (representative examples are shown in Fig. 1a, b), whilst in 4/10 cases the IDH1-R132H-positive leukemic cells accounted for only a fraction of them, ranging between 3% and 70%, strongly suggesting that they represented a subclone. A representative example showing about 5–10% of IDH1-132H-positive leukemic cells in shown in Fig. 1d.
Extended molecular analysis of the 140 cases also detected IDH1 mutations other than p.R132H in 8/140 (6%) cases. In particular: p.R132C in 3/140 cases (2%; 2 NPM1-mutated, 1 NPM1-wt), p.R132G in 2/140 cases (1%; both NPM1-mutated), and p.R132S in 3/140 cases (2%; all NPM1-mutated). Notably, all these eight cases were negative with the mAb specific for IDH1-R132H (Fig. 1g, h).
To further validate the above findings and extend the correlation of IDH1-R132 changes to other mutations, we analyzed at Munich Leukemia Laboratory another independent cohort of IDH1-mutated AML by comprehensive gene sequencing. Our previously described AML cohort [11] comprised 106 IDH1-mutated de novo AML patients, most often showing IDH1-R132H (n = 44/106; 41%) and R132C (39/106; 37%). In this study, we investigated all cases by NGS and gene scan targeting IDH1 and NPM1 beside 25 other genes (Supplemental Information). 62% (66/106) cases of IDH1-mutated patients showed also a NPM1 mutation, 48% a DNMT3A mutation, 23% a FLT3-ITD, 16% a NRAS mutation, and 12% a SRSF2 mutation (Fig. 2a; Supplementary Table 1). All other mutations occurred in <10% of cases. Therefore, we could confirm the high association of IDH1-R132H with NPM1 mutations in this cohort. In fact, 39/44 (89%) IDH1-R132H patients showed a NPM1 mutation, while in only 44% (27/62) of the other IDH1-R132 mutated patients a NPM1 mutation occurred (p < 0.001) (Fig. 2a; Supplementary Table 1).
Fig. 2.
a Molecular and cytogenetic characterization of IDH1-mutated patients. Illustration of all 106 samples, each column represents one patient. All 25 additionally analyzed genes as well as karyotype information are given for each patient. Patients are grouped by IDH1 R132C, R132H, and R132 other. Light gray: wild type, red: mutated, orange: variant of uncertain significance, dark blue: aberrant karyotype, light blue: normal karyotype, white: no data available. The number of additional mutations per patient is illustrated as bar chart above the graph. The mutation frequencies of single genes are given as bar chart at the right. b Spider plot illustrating the mutation frequencies (in %) of ASXL1, NPM1, RUNX1, and SRSF2 mutations for the single groups of IDH1 R132C, R132H, and R132 other
Analysis of further gene mutations and their associations showed that IDH1-R132H was mutually exclusive for RUNX1 (0/44; 0%; p = 0.001), SRSF2 (3/44; 7%; p = 0.104) and ASXL1 (1/44; 2%; p = 0.02 3) and were less frequently mutated compared to IDH1-R132C mutated patients (23%, 21%, and 18%, respectively) (Fig. 2a, supplementary Table 1). These data resulted, therefore, in two different mutation patterns, differentiating IDH1-R132H and R132C mutated AML (Fig. 2b). While R132C shows a more s-AML like genetic, R132H shows a typical de novo AML pattern [12]. The third group of IDH1-mutated patients (other than R132H/C) seemed to be a mixture of both patterns (Fig. 2b). Addressing the prognostic impact of these IDH1-R132 variants showed a slightly worse prognostic impact of IDH1-R132C compared to IDH1-R132H-mutated patients (overall survival: 19.9 versus 24.9 months; Supplementary Figure 1).
Different co-mutation patterns for hotspots within genes has been previously described under various circumstances [13]. As an example, the NPM1 mutation preferentially associates with NRAS-G12/13 but not with NRAS-Q61 [13]. These findings strongly suggest that the functional consequences of hotspot mutations within genes may not be equivalent. At present, no compound NPM1-mutated/IDH1-mutated mouse model has been described.
Is there any utility to have an anti-IDH1-R132-specific antibody in the NGS era? Although, molecular analyses remain the gold standard for the identification of IDH1 mutations, immunohistochemistry may be a useful adjunct to the above techniques, particularly in hematological centers that still use BM biopsies. Under these circumstances, the antibody could be used both at diagnosis and for monitoring of AML after chemotherapy or targeted therapy with IDH1 inhibitors [14]. The antibody would also allow to analyze the genetic lesion at protein level in the tissues and provide information related to the topographical distribution (nearby trabeculae or vessels) of leukemic cells. Moreover, the use of the antibody may be particularly important in cases of “punctio sicca” or myeloid sarcoma, especially when scarce material is available for molecular analyses (e.g. punch biopsies of the skin).
Supplementary information
Acknowledgements
Supported by the ERC Adv Grant 2016 no. 740230 to B.F. and the Associazione Italiana Ricerca Cancro (AIRC) IG 2016 no.18568.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
The online version of this article (10.1038/s41375-018-0299-2) contains supplementary material, which is available to authorized users.
References
- 1.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3:730–41. doi: 10.1158/2159-8290.CD-13-0083. [DOI] [PubMed] [Google Scholar]
- 2.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–34. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–44. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnittger S, Haferlach C, Ulke M, Alpermann T, Kern W, Haferlach T. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116:5486–96. doi: 10.1182/blood-2010-02-267955. [DOI] [PubMed] [Google Scholar]
- 7.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–43. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 8.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 9.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–66. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Martelli MP, Bolli N, Bonasso R, Ghia E, Pallotta MT, et al. Immunohistochemistry predicts nucleophosmin (NPM) mutations in acute myeloid leukemia. Blood. 2006;108:1999–2005. doi: 10.1182/blood-2006-03-007013. [DOI] [PubMed] [Google Scholar]
- 11.Meggendorfer M, Cappelli LV, Walter W, Haferlach C, Kern W, Falini B, et al. IDH1R132, IDH2R140 and IDH2R172 in AML: different genetic landscapes correlate with outcome and may influence targeted treatment strategies. Leukemia. 2018;32:1249–53. doi: 10.1038/s41375-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 12.Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76. doi: 10.1182/blood-2014-11-610543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 2018;378:2386–98. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.