Key Points
Question
Is the improvement in fractional flow reserve after percutaneous coronary intervention (ΔFFR) associated with clinical outcome and symptomatic relief?
Findings
In this analysis of 2 randomized clinical trials, incidence of vessel-oriented clinical events was significantly greater among patients in the lowest tertile of ΔFFR compared with those in the uppermost tertile, and a significant association was observed between ΔFFR and symptomatic relief.
Meaning
These findings suggest that patients in whom only a modest improvement in FFR can be obtained by percutaneous coronary intervention are at higher risk of events and are more likely to have more persistent complaints.
This post hoc analysis of data from randomized clinical trials investigates the clinical value of fractional flow reserve in patients who have undergone percutaneous coronary intervention.
Abstract
Importance
Whether the improvement in myocardial perfusion provided by percutaneous coronary intervention (PCI) is associated with symptomatic relief or improved outcomes has not been well investigated.
Objective
To investigate the prognostic value of the improvement in fractional flow reserve (FFR) after PCI (ΔFFR) on patients’ symptoms and 2-year outcomes.
Design, Setting, and Participants
This study is a post hoc analysis of data from patients undergoing FFR-guided PCI in the randomized clinical trials Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) 1 (NCT00267774; 2009) and FAME 2 (NCT01132495; 2012), with inclusion of 2 years of follow-up data. The FAME 1 trial included patients with multivessel coronary artery disease from 20 medical centers in Europe and the United States. The FAME 2 trial included patients with stable coronary artery disease involving up to 3 vessels from 28 sites in Europe and North America. Lesions from the group in the FAME 1 trial from whom FFR was measured and the group in the FAME 2 trial who received FFR-guided PCI plus medical therapy were analyzed. Data analysis occurred from May 2017 to May 2018.
Interventions
Measure of post-PCI FFR.
Main Outcomes and Measures
Vessel-oriented clinical events at 2 years, a composite of cardiac death, target vessel-associated myocardial infarction, and target vessel revascularization.
Results
This analysis included 639 patients from whom pre-PCI and post-PCI FFR values were available. Of their 837 lesions, 277 were classified into the lowest tertile (ΔFFR≤0.18), 282 into the middle tertile (0.19≤ΔFFR≤0.31), and 278 into the highest tertile (ΔFFR>0.31). Vessel-oriented clinical events were significantly more frequent in the lowest tertile (n = 25 of 277 [9.1%]) compared with the highest tertile (n = 13 of 278 [4.7%]; hazard ratio, 2.01 [95% CI, 1.03-3.92]; P = .04). In addition, a significant association was observed between ΔFFR and symptomatic relief (odds ratio, 1.33 [95% CI, 1.02-1.74]; P = .02).
Conclusions and Relevance
In this analysis of 2 randomized clinical trials, the larger the improvement in FFR, the larger the symptomatic relief and the lower the event rate. This suggests that measuring FFR before and after PCI provides clinically useful information.
Introduction
After percutaneous coronary intervention (PCI), fractional flow reserve (FFR) is generally smaller than 1.0.1 Two recent studies have supported the concept that the higher the post-PCI FFR value is, the better the patient’s condition is. Yet, the potential of any post-PCI FFR value to prognosticate an outcome remains low.2,3 While a final hemodynamic result can be identical, the improvement in FFR value can be different and might have a different meaning for the patient.
We hypothesized that the improvement in FFR (ΔFFR) reflects a decrease in ischemic burden, determines symptomatic relief, and bears prognostic value. We analyzed this using data from 2 previous randomized clinical trials: Fractional Flow Reserve vs Angiography for Multivessel Evaluation (FAME) 1 (NCT00267774; 2009) and FAME 2 (NCT01132495; 2012).
Methods
Data and Outcomes
All lesions from the group of participants in whom FFR was measured in the FAME 1 trials4,5 and from the group who received FFR-guided PCIs in the FAME 2 trial6,7 were included, if the post-PCI FFR had been measured. The primary outcome was a composite end point consisting of vessel-oriented clinical events (VOCEs; defined as cardiac death, revascularization, and myocardial infarction, including periprocedural myocardial infarction) at 2 years of follow-up. All events were adjudicated by an independent clinical event committee that was blinded to FFR values, which assigned the events as vessel-associated or not. In addition, the association between ΔFFR and the Canadian Cardiovascular Society angina class after 1 month was investigated.
The FAME and FAME 2 trials were approved by the institutional review board at each participating center. All patients provided written informed consent.
Statistical Analysis
The lesions were classified into 3 tertiles of ΔFFR. To compare groups, the t test was used for normally distributed continuous variables, the Mann-Whitney tests for nonnormally distributed continuous variables, and the Pearson χ2 test for categorical variables tests. All parameters with a P value of .15 or less were included in a Cox regression model encompassing ΔFFR, post-PCI FFR, and pre-PCI FFR values as categorical variables, using a cutoff value of 0.70. All angiographic characteristics were also used. Subgroup analyses based on the patients treated with second-generation stents (in the FAME 2 trial only) was also performed. A multivariate linear regression was used to identify factors associated with ΔFFR, and a binary logistic regression was used to calculate the probability of symptomatic relief of at least 2 Canadian Cardiovascular Society classes. All events, including cardiac death, have been adjudicated at vessel level by an independent clinical events committee. When the identification of the culprit vessel was not possible or feasible (ie, in cases in which cardiovascular death occurred, no coronary angiography was performed, or non–ST-segment elevation myocardial infarction occurred in patients with multivessel disease), the end point was assigned to all the stenotic vessels of those patients. This was the case in a very small number of events; the culprit vessel could not be identified in 3 cases. The culprit vessel was identified in all cases in which an FFR value was available at baseline. Statistical analysis was carried out using SPSS version 24.0 software (IBM), and figures were created with Prism version 7.0a (GraphPad Software). We considered 2-sided P values <.05 significant. Data analysis for this study occurred from to May 2017 to May 2018.
Results
Patients and Lesions
In the FAME 1 and 2 trials, 874 and 625 lesions were treated according to FFR, respectively. Among these, 504 lesions in 352 patients in FAME 1 and 333 lesions in 287 patients in FAME 2 had available post-PCI FFR measurements. Accordingly, 837 lesions in 639 patients were included in this analysis. A total of 277 lesions were classified in the lowest tertile (ΔFFR ≤0.18), 282 in the middle tertile (ΔFFR, 0.19-0.30), and 278 in the highest tertile (ΔFFR >0.31).
The clinical characteristics of lesions according to the 3 tertiles of ΔFFR are summarized in Table 1. These lesions were found mainly in male patients (667 of 837 lesions [79.7%]%), with a median (interquartile range) patient age of 65 (57-71) years. Significant differences were found between groups with respect to previous PCIs (lowest tertile, 82 of 277 lesions [29.6%]; middle tertile, 63 of 282 lesions [22.3%]; highest tertile, 43 of 278 lesions [15.5%]; P < .001), pre-PCI FFR (median [interquartile range]: lowest tertile, 0.76 [0.72-0.78]; middle tertile, 0.69 [0.63-0.72]; highest tertile, 0.50 [0.41-0.55]; P < .001), and post-PCI FFR (median [interquartile range]: lowest tertile, 0.87 [0.85-0.91]; middle tertile, 0.91 [0.87-0.96]; highest tertile, 0.92 [0.88-0.96]; P < .001).
Table 1. Clinical Characteristics of Lesions in the 3 Tertiles of Change in Fractional Flow Reserve (ΔFFR)a.
| Variable | Lesions, No. (%) | P Value | ||
|---|---|---|---|---|
| Lowest Tertile | Middle Tertile | Highest Tertile | ||
| No. | 277 | 282 | 278 | |
| Male | 216 (78.0) | 235 (83.3) | 216 (77.7) | .17 |
| Age, median (IQR), y | 65 (59-72) | 64 (57-71) | 65 (57-72) | .18 |
| BMI, median (IQR) | 28 (25-30) | 28 (25-30) | 27.70 (25-31) | .84 |
| Hypertension | 195 (70.4) | 191 (67.7) | 178 (64.0) | .28 |
| Dyslipidemia | 208 (75.1) | 212 (75.2) | 211 (75.9) | .97 |
| Diabetes | 72 (26.0) | 74 (26.2) | 56 (20.1) | .16 |
| Smoker | 63 (22.7) | 56 (19.9) | 76 (27.3) | .11 |
| Family history | 107 (38.6) | 116 (41.1) | 130 (46.8) | .14 |
| Previous percutaneous coronary intervention | 82 (29.6) | 63 (22.3) | 43 (15.5) | <.001 |
| Previous myocardial infarction | 113 (40.8) | 101 (35.8) | 92 (33.1) | .16 |
| Fractional flow reserve, median (IQR) | ||||
| Postpercutaneous coronary intervention | 0.87 (0.85-0.91) | 0.91 (0.87-0.96) | 0.92 (0.88-0.96) | <.001 |
| Prepercutaneous coronary intervention | 0.76 (0.72-0.78) | 0.69 (0.63-0.72) | 0.50 (0.41-0.55) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ΔFFR, change in fractional flow reserve; IQR, interquartile range.
The lowest tertile is defined by a ΔFFR of 0.18 or less; the middle tertile, by a ΔFFR of 0.19 to 0.30; and the highest tertile, by a ΔFFR of 0.31 or more.
Angina Class
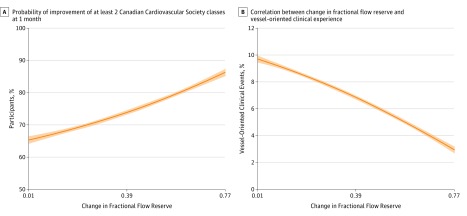
Prior to the index PCI, a significant association was observed between the FFR value in the tightest stenosis evaluated by FFR and the Canadian Cardiovascular Society class. The lower the class (from 0 to IV), the higher the minimal FFR value (median [interquartile range]: class 0, 0.68 [0.613-0.75]; class I, 0.66 [0.505-0.72]; class II, 0.62 [0.50-0.72]; class III, 0.59 [0.45-0.71]; class IV, 0.55 [0.46-0.69]; P < .001). At 1 month, ΔFFR of the tightest stenosis was significantly associated with symptomatic relief via binary logistical regression (odds ratio, 1.33 [95% CI, 1.02-1.74]; P = .02; the Figure, A). When selecting the tightest stenosis in every patient, there were no correlation between pre-PCI FFR values (classified in tertiles) and symptomatic relief of pre-PCI FFR value of the tightest stenosis and symptomatic relief.
Figure. Changes in Patient Population at 1 Month and 2 Years.
A, Probability of improvement of at least 2 Canadian Cardiovascular Society classes at 1 month based on the change in fractional flow reserve. B, Correlation between the change in fractional flow reserve and vessel-oriented clinical events at 2 years.
Outcomes
After 2 years of follow-up, 58 VOCEs occurred; 38 occurred in the left anterior descending artery, 7 in the circumflex coronary artery, and 13 in the right coronary artery. The Figure, B shows the significant association between ΔFFR and the probability of VOCE (P = .01). The number of VOCEs were greater in the lowest tertile (n = 25 of 277 [9.1%]) compared with the highest tertile (n = 13 of 278 [4.7%]; hazard ratio, 2.01 [95% CI, 1.03-3.92]; P = .04; Table 2). When removing from the analysis the lesions to which a pre-PCI FFR value of 0.50 had been inferred, a significant association between ΔFFR and VOCE persisted (hazard ratio, 0.71 [95% CI, 0.56-0.90]; P = .004).
Table 2. Outcomes per Fractional Flow Reserve (ΔFFR) Tertilea.
| End Point | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Lowest Tertile | Middle Tertile | Highest Tertile | Unadjusted | Adjustedb | |
| Lesions, No. | 277 | 282 | 278 | NA | NA |
| Vessel-oriented clinical events | 25 (9.0) | 20 (7.1) | 13 (4.7) | .13 | .01 |
| Deathc | 4 (1.4) | 5 (1.8) | 4 (1.4) | .94 | .69 |
| Myocardial infarction | 5 (1.8) | 6 (2.1) | 4 (1.4) | .83 | .32 |
| Target vessel revascularization | 20 (7.2) | 14 (5.0) | 7 (2.5) | .04 | .002 |
Abbreviation: NA, not applicable.
The lowest tertile is defined by a ΔFFR of 0.18 or less; the middle tertile, by a ΔFFR of 0.19 to 0.30; the highest tertile, by a ΔFFR of 0.31 or more.
The ΔFFR findings were analyzed as a continuous variable in this Cox regression model.
Death is reported per lesion rather than per individual in keeping with previous reports of these data sets.
In a model adjusted for post-PCI FFR values, pre-PCI FFR values, smoking status, history of previous PCI, and positive family history, ΔFFR was the only factor associated with target vessel revascularization (hazard ratio, 0.68 [95% CI, 0.51-0.89]; P = .002) and VOCE (hazard ratio, 0.76 [95% CI, 0.61-0.94]; P = .01). The Figure, B shows the significant association between ΔFFR and the probability of VOCE (hazard ratio, 0.76 [95% CI, 0.61-0.94]; P = .01). When removing from the analysis the lesions to which a pre-PCI FFR value of 0.50 had been inferred, a significant association between ΔFFR and VOCE persisted (hazard ratio, 0.71 [95% CI, 0.56-0.90]; P = .004). Of note, ΔFFR remains a significant factor associated with VOCE when taking into account lesion location, number and length of stents, and presence of tandem stenoses (hazard ratio, 0.78 [95% CI, 0.62-0.97]; P = .02).
The ΔFFR value most closely associated with VOCE was 0.24, with positive and negative likelihood ratios of 1.62 (95% CI, 1.23-2.19) and 0.74 (95% CI, 0.57-0.94), respectively. Among lesions with post-PCI FFR values less than 0.92,3 the rate of VOCEs was significantly higher when ΔFFR was low: 22 occurred in the lowest tertile, 15 in the middle tertile, and 6 in the highest tertile. When taking into account only the FAME 2 results in which patients were treated with second-generation stents, multivariable Cox regression analysis showed that ΔFFR was the only factor significantly associated with VOCEs (hazard ratio, 0.82 [95% CI, 0.73-0.93]; P = .002).
Discussion
This study confirms and quantifies our clinical intuition, namely, that the larger the ΔFFR, the larger the symptomatic improvement. These data extend the findings of the Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina (ORBITA) trial, which showed a physiology-stratified effect of PCI on symptomatic relief,8 to a broader patient population. These data also indicate that the larger the ΔFFR, the lower the rate of VOCEs at 2 years after PCI. Per this analysis, these outcomes are more closely associated with ΔFFR than both pre-PCI FFR and post-PCI FFR values.
Previous Studies
In a small, monocentric study, Kocaman et al9 analyzed 123 consecutive patients with an intermediate lesion of the left anterior descending coronary artery and observed that the major adverse cardiovascular event rate at 3 years was significantly higher when ΔFFR was smaller than 0.10. The results of this study extend these findings to the 3 major coronary arteries, in a larger patient population derived from 2 randomized clinical trials.
In another study based on 220 patients with stenoses, Matsuda et al10 did not find any association between ΔFFR and adverse events. In that study, however, adverse events encompassed nontarget vessel revascularizations and arrhythmias that are not associated with the index artery.
Potential Mechanisms
The potential mechanisms remain largely speculative and multifactorial. First, diffuse coronary atherosclerosis is the most frequent cause of low FFR values after PCI and thus of low ΔFFR. This diffuse involvement has been associated with poor outcomes.11 Second, suboptimal stent deployment and malapposition can contribute to a low post-PCI FFR values and are the main cause of stent failure.12,13 Third, diffuse disease is more likely to be associated with microvascular disease, which can lead to reduced hyperemic flow and relatively high pre-PCI FFR values (that further reduce ΔFFR). Finally, a low FFR value prior to PCI is more likely to be associated with severe symptoms, such that a patient’s perceived improvement will be larger than in the case of a patient with high pre-PCI FFR value. This might contribute to a trend of fewer complaints, less well-controlled angiograms, and finally fewer revascularization procedures.
Limitations
Some limitations have to be accounted for. First, this is a not prespecified post hoc analysis of the randomized clinical trial data. Second, failing to perform a final FFR assessment might introduce a bias. It can be speculated that, at the end of a difficult procedure, the final FFR value was less often measured than after a swift procedure with an excellent angiographic results. Third, pullback with pressure wires was not recorded. Accordingly, it is not possible to distinguish between an in-segment, residual gradient or a diffusely distributed gradient. Finally, in the FAME 2 protocol, operators were allowed to infer an FFR value of 0.50 for a small number of lesions through which the operator did not feel confident advancing a pressure wire.
Excluding these lesions did not change the conclusions of the present analysis. In addition, in this study, the pre-PCI FFR values did not influence the significant association between ΔFFR and VOCEs.
Conclusions
A larger improvement in FFR values after PCI tends to be associated with a more complete symptomatic relief and a lower rate of VOCEs. Measuring FFR before and after PCI provides clinically useful information, because a low ΔFFR appears to be a surrogate marker for a higher risk population. These data might, in the future, be derived from coronary angiography14 when the procedure has been carried out with a regular guide wire.
References
- 1.Tonino PA, Johnson NP. Why Is fractional flow reserve after percutaneous coronary intervention not always 1.0? JACC Cardiovasc Interv. 2016;9(10):1032-1035. doi: 10.1016/j.jcin.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Rimac G, Fearon WF, De Bruyne B, et al. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: a systematic review and meta-analysis. Am Heart J. 2017;183:1-9. doi: 10.1016/j.ahj.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 3.Piroth Z, Toth GG, Tonino PAL, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ Cardiovasc Interv. 2017;10(8):e005233. doi: 10.1161/CIRCINTERVENTIONS.116.005233 [DOI] [PubMed] [Google Scholar]
- 4.Tonino PA, De Bruyne B, Pijls NH, et al. ; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224. doi: 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 5.van Nunen LX, Zimmermann FM, Tonino PA, et al. ; FAME Study Investigators . Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet. 2015;386(10006):1853-1860. doi: 10.1016/S0140-6736(15)00057-4 [DOI] [PubMed] [Google Scholar]
- 6.De Bruyne B, Pijls NH, Kalesan B, et al. ; FAME 2 Trial Investigators . Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease [corrected in N Engl J Med. 2012;367(18):1768]. N Engl J Med. 2012;367(11):991-1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 7.Xaplanteris P, Fournier S, Pijls NHJ, et al. ; FAME 2 Investigators . Five-year outcomes with pci guided by fractional flow reserve. N Engl J Med. 2018;379(3):250-259. doi: 10.1056/NEJMoa1803538 [DOI] [PubMed] [Google Scholar]
- 8.Al-Lamee R, Howard JP, Shun-Shin MJ, et al. Fractional flow reserve and instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation. 2018;138(17):1780-1792. doi: 10.1161/CIRCULATIONAHA.118.033801 [DOI] [PubMed] [Google Scholar]
- 9.Kocaman SA, Sahinarslan A, Arslan U, Timurkaynak T. The delta fractional flow reserve can predict lesion severity and long-term prognosis. Atherosclerosis. 2009;203(1):178-184. doi: 10.1016/j.atherosclerosis.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 10.Matsuda J, Murai T, Kanaji Y, et al. Prevalence and clinical significance of discordant changes in fractional and coronary flow reserve after elective percutaneous coronary intervention. J Am Heart Assoc. 2016;5(12):e004400. doi: 10.1161/JAHA.116.004400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min JK, Dunning A, Lin FY, et al. ; CONFIRM Investigators . Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58(8):849-860. doi: 10.1016/j.jacc.2011.02.074 [DOI] [PubMed] [Google Scholar]
- 12.Adriaenssens T, Joner M, Godschalk TC, et al. ; Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators . Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation. 2017;136(11):1007-1021. doi: 10.1161/CIRCULATIONAHA.117.026788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali ZA, Maehara A, Généreux P, et al. ; ILUMIEN III: OPTIMIZE PCI Investigators . Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388(10060):2618-2628. doi: 10.1016/S0140-6736(16)31922-5 [DOI] [PubMed] [Google Scholar]
- 14.Fearon WF, Achenbach S, Engstrøm T, et al. ; FAST-FFR Study Investigators . Accuracy of fractional flow reserve derived from coronary angiography. Circulation. 2019;139(4):477-484. doi: 10.1161/CIRCULATIONAHA.118.037350 [DOI] [PubMed] [Google Scholar]