Key Points
Question
What is the current burden of cardiovascular disease (CVD) for Chinese residents, and how does it vary across provinces and over time?
Findings
In this analysis of data from the 2016 Global Burden of Disease Study that included the data on mortality, prevalence, and disability-adjusted life-years associated with CVD in mainland China, Hong Kong, and Macao, the relative burden of CVD varied widely at the provincial level. The age-standardized prevalence of CVD increased by 14.7% from 1990 to 2016, but the CVD burden decreased substantially in all provinces and the between-province discrepancy widened over time.
Meaning
The results of this study may help to identify gaps in cardiovascular burden, demonstrating the importance of geo-specific investment in the prevention and control of CVD in China.
This analysis of data from the 2016 Global Burden of Disease Study examines the national and province-level mortality, prevalence, and disability-adjusted life-years associated with cardiovascular disease in China from 1990 to 2016.
Abstract
Importance
Cardiovascular disease (CVD) remains the top cause of death in China. To our knowledge, no consistent and comparable assessments of CVD burden have been produced at subnational levels, and little is understood about the spatial patterns and temporal trends of CVD in China.
Objective
To determine the national and province-level burden of CVD from 1990 to 2016 in China.
Design, Setting, and Participants
Following the methodology framework and analytical strategies used in the 2016 Global Burden of Disease study, the mortality, prevalence, and disability-adjusted life-years (DALYs) of CVD in the Chinese population were examined by age, sex, and year and according to 10 subcategories. Estimates were produced for all province-level administrative units of mainland China, Hong Kong, and Macao.
Exposures
Residence in China.
Main Outcomes and Measures
Mortality, prevalence, and DALYs of CVD.
Results
The annual number of deaths owing to CVD increased from 2.51 million to 3.97 million between 1990 and 2016; the age-standardized mortality rate fell by 28.7%, from 431.6 per 100 000 persons in 1990 to 307.9 per 100 000 in 2016. Prevalent cases of CVD doubled since 1990, reaching nearly 94 million in 2016. The age-standardized prevalence rate of CVD overall increased significantly from 1990 to 2016 by 14.7%, as did rates for ischemic heart disease (19.1%), ischemic stroke (36.6%), cardiomyopathy and myocarditis (23.1%), and endocarditis (26.7%). Substantial reduction in the CVD burden, as measured by age-standardized DALY rate, was observed from 1990 to 2016 nationally, with a greater reduction in women (43.7%) than men (24.7%). There were marked differences in the spatial patterns of mortality, prevalence, and DALYs of CVD overall as well as its main subcategories, including ischemic heart disease, hemorrhagic stroke, and ischemic stroke. The CVD burden appeared to be lower in coastal provinces with higher economic development. The between-province gap in relative burden of CVD increased from 1990 to 2016, with faster decline in economically developed provinces.
Conclusions and Relevance
Substantial discrepancies in the total CVD burden and burdens of CVD subcategories have persisted between provinces in China despite a relative decrease in the CVD burden. Geographically targeted considerations are needed to tailor future strategies to enhance CVD health throughout China and in specific provinces.
Introduction
Cardiovascular disease (CVD) is the largest single contributor to global mortality1 and accounts for more than 40% of deaths in China.2 In 2011, the United Nations formally recognized noncommunicable diseases, including CVD, as a major global health concern and announced a worldwide action plan against these diseases.3 The Sustainable Development Goals (SDGs), released by the United Nations General Assembly in 2015,4 include the target of a reduction in premature mortality owing to noncommunicable diseases by one-third by 2030. In consideration of the SDG goals, the State Council of China subsequently endorsed an important document as a response to disease epidemics, the Medium- to Long-Term Plan for the Prevention and Treatment of Chronic Diseases (2017-2025).5 This plan is aimed at reducing the age-standardized mortality rate of CVD in China by 15% compared with 2015 by 2025. Correspondingly, health authorities in the provincial administrative units of mainland China, all of which are referred to herein as provinces, began efforts to improve health care and reduce CVD risks. A crucial starting point is understanding the levels and trends in the burden of CVD, which is usually measured as incidence, prevalence, mortality, or disability-adjusted life-years (DALYs), to appropriately guide efforts in improving cardiovascular health at both national and subnational levels. However, the fundamental knowledge for decision making remains inadequate. Despite our previous systematic analysis of cause-specific and year-specific mortality by province from 1990 to 2013,2 published studies reporting the burden of CVD are either of limited scope (focusing only on a single subcategory of CVD on a local scale)6,7,8,9,10 or lack analysis of temporal11 and regional12 variations. Systematic evaluation of data collected in vital registries, disease surveillance surveys, health administrative reports, and published reports as well as all available data sources can potentially fill remaining knowledge gaps. The Global Burden of Disease (GBD) study is such an effort, with the aim to continuously produce consistent, transparent, and up-to-date estimates of disease incidence, prevalence, mortality, and other metrics of the disease burden at macro-level (ie, global and national) and meso-level (ie, subnational) geographic scales.13
In close collaboration with the Institute for Health Metrics and Evaluation at the University of Washington in Seattle, the Chinese Center for Disease Control and Prevention joined the 2016 GBD study in conducting a new, comprehensive assessment of morbidity, mortality, and disability patterns from 1990 to 2016 at both national and provincial levels. In this article, we provide new national and subnational estimates of cause-specific mortality, prevalence, and DALYs in CVD, with special attention paid to the related temporal changes and geographic patterns. The results of this study will help to identify gaps in CVD burden and facilitate development of national or subnational responses that can support the health care system in improving CVD health of the Chinese population.
Methods
Framework of the 2016 GBD Study
The 2016 GBD study continuously provides consistent and updated global, regional, and national estimates on the burden of diseases, injuries, and risk factors by integrating all available data related to these.13 The general methods used in the 2016 GBD study, cause-specific methods for CVD, and location-specific methods for China since the 2013 GBD study have been published previously.14,15,16,17,18 Briefly, the 2016 GBD study provided comprehensive and systematic assessments from 1980 to 2016 of age-specific and sex-specific mortality and years of life lost (YLLs) for 264 causes, prevalence and years lived with disability (YLDs) for 328 diseases and injuries, and 84 risk factors in 195 countries and territories. Particularly, subnational assessment in some countries had been introduced since the 2013 GBD study.2 To date, the GBD study has continuously provided subnational analysis for 12 countries.
The ethics committee of the National Center for Chronic and Noncommunicable Disease Control and Prevention of the Chinese Center for Disease Control and Prevention reviewed and approved the study. Informed consent was waived because no identifiable information was included in the analyses. This study complied with the Guidelines for Accurate and Transparent Health Estimates Reporting statement, and the corresponding checklist is presented in section 2 of the eAppendix in the Supplement.
Data Sources for the China Study
Population counts were obtained from the National Bureau of Statistics. Based on the 5 main data sources of our previous publication of the 2013 GBD study,2 mortality data were mainly updated using the new National Mortality Surveillance System, Vital Registration, the Notifiable Infectious Disease Reporting System from 2014 to 2015,15,19 and Maternal and Child Surveillance data.20 New systematic reviews were conducted to identify published scientific reports, unpublished registry data, and health system administrative data on the incidence, prevalence, and distribution of sequelae related to CVD causes. Data from new rounds of important nationwide surveys, such as the Fifth National Health Service Survey21 and the Chronic Disease and Risk Factor Surveillance System22 from 2013 to 2014, were also updated in the 2016 GBD study.
Statistical Analysis
In section 1 of the eAppendix in the Supplement, we provide in detail the list, definitions, and corresponding codes (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10]) of CVD subcategories. Standard methods of the 2016 GBD study were used to estimate mortality, prevalence, YLLs, YLDs, DALYs with 95% uncertainty intervals (UIs), and their time series of CVD (section 1 of the eAppendix in the Supplement). In brief, all-cause and cause-specific mortality of CVD were estimated using the Cause of Death Ensemble model.15 Causes of death that should not be identified as underlying causes of death but have been recorded as the underlying cause of death on death certificates, also known as garbage codes, were redistributed to appropriate ICD-10 codes prior to modeling. A Bayesian meta-regression tool was used to estimate prevalence for each cause and the distribution for severity of its sequalae16; regression models were used to adjust data that did not follow the standard definition for each cause in the direction of case definition–based data.23 Years lived with disability for a specific cause was calculated by multiplying its prevalence with the corresponding disability weights, which have been estimated in several previous worldwide surveys.24,25,26 Years of life lost was calculated by multiplying observed deaths for a specific age by global age-specific reference life expectancy.15 Disability-adjusted life-years for any corresponding subpopulation of a specific cause was the sum of the corresponding YLDs and YLLs17; 95% UIs capturing both random and systematic error in statistical modeling were generated for all estimates. The present study defines significant differences between any 2 estimates as nonoverlap of their 95% UIs. We used the sociodemographic index,27 a composite metric calculated from income per capita, educational attainment, and total fertility rate, to explore the association of CVD burden with socioeconomic development across provinces in China.
Results
CVD Deaths and Mortality Rate
The number of deaths owing to CVD increased from 2.51 million (95% UI, 2.42 million to 2.67 million) in 1990 to 3.97 million (95% UI, 3.88 million to 4.08 million) in 2016 (Table 1). The largest relative growth was seen in peripheral artery disease (PAD) (268.7%), followed by atrial fibrillation and flutter (184.9%), ischemic heart disease (IHD) (184.1%), ischemic stroke (IS) (83.8%), aortic aneurysm (AA) (72.4%), and endocarditis (45.8%); significant decline was observed only in rheumatic heart disease (RHD), which decreased nearly by half (−47.9%).
Table 1. All-Age Deaths and Age-Standardized Mortality Rates for Selected Cardiovascular Disease (CVD) Subcategories and Their Percentage Change by Sex in China, 1990-2016.
| Subcategory | All-Age Deaths, No. in Thousands (95% UI) | Age-Standardized Mortality Rate (95% UI), per 100 000 | ||||
|---|---|---|---|---|---|---|
| 1990 | 2016 | Change, % | 1990 | 2016 | Change, % | |
| All CVD | ||||||
| Male | 1304 (1258-1358) | 2336 (2263-2402) | 79.2 | 479.7 (466.0-495.9) | 383.0 (371.8-393.1) | −20.2 |
| Female | 1204 (1142-1340) | 1638 (1572-1700) | 36.1 | 391.2 (370.5-437.9) | 241.7 (232.1-250.6) | −38.2 |
| Total | 2508 (2422-2672) | 3975 (3878-4076) | 58.5 | 431.6 (416.9-462.7) | 307.9 (300.0-315.5) | −28.7 |
| Rheumatic heart disease | ||||||
| Male | 52 (47-61) | 31 (29-33) | −40.9 | 16.8 (15.2-19.4) | 4.8 (4.5-5.2) | −71.2 |
| Female | 85 (73-103) | 40 (37-44) | −52.2 | 24.0 (20.6-29.6) | 5.6 (5.1-6.1) | −76.6 |
| Total | 137 (123-162) | 71 (67-76) | −47.9 | 20.6 (18.4-24.5) | 5.2 (4.9-5.6) | −74.5 |
| Ischemic heart disease | ||||||
| Male | 325 (305-360) | 981 (945-1013) | 201.8 | 125.3 (118.2-138.0) | 166.7 (161.0-171.9) | 33.1 |
| Female | 281 (260-325) | 742 (707-780) | 163.7 | 97.0 (89.6-113.0) | 112.2 (107.0-117.9) | 15.7 |
| Total | 606 (571-677) | 1723 (1670-1778) | 184.1 | 110.0 (103.4-123.6) | 137.7 (133.7-142.1) | 25.3 |
| Ischemic stroke | ||||||
| Male | 210 (197-227) | 444 (424-463) | 110.9 | 83.3 (77.8-90.3) | 74.0 (70.7-77.4) | −11.2 |
| Female | 186 (171-217) | 285 (265-304) | 53.1 | 62.6 (57.3-73.5) | 42.1 (39.2-45.0) | −32.7 |
| Total | 396 (372-440) | 729 (698-759) | 83.8 | 71.7 (67.0-80.4) | 56.9 (54.4-59.5) | −20.7 |
| Hemorrhagic stroke | ||||||
| Male | 536 (504-593) | 668 (642-695) | 24.7 | 182.6 (171.8-201.4) | 99.7 (95.8-103.8) | −45.4 |
| Female | 455 (415-535) | 393 (367-417) | −13.5 | 140.1 (127.9-166.1) | 54.6 (50.8-57.9) | −61.0 |
| Total | 991 (931-1120) | 1061 (1023-1102) | 7.2 | 159.6 (149.6-182.2) | 76.0 (73.1-79.0) | −52.4 |
| Hypertensive heart disease | ||||||
| Male | 102 (53-120) | 133 (89-148) | 30.2 | 42.7 (23.3-49.8) | 23.8 (15.6-26.4) | −44.2 |
| Female | 112 (62-130) | 108 (68-133) | −3.9 | 39.5 (22.8-45.3) | 16.4 (10.3-20.2) | −58.4 |
| Total | 215 (140-247) | 241 (168-278) | 12.3 | 41.1 (27.6-46.8) | 19.8 (13.8-22.8) | −51.7 |
| Cardiomyopathy and myocarditis | ||||||
| Male | 7 (7-13) | 18 (13-20) | 102.8 | 2.2 (1.9-3.4) | 2.9 (2.1-3.2) | 29.0 |
| Female | 7 (6-13) | 12 (8-14) | 72.1 | 1.9 (1.5-3.2) | 1.9 (1.2-2.2) | 3.2 |
| Total | 16 (14-24) | 30 (23-33) | 88.9 | 2.0 (1.7-3.0) | 2.4 (1.8-2.6) | 15.7 |
| Atrial fibrillation and flutter | ||||||
| Male | 5 (4-6) | 15 (12-18) | 222.2 | 3.0 (2.3-3.9) | 3.2 (2.5-3.9) | 4.9 |
| Female | 9 (7-12) | 23 (19-29) | 165.5 | 3.8 (2.9-5.1) | 3.7 (3.0-4.6) | −2.9 |
| Total | 13 (10-17) | 38 (31-47) | 184.9 | 3.5 (2.7-4.6) | 3.5 (2.8-4.3) | −1.2 |
| Aortic aneurysm | ||||||
| Male | 5 (5-6) | 9 (8-10) | 76.7 | 1.8 (1.6-2.0) | 1.4 (1.3-1.5) | −21.5 |
| Female | 2 (2-3) | 4 (4-4) | 63.4 | 0.8 (0.7-0.9) | 0.6 (0.5-0.6) | −28.1 |
| Total | 8 (7-9) | 13 (12-14) | 72.4 | 1.2 (1.1-1.4) | 1.0 (0.9-1.0) | −23.0 |
| Peripheral artery disease | ||||||
| Male | 0 (0-1) | 1 (1-2) | 250.4 | 0.2 (0.1-0.3) | 0.2 (0.2-0.3) | 36.9 |
| Female | 0 (0-0) | 1 (1-1) | 297.9 | 0.1 (0.1-0.2) | 0.1 (0.1-0.2) | 55.1 |
| Total | 1 (0-1) | 2 (2-3) | 268.7 | 0.1 (0.1-0.2) | 0.2 (0.2-0.2) | 46.6 |
| Endocarditis | ||||||
| Male | 2 (1-3) | 3 (3-5) | 60.3 | 0.7 (0.5-0.9) | 0.6 (0.5-0.8) | −16.1 |
| Female | 2 (2-2) | 2 (2-3) | 29.4 | 0.5 (0.5-0.7) | 0.4 (0.3-0.4) | −30.9 |
| Total | 4 (3-5) | 6 (5-7) | 45.8 | 0.6 (0.5-0.7) | 0.5 (0.4-0.6) | −22.4 |
| Other CVD and circulatory disease | ||||||
| Male | 57 (22-74) | 33 (27-57) | −41.6 | 21.2 (7.7-27.9) | 5.7 (4.6-9.8) | −73.1 |
| Female | 65 (27-77) | 27 (23-52) | −57.9 | 21.0 (8.9-25.3) | 4.1 (3.5-7.8) | −80.6 |
| Total | 122 (58-145) | 61 (53-95) | −50.3 | 21.2 (9.9-25.3) | 4.8 (4.2-7.6) | −77.3 |
Abbreviation: UI, uncertainty interval.
The age-standardized mortality rate fell by 28.7% from 431.6 per 100 000 persons in 1990 to 307.9 per 100 000 in 2016 (Table 1). There was significant reduction in the age-standardized mortality rate in 5 of the 10 subcategories, including RHD, IS, hemorrhagic stroke (HS), hypertensive heart disease (HHD), and AA; only IHD saw a significant increase from 1990 to 2016.
Ischemic heart disease, IS, and HS were the top 3 causes of CVD deaths in 2016 (age-standardized mortality rate: 137.7 per 100 000, 56.9 per 100 000, and 76.0 per 100 000, respectively), accounting for two-thirds of total CVD deaths (Table 1). A much higher mortality rate among men than women was observed in IHD, IS, HS, AA, and endocarditis. Similarly, higher mortality among men than women was observed for IHD, IS, and HS in each province (eTables 1.1-1.4 of the eAppendix in the Supplement).
eFigure 1 of the eAppendix in the Supplement shows maps of the mortality rate for CVD overall and selected subcategories of CVD (ie, IHD, IS, and HS) in 2016. There was wide geographic variation in the age-standardized mortality rate of overall CVD (range, 99.4 to 578.2 per 100 000), IHD (range, 51.4 to 233.2 per 100 000), IS (range, 11.1 to 92.1 per 100 000), and HS (range, 13.3 to 259.4 per 100 000) among provinces. The exact provincial estimates of the total deaths, age-standardized mortality rate, and 95% UIs for these causes are presented in eTables 1.1-1.4 of the eAppendix in the Supplement.
CVD Prevalence
There were an estimated 93.8 million prevalent cases of CVD overall during 2016 in China, more than twice that of 1990 (40.6 million). All subcategories saw significant increases in prevalent cases from 1990 to 2016. The age-standardized prevalence rate for CVD overall increased significantly by 14.7% from 1990 to 2016, as did that of IHD (19.1%), IS (36.6%), cardiomyopathy and myocarditis (23.1%), and endocarditis (26.7%). The only significant reduction was observed in the age-standardized prevalence rate of RHD (−16.2%) (Table 2).
Table 2. All-Age Prevalence and Age-Standardized Prevalence Rates for Selected Cardiovascular Disease (CVD) Subcategories and Their Percentage Change by Sex in China, 1990-2016.
| Subcategorya | All-Age Prevalence, No. in Thousands (95% UI) | Age-Standardized Prevalence Rate (95% UI), per 100 000 | ||||
|---|---|---|---|---|---|---|
| 1990 | 2016 | Change, % | 1990 | 2016 | Change, % | |
| All CVD | ||||||
| Male | 18 959 (18 109-19 776) | 44 108 (42 095-46 097) | 132.7 | 4958.6 (4753.8-5154.7) | 5779.3 (5536.5-6017.5) | 16.6 |
| Female | 21 614 (20 596-22 586) | 49 700 (47 214-52 148) | 129.9 | 5550.4 (5298.7-5795.1) | 6286.9 (5990.4-6575.9) | 13.3 |
| Total | 40 573 (38 699-42 362) | 93 808 (89 384-98 108) | 131.2 | 5265.6 (5036.2-5484.5) | 6037.0 (5773.7-6297.4) | 14.7 |
| Rheumatic heart disease | ||||||
| Male | 642 (582-707) | 757 (689-837) | 17.8 | 112.7 (102.8-123.2) | 96.1 (87.3-106.7) | −14.7 |
| Female | 895 (824-968) | 1098 (1016-1188) | 22.8 | 173.7 (160.9-187.6) | 143.7 (132.2-156.1) | −17.3 |
| Total | 1537 (1411-1674) | 1855 (1709-2020) | 20.7 | 142.8 (132.2-154.5) | 119.6 (109.7-130.6) | −16.2 |
| Ischemic heart disease | ||||||
| Male | 4668 (4443-4888) | 11 410 (10 791-12 004) | 144.4 | 1295.4 (1235.6-1354.6) | 1527.6 (1449.8-1604.6) | 17.9 |
| Female | 4580 (4361-4805) | 11 494 (10 894-12 089) | 151.0 | 1229.4 (1173.1-1287.9) | 1484.7 (1409.3-1556.0) | 20.8 |
| Total | 9248 (8802-9698) | 22 904 (21 735-24 037) | 147.7 | 1265.5 (1208.1-1325.1) | 1507.4 (1432.9-1579.3) | 19.1 |
| Ischemic stroke | ||||||
| Male | 4408 (4017-4828) | 12 372 (11 035-13 879) | 180.7 | 1128.9 (1024.5-1243.5) | 1608.6 (1434.9-1806.7) | 42.5 |
| Female | 4395 (4042-4793) | 11 725 (10 407-13 072) | 166.8 | 1117.8 (1025.8-1217.2) | 1467.4 (1305.9-1637.7) | 31.3 |
| Total | 8803 (8083-9611) | 24 098 (21 465-26 881) | 173.8 | 1123.2 (1023.9-1230.6) | 1533.9 (1373.9-1712.5) | 36.6 |
| Hemorrhagic stroke | ||||||
| Male | 1411 (1298-1545) | 2934 (2643-3279) | 108.0 | 367.5 (335.5-403.2) | 374.4 (337.3-416.6) | 1.9 |
| Female | 1370 (1263-1488) | 2618 (2363-2926) | 91.1 | 348.9 (321.0-379.2) | 330.5 (298.8-368.3) | −5.3 |
| Total | 2781 (2565-3034) | 5552 (5008-6191) | 99.7 | 357.2 (327.6-390.3) | 352.3 (318.0-391.9) | −1.4 |
| Hypertensive heart disease | ||||||
| Male | 652 (557-757) | 1809 (1544-2108) | 177.5 | 204.2 (176.1-236.7) | 252.2 (215.8-292.0) | 23.5 |
| Female | 1075 (932-1234) | 2960 (2582-3397) | 175.5 | 307.8 (267.7-352.3) | 384.1 (336.2-442.8) | 24.8 |
| Total | 1726 (1487-1989) | 4769 (4134-5491) | 176.2 | 259.7 (225.0-299.2) | 321.7 (280.1-370.9) | 23.9 |
| Cardiomyopathy and myocarditis | ||||||
| Male | 141 (127-157) | 237 (216-259) | 68.0 | 26.2 (24.1-28.7) | 32.8 (30.0-35.8) | 25.2 |
| Female | 273 (248-298) | 446 (409-486) | 63.6 | 51.1 (47.2-55.2) | 62.6 (57.5-67.8) | 22.5 |
| Total | 414 (377-452) | 683 (627-745) | 65.1 | 38.4 (35.5-41.5) | 47.3 (43.5-51.2) | 23.1 |
| Atrial fibrillation and flutter | ||||||
| Male | 1611 (1406-1845) | 4049 (3543-4652) | 151.3 | 531.3 (462.8-608.0) | 582.7 (506.8-669.7) | 9.7 |
| Female | 1922 (1675-2211) | 4695 (4098-5391) | 144.2 | 566.0 (493.4-651.7) | 625.9 (544.2-720.5) | 10.6 |
| Total | 3533 (3091-4062) | 8744 (7642-10 052) | 147.5 | 549.4 (477.5-631.2) | 604.5 (525.4-697.2) | 10.0 |
| Peripheral artery disease | ||||||
| Male | 3595 (3102-4185) | 8940 (7743-10 400) | 148.7 | 1107.3 (970.2-1269.9) | 1199.3 (1053.4-1377.7) | 8.3 |
| Female | 5690 (4934-6629) | 13 178 (11 422-15 307) | 131.6 | 1623.0 (1425.6-1873.0) | 1667.8 (1458.0-1925.4) | 2.4 |
| Total | 9285 (8030-10 809) | 22 118 (19 188-25 702) | 138.2 | 1381.6 (1210.7-1592.6) | 1440.6 (1262.7-1657.4) | 4.3 |
| Endocarditis | ||||||
| Male | 10 (9-11) | 22 (20-24) | 111.1 | 2.4 (2.2-2.6) | 3.0 (2.8-3.3) | 26.1 |
| Female | 8 (7-9) | 17 (15-19) | 112.5 | 1.8 (1.7-2.0) | 2.3 (2.1-2.6) | 26.5 |
| Total | 18 (17-20) | 39 (35-43) | 111.7 | 2.1 (1.9-2.3) | 2.7 (2.4-2.9) | 26.7 |
| Other CVD and circulatory disease | ||||||
| Male | 3475 (2970-4049) | 6823 (5895-7931) | 96.3 | 722.3 (627.2-833.3) | 899.2 (782.2-1036.8) | 24.5 |
| Female | 3613 (3109-4203) | 7819 (6680-9129) | 116.4 | 777.2 (674.0-899.1) | 1002.2 (862.9-1160.2) | 28.9 |
| Total | 7089 (6134-8256) | 14 642 (12 631-17 022) | 106.6 | 749.5 (651.3-865.4) | 952.1 (827.5-1099.2) | 27.0 |
Abbreviation: UI, uncertainty interval.
Nonfatal estimates were not produced for aortic aneurysm in the 2016 Global Burden of Disease study.
In 2016, IS had the highest age-standardized prevalence rate (1534 per 100 000), followed by IHD (1507 per 100 000) and PAD (1441 per 100 000). Rates for other subcategories in 2016 were less than 500 per 100 000 except for atrial fibrillation and flutter (604 per 100 000). In contrast to mortality, the prevalence rate was significantly higher among women than men for RHD, HHD, and PAD (Table 2).
eFigure 2 of the eAppendix in the Supplement presents the geographical distribution of the age-standardized prevalence rate of CVD overall and its main subcategories in 2016. Regarding overall CVD, IHD, and IS, high age-standardized prevalence rates were similarly concentrated in northern and northeastern provinces. However, the spatial pattern for HS differed. The highest estimated rates (more than 400 per 100 000) were in Tibet, Qinghai, Hebei, and Henan; the lowest rates (no more than 300 per 100 000) were in coastal provinces, such as Shanghai, Hong Kong, Macao, and Guangdong. eTables 2.1-2.4 of the eAppendix in the Supplement additionally present the estimates of total prevalent cases, age-standardized prevalence rate, and 95% UIs by province.
Temporal Trends of Burden of CVD From 1990 to 2016
Despite a notable rise in the absolute CVD burden, substantial reduction (−33.3%) in the age-standardized rate of CVD DALYs for both sexes combined was observed from 1990 to 2016, with greater reduction among women (−43.7%) than men (−24.7%). The largest percentage reduction was observed for RHD (−77.6%), followed by HHD (−54.8%), HS (−52.6%), AA (−25.8%), and IS (−15.8%). The extent of decrease across causes varied by sex, with a slower decline in men than women (Table 3).
Table 3. All-Age Disability-Adjusted Life-Years (DALYs) and Age-Standardized DALY Rates for Selected Cardiovascular Disease (CVD) Subcategories and Their Percentage Change by Sex in China, 1990-2016.
| Subcategory | All-Age DALYs, No. in Thousands (95% UI) | Age-Standardized DALY Rate (95% UI), per 100 000 | ||||
|---|---|---|---|---|---|---|
| 1990 | 2016 | Change, % | 1990 | 2016 | Change, % | |
| All CVD | ||||||
| Male | 31 811 (30 478-33 367) | 48 292 (46 348-50 152) | 51.8 | 8678.0 (8347.2-9049.2) | 6535.1 (6282.2-6778.2) | −24.7 |
| Female | 26 601 (25 266-29 039) | 29 814 (28 237-31 475) | 12.1 | 7018.1 (6665.8-7695.2) | 3949.2 (3740.9-4165.2) | −43.7 |
| Total | 58 411 (56 154-62 010) | 78 106 (74 991-81 141) | 33.7 | 7818.6 (7526.0-8312.4) | 5217.2 (5014.5-5417.2) | −33.3 |
| Rheumatic heart disease | ||||||
| Male | 1521 (1363-1798) | 685 (638-739) | −55.0 | 356.8 (321.0-417.2) | 91.5 (85.4-98.5) | −74.4 |
| Female | 2372 (2084-2853) | 869 (794-944) | −63.4 | 548.1 (480.0-663.4) | 111.3 (101.7-120.7) | −79.7 |
| Total | 3894 (3523-4575) | 1555 (1467-1649) | −60.1 | 452.0 (407.8-533.1) | 101.5 (95.8-107.5) | −77.6 |
| Ischemic heart disease | ||||||
| Male | 7747 (7242-8691) | 18 674 (17 905-19 366) | 141.0 | 2122.3 (1992.2-2355.3) | 2543.5 (2443.7-2630.8) | 19.9 |
| Female | 5600 (5182-6327) | 10 958 (10 334-11 555) | 95.7 | 1524.7 (1411.8-1734.7) | 1477.2 (1396.1-1555.5) | −3.1 |
| Total | 13 348 (12 571-14 779) | 29 633 (28 646-30 596) | 122.0 | 1818.1 (1715.4-2018.6) | 2002.1 (1938.0-2069.1) | 10.1 |
| Ischemic stroke | ||||||
| Male | 4863 (4465-5266) | 9673 (8942-10 460) | 98.9 | 1469.6 (1356.7-1590.2) | 1347.7 (1250.8-1450.6) | −8.3 |
| Female | 4125 (3728-4669) | 6543 (5770-7320) | 58.6 | 1153.8 (1048.5-1309.9) | 862.3 (764.2-960.3) | −25.3 |
| Total | 8989 (8267-9836) | 16 216 (14 726-17 716) | 80.4 | 1301.1 (1201.4-1422.8) | 1095.9 (999.3-1193.2) | −15.8 |
| Hemorrhagic stroke | ||||||
| Male | 12 947 (12 167-14 363) | 14 613 (14 037-15 171) | 12.9 | 3444.2 (3241.7-3804.9) | 1877.1 (1805.0-1950.6) | −45.5 |
| Female | 9968 (9176-11 509) | 7795 (7300-8244) | −21.8 | 2605.0 (2393.8-3029.1) | 989.5 (926.2-1047.8) | −62.0 |
| Total | 22 915 (21 588-25 606) | 22 408 (21 645-23 221) | −2.2 | 3010.8 (2838.1-3378.5) | 1427.3 (1376.4-1480.4) | −52.6 |
| Hypertensive heart disease | ||||||
| Male | 2095 (1083-2459) | 2279 (1594-2519) | 8.8 | 642.7 (342.7-751.1) | 330.0 (226.4-364.6) | −48.7 |
| Female | 2012 (1043-2366) | 1658 (1146-2046) | −17.6 | 578.1 (311.7-673.8) | 225.6 (154.4-277.6) | −61.0 |
| Total | 4107 (2624-4761) | 3937 (2880-4511) | −4.2 | 609.9 (398.7-703.2) | 276.0 (200.4-316.3) | −54.8 |
| Cardiomyopathy and myocarditis | ||||||
| Male | 425 (361-655) | 523 (404-586) | 23.2 | 78.7 (67.4-118.9) | 76.8 (59.4-86.3) | −2.4 |
| Female | 351 (281-627) | 320 (229-361) | −8.8 | 67.5 (54.6-119.6) | 51.2 (36.6-57.4) | −24.1 |
| Total | 776 (660-1139) | 843 (689-920) | 8.7 | 73.3 (62.8-107.4) | 64.1 (52.5-67.0) | −12.5 |
| Atrial fibrillation and flutter | ||||||
| Male | 192 (144-250) | 494 (374-637) | 157.3 | 70.9 (54.1-90.6) | 75.8 (58.1-96.4) | 6.9 |
| Female | 257 (197-330) | 615 (472-788) | 139.3 | 81.5 (63.3-104.7) | 84.7 (65.6-108.0) | 4.0 |
| Total | 449 (339-582) | 1109 (848-1424) | 147.0 | 76.9 (59.2-98.4) | 80.5 (62.1-102.5) | 4.6 |
| Aortic aneurysm | ||||||
| Male | 124 (111-146) | 195 (181-214) | 57.4 | 32.4 (29.1-37.6) | 25.1 (23.3-27.4) | −22.6 |
| Female | 53 (45-62) | 69 (62-75) | 29.8 | 13.6 (11.5-15.8) | 8.9 (8.0-9.6) | −34.5 |
| Total | 177 (160-205) | 264 (246-285) | 49.1 | 22.7 (20.6-26.2) | 16.9 (15.7-18.1) | −25.8 |
| Peripheral artery disease | ||||||
| Male | 25 (15-40) | 56 (36-86) | 127.6 | 8.6 (5.1-14.0) | 8.3 (5.4-12.8) | −3.7 |
| Female | 36 (18-62) | 71 (39-121) | 96.2 | 11.3 (5.8-19.4) | 9.8 (5.4-16.7) | −13.0 |
| Total | 61 (34-101) | 128 (76-207) | 108.9 | 10.1 (5.6-17.0) | 9.1 (5.4-14.9) | −10.0 |
| Endocarditis | ||||||
| Male | 85 (54-103) | 83 (67-118) | −1.7 | 17.4 (11.3-20.8) | 12.6 (10.3-18.1) | −27.8 |
| Female | 73 (58-96) | 47 (42-58) | −35.5 | 15.0 (12.8-19.4) | 7.4 (6.6-9.5) | −50.9 |
| Total | 157 (122-183) | 130 (113-168) | −17.3 | 16.2 (12.7-18.6) | 10.0 (8.8-13.2) | −38.1 |
| Other CVD and circulatory disease | ||||||
| Male | 1786 (910-2204) | 1015 (825-1438) | −43.2 | 434.4 (203.5-546.3) | 146.8 (120.0-206.8) | −66.2 |
| Female | 1753 (847-2060) | 869 (697-1263) | −50.4 | 419.7 (197.9-494.4) | 121.3 (98.4-177.5) | −71.1 |
| Total | 3539 (2014-4114) | 1884 (1551-2560) | −46.8 | 427.5 (229.5-499.4) | 133.9 (111.4-182.4) | −68.7 |
Abbreviation: UI, uncertainty interval.
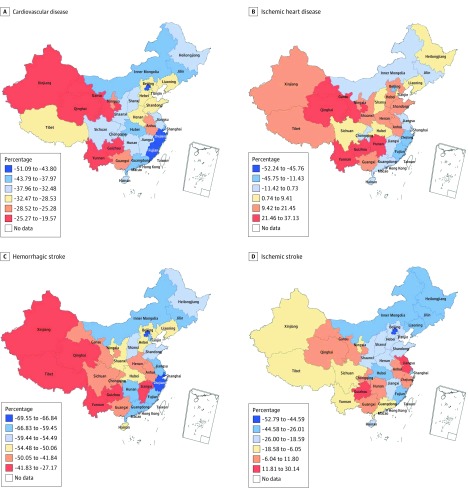
Declines in the age-standardized DALY rate for CVD overall and HS were observed in every province of China from 1990 to 2016; a few provinces saw increased rates of IHD and IS (Figure 1). Improvement in the burden of disease degraded roughly from eastern to western provinces for CVD and its 3 main subcategories. The largest decreases in the disease burden for all CVD combined, IHD, HS, and IS were found in coastal provinces with more developed economies, such as Macao, Beijing, Hong Kong, Fujian, and Shanghai. In contrast, western provinces, such as Tibet, Xinjiang, Qinghai, Yunnan, and Guizhou, had far less decline in the age-standardized DALY rate for CVD and HS and even experienced an increased burden for IHD. We saw substantial reduction in CVD burden in northern provinces, areas heavily affected by IHD and IS. For example, a greater than 30% reduction in age-standardized DALY rate of CVD was found in Jilin, Inner Mongolia, Heilongjiang, and Liaoning. Notably, Jiangsu and Zhejiang, 2 coastal provinces with well-developed economies, experienced increased age-standardized DALY rates of IS.
Figure 1. Map of Age-Standardized Percentage Change in Disability-Adjusted Life-Year Rates for Cardiovascular Disease, Ischemic Heart Disease, Hemorrhagic Stroke, and Ischemic Stroke From 1990 to 2016.
CVD Burden and Spatial Pattern by Selected Causes in 2016
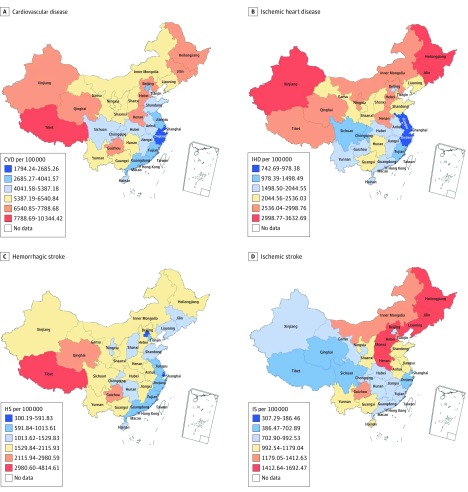
There was wide geographic variation in the age-standardized CVD DALY rate among provinces in 2016 (Figure 2A), ranging from 1794 to 10 344 DALYs per 100 000 persons. Higher burdens were seen in western and northeastern provinces and lower burdens in provinces along the coast from the Bohai Gulf to the South China Sea. A marked north-south discrepancy was observed in the age-standardized DALY rate for IHD (Figure 2B). Of note, the IHD rate was particularly high in northeastern and northwestern border provinces, including Heilongjiang, Jilin, and Xinjiang, nearly 4-fold that of provinces with the lowest burdens, such as Shanghai, Hong Kong, and Zhejiang. The HS burden varied widely across provinces, from 300 to more than 4800 DALYs per 100 000 (Figure 2C). This rate was much lower in eastern coastal provinces; western provinces, including Tibet, Qinghai, Guizhou, and Xinjiang, had the highest burden. Northeastern and northern provinces had the highest age-standardized DALY rates for IS, and western and southeastern provinces had the lowest rates (Figure 2D).
Figure 2. Map of Age-Standardized Disability-Adjusted Life-Year Rates for Cardiovascular Disease (CVD), Ischemic Heart Disease (IHD), Hemorrhagic Stroke (HS), and Ischemic Stroke (IS) in 2016.
Because there is substantial interest in various indexes of CVD disease burden and its main subcategories (ie, IHD, HS, and IS) by different age groups, sex, and provinces, we additionally determined sex-specific and province-specific estimates for the absolute number and age-standardized rate of mortality, prevalence, YLL, YLD, and DALY (eTables 1.1-5.4 of the eAppendix in the Supplement). Percentages of all 10 subcategories of CVD in 2016 by sex and age and by sex and province were also determined for mortality, prevalence, YLL, YLD, and DALY (eFigures 3.1-12.3 of the eAppendix in the Supplement). We used maps and scatterplots to visualize the association of CVD burden with sociodemographic index across provinces (eFigures 13.1-13.4 of the eAppendix in the Supplement).
Discussion
Herein, we present an up-to-date overview of the CVD burden of disease in China, with particular attention paid to temporal trends and spatial patterns. These results demonstrate the importance of geo-specific investment in the prevention and control of CVD in China.
Mortality
As a response to the United Nations SDGs for 2030, the Chinese government issued a plan to reduce CVD mortality by 15% compared with 2015 by 2025.5 From 1990 to 2016, the age-standardized mortality rate of CVD declined by 25% from that before plan implementation. However, it is unreasonable to presume that this declining trend will extend to 2030 and that the SDG goal will be achieved without reinforcing public health interventions. In our previous simulated analysis,28 the one-third reduction target of CVD would only be reached if targets for risk factor intervention were reached beforehand, as proposed in the World Health Organization Global Monitoring Framework.29
Declines in the age-standardized mortality rates of IS and HS accounted for most of the improvement in CVD mortality, offsetting the rise in IHD. Declines in the IS and HS mortality rates were confirmed in a 2017 nationwide retrospective survey on cerebrovascular diseases.11 Factors contributing to these declines include improved health care coverage, upgraded medical technology, an improved public health environment on stroke prevention by the government,30,31 and a decreased predominance of HS over IS.11,32 However, despite these improvements, the age-standardized mortality rate for IHD has continued to increase. The China PEACE study33 reported that the admission rate for ST-segment elevation myocardial infarction grew rapidly from 2001 to 2011, but underuse of guideline-recommended therapies (eg, β-blockers and angiotensin-converting enzyme inhibitors) and use of therapies with unknown effectiveness remained common and had not significantly improved. Inadequate health care professional knowledge, structural inadequacies of care systems, and withdrawal from treatment at terminal status owing to affordability or cultural factors are also responsible for the increased IHD mortality rate.34,35
Prevalence
Reasons for the increased prevalence of CVD are manifold. Besides the 2 key drivers, ie, population growth and aging, other primary drivers include improved medical techniques and cardiovascular care, prolonged life expectancy, and a concurrent declining CVD mortality.2 Increased CVD prevalence can also be partly explained by the rising incidence of stroke11,36 and IHD33 as well as increased lifestyle and metabolic risk factors among younger populations.22,37 An astonishing fact is the current extremely low proportion (0.2%) of ideal cardiovascular health among Chinese adults,38 which calls for urgent responses and targeted public health strategies.
Health care and treatment for the large number of patients with CVD has caused severe economic burden. It was reported that total expenses for cardiovascular hospitalization have increased rapidly since 2004, with an inflation-adjusted annual rate of increase of more than 20%, much faster than the increase in gross domestic product. In 2016, the cost of hospitalization for IS, HS, and acute myocardial infarction was as high as US $13.7 billion.39
We found that nearly one-quarter of individuals living with CVD had at least 1 kind of PAD in 2016. The predominant PAD, eg, lower-extremity atherosclerotic disease and carotid atherosclerosis, shares similar risk factors with arteriosclerotic CVD. In the Chinese population, a reported 30% of patients with IS and 25% of patients with IHD have comorbid lower-extremity atherosclerotic disease.40,41 Peripheral artery disease can also affect the development and outcome of CVD. The lower ankle-brachial index can be used to detect increased risk of all-cause mortality and cardiovascular mortality.42 Carotid intima-media thickness, total area of plaques, number of plaques, and number of segments with plaque are all associated with IHD incidence43; however, PAD is generally neglected in China. Even in Beijing, a city rich in medical resources, only 6% of individuals with PAD have been diagnosed.44 Special attention and greater efforts should be paid to secondary prevention among the very large population with PAD.
Burden of Disease
A nearly 6-fold difference in the total burden of CVD persists among provinces despite marked improvements in CVD burden. Sharp differences in spatial patterns can be seen between the IHD, HS, and IS burdens. Efforts to summarize the causes of these wide variations mainly arise from observations of disparities in lifestyle and metabolic risk factors22,45,46 as well as studies on gaps in cardiovascular care.33,47,48 The CVD burden has declined among all provinces, but the most rapid decline has occurred at the highest levels of development, similar to findings from a global analysis of CVD burden.23 This suggests a deteriorating balance in cardiovascular health within China and demonstrates the importance of increased investment in prevention and treatment of CVD in certain provinces.
Implications
Controlling lifestyle and metabolic risk factors of CVD, reducing their geographical inequity, and increasing the quality of cardiovascular care should be critical points in addressing the daunting CVD burden. Simulation analysis has shown that more than 700 000 deaths among individuals aged 30 to 70 years could be avoided if CVD risk factors were adequately controlled, including smoking, physical inactivity, high body mass index, high fasting glucose levels, high total cholesterol levels, and high systolic blood pressure.28 National surveillance on risk factors of noncommunicable diseases in the past decade shows that compared with residents from eastern China, their counterparts in the west consistently had a higher prevalence of smoking, consumed more salt, had poorer control of hypertension and hyperglycemia, and were less physically active.22,49,50 Besides public health interventions targeting risk factors, efforts on the clinical side are also needed, such as promoting guideline-recommended therapies and use of therapies with evidence-based effectiveness.
The widening gap in health care resources between eastern and western China is another driver of the great disparity in the CVD burden across provinces. Although this is considered one of the key challenges that China must tackle in ongoing health care reform, only slight progress has been seen in the past decade. Further research is needed to better understand other potential reasons for the differences in cause-specific spatial patterns.
Strengths and Limitations
To our knowledge, this study provides the most comprehensive estimates of CVD burden in China at national and provincial levels. The GBD study uses unified and standard methodology involving the most up-to-date advances in data analytical techniques and breakthroughs in epidemiology, making these estimates highly comparable across provinces and time. The heterogeneity across provinces and their temporal trends are perhaps the most important observation regarding the patterns of CVD in China.
Our study has limitations. First, as part of the GBD study, all limitations of the GBD methodology affect this study, which have been described previously14,15,16,17,23,26; herein, we briefly describe some of the most important ones. The GBD study cannot capture the most recent changes in health status owing to time lags in health information reporting by national authorities. Because of the link between death and prevalence, the correlation between uncertainties in YLD and YLL have not been fully explained in the present GBD study, which might cause underestimated uncertainty for DALYs. Comorbidities complicate accuracy in the redistribution of garbage codes and estimation of YLD. Second, despite well-established vital registration in China, data for nonfatal outcomes of CVD are highly insufficient, especially in western provinces. Estimation for prevalence of CVD subcategories and their sequalae are highly dependent on regional patterns and predictors in the model. Results would be more reliable and precise if systematic efforts were made to fill the data gaps. Third, we failed to perform urban-rural stratification of results; this would be highly beneficial for understanding disparities to direct appropriate health policy and programs.
Conclusions
Overall, the CVD burden in China saw substantial declines at national and provincial levels from 1990 to 2016. However, substantial increases in prevalent cases imply high health care costs and warn of even higher costs in the future, given the rapidly aging and growing population and continuing progress in medical techniques. Marked heterogeneity was observed in spatial patterns of mortality, prevalence, and burden of disease for CVD overall and its main subcategories, including IHD, HS, and IS. In particular, the gap in the relative burden of CVD among provinces has widened during the past 2 decades, with slower improvement in provinces with weak economies, mostly in western China. Geographically targeted strategies for CVD control and prevention are needed to reduce negative health outcomes. Our study results will help to identify gaps in cardiovascular health and are valuable when tailoring health priorities and programs to the needs of China and its provinces.
eAppendix.
References
- 1.Sacco RL, Roth GA, Reddy KS, et al. The heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and World Heart Federation. Circulation. 2016;133(23):e674-e690. doi: 10.1161/CIR.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251-272. doi: 10.1016/S0140-6736(15)00551-6 [DOI] [PubMed] [Google Scholar]
- 3.UN General Assembly Political declaration of the high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. http://www.un.org/ga/search/view_doc.asp?symbol=A/66/L.1. Accessed August 21, 2018.
- 4.United Nations Goal 3: ensure healthy lives and promote well-being for all at all ages. http://www.un.org/sustainabledevelopment/health/. Accessed August 21, 2018.
- 5.The State Council of the People’s Republic of China State Council issues plan to prevent chronic diseases. http://english.gov.cn/policies/latest_releases/2017/02/14/content_281475567482818.htm. Accessed August 21, 2018.
- 6.Yang XY, Li XF, Lü XD, Liu YL. Incidence of congenital heart disease in Beijing, China. Chin Med J (Engl). 2009;122(10):1128-1132. [PubMed] [Google Scholar]
- 7.He L, Tang X, Song Y, et al. Prevalence of cardiovascular disease and risk factors in a rural district of Beijing, China: a population-based survey of 58,308 residents. BMC Public Health. 2012;12:34. doi: 10.1186/1471-2458-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong W, Wei X, Liang Y, et al. Urban and rural differences of acute cardiovascular disease events: a study from the population-based real-time surveillance system in Zhejiang, China in 2012. PLoS One. 2016;11(11):e0165647. doi: 10.1371/journal.pone.0165647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XG, Wang YL, Zhang N, et al. Incidence and trends of stroke and its subtypes in Changsha, China from 2005 to 2011. J Clin Neurosci. 2014;21(3):436-440. doi: 10.1016/j.jocn.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 10.Li B, Lou Y, Gu H, et al. Trends in incidence of stroke and transition of stroke subtypes in rural Tianjin China: a population-based study from 1992 to 2012. PLoS One. 2015;10(10):e0139461. doi: 10.1371/journal.pone.0139461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Jiang B, Sun H, et al. ; NESS-China Investigators . Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759-771. doi: 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Hu SS, Kong LZ, et al. ; Editorial Board . Summary of report on cardiovascular diseases in China, 2012. Biomed Environ Sci. 2014;27(7):552-558. [DOI] [PubMed] [Google Scholar]
- 13.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448-457. doi: 10.1056/NEJMra1201534 [DOI] [PubMed] [Google Scholar]
- 14.GBD 2016 Mortality Collaborators Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1084-1150. doi: 10.1016/S0140-6736(17)31833-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151-1210. doi: 10.1016/S0140-6736(17)32152-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GBD 2016 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1260-1344. doi: 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GBD 2016 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345-1422. doi: 10.1016/S0140-6736(17)32366-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Wu X, Lopez AD, et al. An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull World Health Organ. 2016;94(1):46-57. doi: 10.2471/BLT.15.153148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Li X, Zhou M, et al. Under-5 mortality in 2851 Chinese counties, 1996-2012: a subnational assessment of achieving MDG 4 goals in China. Lancet. 2016;387(10015):273-283. doi: 10.1016/S0140-6736(15)00554-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health and Family Planning Commission An Analysis Report of National Health Services Survey in China, 2013. Beijing, China: Chinese Union Medical University Press; 2015. [Google Scholar]
- 22.Chinese Center for Disease Control and Prevention China Chronic Disease and Risk Factor Surveillance 2013. Beijing, China: Military Medical Science Press; 2016. [Google Scholar]
- 23.Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1-25. doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2129-2143. doi: 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haagsma JA, Maertens de Noordhout C, Polinder S, et al. Assessing disability weights based on the responses of 30,660 people from four European countries. Popul Health Metr. 2015;13:10. doi: 10.1186/s12963-015-0042-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712-e723. doi: 10.1016/S2214-109X(15)00069-8 [DOI] [PubMed] [Google Scholar]
- 27.GBD 2015 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603-1658. doi: 10.1016/S0140-6736(16)31460-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Zeng X, Liu J, et al. Can China achieve a one-third reduction in premature mortality from non-communicable diseases by 2030? BMC Med. 2017;15(1):132. doi: 10.1186/s12916-017-0894-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Decisions and list of resolutions. http://apps.who.int/gb/ebwha/pdf_files/WHA65/A65_DIV3-en.pdf. Accessed September 19, 2018.
- 30.Wang W, Wang D, Liu H, et al. Trend of declining stroke mortality in China: reasons and analysis. Stroke Vasc Neurol. 2017;2(3):132-139. doi: 10.1136/svn-2017-000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Hu S, Sang S, Luo L, Yu C. Age-period-cohort analysis of stroke mortality in China: data from the Global Burden of Disease Study 2013. Stroke. 2017;48(2):271-275. doi: 10.1161/STROKEAHA.116.015031 [DOI] [PubMed] [Google Scholar]
- 32.Zhang LF, Yang J, Hong Z, et al. ; Collaborative Group of China Multicenter Study of Cardiovascular Epidemiology . Proportion of different subtypes of stroke in China. Stroke. 2003;34(9):2091-2096. doi: 10.1161/01.STR.0000087149.42294.8C [DOI] [PubMed] [Google Scholar]
- 33.Li J, Li X, Wang Q, et al. ; China PEACE Collaborative Group . ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data [published retraction and replacement appears in Lancet. 2015;385(9966):402]. Lancet. 2015;385(9966):441-451. doi: 10.1016/S0140-6736(14)60921-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du X, Gao R, Turnbull F, et al. ; CPACS Investigators . Hospital quality improvement initiative for patients with acute coronary syndromes in China: a cluster randomized, controlled trial. Circ Cardiovasc Qual Outcomes. 2014;7(2):217-226. doi: 10.1161/CIRCOUTCOMES.113.000526 [DOI] [PubMed] [Google Scholar]
- 35.Ranasinghe I, Rong Y, Du X, et al. ; CPACS Investigators . System barriers to the evidence-based care of acute coronary syndrome patients in China: qualitative analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):209-216. doi: 10.1161/CIRCOUTCOMES.113.000527 [DOI] [PubMed] [Google Scholar]
- 36.Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke. 2008;39(6):1668-1674. doi: 10.1161/STROKEAHA.107.502807 [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515-2523. doi: 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bi Y, Jiang Y, He J, et al. ; 2010 China Noncommunicable Disease Surveillance Group . Status of cardiovascular health in Chinese adults. J Am Coll Cardiol. 2015;65(10):1013-1025. doi: 10.1016/j.jacc.2014.12.044 [DOI] [PubMed] [Google Scholar]
- 39.National Center for Cardiovascular Diseases Report on Cardiovascular Diseases in China, 2016. Beijing, China: Encyclopedia of China Publishing House; 2017. [Google Scholar]
- 40.Wei YD, Hu DY, Zhang RF, et al. Metabolic syndrome complicated by peripheral arterial disease: clinical study of 2115 cases [in Chinese]. Zhonghua Yi Xue Za Zhi. 2006;86(30):2114-2116. [PubMed] [Google Scholar]
- 41.Guocheng L, Liansheng R. Prevalence of peripheral arterial disease and its risk factors in Zhoushan fishery area in Zhejiang Province [in Chinese]. Chin J Geriatr. 2005;24(11):863-865. [Google Scholar]
- 42.Cang Y, Li J, Li YM, et al. Relationship of a low ankle-brachial index with all-cause mortality and cardiovascular mortality in Chinese patients with metabolic syndrome after a 6-year follow-up: a Chinese prospective cohort study. Intern Med. 2012;51(20):2847-2856. doi: 10.2169/internalmedicine.51.7718 [DOI] [PubMed] [Google Scholar]
- 43.Xie W, Liang L, Zhao L, et al. Combination of carotid intima-media thickness and plaque for better predicting risk of ischaemic cardiovascular events. Heart. 2011;97(16):1326-1331. doi: 10.1136/hrt.2011.223032 [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Li XY, He Y, Ni B. A cross-sectional study of peripheral arterial occlusive disease in Wanshoulu area, Beijing [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(3):221-224. [PubMed] [Google Scholar]
- 45.Li Y, Wang L, Feng X, et al. Geographical variations in hypertension prevalence, awareness, treatment and control in China: findings from a nationwide and provincially representative survey. J Hypertens. 2018;36(1):178-187. doi: 10.1097/HJH.0000000000001531 [DOI] [PubMed] [Google Scholar]
- 46.Zhou M, Astell-Burt T, Bi Y, et al. Geographical variation in diabetes prevalence and detection in china: multilevel spatial analysis of 98,058 adults. Diabetes Care. 2015;38(1):72-81. doi: 10.2337/dc14-1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Wang C, Zhao X, et al. ; China National Stroke Registries . Substantial progress yet significant opportunity for improvement in stroke care in China. Stroke. 2016;47(11):2843-2849. doi: 10.1161/STROKEAHA.116.014143 [DOI] [PubMed] [Google Scholar]
- 48.Bi Y, Gao R, Patel A, et al. ; CPACS Investigators . Evidence-based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: results from the Clinical Pathways for Acute Coronary Syndromes in China (CPACS) study. Am Heart J. 2009;157(3):509-516.e1. doi: 10.1016/j.ahj.2008.09.026 [DOI] [PubMed] [Google Scholar]
- 49.Chinese Center for Disease Control and Prevention Report on Chronic Disease Risk Factor Surveillance in China, 2007. Beijing, China: People’s Medical Publishing House; 2010. [Google Scholar]
- 50.Chinese Center for Disease Control and Prevention Report on Chronic Disease Risk Factor Surveillance in China, 2010. Beijing, China: Military Medical Science Press; 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix.