Key Points
Question
What are the coronary atherosclerotic phenotype and prevalence of healed plaques in patients at the extremes of the coronary artery disease spectrum?
Findings
In this cohort study of 105 patients undergoing preintervention optical coherence tomography imaging, patients with recurrent acute coronary syndromes and those with single myocardial infarction followed by long-term stability showed a similar prevalence of thin-cap fibroatheroma, which was significantly lower in patients with long-standing stable angina. Healed coronary plaques were rarely observed in patients with multiple recurrent acute coronary syndromes, while their prevalence was significantly higher in patients with long-term clinical stability.
Meaning
Both atherosclerotic profile and plaque healing may play a role in leading the natural history of patients with coronary artery disease toward either recurrence of acute events or long-term stability.
Abstract
Importance
At one end of the coronary artery disease (CAD) spectrum, there are patients with multiple recurrent acute coronary syndromes (rACS), and at the other end there are those with long-standing clinical stability. Predicting the natural history of these patients is challenging because unstable plaques often heal without resulting in ACS.
Objective
To assess in vivo the coronary atherosclerotic phenotype as well as the prevalence and characteristics of healed coronary plaques by optical coherence tomography (OCT) imaging in patients at the extremes of the CAD spectrum.
Design, Setting, and Participants
This is an observational, single-center cohort study with prospective clinical follow-up. From a total of 823 consecutive patients enrolled in OCT Registry of the Fondazione Policlinico A. Gemelli–IRCCS, Rome, Italy, from March 2009 to February 2016, 105 patients were included in the following groups: (1) patients with rACS, defined as history of at least 3 acute myocardial infarctions (AMIs) or at least 4 ACS with at least 1 AMI; (2) patients with long-standing stable angina pectoris (ls-SAP), defined as a minimum 3-year history of stable angina; and (3) patients with a single unheralded AMI followed by a minimum 3-year period of clinical stability (sAMI). Data were analyzed from January to August 2018.
Exposures
Intracoronary OCT imaging of nonculprit coronary segments.
Main Outcomes and Measures
Coronary plaque features and the prevalence of healed coronary plaques in nonculprit segments as assessed by intracoronary OCT imaging.
Results
Of 105 patients, 85 were men (81.0%); the median (interquartile range) age was 68 (63-75) years. Median (interquartile range) time of clinical stability was 9 (5.0-15.0) years in the ls-SAP group and 8 (4.5-14.5) years in the sAMI group. Patients in the rACS and sAMI groups showed similar prevalence of lipid-rich plaque and thin-cap fibroatheroma, which was significantly higher than in those with ls-SAP (lipid-rich plaque 80.0% [n = 24 of 30] vs 76.3% [n = 29 of 38] vs 37.8% [n = 14 of 37], respectively; P < .001; thin-cap fibroatheroma 40.0% [n = 12 of 30] vs 34.2% [n = 13 of 38] vs 8.1% [n = 3 of 37], respectively; P = .006). Spotty calcifications were more frequently observed in patients with rACS than in those with ls-SAP and sAMI (70.0% [n = 21 of 30] vs 40.5% [n = 15 of 37] vs 44.7% [n = 17 of 38], respectively; P = .04). Healed coronary plaques were rarely observed in patients with rACS, whereas their prevalence was significantly higher in patients with ls-SAP and sAMI (3.3% [n = 1 of 30] vs 29.7% [n = 11 of 37] vs 28.9% [n = 11 of 38], respectively; P = .01).
Conclusions and Relevance
Patients with rACS have a distinct atherosclerotic phenotype compared with those with ls-SAP, including higher prevalence of thin-cap fibroatheroma and lower prevalence of healed coronary plaques, suggesting that atherosclerotic profile and plaque healing may play a role in leading the natural history of patients with CAD.
This study assesses in vivo the coronary atherosclerotic phenotype as well as the prevalence and characteristics of healed coronary plaques by optical coherence tomography imaging in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability.
Introduction
Pathology and imaging studies have demonstrated that plaque rupture and erosion are the most common substrates of acute coronary syndrome (ACS) and sudden cardiac death.1,2,3,4,5 Efforts to identify high-risk patients have focused on advanced imaging methods to detect single vulnerable atherosclerotic plaques such as thin-cap fibroatheroma (TCFA).6 However, the risk of acute myocardial infarction (AMI) or sudden cardiac death related to these lesions is rather low, as observed in the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study and other imaging studies.7,8 These findings are in keeping with numerous pathologic observations demonstrating that many (if not most) plaques destabilize without resulting in a clinical syndrome.9,10 This final event, in fact, conceivably depends on a delicate balance between prothrombotic and thrombosis-resisting factors.6,11 At 1 extreme, thrombus formation associated with plaque rupture or erosion is contained, and plaque healing occurs. At the other extreme, several prothrombotic factors (eg, plaque rupture/erosion, systemic prothrombotic milieu, and inflammatory state) coincide in a perfect storm culminating in ACS.6,11 Based on these observations, healed plaque ruptures or erosions may be considered as a signature of an aborted ACS within the coronary tree. Optical coherence tomography (OCT) has been validated for the detection of healed coronary plaques against histology.12 Yet the prevalence and characteristics of healed coronary plaques in vivo remain unknown. For this purpose, we conducted an observational cohort study in patients at the extremes of the clinical spectrum of coronary artery disease assessed by OCT imaging and coronary angiography: at one extreme, patients with a history of multiple acute coronary events, and at the other extreme, those with long-standing stable coronary artery disease who have never been clinically unstable. To include the full spectrum, we enrolled a third group of patients with single unheralded AMI followed by long-term stability. These 3 groups of patients were followed up for the occurrence of adverse events.
Methods
Study Design, Setting, and Participants
The Fondazione Policlinico A. Gemelli–Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) OCT Registry is a prospectively enrolling registry of patients undergoing OCT imaging during coronary angiography and/or percutaneous coronary intervention (PCI). In this observational cohort study, participants were identified by analyzing admission notes, discharge summaries, and notes of cardiac outpatient clinic of consecutive patients with ACS or stable angina pectoris (SAP) who underwent OCT imaging of target and/or nontarget vessels before PCI and were enrolled in the registry between March 2009 and February 2016. The registry was approved by the institutional review board of Fondazione Policlinico A. Gemelli–IRCCS, and each patient provided written informed consent. Three groups of patients were identified post hoc, characterized, and followed up for clinical adverse events. Data analysis was performed between January 2018 and August 2018. Detailed inclusion and exclusion criteria are reported in eTable 1 in the Supplement.
Group With Recurrent ACS
This group included patients who had at least 3 AMIs or at least 4 ACS (AMI or unstable angina pectoris [UAP]) with at least 1 AMI. An AMI was defined as prolonged (≥30 minutes) typical chest pain and elevated cardiac biomarkers values (rise and/or fall of cardiac troponin or creatine kinase–myocardial band), with or without ST-segment elevation. A UAP was defined as new-onset angina, progressive crescendo pattern of angina, or angina at rest, requiring hospitalization. Acute coronary events needed to have occurred at least 1 month apart from each other to be counted and had not to be related to stent thrombosis.
Group With Uncomplicated Long-standing SAP
This group included patients with a minimum 3-year history of stable angina without any episode suggestive of an acute event. Myocardial ischemia was documented by a functional test (ie, exercise treadmill testing and/or myocardial perfusion imaging). Patients showing Q waves on 12-lead electrocardiogram and/or abnormal echocardiogram (ie, left ventricular ejection fraction <50% and/or wall motion abnormalities) were excluded.
Group With Single Unheralded AMI Followed by Long-term Clinical Stability
This group included patients with a history of a single episode of AMI (sAMI), followed by a minimum 3-year period of clinical stability (stable angina pectoris or asymptomatic), without any further episode suggestive of an acute event. Myocardial ischemia was documented by a functional test (ie, exercise treadmill testing and/or myocardial perfusion imaging).
OCT Analysis
Optical coherence tomography image analysis was performed offline using a proprietary software (LightLab Imaging) by 2 expert investigators who were blinded to clinical and laboratory data; in case of discordance, a consensus reading was achieved with a third senior investigator. Details on OCT image acquisition are reported in the eMethods in the Supplement. Analysis was conducted along the entire OCT pullback after dividing the imaged coronary vessel into consecutive 3-mm segments. In the recurrent ACS (rACS) group, culprit stenosis, identified based on angiographic findings, electrocardiographic changes, and/or left ventricular wall motion abnormalities and confirmed by OCT,13,14 was excluded. Therefore, only nonculprit segments were included in the analysis. Representative OCT images of coronary plaque features are shown in Figure 1. A plaque was defined as a region with a loss of the 3-layered structure (ie, intima, media, and adventitia) of the vessel wall.13,14 Lipid plaque was defined as a diffusely bordered signal-poor region with an overlying signal-rich band, and lipid arc was measured every 1 mm.13,14 Lipid length was measured by calculating the distance between the first and last OCT cross-sections where lipid was observed, and lipid index was derived as the product of mean lipid arc and lipid length.15 A plaque with a lipid arc greater than 90° was defined as lipid rich.13 Fibrous cap thickness was measured 3 times at its thinnest part, and the averaged value was calculated. The TCFA was defined as a lipid-rich plaque covered by a fibrous cap thinner than 65 μm.13,14 Fibrous plaque was defined as a plaque with homogeneous, high back-scattering signal.13,14 Healed coronary plaque was defined as a plaque with at least 1 heterogeneous signal-rich layer of different optical signal intensity located close to the luminal surface and clearly demarcated from underlying tissue.12 Calcifications were defined as signal-poor or heterogeneous areas delimited by sharp borders.13,14 Calcified lesions subtending an arc less than 90° and extending in length for less than 4 mm were classified as spotty calcifications.16 Macrophage accumulation was defined as the presence of signal-rich, distinct, or confluent punctate regions (bright spots) that exceed the intensity of background speckle noise and generate a backward shadowing,14 and the normalized standard deviation of the OCT signal was measured as previously described.17 Intimal vasculature was defined as the presence of small, signal-poor structures with vesicular or tubular shape within the intima without a connection with the vessel lumen, recognized on at least 3 consecutive frames.13,14
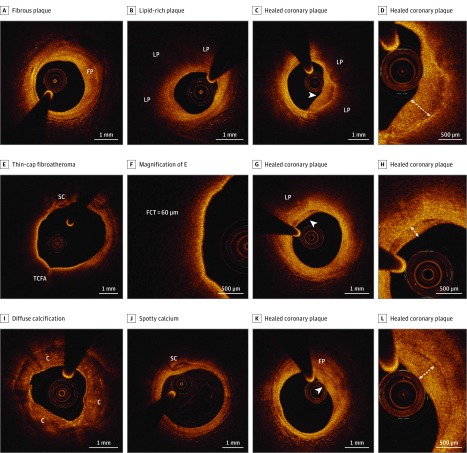
Figure 1. In Vivo Optical Coherence Tomography (OCT) Images of Coronary Plaque Features.
A, Fibrous plaque (FP), appearing as a plaque with homogeneous, high back-scattering signal. B, Lipid-rich plaque, characterized by a diffusely bordered signal-poor lipid pool (LP) extending for more than 90°. C and D, Healed coronary plaque, appearing as a heterogeneous, signal-rich layer of tissue (arrowhead) overlying a lipid-rich plaque; the layered pattern of the OCT signal (onionlike) is highlighted in the in high-power image in D. E, Thin-cap fibroatheroma, defined as a lipid-rich plaque with a fibrous cap thinner than 65 μm, with evidence of spotty calcium (SC). F, High-power image of E, showing a measured fibrous cap thickness (FCT) of 60 μm. G and H, Healed coronary plaque, characterized by a layered pattern of the signals (arrowhead) with underlying lipid plaque (LP). I, Diffuse calcification (C), appearing as a heterogeneous, signal-poor area delimited by sharp borders, extending along the entire circumference of the vessel wall. J, Spotty calcium (SC). K and L, Healed coronary plaque, characterized by a layered pattern of the signals (arrowhead) with underlying fibrous plaque (FP).
Angiographic Analysis
Angiographic analysis was performed by 2 experienced investigators on baseline coronary angiograms acquired during the index procedure. Atherosclerotic disease burden was assessed using the Bogaty and the Gensini scores.18,19 Details of the angiographic analysis are reported in the eMethods of the Supplement. To study angiographic atherosclerotic disease progression in the 3 groups, patients who underwent additional coronary angiography at least 1 year before and/or after the index procedure were extracted from the Fondazione Policlinico A. Gemelli–IRCCS coronary angiography database. ∆ Bogaty and Gensini scores were calculated and compared among the 3 groups.
Clinical Follow-up
Clinical follow-up was obtained at 6 months and then yearly after discharge by telephone call and/or clinical check. The incidence of cardiac death, nonfatal myocardial infarction, rehospitalization owing to UAP, and nontarget vessel revascularization was assessed. Major adverse cardiac events (MACE) were defined as the composite of cardiac death, nonfatal myocardial infarction, and rehospitalization owing to UAP. Cardiac death was ascertained by contacting the primary care physician and/or the hospital where the patient died. Myocardial infarction was diagnosed by detection of raise and/or fall of cardiac biomarkers (troponin or creatine kinase–myocardial band) greater than the 99th percentile of the upper reference limit, plus evidence of myocardial ischemia with at least 1 of the following criteria: typical chest pain; new ST-T changes or new left bundle branch block on electrocardiogram; and/or new loss of viable myocardium or new regional wall motion abnormalities on echocardiogram. A UAP was defined according to the Braunwald Classification.
Statistical Analysis
Categorical variables were expressed as counts (percentages) and compared using the χ2 or Fisher exact test. After assessing data distribution using the Kolmogorov-Smirnov test, continuous variables were expressed as mean (standard deviation) or median (interquartile range [IQR]) and compared using the independent-samples t test or the Mann-Whitney U test. One-way analysis of variance was used for comparisons of continuous data among the 3 groups. Segment-based comparisons were carried out using generalized estimating equations to consider potential cluster effects of multiple segments in a single patient. Survival curves, determined with Kaplan-Meier methods, were compared by means of the log-rank test. Univariate Cox regression analysis was performed to evaluate the association of clinical variables and plaque features with MACE. Age, sex, and the variables exhibiting a P value less than .10 at univariate analysis were entered in a multivariate Cox regression model. Intraobserver and interobserver variability were assessed using κ measure of agreement. All tests were 2-sided. A P value less than .05 was considered statistically significant. All statistical analyses were performed using SPSS, version 21.0 (SPSS, Inc).
Results
Study Participants
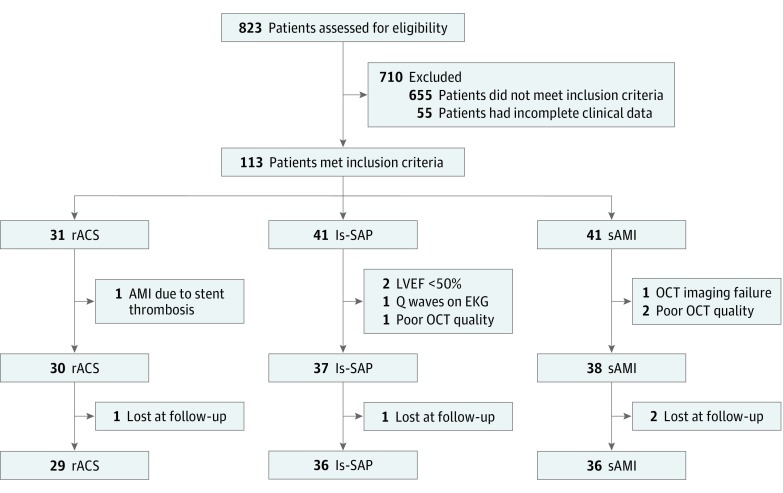
A flowchart of patient entry into the study is provided in Figure 2. Between March 2009 and February 2016, a total of 823 consecutive patients undergoing OCT imaging at the time of diagnostic coronary angiography and/or during PCI were enrolled in the Fondazione Policlinico A. Gemelli–IRCCS OCT Registry. The decision to perform OCT imaging was left at each operator’s choice. Fifty-five patients were excluded owing to incomplete/unclear clinical information related to history of previous AMI and/or duration of clinical stability. A total of 113 patients met inclusion criteria and were eligible for the study. After excluding 8 patients who met at least 1 of the exclusion criteria (details in Figure 2), 105 patients were finally enrolled: 30 in the rACS group, 37 in the long-standing SAP (ls-SAP) group, and 38 in the sAMI group.
Figure 2. Study Flowchart.
EKG indicates electrocardiogram; ls-SAP, long-standing stable angina pectoris; LVEF, left ventricular ejection fraction; OCT, optical coherence tomography; rACS, recurrent acute coronary syndromes; sAMI, single acute myocardial infarction followed by clinical stability.
Twenty-three patients in the rACS group (76.7%) were admitted with ACS, while 7 patients (23.3%) were admitted for SAP or for undergoing a scheduled angiographic follow-up in the absence of symptoms. By definition, all patients in the ls-SAP and sAMI groups were stable at the time of enrollment. In the sAMI group, median duration of clinical stability was 8 years (IQR, 4.5-14.5 years), and in the ls-SAP group, median duration was 9 years (IQR, 5.0-15.0 years). Statin use on admission was reported in 73 of 105 patients (69.5%) and was not different among the 3 groups (rACS n = 20 of 30 [66.7%] vs ls-SAP n = 27 of 37 [73.0%] vs sAMI n = 26 of 38 [68.4%]; P = .84). All clinical and laboratory findings are detailed in Table 1.
Table 1. Clinical and Laboratory Findings.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Overall (N = 105) | rACS (n = 30) | ls-SAP (n = 37) | sAMI (n = 38) | ||
| Age, median (IQR), y | 68 (63-75) | 68 (61-75) | 72 (65-78) | 67 (62-72) | .18 |
| Male sex | 85 (81.0) | 22 (73.3) | 28 (75.7) | 35 (92.1) | .09 |
| Clinical presentation | <.001 | ||||
| ACS | 23 (21.9) | 23 (76.7) | 0 (0.0) | 0 (0.0) | |
| SAP/asymptomatic | 82 (78.1) | 7 (23.3) | 37 (100.0) | 38 (100.0) | |
| Hypertension | 94 (89.5) | 26 (86.7) | 34 (91.9) | 34 (89.5) | .79 |
| Dyslipidemia | 88 (83.8) | 26 (86.7) | 30 (81.1) | 32 (84.2) | .82 |
| Diabetes mellitus | 42 (40.0) | 13 (43.3) | 15 (40.5) | 14 (36.8) | .86 |
| Current smoking | 11 (10.5) | 5 (16.7) | 1 (2.7) | 5 (13.2) | .14 |
| Prior smoking | 63 (60.0) | 18 (60.0) | 20 (54.1) | 25 (65.8) | .58 |
| Family history | 38 (36.2) | 12 (40.0) | 12 (32.4) | 14 (36.8) | .81 |
| Prior PCI | 85 (81.0) | 29 (96.7) | 24 (64.9) | 32 (84.2) | .004 |
| Prior CABG | 12 (11.4) | 7 (23.3) | 2 (5.4) | 3 (7.9) | .05 |
| LVEF, % | 55 (41-60) | 52 (40-59) | 61 (57-65) | 50 (40-59) | .04 |
| BMI, median (IQR) | 27.4 (24.7-30.9) | 29.3 (25.8-32.3) | 27.6 (25.3-30.6) | 25.4 (23.3-29.6) | .32 |
| Serum, mg/dL | |||||
| Creatinine | 0.98 (0.83-1.19) | 1.01 (0.82-1.16) | 0.88 (0.83-1.17) | 1.03 (0.86-1.29) | .42 |
| Glucose | 103 (88-134) | 103 (88-157) | 103 (90-135) | 100 (85-131) | .91 |
| Cholesterol, median (IQR), mg/dL | |||||
| Total | 141 (126-165) | 135 (98-148) | 143 (132-200) | 143 (127-164) | .23 |
| LDL | 74 (56-91) | 73 (51-89) | 74 (55-96) | 75 (59-91) | .85 |
| HDL | 43 (34-52) | 34 (26-47) | 50 (41-59) | 41 (38-45) | .006 |
| Triglycerides | 118 (79-172) | 123 (78-137) | 101 (65-166) | 123 (85-191) | .48 |
| High-sensitivity CRP, mg/L | 3.7 (2.9-15.4) | 11.5 (3.2-38.4) | 3.3 (0.8-7.3) | 3.8 (2.9-14.8) | .23 |
| Discharge medications | |||||
| Aspirin | 102 (97.1) | 30 (100.0) | 36 (97.3) | 36 (94.7) | .43 |
| P2Y12-inhibitor | 76 (72.4) | 23 (76.7) | 23 (62.2) | 30 (78.9) | .22 |
| β-Blockers | 90 (85.7) | 25 (83.3) | 30 (81.1) | 35 (92.1) | .36 |
| ACE-i/ARBs | 83 (79.0) | 25 (83.3) | 29 (78.4) | 29 (76.3) | .77 |
| Statins | 101 (96.2) | 28 (93.3) | 35 (94.6) | 38 (100.0) | .30 |
Abbreviations: ACE-i/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CRP, C-reactive protein; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; ls-SAP, long-standing stable angina pectoris; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary interventions; rACS, recurrent acute coronary syndromes; SAP, stable angina pectoris.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259; creatinine to micromoles per liter, multiply by 76.25; C-reactive protein to nanomoles per liter, multiply by 9.524; glucose to millimoles per liter, multiply by 0.0555; triglycerides to millimoles per liter, multiply by 0.0113.
Angiographic and OCT Findings
A total of 1609 nonculprit segments were analyzed by OCT. The number of analyzed segments was not different among patients in the rACS, sAMI, and ls-SAP groups (median, 14; IQR, 8-18 vs median, 17; IQR, 1122 vs median, 15; IQR, 8-19, respectively; P = .17). Multivessel imaging was performed in 15 patients (14.3%). Angiographic and OCT findings are reported in Table 2.
Table 2. Angiographic and OCT Findings.
| Finding | rACS (n = 30) | ls-SAP (n = 37) | sAMI (n = 38) | P Value | ||
|---|---|---|---|---|---|---|
| rACS vs ls-SAP | rACS vs sAMI | ls-SAP vs sAMI | ||||
| Angiographic findings | ||||||
| Baseline, median (IQR) | ||||||
| Bogaty score | 1.00 (0.77-1.40) | 0.60 (0.35-0.80) | 0.65 (0.49-1.00) | <.001 | .005 | .02 |
| Gensini score | 52.5 (23.0-84.0) | 20.0 (12.0-47.4) | 38.0 (20.0-55.3) | <.001 | .10 | .01 |
| Follow-up median (IQR) | ||||||
| Patients, No. | 22 | 23 | 24 | |||
| Follow-up time, mo | 62.5 (44.6-89.8) | 64.3 (37.2-78.0) | 64.2 (45.4-92.6) | .48 | .55 | .34 |
| ∆ Bogaty score | 0.24 (0.00-0.40) | 0.00 (0.00-0.11) | 0.20 (0.00-0.40) | .01 | .95 | .008 |
| ∆ Gensini score | 5.5 (0.0-20.5) | 1.0 (0.0-4.5) | 5.5 (0.5-16.8) | .03 | .86 | .04 |
| OCT findings | ||||||
| Qualitative analysis, count (%) | ||||||
| Lipid-rich plaque | 24 (80.0) | 14 (37.8) | 29 (76.3) | .001 | .72 | .001 |
| TCFA | 12 (40.0) | 3 (8.1) | 13 (34.2) | .002 | .62 | .006 |
| Fibrous plaque | 21 (70.0) | 34 (91.9) | 27 (71.1) | .02 | .93 | .021 |
| Calcification | 20 (66.7) | 33 (89.2) | 25 (65.8) | .02 | .94 | .02 |
| Spotty calcification | 21 (70.0) | 15 (40.5) | 17 (44.7) | .02 | .04 | .71 |
| Macrophage accumulation | 16 (53.3) | 7 (18.9) | 7 (18.4) | .003 | .003 | .96 |
| Intimal vasculature | 17 (56.7) | 25 (67.6) | 22 (57.9) | .36 | .92 | .39 |
| Quantitative analysis, median (IQR) | ||||||
| Mean lipid arc | 166 (120-231) | 120 (90-150) | 150 (110-180) | <.001 | .07 | .009 |
| Lipid index | 480 (330-650) | 360 (255-450) | 450 (300-540) | <.001 | .07 | .008 |
| Fibrous cap thickness, μm | 100 (70-120) | 150 (100-200) | 110 (90-150) | <.001 | .63 | <.001 |
| NSD, % | 6.95 (6.45-7.53) | 6.50 (6.33-6.78) | 6.60 (6.50-6.70) | .11 | .22 | .46 |
Abbreviations: IQR, interquartile range; ls-SAP, long-standing stable angina pectoris; NSD, normalized standard deviation; rACS, recurrent acute coronary syndromes; sAMI, single acute myocardial infarction followed by long-term clinical stability; TCFA, thin-cap fibroatheroma.
Angiographic atherosclerotic disease burden at the time of index procedure was higher in patients with rACS than in those with ls-SAP and sAMI, as assessed by Bogaty score and Gensini score. In a subgroup of 69 patients, an additional coronary angiography was performed at least 1 year before and/or after the index procedure at the A. Gemelli Hospital. Median follow-up time between the first and last performed coronary angiography was 64.3 months (IQR, 39.7-83.0) and was not different between the 3 groups. Patients with rACS and sAMI showed similar angiographic atherosclerotic disease progression, which was higher than that in patients with ls-SAP, as demonstrated by both ∆ Bogaty score and ∆ Gensini score (Table 2).
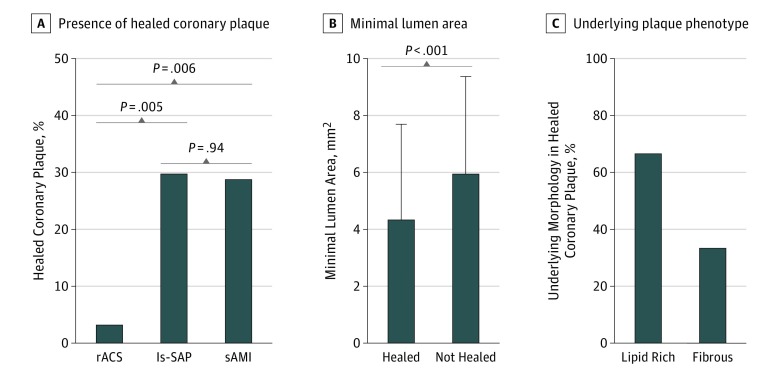
Plaque phenotype was similar in patients with rACS and sAMI, with significantly higher prevalence of lipid-rich plaque and TCFA compared with patients with ls-SAP, while the prevalence of fibrous plaque was similar in patients with rACS and sAMI and higher in those with ls-SAP. Similarly, mean lipid arc and lipid index were significantly higher in patients with rACS and sAMI than in those with ls-SAP, while fibrous cap thickness was thinner. In contrast, spotty calcifications were more frequently observed in patients with rACS than in those with ls-SAP and sAMI (Table 2). The OCT results of segment-based analysis are reported in eTable 2 in the Supplement. Healed coronary plaques were rarely observed in patients with rACS, whereas their prevalence was significantly higher in patients with ls-SAP and sAMI (3.3% [n = 1 of 30] vs 29.7% [n = 11 of 37] vs 28.9% [n = 11 of 38], respectively; P = .01) (Figure 3A). Segments with healed coronary plaques had a significantly smaller mean (SD) minimal lumen area than those without (4.34 [3.38] mm2 vs 5.95 [3.43] mm2; P < .001) (Figure 3B). Underlying plaque in healed coronary lesions was more frequently lipid-rich than fibrous (66.7% [40 of 60 segments] vs 33.3% [20 of 60 segments]) (Figure 3C). Intraobserver and interobserver agreements were good for healed coronary plaques (κ = 0.93 and κ = 0.90, respectively), TCFA (κ = 0.90 and κ = 0.88, respectively), and macrophage accumulation (κ = 0.89 and κ = 0.87, respectively).
Figure 3. Healed Coronary Plaques in Patients With Recurrent Acute Coronary Syndromes (rACS), Long-standing Stable Angina Pectoris (ls-SAP), and Single Acute Myocardial Infarction Followed by Long-term Clinical Stability (sAMI).
A, Prevalence of healed coronary plaques in patients with rACS, ls-SAP, and sAMI. B, Minimal lumen area in segments with and without healed coronary plaques. C, Underlying plaque phenotype in healed coronary plaques.
Clinical Outcome
Clinical follow-up was obtained in 101 patients (96.2%). During a median follow-up period of 36.8 months (IQR, 18.3-56.2 months), 26 MACEs occurred (25.7%): 5 cardiac deaths (5.0%), 10 nonfatal MI (9.9%), and 11 rehospitalization owing to UAP (10.9%). The rate of non-TVR was 17.8% (18 patients) (eTable 3 in the Supplement). The MACE rate was significantly higher in patients with rACS than in those with ls-SAP and sAMI (15 [51.7%] vs 6 [16.7%] vs 5 [13.9%], respectively; P < .001). Major adverse cardiac events were significantly more frequent in patients without than in those with healed coronary plaques (24 [31.2%] vs 2 [8.3%], P = .01) (eFigure, A in the Supplement). This difference was mainly driven by higher rates of nonfatal MI (10 [13.0%] vs 0; P = .04) and rehospitalization owing to UAP (10 [13.0%] vs 1 [4.2%], P = .10). Kaplan-Meier event-free survival curves in patients with and without healed coronary plaques are shown in eFigure, B in the Supplement.
At univariate Cox regression analysis, rACS, prior PCI, TCFA, macrophage accumulation, spotty calcification, and Bogaty score were associated with MACE, while fibrous plaque and healed coronary plaque were inversely associated with MACE (eTable 4 in the Supplement). At multivariate Cox regression analysis, rACS (HR, 3.09; 95% CI, 1.04-9.20; P = .04), TCFA (HR, 2.80; 95% CI, 1.04-7.54; P = .04), and macrophage accumulation (HR, 2.78; 95% CI, 1.13-6.86; P = .03) remained independently associated with MACE, whereas healed coronary plaque (HR, 0.17; 95% CI, 0.03-0.85; P = .03) was independently associated with better clinical outcome (eTable 5 in the Supplement).
Discussion
This study examined subsets of patients at the extremes of the coronary artery disease spectrum using OCT and coronary angiography. At one end were patients who had experienced several acute coronary events (rACS group); at the other end were patients with long-standing stable angina who had always been clinically stable (ls-SAP group). A third group of patients with a single unheralded MI followed by prolonged clinical stability (sAMI group) was also enrolled. We found that patients with rACS had a similar plaque phenotype (ie, prevalence of lipid-rich plaque and TCFA) compared with patients who had experienced an isolated MI followed by long-term clinical stability, suggesting that different mechanisms should act on a similar background of coronary atherosclerosis to determine a distinct susceptibility to acute coronary event in these patients. Of importance, a feature discriminating these 2 groups of patients was the presence of healed coronary plaques (rarely detected in patients with rACS, while observed in one-third of patients with ls-SAP and sAMI).
Coronary Plaque Phenotype and Plaque Healing
The ACS ultimately depends on the disruption of the delicate balance between prothrombotic and thrombosis-resisting factors.6,11 When plaque rupture or erosion occur in a prothrombotic milieu, they likely culminate in a symptomatic acute coronary event; otherwise, if thrombosis-resisting factors prevail, thrombus formation is inhibited and plaque healing occurs.6,11 Because healed coronary plaques are a sort of signature of the prevalence of thrombosis-resisting factors, we hypothesized that they could indeed represent the missing link between plaque morphology and symptomatic acute coronary event. Thanks to its high spatial resolution, OCT is the only in vivo diagnostic modality able to detect healed coronary plaques that typically have a multilayered, onionlike appearance, with 1 or more layers of tissue with different optical signal clearly separated from the underlying plaque.20,21 A 2018 study by Shimokado et al12 validated OCT against histology for the assessment of healed coronary plaques, showing a very good agreement.
One of the main findings of our study is the observation that healed coronary plaques are rarely detected in patients with a history of multiple recurrent ACS, while they are observed in about one-third of patients with a history of long-term clinical stability. Of note, lipid-rich plaques and TCFA were equally frequent in patients with multiple recurrent events (rACS group) and in those with a single unheralded MI followed by long-term clinical stability (sAMI group), whereas they were significantly less common in patients who had never been unstable (ls-SAP group). These results, coupled with the observation of higher atherosclerotic disease burden and angiographic progression in the rACS and sAMI groups compared with the ls-SAP group, suggest that such high-risk plaques may be markers of a more extensive atherosclerotic disease, and that the patient’s response to a thrombogenic trigger may be critical for determining the probability of events over a predisposing background of atherosclerosis. Furthermore, smaller minimal lumen area values measured at sites of healed coronary plaques corroborate the role of subclinical plaque destabilization with subsequent healing in silent stenosis progression. While there is robust evidence supporting a benefit for clinical outcome of strategies addressing the extent and activity of the atherosclerotic disease and thrombosis-promoting risk factors,22,23 no conclusive evidence of incremental risk reduction by lesion-specific treatments (eg, stenting lipid-rich or vulnerable plaques) exists.24 Individual plaque features may therefore have relevant implications in specific settings; for instance, identifying a TCFA in patients with or without a known susceptibility to thrombosis is likely to have different clinical implications. Detection of healed coronary plaques may therefore help identifying those patients who are relatively protected from developing an acute occlusive thrombosis during plaque instability, aiding risk stratification and, potentially, treatment.
Limitations
Our study has some limitations. First, this was a single-center investigation, and the study’s overall population was not large. In addition, the decision to perform OCT imaging was at the operator’s discretion. As such, inherent to this methodologic approach, potential selection bias cannot be excluded. Second, stringent clinical inclusion/exclusion criteria were applied so that, from 823 patients of the initial cohort, after exclusion of patients who did not fill inclusion criteria (n = 655), had incomplete clinical data (n = 55), stent thrombosis (n = 1 in rACS group), LVEF<50% (n = 2 in ls-SAP group), Q waves on EKG (n = 1 in ls-SAP group), and poor OCT imaging quality (n = 1 in ls-SAP group and n = 2 in sAMI group) or OCT imaging failure (n = 1 in sAMI group), only 105 patients were included in the final analysis. However, this approach allowed us to enroll cohorts representing, as far as possible, pure subsets of patients at the extremes of the coronary artery disease spectrum, which was important for the aim of our study. Third, although OCT criteria for detection of healed coronary plaques have been validated against histology, these data derive from a single-center study with a relatively small sample size.12 Larger studies are warranted to further characterize these features and their potential clinical implications. Fourth, assessment of macrophage accumulation by OCT may be influenced by changes in the indices of refraction caused by other plaque components, such as microcalcifications, cholesterol crystals, or internal/external elastic membrane.25 These data should therefore be interpreted with caution and considered as hypothesis-generating. Finally, no systematic 3-vessel imaging was performed. However, in the real-world practice, imaging 3 vessels is impractical and might expose patients to unjustified additional risk.
Conclusions
Patients with rACS and those with sAMI showed a similar prevalence of lipid-rich plaque and TCFA as well as similar angiographic disease burden and progression, which were significantly lower in patients with ls-SAP. Healed coronary plaques, a signature of prior plaque destabilization contained by reparative mechanisms, which are characterized by a multilayered, onionlike appearance at OCT imaging, were rarely observed in patients with rACS, whereas their prevalence was significantly higher in patients with ls-SAP and sAMI. Taken together, these findings suggest that although atherosclerotic disease phenotype and burden are important predisposing factors for acute coronary disease, other factors, including plaque healing, may lead the natural history of a patient toward either recurrence of acute events or long-term clinical stability.
eMethods.
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Segment-Based OCT Analysis.
eTable 3. Clinical Follow-up
eTable 4. Univariate Cox Regression Analysis
eTable 5. Multivariate Cox Regression Analysis
eReferences.
eFigure. Major Adverse Cardiac Event (MACE) Rates and Kaplan-Meier Survival Curves in Patients With and Without Healed Coronary Plaques
References
- 1.Eisen A, Giugliano RP, Braunwald E. Updates on acute coronary syndrome: a review. JAMA Cardiol. 2016;1(6):718-730. doi: 10.1001/jamacardio.2016.2049 [DOI] [PubMed] [Google Scholar]
- 2.Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA. 1999;281(10):921-926. doi: 10.1001/jama.281.10.921 [DOI] [PubMed] [Google Scholar]
- 3.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20(5):1262-1275. doi: 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 4.Niccoli G, Montone RA, Di Vito L, et al. . Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J. 2015;36(22):1377-1384. doi: 10.1093/eurheartj/ehv029 [DOI] [PubMed] [Google Scholar]
- 5.Jia H, Abtahian F, Aguirre AD, et al. . In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748-1758. doi: 10.1016/j.jacc.2013.05.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65(8):846-855. doi: 10.1016/j.jacc.2014.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone GW, Maehara A, Lansky AJ, et al. ; PROSPECT Investigators . A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226-235. doi: 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 8.Ferencik M, Mayrhofer T, Bittner DO, et al. . Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018;3(2):144-152. doi: 10.1001/jamacardio.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke AP, Kolodgie FD, Farb A, et al. . Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103(7):934-940. doi: 10.1161/01.CIR.103.7.934 [DOI] [PubMed] [Google Scholar]
- 10.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart. 1999;82(3):265-268. doi: 10.1136/hrt.82.3.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crea F, Liuzzo G. Pathogenesis of acute coronary syndromes. J Am Coll Cardiol. 2013;61(1):1-11. doi: 10.1016/j.jacc.2012.07.064 [DOI] [PubMed] [Google Scholar]
- 12.Shimokado A, Matsuo Y, Kubo T, et al. . In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis. 2018;275:35-42. doi: 10.1016/j.atherosclerosis.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 13.Prati F, Regar E, Mintz GS, et al. ; Expert’s OCT Review Document . Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401-415. doi: 10.1093/eurheartj/ehp433 [DOI] [PubMed] [Google Scholar]
- 14.Tearney GJ, Regar E, Akasaka T, et al. ; International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT) . Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058-1072. doi: 10.1016/j.jacc.2011.09.079 [DOI] [PubMed] [Google Scholar]
- 15.Sugiyama T, Yamamoto E, Bryniarski K, et al. . Nonculprit plaque characteristics in patients with acute coronary syndrome caused by plaque erosion vs plaque rupture: a 3-vessel optical coherence tomography study. JAMA Cardiol. 2018;3(3):207-214. doi: 10.1001/jamacardio.2017.5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergallo R, Uemura S, Soeda T, et al. . Prevalence and predictors of multiple coronary plaque ruptures: in vivo 3-vessel optical coherence tomography imaging study. Arterioscler Thromb Vasc Biol. 2016;36(11):2229-2238. doi: 10.1161/ATVBAHA.116.307891 [DOI] [PubMed] [Google Scholar]
- 17.Di Vito L, Agozzino M, Marco V, et al. . Identification and quantification of macrophage presence in coronary atherosclerotic plaques by optical coherence tomography. Eur Heart J Cardiovasc Imaging. 2015;16(7):807-813. doi: 10.1093/ehjci/jeu307 [DOI] [PubMed] [Google Scholar]
- 18.Bogaty P, Brecker SJ, White SE, et al. . Comparison of coronary angiographic findings in acute and chronic first presentation of ischemic heart disease. Circulation. 1993;87(6):1938-1946. doi: 10.1161/01.CIR.87.6.1938 [DOI] [PubMed] [Google Scholar]
- 19.Gensini G. Coronary Arteriography. New York, NY: Futura Publishing Company; 1975.
- 20.Otsuka F, Joner M, Prati F, Virmani R, Narula J. Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol. 2014;11(7):379-389. doi: 10.1038/nrcardio.2014.62 [DOI] [PubMed] [Google Scholar]
- 21.Souteyrand G, Arbustini E, Motreff P, et al. . Serial optical coherence tomography imaging of ACS-causing culprit plaques. EuroIntervention. 2015;11(3):319-324. doi: 10.4244/EIJV11I3A59 [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 23.Sabatine MS, Giugliano RP, Wiviott SD, et al. ; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509. doi: 10.1056/NEJMoa1500858 [DOI] [PubMed] [Google Scholar]
- 24.Räber L, Mintz GS, Koskinas KC, et al. ; ESC Scientific Document Group . Clinical use of intracoronary imaging, part 1: guidance and optimization of coronary interventions, an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39(35):3281-3300. doi: 10.1093/eurheartj/ehy285 [DOI] [PubMed] [Google Scholar]
- 25.Phipps JE, Vela D, Hoyt T, et al. . Macrophages and intravascular OCT bright spots: a quantitative study. JACC Cardiovasc Imaging. 2015;8(1):63-72. doi: 10.1016/j.jcmg.2014.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Inclusion and Exclusion Criteria
eTable 2. Segment-Based OCT Analysis.
eTable 3. Clinical Follow-up
eTable 4. Univariate Cox Regression Analysis
eTable 5. Multivariate Cox Regression Analysis
eReferences.
eFigure. Major Adverse Cardiac Event (MACE) Rates and Kaplan-Meier Survival Curves in Patients With and Without Healed Coronary Plaques