Key Points
Question
Does doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash compared with placebo reduce oral mucositis–related pain in patients undergoing radiotherapy for head and neck cancer?
Findings
In this randomized clinical trial that included 275 patients undergoing radiotherapy, the use of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash reduced mucositis pain (defined by the area under the curve and adjusted for baseline pain score) during the first 4 hours after administration by 11.6 and 11.7 points, respectively, compared with 8.7 points for placebo mouthwash; both comparisons with placebo were statistically significant.
Meaning
Doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash may be effective in reducing radiotherapy-related mucositis pain, but further research is needed to assess longer-term efficacy and safety.
Abstract
Importance
Oral mucositis causes substantial morbidity during head and neck radiotherapy. In a randomized study, doxepin mouthwash was shown to reduce oral mucositis–related pain. A common mouthwash comprising diphenhydramine-lidocaine-antacid is also widely used.
Objective
To evaluate the effect of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash for the treatment of oral mucositis–related pain.
Design, Setting, and Participants
A phase 3 randomized trial was conducted from November 1, 2014, to May 16, 2016, at 30 US institutions and included 275 patients who underwent definitive head and neck radiotherapy, had an oral mucositis pain score of 4 points or greater (scale, 0-10), and were followed up for a maximum of 28 days.
Interventions
Ninety-two patients were randomized to doxepin mouthwash (25 mg/5 mL water); 91 patients to diphenhydramine-lidocaine-antacid; and 92 patients to placebo.
Main Outcome and Measures
The primary end point was total oral mucositis pain reduction (defined by the area under the curve and adjusted for baseline pain score) during the 4 hours after a single dose of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash compared with a single dose of placebo. The minimal clinically important difference was a 3.5-point change. The secondary end points included drowsiness, unpleasant taste, and stinging or burning. All scales ranged from 0 (best) to 10 (worst).
Results
Among the 275 patients randomized (median age, 61 years; 58 [21%] women), 227 (83%) completed treatment per protocol. Mucositis pain during the first 4 hours decreased by 11.6 points in the doxepin mouthwash group, by 11.7 points in the diphenhydramine-lidocaine-antacid mouthwash group, and by 8.7 points in the placebo group. The between-group difference was 2.9 points (95% CI, 0.2-6.0; P = .02) for doxepin mouthwash vs placebo and 3.0 points (95% CI, 0.1-5.9; P = .004) for diphenhydramine-lidocaine-antacid mouthwash vs placebo. More drowsiness was reported with doxepin mouthwash vs placebo (by 1.5 points [95% CI, 0-4.0]; P = .03), unpleasant taste (by 1.5 points [95% CI, 0-3.0]; P = .002), and stinging or burning (by 4.0 points [95% CI, 2.5-5.0]; P < .001). Maximum grade 3 adverse events for the doxepin mouthwash occurred in 3 patients (4%); diphenhydramine-lidocaine-antacid mouthwash, 3 (4%); and placebo, 2 (2%). Fatigue was reported by 5 patients (6%) in the doxepin mouthwash group and no patients in the diphenhydramine-lidocaine-antacid mouthwash group.
Conclusions and Relevance
Among patients undergoing head and neck radiotherapy, the use of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash vs placebo significantly reduced oral mucositis pain during the first 4 hours after administration; however, the effect size was less than the minimal clinically important difference. Further research is needed to assess longer-term efficacy and safety for both mouthwashes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02229539
This phase 3 randomized trial compares the effect of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash on oral mucositis–related pain among patients who underwent definitive head and neck radiotherapy.
Introduction
Pain from radiotherapy- and chemoradiotherapy-induced oral mucositis is a substantial adverse effect of treatment for head and neck cancer. Symptoms can be severe, and oral mucositis can lead to hospitalization and requirement of a feeding tube. More than 80% of patients develop oral mucositis during radiotherapy, and mouthwashes and systemic analgesic agents are frequently used to treat the condition.1
Few pharmacological agents or interventions have been shown to effectively reduce the severity of radiotherapy-related oral mucositis and its associated pain.2 However, in single-group pilot studies, the tricyclic antidepressant doxepin (used in a mouthwash preparation) reduced radiotherapy-induced oral mucositis pain.3,4,5,6 Previously, the Alliance for Clinical Trials in Oncology conducted a multi-institutional randomized clinical trial (North Central Cancer Treatment Group N09C6 [Alliance]) that showed a doxepin mouthwash reduced the oral mucositis pain experienced by patients with cancer and was well tolerated.7
A number of commercially available combination mouthwash preparations have long existed for radiotherapy-related oral mucositis pain.8,9 A common preparation with more than 50 variations contains the 3 active ingredients of diphenhydramine, lidocaine, and antacid. Despite the popularity of a diphenhydramine-lidocaine-antacid mouthwash, to our knowledge, no randomized placebo-controlled trials or Cochrane reviews exist to support the use of these preparations.9 The American Academy of Nursing recently advised against using these mixed medications for oral mucositis induced by cancer treatment.10 Therefore, randomized data were needed.
This randomized trial was designed to evaluate whether a single dose of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash was more effective than placebo in reducing oral mucositis pain for patients undergoing radiotherapy to the oral cavity. The patients’ symptoms and adverse event profiles were also assessed.
Methods
The approved versions of the protocol and statistical analysis plans appear in Supplement 1. All local institutional review boards and their ethics committees approved the full protocol and methods for conducting this trial before entry of any patient into the study. Written informed consent was obtained for every participant before administration of any study intervention. The participants did not receive a monetary stipend.
Study Design and Participants
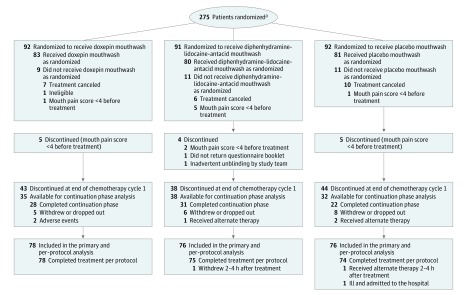
The study was designed as a 3-group, double-blind, placebo-controlled phase 3 randomized trial (Figure 1). To be eligible for the study, patients had to be aged 18 years or older; have an Eastern Cooperative Oncology Group performance status of 0, 1, or 2; have head and neck cancer and be undergoing definitive radiotherapy (including parts of the oral cavity or oropharyngeal area) to a dose of at least 45 Gy; and have had oral mucositis pain rated as at least a 4 on a 0 to 10 scale (0 being no pain; 10 being the worst imaginable pain).
Figure 1. Recruitment, Randomization, and Patient Flow in a Study of Mouthwash to Treat Oral Mucositis Pain.
aThe number of patients approached and assessed for eligibility was not collected because it was not required per protocol.
Cancer had to be confirmed by histological findings and patients had to undergo a physical examination that showed radiotherapy-related oral mucositis. Patients had to be able to complete multiple questionnaires. There was no planned posttreatment evaluation for assessing change in oral mucositis. Race and ethnicity were measured in this study in accordance with the guidelines encouraging diversity of patients with cancer participating in national clinical trials. Fixed categories were used and patients self-reported their racial and ethnic identities.
Exclusion criteria were known allergy to diphenhydramine, lidocaine, antacid, doxepin, or tricyclic antidepressants; use of any antiarrhythmic medication; current diagnosis of untreated or unresolved oral candidiasis or oral herpes simplex virus infection; use of glutamine or sucralfate powders during study registration; and use of cryotherapy for prophylactic mucosal protection within 6 weeks prior to registration.
Randomization
Eligible patients were registered and randomized in a 1:1:1 ratio to receive 1 of the 3 treatments: (1) doxepin mouthwash (25 mg in 5-mL solution); (2) mouthwash composed of 1.7 mL each (5.1-mL total solution) of diphenhydramine (12.5 mg in 5-mL alcohol-free solution), lidocaine (2% viscous solution), and antacid (200 mg of aluminum hydroxide, 200 mg of magnesium hydroxide, and 20 mg of simethicone in 355-mL solution); or (3) placebo mouthwash (2.5 mL of Ora-Sweet SF oral solution and 2.5 mL of water). A period of 14 days between randomization and treatment initiation was allowed.
The doxepin mouthwash and placebo mouthwash had similar transparent appearances, whereas the mixed mouthwash had a thicker texture. However, patients were not told what to expect regarding the consistency or texture of the treatment. Patients were assigned to each treatment based on a computer-generated randomization algorithm using the Pocock and Simon dynamic allocation procedure. Use of this procedure balanced the marginal distribution of the following stratification factors: sex, use of concurrent chemotherapy, patient age (<60 vs ≥60 years), and Radiation Therapy Oncology Group acute radiation morbidity criteria (1 vs 2 vs ≥3). In all 3 groups, patients were instructed to rinse for 1 minute and then spit it out.
Interventions
Patients began protocol treatment within 14 days of enrollment. Protocol therapy consisted of 2 cycles: cycle 1 was mandatory and double-blinded and consisted of a single-dose treatment during 1 day. During cycle 1, coordination of the timing of radiotherapy and administration of the study mouthwashes was not required. Cycle 2 was an optional continuation phase, with a rinse administered every 4 hours for up to 7 days.
Cycle 2 took place immediately after cycle 1 (within 7 days). In a blinded fashion, patients were encouraged to continue treatment with the same study agent for an additional week. During the optional continuation phase, patients could opt out at any time if or when they perceived that the treatment was no longer beneficial. If the patient opted out, he or she could then be unblinded to treatment and receive further intervention for oral mucositis during the course of routine clinical care.
Outcomes
A modified combination of 2 validated tools (the Oral Mucositis Daily Questionnaire and the Oral Mucositis Weekly Questionnaire–Head and Neck Cancer) was used to measure pain, drowsiness, unpleasant taste, and stinging or burning.11,12 The questionnaires were administered in the clinic before administration of the study intervention (baseline) and at 5, 15, 30, and 60 minutes after the rinse. Following discharge from the clinic, patients completed 2 additional sets of questionnaires at 120 and 240 minutes after the rinse.
Additional analgesic use was evaluated 60 minutes after the patient received the study medication. Patients who chose to continue with cycle 2 were given additional booklets with 7 daily questionnaires to complete. All symptom questions in the questionnaires (including pain, drowsiness, taste, and stinging or burning) used a scale from 0 to 10 with 0 being the least burdensome and 10 being the most burdensome. Patients were monitored for adverse events using version 4.0 of the Common Terminology Criteria for Adverse Events (CTCAE). No other mouthwashes were allowed. Patients could take other analgesic agents at least 1 hour before or after the study treatment.
The primary outcome was pain. Secondary outcomes included drowsiness; unpleasant taste; stinging or burning; use of alternative analgesic agents; patient preference for continued therapy; and, during the optional continuation phase, length of time, pain score, and alternative analgesic use.
Sample Size
The primary end point was change in total mouth and throat pain that was measured using the numerical analog scale in the questionnaires and taken at baseline and at 5, 15, 30, 60, 120, and 240 minutes after assigned treatment with doxepin mouthwash, diphenhydramine-lidocaine-antacid mouthwash, or placebo mouthwash. The total pain reduction was calculated by the area under the curve for the mean of mouth and throat pain using a numerical scale. The area under the curve was calculated by proration when there were terminal missing data.
The proration was accomplished by calculating the area under the curve from baseline to the last reported value and dividing this area under the curve by the fraction of the total expected time that was completed.13 If the missing data were intermittent, simple imputation according to the trapezoidal rule was applied to calculate the area under the curve. As per protocol, the time points of 5, 15, 30, 60, 120, and 240 minutes were replaced with a numerical scale of 1, 2, 3, 4, 5, and 6 in the area under the curve calculation to properly weight the more immediate pain scores after treatment. Next, the area under the curve was calculated by subtracting the baseline score from the score at each time point and by summing these differences across the time points.
A study by Leenstra et al7 showed that doxepin mouthwash was associated with an area under the curve reduction for pain of 4.4 points (common SD, 7.0) compared with placebo. In that study, an area under the curve reduction for pain of 3.5 points (ie, half of the SD14) was considered clinically meaningful. In the current trial, the primary analysis was to compare the pain reduction from each of the treatment groups (doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash) with the placebo mouthwash group.
A conservative Bonferroni approach was used to adjust for the 2 treatment vs placebo comparisons. A sample size of 78 patients per group was estimated to provide at least 80% power at a 2-sided type I error rate of 2.5% to detect a difference in the area under the curve for pain reduction of 3.5 points (half of the common SD of 7.0). The sample size was then inflated by 15% to account for cancellations and ineligible patients, resulting in a total sample size of 270 patients.
Statistical Analysis
The primary analysis included all patients who met the eligibility criteria, did not cancel before receiving treatment, had a pain score of 4 points or greater at baseline, and had no major protocol violations. A major protocol violation was defined as a severe deviation from treatment during cycle 1 of initial therapy such that the primary end point was not evaluable. The mean pain scores were calculated, and the area under the curve for pain reduction was adjusted for baseline scores and calculated for each treatment group.
For each treatment group, mean pain scores over time were plotted. Pain reduction was compared between 2 treatment groups (doxepin mouthwash vs placebo mouthwash and diphenhydramine-lidocaine-antacid mouthwash vs placebo mouthwash) using nonparametric Wilcoxon rank sum tests.15 In addition, longitudinal analyses of mouth and throat pain were conducted using the linear mixed model to adjust for baseline covariates.
Similar analyses were conducted for drowsiness, unpleasant taste, and stinging or burning. The incidence of using an additional analgesic agent between 1 and 4 hours after initial mouth rinse, frequency of other adverse events from the oral mouthwash, and helpfulness of the mouthwash for alleviating mouth or throat pain at any time during the previous 4 hours were compared using the χ2 test. The frequencies of patient preference for continued therapy were compared in the same manner. The maximum grade of each adverse event (using the CTCAE) reported at cycle 1 was similarly compared between groups.
The post hoc analyses of the primary end point included a responder analysis with a 3.5-point improvement in the area under the curve as the definition of response using a χ2 test, linear mixed models using site as a random effect, Hodges-Lehmann location-shift estimators of the treatment effect and confidence intervals,15 and comparisons of the change in peak score from baseline and final score from baseline using Wilcoxon rank sum tests. Sensitivity analyses using difference imputation methods for missing values and parallel line plots also were evaluated.16
In addition, an exploratory analysis of the continuation phase (cycle 2) was summarized by treatment group, and scores between groups were compared using the Wilcoxon rank sum test. Other end points, including daily use of additional analgesic agents, frequency of other adverse events, and maximum grade of each adverse event using the CTCAE reported at cycle 2, were similarly analyzed.
The distribution of daily study mouthwash was compared between groups using the Fisher exact test. A post hoc analysis to explore the differences between patients who chose to continue treatment vs those who chose to discontinue treatment was conducted using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables.
The primary end point was tested at a 2-sided significance level of .025, whereas the secondary end points were tested at a 2-sided significance level of .05. Because the study was powered only for the primary end point comparison and no adjustment was made for the significance threshold for the secondary end points, all secondary analyses should be interpreted as exploratory.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. SAS software version 9.4 (SAS Institute Inc) was used for the data analysis. Data quality was monitored and reviewed by the Alliance Statistics and Data Center and by the study chairperson. All analyses were based on the data in the study database with an end date of August 21, 2017. The study was monitored by the Alliance data and safety monitoring board, a National Cancer Institute–approved functioning body.
Results
Accrual Period and Trial Participants
The study was opened on November 1, 2014, and closed to accrual on May 16, 2016. The last patient follow-up was on June 1, 2016. The trial enrolled 275 patients (92 in the doxepin mouthwash group, 91 in the diphenhydramine-lidocaine-antacid mouthwash group, and 92 in the placebo mouthwash group) from 30 US institutions (additional details appear in the eBox in Supplement 2). Forty-five patients (14 in the doxepin mouthwash group, 15 in the diphenhydramine-lidocaine-antacid mouthwash group, and 16 in the placebo mouthwash group) were excluded from the primary analysis (Figure 1).
Thirty-one patients (11%) canceled or were found ineligible before they started treatment and were excluded. Fourteen patients (5%) were excluded because they had a baseline mouth pain score of less than 4 points (5 patients in the doxepin mouthwash group, 2 in the diphenhydramine-lidocaine-antacid mouthwash group, and 5 in the placebo mouthwash group), did not return the questionnaire booklets (1 patient in the diphenhydramine-lidocaine-antacid mouthwash group), or had a randomization issue (1 patient in the diphenhydramine-lidocaine-antacid mouthwash group).
The characteristics of the 45 excluded patients were similar to the rest of analyzed cohort. There were more men who were excluded vs women (96% vs 76%, respectively; P = .001). Of the 230 patients eligible for the primary analysis (78 in the doxepin mouthwash group, 76 in the diphenhydramine-lidocaine-antacid mouthwash group, and 76 in the placebo mouthwash group), 227 patients (78 in the doxepin mouthwash group [100%], 75 in the diphenhydramine-lidocaine-antacid mouthwash group [99%], and 74 in the placebo mouthwash group [97%]) completed treatment per protocol. Of the 227 patients who completed per-protocol treatment, 105 patients (46%) (35 in the doxepin mouthwash group, 38 in the diphenhydramine-lidocaine-antacid mouthwash group, and 32 in the placebo mouthwash group) continued treatment during the optional cycle 2. The baseline characteristics of the patients appear in Table 1.
Table 1. Baseline Characteristics.
| Characteristic | No. (%) of Patients | ||
|---|---|---|---|
| Doxepin Mouthwash (n = 78) |
Diphenhydramine-Lidocaine-Antacid Mouthwash (n = 76) |
Placebo Mouthwash (n = 76) |
|
| Age, median (range), y | 61.5 (26.0-87.0) | 60.0 (23.0-82.0) | 60.0 (31.0-94.0) |
| Race | |||
| White | 75 (96) | 67 (88) | 71 (93) |
| Black | 2 (3) | 7 (9) | 4 (5) |
| Asian | 1 (1) | 1 (1) | 1 (1) |
| Not reported | 0 | 1 (1) | 0 |
| Sex | |||
| Male | 59 (76) | 58 (76) | 57 (75) |
| Female | 19 (24) | 18 (24) | 19 (25) |
| Concurrent chemotherapy | |||
| Yes | 62 (79) | 62 (82) | 64 (84) |
| No | 16 (21) | 14 (18) | 12 (16) |
| Mucous membrane scorea | |||
| 1 | 17 (22) | 16 (21) | 16 (21) |
| 2 | 47 (60) | 48 (63) | 49 (64) |
| ≥3 | 14 (18) | 12 (16) | 11 (15) |
| ECOG performance statusb | |||
| 0 | 37 (47) | 31 (41) | 33 (43) |
| 1 | 40 (51) | 39 (51) | 40 (53) |
| 2 | 1 (1) | 6 (8) | 3 (4) |
| Oral pain score at registrationc | |||
| Mean (SD) | 6.1 (1.8) | 5.9 (1.6) | 5.9 (1.8) |
| Median (range) | 6.0 (4.0-10.0) | 6.0 (4.0-10.0) | 5.5 (4.0-10.0) |
| Current smoking, No./total (%) | |||
| Yes | 12/78 (15) | 10/75 (13) | 10/74 (14) |
| No | 66/78 (85) | 65/75 (87) | 64/74 (86) |
| Current alcohol use, No./total (%) | |||
| Yes | 6/77 (8) | 4/75 (5) | 5/74 (7) |
| No | 71/77 (92) | 71/75 (95) | 69/74 (93) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Evaluated according to the Radiation Therapy Oncology Group acute radiation morbidity scoring criteria: grade 0, no change over baseline; grade 1, injection or mild pain but not requiring analgesics; grade 2, patchy mucositis that may produce inflammatory serosanguinous discharge, moderate pain requiring analgesia, or both; grade 3, confluent fibrinous mucositis that may include severe pain requiring narcotics; and grade 4, ulceration, hemorrhage, or necrosis.
Evaluated as grade 0, fully active, able to carry out all predisease performance without restriction (best); grade 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; grade 2, ambulatory, up and about for more than 50% of waking hours, and capable of all self-care but unable to carry out any work activities; grade 3, capable of only limited self-care and confined to bed or chair for more than 50% of waking hours; and grade 4, completely disabled, cannot carry on any self-care, or totally confined to bed or chair (worst).
Range from 0 to 10; 0 indicates the best; 10, the worst.
Primary Outcome
Total pain during the first 4 hours after treatment (using the mean score as measured by the area under the curve and adjusted for the baseline pain score) decreased by 11.6 points in the doxepin mouthwash group, by 11.7 points in the diphenhydramine-lidocaine-antacid mouthwash group, and by 8.7 points in the placebo mouthwash group (between-group difference for doxepin vs placebo, 2.9 points [95% CI, 0.2-6.0 points], P = .02; and between-group difference for diphenhydramine-lidocaine-antacid vs placebo, 3.0 points [95% CI, 0.1-5.9 points], P = .004).
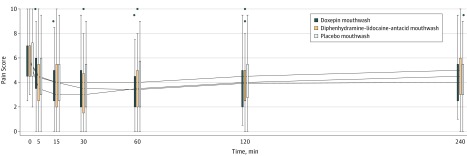
Total pain reduction measured by the area under the curve during the first 4 hours after treatment was higher in the doxepin mouthwash group (median, 10.4 points [interquartile range {IQR}, 4.5-15.3 points]) vs the placebo mouthwash group (median, 8.7 points [IQR, 2.0-13.0 points], P = .02) and higher in the diphenhydramine-lidocaine-antacid mouthwash group (median, 11.5 points [IQR, 5.4-17.0 points]) vs the placebo mouthwash group (median, 8.7 points [IQR, 2.0-13.0 points], P = .004). Pain scores at baseline and during the next 2 hours after the study intervention appear in Figure 2.
Figure 2. Pain Scores Over Time During Cycle 1 for Doxepin, Diphenhydramine-Lidocaine-Antacid, and Placebo Groups.
Pain was measured on a scale from 0 (least burdensome) to 10 (most burdensome). Compared with placebo, both doxepin mouthwash and diphenhydramine-lidocaine-antacid mouthwash resulted in a statistically significant reduction in pain (P = .03 for doxepin mouthwash; P = .004 for diphenhydramine-lidocaine-antacid mouthwash). Boxplots of the pain scores for each group have been staggered at the time points so that they do not overlap. In the boxplots, the solid lines in the middle of the box are the median values, the top of the box is the 75th percentile, the bottom of the box is the 25th percentile, and the lines extending from the boxes extend out to the upper and lower adjacent values. Outliers are represented by the black dots.
Results from the linear mixed model (adjusted for sex, age, concurrent use of chemotherapy, treatment site, and mucous membrane score) showed an area under the curve improvement in pain score of 3.27 points (95% CI, 0.91-5.62 points) in the doxepin mouthwash group compared with the placebo mouthwash group (P = .007) and an improvement of 3.04 points (95% CI, 0.67-5.41 points) in the diphenhydramine-lidocaine-antacid mouthwash group compared with placebo mouthwash group (P = .01).
Only 11 patients (3 in the doxepin mouthwash group, 4 in the diphenhydramine-lidocaine-antacid mouthwash group, and 4 in the placebo mouthwash group) had missing data at any time point. Five of these patients were missing pain scores at only 1 time point, and the other 6 were missing pain scores at only 2 time points. Sensitivity analyses were run imputing these missing values and using different assumptions about the missing data. Because only 1% of all possible pain scores were missing, the results of these sensitivity analyses were almost identical to the original results.
Secondary End Points
Total drowsiness during the first 4 hours after treatment (adjusted for baseline scores as measured by the area under the curve) was 1.5 points (95% CI, 0 to 4.0 points) higher after the doxepin mouthwash was used compared with the placebo mouthwash (P = .03; Table 2). There was no significant difference in drowsiness between the use of the diphenhydramine-lidocaine-antacid mouthwash and the use of the placebo mouthwash (difference, 0 points [95% CI, −3.0 to 0.5 points], P = .35).
Table 2. Adverse Events, Additional Analgesic Use, and Patient Preference.
| Doxepin Mouthwash (n = 78) |
Diphenhydramine-Lidocaine-Antacid Mouthwash (n = 76) |
Placebo Mouthwash (n = 76) |
Doxepin Mouthwash vs Placebo Mouthwash | Diphenhydramine-Lidocaine-Antacid Mouthwash vs Placebo Mouthwash | |||
|---|---|---|---|---|---|---|---|
| Difference (95% CI) | P Value | Difference (95% CI) | P Value | ||||
| Adverse Events | |||||||
| Drowsiness, median (IQR), points | 0 (−2.5 to 3.0) | −1.8 (−7.3 to 0) | 0 (−8.0 to 0) | 1.5 (0 to 4.0)a | .03b | 0 (−3.0 to 0.5)a | .35b |
| Unpleasant taste, median (IQR), pointsc | 3.5 (0 to 8.5) | 2.0 (0 to 6.3) | 0.8 (0 to 3.0) | 1.5 (0 to 3.0)a | .002b | 0 (0 to 1.5)a | .12b |
| Stinging or burning, median (IQR), pointsc | 5.3 (2.5 to 11.0) | 0.8 (0 to 4.8) | 0.5 (0 to 3.0) | 4.0 (2.5 to 5.0)a | <.001b | 0 (0 to 0.5)a | .29b |
| Additional Analgesic Use, No./Total (%) | |||||||
| At 120 min | 10/77 (13.0) | 7/73 (9.6) | 14/70 (20.0) | −7.0 (−23.0 to 9.3)d | .25e | −10.4 (−26.6 to 6.3)d | .08e |
| At 240 min | 13/76 (17.1) | 14/72 (19.4) | 21/72 (29.2) | −12.1 (−27.9 to 4.3)d | .08e | −9.7 (−26.3 to 7.3)d | .17e |
| Patient Preference | |||||||
| Continue mouthwash use, No./total (%) | 45/75 (60.0) | 46/73 (63.0) | 38/71 (53.5) | 6.5 (−10.0 to 22.5)d | .43e | 9.5 (−7.3 to 25.4)d | .25e |
Abbreviation: IQR, interquartile range.
This is an estimate using the Hodges-Lehmann method, which was a post hoc analysis.
Calculated using the Wilcoxon rank sum test.
Starting at 5 to 240 minutes during cycle 1. All adverse events were measured on a scale from 0 (least burdensome) to 10 (most burdensome).
Expressed as percentages.
Calculated using the χ2 test.
Patients using the doxepin mouthwash reported higher frequencies for unpleasant taste than patients in the placebo mouthwash group (between-group difference, 1.5 points [95% CI, 0-3.0 points], P = .002; Table 2). No significant difference for unpleasant taste was observed between the diphenhydramine-lidocaine-antacid mouthwash group compared with the placebo mouthwash group (between-group difference, 0 points [95% CI, 0-1.5 points], P = .12).
Patients reported higher frequencies for stinging or burning after use of the doxepin mouthwash compared with the placebo mouthwash (between-group difference, 4.0 points [95% CI, 2.5-5.0 points], P < .001). No significant difference in stinging or burning was reported between the diphenhydramine-lidocaine-antacid mouthwash group compared with the placebo mouthwash group (between-group difference, 0 points [95% CI, 0-0.5 points], P = .29; Table 2).
After the study intervention, 13 patients (17%) in the doxepin mouthwash group and 14 patients (19%) in the diphenhydramine-lidocaine-antacid mouthwash group used additional analgesic agents compared with 21 patients (29%) in the placebo mouthwash group. Additional analgesic use was not significantly different between the groups (Table 2).
Forty-five patients (60%) in the doxepin mouthwash group, 46 patients (63%) in the diphenhydramine-lidocaine-antacid mouthwash group, and 38 patients (54%) in the placebo mouthwash group reported a preference for continued therapy. These differences were not statistically significant.
Adverse Events
Fatigue was the most commonly reported adverse event. Five patients (6%) using the doxepin mouthwash reported fatigue during cycle 1, whereas no fatigue was reported in the diphenhydramine-lidocaine-antacid mouthwash group or the placebo mouthwash group.
Overall, 3 patients (4%) in the doxepin mouthwash group, 3 (4%) in the diphenhydramine-lidocaine-antacid mouthwash group, and 2 (2%) in the placebo mouthwash group reported a maximum grade 3 adverse event. The other adverse event grades and frequencies were all mild according to the CTCAE and did not differ among groups.
Other End Points
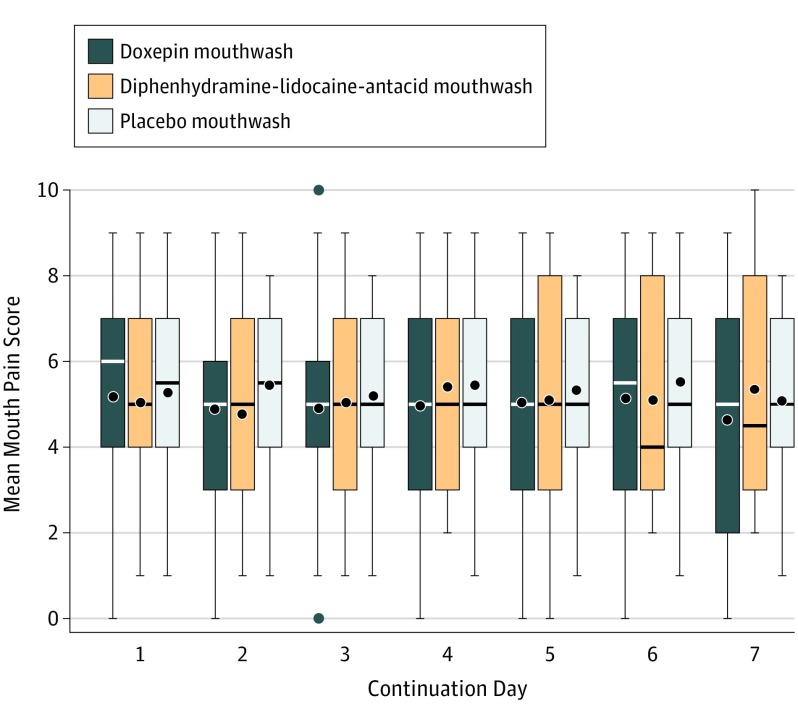
Of the 227 patients who completed cycle 1 treatment, 105 (46%) continued in a blinded fashion. The median length of cycle 2 treatment was 7 days (IQR, 5-7 days) in the doxepin mouthwash group, 6 days (IQR, 3-7 days) in the diphenhydramine-lidocaine-antacid mouthwash group, and 5.5 days (IQR, 2 to 7 days) in the placebo mouthwash group. The median daily mouth pain scores were not significantly different (Figure 3).
Figure 3. Mouth Pain Scores by Continuation Day During Cycle 2 for Doxepin, Diphenhydramine-Lidocaine-Antacid, and Placebo Groups.
Pain was measured on a scale from 0 (least burdensome) to 10 (most burdensome). Boxplots of the pain scores for each group have been staggered at the time points so that they do not overlap. In the boxplots, the solid lines in the middle of the box are the median values, the top of the box is the 75th percentile, the bottom of the box is the 25th percentile, the black dots are mean values, and the lines extending from the boxes extend out to the upper and lower adjacent values. Outliers, which appear above and below doxepin mouthwash at continuation day 3, are represented by the black dots.
Patients reported more unpleasant taste on day 3 with the doxepin mouthwash (difference, 1.0 [95% CI, 0-2.0]) vs placebo mouthwash (P = .007), but not with the diphenhydramine-lidocaine-antacid mouthwash vs the placebo mouthwash. Patients using the doxepin mouthwash daily reported more stinging or burning than those using the placebo mouthwash daily over all days (P ≤ .02) including day 3 (1.8 points [95% CI, 0.8-2.8 points], P < .001), day 4 (1.8 points [95% CI, 0.7-2.5 points], P = .002), day 5 (2.3 points [95% CI, 1.3-3.3 points], P < .001), and day 7 (2.1 points [95% CI, 0.7-3.5 points], P = .004) (eTable 1 in Supplement 2).
The incidence of additional analgesic use during cycle 2 was not significantly different among groups. The types of baseline analgesic medication use included nonsteroidal anti-inflammatory drugs (NSAIDs), drugs that were not NSAIDs (such as acetaminophen), and short-acting and long-acting opioids. The incidence of use by type of medication did not differ significantly among groups for both cycles 1 and 2 (eTable 2 in Supplement 2).
There was no significant difference in pain relief between the doxepin mouthwash and the placebo mouthwash. More mouth or throat pain relief was noted for the diphenhydramine-lidocaine-antacid mouthwash group compared with the placebo mouthwash group on day 1 (97% vs 80%, respectively; between-group difference, 17.3% [95% CI, 2.6%-32.5%], P = .02) and on day 6 (100% vs 81%; between-group difference, 18.8% [95% CI, 0%-38.9%], P = .02). No patients using the doxepin mouthwash had a grade 3 or worse adverse event. Three patients using the diphenhydramine-lidocaine-antacid mouthwash and 2 using the placebo mouthwash experienced a grade 3 adverse event.
Post Hoc Analyses
The Hodges-Lehmann location-shift estimators of the median area under the curve for total pain reduction were higher in the doxepin mouthwash group compared with the placebo mouthwash group (between-group difference, 3.0 [95% CI, 0.5-5.5]) and in the diphenhydramine-lidocaine-antacid mouthwash group compared with the placebo mouthwash group (between-group difference, 3.8 [95% CI, 1.3-6.3]).
Post hoc exploratory responder analyses (with an area under the curve reduction of ≥3.5 points defined as a response) showed that 62 patients (79.5%) in the doxepin mouthwash group, 65 patients (85.5%) in the diphenhydramine-lidocaine-antacid mouthwash group, and 52 patients (68.4%) in the placebo mouthwash group responded to treatment (P = .14 for doxepin vs placebo and P = .02 for diphenhydramine-lidocaine-antacid vs placebo).
Additional post hoc analyses comparing pain reduction at peak score from baseline showed an improvement of 0 points (95% CI, 0 to 0.5 points) in the doxepin mouthwash group vs the placebo mouthwash group (P = .44) and an improvement of 0.5 points (95% CI, 0 to 1.0 points) in the diphenhydramine-lidocaine-antacid mouthwash group vs the placebo mouthwash group (P = .01). Pain reduction at last time point from baseline showed an improvement of 0.5 points (95% CI, 0 to 1.0 points) in the doxepin mouthwash group vs the placebo mouthwash group (P = .03) and an improvement of 0 points (95% CI, −0.5 to 0.5 points) in the diphenhydramine-lidocaine-antacid mouthwash group vs the placebo mouthwash group (P = .48) (eFigures 1 and 2 in Supplement 2).
Of the 30 participating sites, 25 enrolled fewer than 10 patients each. A post hoc linear mixed model (with site as a random effect) was performed and the results were consistent with the primary outcome. There was an area under the curve improvement of 3.27 points (95% CI, 0.91-5.62 points) in the doxepin mouthwash group vs the placebo mouthwash group (P = .007) and of 3.04 points (95% CI, 0.67-5.41 points) in the diphenhydramine-lidocaine-antacid mouthwash group vs the placebo mouthwash group (P = .01).
The median pain score at the end of cycle 1 for patients who chose to discontinue treatment during cycle 2 was higher at 5 points compared with patients who chose to continue treatment at 4 points (difference, 0.5 points [95% CI, 0-1.5 points]; P = .23). There was no statistical difference in other baseline characteristics.
Discussion
In this randomized clinical trial of patients who underwent head and neck radiotherapy, use of either the doxepin mouthwash or the diphenhydramine-lidocaine-antacid mouthwash significantly reduced oral mucositis pain during the first 4 hours after administration compared with the placebo mouthwash. However, the mean differences in pain reduction by the area under the curve for both treatment intervention mouthwashes were less than the minimal clinically important difference of 3.5 points.
There is some suggestion in post hoc analyses that the findings may have been clinically relevant for some patients. For example, the responder analysis showed a significantly better result compared with the placebo mouthwash for the diphenhydramine-lidocaine-antacid mouthwash, but not for the doxepin mouthwash. In addition, the 95% CIs for the primary outcome included the minimal clinically important difference. However, the overall clinical importance of the statistically significant primary findings remains uncertain.
For the doxepin mouthwash, the current study reported similar findings to those of a previous randomized study.7 For the diphenhydramine-lidocaine-antacid mouthwash, the current study provided the first evidence that the mouthwash appeared to be effective for short-term oral mucositis–related pain resulting from radiotherapy.
However, the mean differences in pain reduction by the area under the curve for both treatment intervention mouthwashes were less than the minimal clinically important difference, which was predetermined as 3.5 points. Nonetheless, when the point estimates, 95% CIs, and skewness of the pain scores were taken into consideration, the nonparametric treatment effects were higher with either the doxepin mouthwash or the diphenhydramine-lidocaine-antacid mouthwash compared with the placebo mouthwash, and 3.5 points was still well within the confidence limits. Although the results from additional post hoc analyses were consistent with those of the primary analysis, which included exploratory responder analysis, pain reduction at peak score analysis, and parallel line plotting, these analyses are exploratory by definition and should only be considered hypothesis generating.
In contrast to other topical analgesic agents with only limited local properties, doxepin has effects on both the central and peripheral nervous systems. Although the mechanism of doxepin-mediated pain relief is not completely clear, the anesthetic and analgesic effects of doxepin are probably related to potent sodium channel blocking, which limits the transmission of noxious stimuli in mucosal nociceptors.17
Before this trial was completed, high-quality evidence (placebo-controlled randomized studies) did not exist for the efficacy of the diphenhydramine-lidocaine-antacid mouthwash. Although this gap in knowledge was recognized in multiple clinical practice guidelines,9,18,19 many oncologists still recommended this type of mouthwash to patients. A study by Dodd et al,20 and a small prospective Canadian study by Kuk et al,21 attempted to address this question; however, neither of these trials used a true placebo-based control group and only 1 of the trials was specific to radiotherapy.21 Neither study addressed oral mucositis pain as a definitive end point.
Doxepin mouthwash is an option that can be used for the treatment of radiotherapy-induced oral mucositis pain. The current trial does not provide proof regarding the relative efficacy of doxepin mouthwash vs diphenhydramine-lidocaine-antacid mouthwash because it was not designed to directly compare these 2 agents.
Regarding the components of the diphenhydramine-lidocaine-antacid mouthwash, diphenhydramine is used as an antihistamine and is commonly prescribed for seasonal allergies and pruritus. Lidocaine is an immediate-acting local anesthetic. The third component is an antacid. Therefore, the combined action of these 3 medications is antihistaminic, anesthetic, analgesic, and antiacidic. The beneficial effect of doxepin mouthwash observed in the current trial is consistent with a previous study.7
Additional analgesic use was common during the continuation phase. Even though the diphenhydramine-lidocaine-antacid mouthwash seemed to have a faster onset of pain relief, the doxepin mouthwash may have a more long-lasting effect on symptom control, which is consistent with the decreased need for analgesic use in the doxepin mouthwash group but not in the diphenhydramine-lidocaine-antacid mouthwash group.
Results of the current study suggest that the efficacy of the doxepin mouthwash and the diphenhydramine-lidocaine-antacid mouthwash may also be beneficial for oral mucositis pain during the continuation phase; however, this was not within the scope of the secondary analyses, which were limited and should be considered exploratory only.
Limitations
The study had several limitations. First, the trial was not designed to compare doxepin mouthwash and diphenhydramine-lidocaine-antacid mouthwash directly or to evaluate the combined analgesic effect of both preparations together. At the time of the trial’s conception, there was no randomized placebo-controlled evidence for the diphenhydramine-lidocaine-antacid mouthwash; therefore, it was determined the 2 active mouthwashes should not be compared against each other.
Second, there may have been other factors such as chemotherapy choice, size of the radiotherapy field, and primary site (oral cavity or oropharynx) that could have affected the severity of mucositis pain. However, this study was designed as a pragmatic trial for generalizable knowledge regarding the efficacy and adverse event profiles for these common mouthwashes.
Third, the study was limited to assessing pain control for only up to 4 hours after mouthwash administration. Given the potential for selection bias in the randomized groups, the smaller percentage of patients entering the continuation phase may not have provided reliable data. Although there was no significant difference in the baseline characteristics and the pain scores, it was noted in a post hoc analysis that patients who chose to continue treatment tended to have lower pain scores at the end of cycle 1 than patients who chose not to continue with cycle 2.
Fourth, the time course for pain after radiotherapy may persist for hours and even days, and further studies are necessary to evaluate the repeated use of these mouthwashes every 4 hours as needed, which is now typical in clinical practice.
Fifth, future studies should look at the potential combined effect of doxepin mouthwash and diphenhydramine-based mouthwash in a dose-finding study because drowsiness and fatigue are common adverse events with their use. Evaluation of peak and end of treatment pain sensations should be considered to fully capture the entire spectrum of pain perception by the patient.
Conclusions
Among patients undergoing head and neck radiotherapy, the use of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash compared with placebo significantly reduced oral mucositis pain during the first 4 hours after administration; however, the effect size was less than the minimal clinically important difference. Further research is needed to assess longer-term efficacy and safety for both mouthwashes.
Trial protocol
eBox. Participating Institutions (30 groups)
eTable 1. Unpleasant Taste and Stinging/Burning Sensations for 105 Patients Continuing With Cycle 2
eTable 2. Baseline Analgesic Use During Cycle 1 and Cycle 2 (Continuation Phase)
eFigure 1. Parallel line plot for doxepin vs placebo.
eFigure 2. Parallel line plot for DLA vs placebo.
Data sharing statement
References
- 1.Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. Complications of radiation therapy for head and neck cancers: the patient’s perspective. Cancer Nurs. 2002;25(6):461-467. doi: 10.1097/00002820-200212000-00010 [DOI] [PubMed] [Google Scholar]
- 2.Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2007;(4):CD000978. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL. Management of pain in cancer patients with oral mucositis: follow-up of multiple doses of doxepin oral rinse. J Pain Symptom Manage. 2007;33(2):111-114. doi: 10.1016/j.jpainsymman.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL. Doxepin rinse for management of mucositis pain in patients with cancer: one week follow-up of topical therapy. Spec Care Dentist. 2008;28(2):73-77. doi: 10.1111/j.1754-4505.2008.00015.x [DOI] [PubMed] [Google Scholar]
- 5.Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL. Oral doxepin rinse: the analgesic effect and duration of pain reduction in patients with oral mucositis due to cancer therapy. Anesth Analg. 2006;103(2):465-470. doi: 10.1213/01.ane.0000223661.60471.78 [DOI] [PubMed] [Google Scholar]
- 6.Epstein JB, Truelove EL, Oien H, Allison C, Le ND, Epstein MS. Oral topical doxepin rinse: analgesic effect in patients with oral mucosal pain due to cancer or cancer therapy. Oral Oncol. 2001;37(8):632-637. doi: 10.1016/S1368-8375(01)00005-7 [DOI] [PubMed] [Google Scholar]
- 7.Leenstra JL, Miller RC, Qin R, et al. Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: a phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance]). J Clin Oncol. 2014;32(15):1571-1577. doi: 10.1200/JCO.2013.53.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan A, Ignoffo RJ. Survey of topical oral solutions for the treatment of chemo-induced oral mucositis. J Oncol Pharm Pract. 2005;11(4):139-143. doi: 10.1191/1078155205jp166oa [DOI] [PubMed] [Google Scholar]
- 9.Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010;(8):CD001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Academy of Nursing Choosing Wisely: 2015. http://www.choosingwisely.org/clinician-lists/nursing-magic-mouthwash-for-oral-mucositis/. Accessed November 16, 2017.
- 11.Sonis ST, Eilers JP, Epstein JB, et al. ; Mucositis Study Group . Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer. 1999;85(10):2103-2113. doi: [DOI] [PubMed] [Google Scholar]
- 12.Bellm LA, Cunningham G, Durnell L, et al. Defining clinically meaningful outcomes in the evaluation of new treatments for oral mucositis: oral mucositis patient provider advisory board. Cancer Invest. 2002;20(5-6):793-800. doi: 10.1081/CNV-120002497 [DOI] [PubMed] [Google Scholar]
- 13.Qian W, Parmar MK, Sambrook RJ, Fayers PM, Girling DJ, Stephens RJ; MRC Lung Cancer Working Party . Analysis of messy longitudinal data from a randomized clinical trial. Stat Med. 2000;19(19):2657-2674. [DOI] [PubMed] [Google Scholar]
- 14.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581-585. doi: 10.1586/14737167.4.5.581 [DOI] [PubMed] [Google Scholar]
- 15.Lehmann EL, D’Abrera HJM. Nonparametrics: Statistical Methods Based on Ranks. New York, NY: Springer; 2006. [Google Scholar]
- 16.Schriger DL. Graphic portrayal of studies with paired data: a tutorial. Ann Emerg Med. 2018;71(2):239-246. doi: 10.1016/j.annemergmed.2017.05.033 [DOI] [PubMed] [Google Scholar]
- 17.Sudoh Y, Cahoon EE, Gerner P, Wang GK. Tricyclic antidepressants as long-acting local anesthetics. Pain. 2003;103(1-2):49-55. doi: 10.1016/S0304-3959(02)00375-5 [DOI] [PubMed] [Google Scholar]
- 18.Keefe DM, Schubert MM, Elting LS, et al. ; Mucositis Study Section of the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology . Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820-831. doi: 10.1002/cncr.22484 [DOI] [PubMed] [Google Scholar]
- 19.Rubenstein EB, Peterson DE, Schubert M, et al. ; Mucositis Study Section of the Multinational Association for Supportive Care in Cancer; International Society for Oral Oncology . Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100(9)(suppl):2026-2046. doi: 10.1002/cncr.20163 [DOI] [PubMed] [Google Scholar]
- 20.Dodd MJ, Dibble SL, Miaskowski C, et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(1):39-47. doi: 10.1067/moe.2000.105713 [DOI] [PubMed] [Google Scholar]
- 21.Kuk JS, Parpia S, Sagar SM, et al. A randomized phase III trial of magic mouthwash and sucralfate versus benzydamine hydrochloride for prophylaxis of radiation-induced oral mucositis in head and neck cancer. J Clin Oncol. 2011;29(15):5521. doi: 10.1200/jco.2011.29.15_suppl.5521 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eBox. Participating Institutions (30 groups)
eTable 1. Unpleasant Taste and Stinging/Burning Sensations for 105 Patients Continuing With Cycle 2
eTable 2. Baseline Analgesic Use During Cycle 1 and Cycle 2 (Continuation Phase)
eFigure 1. Parallel line plot for doxepin vs placebo.
eFigure 2. Parallel line plot for DLA vs placebo.
Data sharing statement