Key Points
Question
What is the efficacy of oral semaglutide (3, 7, or 14 mg/d) compared with sitagliptin, 100 mg/d, when added to metformin with or without sulfonylurea in patients with uncontrolled type 2 diabetes?
Findings
In this randomized clinical trial that included 1864 adults, oral semaglutide, 7 mg/d and 14 mg/d, compared with sitagliptin resulted in statistically significantly greater reductions in glycated hemoglobin over 26 weeks (–1.0% and –1.3%, respectively, compared with –0.8%). There was no significant benefit with oral semaglutide, 3 mg/d, compared with sitagliptin.
Meaning
In this trial, oral semaglutide, 7 mg/d and 14 mg/d, when added to metformin with or without sulfonylurea, resulted in greater improvements in glycated hemoglobin than sitagliptin after 26 weeks.
Abstract
Importance
Phase 3 trials have not compared oral semaglutide, a glucagon-like peptide 1 receptor agonist, with other classes of glucose-lowering therapy.
Objective
To compare efficacy and assess long-term adverse event profiles of once-daily oral semaglutide vs sitagliptin, 100 mg added on to metformin with or without sulfonylurea, in patients with type 2 diabetes.
Design, Setting, and Participants
Randomized, double-blind, double-dummy, parallel-group, phase 3a trial conducted at 206 sites in 14 countries over 78 weeks from February 2016 to March 2018. Of 2463 patients screened, 1864 adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea were randomized.
Interventions
Patients were randomized to receive once-daily oral semaglutide, 3 mg (n = 466), 7 mg (n = 466), or 14 mg (n = 465), or sitagliptin, 100 mg (n = 467). Semaglutide was initiated at 3 mg/d and escalated every 4 weeks, first to 7 mg/d then to 14 mg/d, until the randomized dosage was achieved.
Main Outcomes and Measures
The primary end point was change in glycated hemoglobin (HbA1c), and the key secondary end point was change in body weight, both from baseline to week 26. Both were assessed at weeks 52 and 78 as additional secondary end points. End points were tested for noninferiority with respect to HbA1c (noninferiority margin, 0.3%) prior to testing for superiority of HbA1c and body weight.
Results
Among 1864 patients randomized (mean age, 58 [SD, 10] years; mean baseline HbA1c, 8.3% [SD, 0.9%]; mean body mass index, 32.5 [SD, 6.4]; n=879 [47.2%] women), 1758 (94.3%) completed the trial and 298 prematurely discontinued treatment (16.7% for semaglutide, 3 mg/d; 15.0% for semaglutide, 7 mg/d; 19.1% for semaglutide, 14 mg/d; and 13.1% for sitagliptin). Semaglutide, 7 and 14 mg/d, compared with sitagliptin, significantly reduced HbA1c (differences, –0.3% [95% CI, –0.4% to –0.1%] and –0.5% [95% CI, –0.6% to –0.4%], respectively; P < .001 for both) and body weight (differences, –1.6 kg [95% CI, –2.0 to –1.1 kg] and –2.5 kg [95% CI, –3.0 to –2.0 kg], respectively; P < .001 for both) from baseline to week 26. Noninferiority of semaglutide, 3 mg/d, with respect to HbA1c was not demonstrated. Week 78 reductions in both end points were statistically significantly greater with semaglutide, 14 mg/d, vs sitagliptin.
Conclusions and Relevance
Among adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide, 7 mg/d and 14 mg/d, compared with sitagliptin, resulted in significantly greater reductions in HbA1c over 26 weeks, but there was no significant benefit with the 3-mg/d dosage. Further research is needed to assess effectiveness in a clinical setting.
Trial Registration
ClinicalTrials.gov Identifier: NCT02607865
This randomized clinical trial compares the effects of 3-, 7-, and 14-mg/d of oral semaglutide vs sitagliptin on glycated hemoglobin in patients with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea.
Introduction
Achieving and maintaining glycemic control is a key aim for the treatment of type 2 diabetes.1 The recommended target glycated hemoglobin (HbA1c) level of less than 7.0% is, however, not achieved by a sizable proportion of patients.2 Following metformin, many second-line treatment options for type 2 diabetes are available, with choice influenced by a variety of factors including the presence of comorbidities (eg, cardiovascular disease, renal disease), potential body weight effects, safety concerns (eg, risk of hypoglycemia), cost, and patient preferences.1,3
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) and dipeptidyl peptidase 4 (DPP-4) inhibitors are incretin-based therapies for treatment of type 2 diabetes. DPP-4 inhibitors generally have modest glucose-lowering potential and a neutral effect on body weight; in comparison, GLP-1RAs are associated with greater reductions in HbA1c and also reduce body weight.4 Also, while no cardiovascular benefit has been demonstrated for DPP-4 inhibitors,5,6 some GLP-1RAs have been shown to improve cardiovascular outcomes7,8,9 and are recommended for patients with cardiovascular disease.1 Based on their convenient oral administration and safety profile, DPP-4 inhibitors continue to be widely prescribed.10
As peptides, GLP-1RAs are currently administered only via subcutaneous injection. Peptide medications have low oral bioavailability because they are subject to rapid enzymatic and pH-induced proteolytic degradation in the stomach and are poorly absorbed by the gastrointestinal tract.11 To overcome this, an oral tablet of the GLP-1RA semaglutide has been developed through co-formulation with the absorption enhancer sodium N-(8-[2-hydroxylbenzoyl] amino) caprylate.12 Oral semaglutide treatment has shown significant improvements in glycemic control and body weight compared with placebo.12,13
This article reports the results of PIONEER 3, a trial that compared the efficacy, long-term adverse event profile, and tolerability of oral semaglutide with sitagliptin, a widely used DPP-4 inhibitor, as an add-on to metformin with or without sulfonylurea in patients with type 2 diabetes.
Methods
PIONEER 3 was a 78-week, randomized, double-blind, double-dummy, active-controlled, parallel-group, phase 3a trial conducted at 206 sites in 14 countries between February 2016 and March 2018. The trial protocol (see Supplement 2 and statistical analysis plan in Supplement 3) was approved by the institutional review board/independent ethics committees at each site, and the trial was conducted in accordance with International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice guidelines and the Declaration of Helsinki.14,15 All patients provided written informed consent prior to any trial-related intervention.
Patients
Adult patients diagnosed as having type 2 diabetes, with HbA1c levels of 7.0% to 10.5% inclusive and taking a stable dosage of metformin with or without sulfonylurea, were eligible. Exclusion criteria included treatment with any medication for diabetes or obesity 90 days or less before screening (other than metformin, sulfonylurea, or short-term insulin [≤14 days in total]), history of pancreatitis, renal impairment, and proliferative retinopathy or maculopathy requiring acute treatment. Full eligibility criteria are provided in eTable 1 in Supplement 1.
Data on race and ethnicity were recorded in the electronic case report form (fixed categories supplemented by a free-text “other” field and a “not applicable” field) by investigators at screening based on conversations between participants and investigators. These data were collected in part to address regulatory requests to assess efficacy and safety of an investigational product in patients of different races and ethnicities.
Randomization
Patients were randomized 1:1:1:1 to once-daily oral semaglutide (3, 7, or 14 mg) or once-daily oral sitagliptin, 100 mg, for 78 weeks (eFigure 1 in Supplement 1). Treatment codes were assigned by an interactive web response system in an effort to ensure that patients and investigators were blinded to treatment allocation. Randomization was done in blocks of size 8 and stratified by patients’ country of origin (Japanese or non-Japanese) and background medication (metformin with or without sulfonylurea).
Interventions
Because food intake impairs absorption of oral semaglutide, patients were instructed to administer trial products (eAppendix 2 in Supplement 1) in the morning, in a fasting state, with up to half a glass of water (approximately 120 mL) at least 30 minutes before having breakfast or taking any other oral medication (including background glucose-lowering medication). Oral semaglutide treatment was initiated with the 3-mg/d dosage, then escalated to 7 mg/d after 4 weeks and 14 mg/d after a further 4 weeks, until the randomized dosage was achieved; sitagliptin was initiated and maintained at 100 mg/d. Patients also received background metformin with or without sulfonylurea, maintained at the stable, pretrial dosage. Intensification of existing background glucose-lowering medication and/or initiation of new glucose-lowering medication was prescribed as an add-on to randomized treatment for patients with persistent or unacceptable hyperglycemia, based on predefined fasting plasma glucose and/or HbA1c criteria (eTable 2 in Supplement 1). All patients were to continue in the trial even if they prematurely discontinued the trial product and/or received additional glucose-lowering medications. Patients prematurely discontinuing the trial product could receive any glucose-lowering drug, excluding other GLP-1RAs or DPP-4 inhibitors, before final follow-up visit at investigator discretion.
Outcomes
The primary end point was the change in HbA1c, and the key secondary end point was the change in body weight, both from baseline to week 26. All additional secondary end points were assessed at weeks 26, 52 and 78. These included change from baseline in HbA1c and body weight, fasting plasma glucose, mean and mean postprandial increment in self-measured whole-blood glucose 7-time-point profile, body mass index, waist circumference, and fasting lipid profile. Further secondary end points were whether patients achieved HbA1c levels below 7.0% (American Diabetes Association target) and at or below 6.5%; weight loss of at least 5% and at least 10%; composites of (1) HbA1c below 7.0% without hypoglycemia (severe or whole-blood glucose–confirmed symptomatic hypoglycemia [<56 mg/dL {<3.1 mmol/L}]) and without weight gain and (2) HbA1c reduction of at least 1% and weight loss of at least 3%; and time to rescue medication and additional glucose-lowering medication.
Additional patient-reported outcomes included the questionnaire Impact of Weight on Quality of Life–Lite Clinical Trial Version, the Short Form 36 Version 2 health survey (acute version), and the Control of Eating Questionnaire, described in eAppendix 3 in Supplement 1.
Safety assessments included the number of adverse events; number of severe (according to American Diabetes Association classification) or whole-blood glucose–confirmed (<56 mg/dL [<3.1 mmol/L]) episodes of symptomatic hypoglycemia; changes from baseline at weeks 26, 52, and 78 in laboratory parameters (amylase and lipase), vital signs (pulse and systolic and diastolic blood pressure), and eye examination category (weeks 52 and 78 only); and occurrence of antisemaglutide antibodies. An independent external event adjudication committee performed blinded validation of selected adverse events (deaths, acute coronary syndromes, cerebrovascular events, heart failure requiring hospitalization, acute pancreatitis, malignant neoplasms, thyroid diseases [malignant thyroid neoplasms and C-cell hyperplasia], acute kidney injury, and lactic acidosis) according to predefined diagnostic criteria. Semaglutide plasma concentration was determined in approximately 50% of patients but is not reported in this article. Tolerability was assessed as the rate of premature discontinuations of trial product, along with the primary reasons for discontinuation.
Trial Design
Two different scientific questions related to the efficacy objectives were addressed through the definition of 2 estimands (“treatment policy” and “trial product”). Both estimands were defined based on interactions with regulatory agencies, including the US Food and Drug Administration and the European Medicines Agency.
The treatment policy estimand evaluates the treatment effect for all randomized patients regardless of trial product discontinuation or use of rescue medication. This estimand reflects the intention-to-treat principle as defined in ICH guideline E9.16 The estimand reflects the effect of initiating treatment with oral semaglutide compared with initiating treatment with sitagliptin, both potentially followed by either discontinuation of trial product and/or addition of or switch to another glucose-lowering drug.
The trial product estimand evaluates the treatment effect for all randomized patients under the assumption that all patients continued taking trial product for the entire planned duration of the trial and did not use rescue medication. This estimand aims at reflecting the effect of oral semaglutide compared with sitagliptin without the confounding effect of trial product discontinuation or use of rescue medication.
Trial product discontinuation and initiation of rescue medication are postrandomization events that are accounted for by the treatment policy strategy for the treatment policy estimand and by the hypothetical strategy for the trial product estimand, as defined in draft ICH guideline E9 (Revision 1).17 For the treatment policy estimand, the event (trial product discontinuation or initiation of rescue medication) was considered irrelevant and data collected thereafter were included in the estimation. For the trial product estimand, any data collected after the event were discarded and the estimation relies on statistical modeling to estimate the treatment effect under the assumption that the event had not occurred. Further details on the estimands are in eAppendix 4 in Supplement 1.
Sample Size
A sample size of 465 patients per treatment group was calculated to provide 90% power to jointly demonstrate superiority of oral semaglutide, 7 mg/d and 14 mg/d, vs sitagliptin and noninferiority of oral semaglutide, 3 mg/d, vs sitagliptin in reducing HbA1c at week 26.
Statistical Analysis
The assessment of efficacy of oral semaglutide on change in HbA1c and in body weight, both from baseline to week 26, was based on a weighted Bonferroni closed-testing strategy18 to control the overall type I error at a level of .05 (2-sided) for the hypotheses evaluated by the treatment policy estimand. The testing strategy was based on 2 principles: (1) within a dosage level, noninferiority with respect to HbA1c had to be demonstrated before testing for added benefits in terms of superiority with respect to HbA1c or to body weight and (2) noninferiority with respect to HbA1c had to be demonstrated on all higher dosage levels before continuing testing hypotheses on lower dosage levels (eAppendix 4 in Supplement 1).
Because of the potential for type I errors due to multiple comparisons, findings for analyses of additional secondary end points should be interpreted as exploratory.
The treatment policy estimand was estimated by a pattern mixture model using multiple imputation to handle missing week 26 data for both end points. Data collected at week 26, irrespective of premature discontinuation of trial product or initiation of rescue medication, were included in the statistical analysis. Missing data were imputed within groups defined by trial product and treatment status at week 26, and this assumed that the likely values of the missing data were best described by observed responses from patients taking the same trial product and with the same treatment discontinuation status and/or rescue medication use. Both the imputation and the analysis were based on analysis of covariance models with region and background medication as factors and baseline value as a covariate. The results were combined by use of Rubin’s rule.19 Prior to testing for noninferiority, a value of 0.3% (the noninferiority margin) was added to imputed values at week 26 for the oral semaglutide treatment groups only to ensure that imputation of missing values would not increase the likelihood of demonstrating noninferiority.20
The trial product estimand was estimated by a mixed model for repeated measurements with treatment, region, and background medication as categorical fixed effects and baseline value as a covariate, all nested within visit. All data collected at scheduled visits prior to premature trial product discontinuation or initiation of rescue medication were included in the statistical analysis. An unstructured covariance matrix for end-point measurements within the same patient was used.
Three sensitivity analyses were prespecified for the main analysis of the treatment policy estimand. These were (1) a comparator multiple imputation analysis in which missing data in the semaglutide groups were imputed based on the distribution of the week 26 values in the sitagliptin group; (2) an adverse event–determined comparator multiple imputation analysis in which missing data as a result of trial product discontinuation because of adverse events were imputed from the sitagliptin group as described above and the remaining missing data were imputed as in the main analysis; and (3) a tipping-point analysis in which a penalty was added to the imputed values in the semaglutide group. The penalty was increased until the conclusions from the main analyses were reversed. The specific value of the penalty that reversed the conclusion was used to evaluate the robustness of the main analysis results.
Safety and tolerability were assessed using the safety analysis set (all patients exposed to ≥1 dose of trial product) and evaluated both “on treatment” while participants were receiving trial product regardless of rescue medication use and “in trial” while participants remained in the trial regardless of trial product discontinuation or rescue medication use.
Further details on the statistical analyses can be found in eAppendix 4 in Supplement 1. All statistical analyses were prespecified and performed using SAS version 9.4M2 (SAS Institute Inc). The analysis model for binary end points was changed post hoc from logistic regression to a generalized linear model with identity link function.
Results
Patient Disposition and Baseline Characteristics
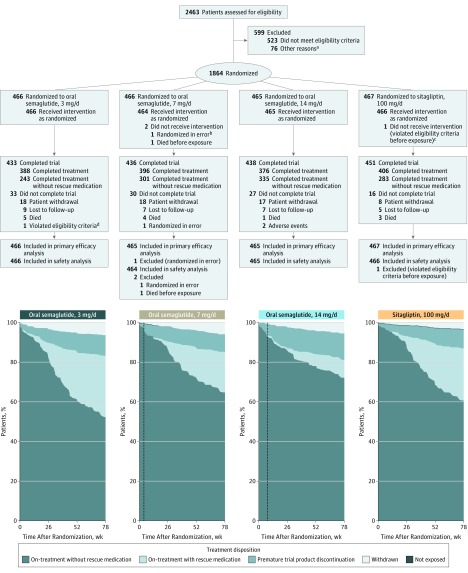
A total of 2463 patients were screened, with 1864 randomized to semaglutide, 3 mg/d (n = 466), 7 mg/d (n = 466), or 14 mg/d (n = 465); or to sitagliptin (n = 467) (Figure 1). The trial was completed by 94.3% of patients (1758/1864). At week 26, rescue medication was initiated by 5.4% (25/466), 2.4% (11/465), and 1.1% (5/465) in the semaglutide 3-mg/d, 7-mg/d, and 14-mg/d groups, respectively, and by 2.8% (13/467) for sitagliptin; these proportions increased throughout the trial (Figure 1 and eTable 2 in Supplement 1). The percentages of patients completing treatment without use of rescue medication were 52.1% (243/466), 64.6% (301/466), 72% (335/465), and 60.6% (283/467) in the semaglutide 3-mg/d, 7-mg/d, and 14-mg/d and the sitagliptin groups, respectively.
Figure 1. Participant Flow and Treatment Disposition Over Time.
Dotted lines in treatment disposition graphs indicate time points at which escalation to randomized dosages was achieved (week 4 in 7-mg/d semaglutide group and week 8 in 14-mg/d semaglutide group).
aOther reasons included patient withdrew consent and/or did not attend scheduled randomization visit (n = 58), study center–related issues (n = 8), incomplete screening data (n = 8), investigator unable to confirm patient eligibility (n = 1), and serious adverse event occurring between screening and randomization (n = 1).
bNo assessments were performed.
cReceived disallowed background medication (nateglinide).
dDid not provide informed consent.
Demographics and baseline disease characteristics were balanced across treatment groups (Table 1). Approximately half (52.8% [984/1864]) of all patients were male, and the majority were white (71.1% [1324/1864]). The mean age was 58 years, with mean values for HbA1c of 8.3%, duration of diabetes of 8.6 years, fasting plasma glucose of 171 mg/dL (9.49) mmol/L, body weight of 91.2 kg, and body mass index of 32.5. All patients were taking background metformin, with approximately half in each treatment group also receiving sulfonylurea (Table 1).
Table 1. Demographics and Baseline Disease Characteristics.
| Characteristics | Oral Semaglutide | Sitagliptin, 100 mg/d (n = 467) | ||
|---|---|---|---|---|
| 3 mg/d (n = 466) | 7 mg/d (n = 465) | 14 mg/d (n = 465) | ||
| Sex, No. (%) | ||||
| Male | 254 (54.5) | 245 (52.7) | 247 (53.1) | 238 (51.0) |
| Female | 212 (45.5) | 220 (47.3) | 218 (46.9) | 229 (49.0) |
| Age, mean (SD), y | 58 (10.0) | 58 (10.0) | 57 (10.0) | 58 (10.0) |
| Race, No. (%) | ||||
| White | 344 (73.8) | 330 (71.0) | 317 (68.2) | 333 (71.3) |
| Black or African American | 38 (8.2) | 38 (8.2) | 45 (9.7) | 39 (8.4) |
| Asian | 56 (12.0) | 69 (14.8) | 61 (13.1) | 59 (12.6) |
| American Indian or Alaska Native | 4 (0.9) | 3 (0.6) | 5 (1.1) | 6 (1.3) |
| Native Hawaiian or other Pacific Islander | 1 (0.2) | 0 | 0 | 0 |
| Other | 13 (2.8) | 11 (2.4) | 20 (4.3) | 12 (2.6) |
| Not applicablea | 10 (2.1) | 14 (3.0) | 17 (3.7) | 18 (3.9) |
| Hispanic or Latino ethnicity | 76 (16.3) | 77 (16.6) | 75 (16.1) | 93 (19.9) |
| Background medication, No. (%) | ||||
| Metformin | 466 (100.0) | 465 (100.0) | 465 (100.0) | 467 (100.0) |
| Sulfonylurea | 220 (47.2) | 218 (46.9) | 220 (47.3) | 219 (46.9) |
| Glimepiride | 93 (20.0) | 88 (18.9) | 107 (23.0) | 97 (20.8) |
| Gliclazide | 47 (10.1) | 59 (12.7) | 51 (11.0) | 53 (11.3) |
| Glibenclamide | 46 (9.9) | 41 (8.8) | 36 (7.7) | 46 (9.9) |
| Glipizide | 33 (7.1) | 30 (6.5) | 26 (5.6) | 23 (4.9) |
| Gliquidone | 1 (0.2) | 0 | 0 | 0 |
| Duration of diabetes, y | ||||
| Mean (SD) | 8.4 (6.1) | 8.3 (5.8) | 8.7 (6.1) | 8.8 (6.0) |
| Median (IQR) | 7.3 (3.9-10.8) | 7.5 (4.4-10.7) | 7.4 (4.0-12.2) | 7.9 (4.3-12.2) |
| Body weight, kg | ||||
| Mean (SD) | 91.6 (22.0) | 91.3 (20.8) | 91.2 (21.7) | 90.9 (21.0) |
| Median (IQR) | 88.5 (76.8-104.5) | 89.8 (76.2-103.9) | 88.5 (75.4-104.6) | 88.9 (76.7-101.6) |
| Body mass indexb | ||||
| Mean (SD) | 32.6 (6.7) | 32.6 (6.4) | 32.3 (6.3) | 32.5 (6.2) |
| Median (IQR) | 31.9 (27.7-36.2) | 32.0 (28.0-36.4) | 31.4 (28.0-36.0) | 31.7 (28.0-35.9) |
| HbA1c, % | ||||
| Mean (SD) | 8.3 (1.0) | 8.4 (1.0) | 8.3 (0.9) | 8.3 (0.9) |
| Median (IQR) | 8.2 (7.5-9.0) | 8.3 (7.6-9.1) | 8.1 (7.5-8.8) | 8.1 (7.5-8.8) |
| Fasting plasma glucose, mg/dL | ||||
| Mean (SD) | 174.2 (50.5) | 170.3 (42.9) | 167.9 (45.1) | 171.8 (41.9) |
| Median (IQR) | 163.4 (141.4-198.2) | 161.8 (142.4-193.9) | 160.1 (135.7-189.9) | 165.5 (142.0-192.3) |
| Estimated glomerular filtration rate, mL/min/1.73 m2c | ||||
| Mean (SD) | 96 (15) | 96 (16) | 95 (16) | 96 (15) |
| Median (IQR) | 96 (87-106) | 97 (88-107) | 97 (86-105) | 98 (87-106) |
| Comorbidities of relevance affecting ≥10% of patients at screening in any treatment group (by MedDRA preferred term), No. (%)d | ||||
| Hypertension | 348 (74.7) | 328 (70.5) | 357 (76.8) | 339 (72.6) |
| Dyslipidemia | 134 (28.8) | 132 (28.4) | 136 (29.2) | 141 (30.2) |
| Obesity | 125 (26.8) | 142 (30.5) | 119 (25.6) | 133 (28.5) |
| Diabetic neuropathy | 127 (27.3) | 102 (21.9) | 115 (24.7) | 129 (27.6) |
| Hyperlipidemia | 104 (22.3) | 99 (21.3) | 94 (20.2) | 102 (21.8) |
| Gallbladder disease | 75 (16.1) | 66 (14.2) | 84 (18.1) | 85 (18.2) |
| Ischemic heart disease | 73 (15.7) | 76 (16.3) | 77 (16.6) | 81 (17.3) |
| Diabetic retinopathy | 73 (15.7) | 73 (15.7) | 74 (15.9) | 81 (17.3) |
| Osteoarthritis | 67 (14.4) | 61 (13.1) | 74 (15.9) | 59 (12.6) |
| Hepatic steatosis | 55 (11.8) | 47 (10.1) | 56 (12.0) | 55 (11.8) |
| Cholecystectomy | 51 (10.9) | 49 (10.5) | 52 (11.2) | 46 (9.9) |
| Cataract | 45 (9.7) | 46 (9.9) | 54 (11.6) | 45 (9.6) |
| Diabetic nephropathy | 46 (9.9) | 43 (9.2) | 52 (11.2) | 40 (8.6) |
| Depression | 37 (7.9) | 47 (10.1) | 36 (7.7) | 32 (6.9) |
Abbreviations: HbA1c, glycated hemoglobin; IQR, interquartile range; MedDRA, Medical Dictionary for Regulatory Activities, version 20.1.
SI conversion factor: To convert fasting plasma glucose from mg/dL to mmol/L, multiply by 0.055.
Not applicable for Brazil and France.
Calculated as weight in kilograms divided by height in meters squared.
Glomerular filtration rate was estimated by the Chronic Kidney Disease Epidemiology Collaboration formula.
Comorbidities include medical history of comorbidities at screening as well as ongoing comorbidities at screening based on data collected in the case report form.
Primary End Point
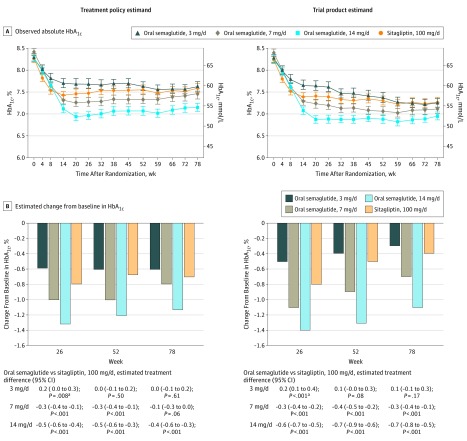
For the treatment policy estimand, the estimated mean changes from baseline in HbA1c at week 26 were –0.6%, –1.0%, and –1.3% for semaglutide, 3, 7, and 14 mg/d, respectively, and –0.8% for sitagliptin. The 7- and 14-mg/d semaglutide dosages were superior to sitagliptin in reducing HbA1c from baseline at week 26 (estimated treatment differences of –0.3% [95% CI, –0.4% to –0.1%; P < .001] and –0.5% [95% CI, –0.6% to –0.4%; P < .001], respectively) (Figure 2 and eFigure 2 in Supplement 1). Noninferiority of the 3-mg/d semaglutide dosage vs sitagliptin could not be demonstrated (estimated treatment difference of 0.2%; 95% CI, 0.1% to 0.3%; P = .09; α = .05). There were similar results for the trial product estimand (Figure 2 and eFigure 2). The robustness of the primary analyses was supported by sensitivity analyses (eTable 3 and eFigure 3 in Supplement 1).
Figure 2. Glycemic Control–Related Efficacy End Points.
HbA1c indicates glycated hemoglobin. Data in panel A are observed absolute mean values (with 95% confidence intervals shown as error bars) for the “in-trial” period (ie, while participants remained in the trial regardless of trial product discontinuation or rescue medication use) and the “on-treatment without rescue observation” period (ie, while patients were receiving trial product without use of rescue medication), and data in panel B are estimated mean changes from baseline by the treatment policy estimand and the trial product estimand at weeks 26, 52 and 78. Unadjusted 2-sided P values are given for the test of no difference. Treatment policy estimand: analysis of covariance using data irrespective of discontinuation of trial product or initiation of rescue medication. Missing values were imputed by a pattern mixture model using multiple imputation. Pattern was defined by randomized trial product and treatment status (premature trial product discontinuation and/or initiation of rescue medication). Trial product estimand: mixed model for repeated measurements. Data collected after discontinuation of trial product or initiation of rescue medication were excluded.
aFavored sitagliptin.
Key Secondary End Point
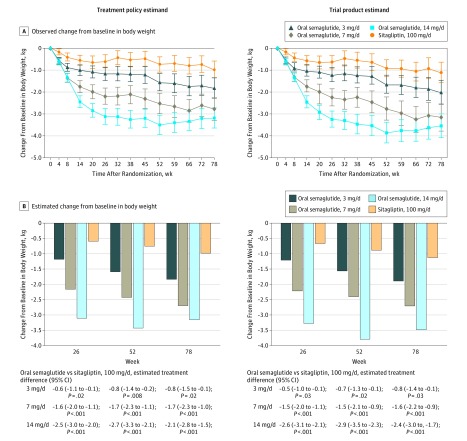
For the treatment policy estimand, the estimated mean changes from baseline in body weight at week 26 were –1.2 kg, –2.2 kg, and –3.1 kg for semaglutide, 3, 7, and 14 mg/d, respectively, and –0.6 kg for sitagliptin. The 7- and 14-mg/d semaglutide dosages were superior to sitagliptin in reducing body weight from baseline at week 26 (estimated treatment differences of –1.6 kg [95% CI, –2.0 to –1.1 kg; P < .001] and –2.5 kg [95% CI, –3.0 to –2.0 kg; P < .001], respectively) (Figure 3 and eFigure 2 in Supplement 1). Because noninferiority with respect to HbA1c was not demonstrated for the 3-mg/d semaglutide dosage, superiority with respect to body weight was not tested. There were similar results at week 26 for the trial product estimand. The primary analyses were supported by the sensitivity analyses (eTable 3 and eFigure 3 in Supplement 1).
Figure 3. Body Weight–Related Efficacy End Points.
Data in panel A are observed mean change from baseline values (with 95% confidence intervals shown as error bars) for the “in-trial” period (ie, while participants remained in the trial regardless of trial product discontinuation or rescue medication use) and the “on-treatment without rescue observation” period (ie, while patients were receiving trial product without use of rescue medication), and data in panel B are estimated mean changes from baseline by the treatment policy estimand and the trial product estimand at weeks 26, 52 and 78. Unadjusted 2-sided P values are given for the test of no difference. Treatment policy estimand: analysis of covariance using data irrespective of discontinuation of trial product or initiation of rescue medication. Missing values were imputed by a pattern mixture model using multiple imputation. Pattern was defined by randomized trial product and treatment status (premature trial product discontinuation and/or initiation of rescue medication). Trial product estimand: mixed model for repeated measurements. Data collected after discontinuation of trial product or initiation of rescue medication were excluded.
Additional Secondary End Points
At week 78, HbA1c reductions from baseline were statistically significantly greater with semaglutide, 7 mg/d (trial product estimand only), and 14 mg/d (both estimands), vs sitagliptin, with no significant differences observed with semaglutide, 3 mg/d (Figure 2 and eFigure 2 in Supplement 1). For both estimands, the body weight reductions at week 78 were statistically significantly greater with all dosages of semaglutide compared with sitagliptin (Figure 3 and eFigure 2). For fasting plasma glucose and mean self-measured whole-blood glucose, the reductions from baseline were significantly greater in the 14-mg/d semaglutide group at weeks 26 and 78 compared with sitagliptin (Table 2). Distributions of HbA1c and fasting plasma glucose values at baseline and weeks 26, 52, and 78 are shown in eFigure 4 in Supplement 1.
Table 2. Additional Secondary End Points.
| End Points | Treatment Policy Estimanda | Trial Product Estimanda | ||||||
|---|---|---|---|---|---|---|---|---|
| Oral Semaglutide | Sitagliptin, 100 mg/d (n = 467) | Oral Semaglutide | Sitagliptin, 100 mg/d (n = 467) | |||||
| 3 mg/d (n = 466) | 7 mg/d (n = 465) | 14 mg/d (n = 465) | 3 mg/d (n = 466) | 7 mg/d (n = 465) | 14 mg/d (n = 465) | |||
| Fasting Plasma Glucose, mg/dL | ||||||||
| Week 26 | ||||||||
| Estimated mean | 157.5 | 149.8 | 140.5 | 155.6 | 160.3 | 150.2 | 136.4 | 157.1 |
| Estimated mean change from baseline | –13.6 | –21.3 | –30.5 | –15.4 | –10.7 | –20.8 | –34.6 | –13.9 |
| Estimated treatment difference vs sitagliptin (95% CI) | 1.9 (–3.6 to 7.3) | –5.9 (–11.4 to –0.3) | –15.1 (–20.6 to –9.7) | 3.2 (–1.9 to 8.3) | –6.9 (–12.0 to –1.8) | –20.7 (–25.8 to –15.6) | ||
| P valueb | .50 | .04 | <.001 | .22 | .008 | <.001 | ||
| Week 52 | ||||||||
| Estimated mean | 155.2 | 149.1 | 138.5 | 153.0 | 162.8 | 149.6 | 137.2 | 158.4 |
| Estimated mean change from baseline | –15.9 | –22.0 | –32.6 | –18.1 | –8.3 | –21.5 | –33.8 | –12.7 |
| Estimated treatment difference vs sitagliptin (95% CI) | 2.2 (–3.3 to 7.7) | –3.9 (–9.7 to 1.9) | –14.5 (–20.0 to –9.1) | 4.4 (–1.1 to 9.9) | –8.8 (–14.1 to –3.5) | –21.2 (–26.5 to –15.9) | ||
| P valueb | .44 | .18 | <.001 | .12 | .001 | <.001 | ||
| Week 78 | ||||||||
| Estimated mean | 154.0 | 153.0 | 140.3 | 156.1 | 162.9 | 152.7 | 139.3 | 161.3 |
| Estimated mean change from baseline | –17.1 | –18.1 | –30.8 | –15.0 | –8.2 | –18.4 | –31.7 | –9.8 |
| Estimated treatment difference vs sitagliptin (95% CI) | –2.1 (–8.0 to 3.9) | –3.1 (–9.3 to 3.1) | –15.8 (–21.7 to –9.9) | 1.6 (–4.5 to 7.7) | –8.6 (–14.5 to –2.7) | –21.9 (–27.7 to –16.1) | ||
| P valueb | .50 | .33 | <.001 | .61 | .004 | <.001 | ||
| 7-Point Self-Measured Whole-Blood Glucose, Mean, mg/dLc | ||||||||
| Week 26 | ||||||||
| Estimated mean | 164.2 | 157.4 | 155.0 | 163.0 | 164.1 | 155.8 | 148.3 | 160.6 |
| Estimated mean change from baseline | –20.0 | –26.8 | –29.3 | –21.2 | –19.0 | –27.3 | –34.8 | –22.6 |
| Estimated treatment difference vs sitagliptin (95% CI) | 1.2 (–3.7 to 6.1) | –5.6 (–10.4 to –0.7) | –8.0 (–13.1 to –2.9) | 3.6 (–1.0 to 8.2) | –4.8 (–9.3 to –0.2) | –12.3 (–16.8 to –7.7) | ||
| P valueb | .63 | .03 | .002 | .13 | .004 | <.001 | ||
| Week 52 | ||||||||
| Estimated mean | 162.5 | 157.3 | 151.1 | 159.5 | 161.6 | 156.1 | 146.9 | 161.9 |
| Estimated mean change from baseline | –21.7 | –26.9 | –33.1 | –24.7 | –21.5 | –27.0 | –36.2 | –21.2 |
| Estimated treatment difference vs sitagliptin (95% CI) | 3.0 (–1.8 to 7.8) | –2.2 (–7.0 to 2.6) | –8.4 (–13.2 to –3.6) | –0.3 (–5.4 to 4.8) | –5.8 (–10.7 to –0.9) | –15.0 (–19.8 to –10.1) | ||
| P valueb | .22 | .37 | .001 | .90 | .02 | <.001 | ||
| Week 78 | ||||||||
| Estimated mean | 161.6 | 158.9 | 153.9 | 161.6 | 164.9 | 157.3 | 150.9 | 160.6 |
| Estimated mean change from baseline | –22.6 | –25.3 | –30.4 | –22.7 | –18.3 | –25.9 | –32.2 | –22.5 |
| Estimated treatment difference vs sitagliptin (95% CI) | 0.0 (–5.0 to 5.1) | –2.6 (–7.9 to 2.6) | –7.7 (–12.7 to –2.7) | 4.3 (–1.1 to 9.6) | –3.4 (–8.5 to 1.8) | –9.7 (–14.8 to –4.7) | ||
| P valueb | .99 | .33 | .003 | .12 | .20 | <.001 | ||
| HbA1c <7.0% | ||||||||
| Week 26 | ||||||||
| Estimated % of patients | 27 | 42 | 55 | 32 | 26 | 44 | 59 | 32 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | –5 (–11 to 1) | 10 (4 to 17) | 23 (17 to 30) | –6 (–12 to –0) | 13 (6 to 19) | 27 (21 to 34) | ||
| P valueb | .07 | <.001 | <.001 | .04 | <.001 | <.001 | ||
| Week 52 | ||||||||
| Estimated % of patients | 27 | 38 | 53 | 31 | 23 | 38 | 57 | 30 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | –4 (–10 to 2) | 7 (0 to 13) | 22 (16 to 28) | –6 (–12 to –0) | 8 (2 to 14) | 27 (21 to 34) | ||
| P valueb | .15 | .04 | <.001 | .04 | .01 | <.001 | ||
| Week 78 | ||||||||
| Estimated % of patients | 27 | 37 | 44 | 29 | 23 | 36 | 47 | 28 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | –2 (–8 to 4) | 8 (2 to 14) | 15 (8 to 21) | –5 (–11 to 1) | 8 (2 to 15) | 19 (13 to 26) | ||
| P valueb | .48 | .01 | <.001 | .09 | .009 | <.001 | ||
| Weight Loss ≥5% | ||||||||
| Week 26 | ||||||||
| Estimated % of patients | 13 | 19 | 30 | 10 | 13 | 20 | 32 | 11 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 3 (–1 to 7) | 9 (4 to 13) | 20 (15 to 25) | 2 (–2 to 6) | 9 (4 to 13) | 21 (16 to 27) | ||
| P valueb | .15 | <.001 | <.001 | .39 | <.001 | <.001 | ||
| Week 52 | ||||||||
| Estimated % of patients | 17 | 27 | 34 | 12 | 17 | 26 | 38 | 12 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 5 (–0 to 9) | 15 (10 to 20) | 22 (16 to 27) | 4 (–1 to 9) | 14 (9 to 20) | 26 (20 to 31) | ||
| P valueb | .06 | <.001 | <.001 | .10 | <.001 | <.001 | ||
| Week 78 | ||||||||
| Estimated % of patients | 21 | 27 | 33 | 14 | 23 | 27 | 36 | 15 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 7 (2 to 12) | 13 (8 to 19) | 19 (13 to 24) | 7 (1 to 14) | 12 (6 to 18) | 21 (15 to 27) | ||
| P valueb | .01 | <.001 | <.001 | .02 | <.001 | <.001 | ||
| HbA1c <7.0% Without Hypoglycemic Episodes and Without Body Weight Gaind | ||||||||
| Week 26 | ||||||||
| Estimated % of patients | 20 | 34 | 46 | 20 | 19 | 35 | 50 | 20 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | –1 (–5 to 4) | 14 (8 to 19) | 26 (20 to 32) | –1 (–6 to 3) | 15 (9 to 20) | 29 (23 to 35) | ||
| P valueb | .80 | <.001 | <.001 | .55 | <.001 | <.001 | ||
| Week 52 | ||||||||
| Estimated % of patients | 20 | 30 | 43 | 20 | 18 | 30 | 46 | 20 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | –0 (–5 to 5) | 10 (5 to 16) | 23 (17 to 29) | –2 (–7 to 3) | 10 (5 to 16) | 26 (20 to 32) | ||
| P valueb | .97 | <.001 | <.001 | .46 | <.001 | <.001 | ||
| Week 78 | ||||||||
| Estimated % of patients | 20 | 31 | 34 | 19 | 17 | 30 | 37 | 19 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 1 (–4 to 6) | 11 (6 to 17) | 15 (9 to 20) | –2 (–7 to 3) | 11 (5 to 16) | 17 (12 to 23) | ||
| P valueb | .80 | <.001 | <.001 | .43 | <.001 | <.001 | ||
| HbA1c Reduction ≥1% With Weight Loss ≥3% | ||||||||
| Week 26 | ||||||||
| Estimated % of patients | 13 | 26 | 37 | 9 | 12 | 27 | 40 | 10 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 4 (–1 to 8) | 17 (12 to 22) | 28 (23 to 33) | 3 (–2 to 7) | 17 (12 to 22) | 30 (25 to 36) | ||
| P valueb | .09 | <.001 | <.001 | .22 | <.001 | <.001 | ||
| Week 52 | ||||||||
| Estimated % of patients | 17 | 24 | 36 | 12 | 15 | 23 | 38 | 11 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 5 (1 to 10) | 12 (7 to 17) | 24 (19 to 30) | 4 (–1 to 8) | 12 (7 to 17) | 27 (21 to 32) | ||
| P valueb | .03 | <.001 | <.001 | .12 | <.001 | <.001 | ||
| Week 78 | ||||||||
| Estimated % of patients, % | 18 | 26 | 34 | 14 | 16 | 24 | 35 | 12 |
| Estimated treatment difference vs sitagliptin, % (95% CI) | 4 (–0 to 9) | 12 (7 to 17) | 20 (14 to 25) | 4 (–1 to 9) | 12 (6 to 17) | 23 (17 to 28) | ||
| P valueb | .08 | <.001 | <.001 | .12 | <.001 | <.001 | ||
Abbreviation: HbA1c, glycated hemoglobin.
SI conversion factor: To convert glucose from mg/dL to mmol/L, multiply by 0.055.
Treatment policy estimand: analysis of covariance for continuous end points and generalized linear model with binomial distribution and identity link for binary end points, using data irrespective of discontinuation of trial product or initiation of rescue medication. Missing values were imputed by a pattern mixture model using multiple imputation. Pattern was defined by randomized trial product and treatment status (premature trial product discontinuation and/or initiation of rescue medication). Trial product estimand: Mixed model for repeated measurements for continuous end points and generalized linear model with binomial distribution and identity link for binary end points. Data collected after discontinuation of trial product or initiation of rescue medication were excluded. For binary end points, missing values were imputed from patients randomized to the same trial product using sequential multiple imputation.
Unadjusted 2-sided P values for the test of no difference.
Self-monitored whole-blood glucose at 7 time points is reported as plasma-equivalent values of capillary whole-blood glucose.
Reported episodes were either severe (defined according to the American Diabetes Association classification) or confirmed by a whole-blood glucose value <56 mg/dL (<3.1 mmol/L), with symptoms consistent with hypoglycemia.
In the 7- and 14-mg/d semaglutide groups, significantly greater proportions of patients achieved HbA1c levels lower than 7.0%, body weight loss of 5% or greater, and the 2 composite outcomes (HbA1c <7.0% without hypoglycemia and without weight gain, and HbA1c reduction ≥1% and weight loss ≥3%) compared with the sitagliptin group (Table 2).
Time to rescue medication and time to additional glucose-lowering medication were statistically significantly longer with semaglutide, 7- and 14-mg/d, compared with sitagliptin (eTable 4 in Supplement 1). All other secondary end points are reported in eTable 5 and eFigures 5 through 7 in Supplement 1.
Adverse Events and Tolerability
The overall proportions of patients experiencing at least 1 adverse event while receiving treatment were similar across treatment groups (Table 3). The most frequent adverse events by system organ class were gastrointestinal disorders in the 14-mg/d semaglutide group and infections and infestations in the 3- and 7-mg/d semaglutide and sitagliptin groups. Among gastrointestinal adverse events, the majority were of mild or moderate severity, and the most common in the 7- and 14-mg/d semaglutide groups was nausea (Table 3 and eFigure 8 in Supplement 1). The number and proportions of serious adverse events during treatment were also similar across treatments.
Table 3. Adverse Events During Treatment With Trial Producta.
| Adverse Events | Oral Semaglutide | Sitagliptin, 100 mg/d (n = 466) | ||
|---|---|---|---|---|
| 3 mg/d (n = 466) | 7 mg/d (n = 464) | 14 mg/d (n = 465) | ||
| Patients experiencing ≥1 adverse event | ||||
| Any adverse eventsb | 370 (79.4) | 363 (78.2) | 370 (79.6) | 388 (83.3) |
| Serious adverse events | 64 (13.7) | 47 (10.1) | 44 (9.5) | 58 (12.4) |
| Severity of any adverse eventb | ||||
| Mild | 323 (69.3) | 318 (68.5) | 321 (69.0) | 340 (73.0) |
| Moderate | 186 (39.9) | 171 (36.9) | 199 (42.8) | 197 (42.3) |
| Severe | 47 (10.1) | 37 (8.0) | 40 (8.6) | 53 (11.4) |
| Adverse events leading to premature trial product discontinuation | 26 (5.6) | 27 (5.8) | 54 (11.6) | 24 (5.2) |
| Severe or whole-blood glucose–confirmed symptomatic hypoglycemiac | 23 (4.9) | 24 (5.2) | 36 (7.7) | 39 (8.4) |
| Most frequent adverse events occurring in ≥5% of patients in any treatment group (by MedDRA preferred term)b | ||||
| Nausea | 34 (7.3) | 62 (13.4) | 70 (15.1) | 32 (6.9) |
| Diarrhea | 45 (9.7) | 53 (11.4) | 57 (12.3) | 37 (7.9) |
| Nasopharyngitis | 53 (11.4) | 49 (10.6) | 47 (10.1) | 47 (10.1) |
| Vomiting | 13 (2.8) | 28 (6.0) | 42 (9.0) | 19 (4.1) |
| Headache | 29 (6.2) | 30 (6.5) | 37 (8.0) | 36 (7.7) |
| Decreased appetite | 8 (1.7) | 14 (3.0) | 32 (6.9) | 14 (3.0) |
| Upper respiratory tract infection | 36 (7.7) | 35 (7.5) | 26 (5.6) | 32 (6.9) |
| Hypertension | 30 (6.4) | 24 (5.2) | 26 (5.6) | 29 (6.2) |
| Back pain | 24 (5.2) | 25 (5.4) | 25 (5.4) | 29 (6.2) |
| Urinary tract infection | 30 (6.4) | 21 (4.5) | 23 (4.9) | 26 (5.6) |
| Arthralgia | 22 (4.7) | 14 (3.0) | 21 (4.5) | 30 (6.4) |
| Influenza | 30 (6.4) | 25 (5.4) | 18 (3.9) | 30 (6.4) |
| Diabetic retinopathy | 27 (5.8) | 24 (5.2) | 16 (3.4) | 27 (5.8) |
| Kaplan-Meier estimated duration of gastrointestinal events, median (IQR), d | ||||
| Nausea | 28.0 (3.0-105.0) | 9.0 (4.0-41.0) | 20.5 (4.0-61.5) | 5.5 (1.5-25.0) |
| Diarrhea | 4.0 (2.0-26.0) | 3.0 (1.0-7.0) | 9.0 (3.0-44.0) | 5.5 (2.0-16.5) |
| Vomiting | 2.0 (1.0-4.0) | 2.0 (1.0-7.0) | 2.0 (1.0-9.0) | 1.0 (1.0-2.0) |
Abbreviations: IQR, interquartile range; MedDRA, Medical Dictionary for Regulatory Activities, version 20.1.
Data are expressed as No. (%) of participants unless otherwise indicated.
Includes serious adverse events.
Episodes of hypoglycemia were reported on a form separate from adverse events. Reported episodes were either severe (defined according to the American Diabetes Association classification) or confirmed by a whole-blood glucose value <56 mg/dL (<3.1 mmol/L), with symptoms consistent with hypoglycemia.
The proportions of patients who prematurely discontinued trial product for any reason were 16.7% (78/466), 15.0% (70/466), and 19.1% (89/465) for the 3-, 7-, and 14-mg/d semaglutide groups, respectively, and 13.1% (61/467) for sitagliptin. Adverse events led to premature discontinuation for 5.6% (26/466), 5.8% (27/464), and 11.6% (54/465) in the 3-, 7-, and 14-mg/d semaglutide groups, respectively, and 5.2% (24/466) for sitagliptin (Table 3), with gastrointestinal adverse events being the primary cause in all treatment groups (eTable 6 in Supplement 1). For a substantial proportion of patients in the 7- and 14-mg/d semaglutide groups who discontinued because of an adverse event, the onset of the causative event occurred during the dosage-escalation period.
Severe or whole-blood glucose–confirmed episodes of symptomatic hypoglycemia were experienced by 4.9% (23/466), 5.2% (24/464), and 7.7% (36/465) of patients in the 3-, 7-, and 14-mg/d semaglutide groups, respectively, and by 8.4% (39/466) in the sitagliptin group (Table 3). These episodes mainly occurred in patients prescribed background metformin with sulfonylurea (eTable 7 in Supplement 1). Diabetic retinopathy–related adverse events were infrequent and similar across all treatment groups (eTable 8 in Supplement 1) and were mostly mild or moderate in severity, were reported at routine eye examinations, and did not require treatment. The frequencies of event adjudication committee–confirmed acute kidney injury, acute pancreatitis, cardiovascular events, and malignant neoplasms were similar across treatment groups (eTable 9 in Supplement 1). Other safety parameters are reported in eTable 10 in Supplement 1.
There were 12 deaths among exposed patients (eTable 9 in Supplement 1): 5 in the 3-mg/d semaglutide group, 3 in the 7-mg/d semaglutide group, 1 in the 14-mg/d semaglutide group, and 3 in the sitagliptin group. No pattern or clustering of causes of death were observed. Very few patients tested positive for antisemaglutide antibodies (eTable 11 in Supplement 1).
Discussion
In this multicenter, randomized clinical trial in patients with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, addition of once-daily oral semaglutide, 7 or 14 mg/d, demonstrated superior reductions from baseline in HbA1c and body weight than sitagliptin after 26 weeks. Noninferiority of oral semaglutide, 3 mg/d, compared with sitagliptin could not be demonstrated with respect to HbA1c reduction from baseline.
The greater effect with oral semaglutide for reducing HbA1c and body weight compared with sitagliptin is consistent with other head-to-head trials that have demonstrated superior glycemic control and weight reduction with GLP-1RAs over DPP-4 inhibitors.21,22,23,24 This glucose-lowering effect was also reflected by the longer time to and less use of rescue medication with the 7- and 14-mg/d oral semaglutide dosages. The results achieved with oral semaglutide may be of clinical relevance, as improved glycemic control is associated with better diabetes-related outcomes,25 and because some patients may prefer oral medications to achieve this improved glycemic control.26 Furthermore, clinically meaningful weight loss contributes to greater glycemic control and reduces cardiovascular risk factors,27 which is particularly beneficial in a population that frequently has comorbid obesity.
The long-term adverse event profile of oral semaglutide in this trial was consistent with the GLP-1RA class as a whole.28 The majority of gastrointestinal adverse events were mild to moderate in severity, with nausea, vomiting, or diarrhea the most frequently observed, and this is consistent with use of subcutaneous semaglutide29 and other GLP-1RAs.24,28,30 Dipeptidyl peptidase 4 inhibitors cause fewer gastrointestinal adverse events than GLP-1RAs,4 but in this trial, similar proportions of patients prematurely discontinued because of these events in the 3- and 7-mg/d (but not 14-mg/d) oral semaglutide groups and the sitagliptin group. Around twice as many patients prematurely discontinued the 14-mg/d oral semaglutide dosage because of any adverse event. Most adverse event–related discontinuations of oral semaglutide occurred during the dosage-escalation period. The dosage escalation was fixed according to the trial protocol, so it may not reflect clinical practice, in which dosage escalation would be based on individualized efficacy and tolerability. It was identified in the oral semaglutide phase 2 trial that initiating oral semaglutide at a low dosage and escalating it slowly improves tolerability and helps minimize gastrointestinal adverse events.12 The proportions of adverse event–related premature discontinuations of the 14-mg/d oral semaglutide dosage were similar to that previously observed with subcutaneous semaglutide, 1.0 mg once weekly,23,31 and in previous trials of other GLP-1RAs.22,24
The doses of oral semaglutide used in this trial are greater than the largest dose of subcutaneous semaglutide assessed in the SUSTAIN program.23,31 As a result of their low oral bioavailability, larger doses of orally administered peptide medications are required to achieve plasma concentrations comparable with those achieved via other routes.
In this trial, 2 different scientific questions related to the efficacy were addressed through the definition of the 2 estimands. Treatment differences vs sitagliptin were smaller at week 78 for the treatment policy estimand compared with the trial product estimand; a significant difference in HbA1c reduction was not shown with the 7-mg/d dosage at this time point, in line with the increasing use of rescue medication with sitagliptin.
The trial product estimand was estimated using a mixed model for repeated measurements, the appropriateness of which relies on the assumption of the missing data mechanism being “missing at random”; ie, that patients who discontinued trial product or initiated rescue medication had evolved similarly to patients still taking the same trial product without rescue medication and having the same covariates and same trajectory prior to the time point of discontinuation of trial product or initiation of rescue medication. While this approach aims at reflecting the treatment effect without the confounding effect of trial product discontinuation and use of rescue medication, residual confounding due to unmeasured factors cannot be ruled out. Initiation of rescue medication was more frequent with the 3-mg/d dosage of oral semaglutide and with sitagliptin, and discontinuation of trial product was more frequent with the 14-mg/d dosage of oral semaglutide.
A strength of this trial was the double-blind design, achieved using a double-dummy approach. This approach was used in an effort to ensure that the comparisons of the trial products were unbiased, as oral semaglutide and sitagliptin tablets are not visually identical. An additional strength was the long duration and high retention rate, which allowed for the long-term efficacy, adverse event profile, and tolerability of oral semaglutide to be investigated.
Limitations
This trial has several limitations. First, the trial had a relatively high discontinuation rate, which was greater with semaglutide compared with sitagliptin and therefore could have influenced the assessment of treatment effect. Second, adherence to treatment was not formally measured in this trial, with patients instead routinely reminded to adhere to trial procedures, and with monitoring of drug accountability. It is therefore unknown if poor adherence to treatment affected the results for patients receiving oral semaglutide, given the importance of correct administration on its absorption.32 Moreover, both discontinuation rates and nonadherence are generally higher outside of a randomized trial. Third, sitagliptin may not be the best active comparator for this trial because DPP-4 inhibitors have modest glucose-lowering effects and minimal effect on body weight compared with GLP-1RAs.4 However, they are widely used and are well tolerated. Other trials within the PIONEER phase 3 program will assess oral semaglutide against other glucose-lowering medications and with flexible dosing of oral semaglutide.
Conclusions
Among adults with type 2 diabetes uncontrolled with metformin with or without sulfonylurea, oral semaglutide, 7 and 14 mg/d, compared with sitagliptin, resulted in significantly greater reductions in HbA1c over 26 weeks, but there was no significant benefit with the 3-mg/d dosage. Further research is needed to assess effectiveness in a clinical setting.
eAppendix 1. PIONEER 3 Investigators
eAppendix 2. Trial Product Administration
eAppendix 3. Description of Patient-Reported Outcomes
eAppendix 4. Estimands
eAppendix 5. Statistical Considerations
eFigure 1. Trial Design
eFigure 2. Boxplots of Observed HbA1c and Body Weight Over Time
eFigure 3. Sensitivity Analyses for Changes From Baseline in HbA1c and Body Weight at Week 26 for the Treatment Policy Estimand
eFigure 4. Distributions of HbA1c and Fasting Plasma Glucose Values at Baseline and Weeks 26, 52 and 78
eFigure 5. Change From Baseline in Impact of Weight on Quality of Life-Lite Clinical Trial Version Questionnaire Scores
eFigure 6. Change From Baseline in Short Form-36 Version 2 (Acute Version) Health Survey Summary Scores
eFigure 7. Change From Baseline in Control of Eating Questionnaire Domain Scores
eFigure 8. Overview of On-Treatment Nausea Events
eTable 1. List of Inclusion and Exclusion Criteria
eTable 2. Rescue Medication and Additional Glucose-Lowering Medication Use
eTable 3. Tipping Point Analyses for Changes From Baseline in HbA1c and Body Weight at Week 26 for the Treatment Policy Estimand
eTable 4. Time to Rescue Medication and Additional Glucose-Lowering Medication
eTable 5. Additional Secondary Endpoints Not Included in the Main Text
eTable 6. On-Treatment Adverse Events Leading to Discontinuation by System Organ Class/Preferred Term
eTable 7. On-Treatment Hypoglycemic Episodes
eTable 8. In-Trial Adverse Events Related to Diabetic Retinopathy
eTable 9. External Event Adjudication Committee–Confirmed Events and Selected In-Trial Adverse Events
eTable 10. Additional Safety Parameters
eTable 11. In-Trial Anti-Semaglutide Antibodies
eReferences
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8):2271-2279. doi: 10.2337/dc12-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of Medical Care in Diabetes—2018, 8: pharmacologic approaches to glycemic treatment. Diabetes Care. 2018;41(suppl 1):S73-S85. doi: 10.2337/dc18-S008 [DOI] [PubMed] [Google Scholar]
- 4.Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(suppl 1):68-76. doi: 10.1111/dom.13137 [DOI] [PubMed] [Google Scholar]
- 5.Scirica BM, Bhatt DL, Braunwald E, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326. doi: 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 6.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79. doi: 10.1001/jama.2018.18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322. doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez AF, Green JB, Janmohamed S, et al. ; Harmony Outcomes Committees and Investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519-1529. doi: 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- 10.Scott LJ. Sitagliptin: a review in type 2 diabetes. Drugs. 2017;77(2):209-224. doi: 10.1007/s40265-016-0686-9 [DOI] [PubMed] [Google Scholar]
- 11.Philippart M, Schmidt J, Bittner B. Oral delivery of therapeutic proteins and peptides: an overview of current technologies and recommendations for bridging from approved intravenous or subcutaneous administration to novel oral regimens. Drug Res (Stuttg). 2016;66(3):113-120. [DOI] [PubMed] [Google Scholar]
- 12.Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318(15):1460-1470. doi: 10.1001/jama.2017.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aroda VR, Rosenstock J, Terauchi Y, et al. Effect and safety of oral semaglutide monotherapy in type 2 diabetes—PIONEER 1 trial. Diabetes. 2018;67(suppl 1):2. doi: 10.2337/db18-2-LB [DOI] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 15.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6 (R1) June 10, 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed December 2018.
- 16.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline: Statistical Principles for Clinical Trials: E9. February 5, 1998. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed March 13, 2019.
- 17.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline: Estimands and Sensitivity Analysis in Clinical Trials: E9 (R1). June 16, 2017. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/E9-R1EWG_Step2_Guideline_2017_0616.pdf. Accessed March 13, 2019.
- 18.Bretz F, Posch M, Glimm E, Klinglmueller F, Maurer W, Rohmeyer K. Graphical approaches for multiple comparison procedures using weighted Bonferroni, Simes, or parametric tests. Biom J. 2011;53(6):894-913. doi: 10.1002/bimj.201000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little RJA, Rubin DB. Statistical Analysis With Missing Data. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 20.Koch GG. Comments on “current issues in non-inferiority trials” by Thomas R. Fleming. Stat Med. 2008;27(3):333-342. doi: 10.1002/sim.2923 [DOI] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Wysham C, Macconell L, et al. ; DURATION-2 Study Group . Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431-439. doi: 10.1016/S0140-6736(10)60590-9 [DOI] [PubMed] [Google Scholar]
- 22.Pratley R, Nauck M, Bailey T, et al. ; 1860-LIRA-DPP-4 Study Group . One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397-407. doi: 10.1111/j.1742-1241.2011.02656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5(5):341-354. doi: 10.1016/S2213-8587(17)30092-X [DOI] [PubMed] [Google Scholar]
- 24.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care. 2014;37(8):2149-2158. doi: 10.2337/dc13-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412. doi: 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385-1399. doi: 10.2147/PPA.S101821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(6):1573-1580. doi: 10.2337/dc13-2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524-536. doi: 10.1111/dom.12849 [DOI] [PubMed] [Google Scholar]
- 29.Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251-260. doi: 10.1016/S2213-8587(17)30013-X [DOI] [PubMed] [Google Scholar]
- 30.Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care. 2015;38(12):2241-2249. doi: 10.2337/dc14-1625 [DOI] [PubMed] [Google Scholar]
- 31.Ahmann AJ, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41(2):258-266. doi: 10.2337/dc17-0417 [DOI] [PubMed] [Google Scholar]
- 32.Buckley ST, Bækdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):eaar7047. doi: 10.1126/scitranslmed.aar7047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. PIONEER 3 Investigators
eAppendix 2. Trial Product Administration
eAppendix 3. Description of Patient-Reported Outcomes
eAppendix 4. Estimands
eAppendix 5. Statistical Considerations
eFigure 1. Trial Design
eFigure 2. Boxplots of Observed HbA1c and Body Weight Over Time
eFigure 3. Sensitivity Analyses for Changes From Baseline in HbA1c and Body Weight at Week 26 for the Treatment Policy Estimand
eFigure 4. Distributions of HbA1c and Fasting Plasma Glucose Values at Baseline and Weeks 26, 52 and 78
eFigure 5. Change From Baseline in Impact of Weight on Quality of Life-Lite Clinical Trial Version Questionnaire Scores
eFigure 6. Change From Baseline in Short Form-36 Version 2 (Acute Version) Health Survey Summary Scores
eFigure 7. Change From Baseline in Control of Eating Questionnaire Domain Scores
eFigure 8. Overview of On-Treatment Nausea Events
eTable 1. List of Inclusion and Exclusion Criteria
eTable 2. Rescue Medication and Additional Glucose-Lowering Medication Use
eTable 3. Tipping Point Analyses for Changes From Baseline in HbA1c and Body Weight at Week 26 for the Treatment Policy Estimand
eTable 4. Time to Rescue Medication and Additional Glucose-Lowering Medication
eTable 5. Additional Secondary Endpoints Not Included in the Main Text
eTable 6. On-Treatment Adverse Events Leading to Discontinuation by System Organ Class/Preferred Term
eTable 7. On-Treatment Hypoglycemic Episodes
eTable 8. In-Trial Adverse Events Related to Diabetic Retinopathy
eTable 9. External Event Adjudication Committee–Confirmed Events and Selected In-Trial Adverse Events
eTable 10. Additional Safety Parameters
eTable 11. In-Trial Anti-Semaglutide Antibodies
eReferences
Trial Protocol
Statistical Analysis Plan
Data Sharing Statement