Key Points
Question
Are the safety and efficacy of a 3-dimensionally printed navigational template guide for localizing small percutaneous lung nodules noninferior to the conventional computed tomography–guided method?
Findings
In this single-center, noninferiority randomized clinical trial of 190 patients, lung nodule localization accuracy using the template-guided approach was noninferior to that using the conventional method. Compared with the conventional approach, the template-guided procedure was significantly faster and significantly decreased patient radiation exposure.
Meaning
The accuracy of the template-guided lung nodule localization appears noninferior to and this method appears safer than the computed tomography–guided method; thus, this new approach may provide patients with an option for undergoing lung nodule localization without computed tomographic scan dependency.
Abstract
Importance
Localization of small lung nodules are challenging because of the difficulty of nodule recognition during video-assisted thoracoscopic surgery. Using 3-dimensional (3-D) printing technology, a navigational template was recently created to assist percutaneous lung nodule localization; however, the efficacy and safety of this template have not yet been evaluated.
Objective
To assess the noninferiority of the efficacy and safety of a 3-D–printed navigational template guide for localizing small peripheral lung nodules.
Design, Setting, and Participants
This noninferiority randomized clinical trial conducted between October 2016 and October 2017 at Shanghai Pulmonary Hospital, Shanghai, China, compared the safety and precision of lung nodule localization using a template-guided approach vs the conventional computed tomography (CT)-guided approach. In total, 213 surgical candidates with small peripheral lung nodules (<2 cm) were recruited to undergo either CT- or template-guided lung nodule localization. An intention-to-treat analysis was conducted.
Interventions
Percutaneous lung nodule localization.
Main Outcomes and Measures
The primary outcome was the accuracy of lung nodule localization (localizer deviation), and secondary outcomes were procedural duration, radiation dosage, and complication rate.
Results
Of the 200 patients randomized at a ratio of 1:1 to the template- and CT-guided groups, most were women (147 vs 53), body mass index ranged from 15.4 to 37.3, the mean (SD) nodule size was 9.7 (2.9) mm, and the mean distance between the outer edge of target nodule and the pleura was 7.8 (range, 0.0-43.9) mm. In total, 190 patients underwent either CT- or template-guided lung nodule localization and subsequent surgery. Among these patients, localizer deviation did not significantly differ between the template- and CT-guided groups (mean [SD], 8.7 [6.9] vs 9.6 [5.8] mm; P = .36). The mean (SD) procedural durations were 7.4 (3.2) minutes for the template-guided group and 9.5 (3.6) minutes for the CT-guided group (P < .001). The mean (SD) radiation dose was 229 (65) mGy × cm in the template-guided group and 313 (84) mGy × cm in CT-guided group (P < .001).
Conclusions and Relevance
The use of the 3-D–printed navigational template for localization of small peripheral lung nodules showed efficacy and safety that were not substantially worse than those for the CT-guided approach while significantly simplifying the localization procedure and decreasing patient radiation exposure.
Trial Registration
ClinicalTrials.gov identifier: NCT02952261
This noninferiority randomized clinical trial conducted at a single center in China compares the safety and efficacy of a 3-dimensionally printed navigational template approach with that of the conventional computed tomography–guided method for localizing small peripheral lung nodules.
Introduction
Because low-dose chest helical computed tomographic (CT) screening has been shown to increase the detection rate of early-stage lung cancer and to reduce the mortality rate in a high risk population, lung cancer screening programs using chest CT have been widely implemented. Such screening has resulted in the detection of more small lung nodules.1,2,3 Small peripheral lung nodules, and those that are nonsolid, can be difficult to localize during video-assisted thoracoscopic surgery (VATS).4 It has been reported that failure to localize pulmonary nodules is the most common reason for conversion to thoracotomy.5 Lung nodules are traditionally recognized by palpation with thoracoscopic instruments or with the surgeon’s finger when there is no pleural indentation or pigmentation.4 However, increased intraoperative lung manipulation may subject the lung to more physical injury. In addition, the lung palpation technique is often time-consuming, experience-dependent, and limited because of high failure rates, especially when the target nodule is nonsolid or located deep beneath the pleural surface.5,6
Therefore, several methods to mark target pulmonary nodules during the preoperative or intraoperative period have been developed, enabling wedge resection of small lung nodules using VATS.7,8,9 Percutaneous localization with a hookwire or microcoil placement, dye marking, or ethiodized oil injection are the most frequently used approaches.10,11,12,13 The critical issue in percutaneous lung nodule localization is how to accurately place the localizer near the target lung nodule. Conventionally, the localization procedure is guided by CT scan. The interventional radiologist measures the length and angle of the localizer from 2-dimensional (2-D) images and manually places the localizer by following the insertion length and angle. However, even experienced operators sometimes require repeated attempts at directing the localizer to achieve accurate nodule localization.
In our previous study,14 we created a navigational template by using 3-D printing technology to facilitate lung nodule localization and to reduce the dosage of the radiation exposure. To investigate the safety and precision of this template-guided lung nodule localization, we conducted the present prospective, noninferiority randomized clinical trial. We hypothesize that the precision of nodule localization using the template-guided method is noninferior to that using the conventional CT-guided method and that the template-guided method would facilitate a shorter localization procedure and reduce patient radiation exposure.
Methods
Study Design
This was a prospective, single-center, noninferiority randomized clinical trial comparing a template-guided method vs the conventional CT-guided method for localization of small lung nodules. The present trial was designed to assess the noninferiority of the precision of the nodule localization using the newly developed template-guided method. The trial protocol is provided in Supplement 1. The study was approved by the Institutional Review Board at Shanghai Pulmonary Hospital and conducted in accordance with the Declaration of Helsinki.15 Participants were informed of any potential risks associated with their participation, and their written informed consent was obtained before they entered the trial. Participants could withdraw their consent at any time during the study without consequence to their medical care.
Inclusion and Exclusion Criteria
Patients with indeterminate lung nodules who were scheduled for VATS lung nodule resection were approached by a trained research assistant (L. Zeyao) to evaluate their eligibility. The 3 main inclusion criteria were that the long-axis diameter of the target lung nodule was less than 20 mm, that the inner edge of the target nodule was at least 2 cm from major arteries or veins to allow for secure nodule excision through wedge resection, and that the patient’s surgeon confirmed with L. Zeyao the necessity for lung nodule localization. The 2 primary exclusion criteria were that the target lung nodule was shielded by the scapula, limiting access by percutaneous localizer, and that there were 2 or more lung nodules that needed to be localized.
Intervention
In our previous study,14 the process of template design and printing was described in detail, and a video clip was provided to demonstrate the template-guided localization technique. Using stereolithography 3-D printing in the present study, the manufacturing process took approximately 6 hours and cost approximately $100 per person (eFigure 1 in Supplement 2).
One of us (L. Zhang) conducted all lung nodule localization procedures, which were monitored by 2 trained research assistants (L.W. and X.K.) on-site to confirm the adherence to standard operative procedures and collect related information. After participants were positioned on the examining table of the CT scanner, they underwent either template- or CT-guided hookwire localization based on their group randomization. After the hookwire placement was confirmed by CT scan, the localizer was released into the lung parenchyma, and the sheath of the hookwire was retrieved. Finally, a CT scan was repeated to evaluate for complications of lung nodule localization.14 All thoracic CT scans were performed using a Brilliance-40 CT scanner (Philips Medical Systems).
Test Arm
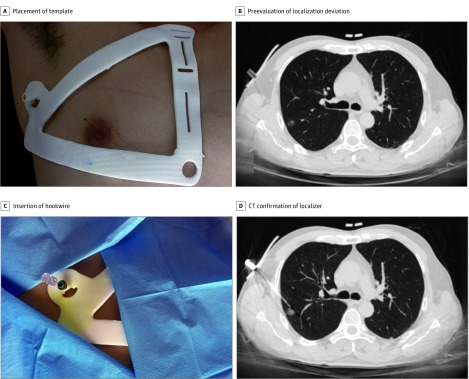
The surgeon (L. Zhang) placed the printed template on the patient’s thoracic wall and then immobilized the template to prevent incidental deviation (Figure 1A). A CT scan was then obtained, and one of us (L.W.) preevaluated the localizer deviation by drawing an imaginary line from the insertion site toward the target nodule on the CT images (Figure 1B). If the distance between this imaginary line and the center of the target nodule was 2 cm or less, a 20-gauge hookwire (PAJUNK) was inserted and released under the guidance of the navigational template during the patient’s deep inspiration after local anesthesia administration (Figure 1C and D). However, if the distance was more than 2 cm beyond the acceptable range of localizer deviation, one of us (L.W.) recorded it as a failure of template-guided localization, and conventional CT-guided localization was performed instead.
Figure 1. Workflow of Template-Guided Lung Nodule Localization.
Preevaluation of localization deviation prior to needle insertion consists of evaluating an imaginary line drawn from the puncture point toward the target nodule. CT indicates computed tomography.
Control Arm
After patients were positioned on the CT table, an initial CT scan was conducted in the area of interest. The hookwire insertion site was determined using the CT gantry laser lights and a metal marker on the skin. The insertion length and angle were estimated on the initial CT images, and the localizer was then inserted without penetrating the pleura. Afterwards, repeated CT scans were obtained to confirm or redirect the localizer to obtain adequate accuracy of nodule localization. On the basis of previous experience, deviations of 2 cm or less between the localizer and the center of the target nodule were considered sufficiently accurate to allow for safe nodule localization. Once the relative position of the hookwire and target nodule was acceptable, the hookwire was deployed into the lung parenchyma.16,17
Primary Outcome
The primary outcome was the accuracy of nodule localization, which was evaluated using the deviation between localizer and the center of the target nodule. The deviation was calculated based on the final CT scan obtained during nodule localization.
Because the localizer and target nodule may not be seen on the same plane of the CT scan, it was difficult to precisely measure the localizer deviation on 2-D CT images. Therefore, we reconstructed the patient’s thorax based on CT images obtained during nodule localization and then accurately calculated the deviation using computer-aided design software (eFigure 2 in Supplement 2). The deviation was represented 3-dimensionally as vertical, anterior-posterior, and horizontal deviations, and the total deviation of the nodule localization was calculated as the square root of the sum of each dimension squared (eFigure 3 in Supplement 2).
Secondary Outcome
The secondary outcomes were the procedural duration, complication rate, and radiation exposure dose. The procedural duration was derived from CT scan data and was calculated as the time between the initial and final scans. Complications associated with lung nodule localization included pneumothorax, pulmonary hemorrhage, and hemoptysis. Radiation exposure was represented as the dose-length product and effective dose.18
Randomization
A computerized randomization list was created using block randomization with a block size of 10, and the results were placed in sealed envelopes by individuals not involved in the present trial. After providing written informed consent, the participants were assigned a random number based on the sequence of their participation. Because of the unfeasibility of masking, the study was not blinded after randomization.
Statistical Analysis
Based on our experience in our previous study,14 the expected difference in localizer deviation between the CT- and template-guided groups was 3.5 mm. Randomization of 140 patients (70 in each group) was needed to obtain 90% power with a 1-sided α of 0.05 and a noninferiority margin of 5 mm. To allow for some dropouts, we planned to include 100 patients per group.
Three tests were used to determine whether differences between the 2 groups were statistically significant: independent-sample t tests for continuous variables that were normally distributed, χ2 tests for categorical variables, and Mann-Whitney tests for continuous variables that were not normally distributed. Owing to the existence of premature randomization,19 an intention-to-treat analysis was performed for all participants who received lung nodule localization and subsequent surgery. All statistical analyses were 2-sided, and P < .05 was considered statistically significant. Analyses were conducted with Stata, version SE/12.0 (StataCorp).
Results
From October 1, 2016, to October 25, 2017, 213 patients were eligible for inclusion, and 200 were randomized at a ratio of 1:1 to the template- and CT-guided groups. Demographic, clinical, and radiologic characteristics are given in Table 1. Participants were mostly female (147 vs 53) and had a body mass index, calculated as weight in kilograms divided by height in meters squared, ranging from 15.4 to 37.3. The mean (SD) nodule size was 9.7 (2.9) mm, and the mean distance between the outer edge of target nodule and the pleura was 7.8 (range, 0.0-43.9) mm.
Table 1. Patient Preoperative Demographic and Clinical Information.
| Characteristic | No. of Patients | P Value | ||
|---|---|---|---|---|
| Total (n = 200) | Template-Guided Group (n = 100) | CT-Guided Group (n = 100) | ||
| Age, mean (SD), y | 53 (13) | 54 (15) | 52 (11) | .45 |
| Sex | ||||
| Men | 53 | 26 | 27 | .87 |
| Women | 147 | 74 | 73 | |
| Smoking history | ||||
| No | 142 | 70 | 72 | .76 |
| Yes | 58 | 30 | 28 | |
| BMI, mean (SD) | 22.9 (2.8) | 22.8 (2.8) | 22.9 (2.9) | .86 |
| FEV1 (% predicted), mean (SD) | 94.0 (13.8) | 94.3 (14.6) | 93.6 (13.0) | .71 |
| Nodule location | ||||
| RUL | 64 | 38 | 26 | .23 |
| RML | 14 | 5 | 9 | |
| RLL | 43 | 17 | 26 | |
| LUL | 47 | 25 | 22 | |
| LLL | 32 | 15 | 17 | |
| Nodule size, mean (SD), mm | 9.7 (2.9) | 10.1 (3.0) | 9.2 (2.7) | .03 |
| Distance from pleura, mean (range), mm | 7.8 (0.0-43.9) | 8.5 (0.0-43.9) | 6.5 (0.0-35.9) | .20 |
| Nodule type | ||||
| Pure GGO | 96 | 41 | 55 | .09 |
| Mixed GGO | 85 | 50 | 35 | |
| Solid | 19 | 9 | 10 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CT, computed tomography; FEV1, forced expiratory volume in 1 s; GGO, ground glass opacity; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
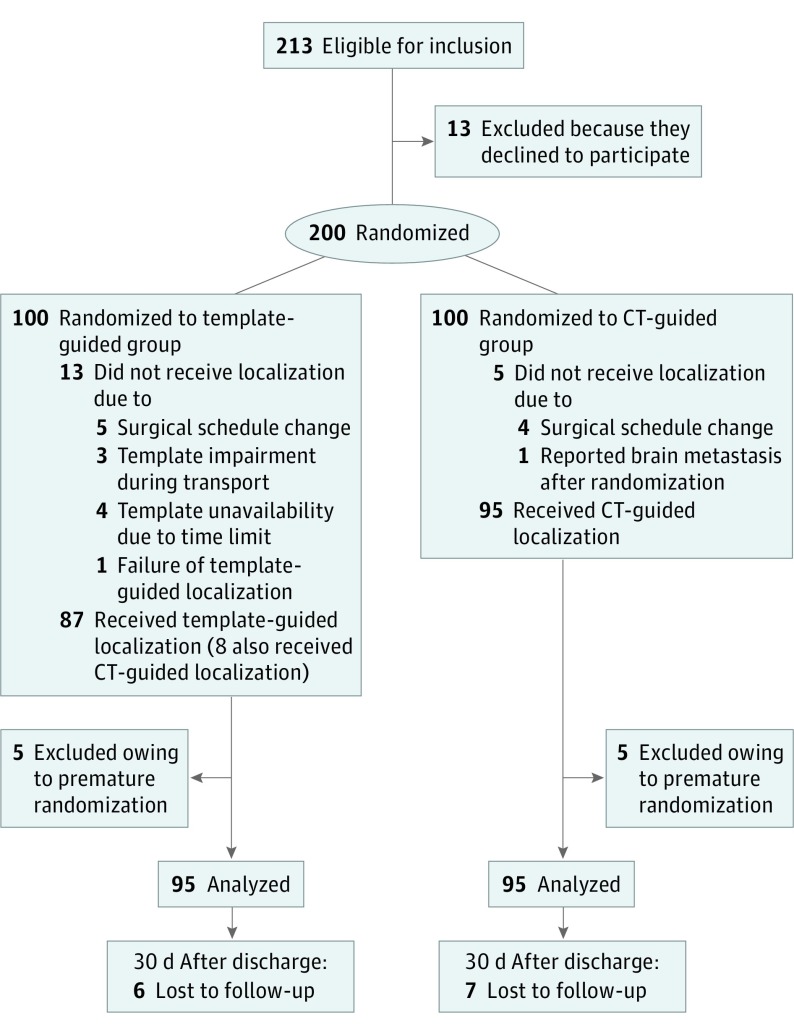
Owing to premature randomization, 10 patients (5 in each treatment group) did not undergo lung nodule localization and subsequent lung resection and were thus excluded from the intention-to-treat analysis.19 Among these 10 excluded patients, 5 had a modified surgical schedule, 3 ultimately declined to receive lung resection, 1 had unexpected brain metastases reported after randomization, and 1 had surgery delay due to menstruation. In addition, because of the limited time from template design to printing (n = 4), template impairment during transportation (n = 3), and estimated localizer deviation beyond 2 cm (n = 1), 8 patients randomized to the template-guided group underwent CT-guided lung nodule localization. A flowchart of the study participants is shown in Figure 2.
Figure 2. Flowchart of the Study Participants.
CT indicates computed tomography.
Characteristics of Lung Nodule Localization
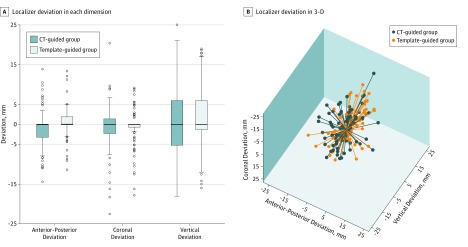
In the intention-to-treat analysis, vertical, coronal, and total deviations were not significantly different between the template- and CT-guided groups. In total, 190 patients underwent either CT- or template-guided lung nodule localization and subsequent surgery. Among these patients, localizer deviation did not significantly differ between the template- and CT-guided groups (mean [SD], 8.7 [6.9] vs 9.6 [5.8] mm; P = .36). Localizer deviation in the anterior-posterior dimension was marginally but significantly larger for patients in the CT-guided group than in the temple-guided group (mean [SD], 2.2 [3.1] vs 3.2 [3.7]; P = .04; Table 2). The 95% CIs for the difference in localizer deviation ranged from −2.7 mm to 1.0 mm, which did not reach the predetermined noninferiority margin of 5 mm. Regarding the 3-D deviation, the localizer mostly deviated in the vertical dimension (Figure 3).
Table 2. Characteristics of Nodule Localization and Surgical Outcomes Between the Template- and CT-Guided Groups.
| Nodule Localization or Surgical Outcome | Intention-to-Treat Analysis | |||
|---|---|---|---|---|
| Total | Template-Guided Group (n = 95) | CT-Guided Group (n = 95) | P Value | |
| Procedural duration, mean (SD), min | 8.5 (3.6) | 7.4 (3.2) | 9.5 (3.6) | <.001 |
| Decubitus position, No. | ||||
| Supine | 109 | 58 | 51 | .30 |
| Lateral | 81 | 37 | 44 | |
| Insertion attempts, No. | ||||
| 1 | 91 | 85 | 6 | <.001 |
| 2 | 86 | 8 | 78 | |
| ≥3 | 13 | 2 | 11 | |
| Deviation, mean (SD), mm | ||||
| Total | 9.2 (6.4) | 8.7 (6.9) | 9.6 (5.8) | .36 |
| Vertical | 6.3 (6.1) | 6.6 (6.5) | 6.0 (5.7) | .52 |
| Anterior-posterior | 2.7 (3.5) | 2.2 (3.1) | 3.2 (3.7) | .04 |
| Coronal | 3.2 (4.3) | 3.0 (3.9) | 3.4 (4.6) | .47 |
| DLP, mean (SD), mGy × cm | 271 (86) | 229 (65) | 313 (84) | <.001 |
| ED, mean (SD), mSv | 3.8 (1.2) | 3.2 (0.9) | 4.4 (1.2) | <.001 |
| Pneumothorax, No. | 27 | 10 | 17 | .15 |
| Hemorrhage, No. | 17 | 6 | 11 | .20 |
| Waiting time, mean (SD), min | 63 (23) | 63 (24) | 63 (21) | .95 |
| Surgical types, No. | ||||
| Wedge resection | 149 | 71 | 78 | .22 |
| Lobectomy | 41 | 24 | 17 | |
| Localizer dislodgement, No. | 5 | 3 | 2 | .65 |
| Nodule size, mean (SD), mma | 6.9 (2.8) | 7.4 (3.0) | 6.5 (2.6) | .03 |
| Margin distance, mean (SD), mm | 18.2 (8.3) | 18.7 (9.2) | 17.6 (7.4) | .35 |
| Frozen section analysis, No. | ||||
| Benign | 15 | 6 | 9 | .28 |
| AAH | 18 | 12 | 6 | |
| AIS | 82 | 39 | 43 | |
| MIA | 34 | 14 | 20 | |
| IA | 41 | 24 | 17 | |
Abbreviations: AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; CT, computed tomography; DLP, dose-length product; ED, effective dose; IA, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma.
Measured on resected specimen.
Figure 3. Localizer Deviation Between the Computed Tomography (CT)– and Template-Guided Groups in 3 Dimensions (3-D).
A, Comparison of the localizer deviation in each dimension. The horizontal line in each box indicates the median, while the top and bottom borders of each box indicate the 75th and 25th percentiles, respectively. The ends of the whiskers above and below each box indicate the minimum and maximum values. The points beyond the whiskers indicate outliers that are beyond the quartiles by 1.5 interquartiles. B, Comparison of the 3-dimensional deviation. Negative values for anterior-posterior, coronal, or vertical deviations indicate that the localizer deviates posteriorly, laterally, or caudally, respectively.
The mean (SD) localizer deviation was 8.2 (6.1) mm and 10.8 (6.4) mm for nodules located in the upper and middle lobes, respectively, vs the lower lobes (P = .006). In the template-guided localization, patients receiving localizer insertion in the lateral position had larger localizer deviations than patients in the supine position (mean [SD], 11.6 [7.3] vs 6.8 [6.3] mm, P = .002). However, in the CT-guided localization, localizer deviation was not correlated with patient position. In addition, there was no significant correlation between body mass index and localizer deviation in either group (r = 0.19 for the CT-guided group, and r = 0.06 for the template-guided group).
Because nodule location and patient decubitus position can contribute to localizer placement accuracy, a subgroup analysis of localizer deviation by these 2 factors was conducted (eFigure 4 in Supplement 2). The location of each nodule was redefined based on its location relative to the carina of the trachea. A nodule above the carina was considered an upper nodule, and a nodule below the carina was considered a lower nodule. For patients with an upper nodule and supine position, template-guided localization was superior to the CT-guided method in terms of localizer deviation (mean [SD], 4.4 [4.3] vs 8.6 [4.1] mm; P < .01).
Radiation exposure was significantly lower for patients in the template-guided group (mean [SD] dose-length product, 229 [65] vs 313 [84] mGy × cm, P < .001; mean [SD] effective dose, 3.2 [0.9] vs 4.4 [1.2] mSv, P < .001) (Table 2). The mean (SD) procedural duration was significantly shorter for patients randomized to the template-guided group (7.4 [3.2] vs 9.5 [3.6] minutes; P < .001). In addition, in the template-guided group, the surgeon (L.Z.) needed fewer insertion attempts to accurately place the localizer, with 85 of 95 patients (89%) having only 1 insertion attempt (Table 2). In terms of complications associated with percutaneous nodule localization, the frequencies of pneumothorax and pulmonary hemorrhage tended to be lower in the template-guided group, but the difference did not reach statistical significance. All complications were asymptomatic, and no special interventions were needed.
Surgical Outcomes and Pathological Analysis
Surgical outcomes and pathological analysis results are summarized in Table 2. Intraoperative localizer dislodgement was observed intraoperatively in 5 patients (2.6%). However, using bleeding on the visceral pleura caused by needle puncture as a guide, lung wedge resection was still successfully performed by VATS. The results of our frozen section analysis found 41 (21.6%) invasive adenocarcinomas, 34 (18.4%) minimally invasive adenocarcinomas, 82 (44.2%) adenocarcinomas in situ, 18 (8.9%) atypical adenomatous hyperplasia, and 15 (6.8%) benign lesions. The margin distance was not significantly different between the 2 groups (mean [SD], 18.7 [9.2] vs 17.6 [7.4] mm for the template- and CT-guided groups, respectively). Following frozen section analysis, 41 patients found to have invasive adenocarcinoma underwent complete lobectomy with systematic lymph node dissection.
There were 13 cases (6.8%) with a discrepancy between the final pathology and frozen section analyses (eTable in Supplement 2). Among these, 1 case with minimally invasive adenocarcinoma on final pathology was originally read as invasive adenocarcinoma on frozen section, and 1 case with a final diagnosis of invasive adenocarcinoma was originally read as minimally invasive adenocarcinoma. Therefore, frozen section diagnosis error had substantial clinical influence on 2 (1.1%) patients, with 1 patient receiving unnecessary radical lobectomy and 1 patient receiving insufficient resection.
Discussion
With an increasing number of small lung nodules being detected by CT screening, the importance of establishing a standard management strategy for this group of lung lesions has emerged.1,8,20 Video-assisted thoracoscopic surgery is a minimally invasive approach used to obtain a definitive tissue diagnosis through wedge resection biopsy, and at the same time, the procedure can be used to provide curative treatment. However, localizing small lung nodules during VATS can be challenging, and failure of nodule localization results in conversion to thoracotomy.5 To mitigate this problem, modalities have been established to preoperatively place a localizer or radiotracer near the target nodule so that surgeons can identify the resection region. Electromagnetic navigational bronchoscopic localization introduces a localizer through the bronchi and can be performed in the operating room just before the surgery.21 However, owing to the high cost and prolonged operative time, this method is not widely applied. Because of its simplicity and independence of special equipment, CT-guided percutaneous lung nodule localization is currently the most commonly performed technique.12 Nevertheless, owing to the difficulty of accurate localizer navigation, this approach may lead to repeated CT scans and several localizer insertion attempts, especially when the practitioner is less experienced.
To simplify the percutaneous lung nodule localization procedure, decrease the associated radiation dosage, or even potentially perform percutaneous localization without the need to depend on CT scan guidance, a novel navigational template was recently created by our group to guide percutaneous lung nodule localization.14 This navigational template created by 3-D printing technology accurately transferred CT-derived spatial information to guide nodule localization. Interventional radiologists do not need to calculate the puncture site from 2-D images or make several attempts to redirect the localizer, thus reducing lung parenchyma injury caused by repeated needle insertions. Prior to planned lung wedge resection, the insertion route of a localizer is designed by the patient’s operating surgeon. Using the information about this insertion route, the navigational template is designed and then imported to the 3-D printer. By setting the insertion length and angle of the localizer, the navigational template facilitates the localization procedure and minimizes performer-related operation discrepancies. In addition, the operating surgeon determines how the lung nodule localization should be performed without being present in the CT room. Therefore, this navigational template-guided approach gives the operating surgeon full control over a patient’s surgical strategy, as the insertion route of the localizer dictates the manipulation of the endostaplers during wedge resection.
To practically apply this navigational template in clinical applications, the critical requirement is a small and stable localizer deviation. Anatomical displacement of the lung during the respiratory cycle is mostly responsible for localizer deviation. Diaphragmatic movement causes lung displacement primarily in the vertical dimension, which likely accounts for localizer deviation occurring more often in this dimension than the other 2 dimensions. In addition, as diaphragmatic movement mostly affects lower lung field ventilation,7 it is usually more difficult to accurately localize nodules in the lower field of the lung than in the upper field. Therefore, the localizer deviation in the present study tended to be larger for nodules in the lower lobe in both the CT- and template-guided groups. We also found that patients undergoing template-guided localization in a lateral position displayed larger localizer deviations than patients in the supine position. This phenomenon could be explained by a patient’s postural alteration between the design and real-world conditions. The navigational template was designed based on the CT scans of patients in the supine position, but patients who require posterior access due to nodule location need to be in the lateral decubitus position during localization. The spatial relationship between the target nodule and thoracic cage may have changed when the patient moved from a supine to a lateral decubitus position, which likely accounted for the increased localizer deviation.
With improved 3-D printer accessibility and advanced segmentation methods, 3-D printing based on imaging data has recently been applied in a variety of medical applications, including surgical planning,22,23,24,25,26,27 medical training,28,29 and prosthetics production.30 The navigational template used in the present trial was initially developed with the intent of achieving percutaneous lung nodule localization without CT assistance. However, when CT confirmation of localizer placement was completely omitted, patients could potentially be faced with the risk that the target lesion might be partially resected or missed at the time of wedge resection. For patients with a nodule in the upper field of the lung who receive percutaneous localization in the supine position, the template-guided localization may be performed without CT confirmation because the localizer deviation is small and stable for this subgroup of patients. The safety of template-guided localization without CT assistance needs to be further validated in future studies.
Limitations
In present study, lung nodule localization guided by standard CT imaging was compared with template-guided localization. The CT fluoroscopic guidance system has been used in interventional radiology and demonstrates high localization accuracy. Therefore, the difference in accuracy between template- and CT fluoroscopy-guided localization is unclear. Furthermore, because the accuracy of nodule localization using the template-guided approach is more likely to be influenced by nodule location and the patient’s decubitus position, future studies should identify the subgroup of patients who are optimal candidates for template-guided localization and compare the efficacy and efficiency of template-guided localization with those of the current best approaches in these patients.
Conclusions
The present randomized clinical trial showed that the accuracy of localizing small lung nodules using a template-guided approach was noninferior to that using conventional CT-guided localization. In addition, the template-guided approach appeared to simplify the procedure and decrease patient radiation exposure.
Trial Protocol
eFigure 1. Design and Printing of the Navigational Template
eFigure 2. Representative Images of Localizer Deviation
eFigure 3. Measurement of Localizer Deviation in 3-D by Tomographic Reconstruction
eFigure 4. Subgroup Analysis of Localizer Deviation by Patients’ Positions and Nodules’ Locations in Template-Guided Lung Nodule Localization
eTable. Comparison of Frozen Section Analysis and Final Pathology
Data Sharing Statement
References
- 1.Church TR, Black WC, Aberle DR, et al. ; National Lung Screening Trial Research Team . Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi: 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manser R, Lethaby A, Irving LB, et al. . Screening for lung cancer. Cochrane Database Syst Rev. 2013;6(6):CD001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gould MK, Tang T, Liu IL, et al. . Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. doi: 10.1164/rccm.201505-0990OC [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Minamiya Y, Matsuzaki I, et al. . Indication for preoperative localization of small peripheral pulmonary nodules in thoracoscopic surgery. J Thorac Cardiovasc Surg. 2002;124(6):1198-1202. doi: 10.1067/mtc.2002.127331 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Nagai K, Yoshida J, et al. . Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115(2):563-568. doi: 10.1378/chest.115.2.563 [DOI] [PubMed] [Google Scholar]
- 6.Tamura M, Oda M, Fujimori H, Shimizu Y, Matsumoto I, Watanabe G. New indication for preoperative marking of small peripheral pulmonary nodules in thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2010;11(5):590-593. doi: 10.1510/icvts.2010.241018 [DOI] [PubMed] [Google Scholar]
- 7.Kolar P, Neuwirth J, Sanda J, et al. . Analysis of diaphragm movement during tidal breathing and during its activation while breath holding using MRI synchronized with spirometry. Physiol Res. 2009;58(3):383-392. [DOI] [PubMed] [Google Scholar]
- 8.Van Schil PE, Asamura H, Rusch VW, et al. . Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur Respir J. 2012;39(2):478-486. doi: 10.1183/09031936.00027511 [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, McDermott S, Mathisen DJ, Shepard JO. Preoperative localization of lung nodules with fiducial markers: feasibility and technical considerations. Ann Thorac Surg. 2017;103(4):1114-1120. doi: 10.1016/j.athoracsur.2016.09.112 [DOI] [PubMed] [Google Scholar]
- 10.Mullan BF, Stanford W, Barnhart W, Galvin JR. Lung nodules: improved wire for CT-guided localization. Radiology. 1999;211(2):561-565. doi: 10.1148/radiology.211.2.r99ma35561 [DOI] [PubMed] [Google Scholar]
- 11.Powell TI, Jangra D, Clifton JC, et al. . Peripheral lung nodules: fluoroscopically guided video-assisted thoracoscopic resection after computed tomography-guided localization using platinum microcoils. Ann Surg. 2004;240(3):481-488. doi: 10.1097/01.sla.0000137132.01881.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CH, Han K, Hur J, et al. . Comparative effectiveness and safety of pre-operative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017;151(2):316-328. doi: 10.1016/j.chest.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zhou J, Zhang J, et al. . Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc. 2011;25(6):1723-1729. doi: 10.1007/s00464-010-1502-3 [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Li M, Li Z, et al. . Three-dimensional printing of navigational template in localization of pulmonary nodule: A pilot study. J Thorac Cardiovasc Surg. 2017;154(6):2113-2119.e7. [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Ichinose J, Kohno T, Fujimori S, Harano T, Suzuki S. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg. 2013;96(4):1203-1208. doi: 10.1016/j.athoracsur.2013.05.026 [DOI] [PubMed] [Google Scholar]
- 17.Klinkenberg TJ, Dinjens L, Wolf RFE, et al. . CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol. 2017;115(7):898-904. doi: 10.1002/jso.24589 [DOI] [PubMed] [Google Scholar]
- 18.Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008;248(3):995-1003. doi: 10.1148/radiol.2483071964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fergusson D, Aaron SD, Guyatt G, Hébert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652-654. doi: 10.1136/bmj.325.7365.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacMahon H, Naidich DP, Goo JM, et al. . Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi: 10.1148/radiol.2017161659 [DOI] [PubMed] [Google Scholar]
- 21.Abbas A, Kadakia S, Ambur V, Muro K, Kaiser L. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg. 2017;153(6):1581-1590. doi: 10.1016/j.jtcvs.2016.12.044 [DOI] [PubMed] [Google Scholar]
- 22.Guibert N, Moreno B, Plat G, Didier A, Mazieres J, Hermant C. Stenting of complex malignant central-airway obstruction guided by a three-dimensional printed model of the airways. Ann Thorac Surg. 2017;103(4):e357-e359. doi: 10.1016/j.athoracsur.2016.09.082 [DOI] [PubMed] [Google Scholar]
- 23.Chang PC. Computer-assisted planning and 3D printing-assisted modeling for chin augmentation. Aesthet Surg J. 2017;38(1):1-10. doi: 10.1093/asj/sjx071 [DOI] [PubMed] [Google Scholar]
- 24.Hermsen JL, Burke TM, Seslar SP, et al. . Scan, plan, print, practice, perform: development and use of a patient-specific 3-dimensional printed model in adult cardiac surgery. J Thorac Cardiovasc Surg. 2017;153(1):132-140. doi: 10.1016/j.jtcvs.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 25.Li B, Wei H, Zeng F, Li J, Xia JJ, Wang X. Application of a novel three-dimensional printing genioplasty template system and its clinical validation: a control study. Sci Rep. 2017;7(1):5431. doi: 10.1038/s41598-017-05417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368(21):2043-2045. doi: 10.1056/NEJMc1206319 [DOI] [PubMed] [Google Scholar]
- 27.Arcieri L, Giordano R, Bellanti E, Chiappino D, Murzi B. Impact of 3D printing on the surgical management of tracheal stenosis associated to pulmonary sling: a case report. J Thorac Dis. 2018;10(2):E130-E133. doi: 10.21037/jtd.2017.12.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Ahn JY, Han M, et al. . Efficacy of a three-dimensional-printed training simulator for endoscopic biopsy in the stomach. Gut Liver. 2018;12(2):149-157. doi: 10.5009/gnl17126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gretsch KF, Lather HD, Peddada KV, Deeken CR, Wall LB, Goldfarb CA. Development of novel 3D-printed robotic prosthetic for transradial amputees. Prosthet Orthot Int. 2016;40(3):400-403. doi: 10.1177/0309364615579317 [DOI] [PubMed] [Google Scholar]
- 30.Zuniga JM, Carson AM, Peck JM, Kalina T, Srivastava RM, Peck K. The development of a low-cost three-dimensional printed shoulder, arm, and hand prostheses for children. Prosthet Orthot Int. 2017;41(2):205-209. doi: 10.1177/0309364616640947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Design and Printing of the Navigational Template
eFigure 2. Representative Images of Localizer Deviation
eFigure 3. Measurement of Localizer Deviation in 3-D by Tomographic Reconstruction
eFigure 4. Subgroup Analysis of Localizer Deviation by Patients’ Positions and Nodules’ Locations in Template-Guided Lung Nodule Localization
eTable. Comparison of Frozen Section Analysis and Final Pathology
Data Sharing Statement