Abstract
Cyclic AMP (cAMP), the prototypical second messenger, has been implicated in a wide variety of (often opposing) physiological processes. It simultaneously mediates multiple, diverse processes, often within a single cell, by acting locally within independently-regulated and spatially-restricted microdomains. Within each microdomain, the level of cAMP will be dependent upon the balance between its synthesis by adenylyl cyclases and its degradation by phosphodiesterases (PDEs). In mammalian cells, there are many PDE isoforms and two types of adenylyl cyclases; the G protein regulated transmembrane adenylyl cyclases (tmACs) and the CO2//pH-, calcium-, and ATP-sensing soluble adenylyl cyclase (sAC). Discriminating the roles of individual cyclic nucleotide microdomains requires pharmacological modulators selective for the various PDEs and/or adenylyl cyclases. Such tools present an opportunity to develop therapeutics specifically targeted to individual cAMP dependent pathways. The pharmacological modulators of tmACs have recently been reviewed, and in this review, we describe the current status of pharmacological tools available for studying sAC.
Keywords: ADCY10, cAMP signaling, Inhibitors
1. Introduction
Dr. Earl Sutherland was awarded the Nobel Prize for identifying cAMP as the mediator of cellular control over metabolic activity (Robison, Butcher, & Sutherland, 1968). Over the 60 years it has been studied, cAMP has been implicated in a wide variety of (often opposing) physiological processes, including different aspects of cell proliferation, apoptosis, differentiation, migration, development, ion transport, pH regulation, and gene expression. Recent advances in cAMP signaling, which have revolutionized our understanding of this ubiquitous second messenger, clarify how this single second messenger could simultaneously mediate so many diverse processes. In cells, cAMP signaling is compartmentalized into multiple, independently-regulated microdomains. Cyclic AMP micro-domains control distinct functions by possessing unique effectors, targets, and means of regulating the concentration of the second messenger [reviewed in (Arora et al., 2013; Desman, Waintraub, & Zippin, 2014; Lefkimmiatis & Zaccolo, 2014). Cyclic AMP is produced from ATP by adenylyl cyclases (ACs), and degraded by catabolizing phosphodiesterases (PDEs). There are two distinct types of AC in mammals; bicarbonate-regulated soluble adenylyl cyclase (sAC, ADCY10) and G protein regulated transmembrane adenylyl cyclases (tmACs; ADCY1–9) (Kamenetsky et al., 2006). TmACs mediate the cAMP response to hormonal signals operating via G protein coupled receptors (GPCR). The idea that hormones regulate distinct cAMP microdomains was proposed over thirty years ago (Buxton & Brunton, 1983, 1986), and the functional significance of membrane-proximal, GPCR-signaling cAMP microdomains were among the first to be appreciated (Davare et al., 2001; Marx et al., 2002). Efforts to selectively target individual, GPCR-defined microdomains include identifying pharmacological reagents selective for individual tmAC isoforms (Brust et al., 2017; Conley et al., 2013; Dessauer et al., 2017; Hayes, Soto-Velasquez, Fowler, Watts, & Roman, 2017; Seifert, Schneider, & Bahre, 2014) as well as numerous efforts to identify selective PDE inhibitors [reviewed in (Francis, Blount, & Corbin, 2011; Houslay, 2010)].
Cyclic AMP targets also reside inside the cell, far from the plasma membrane. The best characterized effector of cAMP, Protein Kinase A (PKA), is tethered to multiple intracellular sites via A Kinase Anchoring Proteins (Pawson & Scott, 1997; Aggarwal-Howarth & Scott, 2017), and the more recently identified effector, Exchange Protein Activated by cAMP (EPAC), is also distributed throughout the interior of cells (Schmidt, Dekker, & Maarsingh, 2013). Both PDEs and adenylyl cyclases ‘sculpt’ intracellular cAMP microdomains to control the activity of these intracellular targets (Fischmeister et al., 2006; Monterisi et al., 2017; Oliveira et al., 2010; Terrin et al., 2006). For example, intracellular microdomains can be ‘fed’ by persistent signaling from G protein stimulated tmACs during endocytosis (Calebiro et al., 2009; Kotowski, Hopf, Seif, Bonci, & von Zastrow, 2011).
A second type of adenylyl cyclase in mammalian cells, sAC, defines an independent source of second messenger at intracellular microdo-mains. sAC is found distributed through the cytoplasm and in cellular organelles (Acin-Perez et al., 2009; Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013; Lefkimmiatis, 2014; Lefkimmiatis, Leronni, & Hofer, 2013; Lefkimmiatis & Zaccolo, 2014; Valsecchi, Konrad, & Manfredi, 2014; Valsecchi, Ramos-Espiritu, Buck, Levin, & Manfredi, 2013; Zippin et al., 2003; Zippin et al., 2004; Zippin, Chadwick, Levin, Buck, & Magro, 2010), including inside the nucleus (Zippin et al., 2004; Zippin, Chadwick, Levin, Buck, & Magro, 2010) and the mitochondrial matrix (Acin-Perez et al., 2011; Acin-Perez, Salazar, Kamenetsky, et al., 2009; Zippin et al., 2003). Inside the matrix, the sAC-defined intramitochondrial cAMP signaling cascade regulates ATP production (Acin-Perez, Salazar, Kamenetsky, et al., 2009; Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013; Lefkimmiatis, 2014; Lefkimmiatis, Leronni, & Hofer, 2013; Lefkimmiatis & Zaccolo, 2014). In the cytoplasm, sAC has been identified as the AC responsible for the cAMP regulating lysosomal acidification (Rahman, Ramos-Espiritu, Milner, Buck, & Levin, 2016), apoptosis (Ladilov & Appukuttan, 2014), and the downstream effects from the trafficking GPCRs corticotropin-releasing hormone receptor (Inda et al., 2016, 2017). Thus, spatial control of cAMP signaling involves at least three distinct types of enzymatic activities which locally modulate levels of the second messenger; PDEs, tmACs, and sAC. Understanding cAMP signal transduction requires tools to distinguish between these enzymatic activities, and in this review we detail the pharmacological tools available for selectively studying sAC.
2. Soluble adenylyl cyclase genetics and biochemistry
There are six unique classes of ACs; however, all mammalian nucleotidyl cyclases (i.e., adenylyl and guanylyl cyclases) come from the same class, Class III, and share homologous catalytic cores. Among mammalian ACs, nine genes encode tmAC isoforms (ADCY1–9) while a tenth gene, ADCY10, encodes soluble AC (sAC) (Kamenetsky et al., 2006; Steegborn, 2014). sAC’s activity was first identified in mammals in the 1970’s (Braun, 1975; Braun, Frank, Dods, & Sepsenwol, 1977) but it was only appreciated to define a separate subfamily of ACs after the cloning of ADCY10 in 1999 (Buck, Sinclair, Schapal, Cann, & Levin, 1999). Both sAC-like and tmAC-like AC enzymes are found throughout the animal kingdom, but mammalian sAC is most similar to ACs found in cyanobacteria (Buck, Sinclair, Schapal, Cann, & Levin, 1999; Kleinboelting, van den Heuvel, & Steegborn, 2014). Because modern day cyanobacteria appear to be indistinguishable from the oldest known forms of life in the fossil record, we speculate sAC represents the evolutionarily ancient source of cAMP in mammalian cells (Cann, Hammer, Zhou, & Kanacher, 2003; Hall et al., 2010; Topal et al., 2012).
Unlike the G protein regulated tmACs, sAC is directly regulated by bicarbonate anions () (Chen et al., 2000; Kleinboelting, van denHeuvel, & Steegborn, 2014). Due to the ubiquitous presence of carbonic anhydrases (CA), which catalyze the instantaneous equilibration of carbon dioxide (CO2), bicarbonate (), and protons, mammalian sAC and its -regulated orthologs serve as Nature’s physiological CO2//pHi sensors (Reviewed in (Blackstone, 2014; Buck & Levin, 2011; Chang & Oude-Elferink, 2014; Levin & Buck, 2015; Tresguerres, 2014; Tresguerres, Barott, Barron, & Roa, 2014; Tresguerres, Buck, & Levin, 2010)). In mammalian cells, via regulation of sAC, CO2//pHi act as signals regulating sperm activation and motility (Hess et al., 2005); intraocular pressure in the eye (Lee et al., 2011); ciliary beat frequency in airway (Chen et al., 2014; Schmid et al., 2007); luminal pH in the epididymis (Pastor-Soler et al., 2003) and most likely in the kidney (Paunescu et al., 2008; Paunescu et al., 2010); the mitochondrial electron transport chain (Acin-Perez et al., 2009); activity dependent feeding of neurons in the brain (Choi et al., 2012); and glucose stimulated insulin release from β cells of the pancreas (Holz, Leech, & Chepurny, 2014; Zippin et al., 2013).
In addition to bicarbonate regulation, sAC activity is directly stimulated by Ca2+ (Jaiswal & Conti, 2003; Kleinboelting et al., 2014; Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003), and it is sensitive to physiological relevant fluctuations in substrate ATP (Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003; Zippin et al., 2013). Thus, while tmACs respond to signals originating in other cells (i.e., hormones and neuro-transmitters acting via GPCRs), sAC functions as an environmental sensor and an integrator of intracellular signals (HCO−, ATP, or Ca2+)(Chen et al., 2000; Kleinboelting et al., 2014; Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003; Steegborn, Litvin, Levin, Buck, & Wu, 2005; Zippin et al., 2013).
2.1. Overall sAC architecture
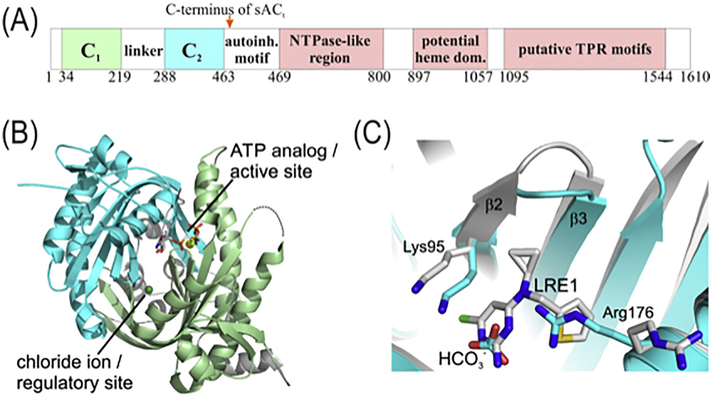
The catalytic cores of sAC and tmACs are homologous and share many common features with respect to structure and catalysis. Both types of enzymes are comprised of two homologous and structurally similar Class III catalytic domains, which dimerize in a head-to-tail fashion to form the pseudo-symmetric Class III catalytic core. Most mammalian genomes have a single sAC locus (ADCY10) with alternative splicing generating a number of sAC isoforms (Levin & Buck, 2015). When initially cloned, two active sAC cDNAs were identified (Buck, Sinclair, Schapal, Cann, & Levin, 1999); the longer cDNA encoded an isoform considered to be full-length (sACfl) while a second cDNA encoded a ‘truncated’ isoform (sACt) (Buck, Sinclair, Schapal, Cann, & Levin, 1999; Jaiswal & Conti, 2001), which corresponds to the protein originally purified from rat testis (Buck, Sinclair, & Levin, 2002; Buck, Sinclair, Schapal, Cann, & Levin, 1999). The sACt isoform comprises the two catalytic domains (C1 and C2), which reside in the ~470 N-terminal residues (Fig. 1A). sACt is sufficient for cAMP formation and the regulatory effects of ATP, calcium and bicarbonate (Steegborn, 2014).
Fig. 1.
Structure and pharmacological inhibition of sAC. (A) Domain architecture of sAC. Residue numbers below the scheme refer to human sAC. (B) Overall structure of the human sAC catalytic core. C1 is shown in green, C2 in cyan, and linker and N-terminus are colored grey. Active site and pseudo-symmetric regulatory site and their respective ligands are labeled. (C) Overlay of the regulatory sites of sAC complexes ith the physiological activator bicarbonate and the pharmacological inhibitor LRE1, respectively. This figure panel was reproduced from (Ramos-Espiritu, Kleinboelting, et al., 2016).
The specific activity of sACt is at least ten times higher than the longer sACfl isoform (~1600 residues), due to inclusion of an autoinhibitory regulatory domain in sACfl (Chaloupka, Bullock, Iourgenko, Levin, & Buck, 2006). The autoinhibitory motif is immediately C-terminal to the C2 catalytic domain (Chaloupka, Bullock, Iourgenko, Levin, & Buck, 2006). It might be part of a larger NTPase domain arrangement, which was postulated based on weak sequence similarities to so-called “Signal Transduction ATPases with Numerous Domains” (STAND) proteins (Danot, Marquenet, Vidal-Ingigliardi, & Richet, 2009). In STAND proteins, an intrinsic NTPase activity controls signaling activity through conformational changes in linker and/or effector domains; examples include the bacterial transcription factor MalT and the apoptosis regulator Apaf-1, whose activating oligomerizations are controlled by ATP.
The region of sACfl which is C terminal to the catalytic domains (sAC-CTR) also features three putative tetratricopeptide repeat (TPR) motifs (Middelhaufe, Leipelt, Levin, Buck, & Steegborn, 2012), which can serve as effector domains in STAND proteins. But STAND-related sAC regulation has yet to be demonstrated experimentally. The only experimentally characterized sAC-CTR domain besides the autoinhibitory module is a heme binding domain (sAC-HD; Fig. 1A) (Middelhaufe, Leipelt, Levin, Buck, & Steegborn, 2012). sAC-HD shows no significant sequence similarity to the NO-recognizing heme domain of the related Class III enzyme soluble guanylyl cyclase (sGC) or to any other known heme proteins (Middelhaufe, Leipelt, Levin, Buck, & Steegborn, 2012). sAC-HD can bind the gaseous signaling molecules NO and CO, but physiological relevance and function of the heme ligand remain to be clari-fied (Steegborn, 2014).
Both sACt and sACfl protein isoforms are expressed in testis (Hess et al., 2005). Molecular cloning and directed PCR experiments identified additional alternatively spliced sAC isoforms from the ADCY10 locus (Geng et al., 2005; Jaiswal & Conti, 2001; Middelhaufe, Leipelt, Levin, Buck, & Steegborn, 2012; Schmid et al., 2007). Reverse-transcription PCR (RT-PCR) reveals that exons are differentially expressed; e.g., while exons encoding the C terminus of sACfl are found to be widely expressed (Geng et al., 2005), exons encoding the Heme binding domain appear to be only expressed in testis and skeletal muscle (Middelhaufe, Leipelt, Levin, Buck, & Steegborn, 2012). In addition, molecular cloning (Geng et al., 2005; Schmid et al., 2007), 5′RACE (Farrell et al., 2008), and the recent mapping of the human proteome (www.proteomicsdb.org) revealed the existence of an alternate sAC promoter generating sAC isoforms missing the first of the two catalytic domains (i.e., sAC-C2 isoforms). cDNAs encoding sAC-C2 isoforms have been cloned from kidney, small intestine (Geng et al., 2005) and bronchial epithelial cells (Schmid et al., 2007), and proteins containing this unique N-terminus are specifically targeted to cilia in bronchial epithelial cells. However, cyclase activity from these sAC-C2 only isoforms has yet to be characterized, and a deeper understanding of sAC isoform expression and function awaits further studies.
2.2. sAC catalytic core structure and regulation
Crystal structures for human sAC catalytic core (hsAC-cat) alone and complexed with a range of ligands (Kleinboelting et al., 2014), as well as prokaryotic sAC-like enzymes in the presence and absence of modulating compounds (Steegborn et al., 2005; Steegborn, Litvin, Levin, Buck, & Wu, 2005; Topal et al., 2012) have added to our understanding of Class III nucleotidyl cyclase features. In particular, these structures reveal nucleotidyl cyclase catalytic mechanism, as well as insights into how sAC is modulated by physiological and pharmacological regulators. The structurally characterized human sAC N-terminus comprises residues 1–469 (Fig. 1B). An N-terminal tail forms a helical subdomain with the ~68 residue linker between C1 (residues 34–219) and C2 (residues 288–463) (Kleinboelting et al., 2014). The catalytic domains both feature the generic Class III fold with a central seven-stranded β-sheet shielded from solvent by three helices and dimerize into the typical, pseudo-heterodimeric Class III AC catalytic core (Fig. 1B) (see (Kleinboelting et al., 2014; Sinha & Sprang, 2006; Steegborn, 2014)). A detailed structure-based comparison of sAC and sAC homologs with tmACs revealed only small differences in overall structure (Kleinboelting, van den Heuvel, & Steegborn, 2014; Steegborn, 2014), with specific differences contributing to the unique regulatory properties of sAC versus tmACs.
The tmACs are prototypically regulated by heterotrimeric G proteins. The activities of most, if not all, tmAC isoforms are stimulated by the GTP-bound alpha subunit of stimulatory G proteins (Gsα), and there is isoform specific regulation by the alpha subunit of the inhibitory class of G proteins (Giα) as well as by the G protein beta gamma (Gβγ) subunits (Dessauer et al., 2017). Thus far, all published (Buck, Sinclair, Schapal, Cann, & Levin, 1999) and unpublished (LRL & JB, unpublished observations) tests exploring G protein regulation of sAC have been negative. A single report showing that the sAC inhibitor KH7 blocked signaling by mitochondrial G Protein coupled cannabinoid receptors postulated G protein regulation of sAC; however, the data can also be explained by indirect regulation of sAC (Hebert-Chatelain et al., 2016). For tmAC activation, the effector region of GTP-bound Gsα subunit (i.e., the switch II helix) inserts into the groove formed by α2′ and the α3′-β4′ loop of the tmAC C2 domain (Tesmer et al., 1999). In sAC, shortened loops between C2 domain α1′ and α2′ as well as α3′ and β3′ seem to preclude Gsα interaction. Similarly, Gαi binds and inhibits tmACs by interacting with the analogous cleft of the tmAC C1 domain, i.e., between the α1/α2 loop and α3/β4 loop (Dessauer, Tesmer, Sprang, & Gilman, 1998). These regions are also significantly different in sAC, in particular the α1/α2 loop is 8 residues longer (Kleinboelting et al., 2014). And importantly, the critical tmAC residues for interacting with Gαi, which are conserved among Gi-inhibited tmACs, are not conserved in sAC. Finally, two regions have been implicated in Gβγ regulation of tmACs; the extended loop between β3′ and α3′ in the tmAC isoform II C2 domain (Brand, Sadana, Malik, Smrcka, & Dessauer, 2015) and/or a region within the C1b region outside of the conserved catalytic core (Brand, Sadana, Malik, Smrcka, & Dessauer, 2015). Both regions are missing in sAC. Thus, all known molecular determinants of heterotrimeric G protein regulation of tmACs are missing in sAC. Another difference between sAC and tmACs is a four-residue insertion in the C2 β2–β3 loop of sAC, which contributes to binding of the physiological sAC activator bicarbonate and to sAC’s insensitivity to the pharmacological tmAC activator forskolin (see below) (Kleinboelting et al., 2014; Steegborn, 2014).
2.2.1. Active site, regulation by Ca2+ and ATP levels
ACs synthesize cAMP by catalyzing formation of a phosphoesterbond between ATP’s α-phosphate group and the 3′ hydroxyl group of its ribose moiety. The active site of sAC, as in tmACs, is located at the pseudo-dimer interface and comprises residues from C1 and C2, including a set of catalytically essential residues conserved between all Class III ACs. Conservation of these residues and of the overall structure as well as all available biochemical data indicate that sAC and tmACs act via the same two-ion mechanism involving a pseudo-binuclear nucleophilic substitution (SN2) at the α-phosphate. Two divalent cations,ionA and ion B, are bound between the two Asp residues (Asp47 and Asp99) conserved in all Class III ACs (Sinha & Sprang, 2006; Steegborn, 2014). Both sites are normally occupied by Mg2+, but the sAC ion B site can also be occupied with Ca2+ (Kleinboelting et al., 2014; Steegborn, Litvin, Levin, Buck, & Wu, 2005). Ion B is coordinated by the ATP β- and γ-phosphates, and replacing Mg2+ with Ca2+ in this site provides them with a stronger interaction partner. Ca2+ thereby increases the substrate affinity of sAC ~10-fold (reflected by a lowering of the Km from 10 mM to 1 mM), leading to increased sAC activity at physiological ATP concentrations (Kleinboelting et al., 2014; Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003; Steegborn, 2014; Steegborn, Litvin, Levin, Buck, & Wu, 2005). This activation mechanism has only been observed for sAC-like AC, while tmAC inhibition by Ca2+ or activation by Ca2+/calmodulin are based on other mechanisms and metal binding sites (Dessauer et al., 2017).
Even in the presence of both Ca2+ and Mg2+, sAC has a 20–50 fold higher Km for its substrate ATP (Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003) than most other ATP utilizing enzymes including all other known mammalian ACs (i.e., the tmACs) (Kamenetsky et al., 2006; Steegborn, 2014). Due to this elevated Km, sAC is not saturated with substrate at physiological ATP concentrations and its activity will thus vary with ATP fluctuations. This ATP-sensor feature of sAC seems physiologically relevant in glucose-sensing β cells of the pancreas, where sAC activity appears to be regulated by glucose-dependent fluctuations in ATP (Zippin et al., 2013). In Class III ACs, the ATP ribose moiety is accommodated in a mainly hydrophobic pocket and forms a polar contact with its ring oxygen to a conserved residue, Asn412 in sAC. In addition, this oxygen interacts with a Ser conserved in tmACs (Ser1028* in tmAC2-C2). In contrast, sAC enzymes have a conservedAla at this position (Ala415*), which prevents this additional polar interaction and contributes to sAC’s reduced ATP affinity in absence of Ca2+ (Kleinboelting et al., 2014; Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003; Steegborn, Litvin, Levin, Buck, & Wu, 2005). Furthermore, sAC’s affinity for ATP may be relatively low because it must undergo a substrate-induced rearrangement. In the apo state, the sAC active site assumes a unique, non-productive conformation. A salt bridge between Arg176 and one of the ion-coordinating residues, Asp99, causes the Asp99-harboring loop to overlap with the ion sites (Kleinboelting et al., 2014). During substrate (ATP/Mg2+) binding, shifts of Arg176 and Asp99 and additional, small rearrangements release the salt bridge and enable ion site formation (Kleinboelting et al., 2014). The energy required for these sAC adjustments is likely provided by the substrate binding energy and thus lowers the affinity of this ligand.
Substrate binding places the adenine in a mostly hydrophobic cleft, which features two polar residues for base recognition. A Lys residue found in all Class III ACs, Lys334 in sAC, recognizes the ring nitrogen 1. It interacts with this base atom in several tmAC/nucleotide analog complexes and its role has been confirmed by mutagenesis in tmACs, mammalian sAC, and bacterial AC. Interestingly, it forms only a water-mediated interaction with the base in the available hsAC-cat complexes with substrate analogs or even unmodified ATP (Kleinboelting et al., 2014) and was suggested to form only upon transition state formation (Kleinboelting et al., 2014; Kleinboelting, van den Heuvel, & Steegborn, 2014; Steegborn, 2014). Interestingly, the direct Lys/base interaction was also not formed in a tmAC/ATP complex crystal structure in a Ca2+-inhibited state (Mou, Masada, Cooper, & Sprang, 2009), supporting the model that this interaction requires catalytic active site rearrangements. A major difference to tmACs in sAC’s adenine binding pocket is the replacement of the second adenine-recognizing residue, an Asp in tmACs, with a functionally equivalent Thr. Since Thr can act as H-bond donor and acceptor, in contrast to the tmAC Asp, this replacement could explain sAC’s slightly lower selectivity for ATP over GTP (Kleinboelting, van den Heuvel, & Steegborn, 2014).
2.2.2. Bicarbonate activation
A sAC complex structure with bicarbonate shows that the activator binds between Lys95 and Arg176 (Fig. 1C) (Kleinboelting et al., 2014). In mammalian ACs, C1 and C2 are homologous, but because C1 and C2 provide different essential catalytic residues, the pseudo-symmetric site (i.e., the site formed at the dimer interface opposite to the active site) in sAC and tmACs lacks essential catalytic residues and is inactive. This pseudo-symmetric site in tmACs binds the non-physiological activator, forskolin (Tesmer, Sunahara, Gilman, & Sprang, 1997). In sAC, the pseudo-symmetric site is tightened through an insertion of four residues in the β2/3* loop and the side chain of the sAC-specific Arg176 (replaced by Ala in tmACs). Due to this insertion, which is conserved among sAC orthologs, the pseudo-symmetric regulatory site is too small to accommodate forskolin. However, it is optimized for accommodation of the small, physiological sAC activator bicarbonate. In the presence of bicarbonate, Arg176 is oriented towards this bicarbonate binding site (BBS) and interacts with the activator. The Arg176 orientation is flipped in apo sAC, where it adopts an inhibitory interaction with Asp99 (Kleinboelting et al., 2014). The bicarbonate-induced rearrangement releases Asp99 and allows for ion site formation and substrate binding (see above). Arg176 is thus assumed to act as a trigger connecting active and regulatory sites, and its reorientation upon bicarbonate binding allows the active site to assume its active conformation (Kleinboelting et al., 2014; Steegborn, 2014). Bicarbonate binding induces some additional, smaller active site rearrangements, many of them also resembling the changes observed upon complex formation with substrate (Kleinboelting et al., 2014; Steegborn, 2014). Kinetically, bicarbonate was shown to mainly affect kcat rather than Km (Litvin,Kamenetsky, Zarifyan, Buck, & Levin, 2003), which is consistent with the model that substrate binding coincides with substrate distortion towards the transition state (Steegborn, 2014).
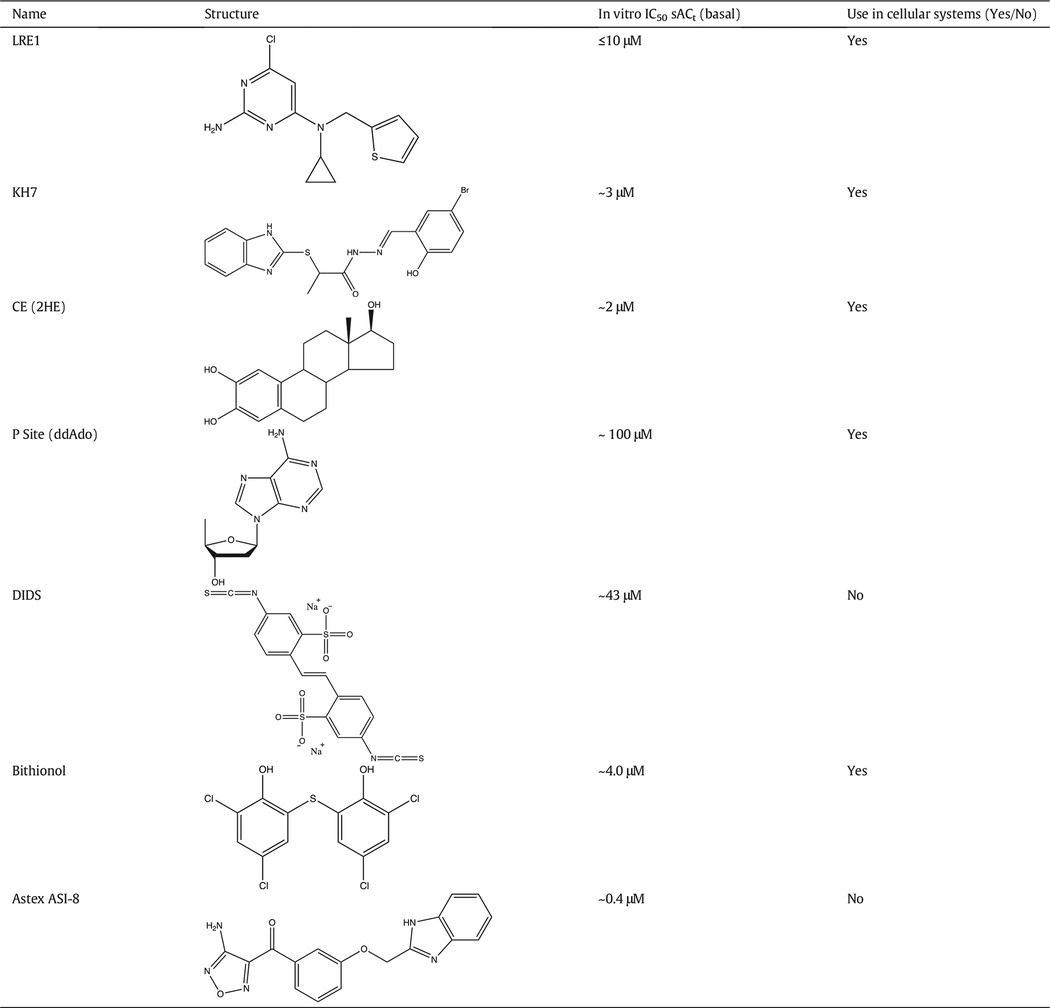
3. Pharmacological inhibitors (Table 1)
Table 1.
Pharmacological inhibitors of sAC.
|
3.1. P site ligands preferably inhibit tmACs
The search for sAC modulating reagents began by exploring whether known inhibitors of tmACs were efficacious against sAC. Adenosine and adenine analogs were among the first identified inhibitors of G protein regulated tmAC activity (Johnson, Yeung, Stubner, Bushfield, & Shoshani, 1989). Because inhibition appeared to be non- or uncompetitive with the substrate ATP, they were initially postulated to inhibit via an undefined P-site inhibitory region. However, crystallographic studies revealed that these inhibitors in fact bind to the nucleotide binding site of the catalytic center, either by themselves or together with the pyro-phosphate product of the cyclization reaction (Tesmer et al., 2000).
They inhibit tmACs by trapping the enzyme in a product complex-like state. Given sAC’s lower affinity for its nucleotide substrate (ATP), it is not surprising that P site inhibitors exhibit reduced affinity for sAC relative to tmACs (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013; Gille et al., 2004; Stessin et al., 2006; Wu et al., 2006). In particular, the cell permeable P-site inhibitor 2′5′-dideoxyadenosine (ddAdo) does not inhibit sAC activity in cellular systems when used at concentrations up to 100 μM (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013). Because P site inhibitors show low tmAC isoform selectivity (Dessauer et al., 2017; Dessauer, Tesmer, Sprang, & Gilman, 1999) they are useful as broad specificity tmAC inhibitors. In fact, ddAdo effectively inhibits cellular tmAC activity at concentrations between 1 and 50 μM; thus, ddAdo selectively inhibits tmAC-dependent signaling (Ramos, Zippin, Kamenetsky, Buck, & Levin, 2008; Stessin et al., 2006; Wu et al., 2006).
3.2. Catechol derivatives of estrogen inhibit sAC and tmACs
Catechol derivatives of estrogen (CEs) are naturally produced steroid metabolites. Both 2-Hydroxyestradiol (2-HE) and 4-Hydroxyestradiol (4-HE) were fortuitously discovered to diminish cAMP production in testis extracts enriched for sAC activity (Braun, 1990). Following the cloning and heterologous expression of sAC, in vitro studies confirmed that CEs inhibit purified mammalian and bacterial sAC proteins with affinities in the low μM range (Steegborn et al., 2005). CE inhibition is noncompetitive with substrate, and crystal structures of CEs with a cyanobacterial sAC ortholog reveal that CEs bind to a partially conserved hydrophobic cleft and chelate essential metal cofactors (Pastor-Soler et al., 2003; Steegborn et al., 2005). Although they had ≥10-fold lower affinities than they had for sAC, CEs also inhibited the in vitro activities of membrane preparations enriched with tmACs types I, II and V. Thus, CEs appear to be general inhibitors of AC activity in vitro. However, in cellular systems, CEs proved to selectively inhibit sAC. Cellular sAC activity is inhibited with an IC50 just slightly above 10 μM while concentrations in excess of 100 μM are required to observe inhibition of tmACs (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013). While CEs do not appreciably regulate estrogen receptors, they exhibit non-genomic effects (Hill, Gebre, Schlicker, Jordan, & Necessary, 2010; Samartzis, Imesch, Twiehaus, Dubey, & Leeners, 2016). These effects are mimicked by the metabolite methoxyestradiol which is inert towards sAC (Steegborn et al., 2005), suggesting CEs modulate additional cellular targets. In early studies of sAC physiology, CEs were the only available sAC selective inhibitors, and CEs were successfully used to identify sAC as a modulator of vacuolar H+ ATPase translocations in response to extracellular pH (Pastor-Soler et al., 2003). CEs remain useful for probing sAC functions, especially to confirm functions identified genetically or using other sAC inhibitors (Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013; Tresguerres et al., 2010; Wang et al., 2016; Wu et al., 2006).
3.3. KH7
To identify selective sAC inhibitors, we performed a small molecule high throughput screen and identified the sAC specific inhibitor, KH7 (Hess et al., 2005). KH7 was shown to be noncompetitive with substrate Mg2+-ATP (Hess et al., 2005), but its binding site has remained uncharacterized. KH7 is cell permeable; both its IC50 in cellular systems and its in vitro IC50 on purified sAC protein is low μM (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013). KH7 has no detectable activity versus tmACs (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013; Ramos, Zippin, Kamenetsky, Buck, & Levin, 2008; Stessin et al., 2006; Wu et al., 2006), and it inhibits sAC in tissues and animals (Choi et al., 2012; Lee et al., 2011; Schmid et al., 2007; Tresguerres et al., 2010; Tresguerres, Levin, Buck, & Grosell, 2010; Zippin et al., 2013). Thus, KH7 quickly became the most widely used pharmacological tool for elucidating sAC functions, and it, along with our defined methodology for using ddAdo to selectively inhibit tmACs (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013), has been widely used by numerous laboratories to identify cAMP signaling functions mediated by sAC (Table 3).
Table 3.
Pharmacologically identified functions of sAC.
As with most pharmacological tools, KH7 is imperfect. As mentioned above, its binding site remains uncharacterized because it has resisted crystallographic characterization, and use of KH7 is limited by an off-target uncoupling effect (Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013; Ramos-Espiritu, Kleinboelting, et al., 2016; Tengholm, 2012) which can cause sAC independent cellular toxicity (Ramos-Espiritu, Kleinboelting, et al., 2016). For these reasons, KH7 use should be restricted to short term assays.
3.4. DIDS - 4,4′ diisothiocyanatostilbene- 2,2′ – disulfonic acid
DIDS is an inhibitor of bicarbonate transporters (Boron, 2001) and while it was shown to inhibit cAMP accumulation in sperm (Visconti, Muschietti, Flawia, & Tezon, 1990), it was unclear if that reflected a direct effect on sAC or a block of bicarbonate import. We found DIDS can directly inhibit sAC activity (Kleinboelting et al., 2014). DIDS inhibition of sAC is competitive with bicarbonate, and co-crystals of DIDS with human sAC show it inhibits sAC activity by interfering with the active site and by blocking bicarbonate access. Due to its limited sAC potency and inhibitory effect on bicarbonate transporters, its use to study specific functions of sAC is limited.
3.5. ASI-8
Fragment screening identified the sAC inhibitor ASI-8 formally known as (4-Aminofurazan-3-yl)-[3-(1H-benzoimidazol-2-ylmethoxy)phenyl]methanone (Saalau-Bethell et al., 2014). The crystal structure of a hsAC-cat/ASI-8 complex reveals that this inhibitor occupies the binding sites for bicarbonate and ATP (Saalau-Bethell et al., 2014). It was reported to inhibit sAC in vitro activity with an IC50 of0.4 μM, but its selectivity for sAC relative to tmACs and efficacy in cellular systems were not reported.
3.6. Bithionol
Over forty years ago, the bisphenol hexachlorophene was identi-fied as an inhibitor of mammalian adenylyl cyclase activity (Mavier, Stengel, & Hanoune, 1976), and we showed recently that it specifically inhibits sAC (Kleinboelting et al., 2016). Both hexachlorophene and the related bisphenol bithionol inhibit sAC by binding to the bicarbonate binding site of the enzyme. In addition to inhibiting bicarbonate-dependent sAC activity via binding to the allosteric bicarbonate regulatory site, bithionol binding induces a rearrangement of the substrate binding residues which affects sAC affinity for ATP. Therefore, these compounds also inhibit sAC activity measured in the absence of bicarbonate. Bithionol and hexachlorophene affect several targets and have pleiotropic effects in cellular systems (Kleinboelting et al., 2016) and thus appear not suitable for probing cellular sAC functions.
3.7. LRE1
Following the success of KH7, we employed a second small molecule screen to identify a new generation of sAC-specific inhibitors with improved pharmacological profile. We screened a more extensive small molecule library using a novel mass spectrometry-based cyclase assay (Ramos-Espiritu, Kleinboelting, et al., 2016). This screen identified LRE1. A hsAC-cat/LRE1 complex structure revealed that the inhibitor binds to sAC’s allosteric regulatory bicarbonate binding site (Fig. 1C), similar to bithionol, and its binding was competitive with bicarbonate (Ramos-Espiritu, Kleinboelting, et al., 2016). Even though LRE1 inhibition was non-competitive with substrate binding, it inhibited basal sAC activity (i.e., measured in the absence of bicarbonate). LRE1 inhibits basal sAC activity via a novel allosteric mechanism; it alters the conformation of the active site and disturbs the proper arrangement of the bound ATP for turnover. Similar to KH7, LRE1 is selective for sAC relative to tmACs (Ramos-Espiritu, Kleinboelting, et al., 2016) and it is efficacious in both cellular systems and in animals (Gandhi et al., 2017). In particular, LRE1 was confirmed to block sAC-specific functions in sperm, mitochondria (Ramos-Espiritu, Kleinboelting, et al., 2016), and the eye (Gandhi et al., 2017). LRE1 does not have the non-sAC mediated mitochondrial uncoupling effect observed with KH7, and it is non-toxic to a variety of cell types for at least 48 h (Ramos-Espiritu, Kleinboelting, et al., 2016). Thus, LRE1 is the preferred sAC specific inhibitor to use for mitochondrial studies and for long-term cellular assays and animal studies.
4. Activators
Our understanding of cyclic nucleotide biology has been propelled by the availability of pharmacological activators of tmACs and guanylyl cyclases. In contrast, there are not good ways to pharmacologically stimulate sAC activity in cells. The plant diterpene, forskolin, binds to the allosteric regulatory site in tmACs to directly stimulate their activity independent of hormonal regulation (Tesmer et al., 1999). sAC is insensitive to forskolin (Buck, Sinclair, Schapal, Cann, & Levin, 1999; Forte, Bylund, & Zahler, 1983) because of its smaller regulator binding site (Kleinboelting et al., 2014). Many of the physiological processes ascribed to cAMP have been revealed, or confirmed, through the use of forskolin, and because sAC is insensitive to forskolin, these processes are thought to be controlled by tmAC-generated cAMP. However, conclusions from studies using forskolin need to be interpreted with caution. Forskolin is an extremely potent activator of tmACs that elevates intracellular cAMP levels to supraphysiological levels. And from the earliest demonstrations that cAMP signaling is spatially restricted, which used micro-injected FRET-based cAMP sensors (Bacskai et al., 1993), forskolin was shown to overwhelm the mechanisms ensuring compartmentalization. Thus, even though forskolin does not stimulate sAC, forskolin could result in elevation of second messenger in sAC-controlled cAMP microdomains. In addition to this lack of selectivity for modulating second messenger production in tmAC-defined microdo-mains, the ability of forskolin to induce supraphysiological levels of cAMP mimics the disease-causing mechanism employed by multiple human pathogens (McDonough & Rodriguez, 2011). Similar to forskolin, cholera toxin from Vibrio cholorae and pertussis toxin from Bordetella pertussis constitutively stimulate host adenylyl cyclase activity. Other bacterial pathogens (i.e., Bacilus anthracis, Pseudomonas aeruginosa, and Yersinia pestis) cause disease by directly injecting a bacterially encoded AC (Gancedo, 2013). Thus, like forskolin, these pathogen-induced stimulators of cAMP result in simultaneous activation of multiple intracellular cAMP signaling microdomains which may actually contribute to their pathogenicity.
Our understanding of cGMP signaling, specifically signaling via the nitric oxide-regulated soluble guanylyl cyclase (sGC), has greatly benefited from two sGC-specific pharmacological activators, YC-1 and BAY 41–8543 (Ramos-Espiritu, Hess, Buck, & Levin, 2011). We tested their efficacy towards sAC. While BAY 41–8543 was inert towards sAC, YC-1 had a complicated relationship with sAC generated cAMP (Ramos-Espiritu, Hess, Buck, & Levin, 2011). Treating cells with YC-1 elevated specifically sAC-generated cAMP, but because there was no evidence YC-1 directly stimulated sAC, its use for probing functions of sAC generated cAMP would be suspect.
The absence of pharmacological activators of sAC is exacerbated by the problems inherent in manipulating the known physiological modulators of sAC. As described above, sAC activity is directly regulated by three intracellular signals (, ATP, and Ca2+) (Chen et al., 2000; Jaiswal & Conti, 2003; Kleinboelting et al., 2014; Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003), but none can be experimentally manipulated without pleiotropic effects. (1) Varying intracellular is accompanied by changes in CO2 or pHi. (2) Intracellular ATP (and ) levels are carefully controlled by the cell, which makes manipulating their levels in cellular systems difficult. And (3) altering Ca2+ levels has broad, pleiotropic effects. Thus, our understanding of sAC and cAMP biology remain hindered by the absence of pharmacological mechanisms for experimentally stimulating sAC activity inside cells.
5. Physiological roles of sAC identified using sAC inhibitors
sAC has been shown to play a role in a number of physiological processes (Buffone, Wertheimer, Visconti, & Krapf, 2014; Chang, Beuers, & Oude Elferink, 2017; Desman, Waintraub, & Zippin, 2014; Ladilov & Appukuttan, 2014; Lee, Marmorstein, & Marmorstein, 2014; Levin & Buck, 2015; Muller, 2016; Schmid, Meili, & Salathe, 2014; Shim, Kim, & Ju, 2017; Stiles, Kapiloff, & Goldberg, 2014; Valsecchi, Konrad, & Manfredi, 2014). A number of sAC’s physiological roles have been identified genetically, via phenotypes observed in genetically engineered mice (Table 2). There are two independently derived sAC knockout (KO) mouse strains; sAC-C1 KO which removes three exons encoding most of the first catalytic domain (Esposito et al., 2004; Hess et al., 2005), and sAC-C2 KO which removes three exons encoding the second catalytic domain (Chen, Martinez, Milner, Buck, & Levin, 2013). A third genetically altered strain harbors loxP sites surrounding the C2 encoding exons for generating tissue-specific sAC KO mice (Watson et al., 2015). Pharmacological inhibitors have also been used to suggest functions for sAC (Table 3). Many pharmacologically identified functions have been confirmed genetically; either the sAC KO or knockdown recapitulates the phenotype observed in the presence of a sAC pharmacological inhibitor, and/or the effects of the inhibitor are not observed in cells, tissue, or animals where sAC is knocked out. In this review, we focus on the pharmacologic and genetic evidence supporting the various confirmed functions for sAC, and we highlight sAC functions where modulators targeting sAC have potential therapeutic applications.
Table 2.
Functions of sAC inferred from sAC knockout mouse phenotypes.
| Male fertility; specifically, sperm capacitation and hyperactivated motility | Esposito et al. (2004), Farrell et al. (2008), Hess etal. (2005), Navarrete et al. (2016), Xie et al. (2006) |
| Regulation oflntraocular Pressure | Lee et al. (2011) |
| Glucose homeostasis; specifically, insulin response to bolus glucose challenge | Zippin et al. (2013) |
| Ciliary beat frequency in airway epithelial cells | Chen et al. (2014) |
| Transendothelial migration of leukocytes; specifically, sAC is required in endothelial cells to support leukocytes traversing the endothelial barrier | Watson et al. (2015) |
| Retinal ganglion cell growth and differentiation | Shaw et al. (2016) |
| Malignant progression was enhanced in chemically-induced carcinogenesis in skin in the absence of sAC | Ramos-Espiritu, Diaz, et al. (2016) |
As mentioned above, sAC functions as a physiological CO2//pH chemosensor (Buck & Levin, 2011; Chang & Oude-Elferink, 2014; Levin & Buck, 2015; Rahman, Buck, & Levin, 2013; Tresguerres, Buck, & Levin, 2010), and its activity is also regulated by calcium (Jaiswal & Conti, 2003; Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003) and physiologically relevant changes in ATP (Litvin, Kamenetsky, Zarifyan, Buck, & Levin, 2003; Zippin et al., 2013). sAC is also likely to be subject to additional allosteric forms of regulation via its autoinhibitory domain and/or its presumptive interaction domains in the C terminal region (sAC-CTR). These uncharacterized regulatory domains appear to be differentially included (or excluded) in alternatively spliced isoforms of sAC. However, because all known inhibitors block sAC’s catalytic center, and all genetic experiments performed thus far have deleted sAC’s catalytic domains (Table 2), the identified functions apply to all catalytically active forms of sAC and do not distinguish among the various spliced forms.
A number of the functions ascribed to sAC (i.e., those associated with specific organelles such as mitochondria and lysosomes) have been demonstrated in multiple cellular contexts and appear to define widely distributed, cellular functions. Other functions are unique to specific cells or tissues.
5.1. General, cellular functions
5.1.1. Mitochondrial matrix
sAC was found to reside inside the mitochondrial matrix where it de-fines a cAMP microdomain which couples activity of the Krebs cycle with flux through the electron transport chain and ATP generation (Di Benedetto, Gerbino, & Lefkimmiatis, 2017; Lefkimmiatis, 2014; Lefkimmiatis & Zaccolo, 2014; Valsecchi, Konrad, & Manfredi, 2014; Valsecchi, Ramos-Espiritu, Buck, Levin, & Manfredi, 2013). The Krebs cycle produces the electrons which feed the electron transport chain to create the electrochemical gradient which drives ATP production. The Krebs cycle also produces CO2 as a by-product. sAC senses the CO2 generated (after nearly instantaneous equilibration into bicarbonate via mitochondrial carbonic anhydrases) and regulates the activity of enzymes in the electron transport chain so their activities matches the output of the Krebs cycle. sAC localization inside the matrix and sAC-dependent regulation of the electron transport chain were first demonstrated by inhibition of sAC using KH7, and confirmed using RNAi-mediated knockdown (Acin-Perez, Salazar, Brosel, et al., 2009). This intramitochondrial sAC-cAMP signaling cascade was subsequently demonstrated using two independently developed FRET-based cAMP sensors (Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013; Lefkimmiatis, Leronni, & Hofer, 2013), and the sAC-dependent regulation of oxidative phosphorylation was confirmed using catechol estrogens (Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013) as well as in sAC knockout (KO) cell lines (Ramos-Espiritu, Kleinboelting, et al., 2016; Valsecchi et al., 2017). Exactly how sAC-derived cAMP regulates oxidative phosphorylation remains under investigation. One consequence of intramitochondrial sAC derived cAMP is phosphorylation of complex IV of the electron transport chain (Acin-Perez, Salazar, Brosel, et al., 2009) and sAC-dependent regulation of complex IV activity was confirmed using LRE1 (Ramos-Espiritu, Kleinboelting, et al., 2016) and sAC KO cell lines (Ramos-Espiritu, Kleinboelting, et al., 2016; Valsecchi et al., 2017). However, whether this phosphorylation is mediated by the cAMP effector, Protein Kinase A (PKA), remains questionable (Di Benedetto, Gerbino, & Lefkimmiatis, 2017; Di Benedetto, Scalzotto, Mongillo, & Pozzan, 2013). Other studies have implicated the cAMP effector, Exchange Protein activated by cAMP (EPAC) as the downstream target of intramitochondrial sAC-generated cAMP (Fazal et al., 2017; Lezoualc’h, Fazal, Laudette, & Conte, 2016; Wang et al., 2016).
5.1.2. Lysosomal pH
Lysosomal enzymes, which degrade proteins, lipids, and polysaccha-rides, are optimally active in an acidic environment; thus, in the majority of cells, the pH of the lysosomal lumen is maintained around 4.7 (Pillay, Elliott, & Dennison, 2002). In immortalized mouse fibroblasts, primary mouse neurons, and human liver cell lines, inhibition of sAC using KH7 resulted in lysosomal acidification defects (Rahman, Ramos-Espiritu, Milner, Buck, & Levin, 2016). This KH7 induced acidification defect was accompanied by decreased degradative capacity of the lysosomes and an accumulation of autophagic vacuoles. The effects of KH7 were genetically confirmed to be sAC-dependent. Specifically, sAC KO neurons and fibroblasts exhibited a lysosomal acidification defect, and the effects of KH7 were specific to WT cells; KH7 had no effect in sAC KO cells. As expected, the lysosomal acidification defects in KH7-treated and sAC KO cells were rescued by the product of sAC, cAMP.
5.1.3. Apoptosis and cellular growth
Using KH7 along with sAC-specific RNAi knockdown, Ladilov and coworkers identified sAC as a regulator of stress-induced apoptosis in coronary endothelial cells (Kumar, Kostin, Flacke, Reusch, & Ladilov, 2009), cardiac myocytes (Appukuttan et al., 2012), and smooth muscle cells (Appukuttan, Kasseckert, Kumar, Reusch, & Ladilov, 2013; Kumar et al., 2014). In these cascades, clinically relevant stresses such as ischemia or acidosis induces translocation of sAC from the cytoplasm to the mitochondria where sAC generated cAMP activates a local pool of Protein Kinase A (PKA) and promotes apoptosis via mitochondrial engagement of Bax or other pro-apoptotic Bcl2 family members (Kumar, Kostin, Flacke, Reusch, & Ladilov, 2009).
The role of sAC in supporting cellular growth is complicated and likely has an as yet undefined cell type specificity (Ladilov & Appukuttan, 2014). In some systems (i.e., skin, breast), inhibition of sAC promotes tumorigenesis (Onodera, Nam, & Bissell, 2014; Ramos-Espiritu, Diaz, et al., 2016), while in others (i.e., prostate), the loss of sAC prevents proliferation (Flacke et al., 2013). Similarly, in cardiac myocytes, inhibition of sAC prevents hypertrophy in response to a number of different stimuli (Schirmer et al., 2018).
5.2. Cell- or tissue-specific functions
5.2.1. Male fertility
Mammalian sperm must acquire the capacity to fertilize the egg asthey transit through the reproductive tract in a process known as capacitation (Yanagimachi, 1994). This process is sAC dependent (Esposito et al., 2004; Hess et al., 2005). Male sAC KO mice are sterile (Esposito et al., 2004; Hess et al., 2005), and sAC KO sperm (Hess et al., 2005; Xie et al., 2006) or WT sperm in the presence of either of two, molecularly distinct, sAC-specific inhibitors KH7 (Hess et al., 2005) or LRE1 (Ramos-Espiritu, Kleinboelting, et al., 2016), exhibit motility defects, have reduced ATP levels, do not capacitate, and fail to fertilize in vitro. Because sAC is absolutely required for male fertility, sAC inhibitors have been considered as potential male contraceptives. However, the widespread distribution of sAC, and the likelihood of pleiotropic effects, complicates this idea.
5.2.2. Intraocular pressure regulation
The anterior portion of the eye is bathed in a transparent watery solution called aqueous humor (AH). AH is constantly produced by the ciliary epithelium, and its pressure, termed the intraocular pressure (IOP), is maintained via outflow through various drainage tissues including the trabecular meshwork. sAC KO mice have elevated IOP and systemic treatment of WT mice with either KH7 (Lee & Marmorstein, 2014) or LRE1 (Gandhi et al., 2017) elevated IOP in WT mice; sAC inhibitors were inert in sAC KO mice confirming their effects are mediated specifically by inhibiting sAC. Ocular hypotony is a rare, but potentially blinding condition where IOP falls to critically low levels. There is currently no effective treatment for hypotony. A safe and effective sAC inhibitor could define a “first-in-its-class” therapeutic strategy for elevating IOP to treat hypotony.
Opposite to hypotony, elevated IOP is a predominant risk factor for glaucoma, in which optic nerve injury leads to vision loss. Many current treatment strategies for glaucoma, including carbonic anhydrase inhibitors (CAIs), work by reducing IOP. CAIs function by altering intracellular concentrations of , but their mechanism of diminishing IOP has remained poorly understood (Shahidullah, Al-Malki, & Delamere, 2011). CAI treatment of fluid-secreting non-pigmented epithelium ciliary body cells transiently increases cAMP production by bicarbonate-regulated sAC (Shahidullah et al., 2014). Because sAC regulates IOP (Gandhi et al., 2017; Lee et al., 2011; Lee & Marmorstein, 2014; Lee, Marmorstein, & Marmorstein, 2014), these data suggest CAI treatment lowers IOP via sAC; i.e., CAIs transiently increase to stimulate sAC activity in ciliary body cells. These data also predict that a sAC activator may define a novel means of lowering IOP defining a new potential therapeutic strategy for treating glaucoma. In both cases, the sAC modulator (i.e., an inhibitor to elevate IOP for treating hypotony or a putative activator to diminish IOP for treating glaucoma) could be administered locally (i.e., in the form of eye drops), which would diminish any potential for pleiotropic effects due to systemic administration.
5.2.3. Ciliary beat in airway epithelia
In addition to conducting air into the lung alveoli for gas exchange,the airways are responsible for cleaning the air of dust and microorganisms. The predominant mechanism for cleaning air is mucocilliary clearance (MCC), in which mucus secreted by the airway epithelia traps inhaled particles while cilia beat from distal to proximal to propel the particle-laden mucus out of the airway (Schmid, Meili, & Salathe, 2014). Both KH7 and CEs were used to demonstrate that sAC, which was shown to specifically localize to airway cilia (Chen et al., 2014), is responsible for the CO2/ dependent regulation of ciliary beat frequency (CBF) (Schmid et al., 2007). sAC dependent regulation of CBF was genetically confirmed in sAC KO mice (Chen et al., 2014).
5.2.4. Leukocyte migration
During inflammation, leukocytes leave the circulation and translocate through the endothelium to reach sites of tissue damage. This transendothelial migration or diapedesis is dependent upon a series of adhesive molecular interactions including the homophilic interaction of CD99 on both the leukocyte and on the endothelial cell. KH7 blocks diapedesis, and it was shown using tissue specific sAC knockout mice that sAC is required for signaling downstream of CD99 specifically in the endothelial cell (Watson et al., 2015).
sAC may play additional roles during inflammation. KH7 was used to explore the role of cAMP in the regulation of the NLRP3-containing inflammasome, a key component leading to the maturation of pro-in-flammatory cytokine interleukin 1β (IL-1β). Both the calcium sensing receptor (CASR) and PGE2 were shown to regulate the NLRP3-inflammasome, and it was thought that both function at least partially by modulating intracellular cAMP concentrations (Lee et al., 2012; Sokolowska et al., 2015). These groups used KH7 to support their conclusion of an involvement of G protein dependent regulation of a tmAC. As detailed above, KH7 is inert against tmACs (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013), which contradicts their conclusions, but raises the interesting possibility that sAC may play an as yet undisclosed role in regulation of the NLRP3-inflammasome. These studies provide a cautionary tale about how it is essential to appreciate the different specificities of the AC inhibitors described here and in Dessauer et al. (2017). They also highlight the importance of using complementary sAC-specific and tmAC-selective inhibitors when exploring the source of cAMP (Bitterman, Ramos-Espiritu, Diaz, Levin, & Buck, 2013), as exemplified in the elucidation of a role for sAC in neuritogenesis.
5.2.5. Neuritogenesis
Cyclic AMP has long been associated with axonal elongation (Roisen,Murphy, Pichichero, & Braden, 1972) and guidance (Lohof, Quillan, Dan, & Poo, 1992; Ming et al., 1997) and studies using both sAC-specific and tmAC-selective inhibitors reveal that different extracellular signals mediate their effects via distinct ACs. As expected, pituitary AC Activating Peptide (PACAP) signals via a prototypical G protein coupled receptor – tmAC signaling cascade; axonal outgrowth in neurons and neuritogenesis in PC12 cells in response to PACAP were blocked by selective inhibition of tmACs using ddAdo, and insensitive to sAC specific inhibition using either KH7, CEs or an siRNA mediated sAC knockdown (Stessin et al., 2006; Wu et al., 2006). In contrast, axonal outgrowth in response to the guidance cue Netrin-1 or the neurotrophin BDNF and neuritogenesis in PC12 cells in response to the neurotrophin NGF were dependent upon sAC-generated cAMP. These responses were unaffected by tmAC-selective concentrations of ddAdo and were blocked by KH7 or CEs as well as by sAC-specific RNAi knockdown or in sAC KO neurons (Corredor et al., 2012; Martinez et al., 2014; Wu et al., 2006). A role for sAC in neuronal differentiation was genetically con-firmed in retinal ganglion cells (Shaw et al., 2016). From these studies, a picture emerges where signals inducing axonal outgrowth via GPCRs is dependent upon tmAC-generated cAMP while neurotrophin- or Netrin-induced outgrowth requires sAC.
Recent work revealed yet another new aspect to spatial regulation of cAMP signaling; GPCRs can also signal via sAC. As mentioned above, there has been a shift away from the paradigm that GPCRs signal exclusively from the plasma membrane; GPCRs elicit sustained signals while trafficking in endosomes (Calebiro et al., 2009; Ferrandon et al., 2009). In their studies of the corticotropin releasing hormone receptor1 (CRHR1), Susana Silberstein’s laboratory found that CRHR1 induced cAMP production via both sAC and tmACs (Inda et al., 2016). CRHR1-induced cAMP production was inhibited by tmAC-selective concentrations of ddAdo, and by the sAC–specific inhibitors KH7 or catechol estrogens. sAC-specific RNAi knockdown also diminished CRHR1 stimulated cAMP. Consistent with both types of cyclases contributing to the cAMP signal, the effects of ddAdo and KH7 or catechol estrogens were additive; thus, because there are no know pan-mammalian cyclase inhibitors, to effectively turn off cAMP production in mammalian cells, one needs to add both sAC- and tmAC-specific inhibitors. When they examined the sustained, internalization-dependent phase of the cAMP response, only KH7 or catechol estrogens blocked cAMP production; ddAdo was inert. Therefore, sustained cAMP responses from CRHR1 on trafficking endosomes is mediated exclusively via sAC, and it is this sAC-dependent cAMP signaling which is responsible for the neuritogenic effects of CRHR1 (Inda et al., 2017). Interestingly, CRHR1 is not the only GPCR which seems to induce both tmAC- and sAC-dependent cAMP signals; β2 adrenergic receptor-dependent short circuit currents in colonic mucosa cells are inhibited by both KH7 and ddAdo implicating both sAC and tmACs (Halm, Zhang, & Halm, 2010).
6. Summary
The discovery of bicarbonate-regulated sAC uncovered previously unappreciated roles for the prototypical second messenger cAMP as the mediator of physiological responses to CO2//pH changes. In addition, the modern recognition that cAMP signaling is compartmentalized into multiple, independently regulated signaling microdomains revealed a requirement for an intracellular source of the second messenger. For these reasons, it is imperative to distinguish between the different types of adenylyl cyclases which synthesize cAMP in mammalian cells. In this review, we summarize the existing pharmacological tools to study sAC and to distinguish sAC-defined cAMP processes from functions mediated downstream from tmACs. Using these reagents, a number of sAC-dependent processes have been identified, and there are numerous possibilities where sAC-directed pharmacological reagents may have therapeutic value.
Acknowledgment
We thank Tom Rossetti for assistance with figures. Work in the Buck-Levin laboratory is supported by grants from National Institutes of Health (GM107442, EY025810, and HD088571) and in the Steegborn lab by Deutsche Forschungsgemeinschaft (STE1701/11).
Abbreviations:
- sAC
soluble adenylyl cyclase
- sACt
sAC-truncated
- sACfl
sAC-full-length
- tmAC
transmembrane adenylyl cyclase
- cAMP
Cyclic AMP
- AC
adenylyl cyclase
- PDE
phosphodiesterase
- GPCR
G- protein-coupled receptors
- STAND
Signal Transduction ATPases with Numerous Domains
- KH7
(+/−)-2-(1H-benzimidazol-2-ylthio)propanoic acid 2-[(5-bromo-2-hydroxyphenyl)methylene]hydrazide
- LRE1
6-chloro-N4-cyclopropyl-N4-((thiophen-3-yl)methyl)pyrimidine-2,4-diamine
- CEs
Catechol derivatives of estrogen
Footnotes
Conflict of interest
Drs. Buck and Levin own equity interest in CEP Biotech which has licensed commercialization of a panel of monoclonal antibodies directed against sAC. Drs. Steegborn, Levin, and Buck are named inventors on use patent applications for LRE1.
References
- Acin-Perez R, Russwurm M, Gunnewig K, Gertz M, Zoidl G, Ramos L, … Steegborn C (2011). A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. The Journal of Biological Chemistry 286, 30423–30432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, & Manfredi G (2009). Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Molecular Medicine 1, 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, & Manfredi G (2009). Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metabolism 9, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal-Howarth S, & Scott JD (2017). Pseudoscaffolds and anchoring proteins: The difference is in the details. Biochemical Society Transactions 45, 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appukuttan A, Flacke JP, Flacke H, Posadowsky A, Reusch HP, & Ladilov Y (2014).Inhibition of soluble adenylyl cyclase increases the radiosensitivity of prostate cancer cells. Biochimica et Biophysica Acta 1842, 2656–2663. [DOI] [PubMed] [Google Scholar]
- Appukuttan A, Kasseckert SA, Kumar S, Reusch HP, & Ladilov Y (2013). Oxysterol-induced apoptosis of smooth muscle cells is under the control of a soluble adenylyl cyclase. Cardiovascular Research 99, 734–742. [DOI] [PubMed] [Google Scholar]
- Appukuttan A, Kasseckert SA, Micoogullari M, Flacke JP, Kumar S, Woste A, … Ladilov Y (2012). Type 10 adenylyl cyclase mediates mitochondrial Bax translocation and apoptosis of adult rat cardiomyocytes under simulated ischaemia/reperfusion. Cardiovascular Research 93, 340–349. [DOI] [PubMed] [Google Scholar]
- Arora K, Sinha C, Zhang W, Ren A, Moon CS, Yarlagadda S, & Naren AP (2013). Compartmentalization of cyclic nucleotide signaling: A question of when, where, and why? Pflugers Archiv: European Journal of Physiology 465, 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao K, & Monje PV (2015). Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination. PLoS One 10, e0116948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, & Tsien RY (1993). Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science 260, 222–226. [DOI] [PubMed] [Google Scholar]
- Battistone MA, Da Ros VG, Salicioni AM, Navarrete FA, Krapf D, Visconti PE, & Cuasnicu PS (2013). Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Molecular Human Reproduction 19, 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, & Buck J (2013). Pharmacological distinction between soluble and transmembrane adenylyl cyclases. The Journal of Pharmacology and Experimental Therapeutics 347, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone NW (2014). sAC as a model for understanding the impact of endosymbiosis on cell signaling. Biochimica et Biophysica Acta 1842, 2548–2554. [DOI] [PubMed] [Google Scholar]
- Boron WF (2001). Sodium-coupled bicarbonate transporters. JOP: Journal of the pancreas 2, 176–181. [PubMed] [Google Scholar]
- Brand CS, Sadana R, Malik S, Smrcka AV, & Dessauer CW (2015). Adenylyl cyclase 5 regulation by gbetagamma involves isoform-specific use of multiple interaction sites. Molecular Pharmacology 88, 758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branham MT, Bustos MA, De Blas GA, Rehmann H, Zarelli VE, Trevino CL, … Tomes CN (2009). Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. The Journal of Biological Chemistry 284, 24825–24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T (1975). The effect of divalent cations on bovine spermatozoal adenylate cyclase activity. Journal of Cyclic Nucleotide Research 1, 271–281. [PubMed] [Google Scholar]
- Braun T (1990). Inhibition of the soluble form of testis adenylate cyclase by catechol estrogens and other catechols. Proceedings of the Society for Experimental Biology and Medicine 194, 58–63. [DOI] [PubMed] [Google Scholar]
- Braun T, Frank H, Dods R, & Sepsenwol S (1977). Mn2+-sensitive, soluble adenylate cyclase in rat testis. Differentiation from other testicular nucleotide cyclases. Biochimica et Biophysica Acta 481, 227–235. [DOI] [PubMed] [Google Scholar]
- Brust TF, Alongkronrusmee D, Soto-Velasquez M, Baldwin TA, Ye Z, Dai M, … Watts VJ (2017). Identification of a selective small-molecule inhibitor of type 1 adenylyl cyclase activity with analgesic properties. Science Signaling 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J, & Levin LR (2011). Physiological sensing of carbon dioxide/bicarbonate/pH via cyclic nucleotide signaling. Sensors (Basel) 11, 2112–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, & Levin LR (2002). Purification of soluble adenylyl cyclase. Methods in Enzymology 345, 95–105. [DOI] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, & Levin LR (1999). Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proceedings of the National Academy of Sciences of the United States of America 96, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffone MG, Wertheimer EV, Visconti PE, & Krapf D (2014). Central role of soluble adenylyl cyclase and cAMP in sperm physiology. Biochimica et Biophysica Acta 1842, 2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton IL, & Brunton LL (1983). Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. The Journal of Biological Chemistry 258, 10233–10239. [PubMed] [Google Scholar]
- Buxton IL, & Brunton LL (1986). Compartmentation of hormone action in adult mammalian cardiomyocytes. Advances in Experimental Medicine and Biology 194, 117–127. [DOI] [PubMed] [Google Scholar]
- Cai Y, Miller CL, Nagel DJ, Jeon KI, Lim S, Gao P, … Yan C (2011). Cyclic nucleotide phosphodiesterase 1 regulates lysosome-dependent type I collagen protein degradation in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology 31, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, … Lohse MJ (2009). Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biology 7, e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann MJ, Hammer A, Zhou J, & Kanacher T (2003). A defined subset of adenylyl cyclases is regulated by bicarbonate ion. The Journal of Biological Chemistry 278, 35033–35038. [DOI] [PubMed] [Google Scholar]
- Chagtoo M, George N, Pathak N, Tiwari S, Godbole MM, & Ladilov Y (2018). Inhibition of intracellular type 10 adenylyl cyclase protects cortical neurons against reper-fusion-induced mitochondrial injury and apoptosis. Molecular Neurobiology 55, 2471–2482. [DOI] [PubMed] [Google Scholar]
- Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, & Buck J (2006). Autoinhibitory regulation of soluble adenylyl cyclase. Molecular Reproduction and Development 73, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Beuers U, & Oude Elferink RP (2017). The emerging role of soluble adenylyl cyclase in primary biliary cholangitis. Digestive Diseases 35, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, Go S, de Waart DR, Munoz-Garrido P, Beuers U, Paulusma CC, & Oude Elferink R (2016). Soluble adenylyl cyclase regulates bile salt-induced apoptosis in human cholangiocytes. Hepatology 64, 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, & Oude-Elferink RP (2014). Role of the bicarbonate-responsive soluble adenylyl cyclase in pH sensing and metabolic regulation. Frontiers in Physiology 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Martinez J, Milner TA, Buck J, & Levin LR (2013). Neuronal expression of soluble adenylyl cyclase in the mammalian brain. Brain Research 1518, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, … Mi MT (2013). Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 9, 2033–2045. [DOI] [PubMed] [Google Scholar]
- Chen X, Baumlin N, Buck J, Levin LR, Fregien N, & Salathe M (2014). A soluble adenylyl cyclase form targets to axonemes and rescues beat regulation in soluble adenylyl cyclase knockout mice. American Journal of Respiratory Cell and Molecular Biology 51, 750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, & Buck J (2000). Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625–628. [DOI] [PubMed] [Google Scholar]
- Choi HB, Gordon GR, Zhou N, Tai C, Rungta RL, Martinez J, … MacVicar BA (2012). Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75, 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Brand CS, Bogard AS, Pratt EP, Xu R, Hockerman GH, … Watts VJ(2013). Development of a high-throughput screening paradigm for the discovery of small-molecule modulators of adenylyl cyclase: Identification of an adenylyl cyclase 2 inhibitor. The Journal of Pharmacology and Experimental Therapeutics 347, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredor RG, Trakhtenberg EF, Pita-Thomas W, Jin X, Hu Y, & Goldberg JL (2012).Soluble adenylyl cyclase activity is necessary for retinal ganglion cell survival and axon growth. The Journal of neuroscience: The Official Journal of the Society for Neuroscience 32, 7734–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danot O, Marquenet E, Vidal-Ingigliardi D, & Richet E (2009). Wheel of life, wheel of death: A mechanistic insight into signaling by STAND proteins. Structure 17, 172–182. [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, … Hell JW (2001). A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293, 98–101. [DOI] [PubMed] [Google Scholar]
- Desman G, Waintraub C, & Zippin JH (2014). Investigation of cAMP microdomains as a path to novel cancer diagnostics. Biochimica et Biophysica Acta 1842, 2636–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauer CW, Tesmer JJ, Sprang SR, & Gilman AG (1999). The interactions of adenylate cyclases with P-site inhibitors. Trends in Pharmacological Sciences 20, 205–210. [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Tesmer JJG, Sprang SR, & Gilman AG (1998). Identification of a gialpha binding site on type V adenylyl cyclase. The Journal of Biological Chemistry 273, 25831–25839. [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Watts VJ, Ostrom RS, Conti M, Dove S, & Seifert R (2017). International union of basic and clinical pharmacology. CI. Structures and small molecule modulators of mammalian adenylyl cyclases. Pharmacological Reviews 69, 93–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Roy D, Majumder GC, Mukherjee B, & Bhattacharyya D (2015). Role of forward-motility-stimulating factor as an extracellular activator of soluble adenylyl cyclase. Molecular Reproduction and Development 82, 1001–1014. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G, Gerbino A, & Lefkimmiatis K (2017). Shaping mitochondrial dynamics:The role of cAMP signalling. Biochemical and Biophysical Research Communications. [DOI] [PubMed] [Google Scholar]
- Di Benedetto G, Scalzotto E, Mongillo M, & Pozzan T (2013). Mitochondrial Ca(2+) uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metabolism 17, 965–975. [DOI] [PubMed] [Google Scholar]
- Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, … Gossen JA (2004). Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proceedings of the National Academy of Sciences of the United States of America 101, 2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J, Ramos L, Tresguerres M, Kamenetsky M, Levin LR, & Buck J (2008). Somatic ‘soluble’ adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex ‘knockout’ mice. PLoS One 3, e3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazal L, Laudette M, Paula-Gomes S, Pons S, Conte C, Tortosa F, … Lezoualc’h F (2017). Multifunctional mitochondrial Epac1 controls myocardial cell death. Circulation Research 120, 645–657. [DOI] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, … Vilardaga JP (2009). Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nature Chemical Biology 5, 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, & Vandecasteele G (2006). Compartmentation of cyclic nucleotide signaling in the heart: The role of cyclic nucleotide phosphodiesterases. Circulation Research 99, 816–828. [DOI] [PubMed] [Google Scholar]
- Flacke JP, Flacke H, Appukuttan A, Palisaar RJ, Noldus J, Robinson BD, … Ladilov Y (2013). Type 10 soluble adenylyl cyclase is overexpressed in prostate carcinoma and controls proliferation of prostate cancer cells. The Journal of Biological Chemistry 288, 3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte LR, Bylund DB, & Zahler WL (1983). Forskolin does not activate sperm adenylate cyclase. Molecular Pharmacology 24, 42–47. [PubMed] [Google Scholar]
- Francis SH, Blount MA, & Corbin JD (2011). Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiological Reviews 91, 651–690. [DOI] [PubMed] [Google Scholar]
- Gancedo JM (2013). Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biological Reviews of the Cambridge Philosophical Society 88, 645–668. [DOI] [PubMed] [Google Scholar]
- Gandhi JK, Roy Chowdhury U, Manzar Z, Buck J, Levin LR, Fautsch MP, &Marmorstein AD (2017). Differential intraocular pressure measurements by tonometry and direct cannulation after treatment with soluble adenylyl cyclase inhibitors. Journal of ocular pharmacology and therapeutics: The Official Journal of the Association for Ocular Pharmacology and Therapeutics 33, 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W, Hill K, Zerwekh JE, Kohler T, Muller R, & Moe OW (2009). Inhibition of osteoclast formation and function by bicarbonate: Role of soluble adenylyl cyclase. Journal of Cellular Physiology 220, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng W, Wang Z, Zhang J, Reed BY, Pak CY, & Moe OW (2005). Cloning and characterization of the human soluble adenylyl cyclase. American Journal of Physiology. Cell Physiology 288, C1305–C1316. [DOI] [PubMed] [Google Scholar]
- Gille A, Lushington GH, Mou TC, Doughty MB, Johnson RA, & Seifert R (2004). Differential inhibition of adenylyl cyclase isoforms and soluble guanylyl cyclase by purine and pyrimidine nucleotides. The Journal of Biological Chemistry 279, 19955–19969. [DOI] [PubMed] [Google Scholar]
- Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, … Pastor-Soler NM (2010). Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. American Journal of Physiology. Renal Physiology 298, F1162–F1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, De Sordi L, Maccallum DM, Topal H, Eaton R, Bloor JW, … Muhlschlegel FA (2010). CO(2) acts as a signalling molecule in populations of the fungal pathogen Candida albicans. PLoS Pathogens 6, e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows KR, Wang H, Edinger RS, Butterworth MB, Oyster NM, Li H, … Pastor-Soler NM (2009). Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. The Journal of Biological Chemistry 284, 5774–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm ST, Zhang J, & Halm DR (2010). beta-Adrenergic activation of electrogenic K+ and Cl− secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. American Journal of Physiology. Gastrointestinal and Liver Physiology 299, G81–G95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MP, Soto-Velasquez M, Fowler CA, Watts VJ, & Roman DL (2017). Identi-fication of FDA-approved small molecules capable of disrupting the calmodulinadenylyl cyclase 8 interaction through direct binding to calmodulin. ACS Chemical Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, … Marsicano G (2016). A cannabinoid link between mitochondria and memory. Nature 539, 555–559. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, … Moss SB (2005). The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Developmental Cell 9, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BJ, Gebre S, Schlicker B, Jordan R, & Necessary S (2010). Nongenomic inhibition of coronary constriction by 17ss-estradiol, 2-hydroxyestradiol, and 2-methoxyestradiol. Canadian Journal of Physiology and Pharmacology 88, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenhorst MI, Lips KS, Kummer W, & Fronius M (2012). Nicotine-induced activation of soluble adenylyl cyclase participates in ion transport regulation in mouse tracheal epithelium. Life Sciences 91, 1009–1012. [DOI] [PubMed] [Google Scholar]
- Holz GG, Leech CA, & Chepurny OG (2014). New insights concerning the molecular basis for defective glucoregulation in soluble adenylyl cyclase knockout mice. Biochimica et Biophysica Acta 1842, 2593–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD (2010). Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends in Biochemical Sciences 35, 91–100. [DOI] [PubMed] [Google Scholar]
- Inda C, Bonfiglio JJ, Dos Santos Claro PA, Senin SA, Armando NG, Deussing JM, & Silberstein S (2017). cAMP-dependent cell differentiation triggered by activated CRHR1 in hippocampal neuronal cells. Scientific Reports 7, 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda C, Dos Santos Claro PA, Bonfiglio JJ, Senin SA, Maccarrone G, Turck CW, & Silberstein S (2016). Different cAMP sources are critically involved in G protein-coupled receptor CRHR1 signaling. The Journal of Cell Biology 214, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivonnet P, Salathe M, & Conner GE (2015). Hydrogen peroxide stimulation of CFTR reveals an Epac-mediated, soluble AC-dependent cAMP amplification pathway common to GPCR signalling. British Journal of Pharmacology 172, 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivonnet P, Unwalla H, Salathe M, & Conner GE (2016). Soluble adenylyl cyclase mediates hydrogen peroxide-induced changes in epithelial barrier function. Respiratory Research 17, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal BS, & Conti M (2001). Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. The Journal of Biological Chemistry 276, 31698–31708. [DOI] [PubMed] [Google Scholar]
- Jaiswal BS, & Conti M (2003). Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proceedings of the National Academy of Sciences of the United States of America 100, 10676–10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Yeung SM, Stubner D, Bushfield M, & Shoshani I (1989). Cation and structural requirements for P site-mediated inhibition of adenylate cyclase. Molecular Pharmacology 35, 681–688. [PubMed] [Google Scholar]
- Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, & Steegborn C (2006). Molecular details of cAMP generation in mammalian cells: A tale of two systems. Journal of Molecular Biology 362, 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi R, Tsuji T, Watanabe O, Yamaguchi K, Furukawa K, Nakamura H, & Aoshiba K (2017). Hypercapnia accelerates adipogenesis: A novel role of high CO2 in exacerbating obesity. American Journal of Respiratory Cell and Molecular Biology 57, 570–580. [DOI] [PubMed] [Google Scholar]
- Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, … Steegborn C (2014). Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate. Proceedings of the National Academy of Sciences of the United States of America 111, 3727–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting S, Ramos-Espiritu L, Buck H, Colis L, van den Heuvel J, Glickman JF, …Steegborn C (2016). Bithionol potently inhibits human soluble adenylyl cyclase through binding to the allosteric activator site. The Journal of Biological Chemistry 291, 9776–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinboelting S, van den Heuvel J, & Steegborn C (2014). Structural analysis of human soluble adenylyl cyclase and crystal structures of its nucleotide complexes-implications for cyclase catalysis and evolution. The FEBS Journal 281, 4151–4164. [DOI] [PubMed] [Google Scholar]
- Kolodecik TR, Shugrue CA, Thrower EC, Levin LR, Buck J, & Gorelick FS (2012).Activation of soluble adenylyl cyclase protects against secretagogue stimulated zymogen activation in rat pancreaic acinar cells. PLoS One 7, e41320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowski SJ, Hopf FW, Seif T, Bonci A, & von Zastrow M (2011). Endocytosis promotes rapid dopaminergic signaling. Neuron 71, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Appukuttan A, Maghnouj A, Hahn S, Peter Reusch H, & Ladilov Y (2014). Suppression of soluble adenylyl cyclase protects smooth muscle cells against oxidative stress-induced apoptosis. Apoptosis 19, 1069–1079. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kostin S, Flacke JP, Reusch HP, & Ladilov Y (2009). Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. The Journal of Biological Chemistry 284, 14760–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladilov Y, & Appukuttan A (2014). Role of soluble adenylyl cyclase in cell death and growth. Biochimica et Biophysica Acta 1842, 2646–2655. [DOI] [PubMed] [Google Scholar]
- Lecuona E, Sun H, Chen J, Trejo HE, Baker MA, & Sznajder JI (2013). Protein kinase A-Ialpha regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO(2) concentrations. American Journal of Respiratory Cell and Molecular Biology 48, 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, … Chae JJ (2012). The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, & Marmorstein AD (2014). Control of outflow resistance by soluble adenylyl cyclase. Journal of Ocular Pharmacology and Therapeutics: The Official Journal of the Association for Ocular Pharmacology and Therapeutics 30, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Marmorstein LY, & Marmorstein AD (2014). Soluble adenylyl cyclase in the eye. Biochimica et Biophysica Acta 1842, 2579–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Tresguerres M, Hess K, Marmorstein LY, Levin LR, Buck J, & Marmorstein AD (2011). Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. The Journal of Biological Chemistry 286, 41353–41358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K (2014). cAMP signalling meets mitochondrial compartments.Biochemical Society Transactions 42, 265–269. [DOI] [PubMed] [Google Scholar]
- Lefkimmiatis K, Leronni D, & Hofer AM (2013). The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. The Journal of Cell Biology 202, 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkimmiatis K, & Zaccolo M (2014). cAMP signaling in subcellular compartments. Pharmacology & Therapeutics 143, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LR, & Buck J (2015). Physiological roles of acid-base sensors. Annual Review of Physiology 77, 347–362. [DOI] [PubMed] [Google Scholar]
- Lezoualc’h F, Fazal L, Laudette M, & Conte C (2016). Cyclic AMP sensor EPAC proteins and their role in cardiovascular function and disease. Circulation Research 118, 881–897. [DOI] [PubMed] [Google Scholar]
- Li S, Allen KT, & Bonanno JA (2011). Soluble adenylyl cyclase mediates bicarbonate-dependent corneal endothelial cell protection. American Journal of Physiology. Cell Physiology 300, C368–C374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin TN, Kamenetsky M, Zarifyan A, Buck J, & Levin LR (2003). Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. The Journal of Biological Chemistry 278, 15922–15926. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, & Poo MM (1992). Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. The Journal of Neuroscience 12, 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannowetz N, Wandernoth PM, & Wennemuth G (2012). Glucose is a pH-dependent motor for sperm beat frequency during early activation. PLoS One 7, e41030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardones P, Chang JC, & Oude Elferink RP (2014). Cyclic AMP and alkaline pH down-regulate carbonic anhydrase 2 in mouse fibroblasts. Biochimica et Biophysica Acta 1840, 1765–1770. [DOI] [PubMed] [Google Scholar]
- Martinez J, Stessin AM, Campana A, Hou J, Nikulina E, Buck J, … Filbin MT (2014).Soluble adenylyl cyclase is necessary and sufficient to overcome the block of axonal growth by myelin-associated factors. The Journal of Neuroscience 34, 9281–9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, Marks AR, & Kass RS(2002). Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295, 496–499. [DOI] [PubMed] [Google Scholar]
- Mavier P, Stengel D, & Hanoune J (1976). Inhibition of adenylate cyclase and ATPase activities from rat liver plasma membrane by hexachlorophene. Biochemical Pharmacology 25, 305–309. [DOI] [PubMed] [Google Scholar]
- McDonough KA, & Rodriguez A (2011). The myriad roles of cyclic AMP in microbial pathogens: From signal to sword. Nature Reviews. Microbiology. 10, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewes M, Nedele J, Schelleckes K, Bondareva O, Lenders M, Kusche-Vihrog K, … Brand E (2017). Salt-induced Na(+)/K(+)-ATPase-alpha/beta expression involves soluble adenylyl cyclase in endothelial cells. Pflugers Archiv: European Journal of Physiology. [DOI] [PubMed] [Google Scholar]
- Middelhaufe S, Leipelt M, Levin LR, Buck J, & Steegborn C (2012). Identification of a haem domain in human soluble adenylate cyclase. Bioscience Reports 32, 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, & Poo MM (1997). cAMP-dependent growth cone guidance by netrin-1. Neuron 19, 1225–1235. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, & Breitbart H (2014). Mitochondrial PKA mediates sperm motility.Biochimica et Biophysica Acta 1840, 3404–3412. [DOI] [PubMed] [Google Scholar]
- Monterisi S, Lobo MJ, Livie C, Castle JC, Weinberger M, Baillie G, … Zaccolo M (2017). PDE2A2 regulates mitochondria morphology and apoptotic cell death via local modulation of cAMP/ PKA signalling. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou TC, Masada N, Cooper DM, & Sprang SR (2009). Structural basis for inhibition of mammalian adenylyl cyclase by calcium. Biochemistry 48, 3387–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA (2016). Transendothelial migration: Unifying principles from the endothelial perspective. Immunological Reviews 273, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Alvau A, Lee HC, Levin LR, Buck J, Leon PM, … Visconti PE (2016). Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Scientific Reports 6, 33589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Garcia-Vazquez FA, Alvau A, Escoffier J, Krapf D, Sanchez-Cardenas C, … Visconti PE (2015). Biphasic role of calcium in mouse sperm capacitation signaling pathways. Journal of Cellular Physiology 230, 1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neviere R, Delguste F, Durand A, Inamo J, Boulanger E, & Preau S (2016). Abnormal mitochondrial cAMP/PKA signaling is involved in sepsis-induced mitochondrial and myocardial dysfunction. International Journal of Molecular Sciences 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols J, Obiako B, Ramila KC, Putinta K, Schilling S, & Sayner SL (2015). Lipopolysaccharide-induced pulmonary endothelial barrier disruption and lung edema: Critical role for bicarbonate stimulation of AC10. American Journal of Physiology. Lung Cellular and Molecular Physiology 309, L1430–L1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiako B, Calchary W, Xu N, Kunstadt R, Richardson B, Nix J, & Sayner SL (2013). Bicarbonate disruption of the pulmonary endothelial barrier via activation of endogenous soluble adenylyl cyclase, isoform 10. American Journal of Physiology. Lung Cellular and Molecular Physiology 305, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Terrin A, Di Benedetto G, Cannon RC, Koh W, Kim M, … Blackwell KT (2010). The role of type 4 phosphodiesterases in generating microdomains of cAMP: Large scale stochastic simulations. PLoS One 5, e11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, Nam JM, & Bissell MJ (2014). Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. The Journal of Clinical Investigation 124, 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, … Breton S (2003). Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. The Journal of Biological Chemistry 278, 49523–49529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, & Brown D (2008). Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. American Journal of Physiology. Renal Physiology 294, F130–F138. [DOI] [PubMed] [Google Scholar]
- Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, … Brown D (2010). cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. American Journal of Physiology. Renal Physiology 298, F643–F654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, & Scott JD (1997). Signaling through scaffold, anchoring, and adaptor proteins. Science 278, 2075–2080. [DOI] [PubMed] [Google Scholar]
- Perez DR, Smagley Y, Garcia M, Carter MB, Evangelisti A, Matlawska-Wasowska K, … Chigaev A (2016). Cyclic AMP efflux inhibitors as potential therapeutic agents for leukemia. Oncotarget 7, 33960–33982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay CS, Elliott E, & Dennison C (2002). Endolysosomal proteolysis and its regulation.The Biochemical Journal 363, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N, Buck J, & Levin LR (2013). pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC). Frontiers in Physiology 4, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N, Ramos-Espiritu L, Milner TA, Buck J, & Levin LR (2016). Soluble adenylyl cyclase is essential for proper lysosomal acidification. The Journal of General Physiology 148, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos LS, Zippin JH, Kamenetsky M, Buck J, & Levin LR (2008). Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. The Journal of General Physiology 132, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Espiritu L, Diaz A, Nardin C, Saviola AJ, Shaw F, Plitt T, … Zippin JH (2016). The metabolic/pH sensor soluble adenylyl cyclase is a tumor suppressor protein. Oncotarget 7, 45597–45607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Espiritu L, Kleinboelting S, Navarrete FA, Alvau A, Visconti PE, Valsecchi F, … Buck J (2016). Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase. Nature Chemical Biology 12, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Espiritu LS, Hess KC, Buck J, & Levin LR (2011). The soluble guanylyl cyclase activator YC-1 increases intracellular cGMP and cAMP via independent mechanisms in INS-1E cells. The Journal of Pharmacology and Experimental Therapeutics 338, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison GA, Butcher RW, & Sutherland EW (1968). Cyclic AMP. Annual Review of Biochemistry 37, 149–174. [DOI] [PubMed] [Google Scholar]
- Roisen FJ, Murphy RA, Pichichero ME, & Braden WG (1972). Cyclic adenosine monophosphate stimulation of axonal elongation. Science 175, 73–74. [DOI] [PubMed] [Google Scholar]
- Ruete MC, Lucchesi O, Bustos MA, & Tomes CN (2014). Epac, Rap and Rab3 act in concert to mobilize calcium from sperm’s acrosome during exocytosis. Cell Communication and Signaling: CCS 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalau-Bethell SM, Berdini V, Cleasby A, Congreve M, Coyle JE, Lock V, … Jhoti H(2014). Crystal structure of human soluble adenylate cyclase reveals a distinct, highly flexible allosteric bicarbonate binding pocket. ChemMedChem 9, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samartzis EP, Imesch P, Twiehaus A, Dubey RK, & Leeners B (2016). The estrogen metabolites 2-methoxyestradiol and 2-hydroxyestradiol inhibit endometriotic cell proliferation in estrogen-receptor-independent manner. Gynecological Endocrinology 32, 529–533. [DOI] [PubMed] [Google Scholar]
- Schirmer I, Bualeong T, Budde H, Cimiotti D, Appukuttan A, Klein N, …Jaquet K (2018). Soluble adenylyl cyclase: A novel player in cardiac hypertrophy induced by isoprenaline or pressure overload. PLoS One 13, e0192322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Meili D, & Salathe M (2014). Soluble adenylyl cyclase in health and disease.Biochimica et Biophysica Acta 1842, 2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Sutto Z, Nlend MC, Horvath G, Schmid N, Buck J, … Salathe M (2007).Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. The Journal of General Physiology 130, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Sutto Z, Schmid N, Novak L, Ivonnet P, Horvath G, … Salathe M (2010). Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. The Journal of Biological Chemistry 285, 29998–30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Dekker FJ, & Maarsingh H (2013). Exchange protein directly activated by cAMP (epac): A multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacological Reviews 65, 670–709. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Nedele J, Guske K, Maase M, Lenders M, Schelleckes M, … Brand E (2014). Soluble adenylyl cyclase in vascular endothelium: Gene expression control of epithelial sodium channel-alpha, Na+/K+-ATPase-alpha/beta, and mineralocorticoid receptor. Hypertension 63, 753–761. [DOI] [PubMed] [Google Scholar]
- Seifert R, Schneider EH, & Bahre H (2014). From canonical to non-canonical cyclic nucleotides as second messengers: Pharmacological implications. Pharmacology & Therapeutics. [DOI] [PubMed] [Google Scholar]
- Shahar S, Wiser A, Ickowicz D, Lubart R, Shulman A, & Breitbart H (2011). Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Human Reproduction 26, 2274–2282. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Al-Malki WH, & Delamere NA (2011). Mechanism of aqueous humor secretion, its regulation and relevance to glaucoma In Rumelt S (Ed.), Glaucoma -Basic and Clinical Concepts. InTech. [Google Scholar]
- Shahidullah M, Mandal A, Wei G, Levin LR, Buck J, & Delamere NA (2014). Nonpigmented ciliary epithelial cells respond to acetazolamide by a soluble adenylyl cyclase mechanism. Investigative Ophthalmology & Visual Science 55, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PX, Fang J, Sang A, Wang Y, Kapiloff MS, & Goldberg JL (2016). Soluble adenylyl cyclase is required for retinal ganglion cell and photoreceptor differentiation. Investigative Ophthalmology & Visual Science 57, 5083–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim MS, Kim KY, & Ju WK (2017). Role of cyclic AMP in the eye with glaucoma.BMB Reports 50, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SC, & Sprang SR (2006). Structures, mechanism, regulation and evolution of class III nucleotidyl cyclases. Reviews of Physiology, Biochemistry and Pharmacology 157, 105–140. [DOI] [PubMed] [Google Scholar]
- Sokolowska M, Chen LY, Liu Y, Martinez-Anton A, Qi HY, Logun C, … Shelhamer JH (2015). Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. Journal of Immunology 194, 5472–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa CM, Zanetti MN, Pocognoni CA, & Mayorga LS (2016). Acrosomal swelling is triggered by cAMP downstream of the opening of store-operated calcium channels during acrosomal exocytosis in human sperm. Biology of Reproduction 94, 57. [DOI] [PubMed] [Google Scholar]
- Steegborn C (2014). Structure, mechanism, and regulation of soluble adenylyl cyclases -similarities and differences to transmembrane adenylyl cyclases. Biochimica et Biophysica Acta 1842, 2535–2547. [DOI] [PubMed] [Google Scholar]
- Steegborn C, Litvin TN, Hess KC, Capper AB, Taussig R, Buck J, … Wu H (2005). A novel mechanism for adenylyl cyclase inhibition from the crystal structure of its complex with catechol estrogen. The Journal of Biological Chemistry 280, 31754–31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegborn C, Litvin TN, Levin LR, Buck J, & Wu H (2005). Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nature Structural & Molecular Biology 12, 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, & Levin LR (2006). Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. The Journal of Biological Chemistry 281, 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles TL, Kapiloff MS, & Goldberg JL (2014). The role of soluble adenylyl cyclase in neurite outgrowth. Biochimica et Biophysica Acta 1842, 2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzabosco M, Fiorotto R, Melero S, Glaser S, Francis H, Spirli C, & Alpini G (2009). Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology 50, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengholm A (2012). Cyclic AMP dynamics in the pancreatic beta-cell. Upsala Journal of Medical Sciences 117, 355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, … Zaccolo M (2006). PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. The Journal of Cell Biology 175, 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesmer JJ, Dessauer CW, Sunahara RK, Murray LD, Johnson RA, Gilman AG, & Sprang SR (2000). Molecular basis for P-site inhibition of adenylyl cyclase. Biochemistry 39, 14464–14471. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, & Sprang SR (1997). Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha. GTPgammaS. Science 278, 1907–1916. [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Johnson RA, Gosselin G, Gilman AG, & Sprang SR(1999). Two-metal-ion catalysis in adenylyl cyclase. Science 285, 756–760. [DOI] [PubMed] [Google Scholar]
- Teves ME, Guidobaldi HA, Unates DR, Sanchez R, Miska W, Publicover SJ, … Giojalas LC (2009). Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS One 4, e8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Sandler S, Gylfe E, & Tengholm A (2011). Glucose- and hormone-induced cAMP oscillations in {alpha}- and {beta}-cells within intact pancreatic islets. Diabetes 60, 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal H, Fulcher NB, Bitterman J, Salazar E, Buck J, Levin LR, … Steegborn C (2012). Crystal structure and regulation mechanisms of the CyaB adenylyl cyclase from the human pathogen Pseudomonas aeruginosa. Journal of Molecular Biology 416, 271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M (2014). sAC from aquatic organisms as a model to study the evolution of acid/base sensing. Biochimica et Biophysica Acta 1842, 2629–2635. [DOI] [PubMed] [Google Scholar]
- Tresguerres M, Barott KL, Barron ME, & Roa JN (2014). Established and potential physiological roles of bicarbonate-sensing soluble adenylyl cyclase (sAC) in aquatic animals. The Journal of Experimental Biology 217, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Buck J, & Levin LR (2010). Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Archiv: European Journal of Physiology 460, 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Levin LR, Buck J, & Grosell M (2010). Modulation of NaCl absorption by [HCO(3)(–)] in the marine teleost intestine is mediated by soluble adenylyl cyclase. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 299, R62–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, & Buck J (2010). Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis.Proceedings of the National Academy of Sciences of the United States of America 107, 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi F, Konrad C, D’Aurelio M, Ramos-Espiritu LS, Stepanova A, Burstein SR, … Manfredi G (2017). Distinct intracellular sAC-cAMP domains regulate ER Ca(2+) signaling and OXPHOS function. Journal of Cell Science 130, 3713–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi F, Konrad C, & Manfredi G (2014). Role of soluble adenylyl cyclase in mitochondria. Biochimica et Biophysica Acta 1842, 2555–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, & Manfredi G (2013). cAMP and mitochondria. Physiology 28, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma MK, Sadasivuni MK, Yateesh AN, Neelima K, Mrudula S, Reddy M, …Somesh BB (2014). Activation of GPR40 attenuates chronic inflammation induced impact on pancreatic beta-cells health and function. BMC Cell Biology 15, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Muschietti JP, Flawia MM, & Tezon JG (1990). Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochimica et Biophysica Acta 1054, 231–236. [DOI] [PubMed] [Google Scholar]
- Wan X, Ru Y, Chu C, Ni Z, Zhou Y, Wang S, … Zhang Y (2016). Bisphenol A accelerates capacitation-associated protein tyrosine phosphorylation of rat sperm by activating protein kinase A. Acta Biochimica et Biophysica Sinica (Shanghai) 48, 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lam CS, Wu F, Wang W, Duan Y, & Huang P (2005). Regulation of CFTR channels by HCO(3)—sensitive soluble adenylyl cyclase in human airway epithelial cells. American Journal of Physiology. Cell Physiology 289, C1145–C1151. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu D, Varin A, Nicolas V, Courilleau D, Mateo P, … Brenner C (2016). A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death. Cell Death & Disease 7, e2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RL, Buck J, Levin LR, Winger RC, Wang J, Arase H, & Muller WA (2015). Endothelial CD99 signals through soluble adenylyl cyclase and PKA to regulate leukocyte transendothelial migration. The Journal of Experimental Medicine 212, 1021–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer E, Krapf D, de la Vega-Beltran JL, Sanchez-Cardenas C, Navarrete F,Haddad D, … Visconti PE (2013). Compartmentalization of distinct cAMP signaling pathways in mammalian sperm. The Journal of Biological Chemistry 288, 35307–35320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, … Jaffrey SR (2006). Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nature Neuroscience 9, 1257–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, … Conti M (2006). Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Developmental Biology 296, 353–362. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R (1994). Mammalian fertilization In Knobil E, & Neill JD (Eds.), The physiology of reproduction (pp. 189–317). New York: Raven Press, Ltd. [Google Scholar]
- Young JJ, Mehdi A, Stohl LL, Levin LR, Buck J, Wagner JA, & Stessin AM (2008). “Soluble” adenylyl cyclase-generated cyclic adenosine monophosphate promotes fast migration in PC12 cells. Journal of Neuroscience Research 86, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ru Y, Shi H, Wang Y, Wu B, Upur H, & Zhang Y (2015). Cholecystokinin receptors regulate sperm protein tyrosine phosphorylation via uptake of HCO3. Reproduction 150, 257–268. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ru Y, Wang C, Wang S, Zhou Z, & Zhang Y (2013). Tripeptidyl peptidase II regulates sperm function by modulating intracellular Ca(2+) stores via the ryanodine receptor. PLoS One 8, e66634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Chadwick PA, Levin LR, Buck J, & Magro CM (2010). Soluble adenylyl cyclase defines a nuclear cAMP microdomain in keratinocyte hyperproliferative skin diseases. The Journal of Investigative Dermatology 130, 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, … Buck J (2003). Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. The FASEB Journal 17, 82–84. [DOI] [PubMed] [Google Scholar]
- Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, … Buck J (2013). CO2/HCO3 (−)- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. The Journal of Biological Chemistry 288, 33283–33291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, … Buck J (2004). Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. The Journal of Cell Biology 164, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]