Abstract
Background:
The role of testosterone (T) in the pathophysiology of affective disorders and anxiety is broadly supported. Evidence suggests that T has anxiolytic and antidepressant properties. One proposed route for the central effects of T is its interaction with the gamma-aminobutyric acid (GABA) system. We explored the relationship between T levels and GABA+ levels in anterior-cingulate (ACC) and the posterior-cingulate (PCC) regions in depressed women, using magnetic resonance spectroscopy (1H-MRS).
Methods:
Twenty-one depressed patients with regularly cycling who were not taking hormonal or psychotropic drugs were recruited. We assessed severity of depression using the Hamilton Depression Rating Scale (HDRS). Blood samples were taken for quantification of free (FT) and total testosterone (TT) on the day of the magnetic resonance (MR) scan. We evaluated GABA+ levels in the PCC and ACC, using the Hadamard Encoding and Reconstruction of MEGA-Edited Spectroscopy (HERMES) sequence. Pearson correlations were used to evaluate the association between FT, TT, GABA+ concentrations, and HDRS scores.
Results:
TT and FT levels were positively correlated with GABA+ levels in the PCC. No correlation was observed between T levels and GABA+ levels in the ACC. The HDRS total scores correlated negatively with FT levels.
Limitations:
Limitations include the cross-sectional evaluation and the lack of a comparative healthy group.
Conclusions:
Our findings suggest that the potential anxiolytic and antidepressant properties of T are related to increased GABA+ levels in the PCC. This observation may contribute to increased understanding of the role of T in depressive and anxiety symptoms in women.
Keywords: Testosterone, Major depressive disorder, Women, GABA, MR spectroscopy
1. Introduction
Major depressive disorder (MDD) is a chronic illness that is approximately twice as common in women as in men. The complexity of this disorder notwithstanding, biological factors are suggested as triggers of a depressive episode in women (Albert, 2015). Similarly, anxiety is highly prevalent in women (Remes et al., 2016), and frequently MDD and anxiety are comorbid, suggesting common pathophysiological elements (Wichers et al., 2009). Gender differences in the presentation of MDD and anxiety disorders have been attributed to hormonal fluctuations, as is observed in studies that explore depressive and anxiety symptoms during periods of hormonal fluctuations, such as puberty, postpartum and perimenopause (Dahl and Gunnar, 2009; Douma et al., 2005; Halbreich, 2010; Flores-Ramos et al., 2014, 2017). A dysregulation in the stress system, which differs between sexes (Fernandez-Guasti et al., 2012; Oyola and Handa, 2017), is a proposed pathway to understand the hormonal influence on mood and anxiety, since it has been observed that differences in the cortisol response to specific stressors depend on sex and circulating hormones (Hollanders et al., 2017; Stephens et al., 2016). The hypothalamic-pituitary-adrenal (HPA) axis plays an important role in the regulation of stress (and its dysregulation in affective disorders) (Herman et al., 2005), which in turn is regulated by neurosteroids, such as testosterone and its metabolites, via GABAergic receptors (Gunn et al., 2015). Animal studies show that cerebrocortical neurosteroid concentrations increase after 1 h of restraint stress exposition (Mele et al., 2004); similarly foot shock stress elicits an increase of neuroactive steroids, which is blocked by abecarnil (a GABAA receptor agonist), suggesting a relationship between brain neuroactive steroids and GABAA receptor function (Barbaccia et al., 1996). Modulation of GABA receptors by neurosteroids has been pro-posed as a “fine tuning” HPA axis function, or in other words, a mechanism to return to homeostasis after an acute stressor (Gunn et al., 2015). In humans, some imaging studies support the role of neuro-steroids in stress regulation; for instance, a resting-state study showed a modulation of amygdala connectivity by neurosteroids (allopregnano-lone and DHEA) (Sripada et al., 2014).
Interaction between the T and GABA systems has been supported by preclinical studies that demonstrate that anxiolytic effects of T are mediated by GABA-A receptors (Fernandez-Guasti and Martinez-Mota, 2005), with a minimal participation of estradiol aromatization (Gutiérrez-García et al., 2009). However, clinical studies of T participation in affective/anxiety symptoms have reported contradictory results that may depend on sex. In men, lower levels of bioavailable and total testosterone were observed in depressed patients compared to healthy males (McIntyre et al., 2006); furthermore beneficial effects of T supplementation have been demonstrated, improving mood and alleviating anxiety (Pope et al., 2000, 2010). In the case of women, data about the relation of T and depression are scarce and paradoxical results have been obtained. One study, based on a community sample, showed lower salivary testosterone levels in females with a current major depressive disorder and other anxiety disorders compared to healthy controls (Giltay et al., 2012).
Reproductive life stage could influence the relationship between testosterone and mood in the menopausal transition, an increased risk of depressive symptoms was related to higher levels of T in Caucasian, but not in African-American women (Milman et al., 2015). Similarly, a follow-up study observed that an increase in T levels was associated to an increased risk of depressive symptoms (Bromberger et al., 2010). Paradoxically, low-dose T supplementation in women seems to improve mood and reduce anxiety in women with treatment-resistant-depression, when used as a complementary therapy (Miller et al., 2009). Testosterone effects on mood also seem to be dose-dependent, with high T levels associated to depression (Rohr, 2002).
Magnetic resonance spectroscopy (MRS), which can measure levels of metabolites in the central nervous system (CNS) (Harris et al., 2017), has been applied in a number of studies of depression, hormones and GABAergic systems. Studies have demonstrated that GABA concentrations are diminished in the occipital cortex (OCC) in depressed patients compared to healthy controls (Sanacora et al., 1999), as well as in the prefrontal cortex (PFC) areas (Hasler et al., 2007). Moreover, in patients that have received an adequate treatment, such as serotonin selective reuptake Inhibitors (SSRI) or electroconvulsive therapy (ECT), normalization of GABA concentrations in the OCC is observed (Sanacora et al., 2002, 2003). Changes in GABA levels across the menstrual cycle have been reported, as well as an influence of the use of hormonal contraceptives (De Bondt et al., 2015), suggesting MRS is sensitive to the impact of hormonal fluctuations on the GABAergic system. To our knowledge, MRS studies have not evaluated the relationship between testosterone levels, mood and GABA levels. There-fore, this work explores testosterone levels in depressed women and their relationship to ACC and PCC GABA levels, based on the hypothesis that a positive relationship will be observed between free and total testosterone and GABA levels in the explored brain regions.
2. Methods
2.1. Participants
Twenty-one female patients with major depressive disorder (MDD), treatment-free and with regular menstrual cycling, participated in this study. Participants were recruited in the outpatient clinic of the National Institute of Psychiatry Ramón de la Fuente Muñiz (INPRFM), in Mexico City. The protocol fulfilled the requirements of the institutional ethical committee and all the participants signed an informed consent.
The MINI International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) was applied to evaluate psychiatric disorders. The inclusion criteria were: women; MDD diagnosis; 18 to 45 years old; regular menstrual cycles; reproductive period according to the Stages of Reproductive Age Workshop (STRAW) (Harlow et al., 2012). Patients with psychiatric comorbidities, except anxiety disorders, were excluded; medical illnesses were also not allowed according to medical examination and laboratory tests. Women using any hormonal-based compound (contraceptives or hormone therapy replacement), anti-epileptic drugs, and/or psychotropic medication were excluded; patients with alcohol use in the last 2 weeks or drug use in the last 6 months were also excluded. Lastly, patients treated with electro-convulsive therapy and transcranial magnetic stimulation in the last year were not included in the present study.
2.2. Procedures
MDD was diagnosed by a trained psychiatrist, according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria (APATFo, 1991). The MINI was applied to confirm the diagnosis and to evaluate comorbidities. A complete clinical evaluation was performed for all the participants, including a menstrual cycle history to select only patients on the −5 to −3 reproductive stages according to STRAW, which means reproductive age and includes women with regular menstrual cycle, and does not include women in the menopausal transition (Soules et al., 2001). We recorded the last day of menstrual period and classified it in follicular or luteal menstrual phases. Severity of depression symptoms was evaluated by the 17-item Hamilton depression rating scale (HDRS-17) (Hamilton, 1960).
2.3. Laboratory test
Blood samples were taken between 7:00 and 9:00 AM. Quantification of hormones (progesterone, estradiol, free and total testosterone) was performed by chemoluminiscence. Additionally thyroid tests were conducted in order to exclude thyroid abnormalities in the participants.
2.4. Magnetic resonance imaging (MRI) study protocol
All the MRI experiments were performed with a 3.0 Tesla scanner (Phillips Medical Systems) equipped with a 32-channel phased-array head coil. Before the MRS scans, a T1-weighted anatomical MRI was acquired for localization purposes with repetition time (TR )= 7.0 ms, and echo time (TE) = 3.5 ms.
MR Spectroscopy data were acquired from voxels centered on the anterior cingulate cortex (3 × 3 × 3 cm3) and the posterior cingulate cortex (3 × 3 × 3 cm3), avoiding the lateral ventricle and skull, as is shown in the Fig. 1. Signals of GABA and glutathione (GSH) were detected using Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) (Saleh et al., 2016). Briefly, HERMES allows the simultaneous J-difference editing of GSH and GABA signals by applying editing pulses to GABA spins (at 1.9 ppm) and GSH spins (at 4.56 ppm) in a Hadamard-encoded editing scheme. Four sub-experiments are performed and the GABA and GSH-edited difference spectrums are calculated. The HERMES sequence was acquired with the following parameters: TR = 2000 ms; TE = 80 ms; 320 signal averages (80 averages for each sub-experiment; 2 kHz spectral width; 2k data-points). Data were processed using Gannet, the GABA Analysis Toolkit, which allows the evaluation of the quality of data, removing rater-de-pendent variance, preventing the significance-based selection of processing parameters and widening access to the non-expert user (Edden et al., 2014). Post-processing frequency and phase correction was applied according to Mikkelsen et al. (2017). GABA values were corrected to account for differential gray matter/white matter/cerebrospinal fluid composition of the voxel (Porges et al., 2017; Harris et al., 2015). GABA levels were quantified relative to the unsuppressed water signal from the same region, corrected for the CSF volume-fraction fCSF by dividing by (1 − fCSF).
Fig. 1.
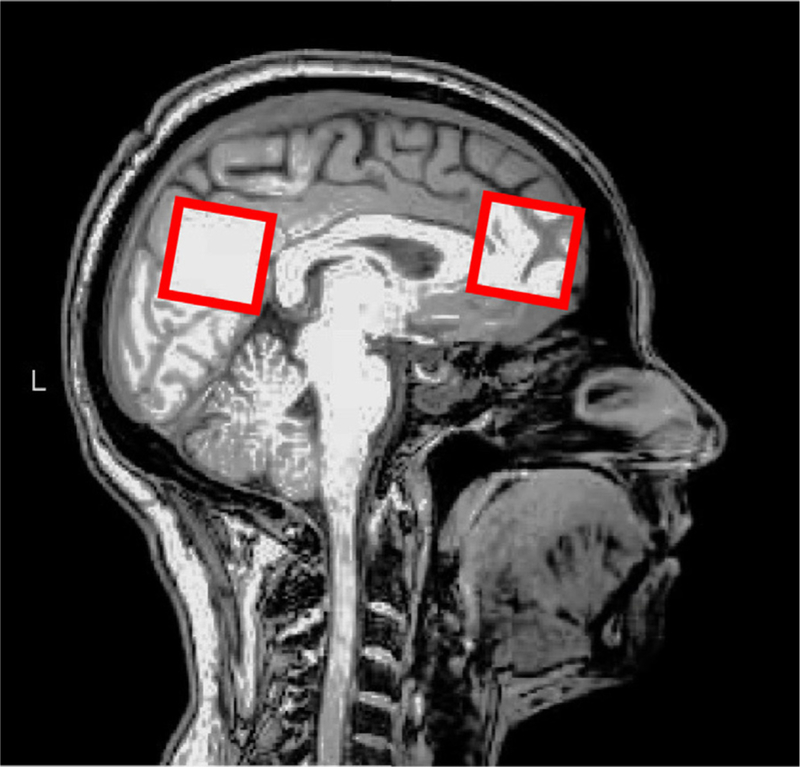
Location of MRS voxels in the anterior (A) and posterior (B) cingulate regions.
Additionally, since the edited signal at 3 ppm includes contributions from GABA, macromolecules and homocarnosine (Rothman et al., 1993), we refer to the signal obtained as GABA+.
2.5. Statistical analysis
Descriptive statistical analysis was carried out, and a two-tailed t-test was applied in order to evaluate differences in GABA+ levels be-tween menstrual cycle phases. Associations between HDRS scores and GABA+ concentrations as well as FT and TT levels were assessed using Pearson correlation coefficient. Level of significance was set at p < 0.05. A Pearson correlation analysis was also carried out with other variables that could influence the results (progesterone, estradiol, number of previous episodes and age at first episode). Lastly, a partial correlation analysis was carried out to analyze the relationship between PCC_ GABA+ levels, ACC GABA+ levels, free testosterone and total testosterone, controlling by HDRS scores. False discovery rate (FDR) (Benjamini and Yekutieli, 2001) method was used to correct the correlations between the main variables according to our hypothesis (FT, TT, PCC_GABA+ and ACC_GABA+) before and after controlling by HDRS. All statistical analyses were carried using the Statistical Package for Social Sciences Software 20th version (SPSS-20).
3. Results
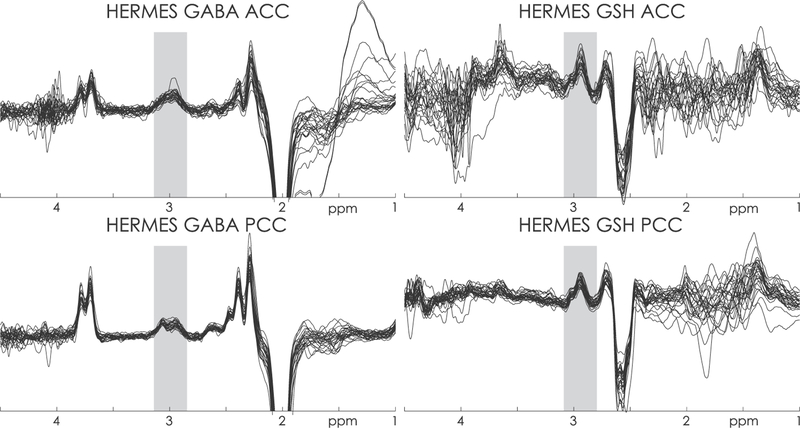
Mean age of the patients was 31.2 (sd 8.18); 12 patients were assessed to be in a follicular phase and 9 in the luteal phase. The clinical and socio-demographic characteristics of the patients are shown in the Table 1. GABA+ levels were normally distributed and are presented as means (sd), as well as testosterone levels. GABA+-edited spectra were successfully acquired in all subjects, as shown in Fig. 2. No differences in the GABA+ concentrations in the PCC nor ACC regions were observed between menstrual phases (p = 0.83; p = 0.55).
Table 1.
Clinical and socio-demographic characteristics of participants.
| Sociodemographic | n = 21 |
|---|---|
| Age (years) | 31.48 ± 9.41 |
| Education (years) | 13.43 ± 4.11 |
| Marital status, n (%) | |
| Single | 12 (57.1) |
| Married | 8 (38.1) |
| Divorced | 1 (4.8) |
| Occupation, n (%) | |
| Student | 8 (38.1) |
| Employed | 5 (23.8) |
| Not employed | 8 (38.1) |
| Economic status, n (%) | |
| Low income | 8 (38.1) |
| Medium income | 8 (38.1) |
| High income | 2 (9.5) |
| Clinic | mean ± sd |
| HDRS | 19.05 ± 3.4 |
| BMI | 27.44 ± 7.4 |
| Total testosterone (ng/ml) | 0.34 ± 0.17 |
| Free testosterone (pg/ml) | 1.38 ± 0.49 |
| ACC_GABA+ levels (mM) | 1.72 ± 1.54 |
| PCC_GABA+ levels (mM) | 1.94 ± 0.70 |
| Menstrual phase, n (%) | |
| Follicular | 12 (57.1) |
sd = standard deviation; HDRS = 17 items hamilton depression rating scale; BMI = body mass index; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; GABA = gamma amino-butyric acid.
Fig. 2.
GABA+ edited difference spectrum from one subject. The GABA+ signal being quantified is marked at 3 ppm.
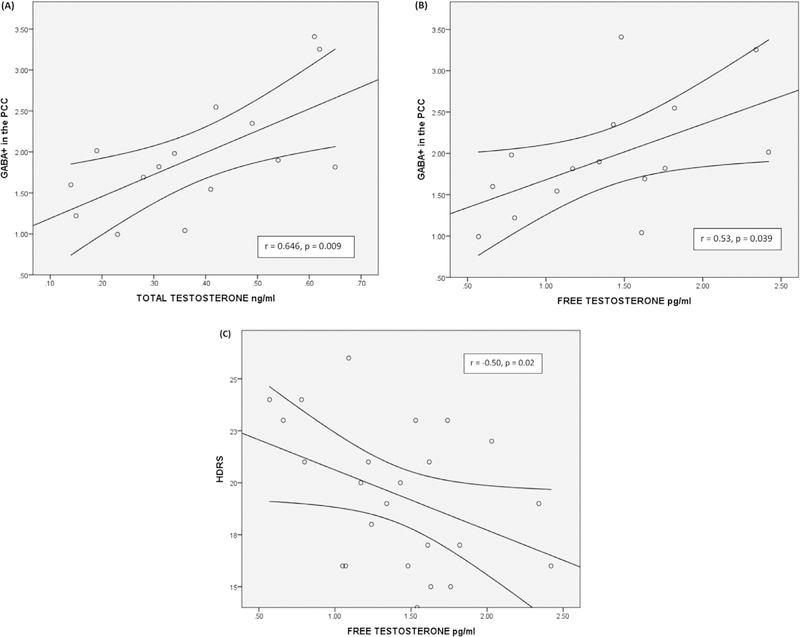
Concentration of GABA+ in the PCC showed significant positive correlations with free (r = 0.53, p = 0.039) (Fig. 3B) and total (r = 0.64, p = 0.009) testosterone levels (Fig. 3A). Estradiol and progesterone levels were not correlated with brain metabolites in the regions of interest. Previous depressive episodes were observed in patients in a range from 0 to 12; we did not observe a relationship between the number of previous depressive episodes and GABA+ levels in the regions of interest. Age at first episode was also not related to GABA+ levels (Table 2a). According to our hypothesis we analyzed the correlation between TT, FT, PCC-GABA+ and ACC-GABA+, and after a correction with the FDR method the only correlation that remained significant was TT and PCC-GABA+. Lastly, HDRS total scores showed a negative relationship to FT (r = −0.50, p = 0.02) (Fig. 3C), but no relationship to TT. The partial correlation analysis between TT, FT, PCC GABA+ and ACC GABA+ controlling by HDRS showed that correlation between PCC GABA+ and FT, and correlation between PCC GABA+ and TT were significant (r = 0.67, p = 0.032; r = 0.813, p = 0.004, respectively) (see Table 2b). After correction with the FDR method, only the correlation between PCC GABA+ and TT remained significant.
Fig. 3.
(A) GABA+ levels in PCC are correlated with total testosterone (r = 0.64; p = 0.009). (B) GABA+ levels in PCC are correlated with free testosterone (r = 0.53; p = 0.039). (C) HDRS total scores showed a negative relationship to FT (r = −0.50, p = 0.02).
Table 2a.
Correlations between GABA levels, hormones and HDRS scores.
| ACC_GABA+ | PCC_GABA+ | PROG ng/ml | Estradiol pg/ml | TT ng/ml | FT pg/ml | HDRS scores | Age at FDE | No. PDE | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ACC_GABA+ | r | 1 | −.127 | −.234 | .401 | .050 | .353 | −.451 | −.161 | .028 |
| p | .709 | .463 | .196 | .877 | .261 | .142 | .616 | .932 | ||
| PCC_GABA+ | r | 1 | .273 | −.442 | .646** | .538 | −.270 | .066 | −.384 | |
| p | .324 | .099 | .009 | .039 | .330 | .816 | .157 | |||
| PROG ng/ml | r | 1 | .246 | .079 | .233 | −.538 | .122 | −.046 | ||
| p | .283 | .734 | .308 | .012 | .599 | .843 | ||||
| Estradiol pg/ml | r | 1 | .140 | −.108 | −.331 | −.280 | .132 | |||
| p | .544 | .642 | .143 | .220 | .570 | |||||
| TT ng/ml | r | 1 | .208 | −.286 | −.202 | −.382 | ||||
| p | .365 | .208 | .381 | .087 | ||||||
| FT pg/ml | r | 1 | −.504 | −.082 | −.187 | |||||
| p | .020 | .724 | .418 | |||||||
| HDRS scores | r | 1 | −.208 | .033 | ||||||
| p | .365 | .886 | ||||||||
| Age at FDE | r | 1 | −.241 | |||||||
| p | .293 | |||||||||
| No. PDE | r | 1 | ||||||||
| p |
Table 2b.
Partial correlation analysis controlling for HDRS scores.
| Control variable | ACC-GABA+ | PCC_GABA+ | TT (ng/ml) | FT (pg/ml) | ||
|---|---|---|---|---|---|---|
| HDRS scores | ACC_GABA+ | r | 1.000 | −0.167 | −0.106 | 0.167 |
| p | 0.645 | 0.770 | 0.644 | |||
| PCC_GABA+ | r | 1.000 | 0.677 | 0.813 | ||
| p | 0.032 | 0.004 | ||||
| TT (ng/ml) | r | 1.000 | 0.560 | |||
| p | 0.092 | |||||
| FT (pg/ml) | r | 1.000 | ||||
| p |
4. Discussion
GABA is the main inhibitory neurotransmitter in the brain with an important role in maintaining excitation/inhibition balance, which is implicated in the pathophysiology of many neurological and psychiatric disorders. Reduced GABA neurotransmission has been observed in patients with MDD, underlining the importance of GABAergic systems in mood and anxiety symptoms. Biochemical measures of GABA in plasma and cerebrospinal fluid demonstrated decreased GABA levels in depressed patients (Luscher et al., 2011; Sanacora and Saricicek, 2007). Additionally, evidence coming from cortical inhibition, neuroimaging and neurophysiological studies converge to implicate GABAergic deficits in the pathophysiology of MDD (Croarkin et al., 2011). MRS studies have shown reduced GABA concentrations mainly in the occipital cortex (OCC, frequently studied for methodological reasons) and the ACC (Pehrson and Sanchez, 2015) as well normalization of GABA levels with appropriate treatment (Sanacora et al., 2002). The lack of a healthy control group in the current study design does not allow us to report group differences in GABA levels in our patients. We did not observe a relation between GABA+ concentrations and HDRS scores, in line with other studies using the same instrument to evaluate depressive symptoms (Wang et al., 2016) or using the Montgomery-Asberg Depression rating scale (Hasler et al., 2007). Other studies suggest that GABA changes are related to the diagnosis of depression, but not to the intensity of depressive symptoms. However, in the present sample of depressed women GABA+ levels showed no correlation to intensity of depressive symptoms measured by HDRS.
The main finding of our study was a correlation between GABA+ concentrations in the PCC and FT and TT serum levels. After correction for multiple comparisons and after controlling for HDRS, the relation between TT and GABA+ concentrations in the PCC remained significant, suggesting that TT has a role in the modulation of GABA concentrations in the brain. Studies that evaluate connectivity in brain regions have reported that depressed patients show more functional connectivity between the PCC and the subgenual-cingulate cortex than healthy controls during the rest periods, and these connectivities are related to behaviors such as rumination and brooding (Berman et al., 2010). Rumination, focusing on symptoms of distress, is an important symptom present in depression, anxiety and self-harm (Nolen-Hoeksema et al., 2008), the same disorders that have been related to testosterone (Sher et al., 2012, 2014; Zhang et al., 2015; Weiner et al., 2004; Kische et al., 2017; McHenry et al., 2014). To our knowledge, no MRS studies have evaluated the relationship between GABA+ levels and testosterone, although one cross-sectional study of CSF GABA has demonstrated that polycystic ovary syndrome patients had higher CSF GABA levels, as well as higher testosterone circulating levels, compared to eumenorrheic ovulatory women (Kawwass et al., 2017). Due to the observation that estradiol fluctuations are related to depressive episodes but also to vulnerability to stress (Freeman et al., 2006; Gordon et al., 2016), some authors have suggested that the effect of testosterone may be due to the conversion of testosterone to estradiol via aromatase. However, some studies have observed a relationship between T and depressive symptoms, and no such relationship for estradiol (Giltay et al., 2012; Milman et al., 2015; Bromberger et al., 2010; Kische et al., 2017). Our results concur with this observation, since we did not observe a significant correlation between estradiol levels and GABA+ concentrations in the explored brain regions, suggesting that anxiolytic and antidepressant effects of T are not related to estradiol conversion, or perhaps indicating a preponderant role of testosterone in GABAergic modulation. A similar finding was reported in postmenopausal women with mild-to-moderate depression, in whom no relationship was observed between estradiol and GABA+ concentrations in the PCC (Wang et al., 2016). Moreover, we find a negative association between free testosterone levels and depressive symptom intensity, suggesting a clinical link between the testosterone/GABA relationship and symptomatology.
The mechanisms underlying the effect of T on mood and anxiety remain unclear; although there is strong preclinical evidence that T and its metabolites can be allosteric modulators on GABAA receptors (McHenry et al., 2014). Testosterone anxiolytic properties are blocked by the administration of picrotoxin (a GABAA receptor antagonist) in female rats, suggesting that testosterone properties are mediated by GABAA receptors (Gutiérrez-García et al., 2009). Moreover, the observation that testosterone metabolites (5-alpha dihydrotestosterone, androsterone and 3-alpha androstandione) have a more potent effect than testosterone, suggests that testosterone needs to be convert to neurosteroids to exert its action (Aikey et al., 2002). Androstanediol, a neurosteroid produced from testosterone, markedly potentiates response to GABA in a concentration-dependent manner (Reddy and Jian, 2010). Exposure to neurosteroids enhances the open-probability of the GABAA receptor, resulting in chloride influx and reducing neuronal excitability.
Menstrual phase is a potential confound, according to the GABA+ differences observed by (Epperson et al., 2012), therefore we compared in our sample the levels of GABA+ in follicular vs luteal phase. We did not find a significant difference in GABA+ levels in the brain regions explored, and therefore we can assume that the observed results were not related to the menstrual cycle phase.
Lastly, we evaluated the number of previous depressive episodes in our patients, in order to correlate it with GABAergic concentrations, without finding any significant relation between those variables. A history of depression seems to be associated to low GABAergic concentrations in brain, which in turn, is associated with an altered neurosteroid profile (Girdler et al., 2012).
Limitations of the present study should be mentioned: first, we did not compare the brain metabolites concentration and testosterone levels with a healthy control group. The comparison between groups could add some information regarding the GABA/testosterone interaction in non-depressed women. Secondly, the cross-sectional evaluation in our patients represents a limitation because hormonal systems are dynamic and differences in the effect of testosterone on mood have been observed when cross-sectional or longitudinal evaluations are done (Kische et al., 2018). Third, a complete evaluation of hormone profile including precursors and metabolites of testosterone could add information about the interrelation between neurosteroids and GABA concentrations. Lastly, the sample size of the present study is small and our results should be taken cautiously, and apply only for women in reproductive age with depressive disorder.
Clinical implications could derive from our results, including the potential role of neurosteroids in alleviating depressive and anxiety symptoms in women, as well as the consideration of GABAergic path-ways in the depressive symptoms associated with hormonal profile in women (including mood changes associated to perimenopause and premenstrual dysphoric disorder). Lastly, considering that new rapid-acting antidepressants act on the GABAergic and glutamatergic systems, neurosteroid participation in the response to antidepressants should be explored.
Future studies about this topic should be conducted with a larger sample size, including control subjects and considering a longitudinal evaluation to better understand T effects on GABAergic systems; additionally, the measurement of T metabolites (such as dehy-drotestosterone) could deliver insight on the effect of testosterone, its metabolites and GABAergic systems.
5. Conclusions
In women with major depression, Testosterone effects could be related to increased GABA+ levels in the PCC. This observation may contribute, at least in part, to an improved understanding of the role of T in depressive and anxiety symptoms in women.
Acknowledgements
This study applies tools developed under NIH grants P41EB015909, R01EB016089, and R01EB023963; RAEE also receives salary support from these grants.
Financial support was received from CONACYT (grant FOSISS-2015-261435), recipient MFR.
MFR is supported by CONACYT (Cátedras de Investigación, project 1683).
Role of the funding source
The funding sponsors had not a role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the present article for publication.
RAEE has received honoraria from Philips Medical Systems, and grant funding from Siemens Medical Solutions, USA.
MFR has received grant support from Consejo Nacional de Ciencia y Tecnología (CONACyT) and has served as a speaker for Lundbeck and Schwabe Pharma. RAEE has received honoraria from Philips Medical Systems, and grant funding from Siemens Medical Solutions, USA.
Footnotes
Disclosures
All other authors have not disclosures to report.
References
- Aikey JL, Nyby JG, Anmuth DM, James PJ, 2002. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav 42 (4), 448–460. [DOI] [PubMed] [Google Scholar]
- Albert PR, 2015. Why is depression more prevalent in women? J. Psychiatry Neurosci 40, 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APATFo, 1991. DSM-IV Options Book: Work in Progress (7/1/91) American Psychiatric Press. [Google Scholar]
- Barbaccia ML, Roscetti G, Bolacchi F, et al. , 1996. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol. Biochem. Behav 54 (1), 205–210. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli D, 2001. The control of the false discovery rate in multiple testing under dependency. Ann. Stat 1165–1188.
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J, 2010. Depression, rumination and the default network. Soc. Cogn. Affect. Neurosci 6 (5), 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Schott LL, Kravitz HM, et al. , 2010. Longitudinal change in re-productive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Arch. Gen. Psychiatry 67 (6), 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ, 2011. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci. Biobehav. Rev 35 (3), 818–825. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR, 2009. Heightened stress responsiveness and emotional re-activity during pubertal maturation: implications for psychopathology. Dev. Psychopathol 21 (1), 1–6. [DOI] [PubMed] [Google Scholar]
- De Bondt T, De Belder F, Vanhevel F, Jacquemyn Y, Parizel PM, 2015. Prefrontal GABA concentration changes in women – influence of menstrual cycle phase, hormonal contraceptive use, and correlation with premenstrual symptoms. Brain Res 1597, 129–138. [DOI] [PubMed] [Google Scholar]
- Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK, 2005. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv. Nurs. Sci 28 (4), 364–375. [DOI] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ, 2014. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J. Magn. Reson. Imaging 40 (6), 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, et al. , 2012. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am. J. Psychiatry 169 (5), 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Fiedler JL, Herrera L, Handa RJ, 2012. Sex, stress, and mood disorders: at the intersection of adrenal and gonadal hormones. Horm. Metab. Res 44 (8), 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L, 2005. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology 30 (8), 762–770. [DOI] [PubMed] [Google Scholar]
- Flores-Ramos PhD M, Silvestri Tomassoni R, Guerrero-López JB, Salinas M, 2017. Evaluation of trait and state anxiety levels in a group of peri-and postmenopausal women. Women Health 1–15. [DOI] [PubMed]
- Flores-Ramos M, Moreno J, Heinze G, Aguilera-Pérez R, Pellicer Graham F, 2014. Gonadal hormone levels and platelet tryptophan and serotonin concentrations in perimenopausal women with or without depressive symptoms. Gynecol. Endocrinol [DOI] [PubMed]
- Freeman EW, Sammel MD, Lin H, Nelson DB, 2006. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch. Gen. Psychiatry 63 (4), 375–382. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Enter D, Zitman FG, et al. , 2012. Salivary testosterone: associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J. Psychosom. Res 72 (3), 205–213. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL, 2012. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology 37 (4), 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS, 2016. Estradiol variability, stressful life events, and the emergence of depressive sympto-matology during the menopausal transition. Menopause 23 (3), 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D, 2015. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front. Neuroendocrinol 36, 28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-García AG, Contreras CM, Vásquez-Hernández DI, Molina-Jiménez T, Jacome-Jacome E, 2009. Testosterone reduces cumulative burying in female Wistar rats with minimal participation of estradiol. Pharmacol. Biochem. Behav 93 (4), 406–412. [DOI] [PubMed] [Google Scholar]
- Halbreich U, 2010. Women’s reproductive related disorders (RRDs). J. Affect. Disord 122 (1–2), 10–13. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, et al. , 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19 (4), 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA, 2015. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 42 (5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Saleh MG, Edden RA, 2017. Edited (1) H magnetic resonance spectroscopy in vivo: methods and metabolites. Magn. Reson. Med 77 (4), 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC, 2007. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 64 (2), 193–200. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H, 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry 29 (8), 1201–1213. [DOI] [PubMed] [Google Scholar]
- Hollanders JJ, van der Voorn B, Rotteveel J, Finken MJJ, 2017. Is HPA axis re-activity in childhood gender-specific? A systematic review. Biol. Sex Differ 8 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawwass JF, Sanders KM, Loucks TL, Rohan LC, Berga SL, 2017. Increased cerebrospinal fluid levels of GABA, testosterone and estradiol in women with poly-cystic ovary syndrome. Hum. Reprod 32 (7), 1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kische H, Gross S, Wallaschofski H, et al. , 2017. Associations of androgens with depressive symptoms and cognitive status in the general population. PLoS One 12 (5), e0177272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kische H, Pieper L, Venz J, et al. , 2018. Longitudinal change instead of baseline testosterone predicts depressive symptoms. Psychoneuroendocrinology 89, 7–12. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N, 2011. The GABAergic deficit hypothesis of major de-pressive disorder. Mol. Psychiatry 16 (4), 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, Kabbaj M, 2014. Sex differences in anxiety and de-pression: role of testosterone. Front. Neuroendocrinol 35 (1), 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Mancini D, Eisfeld BS, et al. , 2006. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology 31 (9), 1029–1035. [DOI] [PubMed] [Google Scholar]
- Mele P, Oberto A, Serra M, et al. , 2004. Increased expression of the gene for the Y1 receptor of neuropeptide Y in the amygdala and paraventricular nucleus of Y1R/LacZ transgenic mice in response to restraint stress. J. Neurochem 89 (6), 1471–1478. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Saleh MG, Near J, et al. , 2017. Frequency and phase correction for multiplexed edited MRS of GABA and glutathione. Magn. Reson. Med [DOI] [PMC free article] [PubMed]
- Miller KK, Perlis RH, Papakostas GI, et al. , 2009. Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS Spectr 14 (12), 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman LW, Sammel MD, Barnhart KT, Freeman EW, Dokras A, 2015. Higher serum total testosterone levels correlate with increased risk of depressive symptoms in Caucasian women through the entire menopausal transition. Psychoneuroendocrinology 62, 107–113. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S, 2008. Rethinking rumination. Perspect. Psychol. Sci 3 (5), 400–424. [DOI] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ, 2017. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress 20 (5), 476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Sanchez C, 2015. Altered gamma-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Des. Dev. Ther 9, 603–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG Jr., Amiaz R, Brennan BP, et al. , 2010. Parallel-group placebo-controlled trial of testosterone gel in men with major depressive disorder displaying an incomplete response to standard antidepressant treatment. J. Clin. Psychopharmacol 30 (2), 126–134. [DOI] [PubMed] [Google Scholar]
- Pope HG Jr., Kouri EM, Hudson JI, 2000. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men: a randomized controlled trial. Arch. Gen. Psychiatry 57 (2), 133–140 discussion 155–136. [DOI] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Lamb DG, et al. , 2017. Impact of tissue correction strategy on GABA-edited MRS findings. Neuroimage 162, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Jian K, 2010. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J. Pharmacol. Exp. Ther 334 (3), 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes O, Brayne C, van der Linde R, Lafortune L, 2016. A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav 6 (7), e00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr UD, 2002. The impact of testosterone imbalance on depression and women’s health. Maturitas 1, S25–S46 41 Suppl. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH, 1993. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc. Natl. Acad. Sci. USA 90 (12), 5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MG, Oeltzschner G, Chan KL, et al. , 2016. Simultaneous edited MRS of GABA and glutathione. Neuroimage 142, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, et al. , 1999. Reduced cortical gamma-amino-butyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 56 (11), 1043–1047. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, et al. , 2003. Increased cortical GABA concentrations in depressed patients receiving ECT. Am. J. Psychiatry 160 (3), 577–579. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH, 2002. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 159 (4), 663–665. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A, 2007. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol. Disord. Drug Targets 6 (2), 127–140. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. , 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 20, 22–33 59 Supplquiz 34–57. [PubMed] [Google Scholar]
- Sher L, Grunebaum MF, Sullivan GM, et al. , 2012. Testosterone levels in suicide attempters with bipolar disorder. J. Psychiatr. Res 46 (10), 1267–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher L, Grunebaum MF, Sullivan GM, et al. , 2014. Association of testosterone levels and future suicide attempts in females with bipolar disorder. J. Affect. Disord 166, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, et al. , 2001. Stages of Reproductive Aging Workshop (STRAW). J. Womens Health Gend. Based Med 10 (9), 843–848. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Welsh RC, Marx CE, Liberzon I, 2014. The neurosteroids allopregna-nolone and dehydroepiandrosterone modulate resting-state amygdala connectivity. Hum. Brain Mapp 35 (7), 3249–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Mahon PB, McCaul ME, Wand GS, 2016. Hypothalamic-pituitary-adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology 66, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang A, Zhao B, et al. , 2016. GABA+ levels in postmenopausal women with mild-to-moderate depression: a preliminary study. Medicine (Baltimore) 95 (39), e4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner CL, Primeau M, Ehrmann DA, 2004. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom. Med 66 (3), 356–362. [DOI] [PubMed] [Google Scholar]
- Wichers M, Geschwind N, Jacobs N, et al. , 2009. Transition from stress sensitivity to a depressive state: longitudinal twin study. Br. J. Psychiatry 195 (6), 498–503. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jia CX, Wang LL, 2015. Testosterone differs between suicide attempters and community controls in men and women of China. Physiol. Behav 141, 40–45. [DOI] [PubMed] [Google Scholar]