Abstract
Thiazolidinediones (TZDs) are oral anti-diabetic drugs that are peroxisome proliferator-activated receptor gamma (PPARγ) agonists and act as insulin sensitizers. The clinical efficacy and durability of the currently available TZDs in improving glycemic control are well established. However, TZDs cause weight gain, which has been thought to be a class effect of TZDs. TZD-associated weight gain may result mainly from increased fat mass and fluid retention and may be in part congruent to the mechanism of action of TZD. Increases in fat mass are almost exclusively limited to subcutaneous fat, while there are no effects or even decreases in visceral fat. Insulin resistance and cardiovascular risk associated with fat accumulation (obesity) depend on body fat distribution, with visceral fat associated with insulin resistance and a greater degree of risk than subcutaneous fat. Therefore, despite TZD-associated weight gain, TZDs are less likely to confer an increased risk of insulin resistance and cardiovascular complications. As patients with diabetes are younger and/or more obese in Korea, TZDs may be a cost-effective treatment option, offering a unique insulin-sensitizing action and good durability for the long-term management of type 2 diabetes.
Keywords: Thiazolidinediones, Weight gain, Body fat distribution
INTRODUCTION
Thiazolidinediones (TZDs) are a class of oral antidiabetic drugs that reduce insulin resistance in peripheral tissues by activating peroxisome proliferator-activated receptor gamma (PPARγ), which is a nuclear receptor.1,2 Two TZD-class drugs, pioglitazone and lobeglitazone, are currently available in Korea. They are also termed glitazones, referring to the use of “glitazone” as a component of their names.
The glucose-lowering effects of TZD monotherapy and combination therapy in type 2 diabetes patients are well documented in the literature.3–7 It is widely accepted that insulin resistance is a major risk factor for the development of metabolic syndrome and cardiovascular disease. For this reason, TZDs, whose mechanism of action leads to reduced insulin resistance, have attracted the attention not only for their well-established hypoglycemic effect but also for their potentially favorable effects on metabolic syndrome and cardiovascular disease. For example, the ACT NOW study conducted in 602 patients with impaired glucose tolerance demonstrated that pioglitazone reduced the incidence of diabetes (hazard ratio [HR], 0.28; 95% confidence interval [CI], 0.16–0.49; P< 0.001).8 In the PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events) study, which is a four-year study of 5,238 patients with type 2 diabetes and a history of macrovascular disease recruited from 19 European countries, pioglitazone was shown to have cardiovascular protective effects (HR, 0.84; 95% CI, 0.72–0.98; P=0.027 for the composite endpoint of all-cause death, nonfatal myocardial infarction, and stroke).9 In 2007, however, Nissen et al.10 reported the findings of a meta-analysis that rosiglitazone potentially increases the risk of myocardial infarction and cardiovascular mortality (myocardial infarction: odds ratio [OR], 1.43; 95% CI, 1.03–1.98; P=0.03; cardiovascular mortality: OR, 1.64; 95% CI, 0.98–2.74; P=0.06). Although numerous questions11 were raised about the reliability of Nissen’s study, rosiglitazone was withdrawn from the European market in 2010 after years of heated discussions. The United States Food and Drug Administration (FDA) restricted access to rosiglitazone in 2011, but removed its prescribing restrictions in 2013 based on a series of studies that mitigated the suspicion of the cardiovascular risks of rosiglitazone.12 However, the use of TZDs is still encumbered with safety issues due to its possible association with increased risks of cardiovascular disease, bladder cancer, fracture, heart failure, and weight gain.
This review paper discussed the mechanism of action of TZDs on weight gain and the so-called “glitazone paradox”, the phenomenon that TZD-associated weight gain improves rather than exacerbates insulin resistance.
Mechanism of action of TZD-associated weight gain
All TZD-class drugs have been associated with weight gain, with a dose-dependent relationship observed between TZD therapy and weight gain.13 TZD-associated weight gain can thus be ascribed to a TZD class effect.13 Previous studies have demonstrated an average weight gain of 3–4 kg over the first six months of TZD therapy and up to 5 kg over a period of 3–5 years.9,14 However, TZD-associated weight gain is determined by multiple factors such as the baseline weight, combination medications for the treatment of diabetes, diet control, exercise compliance, and TZD dose.13 In particular, TZDs are widely prescribed in combination therapy for diabetes, second only to metformin; in such cases, the problem of TZD-induced weight gain may be abated.5
TZD-induced weight gain can be explained by various mechanisms. First, the weight gain may result from increased body fat. As mentioned above, the antidiabetic effect of TZD is mediated by PPARγ. PPARγ activation leads to increased fat mass, especially subcutaneous depots.15,16 Many of the TZD-related studies have reported concurrent findings of increased peripheral subcutaneous fat and decreased visceral fat.17–25 These findings imply that the use of TZDs leads to fat redistribution tending towards increased subcutaneous fat and decreased visceral fat, with weight gain being attributable to the increase in subcutaneous fat. Second, weight gain can result from increased body fluid volume due to water retention. This effect can be explained by a TZD-induced increase in sodium reabsorption in the distal tubules of the kidney.13 It is for this reason that TZDs are contraindicated in diabetic patients with New York Heart Association Class III or IV heart failure.26 Moreover, after the use of TZDs in diabetic patients, care should be taken to check for symptoms of increased body fluid such as edema and dyspnea. Third, improved glycemic control can lead to weight gain. This is not unlike the weight gain after using sulfonylurea (SU) or insulin. The United Kingdom Prospective Diabetes Study (UKPDS) of SU and insulin and the Diabetes Control and Complication Trial (DCCT) of insulin also reported weight gains of 3–5 kg associated with glycemic control.27 Another mechanism of action of TZDs that can lead to weight gain is enhanced appetite. An animal study reported hyperphagia and weight gain in TZD-fed rats.28
The glitazone paradox: the effects of TZDs on body fat distribution (depot-specific effects)
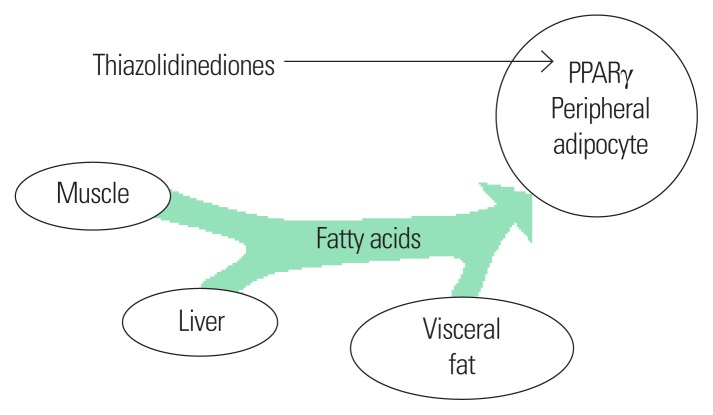
With regard to TZD-associated weight gain, it is not clear how TZD improves rather than exacerbates insulin resistance and how it can lower cardiovascular risk. To better understand the issue, it should be made clear that not all body fat is alike from the perspective of cardiovascular risk. For example, while subcutaneous fat in the hip and thigh areas is not associated with cardiovascular risk, visceral fat such as intrahepatic fat causes insulin resistance and becomes a cardiovascular risk factor.29 As mentioned above, TZDs exert depot-specific effects on regional adiposity (Fig. 1). In other words, they induce body fat redistribution in the direction of reducing (or at least not accumulating) visceral fat and increasing subcutaneous fat. Not only in-vitro and animal studies with TZDs but also numerous clinical studies using imaging techniques such as computed tomography (CT), dual-energy X-ray absorptiometry (DEXA), and magnetic resonance imaging (MRI) as well as anthropometric measures such as waist-to-hip ratio for fat measurement have yielded results supporting this finding.17–25 The results of studies on the effects of TZDs in patients with non-alcoholic fatty liver disease (NAFLD), such as decreased intrahepatic fat levels and fatty liver and liver fibrosis improvement, may be due to similar mechanisms.2,30–32 Although more research is needed to reach definite conclusions, the possible mechanisms by which TZDs exert depot-specific effects are as follows. Activation of PPARγ in subcutaneous adipocytes significantly increases the uptake and esterification of fatty acids, which is stronger than the compensatory fatty acid oxidation. In contrast, activation of PPARγ in visceral adipocytes results in a negligible increase in fatty acid absorption and esterification, and increased fatty acid oxidation. Furthermore, TZDs inhibit the expression of free fatty acid and tumor necrosis factor alpha (TNF-α) and increase adiponectin expression in adipose tissues.33
Figure 1.
Depot-specific effects on regional adiposity of thiazolidinediones.
These mechanisms may explain why the use of TZDs causes weight gain, but improves insulin resistance and does not increase cardiovascular risk. In a randomized controlled trial in 33 patients with type 2 diabetes, subcutaneous fat increased and intrahepatic fat levels decreased by 45% in the rosiglitazone-treated group compared to the levels in the control group. Along with these changes, the mean weight increased relative to the baseline in the rosiglitazone-treated group, but insulin resistance improved.18 A post hoc analysis of the PROactive study revealed that weight gain after use of pioglitazone was associated with improved cardiovascular outcomes.34
CONCLUSION
TZD-induced weight gain contributes to significant improvements in insulin sensitivity, defying the common belief that the higher the weight gain, the higher the insulin resistance. This is ascribable to the depot-specific effects of TZDs on body fat distribution, with subcutaneous fat tending to increase rather than visceral fat, which is associated with increased cardiovascular risk. The recent reports of the effects of TZDs on NAFLD may be due to similar mechanisms.
TZDs have suffered a loss of reputation due to safety warnings against rosiglitazone and pioglitazone in relation to cardiovascular disease and bladder cancer, respectively. This situation is reflected in the low proportion of TZD prescriptions (6.5%) among all antidiabetic drugs as reported in the 2015 Diabetes Fact Sheet.35 However, TZDs offer a range of advantages such as an ideal mechanism of action, efficient blood glucose control with 0.5–1.4% reduction of glycosylated hemoglobin (HbA1c) concentration, good durability, rare hypoglycemic episodes, improved metabolic syndrome, and cardiovascular disease prevention.1,2,14 A review paper comparing the clinical effects and costs of pioglitazone estimated that its use would reduce cardiovascular, metabolic and cancer deaths by 60 per 100,000 population and increase mortality due to hip fracture by 30 deaths per 100,000 population.1 The recent report on the anti-atherosclerosis effects of lobeglitazone also suggests its potential for reducing cardiovascular disease.36 These results suggest the cost-effectiveness of TZDs.
The proportion of diabetes patients with obesity in Korea is steadily increasing.37 A recent community-based study reported that this upward trend is reflected in the increasing problem of insulin resistance relative to insulin secretion.38 Moreover, the increasing prevalence of type 2 diabetes among younger individuals37 highlights the importance of drug durability in consideration of the long journey of diabetes treatment. For these reasons, we should reconsider the use of TZDs.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20:573–91. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205–15. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. Diabetes Care. 2000;23:1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 4.Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI Rosiglitazone Clinical Trials Study Group. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001;86:280–8. doi: 10.1210/jcem.86.1.7157. [DOI] [PubMed] [Google Scholar]
- 5.Rosak C, Petzoldt R, Wolf R, Reblin T, Dehmel B, Seidel D. Rosiglitazone plus metformin is effective and well tolerated in clinical practice: results from large observational studies in people with type 2 diabetes. Int J Clin Pract. 2005;59:1131–6. doi: 10.1111/j.1368-5031.2005.00652.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim SG, Kim DM, Woo JT, Jang HC, Chung CH, Ko KS, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014;9:e92843. doi: 10.1371/journal.pone.0092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin SM, Park CY, Cho YM, Ku BJ, Ahn CW, Cha BS, et al. Lobeglitazone and pioglitazone as add-ons to metformin for patients with type 2 diabetes: a 24-week, multicentre, randomized, double-blind, parallel-group, active-controlled, phase III clinical trial with a 28-week extension. Diabetes Obes Metab. 2015;17:599–602. doi: 10.1111/dom.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–15. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 9.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 11.Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med. 2007;147:578–81. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 12.Carpio GR, Fonseca VA. Update on safety issues related to antihyperglycemic therapy. Diabetes Spectr. 2014;27:92–100. doi: 10.2337/diaspect.27.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilding J. Thiazolidinediones, insulin resistance and obesity: finding a balance. Int J Clin Pract. 2006;60:1272–80. doi: 10.1111/j.1742-1241.2006.01128.x. [DOI] [PubMed] [Google Scholar]
- 14.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 15.Kang JG, Park CY. The actions of PPARγ agonists on the various target organs. Korean J Obes. 2011;20:161–9. doi: 10.7570/kjo.2011.20.4.161. [DOI] [Google Scholar]
- 16.Choi SS, Park J, Choi JH. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 2014;47:599–608. doi: 10.5483/BMBRep.2014.47.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000;283:1695–702. doi: 10.1001/jama.283.13.1695. [DOI] [PubMed] [Google Scholar]
- 18.Carey DG, Cowin GJ, Galloway GJ, Jones NP, Richards JC, Biswas N, et al. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected] Obes Res. 2002;10:1008–15. doi: 10.1038/oby.2002.137. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–91. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 20.Shadid S, Jensen MD. Effects of pioglitazone versus diet and exercise on metabolic health and fat distribution in upper body obesity. Diabetes Care. 2003;26:3148–52. doi: 10.2337/diacare.26.11.3148. [DOI] [PubMed] [Google Scholar]
- 21.Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metabolism. 2005;54:24–32. doi: 10.1016/j.metabol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Hwang YC, Lee EY, Lee WJ, Cha BS, Yoon KH, Park KS, et al. Effects of rosiglitazone on body fat distribution and insulin sensitivity in Korean type 2 diabetes mellitus patients. Metabolism. 2008;57:479–87. doi: 10.1016/j.metabol.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Nam JS, Nam JY, Yoo JS, Cho M, Park JS, Ahn CW, et al. The effect of rosiglitazone on insulin sensitivity and mid-thigh low-density muscle in patients with type 2 diabetes. Diabet Med. 2010;27:30–6. doi: 10.1111/j.1464-5491.2009.02897.x. [DOI] [PubMed] [Google Scholar]
- 24.Bray GA, Smith SR, Banerji MA, Tripathy D, Clement SC, Buchanan TA, et al. Effect of pioglitazone on body composition and bone density in subjects with prediabetes in the ACT NOW trial. Diabetes Obes Metab. 2013;15:931–7. doi: 10.1111/dom.12099. [DOI] [PubMed] [Google Scholar]
- 25.Punthakee Z, Alméras N, Després JP, Dagenais GR, Anand SS, Hunt DL, et al. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: a sub-study of the DREAM trial. Diabet Med. 2014;31:1086–92. doi: 10.1111/dme.12512. [DOI] [PubMed] [Google Scholar]
- 26.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care. 2004;27:256–63. doi: 10.2337/diacare.27.1.256. [DOI] [PubMed] [Google Scholar]
- 27.Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol. 2003;2:33–47. doi: 10.2165/00024677-200302010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Pickavance LC, Tadayyon M, Widdowson PS, Buckingham RE, Wilding JP. Therapeutic index for rosiglitazone in dietary obese rats: separation of efficacy and haemodilution. Br J Pharmacol. 1999;128:1570–6. doi: 10.1038/sj.bjp.0702932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–53. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, et al. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087–93. doi: 10.1002/hep.23116. [DOI] [PubMed] [Google Scholar]
- 33.Laplante M, Festuccia WT, Soucy G, Gélinas Y, Lalonde J, Berger JP, et al. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes. 2006;55:2771–8. doi: 10.2337/db06-0551. [DOI] [PubMed] [Google Scholar]
- 34.Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162:20–6. doi: 10.1016/j.ijcard.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Korean Diabetes Association. Korean diabetes fact sheet 2015. Seoul: Korean Diabetes Association; 2011. [accessed 2016 Sep 12]. Available from: URL: http://www.diabetes.or.kr. [Google Scholar]
- 36.Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015;243:107–19. doi: 10.1016/j.atherosclerosis.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Ha KH, Kim DJ. Trends in the diabetes epidemic in Korea. Endocrinol Metab (Seoul) 2015;30:142–6. doi: 10.3803/EnM.2015.30.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son JW, Park CY, Kim S, Lee HK, Lee YS Insulin Resistance as Primary Pathogenesis in Newly Diagnosed, Drug Naïve Type 2 Diabetes Patients in Korea (SURPRISE) Study Group. Changing clinical characteristics according to insulin resistance and insulin secretion in newly diagnosed type 2 diabetic patients in Korea. Diabetes Metab J. 2015;39:387–94. doi: 10.4093/dmj.2015.39.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]