Ceftolozane-tazobactam (C/T), the combination of a new cephalosporin with a classic β-lactamase inhibitor, is currently considered the most active betalactam antibiotic against P. aeruginosa [1]. Despite several case reports on C/T pharmacokinetics in critically ill patients during continuous renal replacement therapy (CRRT) [2–4], the optimal dose in this clinical scenario still remains unclear [5].
A 68-year-old patient was admitted to our ICU with septic shock (nosocomial peritonitis) and anuric acute renal failure. Broad-spectrum antimicrobial therapy, including C/T and continuous venovenous hemodiafiltration (CVVHD), was initiated, using a polysulphone hemofilter (Fresenius, Germany) with blood flow, dialysate fluid, and replacement fluid rates of 100 mL/min, 2000 mL/h, and 1000 mL/h. The patient received high C/T doses of C/T 2 g/1 g every 8 h (infused over 1 h) while receiving CVVHD, and became apyrexial 7 days after C/T treatment initiation, remaining fever-free for 14 days without any adverse effects related to this drug.
Pre-filter and post-filter blood and ultradiafiltrate samples were obtained during the 8-h dosing interval after the fourth dose. Drug concentrations were measured by high-performance liquid chromatography. Figure 1 and Table 1 show pre- and post-filter plasma concentrations. Pharmacokinetic parameters were calculated (Table 2). Extraction ratios were high for both ceftolozane and tazobactam (49.3% ± 1.8% and 40.5% ± 4.5%). Mean C/T concentrations in the ultrafiltrate were 40 mg/L and 13.5 mg/L, respectively.
Fig. 1.
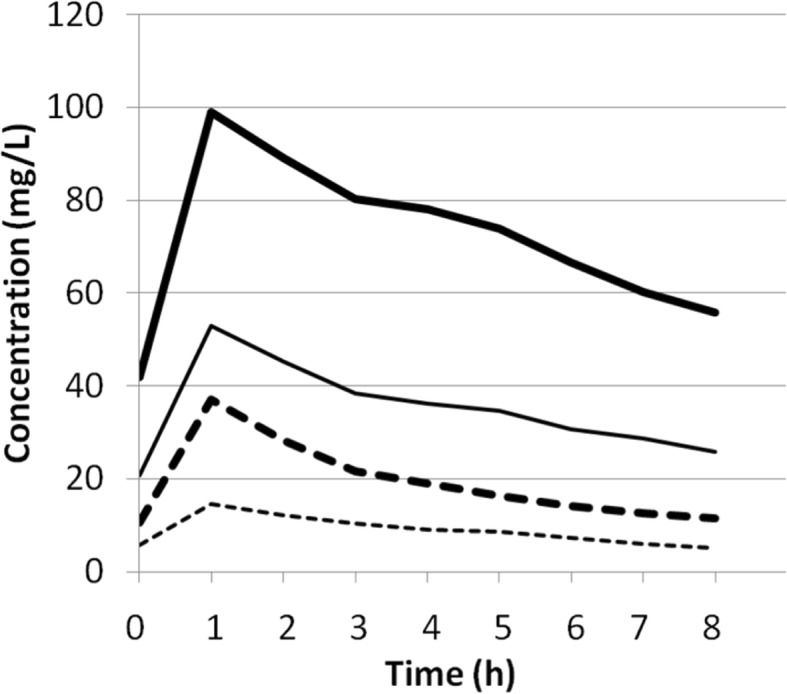
Simulated plasma concentrations versus time curves for ceftolozane and tazobactam. Pre-filter (thick line) and post-filter (fine line) ceftolozane plasma concentrations and pre-filter (thick dotted line) and post-filter (fine dotted line) tazobactam plasma concentrations. (The figure is original for this article)
Table 1.
Concentrations of ceftolozane and tazobactam in pre-filter and post-filter plasma samples obtained after the fourth dose of 2 g/1 g ceftolozane-tazobactam administered as intravenous 1-h infusion
| Sampling time | Ceftolozane (mg/L) | Tazobactam (mg/L) | ||
|---|---|---|---|---|
| Pre-filter | Post-filter | Pre-filter | Post-filter | |
| 0 h (pre dose) | 41.9 | 20.7 | 10.6 | 5.8 |
| 1.5 h post dose | 89.1 | 45.2 | 28.3 | 12.2 |
| 2 h post dose | 80.3 | 38.4 | 21.6 | 10.3 |
| 2.5 h post dose | 77.1 | 36.1 | 19.0 | 9.0 |
| 3 h post dose | 73.8 | 34.7 | 16.3 | 8.2 |
| 5 h post dose | 66.6 | 30.6 | 14.2 | 7.4 |
| 7 h post dose | 60.2 | 28.7 | 12.7 | 6.0 |
| 8 h post dose | 55.8 | 25.8 | 11.4 | 5.1 |
Table 2.
Pharmacokinetic parameters of ceftolozane and tazobactam
| Parameter | Ceftolozane | Tazobactam | ||
|---|---|---|---|---|
| Pre-filter | Post-filter | Pre-filter | Post-filter | |
| Clearance (L/h) | 2.1 | 5.4 | 6.4 | 17.4 |
| Volume of distribution (L) | 53.9 | 97.5 | 108.9 | 194.2 |
| Half-life (h) | 17.9 | 12.6 | 11.9 | 7.8 |
| AUC (h mg/L) | 960 | 373 | 157 | 57.6 |
| Maximum concentration (mg/L) | 99 | 53 | 37 | 14.5 |
| Minimum concentration (mg/L) | 55.9 | 25.8 | 11.4 | 5.1 |
AUC area under the concentration-time curve
We decided on a 3 g/iv dose every 8 h, taking into account two previous studies [3, 4] and a recent study which showed CRRT to be an independent predictor of clinical failure (OR 4.5, 95% CI 1.18–17.39, p = 0.02) when C/T is administered at 1.5 g every 8 h [5].
Ceftolozane and tazobactam are small molecules with low plasma protein binding rates, causing most to be removed during CRRT. Despite the considerable C/T clearance observed in our patients during CVVHD, however, ceftolozane plasma concentrations remained above the MIC, for MICs of up to 8 μg/mL, throughout the dosing interval, assuming 20% protein binding. Given that C/T exhibits linear, dose-proportional pharmacokinetics, a standard C/T dose of 1 g/0.5 g would be expected to maintain ceftolozane levels above the MIC during the entire dosing interval, although tazobactam concentrations could be insufficient, even taking higher pre-filter rather than lower post-filter levels as representative of therapeutic serum levels.
In conclusion, our data underscore that a dosage of 3 g every 8 h can be used safely to prevent the potential harm of underdosing ceftolozane/tazobactam during CRRT; larger studies are however needed to confirm our findings.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All relevant data are within the paper and its supporting information files. All data are fully available without restriction.
Abbreviations
- AUC
Area under the concentration-time curve
- C/T
Ceftolozane-tazobactam
- CRRT
Continuous renal replacement therapy
- CVVHD
Continuous venovenous hemodiafiltration
- HPLC
High-performance liquid chromatography
Authors’ contributions
GA conceived the study, participated in its design, and drafted the manuscript. RF participated in the study design and coordination and helped draft the manuscript. CE performed pharmacokinetics analysis and helped revise the manuscript. SMC, EP, JC, and participated in data analysis and interpretation and helped revise the manuscript. DN and MA participated in the study design and coordination and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study protocol (TC-TCRR-2018) was approved by the local ethics committee (INCLIVA Health Research Institute) and written informed consent obtained from the patients or their relatives prior to study inclusion.
Consent for publication
Written informed consent was obtained from the patient or their relatives for publication of their individual details. The consent form is held by the authors’ institution and is available for review by the Editor-in-Chief.
Competing interests
GA received financial support for speaking at meetings organized on behalf of Astellas, Gilead, Merck Sharp and Dohme (MSD), and Pfizer, as well as unrestricted research grants from Astellas, MSD, and Pfizer. DN received financial support for speaking at meetings organized on behalf of Astellas, MSD, and Pfizer and received unrestricted research grants from Astellas and Pfizer. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gerardo Aguilar, Phone: +34-961-97-35-00, Email: gerardo.aguilar@uv.es.
Rafael Ferriols, Email: ferriols.raf@gmail.com.
Sara Martínez-Castro, Email: saradacuris@hotmail.com.
Carlos Ezquer, Email: cezquer@incliva.es.
Ernesto Pastor, Email: ernesto.pastormz@gmail.com.
José A. Carbonell, Email: joseacarbonell19@hotmail.com
Manuel Alós, Email: alos_man@gva.es.
David Navarro, Email: david.navarro@uv.es.
References
- 1.Montravers P, Bassetti M. The ideal patient profile for new beta-lactam/beta-lactamase inhibitors. Curr Opin Infect Dis. 2018;31(6):587–e93. doi: 10.1097/QCO.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 2.Oliver WD, Heil EL, Gonzales JP, Mehrotra S, Robinett K, Saleeb P, Nicolau DP. Ceftolozane-tazobactam pharmacokinetics in a critically ill patient on continuous venovenous hemofiltration. Antimicrob Agents Chemother. 2015;60(3):1899–1901. doi: 10.1128/AAC.02608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuti JL, Ghazi IM, Quintiliani R, Jr, Shore E, Nicolau DP. Treatment of multidrug-resistant Pseudomonas aeruginosa with ceftolozane/tazobactam in a critically ill patient receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents. 2016;48:342–343. doi: 10.1016/j.ijantimicag.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Bremmer DN, Nicolau DP, Burcham P, Chunduri A, Shidham G, Bauer KA. Ceftolozane/tazobactam pharmacokinetics in a critically ill adult receiving continuous renal replacement therapy. Pharmacotherapy. 2016;36:e30–ee3. doi: 10.1002/phar.1744. [DOI] [PubMed] [Google Scholar]
- 5.Bassetti M, Castaldo N, Cattelan A, Mussini C, Righi E, Tascini C, et al. Ceftolozane/tazobactam for the treatment of serious P. aeruginosa infections: a multicenter nationwide clinical experience. Int J Antimicrob Agents. 2018; [Epub ahead of print]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its supporting information files. All data are fully available without restriction.


