Abstract
Background
Previous studies have suggested that metabolic syndrome (MetS) and its component conditions are linked to the development of many benign or malignant diseases. Some studies have described relationships among metabolic syndrome or diabetes and liver cancer, but not many articles described the relationships between MetS and cirrhosis, acute hepatic failure, end-stage liver disease, and even death. However, liver cancers, cirrhosis, acute hepatic failure, end-stage liver disease, and liver-related mortality—collectively described as liver-related events (LREs)—may have different relationships with MetS. We undertook this meta-analysis to examine the association between MetS and LREs, and to determine whether geographic region or hepatitis B virus (HBV) positivity might influence the association.
Methods
Relevant studies were identified from PubMed, EMBASE, and the Cochrane database. Two reviewers independently searched records from January 1980 to December 2017. The search terms included ‘metabolic syndrome’, ‘diabetes mellitus’, ‘insulin resistance syndrome’, and ‘metabolic abnormalities’, combined with ‘cirrhosis’, ‘hepatic fibrosis ’, ‘hepatocellular carcinoma’, ‘complication’, ‘LRE’, ‘HCC’, ‘liver-related events’, and ‘liver cancer’. No language restriction was applied to the search. We chose the studies reporting an association between MetS and LREs. We used Begg’s and Egger’s tests and visually examined a funnel plot to assess publication bias. All analyses were conducted in Stata 14.0 software.
Results
There were 19 studies (18 cohort and 1 case-control) included in the analysis, with a total of 1,561,457 participants. The subjects’ ages ranged from 18 to 84 years. The combined analysis showed an overall 86% increase risk of LREs in cases with MetS (RR: 1.86,95% CI: 1.56–2.23). The funnel plot was asymmetrical, and the Egger’s test p values showed a publication bias in this meta analysis. However, through the trim and fill method, we obtained a new RR value for LREs with MetS of 1.49 (95% CI: 1.40–1.58, p = 0.000). There was no obvious difference with the two answers, so we concluded that the results were robust. For hepatitis B positive patients, the RR for MetS and LREs was 2.15 (95% CI:1.02–4.53, p = 0.038), but for the hepatitis B negative patients, the RR was 1.85 (95% CI:1.53–2.24, p = 0.000). And for non-Asians, the RR for MetS and LREs was 2.21 (95% CI: 1.66–2.69, p = 0.000), while for Asians, the RR was 1.73 (95% CI: 1.35–2.22, p = 0.000).
Conclusions
This meta-analysis showed that MetS is associated with a moderately increased risk of LREs prevalence. Patients with MetS together with hepatitis B are more likely to develop hepatic events. For non-Asians, MetS is more likely to increase the incidence of LREs.
Keywords: Metabolic syndrome, Diabetes mellitus, Insulin resistance, Metabolic abnormalities, Hepatocellular carcinoma, Cirrhosis, Liver-related events, Meta-analysis
Background
Because of a significant increase in the incidence and mortality of hepatocellular carcinoma (HCC), this cancer has become one of the most common malignancies and a major cause of death worldwide. In recent years, chronic liver disease has become a major cause of death in the United States, causing a large number of deaths every year, according to national life statistics. All the cirrhotic complications, HCC, and/or liver-related mortality were called LREs. And liver-related death is defined as death related to hepatic events [1]. Viral hepatitis and excessive drinking have been identified as the major risk factors for LREs, but risk factors for approximately 5 to 30% of HCC cases remain to be identified [2, 3]. Metabolic syndrome (MetS) patients have a high risk of cardiovascular disease. In addition, there is increasing evidence that patients with chronic liver disease are at risk of a higher rate of diagnosis of MetS or diabetes mellitus [4]. Recent studies have shown that MetS might have additional associations with liver disease. Liver cancer is the most severe liver disease. Although MetS is known to promote liver cancer, there is little evidence of whether MetS is associated with cirrhosis, liver failure, liver fibrosis, and death from liver causes.
MetS has become a major public health focus worldwide, and it is related to the occurrence of obesity and the diabetes pandemic. MetS components are a series of risk factors for cardiovascular diseases and it has become an increasingly severe problem globally [5]. The risk factors comprising MetS include obesity, abnormal blood sugar, Increased blood pressure, elevated triglyceride levels, decreased high-density lipoprotein cholesterol levels [6]. Several studies have described the relationship between MetS and diabetes and liver cancer [5], but few have investigated the LREs. The article intended to address this knowledge gap. Recent cohort studies have attempted to further understand the temporal relationship between these factors and to validate previous findings. However, there are few data on the association of LREs and MetS factors, which include obesity, diabetes and MetS.
In this study, we conducted a systematic review and meta-analysis to assess all available evidence to identify an association between MetS and LREs. We additionally analyzed related factors.
Methods
Search strategy
We used PubMed, EMBASE, and the Cochrane database for literature retrieval. In December 2017, the following search terms were used without language restrictions: MetS, diabetes mellitus, insulin resistance, metabolic abnormalities, cirrhosis, hepatic fibrosis, hepatocellular carcinoma, complication, LRE, HCC, liver-related event, and liver cancer All references cited in these studies were also reviewed to identify other published articles not indexed in the databases. The systematic review process followed established quality standards for reporting of meta-analyses.
Study eligibility
The inclusion criteria for studies in the meta-analysis were as follows: either case-control or cohort design; inclusion of subjects over 18 years old; assessment of the effects of MetS on the risk of liver events; and reporting of relative risk (RR) estimates for LREs in subjects with MetS. Only complete papers and published studies in the medical literature were included. Data from summaries, reviews, editorials, case reports, and letters were excluded. Studies reporting no risk ratio (HR) and 95% confidence intervals (95% CI), and those with participants with incomplete data were also excluded.
Data extraction
The following data were collected from each study: first author’s surname, type of article, year and country of publication, distribution of age and sex, number and characteristics of the participator, definitions of MetS, risk estimates with corresponding confidence intervals (CIs), and all other information. Quality assessment for cohort studies in this meta-analysis was assessed with the Newcastle Ottawa scale (NOS), as recommended by the Cochrane Non-Randomized Studies Methods Working Group. The scale allocates a number of stars ranging from one to nine.
Statistical analysis
We used both fixed- and random-effects models to calculate the pooled RR and 95% CI. We performed an analysis to identify the association between MetS and LREs, and also to analyze any influence of geographic region or HBV positivity. Heterogeneity was assessed with the I2 statistic and p value. Funnel plots and Egger and Begg’s and Mazumdar’s tests were used to assess publication bias. A p value < 0.05 was considered statistically significant [7, 8]. The trim and fill method aims to identify and correct the funnel asymmetry caused by publication bias, it can remove a small sample study that causes asymmetry in the funnel plot, and then estimate the center value of the funnel plot with the symmetrical part after trimming. All statistical analysis was performed in Stata 14.0 software.
Results
Figure 1 details our search steps. A total of 1,561,457 individuals were included in the study. Overall, we identified 8400 studies from different databases, including 4559 studies from PubMed, 173 studies from the Cochrane database, and 3668 studies from EMABASE. After reading the abstract, we initially considered 173 publications to be relevant. Among them, we excluded 25 comments, 2 letters, 7 meta-analysis, 25 comments, 1 unreported HCC, and 94 lacking risk estimates. A total of 19 studies (18 cohort studies and one case-control study) were included in the final analysis [1, 6, 9–25].
Fig. 1.
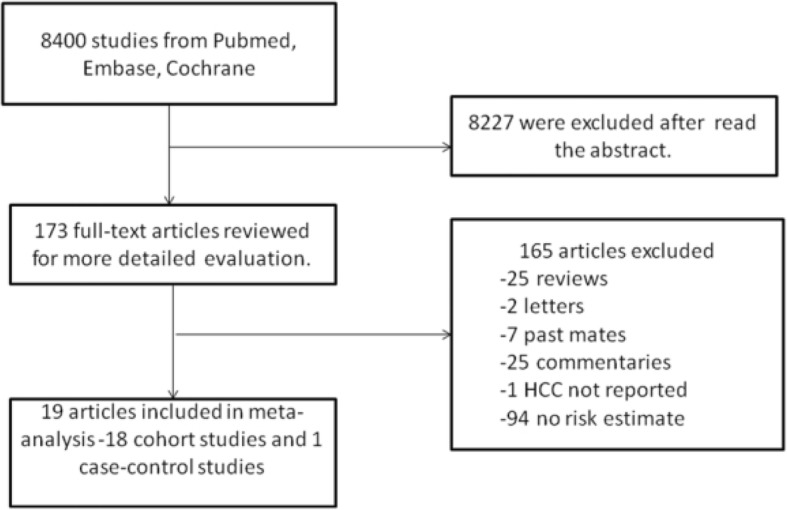
Flow chart of study selection in this systematic review
Association between metabolic syndrome and liver-related events
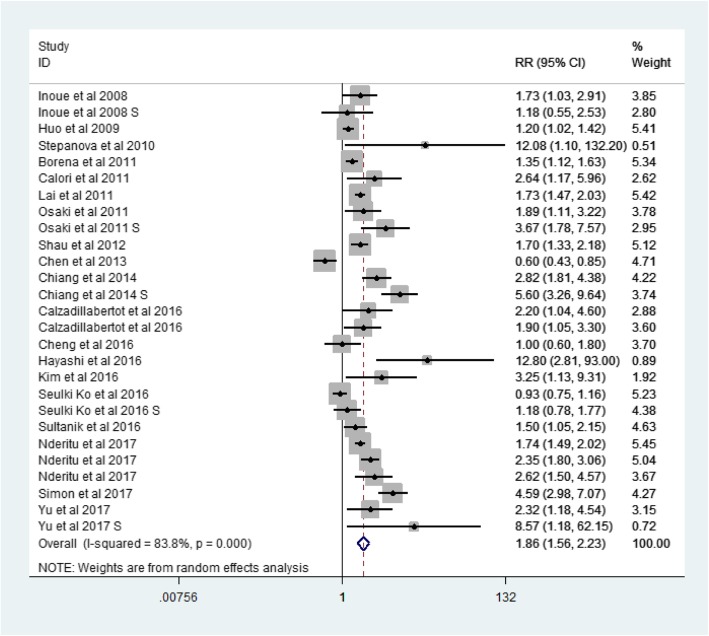
A total of 19 articles were included in this meta analysis. Among the 18 cohort studies, there were 1,546,392 participants, of which 7373 had liver-related events (LREs) (Table 1). The one case-control study was published in 2010 as part of the Third National Health and Nutrition Examination Survey, and it included 2061 liver disease cases and 13,004 controls. The age of participants ranged from 18 to 84 years old. The pooled RR (among 1,561,457 participants from 19 studies) for LREs among subjects with MetS was 1.86 (95% CI, 1.56–2.23) (Fig. 2).
Table 1.
Main characteristic of 19 eligible studies in this meta-analysis
| Study authors, | Country | Type of study | Study population | Age(years) | Male:Female | N-O-Scale | RR,95%CI, and p | Follow-up period | Definition of Metabolic risk factors | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|
| Inoue et al.,2008 [9] | Japan | Cohort (prospective cohort) |
27,724 total Japanese men and women (114 HCC cases) | Men:56.5 years, Women:55.5 years |
9548:18,176 | 8 | M-1.73,1.03–2.91,P=NA F-1.18,0.55–2.51,P=NA |
9–11 years | AHA | Age, sex, study area, smoking status, weekly ethanol intake |
| Huo et al.,2009 [10] | China | Cohort (prospective cohort) |
1713 HCC cases(907 HCC-related died) |
65.4 years | 1313:400 | 9 | Total-1.2,1.02–1.42,P = 0.03 | 18 ± 16 months | WHO | Age, sex |
| Stepanova et al.,2010 [11] | Europe and America | Case-control (retrospective cohort) |
2061 liver disease (331 died) 13,004 controls(177 died) |
Case:41.1 years, Controls:44.4 years |
Case:192:139 Controls:5943:7061 |
8 | Total-12.08,1.1–132.2,P = 0.042 | 12–18 years | WHO | Age, sex, smoking status |
| Borena et al.,2011 [12] | Europe | Cohort (prospective cohort) |
578,700 people from Norway, Austria and Sweden(266 HCC cases) | Men:43.9 years, Women:44.1 years |
289,866:288,834 | 8 | Total-1.35,1.12–1.63,P=NA | Men for 12.8 years, Women for 11.3 years |
WHO | Age, sex, smoking status |
| Calori et al.,2011 [13] | Italy | Cohort (prospective cohort) |
2011 total Italians (34 liver-specific died) | 57 years | 885:1126 | 8 | Total-2.643,1.172–5.957, P < 0.0191 | 15 years | WHO | Age, sex,smoking status, alcohol intake |
| Lai et al.,2011 [14] | China | Cohort (retrospective cohort) |
19,349 total Chinese (224 HCC cases) | 55.5 years | 10,792:8557 | 7 | Total-1.73,1.47–2.03, P=NA | 3–8 years | ICD | Age, sex |
| Osaki et al.,2011 [15] | Japan | Cohort (prospective cohort |
23,625 Japanese men and women(129 HCC cases) | 58.6 years | 8239:15,386 | 7 | M-1.89,1.11–3.22, F-3.67,1.78–7.57, P=NA |
9.1 years | NCEP-ATP III | Age, sex,smoking status, heavy drinking |
| Shau et al.,2012 [16] | China | Cohort (prospective cohort |
931 HCC cases who received surgical resection(321 liver-specific died) | 57.7 years | 679:252 | 9 | Total-1.7,1.33–2.18, P < 0.001 |
5–6 years | NCEP-ATP III | Age,sex,tumor stage |
| Chen et al.,2013 [6] | China | Cohort (prospective cohort |
56,231 total Chinese men and women(262 HCC cases) | 60.9 years | 17,440:38,896 | 6 | Total-0.6,0.43–0.85, P = 0.004 |
5–7 years | AHA | Age, sex, weight, liver function |
| Chiang et al.,2014 [17] | China | Cohort (prospective cohort |
50,080 Chinese men and women (235 HCC-related deaths) | M:54.2 years, F:53.7 years |
23,484:26,596 | 9 | M-2.82,1.81–4.38, P < 0.0001 F-5.60,3.26–9.64, P < 0.0001 |
10 years | WHO | Age,sex,smoking status,alcohol intake |
| Calzadillabertot et al.,2016 [18] | Cuba | Cohort (prospective cohort |
250 compensated HCV-related cirrhosis(28 death and 55 decompensated) | 60 years | 96:154 | 8 | Died-2.2,1.04–4.6, P = 0.04 Decompensated- 1.9,1.05–3.3, P = 0.03 |
22–80 months | WHO | Age,sex,alcohol intake |
| Cheng et al.,2016 [1] | China | Cohort (prospective cohort |
1466 CHB patients(93 hepatic events) | 46 years | 939:527 | 8 | Total-1.0,0.6–1.8, P = 0.939 |
88 ± 20 months | NCEP-ATP III | Age, sex, weight, weekly ethanol intake, liver function |
| Hayashi et al.,2016 [19] | Japan | Cohort (prospective cohort |
474 Japanese non-cirrhotic patients with chronic hepatitis (21HCC cases) | 58 years | 230:244 | 9 | Total-12.8,2.81–93.0, P = 0.0006 |
8 years | HOMA | Age,sex |
| Kim et al.,2016 [20] | Korea | Cohort (prospective cohort |
1696 chronic HBV infected patients (24 HCC cases) |
50 years | 964:732 | 9 | Total-3.25,1.13–9.31, P = 0.028 |
1.0–10.5 years | NCEP-ATP III | Age,sex |
| Seulki Ko et al.,2016 [21] | Korea | Cohort (prospective cohort |
99,565 Koreas men and women(588 HCC cases) | Above 20 years | 61,758:37,807 | 8 | M-0.93,0.75–1.16, P=NA F-1.18,0.78–1.77, P=NA |
10.4 years | WHO | Age,sex,smoking status,alcohol intake |
| Sultanik et al.,2016 [22] | France | Cohort (prospective cohort |
341 HCV patients with cirrhosis(136 HCC cases,ESLD cases,HCC and ESLD cases) | 56 years | 225:116 | 9 | Total-1.5,1.05–2.15, P = 0.03 |
8.75 years | WHO | Age,sex,alcohol intake |
| Nderitu et al., 2017 [23] | Sweden | Cohort (prospective cohort |
509,436 participants (2775 cirrhosis cases,766HCC cases,158 cirrhosis and HCC cases) | 44 years | 272,167:237,269 | 9 | Cirrhosis-1.74,1.49–2.02, HCC-2.35,1.80–3.06, LREs-2.62,1.50–4.57, P=NA |
13.6 years | WHO | Age,sex |
| Simon et al.,2017 [24] | USA | Cohort (prospective cohort |
171,110 Americans men and women (112 HCC cases) | 64.1 years | 50,284:120,826 | 9 | Total-4.59,2.98–7.07, P < 0.0001 |
32 years | National Diabetes Date Group | Age,sex,race,smoking status,alcohol intake |
| Yu et al.,2017 [25] | China | Cohort (prospective cohort |
1690HBV carriers (158HCC cases,126 liver-related died) |
48.4 years | All men | 7 | HCC-2.32,1.18–4.54, P=NA LREs-8.57,1.18–62.15, P=NA |
19 years | Asian and Chinese Criteria | Age,smoking status,alcohol intake |
MetS Metabolic syndrome, HCC hepatocellular carcinoma, RR relative risk, N-O-Scale Newcastle-Ottawa quality assessment scale, LREs Liver-related events, ICD – International Classification of Disease, HOMA Homeostasis Model Assessment, WHO World Health Organization,NCEP-ATP III National Cholesterol Education Program-Adult Treatment Panel III, AHA American Heart Association, M male,F female, NA not applicable
Fig. 2.
Forest plot: Meta-analysis of the association between metabolic syndrome and liver-related events
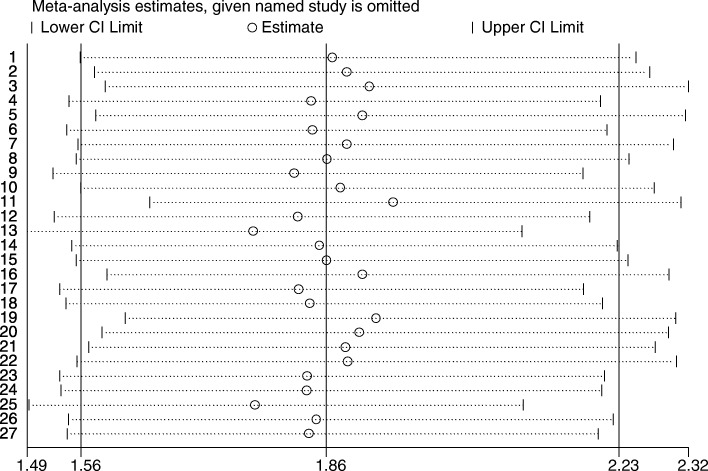
Because of the heterogeneity of the 19 studies (p value for heterogeneity = 0.000, I2 = 83.8%) (Fig. 2), we used the random-effects model to calculate the combined RR (Fig. 3). On the basis of the random-effects model, no article has a big impact on the results.
Fig. 3.
Meta-analysis random-effects estimates
Association between metabolic syndrome and hepatocellular carcinoma
To explore potential sources of heterogeneity, we also performed subgroup analysis for different liver events, such as hepatocellular carcinoma, liver-related death, and cirrhosis.
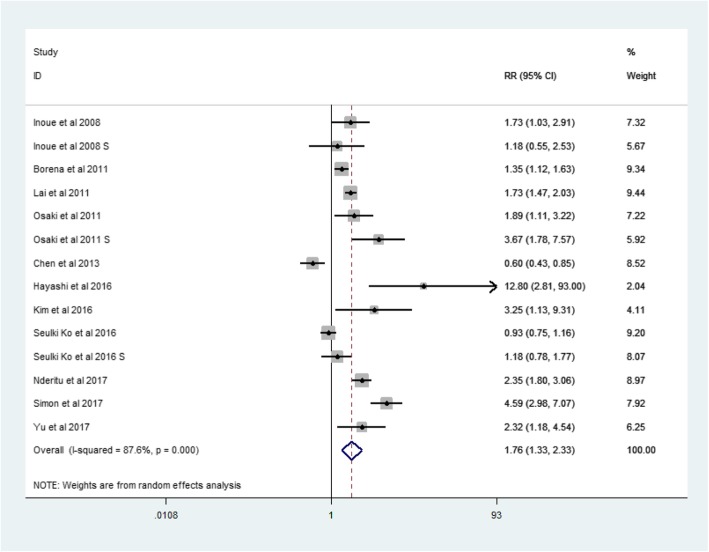
There were 11 studies about hepatocellular carcinoma, which were cohort studies (Table 1). The RR for hepatocellular carcinoma was 1.76 (95% CI: 1.33–2.33, p = 0.000, I2 = 87.6%) (Fig. 4).
Fig. 4.
Forest plot: Meta-analysis of the association between metabolic syndrome and HCC
Association between metabolic syndrome and liver-specific death
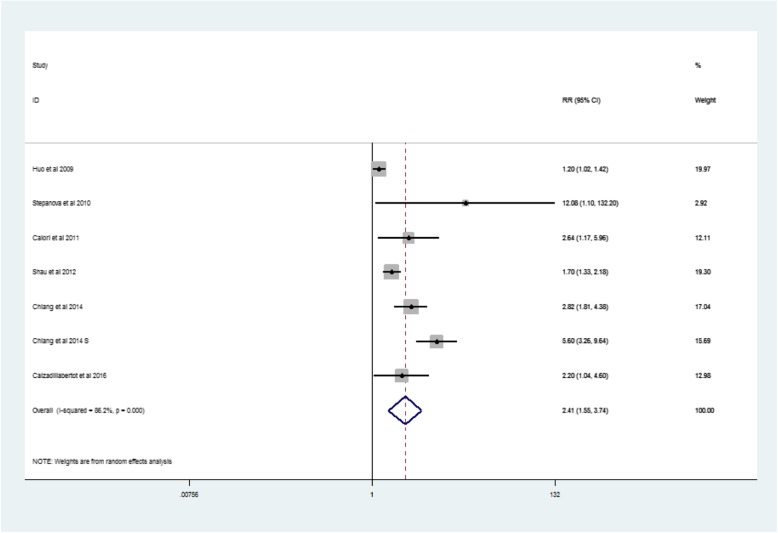
One case-control study and five cohort studies reported risk estimates for liver-specific death (Table 1). The pooled RR for liver-specific deaths was 2.41 (95% CI: 1.55–3.74, p = 0.0005, I2 = 86.2%) (Fig. 5).
Fig. 5.
Forest plot: Meta-analysis of the association between metabolic syndrome and liver-related deaths
Inclusion characteristics
We divided the included population into HBV-positive and HBV-negative groups, and then calculated the relationship between MetS and LREs in each group.
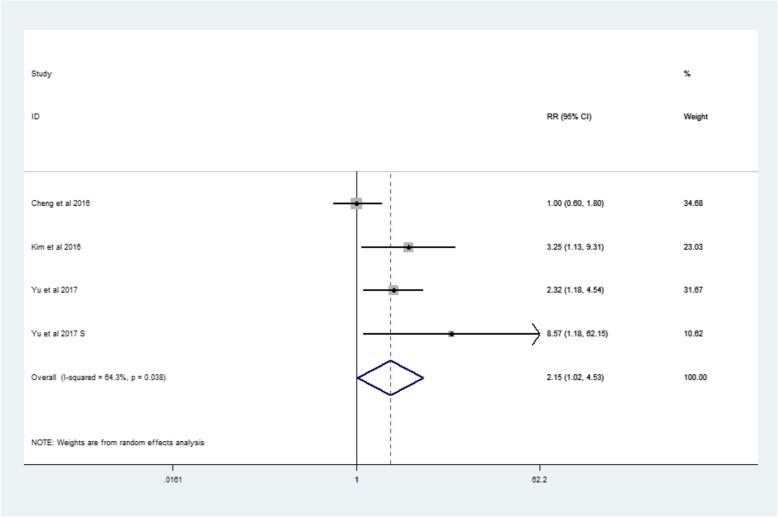
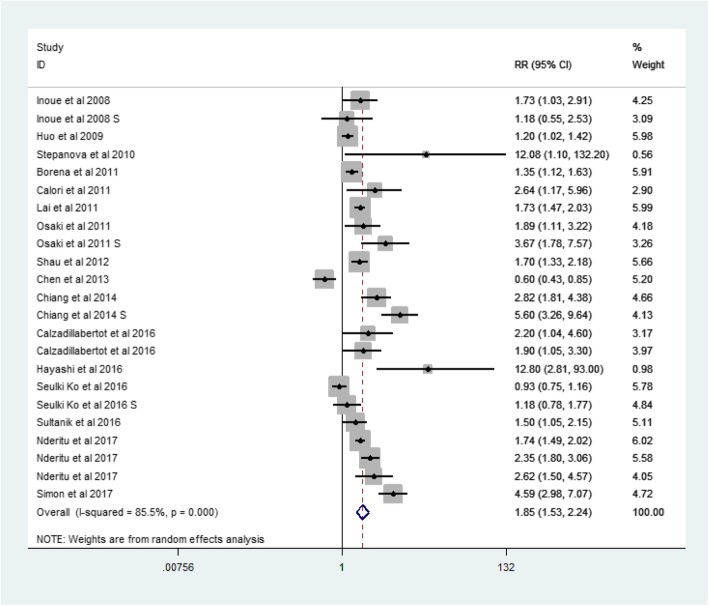
In the HBV-positive group, the RR for MetS and LREs was 2.15 (95% CI: 1.02–4.53, p = 0.038, I2 = 64.3%) (Fig. 6). And in the HBV-negative group, the RR was 1.85 (95% CI: 1.53–2.24, p = 0.000, I2 = 85.5%)(Fig. 7).
Fig. 6.
Forest plot: For HBV-positive cases, the association between metabolic syndrome and liver-related events
Fig. 7.
Forest plot: For HBV-negative cases, the association between metabolic syndrome and liver-related events
Terrain analysis
We divided all the data into Asian and non-Asian regions, and then calculated the relationship between MetS and LREs in each group. We then compared the differences between the groups.
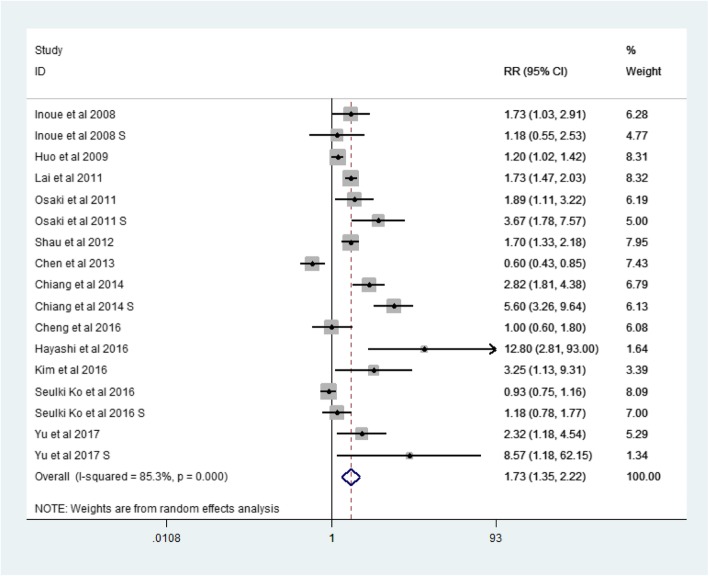
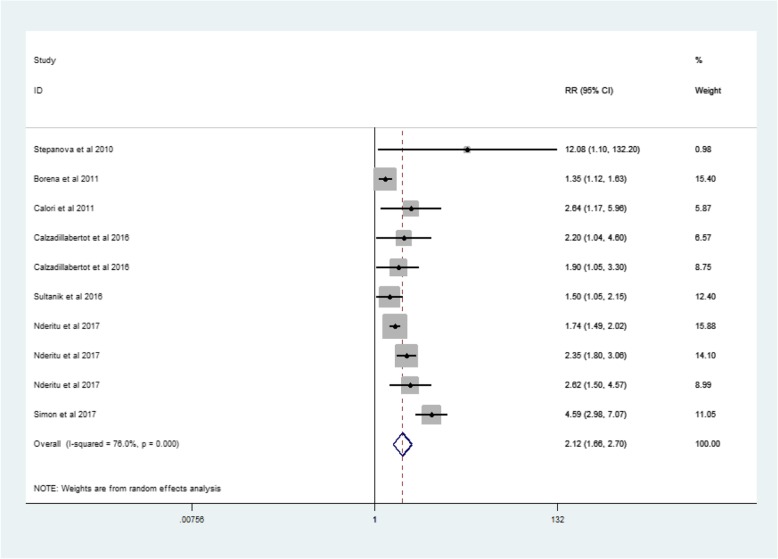
There were 12 articles from Asian countries (Table 1). The RR for MetS and LREs was 1.73 (95% CI: 1.35–2,22, p = 0.000, I2 = 85.3%)(Fig. 8). Then there were 7 studies describing non-Asian populations (Table 1). The RR for MetS and LREs was 2.12(95% CI: 1.66–2.70, p = 0.000, I2 = 76.0%) (Fig. 9) .
Fig. 8.
Forest plot: For Asian, the association between metabolic syndrome and liver-related events
Fig. 9.
Forest plot: For non-Asian, the association between metabolic syndrome and liver-related events
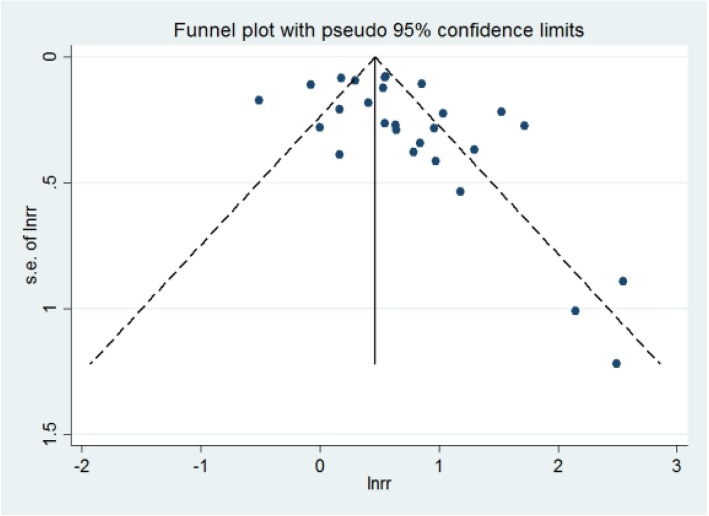
Publication bias
We used the NOS to assess publication quality and determined credible results with 8.2 stars for the 18 cohort studies and 8 stars for the single case-control study (Table 1). Funnel plots were made (Figs. 10) and Egger’s test was performed to assess the publication bias in the meta-analysis. The funnel plot was not completely symmetrical, and the p values of Egger’s test were close to 0.05 (Begg and Mazumdar’s test: p = 0.08; Egger’s test: p = 0.049). Thus, the results suggested that publication bias was present in this meta analysis. Then we use the trim and fill method to correct the funnel asymmetry caused by publication bias, and we get a new RR for MetS and LREs which is 1.49(95% CI: 1.40–1.58, p = 0.000), and there was no obvious difference compared with our previous results.
Fig. 10.
Funnel plots for publication bias
Discussion
In the meta-analysis of 19 studies including 1,561,457 patients with MetS, 16 studies found a statistically significant positive association, while 3 studies showed a negative association. After we removed the negative study indicated by the random-effects model to have affected the results, we also found that MetS significantly increased the risk of LREs. Therefore, the negative study did not change the finding.
The funnel plot was asymmetrical, and the p values of Egger’s test showed a publication bias in this meta analysis. However, through the trim and fill method, we obtained a new RR for LREs with MetS of 1.49 (95% CI: 1.40–1.58, p = 0.000), and there was no obvious difference compared with our previous results. Therefore, we concluded that the results were robust, and MetS is associated with a moderately increased risk of LRE prevalence.
The results also indicated that preexisting MetS confers a statistically significant 1.56- or 2.23-fold increased risk for LREs that is independent of other risk factors, an association also observed for its components. MetS can promote the occurrence of cardiovascular diseases, coronary heart disease and other diseases, and the degree of association between MetS and liver cancer is similar to the former [26, 27]. Nonalcoholic fatty liver disease (NAFLD), including non-alcoholic steatohepatitis (NASH), insulin resistance, and several metabolic abnormalities are closely related, thus suggesting a link between metabolic factors and cancer of the liver.
The findings from our study suggested a 1.33- or 2.33-fold increased risk of HCC development in patients with MetS. In addition, the study also demonstrated a 1.55- or 3.74-fold increased risk of liver-related death in patients with MetS. Our results together indicate that MetS is associated with a moderately increased risk of LRE.
Analysis of related factors showed an overall 115% increase in risk of LREs in HBV-positive cases (RR: 2.15, 95% CI: 1.02–4.53). For HBV-negative cases, the probability indicated a 85% increase in risk (RR: 1.85, 95% CI: 1.53–2.24). This conclusion is consistent with viral hepatitis having been identified as a major risk factors for LREs [2, 3], because over time, patients infected with viral hepatitis develop cirrhosis and, eventually, HCC.
And there was some article showed the pooled RR for HCV subjects with IR was 1.89 (95% CI, 1.54–2.33). It is because the presence of the HCV core protein will increase the level of tumor necrosis factor-α, further leading to proteasomal degradation of the insulin receptor substrate, ultimately altering insulin function and promoting the development of IR. At the same time, IR further causes lipid accumulation in the liver and production of reactive oxygen species, and indirectly activates stellate cells, eventually leading to the occurrence of liver fibrosis [28].
The related factor analysis also indicated that non-Asian MetS populations are more likely than Asian populations to have LREs. There was a 73% increase in risk of LREs for Asians and an 112% increase for non-Asians. The reason for this result is not yet clear, and more research is needed to provide an explanation. It is possible that the economy and the typical non-Western diet in Asia may explain the lower risk of MetS. And for Asian, the HBV vaccine is being recommended widespread. Meanwhile, the incidence of T2DM is lower in the Asian population, and it may have influenced the results. Some studies have reported that up to 90% of obese people in Europe have some degree of fatty degeneration in their liver and overweight increases the risk of HCC. The observed association between excess body weight and the increased risk of liver disease seems to support the reports that liver disease in obese individuals may be mediated through the development of NAFLD and NASH [12].
Our study suggests a relationship between MetS and LREs, but the mechanism that links the two is not fully understood. MetS is likely to be a proxy for other cancer risk factors, such as low physical activity, intake of high caloric food producing a high quantity of heat, high dietary fat intake, low fiber intake, and oxidative stress [29].The mechanisms by which DM induces HCC are related to insulin-like growth factor I (IGF-I) or insulin-like growth factor-binding protein-3 (IGFBP-3) [6]. The presence of insulin resistance and hepatic steatosis may support the hypothesis that diabetes promotes HCC, while the growth of insulin-like growth factor-1, the increase in leptin, the decrease in adiponectin and the imbalance of pro-inflammatory/anti-inflammatory cytokines are supported the appeal opinion [30]. There are several articles that study oxidative stress, cytokine effects, and other factors that contribute to the development of NAFLD. And because of the interaction between many metabolic factors, the study of their contribution to liver disease and liver cancer is more complicated. For example, deposition of free fatty acids and their metabolites in liver tissue is associated with hyperinsulinemia and insulin resistance, and they further promote hepatic steatosis [26].
Moreover, diabetic people have a higher risk of HCV infection, and diabetes is also associated with hyperinsulinemia. Both MetS and LREs are a problem worldwide, and growing evidence shows a relationship between MetS and an increased risk of LREs [31]. Insulin resistance and obesity are often thought to be linked to cancer, and diabetes is an independent prognostic factor for several common human malignancies, such as breast, colorectal, and prostate cancer [32, 33]. Therefore, a link between MetS and cancer is also possible. Previous studies have shown that coexisting MetS in Chronic hepatitis B (CHB) patients increases the risk of different kinds of liver complications, such as liver fibrosis progression and liver cirrhosis [34]. The role of diabetes in liver cancer has been extensively studied, and a variety of biological mechanisms support an association [35–38]. For example, diabetes can affect the recurrence of liver cancer through hyperinsulinemia, hyperglycemia, or chronic inflammation.
A previous meta-analysis has described a relationship between hypertension and cancer, thus suggesting that hypertension is associated with an increased risk of cancer death [26]. That study found that people with an average blood pressure of up to one in five have a cancer risk 2.8 times more than those with the lowest blood pressure. Another meta-analysis has examined the relationship between liver cancer and diabetes. Most studies have shown a significant increase in risk, with a combined risk ratio of 2.5 (95% CI, 1.9–3.2) [39]. The above studies show that hypertension and diabetes as metabolic risk factors are significantly associated with the risk of liver disease, and decreasing the risk of MetS may also contribute to the long-term reduction of liver disease.
There are several limitations that should be considered when interpreting the findings of our meta-analysis. First, there are multiple factors that were not considered in the combined RR analysis, such as dietary habits, HCC family history, and other genetic risk factors, owing to the unavailability of these variables in the original study. Second, for diabetes and liver cancer risk meta-analysis, we referred data from observational studies, while those studies may have lower heterogeneity. Third, because chronic liver disease can also cause diabetes, the link between diabetes and HCC may not be exact. Fourth, different controls on confounding factors also influenced the results. Finally, the meta-analysis was limited to English-language studies, which might have introduced publication bias.
However, our study has several strengths; for example, this article included more data than previous studies and examined links between MetS and cirrhosis, as well as liver-related deaths, among other factors. We also analyzed the geographic region and the population for hepatitis B and other parameters. The all cohort studies in our meta-analysis are of high quality and were able to detect potential associations and eliminate the possibility of recall and selection bias.
Conclusion
In conclusion, this population-based study showed that MetS is an important risk factor for the development of liver disease. Therefore, the control of the globally prevalent MetS may help reduce liver disease. At the same time, the metabolic syndrome and related factors are introduced in the paper. Trying to avoid these risk factors is a better treatment. More well-designed randomized controlled trials or prospective cohort or retrospective cohort studies are urgently needed to improve understanding of this risk.
Acknowledgements
-Not applicable.
Funding
-Not applicable.
Availability of data and materials
-All data generated or analysed during this study are included in this published article, and you can find these data in references [1, 6, 9–25].
Abbreviations
- CHB
Chronic hepatitis B
- CI
Confidence interval
- DM
Diabetes mellitus
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HR
Hazard ratio
- IR
Insulin resistance
- LREs
Liver-related events
- MetS
Metabolic syndrome
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NOS
Newcastle-Ottawa Scale
- RR
Relative risk
Authors’ contributions
-HR, JW, YG and FY search the databases to find related articles, HR and WH analysis those articles and write the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
-Not applicable.
Consent for publication
-Not applicable.
Competing interests
-The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huina Ren, Email: 15223458762@163.com.
Junna Wang, Email: 13709414235@163.com.
Yue Gao, Email: Yue-gaoo@163.com.
Fuwei Yang, Email: m17723508806@163.com.
Wenxiang Huang, Email: wenxiang_huang@hotmail.com.
References
- 1.Cheng YK, Wong WS, Tse YK. Metabolic syndrome increases cardiovascular event but not hepatic event and death in patients with chronic hepatitis B. Hepatology. 2016;64(5):1507. doi: 10.1002/hep.28778. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. CURRENT CONCEPTS Hepatocellular Carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 3.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115(24):5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne CD, Olufadi R, Bruce KD. Metabolic disturbances in non-alcoholic fatty liver disease. Clin Sci. 2009;116(7):539–564. doi: 10.1042/CS20080253. [DOI] [PubMed] [Google Scholar]
- 5.Esposito K, Chiodini P, Colao A. Metabolic syndrome and risk of Cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CT, Chen JY, Wang JH. Diabetes mellitus, metabolic syndrome and obesity are not significant risk factors for hepatocellular carcinoma in an HBV- and HCV-endemic area of southern Taiwan. Kaohsiung J Med Sci. 2013;29(8):451–459. doi: 10.1016/j.kjms.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 9.Inoue M, Noda M, Kurahashi N. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18(3):240–247. doi: 10.1097/CEJ.0b013e3283240460. [DOI] [PubMed] [Google Scholar]
- 10.Huo TI, Hsu CY, Huang YH. Diabetes mellitus as an independent prognostic predictor and its association with renal dysfunction in patients with hepatocellular carcinoma. Liver Int. 2010;30(2):198–207. doi: 10.1111/j.1478-3231.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- 11.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59(10):1410–1415. doi: 10.1136/gut.2010.213553. [DOI] [PubMed] [Google Scholar]
- 12.Borena W, Strohmaier S, Lukanova A. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131(1):193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 13.Calori G, Lattuada G, Ragogna F. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145–152. doi: 10.1002/hep.24356. [DOI] [PubMed] [Google Scholar]
- 14.Lai SW, Chen PC, Liao KF. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: a population-based cohort study. Am J Gastroenterol. 2012;107(1):46. doi: 10.1038/ajg.2011.384. [DOI] [PubMed] [Google Scholar]
- 15.Osaki Y, Taniguchi SI, Tahara A. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. 2012;36(2):141. doi: 10.1016/j.canep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Shau WY, Shao YY, Yeh YC. Diabetes mellitus is associated with increased mortality in patients receiving curative therapy for hepatocellular carcinoma. Oncologist. 2012;17(6):856–862. doi: 10.1634/theoncologist.2012-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang CH, Lee LT, Hung SH. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology. 2014;59(6):2207–2215. doi: 10.1002/hep.27014. [DOI] [PubMed] [Google Scholar]
- 18.Calzadillabertot L, Vilargomez E, Torresgonzalez A. Impaired glucose metabolism increases risk of hepatic decompensation and death in patients with compensated hepatitis C virus-related cirrhosis. Digestive & Liver Disease Official Journal of the Italian Society of Gastroenterology & the Italian Association for the Study of the Liver. 2015;48(3):283–290. doi: 10.1016/j.dld.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T, Ogawa E, Furusyo N. Influence of insulin resistance on the development of hepatocellular carcinoma after antiviral treatment for non-cirrhotic patients with chronic hepatitis C. Infectious Agents & Cancer 2016;11(1):1–10. [DOI] [PMC free article] [PubMed]
- 20.Kim JH, Sinn DH, Gwak GY. Insulin resistance assessment is useful in risk stratification of hepatocellular carcinoma in chronic hepatitis B patients. Journal of Gastroenterology & Hepatology. 2016;32(5). [DOI] [PubMed]
- 21.Ko S, Yoon SJ, Kim D. Metabolic risk profile and Cancer in Korean men and women. Journal of Preventive Medicine & Public Health. 2016;49(3):143–152. doi: 10.3961/jpmph.16.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sultanik P, Kramer L, Soudan D. The relationship between liver stiffness measurement and outcome in patients with chronic hepatitis C and cirrhosis: a retrospective longitudinal hospital study. Aliment Pharmacol Ther. 2016;44(5):505–513. doi: 10.1111/apt.13722. [DOI] [PubMed] [Google Scholar]
- 23.Nderitu P, Bosco C, Garmo H. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: a study in the Swedish AMORIS cohort. Int J Cancer. 2017. [DOI] [PubMed]
- 24.Simon TG, King LY, Chong DQ. Diabetes, metabolic comorbidities and risk of hepatocellular carcinoma: results from two prospective cohort studies. Hepatology. 2017. [DOI] [PMC free article] [PubMed]
- 25.Yu MW, Lin CL, Liu CJ. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: a large cohort study. Gastroenterology. 2017;153(4):1006. doi: 10.1053/j.gastro.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Welzel TM, Graubard BI, Zeuzem S. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-medicare database. J Hepatol. 2011;54(2):463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratziu V, Bellentani S, Cortez-Pinto H. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Jinjuvadia R, Patel R, et al. Insulin resistance is associated with significant liver fibrosis in chronic hepatitis C patients: a systemic review and Meta-analysis.[J] J Clin Gastroenterol. 2015;50(1):80. doi: 10.1097/MCG.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alberti KGMM, Eckel RH, Grundy SM. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 30.Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of Cancer: a systematic review and meta-analysis[J] Diabetes Care. 2012;35(11):2402. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Wang X, Gong G. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 32.Yuhara H, Steinmaus C, Cohen SE. Is diabetes mellitus an independent risk factor for Colon Cancer and rectal Cancer|[quest] Am J Gastroenterol. 2011;106(11):1911. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peairs KS, Barone BB, Snyder CF. Diabetes mellitus and breast Cancer outcomes: a systematic review and Meta-analysis. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 2011;29(1):40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong GL, Wong VW. Commentary: coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B - authors' reply. Aliment Pharmacol Ther. 2014;39(10):1236. doi: 10.1111/apt.12714. [DOI] [PubMed] [Google Scholar]
- 35.Wang YG, Wang P, Wang B. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9(5):e95485. doi: 10.1371/journal.pone.0095485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baffy G. Editorial: hepatocellular carcinoma in type 2 diabetes: more than meets the eye. Am J Gastroenterol. 2012;107(1):53–55. doi: 10.1038/ajg.2011.390. [DOI] [PubMed] [Google Scholar]
- 37.Salmon D, Bani-Sadr F, Loko MA. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: Results from ANRS CO13 HEPAVIH. J Hepatol. 2012;56(4):862–868. doi: 10.1016/j.jhep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Seshasai SR, Kaptoge S, Thompson A. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.HB E–S, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clinical gastroenterology and hepatology : the official clinical practice. journal of the American Gastroenterological Association. 2006;4(3):369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-All data generated or analysed during this study are included in this published article, and you can find these data in references [1, 6, 9–25].