Abstract
Background
Originating from poultry, particularly chickens, Campylobacter jejuni is the leading foodborne pathogen worldwide and a major cause of campylobacteriosis. Isolating C. jejuni is difficult due to its specific growth requirements, the presence of viable but non-culturable bacteria, and because it is often masked by competing flora. Currently, there is no optimized method for isolating C. jejuni from chicken feces. Here, we evaluated the method for isolating C. jejuni from chicken feces using culture-independent sequence-based metagenomics and culture-dependent tools. Further, we assessed changes in microbial communities during microbe isolation to determine how the process can be improved.
Results
Fourteen different variations of C. jejuni isolation procedures were applied to all 35 chicken fecal samples. These variations included using different enrichment broths (without enrichment or enrichment in Bolton or Preston broth), different ratios of sample-to-enrichment broth (1:101, 1:102, and 1:103), and different selective agars (modified charcoal-cefoperazone-deoxycholate agar (mCCDA) or Preston agar). Enrichment during isolation of C. jejuni was evaluated on the basis of microbial diversity and taxonomic composition using metagenomics tools. The effect of selective media was evaluated using a combination of metagenomics and culture-dependent tools. Microbial diversity significantly decreased during the enrichment process, regardless of the type of enrichment broth, with the most significant decrease observed at a feces-to-broth ratio of 1:103. Particularly, in 103-Preston broth, the relative abundance of Campylobacter increased, while extended-spectrum beta-lactamase-producing Escherichia coli, which interfere with Campylobacter isolation, decreased. Metagenomics results were validated by quantitative PCR and culture-dependent analysis. Additionally, selective media affected the isolation results, although microbes with high relative abundance during enrichment were also frequently isolated using culture-dependent methods. Significantly more C. jejuni was isolated from mCCDA than from Preston agar enriched in 103 Preston broth.
Conclusions
Enrichment in Preston broth at a ratio of 1:103 followed by spreading onto mCCDA was the most effective method for isolating C. jejuni. This is the first study to apply metagenomics to evaluate a method for isolating a targeted microbe, C. jejuni, from chicken feces, a source with high microbial contamination. Thus, metagenomics can be applied to improve methods for isolating bacteria that are difficult to separate.
Electronic supplementary material
The online version of this article (10.1186/s40168-019-0680-z) contains supplementary material, which is available to authorized users.
Keywords: Metagenomics, Microbial community analysis, Isolation method, Campylobacter jejuni
Background
Campylobacter spp., particularly Campylobacter jejuni, is a leading foodborne pathogen, worldwide [1]. Approximately nine million people in the European Union and 1.3 million people in the USA suffer from the Campylobacter-borne disease, and the number of infected people increases each year [2, 3]. A commensal organism that naturally colonizes and is asymptomatic in the gastrointestinal tract of poultry, particularly chickens, C. jejuni is a major cause of human campylobacteriosis [4, 5]. Symptoms of C. jejuni infections in humans include gastro-intestinal distress such as abdominal pain and diarrhea, as well as neurological issues including Guillain-Barre and Miller-Fisher syndrome [6]. Therefore, for the prevention and management of campylobacteriosis, isolation of C. jejuni from chicken should be prioritized. However, unlike other common foodborne pathogens, including pathogenic Escherichia coli and Salmonella spp., C. jejuni is difficult to isolate because of its specific growth conditions (i.e., requires a microaerophilic environment) [7]. The presence of viable but nonculturable (VBNC) C. jejuni and the tendency of C. jejuni to be masked by competing microbes, such as extended-spectrum beta-lactamase (ESBL)-producing E. coli, make it challenging to isolate C. jejuni from chickens [8, 9]. The most common methods for isolating C. jejuni from chicken meat are pre-enrichment (4–5 h, microaerophilic conditions, 37 °C) and enrichment (48 h, microaerophilic conditions, 42 °C) procedures in Bolton broth or Preston broth, followed by selection (48 h, microaerophilic conditions, 42 °C) on modified charcoal-cefoperazone-deoxycholate agar (mCCDA) or Preston agar. However, the prevalence of C. jejuni infections varies between studies, as there is no standard method, and outcomes differ depending on the type of enrichment broth and selective agar used [1, 10]. Additionally, most studies were performed using chicken meat. Because Campylobacter is a commensal flora of the gastrointestinal tract in poultry, it is important to isolate Campylobacter from the farm stage (feces of chicken). However, a method for isolating C. jejuni from chicken feces has not been established, and few studies have been performed [11].
The need for an effective method of isolating C. jejuni from chicken meat and feces has increased. Several studies were conducted comparing currently used methods for isolating C. jejuni and improving isolation by adding several antibiotics, such as polymyxin B and triclosan, to the enrichment broth or selective agar [7–9, 12]. However, most studies were carried out using culture-based techniques, which rely on the isolation results of a specific microbe, such as Campylobacter spp. and ESBL-producing E. coli. Changes in the overall microbial community and composition of specific microbes affecting the isolation of C. jejuni during isolation procedure have not been identified. Additionally, evaluation of the enrichment procedure using culture-based techniques, which greatly affects microbe isolation compared to selective procedures, is not possible [13]. Therefore, to accurately compare isolation methods of C. jejuni, studies based on metagenomics using next-generation sequencing are needed.
Currently, various next-generation sequencing (NGS) systems, such as Roche 454 pyrosequencing, Illumina Genome Analyzer (HiSeq, MiSeq), Applied Biosystems SOLiD System, Life Technologies Ion Torrent, and the PacBio RX system, are available in microbiological research fields [14]. Advances in these NGS technologies have enabled comprehensive analyses in microbiome analysis and whole-genome-sequencing analysis [15, 16]. However, most microbiological studies using these approaches have been conducted on the gut microbiome, which is closely linked to host health status [17–19]. Studies have been conducted to identify specific microorganisms based on sequence analysis but only for disease diagnosis [20]. However, disease diagnosis, alone, is not suitable for epidemiologic investigations or analyzing pathogenic factors, such as susceptibility to antibiotics, serotypes, subtypes, and virulence factors. These studies are possible only if the target microbes are isolated. Application of metagenomics approaches can improve the methods used to isolate specific microbes, particularly pathogens, by evaluating changes in microbial communities during pathogen isolation procedures.
The current study was carried out to evaluate the method for isolating C. jejuni from chicken feces through culture-independent sequence-based metagenomics tools and culture-dependent tools. First, the microbial community change, including alpha diversity, beta diversity, and taxonomic composition of chicken feces during the isolation procedures of C. jejuni, was analyzed for a total of seven procedures with different combinations of enrichment broth (enriched in Bolton or Preston broth or a no-enrichment process) and the ratio of sample-to-enrichment media (1:101, 1:102, and 1:103). Second, the isolation results of C. jejuni and competing colonies in a total of 14 procedures, according to the combinations of the above seven procedures and type of selective media (mCCDA and Preston agar), were compared. Finally, the metagenomics results were validated by comparing the sequencing results to the culture-dependent results.
Results
Campylobacter spp. and competing colonies isolated in each procedure determined using culture-dependent tools
Comparison of the isolation results of C. jejuni according to each isolation method of Campylobacter revealed that among samples not subjected to the enrichment process, 45.7% (16/35) of C. jejuni was isolated from mCCDA and 57.1% (20/35) from Preston agar (Fig. 1). In samples enriched in Bolton broth, C. jejuni was not isolated, regardless of the ratio of sample-to-enrichment broth and types of selective agars. In Preston broth-mCCDA procedures, C. jejuni was not isolated from the 1:101 ratio of sample-to-enrichment broth, while 31.4% (11/35) and 97.1% (34/35) of C. jejuni was isolated at ratios of 1:102 and 1:103, respectively. In the Preston broth-Preston agar procedures, C. jejuni was not isolated from the 1:101 and 1:102 ratios of sample-to-broth, while 5.7% (2/35) of C. jejuni was isolated from the 1:103 ratio. The isolation rate of C. jejuni was significantly higher in the 103-Preston broth-mCCDA procedure than in the other procedures (chi-square test, p < 0.05) and significantly higher in the 102-Preston broth-mCCDA procedure than in the other procedures except for the 103-Preston broth-mCCDA procedure (chi-square test, p < 0.05, Table 1).
Fig. 1.
A schematic diagram of the current study. All chicken fecal samples were analyzed to compare isolation methods of C. jejuni using culture-independent sequence-based metagenomics and culture-dependent tools. Metagenomic analysis was performed to investigate the effects of seven enrichment procedures. Six procedures involved enrichment processes (Bolton and Preston broth at 1:101, 1:102, and 1:103, respectively), and one procedure did not. In culture-dependent analysis, a total of 14 procedures were applied for all samples, which were combinations of different enrichment broths (without enrichment process or enriched in Bolton broth or Preston broth), ratio of sample-to-enrichment broth (1:101, 1:102, or 1:103), and selective media (mCCDA or Preston agar). aThe ratio of fecal sample-to-enrichment broth
Table 1.
Culture-dependent isolation results of Campylobacter jejuni and competing microbes in each procedure
| Enrichment type | No enrichment | Bolton broth | Preston broth | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratio | − | 1:101 | 1:102 | 1:103 | 1:101 | 1:102 | 1:103 | ||||||||
| Selective media | mCCDA | Preston agar | mCCDA | Preston agar | mCCDA | Preston agar | mCCDA | Preston agar | mCCDA | Preston agar | mCCDA | Preston agar | mCCDA | Preston agar | |
| Target and competing microbes | C. jejuni | 48.6% (17/35) | 54.3% (19/35) | − | − | − | − | − | − | − | − | 31.4% (11/35)ac | − | 97.1% (34/35)abc | 5.7% (2/35) |
| ESBLs producing E. coli | 48.6% (17/35)a | 8.6% (3/35) | 80.0% (28/35)ac | − | 97.1% (34/35)ac | 2.9% (1/35) | 82.9% (29/35)ac | 54.3% (19/35)bc | 48.6% (17/35)a | − | 42.9% (15/35)a | − | 11.4% (4/35)b | − | |
| P. mirabilis | 42.9% (15/35) | 88.6% (31/35)a | 45.7% (16/35) | 94.3% (33/35)a | 71.4% (24/35) | 91.4% (32/35) | 62.9% (22/35)c | 82.9% (29/35) | 65.7% (23/35) | 94.3% (33/35) | 54.3% (19/35) | 80.0% (28/35)a | 28.6% (10/35)b | 97.1% (34/35) a | |
| Enterococcus spp. | 22.9% (8/35)a | 2.9% (1/35) | − | − | − | − | − | − | 20.0% (7/35)ac | 2.9% (1/35) | 31.4% (11/35)ac | 2.9% (1/35) | 17.1% (6/35) | 8.6% (3/35) | |
A total of 14 procedures were applied for all 35 fecal samples, which were combinations of different enrichment type (without enrichment process or enriched in Bolton broth or Preston broth), ratio of sample-to-enrichment broth (1:101, 1:102, or 1:103), and selective media (mCCDA or Preston agar)
aSignificant differences according to selective media (enriched in the same enrichment broth and the same ratio of sample-to-broth)
bSignificant differences according to the ratio of sample-to-broth (enriched in the same enrichment broth and same selective media)
cSignificant differences according to the type of enrichment broth (enriched in the same ratio of sample-to-broth and the same type of selective media), −, negative result (0%).
Additionally, ESBL-producing E. coli (all isolated E. coli were identified as ESBL-producing), Proteus mirabilis, and Enterococcus spp. were found in each procedure as colonies competing with C. jejuni (Table 1). For ESBL-producing E. coli, the isolation rate was significantly higher in mCCDA than in Preston agar (same enrichment broth type and ratio of sample-to-enrichment broth) and significantly higher in Bolton broth than in Preston broth (same ratio of sample-to-enrichment broth and selective media type) (chi-square test, p < 0.05, respectively). The isolation rate of ESBL-producing E. coli according to the ratio of sample-to-enrichment broth (in the same type of enrichment broth and selective agar) was significantly higher in 103-Bolton broth/Preston agar and significantly lower in 103-Preston broth/mCCDA (chi-square test, p < 0.05, respectively). For P. mirabilis, the isolation rate was significantly higher in Preston agar than in mCCDA (same enrichment broth type and the ratio of sample-to-enrichment broth) (chi-square test, p < 0.05). Enterococcus spp. was not isolated using the procedures enriched in Bolton broth, and the isolation rate was significantly higher in mCCDA than in Preston agar (same enrichment broth type and the ratio of sample-to-enrichment broth) (chi-square test, p < 0.05).
Proportion of competing colonies and its relationship with isolation result of C. jejuni according to each procedure determined using culture-dependent tools
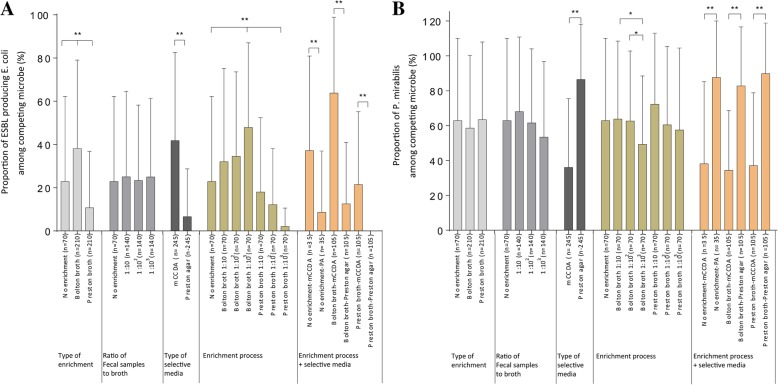
The ratio of the number of each colony to the total number of isolated colonies in the plate except for C. jejuni was used as a criterion for determining the proportion of competing colonies. Comparison of the proportion of each microbe according to enrichment type revealed that the proportions of ESBL-producing E. coli were significantly different (no enrichment process 22.9 ± 39.3%, Bolton broth 38.1 ± 40.9%, Preston broth 10.7 ± 26.1%, one-way ANOVA, p < 0.01), while P. mirabilis was not different (Fig. 2a, b). Comparison of the proportions of each microbe according to the selective media used showed that the proportion of ESBL-producing E. coli (mCCDA 41.8 ± 40.8%, Preston agar 6.9 ± 22.2%, t test, p < 0.01) and P. mirabilis (mCCDA 36.0 ± 39.5%, Preston agar 86.5 ± 31.7%, t test, p < 0.01) was significantly different. Comparison of the proportion of each microbe according to the ratio of sample-to-enrichment broth indicated that the proportion of ESBL-producing E. coli was significantly higher in 103-Bolton broth, while the proportion of P. mirabilis was significantly lower in 103-Bolton broth.
Fig. 2.
Proportion of competing microbes according to isolation procedures of C. jejuni based on culture-dependent tools. Proportion of a ESBL-producing E. coli and b P. mirabilis among competing microbes according to the isolation procedure of C. jejuni (mean ± SEM). Significance was determined by t test and one-way ANOVA. The proportion of ESBL-producing E. coli was significantly different according to the isolation procedure including the type of enrichment broth, selective media, and the combination of different enrichment broths, the ratio of sample-to-enrichment broth, and selective media. The proportion of P. mirabilis was significantly different according to the isolation procedure including the type of selective media and combination of different enrichment broths, the ratio of sample to enrichment broth, and selective agars. *p < 0.05, **p < 0.01
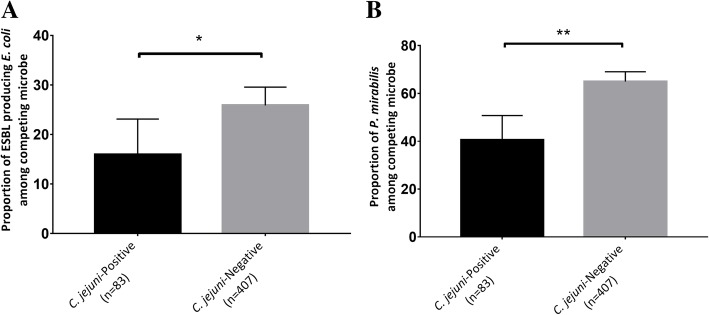
Additionally, the proportion of ESBL-producing E. coli and P. mirabilis significantly differed according to the isolation of C. jejuni in a total of 490 samples by applying 14 methods to each of 35 samples (Fig. 3a, b). In 83 C. jejuni-positive plates and 407 C. jejuni-negative plates, based on culture-dependent tools, the proportions of ESBL-producing E. coli were 15.9 ± 32.8% and 25.9 ± 22.2% ( t test, p < 0.05), respectively, while the proportions of P. mirabilis were 40.5 ± 46.7% and 65.0 ± 42.1%, respectively (t test, p < 0.01).
Fig. 3.
Evaluating the effect of competing flora on the isolation of C. jejuni using culture-dependent tools. The proportion of a ESBL-producing E. coli and b P. mirabilis according to the isolation results of C. jejuni (mean ± SEM). Significance was determined by t test. The proportion of ESBL-producing E. coli and P. mirabilis was significantly higher in C. jejuni-negative fecal samples. *p < 0.05, **p < 0.01
Diversity of microbial communities in chicken feces of each enrichment process determined using metagenomics tools
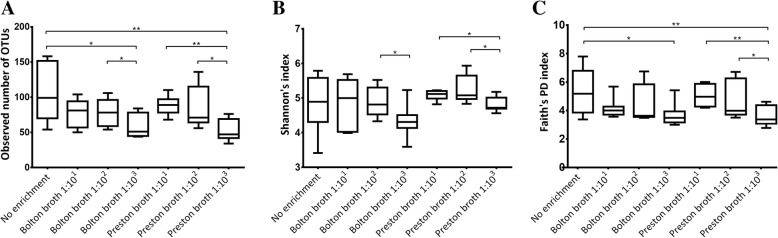
A total of 8,326,000 reads were obtained from 54 selected samples (all seven procedures for seven fecal samples and additional four fecal samples enriched in 103-Preston broth and one additional fecal sample without enrichment) (Additional file 1: Table S1), of which 1,680,254 were valid after being filtered, de-replicated, and de-noised. As enrichment of the fecal samples progressed, the number of operational taxonomic units (OTUs) tended to decrease in the other processes (Fig. 4a). Additionally, the number of OTUs of the fecal samples in the non-enriched process was significantly higher than those in the 103-Bolton (Mann-Whitney U test, p < 0.05) and Preston broth (Mann-Whitney U test, p < 0.01) processes. There was no significant difference in the number of OTUs between each enrichment broth type (same ratio of sample-to-enrichment broth). The Shannon index showed no significant difference between each enrichment broth type (same ratio of sample-to-enrichment broth). However, the Shannon index tended to decrease as the ratio of sample-to-enrichment broth decreased (Fig. 4b), particularly in 103-Bolton and Preston broth (Mann-Whitney U test, p < 0.05 for each). Additionally, there was no difference between each type of enrichment broth in Faith’s phylogenetic diversity, while this value tended to decrease as the ratio of sample-to-enrichment broth decreased (Fig. 4c). Particularly, Faith’s phylogenetic diversity of fecal samples enriched in 103-Preston broth was significantly decreased (Mann-Whitney U test, p < 0.05).
Fig. 4.
Alpha diversity of each seven enrichment procedures using metagenomics tools. a The number of observed operational taxonomic units (OTUs), b Shannon diversity index, and c Faith’s phylogenetic diversity in each procedure (min to max). Significance was determined by Mann-Whitney U test. The number of OTUs, microbial abundance and evenness, and microbial richness of fecal samples significantly decreased in the enrichment process as the ratio of sample-to- enrichment broth decreased. *p < 0.05, **p < 0.01
The microbial communities in fecal samples in each of the seven enrichment processes were compared using a principal coordinates analysis (PCoA) plot based on a weighted UniFrac distance metric and heat map. The chicken feces that had not undergone enrichment, and fecal samples enriched in the Bolton broth process (regardless of the sample- to-broth ratio), were not clustered together. In 101-/102-Preston broth, the enriched fecal samples were not clustered together. However, fecal samples enriched in 103-Preston broth were strongly clustered together (Fig. 5a). In the heat map, as well as the PCoA plot, fecal samples enriched in 103-Preston broth were clustered together, unlike those enriched in Bolton broth or other ratios of sample-to-Preston broth (Fig. 5b). In permutational multivariate analysis (PERMANOVA) and analysis of similarities (ANOSIM) to detect differences between groups, the 103-Preston broth process was significantly different from the other groups (p < 0.001).
Fig. 5.
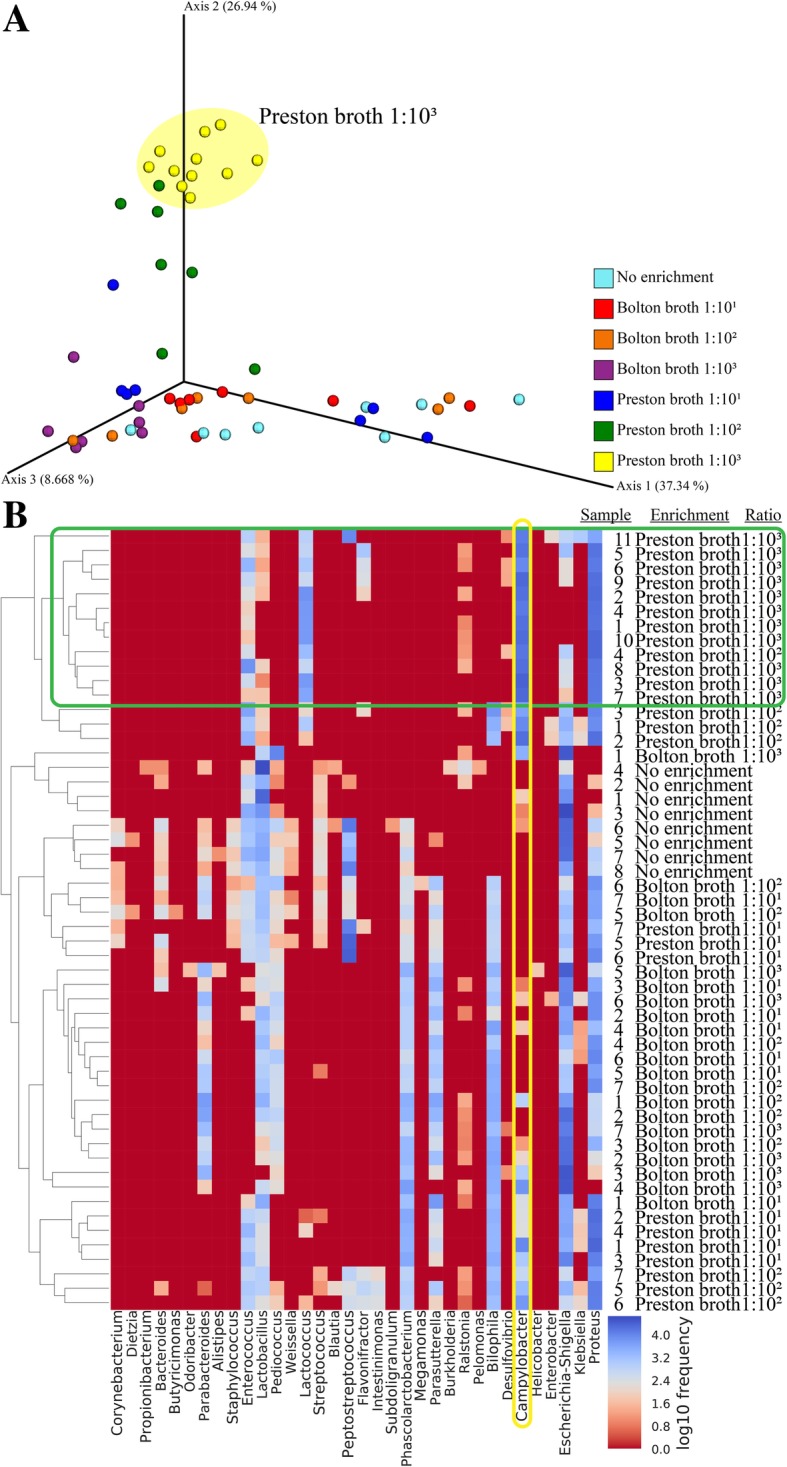
Similarity of the microbial community of fecal samples in each procedure. a Principal coordinates analysis (PCoA) plot of fecal samples (n = 54) in seven procedures based on weighted UniFrac distance metrics and b Heatmap of fecal samples (n = 54) in seven procedures. Fecal samples enriched in Preston broth at 1:103 ratio were clustered together
Composition of core microbial communities in feces of each enrichment process determined using metagenomics tools
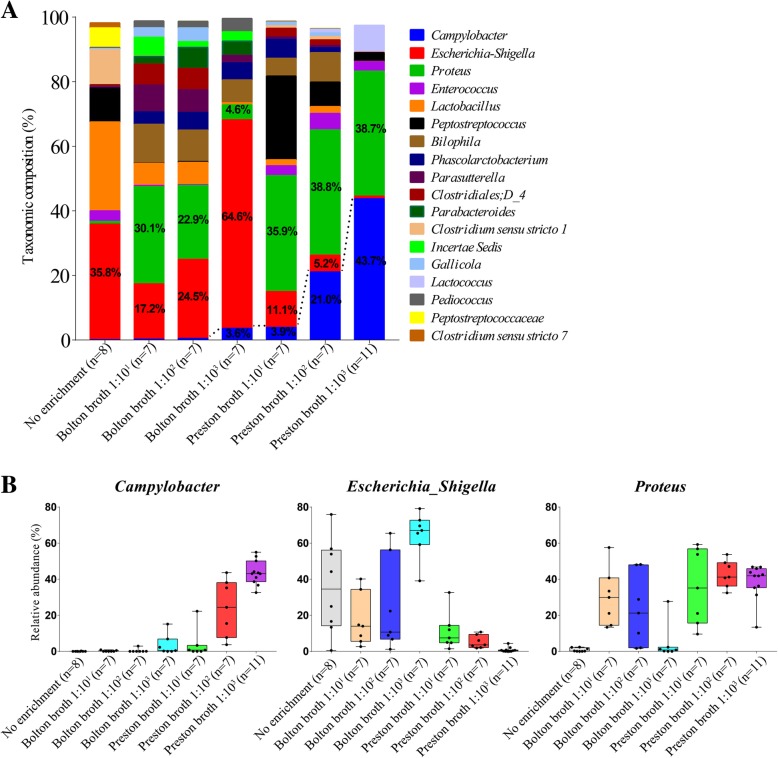
The core taxonomic composition at the genus level (with an average of ≥ 1% in at least one process) is shown in Fig. 6a. At the genus level, 61 genera were identified; among these, 18 genera comprised the core microbiota in all processes and accounted for more than 98% of the total microbiota. In feces without the enrichment process, the predominant core microbiota included Escherichia-Shigella (35.8%), followed by Lactobacillus (27.6%), Clostridium sensu stricto 1 (10.8%), and Peptostreptococcus (10.3%). In the 101-Bolton broth procedure, Proteus (30.1%), followed by Escherichia-Shigella (17.2%) and Bilophila (11.9%), were the predominant core microbiota; in the 102-Bolton broth procedure, Escherichia-Shigella (24.5%) followed by Proteus (22.9%) were the predominant core microbiota; in the 103-Bolton broth process, Escherichia-Shigella (64.6%) was the predominant core microbiota. In the 101-Preston broth process, Proteus (35.9%), followed by Peptostreptococcus (25.9%), and Escherichia-Shigella (11.1%) were the predominant core microbiota; in the 102-Preston broth process, Proteus (38.9%) followed by Campylobacter (21.0%) were the predominant core microbiota; in the 103-Preston broth process, Campylobacter (43.7%), followed by Proteus (38.7%), were the predominant core microbiota.
Fig. 6.
Microbial community of fecal samples in each procedure at the genus level. a Taxonomic composition and b relative abundance (min to max) of Campylobacter, Escherichia, and Proteus of fecal samples in each procedure. The relative abundance of Campylobacter in fecal samples in 103–Preston broth was significantly higher than in other procedures. In the case of Escherichia-Shigella, the relative abundance was reduced, compared to feces, but was still present in large proportions in the process of 101-/102-Bolton broth enrichment. In the case of Proteus, the relative abundance increased during the enrichment process in both enrichment broths, at all ratios of sample-to- broth, with the exception of 103-Bolton broth
Relative abundance of Campylobacter and competing flora in feces of each enrichment process determined using metagenomics tools
The relative abundance of Campylobacter in feces that had not been subjected to the enrichment process and fecal samples enriched in Bolton broth was low, while that in fecal samples enriched in Preston broth tended to increase as the ratio of sample-to-broth decreased (Fig. 6b). In particular, the relative abundance of Campylobacter in fecal samples in 103-Preston broth (43.7%) and 102-Preston broth (21.0%) were significantly higher than in other procedures (Mann-Whitney U test, p < 0.01). The relative abundance of Campylobacter in fecal samples in 103-Preston broth was significantly higher than that in 102-Preston broth (Mann-Whitney U test, p < 0.05). The relative abundance of Escherichia-Shigella in fecal samples in Preston broth was reduced compared to in feces (Fig. 6b). The abundance of these bacteria tended to decrease as the ratio of sample-to-broth decreased during Preston broth enrichment, particularly in 103-Preston broth (Mann-Whitney U test, p < 0.01). However, a substantial increase was observed in fecal samples enriched in 103-Bolton broth (Mann-Whitney U test, p < 0.05) compared to other procedures. In the case of Proteus, the relative abundance was only 0.8% in feces not subjected to the enrichment process, while it was significantly increased in enrichment broth at all ratios of sample-to-broth (Mann-Whitney U test, p < 0.01), except for in 103-Bolton broth (Fig. 6b). The abundance of Proteus in fecal samples in 103-Bolton broth was significantly lower than in other procedures (Mann-Whitney U test, p < 0.01), except for feces that had not been subjected to the enrichment process. The results of linear discriminant analysis effect size (LEfSe) performed to compare 103-Preston broth with other processes (no enrichment and 103-Bolton broth) were also consistent (Additional file 3: Figure S1).
In correlation analysis, Campylobacter was negatively correlated with Proteus (Pearson correlation coefficient (r) − 0.39), and Escherichia-Shigella was also negatively correlated with Proteus (r − 0.82) in Bolton broth, regardless of the ratio of sample-to-broth (Additional file 4: Figure S2A). In Preston broth, regardless of the ratio of sample-to-broth, Campylobacter was negatively correlated with nearly all microbes including Escherichia-Shigella (r − 0.54) (Additional file 4: Figure S2B).
Relationship between competing flora and C. jejuni determined using metagenomics tools and culture-dependent tools
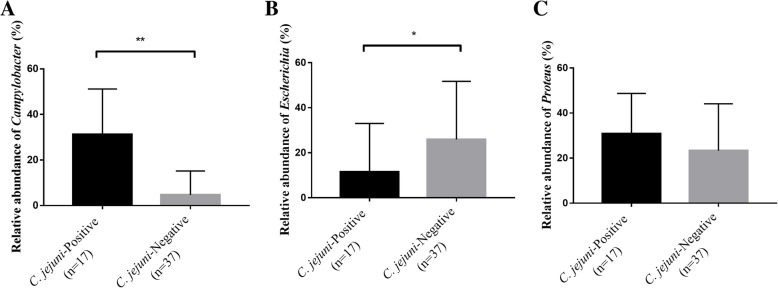
A total of 54 samples analyzed by metagenomics tools were divided into two groups according to the culture results; 17 samples were positive for C. jejuni, and 37 were negative. The relative abundance of Campylobacter was significantly higher in fecal samples from which C. jejuni was detected based on the culture result (t test, p < 0.01, Fig. 7a). However, the relative abundance of Escherichia was significantly lower in fecal samples from which C. jejuni was detected based on the culture result (t test, p < 0.05, Fig. 7b). For Proteus, there was no difference in the relative abundance in the fecal sample according to isolation result of C. jejuni based on the culture result (Fig. 7c).
Fig. 7.
The relative abundance of each microbe according to the isolation results of C. jejuni. The relative abundance of a Campylobacter, b Escherichia, and c Proteus according to the isolation results of C. jejuni based on culture-dependent tools (mean ± SEM). A total of 54 samples analyzed by metagenomics tools were divided into two groups according to the culture results; 17 samples were positive for C. jejuni, and 37 were negative. Significance was determined by t test. The relative abundance of Campylobacter was significantly higher in the fecal sample from which C. jejuni was isolated, while the relative abundance of Escherichia was significantly lower. There was no difference in the relative abundance of Proteus in the fecal sample according to isolation result of C. jejuni. *p < 0.05, **p < 0.01
Validation of metagenomics data using quantitative PCR
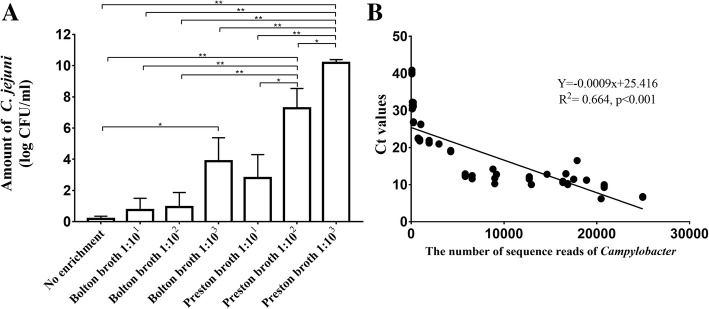
Quantitative PCR (qPCR) of C. jejuni was conducted on all 54 samples to validate the metagenomics data and estimate the levels of C. jejuni in each procedure. First, qPCR was performed using standard strains (NCTC 11168 and ATCC 33560) to confirm the correlation between colony-forming units (CFU) and cycle threshold (Ct) values of C. jejuni (y = − 3.3631x + 44.547, R2 = 0.9118, p < 0.001) (Additional file 5: Figure S3). Next, qPCR was conducted on the DNA used in the current study. The log CFU/ml of C. jejuni in each sample was inferred through the standard curve: 0.2 ± 0.2 (no enrichment, mean ± SEM), 0.7 ± 0.7 (101-Bolton broth), 0.9 ± 0.9 (102-Bolton broth), 3.9 ± 1.5 (103-Bolton broth), 2.8 ± 1.5 (101-Preston broth), 7.3 ± 1.3 (102-Preston broth), and 10.2 ± 0.2 (103-Preston broth) (Fig. 8a). The amount of C. jejuni was significantly higher in fecal samples in 103-Preston broth than in the other procedures (Mann-Whitney U test, p < 0.05). Additionally, the amount of C. jejuni was significantly higher in fecal samples in 102-Preston broth than in the other procedures (Mann-Whitney U test, p < 0.05), except for the 103-Preston/Bolton broth. Furthermore, the correlation between the Ct values from the qPCR results and number of Campylobacter reads from metagenomics results was examined (y = − 0.0009x + 25.416, R2 = 0.664, p < 0.001, Fig. 8b).
Fig. 8.
Quantitative PCR of C. jejuni applied to all 54 samples. a Log CFU/ml of C. jejuni in each procedure (mean ± SEM) inferred from the standard curve and b correlation of metagenomics data and quantitative polymerase chain reaction (qPCR) results. Significance was determined by Mann-Whitney U test. The amount of C. jejuni was significantly higher in fecal samples in 103-Preston broth, 1.0 × 1010.2 ± 0.2, followed by 102-Preston broth (1.0 × 107.3 ± 1.3) and 103-Bolton broth (1.0 × 103.9 ± 1.5). In addition, the cycle threshold (Ct) values from qPCR results and the number of Campylobacter reads from metagenomics results had a high correlation. *p < 0.05, **p < 0.01
Discussion
This is the first study to apply culture-independent sequence-based metagenomics to evaluate C. jejuni isolation methods in chicken feces. Isolation of Campylobacter spp. is difficult because of the specific growth conditions of the bacteria, presence of VBNC bacteria, and masking by competing flora on selective media. Therefore, culture-independent diagnostic tests, which show high sensitivity and rapid diagnosis, were recently applied to detect Campylobacter-infections, particularly by the Centers for Disease Control Foodborne Diseases Active Surveillance Network [21]. However, applying culture-independent diagnostic tests alone without obtaining isolates from culture-dependent methods makes further analyses, such as epidemiological investigation and characterization of strains, impossible. Given this, effective methods for isolating C. jejuni are needed. Numerous studies have been conducted to evaluate and improve the methods of isolating C. jejuni from various food sources using culture-dependent tools [7, 8, 22]. Although food sources are the main cause of human campylobacteriosis, isolation of C. jejuni at the farm level is primarily necessary for managing campylobacteriosis. However, most studies have been carried out using chicken meat with low microbial contamination rather than chicken feces, which is a high microbial contamination source [23, 24]. Therefore, to isolate Campylobacter from feces, a specific method, which differs from that used for food, should be applied. Here, the methods for isolating C. jejuni from chicken feces were evaluated using metagenomics tools and culture-dependent tools.
Based on metagenomics analysis, the current study indicated that feces undergoing an enrichment process in Preston broth at a 1:103 ratio of feces-to-broth were the most effective for isolating C. jejuni. In metagenomics applications to evaluate the isolation method of Campylobacter, microbial diversity and taxonomic composition were calculated. First, the effect of the enrichment process during the isolation of C. jejuni was assessed based on microbial diversity. Based on the alpha diversity, including the number of OTUs, Shannon diversity index which measures species abundance and evenness, and Faith’s phylogenetic diversity index which measures biodiversity, the diversity of the microbial community in the feces was decreased during enrichment compared to fecal samples that were not enriched [25, 26]. The microbial diversity decreased to a greater extent as the ratio of feces-to-broth decreased, particularly when the level of enrichment broth was high compared to the fecal samples. However, at the same ratio of fecal samples-to-each enrichment broth, there was no significant difference in microbial diversity. This suggests that the ratio of feces-to-broth affects microbial diversity, regardless of the type of enrichment broth. This was because during the enrichment process for C. jejuni, only the target microbe selectively grew, and other microbes were inhibited by antibiotics and specific enrichment conditions (microaerophilic conditions and a high incubation temperature of 42 °C). Additionally, the difference in microbial diversity according to the ratio of fecal samples-to-broth may be related to differences in the relative amounts of antibiotics-to-fecal samples. A greater relative amount of antibiotics-to-fecal samples led to greater inhibitory effects of antibiotics on the fecal sample.
Second, the effect of the enrichment process was evaluated based on taxonomic composition. In non-enriched feces, Escherichia-Shigella and Lactobacillus were the most abundant genera. This is similar to results of previous studies showing that Lactobacillus (14.9%) and Escherichia-Shigella (9.0%) are the most abundant bacteria in feces from healthy chickens [27]. However, the original taxonomic composition in the feces changed dramatically during the enrichment process. The relative abundance of Campylobacter (identified as C. jejuni at the species level using metagenomics and culture-based tools) was significantly higher in Preston broth, especially in 102- and 103-Preston broth, than in Bolton broth. In Preston broth, the relative abundance of Campylobacter increased as the ratio of feces-to-broth decreased from 1:101 to 1:103. Furthermore, qPCR was performed to validate the metagenomics data, which varied depending on the type of sequencer used, type of database, and DNA extraction method [14, 28, 29]. The number of reads of C. jejuni from metagenomics data and qPCR results of C. jejuni was highly correlated, suggesting that the metagenomics data in this study was reliable, and the amount of C. jejuni in each enrichment process was deduced. The amount of C. jejuni was high in fecal samples in 103-Preston broth at 1.0 × 1010.2 ± 0.2. This was similar to that for C. jejuni in brain heart infusion broth at 48 h (1.0 × 108–1.0 × 109) [30]. The negative qPCR results for C. jejuni may be due to DNA contamination from other bacteria, or that transcription was below the detection limit.
Differences in the levels of C. jejuni, according to enrichment procedures, were observed because of competing flora, particularly ESBL-producing E. coli, which were influenced by the type of antibiotics in each enrichment broth and by the relative amount of antibiotics-to-feces in each ratio of broth. In general, the amount of background flora affects the isolation result of C. jejuni, often producing false-negative results [31]. Comparison of the two enrichment broth types revealed that polymyxin B effectively controlled ESBL-producing E. coli; this antibiotic is present in Preston broth but not in Bolton broth [9]. Recent studies also showed that ESBL-producing E. coli is poorly resistant to the antibiotics cefoperazone, vancomycin, and trimethoprim, which are present in Bolton broth [8]. Additionally, the growth of C. jejuni in co-cultures of C. jejuni and ESBL-producing E.coli was significantly lower than in the single culture of C. jejuni, indicating that ESBL-producing E. coli acts as a competitive inhibitor leading to a reduction of C. jejuni levels [32]. In our study, the relative abundance of Escherichia (identified as ESBL-producing E. coli using culture-based tools), which was highest in non-enriched feces, showed a more substantial decrease in Preston broth than in Bolton broth at the same ratio of fecal samples-to-enrichment broth and tended to decrease as the ratio of feces-to-Preston broth decreased. Campylobacter was negatively correlated with Escherichia when fecal samples were enriched in Preston broth. Therefore, in the enrichment process of Campylobacter, Preston broth more effectively inhibited ESBL-producing E. coli compared to Bolton broth. Furthermore, a comparison of the ratio of fecal samples-to-enrichment broth of 1:101 to 1:103 in Preston broth revealed that the differences depended on the amount of antibiotics used. The ratio of sample-to-enrichment broth of 1:101, which is the most commonly used ratio for isolating Campylobacter from food sources, did not effectively enrich target microbes in the fecal samples. This means that antibiotics in the enrichment broth may not work effectively in the enrichment process unless the ratio of feces-to-enrichment broth is optimized. Therefore, the relative amount of antibiotics should be high in order to inhibit competing microbes when isolating C. jejuni from feces with higher microbial contamination than in food. Moreover, fecal samples enriched in 103-Preston broth were strongly clustered together in the PCoA plot and heat map, while fecal samples in the other procedures were not clustered with each other. These results suggest that 103-Preston broth has the most constant effect on fecal samples during the enrichment process, including decreasing microbial diversity and changing the microbial composition.
P. mirabilis was a major competing microbe observed during the process of isolating Campylobacter in previous studies [33]. The current study also showed that the relative abundance of Proteus (identified as P. mirabilis at the species level using metagenomics tools and culture-based tools) in the enrichment process increased, compared to fecal samples that were not enriched. This may be because of their high ability to differentiate and survive [34, 35]. However, the relative abundance of Proteus did not affect the relative abundance of Campylobacter, unlike ESBL-producing E. coli. Instead, the relative abundance of Proteus was high in Preston broth at 1:102 and 1:103 ratios, but low in Bolton broth at a 1:103 ratio. This was most likely due to resistance to polymyxin B in Preston broth, but susceptibility to vancomycin in Bolton broth [36]. We also predict that competition occurred between Proteus and ESBL-producing E. coli based on the negative correlation between these two microbes in Bolton broth, although further studies are needed to confirm this.
Analysis of C. jejuni isolation procedures using metagenomics was confirmed using culture-dependent tools. Campylobacter, Escherichia-Shigella, and Proteus sequence reads based on metagenomics tools matched those of isolates obtained by culture-dependent tools. Campylobacter and Proteus were identified as C. jejuni and P. mirabilis at the species level, respectively, and only C. jejuni and P. mirabilis, without other species, were isolated based on culture-dependent tools. For Escherichia-Shigella at the genus level, although species-level identification was not possible, only E. coli was isolated, all of which were identified as ESBL-producing E. coli using culture-dependent methods. The microbes that, using metagenomics, were identified to be present at a relatively higher abundance in each enrichment process were also frequently isolated using culture-dependent methods, regardless of the type of selective media used. The isolation rates of C. jejuni, ESBL-producing E. coli, and P. mirabilis were significantly higher in enriched fecal samples, with a higher relative abundance of all three. Additionally, the inhibitory effect of competing flora in the isolation of C. jejuni, which was determined using metagenomics tools, was confirmed using culture-dependent tools; the proportion of ESBL-producing E. coli and P. mirabilis was significantly higher in C. jejuni-negative fecal samples than in C. jejuni-positive fecal samples. The consistency between the culture-based results and metagenomics results suggests that our evaluation of the isolation method using metagenomics tools was reliable.
The effect of selective media was evaluated based on the use of culture-dependent tools. Using metagenomics alone, only the effects of the enrichment process on the isolation of Campylobacter can be evaluated; however, using a combination of metagenomics and culture-dependent tools enabled evaluation of the enrichment broth and selective media in isolating Campylobacter. Generally, the enrichment step had a greater effect on the isolation of microbes than did the selective media [13]. However, we found that selective media significantly affected target microbe isolation as well. Even if the fecal sample was subjected to the same enrichment process (same type of enrichment broth and ratio of feces-to-enrichment broth), the isolation rate and proportion of microbes differed depending on the selective media used. ESBL-producing E. coli was isolated from mCCDA significantly more frequently than from Preston agar, while P. mirabilis isolation was greater from Preston agar than from mCCDA. This was most likely due to the antibiotics present in the selective media. The antibiotics in mCCDA were similar those in Bolton broth, while the antibiotics in Preston agar were consistent with those in Preston broth. Because of this, Preston agar and broth showed similar inhibitory effects on ESBLs, while mCCDA inhibited Proteus as in Bolton broth. Particularly, Preston agar, which does not inhibit P. mirabilis, made it difficult to isolate C. jejuni because of the swarming differentiation characteristics of P. mirabilis, which colonized the entire plate surface causing a masking phenomenon [37]. Additionally, in the enrichment process in 102- and 103-Preston broth, while the same type of enrichment broth was used, the isolation rate of C. jejuni was significantly higher in Preston agar than in mCCDA. This may be due to a cross-selective inhibition effect of antibiotics in enrichment broth and selective media. The relative abundance of ESBL-producing E. coli decreased during the enrichment process in Preston broth, and P. mirabilis was inhibited in mCCDA, facilitating the isolation of C. jejuni.
Conclusions
This is the first study to apply culture-independent sequence-based metagenomics and culture-dependent tools to evaluate C. jejuni isolation methods from feces, a high-level microbial matrix. The application of metagenomics revealed changes in the microbial community during the microbial isolation procedure, which cannot be detected using previous culture-based analyses. Particularly, changes in the target microbe and competing flora during the enrichment process, and their correlation, were observed. Our results suggest that (1) Preston broth is more effective for isolating C. jejuni from chicken feces compared to Bolton broth, which is widely used to isolate this bacteria from chicken meat; (2) the effectiveness of the enrichment process depends on the ratio of samples to enrichment broth, even in enrichment broth containing effective antibiotics; (3) when isolating microorganisms, although the type and ratio of enrichment broth should be considered, the type of selective agars is also essential. Taken together, we optimized the method for isolating C. jejuni from chicken feces, enriched in Preston broth at a ratio of 1:103 (sample-to-broth) followed by spreading onto mCCDA. This procedure effectively enriched C. jejuni and controlled competing flora, particularly ESBL-producing E. coli and P. mirabilis. This isolation method aids in obtaining Campylobacter isolates effectively, enabling further analyses, which include epidemiological investigation and molecular characterization of strains. This study provides a new perspective and possibilities for applying metagenomics in microbiological research, such as improving diagnostic methods for fastidious bacteria that are difficult to isolate.
Methods
Sampling of chicken feces and applying different isolation methods of C. jejuni
In December 2017, 35 fecal samples from the gastrointestinal tract of chickens at a slaughterhouse were collected. All fecal samples were analyzed to compare isolation methods of C. jejuni (Fig. 1 and Additional file 1: Table S1). A total of 14 procedures were applied for all samples, which were combinations of different enrichment broths (without enrichment process or enriched in Bolton broth or Preston broth), ratio of sample-to-enrichment broth (1:101, 1:102, or 1:103), and selective media (mCCDA or Preston agar). First, one loop (10 μL) of all 35 fecal samples was directly spread onto two different types of selective media, mCCDA (Oxoid Ltd., Hampshire, UK) with a CCDA selective supplement (Oxoid Ltd.) and Preston agar (Oxoid Ltd.) with a Preston Campylobacter selective supplement (Oxoid Ltd.) and lysed horse blood (Oxoid Ltd.) without enrichment. The selective media were microaerobically incubated at 42 °C for 48 h. Second, all 35 fecal samples were enriched in Bolton broth (Oxoid Ltd.) with a Bolton broth selective supplement (Oxoid Ltd.) at different ratios of sample-to-enrichment broth (1:10, 1:102, and 1:103, respectively). Next, the enrichment broths were micro-aerobically incubated at 42 °C for 48 h, followed by plating onto mCCDA and Preston agar at 42 °C for 48 h under microaerobic conditions. Third, all 35 fecal samples were enriched in Preston broth (Oxoid Ltd.) with a Preston broth selective supplement (Oxoid Ltd, Hampshire, UK) at different ratios of sample-to-enrichment broth (1:10, 1:102, and 1:103, respectively). The enrichment broths were microaerobically incubated at 42 °C for 48 h, followed by plating onto mCCDA and Preston agar at 42 °C for 48 h microaerobically.
Identification of bacterial colonies obtained from culture-dependent tools
In 14 different procedures, at least four colonies suspected as Campylobacter spp. were transferred to Müller-Hinton agar (MHA, Oxoid Ltd.) and microaerobically incubated at 42 °C for 48 h. Next, C. jejuni was confirmed by PCR for each colony (Additional file 2: Table S2) [38]. For colonies that did not show typical morphology of Campylobacter spp., an average of 2.47 (range 1–9) colonies with different shapes was transferred to MHA, according to the proportion of colonies constituting the selective agar. The agars were incubated at 42 °C for 48 h under microaerobic conditions. Colonies suspected to be Escherichia coli and Enterococcus faecium/faecalis were confirmed by PCR (Additional file 2: Table 2) [39, 40]. For colonies identified as E. coli, an antibiotic resistance test was performed according to the Clinical and Laboratory Standards Institute guidelines to identify ESBL-producing E. coli. Additionally, colonies not confirmed by PCR (C. jejuni, E. coli, E. faecium, E. faecalis) were identified by 16 s rRNA sequencing using the universal 16S rRNA primers 518F and 805R [41].
DNA extraction and Illumina MiSeq sequencing
Microbial community analysis of feces and enriched fecal samples was performed to investigate the effects of seven enrichment procedures using different types of enrichment broth and ratios of sample-to-enrichment broth. Six procedures involved enrichment processes (Bolton and Preston broth at 1:101, 1:102, and 1:103, respectively), and one procedure did not. At least seven samples per each procedure were selected according to the selection criteria (isolation result of C. jejuni based on the culture-dependent tool), and a total of 54 samples (8 feces and 46 enriched fecal samples) were examined in microbial community analysis (Additional file 1: Table S1). Metagenomic DNA was extracted using a FastDNA SPIN extraction kit (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. DNA amounts and quality were quantified using PicoGreen (Thermo Scientific, Waltham, MA, USA) and a Nanodrop spectrometer (Thermo Scientific). Bacterial DNA amplification was carried out, targeting the V3–V4 regions of the 16S rRNA gene (Additional file 2: Table S2) [42]. Next, to add multiplexing indices and the sequencing adapter, a subsequent amplification was performed. The amplified products were pooled and normalized using PicoGreen. Sequencing was conducted at Macrogen, Inc. (Seoul, Korea), using an Illumina MiSeq (Illumina, San Diego, CA, USA).
Processing of sequencing analysis
Bioinformatic analysis was performed using QIIME2 software (version 2018.2) pipeline [43]. Raw sequence reads were filtered, de-replicated, and de-noised using DADA2’s recommended parameters [44]. Phylogenetic diversity analysis was performed using MAFFT, and a phylogenetic tree was created using the midpoint rooting method with the FastTree plugin [45, 46]. Alpha diversity was measured in QIIME2 with a subsampling depth of 12,001 sequences, and beta diversity was measured in QIIME2 based on the weighted UniFrac distance [47, 48]. Taxonomy assignments were conducted using the SILVA database version 119 [29]. Pearson’s correlation coefficient was calculated in MicrobiomeAnalyst, and a correlation matrix plot was generated using R version 3.5.0 [49]. To identify the bacterial taxa that are differentially abundant in each group’s microbial community, LEfSe analysis was performed using the online Galaxy interface (http://huttenhower.sph.harvard.edu/galaxy/), and the logarithmic linear discriminant analysis score cutoff was set to 2.0.
Quantitative PCR of C. jejuni
Quantitative PCR of C. jejuni was performed using DNA extracted from ATTC 33560 and NCTC 11168 (DNA concentration was 0.0000237–237 ng/μL) and 54 DNA samples subjected to microbial community analysis. hipO was selected as a target gene for distinguishing C. jejuni from other microbes. Primers and probes for qPCR were used with slight modifications from previous studies (Additional file 1: Table S1) [50]. All reactions were performed in a total volume of 25 μL containing 5 μL of DNA, 2.5 μL of primers (0.8 μM of each primer), 0.5 μL of probe (0.2 μM), 12.5 μL of 2x TaqMan Universal Master Mix II (Life Technologies, Carlsbad, CA, USA), and 4.5 μL of distilled water. The amplification conditions were 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s and 58 °C for 1 min. All reactions were run in triplicate using an ABI PRISM 7500 fast real-time PCR system (Life Technologies).
Statistical analysis
Chi-squared test was performed to compare the isolation rates of C. jejuni and competing colonies between procedures. One-way ANOVA and unpaired t test were performed to compare the proportion of competing colonies grown on the plates, for each isolation procedure. In addition, the Mann-Whitney U test was performed to compare microbial communities, including the microbial diversity and taxonomic composition of bacteria in fecal samples, according to enrichment procedures. All statistical analyses were performed in SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) and a p value of < 0.05 was accepted to indicate statistical significance. Additionally, regression analysis was performed using SPSS to correlate and validate the microbiota data. PERMANOVA and ANOSIM were performed to evaluate similarity among groups (based on weighted UniFrac distance).
Additional files
Table S1. Information of samples and isolation results of C. jejuni. CJ: positive for C. jejuni in culture-based results, Blank: negative for C. jejuni in culture-based results. Gray color: samples used for microbial community analysis. A total of 54 samples (at least seven samples per process) were used for microbial community analysis.
Table S2. Primer list for polymerase chain reaction (PCR), quantitative PCR, and bacterial DNA amplification in this study. (DOCX 16 kb)
Figure S1. Bacterial taxa that are differentially abundant in the microbial community of fecal samples in each procedure. A) Linear discriminant analysis effect size (LEfSe) and B) taxonomic cladogram between fecal samples enriched in Preston broth at the 1:103 ratio and not undergoing the enrichment process. C) LEfSe and D) taxonomic cladogram between fecal samples enriched in Preston broth and Bolton broth at the 1:103 ratio. The logarithmic linear discriminant analysis score cutoff was set to 2.0. The relative abundance of Campylobacter in fecal samples in 103-Preston broth was significantly higher than in other procedures, while the relative abundance of Escherichia-Shigella was significantly lower than in other procedures. (TIF 935 kb)
Figure S2. Relationship between microorganisms in microbial community of fecal samples. Correlation plot in A) Bolton broth and B) Preston broth regardless of the ratio of sample- to-enrichment broth. Campylobacter was negatively correlated with Proteus in Bolton broth, while Campylobacter was negatively correlated with Escherichia-Shigella in Preston broth. (TIF 711 kb)
Figure S3. The correlation between colony forming-units and cycle threshold (Ct) values of C. jejuni standard strains (NCTC 11168 and ATCC 33560). (TIF 155 kb)
Acknowledgements
Not applicable.
Funding
This research was supported by a grant from National Research Foundation of Korea Grant funded by the Korean Government (NRF-2018R1A2B6002396) and a grant from the Ministry of Food and Drug Safety (16162MFDS029).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the SRA repository under Project Accession ID of PRJNA 503089.
Abbreviations
- ANOSIM
Analysis of similarities
- CFU
Colony-forming units
- Ct
Cycle threshold
- ESBL
Extended-spectrum beta-lactamase
- LEfSe
Linear discriminant analysis effect size
- mCCDA
Modified charcoal-cefoperazone-deoxycholate agar
- OTU
Operational taxonomic units
- PCoA
Principal coordinates analysis
- PCR
Polymerase chain reaction
- PERMANOVA
Permutational multivariate analysis
- qPCR
Quantitative PCR
- r
Pearson correlation coefficient
- VBNC
Viable but nonculturable
Authors’ contributions
SC conceived and designed the study. JHG, HS, JK, SR, and BJ performed sampling and prepared the manuscript. SHM and JUA analyzed the sequencing data. JK was a major contributor, both in experiments and writing the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Junhyung Kim, Email: tpkimjh@snu.ac.kr.
Jae-Ho Guk, Email: gukjh@snu.ac.kr.
Seung-Hyun Mun, Email: nicemuns@snu.ac.kr.
Jae-Uk An, Email: nsunshine@snu.ac.kr.
Hyokeun Song, Email: hsongmd@snu.ac.kr.
Jinshil Kim, Email: jinsilk1130@naver.com.
Sangryeol Ryu, Email: sangryu@snu.ac.kr.
Byeonghwa Jeon, Email: bjeon@ualberta.ca.
Seongbeom Cho, Email: chose@snu.ac.kr.
References
- 1.Silva J, Leite D, Fernandes M, Mena C, Gibbs PA, Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazards EPB. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011;9(4):2105. doi: 10.2903/j.efsa.2011.2105. [DOI] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dearlove BL, Cody AJ, Pascoe B, Méric G, Wilson DJ, Sheppard SK. Rapid host switching in generalist Campylobacter strains erodes the signal for tracing human infections. ISME J. 2016;10(3):721. doi: 10.1038/ismej.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skarp C, Hänninen M-L, Rautelin H. Campylobacteriosis: the role of poultry meat. Clin Microbiol Infect. 2016;22(2):103–109. doi: 10.1016/j.cmi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Ang C, De Klerk M, Endtz H, Jacobs B, Laman J, Van Der Meche F, Van Doorn P. Guillain-Barré syndrome-and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-GM1 and anti-GQ1b antibodies in rabbits. Infect Immun. 2001;69(4):2462–2469. doi: 10.1128/IAI.69.4.2462-2469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugarte-Ruiz M, Gómez-Barrero S, Porrero M, Alvarez J, Garcia M, Comeron M, Wassenaar T, Dominguez L. Evaluation of four protocols for the detection and isolation of thermophilic Campylobacter from different matrices. J Appl Microbiol. 2012;113(1):200–208. doi: 10.1111/j.1365-2672.2012.05323.x. [DOI] [PubMed] [Google Scholar]
- 8.Chon J-W, Kim Y-J, Kim H-S, Kim D-H, Kim H, Song K-Y, Seo K-H. Supplementation of Bolton broth with triclosan improves detection of Campylobacter jejuni and Campylobacter coli in chicken carcass rinse. Int J Food Microbiol. 2014;181:37–39. doi: 10.1016/j.ijfoodmicro.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Chon J-W, Hyeon J-Y, Yim J-H, Kim J-H, Song K-Y, Seo K-H. Improvement of modified charcoal-cefoperazone-deoxycholate agar by supplementation with a high concentration of polymyxin B for detection of Campylobacter jejuni and C. coli in chicken carcass rinses. Appl Environ Microbiol. 2012;78(5):1624–1626. doi: 10.1128/AEM.07180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adzitey F, Huda N, Rahmat Ali GR. Prevalence and antibiotic resistance of Campylobacter, Salmonella, and L. monocytogenes in ducks: a review. Foodborne Pathog Dis. 2012;9(6):498–505. doi: 10.1089/fpd.2011.1109. [DOI] [PubMed] [Google Scholar]
- 11.Keramas G, Bang DD, Lund M, Madsen M, Bunkenborg H, Telleman P, Christensen CBV. Use of culture, PCR analysis, and DNA microarrays for detection of Campylobacter jejuni and Campylobacter coli from chicken feces. J Clin Microbiol. 2004;42(9):3985–3991. doi: 10.1128/JCM.42.9.3985-3991.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylis C, MacPhee S, Martin K, Humphrey T, Betts R. Comparison of three enrichment media for the isolation of Campylobacter spp. from foods. J Appl Microbiol. 2000;89(5):884–891. doi: 10.1046/j.1365-2672.2000.01203.x. [DOI] [PubMed] [Google Scholar]
- 13.Chon JW, Kim YJ, Kim YJ, Jung JY, Bae D, Khan S, Seo KH, Sung K. Addition of rifampicin to Bolton broth to inhibit extended-spectrum β-lactamase-producing Escherichia coli for the detection of Campylobacter. J Ffood Sci. 2017;82(7):1688–1692. doi: 10.1111/1750-3841.13761. [DOI] [PubMed] [Google Scholar]
- 14.Quail MA, Smith M, Coupland P, Otto TD, Harris SR, Connor TR, Bertoni A, Swerdlow HP, Gu Y. A tale of three next generation sequencing platforms: comparison of Ion Torrent, Pacific Biosciences and Illumina MiSeq sequencers. BMC Genomics. 2012;13(1):341. doi: 10.1186/1471-2164-13-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargis AS, Kalman L, Lubin IM. Assuring the quality of next-generation sequencing in clinical microbiology and public health laboratories. J Clin Microbiol. 2016;54(12):2857–2865. doi: 10.1128/JCM.00949-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Kim W, An J-U, Suh JG, Seong JK, Jeon B-Y, Cho S. Complete genome sequencing and comparative genomic analysis of Helicobacter apodemus isolated from the wild Korean striped field mouse (Apodemus agrarius) for potential pathogenicity. Front Pharmacol. 2018;9:838. doi: 10.3389/fphar.2018.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, An J-U, Kim W, Lee S, Cho S. Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the Illumina MiSeq platform. Gut Pathog. 2017;9(1):68. doi: 10.1186/s13099-017-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong JC, McCallum N, Sintchenko V, Howden BP. Whole genome sequencing in clinical and public health microbiology. Pathology. 2015;47(3):199–210. doi: 10.1097/PAT.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Jennifer Y., Henao Olga L., Griffin Patricia M., Vugia Duc J., Cronquist Alicia B., Hurd Sharon, Tobin-D’Angelo Melissa, Ryan Patricia, Smith Kirk, Lathrop Sarah, Zansky Shelley, Cieslak Paul R., Dunn John, Holt Kristin G., Wolpert Beverly J., Patrick Mary E. Infection with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2012–2015. MMWR. Morbidity and Mortality Weekly Report. 2016;65(14):368–371. doi: 10.15585/mmwr.mm6514a2. [DOI] [PubMed] [Google Scholar]
- 22.Chon J-W, Kim H, Yim J-H, Park J-H, Kim M-S, Seo K-H. Development of a selective enrichment broth supplemented with bacteriological charcoal and a high concentration of polymyxin B for the detection of Campylobacter jejuni and Campylobacter coli in chicken carcass rinses. Int J food Microbiol. 2013;162(3):308–310. doi: 10.1016/j.ijfoodmicro.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers J, Clifton-Hadley F, Marin C, Vidal A. An evaluation of survival and detection of Campylobacter jejuni and C. coli in broiler caecal contents using culture-based methods. J Appl Microbiol. 2010;109(4):1244–1252. doi: 10.1111/j.1365-2672.2010.04748.x. [DOI] [PubMed] [Google Scholar]
- 24.Berndtson E, Danielsson-Tham M-L, Engvall A. Campylobacter incidence on a chicken farm and the spread of Campylobacter during the slaughter process. Int J Food Microbiol. 1996;32(1-2):35–47. doi: 10.1016/0168-1605(96)01102-6. [DOI] [PubMed] [Google Scholar]
- 25.Spellerberg IF, Fedor PJ. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’Index. Global Ecol Biogeogr. 2003;12(3):177–179. doi: 10.1046/j.1466-822X.2003.00015.x. [DOI] [Google Scholar]
- 26.Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conservation. 1992;61(1):1–10. doi: 10.1016/0006-3207(92)91201-3. [DOI] [Google Scholar]
- 27.Yan W, Sun C, Yuan J, Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci Rep. 2017;7:45308. doi: 10.1038/srep45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner Mackenzie B, Waite DW, Taylor MW. Evaluating variation in human gut microbiota profiles due to DNA extraction method and inter-subject differences. Front Microbiol. 2015;6:130. doi: 10.3389/fmicb.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41(D1):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright JA, Grant AJ, Hurd D, Harrison M, Guccione EJ, Kelly DJ, Maskell DJ. Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary-phase physiological switch. Microbiology. 2009;155(1):80–94. doi: 10.1099/mic.0.021790-0. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki W, Taguchi M, Kawai T, Kawatsu K, Sakata J, Inoue K, Misawa N. Comparison of loop-mediated isothermal amplification assay and conventional culture methods for detection of Campylobacter jejuni and Campylobacter coli in naturally contaminated chicken meat samples. Appl Environ Microbiol. 2009;75(6):1597–1603. doi: 10.1128/AEM.02004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazeleger WC, Jacobs-Reitsma WF, den Besten HM. Quantification of growth of Campylobacter and extended spectrum β-lactamase producing bacteria sheds light on black box of enrichment procedures. Front Microbiol. 2016;7:1430. doi: 10.3389/fmicb.2016.01430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaz C, Voss-Rech D, Pozza J, Coldebella A, Silva V. Isolation of Campylobacter from Brazilian broiler flocks using different culturing procedures. Poultry Sci. 2014;93(11):2887–2892. doi: 10.3382/ps.2014-03943. [DOI] [PubMed] [Google Scholar]
- 34.Budding A, Ingham C, Bitter W, Vandenbroucke-Grauls C, Schneeberger P. The Dienes phenomenon: competition and territoriality in swarming Proteus mirabilis. J Bacteriol. 2009;191(12):3892–3900. doi: 10.1128/JB.00975-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justice SS, Hunstad DA, Cegelski L, Hultgren SJ. Morphological plasticity as a bacterial survival strategy. Nat Rev Microbiol. 2008;6(2):162. doi: 10.1038/nrmicro1820. [DOI] [PubMed] [Google Scholar]
- 36.Jasson V, Sampers I, Botteldoorn N, López-Gálvez F, Baert L, Denayer S, Rajkovic A, Habib I, De Zutter L, Debevere J. Characterization of Escherichia coli from raw poultry in Belgium and impact on the detection of Campylobacter jejuni using Bolton broth. Inter J Food Microbiol. 2009;135(3):248–253. doi: 10.1016/j.ijfoodmicro.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Armbruster CE, Mobley HL. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol. 2012;10(11):743. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang G, Clark CG, Taylor TM, Pucknell C, Barton C, Price L, Woodward DL, Rodgers FG. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J Clin Microbiol. 2002;40(12):4744–4747. doi: 10.1128/JCM.40.12.4744-4747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cenci Goga BT, Aquilanti L, Osimani A, Miraglia D, Aloisio F. Identification with multiplex PCR assay of Enterococcus species isolated from dairy products in Umbria, Italy. Vet Res Commun. 2003;27(1):671–674. doi: 10.1023/B:VERC.0000014244.68558.55. [DOI] [PubMed] [Google Scholar]
- 40.Emami NK, Daneshmand A, Naeini SZ, Graystone E, Broom L. Effects of commercial organic acid blends on male broilers challenged with E. coli K88: Performance, microbiology, intestinal morphology, and immune response. Poultry Sci. 2017;96(9):3254–3263. doi: 10.3382/ps/pex106. [DOI] [PubMed] [Google Scholar]
- 41.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh K, Misawa K, Ki K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PloS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lozupone C, Hamady M, Knight R. UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7(1):371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45(W1):W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toplak N, Kovač M, Piskernik S, Možina SS, Jeršek B. Detection and quantification of Campylobacter jejuni and Campylobacter coli using real-time multiplex PCR. J Appl Microbiol. 2012;112(4):752–764. doi: 10.1111/j.1365-2672.2012.05235.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Information of samples and isolation results of C. jejuni. CJ: positive for C. jejuni in culture-based results, Blank: negative for C. jejuni in culture-based results. Gray color: samples used for microbial community analysis. A total of 54 samples (at least seven samples per process) were used for microbial community analysis.
Table S2. Primer list for polymerase chain reaction (PCR), quantitative PCR, and bacterial DNA amplification in this study. (DOCX 16 kb)
Figure S1. Bacterial taxa that are differentially abundant in the microbial community of fecal samples in each procedure. A) Linear discriminant analysis effect size (LEfSe) and B) taxonomic cladogram between fecal samples enriched in Preston broth at the 1:103 ratio and not undergoing the enrichment process. C) LEfSe and D) taxonomic cladogram between fecal samples enriched in Preston broth and Bolton broth at the 1:103 ratio. The logarithmic linear discriminant analysis score cutoff was set to 2.0. The relative abundance of Campylobacter in fecal samples in 103-Preston broth was significantly higher than in other procedures, while the relative abundance of Escherichia-Shigella was significantly lower than in other procedures. (TIF 935 kb)
Figure S2. Relationship between microorganisms in microbial community of fecal samples. Correlation plot in A) Bolton broth and B) Preston broth regardless of the ratio of sample- to-enrichment broth. Campylobacter was negatively correlated with Proteus in Bolton broth, while Campylobacter was negatively correlated with Escherichia-Shigella in Preston broth. (TIF 711 kb)
Figure S3. The correlation between colony forming-units and cycle threshold (Ct) values of C. jejuni standard strains (NCTC 11168 and ATCC 33560). (TIF 155 kb)
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the SRA repository under Project Accession ID of PRJNA 503089.