Patients with SLE experience multiple, varied symptoms and laboratory abnormalities that occur in different combinations, at different points in time.1 A result of the heterogeneity is that studies on SLE employ several, sometimes conflicting, definitions of the illness. Investigators disagree about when SLE begins; how SLE relates to similar and overlapping rheumatic illnesses; whether SLE-like illnesses of known genetic causes count as SLE; and whether the same diagnosis name should apply when investigators describe stratified populations, for instance, SLE with and without renal disease. Inconsistent definitions lead stakeholders—patients, practising physicians, administrators, epidemiologists and investigators—to count different patients and to develop different opinions about the mechanisms and treatment of SLE.
To advocate a consensus vocabulary and conceptual model, in this paper we deconstruct the process of making a diagnosis of SLE by examining its classification and diagnostic criteria, definitions and illness models. We discuss the ways by which stratification biases conclusions and how the purpose for which a stakeholder names a diagnosis determines whom they accept as having this disease.
Definitions
Classification and diagnostic criteria
When in the mid-19th century Cazenave first used the name lupus erythematosus, SLE was a rare and life-threatening illness.2 3 A century later, as new technologies identified more patients,4–10 physicians found SLE to be more clinically diverse, and often less severe, than they once believed. To improve homogeneity of patient populations identified for clinical studies, investigators developed classification criteria that are specific, binary (SLE is present or not) and time limited (valid only for the extent of a study). The homogeneity of classification criteria is implied but not real. The American College of Rheumatology (ACR) criteria allow 330 different combinations of symptoms and laboratory tests to affirm a diagnosis of SLE.11 Classification criteria are insensitive; they exclude patients who, despite disabling and treatable symptoms, fall below criteria thresholds and those who have overlapping or changing forms of SLE, whom practising physicians treat according to rules established for typical SLE.12–21 Diagnostic criteria, which would be more sensitive, if less specific, time independent and scalar rather than binary, do not exist for SLE. (An internet search (23 September 2018) for SLE diagnostic criteria identified references only to classification criteria. Many published papers fail to distinguish diagnostic from classification criteria.21)
Exclusive and inclusive definitions
Many clinical and basic science investigators use an exclusive definition of SLE that accepts only classification criteria-defined patients and rejects (for ethical and practical reasons) patients with dementia, pregnancy, comorbid illness or specific forms of treatment.22–24 Practising physicians use an inclusive definition that gathers under one name all patients with lupus spectrum illness, including those with typical SLE (criteria fulfilling), overlap syndromes (typical SLE associated with another definable autoimmune illness), undifferentiated autoimmune syndrome (UAS) (lupus-like illness that does not fulfil criteria),25–27 SLE-associated antibodies only (diagnostic autoantibodies but no clinical illness)28 and cutaneous SLE (skin disease without systemic manifestations).29
In rheumatology practice, only 35% of patients with lupus spectrum have typical SLE. Compared with patients with atypical forms of lupus spectrum disease, patients with typical SLE differ demographically, have different involved organ systems and receive different treatments.30 31 Although differences between typical and atypical patients may result in different disease mechanisms and outcomes, many SLE studies include atypical patients with neither comment nor subanalysis.32
Studies differ in how they define onset of SLE, which may be first appearance of ANA, anti-DNA antibody, symptoms, ACR criteria or diagnosis by a physician. In animal model studies, onset may be first appearance of glomerulonephritis, anti-DNA antibodies, biomarkers, gene expression profiles or disease-inducing intervention.33–35 A study that defines SLE as first appearance of arthralgia or ANA may enrol patients more than a decade earlier than does one that defines SLE as first fulfilment of ACR criteria.36 Although both studies will speak of SLE, their results cannot be compared.
Stakeholders who use different definitions describe different patients (figure 1). Clinical and basic science investigators usually use exclusive definitions (clear area, centre and bottom); by study design, they include only patients whom a practising physician has already diagnosed to have SLE, that is, after time 2. Patients, practising physicians and some investigators consider SLE to begin at time 1 (shaded area on the left). Symptoms and laboratory abnormalities first occur at the earlier date, when social, environmental and/or endogenous inducers may determine which patient will remain well, develop typical SLE or fall ill with a different lupus spectrum illness (shaded area at the top). Exogenous factors may also determine if, how, and when the clinical phenotype may change (time 4, shaded area at the right). Outcomes are measurable after a diagnosis is assigned, throughout time 3.
Figure 1.
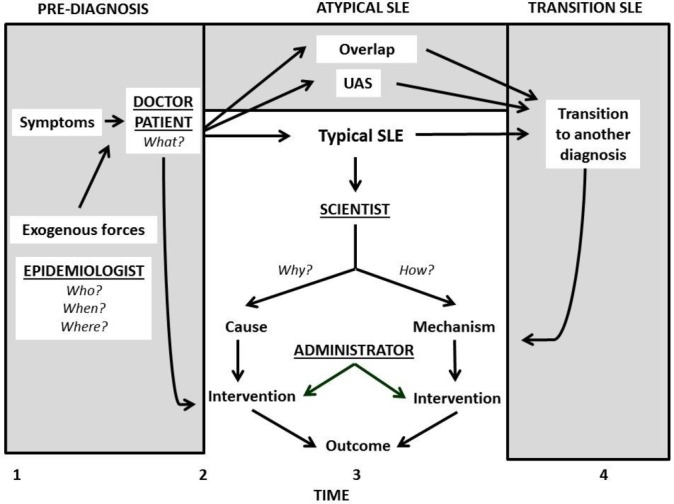
A proposed model of SLE-lupus spectrum illness that considers stakeholders’ definitions and the concept of time. Time 1 is first onset of clinical or laboratory abnormalities, time 2 first diagnosis, time 3 outcome measurement and time 4 transition to another illness. Practising doctors and their patients mostly ask what, epidemiologists who, when and where (to suggest answers to how and why) and investigators how and why. Most scientific research on SLE focuses on typical postdiagnosis SLE (clear area at the centre), but ignores prediagnosis (shaded area left), atypical illness (shaded area top) and transition illness (shaded area right). Practising physicians are more likely to consider SLE to be the entire spectrum (whole figure); epidemiologists, prediagnosis and typical SLE; investigators and administrators only postdiagnosis, typical SLE. UAS, undifferentiated autoimmune syndrome.
Most administrators, patients, practising physicians and some clinical investigators ask the question what. The answer, for which the exclusive definition works best, is the diagnosis name. Most basic and some clinical science investigators ask how, the answer being disease mechanisms, for which the exclusive definition also functions best. Some epidemiological, clinical and basic science investigators, and practising physicians ask why—the aetiology. For this question the inclusive definition is preferred.
Illness models
One can reduce the complexity of making a diagnosis of SLE by using conceptual models. There are two such models, one separate illnesses, the other linear illness; both cluster SLE’s disparate elements. The separate illnesses model posits that typical, overlap, UAS and antibody-only SLE are separate but related illnesses. A strength of this model is that it assigns different, targetable biological mechanisms to each diagnosis. Another strength is that a diagnosis, once made, does not change.37–40 Weaknesses of this model are that, in clinical practice, ambiguous diagnoses occur often, blurring the separating lines, and that diagnoses do sometimes change. Clinical protocols that rigidly adhere to sharp distinctions among diagnoses may remove options available to the treating physician. Another weakness is that insights suggested by evolving phenotypes may be unseen if an investigator believes that change of diagnosis cannot occur.30
The linear illness model posits that UAS, overlap, antibody-only and typical SLE reside within a continuum of a single pathogenic process. This model applies throughout a patient’s lifetime, during which the diagnosis name may change.41–49 The model’s strengths are that it suggests common pathogeneses and flexible treatment protocols for all lupus spectrum illnesses; it highlights potential causes for phenotype changes. A weakness is that boundaries among diagnoses are vague, gathering under one name, like lupus spectrum, patients who have distinctly different phenotypes.
When the cause of a chronic illness is unknown, the separate illnesses model usually applies. Using this model, investigators mostly ask how of narrowly defined populations in order to discover mechanisms that will become the basis for targeted, ameliorative treatments. Investigators who ask why hope to find a single cause for a disease. When why is answered, syndromes will be seen to be different phases of a linear illness and treatment will be directed to prevention or cure.
For example, in the separate illness model, the syndrome consisting of an abnormal venereal disease research laboratory blood test, rash, aortic aneurysm and tabes dorsalis, lacking a known cause, consists of related different illnesses, mechanistically different, with mechanism-based ameliorative treatments. When the cause is known—infection by Treponema pallidum—the separate symptoms are seen to be phases in a linear model of syphilis, amenable to aetiology-based cure. Similarly, anaemia, jaundice and neuropsychiatric symptoms are separate illnesses until deficiency of vitamin B12 is recognised, at which time they become linear illness phases of pernicious anaemia; and pigment change, neuropathy, hepatitis and cytopenia are separate illnesses until arsenic poisoning is found.
The cause(s) of a chronic illness can be one or many things. Whether the cause is infection, deficiency, intoxication or autoimmunity, how one conceptualises the illness is important. The separate illness model favours mechanism-based management, the linear illness model aetiology-based cure. The choice depends mostly on whether the cause is or is not known.
Stratification
When studies stratify SLE populations, conclusions drawn from one subpopulation may differ from those drawn from another. Stratification by clinical and serological phenotypes, demography and habits is qualitative, on disease activity measures quantitative.50–57 Stratification on sex, race, socioeconomic status,58–62 access to medical care, medication choice and adherence,63 willingness to participate in clinical trials, doctor–patient interactions,64 patient preferences and perceptions,65 lifestyle choices,66 67 physician choices,68–71 environmental triggers,72–76 poverty,77 social disparities,78 and life events,79 smoking80 and the gut pathobiont81 all affect manifestations and outcomes in ways that dictate who participates in a study on SLE and in ways that cannot be examined in animal models.82 Stratification on gene expression, quantitative, predicts risk and possibly phenotype83–93; SLE-like illnesses (the autoinflammatory diseases),94 Aicardi-Goutières syndrome,95 96 Canale-Smith syndrome97 and SLE associated with immunodeficiency98 suggest mechanisms for primary illness, and for phenotype diversity. Stratification by molecular biomarkers predicts fulfilment of classification criteria,99 organ involvement and development of SLE in relatives of patients.100–104 Stratifying by time will offer insight about how SLE diagnoses change.105 New computational techniques, like multidimensional models, cluster analyses, machine learning, the word cloud, personalised immunomonitoring and transancestral mapping, are modern ways to stratify.106–114 Many of today’s mechanistic studies compare one dependent against one independent variable. Three-dimensional stratification can quantify combinations of biomarkers, severity indices, phenotypes, microscopic pathology, immunopathology, gene patterns, epidemiological variables, microbiomes or gradations of biological sex. Four-dimensional studies can compare non-calendric variables at different points in time.
Studies on stratified populations of patients with SLE that demonstrate different mechanisms among the groups validate the separate illness model of SLE; studies that identify common mechanisms validate the linear illness model. Although stratification by itself cannot explain the origins of SLE, its ability to show population differences enhances understanding and treatment of the disease.
Stakeholders’ purposes
How different stakeholders use the name SLE and which definition they use depends on the purpose for which they assign the name (table 1).
Table 1.
Types and purposes of SLE definitions used by different stakeholders. The types of definitions, illness models, modes of diagnosis and time considerations are described in the text
| Stakeholder | Purpose of naming a diagnosis | Definition | Illness model | Mode | Time limited? |
| Payer | Determine reimbursement. | Exclusive | Separate | Binary | Yes |
| Administrator | Count affected persons. | Exclusive | Separate | Binary | Yes |
| Epidemiologist | Count affected persons. | Inclusive or exclusive | Separate >linear | Binary | Yes |
| Clinical researcher | Identify homogeneous populations to identify risk, measure outcomes, study mechanisms, develop and test treatments. | Exclusive | Separate | Binary | Yes |
| Basic/translational researcher | Identify affected persons to study mechanisms and/or causes. | Inclusive or exclusive | Separate or linear | Binary or scalar | Yes or no |
| Editor | Classify published articles. | Inclusive or exclusive | Separate or linear | Not considered | Not considered |
| Physician | Identify cause of symptoms, prognosticate and treat. | Inclusive | Linear | Scalar | No |
| Patient | Know prognosis, choose among diagnostic and treatment options. | Inclusive | Linear | Scalar | No |
The purpose for which payers and administrators use the name SLE is to guide reimbursement and regulatory policy. Epidemiologists do so to identify disparities among populations that may identify the exogenous and endogenous factors that drive the illness and that demarcate boundaries by which clinical and basic science researchers can study mechanisms, causes, treatments and outcomes. Office physicians use the name SLE to anchor prognoses, justify interventions and enhance patients’ confidence (and their own). Patients use it to understand their options and their futures. Editors of medical journals use it to flag articles for readers’ attention.
Payers, administrators, clinical researchers and some basic science researchers mostly select the separate illness model and the exclusive, binary and time-limited definition of SLE.115–124 Physicians, patients and other basic researchers choose the inclusive, scalar and time-variable definition and linear illness model. Journal editors consider the definition and disease models irrelevant if the published report can be indexed and identified by a keyword. A result of this choice is that literature and internet searches on SLE yield studies of patients and animals defined in many different ways, with little attention to distinctions among the definitions.
Until recently American physicians used common language diagnosis names in medical charts, biasing recorded diagnoses towards the exclusive definition. Quality monitors did not challenge common language diagnoses, payers reimbursed expenses and patients with ambiguous diagnoses usually did not participate in studies of SLE. New administrative rules require American physicians to use International Classification of Diseases (ICD) code numbers that disregard the uncertainty of lupus spectrum illness.125 126 Because when diagnoses are ambiguous payers often refuse to reimburse costs of SLE-relevant tests and medications, American physicians now assign the ICD code for typical SLE to patients they previously diagnosed with UCTD, overlap or other lupus spectrum disease, and these patients may now participate in studies that select patients by ICD code.
A consensus definition
Although many investigators suggest improvements to the available SLE criteria,22 39 121 127–132 the argument for more precise and more exclusive criteria is circular. Studies that exclude patients who do not fulfil criteria cannot prospectively examine phenomena that antedate diagnosis or that cause patients to develop non-criteria variants within lupus spectrum. Deconstructing the process of diagnosis—its definitions, models, stratifications and purposes—can help solve this problem. A consensus vocabulary is the first step to an agreed concept of SLE, including consensus answers to these questions:
When does SLE begin?
Do persons with autoantibodies only, UAS or overlap illness have ‘SLE’?
Do persons with predisposing genetic abnormalities have ‘SLE’?
Do patients with mild and severe ‘SLE’ have the same illness?
When ‘SLE’ changes course or changes to a different illness, does the change represent alteration of a continuous process or introduction of a new process?
Is SLE a clinical syndrome, having doctor-defined symptoms and specific organ system abnormalities? An abnormal biologic state, defined by laboratory phenomena that may or may not accompany clinical illness? A state of susceptibility, determined by genes and environment? Can it fully subside? What exogenous and/or endogenous factors trigger its onset or its change?
There are no definitive answers to these questions, but they will be better addressed when stakeholders agree on consensus definitions. Which definition, illness model or stratification we choose is less important than is consensus about the vocabulary that describes which patients we study, and to whom the results of our inquiries apply.
Footnotes
Contributors: All authors contributed to, reviewed and approved this document.
Funding: This study was funded by Barbara Volcker Center for Women and Rheumatic Diseases, Rheumatology Research Foundation.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Fritzler MJ, Mahler M. Redefining systemic lupus erythematosus - SMAARTT proteomics. Nat Rev Rheumatol 2018;14:451–2. 10.1038/s41584-018-0035-3 [DOI] [PubMed] [Google Scholar]
- 2. Pierre Louis Alphée Cazenave. 2018. Available from: https://www.revolvy.com/main/index.php?s=Pierre%20Louis%20Alph%C3%A9e%20Cazenave
- 3. Wallace DJ, Lyon I. Pierre Cazenave and the first detailed modern description of lupus erythematosus. Semin Arthritis Rheum 1999;28:305–13. 10.1016/S0049-0172(99)80014-6 [DOI] [PubMed] [Google Scholar]
- 4. Hargraves MM, Richmond H, Morton R. Presentation of two bone marrow elements; the tart cell and the L.E. cell. Proc Staff Meet Mayo Clin 1948;23:25–8. [PubMed] [Google Scholar]
- 5. Holborow EJ, Weir DM, Johnson GD. A serum factor in lupus erythematosus with affinity for tissue nuclei. Br Med J 1957;2:732–4. 10.1136/bmj.2.5047.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robbins WC, Holman HR, Deicher H, et al. Complement fixation with cell nuclei and DNA in lupus erythematosus. Exp Biol Med 1957;96:575–9. 10.3181/00379727-96-23545 [DOI] [PubMed] [Google Scholar]
- 7. Tan EM, Kunkel HG. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol 1966;96:464. [PubMed] [Google Scholar]
- 8. Keil H. Dermatomyositis and systemic lupus erythematosus. II. A comparative study of the essential clinicopathologic features. Arch Intern Med 1940;66:339–83. [Google Scholar]
- 9. Bowie EJ, THOMPSON JH, Pascuzzi CA, et al. Thrombosis in systemic lupus erythematosus despite circulating anticoagulants. J Lab Clin Med 1963;62:416–30. [PubMed] [Google Scholar]
- 10. Nilsson IM, Åstedt B, Hedner U, et al. Intrauterine death and circulating anticoagulant ("antithromboplastin"). Acta Med Scand 1975;197:153–9. 10.1111/j.0954-6820.1975.tb04897.x [DOI] [PubMed] [Google Scholar]
- 11. Combinations and Permutations Calculator. 2018. Available from: http://stattrek.com/online-calculator/combinations-permutations.aspx
- 12. Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res 2015;67:891–7. 10.1002/acr.22583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aringer M, Dörner T, Leuchten N, et al. Toward new criteria for systemic lupus erythematosus—a standpoint. Lupus 2016;25:805–11. 10.1177/0961203316644338 [DOI] [PubMed] [Google Scholar]
- 14. Cohen AS, Reynolds WE, Franklin EC. Preliminary criteria for the classification of systemic lupus erythematosus. Bull Rheum Dis 1971;21:643–8. [Google Scholar]
- 15. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 16. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clough JD, Elrazak M, Calabrese LH. Weighted criteria for the diagnosis of systemic lupus erythematosus. Arch Intern Med 1984;144:281–5. 10.1001/archinte.1984.00350140083013 [DOI] [PubMed] [Google Scholar]
- 18. Costenbader KH, Karlson EW, Mandl LA. Defining lupus cases for clinical studies: the Boston weighted criteria for the classification of systemic lupus erythematosus. J Rheumatol 2002;29:2545–50. [PubMed] [Google Scholar]
- 19. Aringer M, Costenbader KH, Brinks R. Validation of new systemic lupus erythematosus classification criteria. Ann Rheum Dis 2018;77(suppl 2). [Google Scholar]
- 20. Izmirly PM, Buyon JP, Wan I. The incidence and prevalence of adult primary Sjögren’s syndrome in New York County. Arthritis Care Res;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 2014;48-49:10–13. 10.1016/j.jaut.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 22. Dall’Era M, Cisternas MG, Snipes K, et al. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: The California lupus surveillance project. Arthritis Rheumatol 2017;69:1996–2005. 10.1002/art.40191 [DOI] [PubMed] [Google Scholar]
- 23. Aberle T, Bourn RL, Chen H, et al. Use of SLICC criteria in a large, diverse lupus registry enables SLE classification of a subset of ACR-designated subjects with incomplete lupus. Lupus Sci Med 2017;4:e000176 10.1136/lupus-2016-000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izmirly P, Wan I, Sahl S. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York. Arthritis Rheumatol 2017;69:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferreira S, D’Cruz DP, Hughes GRV. Multiple sclerosis, neuropsychiatric lupus and antiphospholipid syndrome: where do we stand? Rheumatology 2005;44:434–42. 10.1093/rheumatology/keh532 [DOI] [PubMed] [Google Scholar]
- 26. Sharp GC. MCTD: a concept which stood the test of time. Lupus 2002;11:333–9. 10.1191/0961203302lu220oa [DOI] [PubMed] [Google Scholar]
- 27. Ungprasert P, Crowson CS, Chowdhary VR, et al. Epidemiology of Mixed Connective Tissue Disease, 1985-2014: A Population-Based Study. Arthritis Care Res 2016;68:1843–8. 10.1002/acr.22872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Z, Casciola-Rosen L, Shah AA, et al. Estimating autoantibody signatures to detect autoimmune disease patient subsets. Biostatistics 2017. 10.1093/biostatistics/kxx061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drenkard C, Parker S, Aspey LD, et al. Racial disparities in the incidence of primary chronic cutaneous lupus erythematosus in the Southeastern United States: the georgia lupus registry. Arthritis Care Res 2018. 10.1002/acr.23578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lockshin MD, Levine AB, Erkan D. Patients with overlap autoimmune disease differ from those with 'pure' disease. Lupus Sci Med 2015;2:e000084 10.1136/lupus-2015-000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jia L, Levine AB, Lockshin MD. American College of Rheumatology criteria for systemic lupus erythematosus exclude half of all systemic lupus erythematosus patients. Arthritis Rheumatol 2017;69:1502–3. 10.1002/art.40120 [DOI] [PubMed] [Google Scholar]
- 32. Jia L, Sevim E, Barbhaiya M. What do we mean when we say SLE? Lupus Science & Medicine 2018;5(Suppl 2):A37. [Google Scholar]
- 33. McCormick N, Marra CA, Sadatsafavi M, et al. Incremental direct medical costs of systemic lupus erythematosus patients in the years preceding diagnosis: A general population-based study. Lupus 2018;27:1247–58. 10.1177/0961203318768882 [DOI] [PubMed] [Google Scholar]
- 34. Li W, Titov AA, Morel L. An update on lupus animal models. Curr Opin Rheumatol 2017;29:434–41. 10.1097/BOR.0000000000000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richard ML, Gilkeson G. Mouse models of lupus: what they tell us and what they don’t. Lupus Sci Med 2018;5:e000199 10.1136/lupus-2016-000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med Overseas Ed 2003;349:1526–33. 10.1056/NEJMoa021933 [DOI] [PubMed] [Google Scholar]
- 37. Doria A, Mosca M, Gambari PF, et al. Defining unclassifiable connective tissue diseases: incomplete, undifferentiated, or both? J Rheumatol 2005;32:213–5. [PubMed] [Google Scholar]
- 38. Mosca M, Tani C, Bombardieri S. Defining undifferentiated connective tissue diseases: a challenge for rheumatologists. Lupus 2008;17:278–80. 10.1177/0961203307088004 [DOI] [PubMed] [Google Scholar]
- 39. Ugarte-Gil MF, Alarcón GS. Incomplete Systemic Lupus Erythematosus: Early Diagnosis or Overdiagnosis? Arthritis Care Res 2016;68:285–7. 10.1002/acr.22663 [DOI] [PubMed] [Google Scholar]
- 40. Daikh DI, Costenbader KH. How Do We Classify “Incomplete Lupus?”. Arthritis Care Res 2017;69:1777–9. 10.1002/acr.23196 [DOI] [PubMed] [Google Scholar]
- 41. Rees F, Doherty M, Lanyon P, et al. Early clinical features in systemic lupus erythematosus: can they be used to achieve earlier diagnosis? A risk prediction model. Arthritis Care Res 2017;69:833–41. 10.1002/acr.23021 [DOI] [PubMed] [Google Scholar]
- 42. Bortoluzzi A, Furini F, Campanaro F, et al. Application of SLICC classification criteria in undifferentiated connective tissue disease and evolution in systemic lupus erythematosus: analysis of a large monocentric cohort with a long-term follow-up. Lupus 2017;26:616–22. 10.1177/0961203316671814 [DOI] [PubMed] [Google Scholar]
- 43. Slight-Webb S, Lu R, Ritterhouse LL, et al. Autoantibody-positive healthy individuals display unique immune profiles that may regulate autoimmunity. Arthritis Rheumatol 2016;68:2492–502. 10.1002/art.39706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Costenbader KH, Schur PH. We Need Better Classification and Terminology for “People at High Risk of or in the Process of Developing Lupus”. Arthritis Care Res 2015;67:593–6. 10.1002/acr.22484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berens-Norman HM, Boackle SA. Editorial: Subduing Lupus: Can Preclinical Autoimmune Disease Be Arrested? Arthritis Rheumatol 2016;68:2357–60. 10.1002/art.39804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. García-González M, Rodríguez-Lozano B, Bustabad S, et al. Undifferentiated connective tissue disease: predictors of evolution into definite disease. Clin Exp Rheumatol 2017;35:739–45. [PubMed] [Google Scholar]
- 47. Munroe ME, Young KA, Kamen DL, et al. Discerning risk of disease transition in relatives of systemic lupus erythematosus patients utilizing soluble mediators and clinical features. Arthritis Rheumatol 2017;69:630–42. 10.1002/art.40004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Vollenhoven RF. Editorial: Who Gets Lupus? Clues to a Tantalizing Syndrome. Arthritis Rheumatol 2017;69:483–6. 10.1002/art.40005 [DOI] [PubMed] [Google Scholar]
- 49. van Steenbergen HW, da Silva JAP, Huizinga TWJ, et al. Preventing progression from arthralgia to arthritis: targeting the right patients. Nat Rev Rheumatol 2018;14:32–41. 10.1038/nrrheum.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muangchan C, van Vollenhoven RF, Bernatsky SR, et al. Treatment Algorithms in Systemic Lupus Erythematosus. Arthritis Care Res 2015;67:1237–45. 10.1002/acr.22589 [DOI] [PubMed] [Google Scholar]
- 51. Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus. Arthritis Care & Research 2011;63(S11):S37–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Isenberg D, Sturgess J, Allen E, et al. Study of flare assessment in systemic lupus erythematosus based on paper patients. Arthritis Care Res 2018;70:98–103. 10.1002/acr.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallace DJ. Editorial: Epratuzumab: Reveille or Requiem? Teachable Moments for Lupus and Sjögren's Syndrome Clinical Trials. Arthritis Rheumatol 2018;70:633–6. 10.1002/art.40427 [DOI] [PubMed] [Google Scholar]
- 54. Pisetsky DS, Spencer DM, Lipsky PE, et al. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis 2018;77:annrheumdis-2017-212599 10.1136/annrheumdis-2017-212599 [DOI] [PubMed] [Google Scholar]
- 55. Lewis MJ, McAndrew MB, Wheeler C, et al. Autoantibodies targeting TLR and SMAD pathways define new subgroups in systemic lupus erythematosus. J Autoimmun 2018;91:1–12. 10.1016/j.jaut.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 56. Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 57. Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005;44:902–6. 10.1093/rheumatology/keh624 [DOI] [PubMed] [Google Scholar]
- 58. Vashisht P, Sayles H, Cannella AC, et al. Generalizability of patients with rheumatoid arthritis in biologic agent clinical trials. Arthritis Care Res 2016;68:1478–88. 10.1002/acr.22860 [DOI] [PubMed] [Google Scholar]
- 59. Merrill JT, Immermann F, Whitley M, et al. The Biomarkers of Lupus Disease Study: A Bold Approach May Mitigate Interference of Background Immunosuppressants in Clinical Trials. Arthritis Rheumatol 2017;69:1257–66. 10.1002/art.40086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barbhaiya M, Feldman CH, Guan H, et al. Race/ethnicity and cardiovascular events among patients with systemic lupus erythematosus. Arthritis Rheumatol 2017;69:1823–31. 10.1002/art.40174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pope JE, Weisman MH, Sjöwall C. Editorial: The Effect of Ethnicity on Cardiovascular Outcomes in Systemic Lupus Erythematosus Is Perhaps Not a Paradox. Arthritis Rheumatol 2017;69:1707–9. 10.1002/art.40173 [DOI] [PubMed] [Google Scholar]
- 62. Falasinnu T, Chaichian Y, Bass MB, et al. The representation of gender and race/ethnic groups in randomized clinical trials of individuals with systemic lupus erythematosus. Curr Rheumatol Rep 2018;20:20:20 10.1007/s11926-018-0728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feldman CH, Broder A, Guan H, et al. Sex Differences in Health Care Utilization, End-Stage Renal Disease, and Mortality Among Medicaid Beneficiaries With Incident Lupus Nephritis. Arthritis Rheumatol 2018;70:417–26. 10.1002/art.40392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yelin E, Yazdany J, Trupin L. Relationship between process of care and a subsequent increase in damage in systemic lupus erythematosus. Arthritis Care Res 2017;69:927–32. 10.1002/acr.22977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Qu H, Shewchuk RM, Alarcón G, et al. Mapping perceptions of lupus medication decision-making facilitators: the importance of patient context. Arthritis Care Res 2016;68:1787–94. 10.1002/acr.22904 [DOI] [PubMed] [Google Scholar]
- 66. Arat S, Lenaerts JL, De Langhe E, et al. Illness representations of systemic lupus erythematosus and systemic sclerosis: a comparison of patients, their rheumatologists and their general practitioners. Lupus Sci Med 2017;4:e000232 10.1136/lupus-2017-000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barbhaiya M, Lu B, Sparks JA, et al. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the Nurses’ Health Study Cohorts. Arthritis Care Res 2017;69:384–92. 10.1002/acr.22945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Golder V, Morand EF, Hoi AY. Quality of care for systemic lupus erythematosus: mind the knowledge gap. J Rheumatol 2017;44:271–8. 10.3899/jrheum.160334 [DOI] [PubMed] [Google Scholar]
- 69. Ryu J, Lee TH. The Waiting Game — Why providers may fail to reduce wait times. N Engl J Med Overseas Ed 2017;376:2309–11. 10.1056/NEJMp1704478 [DOI] [PubMed] [Google Scholar]
- 70. Kim M, Merrill J, Kalunian K, et al. Brief Report: Longitudinal Patterns of Response to Standard of Care Therapy for Systemic Lupus Erythematosus: Implications for Clinical Trial Design. Arthritis Rheumatol 2017;69:785–90. 10.1002/art.40013 [DOI] [PubMed] [Google Scholar]
- 71. Walunas TL, Jackson KL, Chung AH, et al. Disease outcomes and care fragmentation among patients with systemic lupus erythematosus. Arthritis Care Res 2017;69:1369–76. 10.1002/acr.23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rider L, Miller FW, 2017. Environmental factors in pediatric systemic autoimmune diseases. The Rheumatologist. Available from: www.the-rheumatologist.org/article/environmental-factors-pediatric-systemic-autoimmune-diseases/
- 73. Bouziat R, Hinterleitner R, Brown JJ, et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017;356:44–50. 10.1126/science.aah5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fotis L, Shaikh N, Baszis KW, et al. Serologic Evidence of Gut-driven Systemic Inflammation in Juvenile Idiopathic Arthritis. J Rheumatol 2017;44:1624–31. 10.3899/jrheum.161589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zeng P, Chen Z, Klareskog L, et al. Amount of smoking, duration of smoking cessation and their interaction with silica exposure in the risk of rheumatoid arthritis among males: results from the Swedish Epidemiological Investigation of Rheumatoid Arthritis (EIRA) study. Ann Rheum Dis 2018;77:annrheumdis-2017-212145 10.1136/annrheumdis-2017-212145 [DOI] [PubMed] [Google Scholar]
- 76. Barbhaiya M, Hart J, Malspeis S, 2017. Association of Ultraviolet-B Radiation and Risk of SLE Among Women in the Nurses’ Health Studies. Arthritis Rheum. Available from: https://www.rheumatology.org/Learning-Center/Publications-Communications/Abstract-Archives [DOI] [PMC free article] [PubMed]
- 77. Yelin E, Trupin L, Yazdany J. A prospective study of the impact of current poverty, history of poverty, and exiting poverty on accumulation of disease damage in systemic lupus erythematosus. Arthritis Rheumatol 2017;69:1612–22. 10.1002/art.40134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. West KM, Blacksher E, Burke W. Genomics, health disparities, and missed opportunities for the Nation’s research agenda. JAMA 2017;317:1831–2. 10.1001/jama.2017.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roberts AL, Malspeis S, Kubzansky LD, et al. Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women. Arthritis Rheumatol 2017;69:2162–9. 10.1002/art.40222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Barbhaiya M, Tedeschi SK, Lu B, et al. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann Rheum Dis 2018;77:196–202. 10.1136/annrheumdis-2017-211675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Manfredo Vieira S, Hiltensperger M, Kumar V, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018;359:1156–61. 10.1126/science.aar7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reardon S. A mouse’s house may ruin studies. Nature 2016;530:264. [DOI] [PubMed] [Google Scholar]
- 83. Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med Overseas Ed 2011;365:1612–23. 10.1056/NEJMra1100030 [DOI] [PubMed] [Google Scholar]
- 84. YR L, Li J, Zhao SD, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med 2015;21:1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nigrovic PA, Raychaudhuri S, Thompson SD. Review: genetics and the classification of arthritis in adults and children. Arthritis Rheumatol 2018;70:7–17. 10.1002/art.40350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Blegvad C, Egeberg A, Nielsen T, Tind Nielsen TE, et al. Autoimmune disease in children and adolescents with psoriasis: a cross-sectional study in Denmark. Acta Derm Venereol 2017;97:1225–9. 10.2340/00015555-2743 [DOI] [PubMed] [Google Scholar]
- 87. International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN), Harley JB, Alarcón-Riquelme ME, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 2008;40:204–10. 10.1038/ng.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guerra SG, Vyse TJ, Cunninghame Graham DS. The genetics of lupus: a functional perspective. Arthritis Res Ther 2012;14:211 10.1186/ar3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Blair DR, Lyttle CS, Mortensen JM, et al. A nondegenerate code of deleterious variants in Mendelian loci contributes to complex disease risk. Cell 2013;155:70–80. 10.1016/j.cell.2013.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Phillips D, 2015. Multiple autoimmune diseases share same genetic risk factors. Medscape. Available from: http://www.medscape.com/viewarticle/850543
- 91. Boycott KM, Innes AM. When One Diagnosis Is Not Enough. N Engl J Med Overseas Ed 2017;376:83–5. 10.1056/NEJMe1614384 [DOI] [PubMed] [Google Scholar]
- 92. Posey JE, Harel T, Liu P, et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med Overseas Ed 2017;376:21–31. 10.1056/NEJMoa1516767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Raj P, Pichilingue-Reto P, Dozmorov I. Immune repertoire and genetic risk alleles in healthy pediatric populations with autoimmune indicators. Lupus Sci & Med 2018;5(Suppl 2):A58. [Google Scholar]
- 94. Yao Q, Shen B. A systematic analysis of treatment and outcomes of NOD2-associated autoinflammatory disease. Am J Med 2017;130:365.e13–365.e18. 10.1016/j.amjmed.2016.09.028 [DOI] [PubMed] [Google Scholar]
- 95. Gao D, Li T, Li XD, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci U S A 2015;112:E5699–E5705. 10.1073/pnas.1516465112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim H, Sanchez GA, Goldbach-Mansky R. Insights from Mendelian Interferonopathies: Comparison of CANDLE, SAVI with AGS, Monogenic Lupus. J Mol Med 2016;94:1111–27. 10.1007/s00109-016-1465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Drappa J, Vaishnaw AK, Sullivan KE, et al. Fas gene mutations in the canale–Smith syndrome, an inherited lymphoproliferative disorder associated with autoimmunity. N Engl J Med Overseas Ed 1996;335:1643–9. 10.1056/NEJM199611283352204 [DOI] [PubMed] [Google Scholar]
- 98. Demirkaya E, Zhou Q, Smith CK, et al. Brief report: deficiency of complement 1r subcomponent in early-onset systemic lupus erythematosus: the role of disease-modifying alleles in a monogenic disease. Arthritis & Rheumatology 2017;69:1832–9. 10.1002/art.40158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kottyan LC, Zoller EE, Bene J, et al. The IRF5-TNPO3 association with systemic lupus erythematosus has two components that other autoimmune disorders variably share. Hum Mol Genet 2015;24:582–96. 10.1093/hmg/ddu455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Salaman MR, Isenberg DA. The immunological personality of close relatives of SLE patients. Lupus 2017;26:1513–6. 10.1177/0961203317707826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Md YMY, Psarras A, El-Sherbiny YM. Prediction of autoimmune connective tissue disease in an at-risk cohort: prognostic value of a novel two-score system for interferon status. Ann Rheum Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Obermoser G, Pascual V. The interferon-α signature of systemic lupus erythematosus. Lupus 2010;19:1012–9. 10.1177/0961203310371161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Monteith AJ, Kang S, Scott E, et al. Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus. Proc Natl Acad Sci U S A 2016;113:E2142–E2151. 10.1073/pnas.1513943113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kubo S, Nakayamada S, Yoshikawa M, et al. Peripheral immunophenotyping identifies three subgroups based on T cell heterogeneity in lupus patients. Arthritis Rheumatol 2017;69:2029–37. 10.1002/art.40180 [DOI] [PubMed] [Google Scholar]
- 105. Hanly JG, Li Q, Su L, et al. Psychosis in systemic lupus erythematosus. Arthritis Rheumatol. In Press;2019 10.1002/art.40764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mina-Osorio P. Progress in Inflammation Research: Next-Generation Therapies and Technologies for Immune-Mediated Inflammatory Diseases. Switzerland: Springer, 2017. [Google Scholar]
- 107. Cepika AM, Banchereau R, Segura E, et al. A multidimensional blood stimulation assay reveals immune alterations underlying systemic juvenile idiopathic arthritis. J Exp Med 2017;214:3449–66. 10.1084/jem.20170412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Char DS, Shah NH, Magnus D. Implementing Machine Learning in Health Care - Addressing Ethical Challenges. N Engl J Med 2018;378:981–3. 10.1056/NEJMp1714229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tag cloud. 2018. Available from: https://en.wikipedia.org/wiki/Tag_cloud
- 110. Mok CC. Systemic lupus erythematosus: Withdrawing standard of care therapies in SLE trials? Nat Rev Rheumatol 2017;13(6):328–330. 10.1038/nrrheum.2017.59 [DOI] [PubMed] [Google Scholar]
- 111. Barturen G, Beretta L, Cervera R, et al. Moving towards a molecular taxonomy of autoimmune rheumatic diseases. Nat Rev Rheumatol 2018;14:75–93. 10.1038/nrrheum.2017.220 [DOI] [PubMed] [Google Scholar]
- 112. Weckerle CE, Franek BS, Kelly JA, et al. Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum 2011;63:1044–53. 10.1002/art.30187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Langefeld CD, Ainsworth HC, Cunninghame Graham DS, et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat Commun 2017;8:16021 10.1038/ncomms16021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bernstein S, 2018. Experts discuss the latest precision medicine research. The Rheumatologist. Available from: http://www.the-rheumatologist.org/article/experts-discuss-latest-precision-medicine-research-2/?singlepage=1&theme=print-friendly
- 115. Castrejón I, Tani C, Jolly M. Indices to assess patients with systemic lupus erythematosus in clinical trials, long-term observational studies, and clinical care. Clin Exp Rheumatol 2014;32(Suppl. 85):S85–S95. [PubMed] [Google Scholar]
- 116. Amezcua-Guerra LM, Higuera-Ortiz V, Arteaga-García U, et al. Performance of the 2012 systemic lupus International collaborating clinics and the 1997 American college of rheumatology classification criteria for systemic lupus erythematosus in a real-life scenario. Arthritis Care Res 2015;67:437–41. 10.1002/acr.22422 [DOI] [PubMed] [Google Scholar]
- 117. Inês L, Silva C, Galindo M. Classification of systemic lupus erythematosus. Arthritis Care & Research 2015;67:437–41. [DOI] [PubMed] [Google Scholar]
- 118. Inês L, Silva C, Galindo M, et al. Classification of systemic lupus erythematosus: Systemic lupus international collaborating clinics versus american college of rheumatology criteria. A comparative study of 2,055 patients from a real-Life, international systemic lupus erythematosus cohort. Arthritis Care Res 2015;67:1180–5. 10.1002/acr.22539 [DOI] [PubMed] [Google Scholar]
- 119. Isenberg D, Sturgess J, Allen E, et al. Study of flare Assessment in systemic lupus erythematosus based on paper patients. Arthritis Care Res 2018;70:98–103. 10.1002/acr.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sanz I. New perspectives in rheumatology: may you live in interesting times: challenges and opportunities in lupus research. Arthritis Rheumatol 2017;69:1552–9. 10.1002/art.40109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Aberle T, Bourn RL, Munroe ME, et al. Clinical and serologic features in patients with incomplete lupus classification versus systemic lupus erythematosus patients and controls. Arthritis Care Res 2017;69:1780–8. 10.1002/acr.23201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Raymond SC, Hanrahan LM, Merrill J. Lupus classification criteria effort is going in the wrong direction. Dermatology News, 2017. [Google Scholar]
- 123. Merrill JT, Manzi S, Aranow C, et al. Lupus community panel proposals for optimising clinical trials: 2018. Lupus Sci Med 2018;5:e000258 10.1136/lupus-2018-000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. International Statistical Classification of Diseases and Related Health Problems. 2018. Available from: https://en.wikipedia.org/wiki/International_Statistical_Classification_of_Diseases_and_Related_Health_Problems
- 125. Systemic lupus erythematosus, unspecified. 2018. Available from: http://www.icd10data.com/ICD10CM/Codes/M00-M99/M30-M36/M32-/M32.9
- 126. Leuchten N, Milke B, Winkler-Rohlfing B. on behalf of the SLE Classification Criteria Steering Committee. Early symptoms of systemic lupus erythematosus (SLE) recalled by 339 SLE patients. Lupus 2018;27:1431–6. [DOI] [PubMed] [Google Scholar]
- 127. Lu R, Munroe ME, Guthridge JM, et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun 2016;74:182–93. 10.1016/j.jaut.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mosca M, Costenbader KH, Johnson SR. New SLE classification criteria for clinical research are being developed, sponsored by EULAR and ACR. How Do Patients with Newly Diagnosed Systemic Lupus Erythematosus Present? A Multicenter Cohort of Early Systemic Lupus Erythematosus to Inform the Development of New Classification Criteria. Arthritis Rheumatol 2018. Epub ahead of print 23 Jul 2018. [DOI] [PubMed] [Google Scholar]
- 129. Munroe ME, Lu R, Zhao YD, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis 2016;75:2014–21. 10.1136/annrheumdis-2015-208140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Olsen NJ, McAloose C, Carter J, et al. Clinical and immunologic profiles in incomplete lupus erythematosus and improvement with hydroxychloroquine treatment. Autoimmune Dis 2016;2016:1–9. 10.1155/2016/8791629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Schmajuk G, Hoyer BF, Aringer M, et al. multicenter Delphi exercise to identify important key items for classifying systemic lupus erythematosus. Arthritis Care Res 2018;70:1488–94. 10.1002/acr.23503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tedeschi SK, Johnson SR, Boumpas D, et al. Developing and refining new candidate criteria for systemic lupus erythematosus classification: An international collaboration. Arthritis Care Res 2018;70:571–81. 10.1002/acr.23317 [DOI] [PMC free article] [PubMed] [Google Scholar]


