Abstract
Since the discovery of scarless fetal skin wound healing, research in the field has expanded significantly with the hopes of advancing the finding to adult human patients. There are several differences between fetal and adult skin that have been exploited to facilitate scarless healing in adults including growth factors, cytokines, and extracellular matrix substitutes. However, no one therapy, pathway, or cell subtype is sufficient to support scarless wound healing in adult skin. More recently, products that contain or mimic fetal and adult uninjured dermis were introduced to the wound healing market with promising clinical outcomes. Through our review of the major experimental targets of fetal wound healing, we hope to encourage research in areas that may have a significant clinical impact. Additionally, we will investigate therapies currently in clinical use and evaluate whether they represent a legitimate advance in regenerative medicine or a vulnerary agent.
Keywords: fetal wound healing, fibrosis, regenerative medicine, scarless, scarring, wound healing
1 ∣. INTRODUCTION
Though skin scarring is the normal and inevitable outcome of a full thickness dermal injury, many people fail to recognize the implications of fibrosis over true regenerative healing. In the skin and most organs, large enough insults in the form of traumatic wounds, surgical incisions, burns, major disease processes, or infections result in the deposition of collagen and recolonization with local cells that fail to recreate normal organ architecture. In the kidney, scarring of the nephrons leads to irreversible changes in glomerular filtration rate. In the liver, fibrosis leads to gradual accumulation of serum toxins as the organ loses its enzymatic ability. In the skin, scarring results in disfigurement, contractures, and at times severe psychological trauma. Skin healing can be “normal,” resulting in a fibrotic and organized scar devoid of sensory, hair, and glandular appendages. Alternatively, the healing can be dysfunctional, leading to the more serious consequences of under and over healing, including ulceration and fibroproliferative scarring.
Skin scarring constitutes an enormous burden on individual patients and society. Every year, 100 million patients acquire scars from surgery in the developed world (Brown, McKenna, Siddhi, McGrouther, & Bayat, 2008). Patients with visible scars, particularly on the face, suffer from social stigma and psychological trauma (Brown et al., 2008; Hunt, Burden, Hepper, Stevenson, & Johnston, 2006). In the United States 500,000 patients per year are treated for burns, many of which leave scars and painful contractures that require major surgery (Asuku, Ibrahim, & Ijekeye, 2008; Egeland, More, Buchman, & Cederna, 2008). The total annual cost of medical care for burns in the United States is $7.5 billion (Finkelstein, Corso, & Miller, 2006), and much of this cost is related to the treatment of burn scars and contractures. Children are particularly affected by scarring, and can suffer from long-term physical dysfunction (Esselman, 2007; Sheridan et al., 2000) and psychological harm (Robert et al., 1999; Thomas et al., 2012; Van Loey & Van Son, 2003). Expectedly, there is an enormous market for consumer anti-scarring treatments, estimated at $12 billion annually in the United States (Sen et al., 2009). However, most commercial wound healing products fail to initiate skin regeneration.
In the 1970s, experiments in fetal lambs demonstrated the ability to heal skin without scarring in early gestation (Burrington, 1971). Since this discovery, several other animals including sheep, rats, mice, pigs, monkeys, and humans have demonstrated the same remarkable ability (Gurtner, Werner, Barrandon, & Longaker, 2008; Rowlatt, 1979). In each instance, cutaneous wounds made prior to the transition to the adult skin scarring phenotype resulted in complete organ regeneration, including the development of dermal appendages (Walmsley et al., 2015). In a subsequent series of reciprocal translocation experiments, tissue transplanted between postnatal and fetal animals implicated a cell intrinsic mechanism to the scarless wound healing phenomenon (Longaker et al., 1994; Lorenz et al., 1992). Since then, characterizing fetal wound healing has achieved deserved attention with the hopes of identifying the cells or cell signaling pathways that drive scarless wound healing in adult tissue (Buchanan, Longaker, & Lorenz, 2009; McPherson et al., 1988; Nwomeh, Liang, Diegelmann, Cohen, & Yager, 1998; Shah, Foreman, & Ferguson, 1995; Walmsley et al., 2015; Whyte et al., 2013; You & Han, 2014). For patients, scarless skin wound healing would allow complete functional recovery from burns, surgical incisions, and major tissue loss from trauma or other causes. In the pediatric population, this would mean contractures limiting normal growth and development could be avoided. As such, research into the mechanism behind fetal scarless wound healing has expanded significantly.
Below, we discuss the known mechanisms of adult fibrosis and fetal scarless wound healing. From there, we will investigate the growth factors, cytokines, enzymes, and cell-based strategies that attempt to achieve scarless wound healing in adults; whether through fetal mimicry or dampening the fibrotic response. Through our discussion, we hope to elucidate promising clinical targets and critically evaluate therapies currently in practice.
2 ∣. MAMMALIAN SKIN ANATOMY AND EMBRYOLOGY
To understand skin wound healing, a basic understanding of skin anatomy and embryology is necessary. The skin, or integumentary system, is a complex organ that provides a moisture and microbial barrier to the outside world. It is divided into several basic layers that can then be subdivided further by cellular differentiation and cell types.
The most superficial layer of the skin is the epidermis. The epidermis is formed from a thin layer of embryonic ectoderm. After neuralization occurs in the fetus, the single cell layer of simple ectoderm expands to become a simple squamous epithelium known as the periderm. Eventually, the differentiating epithelium stratifies into distinct layers; the stratum germinativum/basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum (also known as the horny layer, or cornified layer). The outermost layer (stratum corneum) sheds and sloughs off in adults. In the fetus, the development of the stratum corneum leads to the eventual shedding of the periderm.
The epidermis contains a deep layer of cells, known as basal cells, that serve as a stem cell reservoir for the development and differentiation of keratinocytes. Keratinocytes get their name from keratin, the characteristic protein of the epidermis. Keratinocytes begin their lifecycle at the basement membrane and gradually migrate up through the epidermis to the surface of the skin; where they expel their nuclei and flatten to form a stratified squamous epithelium.
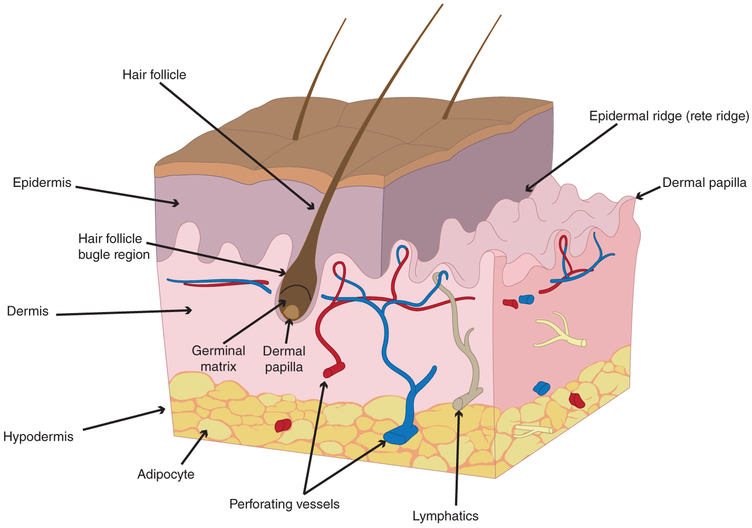
Below the epidermis lies the dermis, a thick layer rich in extracellular matrix (ECM) proteins. The dermis houses the base of epidermal appendages, such as hair follicles, sweat glands, and also houses dermal fibroblasts. Fibroblasts deposit both normal and scar ECM proteins, which give the skin its tensile strength. Additionally, nerves and muscles are found in the dermis. The dorsal, ventral, and cranial dermis have different embryonic origins, though all mesodermal. The ventral dermis is derived from the somatic layer of lateral plate mesoderm while the cranial dermis is derived from the neural crest. In the dorsal dermis, cells are derived from the dermamyotome subdivision of somites. During fetal development, the dermis develops upward projections toward the epidermis known as dermal papillae, while the epidermis develops downward projections into the dermis, known as epidermal ridges (rete ridges).
From the epidermal ridges, several specialized epidermal structures develop, including hair, sebaceous glands, accrine sweat glands, apocrine sweat glands, mammary glands, teeth, and nails. Sebaceous glands secrete sebum, an oily substance that lubricates the skin and hair. Accrine sweat glands are widely distributed and function to secrete thermoregulatory sweat, while apocrine sweat glands secrete odorous substances and are located mostly in the axilla and pubic regions in humans.
Hair is formed by an epidermal ridge that is indented at the bottom by a dermal papilla. Overlying the dermal papilla is the germinal matrix, a proliferating ectoderm that forms the base of the hair bulb. The germinal matrix undergoes a specialized keratinization process which leads to the development of a hair shaft that protrudes through the inner and outer epidermal root sheath. Hair color is produced by melanocytes secreting melanin at the base of the hair bulb. Progenitor cells for the hair follicle are located within the hair follicle bulge region. These cells differentiate and migrate from the bulge downward into the follicle to participate in hair follicle growth phases.
Sebaceous glands in most parts of the body are associated with and bud off of the hair follicle shaft as a diverticula. The gland then expands into the dermis where it branches to form secretory acini or alveoli. In sebaceous glands, the proliferating stem cell population resides in the basal layer. The basal layer stem cells then divide and differentiate to supply the gland with a constant source of new mature secretory epithelial cells.
Below the dermis is the hypodermis. The hypodermis is a layer of subcutaneous fat containing the skin’s perforating blood vessels and adipose tissue. Blood vessels in the skin arise from deeper muscle tissue and then penetrate the dermis and epidermis. In the dermis and epidermis, blood vessels give off fine capillary tributaries to supply skin cells with necessary oxygen and nutrients.
In the skin, other specialized resident cells include Langerhans cells (which function as antigen-presenting cells) and melanocytes (which give the skin and hair color from the production of melanin). Melanin also serves as a natural sunscreen. Merkel cells also are present in the epidermis; where they act as pressure-sensitive mechanoreceptors. Lastly, scattered inflammatory cells like macrophages and mast cells scavenge debris and perform additional roles as resident antigen-presenting cells (Figure 1; Driskell et al., 2013; Forni, Trombetta-Lima, & Sogayar, 2012; Fuchs, 2007; Ge et al., 2017; Schoenwolf & Larsen, 2009; Zhu et al., 2014).
FIGURE 1.
Anatomy of human skin. The most superficial layer of the skin is the epidermis, followed by the dermis, and then hypodermis. Also depicted in this figure is a specialized skin structure: the hair follicle. Note the dermal papilla, germinal matrix, and bulge regions
3 ∣. ADULT SKIN HEALING
Our understanding of the formation of scar tissue is inseparably linked to the process of normal wound healing. In clinical and experimental practice, specific elements of the wound healing process are usually targeted to reduce scar formation. However, it is likely that every event in the wound healing cascade contributes in some way to the eventual formation of scar tissue.
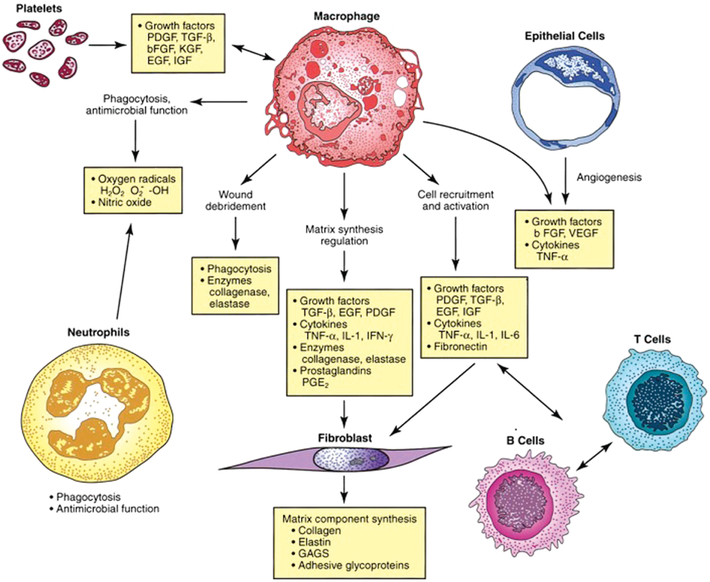
Human skin wound healing is often described in three overlapping stages: inflammation, proliferation, and remodeling (Gurtner et al., 2008). The inflammatory phase involves the influx of a diverse array of cells, including neutrophils, macrophages, and lymphocytes, that cleanse the wound space of bacteria, debris, and dead cells (Gurtner et al., 2008). These cells also release a mixture of cytokines that regulate the behavior of other cells and allow the wound healing process to continue (Box 1).
BOX 1. IMPORTANCE OF MACROPHAGES IN WOUND HEALING.
Not mentioned in the introduction of adult skin healing is the importance of macrophages in wound healing. Eliminating inflammatory cells has minimal effect on wound healing, except in the case of macrophages. Macrophages are thought to have a regulatory role in the process of wound healing. Macrophages appear to be responsible for recruiting inflammatory cells into the wound, for initiating dermal proliferation, and for determining collagen subtype deposition.
The inflammatory phase is followed by the proliferative phase, during which fibroblasts create and organize collagen and other ECM molecules to form new tissue. In adult wounds, new collagen is laid down in parallel tightly spaced bundles. This is a hallmark of scar tissue, and is quite unlike uninjured tissue, where collagen exists in a looser, basket-weave configuration (van Zuijlen et al., 2003). Another characteristic of human scar tissue is that the dermal appendages in normal skin, including hair follicles and sweat glands, do not regenerate and are not present (Gurtner et al., 2008). The absence of these appendages may have implications for the regeneration of tissue during healing, since stem cells residing within these structures contribute to skin regeneration under homeostatic and injured conditions (Plikus et al., 2012; Figure 2).
FIGURE 2.
Interaction of cellular and humoral factors in wound healing. Note the key role of the macrophage. FGF2 = basic fibroblast growth factor; EGF = epidermal growth factor; GAGs = glycosaminoglycans; H2O2 = hydrogen peroxide; IFNG = interferon-gamma; IGF = insulin-like growth factor; IL1 = interleukin-1; IL6 = interleukin-6; KGF = keratinocyte growth factor; O2− = superoxide; -OH = hydroxyl radical; PDGF = platelet-derived growth factor; PGE2 = prostaglandin E2; TGFB = transforming growth factor-beta; TNFA = tumor necrosis factor-alpha; VEGF = vascular endothelial growth factor (Reprinted with permission from Townsend Beauchamp, Evers, and Mattox (2017). Copyright 2017 Elsevier)
The final remodeling phase of wound healing takes place over months to years and involves the gradual structural evolution of the scar tissue. Scar tissue initially contains high levels of type III collagen, but during the remodeling phase it is gradually replaced with type I collagen (Monaco & Lawrence, 2003). Scars that are initially raised, hyperpigmented, and pruritic may lose these characteristics over time (Baum & Arpey, 2005). Additionally, scars contract to reduce their overall size. Lastly, the tensile strength of scarred skin increases as remodelling occurs, but never reaches the full strength of uninjured skin (Levenson et al., 1965).
4 ∣. FETAL SCARLESS HEALING
Surgical incisions in a mammalian fetus heal rapidly without scar formation (Burrington, 1971) and are nearly indistinguishable from uninjured tissue (Beanes et al., 2002). In 1979, the same observation was reported in human fetuses (Rowlatt, 1979). Fetal scarless healing was likely seen as a minor biological curiosity until the 1980s and 1990s, when it was recognized as a study vehicle through which scarless adult healing could be achieved (Adzick & Longaker, 1992). The most significant advances were made in the late 1980s through a series of experiments delineating the differences between adult and fetal wound healing in lambs (Longaker et al., 1990, 1991; Longaker, Chiu, et al., 1989; Longaker, Harrison, Crombleholme, et al., 1989; Longaker, Harrison, Langer, et al., 1989; Longaker, Whitby, et al., 1989). An initial hypothesis was that factors present in the fetal environment but extrinsic to the fetal tissue, such as complete sterility, growth factor-rich amniotic fluid, and low oxygen tension were responsible for scarless healing (Longaker & Adzick, 1991). However later experiments showed that these factors are not sufficient, and that scarless healing is intrinsic to fetal tissue (Longaker et al., 1994). Current efforts in the field of scarless healing are aimed at identifying factors intrinsic to fetal tissue that allow for scarless healing.
Inherently, fetal wound healing is difficult to study because the fetus develops in the protective and closed environment of the uterus. Fetal animal models are thus used extensively because of the difficulties in studying healing in human fetuses (Adzick & Longaker, 1991). Early studies in fetal wound healing noted several histological and chemical differences between healing fetal and adult wounds (Longaker & Adzick, 1991). Healing fetal wounds contain far fewer inflammatory cells compared with adult wounds (Longaker et al., 1990). The absence of these cells likely results in a very different microenvironment with respect to cytokine and growth factor expression, and this may influence fibroblasts to lay down ECM molecules in a regenerative rather than scarring pattern. Hyaluronic acid is present in greater concentrations in fetal wounds (Longaker et al., 1989) and the ECM molecule fibronectin is deposited more rapidly (Longaker et al., 1989). These molecules likely interact with cells involved in healing and may encourage a regenerative phenotype. There is also a higher level of matrix metalloproteinase activity in fetal wounds, which may allow for early remodeling of skin wounds and scar collagen (Dang et al., 2003).
Fibroblasts deposit ECM molecules, whose configuration is an important element of regenerated versus scarred tissue. Therefore, it is possible that changes in fibroblasts populations or fibroblast phenotype in the wound play a role in the loss of scarless healing. In vitro studies show that fetal fibroblasts express more collagen III and IV compared with adult fibroblasts (Larson, Longaker, & Lorenz, 2010; Table 1). Additionally, fetal fibroblasts are capable of proliferating while simultaneously depositing collagen, whereas adult fibroblasts undergo proliferation in the wound and then later deposit collagen (Larson et al., 2010).
TABLE 1.
Key differences between adult and fetal wound healing
| Fetal wounds | Adult wounds | |
|---|---|---|
| Inflammation (Longaker et al., 1994; Lorenz et al., 1992) | Few or no inflammatory cells | Many inflammatory cells |
| Hyaluronic acid (Nwomeh et al., 1998; Shah et al., 1995) | High, prolonged levels, promotes cellular movement | Lower levels, inhibits cellular movement |
| Collagen (Gurtner et al., 2008; McPherson et al., 1988) | Elevated ratio of type III compared to that of type I | Elevated ratio of type I compared to that of type III |
| Keratin (Lorenz et al., 1992; Rowlatt, 1979) | K8 and K19 are present during fetal development | Absence of K8 and K19 |
| Mast cells (Gurtner et al., 2008; Walmsley et al., 2015) | Low numbers and less mature | High numbers, more mature |
| TGF-β (Burrington, 1971; Rowlatt, 1979; Sen et al., 2009) | Low expression of TGF-β1 and TGF-β2 | High expression of TGF-β1 and TGF-β2 |
| Stem cells (Sen et al., 2009) | Higher levels of MSCs at injury sites with accompanying E-cadherin-positive cells | Lower levels of MSCs at injury sites without accompanying E-cadherin-positive cells |
MSCs = mesenchymal stem cells (Reprinted with permission from Walmsley et al., 2015. Copyright 2015 LWW).
Rinkevich et al. recently reported a population of fibroblasts, defined by embryonic expression of the gene engrailed 1 (Enl) that are responsible for the production of normal and scar collagen in the dorsal skin of adult mice (Rinkevich et al., 2015). Initial comparisons of gene expression between En1-derived and En1-negative fibroblast populations revealed differences in a limited number of genes, including Hoxc10 (homeobox C10), Slit2 (slit guidance ligand 2), Foxp1 (forkhead box P1), Lepr (leptin receptor R), Mylk (myosin light chain kinase), and Acta1 (actin alpha 1). Of note, En1 is only expressed early in development, and allows only for lineage tracing these cells. However, CD26 identifies the same population of En1-derived cells in adulthood, with 94% of En1-derived fibroblasts expressing CD26. Further studies will determine the extent to which these transcriptional differences contribute to regenerative versus scarring healing (Box 2).
BOX 2. TARGETING CD26 IN EN1 DERIVED FIBROBLASTS.
CD26 is a major identifying cell surface marker in the scar fibroblasts derived from En1 embryonic skin cells. When CD26 is targeted with a specific inhibitor, diprotin A, wounds will heal with significantly diminished scar collagen production. CD26 may also identify human scar fibroblasts and could be used as a therapeutic target.
5 ∣. EVIDENCE OF ADULT SCARLESS WOUND HEALING
In adult mammals, the oral mucosa represents a model of regenerative healing, where injury results in minimal to no scar formation. While inflammation at the wound bed is known to contribute to the formation of a scar, the oral mucosa, and fetal skin are relatively immune privileged during wound healing (Walraven, Talhout, Beelen, van Egmond, & Ulrich, 2016). Additionally, in the oral cavity, salivary mucins act as barriers protecting the underlying mucosa from mechanical damage and direct interaction with pathogens and other harmful substances (Amerongen & Veerman, 2002; Wong et al., 2009).
Analysis of healthy human oral mucosa reveals significantly lower numbers of neutrophils and macrophages compared with healthy skin (Glim, Beelen, Niessen, Everts, & Ulrich, 2015). The attenuated inflammatory microenvironment of the oral mucosa may partially explain the reduction in scar formation. But also, ECM composition prior to wounding may be a major contributor to scarless healing. Relative to skin, the oral mucosa exhibits increased fibronectin and its splice variant ED-A (Glim, Everts, Niessen, Ulrich, & Beelen, 2014). Interestingly, the pro-fibrotic myofibroblast ACTA2 (smooth muscle alpha actin) expression relies on cell surface ED-A fibronectin interaction and TGFB1 to initiate scar contraction (Tomasek, Gabbiani, Hinz, Chaponnier, & Brown, 2002). Conversely, the presence of elastin is more pronounced in the skin than the oral mucosa.
6 ∣. TARGETS OF SCARLESS SKIN REGENERATION
6.1 ∣. Growth factors
6.1.1 ∣. Wingless-type signaling
Wingless-type (WNT) signaling involves many highly evolutionarily conserved cell signaling pathways that control various phases of embryonic development. Though WNT activates three separate and independent signaling cascades, canonical WNT signaling has been the major focus of wound healing research and scarless fetal healing. This class of secreted glycoproteins, of which humans have 19 individual subtypes, acts on cells locally to regulate proliferation and differentiation (Leavitt et al., 2016). In the most basic signaling cascade, WNT binds to the frizzled cell receptor on WNT-responsive cells to initiate the transcriptional activation of β-catenin, a protein that once translocated to the nucleus in high concentrations forms a protein complex with TCF/LEF (T-cell factor/lymphoid enhancer factor). TCF then activates transcription of several growth factors involved in embryonic development, organogenesis, and tissue repair and regeneration (Houschyar et al., 2015).
In adult dermal tissue, many WNT proteins (WNT 1, 3, 4, 5, and 10)—both canonical and otherwise—are activated during normal wound healing, similar to cell proliferation signals for dermal fibroblasts and keratinocytes (Whyte et al., 2013). WNT responsive cells in dermal tissue include hair follicle bulge cells, basal interfollicular epidermal cells, and dermal fibroblasts. In each of these locations, WNT responsive cells represent a population capable of recruitment for re-epithelialization and the development of granulation tissue (Reya & Clevers, 2005). In mouse fetal skin, WNT4, WNT5a, and WNT11 are expressed in the dermis and play important roles in hair follicle morphogenesis (Whyte et al., 2013). Additionally, WNTs interact with a number of other important embryonic proteins, including SHH (sonic hedgehog), BMP (bone morphogenic protein), and NOTCH (Reya & Clevers, 2005).
Recently, the study of wound-induced hair follicle neogenesis, a phenomenon restricted to murine skin wound healing, revealed the role of FGF9 (fibroblast growth factor 9) and WNT2A in murine dermal wound healing (Gay et al., 2013). Through a signaling cascade initiated by resident dermal γδ T cells, mice can develop new hair follicles in the base of a scar likely through initiating a communication between epidermal keratinocytes and dermal fibroblasts (Gay et al., 2013). Coming from these studies, recombinant WNT proteins have emerged as a potential therapeutic (Whyte et al., 2013). Specifically, WNT3A has shown promising results as a vulnerary agent, where several studies demonstrated its ability to enhance healing of full-thickness cutaneous wounds (Whyte et al., 2013). However, WNT’s usual obligate palmitate appendage often renders the protein hydrophobic and therefore unreliable when delivered to living tissues (Clevers, Loh, & Nusse, 2014). While still an attractive study candidate for scarless wound healing, reliable recombinant WNTs are difficult to generate (Carthy, Engstrom, Heldin, & Moustakas, 2016).
6.1.2 ∣. Transforming growth factor beta
The transforming growth factor beta (TGFB) superfamily receives attention for its ability as a ubiquitous growth factor in mammalian wound healing. The TGFB group has three major subtypes, 1, 2, and 3, each intimately involved in the process of cell recruitment, proliferation, differentiation, migration, and potentially immune modulation (Lichtman, Otero-Vinas, & Falanga, 2016). These proteins, while having striking structural homology, are thought to exhibit different and potentially competing actions in the wound environment. In vivo, the TGFB subtypes have specific temporal and spatial distributions—potentially indicating their individual roles in wound healing and regenerative repair. Also, an important part of TGFB function is that it can bind to and be stored on the ECM, where until cleaved by serum proteases, it remains quiescent and as an indolent reservoir for augmenting wound healing when provoked by injury (Lichtman et al., 2016).
The TGFB subtypes 1 and 3 are perhaps the most important in wound healing, with TGFB1 being involved in the scarring mechanism and implicated in the development of systemic sclerosis, while TGFB3 is thought to be anti-fibrotic and pro-regenerative. TGFB1 also plays an important role in the early phases of adult wound healing after being released from activated platelets. Once released, TGFB1 recruits macrophages and other inflammatory cells to the wound where it functions as a pro-inflammatory mediator, ultimately perpetuating the fibrotic healing mechanism. TGFB3, however, may play a role in fetal scarless wound healing (Lichtman et al., 2016). In the adult wound environment, TGFB3 appears in the early and later phases of wound healing where it aids in macrophage recruitment, ECM deposition, and may reduce cell proliferation. In the fetal environment, many studies revealed elevated levels of TGFB3 relative to the amount of TGFB1 and TGFB2 seen in adult wound environments, potentially pointing to TGFB3 as a pro-regenerative cellular signal (Occleston et al., 2011). However, recent studies highlight the importance of each of the TGFB subtypes in adult and fetal wound healing. While proportions of TGFB subtypes do differ in fetal and adult wounds, attempting to alter these ratios does not significantly impact adult wound healing. In part, this may be because the TGFB subtypes have different functions in adult and fetal healing that are not explained by concentration or ratio alone (Penn, Grobbelaar, & Rolfe, 2012). Yet, adult wounds treated with TGFB1 inhibitors tend to heal with an improved appearance and smaller resultant scars, while those treated with recombinant TGFB3 reveal the same outcome (Shah et al., 1995).
Attempts at enhancing expression or delivery of TGFB proteins are unsuccessful to date. Between 2008 and 2011, Avotermin received press as a recombinant TGFB3 for improved scar formation in acute wounds and scar revisions (So et al., 2011). Unfortunately, this product failed phase III clinical trials and has subsequently been abandoned. Ultimately, study of the TGFB pathway is necessary for achieving a complete understanding of the wound healing mechanism. But given the involvement of TGFB in a number of complex cellular pathways (Hameedaldeen, Liu, Batres, Graves, & Graves, 2014), targeting TGFB as a wound repair therapeutic is problematic.
6.1.3 ∣. Fibroblast growth factor 9
Another important group of growth factors in wound healing are the fibroblast growth factors (FGFs). FGFs target fibroblasts and several other cell types involved in wound healing to induce proliferation, differentiation, and cell migration. In humans, 20 fibroblast growth factor subtypes exist, each structurally similar to the next but with a distinct biological function (Ornitz & Itoh, 2015). As FGF receptors are tyrosine kinases, they can directly influence intracellular activity, making them important in wound healing research (Potthoff, Kliewer, & Mangelsdorf, 2012).
In the fetus, FGFs are highly involved in organogenesis and cell differentiation. FGFs also control cell signaling cascades that influence the development of many organs including the limbs, lungs, brain, inner ear, liver, and pancreas (Ornitz & Itoh, 2015; Potthoff et al., 2012). Locally in the adult, FGFs influence wound healing by creating feedback loops to initiate fibroblast growth and differentiation. As is commonly the case, FGFs are closely associated with the ECM and are usually bound to heparin sulfate proteoglycans, limiting the growth factor’s systemic potential (Ornitz & Itoh, 2015).
Though many FGFs are involved in wound healing and embryonic development, FGF9 has emerged as an exciting new target due to its role in wound-induced hair follicle neogenesis (Gay et al., 2013). Activation of cells by FGF9 often leads to downstream upregulation of other FGFs, including FGF10, and also upregulation of the WNT2A/β-catenin pathway (Ornitz & Itoh, 2015). Through these signaling cascades, FGF9 may be able to re-establish a communication between dermal and epidermal tissues, resulting in invagination of the epidermis and the eventual development of new hair placodes (Gay et al., 2013). As a result of these findings, current experimental treatments with FGF9 focus on male patterned baldness (Fan et al., 2011). However, FGF9 may also be an important new target for wound healing since it is the only small molecule capable of regenerating skin appendages.
6.1.4 ∣. Hypoxia inducible factor 1 alpha subunit
The transcription factor hypoxia-inducible factor-1 (HIF1), composed of a dimer of an alpha and a beta subunit, plays a major role in cellular adaptation and survival under hypoxic stress. The subunits of HIF1 bind together to acquire transcriptional properties, allowing it to regulate the transcriptional activity of hundreds of genes that promote cell survival in hypoxic conditions. A master regulator of oxygen homeostasis, HIF1 acts predominantly under hypoxic conditions, such as in the wound bed with its disrupted vascular supply. The HIF1B subunit is constitutively expressed whereas the HIF1A subunit is oxygen regulated (Semenza, 2011a). Regulation of HIF1 is thus determined by the rapid posttranslational degradation or stabilization of the HIF1A subunit (Semenza, 2010). In normal tissue oxygen conditions, HIF1A is rapidly and continuously degraded following translation (Tamama et al., 2011). Tissue hypoxia, however, induces a sustained increase in the expression of HIF1A. Levels of HIF1A protein increase exponentially as oxygen concentration declines (Semenza, 2011b).
Pathologic scars, including keloid and hypertrophic scars, are the result of an excessive fibrotic response that surpasses the rate of remodeling (Craig, 1975). Biopsies of these pathologic scars often demonstrate elevated levels of growth factors and upregulation of their receptors (Schmid, Itin, Cherry, Bi, & Cox, 1998). Consistent elevation of HIF1A protein levels resulting from increased transcription and translation, followed by stabilization of the HIF1A subunit, is observed in keloid and scleroderma tissues compared with normal skin (Distler et al., 2007), making this a potential target in wound healing and remodeling.
The complex nature of the HIF1 pathway and its critical role in cellular oxygen homeostasis limits the potential for molecular-targeted inhibition for applications in scarless wound healing. Many compounds described as HIF1A inhibitors are only effective at anti-proliferative and cytotoxic concentrations, limiting the clinical relevance of such studies (Giaccia, Siim, & Johnson, 2003; Semenza, 2003; Welsh & Powis, 2003). Translation of targeted HIF1A inhibition for scarless wound healing necessitates that future studies evaluate therapeutically useful HIF1A inhibitors and exclude cytotoxic compounds.
6.1.5 ∣. Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is a key factor in angiogenesis, another important component of wound healing. It is a homodimeric glycoprotein that regulates the permeability of blood vessels. In pathologic scars, expression of VEGF and the density of blood vessels tend to be higher than in normal skin (Bock, Schmid-Ott, Malewski, & Mrowietz, 2006; Ong et al., 2007). Several recent studies suggest a link between the surplus capillary growth seen in healing wounds and scar formation. In one such study, neutralization of VEGF via antibody treatment caused an approximately 50% reduction in peak wound vascularity in adult skin wounds. These treated wounds closed normally and showed a significant reduction in wound scar width (Wilgus, Ferreira, Oberyszyn, Bergdall, & Dipietro, 2008). More recent studies suggest an association of robust capillary growth with the development of keloids (Mogili et al., 2012). Moreover, a recent comparison of capillary content in human normotrophic and hypertrophic scars demonstrated that hypertrophic scar formation is associated with higher levels of angiogenesis (van der Veer et al., 2011). This evidence has led to the suggested use of anti-angiogenic therapy to reduce scar formation (Diao, Xia, & Guo, 2010; Wilgus et al., 2008).
Bevacizumab (Avastin, Roche Pharma, Basel, Switzerland) is an anti-VEGF-A monoclonal antibody. It is the first angiogenesis inhibitor approved by the U.S. Food and Drug Administration for the prevention of tumor growth and it is widely used in the management of diabetic retinopathy. Although bevacizumab reduces scar formation, it does have adverse effects including headache, high blood pressure, increased risk of bleeding, and intestinal perforation. Further research should be performed to reveal the mechanisms underlying its effect on cutaneous scar formation and to ensure that bevacizumab may be used as an anti-scarring therapy, without inducing these or other adverse effects.
6.2 ∣. Cytokines
6.2.1 ∣. Tumor necrosis factor-alpha
Tumor necrosis factor-alpha (TNFA) is a cytokine chiefly produced by macrophages that plays a critical role in the inflammatory response. In the context of wound healing, TNFA secreted by macrophages leads to the recruitment of inflammatory cells such as neutrophils (Olutoye, Zhu, Cass, & Smith, 2005). Interestingly, TNFA levels are elevated in chronic wounds (Mast & Schultz, 1996), suggesting that it may have a detrimental effect on healing and may perpetuate inflammation and inhibit tissue growth. However, the direct effects of elevated TNFA levels on healing are unclear, with some studies suggesting impairment of healing processes (Rawdanowicz, Hampton, Nagase, Woolley, & Salamonsen, 1994; So, Ito, Sato, Mori, & Hirakawa, 1992; Unemori, Hibbs, & Amento, 1991) and others suggesting pro-healing effects (Barrientos, Stojadinovic, Golinko, Brem, & Tomic-Canic, 2008; Brauchle, Angermeyer, Hubner, & Werner, 1994; Kristensen et al., 1993). Inhibition of TNFA signaling using a neutralizing antibody improved the rate of re-epithelialization in a mouse wound model (Ashcroft et al., 2012). Unfortunately, the effects of TNFA modulation on human wound healing are less studied.
In a non-controlled human trial, patients with non-healing wounds that failed to respond to standard therapies were treated with TNFA inhibitor infliximab. The majority of test subjects experienced improved wound closure within 8 weeks (Streit, Beleznay, & Braathen, 2006). In a separate non-controlled study, patients with chronic venous leg ulcers were treated with the TNFA inhibitor adalimumab (Fox et al., 2016). Several of the wounds demonstrated healing during the study period, and the degree of healing correlated with a reduction of TNF staining seen on immunohistochemical examination of wound biopsies. From these studies, TNFA inhibition may be useful in the treatment of chronic wounds; however, high-quality randomized trials are needed before any conclusion can be made about its efficacy, particularly in acute wound healing.
6.2.2 ∣. Interleukins
Interleukins are cytokines that regulate the function of immune cells. As such, they are ideal targets to mediate inflammation and ultimately wound healing. Interleukin-1 (IL1) is a key regulator of the acute phase of inflammation (McKay & Leigh, 1991). Studies have repeatedly demonstrated its ability to induce inflammatory cell and keratinocyte migration (Beck, Habicht, Benach, & Miller, 1986; Granstein, Margolis, Mizel, & Sauder, 1986; Sauder et al., 1989) and fibroblast proliferation (Schmidt, Mizel, Cohen, & Green, 1982). IL1 also promotes the production of pro-fibrotic cytokines like TGFB and IL6 (Aoki et al., 2006). As such, pharmacologic antagonism of the IL1 receptor (IL1R) could prove beneficial in the development of scarless wound healing products. To date, studies show that targeting the IL1R with antagonists or antibodies reduces fibrosis and accelerates reepithelization in mice in vivo (Mirza, Fang, Ennis, & Koh, 2013; Thomay et al., 2009; Yan et al., 2016) but these products have yet to be tested in human subjects.
Another interleukin of interest is the anti-inflammatory and anti-fibrotic cytokine IL10. Several studies demonstrate that fetal skin expresses increased IL10 relative to adult skin (Gordon et al., 2008; Liechty, Kim, Adzick, & Crombleholme, 2000; Yamamoto, Eckes, & Krieg, 2001) and specifically, that IL10 is necessary for scarless fetal skin repair (Liechty et al., 2000). Early studies demonstrated the ability of IL10 to downregulate pro-inflammatory cytokines like IL6 and IL8 (Gordon et al., 2008), while more recent evidence describes fibroblast-specific STAT3 (signal transducer and activator of transcription 3)-dependent regulation of hyaluronan metabolism (Balaji et al., 2016). Ultimately, most evidence points to IL10 as a mediator of ECM remodeling (Balaji et al., 2016; Gordon et al., 2008; Reitamo, Remitz, Tamai, & Uitto, 1994), an area with increasing research interest.
In clinical trials, IL10 has been shown to reduce scar formation in murine (Gordon et al., 2008; Kieran et al., 2013; Peranteau et al., 2008) and human research subjects (Kieran et al., 2013, 2014). Though the ideal timing and dosing of IL10 administration has not yet been determined in humans, studies suggest that scar appearance is optimized with low concentrations of IL10 administered multiple times over the course of scar maturation, likely influencing scar remodeling over time (Kieran et al., 2013, 2014). Additional studies could further legitimatize IL10 as a wound healing therapy.
6.3 ∣. Enzymes
6.3.1 ∣. Lysyl oxidase
Lysyl oxidase (LO) is secreted as a 50 kDa proenzyme and then proteolytically cleaved to the 32 kDa active enzyme. It catalyses inter- and intra-molecular crosslinking in collagen and elastin by oxidatively deaminating lysyl residues in these proteins into peptidyl aldehydes (Kagan & Li, 2003). These aldehydes can then spontaneously condense with one another to yield a variety of covalent cross-linkages. Previous studies have reported increased lysyl oxidase expression associated with increased scar formation, congruent with the known role of lysyl oxidase in fibrotic cutaneous disorders such as scleroderma and hypertrophic scar formation (Fushida-Takemura et al., 1996). Lower lysyl oxidase expression in fetal scarless wounds likely results in less cross-linking and thus less accumulation of collagen.
LO is regulated by a variety of factors including serum conditions, TGFB1, and shear stress (Ando et al., 1996; Boak et al., 1994; Gacheru et al., 1997). A commonly used inhibitor of LO is BAPN (β-aminopropionitrile) (Tang, Trackman, & Kagan, 1983). Other inhibitors include diamines, heparin, amino nitrites, semicarbazides, and hydrazines (Gavriel & Kagan, 1988; Udupa, 1995). Molecular inhibition of LO during wound healing reportedly results in diminished tensile strength in addition to collagen content. However, further studies are necessary to evaluate the efficacy of these compounds, as they have yet to be tested using appropriate in vivo models of excisional wound healing or hypertrophic scar formation.
6.3.2 ∣. Matrix metalloproteinases
Studies to date have thoroughly evaluated the role of matrix metalloproteinases (MMP) in scarless fetal wound healing. MMPs are proteases that play a significant role in ECM remodeling (Lee & Murphy, 2004). Since the fetal ECM is distinct from the adult ECM (Chin et al., 2001; Lovvorn et al., 1999), it follows that fetal MMP expression differs from that of the adult. The expression of MMPs also differs between uninjured and wounded skin (Chen, Fu, Ge, Sun, & Sheng, 2007; Dang et al., 2003). For example, MMP2 (gelatinase A), MMP9 (gelatinase B), known as the type IV collagenases, degrade collagen in the basement membrane in addition to other minor cleavage roles in the ECM. MMP2 and MMP9 expression is low in the uninjured skin of the early fetus, and high in the adult (Chen et al., 2007), but the opposite is true in wounded skin (Dang et al., 2003). In addition, wound healing studies in adult Mmp9 knockout mice did not show any impairment in cutaneous wound healing (Liu et al., 1998), highlighting the complexity of scar formation in adult skin.
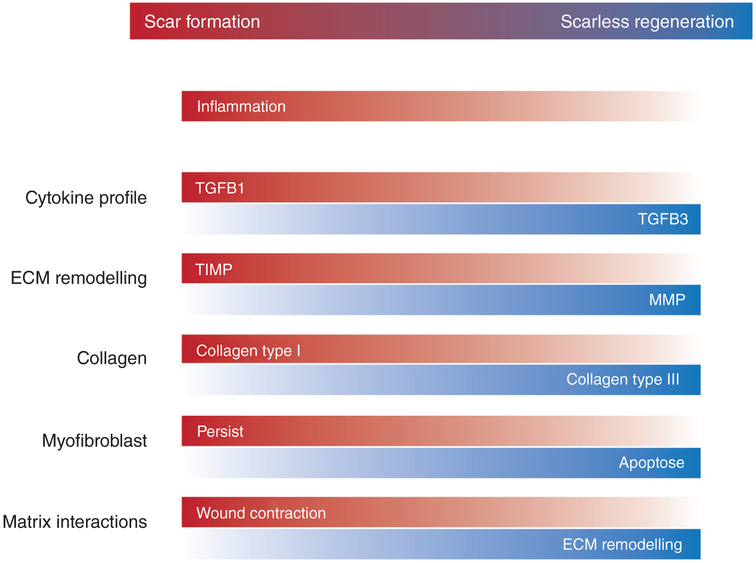
Research efforts demonstrate that MMP regulation can improve hypertrophic scarring models in rabbits (Li, Kilani, Rahmani-Neishaboor, Jalili, & Ghahary, 2014; Wang et al., 2014). Though there are many studies that characterize differences in MMP composition in both animals and humans (Gawronska-Kozak, 2011; Manuel & Gawronska-Kozak, 2006; Tanriverdi-Akhisaroglu, Menderes, & Oktay, 2009; Witte et al., 1998), clinical trials in humans have yet to be performed. Overall, while MMPs appear to be a promising target, the complexity of their interactions with each other and at different times in the wound healing process may prove challenging when developing a therapy (Figure 3).
FIGURE 3.
Features of reparative scar formation and scarless regeneration in wound healing. ECM = extracellular matrix, MMP = matrix metalloproteinase; TGFB = transforming growth factor beta; TIMP = tissue inhibitor of metalloproteinase (Reprinted with permission from Leavitt et al., 2016. Copyright 2016 Springer)
6.4 ∣. ECM Components
6.4.1 ∣. Hyaluronic acid
Hyaluronic acid (HA) is a glycosaminoglycan present in normal and wounded ECM and is distinguishable by its large molecular size (Vigetti et al., 2014). Since HA is highly expressed in fetal wounds compared to adult wounds, this molecule may be useful as a therapeutic agent to reduce scarring. Almost all human cells express the surface receptor CD44 that binds to HA as well as to other ECM molecules (Litwiniuk, Krejner, Speyrer, Gauto, & Grzela, 2016). In their interaction, HA and CD44 may modulate the inflammatory response of immune cells as well as promote fibroblast migration into wounded tissue (Litwiniuk et al., 2016). In vitro studies also indicate that HA directly modulates the expression of genes related to ECM production in fibroblasts (Mast, Diegelmann, Krummel, & Cohen, 1993).
Because of the central role played by hyaluronic acid in wound healing, there is widespread interest in its use as a therapeutic agent. Several commercial products utilize HA and its derivatives, particularly in the setting of chronic non-healing wounds. In one randomized trial, 34 patients with diabetic foot ulcers were treated with either an HA-based dressing or a conventional gauze dressing (Lee, Han, Choi, Chung, & Lee, 2016). Subjects treated with HA demonstrated greater rates of complete healing within the study period, as well as greater healing velocity. In a separate study, patients with second-degree burns were randomized to receive a topical cream containing either HA plus silver sulfadiazine (SSD) versus SSD alone (Costagliola & Agrosi, 2005). Those treated with the HA-SSD cream experienced shorter times to re-epithelialization. Several observational studies without control arms have shown satisfactory healing rates in wounds treated with HA products (Caravaggi, Grigoletto, & Scuderi, 2011; Gravante et al., 2007; Voinchet, Vasseur, & Kern, 2006), but it is impossible to determine from these studies whether the HA product truly improves healing.
Based on the important role that HA plays in wound healing, and evidence of a beneficial effect in chronic wounds, it is likely that HA-based products will serve as important tools for clinicians caring for patients with chronic wounds. To date, however, there is far less evidence that HA may be used in normally healing wounds to reduce scarring, and it has been incapable of promoting regeneration in adult skin.
6.4.2 ∣. Type III Collagen
Histologic examinations of fetal wounds and skin consistently demonstrate a higher ratio of Type III to Type I collagen (Beanes et al., 2002). Type III collagen (COL3) is homotrimeric fibril-forming collagen believed to regulate Type I collagen (COL1) fibril diameter, altering the physical properties of tissue (Liu, Wu, Byrne, Krane, & Jaenisch, 1997). Recent studies in mice show that inhibiting COL3 leads to increased scarring potentially through excess COL1 deposition or organization (Volk, Wang, Mauldin, Liechty, & Adams, 2011). Additionally, hypertrophic scarring is associated with excess COL1 deposition (Colwell, Phan, Kong, Longaker, & Lorenz, 2005). In practice, collagen can be used to augment the wounded ECM through the use of tissue engineered scaffolds that allow cells to attach, grow, and differentiate (Hsu, Hung, Liou, & Shen, 2010). COL1 may also be used as a therapy itself to coat nanofiber matrices providing accelerated wound healing (Rho et al., 2006). However, COL3 might be a more appropriate target for reduced or scarless wound healing. To consider COL3 or COL1 alone as a clinical target may not be relevant due to the density of cell signaling molecules, cells, and other matrix proteins that augment and regulate cellular signaling, cytokine release, cell migration, and scar maturation within the ECM (Vorotnikova et al., 2010). However, the histological assessment of COL1 and COL3 remains the standard of measuring outcomes in scarring research today (Cuttle et al., 2005; Gawronska-Kozak & Kirk-Ballard, 2013).
6.5 ∣. Cell surface receptors
6.5.1 ∣. Integrins
ECM adhesion proteins are particularly important for facilitating responses to the wound environment by embedding cells in the neo-ECM following cutaneous injury. Of the many adhesion proteins on the cell surface, the integrin family of receptors is the most abundant facilitator of cell-ECM interactions. Integrins are heterodimeric glycoproteins with two subunits, one comprised of 18 α-subunits and one of eight β-subunits (Eckes, Nischt, & Krieg, 2010). Interestingly, integrin protein expression differs in fetal fibroblasts and adult fibroblasts (Cass et al., 1998). In the fetus, there is increased α2 subunit expression coupled with decreased α1 and 3 expression when compared to the adult (Moulin & Plamondon, 2002).
In response to injury in adult skin, integrin expression is modified and upregulated to mediate fibroblast and keratinocyte adhesion and migration in addition to epidermal proliferation (DiPersio, Zheng, Kenney, & Van De Water, 2016). Notably, the β1 integrin is necessary for keratinocyte migration and wound closure. However, redundancy in integrin receptors makes it difficult to determine the specific roles of individual integrins in vivo (Kenny & Connelly, 2015).
Though it is difficult to target integrins themselves, studies demonstrate successful suppression of downstream signaling. The addition of lumican, a small leucine-rich proteoglycan (SLRP) detected in the ECM, was shown to suppress hypertrophic scarring in rabbits by inhibiting collagen-integrin α2β1-FAK signaling (Zhao et al., 2016). Though current knowledge is limited, future investigations into modulation of integrin adhesion protein activity could be used to improve cutaneous wound repair.
6.5.2 ∣. CD26
CD26, also known as DPP4 (dipeptidyl peptidase 4) is a cell surface marker that is implicated in fibrotic conditions in various organs including liver (Kaji et al., 2014) and kidney (Min et al., 2014). Rinkevich et al. (2015) showed that a lineage of fibroblasts that form scar collagen in healing wounds highly express CD26, while the fibroblasts that form normal collagen in uninjured skin do not. Inhibition of CD26 with a small molecule inhibitor reduced scar formation after excisional wounding without slowing the rate of healing. Interestingly, elevated CD26 levels are also seen in burn exudate in human patients (Prager, Sabeh, Baxter, Atiles, & Hartline, 1994), but there is little human evidence of CD26 expression in scar forming fibroblasts. From these studies, CD26 is emerging as a new clinical target in wound healing. Though its role is not well understood, it may be an attractive target for reducing scar formation. To date, no studies on CD26 inhibition in the setting of human wound healing have been performed.
6.6 ∣. Tissue and cell therapy
In the clinic, scaffolds and cell therapies are often used in the setting of refractory chronic wounds and acute burn injuries (burns less than 1 week after injury, status post- or pre-debridement). For acute wounds (clean noncontaminated wounds, less than 48 hr after injury), reliance on engineered products is less prevalent. In general, engineered products fall into any one of several categories: they may be synthetic or natural, two or three dimensional (3D), and may or may not involve the use of autologous or transplanted cells.
6.6.1 ∣. Two- and three-dimensional scaffolds
Scaffolds mimicking the fetal or adult ECM can be used to recapitulate the fetal wound healing environment. Often, it is extrapolated that the loss of normal three-dimensional architecture may play a significant role in signaling scar tissue formation. In contrast, in the fetus where organizational cues abound, tissue can be regenerated without apparent healing defects. As mentioned in prior sections, the ideal scaffold should provide adequate structural and mechanical support while also allowing for cellular adhesion, proliferation, and differentiation (Zhong, Zhang, & Lim, 2010). In general, scaffolds can be divided by material composition into natural polymers, synthetic polymers, and composites.
Two-dimensional scaffolds are often used to aid in debridement, and/or to deliver therapeutic compounds. Most often, two-dimensional scaffolds are used in the setting of chronic wounds, where chronic skin barrier breakdown makes healing difficult. Three-dimensional scaffolds are used in slightly more physiologically challenging scenarios, such as burn injuries, where there may not be enough soft tissue available to cover an injury with an autologous tissue transplant. In these settings, scaffolds that mimic the three-dimensional architecture of skin are instrumental, and can serve as a medium to accept and protect skin cell allografts. Finally, three-dimensional scaffolds such as acellular dermal matrices are valuable in situations of extreme soft tissue damage and loss, and also in situations where tensile strength preservation is paramount, for instance, in hernia repair.
As mentioned before, collagen, a natural polymer, may be used to create ECM scaffolds. Other advancements in the fields of tissue engineering and biochemistry led to the creation of synthetic matrices as well (Benedetti et al., 1993). One such material used for matrices and scaffolds is semisynthetic HYAFF, which is biocompatible and biodegradable (Milella et al., 2002). With the ability to be fashioned into different structures depending on the therapeutic need, synthetic matrices can be used for sustained drug release, delivery of small molecules such as growth factors, and application of cells to any injured area (Zavan et al., 2009). This has proved invaluable in burn victims, allowing for cultured epithelial autografts to be applied (Atiyeh & Costagliola, 2007). One of the latest developments has been the use of siRNA within a silicon bilayer to block the TGFB1-mediated pathway, resulting in downregulation of COL1, COL3, and ACTA2—ultimately leading to inhibited scar formation during skin regeneration (Liu et al., 2013). Also, since the development of 3D printing technology, the ability of 3D scaffolds to accurately mimic skin has improved significantly (Michael et al., 2013). Advancements in 3D printing has led to more complex natural and synthetic matrix production, but most excitingly, 3D printing has allowed for the development of 3D scaffolds comprised of printed cells. Several studies used 3D printers to develop scaffolds entirely comprised of adult keratinocytes and fibroblasts. Potentially, this technology could also harness and incorporate stem cell populations with the aim of ongoing tissue renewal and scarless healing (Abaci, Guo, Doucet, Jackow, & Christiano, 2017; Kim, Lee, Gao, & Cho, 2017; Lee et al., 2014; Michael et al., 2013). With their promising clinical results, two dimensional and 3D scaffolds, whether synthetic or otherwise, continue to find new applications in wound healing (Table 2).
TABLE 2.
Clinically relevant biomaterials for wound healing
| Substrate | Origin | Composition | Clinical examples |
|---|---|---|---|
| ECM substitutes | Animal or plant tissue | Single to multilayer films with variable 3D architecture | Beriplast, Biocol, Hyaff, Hycoat, Hyalomatrix, Promogran, Puraply |
| Acellular dermal matrices | Porcine or human | Decellularized skin, chemically treated to achieve sterility and improve tensile strength | AlloDerm, DermaSpan, FlexHD, Surgimend |
| Synthetic compounds | Electrospinning, salt lithography, soft lithography stamping, solid free-form fabrication | Single and multi-compound materials can be impregnated with pharmaceuticals | Biobrane, Dermagraft, Integra |
| Cultured stem cells | Human amniotic membrane, dermal fibroblasts, BM-MSCs, ASCs, neonatal prepuce | Most often cells are embedded or seeded onto cultured scaffolds | Apligraf, denovoSkin, denovoDerm,Epicel, EpiFix, OrCel, StrataGraft, Transcyte, Triscover |
Table includes data from Hu et al. (2015) and Varkey, Ding, and Tredget (2015). ASC = adipose-derived stromal cell; BM = bone marrow; ECM = extracellular matrix; MSC = mesenchymal stem cell (Reprinted with permission from Moore, Marshall, & Longaker, 2017. Copyright 2017 MDPI). Link to license https://creativecommons.org/licenses/by/4.0/.
6.6.2 ∣. Neonatal stem cell therapy
Of the possible neonatal cell sources, umbilical cord epithelium (UCE), human amniotic epithelial cells, and human foreskin fibroblasts (HFF) have emerged as the most promising cell-based therapies for scarless wound healing. The UCE is structurally similar to the epidermis of the early fetus (Mizoguchi, Ikeda, Suga, & Ogawa, 2000). UCE can grow into a multilayered stratified epithelium (similar to cutaneous skin) when cultured or upon transplantation in vivo (Mizoguchi, Suga, Sanmano, Ikeda, & Ogawa, 2004). Surrounding the UCE is a gelatinous ECM known as Warton’s Jelly (WJ). Mesenchymal stem cells (MSC) isolated from WJ have been shown to promote wound healing through paracrine signaling (Arno et al., 2014).
More recently, human amniotic epithelial cells were identified as another source of stem cells capable of forming tissue-engineered skin with similar morphology to normal skin (Jiang, Chen, & Lu, 2016). Also, HFF are used in allogeneic grafting. They can be seeded onto scaffolds and applied directly to wounds to impact chronic or acute wound healing. Transfected HFF from newborn males may have a potential role in scarless healing, as they can be grafted into wounds to increase expression of TGFB3 (Mahmoudi Rad, Mahmoudi Rad, & Mirdamadi, 2015). HFF have been successfully used to form induced pluripotent stem cells, also increasing their potential therapeutic range (Skrzypczyk, Giri, & Bader, 2016). Shown to have more superior scarless healing capacity than HFF, fetal cells used to construct fetal dermal matrices have marked upregulation in genes responsible for scarless healing (Pouyani, Papp, & Schaffer, 2012). As the technology advances, the use of allogenic cells from fetal tissue may have increasing significance, particularly in diseased individuals in whom the burden of scarless wound healing by another mechanism may be too prolonged or taxing (Figure 4).
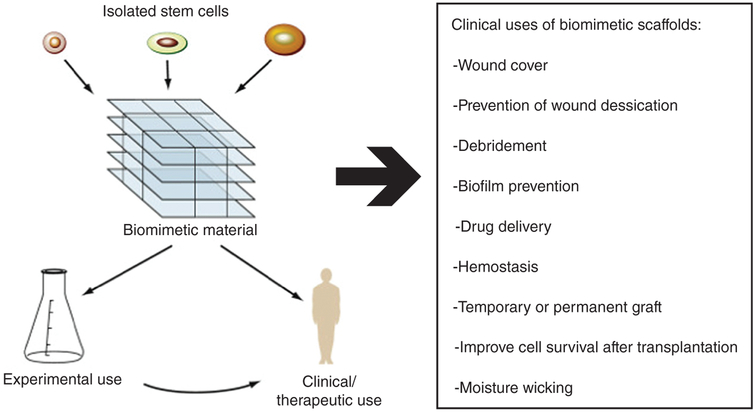
FIGURE 4.
Biomimetic materials are engineered to create favorable stem cell niches for in vitro experimental stem cell biology studies and for clinical use in regenerative medicine applications. Because all stem cells are exquisitely sensitive to environmental cues, the bioengineering component of regenerative medicine will be crucial to modulate and control stem cell behavior to allow effective cell-based therapies to be used clinically (Reprinted with permission from Townsend et al., 2017. Copyright 2017 Elsevier)
6.6.3 ∣. Adult stem cell therapy
With the ability of self-renewal and multilineage differentiation, MSCs are an attractive therapeutic consideration for many chronic diseases (Ding, Shyu, & Lin, 2011). MSCs have been shown to accelerate wound closure, reduce inflammation, improve angiogenesis, and positively augment the deposition of the ECM (Lee, Ayoub, & Agrawal, 2016). Some sources of stem cells for wound healing include: WJ, amnion, adipose tissue, and bone marrow. Directly compared, human adipose-derived stem cells (ADSC) have shown comparative results to human amniotic MSCs in inducing dermal fibroblast response to cutaneous wounds (Liu et al., 2013). Clinically more accessible and as efficacious, ADSC are being used in human clinical trials to assess their effect in wound healing (National Clinical Trial, NCT02590042). The future use of MSCs in scarless healing will depend largely on our understanding of how MSCs function in their niche environment (Wong, Gurtner, & Longaker, 2013). Additionally, the use of biological scaffolds in combination with the application of MSCs may allow for improved cellular migration, proliferation, and differentiation. However, the mechanical and structural components of these scaffolds will affect how the impregnated MSCs function, therefore making careful development paramount (Wang et al., 2010).
6.7 ∣. Targeting Mechanical Forces
Tension, compression, shear, and osmotic forces play an important role in cutaneous wound healing outcomes (Agha, Ogawa, Pietramaggiori, & Orgill, 2011). For example, incisions made parallel to Langer lines (naturally occurring bands of tension in human skin) experience reduced tension and typically heal with thinner scars containing less scar collagen than those placed perpendicular (Langer, 1978; Meyer & McGrouther, 1991; Silver, Siperko, & Seehra, 2003). In addition, areas of the body subjected to increased mechanical force, such as the back, sternum, and joints exhibit increased scar formation when compared to areas that experience reduced mechanical force, such as skin on the eyelid (Meyer & McGrouther, 1991; Wray, 1983).
Specifically, mechanical forces influence cell gene expression, proliferation, and migration (Chiquet, Tunc-Civelek, & Sarasa-Renedo, 2007; Eckes et al., 2006; Kadi, Fawzi-Grancher, Lakisic, Stoltz, & Muller, 2008; Khetan et al., 2013; Yang, Tibbitt, Basta, & Anseth, 2014). These extracellular cues induce changes in mechanosensors (including FAK-SRC [the dual focal adhesion-tyrosine kinase complex], ROCK [Rho associated coiled-coil containing protein kinase], MAPK1 [mitogen-activated protein kinase 1], and PI3K [phosphoinositide 3-kinase] calcium/calcineurin) and ultimately influence gene expression (Das et al., 2015; Januszyk et al., 2014; Kenny & Connelly, 2015; Takada, Furuya, & Sokabe, 2014; Wang et al., 2015; Wong, Akaishi, Longaker, & Gurtner, 2011; Wong, Longaker, & Gurtner, 2012). Fibroblasts subjected to mechanical cues (e.g., suction, cyclical stretching) in vitro have been shown to exhibit increased proliferation, upregulation of genes typically expressed by fibroblasts (e.g., collagen 1alpha 1, FGF2, TGFB), increased migration, and reduced apoptosis via mechanisms related to the ITG and WNT mechanotransduction pathways (Huang et al., 2013; Lu et al., 2011; Webb et al., 2006). In vivo, mechanical stress in the early phases of cutaneous wound healing reduces AKT-dependent apoptosis, resulting in hypertrophic scaring (Aarabi et al., 2007).
The mechanotransduction signaling pathway is an ideal target for researchers attempting to achieve the scarless ideal partly because these same forces are not in play in the relatively immobile fetal environment. Techniques to offload mechanical stresses in the wound environment, such as Embrace Advanced Scar Therapy®, have been shown to reduce scar formation in pigs, and humans (Aarabi et al., 2007; Agha et al., 2011; Huang et al., 2013; Huang, Holfeld, Schaden, Orgill, & Ogawa, 2013; Lim et al., 2014; Longaker et al., 2014; Lu et al., 2011; Wang et al., 2015; Webb et al., 2006; Wong et al., 2011; Wong, Beasley, et al., 2013; Wong, Gurtner, et al., 2013). Further research into the field of mechanotransduction in wound healing could offer exciting new insights into the mechanism of scarless healing.
7 ∣. CONCLUSION
By discussing the major experimental and clinical targets aimed at achieving scarless wound healing, it is evident that (1) none have been successful in regenerating all dermal appendages and (2) most can reduce scar thickness and appearance, but will not produce histological or functional similarity to uninjured skin. The study of fetal wound healing offers promising insights, with many obvious dissimilarities between fetal and adult wounds. However, few clinical tools mimic the fetal wound healing environment to reduce the adult fibrosis mechanism or cell signaling response. For instance, dermal substitutes often mimic adult uninjured skin but do not often contain the growth factors, cytokines, or other components of developing skin. Additionally, targeting components of adult healing, like inflammation, focuses our attention on adult cells instead of the few fetal inflammatory cells involved in in utero wound healing. Novel technology, such as tension offloading, and new molecular targets, such as CD26, offer promising advancements in our understanding of the adult skin healing mechanism. Ultimately, it is unlikely that dermal regeneration in adults will be accomplished without a complete understanding of dermal development in the fetus and of fetal scarless wound healing.
ACKNOWLEDGMENTS
A.L.M., MD’s research is supported by the Society of University Surgeons Resident Scholar Award as well as the Stanford University Tissue and Transplant Engineering Center of Excellence Program Fellowship. C.D.M., MD is supported by the American College of Surgeons Resident Research Scholarship, the Stanford University Child Health Research Institute, and the Stanford University Transplant and Tissue Engineering Center of Excellence. L.A.B., BA is a Howard Hughes Medical Institute Medical Research Fellow. Special Thanks to Tripp Leavitt, MD for his artistic contributions to our group. A.L.M., MD was the primary author, editor, and researcher. C.D.M., MD contributed the next most significant authorship. The remaining authors (Barnes, Murphy, Ransom) contributed equally to the manuscript and thus are listed in alphabetical order. M.T.L., MD MBA was the senior author, editor, and corresponding author.
Funding information
Stanford University Transplant and Tissue Engineering Center of Excellence; Stanford University Child Health Research Institute; American College of Surgeons Resident Research Scholarship; Stanford University Tissue and Transplant Engineering Center of Excellence Program Fellowship; Society of University Surgeons Resident Scholar Award
Footnotes
CONFLICT OF INTEREST
Michael T. Longaker is a founder and has an equity position in Neodyne Biosciences, Inc.
FURTHER READING
Atala, A., Lanza, R., Thomson, J., & Nerem, R. (2011). Scarless wound healing. In Principles of regenerative medicine (2nd ed.). London: Elsevier.
This article is categorized under:
Adult Stem Cells, Tissue Renewal, and Regeneration > Regeneration Plant Development > Cell Growth and Differentiation Adult Stem Cells, Tissue Renewal, and Regeneration > Environmental Control of Stem Cells
REFERENCES
- Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, … Gurtner GC (2007). Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. The FASEB Journal, 21, 3250–3261. [DOI] [PubMed] [Google Scholar]
- Abaci HE, Guo Z, Doucet Y, Jackow J, & Christiano A (2017). Next generation human skin constructs as advanced tools for drug development. Experimental Biology and Medicine (Maywood, N.J.) 242, 1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzick NS, & Longaker MT (1991). Animal models for the study of fetal tissue repair. The Journal of Surgical Research, 51, 216–222. [DOI] [PubMed] [Google Scholar]
- Adzick NS, & Longaker MT (1992). Scarless fetal healing. Therapeutic implications. Annals of Surgery, 215, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha R, Ogawa R, Pietramaggiori G, & Orgill DP (2011). A review of the role of mechanical forces in cutaneous wound healing. The Journal of Surgical Research, 171, 700–708. [DOI] [PubMed] [Google Scholar]
- Amerongen AV, & Veerman EC (2002). Saliva: The defender of the oral cavity. Oral Diseases, 8, 12–22. [DOI] [PubMed] [Google Scholar]
- Ando J, Tsuboi H, Korenaga R, Takahashi K, Kosaki K, Isshiki M, … Kamiya A (1996). Differential display and cloning of shear stress-responsive messenger RNAs in human endothelial cells. Biochemical and Biophysical Research Communications, 225, 347–351. [DOI] [PubMed] [Google Scholar]
- Aoki H, Ohnishi H, Hama K, Ishijima T, Satoh Y, Hanatsuka K, … Sugano K (2006). Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. American Journal of Physiology. Cell Physiology, 290, C1100–C1108. [DOI] [PubMed] [Google Scholar]
- Arno AI, Amini-Nik S, Blit PH, Al-Shehab M, Belo C, Herer E, … Jeschke MG (2014). Human Wharton’s jelly mesenchymal stem cells promote skin wound healing through paracrine signaling. Stem Cell Research & Therapy, 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Jeong MJ, Ashworth JJ, Hardman M, Jin W, Moutsopoulos N, … Wahl SM (2012). Tumor necrosis factor-alpha (TNF-alpha) is a therapeutic target for impaired cutaneous wound healing. Wound Repair and Regeneration, 20, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuku ME, Ibrahim A, & Ijekeye FO (2008). Post-burn axillary contractures in pediatric patients: A retrospective survey of management and outcome. Burns, 34, 1190–1195. [DOI] [PubMed] [Google Scholar]
- Atiyeh BS, & Costagliola M (2007). Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns, 33, 405–413. [DOI] [PubMed] [Google Scholar]
- Balaji S, Wang X, King A, Le LD, Bhattacharya SS, Moles CM, … Keswani SG (2016). Interleukin-10-mediated regenerative postnatal tissue repair is dependent on regulation of hyaluronan metabolism via fibroblast-specific STAT3 signaling. The FASEB Journal, 31, 868–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, & Tomic-Canic M (2008). Growth factors and cytokines in wound healing. Wound Repair and Regeneration, 16, 585–601. [DOI] [PubMed] [Google Scholar]
- Baum CL, & Arpey CJ (2005). Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatologic Surgery, 31, 674–686 discussion 686. [DOI] [PubMed] [Google Scholar]
- Beanes SR, FY H, Soo C, Dang CM, Urata M, Ting K, … Lorenz HP (2002). Confocal microscopic analysis of scarless repair in the fetal rat: Defining the transition. Plastic and Reconstructive Surgery, 109, 160–170. [DOI] [PubMed] [Google Scholar]
- Beck G, Habicht GS, Benach JL, & Miller F (1986). Interleukin 1: A common endogenous mediator of inflammation and the local Shwartzman reaction. Journal of Immunology, 136, 3025–3031. [PubMed] [Google Scholar]
- Benedetti L, Cortivo R, Berti T, Berti A, Pea F, Mazzo M, … Abatangelo G (1993). Biocompatibility and biodegradation of different hyaluronan derivatives (Hyaff) implanted in rats. Biomaterials, 14, 1154–1160. [DOI] [PubMed] [Google Scholar]
- Boak AM, Roy R, Berk J, Taylor L, Polgar P, Goldstein RH, & Kagan HM (1994). Regulation of lysyl oxidase expression in lung fibroblasts by transforming growth factor-beta 1 and prostaglandin E2. American Journal of Respiratory Cell and Molecular Biology, 11, 751–755. [DOI] [PubMed] [Google Scholar]
- Bock O, Schmid-Ott G, Malewski P, & Mrowietz U (2006). Quality of life of patients with keloid and hypertrophic scarring. Archives of Dermatological Research, 297, 433–438. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Angermeyer K, Hubner G, & Werner S (1994). Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene, 9, 3199–3204. [PubMed] [Google Scholar]
- Brown BC, McKenna SP, Siddhi K, McGrouther DA, & Bayat A (2008). The hidden cost of skin scars: Quality of life after skin scarring. Journal of Plastic, Reconstructive & Aesthetic Surgery, 61, 1049–1058. [DOI] [PubMed] [Google Scholar]
- Buchanan EP, Longaker MT, & Lorenz HP (2009). Fetal skin wound healing. Advances in Clinical Chemistry, 48, 137–161. [DOI] [PubMed] [Google Scholar]
- Burrington JD (1971). Wound healing in the fetal lamb. Journal of Pediatric Surgery, 6, 523–528. [DOI] [PubMed] [Google Scholar]
- Caravaggi C, Grigoletto F, & Scuderi N (2011). Wound bed preparation with a dermal substitute (Hyalomatrix(R) PA) facilitates re-epithelialization and healing: Results of a multicenter, prospective, observational study on complex chronic ulcers (the FAST study). Wounds, 23, 228–235. [PubMed] [Google Scholar]
- Carthy JM, Engstrom U, Heldin CH, & Moustakas A (2016). Commercially available preparations of recombinant Wnt3a contain non-Wnt related activities which may activate TGF-beta signaling. Journal of Cellular Biochemistry, 117, 938–945. [DOI] [PubMed] [Google Scholar]
- Cass DL, Bullard KM, Sylvester KG, Yang EY, Sheppard D, Herlyn M, & Adzick NS (1998). Epidermal integrin expression is upregulated rapidly in human fetal wound repair. Journal of Pediatric Surgery, 33, 312–316. [DOI] [PubMed] [Google Scholar]
- Chen W, Fu X, Ge S, Sun T, & Sheng Z (2007). Differential expression of matrix metalloproteinases and tissue-derived inhibitors of metalloproteinase in fetal and adult skins. The International Journal of Biochemistry & Cell Biology, 39, 997–1005. [DOI] [PubMed] [Google Scholar]
- Chin GS, Lee S, Hsu M, Liu W, Kim WJ, Levinson H, & Longaker MT (2001). Discoidin domain receptors and their ligand, collagen, are temporally regulated in fetal rat fibroblasts in vitro. Plastic and Reconstructive Surgery, 107, 769–776. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Tunc-Civelek V, & Sarasa-Renedo A (2007). Gene regulation by mechanotransduction in fibroblasts. Applied Physiology, Nutrition, and Metabolism, 32, 967–973. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, & Nusse R (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science, 346, 1248012. [DOI] [PubMed] [Google Scholar]
- Colwell AS, Phan TT, Kong W, Longaker MT, & Lorenz PH (2005). Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plastic and Reconstructive Surgery, 116, 1387–1390 discussion 1391-1382. [DOI] [PubMed] [Google Scholar]
- Costagliola M, & Agrosi M (2005). Second-degree burns: A comparative, multicenter, randomized trial of hyaluronic acid plus silver sulfadiazine vs. silver sulfadiazine alone. Current Medical Research and Opinion, 21, 1235–1240. [DOI] [PubMed] [Google Scholar]
- Craig RD (1975). Collagen biosynthesis in normal human skin, normal and hypertrophic scar and keloid. European Journal of Clinical Investigation, 5, 69–74. [DOI] [PubMed] [Google Scholar]
- Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, & Hayes MT (2005). Collagen in the scarless fetal skin wound: Detection with picrosirius-polarization. Wound Repair and Regeneration, 13, 198–204. [DOI] [PubMed] [Google Scholar]
- Dang CM, Beanes SR, Lee H, Zhang X, Soo C, & Ting K (2003). Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plastic and Reconstructive Surgery, 111, 2273–2285. [DOI] [PubMed] [Google Scholar]
- Das T, Safferling K, Rausch S, Grabe N, Boehm H, & Spatz JP (2015). A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nature Cell Biology, 17, 276–287. [DOI] [PubMed] [Google Scholar]
- Diao JS, Xia WS, & Guo SZ (2010). Bevacizumab: A potential agent for prevention and treatment of hypertrophic scar. Burns, 36, 1136–1137. [DOI] [PubMed] [Google Scholar]
- Ding DC, Shyu WC, & Lin SZ (2011). Mesenchymal stem cells. Cell Transplantation, 20, 5–14. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Zheng R, Kenney J, & Van De Water L (2016). Integrin-mediated regulation of epidermal wound functions. Cell and Tissue Research, 365, 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JH, Jungel A, Pileckyte M, Zwerina J, Michel BA, Gay RE, et al. (2007). Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis and Rheumatism, 56, 4203–4215. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, … Watt FM (2013). Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature, 504, 277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes B, Nischt R, & Krieg T (2010). Cell-matrix interactions in dermal repair and scarring. Fibrogenesis & Tissue Repair, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M, & Krieg T (2006). Mechanical tension and integrin alpha 2 beta 1 regulate fibroblast functions. The Journal of Investigative Dermatology. Symposium Proceedings, 11, 66–72. [DOI] [PubMed] [Google Scholar]
- Egeland B, More S, Buchman SR, & Cederna PS (2008). Management of difficult pediatric facial burns: Reconstruction of burn-related lower eyelid ectropion and perioral contractures. The Journal of Craniofacial Surgery, 19, 960–969. [DOI] [PubMed] [Google Scholar]
- Esselman PC (2007). Burn rehabilitation: An overview. Archives of Physical Medicine and Rehabilitation, 88, S3–S6. [DOI] [PubMed] [Google Scholar]
- Fan C, Luedtke MA, Prouty SM, Burrows M, Kollias N, & Cotsarelis G (2011). Characterization and quantification of wound-induced hair follicle neogenesis using in vivo confocal scanning laser microscopy. Skin Research and Technology, 17, 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein E, Corso PS, & Miller TR (2006). The incidence and economic burden of injuries in the United States. Oxford, New York: Oxford University Press. [Google Scholar]
- Forni MF, Trombetta-Lima M, & Sogayar MC (2012). Stem cells in embryonic skin development. Biological Research, 45, 215–222. [DOI] [PubMed] [Google Scholar]
- Fox JD, Baquerizo-Nole KL, Keegan BR, Macquhae F, Escandon J, Espinosa A, … Kirsner RS (2016). Adalimumab treatment leads to reduction of tissue tumor necrosis factor-alpha correlated with venous leg ulcer improvement: A pilot study. International Wound Journal, 13, 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E (2007). Scratching the surface of skin development. Nature, 445, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushida-Takemura H, Fukuda M, Maekawa N, Chanoki M, Kobayashi H, Yashiro N, … Ooshima A (1996). Detection of lysyl oxidase gene expression in rat skin during wound healing. Archives of Dermatological Research, 288, 7–10. [DOI] [PubMed] [Google Scholar]
- Gacheru SN, Thomas KM, Murray SA, Csiszar K, Smith-Mungo LI, & Kagan HM (1997). Transcriptional and post-transcriptional control of lysyl oxidase expression in vascular smooth muscle cells: Effects of TGF-beta 1 and serum deprivation. Journal of Cellular Biochemistry, 65, 395–407. [DOI] [PubMed] [Google Scholar]
- Gavriel P, & Kagan HM (1988). Inhibition by heparin of the oxidation of lysine in collagen by lysyl oxidase. Biochemistry, 27, 2811–2815. [DOI] [PubMed] [Google Scholar]
- Gawronska-Kozak B (2011). Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biology, 30, 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawronska-Kozak B, & Kirk-Ballard H (2013). Cyclosporin a reduces matrix metalloproteinases and collagen expression in dermal fibroblasts from regenerative FOXN1 deficient (nude) mice. Fibrogenesis & Tissue Repair, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, … Cotsarelis G (2013). Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nature Medicine, 19, 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Gomez NC, Adam RC, Nikolova M, Yang H, Verma A, et al. (2017). Stem cell lineage infidelity drives wound repair and cancer. Cell, 169, 636, e614–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccia A, Siim BG, & Johnson RS (2003). HIF-1 as a target for drug development. Nature Reviews. Drug Discovery, 2, 803–811. [DOI] [PubMed] [Google Scholar]
- Glim JE, Beelen RH, Niessen FB, Everts V, & Ulrich MM (2015). The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Archives of Oral Biology, 60, 272–281. [DOI] [PubMed] [Google Scholar]
- Glim JE, Everts V, Niessen FB, Ulrich MM, & Beelen RH (2014). Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Archives of Oral Biology, 59, 1048–1055. [DOI] [PubMed] [Google Scholar]
- Gordon A, Kozin ED, Keswani SG, Vaikunth SS, Katz AB, Zoltick PW, … Crombleholme TM (2008). Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10prevents scar formation. Wound Repair and Regeneration, 16, 70–79. [DOI] [PubMed] [Google Scholar]
- Granstein RD, Margolis R, Mizel SB, & Sauder DN (1986). In vivo inflammatory activity of epidermal cell-derived thymocyte activating factor and recombinant interleukin 1 in the mouse. The Journal of Clinical Investigation, 77, 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravante G, Delogu D, Giordan N, Morano G, Montone A, & Esposito G (2007). The use of Hyalomatrix PA in the treatment of deep partial-thickness burns. Journal of Burn Care & Research, 28, 269–274. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, & Longaker MT (2008). Wound repair and regeneration. Nature, 453, 314–321. [DOI] [PubMed] [Google Scholar]
- Hameedaldeen A, Liu J, Batres A, Graves GS, & Graves DT (2014). FOXO1, TGF-beta regulation and wound healing. International Journal of Molecular Sciences, 15, 16257–16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houschyar KS, Momeni A, Pyles MN, Maan ZN, Whittam AJ, & Siemers F (2015). Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis, 11, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FY, Hung YS, Liou HM, & Shen CH (2010). Electrospun hyaluronate-collagen nanofibrous matrix and the effects of varying the concentration of hyaluronate on the characteristics of foreskin fibroblast cells. Acta Biomaterialia, 6, 2140–2147. [DOI] [PubMed] [Google Scholar]
- Huang C, Holfeld J, Schaden W, Orgill D, & Ogawa R (2013). Mechanotherapy: Revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends in Molecular Medicine, 19, 555–564. [DOI] [PubMed] [Google Scholar]
- Huang C, Miyazaki K, Akaishi S, Watanabe A, Hyakusoku H, & Ogawa R (2013). Biological effects of cellular stretch on human dermal fibroblasts. Journal of Plastic, Reconstructive & Aesthetic Surgery, 66, e351–e361. [DOI] [PubMed] [Google Scholar]
- Hunt O, Burden D, Hepper P, Stevenson M, & Johnston C (2006). Self-reports of psychosocial functioning among children and young adults with cleft lip and palate. The Cleft Palate-Craniofacial Journal, 43, 598–605. [DOI] [PubMed] [Google Scholar]
- Januszyk M, Wong VW, Bhatt KA, Vial IN, Paterno J, Longaker MT, & Gurtner GC (2014). Mechanical offloading of incisional wounds is associated with transcriptional downregulation of inflammatory pathways in a large animal model. Organogenesis, 10, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LW, Chen H, & Lu H (2016). Using human epithelial amnion cells in human de-epidermized dermis for skin regeneration. Journal of Dermatological Science, 81, 26–34. [DOI] [PubMed] [Google Scholar]
- Kadi A, Fawzi-Grancher S, Lakisic G, Stoltz JF, & Muller S (2008). Effect of cyclic stretching and TGF-beta on the SMAD pathway in fibroblasts. Bio-medical Materials and Engineering, 18, S77–S86. [PubMed] [Google Scholar]
- Kagan HM, & Li W (2003). Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. Journal of Cellular Biochemistry, 88, 660–672. [DOI] [PubMed] [Google Scholar]
- Kaji K, Yoshiji H, Ikenaka Y, Noguchi R, Aihara Y, Douhara A, … Fukui H (2014). Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. Journal of Gastroenterology, 49, 481–491. [DOI] [PubMed] [Google Scholar]
- Kenny FN, & Connelly JT (2015). Integrin-mediated adhesion and mechano-sensing in cutaneous wound healing. Cell and Tissue Research, 360, 571–582. [DOI] [PubMed] [Google Scholar]
- Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, & Burdick JA (2013). Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials, 12, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran I, Knock A, Bush J, So K, Metcalfe A, Hobson R, … Ferguson M (2013). Interleukin-10 reduces scar formation in both animal and human cutaneous wounds: Results of two preclinical and phase II randomized control studies. Wound Repair and Regeneration, 21, 428–436. [DOI] [PubMed] [Google Scholar]
- Kieran I, Taylor C, Bush J, Rance M, So K, Boanas A, … Ferguson M (2014). Effects of interleukin-10 on cutaneous wounds and scars in humans of African continental ancestral origin. Wound Repair and Regeneration, 22, 326–333. [DOI] [PubMed] [Google Scholar]
- Kim BS, Lee JS, Gao G, & Cho DW (2017). Direct 3D cell-printing of human skin with functional transwell system. Biofabrication, 9, 025034. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, & Breathnach SM (1993). Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: Epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clinical and Experimental Immunology, 94, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer K (1978). Anatomy and physiology of skin .1. Cleavability of cutis. British Journal of Plastic Surgery, 31, 3–8.342028 [Google Scholar]
- Larson BJ, Longaker MT, & Lorenz HP (2010). Scarless fetal wound healing: A basic science review. Plastic and Reconstructive Surgery, 126, 1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, & Longaker MT (2016). Scarless wound healing: Finding the right cells and signals. Cell and Tissue Research, 365, 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DE, Ayoub N, & Agrawal DK (2016). Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Research & Therapy, 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Han SH, Choi WJ, Chung KH, & Lee JW (2016). Hyaluronic acid dressing (Healoderm) in the treatment of diabetic foot ulcer: A prospective, randomized, placebo-controlled, single-center study. Wound Repair and Regeneration, 24, 581–588. [DOI] [PubMed] [Google Scholar]
- Lee MH, & Murphy G (2004). Matrix metalloproteinases at a glance. Journal of Cell Science, 117, 4015–4016. [DOI] [PubMed] [Google Scholar]
- Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, … Karande P (2014). Design and fabrication of human skin by three-dimensional bioprinting. Tissue Engineering. Part C, Methods, 20, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson SM, Geever EF, Crowley LV, Oates JF 3rd, Berard CW, & Rosen H (1965). The healing of rat skin wounds. Annals of Surgery, 161, 293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kilani RT, Rahmani-Neishaboor E, Jalili RB, & Ghahary A (2014). Kynurenine increases matrix metalloproteinase-1 and −3 expression in cultured dermal fibroblasts and improves scarring in vivo. The Journal of Investigative Dermatology, 134, 643–650. [DOI] [PubMed] [Google Scholar]
- Lichtman MK, Otero-Vinas M, & Falanga V (2016). Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair and Regeneration, 24, 215–222. [DOI] [PubMed] [Google Scholar]
- Liechty KW, Kim HB, Adzick NS, & Crombleholme TM (2000). Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. Journal of Pediatric Surgery, 35, 866–873. [DOI] [PubMed] [Google Scholar]
- Lim AF, Weintraub J, Kaplan EN, Januszyk M, Cowley C, McLaughlin P, … Longaker MT (2014). The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plastic and Reconstructive Surgery, 133, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, & Grzela T (2016). Hyaluronic acid in inflammation and tissue regeneration. Wounds, 28, 78–88. [PubMed] [Google Scholar]
- Liu X, Ma L, Liang J, Zhang B, Teng J, & Gao C (2013). RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials, 34, 2038–2048. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang Z, Wang R, Zhao F, Shi P, Jiang Y, & Pang X (2013). Direct comparison of the potency of human mesenchymal stem cells derived from amnion tissue, bone marrow and adipose tissue at inducing dermal fibroblast responses to cutaneous wounds. International Journal of Molecular Medicine, 31, 407–415. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Krane S, & Jaenisch R (1997). Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proceedings of the National Academy of Sciences of the United States of America, 94, 1852–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, & Senior RM (1998). Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. The Journal of Experimental Medicine, 188, 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, & Adzick NS (1991). The biology of fetal wound healing: A review. Plastic and Reconstructive Surgery, 87, 788–798. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, & Stern R (1991). Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Annals of Surgery, 213, 292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, Chiu ES, Harrison MR, Crombleholme TM, Langer JC, Duncan BW, … Stern R (1989). Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Annals of Surgery, 210, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, Harrison MR, Crombleholme TM, Langer JC, Decker M, Verrier ED, … Stern R (1989). Studies in fetal wound healing: I. A factor in fetal serum that stimulates deposition of hyaluronic acid. Journal of Pediatric Surgery, 24, 789–792. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Harrison MR, Langer JC, Crombleholme TM, Verrier ED, Spendlove R, & Stern R (1989). Studies in fetal wound healing: II. A fetal environment accelerates fibroblast migration in vitro. Journal of Pediatric Surgery, 24, 793–797; discussion 798. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Rohrich RJ, Greenberg L, Furnas H, Wald R, Bansal V, … Gurtner GC (2014). A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plastic and Reconstructive Surgery, 134, 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, … Harrison MR (1990). Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. Journal of Pediatric Surgery, 25, 63–68; discussion 68–69. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Ferguson MW, Harrison MR, Crombleholme TM, Langer JC, … Stern R (1989). Studies in fetal wound healing: III. Early deposition of fibronectin distinguishes fetal from adult wound healing. Journal of Pediatric Surgery, 24, 799–805. [DOI] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, & Adzick NS (1994). Adult skin wounds in the fetal environment heal with scar formation. Annals of Surgery, 219, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, & Adzick NS (1992). Scarless wound repair: A human fetal skin model. Development, 114, 253–259. [DOI] [PubMed] [Google Scholar]
- Lovvorn HN 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, & Adzick NS (1999). Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. Journal of Pediatric Surgery, 34, 218–223. [DOI] [PubMed] [Google Scholar]
- Lu F, Ogawa R, Nguyen DT, Chen B, Guo D, Helm DL, … Orgill DP (2011). Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Annals of Plastic Surgery, 66, 296–300. [DOI] [PubMed] [Google Scholar]
- Mahmoudi Rad M, Mahmoudi Rad N, & Mirdamadi Y (2015). Expression of TGF-beta3 in isolated fibroblasts from foreskin. Reports of Biochemistry and Molecular Biology, 3, 76–81. [PMC free article] [PubMed] [Google Scholar]
- Manuel JA, & Gawronska-Kozak B (2006). Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biology, 25, 505–514. [DOI] [PubMed] [Google Scholar]
- Mast BA, Diegelmann RF, Krummel TM, & Cohen IK (1993). Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix, 13, 441–446. [DOI] [PubMed] [Google Scholar]
- Mast BA, & Schultz GS (1996). Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair and Regeneration, 4, 411–420. [DOI] [PubMed] [Google Scholar]
- McKay IA, & Leigh IM (1991). Epidermal cytokines and their roles in cutaneous wound healing. The British Journal of Dermatology, 124, 513–518. [DOI] [PubMed] [Google Scholar]
- McPherson JM, Ledger PW, Ksander G, Sawamura SJ, Conti A, Kincaid S, … Clark RA (1988). The influence of heparin on the wound healing response to collagen implants in vivo. Collagen and Related Research, 8, 83–100. [DOI] [PubMed] [Google Scholar]
- Meyer M, & McGrouther DA (1991). A study relating wound tension to scar morphology in the pre-sternal scar using Langers technique. British Journal of Plastic Surgery, 44, 291–294. [DOI] [PubMed] [Google Scholar]
- Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, … Reimers K (2013). Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One, 8, e57741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella E, Brescia E, Massaro C, Ramires PA, Miglietta MR, Fiori V, & Aversa P (2002). Physico-chemical properties and degradability of non-woven hyaluronan benzylic esters as tissue engineering scaffolds. Biomaterials, 23, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Min HS, Kim JE, Lee MH, Song HK, Kang YS, Lee MJ, … Cha DR (2014). Dipeptidyl peptidase IV inhibitor protects against renal interstitial fibrosis in a mouse model of ureteral obstruction. Laboratory Investigation, 94, 598–607. [DOI] [PubMed] [Google Scholar]
- Mirza RE, Fang MM, Ennis WJ, & Koh TJ (2013). Blocking interleukin-1beta induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes, 62, 2579–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi M, Ikeda S, Suga Y, & Ogawa H (2000). Expression of cytokeratins and cornified cell envelope-associated proteins in umbilical cord epithelium: A comparative study of the umbilical cord, amniotic epithelia and fetal skin. The Journal of Investigative Dermatology, 115, 133–134. [DOI] [PubMed] [Google Scholar]
- Mizoguchi M, Suga Y, Sanmano B, Ikeda S, & Ogawa H (2004). Organotypic culture and surface plantation using umbilical cord epithelial cells: Morphogenesis and expression of differentiation markers mimicking cutaneous epidermis. Journal of Dermatological Science, 35, 199–206. [DOI] [PubMed] [Google Scholar]
- Mogili NS, Krishnaswamy VR, Jayaraman M, Rajaram R, Venkatraman A, & Korrapati PS (2012). Altered angiogenic balance in keloids: A key to therapeutic intervention. Translational Research, 159, 182–189. [DOI] [PubMed] [Google Scholar]
- Monaco JL, & Lawrence WT (2003). Acute wound healing an overview. Clinics in Plastic Surgery, 30, 1–12. [DOI] [PubMed] [Google Scholar]
- Moore AL, Marshall CD, & Longaker MT (2017). Minimizing skin scarring through biomaterial design. Journal of Functional Biomaterials, 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin V, & Plamondon M (2002). Differential expression of collagen integrin receptor on fetal vs. adult skin fibroblasts: Implication in wound contraction during healing. The British Journal of Dermatology, 147, 886–892. [DOI] [PubMed] [Google Scholar]
- Hu MS, Leavitt T, Malhotra S, Duscher D, Pollhammer MS, Walmsley GG, et al. (2015). Stem cell-based therapeutics to improve wound healing. Plastic Surgery International, 2015, 383581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, & Yager DR (1998). Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair and Regeneration, 6, 127–134. [DOI] [PubMed] [Google Scholar]
- Occleston NL, O’Kane S, Laverty HG, Cooper M, Fairlamb D, Mason T, … Ferguson MW (2011). Discovery and development of avotermin (recombinant human transforming growth factor beta 3): A new class of prophylactic therapeutic for the improvement of scarring. Wound Repair and Regeneration, 19(Suppl 1), s38–s48. [DOI] [PubMed] [Google Scholar]
- Olutoye OO, Zhu X, Cass DL, & Smith CW (2005). Neutrophil recruitment by fetal porcine endothelial cells: Implications in scarless fetal wound healing. Pediatric Research, 58, 1290–1294. [DOI] [PubMed] [Google Scholar]
- Ong CT, Khoo YT, Tan EK, Mukhopadhyay A, Do DV, Han HC, … Phan TT (2007). Epithelial-mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion. The Journal of Pathology, 211, 95–108. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, & Itoh N (2015). The fibroblast growth factor signaling pathway. Wiley Interdisciplinary Reviews: Developmental Biology, 4, 215–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, & Rolfe KJ (2012). The role of the TGF-beta family in wound healing, burns and scarring: A review. International Journal of Burns and Trauma, 2, 18–28. [PMC free article] [PubMed] [Google Scholar]
- Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, & Liechty KW (2008). IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. The Journal of Investigative Dermatology, 128, 1852–1860. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, & Cotsarelis G (2012). Epithelial stem cells and implications for wound repair. Seminars in Cell & Developmental Biology, 23, 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, & Mangelsdorf DJ (2012). Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes & Development, 26, 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyani T, Papp S, & Schaffer L (2012). Tissue-engineered fetal dermal matrices. In Vitro Cellular & Developmental Biology. Animal, 48, 493–506. [DOI] [PubMed] [Google Scholar]
- Prager MD, Sabeh F, Baxter CR, Atiles L, & Hartline B (1994). Dipeptidyl peptidase IV and aminopeptidase in burn wound exudates: Implications for wound healing. The Journal of Trauma, 36, 629–633. [DOI] [PubMed] [Google Scholar]
- Rawdanowicz TJ, Hampton AL, Nagase H, Woolley DE, & Salamonsen LA (1994). Matrix metalloproteinase production by cultured human endometrial stromal cells: Identification of interstitial collagenase, gelatinase-A, gelatinase-B, and stromelysin-1 and their differential regulation by interleukin-1 alpha and tumor necrosis factor-alpha. The Journal of Clinical Endocrinology and Metabolism, 79, 530–536. [DOI] [PubMed] [Google Scholar]
- Reitamo S, Remitz A, Tamai K, & Uitto J (1994). Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. The Journal of Clinical Investigation, 94, 2489–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, & Clevers H (2005). Wnt signalling in stem cells and cancer. Nature, 434, 843–850. [DOI] [PubMed] [Google Scholar]
- Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, … Min BM (2006). Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials, 27, 1452–1461. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, et al. (2015). Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science, 348, aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert R, Meyer W, Bishop S, Rosenberg L, Murphy L, & Blakeney P (1999). Disfiguring burn scars and adolescent self-esteem. Burns, 25, 581–585. [DOI] [PubMed] [Google Scholar]
- Rowlatt U (1979). Intrauterine wound healing in a 20 week human fetus. Virchows Archiv. A, Pathological Anatomy and Histology, 381, 353–361. [DOI] [PubMed] [Google Scholar]
- Sauder DN, Orr FW, Matic S, Stetsko D, Parker KP, Chizzonite R, & Kilian PL (1989). Human interleukin-1 alpha is chemotactic for normal human keratinocytes. Immunology Letters, 22, 123–127. [DOI] [PubMed] [Google Scholar]
- Schmid P, Itin P, Cherry G, Bi C, & Cox DA (1998). Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. The American Journal of Pathology, 152, 485–493. [PMC free article] [PubMed] [Google Scholar]
- Schmidt JA, Mizel SB, Cohen D, & Green I (1982). Interleukin 1, a potential regulator of fibroblastproliferation. Journal of Immunology, 128, 2177–2182. [PubMed] [Google Scholar]
- Schoenwolf GC, & Larsen WJ (2009). Larsen’s human embryology (4th ed.). Philadelphia, PA: Elsevier/Churchill Livingstone. [Google Scholar]
- Semenza GL (2003). Targeting HIF-1 for cancer therapy. Nature Reviews. Cancer, 3, 721–732. [DOI] [PubMed] [Google Scholar]
- Semenza GL (2010). Oxygen homeostasis. Wiley Interdisciplinary Reviews. Systems Biology and Medicine, 2, 336–361. [DOI] [PubMed] [Google Scholar]
- Semenza GL (2011a). Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. American Journal of Physiology. Cell Physiology, 301, C550–C552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL (2011b). Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harbor Symposia on Quantitative Biology, 76, 347–353. [DOI] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, … Longaker MT (2009). Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration, 17, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Foreman DM, & Ferguson MW (1995). Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. Journal of Cell Science, 108(Pt 3), 985–1002. [DOI] [PubMed] [Google Scholar]
- Sheridan RL, Hinson MI, Liang MH, Nackel AF, Schoenfeld DA, Ryan CM, … Tompkins RG (2000). Long-term outcome of children surviving massive burns. JAMA, 283, 69–73. [DOI] [PubMed] [Google Scholar]
- Silver FH, Siperko LM, & Seehra GP (2003). Mechanobiology of force transduction in dermal tissue. Skin Research and Technology, 9, 3–23. [DOI] [PubMed] [Google Scholar]
- Skrzypczyk A, Giri S, & Bader A (2016). Generation of induced pluripotent stem cell line from foreskin fibroblasts. Stem Cell Research, 17, 572–575. [DOI] [PubMed] [Google Scholar]
- So K, McGrouther DA, Bush JA, Durani P, Taylor L, Skotny G, … Ferguson MW (2011). Avotermin for scar improvement following scar revision surgery: A randomized, double-blind, within-patient, placebo-controlled, phase II clinical trial. Plastic and Reconstructive Surgery, 128, 163–172. [DOI] [PubMed] [Google Scholar]
- So T, Ito A, Sato T, Mori Y, & Hirakawa S (1992). Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biology of Reproduction, 46, 772–778. [DOI] [PubMed] [Google Scholar]
- Streit M, Beleznay Z, & Braathen LR (2006). Topical application of the tumour necrosis factor-alpha antibody infliximab improves healing of chronic wounds. International Wound Journal, 3, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H, Furuya K, & Sokabe M (2014). Mechanosensitive ATP release from hemichannels and Ca(2)(+) influx through TRPC6 accelerate wound closure in keratinocytes. Journal of Cell Science, 127, 4159–4171. [DOI] [PubMed] [Google Scholar]
- Tamama K, Kawasaki H, Kerpedjieva SS, Guan J, Ganju RK, & Sen CK (2011). Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. Journal of Cellular Biochemistry, 112, 804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SS, Trackman PC, & Kagan HM (1983). Reaction of aortic lysyl oxidase with beta-aminopropionitrile. The Journal of Biological Chemistry, 258, 4331–4338. [PubMed] [Google Scholar]
- Tanriverdi-Akhisaroglu S, Menderes A, & Oktay G (2009). Matrix metalloproteinase-2 and −9 activities in human keloids, hypertrophic and atrophic scars: A pilot study. Cell Biochemistry and Function, 27, 81–87. [DOI] [PubMed] [Google Scholar]
- Thomas CR, Russell W, Robert RS, Holzer CE, Blakeney P, & Meyer WJ (2012). Personality disorders in young adult survivors of pediatric burn injury. Journal of Personality Disorders, 26, 255–266. [DOI] [PubMed] [Google Scholar]
- Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, … Albina JE (2009). Disruption of interleukin-1 signaling improves the quality of wound healing. The American Journal of Pathology, 174, 2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, & Brown RA (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews. Molecular Cell Biology, 3, 349–363. [DOI] [PubMed] [Google Scholar]
- Townsend C, Beauchamp D, Evers M, & Mattox KL (2017). Wound healing In Leong C, Murphy KD, & Phillips LG (Eds), Sabiston textbook of surgery: The biological basis of modern surgical practice (Chapter 6, 20th ed., pp. 130–162). Philadelphia, PA: Elsevier. [Google Scholar]
- Udupa SL (1995). Inhibition of lysyl oxidase by isoniazid and its effect on wound healing. Indian Journal of Experimental Biology, 33, 278–280. [PubMed] [Google Scholar]
- Unemori EN, Hibbs MS, & Amento EP (1991). Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. The Journal of Clinical Investigation, 88, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer WM, Niessen FB, Ferreira JA, Zwiers PJ, de Jong EH, Middelkoop E, & Molema G (2011). Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair and Regeneration, 19, 292–301. [DOI] [PubMed] [Google Scholar]
- Van Loey NE, & Van Son MJ (2003). Psychopathology and psychological problems in patients with burn scars: Epidemiology and management. American Journal of Clinical Dermatology, 4, 245–272. [DOI] [PubMed] [Google Scholar]
- van Zuijlen PP, Ruurda JJ, van Veen HA, van Marle J, van Trier AJ, Groenevelt F, … Middelkoop E (2003). Collagen morphology in human skin and scar tissue: No adaptations in response to mechanical loading at joints. Burns, 29, 423–431. [DOI] [PubMed] [Google Scholar]
- Varkey M, Ding J, & Tredget EE (2015). Advances in skin substitutes-potential of tissue engineered skin for facilitating anti-fibrotic healing. Journal of Functional Biomaterials, 6, 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigetti D, Karousou E, Viola M, Deleonibus S, De Luca G, & Passi A (2014). Hyaluronan: Biosynthesis and signaling. Biochimica et Biophysica Acta, 1840, 2452–2459. [DOI] [PubMed] [Google Scholar]
- Voinchet V, Vasseur P, & Kern J (2006). Efficacy and safety of hyaluronic acid in the management of acute wounds. American Journal of Clinical Dermatology, 7, 353–357. [DOI] [PubMed] [Google Scholar]
- Volk SW, Wang Y, Mauldin EA, Liechty KW, & Adams SL (2011). Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells, Tissues, Organs, 194, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, … Braunhut SJ (2010). Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biology, 29, 690–700. [DOI] [PubMed] [Google Scholar]
- Walmsley GG, Maan ZN, Wong VW, Duscher D, MS H, Zielins ER, et al. (2015). Scarless wound healing: Chasing the holy grail. Plastic and Reconstructive Surgery, 135, 907–917. [DOI] [PubMed] [Google Scholar]
- Walraven M, Talhout W, Beelen RH, van Egmond M, & Ulrich MM (2016). Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair and Regeneration, 24, 533–541. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Zhang N, Wang C, Herrler T, & Li Q (2015). An updated review of mechanotransduction in skin disorders: Transcriptional regulators, ion channels, and microRNAs. Cellular and Molecular Life Sciences, 72, 2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Dong Y, Geng S, Su H, Ge W, & Zhen C (2014). Photodynamic therapy inhibits the formation of hypertrophic scars in rabbit ears by regulating metalloproteinases and tissue inhibitor of metalloproteinase-1. Clinical and Experimental Dermatology, 39, 196–201. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang M, Lu W, Zhang X, Ma D, Rong X, … Jin Y (2010). Cross-linked collagen-chondroitin sulfate-hyaluronic acid imitating extracellular matrix as scaffold for dermal tissue engineering. Tissue Engineering. Part C, Methods, 16, 269–279. [DOI] [PubMed] [Google Scholar]
- Webb K, Hitchcock RW, Smeal RM, Li W, Gray SD, & Tresco PA (2006). Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. Journal of Biomechanics, 39, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Welsh SJ, & Powis G (2003). Hypoxia inducible factor as a cancer drug target. Current Cancer Drug Targets, 3, 391–405. [DOI] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Liu B, Manzano WR, Evans ND, Dhamdhere GR, … Helms JA (2013). Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One, 8, e76883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, & Dipietro LA (2008). Regulation of scar formation by vascular endothelial growth factor. Laboratory Investigation, 88, 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MB, Thornton FJ, Kiyama T, Efron DT, Schulz GS, Moldawer LL, & Barbul A (1998). Metalloproteinase inhibitors and wound healing: A novel enhancer of wound strength. Surgery, 124, 464–470. [PubMed] [Google Scholar]
- Wong JW, Gallant-Behm C, Wiebe C, Mak K, Hart DA, Larjava H, & Hakkinen L (2009). Wound healing in oral mucosa results in reduced scar formation as compared with skin: Evidence from the red Duroc pig model and humans. Wound Repair and Regeneration, 17, 717–729. [DOI] [PubMed] [Google Scholar]
- Wong VW, Akaishi S, Longaker MT, & Gurtner GC (2011). Pushing back: Wound mechanotransduction in repair and regeneration. The Journal of Investigative Dermatology, 131, 2186–2196. [DOI] [PubMed] [Google Scholar]
- Wong VW, Beasley B, Zepeda J, Dauskardt RH, Yock PG, Longaker MT, & Gurtner GC (2013). A mechanomodulatory device to minimize incisional scar formation. Advances in Wound Care (New Rochelle), 2, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Gurtner GC, & Longaker MT (2013). Wound healing: A paradigm for regeneration. Mayo Clinic Proceedings, 88, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Wong VW, Longaker MT, & Gurtner GC (2012). Soft tissue mechanotransduction in wound healing and fibrosis. Seminars in Cell & Developmental Biology, 23, 981–986. [DOI] [PubMed] [Google Scholar]
- Wray RC (1983). Force required for wound closure and scar appearance. Plastic and Reconstructive Surgery, 72, 380–382. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Eckes B, & Krieg T (2001). Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: Differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochemical and Biophysical Research Communications, 281, 200–205. [DOI] [PubMed] [Google Scholar]
- Yan C, Gao N, Sun H, Yin J, Lee P, Zhou L, … Yu FS (2016). Targeting imbalance between IL-1beta and IL-1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. The American Journal of Pathology, 186, 1466–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Tibbitt MW, Basta L, & Anseth KS (2014). Mechanical memory and dosing influence stem cell fate. Nature Materials, 13, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You HJ, & Han SK (2014). Cell therapy for wound healing. Journal of Korean Medical Science, 29, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavan B, Vindigni V, Vezzu K, Zorzato G, Luni C, Abatangelo G, … Cortivo R (2009). Hyaluronan based porous nano-particles enriched with growth factors for the treatment of ulcers: A placebo-controlled study. Journal of Materials Science. Materials in Medicine, 20, 235–247. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Li X, Xu X, He Z, Cui L, & Lv X (2016). Lumican alleviates hypertrophic scarring by suppressing integrin-FAK signaling. Biochemical and Biophysical Research Communications, 480, 153–159. [DOI] [PubMed] [Google Scholar]
- Zhong SP, Zhang YZ, & Lim CT (2010). Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology, 2, 510–525. [DOI] [PubMed] [Google Scholar]
- Zhu XJ, Liu Y, Dai ZM, Zhang X, Yang X, Li Y, et al. (2014). BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genetics, 10, e1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]