Almost all individuals exhibit sensory eye dominance, one neural basis of which is unequal interocular inhibition. Sensory eye dominance can impair binocular functions that depend on both excitatory and inhibitory mechanisms [1–3]. We developed a ‘push-pull’ perceptual learning protocol that simultaneously affects the excitatory and inhibitory networks to reduce sensory eye dominance and improve stereopsis in adults with otherwise normal vision [4]. The push-pull protocol provides a promising clinical paradigm for treating the extreme sensory eye dominance in amblyopia (‘lazy eye’). The prevailing standard of care does not directly treat sensory eye dominance; instead, selected excitatory functions in the amblyopic eye are stimulated while the strong eye is patched, on the assumption that recovery of the weak eye’s excitatory functions rebalances the eyes. Patching the strong eye does not directly address interocular inhibition; in contrast, the push-pull protocol by design excites the weak eye, while completely inhibiting the strong eye’s perception to recalibrate the interocular balance of excitatory and inhibitory interactions. Here, we show that three adult amblyopes who trained on the push-pull protocol gained longstanding improvements in interocular balance and stereopsis. Our findings provide a proof-of-concept and evidence that push-pull learning leads to long-term plasticity.
During the push-pull training, attentional cueing causes the rivaling half-image at corresponding retinal points in the amblyopic eye to be perceived (push), while the half-image in the strong eye is perceptually suppressed (pull) (Figure 1A). We measured relative sensory eye dominance (with binocular rivalry stimulus), monocular contrast threshold and stereoacuity ([3,4]; Supplemental Information) in the pre-training and post-training phases to reveal the learning effects (pre versus post). The observers were retested 4–8 months after the training ended for evaluation of learning retention (pre versus retain).
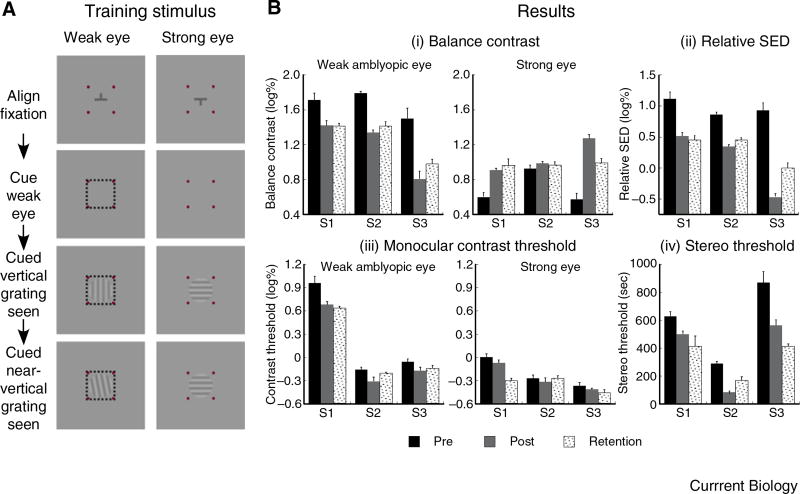
Figure 1. Push-pull training for treating amblyopia.
(A) Key presentation sequence in the push-pull training protocol. The observer achieves binocular alignment with the nonius fixation target. An attention cue (square frame) is presented to the weak amblyopic eye to pave the way for the two subsequent half-images of the binocular rivalry gratings viewed by the amblyopic eye to be perceived (dominant). This allows the observer to perform a secondary task of discriminating whether the first (vertical) or second (near-vertical) grating has a more counter-clockwise orientation. Alternatively, the secondary task can be contrast discrimination (Supplemental). Observers S1, S2 and S3, respectively, underwent 15, 15 and 7 training sessions (~1.5 hours/session). (B) Bar graphs plotting the data of each observer (S1, S2 and S3) measured immediately before (pre) and after (post) the training phase, and 4–8 months after the training ended (retention). (i) The balance contrast values of the amblyopic eye (left graph) are lower after the training and are retained while those of the strong eye (right graph) either become higher or remain unchanged. (ii) Relative sensory eye dominance, defined as the difference between the weak and strong eyes’ balance contrast values, is reduced after training and retained. (iii) The monocular contrast threshold of the weak eye (left graph) either becomes lower or remains unchanged after training while that of the strong eye (right graph) is unchanged after training. Note: The contrast threshold is defined in “log unit of percent contrast” wherein 10% contrast equates to 1 log unit. (iv) Stereo threshold is reduced after training and retained.
Figure 1B(i) shows the weak eye’s balance contrast reduces significantly post-training, indicating increased strength of the amblyopic eye’s channel (S1: t(8) = 3.089, p < 0.015; S2: t(8) = 12.703, p < 0.001; S3: t(8) = 4.895, p = 0.001). The learning effect is retained (S1: t(8) = 3.531, p < 0.008; S2: t(7) = 7.655, p < 0.001; S3: t(8) = 4.215, p < 0.003). The strong eye’s balance contrast increases significantly post-training for observers S1 (t(8) = −5.520, p = 0.001) and S3 (t(8)= −9.163, p < 0.001), and the learning effect is retained (S1: t(8) = −4.169, p = 0.003; S3: t(8) = −5.036, p = 0.001). For observer S2, the increase in the strong eye’s balance contrast is insignificant (t(8) = −1.341, p = 0.217) and remains unchanged during retention testing (t(7) = −0.701, p = 0.506). The relative sensory eye dominance significantly reduces post-training (S1: t(8) = 4.632, p = 0.002; S2: t(8) = 12.321, p < 0.001; S3: t(8) = 10.420, p < 0.001), and the learning effect is retained (S1: t(8) = 4.960, p = 0.001; S2: t(7) = 7.940, p < 0.001; S3: t(8) = 6.047, p < 0.001) (Figure 1B(ii)). These findings reveal that the push-pull training improves interocular balance and induces a sustained learning effect.
Figure 1B(iii) shows the training significantly reduces the amblyopic eye’s contrast threshold for observers S1 (t(6) = 3.032, p = 0.023) and S2 (t(6) = 2.553, p = 0.043), with significant retention for S1 (t(6) = 3.732, p = 0.010) but not S2 (t(6) = 1.377, p = 0.218). The reduced contrast thresholds in S1 and S2 cannot entirely account for the changes in sensory eye dominance. The learning effect for S3 is insignificant (t(6) = 1.901, p = 0.106) and remains unchanged during retention testing (t(6) = 1.559, p = 0.170). For all observers, the strong eye’s contrast threshold remains unchanged (p > 0.05). Notably, the improvement in the weak eye mirrors that by others who exclusively train the amblyopic eye, with the main goal of improving monocular visual functions [5–8]. But there is a significant difference in our case, in that the improvement is achieved with emphasis on the total perceptual inhibition of the strong eye while forcing excitation of the amblyopic eye. Doing so recalibrates the interocular balance of the excitatory and inhibitory interactions.
Figure 1B(iv) reveals the stereo threshold significantly reduces post-training (S1: t(8) = 3.371, p = 0.010; S2: t(7) = 11.186, p < 0.001; S3: t(8) = 3.567, p = 0.007), and the learning effect is retained (S1: t(8) = 2.447, p = 0.040; S2: t(6) = 4.055, p < 0.007; S3: t(8) = 5.826, p < 0.001). This learning effect on stereopsis and its retention parallels that of sensory eye dominance (Figure 1B(ii)). As no stereopsis or binocular fusion training was implemented, the stereopsis improvement is likely due to reduction in sensory eye dominance.
In summary, the push-pull protocol holds promise as a novel amblyopia treatment because it significantly reduces sensory eye dominance and enhances stereopsis. The learning effects last more than four months after the training ends, indicating the push-pull protocol induces long-term cortical plasticity. This study extends our previous perceptual learning studies on non-amblyopes who have smaller sensory eye dominance and suffer less degradation in stereopsis [4]. Our push-pull training is unique as it forces total perceptual suppression of the strong eye while promoting excitatory signals in the weak eye. It is unlike other forms of effective binocular treatments that promote balanced excitatory signals in both eyes through controlled computer stimulation (for example [9]), or filters, and those monocular treatments that excite the weak eye alone [5–7], which also variously achieve improvements in binocular and monocular vision. The push-pull protocol is also conceptually different because the perceptual learning is primarily accomplished by capitalizing on the inhibitory mechanism during binocular rivalry. More generally, our push-pull protocol, which directly inhibits the strong eye’s perception, provides strong human psychophysical evidence that underscores the role of inhibitory activities in cortical plasticity [10].
Supplementary Material
Acknowledgments
This study was supported in part by a grant from the National Institutes of Health Grant R01-EY015804 to Z.J.H. and T.L.O. and the Commonwealth of Pennsylvania, Ben Franklin Technology Development Authority to T.L.O.
Footnotes
Supplemental information includes experimental procedures, references and two figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.03.004.
References
- 1.Sengpiel F, Blakemore C, Kind PC, Harrad R. Interocular suppression in the visual cortex of strabismic cats. J. Neurosci. 1994;14:6855–6871. doi: 10.1523/JNEUROSCI.14-11-06855.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su Y, He ZJ, Ooi TL. Coexistence of binocular integration and suppression determined by surface border information. Proc. Natl. Acad. Sci. USA. 2009;106:15990–15995. doi: 10.1073/pnas.0903697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu JP, He ZJ, Ooi TL. A binocular perimetry study of the causes and implications of sensory eye dominance. Vis. Res. 2011;51:2386–2397. doi: 10.1016/j.visres.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu JP, He ZJ, Ooi TL. Effectively reducing sensory eye dominance with a push–pull perceptual learning protocol. Curr. Biol. 2010;20:1864–1868. doi: 10.1016/j.cub.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc. Natl. Acad. Sci. USA. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi DM, Li RW. Improving the performance of the amblyopic visual system. Phil. Trans. R. Soc. B. 2009;364:399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata K, Kawato M, Watanabe T, Sasaki Y. Monocular deprivation boosts long-term visual plasticity. Curr. Biol. 2012;22:R291–R292. doi: 10.1016/j.cub.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess RF, Mansouri BM, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restor. Neurol. Neurosci. 2010;28:793–802. doi: 10.3233/RNN-2010-0550. [DOI] [PubMed] [Google Scholar]
- 10.Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, et al. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J. Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.