Abstract
The aim of this study was to evaluate the kinetic parameters and the production of metabolites of 13 novel yeasts isolated from a distillery and fruits, and Saccharomyces cerevisiae CAT-1, cultivated in fructose-based medium. The yeasts with the highest µmax were obtained from must, Pichia kudriavzevii BB2, P. kudriavzevii BB1, and S. cerevisiae BB9 (0.47–0.49 h−1). S. cerevisiae CAT-1 (3.02 g gDCM−1 h−1), S. cerevisiae BB9 (3.01 g gDCM−1 h−1), and Candida glabrata Recol 41 (2.52 g gDCM−1 h−1) stood out in terms of µS. C. parapsilosis Recol 29, and Rhodotorula mucilaginosa Recol 03 strains showed the highest YX/S (0.30 and 0.28 gDCM g−1, respectively). C. glabrata Recol 10 and S. cerevisiae BB9 strains stood out for their higher substrate conversion rates into ethanol (0.44 and 0.41 gEth gS−1, respectively). R. mucilaginosa Recol 03 presented the poorest performance in substrate consumption (0.87 g gDCM−1 h−1), while the strains isolated from must and C. glabrata Recol 10 showed the highest ethanol production and the C. parapsilosis Recol 29 showed the highest biomass conversion.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1721-9) contains supplementary material, which is available to authorized users.
Keywords: Fructose, Fermentation, Yeast, Kinetic parameters, Physiology
Introduction
Saccharomyces cerevisiae is a well-known industrial yeast strain (Shafaghat et al. 2009), which is used in the production of ethanol, alcoholic beverages, biomass, and metabolic products (Cardona and Sánchez 2007; Teixeira et al. 2009) and as a source of β-glucan (Kogan and Kocher 2007). In Brazil, the S. cerevisiae strains CAT-1 and PE-2 are commonly used in the production of ethanol (Romani et al. 2015), representing 80% of the yeast marketed for fuel ethanol in this country (Basso et al. 2011). Nevertheless, bioprospecting of novel strains and their physiological characterization is important for obtaining not only microorganisms that have outstanding fermentative potential, but also other biotechnological applications. This strategy has been successfully implemented due to the great biodiversity in the Brazilian biomes (Silva and Fonseca 2016).
Brazilian Midwest region, owing to its environmental characteristics, temperature, humidity, and soil, favors the culture of microorganisms, such as yeasts, and is, therefore, a suitable environment for bioprospecting for new strains. Moreover, the region has several mills and distilleries, where an intensive succession of S. cerevisiae strains occurs in the fermentation broth during the fermentation process, and in some cases the original yeast is completely replaced by wild strains (Silva-Filho et al. 2005; Batistote et al. 2010). These wild strains can be isolated and studied to obtain information about their characteristics and possible applications. In addition to must, yeast strains can be isolated from several environments, soil, fruits, and other sources (Silva et al. 2011; Nyanga et al. 2012; Freire et al. 2014).
Among the non-Saccharomyces yeasts with potential fermentative applications, Pichia kudriavzevii, also identified as Issatchenkia orientalis stands out (Chan et al. 2012). It was isolated from several types of soil substrates (Limtong et al. 2009), fruits (Nyanga et al. 2012) and musts (Freire et al. 2014). It is considered as ethanol-, osmo- and thermotolerant (Kitagawa et al. 2010) and was utilized for the production of wine with reduced alcohol content (Contreras et al. 2015) and for the production of ethanol under different conditions, e.g., high temperature and ethanol concentration (Dhaliwal et al. 2011). Candida glabrata is capable of fermenting glucose and trehalose (Silva et al. 2012). Its thermotolerance and close evolutionary relationship to S. cerevisiae have also been reported (Wang et al. 2013). Also, Candida parapsilosis can ferment xylitol (Nolleau et al. 1995), despite its inability to ferment maltose (Silva et al. 2012). Both C. glabrata and C. parapsilosis produce amylase enzymes (Oliveira et al. 2015). Meyerozyma guilliermondii, also known as Candida guilliermondii (Kurtzman and Suzuki 2010), was isolated from Malvar (Vitis vinifera cv. L.) (Cordero-Bueso et al. 2013). It is effective for the bioconversion of xylose to xylitol (Papon et al. 2013). Despite being a food spoilage yeast, Rhodotorula mucilaginosa has been used in winemaking experiments in consortium with S. cerevisiae to improve the aroma of wines (Albertyn et al. 2014; Wang et al. 2017). Moreover, this species has the potential to produce xylitol (Bura et al. 2012).
It is desirable that yeast can metabolize the broader spectrum of substrates. Fructose is a monosaccharide fermentable by yeast, despite its preference for the consumption of glucose (Berthels et al. 2004). Yeasts that use fructose are important for sugarcane broth fermentation because sucrose breaks down into glucose and fructose. Thus, there may be lower productivity if residual fructose levels in the broth are high (Jasman et al. 2012). Also, fructose consumption profiles can be used to identify yeast strains that prefer fructose sugar (Tronchoni et al. 2009), and the search for fructophilic yeasts is important for the wine industry (Jones et al. 2005). The metabolism and regulatory effect of fructose on S. cerevisiae have been reported to be similar to those of glucose (Anjos et al. 2013), but the consumption of fructose by some strains can be slower and may even be incomplete (Berthels et al. 2004; Wang et al. 2004). Studies of substrate consumption and microbial growth provide an understanding of microbial metabolism under specific cultivation conditions (Gomez-Flores et al. 2015).
Studies concerning the kinetic parameters from fructose-based media are scarce in the literature (Orlowski and Barford 1991; Andersen et al. 2003; Wang et al. 2004; Shafaghat et al. 2009; Fonseca et al. 2013; Kumdam et al. 2013). There are several yeasts capable of assimilating/fermenting many sugars. However, as most of these studies are carried out on complex media, where sugars are present in a mixture, at different concentrations, it becomes hard to evidence the influence of every single sugar on the metabolism. In this context, experiments in minimal medium using a sole carbon source would provide physiological information on the prospected yeasts.
Thus, this work aimed to evaluate the kinetic parameters and production of metabolites of three yeasts isolated from a sugar and alcohol mill in Brazilian Midwest region and ten yeasts isolated from typical fruits of this region, as well as the S. cerevisiae industrial strain CAT-1, using fructose as the only carbon source.
Materials and methods
Microorganisms and maintenance
The yeast strains used were Saccharomyces cerevisiae industrial strain CAT-1, donated by the São Fernando Sugar and Alcohol Distillery in Dourados, MS, Brazil, and 13 yeasts isolated from the Brazilian Midwest region: 3 yeasts isolated from a sugar and alcohol mill in the municipality of Barra do Bugres, MT, Brazil, Pichia kudriavzevii BB1, P. kudriavzevii BB2, and S. cerevisiae BB9 (Silva et al. 2011), and 10 yeasts isolated from fruits, identified as Rhodotorula mucilaginosa Recol 03, Meyerozyma guilliermondii Recol 09, Candida glabrata Recol 10, Candida parapsilosis Recol 12, Candida parapsilosis Recol 29, Candida parapsilosis Recol 37, Pichia kudriavzevii Recol 39, Candida glabrata Recol 41, Candida glabrata Recol 43, and Pichia kudriavzevii Recol 44 (Camargo et al. 2018).
The strains Recol 03, Recol 09, Recol 10, and Recol 12 were obtained from cherry of the Rio Grande (Eugenia involucrata), Recol 29 from uvaia (Eugenia pyriformis), Recol 37 from ubajay (Hexachlamys edulis), Recol 39 and Recol 41 from acerola (Malpighia glabra), and Recol 43 and Recol 44 from pequi (Caryocar brasiliense) (Camargo et al. 2018).
Stock aliquots of 1 ml of the yeasts used here were obtained from stock cultures kept in 20% glycerol (w v−1) at − 80 °C (Fonseca et al. 2013).
Minimal medium for pre-cultures and cultures
The minimal medium contained, per liter of distilled water: (NH4)2SO4, 5.0 g; KH2PO4, 3.0 g; MgSO4.7H2O, 0.5 g; 1 ml of trace elements (prepared in demineralized water, containing, per liter: EDTA, 15 g; ZnSO4.7H2O, 4.5 g; MnCl2.2H2O, 1 g; CoCl2.6H2O, 0.3 g; CuSO4.5H2O, 0.3 g; Na2MoO4.2H2O, 0.4 g; CaCl2.2H2O, 4.5 g; FeSO4.7H2O, 3.0 g; H3BO3, 1.0 g; KI, 0.1 g), with a final pH of 6.0. This medium was autoclaved (121 °C, 15 min.), followed by the addition of 1 ml l−1 of filter-sterilized vitamin solution (prepared in demineralized water containing, per liter: D-biotin, 0.05 g; calcium pantothenate, 1.0 g; nicotinic acid, 1.0 g; myo-inositol, 25 g; thiamine chloride, 1.0 g; pyridoxine, 1.0 g; and para-aminobenzoic acid, 0.20 g) (Verduyn et al. 1992).
Culture conditions
For the preparation of the experiments, a stock aliquot was used to inoculate a dish containing about 20 ml of sterile YPD medium (1% yeast extract, 2% peptone, 2% glucose, and 2% agar) for 48 h at 30 °C.
For the pre-cultures, a loop ful of cells was transferred from the dish to an Erlenmeyer flask (500 ml) containing 250 ml of sterile minimal medium (Verduyn et al. 1992), with pH adjusted to 6.0. Fructose was previously sterilized separately to a concentration of 10 g l−1. Then the pre-cultures were incubated at 30 °C in an orbital shaker (Marconi, Brazil) operating at 200 rpm until an optical density (OD) (λ = 600 nm) of 5 ± 1 was achieved and were measured in a spectrophotometer (Biospectro, Brazil).
Starting from an aliquot of a given amount of the pre-culture for an initial OD (λ = 600 nm) of 0.1, the cultures were performed in duplicate, in media and flasks identical to those used in pre-culturing.
The main cultures were performed at 30 °C in an orbital shaker (Marconi, Brazil) at 200 rpm until the sugar was consumed. A volume of 3 ml of sample was collected at 1, 2, and 3 h, and then in 30-min intervals from 3 h of cultivation to determine the dry weight and concentration of extracellular metabolites. The pH was obtained by potentiometric measurements (Hanna) during cultivations.
Quantification of extracellular metabolites and determination of biomass
Samples were collected and centrifuged in a refrigerated microcentrifuge (NT805, Brazil) (5 min, 17,609g, 5 °C). The supernatant was used to determine the residual substrate, ethanol, glycerol, and organic acids in an Agilent 1290 UPLC system equipped with a Rezex ROA-Organic Acid H + (8%) HPLC column (Phenomenex). The mobile phase was trifluoroacetic acid (TFA) at 5 mM, at a flow rate of 0.6 ml min−1, temperature of 55 °C, and an injected volume of 20 µL. These compounds were detected by an Agilent 1260 refractive index detector (RID) coupled to a data acquisition module (Nascimento et al. 2016).
The biomass sediment obtained after centrifugation of the sample was oven-dried (105 °C) until it reached a constant weight. The dry cell mass (g l−1) was determined from the quotient of the difference in weights over the volume of centrifuged medium. Biomass (X) was also determined indirectly by OD measurements taken in a spectrophotometer (Biospectro, Brazil) at 600 nm. To this end, the measured absorbance values were converted into mass values using a linear relation (OD units per gram of dry biomass) determined for each experiment.
Determination of kinetic parameters
The exponential growth phase was identified as the linear region of the plot of ln (OD) by the time for the culture data. The maximum specific growth rate (µmax) was determined as the slope of this straight line and the doubling time (DT) by the quotient of ln (2) by µmax. An evaluation was made of the maximum formation of biomass (Xmax) obtained from the calibration curve.
The biomass yield (YX/S) was determined as the slope of the line obtained by plotting the cell concentration (X) as a function of substrate concentration (S), and the product (ethanol, glycerol, and acetate) yield (YP/S) was calculated based on the concentration of the product (P) relative to the concentration of the substrate (S). The specific substrate uptake rate (qS) was calculated according to Eq. 1:
| 1 |
Statistical analyses
The results were subjected to an analysis of variance (ANOVA) with 5% probability by the Tukey test, and to the principal component analysis (PCA), using the software Statistica version 6.0 (StatSoft, USA).
Results and discussion
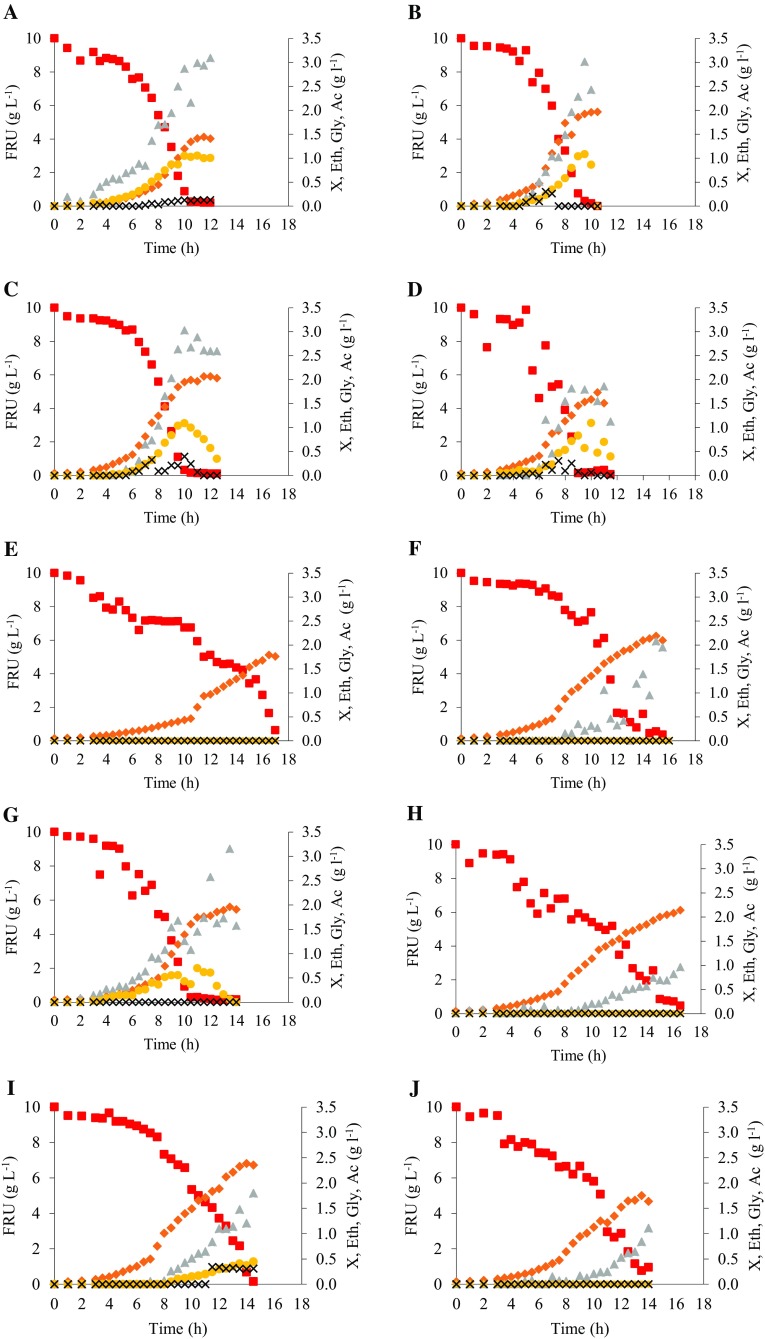
Table 1 shows the growth kinetic parameters during the exponential phase of the cultures. Figure 1 presents the kinetics of growth, metabolite formation and substrate consumption during batch cultures with fructose (10 g l−1) as the sole carbon source.
Table 1.
Kinetic parameters of the cultures, using fructose as carbon source with 13 different yeasts and the S. cerevisiae industrial strain CAT-1
| Strain | µ max | DT | q S | Y X/S | Y Eth/S | Y Gly/S | Y Ac/S | X max | pHa | T |
|---|---|---|---|---|---|---|---|---|---|---|
| (h−1) | (h) | (g gDCM−1 h−1) | (gDCM g S−1) | (gEth g S−1) | (gGly g S−1) | (gAc g S−1) | (g l−1) | (h) | ||
| S. c. CAT-1 | 0.45 ± 0.00b | 1.52 ± 0.01f | 3.02 ± 0.03a | 0.15 ± 0.00c | 0.33 ± 0.00a,b,c | 0.10 ± 0.00a | 0.01 ± 0.00c | 1.44 ± 0.01 h | 5.0 | 12.0 |
| P. k. BB1 | 0.48 ± 0.01a,b | 1.42 ± 0.03f | 2.47 ± 0.02a,b,c | 0.19 ± 0.01b,c | 0.27 ± 0.00a,b,c,d | 0.04 ± 0.00b | 0.11 ± 0.00a | 1.98 ± 0.01d,e | 3.5 | 10.5 |
| P. k. BB2 | 0.49 ± 0.00a | 1.38 ± 0.01f | 2.43 ± 0.00a,b,c | 0.20 ± 0.00b,c | 0.33 ± 0.00a,b,c | 0.10 ± 0.00a | 0.03 ± 0.00b,c | 2.07 ± 0.03c,d | 3.9 | 12.5 |
| S. c. BB9 | 0.47 ± 0.02a,b | 1.46 ± 0.09f | 3.01 ± 0.34a | 0.15 ± 0.00c | 0.41 ± 0.03a,b | 0.01 ± 0.01c | 0.06 ± 0.01b | 1.73 ± 0.00g | 4.9 | 11.5 |
| R. m. Recol 03 | 0.24 ± 0.01g | 2.88 ± 0.08a | 0.87 ± 0.10e | 0.28 ± 0.05a,b | 0 ± 0e | 0 ± 0c | 0 ± 0c | 1.79 ± 0.01f,g | 5.8 | 17.0 |
| M. g. Recol 09 | 0.34 ± 0.01d,e,f | 2.03 ± 0.08b,c | 1.51 ± 0.13d,e | 0.22 ± 0.01a,b,c | 0.26 ± 0.02a,b,c,d | 0 ± 0c | 0 ± 0c | 2.18 ± 0.05b,c | 5.8 | 15.5 |
| C. g. Recol 10 | 0.36 ± 0.00c,d,e | 1.89 ± 0.00c,d | 1.97 ± 0.02b,c,d | 0.18 ± 0.00b,c | 0.44 ± 0.02a | 0.05 ± 0.00b | 0 ± 0c | 1.96 ± 0.01d,e | 5.8 | 14.0 |
| C. p. Recol 12 | 0.32 ± 0.00e,f | 2.13 ± 0.01b | 1.44 ± 0.04d,e | 0.22 ± 0.01a,b,c | 0.22 ± 0.02b,c,d | 0 ± 0c | 0c ± 0 | 2.14 ± 0.00c | 5.8 | 16.5 |
| C. p. Recol 29 | 0.44 ± 0.00b | 1.54 ± 0.00e,f | 1.45 ± 0.08d,e | 0.30 ± 0.01a | 0.22 ± 0.02b,c,d | 0.05 ± 0.00b | 0.03 ± 0.03b,c | 2.36 ± 0.06a | 5.3 | 14.5 |
| C. p. Recol 37 | 0.37 ± 0.01c,d | 1.85 ± 0.04c,d | 1.85 ± 0.28b,c,d | 0.20 ± 0.02b,c | 0.17 ± 0.06c,d,e | 0 ± 0c | 0 ± 0c | 1.75 ± 0.04g | 5.4 | 14.0 |
| P. k. Recol 39 | 0.37 ± 0.01c,d | 1.83 ± 0.04d | 1.89 ± 0.17b,c,d | 0.20 ± 0.01b,c | 0.27 ± 0.08a,b,c,d | 0 ± 0c | 0 ± 0c | 1.98 ± 0.02d,e | 5.6 | 15.0 |
| C. g. Recol 41 | 0.40 ± 0.00c | 1.72 ± 0.00d,e | 2.52 ± 0.60a,b | 0.14 ± 0.06c | 0.32 ± 0.02a,b,c | 0 ± 0c | 0 ± 0c | 1.91 ± 0.01e,f | 5.6 | 13.5 |
| C. g. Recol 43 | 0.31 ± 0.00f | 2.19 ± 0.03b | 1.65 ± 0.05c,d,e | 0.19 ± 0.01b,c | 0.34 ± 0.05a,b,c | 0.05 ± 0.00b | 0 ± 0c | 2.27 ± 0.03a,b | 5.6 | 14.0 |
| P. k. Recol 44 | 0.31 ± 0.01f | 2.17 ± 0.07b | 1.54 ± 0.00d,e | 0.20 ± 0.01b,c | 0.12 ± 0.03d,e | 0 ± 0c | 0 ± 0c | 1.80 ± 0.01f,g | 5.8 | 12.5 |
µmax maximum specific growth rate, DT doubling time, qS specific substrate uptake rate, YX/S biomass yield, YEth/S ethanol yield, YGly/S glycerol yield, YAc/S acetate yield, Xmax maximum concentration of cells formed, DCM dry cell mass, T culture time, S. c. CAT-1 S. cerevisiae CAT-1, P. k. BB1 P. kudriavzevii BB1, P. k. BB2 P. kudriavzevii BB2, S. c. BB9 S. cerevisiae BB9, R. m. Recol 03 R. mucilaginosa Recol 03, M. g. Recol 09 M. guilliermondii Recol 09, C. p. Recol 29 C. parapsilosis Recol 29, C. p. Recol 37 C. parapsilosis Recol 37, C. s. Recol 41 Candida sp. Recol 41, C. g. Recol 43 C. glabrata Recol 43. A, pH at mid-exponential phase
*Data presented as the mean ± standard deviation of the two replicates
a, b, c, d, e, f, g, hThe same letters in the same column indicate no significant difference (P > 0.05)
Fig. 1.
Kinetics of growth, metabolite formation, and sugar consumption during cultivations using fructose (10 g l−1) as a carbon source. a S. cerevisiae CAT-1; b. P. kudriavzevii BB1; c. P. kudriavzevii BB2; d. S. cerevisiae BB9; e. R. mucilaginosa Recol 3; f. M. guilliermondii Recol 9; g. C. glabrata Recol 10; h. C. parapsilosis Recol 12; i. C. parapsilosis Recol 29; j. C. parapsilosis Recol 37; k. P. kudriavzevii Recol 39; l. C. glabrata Recol 41; m. C. glabrata Recol 43; n. P. kudriavzevii Recol 44. Fru (red square): fructose; X (orange diamond): cell concentration; Eth (gray triangle): ethanol; Gly (yellow circle): glycerol; Ac (×): acetate; DCM: dry cell mass. Data are presented as the mean of the two replicates
All results were obtained using a minimal medium with fructose as the sole carbon source. All the strains showed successful growth in the evaluated medium.
The maximum specific growth rate (µmax) is considered an important parameter for selecting strains for bioprocesses (Gomes et al. 2007). The strains isolated from must, P. kudriavzevii BB2, P. kudriavzevii BB1, and S. cerevisiae BB9, showed the highest µmax (between 0.47 and 0.49 h−1), followed by S. cerevisiae industrial strain CAT-1 (0.45 h−1) (Table 1), without a statistically significant difference between them (P < 0.05), but with respect to those isolated from fruit presenting a significant difference (P > 0.05). Among the strains isolated from fruit, C. parapsilosis Recol 29 strain showed the highest µmax (0.44 h−1), while the other strains presented a µmax of 0.24–0.40 h−1 (Table 1).
Shafaghat et al. (2009) studied the S. cerevisiae strain PTCC 24,860 with a concentration of 35g fructose l−1 and found a µmax of 0.42 h−1 at 30 °C. The yeast Kluyveromyces marxianus CBS 6556 was also evaluated in fructose (10 g l−1) and presented a µmax of 0.41 h−1 at 30 °C (Fonseca et al. 2013). R. mucilaginosa ATCC 26,423 in fructose (10 g l−1) showed µmax of 0.20 ± 0.008 h−1 at 200 rpm and 25 °C (Andersen et al. 2003). In these tests, the yeasts stood out for their good adaptation to the medium.
About the specific substrate uptake rate (qS), the strains that stood out were S. cerevisiae CAT-1 (3.02 g gDCM−1 h−1), S. cerevisiae BB9 (3.01 g gDCM−1 h−1) and C. glabrata Recol 41 (2.52 g gDCM−1 h−1). K. marxianus CBS 6556 in fructose (10 g l−1), e.g., presented qS of 0.86 g gDCM−1 h−1 at 30 °C (Fonseca et al. 2013) at very similar conditions. The yeast S. cerevisiae CAT-1, C. glabrata Recol 41, and the strains isolated from must in this work showed considerably higher values than those observed for the other isolates (Table 1), indicating good adaptation to the substrate.
Table 1 also describes the biomass yield (YX/S). It is noteworthy that the C. glabrata Recol 41 strain showed a lower YX/S (0.14 gDCM g−1), followed by S. cerevisiae CAT-1, and S. cerevisiae BB9 (0.15 gDCM g−1), indicating a greater propensity for the production of metabolites. In contrast, the highest YX/S values were found in C. parapsilosis Recol 29 (0.30 gDCM g−1) and R. mucilaginosa Recol 03 (0.28 gDCM g−1).
Wang et al. (2004) studied the S. cerevisiae CCTCC M201022 strain used in the fermentation of apple wine, using fructose (100 g l−1), and found a YX/S of 0.1922 gDCM g−1, which is close to the values found in this work, except for the R. mucilaginosa Recol 03 and C. parapsilosis Recol 29 strains, which presented values above 0.28 gDCM g−1. However, Fonseca et al. (2013) reported even higher values for K. marxianus (0.49 gDCM g−1) in fructose (10 g l−1). It can be stated that the strains with higher YX/S are favorable for the production of biomass, as is described for K. marxianus (Bellaver et al. 2004; Fonseca et al. 2013).
Table 1 describes the data on metabolite formation of the strains under study. These cultures showed the formation of ethanol, glycerol, and acetate.
As for the formation of ethanol, the C. glabrata Recol 10 and S. cerevisiae BB9 strains stood out for their high ethanol yields (YEth/S), 0.44 and 0.41 gEth g−1, respectively (Table 1). It should be noted that the YEth/S of these strains was higher than that of S. cerevisiae industrial yeast CAT-1, despite without significant difference (P < 0.05).
Kumdam et al. (2013) evaluated ethanol production from Debaryomyces nepalensis NCYC 3413 yeast, using fructose (100 g l−1), and observed a YEth/S of 0.12 g g−1. A YEth/S of 0.413 g g−1 was reported for S. cerevisiae PTCC 24,860 in fructose (35 g l−1) (Shafaghat et al. 2009).
Glycerol was produced in small amounts with the strains S. cerevisiae CAT-1, P. kudriavzevii BB1, P. kudriavzevii BB2, S. cerevisiae BB9, C. glabrata Recol 10, C. parapsilosis Recol 29, and C. glabrata Recol 43 (Table 1; Fig. 1a, b, c, d, g, i, m). Acetate was only detected in the strains S. cerevisiae CAT-1, P. kudriavzevii BB1, P. kudriavzevii BB2, S. cerevisiae BB9, and C. parapsilosis Recol 29 (Table 1; Fig. 1a, b, c, d, i). No other metabolite was observed in the cultures used in the study.
Glycerol is known for its protective function for cells, and its formation is a response to stress (Fonseca et al. 2013). The low levels of glycerol synthesis obtained in this study may be attributed to an active metabolism of ethanol formation, which indicates a better performance of yeast fermentation (Ramos et al. 2013). Acetate is a stress- and death-inducing agent produced en route to alcoholic fermentation. It can have negative effects in industrial fermentation processes, e.g., wine production or bioethanol production (Giannattasio et al. 2013).
The four strains that produced acetate were also the same with higher maximum specific growth rates, the values of which were statistically different from that obtained with the other strains, showing a clear relation of growth rate and acetic formation (Table 1). In this sense, the acetic fermentation may be related to an overflow of the carbon flux at the pyruvate level during respiratory metabolism of these yeasts due to a limitation in the enzymatic machinery (Sonnleitner and Kappeli 1986; Castrillo and Ugalde 1993; Fonseca et al. 2013). So, the strain-dependent differences in acetic acid production reflect different regulation of the pyruvate dehydrogenase bypass that a higher acetaldehyde dehydrogenase activity and/or a lower acetyl-CoA synthetase activity could lead to a higher intracellular acetate pool (Postma et al. 1989; Pronk et al. 1996; Della-Bianca et al. 2014).
The absence of acetate production in the other strains can be explained in the same way. In this case, the lower growth rates were responsible for maintaining an oxidative metabolism, without trigger out acetic acid fermentation. The overflow can in part explain the ethanolic fermentation observed for the different strains. However, it may also be related to a certain degree of catabolite repression and/or nutrient limitation (Fonseca et al. 2013).
Metabolites formation can also be evidenced in terms of pH variation (Table 1). It was observed that pH decrease did not affect growth, as the fructose was exhausted during cultivations (Fig. 1).
The R. mucilaginosa Recol 03 strain showed no formation of any extracellular metabolite (Fig. 1e), despite showing lower values of µmax and qS, indicating its difficulty in adapting to the substrate and to the conditions evaluated here. However, the strain was the only one that maintained a purely oxidative metabolism. If there was an oxygen limitation, it probably would have synthesized ethanol. In addition, it may simply be that this is a strain extremelly regulated (Crabtree negative) that coincidentally presented the lower µmax.
PCA analysis resulted in a two-component model that described 74.27% of the total data variation. The first main component (PC 1) represented 57.32% of the data variability and the second main component (PC 2) represented 16.95% of the data variability (Fig. 2).
Fig. 2.
Principal components analysis (PCA) of the kinetic parameters of S. cerevisiae CAT-1 and 13 different yeasts isolated from the Brazilian Mid-West region cultured in fructose substrate, in planes formed by axes of Factor 1 and Factor 2. S. c. CAT-1: S. cerevisiae CAT-1; P. k. BB1: P. kudriavzevii BB1; P. k. BB2: P. kudriavzevii BB2; S. c. BB9: S. cerevisiae BB9; R. m. Recol 03: R. mucilaginosa Recol 03; M. g. 09: M. guilliermondii Recol 09; C. g. 10: C. glabrata Recol 10; C. p. 12: C. parapsilosis Recol 12; C. p. 29: C. parapsilosis Recol 29; C. p. 37: C. parapsilosis Recol 37; P. k. 39: P. kudriavzevii Recol 39; C. g. 41: C. glabrata Recol 41; C. g. 43: C. glabrata Recol 43; P. k. 44: P. kudriavzevii Recol 44
Table 2 presents the most influent loads for PC1 and PC2. μmax, DT, and qS presented the most influential loads for PC1, and YX/S, Xmax, and pH for PC2.
Table 2.
Load of the variables based on correlations for the two main components (PC1 and PC2) in fructose
| Variable | PC 1 | PC 2 |
|---|---|---|
| µ max | 0.931371 | − 0.221825 |
| DT | − 0.907124 | 0.141832 |
| q S | 0.924355 | 0.343720 |
| Y X/S | − 0.617464 | − 0.716488 |
| Y Eth/S | 0.687084 | 0.235042 |
| Y Gly/S | 0.596856 | − 0.210572 |
| Y Ac/S | 0.685829 | − 0.484238 |
| X max | − 0.263473 | − 0.672494 |
| pH | − 0.798218 | 0.451734 |
| T | − 0.894729 | − 0.061277 |
Values marked in bold indicate significant correlations
PC1 first main component, PC2 second main component, µmax maximum specific growth rate, DT doubling time, qS specific substrate uptake rate, YX/S biomass yield, YEth/S ethanol yield, YGly/S glycerol yield, YAc/S acetate yield, Xmax maximum concentration of cells formed, pH hydrogenic potential, T culture time
The comparison of the PC1 x PC2 data is presented in Fig. 2. In this PCA, four different groups were formed. S. cerevisiae CAT-1, S. cerevisiae BB9, C. glabrata Recol 10, and C. glabrata Recol 41 formed a group influenced greater by YEth/S and qS. Another group was formed by the P. kudriavzevii BB1 and P. kudriavzevii BB2 strains, mainly influenced by the formation of glycerol and acetate, beyond a higher μmax. With respect to this last formed group, it was observed that it is also influenced by the lower pH value.
R. mucilaginosa Recol 03, M. guilliermondii Recol 09, C. parapsilosis Recol 12, C. parapsilosis Recol 29, and C. glabrata Recol 43 formed a group related to the production of biomass (Xmax and YX/S). C. parapsilosis Recol 37, P. kudriavzevii Recol 39, and P. kudriavzevii Recol 44 were influenced by the DT parameter.
Summarizing, this study allowed the evaluation of the kinetic parameters of the novel isolated strains to have a comparison between themselves and with other strains cultivated in similar conditions, as reported in the literature. The observations here underline some potential biotechnological applications that with further investigations may lead to industrial applications.
Conclusions
All the tested strains were able to consume the fructose substrate. However, R. mucilaginosa Recol 03 showed great difficulty in fructose consumption, in addition to its inability to synthesize ethanol, glycerol, and organic acids. Potential applications related to the fructose metabolism were evidenced for different yeasts under aerobic batch conditions, e.g., the strains isolated from broth (P. kudriavzevii BB1, P. kudriavzevii BB2, and S. cerevisiae BB9) and the C. glabrata Recol 10 isolated from cherry of Rio Grande (Eugenia involucrata) presented the best performance in ethanol production, while Candida parapsilosis Recol 29 showed the highest biomass yield among the evaluated strains.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the Pro-rectory of Research and Post-graduation of the Federal University of Grande Dourados and the Brazilian research funding agencies CAPES, CNPq, and FUNDECT for their financial support.
Abbreviations
- qS
Specific substrate uptake rate
- µmax
Maximum specific growth rate
- YX/S
Biomass yield
- YP/S
Product yield
- Xmax
Maximum cell concentration
Compliance with ethical standards
Conflict of interest
The authors declare no competing financial interest.
Contributor Information
Cinthia Aparecida de Andrade Silva, Phone: (55 67) 3410-2227, Email: cinthiaandr@yahoo.com.br.
Marta Lígia Oka, Phone: (55 67) 3410-2227, Email: martaligiaoka@gmail.com.
Gustavo Graciano Fonseca, Phone: (55 67) 3410-2227, Email: ggf@ufgd.edu.br.
References
- Albertyn J, Pohl CH, Viljoen BC. Rhodotorula. In: Batt CA, Tortorello ML, editors. Encyclopedia of Food Microbiology. 2. Cambridge: Academic Press; 2014. pp. 291–295. [Google Scholar]
- Andersen D, Renshaw JC, Wiebe MG. Rhodotorulic acid production by Rhodotorula mucilaginosa. Mycol Res. 2003;107:949–956. doi: 10.1017/S0953756203008220. [DOI] [PubMed] [Google Scholar]
- Anjos J, De-Sousa HR, Roca C, Cássio F, Luttik M, Pronk JT, Salema-Oom M, Gonçalves P. Fsy1, the sole hexose-proton transporter characterized in Saccharomyces yeasts, exhibits a variable fructose: H+ stoichiometry. Biochim Biophys Acta. 2013;1828:201–207. doi: 10.1016/j.bbamem.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Basso LC, Basso TO, Rocha SN. Bernardes MAS -recent developments biofuel productionand prospects. Rijeka: Intech; 2011. Ethanol production in Brazil: the industrial process and its impact on yeast fermentation; pp. 85–100. [Google Scholar]
- Batistote M, Cardoso CAL, Ramos DD, Ernandes JR. Desempenho de leveduras obtidas em indústrias de Mato Grosso do Sul na produção de etanol em mosto a base de cana de açúcar. Ciên Nat. 2010;32:83–95. [Google Scholar]
- Bellaver LH, De Carvalho NMB, Abrahão-Neto J, Gombert AK. Ethanol formation and enzyme activities around glucose-6-phosphate in Kluyveromyces marxianus CBS 6556 exposed to glucose or lactose excess. FEMS Yeast Res. 2004;4:691–698. doi: 10.1016/j.femsyr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Berthels NJ, Otero RRC, Bauer FF, Thevelein JM, Pretorius IS. Discrepancy in glucose and fructose utilization during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 2004;4:683–689. doi: 10.1016/j.femsyr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Bura R, Vajzovic A, Doty SL. Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: production of xylitol and ethanol. J Ind Microbiol Biotechnol. 2012;39:1003–1011. doi: 10.1007/s10295-012-1109-x. [DOI] [PubMed] [Google Scholar]
- Camargo JZ, Nascimento VM, Stefanello I, Silva CAA, Gonçalves FA, Perdomo IC, Fonseca GG. Biochemical evaluation, molecular characterization and identification of novel yeast strains isolated from Brazilian savannah fruits, chicken litter and a sugar and alcohol mill with biotechnological potential for biofuel and food industries. Biocatal Agric Biotechnol. 2018;16:390–399. doi: 10.1016/j.bcab.2018.09.006. [DOI] [Google Scholar]
- Cardona CA, Sánchez OJ. Fuel ethanol production: process design trends and integration opportunities. Bioresour Technol. 2007;98:2415–2457. doi: 10.1016/j.biortech.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Castrillo JI, Ugalde UO. Patterns of energy metabolism and growth kinetics of Kluyveromyces marxianus in whey chemostat culture. Appl Microbiol Biotechnol. 1993;40:386–393. doi: 10.1007/BF00170398. [DOI] [Google Scholar]
- Chan GF, Gan HM, Ling HL, Rashid NAA. Genome sequence of Pichia kudriavzevii M12, a potential producer of bioethanol and phytase. Eukaryot Cell. 2012;11:1300–1301. doi: 10.1128/EC.00229-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A, Hidalgo C, Schmidt S, Henschke PA, Curtin C, Varela C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int J Food Microbiol. 2015;205:7–15. doi: 10.1016/j.ijfoodmicro.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Cordero-Bueso G, Esteve-Zarzoso B, Cabellos JM, Gil-Díaz M, Arroyo T. Biotechnological potential of non-Saccharomyces yeasts isolated during spontaneous fermentations of Malvar (Vitis vinifera cv. L.) Eur Food Res Technol. 2013;236:193–207. doi: 10.1007/s00217-012-1874-9. [DOI] [Google Scholar]
- Della-Bianca BE, de Hulster E, Pronk JT, van Maris AJ, Gombert AK. Physiology of the fuel ethanol strain Saccharomyces cerevisiae PE-2 at low pH indicates a context-dependent performance relevant for industrial applications. FEMS Yeast Res. 2014;14:1196–1205. doi: 10.1111/1567-1364.12217. [DOI] [PubMed] [Google Scholar]
- Dhaliwal SS, Oberoi HS, Sandhu SK, Nanda D, Kumar D, Uppal SK. Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresour Technol. 2011;102:5968–5975. doi: 10.1016/j.biortech.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Fonseca GG, De Carvalho NMB, Gombert AK. Growth of the yeast Kluyveromyces marxianus CBS 6556 on different sugar combinations as sole carbon and energy source. Appl Microbiol Biotechnol. 2013;97:5055–5067. doi: 10.1007/s00253-013-4748-6. [DOI] [PubMed] [Google Scholar]
- Freire AL, Ramos CL, De Almeida EG, Duarte WF, Schwan RF. Study of the physicochemical parameters and spontaneous fermentation during the traditional production of yakupa, an indigenous beverage produced by Brazilian Amerindians. World J Microbiol Biotechnol. 2014;30:567–577. doi: 10.1007/s11274-013-1476-0. [DOI] [PubMed] [Google Scholar]
- Giannattasio S, Guaragnella N, Ždralević M, Marra E. Molecular mechanisms of Saccharomyces cerevisiae stress adaptation and programmed cell death in response to acetic acid. Front Microbiol. 2013;4:33. doi: 10.3389/fmicb.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FCO, Silva CLC, Marini MM, Oliveira ES, Rosa CA. Use of selected indigenous Saccharomyces cerevisiae strains for the production of the traditional cachaça in Brazil. J Appl Microbiol. 2007;103:2438–2447. doi: 10.1111/j.1365-2672.2007.03486.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Flores M, Nakhla G, Hafez H. Microbial kinetics of Clostridium termitidis on cellobiose and glucose for biohydrogen production. Biotechnol Lett. 2015;37:1965–1971. doi: 10.1111/j.1365-2672.2007.03486.x. [DOI] [PubMed] [Google Scholar]
- Jasman J, Prijambada ID, Hidayat C, Widianto D. Selection of yeast strains for ethanol fermentation of glucose-fructose-sucrose mixture. Indones J Biotechnol. 2012;17:114–120. doi: 10.22146/ijbiotech.16001. [DOI] [Google Scholar]
- Jones GV, White MA, Cooper OR, Storchmann K. Climate change and global wine quality. Clim Chang. 2005;73:319–343. doi: 10.1007/s10584-005-4704-2. [DOI] [Google Scholar]
- Kitagawa T, Tokuhiro K, Sugiyama H, Kohda K, Isono N, Hisamatsu M, Takahashi H, Imaeda T. Construction of a β-glucosidase expression system using the multistress-tolerant yeast Issatchenkia orientalis. Appl Microbiol Biotechnol. 2010;87:1841–1853. doi: 10.1007/s00253-010-2629-9. [DOI] [PubMed] [Google Scholar]
- Kogan G, Kocher A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest Sci. 2007;109:161–165. doi: 10.1016/j.livsci.2007.01.134. [DOI] [Google Scholar]
- Kumdam H, Murthy SN, Gummadi SN. Production of ethanol and arabitol by Debaryomyces nepalensis: influence of process parameters. AMB Expr. 2013;3:1–13. doi: 10.1186/2191-0855-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman CP, Suzuki M. Meyerozyma. In: Kurtzman C, Fell JW, Boekhout T, editors. The yeasts: a taxonomic study. 5. Amsterdam: Elsevier; 2010. pp. 621–624. [Google Scholar]
- Limtong S, Yongmanitchai W, Kawasaki H, Fujiyama K. Wickerhamomyces edaphicus sp. nov. and Pichia jaroonii sp. nov., two ascomycetous yeast species isolated from forest soil in Thailand. FEMS Yeast Res. 2009;9:504–510. doi: 10.1111/j.1567-1364.2009.00488.x. [DOI] [PubMed] [Google Scholar]
- Nascimento VM, Silva LF, Gomez JGC, Fonseca GG. Growth of Burkholderia sacchari LFM 101 cultivated in glucose, sucrose and glycerol. Sci Agric. 2016;73:429–433. doi: 10.1590/0103-9016-2015-0196. [DOI] [Google Scholar]
- Nolleau V, Preziosi-Belloy L, Navarro JM. The reduction of xylose to xylitol by Candida guilliermondii and Candida parapsilosis: incidence of oxygen and pH. Biotechnol Lett. 1995;17:417–422. doi: 10.1007/BF00130800. [DOI] [Google Scholar]
- Nyanga LK, Nout MJ, Smid EJ, Boekhout T, Zwietering MH. Yeasts preservation: alternatives for lyophilisation. World J Microbiol Biotechnol. 2012;28:3239–3244. doi: 10.1007/s11274-012-1118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira APA, Silvestre MA, Alves-Prado HF, Rodrigues A, Paz MF, Fonseca GG, Leite RSR. Bioprospecting of yeasts for amylase production in solid state fermentation and evaluation of the catalytic properties of enzymatic extracts. Afr J Biotechnol. 2015;14:1215–1223. doi: 10.5897/AJB2014.14062. [DOI] [Google Scholar]
- Orlowski JH, Barford JP. Direct uptake of sucrose by Saccharomyces cerevisiae in batch and continuous culture. J Gen Appl Microbiol. 1991;37:215–218. doi: 10.2323/jgam.37.215. [DOI] [Google Scholar]
- Papon N, Savini V, Lanoue A, Simkin AJ, Crèche J, Giglioli-Guivarc’h N, Clastre M, Courdavault V, Sibirny AA. Candida guilliermondii: biotechnological applications, perspectives for biological control, emerging clinical importance and recent advances in genetics. Curr Genet. 2013;59:73–90. doi: 10.1007/s00294-013-0391-0. [DOI] [PubMed] [Google Scholar]
- Postma E, Verduyn C, Scheffers WA, Van Dijken JP. Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol. 1989;55:468–477. doi: 10.1128/aem.55.2.468-477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk JT, Yde Steensma H, Van Dijken JP. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast. 1996;12:1607–1633. doi: 10.1002/(SICI)1097-0061(199612)12:16<1607::AID-YEA70>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ramos CL, Duarte WF, Freire AL, Dias DR, Eleutherio ECA, Schwan RF. Evaluation of stress tolerance and fermentative behavior of indigenous Saccharomyces cerevisiae. Braz J Microbiol. 2013;44:935–944. doi: 10.1590/S1517-83822013005000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani A, Pereira F, Johansson B, Domingues L. Metabolic engineering of Saccharomyces cerevisiae ethanol strains PE-2 and CAT-1 for efficient lignocellulosic fermentation. Bioresour Technol. 2015;179:150–158. doi: 10.1016/j.biortech.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Shafaghat H, Najafpour GD, Reazaei PS, Sharifzadeh M. Growth kinetics and ethanol productivity of Saccharomyces cerevisiae PTCC 24860 on various carbon sources. World Appl Sci J. 2009;7:140–144. [Google Scholar]
- Silva CAA, Fonseca GG. Brazilian savannah fruits: characteristics, properties, and potential applications. Food Sci Biotechnol. 2016;25:1225–1232. doi: 10.1007/s10068-016-0195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RO, Batistote M, Cereda MP. Wild strains of fermenting yeast isolated of sugar cane juice from an alcohol distillery from Mato Grosso, Brazil. J Biotechnol Biodivers. 2011;2:22–27. [Google Scholar]
- Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- Silva-Filho EA, Santos SKB, Resende AM, Morais JOF, Morais Júnior MA, Simões DA. Yeast population dynamics on industrial fuel ethanol fermentation processes assessed by PCR finger printing. Anton Leew. 2005;88:13–23. doi: 10.1007/s10482-004-7283-8. [DOI] [PubMed] [Google Scholar]
- Sonnleitner B, Kappeli O. Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity: formulation and verification of a hypothesis. Biotechnol Bioeng. 1986;28:927–937. doi: 10.1002/bit.260280620. [DOI] [PubMed] [Google Scholar]
- Teixeira MC, Raposo LR, Mira NP, Lourenço AB, Sá-Correia I. Genome-wide identification of Saccharomyces cerevisiae genes required for maximal tolerance to ethanol. Appl Environ Microbiol. 2009;75:5761–5772. doi: 10.1128/AEM.00845-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchoni J, Gamero A, Arroyo-López FN, Barrio E, Querol A. Differences in the glucose and fructose consumption profiles in diverse Saccharomyces wine species and their hybrids during grape juice fermentation. Int J Food Microbiol. 2009;134:237–243. doi: 10.1016/j.ijfoodmicro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Verduyn C, Postma E, Scheffers WA, Van Dijken JP. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration an alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- Wang D, Xu Y, Hu J, Zhao G. Fermentation kinetics of different sugars by the apple wine yeast Saccharomyces cerevisiae. J Inst Brew. 2004;110:340–346. doi: 10.1002/j.2050-0416.2004.tb00630.x. [DOI] [Google Scholar]
- Wang X, Ike M, Shiroma R, Tokuyasu K, Sakakibara Y. Expression of neutral β-glucosidase from Scytalidium thermophilum in Candida glabrata for ethanol production from alkaline-pretreated rice straw. J Biosci Bioeng. 2013;116:362–365. doi: 10.1016/j.jbiosc.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Wang XC, Li AH, Dizy M, Ullah N, Sun WX, Tao YS. Evaluation of aroma enhancement for ‘‘Ecolly” dry white wines by mixed inoculation of selected Rhodotorula mucilaginosa and Saccharomyces cerevisiae. Food Chem. 2017;228:550–555. doi: 10.1016/j.foodchem.2017.01.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.