Abstract
Experience with delamanid (Dlm) is limited, particularly among HIV-positive individuals. We describe early efficacy and safety data from a programmatic setting in South Africa.
This was a retrospective cohort study of patients receiving Dlm-containing treatment regimens between November 2015 and August 2017. We report 12-month interim outcomes, sputum culture conversion (SCC) by months 2 and 6, serious adverse events (SAEs) and QT intervals corrected using the Frederica formula (QTcF).
Overall, 103 patients were initiated on Dlm; 79 (77%) were HIV positive. The main indication for Dlm was intolerance to second-line anti-tuberculosis (TB) drugs (n=58, 56%). There were 12 months of follow-up for 46 patients; 28 (61%) had a favourable outcome (cure, treatment completion or culture negativity). Positive cultures were found for 57 patients at Dlm initiation; 16 out of 31 (52%) had SCC within 2 months and 25 out of 31 (81%) within 6 months. There were 67 SAEs reported in 29 patients (28%). There were four instances of QTcF prolongation >500 ms in two patients (2%), leading to permanent discontinuation in one case; however, no cardiac arrhythmias occurred.
This large cohort of difficult-to-treat patients receiving Dlm for rifampicin-resistant TB treatment in a programmatic setting with high HIV prevalence had favourable early treatment response and tolerated treatment well. Dlm should remain available, particularly for those who cannot be treated with conventional regimens or with limited treatment options.
Short abstract
Patients with rifampicin-resistant TB treated with delamanid had good treatment response and cardiotoxicity was rare http://ow.ly/bVQu30jGPVJ
Introduction
In 2016, an estimated 600 000 new cases of rifampicin-resistant (RR) tuberculosis (TB), defined as Mycobacterium tuberculosis with demonstrated resistance to at least rifampicin, including mono- and poly-resistant strains of TB, multidrug-resistant (MDR) TB and extensively drug-resistant TB, emerged globally [1, 2]. This represents a significant public health threat [3]. RR-TB requires prolonged treatment with multiple toxic agents, resulting in poor treatment outcomes [4, 5].
A novel anti-TB agent from the nitroimidazole class, delamanid (Dlm) (Deltyba, Otsuka Pharmaceuticals, Rockville, MD, USA), was recommended for use in RR-TB by the World Health Organization (WHO) in 2014 after receiving conditional approval from the European Medicines Agency in 2013 [6, 7]. These recommendations were based on phase 2B trial data, which demonstrated efficacy and safety of the drug [8, 9]. Additionally, Dlm has no drug–drug interactions when given with antiretroviral therapy (ART), including efavirenz (EFV) [10, 11]. Despite these promising results and early recommendations, uptake of Dlm globally has been poor, with only 976 persons having received the drug under programmatic conditions as of October 2017 [12].
At the 48th Union World Conference on Lung Health, preliminary phase 3 clinical trial data were presented, in which Dlm was compared with placebo when added to a multidrug backbone regimen for 6 months. The primary endpoint was time to sputum culture conversion (SCC) measured over 6 months. Patients in the Dlm arm had SCC 6–13 days faster over 6 months than patients in the placebo arm, depending on three different analytic methods used (p-values of 0.0052–0.0562). Long-term outcomes at 24 months, which was a secondary endpoint, did not differ significantly between the Dlm and placebo arms, although the study was not powered to detect differences in final outcomes. Safety data were encouraging because only 5.3% of patients in the Dlm arm experienced a QT interval (corrected using the Frederica formula (QTcF)) >500 ms (Otsuka, unpublished results of delamanid trial 213 presented at the 48th Union Conference on Lung Health, 2017). These results have called into question the role of Dlm in the treatment of RR-TB. There is an urgent need for data on Dlm use under programmatic conditions to better define its role in the treatment of RR-TB, especially among populations that were under-represented in the phase 3 trial, e.g. adolescent patients and patients with HIV, extrapulmonary TB or intolerance to the drugs in the standard MDR regimen [13]. In fact, the WHO recently released a position statement on the use of Dlm for the treatment of RR-TB stating that use should be continued as indicated and that more data are needed from programmatic settings [14].
In South Africa there were close to 20 000 cases of RR-TB confirmed in 2016 [15]. Bedaquiline (Bdq) is registered in the country and is widely used, along with other repurposed drugs such as linezolid and clofazimine (Cfz), to treat RR-TB in line with WHO recommendations [16, 17]. To date, Dlm is not registered or readily available in South Africa outside of an expanded access programme with strict criteria [18]. In 2015, Médecins Sans Frontières (MSF), a humanitarian non-governmental organisation, was able to procure Dlm for a subset of patients with RR-TB in a decentralised treatment programme in Khayelitsha, South Africa. The aim of this study was to describe early efficacy and safety of Dlm-containing RR-TB treatment regimens in a cohort of patients with high rates of HIV co-infection.
Methods
Study design
This was a retrospective, descriptive cohort study of patients with RR-TB who received Dlm within an individualised treatment regimen, using routinely collected programmatic data.
Setting
Khayelitsha is a peri-urban township outside of Cape Town, South Africa, with a population of ∼450 000 people, most of whom reside in informal structures. The annual case notification rate of RR-TB is 55 per 100 000 population, the HIV prevalence among RR-TB patients is 70% and patients with RR-TB are managed mostly as outpatients across 11 primary healthcare facilities, as previously described [19]. Most patients treated for RR-TB receive self-administered treatment, accompanied by enhanced adherence support from a dedicated team of counsellors.
Participants
All patients initiated on Dlm in Khayelitsha between November 2015 and August 2017 were included in the study. Indications for Dlm included 1) intolerance to specific second-line (SL) drugs in the treatment regimen, e.g. fluoroquinolone-susceptible RR-TB but high risk of hearing loss; 2) limited options to design a regimen with at least five effective drugs; and 3) patients in whom previous RR-TB treatment had failed. Patients were further eligible to receive the combination of Bdq and Dlm if there was 1) the inability to construct a regimen with at least four effective drugs; 2) suspected resistance due to previous drug exposure; and 3) intolerance to drugs in the MDR regimen. Because patients on the combination of Bdq and Dlm have been previously reported on, we did not assess safety or efficacy outcomes for these patients separately [20].
Criteria that were considered when designing a Dlm-containing RR-TB treatment regimen were HIV co-infection with EFV-based ART (therefore Bdq was contraindicated); allergy, intolerance or prior treatment with Bdq; >2 months exposure to Cfz; age ≤18 years; pregnancy; extensive drug resistance; and high risk of treatment failure (e.g. diabetes and extensive cavitation).
Patient monitoring
As per WHO recommendations [6], Dlm was administered for a period of 6 months, although some patients required prolonged administration of the drug. Reasons for prolonged administration of Dlm included interruptions in Dlm throughout the course of treatment or the lack of other therapeutic options to devise a treatment regimen with at least four effective drugs following the completion of the intensive phase of treatment with Dlm. Clinical monitoring of patients on Dlm occurred monthly, and ECG monitoring was carried out every 2 weeks for the first 12 weeks and monthly thereafter. In cases of QTcF prolongation (>500 ms), patients were managed according to WHO guidelines and local expert advice.
Study outcomes
Standard WHO definitions were used for RR-TB treatment outcomes, including success, loss to follow-up (LTFU), death and treatment failure [21]. Favourable outcomes were defined as cure, treatment completion or culture negativity if still on treatment.
Efficacy
Efficacy was assessed using SCC measured 2 and 6 months after Dlm initiation (conducted on liquid media using a mycobacteria growth indicator tube, BACTEC MGIT 960; Becton Dickinson, Sparks, MD, USA). SCC was defined as two consecutive negative cultures taken at least 30 days apart in a patient with a positive specimen at baseline [21]. Sputum reversion to positive was defined as two consecutive positive cultures at least 28 days apart occurring after SCC. Baseline refers to when Dlm was added to the regimen, even if background RR-TB treatment was ongoing.
Safety
Serious adverse events (SAEs) were defined according to the International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines as events that resulted in death, were life-threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability/incapacity or congenital anomalies/birth defects [22].
We used QTcF in our cohort [23]. QTcF prolongation was defined as any absolute QTcF interval ≥500 ms or as any QTcF interval increase of >60 ms from the baseline.
Data sources, analysis and statistics
Data were obtained from the South African Electronic Drug-Resistant Tuberculosis Register (EDRWeb.net), RR-TB registers, patients' medical records (including ECG readings) and the National Health Laboratory Service Database (TrakCare). Data on SAEs were obtained from the central MSF Pharmacovigilance unit in Geneva, Switzerland, which compiles SAE reports from the MSF clinical field team within 24 h of becoming aware of the event. Data entered in EDRWeb were validated using information captured in patient medical records and RR-TB registers.
Clinical and demographic characteristics were stratified by HIV status. Categorical variables were described using proportions and continuous variables using medians and interquartile range (IQR). Chi-squared or Fisher's tests and Wilcoxon rank-sum tests were used to determine differences in clinical and demographic characteristics based on HIV status. Interim outcomes for patients who had 12 months of follow-up and culture conversion by 2 and 6 months were reported. A Kaplan–Meier curve was used to show time to 6-month culture conversion; data were censored for LTFU and mortality. Bivariate logistic regression and Cox proportional hazards models were used to determine the strength of association between culture conversion and patient characteristics. SAEs were reported using descriptive statistics. Changes in QTcF values over time at the cohort level were reported as the median of the difference between each follow-up time point and the baseline value for each individual. Box plots were used to show the median QTcF values over time and the median change from baseline for the 6-month follow-up period. Factors were considered statistically significant if there was a two-tailed p-value of <0.05. Stata version 14 (College Station, TX, USA) was used for statistical analysis.
Ethics
Ethical approval for this study was obtained from the University of Cape Town Human Research Ethics Committee (HREC 499/2011). The study fulfilled the MSF Ethics Review Board (Geneva, Switzerland) exemption criteria for a posteriori analyses of routinely collected clinical data. Patients provided written informed consent to receive Dlm.
Results
Patient and treatment characteristics
Overall, 103 patients were given Dlm for RR-TB treatment. Of these, 79 patients (77%) were HIV positive (table 1). The median (IQR) CD4 count was 141 (61–252) cells·mm−3 (n=45; 57% with CD4 count <200 cells·mm−3) and 72 HIV-positive patients (91%) were on ART at Dlm initiation. The seven patients not on ART at Dlm initiation were started on ART within a median (IQR) of 1.9 (1.0–8.0) weeks after Dlm initiation. The majority of patients had pulmonary TB (n=97, 94%); of the five patients with extrapulmonary TB, two had peri-anal abscesses and the remaining three had disseminated TB, a pericardial and pleural effusion, and a pleural effusion, respectively. One patient with both pulmonary and extrapulmonary TB had disseminated TB. The median (IQR) time from RR-TB treatment to Dlm initiation was 1.5 (0.8–3.2) months and the total time on Dlm was 6 (3.6–7.8) months; 54 patients (52%) received treatment with Dlm for >6 months.
TABLE 1.
Clinical and demographic characteristics, stratified by HIV status, of patients treated with rifampicin-resistant tuberculosis regimens containing delamanid from November 1, 2015 to August 31, 2017, in Khayelitsha, South Africa
| Variable | Total patients | HIV positive | HIV-negative | p-value |
| Patients | 103 (100.0) | 79 (100.0) | 24 (100.0) | – |
| Age at Dlm initiation years | 34 (28–43) | 35 (30–43) | 31 (21–40) | 0.073 |
| <18 years of age | 8 (8.8) | 3 (3.8) | 5 (20.8) | 0.016 |
| Male | 63 (61.2) | 47 (59.5) | 16 (66.7) | 0.53 |
| Body mass index at Dlm initiation kg·m−2 | 20.4 (17.7–23.6)+ | 20.4 (17.8–23.6)§ | 20.2 (17.6–23.7)ƒ | 0.92 |
| QTcF at Dlm initiation ms | 412.8 (394.0–433.0)## | 412.6 (394.0–436.0) | 414.0 (394.0–427.0)## | 0.77 |
| Albumin at Dlm initiation g·L−1 | 35.0 (29.0–39.0)¶¶ | 35.0 (29.0–38.0)++ | 38.0 (29.5–40.0)§§ | 0.087 |
| Diabetic | 4 (3.9) | 1 (1.3) | 3 (12.5) | 0.039 |
| CD4 count at Dlm initiation cells·mm−3 | 141.0 (61.0–252.0)ƒƒ | – | ||
| Antiretroviral therapy at Dlm initiation | 72 (91.1) | – | ||
| RR-TB disease classification | ||||
| Presumed RR-TB# | 1 (1.0) | 0 (0.0) | 1 (4.2) | – |
| GeneXpert unconfirmed¶ | 3 (2.9) | 2 (2.5) | 1 (4.2) | – |
| Rifampicin mono-resistant TB | 21 (20.4) | 18 (22.8) | 3 (12.5) | – |
| Multidrug-resistant TB | 41 (39.8) | 36 (45.5) | 5 (20.8) | – |
| Pre-XDR-TB injectable | 5 (4.8) | 4 (5.1) | 1 (4.2) | – |
| Pre-XDR-TB fluoroquinolone | 15 (14.6) | 7 (8.9) | 8 (33.3) | – |
| XDR-TB | 17 (16.5) | 12 (15.2) | 5 (20.8) | 0.014 |
| Previous TB treatment history | ||||
| None | 44 (42.7) | 34 (43.0) | 10 (41.6) | – |
| First-line | 43 (41.8) | 36 (45.6) | 7 (29.2) | – |
| Second-line | 16 (15.5) | 9 (11.4) | 7 (29.2) | 0.085 |
| Disease site | ||||
| Pulmonary | 97 (94.2) | 74 (93.7) | 23 (95.8) | – |
| Extrapulmonary | 5 (4.8) | 4 (5.0) | 1 (4.2) | – |
| Both | 1 (1.0) | 1 (1.3) | 0 (0.0) | 1.0 |
| Culture status at Dlm initiation | ||||
| Negative | 42 (40.8) | 35 (44.3) | 7 (29.2) | – |
| Positive | 58 (56.3) | 42 (53.2) | 16 (66.7) | – |
| Contaminated | 2 (1.9) | 2 (2.5) | 0 (0.0) | – |
| Not done | 1 (1.0) | 0 (0.0) | 1 (4.1) | 0.16 |
| Indication for Dlm | ||||
| Intolerance | 58 (56.3) | 48 (60.8) | 10 (41.7) | – |
| Limited therapeutic options | 38 (36.9) | 27 (34.2) | 11 (45.8) | – |
| Treatment failure | 7 (6.8) | 4 (5.0) | 3 (12.5) | 0.16 |
| Time from RR-TB treatment to Dlm initiation months | 1.5 (0.8–3.2) | 1.5 (0.8–3.2) | 1.7 (1.2–4.7) | 0.35 |
| Received >6 months of Dlm | 54 (52.4) | 39 (49.4) | 15 (62.5) | 0.26 |
Data are presented as n (%) or median (interquartile range). Bold text indicates significance at p<0.05. Dlm: delamanid; QTcF: QT corrected using the Frederica formula; RR: rifampicin-resistant; TB: tuberculosis; XDR: extensively drug-resistant. #: RR-TB diagnosed on presumption without bacteriological confirmation (often children); ¶: GeneXpert diagnosed RR-TB with no follow-up second-line testing to confirm resistance; +: eight missing baseline body mass index; §: seven missing baseline body mass index; ƒ: one missing baseline body mass index; ##: one missing baseline QTcF; ¶¶: nine missing baseline albumin; ++: five missing baseline albumin; §§: four missing baseline albumin; ƒƒ: six missing baseline CD4 count.
The differences based on HIV status are shown in table 1. A higher proportion of HIV-negative patients were <18 years of age or diabetic when compared with HIV-positive patients (21% versus 4% and 12% versus 1%, p<0.05); additionally, more HIV-negative patients had MDR-TB plus additional SL resistance than did HIV-positive patients (58% versus 29%, p<0.05).
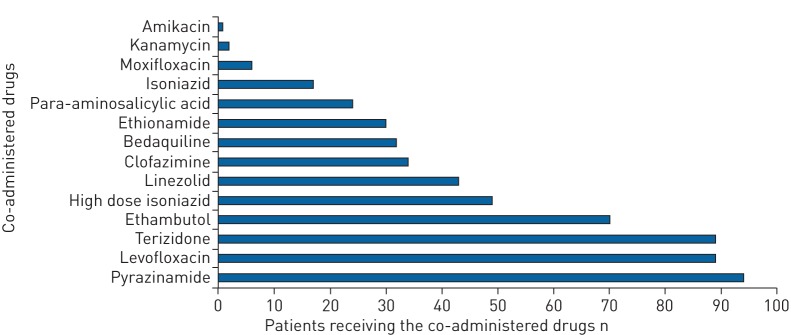
The median number of drugs included in RR-TB regimens at Dlm initiation was six (IQR 6–7) including Dlm. The most commonly co-administered drugs at Dlm initiation included pyrazinamide (91%), levofloxacin (86%), terizidone (86%) and ethambutol (70%) (figure 1). Bdq was co-administered in 32 patients (31%). The majority of patients (97%) received at least one other QT-prolonging drug in addition to Dlm, as follows: Bdq and Cfz (n=24, 23%), Cfz only (n=10, 10%), Bdq only (n=8, 8%), and moxifloxacin only (n=6, 6%). Clinical and demographic characteristics for the 32 patients treated with regimens containing both Dlm and Bdq stratified by HIV status can be seen in table 2.
FIGURE 1.
Number of rifampicin-resistant tuberculosis drugs co-administered at delamanid initiation between November 1, 2015 and August 31, 2017 in Khayelitsha, South Africa.
TABLE 2.
Clinical and demographic characteristics, stratified by HIV status, of patients treated with rifampicin-resistant tuberculosis regimens containing delamanid and bedaquiline from November 1, 2015, to August 31, 2017, in Khayelitsha, South Africa
| Variable | Total patients | HIV positive | HIV-negative | p-value |
| Patients | 32 (100.0) | 18 (100.0) | 14 (100.0) | |
| Age at Dlm initiation years | 35 (30–43) | 36 (31–43) | 32 (25–42) | 0.32 |
| <18 years of age | 2 (6.3) | 0 (0.0) | 2 (14.3) | |
| Male | 19 (59.4) | 10 (55.6) | 9 (64.3) | 0.62 |
| Body mass index at Dlm initiation kg·m−2 | 20.8 (19.5–24.4)# | 20.5 (19.5–24.9) | 21.7 (19.6–23.6)# | 0.86 |
| QTcF at Dlm initiation ms | 410.5 (387.5–429.0) | 405.0 (383.0–432.0) | 415.5 (404.0–426.0) | 0.63 |
| Diabetic | 2 (6.3) | 0 (0.0) | 2 (14.3) | 0.18 |
| CD4 count at Dlm initiation cells·mm−3 | 91.0 (55.0–215.0)¶ | – | ||
| Antiretroviral therapy at Dlm initiation | 16 (88.9) | – | ||
| RR-TB disease classification | ||||
| Rifampicin mono-resistant TB | 2 (6.3) | 2 (11.1) | 0 (0.0) | – |
| Multidrug-resistant TB | 3 (9.4) | 1 (5.6) | 2 (14.3) | – |
| Pre-XDR-TB injectable | 1 (3.1) | 1 (5.6) | 0 (0.0) | – |
| Pre-XDR-TB fluoroquinolone | 12 (37.5) | 5 (27.8) | 7 (50.0) | – |
| XDR-TB | 14 (43.7) | 9 (50.0) | 5 (35.7) | 0.46 |
| Previous TB treatment history | ||||
| None | 9 (28.1) | 3 (16.7) | 6 (42.9) | – |
| First-line | 12 (37.5) | 8 (44.4) | 4 (28.6) | – |
| Second-line | 11 (34.4) | 7 (38.9) | 4 (28.6) | 0.26 |
| Disease site | ||||
| Pulmonary | 30 (93.8) | 17 (94.4) | 13 (92.9) | – |
| Extrapulmonary | 2 (4.8) | 1 (5.6) | 1 (7.1) | 1.0 |
| QTcF-prolonging drugs co-administered+ | ||||
| Clofazimine | 24 (75.0) | 13 (72.2) | 11 (78.6) | 1.0 |
| Moxifloxacin | 0 (0.0) | 0 (0.0%) | 0 (0.0) | – |
Data are presented as n (%) or median (interquartile range). Dlm: delamanid; QTcF: QT corrected using the Frederica formula; RR: rifampicin-resistant; TB: tuberculosis; XDR: extensively drug-resistant. #: one missing baseline body mass index; ¶: one missing baseline CD4 count; +: numbers are not mutually exclusive.
Efficacy
Interim outcomes
The median (IQR) follow-up time for the 103 patients included in this study was 7.8 (4.6–12.9) months. Overall, outcomes of LTFU, death, cure or treatment completion, and failure of the SL regimen occurred after Dlm initiation within a median (IQR) of 2.3 (0.9–3.8) months in 12 patients (12%), 3.1 (1.0–7.6) months in 11 patients (11%), 10.3 (7.1–16.6) months in seven patients (7%) and 10.9 (8.1–12.6) months in two patients (2%). At the time of this analysis, 57 patients (55%) were still on RR-TB treatment with or without Dlm and culture negative; 64 patients (62%) had a favourable outcome.
Overall, 46 patients (45%) had 12 months of follow-up; of these patients, 28 (61%) had a favourable outcome, including either treatment completion and cure or culture negativity; seven (15%) were LTFU, five (11%) died, two (4%) were declared to have a failure in treatment, one (2%) was transferred out and the remaining three (7%) were still on RR-TB treatment (two culture positive and one with an unknown culture status). There was no significant difference in 12-month outcomes based on HIV status (p>0.05; table 3).
TABLE 3.
12-month outcomes, stratified by HIV status, for patients receiving rifampicin-resistant tuberculosis regimens including delamanid from November 1, 2015, to September 30, 2016, in Khayelitsha, South Africa
| Patients | HIV positive | HIV-negative |
Time from Dlm initiation to final outcome months |
|
| Patients | 46 (100.0) | 35 (100.0) | 11 (100.0) | 8.1 (2.0–12.6) |
| Still on treatment | ||||
| Culture positive | 2 (4.3) | 2 (5.7) | 0 (0.0) | –# |
| Culture negative | 21 (45.7) | 14 (40.0) | 7 (63.6) | –# |
| Culture status unknown | 1 (2.2) | 1 (2.9) | 0 (0.0) | –# |
| Cured or completed treatment | 7 (15.2) | 6 (17.1) | 1 (9.1) | 10.3 (7.1–16.6) |
| Lost to follow-up | 7 (15.2) | 6 (17.1) | 1 (9.1) | 7.0 (1.1–10.6) |
| Died | 5 (10.9) | 4 (11.5) | 1 (9.1) | 2.0 (1.4–13.6) |
| Treatment failure | 2 (4.3) | 2 (5.7) | 0 (0) | 10.4 (8.1–12.6) |
| Transferred out | 1 (2.2) | 0 (0.0) | 1 (9.1) | 5.9 (5.9–5.9) |
Data are presented as n (%) or median (interquartile range). Dlm: delamanid. #: rifampicin-resistant tuberculosis (RR-TB) treatment was ongoing in these patients and therefore time to a final outcome could not be calculated.
Culture conversion
At Dlm initiation, 58 out of 103 patients (56%) had positive cultures; 57 (98%) of those had pulmonary TB. Time to culture conversion is displayed in figure 2 for these patients. Of these 57 patients, 31 (54%) had culture data available for months 2 and 6; 16 (52%) had SCC within 2 months and 25 (81%) within 6 months. SCC and time to SCC did not differ based on HIV status, RR-TB resistance profile or indication for Dlm (p>0.05).
FIGURE 2.
Time to culture conversion among patients with pulmonary tuberculosis initiated on rifampicin-resistant tuberculosis treatment regimens including delamanid (Dlm) before January 1, 2017, in Khayelitsha, South Africa (n=57).
Of the 25 patients with SCC within 6 months, three (12%) reverted to culture-positive status, at 2.2, 3.6 and 4.0 months after the date of culture conversion, which was 5.0, 7.1 and 4.9 months after Dlm initiation, respectively. All of these patients were HIV positive and still on Dlm at the time of reversion.
Safety
SAEs
There were 67 SAEs reported in 29 patients (28%); each patient had a median of two (IQR 1–3) SAEs. Of these, 22 (33%) were attributed to Dlm. The most common SAEs were QT prolongation (n=7; 100% attributed to Dlm) and vomiting (n=4; 75% attributed to Dlm). There were three hepatic SAEs of which 67% were attributed to Dlm.
QTcF safety profile
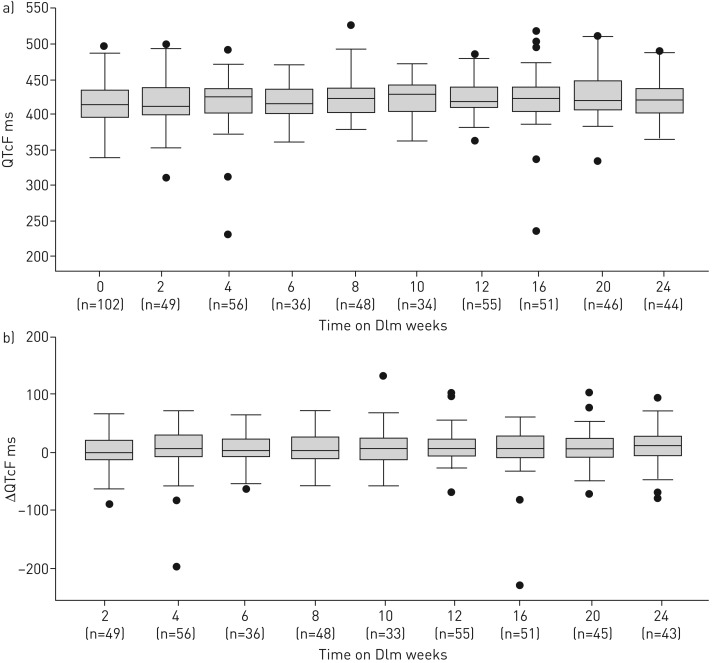
The median (IQR) QTcF values observed during the monitoring visits can be seen in figure 3a. Overall, there were four instances of QTcF prolongation >500 ms in two patients (2%); in one of these patients Dlm was permanently withdrawn owing to QTcF prolongation.
FIGURE 3.
a) Median QT intervals corrected using the Frederica formula (QTcF) (interquartile range) at weeks 0–24 after delamanid (Dlm) initiation, regardless of time on Dlm, among patients treated for rifampicin-resistant tuberculosis from November 1, 2015 to August 31, 2017 in Khayelitsha, South Africa. b) Median change from baseline QTcF (interquartile range) at weeks 2–24 after Dlm initiation, regardless of time on Dlm, among patients treated for rifampicin-resistant tuberculosis from November 1, 2015 to August 31, 2017, in Khayelitsha, South Africa.
Median (IQR) QTcF change from baseline values for each monitoring visit can be seen in figure 3b. There were 14 instances in which the QTcF value increased >60 ms from baseline in nine patients (9%); none of these instances led to known cardiac arrhythmias or the permanent discontinuation of Dlm, and none led to QTcF >500 ms. Eight of these patients were receiving other potentially QT-prolonging drugs along with Dlm: Bdq only (n=1), Cfz only (n=2), and Bdq and Cfz (n=5). A significantly higher proportion of patients on combination treatment with Bdq, Cfz and Dlm experienced QTcF increases >60 ms from baseline (5 of 24, 21%) compared to those not on all three of these drugs (4 of 79, 5%) (risk ratio 4.1, 95% CI 1.2–14.1, p=0.030).
Discussion
We report on a cohort of 103 patients who received Dlm for the treatment of RR-TB in a routine programmatic setting. Notably, more than one third of patients in the cohort were infected with highly resistant strains, more than three out of four patients were HIV positive, and eight patients were aged ≤18 years; these populations have been under-represented in clinical trials.
The high rates of culture conversion in this population are encouraging, and are similar to rates reported among RR-TB patients treated with Dlm-containing regimens in other programmatic and trial settings (67.6–94.4%) [24–26]. Data from a programmatic setting in South Korea showed 24-week SCC rates of 94.4% and 92.9% in solid and liquid media, respectively, which are higher than our SCC rates; however, there are setting-specific differences that likely affected the variability in these findings [25]. In our study, one of the three patients who reverted to culture positive after initial conversion to negative was given Dlm within an optimised regimen following prior RR-TB treatment failure, and therefore was already at considerably higher risk of a poor outcome [27]. This highlights the need for wider access to more effective treatment regimens, including new drugs, at the initial diagnosis of RR-TB. Treatment with Dlm for >6 months might assist in improving long-term outcomes for RR-TB patients with poor early treatment response [28, 29].
Overall, 11% of patients treated with RR-TB regimens including Dlm died. These findings are similar to what has previously been reported in a study of patients treated with Dlm-containing regimens; of 53 patients, 13.2% died by 6 months [24]. Previous reports have found high rates of mortality among RR-TB patients with HIV co-infection, low CD4 counts, SL drug resistance and prior TB treatment histories; thus, our findings are not surprising [30, 31]. Time to death was rapid, within ∼3 months, and may have been due to severe and extensive disease at time of Dlm initiation because Dlm was often offered to those patients with limited treatment options. Data on final treatment outcomes for 16 RR-TB patients treated with Dlm-containing regimens in a programmatic setting in Latvia showed cure rates of 84.2% and LTFU rates of 15.8% [32]. We need a longer follow-up period for patients enrolled in our cohort before we can make comparisons between our findings and those previously reported. Our data further highlight the need for early diagnosis and prompt initiation of robust treatment regimens, improved integrated management of HIV/TB, and management of other risk factors for poor outcomes at the time of RR-TB diagnosis and throughout follow-up.
Overall, few patients (2%) experienced QTcF prolongation >500 ms when treated with Dlm in combination with other potential cardio-toxic drugs and less than half experienced SAEs. These data are supported by the recent phase 3 Dlm trial results in which only 5.3% of patients who received Dlm experienced QTcF prolongation and Dlm was reported to have a favourable safety profile (unpublished results of delamanid trial 213 presented at the 48th Union Conference on Lung Health, 2017). Other case reports and studies from programmatic settings utilising Dlm in RR-TB treatment regimens have reported relatively low rates of QTcF prolongation >500 ms or increase >60 ms when Dlm was co-administered with other cardio-toxic drugs (3.7–17.0%) [13, 20, 24–26, 32–34]. In the current study there appeared to be an increased risk of QT interval change >60 ms from baseline when a combination of cardio-toxic drugs, including Dlm, were administered. However, none of the episodes reported here led to the permanent discontinuation of Dlm and this population included patients treated with multiple drugs, all of which are toxic and can cause SAEs. A multi-site study of 28 patients who received RR-TB regimens containing the combination of Bdq and Dlm similarly found that no patients had a QTcF >500 ms and there were four patients (14%) who experienced six instances of a QTcF increase >60 ms from baseline. Again, none of these instances led to permanent discontinuation of Bdq or Dlm [20]. One of these instances in a patient from South Africa is also reported on in this study. Given that QTcF prolongation mostly occurred after the eighth week of treatment with Dlm in the current study, the intensive ECG monitoring following Dlm initiation may be overcautious. Less than one third of our cohort experienced SAEs, very few of which were attributed to Dlm, so our data further support previous findings regarding the favourable safety profile of Dlm [25, 32]. Similar to the favourable safety profile of Bdq, Dlm performed well in RR-TB treatment regimens, with and without Bdq [35–37]. A systematic review of the evidence regarding Bdq-containing RR-TB treatment regimens showed that Bdq was discontinued in 3.4% of patients owing to adverse events and in 0.6% of patients owing to QTc prolongation [37].
The first limitation of this study was the retrospective, descriptive cohort design, with a relatively small sample size. There was no control group, and final treatment outcomes are still pending for the majority of patients in this cohort; therefore, statistical inferences for the outcomes of interest could not be made. Multivariate analyses could not be performed, particularly for the analysis of culture conversion, owing to the small sample size. There were missing data, particularly on QT intervals, which might have resulted in an underestimation of QTcF prolongation in this cohort. This does, however, reflect the routine monitoring that is carried out in overburdened and under-resourced facilities in this programmatic setting. Nevertheless, there were no instances of clinically significant cardiac arrhythmias or unexplained sudden deaths in this cohort.
This is one of the largest reported cohorts of patients receiving Dlm for RR-TB treatment outside of clinical trial settings, but the generalisability of these findings is limited. Further analyses should include larger numbers of patients and longer follow-up to draw conclusions on final outcomes for patients receiving Dlm in programmatic settings. Additionally, studies are needed to further delineate the possible role of Dlm in populations with limited treatment options, including children, pregnant women, patients with drug intolerances and patients who need to remain on EFV-containing regimens.
Overall, this cohort, which included a large proportion of HIV-infected individuals and patients with extensive disease, had favourable early treatment response and tolerated Dlm-containing regimens reasonably well. Our data suggest that Dlm should remain available for RR-TB patients, particularly those who cannot be treated with conventional regimens and those with limited treatment options.
Acknowledgements
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization. The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medécins Sans Frontières (MSF). The specific SORT IT programme that resulted in this publication was implemented by MSF, Brussels Operational Centre, Luxembourg, Luxembourg, and the Centre for Operational Research, The Union, Paris, France. Mentorship and the coordination/facilitation of these SORT IT workshops were provided through the Centre for Operational Research, The Union, Paris, France; the Operational Research Unit (LuxOR); AMPATH, Eldoret, Kenya; The Institute of Tropical Medicine, Antwerp, Belgium; The Centre for International Health, University of Bergen, Norway; and The National Institute for Medical Research, Muhimbili Medical Research Centre, Dar es Salaam, Tanzania. The authors would like to acknowledge the Provincial Government of the Western Cape and Cape Town City Health for supporting and managing the RR-TB programme and for collaborating with MSF in the provision of delamanid to RR-TB patients.
Footnotes
This article was republished online on July 5, 2018 to address a minor inconsistency between data referring to the number of HIV-positive patients on ART at Dlm initiation in table 1 and the results section.
Author contributions: E. Mohr, S. Ade, N. Alikhanova and P. Isaakidis conceived and designed the study. J. Hughes, A. Reuter and J. Furin provided clinical services. E. Mohr, J. Daniels, J. Hughes and A. Reuter collected study data. L. Trivino Duran, V. De Avezedo, Y. Kock and A. Shroufi supervised the implementation of medical activities. E. Mohr, G. Benedetti, J. Edwards and P. Isaakidis performed the analysis. E. Mohr, H. Cox, J. Furin and P. Isaakidis interpreted the results and drafted the manuscript. All authors contributed to the writing of the manuscript. E. Mohr and P. Isaakidis undertook the manuscript revisions. All the authors have read and approved the final manuscript.
Conflict of interest: None declared.
Support statement: Delamanid was procured by MSF through the Global Drug Facility. The analysis and writing of the study were completed with the support of the SORT IT programme, which was funded by the Department for International Development (UK), The Union, MSF and La Fondation Veuve Emile Metz-Tesch (Luxembourg). La Fondation Veuve Emile Metz-Tesch supported open access publications costs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Falzon D, Schünemann HJ, Harausz E, et al. World Health Organization treatment guidelines for drug-resistant tuberculosis, 2016 update. Eur Respir J 2017; 49: 1602308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2017 http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1 Date last updated: 2017. Date last accessed: December 1, 2017.
- 3.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010; 375: 1830–1843. [DOI] [PubMed] [Google Scholar]
- 4.Migliori GB, Sotgiu G, Gandhi NR, et al. Drug resistance beyond extensively drug-resistant tuberculosis: individual patient data meta-analysis. Eur Respir J 2013; 42: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagwa EL, Mantel-Teeuwisse AK, Ruswa NC. Occurrence and clinical management of moderate-to-severe adverse events during drug-resistant tuberculosis treatment: a retrospective cohort study. J Pharm Policy Pract 2014; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. The Use of Delamanid in the Treatment of Multidrug-resistant Tuberculosis: Interim Policy Guidance http://apps.who.int/iris/bitstream/10665/137334/1/WHO_HTM_TB_2014.23_eng.pdf Date last updated: 2014. Date last accessed: January 1, 2015. [PubMed]
- 7.European Medicines Agency. Deltyba www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002552/human_med_001699.jsp&mid=WC0b01ac058001d124. Date last updated: November 13, 2017. Date last accessed: December 1, 2017.
- 8.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis 2011; 15: 949–954. [DOI] [PubMed] [Google Scholar]
- 9.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012; 366: 2151–2160. [DOI] [PubMed] [Google Scholar]
- 10.Mallikaarjun S, Wells C, Petersen C, et al. Delamanid coadministered with antiretroviral drugs or antituberculosis drugs shows no clinically relevant drug-drug interactions in healthy subjects. Antimicrob Agents Chemother 2016; 60: 5976–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotgiu G, Pontali E, Centis R, et al. Delamanid (OPC-67683) for treatment of multi-drug-resistant tuberculosis. Expert Rev Anti Infect Ther 2015; 13: 305–315. [DOI] [PubMed] [Google Scholar]
- 12.Drug-Resistant TB Scale-Up Treatment Action Team. DR-TB Stat: Country Updates http://drtb-stat.org/country-updates/. Date last updated: October, 2017. Date last accessed: November 15, 2017.
- 13.Drug-Resistant TB Scale-Up Treatment Action Team 2017. Phase III clinical trial results at the 48th Union World Conference on Lung Health: Implications for the Field http://drtb-stat.org/wp-content/uploads/2017/11/Updated_November_12_2017_STAT_phaseIII_Union_summary-002.pdf Date last updated: November 12, 2017. Date last accessed: November 15, 2017.
- 14.World Health Organization. WHO Position Statement on the Use of Delamanid for Multidrug-resistant Tuberculosis: Expedited Review of the Phase III Clinical Trial of Delamanid Added to an Optimised Background MDR-TB Regimen www.who.int/tb/publications/2018/WHOPositionStatementDelamanidUse.pdf?ua=1 Date last updated: January, 2018. Date last accessed: January 31, 2018.
- 15.World Health Organization. South Africa: Tuberculosis Profile https://extranet.who.int/sree/Reports?op=Replet& name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=ZA&outtype=PDF. Date last updated: 2016. Date last accessed: October 1, 2017.
- 16.Ndjeka N, Conradie F, Schnippel K, et al. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis 2015; 19: 979–985. [DOI] [PubMed] [Google Scholar]
- 17.Borisov SE, Dheda K, Enwerem M, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J 2017; 49: 1700387. [DOI] [PubMed] [Google Scholar]
- 18.Lessem E, Cox H, Daniels C, et al. Access to new medications for the treatment of drug-resistant tuberculosis: patient, provider and community perspectives. Int J Infect Dis 2015; 32: 56–60. [DOI] [PubMed] [Google Scholar]
- 19.Cox H, Hughes J, Daniels J, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis 2014; 18: 441–448. [DOI] [PubMed] [Google Scholar]
- 20.Ferlazzo G, Mohr E, Chinmay L, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis 2018; 18: 536–544. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Definitions and Reporting Framework for Tuberculosis–2013 Revision http://apps.who.int/iris/handle/10665/79199 Date last updated: 2013. Date last accessed: January 1, 2014.
- 22.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Harmonised Tripartite Guideline: Clinical Safety Data Management: Definitions and Standards for Expedited Reporting E2A www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf Date last updated: October 27, 1994. Date last accessed: December 15, 2017.
- 23.Vandenberk B, Vandael E, Robyns T, et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc 2016; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewison C, Ferlazzo G, Avaliani Z, et al. Six-month response to delamanid treatment in MDR TB patients. Emerg Infect Dis 2017; 23: 1746–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok J, Kang H, Hwang SH, et al. Interim outcomes of delamanid for the treatment of MDR- and XDR-TB in South Korea. J Antimicrob Chemother 2018; 73: 503–508. [DOI] [PubMed] [Google Scholar]
- 26.Hafkin J, Hittel N, Martin A, et al. Early outcomes in MDR-TB and XDR-TB patients treated with delamanid under compassionate use. Eur Respir J 2017; 50: 1700311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brust JCM, Gandhi NR, Carrara H, et al. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis 2010; 14: 413–419. [PMC free article] [PubMed] [Google Scholar]
- 28.Maryandyshev A, Pontali E, Tiberi S, et al. Bedaquiline and delamanid combination treatment of 5 patients with pulmonary extensively drug-resistant tuberculosis. Emerg Infect Dis 2017; 23: 1718–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guglielmetti L, Barkane L, Le Du D, et al. Safety and efficacy of exposure to bedaquiline-delamanid in MDR-TB: a case series from France and Latvia. Eur Respir J 2018; 51: 1702550. [DOI] [PubMed] [Google Scholar]
- 30.Farley JE, Ram M, Pan W, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS One 2011; 6: e20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010; 51: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuksa L, Barkane L, Hittel N, et al. Final treatment outcomes of multidrug- and extensively drug-resistant tuberculosis patients in Latvia receiving delamanid-containing regimens. Eur Respir J 2017; 50: 1701105. [DOI] [PubMed] [Google Scholar]
- 33.Yoon H, Jo K, Nam G, et al. Clinical significance of QT prolonging drug use in patients with MDRTB or NTM disease. Int J Tuberc Lung Dis 2017; 21: 996–1001. [DOI] [PubMed] [Google Scholar]
- 34.Tadolin IM, Lingtsang R, Tiberi S, et al. Cardiac safety of extensively drug-resistant tuberculosis regimens including bedaquiline, delamanid and clofazimine. Eur Respir J 2016; 48: 1527–1529. [DOI] [PubMed] [Google Scholar]
- 35.Migliori GB, Pontali E, Sotgiu G, et al. Combined use of delamanid and bedaquiline to treat multidrug-resistant and extensively drug-resistant tuberculosis: a systematic review. Int J Mol Sci 2017; 18: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadolini M, Lingtsand R, Tiberi S, et al. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur Respir J 2016; 48: 935–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pontali E, Sotgiu G, Ambrosio LD, et al. Cardiac safety of bedaquiline: a systematic and critical analysis of the evidence. Eur Respir J 2017; 50: 1701462. [DOI] [PubMed] [Google Scholar]