Abstract
Background and Purpose—
Four-dimensional phase-contrast magnetic resonance imaging enables quantification of blood flow rate (BFR; mL/min) in multiple cerebral arteries simultaneously, making it a promising technique for hemodynamic investigation in patients with stroke. The aim of this study was to quantify the hemodynamic disturbance and the compensatory pattern of collateral flow in patients with symptomatic carotid stenosis.
Methods—
Thirty-eight patients (mean, 72 years; 27 men) with symptomatic carotid stenosis (≥50%) or occlusion were investigated using 4-dimensional phase-contrast magnetic resonance imaging. For each patient, BFR was measured in 19 arteries/locations. The ipsilateral side to the symptomatic carotid stenosis was compared with the contralateral side.
Results—
Internal carotid artery BFR was lower on the ipsilateral side (134±87 versus 261±95 mL/min; P<0.001). BFR in anterior cerebral artery (A1 segment) was lower on ipsilateral side (35±58 versus 119±72 mL/min; P<0.001). Anterior cerebral artery territory bilaterally was primarily supplied by contralateral internal carotid artery. The ipsilateral internal carotid artery mainly supplied the ipsilateral middle cerebral artery (MCA) territory. MCA was also supplied by a reversed BFR found in the ophthalmic and the posterior communicating artery routes on the ipsilateral side (−5±28 versus 10±28 mL/min, P=0.001, and −2±12 versus 6±6 mL/min, P=0.03, respectively). Despite these compensations, BFR in MCA was lower on the ipsilateral side, and this laterality was more pronounced in patients with severe carotid stenosis (≥70%). Although comparing ipsilateral MCA BFR between stenosis groups (<70% and ≥70%), there was no difference (P=0.95).
Conclusions—
With a novel approach using 4-dimensional phase-contrast magnetic resonance imaging, we could simultaneously quantify and rank the importance of collateral routes in patients with carotid stenosis. An important observation was that contralateral internal carotid artery mainly secured the bilateral anterior cerebral artery territory. Because of the collateral recruitment, compromised BFR in MCA is not necessarily related to the degree of carotid stenosis. These findings highlight the importance of simultaneous investigation of the hemodynamics of the entire cerebral arterial tree.
Keywords: carotid stenosis; circle of Willis; humans; magnetic resonance imaging, cine; middle cerebral artery
Patients with ischemic stroke or a transient ischemic attack caused by severe carotid stenosis are candidates for carotid endarterectomy. The imaging investigation today is mainly based on the degree of stenosis in the symptomatic carotid artery.1,2 Perfusion studies have shown that the relation between the degree of carotid stenosis or occlusion is not always related to cerebral hypoperfusion because collateral recruitment plays an important role in preserving cerebral perfusion in patients with steno-occlusive disease of the carotids.3–5 The primary collaterals within the circle of Willis (CW) are considered as main collaterals, that is, the anterior communicating artery and the posterior communicating artery (PCoA),3,6 whereas the recruitment of collaterals from leptomeningeal and external carotid arteries has been linked to poor hemodynamic status.7,8 Further, the recruitment of collaterals and the clinical manifestations of carotid artery disease are highly variable because of anatomic variations in CW and variations of the contralateral or intracranial stenosis. Therefore, there is an increasing need to develop noninvasive imaging techniques that can give valuable information about the intracerebral impact of carotid stenosis.
Quantitative measurements of absolute blood flow rates (BFRs), in units of milliliters per minute along the main cerebral arteries, can be performed by using phase-contrast magnetic resonance imaging (PCMRI).9 By using this technique, we have demonstrated that in healthy subjects, BFRs are distributed symmetrically in the carotids and along the cerebral arteries without signs of lateralization or influence by subject age or sex.10 Recent developments in 4-dimensional (4D) PCMRI technique make it possible to simultaneously assess BFR in all cerebral arteries, and the magnetic resonance imaging (MRI) sequence takes about 10 minutes, which makes this technique a promising method for studying the hemodynamic disturbance and the collaterals in patients with carotid stenosis.11,12
The collateral function of CW is well described; however, techniques are needed that can give an absolute quantification of BFR in a specific artery in milliliters per minute.13,14 Using Doppler ultrasound or computed tomography (CT), only an indirect estimation of cerebral blood flow is given.
We aimed to evaluate the hemodynamic disturbance and the compensatory pattern of collateral flow caused by symptomatic carotid stenosis by measuring BFR in 17 cerebral arteries and in the ophthalmic arteries (OAs) in 38 patients.
Methods
Data supporting the findings of this study are available from the corresponding author on reasonable request.
Patients
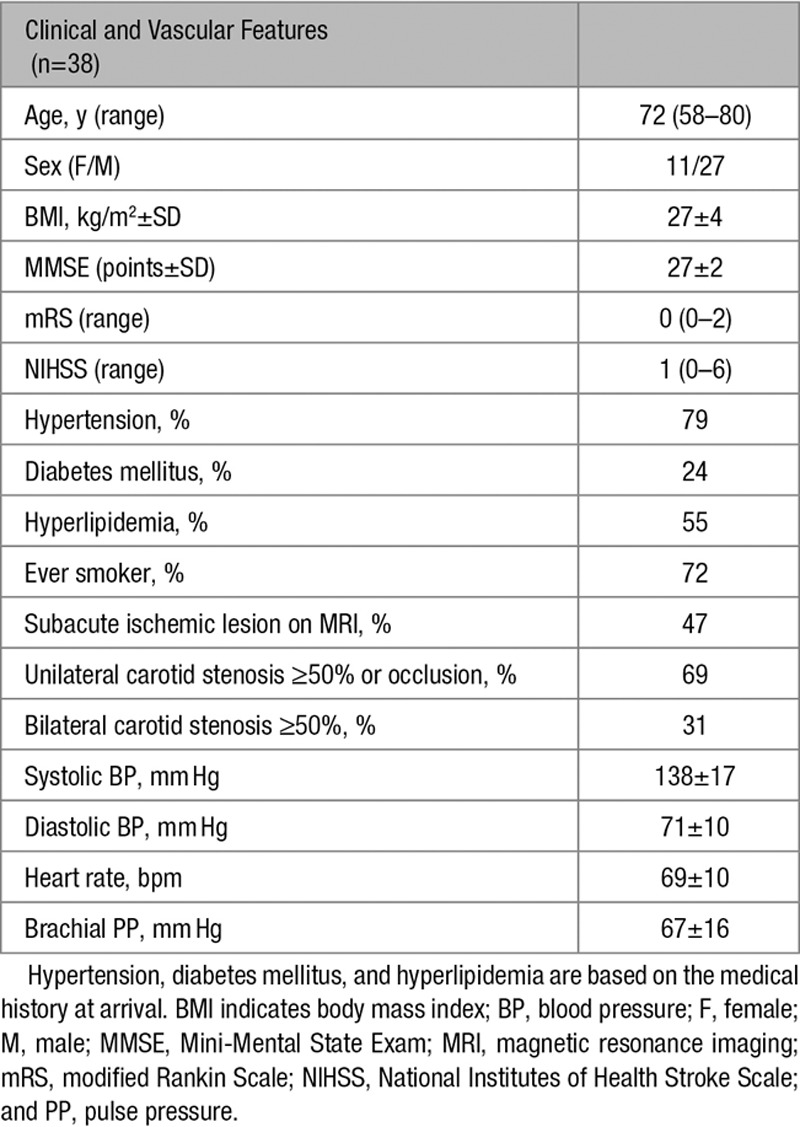
Patients with ischemic stroke or a transient ischemic attack, with corresponding carotid stenosis ≥50% and who were eligible for carotid artery endarterectomy, were admitted to the tertiary stroke referral center at the Umeå University Hospital during 2012 to 2015. Patients were offered to participate in this prospective single-center study and to undergo MRI with 4D PCMRI. Inclusion criteria were modified Rankin Scale score <315 and a Mini-Mental State Exam >2316 points. Patients with severe aphasia, contralateral carotid occlusions, intracranial carotid stenosis, atrial fibrillation, previous ischemic events, or other neurological diseases affecting the central nervous system or contraindications for MRI were excluded. The study population consisted of 38 patients (27 men) with a mean age of 72±6 years with symptomatic carotid stenosis ≥50%, with or without contralateral nonsymptomatic carotid stenosis. Eleven patients had a symptomatic stenosis <70% with contralateral stenosis <50%, 15 had a symptomatic stenosis (n=14) or occlusion (n=1) of 70% to 100% with contralateral stenosis <50%, and 12 had bilateral stenosis >50%. Oral and written information about the study was given to all included patients, and written consent was obtained from all patients. The Ethical Review Board of the Umeå University approved the study, and it was performed in accordance with the guidelines of the Declaration of Helsinki. The characteristics of the patients are summarized in Table 1. The mean degree of stenosis was 76±14% in the symptomatic side and 39±27% on the contralateral side.
Table 1.
Clinical and Vascular Features of the Included Patients
Clinical Investigation
A Doppler ultrasound examination of the carotids was performed on all patients. Stenosis grading was performed by translating the highest peak systolic velocity in the stenosis to the degree of stenosis.17 Thirty-one patients (82%) underwent CT angiography. Carotid stenosis was graded according to NASCET (North American Symptomatic Carotid Endarterectomy Trial).18 Seven patients were not examined by CT angiography, usually due to renal failure or iodine allergy. The carotid stenosis grading in this study was primarily based on CT angiography (31 patients) and Doppler ultrasound measurement in the remaining 7 patients.
MRI Investigation
The MRI examination was performed within a median of 6 days (range, 2–60 days) from symptom onset. A 3T MRI scanner (GE Discovery MR 750; Waukesha, WI) with a 32-channel head coil was used. Using 4D PCMRI, blood flow velocities were obtained for the cerebral arteries. The scan time was ≈9 minutes. Two different velocity encoding settings were used, one set at 110 cm/s for investigation of the main cerebral arteries and 40 cm/s for investigation of the OA. The following parameters were used: number of radial projections, 16 000; acquisition resolution, 300×300×300; imaging volume, 220×220×220 mm; reconstruction resolution, 320×320×320 mm; and voxel size, 0.7×0.7×0.7 mm3. The scan time was ≈9 minutes; repetition time / echo time, 6.5/2.7 ms; flip angle, 8°; and bandwidth, 166.67 kHz.
Imaging Analysis
Flow analysis was performed in Matlab (The Mathworks, Natick, MA) using software developed in house for calculating BFRs in milliliters per minute.19 Blood flow measurements were performed on deidentified images, and the observers were blinded for the side and degree of carotid stenosis, medical history, age, and sex of the patient. Internal carotid artery (ICA) BFR was measured at level of C3–C4 segment, the vertebral artery, intracranially at the level of V4 segment, and in the basilar artery below the superior cerebellar artery. Middle cerebral artery (MCA) was measured at M1 level. BFR in anterior cerebral arteries (ACAs) was measured at A1 level (ACA1) and in the A2 segment (ACA2), measured distal to the anterior communicating artery. The posterior cerebral artery was measured at P1 level (PCA1), that is, proximal to the aperture of the PCoA. The P2 segment of posterior cerebral artery (PCA2) was measured distal to the aperture of PCoA. OA was measured close to the branching off from ICA. Total cerebral blood flow (tCBF) was calculated by adding the BFR of the 2 ICAs and the 2 vertebral arteries. BFR was calculated independently by 2 investigators (L.Z. and A.W.). The BFR was measured in ICA, MCA, ACA1, PCA1, PCA2, vertebral artery, and basilar artery. The mean of the 2 investigators’ values for each artery was used. If a difference >20% in BFR was found, a consensus measurement was performed, that is, the 2 investigators measured the artery BFR together. About 9% of the measurements were consensus measurements, most commonly for vertebral artery and PCA2. Finally, BFR in PCoA, OA, and ACA2 was measured by the 2 investigators together. BFR measurement in ACA2 was performed using manual segmentation because the arteries on the right and left side were too close to each other.
Statistical Analysis
A paired t test was used to compare the mean BFR between the ipsilateral (carotid stenosis) side and the contralateral side. An independent t test was used to compare the BFR in cerebral arteries in patients with and without subacute ischemia. The patients were dichotomized into 2 subgroups: symptomatic moderate carotid stenosis <70% and symptomatic carotid stenosis ≥70%.1,20 An independent t test was performed to compare differences in BFR between subgroups of patients, whereas a paired t test was used to compare ipsilateral/contralateral differences. If an artery was either invisible or hypoplastic and its blood flow was not measurable, the flow in that artery was set as 0 mL/min. Values were expressed as mean±SD, and the statistical significance threshold was set as P<0.05. The data were analyzed using SPSS statistics, version 25 (IBM, Chicago, IL).
Results
BFR Measurements
Mean BFR for each artery, on both ipsilateral and contralateral side, is presented in Table 2. ICA BFR was lower in the symptomatic side than in the contralateral side. The main branches of ICA had decreased or reversed BFR on the ipsilateral side compared with the contralateral side: OA, PCoA, ACA, and MCA (Table 2). A reversed BFR was found in the OA on the ipsilateral side in 9 patients, and BFR was not detectable in 17 patients (45%), indicating a flow close to zero. In the PCoA, a reversed BFR was found on the ipsilateral side in 9 patients. A decrease in BFR was also found in ACA2 on the ipsilateral side when compared with the contralateral side. In the posterior circulation, BFR in PCA1 was higher on the ipsilateral side than on the contralateral side (Table 2).
Table 2.
Mean BFR±SD (mL/min) for Cerebral Arteries Based on Ipsilateral and Contralateral Side
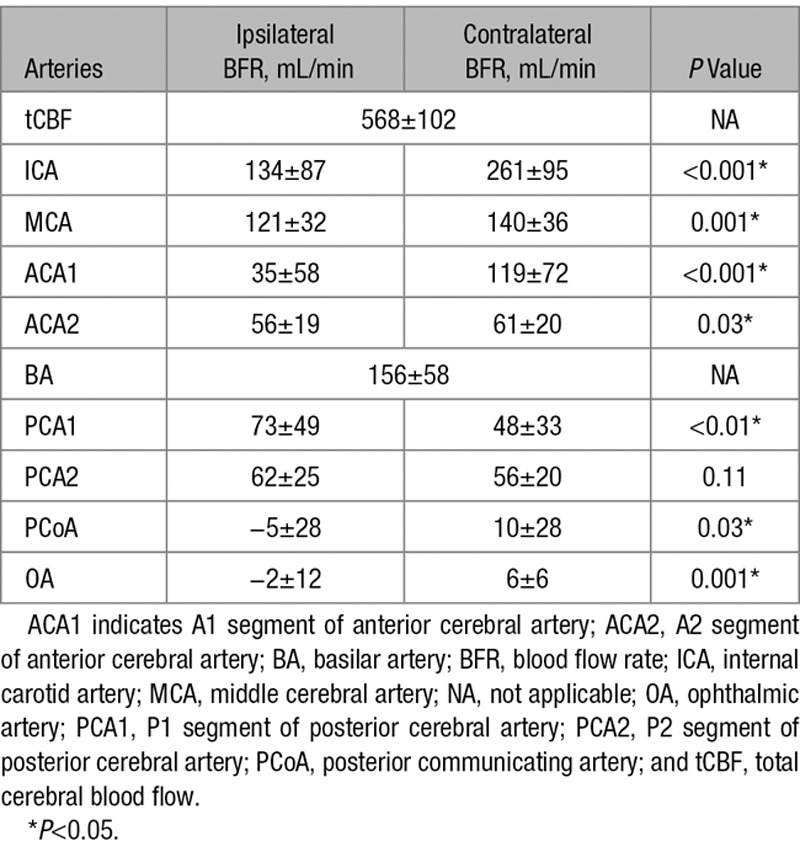
The sum of ACA2, MCA, and PCA2 was lower (P=0.001) on the ipsilateral (239±48 mL/min) than on the contralateral side (256±58 mL/min). This discrepancy was mainly caused by MCA flow because the sum of ACA2 and PCA2 was 118±31 mL/min on the ipsilateral side and 117±30 mL/min on the contralateral side (P=0.79).
The mean value of BFR in ACA on the ipsilateral side and in ACA on the contralateral side was (ACAipsilateral+ACAcontralateral)/2=77±19 mL/min. Seven patients had a reversed BFR in the ipsilateral ACA with a mean BFR of −53±44 mL/min, whereas no patient had a reversed BFR in the contralateral ACA. Of the 31 patients with anterograde BFR in ACA on the ipsilateral side, 13 (42%) had twice as high BFR in the contralateral ACA than in the ipsilateral side ACA.
When comparing patients with and without subacute ischemic lesions, no difference was found in BFR between cerebral arteries, including OA and tCBF (P>0.1).
Distribution of tCBF
Figure 1 illustrates the relative distribution (in percentage) of tCBF in CW for patients with carotid stenosis based on ipsilateral and contralateral side.
Figure 1.
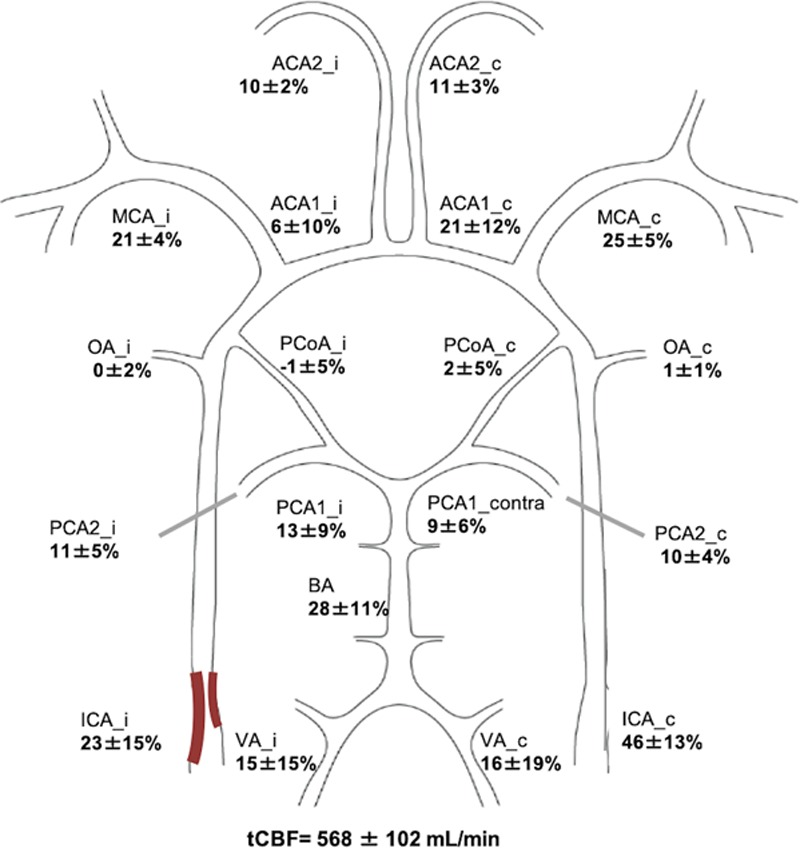
Total cerebral blood flow (tCBF) distribution in patients with symptomatic carotid stenosis using 4-dimensional phase-contrast magnetic resonance imaging. The mean degree of stenosis was 76±14% in the symptomatic side and 39±27% on the contralateral side. Means and SDs for the distribution of tCBF (percentage) in the circle of Willis in patients with carotid stenosis based on ipsilateral (stenos) side (i) and contralateral side (c). ACA1 indicates A1 segment of anterior cerebral artery; ACA2, A2 segment of anterior cerebral artery; BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; OA, ophthalmic artery; PCA1, P1 segment of posterior cerebral artery; PCA2, P2 segment of posterior cerebral artery; PCoA, posterior communicating artery; and VA, vertebral artery.
BFR Comparison in Patients With Severe and Moderate Carotid Stenosis
Table 3 demonstrates the differences in BFR when comparing patients with moderate symptomatic carotid stenosis (<70%) versus severe symptomatic stenosis (≥70%). In patients with severe carotid stenosis, there was a lower BFR in ipsilateral ICA and ACA1 than in patients with moderate carotid stenosis. In addition, the BFR was higher in the contralateral ACA1, ipsilateral PCA1, PCA2, and in basilar artery (Table 3).
Table 3.
Mean Blood Flow Rate±SD (mL/min) for Cerebral Arteries
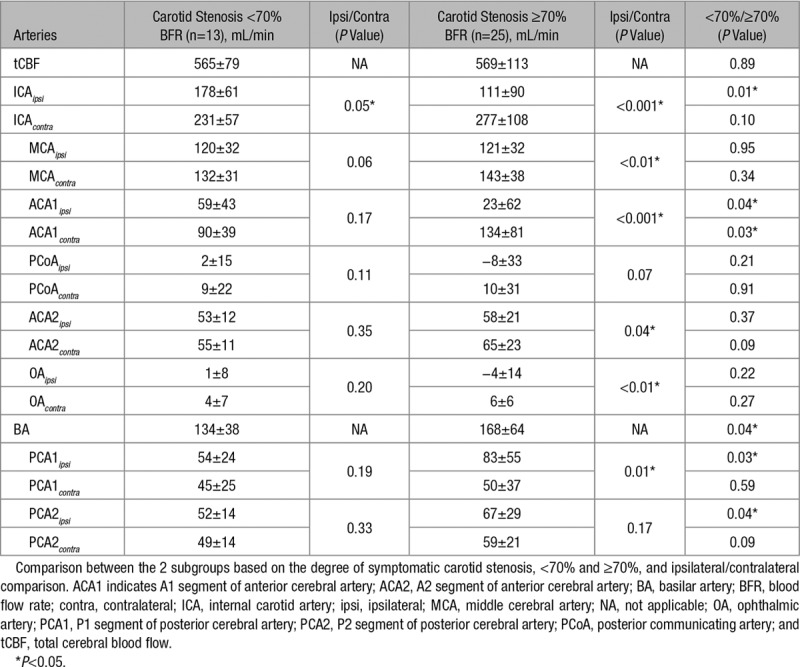
When excluding patients with contralateral carotid stenosis ≥50%, there was still a lower BFR in ipsilateral ICA (105±94 versus 186±59 mL/min; P=0.01) and a higher BFR in contralateral ACA1 (144±84 versus 85±38 mL/min; P=0.03) in patients with severe stenosis (n=15) than in patients with a moderate stenosis (n=11).
Table 3 also demonstrates the BFR comparison between ipsilateral and contralateral sides in the subgroups. In patients with moderate stenosis, a decreased ICA BFR was seen in ipsilateral compared with contralateral. In patients with severe stenosis, a decreased BFR was found in ipsilateral ICA, MCA, ACA1, ACA2, OA, and PCA1 (Table 3).
Discussion
The importance of collateral flow in patients with carotid stenosis is well known. However, a comprehensive quantification of the whole collateral recruitment in the CW is needed. By using 4D PCMRI, a new platform for investigating and understanding the collaterals has been presented in the present study.
There were 4 major findings: (1) 4D PCMRI could simultaneously quantify BFR in individual cerebral arteries, noninvasively and without anatomic restrictions. Thus, the present study demonstrates a new way to map, quantify, and understand the cerebrovascular impact of a significant carotid stenosis and the compensatory mechanism of the collaterals, which was previously difficult to achieve using 1 modality. (2) Contralateral ICA through ACA1 was the major collateral in BFR equalization in patients with carotid artery stenosis, mainly supplying the ACA territory bilaterally. (3) BFR was decreased or reversed in all branches of the carotid artery on the ipsilateral (carotid stenosis) side compared with the contralateral side, most pronounced in patients with severe stenosis. (4) BFR in distal cerebral arteries (MCA and ACA2) on the ipsilateral side was compensated by collaterals. However, this compensatory mechanism failed to provide a fully symmetrical distribution in patients with a severe carotid stenosis ≥70%.
Technical Considerations
PCMRI can quantify and provide a direct measurement of cerebral BFR in milliliters per minute noninvasively, that is, it does not require the use of ionizing radiation, radionuclide injection, or contrast.21,22 We recently validated our 4D PCMRI flow measurements23 against high-resolution 2-dimensional PCMRI (0.35×0.35 mm)—an accurate and established method for BFR assessment.24–26 In summary, that study supports the accuracy of our implementation of 4D PCMRI flow across a wide range of lumen cross section diameters and flow rates. There are several advantages with the use of 4D PCMRI compared with 2-dimensional PCMRI: there is no need for preselection of regions of interest, all cerebral arteries can be measured simultaneously,11 and the scanning time is reasonable. However, postprocessing data analysis is still mostly investigational and takes a long time. The cerebral perfusion at a specific region of the brain can be investigated with perfusion methods (ie, MRI, CT, or positron emission tomography); however, the collateral pathways cannot be quantified or visualized by using these techniques. Doppler ultrasound techniques provide surrogate markers of blood flow, that is, velocity, or angiographic techniques that visualize flow patterns. In contrast, 4D PCMRI provides a quantitative method to measure blood reaching any region of the brain, and it is not limited to only the proximal branches of CW.21,22 Altogether, 4D PCMRI can be developed into a feasible method for describing the impact of a carotid stenosis on cerebral blood flow, and this method indicates critical thresholds preoperatively in patients with carotid stenosis, which were previously difficult to measure using only 1 modality.
Blood Flow Difference in ICAs
In the carotid arteries, BFR averaged 51% less on the ipsilateral (carotid stenosis) side than on the contralateral side. A severe carotid stenosis can constrict the artery and reduce BFR, thus creating a pressure gradient and poststenotic pressure loss that affects BFR distribution in CW.27,28 Hemodynamically significant carotid stenosis is described as a decrease in luminal diameter of a carotid artery by 70% to 80%.2,20,29 The relation between the degree of carotid stenosis and blood flow in the ICA, according to the Spencer curve, is not linear because the blood flow drastically drops with a severe stenosis.30 To investigate the effect of severe carotid stenosis on cerebral BFR, we chose to dichotomize the cohort of patients into 2 subgroups, <70% and ≥70% stenosis (Table 3). We found that severe carotid stenosis led to a decreased BFR in ipsilateral ICA and ACA1, whereas BFR increased through the collateral routes. The effect of severe carotid stenosis was not seen in ipsilateral MCA. This could be because of the compensatory mechanisms that work together to maintain cerebral perfusion by regulating inflow from collaterals or decreased outflow as discussed in the following sections.31,32
Contribution of OA
The OA is the first branch of ICA. A reversed blood flow in OA has been associated with cerebral hemodynamic compromise because of nonfunctioning primary collaterals (anterior communicating artery and PCoA).33,34 In cases of low ICA BFR, OA BFR is also low and will ultimately become reversed. A reversed, or not detectable, OA BFR was found in almost 70% of the patients in this study. In addition to its collateral function,35,36 OA may function as an indicator of low poststenotic pressures and low BFR, that is, a high degree of carotid stenosis. This could be demonstrated as a decreased BFR in ipsilateral OA in patients with severe carotid stenosis when compared with the contralateral side.
PCoA and the Posterior Circulation as Collaterals
The PCoA is the second branch of ICA and has several anatomic variations (absent, hypoplastic, and fetal type).37 In patients with pathologically decreased blood flow in ICA, a reversed blood flow in PCoA (posterior-anterior distribution) can serve as a collateral. In patients with carotid artery occlusion, small PCoA (<1 mm), or its absence, has been related to an increased risk of ischemic stroke.38 Quantification of BFR in PCoA, as in this study, is a more precise method than indirect markers of BFR, such as diameter and velocity measurements. A reversed BFR of −5 mL/min in the PCoA on the ipsilateral side gives a contribution of 15 mL/min to the ICA blood flow (PCoA on the contralateral side was 10 mL/min in the anterior-to-posterior direction; Table 2). When comparing PCoA BFR in patients with severe versus moderate carotid stenosis (Table 3), there was no difference, suggesting other factors (anatomic variation or other stenoses) regulate the collateral route through PCoA. The finding of increased BFR in ipsilateral PCA1, however, supports the importance of the posterior circulation as collateral for patients with severe carotid stenosis. This occurs either through the CW or via leptomeningeal routes, and 1 study suggests that increased BFR in PCA2 indicates leptomeningeal routes (Table 3).39
ACA—the Main Collateral Route
The anterior communicating artery connects the 2 ACA1s and serves as a collateral route for the anterior circulation. ACA1 on the ipsilateral side had a decreased or reversed BFR, whereas BFR was increased on the contralateral side. Comparing patients with severe versus moderate carotid stenosis, we found that the difference in BFR between the 2 ACA1s is directly related to the degree of stenosis, that is, moderate carotid stenosis will not necessarily affect BFR in ACA1. Although asymmetrical BFR was found in ACA1 for the whole group and especially in patients with severe stenosis, the mean BFR (adding ipsilateral+contralateral sides) was 77 to 79 mL/min (Figure 2; Table 3), which is comparable to values reported in studies including healthy subjects.10 This suggests that the collateral contribution from the contralateral ACA1 was adequate to maintain a sufficient BFR to supply both ACA territories in patients with and without severe carotid stenosis. Seven patients (18%) had a reversed BFR in ACA1 on the ipsilateral side, and another 13 (42%) had an asymmetrical blood flow in the ACA1 segments. Reversed blood flow in ipsilateral ACA1 in patients with carotid stenosis is a well-described finding and has mostly been reported in transcranial doppler studies.36,40 Our findings emphasize the importance of considering ACA collateral recruitment as a gradual process. By only looking for the presence of reversed blood flow when assessing the collateral function of ACA1, we are making a crude binary simplification because this study shows that the contralateral A1 compensates by bilaterally supplying the ACA territory.
Figure 2.
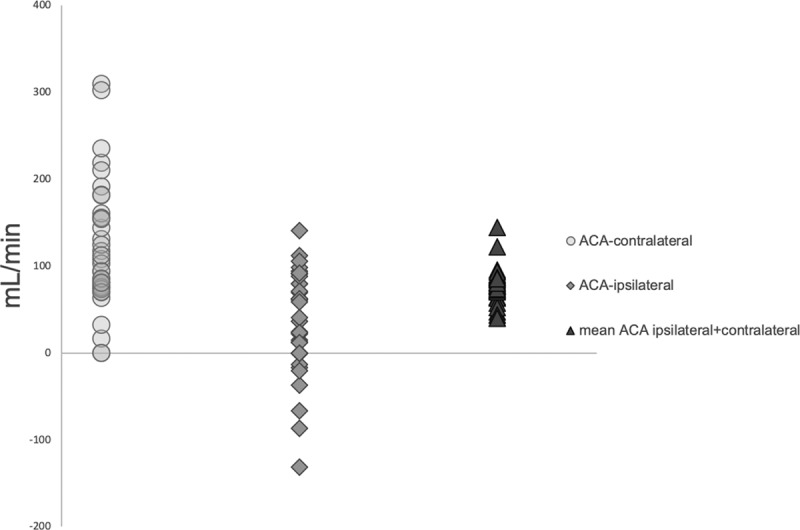
Blood flow rate in anterior cerebral artery (ACA). Blood flow rate in A1 segment of anterior cerebral artery in patients with symptomatic carotid stenosis, based on the ipsilateral side to the carotid stenosis side, contralateral side, and mean ACA blood flow rate.
MCA BFR Is Compromised
The last branch of ICA is MCA, where BFR laterality was less (15%) when compared with the laterality found between the ICAs (51%). This indicates the presence of compensatory mechanisms, however, without the ability to provide a fully symmetrical distribution of BFR between the sides, as seen in healthy subjects.10,41 Patients with severe carotid stenosis did not have lower BFR in ipsilateral MCA than in patients with moderate carotid stenosis. This finding is consistent with previous PCMRI14 and positron emission tomography studies,4 suggesting that the degree of carotid stenosis alone cannot be used to predict distal cerebral blood flow because plaque length and compensatory collateral blood flow are variables that affect the cerebral blood flow as well. However, severe carotid stenosis resulted in asymmetrical BFR distribution in MCA. That is, although the degree of carotid stenosis did not affect the ipsilateral BFR in MCA, it affected the ipsilateral/contralateral distribution where a higher BFR was seen in the contralateral MCA. Our results emphasize the importance to study the distribution of tCBF and quantify BFR in cerebral arteries, for a better description of cerebral hemodynamics. Decreased BFR in ipsilateral MCA highlights the hemodynamic disturbance in ischemic events in patients with symptomatic carotid stenosis.42 From a hemodynamic perspective, the reduced BFR in ipsilateral MCA (compared with the contralateral side) can be explained by an expected slightly lower blood pressure at the branching of MCA from ICA. This expected pressure difference will be the driving force for the collateral flow from the contralateral side toward the ipsilateral side. With a lower blood pressure at the MCA follows a lower perfusion pressure and thus an unsymmetrical MCA BFR. We acknowledge that this explanation disregards the effects of autoregulation that locally can reduce the peripheral resistance on the ipsilateral side and normalize the perfusion. In addition, ischemic lesions reduce the perfused brain volume on the ipsilateral side to a carotid stenosis, contributing to a reduced BFR.
MCA and ACA Territory on the Ipsilateral Side to a Symptomatic Carotid Stenosis Is at Risk
Mean tCBF among patients in this study (568 mL/min) was decreased compared with healthy subjects10 and comparable to tCBF in patients with vascular diseases.43,44 tCBF was highly correlated to BFR in MCA and ACA2 on the ipsilateral side, that is, the effect of low tCBF is seen as lower BFR in these arteries. There is a pressure difference, with a lower blood pressure in the ipsilateral side, which is the driving force for collateral flow toward that side. A low blood pressure leads to a lower perfusion pressure and thus a reduced BFR. The strong correlation between tCBF and ipsilateral side MCA and ACA1 flow supports a reduced perfusion pressure as the cause of reduced MCA flow. Conditions such as heart failure, hypotension, or bradycardia can precipitate the risk of ischemic events in this group of patients.45–47 By quantifying the collateral pathways, we can understand the mechanisms that lie behind the cerebral hypoperfusion, which is seen in some, but not all, patients with carotid stenosis.
Limitations of This Study
The sample size of this study was small, and the included patients were heterogenous. However, our results reflect routine clinical practice for consecutive patients planned for carotid artery endarterectomy with a symptomatic carotid stenosis ≥50%, with or without contralateral stenosis. In spite of this, we found clear and distinct results, which give insights into collateral compensatory mechanisms in patients with symptomatic carotid stenosis. It is likely that in a more selected group that excludes contralateral carotid stenosis patients, the collateral recruitment pattern would be even more distinct. Therefore, the results must be considered in the context of its limitations and should be validated in a larger cohort of patients. The BFRs can be affected by various physiological parameters such as blood pressure, age, and effects from medications. We note that, similar to most measurement techniques, there is likely a lower threshold below which flow cannot be distinguished from noise. Although this limits the ability to firmly establish a complete absence of flow in the investigated artery, it would not alter the description and interpretation of alternate collateral pathways provided in this study. In addition, 4D PCMRI can be distorted in the region of the orbit. This signal loss can limit the ability to detect and measure flow rate in OA. Importantly, this effect is expected to have an equal impact on the ipsilateral and contralateral side. Therefore, our results regarding OA flow direction should still be valid.
Conclusions
With a novel approach using 4D PCMRI technique, we could simultaneously quantify and rank the importance of collateral routes in patients with carotid stenosis. Thus, the present study demonstrates a new way to map and understand the hemodynamic disturbances of a stenosis and the compensatory mechanism of the collaterals, which has been previously difficult to achieve using a single modality. An important observation was that contralateral ICA mainly secured the bilateral ACA territory and not only MCA on the ipsilateral side to the stenosis. Because of the collateral recruitment, compromised BFR in MCA is not necessarily related to the degree of carotid stenosis. These findings highlight the importance of simultaneously investigating the entire cerebral arterial tree.
Acknowledgments
We give special thanks to research nurse Kristin Nyman, Master of Nursing Science, for her great assistance during this study.
Sources of Funding
The following financial support has been conducted for the research, authorship, and publication of this article: the Swedish Research Council, grant numbers 2015–05616; 2017–04949; the County Council of Västerbotten; and the Swedish Heart and Lung Foundation, grant number 20140592.
Disclosures
None.
Supplementary Material
References
- 1.Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Mrc European carotid surgery trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European carotid surgery trialists’ collaborative group. Lancet. 1991;337:1235–1243. [PubMed] [Google Scholar]
- 3.Hoksbergen AW, Fülesdi B, Legemate DA, Csiba L. Collateral configuration of the circle of Willis: transcranial color-coded duplex ultrasonography and comparison with postmortem anatomy. Stroke. 2000;31:1346–1351. doi: 10.1161/01.str.31.6.1346. [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ, Press GA, Grubb RL, Jr, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med. 1987;106:27–34. doi: 10.7326/0003-4819-106-1-27. [DOI] [PubMed] [Google Scholar]
- 5.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Investigators. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluytmans M, van der Grond J, van Everdingen KJ, Klijn CJ, Kappelle LJ, Viergever MA. Cerebral hemodynamics in relation to patterns of collateral flow. Stroke. 1999;30:1432–1439. doi: 10.1161/01.str.30.7.1432. [DOI] [PubMed] [Google Scholar]
- 7.Klijn CJ, Kappelle LJ. Haemodynamic stroke: clinical features, prognosis, and management. Lancet Neurol. 2010;9:1008–1017. doi: 10.1016/S1474-4422(10)70185-X. doi: 10.1016/S1474-4422(10)70185-X. [DOI] [PubMed] [Google Scholar]
- 8.Norrving B, Nilsson B, Risberg J. rCBF in patients with carotid occlusion. Resting and hypercapnic flow related to collateral pattern. Stroke. 1982;13:155–162. doi: 10.1161/01.str.13.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Enzmann DR, Ross MR, Marks MP, Pelc NJ. Blood flow in major cerebral arteries measured by phase-contrast cine MR. AJNR Am J Neuroradiol. 1994;15:123–129. [PMC free article] [PubMed] [Google Scholar]
- 10.Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015;35:648–654. doi: 10.1038/jcbfm.2014.241. doi: 10.1038/jcbfm.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging. 2012;36:1015–1036. doi: 10.1002/jmri.23632. doi: 10.1002/jmri.23632. [DOI] [PubMed] [Google Scholar]
- 12.Turski P, Edjlali M, Oppenheim C. Fast 4D flow MRI re-emerges as a potential clinical tool for neuroradiology. AJNR Am J Neuroradiol. 2013;34:1929–1930. doi: 10.3174/ajnr.A3664. doi: 10.3174/ajnr.A3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Laar PJ, van der Grond J, Moll FL, Mali WP, Hendrikse J. Hemodynamic effect of carotid stenting and carotid endarterectomy. J Vasc Surg. 2006;44:73–78. doi: 10.1016/j.jvs.2006.03.023. doi: 10.1016/j.jvs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Shakur SF, Hrbac T, Alaraj A, Du X, Aletich VA, Charbel FT, et al. Effects of extracranial carotid stenosis on intracranial blood flow. Stroke. 2014;45:3427–3429. doi: 10.1161/STROKEAHA.114.006622. doi: 10.1161/STROKEAHA.114.006622. [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Hansen F, Bergqvist D, Lindblad B, Lindh M, Mätzsch T, Länne T. Accuracy of duplex sonography before carotid endarterectomy–a comparison with angiography. Eur J Vasc Endovasc Surg. 1996;12:331–336. doi: 10.1016/s1078-5884(96)80252-8. [DOI] [PubMed] [Google Scholar]
- 18.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- 19.Wåhlin A, Ambarki K, Birgander R, Wieben O, Johnson KM, Malm J, et al. Measuring pulsatile flow in cerebral arteries using 4D phase-contrast MR imaging. AJNR Am J Neuroradiol. 2013;34:1740–1745. doi: 10.3174/ajnr.A3442. doi: 10.3174/ajnr.A3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Reutern GM, Goertler MW, Bornstein NM, Del Sette M, Evans DH, Hetzel A, et al. Neurosonology Research Group of the World Federation of Neurology. Grading carotid stenosis using ultrasonic methods. Stroke. 2012;43:916–921. doi: 10.1161/STROKEAHA.111.636084. doi: 10.1161/STROKEAHA.111.636084. [DOI] [PubMed] [Google Scholar]
- 21.Bammer R, Hope TA, Aksoy M, Alley MT. Time-resolved 3D quantitative flow MRI of the major intracranial vessels: initial experience and comparative evaluation at 1.5T and 3.0T in combination with parallel imaging. Magn Reson Med. 2007;57:127–140. doi: 10.1002/mrm.21109. doi: 10.1002/mrm.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stankovic Z. Four-dimensional flow magnetic resonance imaging in cirrhosis. World J Gastroenterol. 2016;22:89–102. doi: 10.3748/wjg.v22.i1.89. doi: 10.3748/wjg.v22.i1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunås T, Holmgren M, Wåhlin A, Malm J, Eklund A. Accuracy of blood flow assessment in cerebral arteries with 4d flow MRI: evaluation with three segmentation methods [published online January 14, 2019]. J Magn Reson Imaging. doi: 10.1002/jmri.26641. doi: 10.1002/jmri.26641. https://onlinelibrary.wiley.com/doi/full/10.1002/jmri.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambarki K, Hallberg P, Jóhannesson G, Lindén C, Zarrinkoob L, Wåhlin A, et al. Blood flow of ophthalmic artery in healthy individuals determined by phase-contrast magnetic resonance imaging. Invest Ophthalmol Vis Sci. 2013;54:2738–2745. doi: 10.1167/iovs.13-11737. doi: 10.1167/iovs.13-11737. [DOI] [PubMed] [Google Scholar]
- 25.Spilt A, Box FM, van der Geest RJ, Reiber JH, Kunz P, Kamper AM, et al. Reproducibility of total cerebral blood flow measurements using phase contrast magnetic resonance imaging. J Magn Reson Imaging. 2002;16:1–5. doi: 10.1002/jmri.10133. doi: 10.1002/jmri.10133. [DOI] [PubMed] [Google Scholar]
- 26.Wåhlin A, Ambarki K, Hauksson J, Birgander R, Malm J, Eklund A. Phase contrast MRI quantification of pulsatile volumes of brain arteries, veins, and cerebrospinal fluids compartments: repeatability and physiological interactions. J Magn Reson Imaging. 2012;35:1055–1062. doi: 10.1002/jmri.23527. doi: 10.1002/jmri.23527. [DOI] [PubMed] [Google Scholar]
- 27.Anderson HV, Roubin GS, Leimgruber PP, Cox WR, Douglas JS, Jr, King SB, III, et al. Measurement of transstenotic pressure gradient during percutaneous transluminal coronary angioplasty. Circulation. 1986;73:1223–1230. doi: 10.1161/01.cir.73.6.1223. [DOI] [PubMed] [Google Scholar]
- 28.Hendrikse J, Hartkamp MJ, Hillen B, Mali WP, van der Grond J. Collateral ability of the circle of Willis in patients with unilateral internal carotid artery occlusion: border zone infarcts and clinical symptoms. Stroke. 2001;32:2768–2773. doi: 10.1161/hs1201.099892. [DOI] [PubMed] [Google Scholar]
- 29.Chang YJ, Golby AJ, Albers GW. Detection of carotid stenosis. From NASCET results to clinical practice. Stroke. 1995;26:1325–1328. doi: 10.1161/01.str.26.8.1325. [DOI] [PubMed] [Google Scholar]
- 30.Spencer MP, Reid JM. Quantitation of carotid stenosis with continuous-wave (C-W) Doppler ultrasound. Stroke. 1979;10:326–330. doi: 10.1161/01.str.10.3.326. [DOI] [PubMed] [Google Scholar]
- 31.Fang H, Song B, Cheng B, Wong KS, Xu YM, Ho SS, et al. Compensatory patterns of collateral flow in stroke patients with unilateral and bilateral carotid stenosis. BMC Neurol. 2016;16:39. doi: 10.1186/s12883-016-0560-0. doi: 10.1186/s12883-016-0560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartkamp NS, Hendrikse J, van der Worp HB, de Borst GJ, Bokkers RP. Time course of vascular reactivity using repeated phase-contrast MR angiography in patients with carotid artery stenosis. Stroke. 2012;43:553–556. doi: 10.1161/STROKEAHA.111.637314. doi: 10.1161/STROKEAHA.111.637314. [DOI] [PubMed] [Google Scholar]
- 33.Hofmeijer J, Klijn CJ, Kappelle LJ, Van Huffelen AC, Van Gijn J. Collateral circulation via the ophthalmic artery or leptomeningeal vessels is associated with impaired cerebral vasoreactivity in patients with symptomatic carotid artery occlusion. Cerebrovasc Dis. 2002;14:22–26. doi: 10.1159/000063719. doi: 10.1159/000063719. [DOI] [PubMed] [Google Scholar]
- 34.Smith HA, Thompson-Dobkin J, Yonas H, Flint E. Correlation of xenon-enhanced computed tomography-defined cerebral blood flow reactivity and collateral flow patterns. Stroke. 1994;25:1784–1787. doi: 10.1161/01.str.25.9.1784. [DOI] [PubMed] [Google Scholar]
- 35.van Everdingen KJ, Visser GH, Klijn CJ, Kappelle LJ, van der Grond J. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol. 1998;44:167–176. doi: 10.1002/ana.410440206. doi: 10.1002/ana.410440206. [DOI] [PubMed] [Google Scholar]
- 36.Schneider PA, Rossman ME, Bernstein EF, Ringelstein EB, Otis SM. Noninvasive assessment of cerebral collateral blood supply through the ophthalmic artery. Stroke. 1991;22:31–36. doi: 10.1161/01.str.22.1.31. [DOI] [PubMed] [Google Scholar]
- 37.Krabbe-Hartkamp MJ, van der Grond J, de Leeuw FE, de Groot JC, Algra A, Hillen B, et al. Circle of Willis: morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. 1998;207:103–111. doi: 10.1148/radiology.207.1.9530305. doi: 10.1148/radiology.207.1.9530305. [DOI] [PubMed] [Google Scholar]
- 38.Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, et al. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994;330:1565–1570. doi: 10.1056/NEJM199406023302204. doi: 10.1056/NEJM199406023302204. [DOI] [PubMed] [Google Scholar]
- 39.Ruland S, Ahmed A, Thomas K, Zhao M, Amin-Hanjani S, Du X, et al. Leptomeningeal collateral volume flow assessed by quantitative magnetic resonance angiography in large-vessel cerebrovascular disease. J Neuroimaging. 2009;19:27–30. doi: 10.1111/j.1552-6569.2008.00249.x. doi: 10.1111/j.1552-6569.2008.00249.x. [DOI] [PubMed] [Google Scholar]
- 40.Lindegaard KF, Bakke SJ, Grolimund P, Aaslid R, Huber P, Nornes H. Assessment of intracranial hemodynamics in carotid artery disease by transcranial Doppler ultrasound. J Neurosurg. 1985;63:890–898. doi: 10.3171/jns.1985.63.6.0890. doi: 10.3171/jns.1985.63.6.0890. [DOI] [PubMed] [Google Scholar]
- 41.Amin-Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT. Effect of age and vascular anatomy on blood flow in major cerebral vessels. J Cereb Blood Flow Metab. 2015;35:312–318. doi: 10.1038/jcbfm.2014.203. doi: 10.1038/jcbfm.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 43.van Es AC, van der Grond J, ten Dam VH, de Craen AJ, Blauw GJ, Westendorp RG, et al. PROSPER Study Group. Associations between total cerebral blood flow and age related changes of the brain. PLoS One. 2010;5:e9825. doi: 10.1371/journal.pone.0009825. doi: 10.1371/journal.pone.0009825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI SMART Study Group. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol. 2012;71:825–833. doi: 10.1002/ana.23554. doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- 45.Powers WJ. Stroke: misery perfusion in cerebrovascular disease–is it important? Nat Rev Neurol. 2012;8:479–480. doi: 10.1038/nrneurol.2012.147. doi: 10.1038/nrneurol.2012.147. [DOI] [PubMed] [Google Scholar]
- 46.Powers WJ, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Benign prognosis of never-symptomatic carotid occlusion. Neurology. 2000;54:878–882. doi: 10.1212/wnl.54.4.878. [DOI] [PubMed] [Google Scholar]
- 47.Grubb RL, Jr, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 1998;280:1055–1060. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]


