Ulipristal acetate is associated with significant improvements in health-related quality of life and symptom severity compared with placebo in women with symptomatic uterine leiomyomas.
Abstract
OBJECTIVE:
To investigate effects of ulipristal acetate on health-related quality of life (QOL) and symptom severity in women with symptomatic uterine leiomyomas and abnormal uterine bleeding.
METHODS:
Women were randomized to ulipristal (5 mg, 10 mg) or placebo in two phase 3, multicenter, double-blind, placebo-controlled trials (VENUS I and II). Health-related QOL and symptom severity were assessed at baseline, and over one (VENUS I and II) and two (VENUS II) 12-week treatment courses using the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire. In pooled VENUS I and II data, change from baseline to the end of the first course for each Uterine Fibroid Symptom Health-Related Quality of Life scale was analyzed, including a Revised Activities subscale that measured physical and social activities. The proportion of women achieving meaningful change in the Symptom Severity (20 or more points), Health-Related QOL Total (20 or more points), and Revised Activities (30 or more points) scales was calculated. In VENUS II data, change from baseline to the end of each course in each scale was analyzed for each treatment arm.
RESULTS:
In pooled analyses, the intent-to-treat population included 589 patients (placebo, n=169; ulipristal 5 mg, n=215; ulipristal 10 mg, n=205). Significantly greater improvements from baseline in all Uterine Fibroid Symptom Health-Related Quality of Life scales were observed with both ulipristal doses compared with placebo (P<.001). A meaningful change in Revised Activities was achieved by 51 patients receiving placebo (34.9%), compared with 144 (73.5%; OR 5.0 [97.5% CI 2.9–8.6]) and 141 (80.6%; OR 7.9 [97.5% CI 4.3–14.6]) patients receiving ulipristal 5 mg, and 10 mg, respectively. In VENUS II, at end of courses 1 and 2, both ulipristal doses demonstrated significant improvements from baseline compared with placebo for all Uterine Fibroid Symptom Health-Related Quality of Life scales (P<.01). Mean Revised Activities scores showed that beneficial ulipristal effects were maintained in course 2, and improvements occurred on switching to ulipristal; results for other scales were similar.
CONCLUSION:
Ulipristal was associated with significant improvements in health-related QOL and symptom severity compared with placebo for women with symptomatic uterine leiomyomas.
CLINICAL TRIAL REGISTRATION:
FUNDING SOURCE:
Allergan plc, Dublin, Ireland.
Uterine leiomyomas are a common and significant health issue for women, with an estimated cumulative incidence by age 50 years of more than 80% in black women and nearly 70% in white women.1 Up to 50% of women with uterine leiomyomas are symptomatic,2 with clinical symptoms that can include abnormal uterine bleeding (AUB), anemia, abdominal pressure and pain, increased urinary frequency, and infertility.1–5 Uterine leiomyoma symptoms interfere with daily physical and social activities, and negatively affect women's well-being and health-related quality of life (QOL).6–8
Uterine leiomyomas are the leading indication for hysterectomy in the United States,9,10 but studies have shown that most women with uterine leiomyomas would prefer minimally invasive or noninvasive treatment options.6,11 There are no pharmacologic treatments indicated outside of preoperative use for women with symptomatic uterine leiomyomas.
Ulipristal acetate is an orally administered selective progesterone receptor modulator12–15 that acts on the progesterone receptors of the endometrium, leiomyoma tissue, and pituitary gland to reduce bleeding and uterine leiomyoma size.16–21 Ulipristal has demonstrated efficacy compared with placebo in clinical trials.17,22,23 Two pivotal phase 3 studies—VENUS I and VENUS II—confirmed the efficacy of ulipristal for the treatment of women with symptomatic uterine leiomyomas: in both studies, rate of and time to amenorrhea was superior for ulipristal 5 mg or 10 mg compared with placebo (P<.001).24,25
The objective of this analysis was to provide a more robust and in-depth investigation of the effects of ulipristal on health-related QOL and symptom severity for patients in the VENUS I and VENUS II studies.
ROLE OF THE FUNDING SOURCE
The study was sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Kevin De-Voy, MSc, on behalf of Complete HealthVizion, and by Laura Gibbons, PhD, of Complete HealthVizion, and funded by Allergan plc, Dublin, Ireland. Neither honoraria nor payments were made for authorship. Allergan will share de-identified patient-level data and study-level data including protocols and clinical study reports for phase 2–4 trials completed after 2008 that are registered to ClinicalTrials.gov or EudraCT, have received regulatory approval in the United States or the European Union in a given indication, and the primary manuscript from the trial has been published. To request access to the data, the researcher must sign a data-use agreement, and any shared data are to be used for noncommercial purposes. More information can be found at http://www.allerganclinicaltrials.com/.
The authors had access to relevant aggregated study data and other information (such as study protocol, analytic plan and report, validated data table, and clinical study report) required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. Allergan (the sponsor) played a role in the design, execution, analysis, reporting, and funding of these studies. The authors' personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
METHODS
VENUS I (UL1309; ClinicalTrials.gov: NCT02147197)24 and VENUS II (UL1208; ClinicalTrials.gov: NCT02147158)25 were phase 3, randomized, placebo-controlled, multicenter studies designed to evaluate the efficacy and safety of ulipristal for the treatment of AUB associated with uterine leiomyomas. Each institution obtained approval from a central or local Institutional Review Board (IRB) before study initiation, and written informed consent was acquired from all study participants. The institutions involved were approved by one of the following IRBs: Shulman Associates IRB, Western IRB, Medical University of South Carolina IRB, Hamilton Integrated Research Ethics Board, Ottawa Health Science Network Research Ethics Board, Chesapeake IRB, and Virtua General IRB. VENUS I assessed a single 12-week treatment course of ulipristal, whereas VENUS II evaluated intermittent therapy with two 12-week treatment courses separated by a drug-free interval of two menses. Both studies included a 12-week, drug-free, follow-up period.
In VENUS I, patients were randomized in a 1:1:1 ratio to receive either ulipristal 5 mg, ulipristal 10 mg, or placebo orally, once daily (Fig. 1A).24 In VENUS II, patients were randomized to one of six treatment arms in a 1:1:2:1:2:1 ratio, with course 1, course 2 dosing of placebo, ulipristal 5 mg; placebo, ulipristal 10 mg; ulipristal 5 mg, 5 mg; ulipristal 5 mg, placebo; ulipristal 10 mg, 10 mg; ulipristal 10 mg, placebo (Fig. 1B).25 The treatment arms of the individual studies were pooled (Fig. 1C).
Fig. 1. Treatment randomization in VENUS I (A) and VENUS II (B) and subsequent pooling of treatment groups (C). UPA, ulipristal acetate.
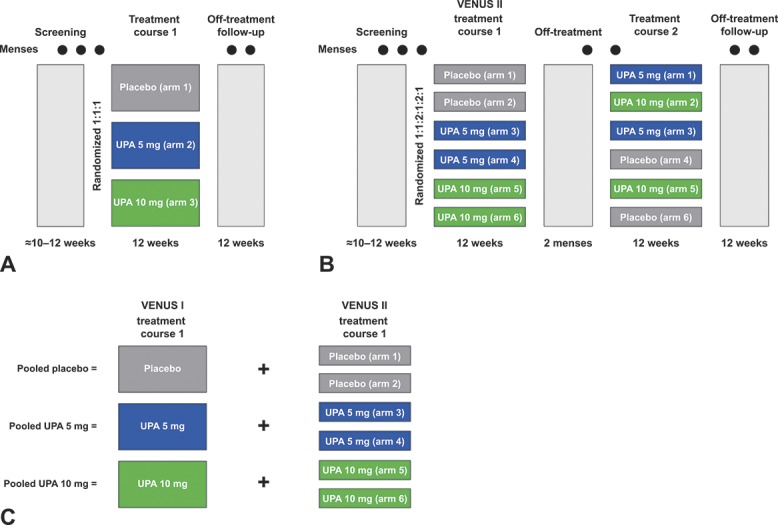
Lukes. Ulipristal for Uterine Leiomyomas: Quality of Life. Obstet Gynecol 2019.
Patient inclusion and exclusion criteria (the same for VENUS I and II) have been described elsewhere.24,25 In brief, key inclusion criteria included premenopausal women aged 18–50 years who had experienced cyclic (between 22 and 35 menstrual cycle length days) AUB, had menstrual blood loss of 80 mL or greater (measured using alkaline hematin), and had at least one discrete leiomyoma observable by transvaginal ultrasound examination. Key exclusion criteria included a history of uterine surgery that would interfere with the study endpoints, known coagulation disorder, and a history of or current, uterine, cervix, ovarian, or breast cancers. Patients with alanine transaminase, aspartate transaminase, alkaline phosphatase, or total bilirubin two or more times the upper limit of normal at screening were excluded.
The Uterine Fibroid Symptom Health-Related Quality of Life questionnaire 1-month recall version is a 37-item questionnaire developed and validated specifically to evaluate the symptoms of uterine leiomyomas and their effect on health-related QOL for women with uterine leiomyomas (Coyne KS, Harrington A, Currie BM, Chen J, Gillard P, Spies JB. Psychometric validation of the 1-month recall uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Poster PRM87 presented at ISPOR 23rd annual international meeting, May 19–23, 2018, Baltimore, MD.).26–29 The Uterine Fibroid Symptom and Health-Related Quality of Life scales were transformed into scores that range from 0–100, with higher scores indicating better health-related QOL; for the Symptom Severity scale, higher scores signified greater symptom severity.
Mean change from baseline at the end of treatment course 1 on the Revised Activities subscale score of the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire was a prespecified secondary endpoint in VENUS I and II. Mean change from baseline at end of treatment (VENUS I) or end of treatment courses 1 and 2 (VENUS II) on the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire Symptom Severity, Health-Related QOL Total scale, and the other health-related QOL subscale scores were other efficacy measurements.
VENUS I and VENUS II were considered suitable for pooling based on similarities in study design, treatment, study population, and endpoints; the pooled analysis was a planned secondary analysis agreed to with the U.S. Food and Drug Administration in June 2016. Changes from baseline to end of treatment for each Uterine Fibroid Symptom Health-Related Quality of Life questionnaire scale score were analyzed using pooled data from VENUS I and VENUS II (treatment course 1 only). Changes from baseline to end of treatment courses 1 and 2 for each Uterine Fibroid Symptom Health-Related Quality of Life questionnaire scale score were analyzed using data from VENUS II for each treatment arm.
Responder thresholds were determined in a separate study using a triangulation approach considering distribution-based (eg, effect size), clinical relevancy-based (eg, absence of bleeding and controlled bleeding), and anchor-based analyses (eg, patient perception of change) (Coyne KS, Harrington A, Currie BM, Mo Y, Gillard P, Spies JB. A meaningful response on the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL) [abstract]. Fertil Steril 2018;110(suppl):e135–6.). A responder threshold of at least a 20-point improvement from baseline was determined to be a meaningful improvement on the Symptom Severity and Health-Related QOL Total scales, and at least a 30-point improvement on the Revised Activities subscale (Coyne et al, Fertil Steril 2018;110(suppl):e135–6.).
The intent-to-treat population, which included all randomized patients, was the primary population for all analyses. Observed cases were also used, including assessments collected at each scheduled visit; this visit type was used in by-visit analyses of the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire data. Patient disposition, demographics, and other baseline characteristics for the pooled data set were analyzed using descriptive statistics. In pooled analyses, the P value of the least-square mean difference between ulipristal treatment and placebo was from an analysis of covariance model with treatment as the main effect, and study, baseline value, and pooled center as covariates. Least-square means are adjusted for the terms in the model (study, baseline value, and pooled center) and are less sensitive to missing data. In VENUS II, the difference between change from baseline to end of treatment course 1 and change from baseline to end of treatment course 2 was analyzed using the paired t-test in each treatment arm. All statistical tests were two-sided hypothesis tests performed at the 2.5% level of significance, that is, an a priori P value of .05 was set unless otherwise mentioned. All P values were considered nominal as a measure of strength of association between the endpoint and the treatment effect.
Responder analyses for the Symptom Severity and Health-Related QOL Total scales, and the Revised Activities subscale, were performed post hoc in the pooled VENUS I and II population (treatment course 1 only). The P value from the Cochran-Mantel-Haenszel test compared odds ratios (ORs) for responder status between different treatment arms, controlling for pooled center.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? Allergan will share de-identified patient-level data and study-level data including protocols and clinical study reports for phase 2–4 trials completed after 2008 that are registered to ClinicalTrials.gov or EudraCT, have received regulatory approval in the United States or the European Union in a given indication, and the primary manuscript from the trial has been published.
What data in particular will be shared? See above.
What other documents will be available? See above.
When will data be available (start and end dates)? See above.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? To request access to the data, the researcher must sign a data-use agreement, and any shared data are to be used for noncommercial purposes. More information can be found at http://www.allerganclinicaltrials.com/.
RESULTS
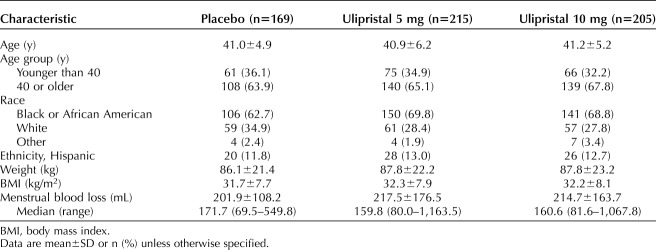
In total, 589 patients were included in both studies (placebo, n=169; ulipristal 5 mg, n=215; ulipristal 10 mg, n=205). Demographic and baseline characteristics were similar across treatment arms (Table 1). In the total population, mean (SD) age was 41.1 (5.5) years, 67.4% of women (n=397) were black or African American, mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was 32.1 (7.9), and mean (SD) menstrual blood loss was 212.1 (155.1) mL. At baseline, mean (SD) Uterine Fibroid Symptom Health-Related Quality of Life questionnaire Revised Activities subscale scores in the placebo, ulipristal 5-mg, and 10-mg groups were 30.7 (25.3), 28.1 (23.8), and 32.0 (26.2), respectively. At baseline, mean (SD) health-related QOL Total scores in the placebo, ulipristal 5-mg, and 10-mg groups were 33.2 (22.0), 32.3 (20.8), and 35.5 (22.6), respectively.
Table 1.
Baseline Demographic and Clinical Characteristics: Pooled VENUS I and VENUS II
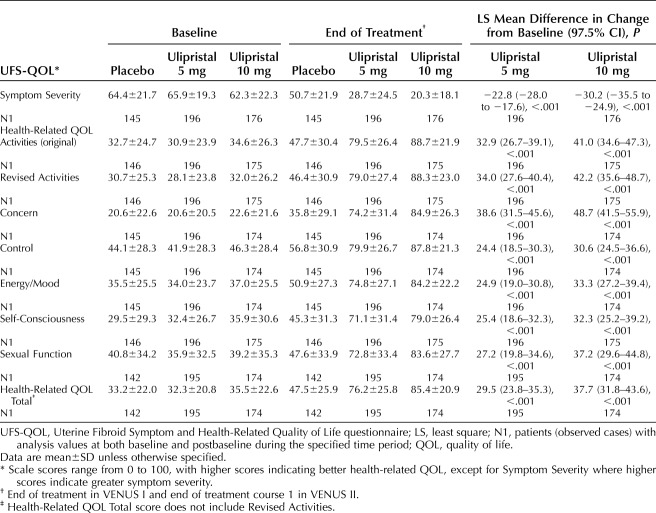
For the pooled analysis, at the end of treatment, significantly greater improvements in Symptom Severity from baseline were observed for both ulipristal doses compared with placebo (Table 2). The least-square mean differences on the Symptom Severity scale score in the ulipristal 5-mg and 10-mg groups compared with placebo (97.5% CI) were −22.8 (−28.0 to −17.6) and −30.2 (−35.5 to −24.9) (both P<.001).
Table 2.
Secondary and Other Efficacy Endpoints: Baseline, End of Treatment, and Least Square Mean Difference in Change From Baseline in Uterine Fibroid Symptom Health-Related Quality of Life Questionnaire Scale Scores (Pooled VENUS I and VENUS II)
Significantly greater improvements from baseline in Uterine Fibroid Symptom Health-Related Quality of Life questionnaire health-related QOL Total score were observed for both ulipristal doses compared with placebo at end of treatment (Table 2): least-square mean differences in the ulipristal 5-mg and 10-mg groups compared with placebo (97.5% CI) were 29.5 (23.8–35.3) and 37.7 (31.8–43.6) (both P<.001). At end of treatment, significantly greater improvements from baseline in all health-related QOL subscale scores were observed for both ulipristal doses compared with placebo (Table 2). For example, the least-square mean differences in the Activities subscale score in the ulipristal 5-mg and 10-mg groups compared with placebo (97.5% CI) were 32.9 (26.7–39.1) and 41.0 (34.6–47.3) (both P<.001).
Significantly greater improvements from baseline in physical and social activities, as measured by the Revised Activities subscale, were observed for both ulipristal doses compared with placebo at end of treatment (Table 2). The least-square mean differences on the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire Revised Activities subscale score in the ulipristal 5-mg and 10-mg groups compared with placebo (97.5% CI) were 34.0 (27.6–40.4) and 42.2 (35.6–48.7) (both P<.001).
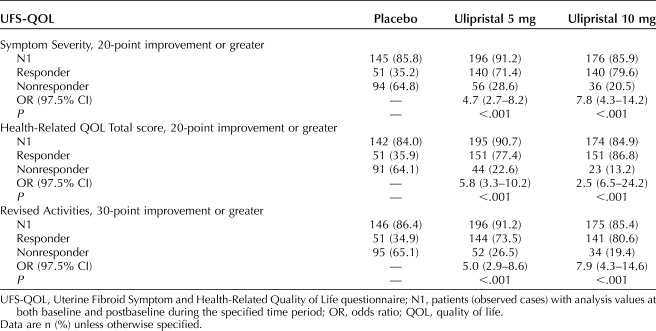
An improvement for Symptom Severity of 20 or more points was achieved among 35.2% of patients (n=51) in the placebo group, 71.4% (n=140) in the ulipristal 5-mg group (OR 4.7; 97.5% CI 2.7–8.2), and 79.6% (n=140) in the ulipristal 10-mg group (OR 7.8; 97.5% CI 4.3–14.2) (Table 3). A Health-Related QOL Total score improvement of 20 or more points was achieved by 35.9% of patients (n=51) in the placebo group, 77.4% (n=151) in the ulipristal 5-mg group (OR 5.8; 97.5% CI 3.3–10.2), and 86.8% (n=151) in the ulipristal 10-mg group (OR 12.5; 97.5% CI 6.5–24.2) (Table 3). An improvement of 30 or more points for the Revised Activities subscale was achieved by 34.9% of patients (n=51) in the placebo group, 73.5% (n=144) in the ulipristal 5-mg group (OR 5.0; 97.5% CI 2.9–8.6), and 80.6% (n=141) in the ulipristal 10-mg group (OR 7.9; 97.5% CI 4.3–14.6) (Table 3 and Fig. 2). A lack of improvement in the Revised Activities subscale was reported by approximately 25%, 8%, and 5% of patients in the placebo, ulipristal 5-mg, and ulipristal 10-mg groups, respectively (Fig. 2).
Table 3.
Post Hoc Analysis: Meaningful Improvements in the Uterine Fibroid Symptom Health-Related Quality of Life Questionnaire Symptom Severity (20 or More Points), Health-Related Quality of Life Total (20 or More Points), and Revised Activities (30 or More Points) Scale Scores (Pooled VENUS I and VENUS II)
Fig. 2. Cumulative distribution function for change from baseline in Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire (UFS-QOL) Revised Activities subscale score at end of treatment (end of treatment in VENUS I and end of treatment course 1 in VENUS II): pooled VENUS I and VENUS II. The vertical line denotes the responder threshold. A responder is defined as a patient who achieved 30 or more points of improvement in change from baseline on the Revised Activities subscale score of the UFS-QOL at end of treatment. UPA, ulipristal acetate.
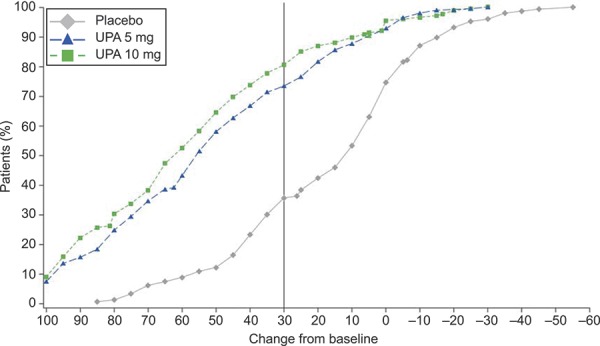
Lukes. Ulipristal for Uterine Leiomyomas: Quality of Life. Obstet Gynecol 2019.
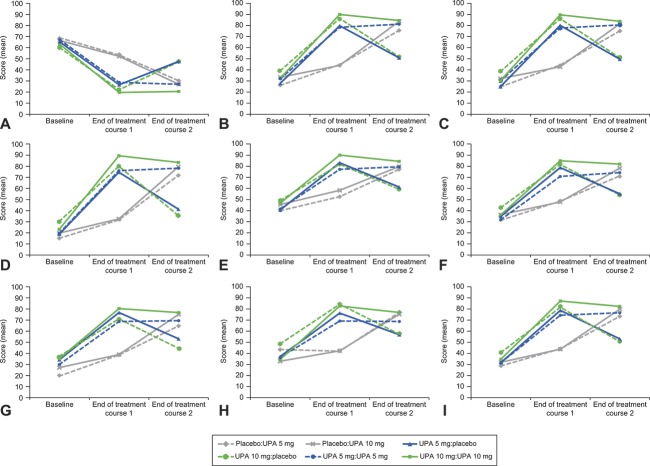
For VENUS II, at the end of both treatment courses, both ulipristal doses showed greater improvement compared with placebo in change from baseline on the Symptom Severity scale score (P<.001), Uterine Fibroid Symptom Health-Related Quality of Life questionnaire Health-Related QOL Total score (P<.001), and all other Uterine Fibroid Symptom Health-Related Quality of Life questionnaire subscale scores (treatment course 1: P<.001; treatment course 2: P<.01). For example, a significant improvement in the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire Revised Activities subscale score was observed for both ulipristal doses compared with placebo (both P<.001) at the end of both treatment courses (Fig. 3). Significant improvements in Uterine Fibroid Symptom Health-Related Quality of Life questionnaire scale scores were observed on switching from placebo in treatment course 1 to ulipristal in treatment course 2 (Fig. 3). In contrast, women receiving ulipristal in treatment course 1 who switched to placebo in treatment course 2 had a significant loss of health-related QOL on the Uterine Fibroid Symptom Health-Related Quality of Life questionnaire scales, although these values did not return to baseline (Fig. 3).
Fig. 3. Baseline and end of treatment courses 1 and 2 Uterine Fibroid Symptom and Health-Related Quality of Life questionnaire scale scores in the treatment arms of VENUS II. Symptom Severity (A), Activities (original) (B), Revised Activities (C), Concern (D), Control (E), Energy/Mood (F), Self-Consciousness (G), Sexual Function (H), and Health-Related Quality of Life Total (I). P values are from the paired t-test comparing the mean difference between baseline and end of treatment course 1 and baseline and end of treatment course 2. P<.001 in the following treatment arms: placebo:UPA 5 mg, placebo:UPA 10 mg, UPA 5 mg:placebo, and UPA 10 mg:placebo, for all scales except control, UPA 10 mg:placebo (P=.001) and sexual function, UPA 5 mg:placebo (P=.004). P≥.05 in the following treatment arms: UPA 5 mg:UPA 5 mg and UPA 10 mg:UPA 10 mg, for all scales except Activities (original), P=.046, Revised Activities, P=.041, and Control, P=.003, in the UPA 10 mg:UPA 10 mg arm. Placebo:UPA 5 mg, n=39–40; placebo:UPA 10 mg, n=36–38; UPA 5 mg:placebo, n=38–40; UPA 10 mg:placebo, n=27; UPA 5 mg:UPA 5 mg, n=77; UPA 10 mg:UPA 10 mg, n=73–74. N indicates patients with analysis values at both baseline and postbaseline during the specified time period. Revised Activities subscale score is not included in the Health-Related Quality of Life Total score. UPA, ulipristal acetate.
Lukes. Ulipristal for Uterine Leiomyomas: Quality of Life. Obstet Gynecol 2019.
DISCUSSION
Women's health-related QOL and well-being, including daily physical and social activities, are known to be negatively affected by the symptoms of uterine leiomyomas.6–8 Women with uterine leiomyomas experience significantly lower health-related QOL than healthy control individuals, as measured by the Short-Form 36 health survey.26,29 Women with severe uterine leiomyoma symptoms had significantly worse health-related QOL measured by Uterine Fibroid Symptom Health-Related Quality of Life questionnaire scores than women with mild or moderate symptoms. Uterine Fibroid Symptom Health-Related Quality of Life questionnaire subscale scores also worsened as the number of symptoms increased.30
Hysterectomy continues to be the most common surgical treatment for uterine leiomyomas, with rates of 21–53% in the United States.31 With no pharmacologic treatments indicated other than for preoperative therapy for women with symptomatic uterine leiomyomas, many are used off-label, including gonadotropin-releasing hormone agonists and tranexamic acid,32–34 nonsteroidal antiinflammatories, levonorgestrel intrauterine devices, and oral and nonoral combination contraceptives.33–36 Thus, there is a significant unmet need for oral therapy for the treatment of uterine leiomyomas that is effective and safe, and can be used both presurgery and postsurgery.
Ulipristal is the first oral therapy to demonstrate efficacy and safety in the treatment of uterine leiomyomas in multiple phase 3 studies: PEARL I–IV17,18,22,23,37 and VENUS I and II.24,25 This analysis shows that, in addition to its demonstrated efficacy and safety, ulipristal is effective at improving the health-related QOL and symptom severity of women with symptomatic uterine leiomyomas, including their ability to engage in physical and social activities.
The population in the pooled VENUS I and VENUS II studies is representative of women with uterine leiomyomas in the U.S. general population, and reflects the higher incidence of uterine leiomyomas in black or African American women, which is two to three times higher than in white women after accounting for age and other risk factors.38,39 Treatment with ulipristal 5 mg and 10 mg improved patients' symptoms and health-related QOL in this population. Although a small proportion of patients experienced no change or some worsening in these outcomes, the majority of women reported clear improvements, for example, more than 70% of patients in the ulipristal treatment arms achieved a meaningful improvement of 30 or more points on the Revised Activities subscale (a 0–100 scale).
The VENUS II analysis showed that the beneficial effects of ulipristal were not completely maintained when women switched to placebo. In contrast, women who started on placebo achieved improvement in Uterine Fibroid Symptom Health-Related Quality of Life questionnaire scores after one treatment course of ulipristal and, for women who received ulipristal in both treatment courses, the benefits of ulipristal were maintained from one treatment course to the next.
The strengths of this health-related QOL analysis include the use of a validated instrument26,27,29 and the advantages of pooling studies with very similar designs. Limitations include that the pooled analyses only assessed up to two treatment courses; therefore, the effect of treatment over an extended period of time was not investigated.
In conclusion, ulipristal treatment was associated with significant improvements in health-related QOL and symptom severity compared with placebo for women with symptomatic uterine leiomyomas. Improvements in health-related QOL, taken together with the significant improvements in amenorrhea, suggest that ulipristal is a promising, noninvasive treatment option for women suffering from symptomatic uterine leiomyomas.
Footnotes
Sponsored by Allergan plc, Dublin, Ireland. Writing and editorial assistance was provided to the authors by Kevin De-Voy, MSc, on behalf of Complete HealthVizion, and by Laura Gibbons, PhD, of Complete HealthVizion, and funded by Allergan plc, Dublin, Ireland. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Financial Disclosure Dr. Lukes has been involved in consulting and advisory boards for the following entities: AbbVie, Myovant, Allergan plc; and has received research grants from Allergan, AbbVie, Merck, Bayer, GlaxoSmithKline, Ferring, Inovio, Sequoia, and Myovant. Dr. Soper has received grants from Allergan, AbbVie, and Bayer. Drs. Harrington, Sniukiene, Mo, and Gillard are employees of Allergan plc. Dr. Shulman has been involved in speaking, advising, consulting, and educational programs for the following entities: AHM LLC, AMAG Pharmaceuticals; Bayer AG; Celula Inc.; Connect Healthcare Inc.; Cory Paeth LLC, Counsyl; Allergan plc; Grey Healthcare Group; Health Advances LLC; Interpublic Group; IntraMed; Meeting Logistics LLC; Merck & Co. Inc.; Natera Inc.; NeoSeq; PharmaWrite LLC; Previvo Genetics LLC; Progenity Inc.; Qiagen; Roche Diagnostics Corporation; Sera Prognostics; and Vermillion.
Presented in part at the American Society for Reproductive Medicine's Annual Meeting, October 6–10, 2018, Denver, Colorado.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B327.
Figure.

No available caption
REFERENCES
- 1.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. [DOI] [PubMed] [Google Scholar]
- 2.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. [DOI] [PubMed] [Google Scholar]
- 3.Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, Dodd SL, Maroulis C, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol 2010;152:96–102. [DOI] [PubMed] [Google Scholar]
- 4.Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health 2014;6:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tropeano G, Amoroso S, Scambia G. Non-surgical management of uterine fibroids. Hum Reprod Update 2008;14:259–74. [DOI] [PubMed] [Google Scholar]
- 6.Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol 2013;209:319.e1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghant MS, Sengoba KS, Recht H, Cameron KA, Lawson AK, Marsh EE. Beyond the physical: a qualitative assessment of the burden of symptomatic uterine fibroids on women's emotional and psychosocial health. J Psychosom Res 2015;78:499–503. [DOI] [PubMed] [Google Scholar]
- 8.Laberge PY, Vilos GA, Vilos AG, Janiszewski PM. Burden of symptomatic uterine fibroids in Canadian women: a cohort study. Curr Med Res Opin 2016;32:165–75. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin-Tommaso SK. Alternatives to hysterectomy: management of uterine fibroids. Obstet Gynecol Clin North Am 2016;43:397–413. [DOI] [PubMed] [Google Scholar]
- 10.Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol 2008;198:34.e1–7. [DOI] [PubMed] [Google Scholar]
- 11.Fennessy FM, Kong CY, Tempany CM, Swan JS. Quality-of-life assessment of fibroid treatment options and outcomes. Radiology 2011;259:785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchard P, Chabbert-Buffet N, Fauser BCJM. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril 2011;96:1175–89. [DOI] [PubMed] [Google Scholar]
- 13.Spitz IM. Progesterone receptor antagonists. Curr Opin Investig Drugs 2006;7:882–90. [PubMed] [Google Scholar]
- 14.Wagenfeld A, Bone W, Schwede W, Fritsch M, Fischer OM, Moeller C. BAY 1002670: a novel, highly potent and selective progesterone receptor modulator for gynaecological therapies. Hum Reprod 2013;28:2253–64. [DOI] [PubMed] [Google Scholar]
- 15.Wagenfeld A, Saunders PTK, Whitaker L, Critchley HOD. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets 2016;20:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biglia N, Carinelli S, Maiorana A, D'Alonzo M, Lo Monte G, Marci R. Ulipristal acetate: a novel pharmacological approach for the treatment of uterine fibroids. Drug Des Devel Ther 2014;8:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012;366:409–20. [DOI] [PubMed] [Google Scholar]
- 18.Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012;366:421–32. [DOI] [PubMed] [Google Scholar]
- 19.Horak P, Mara M, Dundr P, Kubinova K, Kuzel D, Hudecek R, et al. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol 2012;2012:436174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruo T, Ohara N, Yoshida S, Nakabayashi K, Sasaki H, Xu Q, et al. Lessons learned from the preclinical drug discovery of asoprisnil and ulipristal for non-surgical treatment of uterine leiomyomas. Expert Opin Drug Discov 2011;6:897–911. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, et al. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med 2010;28:260–73. [DOI] [PubMed] [Google Scholar]
- 22.Donnez J, Donnez O, Matule D, Ahrendt H-J, Hudecek R, Zatik J, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016;105:165–73.e4. [DOI] [PubMed] [Google Scholar]
- 23.Donnez J, Hudecek R, Donnez O, Matule D, Arhendt H-J, Zatik J, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2015;103:519–27.e3. [DOI] [PubMed] [Google Scholar]
- 24.Simon JA, Catherino W, Segars JH, Blakesley RE, Chan A, Sniukiene V, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas. A randomized controlled trial. Obstet Gynecol 2018;131:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JH, Soper D, Lukes A, Gee P, Kimble T, Kroll R, et al. Ulipristal acetate for treatment of uterine leiomyomas. A randomized controlled trial. Obstet Gynecol 2018;132:1241–51. [DOI] [PubMed] [Google Scholar]
- 26.Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health 2012;15:135–42. [DOI] [PubMed] [Google Scholar]
- 27.Coyne KS, Soliman AM, Margolis MK, Thompson CL, Chwalisz K. Validation of the 4 week recall version of the uterine fibroid symptom and health-related quality of life (UFS-QOL) questionnaire. Curr Med Res Opin 2017;33:193–200. [DOI] [PubMed] [Google Scholar]
- 28.Harding G, Coyne KS, Thompson CL, Spies JB. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health Qual Life Outcomes 2008;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spies JB, Coyne K, Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300. [DOI] [PubMed] [Google Scholar]
- 30.Soliman AM, Margolis MK, Castelli-Haley J, Fuldeore MJ, Owens CD, Coyne KS. Impact of uterine fibroid symptoms on health-related quality of life of US women: evidence from a cross-sectional survey. Curr Med Res Opin 2017;33:1971–8. [DOI] [PubMed] [Google Scholar]
- 31.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alternatives to hysterectomy in the management of leiomyomas. ACOG Practice Bulletin No. 96. American College of Obstetricians and Gynecologists. Obstet Gynecol 2008;112:387–400. [DOI] [PubMed] [Google Scholar]
- 33.Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci 2012;19:339–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao X, Stewart EA, Laughlin-Tommaso SK, Heien HC, Borah BJ. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG 2017;124:322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriplani A, Srivastava A, Kulshrestha V, Kachhawa G, Agarwal N, Bhatla N, et al. Efficacy of ormeloxifene versus oral contraceptive in the management of abnormal uterine bleeding due to uterine leiomyoma. J Obstet Gynaecol Res 2016;42:1744–52. [DOI] [PubMed] [Google Scholar]
- 36.Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynaecol Obstet 2011;112:126–30. [DOI] [PubMed] [Google Scholar]
- 37.Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BCJM, et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014;101:1565–73. e1–18. [DOI] [PubMed] [Google Scholar]
- 38.Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet 2015;131:117–22. [DOI] [PubMed] [Google Scholar]
- 39.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967–73. [DOI] [PubMed] [Google Scholar]