Supplemental Digital Content is Available in the Text.
Key Words: opioid, pain, musculoskeletal, orthopaedic trauma
Abstract
Purpose:
We aimed to produce comprehensive guidelines and recommendations that can be utilized by orthopaedic practices as well as other specialties to improve the management of acute pain following musculoskeletal injury.
Methods:
A panel of 15 members with expertise in orthopaedic trauma, pain management, or both was convened to review the literature and develop recommendations on acute musculoskeletal pain management. The methods described by the Grading of Recommendations Assessment, Development, and Evaluation Working Group were applied to each recommendation. The guideline was submitted to the Orthopaedic Trauma Association (OTA) for review and was approved on October 16, 2018.
Results:
We present evidence-based best practice recommendations and pain medication recommendations with the hope that they can be utilized by orthopaedic practices as well as other specialties to improve the management of acute pain following musculoskeletal injury. Recommendations are presented regarding pain management, cognitive strategies, physical strategies, strategies for patients on long term opioids at presentation, and system implementation strategies. We recommend the use of multimodal analgesia, prescribing the lowest effective immediate-release opioid for the shortest period possible, and considering regional anesthesia. We also recommend connecting patients to psychosocial interventions as indicated and considering anxiety reduction strategies such as aromatherapy. Finally, we also recommend physical strategies including ice, elevation, and transcutaneous electrical stimulation. Prescribing for patients on long term opioids at presentation should be limited to one prescriber. Both pain and sedation should be assessed regularly for inpatients with short, validated tools. Finally, the group supports querying the relevant regional and state prescription drug monitoring program, development of clinical decision support, opioid education efforts for prescribers and patients, and implementing a department or organization pain medication prescribing strategy or policy.
Conclusions:
Balancing comfort and patient safety following acute musculoskeletal injury is possible when utilizing a true multimodal approach including cognitive, physical, and pharmaceutical strategies. In this guideline, we attempt to provide practical, evidence-based guidance for clinicians in both the operative and non-operative settings to address acute pain from musculoskeletal injury. We also organized and graded the evidence to both support recommendations and identify gap areas for future research.
BACKGROUND
Drug overdose deaths have become an epidemic in the United States. In the past 15 years, deaths related to drug overdoses in the United States have tripled, mostly because of the increase in opioid-related deaths.1,2 In the same period, almost half a million people have died of prescription drug overdoses.1,2 Opioids, including prescription drugs and heroin, are involved in 61% of drug overdose deaths.3 The rate of increase in deaths from commonly prescribed opioids has slowed slightly in the past few years, whereas death rates from the synthetic opioids fentanyl and heroin have increased by 72% and 21%, respectively.3 This epidemic has taken a significant toll on the health of the nation, with emerging findings that opioid-related deaths have led to a 0.21-year reduction in average life expectancy—contributing to the overall decrease in life expectancy from 2014 to 2015.4
The increase in opioid overdose deaths aligns with a proportional increase in opioid prescribing rates. Opioid prescriptions increased substantially from 2006 to 20125 with a desired focus on treating patient pain. Family medicine physicians overall provide the most opioids of any specialty; however, orthopaedic surgeons prescribe 7.7% of prescriptions despite representing only 2.5% of physicians.6 The increase in opioid prescriptions was unfortunately not associated with the anticipated reduction of reported pain among Americans.7 Without an improvement in patient outcomes, these prescriptions are needlessly associated with a high risk of abuse. Adding to the problem of oversupply for needs, many opioids go unused following orthopaedic surgery,8,9 creating the possibility of nonmedical usage or diversion. Furthermore, of the patients who receive a first opioid prescription of any duration, 21% progress to receiving more prescriptions episodically and 6% progress to long-term use.10 Up to half of patients who take opioids for at least 3 months remain on opioids 5 years later and are likely to become lifelong users.11–13 Therefore, changing prescribing habits has been a high priority.
Because of the increasing recognition of the opioid crisis, several professional societies, health care systems, pharmacies, insurance companies, and governmental organizations have released guidelines and toolkits for the safe prescribing of opioids. Although some of these guidelines address certain aspects of pain from musculoskeletal conditions, many are focused on the management of chronic pain, and unfortunately, few give concrete examples of practical methods and prescribing practices that can be easily implemented when caring for acute musculoskeletal injuries. Thus, we aimed to produce comprehensive guidelines and recommendations that can be used by orthopaedic practices and other specialties to improve the management of acute pain following musculoskeletal injury.
METHODS
Panel and Target Audience
This guideline aims to provide evidence-based recommendations for the management of acute musculoskeletal pain. A panel of 15 members with expertise in orthopaedic trauma, pain management, or both was convened to review the literature and develop recommendations on acute musculoskeletal pain management. Chronic pain is outside the scope of this guideline.
Literature Review
The panel met in person in October 2017 to define the scope of the guideline and identify important topics for inclusion. The topics included cognitive strategies, physical modalities, opioid safety and effectiveness, multimodal pharmaceutical strategies, medical assistance therapy, nonsteroidal anti-inflammatory drugs and fracture healing, nerve/regional/field blocks, pain and sedation assessment strategies, and health care system strategies. One or 2 panel members were assigned to draft recommendations for each topic area. Literature searches were conducted through September 2018. Information about each included article is available in the Supplemental Digital Content 1 (see Table, http://links.lww.com/JOT/A648).
Grading Process
The methods described by the Grading of Recommendations Assessment, Development, and Evaluation Working Group were applied to each recommendation.14 This method yields a grade for the strength of the recommendation and a grade for the quality of the evidence. The grading of the evidence was based on the study designs, number of studies, sample sizes, and consistency of results among different studies. The panel assigned recommendations as “strong” (practices in which benefits are sure to outweigh potential harms) or “conditional” (the evidence was weaker or if the benefits do not significantly outweigh potential harms).
Approval of Guideline
Recommendations from each topic area were combined to produce a comprehensive guideline for management of acute musculoskeletal pain. All panel members reviewed and revised the combined guideline. The guideline was submitted to the Orthopaedic Trauma Association for review and was approved on October 16, 2018.
Best Practice and Pain Management Recommendations
Because of the increasing recognition of the opioid crisis, several professional societies, health care systems, pharmacies, insurance companies, and governmental organizations have released guidelines and toolkits for the safe prescribing of opioids.3,15–39 Although some of these guidelines address certain aspects of pain from musculoskeletal conditions, many are focused on the management of chronic pain, and few give concrete examples of practical methods and prescribing practices that can be easily implemented when caring for acute musculoskeletal injuries.
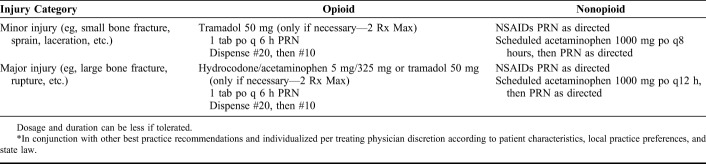
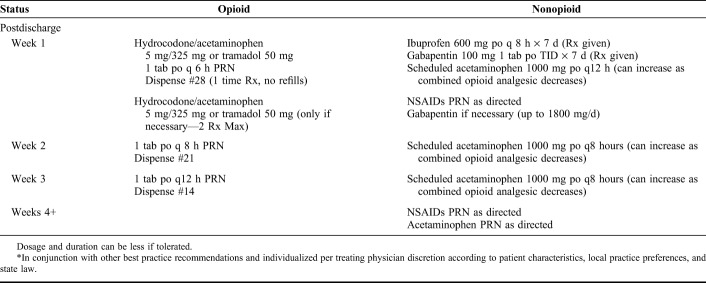
We provide best practice recommendations and pain medication recommendations (Tables 1–4) with the hope that they can be used by orthopaedic practices and other specialties (eg, primary care and emergency medicine) to improve the management of acute pain following musculoskeletal injury. The best practice recommendations for acute pain management following musculoskeletal injury are supplemented with the corresponding in-depth reviews presented in this article. The pain medication recommendations are divided into 3 clinical scenarios—major musculoskeletal injury procedure (eg, operative fixation of long bone or complex joint fracture, extensive soft tissue injury or surgery, etc.), minor musculoskeletal injury procedure (eg, operative fixation of small bone or simple joint fracture, minimal soft tissue dissection or surgery, etc.), and nonoperative musculoskeletal injury (eg, closed management of injury, laceration repair, etc.). The best practice recommendations and the pain management recommendations are meant to be used in conjunction with each other and should be individualized per treating physician discretion according to patient characteristics, local practice preferences, and applicable state laws.
TABLE 1.
Best Practice Recommendations* for Alleviation of Acute Pain After Musculoskeletal Injury

TABLE 4.
Pain Medication Recommended Taper* Following a Nonoperative Musculoskeletal Injury (eg, Closed Management of Injury, Laceration Repair, etc.)
TABLE 2.
Pain Medication Recommended Taper* Following a Major Musculoskeletal Injury Procedure (eg, Operative Fixation of Long Bone or Complex Joint Fracture, Extensive Soft Tissue Injury or Surgery, etc.)
TABLE 3.
Pain Medication Recommended Taper* Following a Minor Musculoskeletal Injury Procedure (eg, Operative Fixation of Small Bone or Simple Joint Fracture, Minimal Soft Tissue Injury or Surgery, etc.)
RECOMMENDATIONS
Cognitive and Emotional Strategies
• The panel recommends discussing alleviation of pain, expected recovery course, and patient experience at all encounters (strong recommendation, moderate-quality evidence).
• The panel recommends connecting patients with pain that is greater or more persistent than expected and patients with substantial symptoms of depression, anxiety, or posttraumatic stress or less effective coping strategies (greater catastrophic thinking and lower self-efficacy) to psychosocial interventions and resources (strong recommendation, low-quality evidence).
• The panel recommends that clinicians consider using anxiety-reducing strategies to increase self-efficacy and promote peace of mind with patients like aromatherapy, music therapy, or cognitive behavioral therapy (strong recommendation, low-quality evidence).
Nociception and Pain
Nociception is the physiology of actual or potential tissue damage. Pain is the unpleasant thoughts, emotions, and behaviors that accompany nociception. There is wide variation in pain intensity for a given nociception.40 Pain catastrophizing is an ineffective coping strategy characterized by unhelpful preparation for the worst including rumination and helplessness.41 Greater catastrophic thinking is consistently associated with greater pain intensity.42 Increased symptoms of anxiety and depression and greater alcohol use are also associated with higher pain intensity, whereas self-efficacy and fewer symptoms of depression are associated with less pain.43–45
Studies of musculoskeletal injuries, including ankle sprains and fractures, have found no association between pain intensity and degree of nociception (injury severity). Variations in pain intensity and magnitude of limitations are accounted for more by measures of psychosocial aspects of illness than by measures of pathophysiology.44,46–53
There are also cultural differences in pain intensity and alleviation of pain with medication. Studies document good pain relief using nonopioid medication in patients recovering from fracture surgery in The Netherlands and Vietnam.54–57 In the United States, however, patients who take more opioids in the hospital after fracture surgery have more pain and less satisfaction with alleviation of pain.43–45 These findings suggest that psychological factors play a significant role in the intensity of pain for a given nociception.
Persistent pain in the absence of infection or implant problems correlates with psychosocial factors.53,56,58–77 Pain intensity, magnitude of limitations, and continued opioid use are associated with greater symptoms of depression or posttraumatic stress disorder and less effective coping strategies (eg, greater catastrophic thinking).
Chronic pain is defined as pain lasting beyond the usual course of healing or more than 3–6 months, which affects the individual's daily functioning and well-being.78 Several nonmodifiable risk factors have been identified for the development of chronic pain including female sex, age >65 years, intense acute pain, and low socioeconomic status. Several modifiable risk factors have also been identified including greater pain catastrophizing, greater pain-related fear, and greater symptoms of anxiety, depression, and posttraumatic stress disorder. Identifying and addressing psychosocial factors may limit persistent pain.
Psychosocial Interventions
A notable portion of trauma patients have substantial symptoms of anxiety, depression, and post-traumatic stress disorder months after injury. Giving opioids for pain that is more intense and disabling than expected might represent a misdiagnosis and mistreatment of stress, distress, and less effective coping strategies.
Initial studies of psychosocial interventions to limit psychological distress and improve comfort and ability have had mixed results.62,79–94 The goals of these interventions are to improve overall mental health and decrease rates and severity of depression, anxiety, and posttraumatic stress disorder. Interventions studied include cognitive behavior therapy, self-management interventions and training, educational information access, peer support, and online social networking. Cognitive behavioral interventions have positive effects on pain relief in some trials.58,95,96 There is also evidence that web-based cognitive behavioral therapy is effective.97–99 Meta-analyses of music therapy demonstrate decreased anxiety and better sleep in the setting of chronic medical illness.100 Music therapy has also demonstrated positive effects on pain relief and opioid dose reduction. Similarly, systematic reviews of aromatherapy have demonstrated anxiolytic effects101 and pain reduction.102 Further research on the utility of various interventions can help elucidate the most effective resources for trauma patients.
Physical Strategies
TENS
• The panel recommends the use of transcutaneous electrical stimulation (TENS) as an adjunct to other immediate postinjury or postoperative pain treatments (strong recommendation, low-quality evidence).
• The panel can neither recommend nor discourage a specific TENS device or protocol. Regimens that incorporate suboptimal frequencies not approaching a “subnoxious or maximal tolerable/painful” setting lack effective pain modulation and should be avoided (conditional recommendation, low-quality evidence).
TENS attempts to modulate pain through delivery of low-voltage electric currents over the skin from a small portable device. The stimulation of large diameter peripheral afferent nerve fibers is believed to reduce pain by activating opioid receptors through an endogenous descending inhibitory pathway.103 The contraindications to the use of TENS include the presence of a pacemaker or implanted defibrillator, broken skin at the site of application, or significant lymphedema.
There are mixed results on the adjunctive use of TENS to modulate pain, largely due to a relative paucity of high-quality trials and significant interstudy heterogeneity due to the lack of any specific standardized treatment protocols. The panel's literature review was restricted to TENS studies within the last 20 years.
The American Pain Society's 2016 Clinical Practice Guideline for the management of postoperative pain recommends the consideration of TENS as an adjunctive modality with treatments directed near the surgical wound. The review panel found insufficient evidence for specific TENS regimens but emphasized that positive effects were stronger when optimal predefined stimulation parameters were used.103
A meta-analysis (21 randomized clinical trials, RCTs) of TENS as an adjunct to reduce postoperative analgesic consumption found that the effectiveness may depend on the current amplitude. The authors only included studies that report a “strong and/or definite subnoxious, and/or maximal nonpainful, and/or maximal tolerable” stimulation with currents >15 mA or a pulse frequency of 1–8 Hz (acupuncture-like TENS; ALTENS) or 25–150 Hz (TENS). The review found TENS (vs. placebo TENS) around the surgical wound significantly reduced postoperative analgesic consumption by 26.5% (range −6% to 51%): subnoxious stimulation reduced opioid consumption by 35.5%, whereas nonspecific trials yielded less effect (4.1% reduction). Overall difference in analgesic consumption favored TENS versus placebo with optimal median frequencies at 2 Hz for ALTENS or 85 Hz for TENS.104
The effectiveness of TENS within the orthopaedic literature is limited by nonstandardized clinical trials often without reported or consistent TENS treatment protocols. Adjunctive TENS use within the immediate postoperative period after a total knee arthroplasty (TKA) postulates a trend toward favorable mean weighted reduction in opioid consumption versus placebo TENS or standard care (3 meta-analyses and 1 RTC).105 One systematic review and meta-analysis found that TENS decreased pain severity at 1, 2, and 6 months after TKA, but this was based on low-quality studies.105 Interestingly, both TENS and placebo TENS (45-second cutoff) were found to decrease postoperative TKA pain with active extension and fast walking, highlighting a potential placebo effect that subsided by 6 weeks postoperatively versus standard treatment.106 A prospective double-blind randomized trial on arthroscopic rotator cuff repair found TENS to significantly reduce immediate postoperative opioid use by 25% at both 48 hours and 1 week.107 These results are moderately consistent with the nonorthopaedic literature where TENS decreased postoperative opioid analgesic requirements (by 53% with mixed frequencies vs. 35% with high-frequency and 32% with low-frequency settings) and opioid-related side effects when used as an adjunct to patient-controlled analgesia (PCA) after lower abdominal gynecological surgery.108 In contrast, although TENS was determined useful after thoracic surgical procedures (only when less invasive approaches yield mild to moderate postoperative pain), TENS was ineffective for severe pain with invasive approaches.109
A meta-analysis (27 RCTs) of 6 different types of electrical stimulation determined that interferential current, a less common modality, was the only treatment to effectively modulate pain intensity and change pain visual analog scale (VAS) scores (standardized mean difference = 2.06, 95% CI: 1.1–3.19), that the effect of high-frequency TENS was uncertain, and that low-frequency TENS was not effective.110
In conclusion, our systematic review indicates that TENS, when applied using strong, subpainful frequencies, is an effective multimodal adjunct to modulate acute orthopaedic injury and postoperative pain. Recent publications demonstrate a substantial degree of interstudy heterogeneity, most notably inconsistent descriptions of both TENS dosing intensities and standardized outcome measures. The long-term tolerance of the same dose TENS parameters and strategies to prolong its effect is largely unknown. Higher-quality clinical trials are necessary to provide stronger evidence in favor of TENS as a consistent treatment for acute pain and perioperative pain modulation.
Cryotherapy
• The panel recommends the use of cryotherapy for acute musculoskeletal injury and the postsurgical orthopaedic patient as an adjunct to other postoperative pain treatments (conditional recommendation, low-quality evidence).
• The panel cannot recommend a specific cryotherapy delivery modality or protocol (no recommendation, limited evidence).
Cryotherapy is the application of an external cold source in which the desired effect is a drop in tissue temperature. Cold sources that have historically been used include ice bags, cold gel packs, ice massage, cold water submersion, gaseous cryotherapy, and continuous-flow cryotherapy devices with and without pneumatic compression. Basic science studies have shown that the biologic effects of cold therapies are multifactorial. A decrease in tissue temperature results in decreased tissue edema and microvascular permeability,111,112 reduced delivery of inflammatory mediators,112–116 reduced blood flow via vasoconstriction,116–120 overall net decrease in tissue metabolic demand, and subsequent hypoxic injury.116–118,120 In addition, the decrease in tissue temperature has been shown to increase the threshold of painful stimuli and increase the tolerance to pain.121
Multiple studies have looked at the efficacy of cryotherapy in the postoperative orthopaedic patient for various anatomic areas including the knee, hip, shoulder, foot and ankle, wrist, and hand. Among the studies that evaluated cryotherapy versus a noncryotherapy control, 10 randomized controlled trials and 2 meta-analyses have shown a significant benefit for pain control.105,122–132 Contrary to this, there have been 8 randomized controlled trials that have shown no benefit to cryotherapy compared with a noncryotherapy control.133–140 Many studies have also looked at cryotherapy's ability to decrease opioid consumption compared with a noncryotherapy control. Of these studies, 11 have shown a significant decrease in pain medication consumption105,123,125–127,129,131–133,138,141 compared with 5 studies showing no difference.134–136,139,140
Many randomized controlled trials have compared continuous-flow cryotherapy devices to ice bags or packs. Nine studies have failed to show a difference in pain scores,142–150 whereas 5 studies have shown improved pain with continuous-flow cryotherapy.151–155 No studies have shown superior pain control with ice bags or packs compared with continuous cryotherapy.
There are also inconclusive results pertaining to the difference in pain medication consumption when comparing continuous-flow cryotherapy with ice bags or packs. Five studies have demonstrated a decreased need for opioids with continuous cryotherapy,148,150,151,154,156 one study showed a lower consumption of pain medication with the use of ice packs,157 and 5 RCTs failed to show a difference between these 2 cryotherapy modalities.142,145,147,149,158 It is possible that continuous-flow cryotherapy results in a higher patient satisfaction with the cryotherapy treatments142,148,150 and that there may also be a benefit to continuous-flow cryotherapy at night.159 It is important to note the methodologic variability within the cryotherapy literature. Variables such as cryotherapy source, temperature, duration, and frequency can vary drastically from treatment groups in the same study, as well as study to study, making the assessment on the magnitude of effect difficult to determine. Because of the current literature's methodological heterogeneity, we are unable to favor 1 method of cryotherapy application, protocol, or both.
Like most therapeutic interventions, cryotherapy can result in complications. Nerve palsies have been reported in the literature, mostly involving more superficial nerves such as the peroneal nerve, lateral femoral cutaneous nerve, ulnar nerve, and supraclavicular nerve. Care must be taken to provide sufficient insulation between the skin and the cryotherapy source, especially in patients with minimal subcutaneous fat. Nerve injuries can range from brief paresthesias to complete axonotmesis.160,161 Frostbite has also been a concern but, to our knowledge, has not been reported as a result of cryotherapy after an orthopaedic procedure.
Overall, the body of literature provides preliminary support for use of cryotherapy for acute pain management. However, future studies should focus on determining the most efficacious method of application and protocol for cryotherapy.
Opioid Safety and Effectiveness
• The panel endorses that all opioids used for pain carry a risk of misuse. Opioids are also associated with adverse clinical events. Patient comfort and safety must be carefully balanced when prescribing opioids. Because of the potential for misuse of all opioids, the panel recommends that the prescriber should use the lowest effective dose for the shortest period possible (strong recommendation, high-quality evidence).
• The panel recommends not prescribing benzodiazepines in conjunction with opioids because of the significant risks of inconsistent sedation and potential for misuse (strong recommendation, high-quality evidence).
• The panel recommends avoiding long-acting opioids in the acute setting (strong recommendation, moderate-quality evidence).
• The panel recommends prescribing precisely. Commonly written prescriptions with ranges of dose and duration can allow tripling of daily dose to levels consistent with adverse events (strong recommendation, low-quality evidence).
Opioids are the most commonly used medications for treatment of most severe pain conditions.162 All opioids come with some level of safety concern. Regardless of the formulation used, there is always a risk of adverse events, as well as abuse, addiction, or both. The number and severity of adverse events from opioids are related to their potency, half-life, and mode of use.
The number of milligrams in the dosage is not an indication of how strong the medication might be. Potent opioids (eg, fentanyl is 50–100 times as potent as morphine) increase the number and severity of events. Although oxymorphone and oxycodone are about equally effective in treating pain, more adverse events are seen with oxymorphone because of its higher potency.163 Oxymorphone has 3–7 times the efficacy of morphine, whereas oxycodone is only 1.5 times greater. Currently, immediate-release opioids are prescribed at a significantly higher rate than extended-release options.164 These extended-release medications result in a 4.6-fold higher abuse rate and a 6.1 times increased diversion potential.164 The risk of addiction and abuse also has a strong correlation with the length of time the opioids are prescribed. Although some patients may become addicted after long-term therapy, a significantly larger proportion will show behavior of medication misuse and illicit drug use.165
The main formulations on the market have vastly different pharmacokinetics. Immediate-release opioids, which cause serum opioid levels to rapidly increase and decrease with a shorter half-life, have a shorter period of pain relief. Long-acting (“continued-release” tablets) may deliver opioids for a longer period, but the amount of opioid absorbed is less per unit of time. This results in less fluctuation in serum drug levels, keeping opioid concentration in the therapeutic range.166 For the inpatient setting, long-acting opioids may have the same effectiveness as short-acting opioids when used as monotherapy, but given newer multimodal pain management regimens, this is not recommended current practice.167 Both short-acting and long-acting opioids have been shown to be effective in treating pain and increasing quality of sleep, with the main difference being that the number of pills prescribed will be higher in the short-acting group.168–170 Other drug formulations have been created to include supposed abuse deterrent properties, but in actuality may have a similar profile in regard to effectiveness and adverse events.171 Combining opioids with other drugs has been shown to be more effective in managing pain than opioids alone. More specifically, combining opioids with nonsteroidal anti-inflammatory drugs (NSAIDs) has been shown to be more effective than opioids alone.172 Benzodiazepines do not have this beneficial synergy. Taking any of these formulations with food does not change the maximum dose of the medication delivered, although when taken after a high fat meal, the time to maximum concentration is delayed.173
The literature comparing the difference of the safety and efficacy of opiates for the treatment of pain in acutely injured musculoskeletal patients is scarce. The majority of the literature on safety and efficacy of opioids is in regard to chronic pain from both malignant and nonmalignant conditions. The evidence in these areas is not strong.162 There is very little in the literature discussing safety and efficacy in the short-term postinjury setting. Hence, the appropriate dose for specific injuries or conditions is not well defined. Standard prescribing habits seem to routinely provide an excess amount of medication. A recent study found that 81% of patients took 20 or fewer pills after knee arthroscopy.174 A study of opioid use by 250 patients who had undergone elective outpatient upper extremity surgery showed that although all patients were prescribed opioids for 30 days (30 pills), 52% used their prescription for pain control for only 2 days or less. On average, each patient took 11 pills, leaving 19 pills unused. With fewer pills prescribed, there was a 79% reduction of leftover pills in the community, thus decreasing the potential for diversion.175
Leaders in musculoskeletal care need to develop specific strategies based on burden of disease. Other nonopioid medications should be used with an intent to obtain balanced patient comfort and safety. Some data have shown that the risk of dependency increases significantly with increasing duration of use.176 Every effort should be made to minimize prescription length.
The main cause of death in patients using opioids is respiratory depression. This can occur with any opioid regardless of the type or formulation. This deadly complication is dose and concentration dependent with many other variables such as opioid tolerance, body mass index, respiratory disease, obstructive sleep apnea, and concomitant medications. Patients with a history of opioid use are expected to require more opioids for adequate pain relief while experiencing fewer adverse events due to tolerance.166,177 Common non–life-threatening side effects seen in approximately 10% of patients prescribed immediate-release opioids are pruritus, nausea, vomiting, dizziness, headache, and somnolence.178,179 Addiction and abuse are complications often seen by psychiatrists or psychologists. Despite early, unsubstantiated claims of improved safety with long-acting opioids,180 the relative abuse and addiction potential with short-acting or long-acting opioids remains a question. Some evidence suggests that there is no difference in illicit drug use, misuse, or both when comparing long-acting versus short-acting opioids, suggesting that prescribing long-acting opioids will not reduce abuse potential.181 A contradictory study showed less drug-seeking behavior with extended-release formulations.182 Benzodiazepines should not be prescribed in conjunction with opioids because the risk of overdose and death increases significantly. There is a 3.9 times risk of overdose due to respiratory depression when opioids and benzodiazepines are prescribed at the same time.183
Combination Pharmaceutical Strategies
Multimodal Analgesia
• The panel recommends the use of multimodal analgesia (MMA) as opposed to opioid monotherapy for pain control (strong recommendation, moderate-quality evidence).
• The panel recommends the use of periarticular injections as an adjunct to pain management that improves pain control postoperatively (strong recommendation, moderate-quality evidence).
• The panel cannot recommend specific MMA regimens at this time without further scientific evidence. MMA should be tailored to patients' injuries and medical comorbidities (strong recommendation, very low–quality evidence).
MMA, also referred to as balanced analgesia, is the use of multiple analgesic medications (opioid and nonopioid) and nonpharmacologic interventions designed to affect peripheral and or central nervous system loci in the pain pathway.103 Benefits of this treatment paradigm include potentiation of multiple medication effects and greater pain control without relying on any 1 class of medication. MMA therefore mitigates the risk profile of each medication, while allowing for synergistic pain control from different classes of medication. Successful postoperative MMA may include psychotherapy, physical therapy, NSAIDs, acetaminophen, gabapentinoids, regional anesthesia (single shot or peripheral nerve catheters), local injections, and opioids. Recent reviews,184 meta-analyses,185 and RCTs186 have shown that MMA is effective in the perioperative period. There is, however, a paucity of literature in the orthopaedic trauma population, and therefore, literature from other subspecialties and surgical fields was included.
The majority of the orthopaedic literature addresses the arthroplasty population (14 articles). These articles addressed the following 3 main clinical trial questions: (1) comparison of different periarticular injections, (2) oral or “standard” medication regimen versus addition of a peripheral nerve block (covered in later section), and (3) oral or “standard” medication regimen versus MMA.
Four studies compared “standard” medication regimens versus MMA. For example, additions to MMA strategies include gabapentin187 and duloxetine.188 Gabapentin seemed to decrease pain scores, but not opioid consumption,187 whereas duloxetine decreased opioid consumption, but not pain scores.189
Finally, 2 studies evaluated the cost-effectiveness of MMA in arthroplasty patients. In both cases, the use of multimodal therapy decreased hospital costs, directly related to medication, and overall hospital costs for patient stay.190,191
There is limited literature regarding the use of MMA in other nontrauma orthopaedic subspecialties. Two articles evaluated the use of MMA in foot and ankle surgery where MMA decreased length of stay192 and decreased pain in the first 24 hours after surgery.193 In spine surgery, the addition of MMA to a standard PCA regimen, decreased opioid use and improved mobilization.194 When compared with intravenous (IV) medication only, MMA decreased VAS scores at all time points following lumbar fusion surgery.195
In orthopaedic trauma, addition of periarticular injection to standard pain control for hip hemiarthroplasty improved VAS scores and reduced opioid usage early in the postoperative course.196 Surgical site injection also improved pain for femoral fracture patients.197 In the upper extremity, MMA compared with PCA showed additional need for pain rescue in the PCA group and lower patient satisfaction.198 In a study of emergency department (ED) fracture patients, IV morphine or IV Tylenol + oral oxycodone was equally effective for pain control in the first hour after administration. However, patients in the IV morphine group did have less nausea and site itching.199
The use of corticosteroids for postoperative pain has been validated in the literature in other specialties in medicine. As with other medications, there are risks associated with the use of corticosteroids. Systemic side effects often associated with long-term therapy include the following: Cushingoid appearance, hirsutism, exophthalmos, hypertension, arrhythmias, gastritis, osteoporosis, avascular necrosis, dysphoria, and hypokalemia just to name a few. From a postoperative perspective, concerns include a decrease or delay in wound healing potential and infection. There are no data to indicate that short-term use of corticosteroids causes an increase in infection. It is not recommended to use corticosteroids in patients older than 60 years and in immunocompromised patients because some data suggest that there is an increase in healing time.200 An increase in blood glucose 24 hours after surgery should be expected and has not been associated with an increase in the rate of infection.201
Corticosteroids given orally or IV can decrease the use of opioid analgesics by 50%.202 Benefits of corticosteroids include a decrease in postoperative nausea, decrease in opioid requirements, decrease in the length of hospital stay, and more complete pain relief.203,204 The smallest dose that is effective should be prescribed. Doses ranging from 15 mg of dexamethasone to 0.1 mg/kg have been shown to be effective with no complications.201,203,205–207 A meta-analysis of perioperative use corticosteroids concludes that an “intermediate-dose dexamethasone (0.11–0.2 mg/kg) is a safe and effective multimodal pain strategy after surgical procedures. The preoperative administration of the drug provides a greater effect on postoperative pain.”201 Physicians should consider perioperative dosing of corticosteroids in low-risk patients, especially in patients at risk of dependency.
Managing Acute Pain for Patients on Long-Term Opioids at Presentation
The panel recommends that perioperative analgesia should be managed with a MMA regimen in all opioid-tolerant patients (strong recommendation, moderate-quality evidence).
• The panel recommends coordinating with acute pain service (APS) (or addiction medicine or psychiatry depending on resources) when inpatient and the patient's prescriber when outpatient to ensure that there is only 1 prescriber for patients on medication-assisted therapy (methadone, buprenorphine, or naltrexone), patients using illicit opioids, or patients misusing prescription opioids (strong recommendation, moderate-quality evidence).
Opioid-tolerant patients present a clinical challenge to effective perioperative pain management. These patients have a medical condition and should be treated with the same respect and dignity as a patient with any other presurgical medical condition. Developed nations have observed a large increase in the number of opioid-tolerant patients over the last decade.103,208 In the United States, a combination of expanding heroin abuse, pain control metrics, and pharmacologic development of long-acting opioids has resulted in a dramatic increase in the number of opioid-tolerant patients. Managing perioperative pain in the opioid-tolerant patient is both a medical and a social challenge. Opioid-tolerant patients are at an increased risk of receiving inadequate perioperative analgesia.103 This risk exists as the result of (1) a social stigmatization of opioid prescription and consumption209; (2) concerns for drug-seeking behavior210 or relapse of recovering addicts, or both; and (3) an incomplete understanding of opioid agonist and opioid replacement therapy pharmacokinetics.211
Opioid-tolerant patients present with 1 of the following 3 clinical scenarios: (1) scheduled, prescribed opioid (short-acting or long-acting) regimens; (2) prescribed medical assisted therapy (methadone and buprenorphine); and (3) illegal consumption of prescription or nonprescription opioids.212 Each patient can be further subdivided into those who are actively experiencing acute pain in an emergent setting (secondary to trauma) or whose treatment necessitates elective surgery (nonunion, malunion, infection, and hardware removal). The care of these patients can be difficult, and there is little literature to guide treatment.
At the time of this publication, there are a limited number of observational studies examining acute perioperative pain management in the opioid-tolerant patient. However, care must be taken when managing these patients. In 2 studies on orthopaedic trauma populations, it has been shown that patients on opioids are at a higher risk of receiving prescriptions from multiple prescribers in the postoperative period, which leads to more prescriptions, higher doses, and longer duration of opioid use.213,214 What follows is a review of available literature and clinical recommendations for perioperative analgesia in the opioid-tolerant patient.
It is critical to identify opioid users immediately after injury or in the preoperative period to avoid uncontrolled acute pain. Physicians should obtain information on type, dose, frequency, and last consumption of all opioids, which will allow conversion to morphine equivalent doses. The opioid-tolerant patient experiences pain, physiologically, differently than the opioid-naive patient103,211,215–217 because of the following:
a. Cross tolerance occurs between different opioids
-
b. Increased sensitivity to natural and experimental pain.103,211,218,219
c. High-affinity partial μ-agonist and antagonist block the effect of standard opioids. When these medications are used, patients require high opioid doses to displace competitive medications before analgesia takes effect.
The following sections provide brief recommendations for specific populations of opioid-tolerant patients, including those taking chronic short-acting opioid therapy, those using illicit opioids, and those taking methadone, buprenorphine, or naltrexone.
Chronic Short-Acting Opioid Therapy
Perioperative pain management of patients consuming routine and scheduled oral opioids should include the following:
-
1. Instructions to continue baseline medication the morning of surgery through the postoperative period.220
2. Titrate short-acting μ-agonist to effective pain control.
3. When oral medications cannot be consumed, the 24-hour morphine equivalent dose should be calculated for conversion to IV management until oral medications can be reinstituted.215
Illicit Opioids
Perioperative pain management is further complicated by inaccurate consumption history and variation in strength of illicit drugs:
If available, consult addiction medicine, APS, or psychiatry.103
Methadone (Slow-Release Oral Morphine or Opioid Agonist)
Perioperative pain management of patients consuming methadone should include the following215:
1. If available, consult addiction medicine, APS, or psychiatry.103
2. Continue baseline methadone throughout the perioperative period including the morning of surgery.
-
3. If unable to take oral medications, convert the 24-hour dose to IV methadone according to the conversion chart and administer in 2–4 divided doses.
a. Pharmacokinetics of methadone are influenced by CYP450 and CYP3A4 metabolism and may also vary based on the patient's own metabolism. Consult a pharmacist or APS specialist for conversion to the appropriate morphine equivalent dose.222
4. Supplement perioperative pain with short-acting agonist.
5. Close respiratory monitoring due to combined effects.
6. Educate the patient on acute opioid taper.
Buprenorphine [Partial μ-Agonist Alone or Mixed With Kappa Antagonist (Naloxone)]
Addiction medicine, APS, or psychiatry (depending on local resources and expertise) should be consulted when managing patients on buprenorphine, which is commonly administered transdermally for chronic pain and sublingually for substitution in opioid abusers.215,223–225 Owing to the medication high affinity for Mu receptors and kappa antagonist effect, other agonists may have limited analgesia effect and typically require high doses to achieve affect. For this reason, close respiratory monitoring is required when using short- and long-acting opioids.
Perioperative pain management of patients consuming buprenorphine will vary according to the clinical setting:
-
1. Elective surgery
-
a. Mild to moderate pain
i. Consider management with increased doses of buprenorphine (when low doses are prescribed at baseline)
ii. Continue buprenorphine and add short-acting μ-agonist
-
b. Moderate to severe pain
-
i. Discontinue 72 hours before surgery and convert to short-acting agonist.
1. Higher-than-expected doses are anticipated for analgesia for 3 to 4 days while buprenorphine is cleared from the body
2. Reassess analgesia daily and expect to decrease full agonist between days 3 and 4
3. Manage acute pain with a tapering regimen
ii. The patient should be opioid-free for 24 hours before restarting buprenorphine to avoid withdrawal.
-
-
-
2. In acute traumatic presentation
a. Conversion to methadone according to conversion tables and titrate dose to effect
-
b. When clinical presentation does not afford conversion and titration, recommend aggressive acute titration to full opioid agonist.
i. High doses are required to displace high-affinity buprenorphine from μ-receptors
ii. Requires continuous cardiopulmonary monitoring
Naltrexone (Opioid Antagonist Often Used to Limit Relapse Following Opioid Dependence Rehabilitation)
Because of its antagonist mechanism, naltrexone creates a difficult clinical scenario, particularly in the acute traumatic setting. Naltrexone reduces opioid sensitivity by blocking receptors, but also upregulates μ-receptors. During initial treatment of postinjury and perioperative pain, a patient may not be sensitive to a short-acting μ-agonist and may require many times the normal dose.226 After 2 weeks, sensitivity to opioids may increase, risking overdose. When the acute pain period is over, and naltrexone is restarted, it carries the risk of inducing withdrawal. Therefore, the recommendation is to consult addiction medicine, APS, or psychiatry.
NSAIDs and Fracture Healing
• The panel recommends for the routine use of NSAIDs as part of a comprehensive analgesic plan for operative and nonoperative fracture care (strong recommendation, low-quality evidence).
One of the major barriers to using non-narcotic analgesics in orthopaedic trauma has been the reluctance to use NSAIDs in the setting of fracture or arthrodesis surgery of any kind. For decades, NSAIDs were avoided because of fears about bone healing. However, a review of the evidence has found the data on the effect of NSAIDs on bone healing too conflicting to make a clinical recommendation one way or the other.227–229 Given the proven track record of NSAIDs in alleviating musculoskeletal pain, withholding NSAIDs from our analgesic armamentarium is a significant disadvantage. Under the current circumstances, the basis of this prohibition merits a critical review.
The basic science studies have been conflicting at best. The most rigorous basic science studies are animal models of spinal fusion, whereas fracture healing models yielded mixed results at best.230 End points for animal studies demonstrated that NSAIDs contributed to reduced mechanical strength (as bone stiffness and load to failure) and delayed time to union.231,232 Nonetheless, this lack of clarity has re-enforced the perception of a deleterious effect. Further animal studies attempted to examine what the possible mechanism of action could be and tried to establish whether there was a lesser impact from COX-2–specific inhibitors compared with indomethacin in the animal setting, again with mixed results.232,233
Clinical studies are similarly unclear, but 4 of the clinical studies should be examined critically because they are frequently cited when raising alarm over NSAIDs in fracture healing. Giannoudis et al234 used a retrospective case–control model to compare femoral shaft fractures that had not healed to a group that healed successfully. The use of NSAIDS was reported to increase the odds of nonunion by 10.7 times (95% CI: 3.55–33.23), but the study was small and underpowered (sample size of 32 patients), NSAID use was severely underrepresented in the control group, and this same sample showed no effect of smoking. Furthermore, by starting with a group of 32 nonunited diaphyseal femur fractures, investigators may well have been preselecting the group most likely to take NSAIDs (for the pain of nonunion). Bhattacharyya et al235 point out exactly this bias when discussing their finding of higher NSAID use in the subset of humerus fractures that were treated closed and did not heal. To avoid selection bias, Bhattacharyya's group queried Medicare data (1995–2000) from 2 states for patients with a humeral shaft fracture. Starting with nearly 10,000 records, they found 104 patients (1.1%) with a nonunion. They reported that patients who used NSAIDs or opioids within the first 90 days after fracture had relative risks for nonunion of 3.7 (95% CI: 2.4–5.6) or 1.6 (95% CI: 1.1–2.5), respectively.235 More recently, Jeffcoach and coworkers retrospectively reviewed long bone fractures over a 2-year period at a single trauma center. The patients who had a long bone fracture and received NSAIDs during the inpatient postoperative days (12% of 1901 patients) had an odds ratio for a complication (nonunion, malunion, and infection) of 2.17 (1.15–4.10).236 In a well-designed, prospective randomized trial on different durations of indomethacin treatment (3 days, 1 week, or 6 weeks) for prophylaxis of heterotopic ossification, Sagi et al237 showed that at 6 months after surgery, the highest incidence of nonunion of the posterior acetabular wall (67%) occurred in the group with the longest duration (6 weeks) of indomethacin use. Although there were only 13 patients in this group and that raises concerns over adequate power, the rate of nonunion of the posterior wall in all groups was surprisingly high.
Although isolated clinical investigations such as these have been cited as evidence to withhold NSAIDs during fracture treatment, this conclusion is not supported by a critical examination of the existing literature. Two recent comprehensive meta-analyses by Kurmis et al229 and Marquez-Lara et al238 have concluded that although some animal studies may raise a concern, there is no high-quality literature support for NSAID inhibition of fracture healing in the clinical setting. Ultimately, these critical evaluations of the existing clinical literature must stand as the cornerstones of our practice guideline recommendations on this issue.
Based on the unknown clinical role of opioids on fracture healing, recent investigations have tried to examine a potential effect of opiate analgesics on fracture healing. Morphine has been demonstrated to inhibit osteocalcin in vitro.239 Chrastil et al240 used a rat model to examine opioid influence on femur fractures and found that animals treated with opiate analgesia formed callus in greater volume, but that this callus was more disorganized and mechanically weaker than the control animals. Opiate-induced androgen deficiency syndrome describes the naturally occurring reduction in serum testosterone seen clinically with both acute and chronic opioid administration,241 and Brinker et al242 have previously demonstrated hypogonadism to be among the metabolic abnormalities identified in patients with nonunion. Chrastil et al243 attempted to determine whether supplemental testosterone might be used to mitigate the effects of opioids on callus formation and strength, but they found that supplemental testosterone was ineffective for this purpose. This study casts doubt on the theory that the effect of opioids on bone healing is solely mediated by hypogonadism because the opioid-treated animals demonstrated a decrease in serum testosterone, but still had impaired callus formation despite administration of supplemental exogenous testosterone. Overall, any conclusions on the role of opioids in bone healing are very preliminary and have not been corroborated with quality clinical studies, but given its potential impact on clinical practice, the field certainly merits further bench and clinical investigation.
With regard to the effectiveness of NSAIDs for pain control, there are now some head-to-head clinical comparisons available between NSAIDs and opioids for the acute management of musculoskeletal complaints in both the pediatric244 and adult245,246 populations. To date, these studies have demonstrated NSAIDs to provide equally effective analgesia.
To summarize, there is simply no conclusive clinical evidence to prohibit the use of NSAIDs in fracture care. Furthermore, risks to the population from oral opioid use, and the prolonged use after resolution of musculoskeletal injury, are well established. NSAIDs also provide effective analgesia in the setting of musculoskeletal pain.247 Taking all these factors and the existing clinical evidence into account, we recommend the routine use of NSAIDs as part of a comprehensive analgesic plan for operative and nonoperative fracture care.
Nerve/Regional/Field Blocks
This section is organized around the following 3 periods: (1) during a hospital admission before fracture surgery, (2) intraoperatively and the immediate postoperative period, and (3) the remote (>3 months) postoperative period. In each of these temporal periods, in relation to fracture surgery, we asked what is the evidence that nerve, or regional, or field blocks improve pain control and decrease use of opioids?
During a Hospital Admission Before Fracture Surgery
• The panel recommends that regional nerve blocks (femoral nerve or fascia iliaca) should be placed in patients with acute hip fractures at the time of presentation to the ED (strong recommendation, high-quality evidence).
The evidence for this recommendation is confined to hip fracture patients. Multiple studies show that nerve blocks placed in the ED can be accomplished by trained personal with minimal risks or complications.248–258 These blocks have consistently been found to be effective in comparison to standard of care (parenteral opioids alone) in decreasing opioid use and improving patient's pain in the preoperative period.248,251,252,254,256,257 These results have been confirmed in multiple RCTs, and some of these studies are placebo controlled with blinded assessment of the outcome.252,253,257 Although there is high-quality evidence for these benefits of nerve blocks, instituting routine nerve blocks for hip fracture patients cannot be accomplished by the surgeon in isolation. System-wide changes in practice with involvement of other care providers (emergency medicine and anesthesia) are required.
There are other possible benefits of ED regional nerve blocks for hip fracture patients. One randomized controlled trial (RCT) found that these blocks decrease the incidence of delirium in hip fracture patients who are at an intermediate risk of this condition.257 Another RCT found a functional postoperative benefit in the hospital (walking distance and stair climbing ability) that lasted until 6 weeks after surgery.256 There is less strength of evidence for these benefits because they have only been assessed in 1 study each.
The nerve block technique has varied between studies. Some studies have used a 3-in-1 femoral nerve block (FNB), whereas others recommend a fascia iliaca block. Most studies recommend ultrasound guidance for either type of block.249,255 The fascia iliaca compartment block requires less precision and is probably more easily learned. The location is more remote from the neurovascular bundle and thus nearly eliminates the risk of intraarterial injection. Femoral nerve and fascia iliaca blocks have also been shown to have similar efficacy in TKA patients.250 Recommended training has been 30 minutes of didactic training, followed by variable periods of practice and supervised clinical performance. This short duration of training, however, may assume preexisting ultrasound skills.249,252
Five studies have compared “standard” preoperative MMA to the addition of a nerve block. Addition of an FNB to preoperative oxycontin and celecoxib did not make a difference in TKA patients.259 YaDeau et al,260 however, showed lower VAS pain scores with addition of an FNB to standard epidural anesthesia. Divella's group evaluated resting and dynamic VAS scores for 3 days after total hip arthroplasty. Pain control was oxycontin and acetaminophen versus continuous epidural levobupivacaine. Resting VAS scores between the 2 groups were similar for days 1 and 2, but VAS scores were significantly lower on day 3 for patients in the oxycontin group. Dynamic VAS scores for the oxycontin group were higher on day 1 and lower on day 3.261 The use of general anesthesia (GA) with preoperative oxycodone and celecoxib versus intrathecal bupivacaine, morphine, and clonidine showed higher pain scores, faster time to first rescue medication need, and longer length of stay in the GA group.262 Addition of multimodal postoperative pain medication (including oxycodone, tramadol, and ketorolac) compared with parenteral PCA showed less narcotic consumption, lower pain scores, and higher satisfaction and higher physical therapy goal achievement in the MMA group.263
The studies reviewed have not reported any complications of blocks, but most admit that the study was not powered to detect rare complications. Clinicians should be aware of the possibility of complications such as inadvertent intravascular injection, infection, intraneural injection, and masking symptoms of compartment syndrome.251 All studies report a rapid onset of pain relief from these blocks; however, the effect is often not complete, and adjunctive analgesics are often necessary.252
Intraoperatively and the Immediate Postoperative Period
• The panel recommends that clinicians consider local or regional block anesthesia during operative treatment of fractures and as part of the postoperative multimodality pain control regimen (strong recommendation, high-quality evidence).
• The panel recommends that if a block is going to be performed for intraoperative and postoperative pain control, a continuous catheter be considered over a single-shot block to better facilitate postoperative pain control and diminish rebound pain (conditional recommendation; moderate-quality evidence).
The use of peripheral anesthesia via local injections, field blocks, single-shot regional blocks, and indwelling catheter regional blocks have all been shown to decrease pain scores and opioid consumption in the immediate and short-term perioperative period. The bulk of these data comes from the arthroplasty literature with contributing articles from the sports medicine, foot and ankle, and trauma literature.264 The data outside the orthopaedic literature are even more robust. Problems with these lower extremity blocks include a possible increase in rate of falls and rebound pain that has been reported in some studies.
Five articles have compared various periarticular injections. Early postoperative pain scores and opioid usage were lower with continuous femoral nerve catheter plus sciatic block than with periarticular injection with ropivacaine or liposomal bupivacaine.265 Ng et al,266 however, found equivalent outcomes with femoral nerve catheter versus periarticular injection. In addition, periarticular injection alone was not superior to postoperative epidural analgesia for pain control.267 The addition of periarticular liposomal bupivacaine to a periarticular injection cocktail was more effective than ropivacaine at 6 and 12 hours postoperatively; however, intrathecal morphine was more effective at 6 hours.268 Addition of ropivacaine and ketorolac to a periarticular injection cocktail improved postoperative pain control.269
In 1 RCT, a significant decrease in opioid consumption and better pain scores was found at 48 hours after hip arthroscopy in patients who received an FNB versus GA. However, the FNB group had a significant increase in the rate of falls compared with the GA group, highlighting one of the risks of this type of anesthesia, which in part accounts for its moderate recommendation.270
In another RCT, the benefit of local injection was assessed. A significant decrease in pain scores and opioid consumption was found for 8 hours and trended less over 48 hours in patients receiving a local injection compared with GA alone for femur fractures. The injection (containing ropivacaine, morphine, and epinephrine) was administered at the time of surgical fixation of the fracture. There were no complications attributed to the local injection itself.197
Preoperative sciatic or popliteal continuous peripheral nerve block (CPNB) was compared with postoperative PCA in a retrospective study of patients undergoing fixation of talus and calcaneal fractures. Although Numerical Rating Scale pain scores, duration of stay, and side effects were equivalent in the 2 groups over 72 hours, morphine equivalent consumption on postoperative day 1 by the PCA patients was 30-fold that of the CPNB patients.271
A single-shot popliteal (SSP) block was compared with an intraoperative ankle block in an RCT of patients undergoing elective forefoot surgery. The length of block time in the popliteal block group was 44% longer than the ankle block group. Although the patient satisfaction and perceived effectiveness with both types of blocks were similar, the popliteal block group showed significantly lower VAS pain scores the night after surgery and throughout the next morning.272
In an RCT of patients undergoing open reduction and internal fixation of distal radius fractures, GA patients needed more IV pain medications in the post-anesthesia care unit compared with those who received a single-shot brachial plexus block. In the 12–24 hours after surgery, patients who received the block showed a more aggressive increase in VAS scores and narcotic use consistent with the block wearing off and the patients experiencing rebound pain. Ultimately, the GA group had a statistically significantly higher total narcotic use at 72 hours compared with the block group.273
Peripheral anesthesia in the form of a block can be administered either via a single-shot injection or by placing a catheter that has the ability to deliver anesthetic around the nerve in a continuous fashion until the catheter is removed. Rebound pain is the pain a patient experiences when the block wears off and can be quite significant. This is typically because the patient has not been taking other postoperative pain medications because of low pain scores during the duration that the block has been in effect.
Goldstein et al274 addressed the problem of rebound pain phenomenon and were one of the first groups to write about this effect. They compared an SSP block with GA in an RCT of patients undergoing fixation of ankle fractures. Significantly lower pain scores were reported for the block group at 2, 4, and 8 hours after surgery, but significantly better pain scores were found in the GA group from 8 to 24 hours.
There is some evidence that continuous catheters control pain for a longer duration of time and may help diminish rebound pain by allowing the patient to get farther in the recovery process. In 1 RCT, an SSP block was compared with a CPNB in patients undergoing fixation of unstable ankle fractures. The CPNB catheter was removed at 48 hours. Over the first 72 hours, patients in the CPNB group took significantly fewer oral narcotics and had lower pain scores.275 Another study of patients undergoing open fixation for calcaneal fractures compared controls (no regional blocks) versus a single-shot block or against a continuous popliteal nerve block. In the 36 hours after surgery, the patients in the continuous block used significantly fewer IV narcotics than did the other 2 groups. However, a limitation of this study was that their postoperative pain protocol changed multiple times during the course of the study.276
Remote (>3 Months) Postoperative Period
• The panel makes no recommendations for this period because we were unable to find any data to guide us on whether regional or local anesthesia performed before, during, or in the immediate postoperative period has any effect on improving pain scores or decreasing opioid consumption at this time frame (no recommendation, no evidence).
Pain/Sedation Assessment
Inpatient Pain Assessment
• The panel recommends regular assessment of pain for both inpatients and outpatients to evaluate the need for initiation or continuation of opioid therapy (strong recommendation, low-quality evidence).
Effective January 1, 2018, the Joint Commission required new and revised pain assessment and management standards to improve quality and safety of care.277 The requirements speak to (1) prioritization of pain assessment and management as an organizational priority, (2) establishment of medical staff in leadership roles to address performance improvement activities related to patient safety, (3) assessment and management of patient pain and minimization of risks associated with treatment with opioids, (4) data collection to monitor performance related to patient safety, and (5) compilation and analysis of data to inform continued performance improvement.
Inpatient Pain Assessment
• The panel recommends that sedation assessment be conducted by nursing staff on all inpatients before and after administration of an opioid medication (strong recommendation, low-quality evidence).
In 2012, the Joint Commission issued a warning regarding adverse drug events associated with opioid analgesics, most importantly respiratory depression, among patients in the inpatient hospital setting.278 The incidence of opioid-induced respiratory depression ranges from 0.1% to 37%.279 Nurses are typically the first to detect respiratory depression.280 One cause of opioid-related adverse events, however, is inadequate monitoring of patients administered opioids, occurring in about a third of cases.278,280 Patient monitoring includes sedation assessments, frequency and quality of respirations, and electronic methods such as pulse oximetry. A survey of nurses belonging to the American Society for Pain Management Nursing281 indicated that nurses find sedation scales and watching the patient to be more useful than electronic methods. However, although there is no evidence to inform the frequency of monitoring, sedation scale scores should be a major consideration in the decision to administer opioids for pain management. It is important to monitor sedation because it is an indicator of impending opioid-induced respiratory depression; detecting oversedation can prevent a more clinically significant adverse event. The Pasero282 Opioid-induced Sedation Scale283 (Table 5), which has been validated for assessing sedation during opioid administration,284 is an example of a tool that can be used by nurses to assess patients before and after administration of prescription opioids.
TABLE 5.
Pasero283 Opioid-Induced Sedation Scale With Intervention
Naloxone
• The panel recommends coprescribing of naloxone when factors that increase the risk of overdose are present (strong recommendation, low-quality evidence).
For patients prescribed opioids, risk mitigation strategies are an important consideration. Although limited evidence exists on the outcomes of prescribing naloxone in combination with opioids, distribution via community-based harm reduction programs has demonstrated a decreased risk of death due to opioid overdose.285–288 Most programs, however, have been conducted with illicit use populations with a focus on harm reduction as opposed to a patient safety focus for patients prescribed opioids for acute or chronic conditions. The Centers for Disease Control and Prevention Guideline for Prescribing Opioids for Chronic Pain25 recommends coprescribing or offering naloxone to patients with an increased risk of opioid overdose who are prescribed opioids. These risk factors include history of overdose or substance use disorder, opioid dosages ≥50 MME/d, or coprescribing with benzodiazepines.
System Strategies
Prescription Drug Monitoring Programs
• The panel recommends that all prescribers register to gain access to their state's Prescription Drug Monitoring Program (PDMP) and regularly query the PDMP before prescribing opioids (strong recommendation, low-quality evidence).
PDMPs are databases that track scheduled medications dispensed from pharmacies. The databases were developed to reduce prescription drug misuse and diversion. The conceptual model of PDMPs assumes that increased monitoring of opioid prescriptions is associated with changes in opioid prescribing behavior, opioid diversion and supply, and opioid-related morbidity and mortality.289 Numerous unintended consequences of PDMPs have been described in the literature and include the following: (1) potential decrease in legitimate prescribing, (2) patient privacy concerns, (3) inability to connect patients with known aberrant use to resources, (4) potential increase in illegal prescription drug activity or users switching to other substances such as heroin, (5) further reduced patient visit time due to time required to check PDMP, and (6) potential decrease in patient satisfaction ratings.290 Finally, PDMPs vary tremendously from state to state based on (1) the number of schedules included, (2) the frequency of updates, (3) housing entities, (4) accessibility, (5) access requirements, (6) reactive and proactive reporting, (7) associated prescriber education, and (8) interstate data sharing.290
Four reviews of PDMPs have been published to date,289–292 with the most recent one synthesizing articles published through 2015.289 Worley et al concluded that PDMPs were associated with lower substance abuse treatment admission rates, fewer opioid prescriptions, less diversion, and less “doctor shopping.” The authors acknowledge, however, that results depend on the specific components of each unique PDMP and that evidence is limited.291 Haegerich et al292 believe PDMPs to be effective, but that effect sizes from the articles they reviewed were generally very low and may depend on specific PDMP components such as mandatory review or proactive reporting. Gugelmann et al290 concluded that PDMPs seem to have benefits including reduced per capita supply of opioids and fewer incidents reported to poison control centers; however, there are also studies showing no effect. Finally, Finley et al289 found no consistent pattern, with efficacy varying by state.
Several articles on PDMP efficacy have been published since 2015, and the results have been mixed as well. The Florida PDMP was associated with a 25% decrease in oxycodone-caused deaths,293 but a multistate study found that PDMPs were not associated with reduction in overdose deaths and were, in fact, sometimes associated with increased mortality from nonprescription opioid drugs, such as heroin.294 There was also evidence of increased ED visits for heroin overdoses in New York, whereas visits for prescription opioid overdose leveled.295 In contrast, Dowell et al25 found “relatively large but statistically insignificant reductions” in heroin overdose deaths, indicating that perhaps a decrease in opioids does not lead to an increase in heroin use.
Three studies on PDMP implementation found no association with decreased opioid prescribing,296–298 whereas 3 others found that PDMP implementation reduced opioid prescriptions25,299,300 and overdose deaths.25 Some studies found PDMPs to be effective in specific groups, such as patients with multiple provider episodes (ie, “doctor shopping”) whose prescribers were sent an unsolicited report by the state,301 Medicare Part D enrollees,302 and Medicaid patients.299 Finally, because of the variability in PDMPs by state, 1 study rated the strength of the PDMP and found that a 1% increase in PDMP strength was associated with a 1% decrease in overdose deaths, indicating room for improvement in outcomes for PDMPs of lower strength.303
Although the literature remains inconsistent, PDMPs are a promising intervention, especially when the PDMPs are of robust strength. We recommend checking the PDMP before prescribing. Steps must be taken, however, to alleviate the potential consequences of curtailing prescribing based on the results of a PDMP search, particularly the potential for patients to switch to heroin. Therefore, we recommend referring patients to behavioral health and addiction medicine if the PDMP indicates aberrant behaviors. Furthermore, the evidence does demonstrate that PDMPs are not a panacea for preventing prescription opioid misuse, abuse, and diversion.
Prescriber and Patient Education
• The panel recommends that departments support opioid education efforts for prescribers and patients (strong recommendation, moderate-quality evidence).
Physicians often lack training in pain management and addiction; 59% of physicians report medical school preparation regarding chronic pain treatment as “fair” or “poor,”304 and median instruction time spent on pain education in US medical schools is 11.1 hours compared with 27.6 hours in Canada.305 After graduate medical education, only 5 states (CT, IA, MD, SC, and TN) require physicians to obtain periodic continuing medical education (CME) on prescribing, substance use disorders, or pain management.306
The effectiveness of educational interventions for physicians is strong. A synthesis of reviews on CME education finds that studies on CME interventions consistently show improvement in both physician performance and patient health outcomes.307 The most effective CME sessions are interactive, use multiple methods, involve multiple exposures, and are longer.307 After New Mexico began requiring CME in 2012–2013 about pain and addiction along with required PDMP registration and query, the state saw statistically significantly increased physician knowledge, self-efficacy, and attitudes, as well as a decrease in both statewide morphine milligram equivalents dispensed and drug overdose deaths.308 Online educational interventions have been moderately effective.309 Education in conjunction with clinical decision support is also effective at changing naloxone prescribing rates.310
Other strategies described in the literature include brief one-on-one physician education,311,312 development and dissemination of guidelines and policies,313,314 and Risk Evaluation and Mitigation Strategy.315 Public health detailing is an approach based on the pharmaceutical sales strategy, by which messages are pushed using brief one-on-one educational visits during the normal workflow. Staten Island saw a reduction in high-dose prescribing and stabilizing of days' supply after implementing this strategy.311 Similarly, an ED in Australia delivered one-on-one education via a clinical champion and was very effective at improving information given to patients, increasing notifications sent to general practitioners, reducing total dose prescribed, and incorporating nonopioid therapies.312 This approach is, however, resource intensive and has a limited scope of impact.
Development of department guidelines, policies, or both is another option. Hill et al described an intervention within surgical specialties at an academic medical center, which included dissemination of operation-specific opioid prescribing guidelines. This intervention significantly reduced the number of pills prescribed.313 When a similar approach was implemented in the ED setting, the number of patients prescribed opioids and number of pills prescribed decreased by 40% and 15%, respectively, with reductions sustained over 2.5 years.314 Finally, Risk Evaluation and Mitigation Strategies developed by the FDA in 2007 required pharmaceutical manufacturers to take steps to reduce risks associated with the medication. Strategies can include medication guides for patients, clinician education, and physician certification.316 Both immediate-release and extended-release opioids are now subject to these regulations.317 Thus, manufacturers are required to fund continuing education regarding opioid prescribing. Overall, the resulting SCOPE of Pain educational program has been shown to increase physician knowledge and reported intention to change practice.318 The SCOPE of Pain program has also implemented a “train the trainer” approach, which facilitates wide dissemination of information.315 Physicians are advised to be aware of potential conflicts of interest when attending pharmaceutical company–funded sessions.319
Overall, education is a necessary, but insufficient, approach to improving prescribing and patient outcomes. In addition, the literature is mostly limited to opioids for chronic pain management rather than acute or postsurgical pain. Regardless, we recommend supporting opioid education efforts both in graduate medical education and through continuing education.
Literature that focuses on evaluating the effects of patient education is limited, but the few studies conducted support effective patient education. Strategies included educational pamphlets,320–322 web-based interactive education,323 and clinician-delivered education.324,325 All interventions that included knowledge as an outcome demonstrated a significant effect,320,322,323,325 and many studies observed changes in risky behaviors, such as sharing pills,320,323 pill storage,320 saving and disposal of pills,320,321,323,324 driving,322 and taking more medication than prescribed.323
Clinical Decision Support
• The panel recommends that prescribers, to the extent possible, develop, support, or both the implementation of clinical decision support regarding opioid prescribing in the electronic medical record (strong recommendation, low-quality evidence).
We reviewed the literature on the impact of clinical informatics interventions on opioid prescribing. A total of 14 articles were identified that included prescribing outcomes, and the quality of the evidence was low. Most of the studies used study designs that did not have any concurrent control group. This is a significant weakness because of the national attention surrounding the opioid crisis currently in lay press, politics, and medicine. Without concurrent controls, the effects seen after implementation of these interventions could be overestimates if prescribing was already decreasing due to the current climate around opioids. There were, however, 2 randomized controlled trials that demonstrated an effect on some outcomes.326,327 Most of the 14 studies included patients in the ED326,328–331 or specifically for patients receiving chronic opioid therapy.327,332–334 Only 1 study assessed clinical decision support in an orthopaedic surgery population.335
There is a gap in the literature surrounding acute pain outside of the emergency department, other than after cesarean section336 and following hand surgery.335 This is an important area of research because a short course of opioid treatment for acute pain can often result in chronic opioid therapy.10
All these studies were conducted in urban settings or across a wide area including both urban and rural settings. It is critical to study these interventions in rural areas because they are substantially burdened with this epidemic.337 In addition, prescriber response to these interventions may differ in outlying hospitals and in practices that are not part of an academic hospital where prescribers are consistently exposed to new literature, new techniques, and other clinical innovations. In addition, numerous articles were identified that described clinical decision support regarding opioids but did not report on outcomes of the intervention.
Although these feasibility and implementation articles are important for fully describing interventions, decisions cannot be made regarding continuation, iterative improvement, or adoption of the intervention by another institution without evidence of efficacy. The lack of follow-up outcome articles could represent publication bias, whereby articles in the literature are more likely to have been effective. For example, only 1 study found no effect of the intervention,331 whereas the rest of the interventions were effective,328,329,332–334,336,338,339 or mixed (had effect on some outcomes but not all).326,327,330,335,340 Finally, most studies included outcomes associated with prescriptions (ie, number of prescriptions, number of pills, average dose, number of risky concurrent prescriptions for opioids with benzodiazepines, and number of extended-release prescriptions).326,328,330,331,335,336,338–340 Others measured outcomes associated with safe prescribing (ie, urine drug screens, treatment agreement, functional assessments, risk assessments, and documented diagnosis).327,329,332–334 The conceptual framework implicitly presented is that these interventions lead to safer prescribing practices that lead to fewer high-risk prescriptions that in turn ultimately reduce the risk of misuse, abuse, or diversion of prescription opioids. However, no studies measured rates of overdose, opioid use disorder, or other outcomes to demonstrate this pathway.
Despite the low-quality evidence, we strongly recommend pursuing clinical decision support to the extent possible. Potential approaches include power plans/order sets,331,335,340 dashboards,332,338,339 risk assessment and screening,327,329,333 alerts,326,328,330 and other decision support.334,336,339
Order set interventions could include recommended pain management regimens and dosing based on patient characteristics,340 prepopulating the dosing at a minimum rather than a range (ie, 1 pill 4× per day rather than 1–2 pills 4–6 times per day),335 and including nonopioid medication options.335
Dashboards are useful for tracking physician adherence to guidelines and protocols. They are particularly useful because they provide actionable information to the prescriber.341 For example, a prescriber can see what patients are due for a certain screening and conduct the appropriate screening at the patient's next visit. Dashboards can also promote transparency, accountability, and natural competition by which prescribers compare their statistics with those of their partners, leading to improved performance.342 Dashboards vary in the metrics tracked (eg, urine drug screens, pain agreements, functional status assessment, visits with behavioral health providers, high-dose opioids, and concurrent opioids and benzodiazepines).332,338 Dashboards also vary regarding the level of integration into workflow. Some are housed on the intranet for prescribers to access on demand,332 whereas others are “pushed” to prescribers at defined time intervals.332,338
Many risk assessment tools are accessible that indicate the risk of opioid abuse, misuse, and diversion. Available tools include the Opioid Risk Tool,343 the Screener and Opioid Assessment for Patients with Pain,344 the Drug Abuse Screening Test,345 the Brief Risk Interview,346 and the Current Opioid Misuse Measure.347 In addition, guidelines recommend that providers screen patients before prescribing opioids, although the Centers for Disease Control and Prevention guidelines caution against placing full confidence in the sensitivity and specificity of these screening tools because consequences of underestimation or overestimation of risk can be significant.348 An electronic risk assessment program called Pain Assessment Interview Network, Clinical Advisory System (PainCAS)327,333 is completed by the patient before their visit, either at home or on registration at the clinic, and includes the Screener and Opioid Assessment for Patients with Pain and Current Opioid Misuse Measure, both validated instruments. Once completed, administrative staff uploads the report to that patient's electronic medical record. Another electronic assessment is a short 3-item screening for tobacco, alcohol, and drug use that is programmed into the electronic triage tool in the ED.329 These studies report a significant increase in screening and documentation; however, their use does not seem to alter patient clinical outcomes.
Alerts were originally developed to reduce adverse drug events by alerting the provider to contraindications or allergies associated with medications.349–351 Since then, alerts have been developed for additional situations, including opioid risk. It is critical when developing alerts to ensure information is meaningful and does not trigger at unacceptable rates, thus causing “alert fatigue.”352 Alerts may include patient risk factors,328 suggest nonopioid medications or nonpharmaceutical modalities,328 inform the prescriber that the patient was referred to pain management,330 or inform the prescriber that the patient has an existing opioid care plan.326
Other examples of decision support implemented in the included articles include “smart set” documentation, a patient-facing tablet decision aid, and comprehensive prescribing tools. “Smart set” documentation standardizes practices by walking prescribers through the appropriate prescribing policies.334 Similarly, another study described implementation of a large set of decision aids into the electronic medical record as part of Safe and Appropriate Opioid Prescribing Program.339 Aids included medication menus, medication alerts, preferred and maximum doses, links to guidelines, prompts for alternative treatments and medications, patient treatment agreements, and a link to the PDMP. Finally, 1 article discussed a patient-facing decision aid in which patients used a tablet-based decision tool to learn about postcesarean pain and oxycodone to guide her in making decisions about the number of pills she wanted.336
These approaches are promising interventions to improve patient safety and reduce opioid prescribing. Many of these interventions included multiple components in addition to the electronic tool such as pocket cards, educational sessions, prescribing policies, care plans, and patient-facing pain policies.326,328,335,339,340 Although a multipronged intervention has a greater likelihood of success, it is challenging to identify the unique contribution of the electronic tool in each case.
CONCLUSIONS
Balancing comfort and patient safety following acute musculoskeletal injury is possible when using a true multimodal approach including cognitive, physical, and pharmaceutical strategies. In this document, we attempt to provide practical, evidence-based guidance for clinicians in both the operative and nonoperative settings to address acute pain from musculoskeletal injury. We also organized and graded the evidence to both support recommendations and identify gap areas for future research.
ACKNOWLEDGMENTS
The authors acknowledge the following individuals who helped in the development and preparation of these Clinical Practice Guidelines: Donald T. Kirkendall, ELS (a contracted medical editor).
APPENDIX 1. Members of the Orthopaedic Trauma Association Musculoskeletal Pain Task Force
Kristin R. Archer, PhD, DPT: Department of Physical Medicine and Rehabilitation, Vanderbilt University Medical Center, Nashville, TN. Basem Attum, MD: Department of Orthopaedic Surgery, University of Louisville School of Medicine, Louisville, KY. Chad Coles, MD: Department of Orthopaedic Surgery, Dalhousie University School of Medicine, Halifax, Nova Scotia, Canada. Jarrod Dumpe, MD: Department of Orthopaedic Surgery, Navicent Health, Macon, GA. Edward Harvey, MD: Division of Orthopaedic Surgery, McGill University Health Centre, Montreal, QC, Canada. Thomas Higgins, MD: Department of Orthopaedic Surgery, University of Utah, Salt Lake City, UT. Joseph Hoegler, MD: Department of Orthopaedic Surgery, Henry Ford Hospital; Detroit, MI. Jane Z. Liu, MD: Department of Orthopaedic Surgery, Case Western Reserve University, Cleveland, OH. Jason Lowe, MD: Department of Orthopaedics, Banner Health University of Arizona, Tucson, AZ. Christiaan Mamczak, DO: Orthopaedics and Sports Specialists, Beacon Health System; South Bend, IN. J. Lawrence Marsh, MD: Department of Orthopaedics and Rehabilitation, University of Iowa Health Care, Iowa City, IA. Anna N. Miller, MD: Division of Orthopaedic Trauma, Washington University Orthopaedics, St. Louis, MO. William Obremskey, MD: Orthopaedic Surgery and Rehabilitation, Vanderbilt University Medical Center, Nashville, TN. Michael Ransone, MD: Department of Orthopaedic Surgery, Carolinas Medical Center, Charlotte, NC. William Ricci, MD: Orthopaedic Trauma Service, Hospital For Special Surgery, New York City, NY. David Ring, MD: Institute of Reconstructive Plastic Surgery of Central Texas, Austin, TX. Babar Shafiq, MD: Department of Orthopaedic Surgery, Johns Hopkins School of Medicine, Baltimore, MD.
Footnotes
D. Ring reports royalties from Skeletal Dynamics and Wright Medical, Deputy Editor for Clinical Orthopaedics and Related Research and Journal of Orthopaedic Trauma, and expert testimony. K. R. Archer reports APTA, Palladian Health, Pacira, and NeuroPoint Alliance, Inc. C. Mamczak reports consulting from Smith & Nephew, speakers bureau for Smith & Nephew and AO North America, publishing royalties for Springer-Verlag and Lippincott, and Journal Review for Journal of Orthopaedic Trauma. T. Higgins reports consulting from DePuy Synthes and Imagen and stock ownership in SMV Holdings, OrthoGrid, and NT nPhase. B. Attum reports consulting for Synthes and research support from Arthrex. E. Harvey reports Editor in Chief of Canadian Journal of Surgery, Orthopaedic Trauma Association Basic Science Focus Forum Supplement, Editorial Board of OTA International, CMO of Greybox Solutions, Co-Founder Head of Medical Innovation for NXTSens Inc, Co-Founder CMO Chairman of Board of Directors of MY01 Inc., and Medical Device Advisor for Wavelite Inc. E. Harvey receives institutional support from J & J (Depuy Synthes) and Stryker and is a board/committee member of the Orthopaedic Trauma Association, Canadian Orthopaedic Association, and CIHR-IAB. J. Lowe reports consulting for Stryker. The remaining authors report no conflict of interest.
Members of the Orthopaedic Trauma Association Musculoskeletal Pain Task Force are listed in Appendix 1.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jorthotrauma.com).
REFERENCES
- 1.Rudd R, Aleshire N, Zibbell J, et al. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–1382. [DOI] [PubMed] [Google Scholar]
- 2.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Pocket guide: tapering opioids for chronic pain. Available at: https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed January 15, 2018.
- 4.Dowell D, Arias E, Kochanek K, et al. Contribution of opioid-involved poisoning to the change in life expectancy in the United States, 2000–2015. JAMA. 2017;318:1065–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Prescribing data. Available at: www.cdc.gov/drugoverdose/data/prescribing.html. Accessed September 20, 2017.
- 6.Volkow ND, McLellan TA, Cotto JH. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daubresse M, Chang H, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care. 2013;51:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim N, Matzon JL, Abboudi J, et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am. 2016;98:e89. [DOI] [PubMed] [Google Scholar]
- 10.Hooten WM, St Sauver JL, McGree ME, et al. Incidence and risk factors for progression from short-term to episodic or long-term opioid prescribing: a population-based study. Mayo Clin Proc. 2015;90:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braden J, Fan M, Edlund M, et al. Trends in use of opioids by noncancer pain type 2000–2005 among Arkansas Medicaid and HealthCore enrollees: results from the TROUP study. J Pain. 2008;9:1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin BC, Fan MY, Edlund MJ, et al. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med. 2011;26:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GRADE Working Group. The grading of recommendations assessment, development and evaluation. Available at: http://www.gradeworkinggroup.org/. Accessed October 15, 2017.
- 15.American Academy of Family Physicians. AAFP chronic pain management toolkit. Available at: https://www.aafp.org/dam/AAFP/documents/patient_care/pain_management/cpm-toolkit.pdf. Accessed August 22, 2018.
- 16.American Academy of Orthopaedic Surgeons. Pain relief toolkit. Available at: https://www.aaos.org/Quality/PainReliefToolkit/?ssopc=1. Accessed October 15, 2017.
- 17.American Academy of Pain Medicine. Use of opioids for the treatment of chronic pain: a statement from the American academy of pain medicine. Available at: http://www.painmed.org/files/use-of-opioids-for-the-treatment-of-chronic-pain.pdf. Accessed October 1, 2017.
- 18.American College of Obstetricians and Gynecologists. ACOG committee opinion summary. Opioid use and opioid use disorder in pregnancy. Available at: https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Opioid-Use-and-Opioid-Use-Disorder-in-Pregnancy. Accessed October 1, 2017.
- 19.American Society of Addiction Medicine. ASAM National practice guideline for the use of medications in the treatment of addiction involving opioid use. Available at: https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. Accessed October 1, 2017. [DOI] [PMC free article] [PubMed]
- 20.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American society of Anesthesiologists Task Force on acute pain management. Anesthesiology. 2012;116:248–273. [DOI] [PubMed] [Google Scholar]
- 21.American Society of Anesthesiologists Task Force on Neuraxial Opioids, American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration: an updated report by the American society of Anesthesiologists Task Force on neuraxial opioids and the American society of regional anesthesia and pain medicine. Anesthesiology. 2016;124:535–552. [DOI] [PubMed] [Google Scholar]
- 22.Busse JW, Guyatt G, Carrasco A, et al. The 2017 Canadian guideline for opioids for chronic non-cancer pain. Available at: http://nationalpaincentre.mcmaster.ca/documents/Opioid%20GL%20for%20CMAJ_01may2017.pdf. Accessed October 15, 2017.
- 23.Centers for Disease Control and Prevention. Calculating total daily dose of opioids for safer dosage. Available at: https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf. Accessed August 22, 2018.
- 24.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowell D, Haegerich T, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 26.Federation of State Medical Boards of the United States I. Model policy on the use of opioid analgesics in the treatment of chronic pain. Available at: https://www.fsmb.org/Media/Default/PDF/Advocacy/Opioid%20Guidelines%20As%20Adopted%20April%202017_FINAL.pdf. Accessed October 1, 2017.
- 27.Federation of State Medical Boards of the United States I. Guidelines for the chronic use of opioid analgesics. Available at: https://www.fsmb.org/Media/Default/PDF/Advocacy/Opioid%20Guidelines%20As%20Adopted%20April%202017_FINAL.pdf. Accessed June 1, 2017.
- 28.Hauk L. Management of chronic pain and opioid misuse: a position paper from the AAFP. Am Fam Physician. 2017;95:458–459. [PubMed] [Google Scholar]
- 29.ICSI Patient Advisory Council. Pain: assessment, non-opioid treatment approaches and opioid management guideline (2016). Available at: https://www.icsi.org/_asset/ypv5rn/2016painsealevidence.pdf. Accessed October 1, 2017.
- 30.Intermountain Healthcare. Clinical guideline: acute pain opioid prescribing guidelines. Available at: https://intermountainphysician.org/Documents/AcutePainOpioidPrescribing_FINAL.pdf. Accessed October 1, 2017.
- 31.Massachusetts Medical Society. Opioid therapy and physician communication guidelines. Available at: http://www.massmed.org/opioid-guidelines/#.W43RqH4nbiw. Accessed October 1, 2017.
- 32.American Society of Anesthesiologists Task Force on Neuraxial Opioids. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218–230. [DOI] [PubMed] [Google Scholar]
- 33.Oregon Pain Guidance Group. Oregon acute pain flow sheet for the evaluation and treatment of acute pain. Available at: https://www.oregonpainguidance.org/app/content/uploads/2016/05/Acute-and-Chronic-Pain-flow-sheets.pdf. Accessed August 22, 2018.
- 34.Pennsylvania Orthopaedic Society. Opioid recommendations for acute pain. Available at: https://www.paorthosociety.org/resources/Documents/POS%20Opioid%20Statement%20and%20Recommendations%20Final.pdf. Accessed August 28, 2018.
- 35.Substance Abuse and Mental Health Services Administration. SAMHSA opioid overdose prevention toolkit. Available at: https://store.samhsa.gov/shin/content//SMA16-4742/SMA16-4742.pdf. Accessed October 1, 2017.
- 36.U.S. Department of Veterans Affairs. Pain management opioid safety educational guide. Available at: https://www.va.gov/PAINMANAGEMENT/docs/OSI_1_Toolkit_Pain_Educational_Guide.pdf. Accessed October 1, 2017.
- 37.U.S. Department of Veterans Affairs. Pain management opioid taper decision tool: a VA clinician's guide. Available at: https://www.pbm.va.gov/AcademicDetailingService/Documents/Pain_Opioid_Taper_Tool_IB_10_939_P96820.pdf. Accessed October 1, 2017.
- 38.Washington State Agency Medical Directors' Group. Interagency guideline on prescribing opioids for pain. Available at: http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed October 1, 2017.
- 39.World Health Organization. Scoping document for WHO guidelines for the pharmacological treatment of persisting pain in adults with medical illnesses. Available at: http://www.who.int/medicines/areas/quality_safety/Scoping_WHO_GLs_PersistPainAdults_webversion.pdf. Accessed October 1, 2017. [PubMed]
- 40.Menendez ME, Ring D. Factors associated with greater pain intensity. Hand Clin. 2016;32:27–31. [DOI] [PubMed] [Google Scholar]
- 41.Golkari S, Teunis T, Ring D, et al. Changes in depression, health anxiety, and pain catastrophizing between enrollment and 1 Month after a radius fracture. Psychosomatics. 2015;56:652–657. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- 43.Archer KR, Castillo RC, Wegener ST, et al. Pain and satisfaction in hospitalized trauma patients: the importance of self-efficacy and psychological distress. J Trauma Acute Care Surg. 2012;72:1068–1077. [DOI] [PubMed] [Google Scholar]
- 44.Bot AG, Bekkers S, Arnstein PM, et al. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472:2542–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nota SP, Spit SA, Voskuyl T, et al. Opioid use, satisfaction, and pain intensity after orthopedic surgery. Psychosomatics. 2015;56:479–485. [DOI] [PubMed] [Google Scholar]
- 46.Bot AG, Bekkers S, Herndon JH, et al. Determinants of disability after proximal interphalangeal joint sprain or dislocation. Psychosomatics. 2014;55:595–601. [DOI] [PubMed] [Google Scholar]
- 47.Briet JP, Houwert RM, Hageman M, et al. Factors associated with pain intensity and physical limitations after lateral ankle sprains. Injury. 2016;47:2565–2569. [DOI] [PubMed] [Google Scholar]
- 48.Das De S, Vranceanu AM, Ring DC. Contribution of kinesophobia and catastrophic thinking to upper-extremity-specific disability. J Bone Joint Surg Am. 2013;95:76–81. [DOI] [PubMed] [Google Scholar]
- 49.Farzad M, Asgari A, Dashab F, et al. Does disability correlate with impairment after hand injury? Clin Orthop Relat Res. 2015;473:3470–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finger A, Teunis T, Hageman MG, et al. Association between opioid intake and disability after surgical management of ankle fractures. J Am Acad Orthop Surg. 2017;25:519–526. [DOI] [PubMed] [Google Scholar]
- 51.Kadzielski JJ, Bot AG, Ring D. The influence of job satisfaction, burnout, pain, and worker's compensation status on disability after finger injuries. J Hand Surg Am. 2012;37:1812–1819. [DOI] [PubMed] [Google Scholar]
- 52.Teunis T, Bot AG, Thornton ER, et al. Catastrophic thinking is associated with finger stiffness after distal radius fracture surgery. J Orthop Trauma. 2015;29:e414–420. [DOI] [PubMed] [Google Scholar]
- 53.Vranceanu A, Bachoura A, Weening A, et al. Psychological factors predict disability and pain intensity after skeletal trauma. J Bone Joint Surg Am. 2014;96:e20. [DOI] [PubMed] [Google Scholar]
- 54.Carragee EJ, Vittum D, Truong TP, et al. Pain control and cultural norms and expectations after closed femoral shaft fractures. Am J Orthop (Belle Mead NJ). 1999;28:97–102. [PubMed] [Google Scholar]
- 55.Helmerhorst GT, Lindenhovius AL, Vrahas M, et al. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43:1958–1961. [DOI] [PubMed] [Google Scholar]
- 56.Helmerhorst GT, Vranceanu AM, Vrahas M, et al. Risk factors for continued opioid use one to two months after surgery for musculoskeletal trauma. J Bone Joint Surg Am. 2014;96:495–499. [DOI] [PubMed] [Google Scholar]
- 57.Lindenhovius AL, Helmerhorst GT, Schnellen AC, et al. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and The Netherlands. J Trauma. 2009;67:160–164. [DOI] [PubMed] [Google Scholar]
- 58.Archer KR, Abraham CM, Obremskey WT. Psychosocial factors predict pain and physical health after lower extremity trauma. Clin Orthop Relat Res. 2015;473:3519–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Archer KR, Abraham CM, Song Y, et al. Cognitive-behavioral determinants of pain and disability two years after traumatic injury: a cross-sectional survey study. J Trauma Acute Care Surg. 2012;72:473–479. [DOI] [PubMed] [Google Scholar]
- 60.Archer KR, Heins SE, Abraham CM, et al. Clinical significance of pain at hospital discharge following traumatic orthopedic injury: general health, depression, and PTSD outcomes at 1 year. Clin J Pain. 2016;32:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castillo R, MacKenzie E, Wegener S, et al. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. [DOI] [PubMed] [Google Scholar]
- 62.Castillo R, Wegener S, Heins S, et al. Longitudinal relationships between anxiety, depression, and pain: results from a two-year cohort study of lower extremity trauma patients. Pain. 2013;154:2860–2866. [DOI] [PubMed] [Google Scholar]
- 63.Clay FJ, Watson WL, Newstead SV, et al. A systematic review of early prognostic factors for persisting pain following acute orthopedic trauma. Pain Res Manag. 2012;17:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crichlow RJ, Andres PL, Morrison SM, et al. Depression in orthopaedic trauma patients. Prevalence and severity. J Bone Joint Surg Am. 2006;88:1927–1933. [DOI] [PubMed] [Google Scholar]
- 65.Edwards RR, Dworkin RH, Sullivan MD, et al. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17:T70–T92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gopinath B, Jagnoor J, Nicholas M, et al. Presence and predictors of persistent pain among persons who sustained an injury in a road traffic crash. Eur J Pain. 2015;19:1111–1118. [DOI] [PubMed] [Google Scholar]
- 67.Hanley MA, Jensen MP, Ehde DM, et al. Psychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb pain. Disabil Rehabil. 2004;26:882–893. [DOI] [PubMed] [Google Scholar]
- 68.McCarthy ML, MacKenzie EJ, Edwin D, et al. Psychological distress associated with severe lower-limb injury. J Bone Joint Surg Am. 2003;85-A:1689–1697. [DOI] [PubMed] [Google Scholar]
- 69.Nota SP, Bot AG, Ring D, et al. Disability and depression after orthopaedic trauma. Injury. 2015;46:207–212. [DOI] [PubMed] [Google Scholar]
- 70.Ponsford J, Hill B, Karamitsios M, et al. Factors influencing outcome after orthopedic trauma. J Trauma. 2008;64:1001–1009. [DOI] [PubMed] [Google Scholar]
- 71.Schweininger S, Forbes D, Creamer M, et al. The temporal relationship between mental health and disability after injury. Depress Anxiety. 2015;32:64–71. [DOI] [PubMed] [Google Scholar]
- 72.Soberg HL, Bautz-Holter E, Roise O, et al. Mental health and posttraumatic stress symptoms 2 years after severe multiple trauma: self-reported disability and psychosocial functioning. Arch Phys Med Rehabil. 2010;91:481–488. [DOI] [PubMed] [Google Scholar]
- 73.van Leeuwen WF, van der Vliet QM, Janssen SJ, et al. Does perceived injustice correlate with pain intensity and disability in orthopaedic trauma patients? Injury. 2016;47:1212–1216. [DOI] [PubMed] [Google Scholar]
- 74.Warren AM, Foreman ML, Bennett MM, et al. Posttraumatic stress disorder following traumatic injury at 6 months: associations with alcohol use and depression. J Trauma Acute Care Surg. 2014;76:517–522. [DOI] [PubMed] [Google Scholar]
- 75.Wegener S, Castillo R, Haythornthwaite J, et al. Psychological distress mediates the effect of pain on function. Pain. 2011;152:1349–1357. [DOI] [PubMed] [Google Scholar]
- 76.Williams AE, Newman JT, Ozer K, et al. Posttraumatic stress disorder and depression negatively impact general health status after hand injury. J Hand Surg Am. 2009;34:515–522. [DOI] [PubMed] [Google Scholar]
- 77.Zatzick DF, Jurkovich GJ, Fan MY, et al. Association between posttraumatic stress and depressive symptoms and functional outcomes in adolescents followed up longitudinally after injury hospitalization. Arch Pediatr Adolesc Med. 2008;162:642–648. [DOI] [PubMed] [Google Scholar]
- 78.Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Washington, DC: IASP Press; 1994. [Google Scholar]
- 79.Andersson AL, Dahlback LO, Bunketorp O. Psychosocial aspects of road traffic trauma—benefits of an early intervention? Injury. 2005;36:917–926. [DOI] [PubMed] [Google Scholar]
- 80.Berube M, Choiniere M, Laflamme YG, et al. Acute to chronic pain transition in extremity trauma: a narrative review for future preventive interventions (part 1). Int J Orthop Trauma Nurs. 2016;23:47–59. [DOI] [PubMed] [Google Scholar]
- 81.Berube M, Choiniere M, Laflamme YG, et al. Acute to chronic pain transition in extremity trauma: a narrative review for future preventive interventions (part 2). Int J Orthop Trauma Nurs. 2017;24:59–67. [DOI] [PubMed] [Google Scholar]
- 82.Berube M, Gelinas C, Martorella G, et al. A hybrid web-based and in-person self-management intervention to prevent acute to chronic pain transition after major lower extremity trauma (iPACT-E-Trauma): protocol for a pilot single-blind randomized controlled trial. JMIR Res Protoc. 2017;6:e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bisson JI, Shepherd JP, Joy D, et al. Early cognitive-behavioural therapy for post-traumatic stress symptoms after physical injury. Randomised controlled trial. Br J Psychiatry. 2004;184:63–69. [DOI] [PubMed] [Google Scholar]
- 84.Campbell L, Kenardy J, Andersen T, et al. Trauma-focused cognitive behaviour therapy and exercise for chronic whiplash: protocol of a randomised, controlled trial. J Physiother. 2015;61:218. [DOI] [PubMed] [Google Scholar]
- 85.Castillo RC, Raja SN, Frey KP, et al. Improving pain management and long-term outcomes following high-energy orthopaedic trauma (pain study). J Orthop Trauma. 2017;31(suppl 1):S71–s77. [DOI] [PubMed] [Google Scholar]
- 86.Chad-Friedman E, Talaei-Khoei M, Ring D, et al. First use of a brief 60-second mindfulness exercise in an orthopedic surgical practice; results from a pilot study. Arch Bone Jt Surg. 2017;5:400–405. [PMC free article] [PubMed] [Google Scholar]
- 87.De Silva M, Maclachlan M, Devane D, et al. Psychosocial interventions for the prevention of disability following traumatic physical injury. Cochrane Database Syst Rev. 2009;Cd006422. 10.1002/14651858.CD006422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goudie S, Dixon D, McMillan G, et al. Is use of a psychological workbook associated with improved disabilities of the arm, shoulder and hand scores in patients with distal radius fracture? Clin Orthop Relat Res. 2018;476:832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holmes A, Hodgins G, Adey S, et al. Trial of interpersonal counselling after major physical trauma. Aust N. Z J Psychiatry. 2007;41:926–933. [DOI] [PubMed] [Google Scholar]
- 90.Pirente N, Blum C, Wortberg S, et al. Quality of life after multiple trauma: the effect of early onset psychotherapy on quality of life in trauma patients. Langenbecks Arch Surg. 2007;392:739–745. [DOI] [PubMed] [Google Scholar]
- 91.Turpin G, Downs M, Mason S. Effectiveness of providing self-help information following acute traumatic injury: randomised controlled trial. Br J Psychiatry. 2005;187:76–82. [DOI] [PubMed] [Google Scholar]
- 92.Vranceanu A, Hageman M, Strooker J, et al. A preliminary RCT of a mind body skills based intervention addressing mood and coping strategies in patients with acute orthopaedic trauma. Injury. 2015;46:552–557. [DOI] [PubMed] [Google Scholar]
- 93.Zatzick D, Roy-Byrne P, Russo J, et al. A randomized effectiveness trial of stepped collaborative care for acutely injured trauma survivors. Arch Gen Psychiatry. 2004;61:498–506. [DOI] [PubMed] [Google Scholar]
- 94.Zatzick DF, Roy-Byrne P, Russo JE, et al. Collaborative interventions for physically injured trauma survivors: a pilot randomized effectiveness trial. Gen Hosp Psychiatry. 2001;23:114–123. [DOI] [PubMed] [Google Scholar]
- 95.Ong AD, Zautra AJ, Reid MC. Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychol Aging. 2010;25:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh MV, Armstrong TW, Poritz J, et al. Resilience, pain interference, and upper limb loss: testing the mediating effects of positive emotion and activity restriction on distress. Arch Phys Med Rehabil. 2016;97:781–787. [DOI] [PubMed] [Google Scholar]
- 97.Eccleston C, Fisher E, Craig L, et al. Psychological therapies (Internet-delivered) for the management of chronic pain in adults. Cochrane Database Syst Rev. 2014;Cd010152. 10.1002/14651858.CD010152.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Macea DD, Gajos K, Daglia Calil YA, et al. The efficacy of web-based cognitive behavioral interventions for chronic pain: a systematic review and meta-analysis. J Pain. 2010;11:917–929. [DOI] [PubMed] [Google Scholar]
- 99.Palermo TM, Eccleston C, Lewandowski AS, et al. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bradt J, Dileo C, Potvin N. Music for stress and anxiety reduction in coronary heart disease patients. Cochrane Database Syst Rev. 2013;Cd006577. 10.1002/14651858.CD006577.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee YL, Wu Y, Tsang HW, et al. A systematic review on the anxiolytic effects of aromatherapy in people with anxiety symptoms. J Altern Complement Med. 2011;17:101–108. [DOI] [PubMed] [Google Scholar]
- 102.Lakhan SE, Sheafer H, Tepper D. The effectiveness of aromatherapy in reducing pain: a systematic review and meta-analysis. Pain Res Treat. 2016;2016:8158693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17:131–157. [DOI] [PubMed] [Google Scholar]
- 104.Bjordal J, Johnson M, Ljunggreen A. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. [DOI] [PubMed] [Google Scholar]
- 105.Tedesco D, Gori D, Desai KR, et al. Drug-free interventions to reduce pain or opioid consumption after total knee arthroplasty: a systematic review and meta-analysis. JAMA Surg. 2017;152:e172872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rakel BA, Zimmerman MB, Geasland K, et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo-controlled trial. Pain. 2014;155:2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahure SA, Rokito AS, Kwon YW. Transcutaneous electrical nerve stimulation for postoperative pain relief after arthroscopic rotator cuff repair: a prospective double-blinded randomized trial. J Shoulder Elbow Surg. 2017;26:1508–1513. [DOI] [PubMed] [Google Scholar]
- 108.Hamza MA, White PF, Ahmed HE, et al. Effect of the frequency of transcutaneous electrical nerve stimulation on the postoperative opioid analgesic requirement and recovery profile. Anesthesiology. 1999;91:1232–1238. [DOI] [PubMed] [Google Scholar]
- 109.Benedetti F, Amanzio M, Casadio C, et al. Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Ann Thorac Surg. 1997;63:773–776. [DOI] [PubMed] [Google Scholar]
- 110.Zeng C, Li H, Yang T, et al. Electrical stimulation for pain relief in knee osteoarthritis: systematic review and network meta-analysis. Osteoarthritis Cartilage. 2015;23:189–202. [DOI] [PubMed] [Google Scholar]
- 111.Deal D, Tipton J, Rosencrance E, et al. Ice reduces edema: a study of microvascular permeability in rats. J Bone Joint Surg. 2002;84-A:1573–1578. [PubMed] [Google Scholar]
- 112.Schaser KD, Stover JF, Melcher I, et al. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61:642–649. [DOI] [PubMed] [Google Scholar]
- 113.Kenjo T, Kikuchi S, Konno S. Cooling decreases fos-immunoreactivity in the rat after formalin injection. Clin Orthop Rel Res. 2002;394:271–277. [DOI] [PubMed] [Google Scholar]
- 114.Schaser KD, Disch AC, Stover JF, et al. Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med. 2007;35:93–102. [DOI] [PubMed] [Google Scholar]
- 115.Scumpia PO, Sarcia PJ, Kelly KM, et al. Hypothermia induces anti-inflammatory cytokines and inhibits nitric oxide and myeloperoxidase-mediated damage in the hearts of endotoxemic rats. Chest. 2004;125:1483–1491. [DOI] [PubMed] [Google Scholar]
- 116.Stalman A, Berglund L, Dungnerc E, et al. Temperature-sensitive release of prostaglandin E(2) and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93:1961–1968. [DOI] [PubMed] [Google Scholar]
- 117.Ho S, Coel M, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537–540. [DOI] [PubMed] [Google Scholar]
- 118.Ho S, Illgen R, Meyer R, et al. Comparison of various icing times in decreasing bone metabolism and blood flow in the knee. Am J Sports Med. 1995;23:74–76. [DOI] [PubMed] [Google Scholar]
- 119.Knobloch K, Grasemann R, Jagodzinski M, et al. Changes of Achilles midportion tendon microcirculation after repetitive simultaneous cryotherapy and compression using a Cryo/Cuff. Am J Sports Med. 2006;34:1953–1959. [DOI] [PubMed] [Google Scholar]
- 120.White GE, Wells GD. Cold-water immersion and other forms of cryotherapy: physiological changes potentially affecting recovery from high-intensity exercise. Extrem Physiol Med. 2013;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Algafly A, George K. The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med. 2007;41:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adie S, Kwan A, Naylor J, et al. Cryotherapy following total knee replacement. Cochrane Database Syst Rev. 2012;CD007911. 10.1002/14651858.CD007911.pub2. [DOI] [PubMed] [Google Scholar]
- 123.Brandsson S, Rydgren B, Hedner T, et al. Postoperative analgesic effects of an external cooling system and intra-articular bupivacaine/morphine after arthroscopic cruciate ligament surgery. Knee Surg Sports Traumatol Arthrosc. 1996;4:200–205. [DOI] [PubMed] [Google Scholar]
- 124.Kuyucu E, Bulbul M, Kara A, et al. Is cold therapy really efficient after knee arthroplasty? Ann Med Surg (Lond). 2015;4:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Levy A, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Rel Res. 1993;297:174–178. [PubMed] [Google Scholar]
- 126.Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17:718–722. [DOI] [PubMed] [Google Scholar]
- 127.Ohkoshi Y, Ohkoshi M, Nagasaki S, et al. The effect of cryotherapy on intraarticular temperature and postoperative care after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:357–362. [DOI] [PubMed] [Google Scholar]
- 128.Raynor MC, Pietrobon R, Guller U, et al. Cryotherapy after ACL reconstruction: a meta-analysis. J Knee Surg. 2005;18:123–129. [DOI] [PubMed] [Google Scholar]
- 129.Saito N, Horiuchi H, Kobayashi S, et al. Continuous local cooling for pain relief following total hip arthroplasty. J Arthroplasty. 2004;19:334–337. [DOI] [PubMed] [Google Scholar]
- 130.Singh H, Osbahr D, Holovacs T, et al. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective, randomized investigation. J Shoulder Elbow Surg. 2001;10:522–525. [DOI] [PubMed] [Google Scholar]
- 131.Speer K, Warren R, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5:62–68. [DOI] [PubMed] [Google Scholar]
- 132.Webb JM, Williams D, Ivory JP, et al. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998;21:59–61. [DOI] [PubMed] [Google Scholar]
- 133.Barber F, McGuire D, Click S. Continuous-flow cold therapy for outpatient anterior cruciate ligament reconstruction. Arthroscopy. 1998;14:130–135. [DOI] [PubMed] [Google Scholar]
- 134.Daniel DM, Stone ML, Arendt DL. The effect of cold therapy on pain, swelling, and range of motion after anterior cruciate ligament reconstructive surgery. Arthroscopy. 1994;10:530–533. [DOI] [PubMed] [Google Scholar]
- 135.Gibbons C, Solan M, Ricketts D. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25:250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kullenberg B, Ylipaa S, Soderlund K, et al. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21:1175–1179. [DOI] [PubMed] [Google Scholar]
- 137.Wittig-Wells D, Johnson I, Samms-McPherson J, et al. Does the use of a brief cryotherapy intervention with analgesic administration improve pain management after total knee arthroplasty? Orthop Nurs. 2015;34:148–153. [DOI] [PubMed] [Google Scholar]
- 138.Holmstrom A, Hardin BC. Cryo/Cuff compared to epidural anesthesia after knee unicompartmental arthroplasty: a prospective, randomized and controlled study of 60 patients with a 6-week follow-up. J Arthroplasty. 2005;20:316–321. [DOI] [PubMed] [Google Scholar]
- 139.Dervin GF, Taylor DE, Keene GC. Effects of cold and compression dressings on early postoperative outcomes for the arthroscopic anterior cruciate ligament reconstruction patient. J Orthop Sports Phys Ther. 1998;27:403–406. [DOI] [PubMed] [Google Scholar]
- 140.Edwards DJ, Rimmer M, Keene GC. The use of cold therapy in the postoperative management of patients undergoing arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med. 1996;24:193–195. [DOI] [PubMed] [Google Scholar]
- 141.Walker RH, Morris BA, Angulo DL, et al. Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation, and continuous cooling pad following total knee arthroplasty. J Arthroplasty. 1991;6:151–156. [DOI] [PubMed] [Google Scholar]
- 142.Bech M, Moorhen J, Cho M, et al. Device or ice: the effect of consistent cooling using a device compared with intermittent cooling using an ice bag after total knee arthroplasty. Physiother Can. 2015;67:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Demoulin C, Brouwers M, Darot S, et al. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55:229–240. [DOI] [PubMed] [Google Scholar]
- 144.Desteli EE, Imren Y, Aydin N. Effect of both preoperative andpostoperative cryoceutical treatment on hemostasis and postoperative pain following total knee arthroplasty. Int J Clin Exp Med. 2015;8:19150–19155. [PMC free article] [PubMed] [Google Scholar]
- 145.Kraeutler MJ, Reynolds KA, Long C, et al. Compressive cryotherapy versus ice-a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24:854–859. [DOI] [PubMed] [Google Scholar]
- 146.Ruffilli A, Castagnini F, Traina F, et al. Temperature-controlled continuous cold flow device after total knee arthroplasty: a randomized controlled trial study. J Knee Surg. 2017;30:675–681. [DOI] [PubMed] [Google Scholar]
- 147.Smith J, Stevens J, Taylor M, et al. A randomized, controlled trial comparing compression bandaging and cold therapy in postoperative total knee replacement surgery. Orthop Nurs. 2002;21:61–66. [DOI] [PubMed] [Google Scholar]
- 148.Su E, Perna M, Boettner F, et al. A prospective, multi-center, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94-B:153–156. [DOI] [PubMed] [Google Scholar]
- 149.Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty? Clin Orthop Rel Res. 2014;472:3417–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whitelaw GP, DeMuth KA, Demos HA, et al. The use of the Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8:28–30; discussion 30–21. [PubMed] [Google Scholar]
- 151.Barber FA. A comparison of crushed ice and continuous flow cold therapy. Am J Knee Surg. 2000;13:97–101; discussion 102. [PubMed] [Google Scholar]
- 152.Meyer-Marcotty M, Jungling O, Vaske B, et al. Standardized combined cryotherapy and compression using Cryo/Cuff after wrist arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:314–319. [DOI] [PubMed] [Google Scholar]
- 153.Schinsky M, McCune C, Bonomi J. Multifaceted comparison of two cryotherapy devices used after total knee arthroplasty: cryotherapy device comparison. Orthop Nurs. 2016;35:309–316. [DOI] [PubMed] [Google Scholar]
- 154.Schroder D, Passler H. Combination of cold and compression after knee surgery. A prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 1994;2:158–165. [DOI] [PubMed] [Google Scholar]
- 155.Song M, Sun X, Tian X, et al. Compressive cryotherapy versus cryotherapy alone in patients undergoing knee surgery: a meta-analysis. Springerplus. 2016;5:1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cohn B, Draeger R, Jackson D. The effects of cold therapy in the postoperative management of pain in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17:344–349. [DOI] [PubMed] [Google Scholar]
- 157.Healy W, Seidman J, Pfeifer B, et al. Cold compressive dressing after total knee arthroplasty. Clin Orthop Rel Res. 1994;299:143–146. [PubMed] [Google Scholar]
- 158.Konrath GA, Lock T, Goitz HT, et al. The use of cold therapy after anterior cruciate ligament reconstruction. A prospective, randomized study and literature review. Am J Sports Med. 1996;24:629–633. [DOI] [PubMed] [Google Scholar]
- 159.Woolf S, Barfield W, Merrill K, et al. Comparison of a continuous temperature-controlled cryotherapy device to a simple icing regimen following outpatient knee arthroscopy. J Knee Surg. 2008;21:15–19. [DOI] [PubMed] [Google Scholar]
- 160.Bassett F, Kirkpatrick J, Engelhardt D, et al. Cryotherapy-induced nerve injury. Am J Sports Med. 1992;20:516–518. [DOI] [PubMed] [Google Scholar]
- 161.Moeller J, Monroe J, McKeag D. Cryotherapy-induced common peroneal Nerve palsy. Clin J Sport Med. 1997;7:212–216. [DOI] [PubMed] [Google Scholar]
- 162.Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res. 2013;6:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gimbel J, Ahdieh H. The efficacy and safety of oral immediate-release oxymorphone for postsurgical pain. Anesth Analg. 2004;99:1472–1477. [DOI] [PubMed] [Google Scholar]
- 164.Iwanicki JL, Severtson SG, McDaniel H, et al. Abuse and diversion of immediate release opioid analgesics as compared to extended release formulations in the United States. PLoS One. 2016;11:e0167499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459. [DOI] [PubMed] [Google Scholar]
- 166.Amabile CM, Bowman BJ. Overview of oral modified-release opioid products for the management of chronic pain. Ann Pharmacother. 2006;40:1327–1335. [DOI] [PubMed] [Google Scholar]
- 167.de Beer JdV, Winemaker MJ, Donnelly GA, et al. Efficacy and safety of controlled-release oxycodone and standard therapies for postoperative pain after knee or hip replacement. Can J Surg. 2005;48:277. [PMC free article] [PubMed] [Google Scholar]
- 168.Aqua K, Gimbel JS, Singla N, et al. Efficacy and tolerability of oxymorphone immediate release for acute postoperative pain after abdominal surgery: a randomized, double-blind, active-and placebo-controlled, parallel-group trial. Clin Ther. 2007;29:1000–1012. [DOI] [PubMed] [Google Scholar]
- 169.Hale ME, Fleischmann R, Salzman R, et al. Efficacy and safety of controlled-release versus immediate-release oxycodone: randomized, double-blind evaluation in patients with chronic back pain. Clin J Pain. 1999;15:179–183. [DOI] [PubMed] [Google Scholar]
- 170.Kaplan R, Parris W, Citron ML, et al. Comparison of controlled-release and immediate-release oxycodone tablets in patients with cancer pain. J Clin Oncol. 1998;16:3230–3237. [DOI] [PubMed] [Google Scholar]
- 171.Wen W, Taber L, Lynch S, et al. 12-Month safety and effectiveness of once-daily hydrocodone tablets formulated with abuse-deterrent properties in patients with moderate to severe chronic pain. J Opioid Manag. 2015;11:339–356. [DOI] [PubMed] [Google Scholar]
- 172.Singla N, Pong A, Newman K, et al. Combination oxycodone 5 mg/ibuprofen 400 mg for thetreatment of pain after abdominal or pelvic surgery in women: a randomized, double-blind, placebo-and active-controlled parallel-group study. Clin Ther. 2005;27:45–57. [DOI] [PubMed] [Google Scholar]
- 173.Devarakonda K, Morton T, Margulis R, et al. Pharmacokinetics and bioavailability of oxycodone and acetaminophen following single-dose administration of MNK-795, a dual-layer biphasic IR/ER combination formulation, under fed and fasted conditions. Drug Des Devel Ther. 2014;8:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wojahn RD, Bogunovic L, Brophy RH, et al. Opioid consumption after knee arthroscopy. J Bone Joint Surg Am. 2018;100:1629–1636. [DOI] [PubMed] [Google Scholar]
- 175.Rodgers J, Cunningham K, Fitzgerald K, et al. Opioid consumption following outpatient upper extremity surgery. J Hand Surg. 2012;37:645–650. [DOI] [PubMed] [Google Scholar]
- 176.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mildh LH, Scheinin H, Kirvelä OA. The concentration-effect relationship of the respiratory depressant effects of alfentanil and fentanyl. Anesth Analg. 2001;93:939–946. [DOI] [PubMed] [Google Scholar]
- 178.Barrett T, Kostenbader K, Nalamachu S, et al. Safety and tolerability of biphasic immediate‐release/extended‐release oxycodone/acetaminophen tablets: analysis of 11 clinical trials. Pain Pract. 2016;16:856–868. [DOI] [PubMed] [Google Scholar]
- 179.Ferrell B, Wisdom C, Wenzl C, et al. Effects of controlled-released morphine on quality of life for cancer pain. Oncol Nurs Forum. 1989;16:521–526. [PubMed] [Google Scholar]
- 180.Jones JP. United States of America v. The Purdue Frederick Company, Inc., et al. United States District Court for the Western District of Virginia Abingdon Division. 1st ed; 2007:07CR0029. [Google Scholar]
- 181.Manchikanti L, Manchukonda R, Pampati V, et al. Evaluation of abuse of prescription and illicit drugs in chronic pain patients receiving short-acting (hydrocodone) or long-acting (methadone) opioids. Pain Physician. 2005;8:257–261. [PubMed] [Google Scholar]
- 182.Morton T, Kostenbader K, Montgomery J, et al. Comparison of subjective effects of extended-release versus immediate-release oxycodone/acetaminophen tablets in healthy nondependent recreational users of prescription opioids: a randomized trial. Postgrad Med. 2014;126:20–32. [DOI] [PubMed] [Google Scholar]
- 183.Park TW, Saitz R, Ganoczy D, et al. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with Nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–697. [DOI] [PubMed] [Google Scholar]
- 185.Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102:248–257. [DOI] [PubMed] [Google Scholar]
- 186.Rafiq S, Steinbruchel DA, Wanscher MJ, et al. Multimodal analgesia versus traditional opiate based analgesia after cardiac surgery, a randomized controlled trial. J Cardiothorac Surg. 2014;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rasmussen ML, Mathiesen O, Dierking G, et al. Multimodal analgesia with gabapentin, ketamine and dexamethasone in combination with paracetamol and ketorolac after hip arthroplasty: a preliminary study. Eur J Anaesthesiol. 2010;27:324–330. [DOI] [PubMed] [Google Scholar]
- 188.YaDeau JT, Brummett CM, Mayman DJ, et al. Duloxetine and subacute pain after knee arthroplasty when added to a multimodal analgesic regimen: a randomized, placebo-controlled, triple-blinded trial. Anesthesiology. 2016;125:561–572. [DOI] [PubMed] [Google Scholar]
- 189.Chisholm MF, Cheng J, Fields KG, et al. Perineural dexamethasone with subsartorial saphenous nerve blocks in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Duncan CM, Hall Long K, Warner DO, et al. The economic implications of a multimodal analgesic regimen for patients undergoing major orthopedic surgery: a comparative study of direct costs. Reg Anesth Pain Med. 2009;34:301–307. [DOI] [PubMed] [Google Scholar]
- 191.Maiese BA, Pham AT, Shah MV, et al. Hospitalization costs for patients undergoing orthopedic surgery treated with intravenous acetaminophen (IV-APAP) plus other IV analgesics or IV opioid monotherapy for postoperative pain. Adv Ther. 2017;34:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Michelson JD, Addante RA, Charlson MD. Multimodal analgesia therapy reduces length of hospitalization in patients undergoing fusions of the ankle and hindfoot. Foot Ankle Int. 2013;34:1526–1534. [DOI] [PubMed] [Google Scholar]
- 193.Fredrickson Fanzca MJ, Danesh-Clough TK, White R. Adjuvant dexamethasone for bupivacaine sciatic and ankle blocks: results from 2 randomized placebo-controlled trials. Reg Anesth Pain Med. 2013;38:300–307. [DOI] [PubMed] [Google Scholar]
- 194.Mathiesen O, Dahl B, Thomsen BA, et al. A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J. 2013;22:2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim SI, Ha KY, Oh IS. Preemptive multimodal analgesia for postoperative pain management after lumbar fusion surgery: a randomized controlled trial. Eur Spine J. 2016;25:1614–1619. [DOI] [PubMed] [Google Scholar]
- 196.Kang H, Ha YC, Kim JY, et al. Effectiveness of multimodal pain management after bipolar hemiarthroplasty for hip fracture: a randomized, controlled study. J Bone Joint Surg Am. 2013;95:291–296. [DOI] [PubMed] [Google Scholar]
- 197.Koehler D, Marsh JL, Karam M, et al. Efficacy of surgical-site, multimodal drug injection following operative management of femoral fractures: a randomized controlled trial. J Bone Joint Surg Am. 2017;99:512–519. [DOI] [PubMed] [Google Scholar]
- 198.Lee SK, Lee JW, Choy WS. Is multimodal analgesia as effective as postoperative patient-controlled analgesia following upper extremity surgery? Orthop Traumatol Surg Res. 2013;99:895–901. [DOI] [PubMed] [Google Scholar]
- 199.Zare MA, Ghalyaie AH, Fathi M, et al. Oral oxycodone plus intravenous acetaminophen versus intravenous morphine sulfate in acute bone fracture pain control: a double-blind placebo-controlled randomized clinical trial. Eur J Orthop Surg Traumatol. 2014;24:1305–1309. [DOI] [PubMed] [Google Scholar]
- 200.Grumbine N, Dobrowolski C, Bernstein A. Retrospective evaluation of postoperative intralesional steroid injections on wound healing. J Foot Ankle Surg. 1998;37:135–144. [DOI] [PubMed] [Google Scholar]
- 201.De Oliveira GS, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative PainA meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588. [DOI] [PubMed] [Google Scholar]
- 202.Vargas JH, III, Ross DG. Corticosteroids and anterior cruciate ligament repair. Am J Sports Med. 1989;17:532–534. [DOI] [PubMed] [Google Scholar]
- 203.Aasboe V, Raeder JC, Groegaard B. Betamethasone reduces postoperative pain and nausea after ambulatory surgery. Anesth Analg. 1998;87:319–323. [DOI] [PubMed] [Google Scholar]
- 204.Glasser RS, Knego RS, Delashaw JB, et al. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg. 1993;78:383–387. [DOI] [PubMed] [Google Scholar]
- 205.Karst M, Kegel T, Lukas A, et al. Effect of celecoxib and dexamethasone on postoperative pain after lumbar disc surgery. Neurosurgery. 2003;53:331–337. [DOI] [PubMed] [Google Scholar]
- 206.Waldron N, Jones C, Gan T, et al. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2012;110:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Curda G. Postoperative analgesic effects of dexamethasone sodium phosphate in bunion surgery. J Foot Surg. 1983;22:187–191. [PubMed] [Google Scholar]
- 208.Coluzzi F, Mattia C, Savoia G, et al. Postoperative pain surverys in Italy from 2006 and 2012: (POPSI and POPSI-2). Eur Rev Med Pharmacol Sci. 2015;19:4261–4269. [PubMed] [Google Scholar]
- 209.van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131:23–35. [DOI] [PubMed] [Google Scholar]
- 210.McCaffery M, Grimm MA, Pasero C, et al. On the meaning of “drug seeking”. Pain Manag Nurs. 2005;6:122–136. [DOI] [PubMed] [Google Scholar]
- 211.Compton P, Charuvastra C, Kintaudi K, et al. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–245. [DOI] [PubMed] [Google Scholar]
- 212.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National findings. NSDUH Series H-41.2011. Rockville, MD: US Department of Health and Human Services. 2011. [Google Scholar]
- 213.Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95:1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morris BJ, Zumsteg JW, Archer KR, et al. Narcotic use and postoperative doctor shopping in the orthopaedic trauma population. J Bone Joint Surg Am. 2014;96:1257–1262. [DOI] [PubMed] [Google Scholar]
- 215.Coluzzi F, Bifulco F, Cuomo A, et al. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017;13:1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kantor TG, Cantor R, Tom E. A study of hospitalized surgical patients on methadone maintenance. Drug Alcohol Depend. 1980;6:163–173. [DOI] [PubMed] [Google Scholar]
- 217.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. [DOI] [PubMed] [Google Scholar]
- 218.Chapman RC, Donaldson G, Davis J, et al. Postoperative pain patterns in chronic patin patients: a pilot study. Pain Med. 2009;10:481–487. [DOI] [PubMed] [Google Scholar]
- 219.Roeckel LA, Le Coz GM, Gaveriauz-Ruff C, et al. Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience. 2016;338:160–182. [DOI] [PubMed] [Google Scholar]
- 220.Richebe P, Beaulieu P. Perioperative pain management in the patient treated with opioids: continuing professional development. Can J Anesthesiol. 2009;56:969–981. [DOI] [PubMed] [Google Scholar]
- 221.Frolich MA, Giannotti A, Modell JH. Opioid overdose in a patient using a fentanyl patch during treatment with a warming blanket. Anesthesia. 2001;93:647–648. [DOI] [PubMed] [Google Scholar]
- 222.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine and other frequently presecribed medications: a review. Am J Addict. 2010;19:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Childers JW, Arnold RM. Treatment of pain in patients taking Buprenorphine for opioid addicition #221. J Palliat Med. 2012;15:613–614. [DOI] [PubMed] [Google Scholar]
- 224.Gervirtz C, Frost E, Bryson E. Perioperative implications od Buprenorphine maintenance treatment for opioid addicition. Anesth Clin. 2011;49:147–155. [DOI] [PubMed] [Google Scholar]
- 225.Vadivelu N, Mitra S, Kaye AD, et al. Perioperative analgesia and challenges in the drug- addicted and drug-dependent patient. Best Pract Res Clin Anaesthesiol. 2014;28:91–101. [DOI] [PubMed] [Google Scholar]
- 226.Dean RL, Todtenkopf MS, Deaver DR, et al. Overriding the blockade of antinociceptive actions of opioids in rats treated with extended release naltrexone. Pharmacol Biochem Behav. 2008;89:515–522. [DOI] [PubMed] [Google Scholar]
- 227.Borgeat A, Ofner C, Saporito A, et al. The effect of nonsteroidal anti-inflammatory drugs on bone healing in humans: a qualitative, systematic review. J Clin Anesth. 2018;49:92–100. [DOI] [PubMed] [Google Scholar]
- 228.Dodwell ER, Latorre JG, Parisini E, et al. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87:193–202. [DOI] [PubMed] [Google Scholar]
- 229.Kurmis AP, Kurmis TP, O'Brien JX, et al. The effect of nonsteroidal anti-inflammatory drug administration on acute phase fracture-healing: a review. J Bone Joint Surg Am. 2012;94:815–823. [DOI] [PubMed] [Google Scholar]
- 230.Geusens P, Emans PJ, de Jong JJ, et al. NSAIDs and fracture healing. Curr Opin Rheumatol. 2013;25:524–531. [DOI] [PubMed] [Google Scholar]
- 231.Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg. 2004;12:139–143. [DOI] [PubMed] [Google Scholar]
- 232.Murnaghan M, Li G, Marsh DR. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: an inhibition of angiogenesis? J Bone Joint Surg Am. 2006;88(suppl 3):140–147. [DOI] [PubMed] [Google Scholar]
- 233.Brown KM, Saunders MM, Kirsch T, et al. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86-A:116–123. [DOI] [PubMed] [Google Scholar]
- 234.Giannoudis PV, MacDonald DA, Matthews SJ, et al. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br. 2000;82:655–658. [DOI] [PubMed] [Google Scholar]
- 235.Bhattacharyya T, Levin R, Vrahas MS, et al. Nonsteroidal antiinflammatory drugs and nonunion of humeral shaft fractures. Arthritis Rheum. 2005;53:364–367. [DOI] [PubMed] [Google Scholar]
- 236.Jeffcoach DR, Sams VG, Lawson CM, et al. Nonsteroidal anti-inflammatory drugs' impact on nonunion and infection rates in long-bone fractures. J Trauma Acute Care Surg. 2014;76:779–783. [DOI] [PubMed] [Google Scholar]
- 237.Sagi HC, Jordan CJ, Barei DP, et al. Indomethacin prophylaxis for heterotopic ossification after acetabular fracture surgery increases the risk for nonunion of the posterior wall. J Orthop Trauma. 2014;28:377–383. [DOI] [PubMed] [Google Scholar]
- 238.Marquez-Lara A, Hutchinson ID, Nunez F, Jr, et al. Nonsteroidal anti-inflammatory drugs and bone-healing: a systematic review of research quality. JBJS Rev. 2016;4. 10.2106/JBJS.RVW.O.00055. [DOI] [PubMed] [Google Scholar]
- 239.Perez-Castrillon JL, Olmos JM, Gomez JJ, et al. Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology. 2000;72:187–194. [DOI] [PubMed] [Google Scholar]
- 240.Chrastil J, Sampson C, Jones KB, et al. Postoperative opioid administration inhibits bone healing in an animal model. Clin Orthop Relat Res. 2013;471:4076–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician. 2012;15:ES145–156. [PubMed] [Google Scholar]
- 242.Brinker MR, O'Connor DP, Monla YT, et al. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–570. [DOI] [PubMed] [Google Scholar]
- 243.Chrastil J, Sampson C, Jones KB, et al. Evaluating the affect and reversibility of opioid-induced androgen deficiency in an orthopaedic animal fracture model. Clin Orthop Relat Res. 2014;472:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Poonai N, Bhullar G, Lin K, et al. Oral administration of morphine versus ibuprofen to manage postfracture pain in children: a randomized trial. CMAJ. 2014;186:1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Beaudoin FL, Gutman R, Merchant RC, et al. Persistent pain after motor vehicle collision: comparative effectiveness of opioids vs nonsteroidal antiinflammatory drugs prescribed from the emergency department-a propensity matched analysis. Pain. 2017;158:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pollack CV, Jr, Diercks DB, Thomas SH, et al. Patient-reported outcomes from A National, prospective, observational study of emergency department acute pain management with an intranasal Nonsteroidal anti-inflammatory drug, opioids, or both. Acad Emerg Med. 2016;23:331–341. [DOI] [PubMed] [Google Scholar]
- 247.Ong CK, Seymour RA, Lirk P, et al. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110:1170–1179. [DOI] [PubMed] [Google Scholar]
- 248.Beaudoin FL, Haran JP, Liebmann O. A comparison of ultrasound-guided three-in-one femoral nerve block versus parenteral opioids alone for analgesia in emergency department patients with hip fractures: a randomized controlled trial. Acad Emerg Med. 2013;20:584–591. [DOI] [PubMed] [Google Scholar]
- 249.Beaudoin FL, Nagdev A, Merchant RC, et al. Ultrasound-guided femoral nerve blocks in elderly patients with hip fractures. Am J Emerg Med. 2010;28:76–81. [DOI] [PubMed] [Google Scholar]
- 250.Brisbane Orthopaedic & Sports Medicine Centre Writing Committee, McMeniman TJ, McMeniman PJ, et al. Femoral nerve block vs fascia iliaca block for total knee arthroplasty postoperative pain control: a prospective, randomized controlled trial. J Arthroplasty. 2010;25:1246–1249. [DOI] [PubMed] [Google Scholar]
- 251.Fletcher AK, Rigby AS, Heyes FL. Three-in-one femoral nerve block as analgesia for fractured neck of femur in the emergency department: a randomized, controlled trial. Ann Emerg Med. 2003;41:227–233. [DOI] [PubMed] [Google Scholar]
- 252.Foss NB, Kristensen BB, Bundgaard M, et al. Fascia iliaca compartment blockade for acute pain control in hip fracture patients: a randomized, placebo-controlled trial. Anesthesiology. 2007;106:773–778. [DOI] [PubMed] [Google Scholar]
- 253.Godoy-Monzon D, Vazquez J, Jauregui JR, et al. Pain treatment in post-traumatic hip fracture in the elderly: regional block vs. systemic non-steroidal analgesics. Int J Emerg Med. 2010;3:321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Haddad FS, Williams RL. Femoral nerve block in extracapsular femoral neck fractures. J Bone Joint Surg Br. 1995;77:922–923. [PubMed] [Google Scholar]
- 255.Haines L, Dickman E, Ayvazyan S, et al. Ultrasound-guided fascia iliaca compartment block for hip fractures in the emergency department. J Emerg Med. 2012;43:692–697. [DOI] [PubMed] [Google Scholar]
- 256.Morrison RS, Dickman E, Hwang U, et al. Regional Nerve blocks improve pain and functional outcomes in hip fracture: a randomized controlled trial. J Am Geriatr Soc. 2016;64:2433–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mouzopoulos G, Vasiliadis G, Lasanianos N, et al. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. J Orthop Traumatol. 2009;10:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yun MJ, Kim YH, Han MK, et al. Analgesia before a spinal block for femoral neck fracture: fascia iliaca compartment block. Acta Anaesthesiol Scand. 2009;53:1282–1287. [DOI] [PubMed] [Google Scholar]
- 259.Beaupre LA, Johnston DB, Dieleman S, et al. Impact of a preemptive multimodal analgesia plus femoral nerve blockade protocol on rehabilitation, hospital length of stay, and postoperative analgesia after primary total knee arthroplasty: a controlled clinical pilot study. ScientificWorldJournal. 2012;2012:273821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.YaDeau JT, Cahill JB, Zawadsky MW, et al. The effects of femoral nerve blockade in conjunction with epidural analgesia after total knee arthroplasty. Anesth Analg. 2005;101:891–895, table of contents. [DOI] [PubMed] [Google Scholar]
- 261.Divella M, Cecconi M, Fasano N, et al. Pain relief after total hip replacement: oral CR oxycodone plus IV paracetamol versus epidural levobupivacaine and sufentanil. A randomized controlled trial. Minerva Anesthesiol. 2012;78:534–541. [PubMed] [Google Scholar]
- 262.Harsten A, Hjartarson H, Werner MU, et al. General anaesthesia with multimodal principles versus intrathecal analgesia with conventional principles in total knee arthroplasty: a consecutive, randomized study. J Clin Med Res. 2013;5:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29:329–334. [DOI] [PubMed] [Google Scholar]
- 264.Moucha CS, Weiser MC, Levin EJ. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24:60–73. [DOI] [PubMed] [Google Scholar]
- 265.Amundson AW, Johnson RL, Abdel MP, et al. A three-arm randomized clinical trial comparing continuous femoral plus single-injection sciatic peripheral nerve blocks versus periarticular injection with ropivacaine or liposomal bupivacaine for patients undergoing total knee arthroplasty. Anesthesiology. 2017;126:1139–1150. [DOI] [PubMed] [Google Scholar]
- 266.Ng FY, Ng JK, Chiu KY, et al. Multimodal periarticular injection vs continuous femoral nerve block after total knee arthroplasty: a prospective, crossover, randomized clinical trial. J Arthroplasty. 2012;27:1234–1238. [DOI] [PubMed] [Google Scholar]
- 267.Jules-Elysee KM, Goon AK, Westrich GH, et al. Patient-controlled epidural analgesia or multimodal pain regimen with periarticular injection after total hip arthroplasty: a randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 2015;97:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Barrington JW, Emerson RH, Lovald ST, et al. No difference in early analgesia between liposomal bupivacaine injection and intrathecal morphine after TKA. Clin Orthop Relat Res. 2017;475:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kelley TC, Adams MJ, Mulliken BD, et al. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28:1274–1277. [DOI] [PubMed] [Google Scholar]
- 270.Xing JG, Abdallah FW, Brull R, et al. Preoperative femoral nerve block for hip arthroscopy: a randomized, triple-masked controlled trial. Am J Sports Med. 2015;43:2680–2687. [DOI] [PubMed] [Google Scholar]
- 271.Luiten WE, Schepers T, Luitse JS, et al. Comparison of continuous nerve block versus patient-controlled analgesia for postoperative pain and outcome after talar and calcaneal fractures. Foot Ankle Int. 2014;35:1116–1121. [DOI] [PubMed] [Google Scholar]
- 272.Schipper ON, Hunt KJ, Anderson RB, et al. Ankle block vs single-shot popliteal Fossa block as primary anesthesia for forefoot operative procedures: prospective, randomized comparison. Foot Ankle Int. 2017;38:1188–1191. [DOI] [PubMed] [Google Scholar]
- 273.Galos DK, Taormina DP, Crespo A, et al. Does brachial plexus blockade result in improved pain scores after distal radius fracture fixation? A randomized trial. Clin Orthop Relat Res. 2016;474:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Goldstein RY, Montero N, Jain SK, et al. Efficacy of popliteal block in postoperative pain control after ankle fracture fixation: a prospective randomized study. J Orthop Trauma. 2012;26:557–561. [DOI] [PubMed] [Google Scholar]
- 275.Ding DY, Manoli A, III, Galos DK, et al. Continuous popliteal sciatic nerve block versus single injection nerve block for ankle fracture surgery: a prospective randomized comparative trial. J Orthop Trauma. 2015;29:393–398. [DOI] [PubMed] [Google Scholar]
- 276.Hunt KJ, Higgins TF, Carlston CV, et al. Continuous peripheral nerve blockade as postoperative analgesia for open treatment of calcaneal fractures. J Orthop Trauma. 2010;24:148–155. [DOI] [PubMed] [Google Scholar]
- 277.The Joint Commission. New and revised pain assessment and management standards. Perspect (Montclair). 2018;38:17–18. [Google Scholar]
- 278.The Joint Commission. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1–5. [PubMed] [Google Scholar]
- 279.Cashman J, Dolin S. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–223. [DOI] [PubMed] [Google Scholar]
- 280.Lee L, Caplan R, Stephens L, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Pain Med. 2015;122:659–665. [DOI] [PubMed] [Google Scholar]
- 281.Jungquist CR, Willens JS, Dunwoody DR, et al. Monitoring for opioid-induced advancing sedation and respiratory depression: ASPMN membership survey of current practice. Pain Manag Nurs. 2014;15:682–693. [DOI] [PubMed] [Google Scholar]
- 282.Pasero C. The perianesthesia nurse's role in the prevention of opioid-related sentinel events. J Perianesth Nurs. 2013;28:31–37. [DOI] [PubMed] [Google Scholar]
- 283.Pasero C. Assessment of sedation during opioid administration for pain management. J Perianesth Nurs. 2009;24:186–190. [DOI] [PubMed] [Google Scholar]
- 284.Nisbet AT, Mooney-Cotter F. Comparison of selected sedation scales for reporting opioid-induced sedation assessment. Pain Manag Nurs. 2009;10:154–164. [DOI] [PubMed] [Google Scholar]
- 285.Coffin P, Sullivan S. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158:1–9. [DOI] [PubMed] [Google Scholar]
- 286.Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community-based naloxone distribution program. Addiction. 2015;110:1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Walley A, Xuan Z, Hackman H, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:F174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Clark A, Wilder C, Winstanley E. A systematic review of community opioid OverdosePrevention and Naloxone distribution programs. J Addict Med. 2014;8:153–163. [DOI] [PubMed] [Google Scholar]
- 289.Finley EP, Garcia A, Rosen K, et al. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res. 2017;17:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gugelmann H, Perrone J, Nelson L. Windmills and pill mills: can PDMPs tilt the prescription drug epidemic? J Med Toxicol. 2012;8:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Worley J. Prescription drug monitoring programs, a response to doctor shopping: purpose, effectiveness, and directions for future research. Issues Ment Health Nurs. 2012;33:319–328. [DOI] [PubMed] [Google Scholar]
- 292.Haegerich T, Paulozzi L, Manns B, et al. What we know, and don't know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Delcher C, Wagenaar AC, Goldberger BA, et al. Abrupt decline in oxycodone-caused mortality after implementation of Florida's prescription drug monitoring program. Drug Alcohol Depend. 2015;150:63–68. [DOI] [PubMed] [Google Scholar]
- 294.Nam Y, Shea D, Shi Y, et al. State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care. 2017;23:297–303. [PubMed] [Google Scholar]
- 295.Brown R, Riley MR, Ulrich L, et al. Impact of New York prescription drug monitoring program, I-STOP, on statewide overdose morbidity. Drug Alcohol Depend. 2017;178:348–354. [DOI] [PubMed] [Google Scholar]
- 296.McAllister MW, Aaronson P, Spillane J, et al. Impact of prescription drug-monitoring program on controlled substance prescribing in the ED. Am J Emerg Med. 2015;33:781–785. [DOI] [PubMed] [Google Scholar]
- 297.Lin HC, Wang Z, Boyd C, et al. Associations between statewide prescription drug monitoring program (PDMP) requirement and physician patterns of prescribing opioid analgesics for patients with non-cancer chronic pain. Addict Behav. 2018;76:348–354. [DOI] [PubMed] [Google Scholar]
- 298.Deyo RA, Hallvik SE, Hildebran C, et al. Association of prescription drug monitoring program use with opioid prescribing and health outcomes: a comparison of program users and Non-users. J Pain. 2017;19:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wen H, Schackman BR, Aden B, et al. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to Medicaid enrollees. Health Aff (Millwood). 2017;36:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Moyo P, Simoni-Wastila L, Griffin BA, et al. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 US States. Addiction. 2017;112:1784–1796. [DOI] [PubMed] [Google Scholar]
- 301.Young LD, Kreiner PW, Panas L. Unsolicited reporting to prescribers of opioid analgesics by a state prescription drug monitoring program: an observational study with matched comparison group. Pain Med. 2017;19:1396–1407. [DOI] [PubMed] [Google Scholar]
- 302.Yarbrough CR. Prescription drug monitoring programs produce a limited impact on painkiller prescribing in Medicare Part D. Health Serv Res. 2017;53:671–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pardo B. Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction. 2017;112:1773–1783. [DOI] [PubMed] [Google Scholar]
- 304.Yanni LM, McKinney-Ketchum JL, Harrington SB, et al. Preparation, confidence, and attitudes about chronic noncancer pain in graduate medical education. J Grad Med Educ. 2010;2:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Mezei L, Murinson BB; Johns Hopkins Pain Curriculum Development T. Pain education in North American medical schools. J Pain. 2011;12:1199–1208. [DOI] [PubMed] [Google Scholar]
- 306.Davis C, Carr D. Physician continuing education to reduce opioid misuse, abuse, and overdose: many opportunities, few requirements. Drug Alcohol Depend. 2016;163:100–107. [DOI] [PubMed] [Google Scholar]
- 307.Cervero R, Gaines J. The impact of CME on physician performance and patient health outcomes: an updated synthesis of systematic reviews. J Contin Educ Health Prof. 2015;35:131–138. [DOI] [PubMed] [Google Scholar]
- 308.Katzman J, Comerci G, Landen MG, et al. Rules and values: a coordinated regulatory and educational approach to the public health crises of chronic pain and addiction. Am J Public Health. 2014;104:1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Trudeau KJ, Hildebrand C, Garg P, et al. A randomized controlled trial of the effects of online pain management education on primary care providers. Pain Med. 2017;18:680–692. [DOI] [PubMed] [Google Scholar]
- 310.Behar E, Rowe C, Santos G, et al. Academic detailing pilot for Naloxone prescribing among primary care providers in san Francisco. Fam Med. 2017;49:122–126. [PubMed] [Google Scholar]
- 311.Kattan J, Tuazon E, Paone D, et al. Public health detailing-A successful strategy to promote judicious opioid analgesic prescribing. Am J Public Health. 2016;106:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Donaldson SR, Harding AM, Taylor SE, et al. Evaluation of a targeted prescriber education intervention on emergency department discharge oxycodone prescribing. Emerg Med Aust. 2017;29:400–406. [DOI] [PubMed] [Google Scholar]
- 313.Hill M, Stucke R, McMahon M, et al. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2017;267:468–472. [DOI] [PubMed] [Google Scholar]
- 314.Osborn S, Yu J, Williams B, et al. Changes in provider prescribing patterns after implementation of an emergency department prescription opioid policy. J Emerg Med. 2017;52:538–546. [DOI] [PubMed] [Google Scholar]
- 315.Zisblatt L, Hayes SM, Lazure P, et al. Safe and competent opioid prescribing education: increasing dissemination with a train-the-trainer program. Subst Abus. 2017;38:168–176. [DOI] [PubMed] [Google Scholar]
- 316.Brooks M. Mitigating the safety risks of drugs with a focus on opioids: are risk evaluation and mitigation strategies the answer? Mayo Clin Proc. 2014;89:1673–1684. [DOI] [PubMed] [Google Scholar]
- 317.Gottlieb S. FDA Takes Important Steps to Stem the Tide of Opioid Misuse and Abuse. U.S. Food and Drug Administration; 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/FDAVoices/ucm612011.htm. Accessed September 28, 2017. [Google Scholar]
- 318.Alford D, Zisblatt L, Ng P, et al. SCOPE of pain: an evaluation of an opioid risk evaluation and mitigation strategy continuing education program. Pain Med. 2015;17:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Davis D. CME and the pharmaceutical industry: two worlds, three views, four steps. CMAJ. 2004;171:149–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.de la Cruz M, Reddy A, Balankari V, et al. The impact of an educational program on patient practices for safe use, storage, and disposal of opioids at a comprehensive cancer center. Oncologist. 2017;22:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rose P, Sakai J, Argue R, et al. Opioid information pamphlet increases postoperative opioid disposal rates: a before versus after quality improvement study. Can J Anesth. 2016;63:31–37. [DOI] [PubMed] [Google Scholar]
- 322.McCarthy DM, Wolf MS, McConnell R, et al. Improving patient knowledge and safe use of opioids: a randomized controlled trial. Acad Emerg Med. 2015;22:331–339. [DOI] [PubMed] [Google Scholar]
- 323.McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addict Behav. 2013;38:2230–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Hero JO, McMurtry C, Benson J, et al. Discussing opioid risks with patients to reduce misuse and abuse: evidence from 2 surveys. Ann Fam Med. 2016;14:575–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Waszak D, Mitchell A, Ren D, et al. A quality improvement project to improve education provided by nurses to ED patients prescribed opioid analgesics at discharge. J Emerg Nurs. 2017;44:336–344. [DOI] [PubMed] [Google Scholar]
- 326.Rathlev N, Almomen R, Deutsch A, et al. Randomized controlled trial of electronic care plan alerts and resource utilization by high frequency emergency department users with opioid use disorder. West J Emerg Med. 2016;17:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Butler SF, Zacharoff KL, Charity S, et al. Impact of an electronic pain and opioid risk assessment program: are there improvements in patient encounters and clinic Notes? Pain Med. 2016;17:2047–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Gugelmann H, Shofer FS, Meisel ZF, et al. Multidisciplinary intervention decreases the use of opioid medication discharge packs from 2 urban EDs. Am J Emerg Med. 2013;31:1343–1348. [DOI] [PubMed] [Google Scholar]
- 329.Johnson JA, Woychek A, Vaughan D, et al. Screening for at-risk alcohol use and drug use in an emergency department: integration of screening questions into electronic triage forms achieves high screening rates. Ann Emerg Med. 2013;62:262–266. [DOI] [PubMed] [Google Scholar]
- 330.Kahler ZP, Musey PI, Schaffer JT, et al. Effect of a “No superuser opioid prescription” policy on ED visits and statewide opioid prescription. West J Emerg Med. 2017;18:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zwank MD, Kennedy SM, Stuck LH, et al. Removing default dispense quantity from opioid prescriptions in the electronic medical record. Am J Emerg Med. 2017;35:1567–1569. [DOI] [PubMed] [Google Scholar]
- 332.Anderson D, Zlateva I, Khatri K, et al. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Butler SF, Zacharoff K, Charity S, et al. Electronic opioid risk assessment program for chronic pain patients: barriers and benefits of implementation. Pain Pract. 2014;14:E98–e105. [DOI] [PubMed] [Google Scholar]
- 334.Canada R, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1–E8. [PubMed] [Google Scholar]
- 335.Stanek J, Renslow M, Kalliainen L. The effect of an educational program on opioid prescription patterns in hand surgery: a quality improvement program. J Hand Surg Am. 2015;40:341–346. [DOI] [PubMed] [Google Scholar]
- 336.Prabhu M, McQuaid-Hanson E, Hopp S, et al. A shared decision-making intervention to guide opioid prescribing after cesarean delivery. Obstet Gynecol. 2017;130:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Mack K, Jones C, Ballesteros M. Illicit drug use, illicit drug use disorders, and drug overdose deaths in metropolitan and nonmetropolitan areas—United States. MMWR Surveill Summ. 2017;66:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Lin LA, Bohnert ASB, Kerns RD, et al. Impact of the opioid safety initiative on opioid-related prescribing in veterans. Pain. 2017;158:833–839. [DOI] [PubMed] [Google Scholar]
- 339.Losby J, Hyatt J, Kanter M, et al. Safer and more appropriate opioid prescribing: a large healthcare system's comprehensive approach. J Eval Clin Pract. 2017;6:1–7. [DOI] [PubMed] [Google Scholar]
- 340.Akce M, Suneja A, Genord C, et al. A multifactorial intervention for hospital opioid management. J Opioid Manag. 2014;10:337–344. [DOI] [PubMed] [Google Scholar]
- 341.Koopman R, Kochendorfer K, Moore J, et al. A diabetes dashboard and physician efficiency and accuracy in accessing data needed for high-quality diabetes care. Ann Fam Med. 2011;9:398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Weiner J, Balijepally V, Tanniru M. Integrating strategic and operational decision making using data-driven dashboards: the case of St. Joseph mercy oakland hospital. J Healthc Manag. 2015;60:319–330. [PubMed] [Google Scholar]
- 343.Webster L, Webster R. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6:432–442. [DOI] [PubMed] [Google Scholar]
- 344.Butler SF, Budman S, Fernandez K, et al. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med. 2009;3:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. J Subst Abuse Treat. 2007;32:189–198. [DOI] [PubMed] [Google Scholar]
- 346.Jones T, Lookatch S, Grant P, et al. Further validation of an opioid risk assessment tool: the Brief Risk Interview. J Opioid Manag. 2014;10:353–364. [DOI] [PubMed] [Google Scholar]
- 347.Butler SF, Budman S, Fernandez K, et al. Development and validation of the current opioid misuse measure. Pain. 2007;130:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Dowell D, Zhang K, Noonan RK, et al. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Aff (Millwood). 2016;35:1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ammenwerth E, Schnell-Inderst P, Machan C, et al. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inf Assoc. 2008;15:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Nuckols T, Smith-Spangler C, Morton S, et al. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev. 2014;3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kaushal R, Shojania K, Bates D. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;263:1409–1416. [DOI] [PubMed] [Google Scholar]
- 352.Genco EK, Forster JE, Flaten H, et al. Clinically inconsequential alerts: the characteristics of opioid drug alerts and their utility in preventing adverse drug events in the emergency department. Ann Emerg Med. 2016;67:240–248. e243. [DOI] [PMC free article] [PubMed] [Google Scholar]