Synthesis of pooled data on herbal medicinal products used during and after pregnancy highlights the need for robust safety studies.
Abstract
OBJECTIVE:
To report the incidence and nature of herbal medicinal products' adverse events and herb–drug interactions used by some pregnant and postnatal women.
DATA SOURCES:
The Allied and Complementary Medicine Database, the Cumulative Index to Nursing and Allied Health Literature, EMBASE, the Cochrane Library, MEDLINE, Scopus, Web of Science, and ClinicalTrials.gov were searched from inception until August 2018.
METHODS OF STUDY SELECTION:
Any studies reporting adverse events, herb–drug interactions or absence thereof associated with herbal medicinal products used during pregnancy or the postnatal period were included. Conference abstracts, pilot studies, and nonhuman studies were excluded. All included studies were critically appraised by two independent reviewers.
TABULATION, INTEGRATION AND RESULTS:
Database searches retrieved 3,487 citations. After duplicate removal and review of titles, abstracts, and full-text, 115 articles were critically appraised. After excluding irrelevant and low-quality articles, 74 articles were included for data extraction and synthesis. Adverse drug reactions, congenital malformations, fetal growth retardation or herb–drug interactions were the primary study objective reported by 19 of the 74 included studies, 16 cohort studies, one cross-sectional survey, and two randomized controlled trials. A total of 47 herbal medicinal products and 1,067,071 women were included in this review. Use of almond oil was associated with preterm birth (odds ratio 2.09, 95% CI 1.07–4.08), oral raspberry leaf was associated with cesarean delivery (adjusted odds ratio [AOR] 3.47, 95% CI 1.45–8.28); heavy licorice use was associated with early preterm birth by 3.07-fold (95% CI 1.17–8.05). African herbal medicine mwanaphepo was associated with maternal morbidity (AOR 1.28; 95% CI 1.09–1.50), and neonatal death or morbidity. Fourteen studies reported absence of adverse events. Four studies reported herb–drug interactions, but none studied adverse events arising from them.
CONCLUSION:
The use of herbal medicinal products during pregnancy and the postnatal period should be discouraged until robust evidence of safety is available.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO, CRD42017081058.
Herbal medicinal products are any plant-derived product (ie, leaves, roots, flowers), in any form, taken as a preventive or curative treatment.1 Although herbal medicinal products have been used for centuries in Asia, Africa, and Latin America, it is only over the past decades that use has attracted attention in the Western world.2 Despite the lack of robust efficacy and safety data, the use of herbal medicinal products is widespread and increasing throughout North America and Europe.3–7
Women have been identified as the major users of herbal medicinal products, both for maintenance of health and treatment of disease.8–12 This widespread use extends into pregnancy, where reportedly between 10 and 74% of pregnant women in Africa, Australia, Europe, the United Kingdom, and the United States use herbal medicinal products.13–23 In the United Kingdom, approximately 40% of pregnant women use herbal medicinal products to treat pregnancy related problems or as nutritional supplements to better pregnancy outcomes.16,17 This use of herbal medicinal products appears to extend into the postnatal period with 31% of breastfeeding women reporting the use of complementary and alternative medicines, including herbal medicinal products, to treat a variety of ailments24 or to improve milk flow.25
A further potential issue is that of herb–drug interactions. Data from Australia, Europe and North and South America suggest that 12–81% of pregnant women use prescription medicines.26–30 It is estimated that between 2.5% and 20.3% of these pregnant women also use herbal medicinal products.31–34 This concurrent use of herbal medicinal products and prescribed medicines gives rise to the possibility of herb–drug interactions with the potential to harm the mother-fetus dyad.35,36
Owing to a variety of reasons, including a lack of appropriately designed and powered studies, low reporting rates to inadequate regulatory supervision, and the widely held belief that herbal medicinal products are natural and hence safe, the prevalence of adverse events and herb–drug interactions associated with herbal medicinal product use is unclear. Because there are reports of severe adverse events such as perinatal stroke,37 severe hyponatremia,38 and lead poisoning,39–41 it is important to explore the prevalence of these and other adverse outcomes.
Previous published systematic reviews on the safety of herbal medicinal products have focused on randomized controlled trials, and excluded cohort studies or case reports.42–49 Furthermore, these systematic reviews have been assessed as being of low quality.50,51 Currently there are no published systematic reviews on adverse events and herb–drug interactions associated with herbal medicinal product use during pregnancy and the postnatal period. The aim of this systematic review was to retrieve primary literature reporting the incidence and nature of adverse events and herb–drug interactions, to determine whether herbal medicinal product use during pregnancy and the postnatal period is associated with adverse maternal or child outcomes.
SOURCES
The Allied and Complementary Medicine Database, the Cumulative Index to Nursing and Allied Health Literature, EMBASE, the Cochrane Library, MEDLINE, Scopus, Web of Science, and ClinicalTrials.gov were searched from inception until August 2018. Only publications in English were included.
A three-step search strategy was applied. An initial limited search of MEDLINE was done, followed by analysis of the text words contained in the title and abstract, and of the index terms used to describe the article. A second search using all identified keywords and index terms was done across all included databases. Thirdly, the reference list of all identified articles and reports was searched for additional studies. The following search string was used:
(antenatal* OR prenatal* OR pregnan* OR postnatal* OR “postpartum*” OR “puerperium*” OR “breastfeeding*” OR “breast feeding*” OR lactati* OR maternal) AND (“herbal medicine*” OR “medicinal herb*” OR “herbal therap*” OR phytotherap* OR “traditional medicine*” OR “herb*” OR “galactagogue herb*” OR “herb* galactagogue*” OR “medicinal plant*” OR “botanical*” OR food supplement* OR liquorice* OR licorice* OR stevia OR senna) AND (“safe*” OR “adverse effect*” OR “adverse event*” OR “adverse reaction*” OR “side effect*” OR “adverse drug* reaction*” OR “drug* interaction*” OR “herb drug interaction*” OR “herb-drug interaction*” OR “drug herb interaction*” OR “drug-herb interaction*” OR “drug* hypersensitivity*” OR hypersensitivity* OR “unwanted effect*” OR “undesired effect*” OR “unwanted reaction*” OR “undesired reaction*”) NOT (vitamin* OR animal product* OR rat* OR mouse OR rabbit* OR mice OR sheep OR chick* OR pig* OR dog* OR sow* OR cow* OR monkey* OR agricultural* OR “veterinary*” OR “animal* model*” OR “in vitro” OR “cell model*”).
STUDY SELECTION
We included human studies that focused on pregnant or postnatal women. Randomized controlled trials, nonrandomized comparative studies, meta-analysis, observational studies, mixed-methodology studies, case reports and case series were considered for inclusion. Only studies that reported adverse events (including side effects, adverse drug reactions, malformations, or adverse birth outcomes), or absence thereof, or herb–drug interactions were included.
We excluded the following types of articles: conference or symposium abstracts; preliminary reports; pilot studies; correspondence articles; studies focusing on homeopathic treatments; or other alternative treatments (eg, aromatherapy, acupuncture, relaxation therapy); and low-quality case reports (see Article Quality Assessment [Appendix 1, available online at http://links.lww.com/AOG/B334]). The shape, active components, and molecular mechanisms of the herbal medicinal products were not a review objective and will not be discussed in this article.
Only studies reporting any safety issue arising from herbal medicinal products used during pregnancy or the postnatal period in any country, adverse events or herb–drug interactions were assessed. Studies comparing herbal medicines with a placebo, positive controls, or no comparator were also included.
Quality assessments were conducted by two independent reviewers (Y.M.B. and D.S.) using modified versions of the Critical Appraisal Skills Programme quality assessment tool for randomized controlled trials52 and cohort studies53 and the Joanna Briggs Institute Checklist for case reports.54 High quality was stated as at least 14 out of 17 points for interventional studies, at least 11 out of 14 points for observational studies, and at least 7 out of 8 points for case reports. These cut-points were established by the review team considering the most relevant items of the checklists. All interventional and observational studies were included irrespective of quality score. Only high-quality case reports were included.
A tailored spreadsheet was prepared for data extraction and data synthesis. All studies identified during the database search were assessed for relevance to the review protocol and quality assessed based on information from the title, abstract and full text review by two independent reviewers (Y.M.B and D.S.). A third reviewer was consulted if consensus could not be reached (J.S.M.). Where information was missing from the studies, contact with authors was attempted, where practical, via email; however, no responses were received.
The following key data were extracted from selected literature: details of the authors; country of publication; year of publication; herbal medicinal product; study population; setting; recruitment; incidence; nature of adverse events; and herb–drug interactions (classification, severity, patient outcomes). Data extracted for trials were the generation of allocation sequence, concealment of allocation, outcome measures, and other risks of bias. For cohort studies, the data extracted were appropriateness of exposed and control recruitment, inclusion and exclusion criteria clearly stated, appropriate validation of exposure, appropriate analyses, and enough follow-up information.
Owing to lack of study homogeneity, a meta-analysis was not appropriate; therefore, a narrative synthesis of the results was conducted. Where available, odds ratios (ORs) were reported and presented in a forest plot for descriptive purposes only, no meta-analysis was conducted.
A systematic review protocol was registered by PROSPERO.55 The PRISMA checklist was used to guide the reporting of the systematic review.
RESULTS
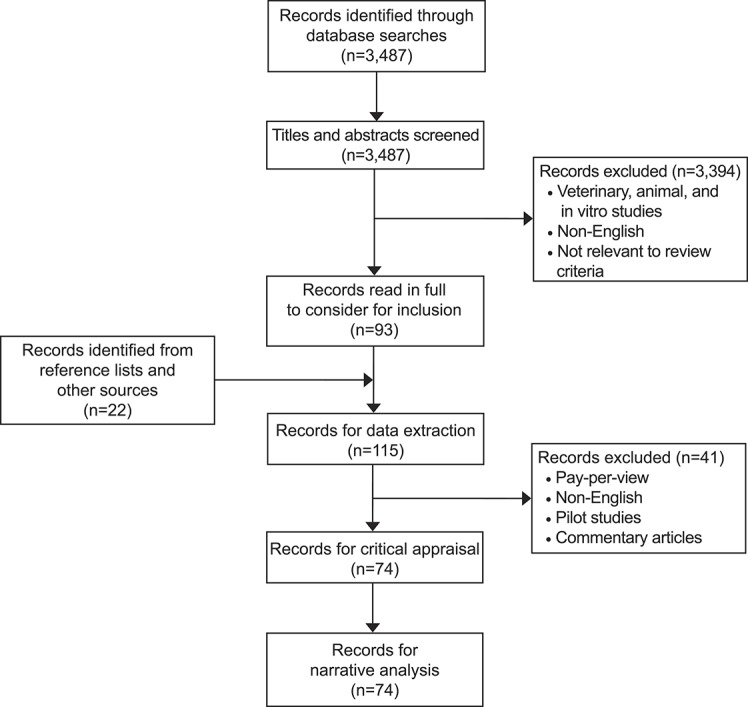
Database searches retrieved a total of 3,487 citations. After removal of duplicates, title and abstract screening, and full-text screening, 115 articles were critically appraised. Only 74 articles were included for data extraction, synthesis and narrative analysis (Fig. 1). Included studies were performed in 24 different countries. Twenty-nine interventional studies, 26 observational studies, and 19 case reports were reviewed.
Fig. 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart.
Muñoz Balbontín. Safety of Herbal Medicines During and After Pregnancy. Obstet Gynecol 2019.
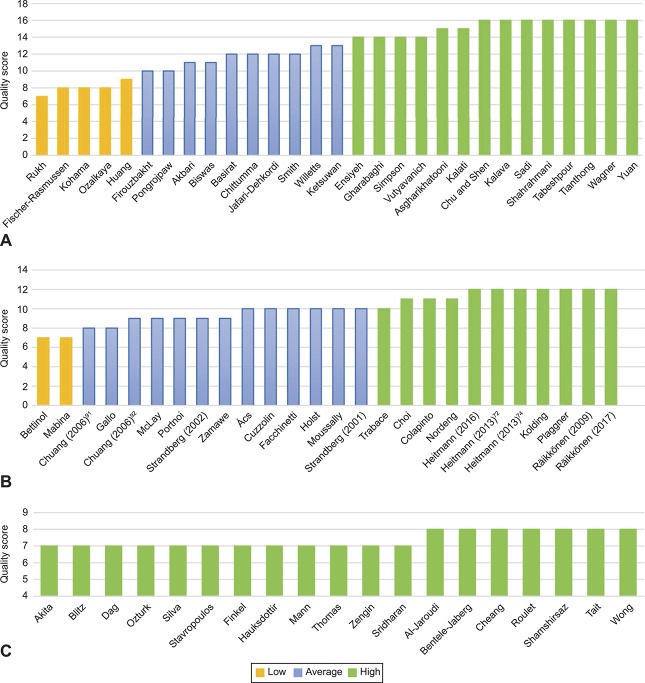
Fourteen interventional56–69 and 11 observational studies34,70–79 were graded as high quality; 10 interventional80–89 and 13 observational studies14,31,90–100 were graded average quality, and five interventional101–105 and two observational studies24,106 were graded poor quality. These poor-quality studies were still included in the review, only low-quality case reports were excluded (Fig. 2).
Fig. 2. Quality scores. Interventional studies (n=29) (A), observational studies (n=26) (B), and case reports (n=19) (C).
Muñoz Balbontín. Safety of Herbal Medicines During and After Pregnancy. Obstet Gynecol 2019.
Excluding case reports, specific safety concerns, such as adverse drug reactions, congenital malformations, fetal growth restriction and herb–drug interactions, were the primary study objective reported by 19 studies, 16 cohort studies,31,34,70–75,77–79,92,95,96,98,100 one cross-sectional survey,76 and two randomized controlled trials.64,68
A total of 47 herbal medicinal products and 1,067,071 women were included in this review. Sample size ranged from 27 to 500 women in interventional studies, 187 to 860,215 women in observational studies, and one to five women in case reports. Seven studies collected data during the first trimester of pregnancy;71,82,85,88,96,105,107 10 during the second trimester;41,58,67,81,83,84,87,89,101,108 12 during the third trimester;38,62,64,80,91,92,100,102,103,106,109,110 five throughout pregnancy;69,70,75,90,95 five around the time of labor (a few hours before or after delivery);37,59–61,77 nine throughout pregnancy and the postnatal period;14,31,39,72–74,78,79,111 three during the third trimester and the postnatal period;40,112,113 20 during the postnatal period only;24,34,56,63,65,66,68,76,86,94,97–99,104,114–119 and two did not specify the precise point during pregnancy of data collection.57,93
The methods used to identify adverse events and herb–drug interactions were clinical examination;37–41,56,57,59,60,63,70,81,82,105,107–112,114–119 patient interviews;61,65,78,86,93,100,106 patient questionnaires;24,31,34,72–74,76,85,90,94,95,97–99 diary cards;62,66,68 laboratory or imaging studies (ie, hematologic and biochemical results, ultrasonography);71,79,102,120 and review of medical records.14,64,65,75,77,88,91,92,96,100,113 Eleven studies did not mention how adverse events or herb–drug interactions were identified.58,67,69,80,83,84,87,89,101,103,104
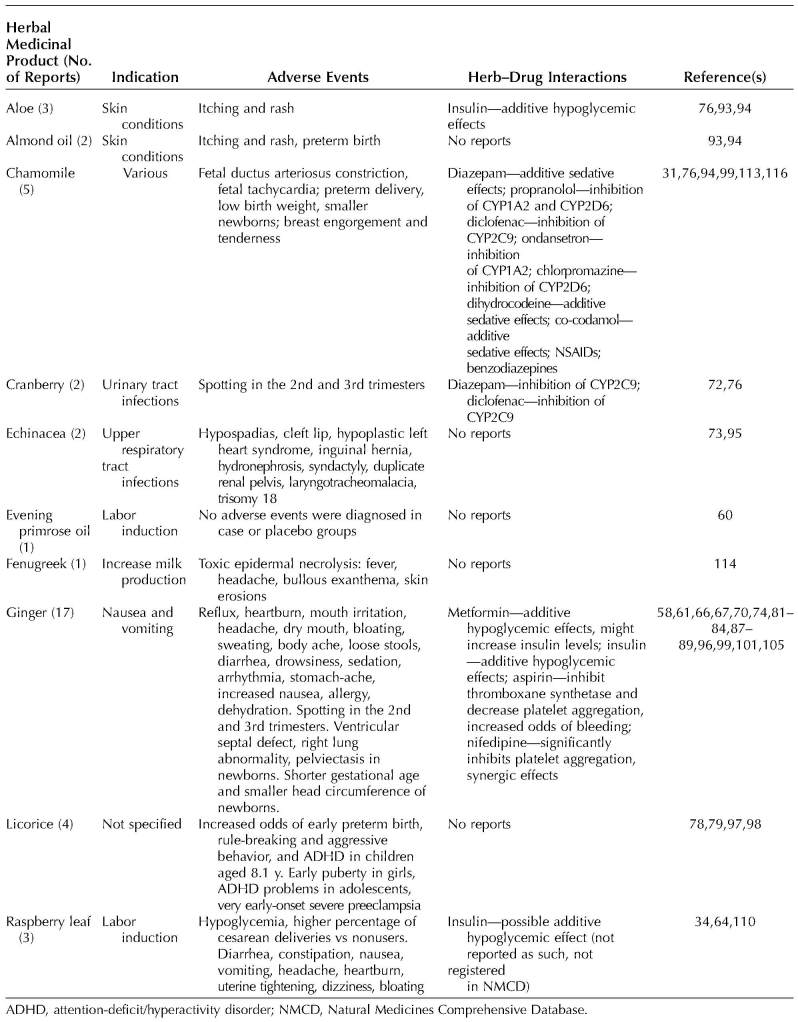
Thirty-one studies identified and reported the incidence of adverse events.14,24,56,61,64–70,72–75,81–84,86–89,93–96,99,100,105,106 Fourteen studies reported that no adverse events were observed57,58,60,62,63,68,71,80,85,101–104,118 (see Appendixes 1–4, available online at http://links.lww.com/AOG/B334, for details). Table 1 reports the most commonly used herbal medicinal products and their reported safety issues.
Table 1.
Reported Safety Issues of the Most Commonly Used Herbal Medicinal Products During Pregnancy and the Postnatal Period
The most frequently reported adverse drug reaction, for all assessed herbal medicinal products, was gastrointestinal complaints. One study comparing the use of capsaicin-containing chili to placebo for the treatment of gestational diabetes mellitus demonstrated a higher rate (60%) of loose stools, gastrointestinal irritation, and diarrhea in women using the study drug compared with none in the placebo group.69 Otherwise, the rates of adverse drug reactions were the same in all interventional studies in the study drug compared with comparator.
Topical use of almond oil during the third trimester to avoid stretch marks was associated with preterm birth (birth before week 37 of pregnancy) (OR 2.09, 95% CI 1.07–4.08).94 When chamomile was used as a substitute for caffeinated tea in the second and third trimester, it was associated with fetal ductus arteriosus constriction, determined by fetal echocardiography in a case report,120 and breast engorgement and a significant (50%) increase in milk production, when used during the postnatal period in another case report.116 Chamomile use during the third trimester was reported in one study to be associated with a higher incidence of preterm birth (P<.002), shorter newborns (P<.05), and low birth weight (P<.002)99 compared with nonusers, whereas a similar study reported no significant increased odds of low birth weight (OR 2.1; 95% CI: 0.99–4.60).94
Ginger is used to treat nausea. The most frequently reported adverse drug reactions were esophageal reflux,89 heartburn,61,66,67,81,83,84,87,89 abdominal discomfort67,84 and increased nausea.84 Heartburn and reflux (n=4), an allergic reaction (n=1), and dehydration (n=1) were reported to be severe enough for study withdrawal in one average-quality, randomized controlled trial that included 48 women who were up to 20 weeks pregnant and were taking ginger.89 The use of ginger throughout pregnancy was associated with a nonsignificant increase in the incidence of stillbirths (OR 7.8, 95% CI 0.9–70.3)70 and a significant decrease in gestational age at delivery and neonatal head circumference (P<.05 and P<.002, respectively)99 compared with nonusers. The use of ginger (7.8% vs 5.8% nonusers [P=.007])74 and cranberry (9.7% vs 5.8% nonusers [P<.001])72 after week 17 of pregnancy was associated with vaginal bleeding (spotting) during 2nd and 3rd trimesters.
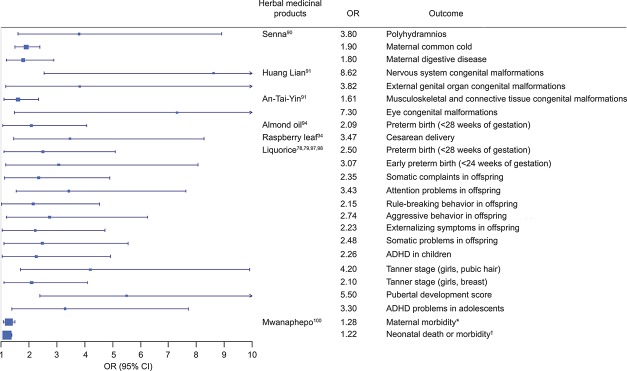
Licorice candy consumption in the second trimester (18 weeks of gestation) was associated with severe, very early-onset preeclampsia in a case report.108 Heavy licorice candy consumption (greater than 500 mg/wk) throughout pregnancy was associated with preterm birth and early preterm birth (birth before week 34 of pregnancy) (adjusted odds ratio [AOR] 2.5, 95% CI 1.1–5.197; AOR 3.07, 95% CI 1.17–8.05,98 respectively) compared with nonusers. Moreover, heavy licorice candy consumption in pregnancy was associated with a variety of psychosocial issues during childhood78 and markers of early puberty for girls 12 years of age79 in the offspring (Fig. 3).
Fig. 3. Forest plot for adverse events of herbal medicinal products. *Maternal morbidity includes cesarean delivery, assisted vaginal delivery, premature rupture of membranes, any postnatal morbidity, and any delivery problem. †Neonatal death or morbidity includes neonatal death, meconium-stained liquor, low birth weight, preterm birth, and any neonatal morbidity. OR, odds ratio; ADHD, attention deficit hyperactivity disorder.
Muñoz Balbontín. Safety of Herbal Medicines During and After Pregnancy. Obstet Gynecol 2019.
Mwanaphepo, an African herbal medicine used for labor induction, was associated with maternal morbidity (ie, emergency cesarean delivery, assisted vaginal delivery, premature rupture of membranes, any postnatal morbidity and any delivery problem) (AOR 1.28; 95% CI 1.09–1.50) and neonatal death or morbidity (ie, neonatal death, meconium-stained liquor, low birth weight, preterm birth and any neonatal morbidity) (AOR 1.22; 95% CI 1.06–1.40).100
Raspberry leaf, when used to induce and shorten labor, was associated with cesarean delivery (AOR 3.47, 95% CI 1.45–8.28).34 Maternal hypoglycemia was reported in one case report.110 Gastrointestinal complaints (28%), headache (1%), uterine tightening (2%), and dizziness (1%) were reported in one randomized controlled trial.64 Senna use during pregnancy was associated with polyhydramnios (OR 3.8, 95% CI 1.6–8.9), weakly associated with influenza or common cold (OR 1.9, 95% CI 1.5–2.4), and acute digestive maternal diseases (OR 1.8, 95% CI 1.2–2.89).90
Hepatotoxicity was reported in six case reports associated with: three different lead-contaminated Ayurvedic medications used to maintain pregnancy,39–41 a herbal tea contaminated with senecionine,119 a lead-contaminated mountain germander infusion,115 and a lead-contaminated fennel and cumin infusion117 each used to increase milk production in the postnatal period.
No significant difference in the incidence of congenital malformations between case and comparator groups has been reported for ginger,70,74,96 echinacea,73,95 cranberry,72 senna,90 and St. John's wort.75 However, Chuang and colleagues reported an association between Huang Lian (Rhizoma coptidis for skin conditions) use during the first trimester and nervous system congenital malformations AOR 8.62, 95% CI 2.54–29.24) and external genital organ congenital malformations (AOR 3.82, 95% CI 1.18–12.40) in the offspring.91 Moreover, use of An-Tai-Yin (Angelica sinensis) and parsley (for prevention of miscarriages) during the first trimester was associated with musculoskeletal and connective tissue congenital malformations (AOR 1.61, 95% CI 1.10–2.36), and eye congenital malformations (AOR 7.30, 95% CI 1.47–36.18) in the offspring.91 Considering the wide CIs reported in these studies, these effect sizes should be considered weak.
Four studies reported herb–drug interactions, but none studied adverse events arising from them. Herb–drug interactions were reported in two cross-sectional surveys34,76 and two prospective cohort studies.14,31 Of these, only three reported their incidence,31,34,76 two reported how they were assessed,31,76 and none reported the incidence of adverse events arising from the identified herb–drug interactions.
Reported herb–drug interactions involved aloe, chamomile, cranberry, ginger, ginseng, common sage, iron-rich herbs and dandelion (see Appendixes 1–4, http://links.lww.com/AOG/B334, for details). Chamomile used alongside prescribed medicines such as diazepam, propranolol, diclofenac, ondansetron, chlorpromazine, dihydrocodeine, and co-codamol was associated with potentially severe herb–drug interactions owing to possible additive sedative effects.34,76 One randomized controlled trial reported 1.6% (1/61) of their participants took other medications alongside ginger,83 and a cross-sectional survey reported ginger was taken with metformin, insulin, aspirin, and nifedipine and could be associated with herb–drug interactions.76 One prospective cohort study reported no increased odds of adverse pregnancy outcomes when cranberry was used together with antibiotics to treat urinary tract infections.72 One case report110 mentioned additive glucose lowering effects when insulin (Lispro) and raspberry leaf were taken simultaneously but did not address this as a potential herb–drug interaction.
The role of herb–drug interactions in the causation of adverse events in pregnant woman has not been assessed. A full list of herbal medicinal products and associated adverse events and herb–drug interactions are available in Appendixes 1–4 (http://links.lww.com/AOG/B334).
DISCUSSION
Robust studies that aim to identify and study the causality of adverse events or herb–drug interactions and associated adverse events arising during pregnancy and the postnatal period as a primary objective are not currently available. Current data suggest that herbal medicinal products such as almond oil, chamomile, licorice, and raspberry leaf used during pregnancy may be associated with adverse maternal and perinatal outcomes or toxicity from contaminants.
Studies focusing on herb–drug interactions are few, although of average to high quality, and did not assess or report adverse events arising from identified herb–drug interactions. Moreover, available evidence demonstrates that widely used herbal medicinal products such as topical almond oil,94 chamomile,120 ginger,58,61,66,67,70,74,81–84,87–89,96,99,101,105 licorice,78,79,97,98 and raspberry leaf34 may be associated with adverse perinatal outcomes. Although the majority of available studies were graded as average and many underpowered, potentially harmful adverse events arising from use of specific herbal medicinal products have been reported (Fig. 3).
The daily use of topical almond oil for stretch marks during the third trimester has been reported to increase the odds of preterm birth.94 The authors hypothesized that continuous rubbing of the belly might stimulate premature myometrial contractions and that components of almond oil might act as prostaglandin precursors. However, only 168 women were exposed to almond oil and the data were collected retrospectively, therefore providing underpowered assumptions and introducing recall bias. Moreover, dosage, surface area of application and almond formulation used were not provided and therefore causality cannot be objectively demonstrated.
The evidence for chamomile during pregnancy reports increased odds of preterm delivery,99 reduced length of newborns, and low birth weight.94 However, both studies were underpowered and of average quality. Chamomile has also been identified as a common source of potentially severe herb–drug interactions when used concurrently with prescribed medicines.34,76 Considering the scarce evidence available, chamomile should be used with caution during pregnancy.
The use of ginger during early pregnancy has been associated with a variety of mild to severe, non–dose-dependent adverse drug reactions, ranging from dry mouth to worsening of nausea and dehydration89; use during late pregnancy has been associated with bleeding or spotting during the second and third trimesters,74 prematurity, and reduced head circumference at birth.99 It has to be noted that formulation, dose, and exposure period were not standardized in these studies. Ginger has also been identified as a source of potentially significant herb–drug interactions with insulin, metformin, and nifedipine, medicines commonly used during pregnancy.76
The use of raspberry leaf has been associated with cesarean delivery by 3.5-fold34; however, this study involved only 34 exposed women. Raspberry leaf has also been associated with hypoglycemia when used with insulin.110 Until there are more safety data, it could be suggested that raspberry leaf should not be used for labor induction because its adverse effects may outweigh its perceived benefit.
Heavy licorice consumption (500 mg glycyrrhizin/wk) throughout pregnancy was reported to increase the odds of preterm birth (AOR 2.5, 95% CI 1.1–5.1)97 and early preterm birth (AOR 3.07, 95% CI 1.17–8.05).98 However, these studies were of average quality, used retrospective data and did not validate exposure. Nonetheless, they had adequate sample sizes and considered validated outcomes. Moreover, glycyrrhizin, the active component in licorice, is recognized to cause developmental issues.78,79,121
The traditional use of mwanaphepo to induce labor in Malawi was associated with maternal morbidity (ie, emergency cesarean delivery on, assisted vaginal delivery, PROM, among others) (AOR 1.28; 95% CI 1.09–1.50) and neonatal death or morbidity (including neonatal death, meconium-stained amniotic fluid, LBW, PTB, among others) (AOR 1.22, 95% CI 1.06–1.40).100 This average quality cross-sectional analysis used grouped retrospective data, which may introduce bias and error,122 and did not validate exposure to the herbal medicinal products. Neither the dosage nor specific preparation of mwanaphepo were reported. Considering the latter, the reported effect sizes in this study should be considered weak.
The absence of adverse events were reported in 11 interventional studies assessing evening primrose oil capsules,60 dill infusion for labor induction,80 red sage for oligohydramnios,57 ginger for nausea and vomiting during pregnancy,58,101 Chinese herbal medicine for intra-uterine growth restriction,102 quince for nausea and vomiting in pregnancy,85 pine bark103 and 1% green tea ointment63 for episiotomy wound healing, saffron for labor induction,62 and a polyherbal infusion to increase milk production.104 One prospective cohort study assessing tea (black green, and herbal) consumption during pregnancy and adverse birth outcomes also reported absence of adverse events.71 More than half of these studies were of poor101–104 or average quality.80,85 Moreover, those studies deemed to be of high quality suffered from a variety of methodologic issues, including small sample sizes,58,62 absence of a power calculation,60 short exposure period,58 and failure to validate exposure.71 Therefore, these studies should not be taken as evidence of safety based on absence of harm, and further robust studies are required.
This review includes all types of studies (interventional, observational and case reports) that report adverse events or herb–drug interactions arising from herbal medicines (including polyherbals) taken during pregnancy and the postnatal period. All interventional and observational studies were included, regardless of quality. However, only high-quality case reports were included because they are considered low scientific evidence per se. Non-English language articles were excluded, possibly leaving out important information from nontranslated articles. Owing to the large number of herbal medicinal products available worldwide and the lack of standardization in reporting the names of herbal medicinal products, it is possible that relevant literature was omitted and that not all herbal medicinal products were reported.
Collective evidence confirms that adverse events and herb–drug interactions arising from herbal medicinal products used during pregnancy and the postnatal period are under-studied and under-reported. Herb–drug interactions are not reported or investigated in the majority of studies. This is of concern given the high prevalence of use of herbal and conventional medicines during and after pregnancy.
The evidence-based use of herbal medicines needs to be backed by robust scientific studies. Currently, there is not enough information to recommend the safe use of herbal medicinal products during pregnancy and the postnatal period. Most herbal medicinal products are recommended and used on the grounds of tradition, historic or anecdotal evidence. Adverse events are generally under-reported to clinical staff, also the use of herbal medicinal products. The U.S. Food and Drug Administration, the European Medicines Agency (European Union), and the Medicines and Healthcare Products Regulatory Agency (United Kingdom) do not subject herbal medicinal products to the same quality standards as medicines. Stricter pharmacovigilance measures should be taken to avoid possible harm arising from current herbal medicinal product use during pregnancy and the postnatal period. Considering the 30 years of evidence of possible harm presented, we conclude herbal medicinal products should not de recommended during pregnancy until robust evidence of safety is available.
Footnotes
Financial Disclosure Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) sponsored Dr. Muñoz Balbontín's doctoral studies. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B335.
REFERENCES
- 1.Merriam-Webster.com. Herbal medicine. Available at: https://www.merriam-webster.com/dictionary/herbal%20medicine. Retrieved September 28, 2018.
- 2.Koren G, Randor S, Martin S, Danneman D. Maternal ginseng use associated with neonatal androgenization. JAMA 1990;264:2866. [PubMed] [Google Scholar]
- 3.Falci L, Shi Z, Greenlee H. Multiple chronic conditions and use of complementary and alternative medicine among US adults: results from the 2012 National Health Interview Survey. Prev Chronic Dis 2016;13:E61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashrash M, Schommer JC, Brown LM. Prevalence and predictors of herbal medicine use among adults in the United States. J Patient Exp 2017;4:108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 1998;280:1569–75. [DOI] [PubMed] [Google Scholar]
- 6.Pokladnikova J, Selke-Krulichova I. Prevalence of complementary and alternative medicine use in the general population in the Czech Republic. Forsch Komplementmed 2016;23:22–8. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Wang C, Tsai M, Huang W, Kennedy J. Trend and pattern of herb and supplement use in the United States: results from the 2002, 2007, and 2012 national health interview surveys. Evid Based Complement Altern Med 2014;2014:872320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep 2015;79:1–16. [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Complementary and Integrative Health, National Institutes of Health. Complementary and alternative medicine: what people aged 50 and older discuss with their health care providers: AARP and NCCAM survey report (2010). Bethesda (MD): National Institutes of Health; 2011. [Google Scholar]
- 10.Eardley S, Bishop FL, Prescott P, Cardini F, Brinkhaus B, Santos-Rey K, et al. A systematic literature review of complementary and alternative medicine prevalence in EU. Forsch Komplementmed 2012;19(suppl 2):18–28. [DOI] [PubMed] [Google Scholar]
- 11.Graham RE, Ahn AC, Davis RB, O'Connor BB, Eisenberg DM, Phillips RS. Use of complementary and alternative medical therapies among racial and ethnic minority adults: results from the 2002 National Health Interview Survey. J Natl Med Assoc 2005;97:535–45. [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt KJ, Coelho HF, Wider B, Perry R, Hung SK, Terry R, et al. Complementary and alternative medicine use in England: results from a national survey. Int J Clin Pract 2010;64:1496–502. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy DA, Lupattelli A, Koren G, Nordeng H. Herbal medicine use in pregnancy: results of a multinational study. BMC Complement Altern Med 2013;13:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holst L, Nordeng H, Haavik S. Use of herbal drugs during early pregnancy in relation to maternal characteristics and pregnancy outcome. Pharmacoepidemiol Drug Saf 2008;17:151–9. [DOI] [PubMed] [Google Scholar]
- 15.Adams J, Lui CW, Sibbritt D, Broom A, Wardle J, Homer C, et al. Women's use of complementary and alternative medicine during pregnancy: a critical review of the literature. Birth 2009;36:237–45. [DOI] [PubMed] [Google Scholar]
- 16.Pallivalappila AR, Stewart D, Shetty A, Pande B, Singh R, Mclay JS. Complementary and alternative medicine use during early pregnancy. Eur J Obstet Gynecol Reprod Biol 2014;181:251–5. [DOI] [PubMed] [Google Scholar]
- 17.Pallivalapila AR, Stewart D, Shetty A, Pande B, Singh R, McLay JS. Use of complementary and alternative medicines during the third trimester. Obstetrics Gynecol 2015;125:204–11. [DOI] [PubMed] [Google Scholar]
- 18.Forster DA, Denning A, Wills G, Bolger M, McCarthy E. Herbal medicine use during pregnancy in a group of Australian women. BMC Pregnancy Childbirth 2006;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westfall RE. Herbal medicine in pregnancy and childbirth. Adv Ther 2001;18:47–55. [DOI] [PubMed] [Google Scholar]
- 20.Hall HG, McKenna LG, Griffiths DL. Midwives' support for complementary and alternative medicine: a literature review. Women and Birth 2012;25:4–12. [DOI] [PubMed] [Google Scholar]
- 21.Nordeng H, Havnen GC. Use of herbal drugs in pregnancy: a survey among 400 Norwegian women. Pharmacoepidemiol Drug Saf 2004;13:371–80. [DOI] [PubMed] [Google Scholar]
- 22.Mekuria AB, Erku DA, Gebresillassie BM, Birru EM, Tizazu B, Ahmedin A. Prevalence and associated factors of herbal medicine use among pregnant women on antenatal care follow-up at University of Gondar referral and teaching hospital, Ethiopia: a cross-sectional study. BMC Complement Altern Med 2017;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laelago T, Yohannes T, Lemango F. Prevalence of herbal medicine use and associated factors among pregnant women attending antenatal care at public health facilities in Hossana Town, Southern Ethiopia: facility based cross sectional study. Arch Public Health 2016;74:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettiol A, Niccolò L, Marconi E, Crescioli G, Bonaiuti R, Maggini V, et al. The use of complementary and alternative medicines during breastfeeding: results from the Herbal supplements in Breastfeeding InvesTigation (HaBIT). Br J Clin Pharmacol 2018;84:2040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turkyilmaz C. Effect of galactagogue herbal tea on breast milk production. In: Zibadi S, Watson RR, Preedy VR, editors. Handbook of dietary and nutritional aspects of human breast milk. Wageningen (The Netherlands): Wageningen Academic Publishers; 2013. p. 615–30. [Google Scholar]
- 26.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol 2011;205:51.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupattelli A, Spigset O, Twigg MJ, Zagorodnikova K, Mardby AC, Moretti ME, et al. Medication use in pregnancy: a cross-sectional, multinational web-based study. BMJ Open 2014;4:e004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephansson O, Granath F, Svensson T, Haglund B, Ekbom A, Kieler H. Drug use during pregnancy in Sweden—assessed by the prescribed drug register and the medical birth register. Clin Epidemiol 2011;3:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee E, Maneno MK, Smith L, Weiss SR, Zuckerman IH, Wutoh AK, et al. National patterns of medication use during pregnancy. Pharmacoepidemiol Drug Saf 2006;15:537–45. [DOI] [PubMed] [Google Scholar]
- 30.Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf 2011;20:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moussally K, Berard A. Exposure to specific herbal products during pregnancy and the risk of low birth weight. Altern Ther Health Med 2012;18:36–43. [PubMed] [Google Scholar]
- 32.Mothupi MC. Use of herbal medicine during pregnancy among women with access to public healthcare in Nairobi, Kenya: a cross-sectional survey. BMC Complement Altern Med 2014;14:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabatabaee M. Use of herbal medicine among pregnant women referring to Valiasr Hospital in Kazeroon, Fars, South of Iran. J Med Plants 2011;10:96–108. [Google Scholar]
- 34.Nordeng H, Bayne K, Havnen GC, Paulsen BS. Use of herbal drugs during pregnancy among 600 Norwegian women in relation to concurrent use of conventional drugs and pregnancy outcome. Complement Ther Clin Pract 2011;17:147–51. [DOI] [PubMed] [Google Scholar]
- 35.Palmer ME, Haller C, McKinney PE, Klein-Schwartz W, Tschirgi A, Smolinske SC, et al. Adverse events associated with dietary supplements: an observational study. Lancet 2003;361:101–6. [DOI] [PubMed] [Google Scholar]
- 36.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 2009;69:1777–98. [DOI] [PubMed] [Google Scholar]
- 37.Finkel RS, Zarlengo KM. Blue cohosh and perinatal stroke. N Engl J Med 2004;351:302–3. [DOI] [PubMed] [Google Scholar]
- 38.Blitz MJ, Smith-Levitin M, Rochelson B. Severe hyponatremia associated with use of black cohosh during prolonged labor and unsuccessful home birth. AJP Rep 2016;6:121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong A, Dargan P, Koutsogiannis Z, Sokol J, Ramkrishna J, Greene SL. Chronic ayurvedic medicine use associated with major and fatal congenital abnormalities. Med J Aust 2015;203:443–4. [DOI] [PubMed] [Google Scholar]
- 40.Tait PA, Vora A, James S, Fitzgerald DJ, Pester BA. Severe congenital lead poisoning in a preterm infant due to a herbal remedy. Med J Aust 2002;177:193–5. [DOI] [PubMed] [Google Scholar]
- 41.Shamshirsaz AA, Yankowitz J, Rijhsinghani A, Greiner A, Holstein SA, Niebyl JR. Severe lead poisoning caused by use of health supplements presenting as acute abdominal pain during pregnancy. Obstet Gynecol 2009;114:448–50. [DOI] [PubMed] [Google Scholar]
- 42.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001;61:2163–75. [DOI] [PubMed] [Google Scholar]
- 43.Ulbricht C, Armstrong J, Basch E, Basch S, Bent S, Dacey C, et al. An evidence-based systematic review of aloe vera by the natural standard research collaboration. J Herbal Pharmacother 2007;7:279–323. [DOI] [PubMed] [Google Scholar]
- 44.Ulbricht C, Conquer J, Costa D, Hamilton W, Higdon ERB, Isaac R, et al. An evidence-based systematic review of Senna (Cassia senna) by the natural standard research collaboration. J Diet Suppl 2011;8:189–238. [DOI] [PubMed] [Google Scholar]
- 45.Ulbricht C, Basch E, Burke D, Cheung L, Ernst E, Giese N, et al. Fenugreek (Trigonella foenum-graecum L. Leguminosae): an evidence-based systematic review by the natural standard research collaboration. J Herbal Pharmacother 2007;7:143–77. [DOI] [PubMed] [Google Scholar]
- 46.Dugoua JJ, Seely D, Perri D, Koren G, Mills E. Safety and efficacy of black cohosh (Cimicifuga racemosa) during pregnancy and lactation. Can J Clin Pharmacol 2006;13:e257–61. [PubMed] [Google Scholar]
- 47.Dugoua JJ, Perri D, Seely D, Mills E, Koren G. Safety and efficacy of blue cohosh (Caulophyllum thalictroides) during pregnancy and lactation. Can J Clin Pharmacol 2008;15:e66–73. [PubMed] [Google Scholar]
- 48.Dugoua JJ, Seely D, Perri D, Koren G, Mills E. Safety and efficacy of chastetree (Vitex Agnus-Castus) during pregnancy and lactation. Can J Clin Pharmacol 2008;15:e74–9. [PubMed] [Google Scholar]
- 49.Dugoua JJ, Seely D, Perri D, Mills E, Koren G. Safety and efficacy of cranberry (Vaccinium Macrocarpon) during pregnancy and lactation. Can J Clin Pharmacol 2008;15:e80–6. [PubMed] [Google Scholar]
- 50.Posadzki P, Watson LK, Ernst E. Adverse effects of herbal medicines: an overview of systematic reviews. Clin Med (Lond) 2013;13:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol 2013;75:603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Critical Appraisal Skills Programme (CASP). CASP checklist for randomised controlled trials. Available at: https://casp‐uk.net/wp‐content/uploads/2018/03/CASP‐Randomised‐Controlled‐Trial‐Checklist‐2018_fillable_form.pdf. Retrieved December 1, 2017. [Google Scholar]
- 53.Critical Appraisal Skills Programme (CASP). CASP checklist for cohort studies. Available at: https://casp‐uk.net/wp‐content/uploads/2018/01/CASP‐Cohort‐Study‐Checklist_2018.pdf. Retrieved December 1, 2017. [Google Scholar]
- 54.The Joanna Briggs Institute. JBI critical appraisal checklist for case reports. Available at: http://joannabriggs.org/assets/docs/critical-appraisal-tools/JBI_Critical_Appraisal-Checklist_for_Case_Reports2017.pdf. December 1, 2017. [Google Scholar]
- 55.Muñoz Balbontín Y, Stewart D, Shetty A, McLay J. Safety issues associated with herbal medicines use during pregnancy and the postnatal period: a systematic review protocol. PROSPERO. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017081058. Retrieved March 28, 2018. [Google Scholar]
- 56.Asgharikhatooni A, Bani S, Hasanpoor S, Alizade SM, Javadzadeh Y. The effect of equisetum arvense (horse tail) ointment on wound healing and pain intensity after episiotomy: a randomized placebo-controlled trial. Iran Red Crescent Med J 2015;17:e25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu HN, Shen MJ. Treating oligohydramnios with extract of Salvia miltiorrhiza: a randomized control trial. Ther Clin Risk Manag 2008;4:287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ensiyeh J, Sakineh MA. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomised controlled trial. Midwifery 2009;25:649–53. [DOI] [PubMed] [Google Scholar]
- 59.Gharabaghi PM, Tabatabei F, Fard SA, Sayyah-Melli M, Ouladesahebmadarek E, Del Azar A, et al. Evaluation of the effect of preemptive administration of Rosa damascena extract on post-operative pain in elective cesarean sections. Afr J Pharm Pharmacol 2011;5:1950–5. [Google Scholar]
- 60.Kalati M, Kashanian M, Jahdi F, Naseri M, Haghani H, Sheikhansari N. Evening primrose oil and labour, is it effective? A randomised clinical trial. J Obstet Gynaecol 2018;38:488–92. [DOI] [PubMed] [Google Scholar]
- 61.Kalava A, Darji SJ, Kalstein A, Yarmush JM, Schianodicola J, Weinberg J. Efficacy of ginger on intraoperative and postoperative nausea and vomiting in elective Cesarean section patients. Eur J Obstet Gynecol Reprod Biol 2013;169:184–8. [DOI] [PubMed] [Google Scholar]
- 62.Sadi R, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Javadzadeh Y, Ahmadi-Bonabi A. Effect of saffron (Fan Hong Hua) on the readiness of the uterine cervix in term pregnancy: a placebo-controlled randomized trial. Iran Red Crescent Med J 2016;18:e27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahrahmani H, Kariman N, Jannesari S, Rafieian-Kopaei M, Mirzaei M, Sahar Ghalandari S, et al. The effect of green tea ointment on episiotomy pain and wound healing in primiparous women: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res 2018;32:522–30. [DOI] [PubMed] [Google Scholar]
- 64.Simpson M, Parsons M, Greenwood J, Wade K. Raspberry leaf in pregnancy: its safety and efficacy in labor. J Midwifery Womens Health 2001;46:51–9. [DOI] [PubMed] [Google Scholar]
- 65.Tabeshpour J, Sobhani F, Sadjadi SA, Hosseinzadeh H, Mohajeri SA, Rajabi O, et al. A double-blind, randomized, placebo-controlled trial of saffron stigma (Crocus sativus L.) in mothers suffering from mild-to-moderate postpartum depression. Phytomedicine 2017;36:145–52. [DOI] [PubMed] [Google Scholar]
- 66.Tianthong W, Phupong V. A randomized, double-blind, placebo-controlled trial on the efficacy of ginger in the prevention of abdominal distention in post cesarean section patients. Sci Rep 2018;8:6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vutyavanich T, Kraisarin T, Ruangsri R. Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial. Obstet Gynecol 2001;97:577–82. [DOI] [PubMed] [Google Scholar]
- 68.Wagner CL, Boan AD, Marzolf A, Finch CW, Morella K, Guille C, et al. The safety of mother's milk tea: results of a randomized double-blind, controlled study in fully breastfeeding mothers and their infants. J Hum Lact 2018:0890334418787474. [DOI] [PubMed] [Google Scholar]
- 69.Yuan LJ, Qin Y, Wang L, Zeng Y, Chang H, Wang J, et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin Nutr 2016;35:388–93. [DOI] [PubMed] [Google Scholar]
- 70.Choi JS, Han JY, Ahn HK, Lee SW, Koong MK, Velazquez-Armenta EY, et al. Assessment of fetal and neonatal outcomes in the off spring of women who had been treated with dried ginger (Zingiberis rhizoma siccus) for a variety of illnesses during pregnancy. J Obstet Gynaecol 2015;35:125–30. [DOI] [PubMed] [Google Scholar]
- 71.Colapinto CK, Arbuckle TE, Dubois L, Fraser W. Tea consumption in pregnancy as a predictor of pesticide exposure and adverse birth outcomes: the MIREC Study. Environ Res 2015;142:77–83. [DOI] [PubMed] [Google Scholar]
- 72.Heitmann K, Nordeng H, Holst L. Pregnancy outcome after use of cranberry in pregnancy—the Norwegian mother and child cohort study. BMC Complement Altern Med 2013;13:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heitmann K, Havnen GC, Holst L, Nordeng H. Pregnancy outcomes after prenatal exposure to echinacea: the Norwegian mother and child cohort study. Eur J Clin Pharmacol 2016;72:623–30. [DOI] [PubMed] [Google Scholar]
- 74.Heitmann K, Nordeng H, Holst L. Safety of ginger use in pregnancy: results from a large population-based cohort study. Eur J Clin Pharmacol 2013;69:269–77. [DOI] [PubMed] [Google Scholar]
- 75.Kolding L, Pedersen LH, Henriksen TB, Olsen J, Grzeskowiak LE. Hypericum perforatum use during pregnancy and pregnancy outcome. Reprod Toxicol 2015;58:234–7. [DOI] [PubMed] [Google Scholar]
- 76.McLay J, Izzati N, Pallivalapilla A, Shetty A, Pande B, Rore C, et al. Pregnancy, prescription medicines and the potential risk of herb-drug interactions: a cross-sectional survey. BMC Complement Altern Med 2017;17:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plangger N, Rist L, Zimmermann R, Von Mandach U. Intravenous tocolysis with Bryophyllum pinnatum is better tolerated than beta-agonist application. Eur J Obstet Gynecol Reprod Biol 2006;124:168–72. [DOI] [PubMed] [Google Scholar]
- 78.Raikkonen K, Pesonen AK, Heinonen K, Lahti J, Komsi N, Eriksson JG, et al. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Obstet Gynecol Surv 2010;65:84–6. [DOI] [PubMed] [Google Scholar]
- 79.Raikkonen K, Martikainen S, Pesonen AK, Lahti J, Heinonen K, Pyhala R, et al. Maternal licorice consumption during pregnancy and pubertal, cognitive, and psychiatric outcomes in children. Am J Epidemiol 2017;185:317–28. [DOI] [PubMed] [Google Scholar]
- 80.Akbari M, Javadnoori M, Siahpoosh A, Afshari P, Haghighi MH, Lake E. Comparison the effect of anethum graveolens and oxytocin on induction of labor in term pregnancy: a randomized clinical trial. Jundishapur J Nat Pharm Prod 2016;11:e27876. [Google Scholar]
- 81.Basirat Z, Moghadamnia AA, Kashifard M, Sarifi-Razavi A. The effect of ginger biscuit on nausea and vomiting in early pregnancy. Acta Med Iran 2009;47:51–6. [Google Scholar]
- 82.Biswas SC, Dey R, Kamliya GS, Bal R, Hazra A, Tripathi SK. A single-masked, randomized, controlled trial of ginger extract in the treatment of nausea and vomiting of pregnancy. J Int Med Sci Acad 2011;24:167–9. [Google Scholar]
- 83.Chittumma P, Kaewkiattikun K, Wiriyasiriwach B. Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: a randomized double-blind controlled trial. J Med Assoc Thai 2007;90:15–20. [PubMed] [Google Scholar]
- 84.Firouzbakht M, Nikpour M, Jamali B, Omidvar S. Comparison of ginger with vitamin B6 in relieving nausea and vomiting during pregnancy. Ayu 2014;35:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jafari-Dehkordi E, Hashem-Dabaghian F, Aliasl F, Aliasl J, Taghavi-Shirazi M, Sadeghpour O, et al. Comparison of quince with vitamin B6 for treatment of nausea and vomiting in pregnancy: a randomised clinical trial. J Obstet Gynaecol 2017:1–5. [DOI] [PubMed] [Google Scholar]
- 86.Ketsuwan S, Baiya N, Paritakul P, Laosooksathit W, Puapornpong P. Effect of herbal compresses for maternal breast engorgement at postpartum: a randomized controlled trial. Breastfeed Med 2018;13:361–5. [DOI] [PubMed] [Google Scholar]
- 87.Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. J Med Assoc Thai 2007;90:1703–9. [PubMed] [Google Scholar]
- 88.Smith C, Crowther C, Willson K, Hotham N, McMillian V. A randomized controlled trial of ginger to treat nausea and vomiting in pregnancy. Obstet Gynecol 2004;103:639–45. [DOI] [PubMed] [Google Scholar]
- 89.Willetts KE, Ekangaki A, Eden JA. Effect of a ginger extract on pregnancy-induced nausea: a randomised controlled trial. Aust N Z J Obstet Gynaecol 2003;43:139–44. [DOI] [PubMed] [Google Scholar]
- 90.Ács N, Bánhidy F, Puhó EH, Czeizel AE. Senna treatment in pregnant women and congenital abnormalities in their offspring—a population-based case–control study. Reprod Toxicol 2009;28:100–4. [DOI] [PubMed] [Google Scholar]
- 91.Chuang CH, Doyle P, Wang JD, Chang PJ, Lai JN, Chen PC. Herbal medicines used during the first trimester and major congenital malformations—an analysis of data from a pregnancy cohort study. Drug Saf 2006;29:537–48. [DOI] [PubMed] [Google Scholar]
- 92.Chuang CH, Lai JN, Wang JD, Chang PJ, Chen PC. Use of Coptidis Rhizoma and foetal growth: a follow-up study of 9895 pregnancies. Pharmacoepidemiol Drug Saf 2006;15:185–92. [DOI] [PubMed] [Google Scholar]
- 93.Cuzzolin L, Francini-Pesenti F, Verlato G, Joppi M, Baldelli P, Benoni G. Use of herbal products among 392 Italian pregnant women: focus on pregnancy outcome. Pharmacoepidemiol Drug Saf 2010;19:1151–8. [DOI] [PubMed] [Google Scholar]
- 94.Facchinetti F, Pedrielli G, Benoni G, Joppi M, Verlato G, Dante G, et al. Herbal supplements in pregnancy: unexpected results from a multicentre study. Hum Reprod 2012;27:3161–7. [DOI] [PubMed] [Google Scholar]
- 95.Gallo M, Sarkar M, Au W, Pietrzak K, Comas B, Smith M, et al. Pregnancy outcome following gestational exposure to echinacea—a prospective controlled study. Arch Intern Med 2000;160:3141–3. [DOI] [PubMed] [Google Scholar]
- 96.Portnoi G, Chng LA, Karimi-Tabesh L, Koren G, Tan MP, Einarson A. Prospective comparative study of the safety and effectiveness of ginger for the treatment of nausea and vomiting in pregnancy. Obstet Gynecol 2003;189:1374–7. [DOI] [PubMed] [Google Scholar]
- 97.Strandberg TE, Jarvenpaa AL, Vanhanen H, McKeigue PM. Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol 2001;153:1085–8. [DOI] [PubMed] [Google Scholar]
- 98.Strandberg TE, Andersson S, Jarvenpaa AL, McKeigue PM. Preterm birth and licorice consumption during pregnancy. Am J Epidemiol 2002;156:803–5. [DOI] [PubMed] [Google Scholar]
- 99.Trabace L, Tucci P, Ciuffreda L, Matteo M, Fortunato F, Campolongo P, et al. “Natural” relief of pregnancy-related symptoms and neonatal outcomes: above all do no harm. J Ethnopharmacol 2015;174:396–402. [DOI] [PubMed] [Google Scholar]
- 100.Zamawe C, King C, Jennings HM, Fottrell E. Associations between the use of herbal medicines and adverse pregnancy outcomes in rural Malawi: a secondary analysis of randomised controlled trial data. BMC Complement Altern Med 2018;18:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer-Rasmussen W, Kjaer SK, Dahl C, Asping U. Ginger treatment of hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol 1991;38:19–24. [DOI] [PubMed] [Google Scholar]
- 102.Huang G, Shu Y, Ye W. Clinical study on effect of Chinese herbal medicine for supplementing kidney and qi and activating blood circulation in treating intrauterine growth retardation of fetus [Chinese]. Zhongguo Zhong Xi Yi Jie He Za Zhi 1999;19:466–9. [PubMed] [Google Scholar]
- 103.Kohama T, Inoue M. Pycnogenol alleviates pain associated with pregnancy. Phytother Res 2006;20:232–4. [DOI] [PubMed] [Google Scholar]
- 104.Ozalkaya E, Aslandogdu Z, Ozkoral A, Topcuoglu S, Karatekin G. Effect of a galactagogue herbal tea on breast milk production and prolactin secretion by mothers of preterm babies. Niger J Clin Pract 2018;21:38–42. [DOI] [PubMed] [Google Scholar]
- 105.Rukh L, Nazar H, Usmanghani K. Efficacy of Gingocap as compared to pyridoxine in the treatment of nausea and vomiting during pregnancy. Pakistan J Pharm Sci 2016;29:1937–43. [PubMed] [Google Scholar]
- 106.Mabina MH, Pitsoe SB, Moodley J. The effect of traditional herbal medicines on pregnancy outcome. The King Edward VIII Hospital experience. S Afr Med J 1997;87:1008–10. [PubMed] [Google Scholar]
- 107.Al-Jaroudi D, Kaddour O, Al-Amin N. Risks of myrrh usage in pregnancy. JBRA Assist Reprod 2016;20:257–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hauksdottir D, Sigurjonsdottir HA, Arnadottir M, Geirsson RT. Severe, very early onset pre-eclampsia associated with liquorice consumption. Hypertens Pregnancy 2015;34:221–6. [DOI] [PubMed] [Google Scholar]
- 109.Akita H, Sowa J, Makiura M, Akamatsu H, Matsunaga K. Maculopapular drug eruption due to the Japanese herbal medicine Kakkonto (kudzu or arrowroot decoction). Contact Derm 2003;48:348–9. [DOI] [PubMed] [Google Scholar]
- 110.Cheang KI, Nguyen TT, Karjane NW, Salley KES. Raspberry leaf and hypoglycemia in gestational diabetes mellitus. Obstet Gynecol 2016;128:1421–4. [DOI] [PubMed] [Google Scholar]
- 111.Ozturk Z, Kalayci CC. Pregnancy outcomes in psychiatric patients treated with passiflora incarnata. Complement Therapies Med 2018;36:30–2. [DOI] [PubMed] [Google Scholar]
- 112.Stavropoulos K, Sotiriadis A, Patoulias D, Imprialos K, Dampali R, Athyros V, et al. Pseudohyperaldosteronism due to mumijo consumption during pregnancy: a licorice-like syndrome. Gynecol Endocrinol 2018;36:1019–21. [DOI] [PubMed] [Google Scholar]
- 113.Jones TK, Lawson BM. Profound neonatal congestive heart failure caused by maternal consumption of blue cohosh herbal medication. J Pediatr 1998;132:550–2. [DOI] [PubMed] [Google Scholar]
- 114.Bentele-Jaberg N, Guenova E, Mehra T, Naegeli M, Chang Y, Cozzio A, et al. The phytotherapeutic fenugreek as trigger of toxic epidermal necrolysis. Dermatology 2015;231:99–102. [DOI] [PubMed] [Google Scholar]
- 115.Dag M, Ozturk Z, Aydnl M, Koruk I, Kadayfc A. Postpartum hepatotoxicity due to herbal medicine Teucrium polium. Ann Saudi Med 2014;34:541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silva FV, Dias F, Costa G, Campos MDG. Chamomile reveals to be a potent galactogogue: the unexpected effect. J Maternal-Fetal Neonatal Med 2018;31:116–18. [DOI] [PubMed] [Google Scholar]
- 117.Zengin S, Oktay MM, Kamalak M, Al B, Yildirim C, Büyükaslan H. Acute hepatitis associated with the use of herbal tea (Fennel and cumin). J Clin Anal Med 2015;6:781–3. [Google Scholar]
- 118.Mann BR, Zhang H. Improvement in lactation with traditional Chinese medicine and western herbal medicine: a case study. J Chin Med 2014;105:50–5. [Google Scholar]
- 119.Roulet M, Laurini R, Rivier L, Calame A. Hepatic veno-occlusive disease in newborn infant of a woman drinking herbal tea. J Pediatr 1988;112:433–6. [DOI] [PubMed] [Google Scholar]
- 120.Sridharan S, Archer N, Manning N. Premature constriction of the fetal ductus arteriosus following the maternal consumption of camomile herbal tea. Ultrasound Obstet Gynecol 2009;34:358–9. [DOI] [PubMed] [Google Scholar]
- 121.Raikkonen K, Seckl JR, Heinonen K, Pyhala R, Feldt K, Jones A, et al. Maternal prenatal licorice consumption alters hypothalamic-pituitary-adrenocortical axis function in children. Psychoneuroendocrinology 2010;35:1587–93. [DOI] [PubMed] [Google Scholar]
- 122.Bliese PD, Hanges PJ. Being both too liberal and too conservative: the perils of treating grouped data as though they were independent. Organ Res Methods 2004;7:400–17. [Google Scholar]