Supplemental Digital Content is available in the text.
Keywords: poly(ADP-ribose) polymerase inhibitors, QTc study, QT interval prolongation, talazoparib
Abstract
The aims of this study were (i) to evaluate the effect of talazoparib (1 mg once daily) on cardiac repolarization in patients with advanced solid tumors by assessing corrected QT interval (QTc) and (ii) to examine the relationship between plasma talazoparib concentration and QTc. In this open-label phase 1 study, patients had continuous 12-lead ECG recordings at baseline followed by time-matched continuous ECG recordings and collection of talazoparib plasma pharmacokinetic samples predose and at 1, 2, 4, and 6 h postdose on treatment days 1 and 22 and before talazoparib administration on day 2. ECG recordings were submitted for independent central review where triplicate 10-s ECGs, extracted up to 15 min before pharmacokinetic samples, were assessed for RR, PR, QRS, and QT intervals and ECG morphology. QT interval was corrected for heart rate using Fridericia’s (QTcF) and Bazett’s (QTcB) formulae. Linear mixed-effects modeling was used to examine the relationship between QTc and RR interval change from baseline and plasma talazoparib concentration. Thirty-seven patients received talazoparib. Mean change in QTcF from time-matched baseline ranged from −3.5 to 6.9 ms, with the greatest change 1 h postdose on day 22. No clinically relevant changes in PR, QRS, QTcB, QTcF, or RR intervals, heart rate, or ECG morphology were observed. No concentration-dependent effect on heart rate or QTc was observed. No deaths, permanent treatment discontinuations due to adverse events were reported. Talazoparib (1 mg once daily) had no clinically relevant effects on cardiac repolarization.
Introduction
Talazoparib is a potent, orally bioavailable, small molecule poly(ADP-ribose) polymerase (PARP) inhibitor that shows cytotoxicity in various cancers associated with defective cellular DNA damage repair. Talazoparib’s mechanism of action includes inhibition of PARP1 and PARP2 enzymes, which play an instrumental role in detection and repair of single-strand DNA damage 1, and PARP trapping, in which PARP protein bound to a PARP inhibitor remains durably associated with DNA, preventing DNA repair, replication, and transcription 2. As cells with mutations in breast cancer susceptibility genes 1 or 2 (BRCA1/2) have an impaired DNA double-strand break repair mechanism, tumors with BRCA1/2 mutations are highly dependent on the single-strand repair pathway, regulated by PARP 3,4. As such, PARP inhibitors selectively destroy BRCA1/2-positive tumor cells with relevant recombination pathway defects through accumulated, irreparable DNA damage 5.
In a first-in-human phase I trial (NCT01286987), 1 mg once daily (QD) was established as the talazoparib maximum tolerated dose. Dosing talazoparib at the maximum tolerated dose led to confirmed objective responses (per Response Evaluation Criteria in Solid Tumors, version 1.1) in patients with germline BRCA-mutated breast [7/14 (50%)], ovarian [5/12 (42%)], and pancreatic cancers [2/10 (20%)] 6. The most common adverse events were transient, reversible cytopenias.
Talazoparib was approved for use by the US Food and Drug Administration for the treatment of adults with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm) human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer. This approval was based on data from the phase 3 EMBRACA trial (NCT01945775) conducted in 431 patients with HER2-negative advanced breast cancer and germline BRCA mutation. Patients treated with talazoparib (1 mg QD) had significantly longer median progression-free survival than those receiving physician’s choice of chemotherapy (8.6 vs. 5.6 months, respectively; 95% confidence ratio: 0.41–0.71; hazard ratio: 0.54; P<0.0001) 7. Grades 3–4 AEs were primarily hematologic and occurred in 55 and 38% of patients on talazoparib and physician’s choice of chemotherapy, respectively, although only 1.4% of patients in the talazoparib arm permanently discontinued because of a hematologic adverse event 8,9. Patients who received talazoparib had significant overall improvements in patient-reported global health status/quality of life scores and a significantly greater delay in time to clinical deterioration 10.
It is widely recognized that some agents can delay cardiac repolarization, observed as a prolonged QT interval on an ECG. Delays in cardiac repolarization increase the risk of life-threatening cardiac arrhythmias, such as torsades de pointes, which can lead to sudden cardiac death 11. As recommended by the International Conference on Harmonization (ICH) guidelines, drugs in clinical development are therefore required to undergo a well-controlled, thorough QT/corrected QT (QTc) clinical study (i.e. TQT study) to evaluate the risk of QT interval prolongation and cardiac arrhythmias during long-term use 12.
TQT studies are generally single-dose crossover studies in healthy volunteers and include a placebo arm, active controls, and higher than normal doses of an investigational agent to achieve supratherapeutic concentrations. However, it is not always possible to undertake a TQT study because of practical or ethical concerns. For instance, the potential for toxicity when evaluating some anticancer drugs can preclude running studies in healthy volunteers and using supratherapeutic doses of investigational drugs. In addition, the need to omit a placebo group for ethical reasons when running QTc evaluations in cancer patients may necessitate an alternative approach 13. In these cases, evaluating the effect of the therapeutic dose of an investigational agent on QTc using a reduced study design is considered an acceptable alternative to the TQT study when supported by additional exposure–response analyses of the concentration-QTc data 14.
Although in-vitro safety pharmacology assessments, preclinical animal toxicity studies, and routine pharmacovigilance identified no evidence of QTc prolongation or arrhythmogenic effects with talazoparib (Pfizer Inc., New York City, New York, USA, data on file), a more rigorous evaluation of the potential effect of talazoparib on QTc prolongation was required to satisfy ICH recommendations. The purpose of the current study was therefore to provide a definitive assessment of the effects of talazoparib on cardiac electrophysiology by evaluating the risk of QTc prolongation using serial ECG recordings with time-matched baselines and time-matched pharmacokinetic (PK) measurements. As talazoparib is mutagenic in vitro and in vivo and the therapeutic dose of 1 mg QD is the maximum tolerated dose, a traditional TQT study would not have been ethical or practical. Therefore, this study was carried out in patients with advanced solid tumors and no standard treatment options with talazoparib administered at the therapeutic dose of 1 mg QD in reduced study design.
Participants and methods
Study design, patients, and treatments
This open-label phase 1 safety study was conducted at seven centers in the USA. Eligible patients were older than or equal to 18 years with histologically or cytologically confirmed advanced solid tumors, Eastern Cooperative Oncology Group (ECOG) performance status of up to 2, estimated life expectancy of at least 3 months, and no standard treatment options. Patients were excluded if they had received antineoplastic therapies or investigational agents within 21 days, or major surgery within 14 days, before baseline. Other exclusion criteria included ongoing toxicity from previous treatment, electrolyte disturbance, myelodysplastic syndrome, other hematologic malignancies, or clinically significant cardiovascular disease.
The study design is detailed in Fig. 1. Eligible patients had continuous 12-lead ECG recordings at baseline (day −1), followed by a collection of time-matched plasma samples for talazoparib PK analysis and continuous ECG recordings predose and at 1, 2, 4, and 6 h postdose on days 1 and 22 and before talazoparib administration on day 2. At baseline, computed tomography or MRI scans were conducted to assess disease stage, as appropriate for the type of cancer and sites of disease. Talazoparib 1 mg QD was administered in the presence of study personnel on day 1 and within an hour of the time of the day 1 dose on days 2 and 22. Patients self-administered the drug on days 3–21 at approximately the same time as the day 1 dose. Patients were instructed to fast for at least 6 h before and 2 h after talazoparib dosing on days 1 and 22; on other days, talazoparib could be taken with or without food.
Fig. 1.

Study design. aSingle (not continuous) ECG performed. bUnless continuing in extension study. cContinuous ECG starting at time 0 (corresponding to day 1 dosing time) for 6 h. 1Conducted within 72 h of day –1 (time 0); 2ECG recording started 45 min before time 0 and continued for 6 h postdose; 330-min continuous ECG before administration of talazoparib; 4Steady state continuous 12-lead ECG recording, starting 45 min before talazoparib administration, and continuing for 6 h postdose; 5Blood samples collected predose and 1, 2, 4, and 6 h postdose; 6Collected before administration of talazoparib.
This study was carried out in accordance with the ethical principles of the Declaration of Helsinki and in compliance with all ICH Good Clinical Practice Guidelines. The protocol was reviewed and approved by institutional review boards at each participating center, and all patients provided written informed consent.
Endpoints and assessments
ECG assessments
Timing and duration of ECGs assessments are presented in Fig. 1. Continuous 12-lead ECG recordings were obtained digitally after 10 min lying supine, using a Mortara Instrument (Mortara Instrument Inc., Milwaukee, Wisconsin, USA) H-12+ ECG continuous 12-lead digital recorder. ECG data from continuous 12-lead ECG recordings were submitted for independent central review to eResearch Technology Inc. (Philidelphia, Pennsylvania, USA) where triplicate 10-s ECGs were extracted from within a 5-min time window at prespecified, time-matched timepoints starting 15 min before collection of each PK sample. Interval duration measurements were collected using computer-assisted caliper placements on three consecutive beats and analyzed centrally for lead and beat placement before further manual adjudication by a cardiologist using an electronic caliper system applied on a computer screen. The cardiologist verified the PR, QRS, QT, and RR interval durations and performed morphology analysis, noting T–U wave complexes that were compatible with an effect on cardiac repolarization. QTcB, QTcF, and heart rate were derived by standard calculations.
Pharmacokinetic assessments
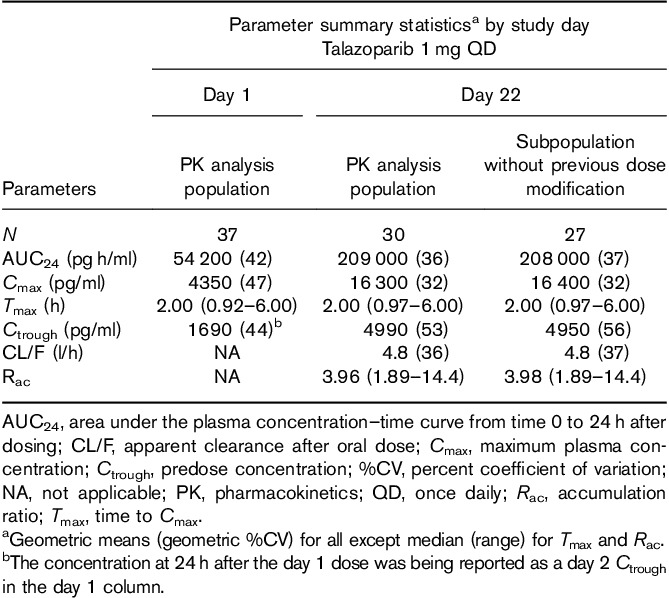
Blood samples for determination of talazoparib concentrations in plasma were collected at times specified in Fig. 1. Talazoparib PK parameters [maximum concentration (Cmax), time to reach Cmax (Tmax), area under the concentration–time curve at 24 h (AUC24), accumulation ratio (Rac), apparent oral clearance (CL/F), and predose concentration (Ctrough)] were calculated on the basis of actual sample collection time for each patient on days 1 and 22, using standard noncompartmental analyses of concentration–time data.
Plasma talazoparib concentration was evaluated using a validated, sensitive, and specific high-performance liquid chromatography–tandem mass spectrometry method (Alliance Pharma Inc., Malvern, Pennsylvania, USA) as described previously 6. Calibration standard responses were linear over the range of 25.0–25 000 pg/ml using weighted (l/concentration2) linear least squares regression. Samples with concentrations above the upper limit of quantification were diluted into calibration range. The lower limit of quantification for talazoparib was 25.0 pg/ml.
Safety assessments
Safety assessment included adverse events, clinical laboratory tests, physical examination, vital signs, and ECGs. Treatment interruption and discontinuation were also recorded. The duration of adverse event reporting is presented in Fig. 1. Assessments of note included adverse events of special interest (myelodysplastic syndrome and acute myeloid leukemia) and liver function tests.
Serious adverse events were those that resulted in persistent or significant incapacity or substantial disruption of normal functioning, were life-threatening, or resulted in death. Abnormal liver function was considered a serious adverse event if aspartate aminotransferase (AST) or alanine aminotransferase (ALT) was more than or equal to 3×upper limit of normal (ULN) (or >5×ULN if baseline ALT or AST was >3×ULN); total bilirubin was more than 2×ULN or international normalized ratio was more than 1.5; or AST or ALT was more than or equal to 3×ULN with signs and symptoms consistent with hepatitis and/or eosinophilia (≥500 eosinophils/µl).
Safety follow-up
Safety follow-up was to occur 30 days after the final dose of talazoparib or before starting a new anticancer drug, whichever came first. Safety follow-up assessments included vital signs, physical examination, ECOG performance status, adverse event and medication review, serum chemistry and hematology, and, in most patients, a single 12-lead ECG. Eligible patients who continued talazoparib in the open-label extension study (NCT02921919) within 30 days of the final study dose were excluded from safety follow-up and instead had a single 12-lead ECG on enrollment in the extension study.
Statistical analysis
The primary analyses were designed to evaluate the effects of talazoparib on QT interval corrected for heart rate using Fridericia’s correction formula [QTcF; calculated as QT/(RR)1/3] 15, the prespecified primary endpoint, and to describe the relationship between plasma talazoparib concentration and change from time-matched baseline in QTcF. Secondary analyses included time-matched changes from baseline in QT interval corrected using Bazett’s formula [QTcB; calculated as QT/(RR)1/2] 16, heart rate, PR and QRS intervals, and ECG morphology.
Sample size determination
At least 27 evaluable patients were required to provide 80% power to reject the null hypothesis if mean change from baseline in QTcF was at least 10 ms with one-sided 5% level of significance, assuming a SD of 20 ms. Allowing for a 10% dropout rate, enrollment was planned for 30 evaluable patients.
ECG analysis
ECG analyses were carried out using the ECG analysis population, defined as all patients who received at least one dose of talazoparib and had at least one baseline and one on-treatment ECG reading. Central tendency analyses were carried out using a time-matched analysis of all ECG interval parameter changes (HR, PR, QRS, QT, QTcF, and QTcB), where the change between time-matched baseline and postdose ECGs were calculated for each patient at each planned nominal timepoint on days 1, 2, and 22. Descriptive statistics were used to summarize the ECG variables and changes from baseline for each timepoint of the analysis. In addition, two-sided 90% confidence intervals were calculated for the time-matched change from baseline data using PROC MEANS. On the basis of regulatory guidance for oncologic agents, if the upper limit of the two-sided 90% confidence interval for change in QTc from time-matched baseline was less than 20 ms, it would be interpreted to mean that the 1 mg dose of talazoparib had no clinically meaningful effect on QTc interval 12.
ECG interval values were considered outliers if they met predetermined cutoff values for heart rate (<50 bpm and ≥25% decrease from baseline mean heart rate or >100 bpm and ≥25% increase from baseline); PR interval (>200 ms and ≥25% increase from baseline); QRS interval (>100 ms and ≥25% increase from baseline); QT interval (>500 ms and baseline interval was ≤500 ms); QTcB and QTcF (per QT interval or if >480 ms when baseline was ≤480 or >450 ms when baseline was ≤450 ms). Outlier analysis was considered exploratory because the study was not powered for this purpose.
ECG waveforms were centrally interpreted for morphologic change by an independent cardiologist. New onset of the following disturbances were to be recorded: atrial fibrillation or flutter, second-degree or third-degree heart block, complete right or left bundle branch block, ST segment depression or elevation, T wave abnormalities (negative T waves only), myocardial infarction (new pathologic Q waves), and new abnormal U waves.
Pharmacokinetic/pharmacodynamic analysis
The PK analysis population was defined as all patients who had sufficient concentration data to derive at least one PK parameter. PK parameters were summarized descriptively by day for the PK population and, among patients with reported PK parameters on day 22, a subcategory of patients with no dose modifications was identified. Descriptive statistics were calculated for PK parameters available from three or more patients.
PK/PD analysis was performed on the PK/PD analysis population, defined as all patients in the ECG analysis population with one or more time-matched pair of talazoparib plasma concentration and ECG measurements obtained at the same timepoint (±30 min). As recommended in published guidance 17, a prespecified linear mixed-effects model was used to examine the relationship between the change from baseline in RR intervals and QTc intervals (QTcF and QTcB) and plasma concentration of talazoparib using the following equation: Ylkt=μl+pt+θClkt+Wk+DkCkt+ɛlkt, where the dependent variable ΔQTc or ΔRR-interval (Ylkt) is for the l-th treatment, k-th patient and t-th timepoint; μl is the treatment-specific intercept; pt is the time effect on the intercept; θ is the slope; C is the concentration; Wk is the random patient effect on the intercept; Dk is the random patient effect on the slope; and εlkt is the residual error. If the model did not converge, it was to be reapplied without the random patient effects on plasma concentration. A slope P value less than 0.05 would be interpreted to indicate a linear relationship. The mean maximum effect was calculated as 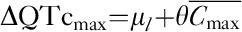 , where
, where 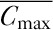 was the mean of the Cmax on day 22 in the population without dose modifications. The upper one-sided 95% confidence interval was obtained from the upper two-sided 90% confidence interval obtained using a nonparametric bootstrap method. All calculations were performed using SAS, version 9.4 (SAS Institute Inc, Cary, North Carolina, USA) (or later), and modeling was performed using SAS PROC MIXED (SAS Institute Inc, Cary, North Carolina, USA) with Satterthwaite degrees of freedom.
was the mean of the Cmax on day 22 in the population without dose modifications. The upper one-sided 95% confidence interval was obtained from the upper two-sided 90% confidence interval obtained using a nonparametric bootstrap method. All calculations were performed using SAS, version 9.4 (SAS Institute Inc, Cary, North Carolina, USA) (or later), and modeling was performed using SAS PROC MIXED (SAS Institute Inc, Cary, North Carolina, USA) with Satterthwaite degrees of freedom.
Safety analysis
The safety population comprised all patients who received any amount of talazoparib. All safety analyses were performed using the safety population, except ECG and PK/PD analyses, which were performed using their respective analysis populations. The safety of talazoparib was evaluated according to the incidence and severity of adverse events, incidence of dose modifications, permanent treatment discontinuations, and clinically significant changes in vital signs, ECGs, and clinical laboratory values. An adverse event was considered treatment emergent if the onset occurred on or after the administration of study drug.
Results
Study patients
A total of 38 patients were enrolled, 37 received talazoparib and 31 (83.8%) completed the study. All analysis populations comprised of 37 patients. Six (16%) patients discontinued talazoparib because of patient choice (three), disease progression (two), or adverse events [one serious adverse event considered by the investigator to be unrelated to talazoparib treatment lead to a temporary treatment discontinuation as the patient resumed treatment with talazoparib in the open-label extension study (NCT02921919)]. In all, 30/37 (81%) treated patients continued talazoparib treatment in the open-label extension study.
Baseline demographic and disease characteristics are summarized in Supplementary Table S1 (Supplemental digital content 1, http://links.lww.com/ACD/A302). Patients were predominantly White [31/37 (84%)] and female [23 (62%)], with a median age of 62 years. Concomitant use of agents known to prolong the QT interval was not prohibited, and multiple agents classified by Arizona Center for Education and Research on Therapeutics as having ‘Known’ or ‘Possible’ risk for QT interval prolongation and torsades de pointes were used in this study, as described in Supplementary Table S1 (Supplemental digital content 1, http://links.lww.com/ACD/A302) 18. The most common primary tumor sites were breast [12 (32%)], ovary [seven (19%)], and prostate [six (16%)]. More than half (54%) of patients had a baseline ECOG performance status of 1.
Plasma PK of talazoparib
Peak plasma talazoparib concentration has reached a median of 2 h after single or multiple dosing, and concentration increased with continued once-daily administration (Table 1). Among patients with no previous dose modifications, the mean apparent oral clearance (Cl/F) was 4.8 l/h and accumulation ratio (Rac) was 3.98. Talazoparib exposures were consistent with those observed in previous clinical studies dosing talazoparib (1 mg QD) in patients with advanced cancer 6.
Table 1.
Pharmacokinetics of plasma talazoparib
ECG analyses
Evaluation of ECG correction methods
Based on visual inspection of the QTc‘X’ vs. RR interval plots and the slope values generated for the two correction methods, QTcF provided the better correction for the effect of heart rate on QT interval for the ECG analysis population, with a close to flat slope, confirming the appropriateness of the prespecified use of QTcF as the primary QT correction method for this study (Supplementary Fig. S1, Supplemental digital content 1, http://links.lww.com/ACD/A302). The relationship between raw QT interval and RR interval showed a steep positive slope (slope P<0.0001), as expected based on normal physiology. Fridericia’s method effectively corrected for RR interval, as showed by a nearly flat QT interval/RR interval slope (slope P=0.20). By contrast, Bazett’s correction was confirmed as less appropriate for analyzing these data, showing a steep negative slope (slope P<0.0001).
Central tendency analyses: change from time-matched baseline in ECG parameters
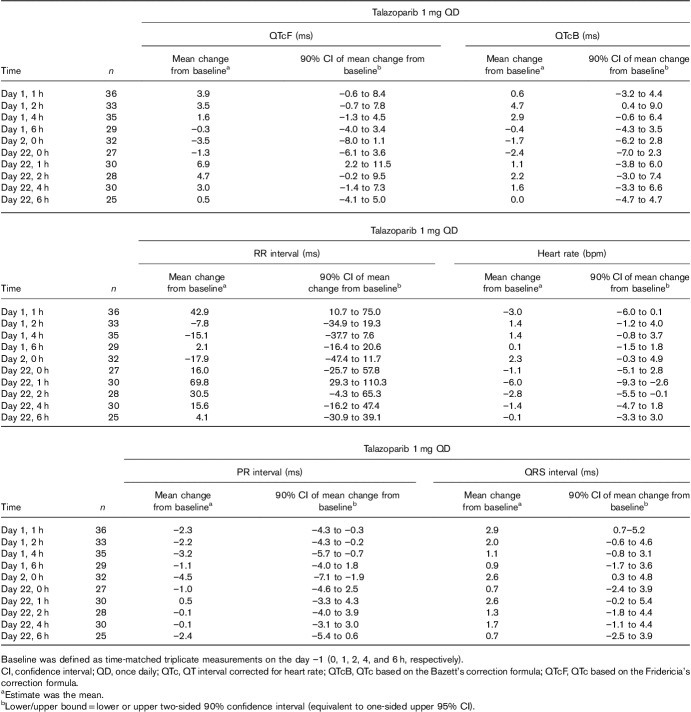
Following treatment with talazoparib 1 mg QD, the mean changes from time-matched baseline for QTcF ranged from –3.5 to 6.9 ms, with the largest value occurring 1 h postdose on day 22 (Table 2). The mean changes from time-matched baseline for QTcB ranged from –2.4 to 4.7 ms, with the largest value reported at 2 h postdose on day 1. The upper limits of the one-sided 95% confidence interval for the mean change from time-matched baseline for QTcF and QTcB were less than 12 ms at all nominal ECG collection timepoints. No clinically relevant changes from time-matched baseline values were observed in the ECG analysis population for the PR interval, RR interval, QRS interval, or heart rate.
Table 2.
Mean change from time-matched baseline in ECG parameters: central tendency analyses (ECG analysis population)
Outlier analyses: categorical summaries of maximum postbaseline value and maximum increase from time-matched baseline in ECG parameters
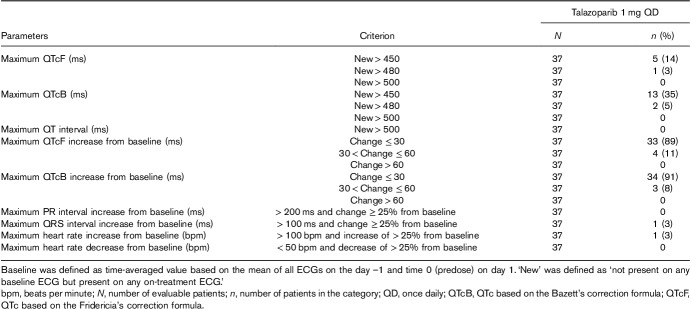
In the ECG analysis population, no patients had a maximum postbaseline QTcF, QTcB, or QT interval of more than 500 ms or a maximum increase from time-matched baseline in QTcF or QTcB of more than 60 ms (Table 3). One (3%) patient had a new QTcF value and two (5%) patients had a new QTcB value between 480 and less than or equal to 500 ms. One (3%) patient was a QRS outlier and another a tachycardic heart rate outlier. No new morphological abnormalities were observed within the ECG analysis population.
Table 3.
Maximum postbaseline and maximum change from baseline in ECG parameters (outlier analysis; ECG analysis population)
Exposure–response (PK/PD) analysis
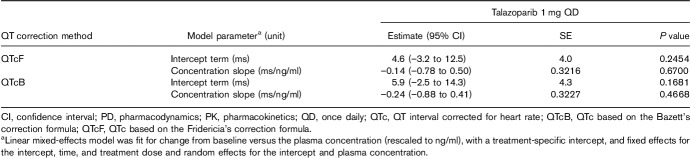
In all, 369 triplicate ECGs and 372 talazoparib concentrations were obtained from 37 patients, generating 332 matched PK-ECG pairs that were used to evaluate the RR concentration, QTcF concentration, and QTcB concentration relationships. Raw PK concentrations were rescaled from pg/ml units to ng/ml for these analyses. The slopes (95% confidence interval) of the lines showing the relationship between talazoparib concentration and RR [slope=0.33 (–4.22 to 4.88) ms/ng/ml; P=0.88], QTcF [–0.14 (–0.78 to 0.50) ms/ng/ml; P=0.67], and QTcB [0.24 (–0.88 to 0.41) ms/ng/ml; P=0.47] were all not statistically different from zero, indicating that talazoparib did not have a concentration-dependent effect on heart rate and QTc (Tables 4 and 5, Fig. 2a and b).
Table 4.
Change from baseline in RR interval by talazoparib plasma concentration (PK/PD analysis population)
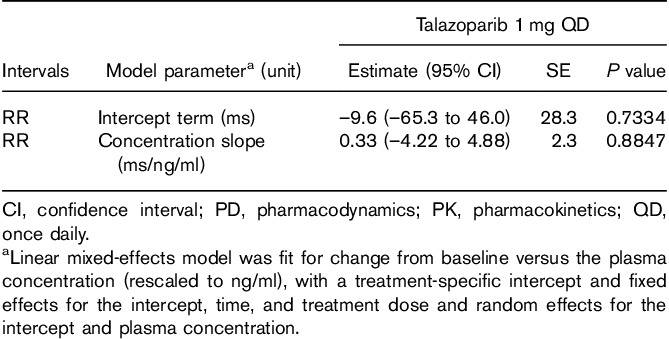
Table 5.
Change from baseline in corrected QT interval versus talazoparib plasma concentration (PK/PD analysis population)
Fig. 2.
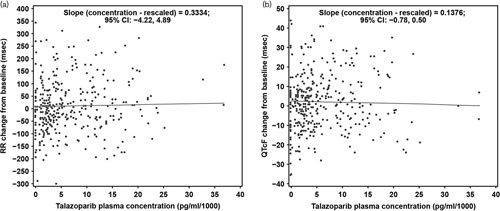
Change from baseline in (a) RR and (b) QTcF versus plasma talazoparib concentration. CI, confidence interval; QTc, QT interval corrected for heart rate; QTcF, QTc based on the Fridericia’s correction formula.
At the mean steady-state talazoparib Cmax (17.2 ng/ml), the PK/PD model predicted mean change from baseline QTc was 2.4 ms with a one-sided upper 95% confidence interval of 4.64 ms for QTcF and 2.1 ms with a one-sided upper 95% confidence interval of 4.59 ms for QTcB, respectively (Supplementary Table S2, Supplemental digital content 1, http://links.lww.com/ACD/A302).
Analysis of the PK/PD model diagnostic plots showed a good model fit, supporting the built-in assumptions of the prespecified model, with model convergence. Data plots did not show any evidence of hysteresis, though the study had limited data to allow detection of hysteresis.
Safety
In all, 17 (46%) patients received talazoparib treatment for 22 days, per protocol. Sixteen (43%) patients had less than 22 treatment days due to discontinuation (n=6) or reduced treatment duration (21 days, n=8; 20 days, n=2), and four (11%) received more than 22 days treatment. Four patients had dose interruptions due to adverse events, of which two (both grade 3 fatigue) were considered by the investigator to be treatment-related.
Overall, 28/37 (76%) patients experienced at least one adverse event, 17 (46%) reported at least one treatment-related adverse event, and nine (24%) had at least one grade 3 adverse events, of which three (8%) were considered treatment-related. Three (8%) patients experienced five serious adverse events, only one of which (anemia in a single patient) was considered treatment-related. The most commonly reported treatment-related adverse events (occurring in ≥5% of patients) were cytopenias [seven (19%)], fatigue [six (16%)], nausea [five (14%)], diarrhea [four (11%)] and vomiting [two (5%)]; no adverse events related to cardiac electrophysiological disturbance were reported. No deaths, permanent treatment discontinuations due to adverse events, grade 4 or 5 adverse events, or adverse events of special interest were reported.
Discussion
Before conducting this study, the effect of talazoparib on cardiac repolarization had been studied in in-vitro safety pharmacology assessments, preclinical animal toxicity studies in beagle dogs, and routine pharmacovigilance in human clinical trials. In-vitro studies indicated that talazoparib has a low potential for significant inhibition of human Ether-à-go-go-related gene (hERG) potassium channels (Pfizer Inc., data on file), which play a central role in cardiac repolarization 19. During 28-day and 13-week preclinical toxicity studies of talazoparib in beagle dogs, ECG evaluations showed no drug-related changes in several measures of cardiac electrical activity (PR, QRS, QT, QTc, or RR intervals and heart rate) and no rhythm abnormalities or qualitative ECG changes (Pfizer Inc., data on file). In a first-in-human phase 1 trial of once-daily talazoparib in patients with advanced or recurrent solid tumors, of the 96 patients with ECG recordings at baseline and postbaseline, one patient had a postbaseline QTcF of more than 500 ms, and one had an increase from baseline in QTcF of more than 60 ms (Pfizer Inc., data on file). These observations collectively indicated that talazoparib had a low risk of QT prolongation or proarrhythmic effects. Consistent with previous assessments, the present study, which served as the definitive evaluation of the potential effects of talazoparib on cardiac repolarization at the approved therapeutic dose of 1 mg QD, showed no clinically significant effect of talazoparib on heart rate, atrioventricular conduction, or cardiac repolarization in patients with advanced solid tumors.
On the basis of the ICH E14 guideline, the threshold level of regulatory concern for QTc prolongation is that the upper bound of the one-sided 95% confidence interval around the largest time-matched mean effect on QTc is less than 10 ms 12 while the threshold level of less than 20 ms is widely accepted for oncology drugs. Following treatment with talazoparib 1 mg QD, the upper limits of the one-sided 95% confidence interval for the mean change from time-matched baseline for QTcF and QTcB were less than 12 ms at all nominal ECG collection timepoints. No patients had a postbaseline absolute mean maximum QTcF or QTcB of more than 500 ms or an increase from time-matched baseline in QTcF or QTcB of more than 60 ms in the ECG analysis population. One (3%) patient had a new QTcF value and two (5%) patients had a new QTcB value between 480 and less than or equal to 500 ms. No clinically relevant changes in PR, RR, or QRS intervals or heart rate were observed, no new ECG morphological abnormalities were observed, and no adverse events as defined by the MedDRA System Organ Class Cardiac preferred terms were reported in the study. Collectively, these results indicate a lack of clinically relevant effect of talazoparib on QTc.
Further evidence of the lack of QT prolongation effect of talazoparib was showed by exposure–response (concentration-QTc) modeling. The prespecified linear mixed-effects analysis was conducted per recommendations published by the US Food and Drug Administration Division of Pharmacometrics 17 and was adequate to describe the relationship between talazoparib concentrations and QTc or RR interval. Incorporating nominal time as a factor variable on the intercept in the model removes the potential effect of circadian rhythm on QT interval. Such models with time as a factor variable provide similar accuracy of the slope estimates when compared with complex biological models with circadian functions, which require more extensive ECG sampling for precise estimation of the model parameters 20. Results from this analysis showed that talazoparib did not appear to have a concentration-dependent effect on the heart rate or QTc, as the predicted upper bound of the one-sided 95% confidence interval for the increase in QTc at the mean steady-state talazoparib maximum concentrations at the therapeutic dose was less than 5 ms for both QTcF and QTcB. Taken together, these findings indicate a lack of clinically relevant effect of talazoparib on QTc.
Talazoparib 1 mg QD was generally well tolerated with manageable toxicities overall, no deaths, no dose reductions, no permanent discontinuations because of adverse events, and no adverse events of special interest reported.
The methodology used in this study followed industry standards as published within ICH guidance, and the appropriateness of our methodology was supported by several analyses carried out during the study. ECG waveforms were selected and read by a cardiologist at an independent central lab using a manual adjudication methodology to minimize bias. Supplemental analyses consistently validated the appropriateness of the assumptions that were built into the study design and analysis plan. In the ECG analysis population, QTcF was established as the better correction factor to account for the effect of heart rate on the QT interval, validating the selection of QTcF as the prespecified primary QTc endpoint. Analysis of the PK/PD model data showed a good model fit with model convergence, and data plots did not show any evidence of hysteresis, supporting the built-in assumptions of the prespecified linear mixed-effects model. The slope (95% confidence interval) of the RR concentration relationship was not statistically different from 0 (P=0.88), indicating that talazoparib did not have a concentration-dependent effect on the heart rate and validating the analysis of the effect of talazoparib on the QT interval using fixed correction methods to account for the effect of heart rate on the QT interval (i.e. QTcF and QTcB). In addition, the observed steady-state talazoparib PK (geometric mean AUC24, Cmax, CL/F, and median Rac) in the subpopulation without previous dose modifications were consistent with previous studies in advanced cancer patients receiving talazoparib 1 mg QD 6, indicating that this study achieved steady-state talazoparib exposures typically associated with the therapeutic dose. Collectively, these supplemental analyses support the validity of the methodology used in this study to conclude that talazoparib does not have a clinically relevant effect on QTc prolongation at the approved therapeutic dose of 1 mg QD.
One limitation of the current study is the lack of inclusion of higher doses to achieve supratherapeutic talazoparib concentrations in the patient population. As discussed previously, dose-limiting toxicity profiles associated with many oncology drugs preclude administration of doses higher than the maximum tolerated dose (for talazoparib, 1 mg QD) in cancer patients. Thus, it may be possible that patient populations that experience higher than typical talazoparib systemic exposures, such as patients with renal impairment or those taking concomitant medications that are strong P-GP inhibitors 21, may have a higher risk of QT interval prolongation with talazoparib treatment than is described by the current study’s results. However, given that no statistically significant relationship between talazoparib concentration and change from time-matched baseline QTc was established in the PK/PD analyses conducted for this study, the risk of QTc prolongation in populations with higher systemic exposure to talazoparib would be expected to similar to what is reported here.
Conclusion
Talazoparib did not have a clinically relevant effect on QTc prolongation at the therapeutic dose of 1 mg QD in patients with advanced solid tumors. The drug was generally well tolerated, and no patients permanently discontinued treatment because of adverse events.
Data sharing
Upon request, and subject to certain criteria, conditions, and exceptions (https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (i) for indications that have been approved in the USA and/or EU or (ii) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, by a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.anti-cancerdrugs.com.
Acknowledgements
This study was sponsored by Medivation, which was acquired by Pfizer in September 2016. The authors thank the patients who participated in this study, their families, the study coordinators, and the support staff at the clinical sites. The authors acknowledge the contributions of Sunil Babu, MD, William Lawler, MD, and David Chan, MD, for their roles in patient enrollment and data collection acting as site principal investigators. The authors also acknowledge Robert B. Kleiman, MD, for overseeing the independent central reading, analysis, and interpretation of the ECG data at eResearch Technology Inc. The authors acknowledge Anh Nguyen, PharmD, for her contributions to the study design, conduct, and performance of the noncompartmental analyses to generate the reported talazoparib PK parameters. Editorial and medical writing support was provided by Michael Weaver, PhD, Edwin Thrower, PhD, and Mary Kacillas of Ashfield Healthcare Communications (Middletown, Connecticut, USA) and was funded by Pfizer.
Conflicts of interest
J.H., J.C., A.P., and D.W. are employees of Pfizer Inc. and own stock in Pfizer Inc. R.M. has received honoraria for participation in Pfizer speakers’ bureau. L.N. is a former employee of Pfizer Inc. Z.A.W. has received research funding from Medivation. For the remaining authors, there are no conflicts of interest.
Footnotes
Justin Hoffman and Jayeta Chakrabarti contributed equally to the writing of this article.
References
- 1.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer 2010; 10:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012; 72:5588–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol 2011; 5:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol 2011; 3:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science 2017; 355:1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov 2017; 7:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018; 379:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonçalves A, Eiermann W, Rugo HS, Ettl J, Hurvitz SA, Yerushalmi R, et al. EMBRACA: efficacy and safety in comparing talazoparib (TALA) with physician’s choice of therapy (PCT) in patients (pts) with advanced breast cancer (ABC) and a germline BRCA mutation: BRCA1/BRCA2 subgroup analysis. Ann Oncol 2018; 29 (Suppl 8):viii90–viii121. [Google Scholar]
- 9.Elmeliegy M, Yu Y, Litton KJ, Turner NC, Czibere A, Wilson GG, et al. Exposure-safety analyses in breast cancer patients with germline BRCA1/2 mutations receiving talazoparib in EMBRACA and ABRAZO trials. Ann Oncol 2018; 29 (Suppl 8):viii90–viii121. [Google Scholar]
- 10.Ettl J, Quek RGW, Lee KH, Rugo HS, Hurvitz S, Goncalves A, et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol 2018; 29:1939–1947. [DOI] [PubMed] [Google Scholar]
- 11.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004; 350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 12.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. Guideline E14. Current step 4 version. 2005. Available at: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. [Accessed 29 August 2018].
- 13.Durairaj C, Ruiz-Garcia A, Gauthier ER, Huang X, Lu DR, Hoffman JT, et al. Palbociclib has no clinically relevant effect on the QTc interval in patients with advanced breast cancer. Anticancer Drugs 2018; 29:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darpo B, Sarapa N, Garnett C, Benson C, Dota C, Ferber G, et al. The IQ-CSRC prospective clinical Phase 1 study: ‘Can early QT assessment using exposure response analysis replace the thorough QT study?’. Ann Noninvasive Electrocardiol 2014; 19:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridericia LS. The duration of systole in the electrocardiogram of normal subjects and of patients with heart disease. Acta Medica Scandinavica 1920; 53:469–486. [Google Scholar]
- 16.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart 1920; 7:353–370. [Google Scholar]
- 17.Zhu H, Wang Y, Gobburu JV, Garnett CE. Considerations for clinical trial design and data analyses of thorough QT studies using drug-drug interaction. J Clin Pharmacol 2010; 50:1106–1111. [DOI] [PubMed] [Google Scholar]
- 18.QT drugs list. Available at: http://www.Crediblemeds.org. [Accessed 13 December 2018].
- 19.Villoutreix BO, Taboureau O. Computational investigations of hERG channel blockers: new insights and current predictive models. Adv Drug Deliv Rev 2015; 86:72–82. [DOI] [PubMed] [Google Scholar]
- 20.Huh Y, Hutmacher MM. Evaluating the use of linear mixed-effect models for inference of the concentration-QTc slope estimate as a surrogate for a biological QTc model. CPT Pharmacometrics Syst Pharmacol 2015; 4:e00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfizer Inc. Talzenna (talazoparib) [prescribing information]. New York, NY: Pfizer Inc; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.anti-cancerdrugs.com.