Abstract
Purpose
The purpose of this study was to evaluate the functional and anatomical outcomes of combined phacovitrectomy with multifocal intraocular lens (mfIOL) implantation.
Methods
Retrospective, interventional, non-comparative case series of six eyes that received a combined phacoemulsification surgery with apodized, diffractive mfIOL implantation for cataract and pars plana vitrectomy (PPV) with membrane peeling for epiretinal membrane (ERM). Outcome measures included distance and near visual acuities (DVA and NVA), central macular thickness (CMT), intraocular pressure (IOP), and disruption of external limiting membrane (ELM) or inner-segment outer-segment (IS/OS) junction.
Results
Mean logMAR glare DVA improved from 0.40 (Snellen 20/50) preoperatively to a mean uncorrected DVA of 0.038 (Snellen 20/22) (P=0.004) at 6 months after surgery. All eyes achieved NVA of J2 or better by 12 months postoperatively. Median CMT improved by 10 µm and mean IOP increased by 1 mmHg at 12 months postoperatively. Percentage of patients with ELM or IS/OS disruptions decreased from 66.7% to 33.3%. Two eyes demonstrated residual metamorphopsia on Amsler grid testing postoperatively. Postoperatively, four eyes required laser capsulotomy and one required LASEK for refractive correction.
Conclusion
Combined phacovitrectomy, membrane peeling, and mfIOL implantation improved VA in patients with idiopathic ERM. Multifocality was achieved, but final visual outcome was delayed due to posterior capsular opacification and macular healing.
Keywords: cataract, combined, epiretinal membrane, multifocal intraocular lens, phacoemulsification, phacovitrectomy
Background
Cataract extraction with apodized, diffractive multifocal intraocular lens (mfIOL) implantation has proven to be an effective option for providing patients functional vision at varying distances using different points of focus and has thus been increasingly utilized with conventional cataract surgery.1–6 The increasing employment of mfIOL implantation is a result of patient desires to address presbyopia and reduce spectacle dependence.3
Cataract and vitreoretinal disease often coexist, and several studies have demonstrated the safety and efficacy of combined phacoemulsification and vitrectomy (ie, phacovitrectomy).7–14 The combined procedure offers several potential benefits such as faster visual recovery, reduced costs, improved patient convenience, equivalent or even reduced rates of complications compared to two separate surgeries, and circumventing the acceleration of cataract associated with vitrectomy. From the retinal surgeon’s perspective, the combined procedure allows for improved retinal visualization.15–17
Despite the increased popularity of using multifocal lenses during cataract surgery, the presence of retinal disease, particularly macular disease, has historically been a relative contraindication for the use of a multifocal lens. Multifocal IOLs may compound the reduction of contrast sensitivity in eyes with macular pathology and may leave the patient experiencing certain dysphotopsias (ie, halo, flicker, and glare disability).18,19 Recent work has demonstrated that patients with poorer preoperative visual acuity from macular pucker may have greater levels of relative visual acuity improvement but greater residual vision deficits.20–22 Conversely, more favorable absolute visual acuity outcomes can be achieved among patients with preoperative visual acuities better than 20/50, suggesting this population may be candidates for mfIOLs.20–22
There is a paucity of data in the ophthalmic literature reporting the results of using a multifocal lens during these combined procedures. We aimed to evaluate short-term functional and anatomical outcomes in selected patients undergoing combined mfIOL implantation for cataract and macular surgery for epiretinal membrane (ERM) from our pilot study.
Methods
A single-center, retrospective, interventional, non-comparative chart review of combined surgeries, with the University of Cincinnati Institutional Review Board approval, was performed. The review included all eyes treated at the Cincinnati Eye Institute (CEI) ambulatory surgery center from August 1, 2013 to August 1, 2016, who received combined cataract surgery with mfIOL implantation and vitreoretinal surgery. Surgical indication for mfIOL implantation included visually significant cataract based on functional impairment and glare testing, as well as patient request for an mfIOL. Vitreoretinal surgical indication was restricted to ERM causing metamorphopsia, visual distortion, and/or decreased visual function. Inclusion criteria included eyes that underwent same-day combined phacoemulsifcation cataract extraction with mfIOL implantation and pars plana vitrectomy (PPV) with membrane peeling for ERM, had ≥3 months of follow up, and did not have a history of other macular pathology (eg, cystoid macular edema [CME], diabetic macular edema) or laser treatment, surgery, or intravitreal injections during the 90-day postoperative period that would otherwise confound surgical results. Six eyes were identified and analyzed based on the inclusion criteria.
Cases reviewed were performed by one of two vitreoretinal surgeons (CDR or REF) and a corneal and refractive specialist (MES) at the CEI. All patients provided informed consent for surgery, and procedures were performed in accordance with the Declaration of Helsinki. The risks and benefits of using an mfIOL compared to monofocal intraocular lens (IOL) in the setting of macular pathology, including dysphotopsias, were discussed in detail with patients prior to IOL selection, and each patient selected mfIOL over other IOL choices. An apodized, diffractive mfIOL with a +3 addition power (AcrySof IQ ReSTOR® SN6AD1; Alcon Laboratories, Inc., Fort Worth, TX, USA) was used in all cases. Retrobulbar or peribulbar anesthesia was used for all procedures. The lens was placed in each eye using standard phacoemulsification techniques (eg, two-handed, in-the-bag technique) without complication. After placing and centering the IOL into the capsular bag, all viscoelastic materials were removed from the anterior chamber; the incisions were closed either with a 10–0 nylon suture or hydration alone. The retinal surgeon then performed PPV with the Constellation Vitrectomy System® (Alcon Laboratories, Inc.) machine through both a direct contact lens (Dutch Ophthalmic) and a non-contact wide angle viewing system (Volk Merlin® and Occulus BIOM® surgical systems) with membrane peeling for ERM using standard techniques, with or without the removal of internal limiting membrane (ILM) assisted by indocyanine green (ICG) dye mixed in 5% dextrose solution. A complete scleral depressed examination of the retina was performed. Intraoperative medications were limited to DisCoVisc®, OcuCoat®, ICG in 5% dextrose, cefazolin, and lidocaine bicarbonate. Postoperative topical ophthalmic medications included bromfenac, difluprednate, flurbiprofen, loteprednol, cyclosporine, moxifloxacin, and prednisolone acetate 1%.
Data reviewed from medical charts included preoperative best-corrected visual acuities (BCVA), preoperative glare BCVA, uncorrected distance visual acuities (UCDVA), near visual acuities (NVA), preoperative keratometries (K1 and K2), IOL powers, axial lengths, spectral domain optical coherence tomography (SD-OCT) data, Amsler grid data, refractive outcomes, patient histories, clinical examination findings, details of surgical procedures, perioperative and postoperative medications, surgical complications, and postoperative procedures. Preoperative uncorrected NVA was unavailable, except in one patient. Postoperative DVA was recorded as BCVA unless unavailable, in which uncorrected DVA was obtained. Postoperative uncorrected NVA was used unless unavailable, in which case NVA with correction was recorded.
Primary outcomes included uncorrected DVA and NVA, and central macular thickness (CMT) measured by SD-OCT. Secondary outcomes included intraocular pressure (IOP) and anatomic disruption of external limiting membrane (ELM) or inner-segment/outer-segment (IS/OS) junction of the retina visualized on Heidelberg Spectralis® HRA-OCT. ELM and IS/OS band disruptions were defined as a reduced intensity or focal disruption of the band on the horizontal 6 mm foveal raster scan or cube scans. Disruptions were recorded as qualitative categorical descriptions (ie, the presence of a disruption indicated a positive value) rather than quantifying the percentage of the band damaged.
Outcomes were measured preoperatively, and at postoperative intervals of 1 day, 1 week, 1 month, 3 months, 6 months, and 12 months. If a postoperative visit at the desired time interval was unavailable, the closest time point was used. Postoperative procedures and complications were also recorded.
Data were consolidated and statistical analysis performed with Microsoft Excel® 2017 (Microsoft Corporation, Redmond, WA, USA). Visual acuity was recorded as a Snellen value and then converted to logMAR scale for statistical analysis. Paired t-test analysis was performed to determine if changes in outcome variables before and after the combined procedure were statistically significant.
Results
The pilot series consisted of six eyes from five patients undergoing same-day combined macular and phacoemulsification surgeries. Baseline demographic information and preoperative clinical features of each case are summarized in Table 1. Three male and two female patients with a mean age of 64.3 years (range 58.6–73.8 years) were included. Mean follow-up time was 540.3 days (range 99–1149 days, standard deviation=461 days). The severity of each case’s cataract and ERM was graded clinically. In all six cases, the visual complaints were attributed to both cataract and ERM. Past ocular history can be found in Table 1. Mean IOL power was 14.3 D (range 8–20 D). The final mean spherical equivalent achieved after the surgery was -0.50±0.27 D.
Table 1.
Patient demographics and clinical features
| Case | Age (Y)* | Sex | Eye | Pre-op K1 | Pre-op K2 | Axial length (mm) | Pre-op cataract grade | Retinal surgery indication | ERM grade | Past ocular history |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62.6 | M | OD | 43.47 | 44.51 | 28.03 | NC2, NO2, C2, P1 (axial) | ERM | 2+ | Cataract, ERM, RD |
| 2 | 58.6 | F | OS | 45.09 | 45.78 | 25.27 | PC1.5 | ERM | 3+ | Cataract, chalazion, ERM, mild astigmatism |
| 3 | 73.9 | M | OD | 40.24 | 41.49 | 25.22 | NC2, NO1 | ERM | 2+ | Cataract, ERM, PVD |
| 4 | 60.7 | M | OS | 42.95 | 43.02 | 26.41 | NC2, NO1, P1 (axial) | ERM | 3+ | Cataract, ERM, PVD |
| 5 | 61 | M | OD | 42.67 | 43.10 | 26.45 | NC1, NO2, P1 (axial) | ERM | 1–2+ | Cataract, ERM, PVD |
| 6 | 68.9 | F | OS | 43.71 | 44.59 | 24.05 | C3, NC2, NO2 | ERM | 2+ | Astigmatism, cataract, ERM, lamellar macular hole |
Note:
Age at time of procedure.
Abbreviations: ERM, epiretinal membrane; K, keratometry; OD, right eye; OS, left eye; PVD, posterior vitreous detachment; RD, retinal detachment; Y, years.
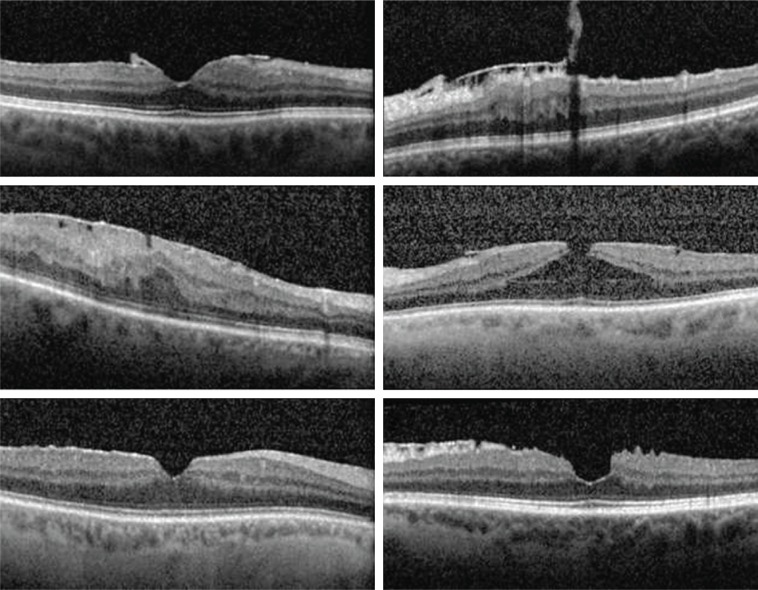
Surgical data are presented in Table 2. In all but one case, the ILM of the retina was removed with the ERM. PPV was performed using 25-gauge instrumentation in five eyes and 27-gauge instrumentation in one eye. Preoperative OCT images of the six cases’ foveas are illustrated in Figure 1. Four of the six cases reported metamorphopsia preoperatively, but only two had documented abnormal Amsler grids preoperatively. While no patients complained of metamorphopsia after surgery, two cases still demonstrated residual metamorphopsia on Amsler grid testing postoperatively at a mean of 418.5 days after the combined surgery. The two cases with abnormal recorded preoperative Amsler grids illustrated reductions in the degree of metamorphopsia on Amsler grid postoperatively.
Table 2.
Surgical data
| Case | Eye | Follow up (days) | IOL power (D) | Gauge PPV | MP stain | ILM peeling | Subsequent procedures |
|---|---|---|---|---|---|---|---|
| 1 | OD | 1,149 | 8 | 25 | ICG | Yes | N/A |
| 2 | OS | 1,025 | 13 | 25 | ICG | Yes | YAG laser posterior capsulotomy; LASEK |
| 3 | OD | 600 | 20 | 25 | ICG | No | YAG laser posterior capsulotomy |
| 4 | OS | 237 | 13.5 | 25 | ICG | Yes | YAG laser posterior capsulotomy |
| 5 | OD | 132 | 12.5 | 27 | ICG | Yes | YAG laser posterior capsulotomy |
| 6 | OS | 99 | 19 | 25 | ICG | Yes | N/A |
Abbreviations: D, diopter; ICG, indocyanine green; ILM, internal limiting membrane; IOL, intraocular lens; MP, membrane peeling; OD, right eye; OS, left eye; PPV, pars plana vitrectomy.
Figure 1.
Ocular coherence tomography images of the six cases demonstrating foveal preoperative raster scans of the epiretinal membrane of each eye.
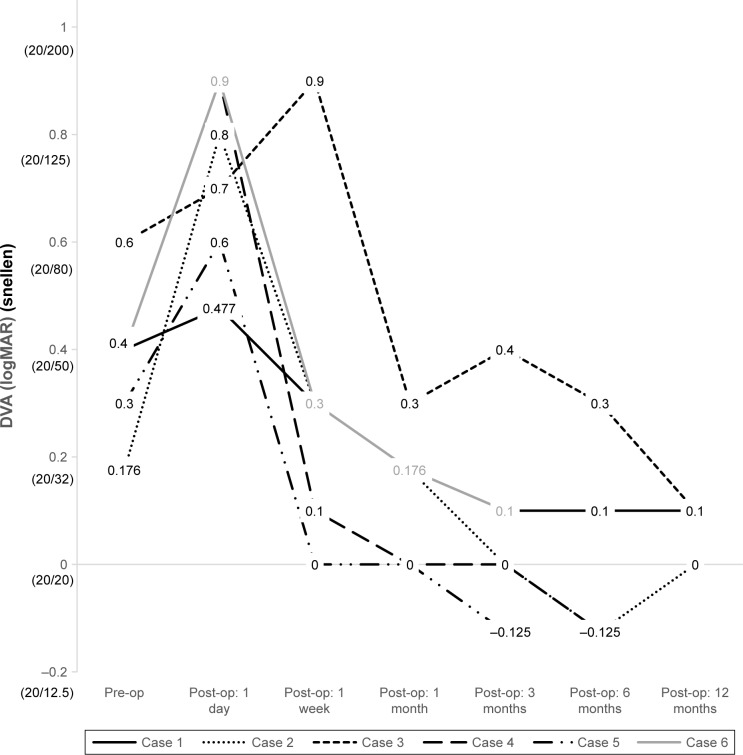
The DVA and CMT measured from each case were plotted over time to help illustrate trends (Figures 2 and 3). Although mean preoperative BCVA was near normal at 0.063 logMAR (Snellen 20/23), preoperative glare acuities were significantly impaired at logMAR 0.40 (Snellen 20/50). Similarly, the mean 25% contrast VA was measured as logMAR 0.36 (Snellen 20/47). Postoperatively, uncorrected distance visual acuity improved to 0.038 (Snellen 20/22) (P=0.004) at 6 months and 0.067 (Snellen 20/23) (P=0.04) at 12 months after index operation (Table 3). All patients achieved J2 or better uncorrected NVA by 12 months postoperatively.
Figure 2.
Individual preoperative and postoperative DVA in six eyes undergoing combined cataract extraction with multifocal IOL implantation and PPV with membrane peeling.
Abbreviations: DVA, distance visual acuity; IOL, intraocular lens; PPV, pars plana vitrectomy.
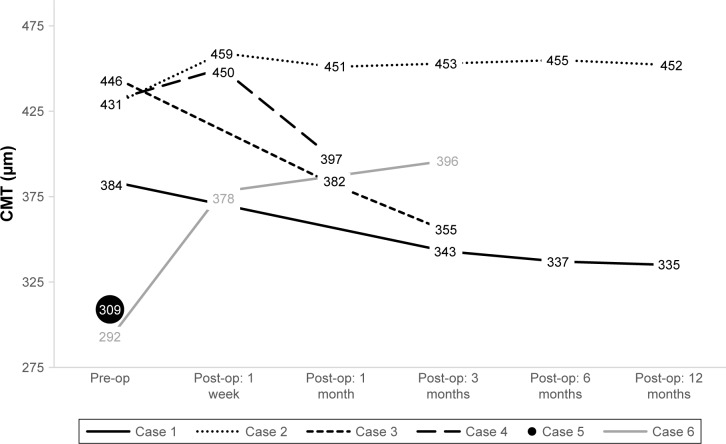
Figure 3.
Individual preoperative and postoperative CMT in six eyes undergoing combined cataract extraction with multifocal IOL implantation and PPV with membrane peeling.
Abbreviations: CMT, central macular thickness; IOL, intraocular lens; PPV, pars plana vitrectomy.
Table 3.
Primary and secondary surgical outcomes (mean values)
| Pre-op | Post-op: 1 day | Post-op: 1 week | Post-op: 1 month | Post-op: 3 months | Post-op: 6 months | Post-op: 12 months | |
|---|---|---|---|---|---|---|---|
| DVA (logMAR) | 0.063±0.07 (Snellen 20/23) | 0.73±0.17 (Snellen 20/107) | 0.317±0.31 (Snellen 20/41) | 0.138±0.12 (Snellen 20/27) | 0.079±0.18 (Snellen 20/24) | 0.038±0.21 (Snellen 20/22) | 0.067±0.06 (Snellen 20/23) |
| NVA (logMAR) | 0.233±0.39 (Snellen 20/34) | N/A | N/A | 0.25±0.38 (Snellen 20/36) | 0.16±0.31 (Snellen 20/29) | 0.415±0.44 (Snellen 20/52) | 0.033±0.06 (Snellen 20/22) |
| CMT (µm) | 381.67±66.4 | N/A | 429±44.4 | 410±36.29 | 386.75±49.66 | 396±83.44 | 393.5±82.73 |
| IOP (mmHg) | 14.33±3.2 | 9.17±4.0 | 17±4.1 | 16.33±2.9 | 13.83±1.8 | 16±2.6 | 15.33±2.3 |
| ELM and IS/OS Disruptions (%)* | 66.67 | N/A | 100 | 66.67 | 100 | 100 | 33.33 |
Note:
Percentage of eyes with ELM and IS/OS disruptions visualized on spectral domain optical coherence tomography.
Abbreviations: CMT, central macular thickness; DVA, distance visual acuity; ELM and IS/OS, external limiting membrane and inner-segment outer-segment junction; IOP, intraocular pressure; NVA, near visual acuity.
Regarding subjective symptoms, three out of six cases reported blurry vision and difficulty reading in low light or small print postoperatively. Subjective blurry vision in one case reportedly improved after YAG capsulotomy for symptomatic posterior capsular opacification (PCO), and one improved with LASEK. Three eyes also experienced reported dysphotopsias, including glare, halos, or streaks of light. Despite these subjective symptoms, patients described satisfaction with their visual result in five out of six eyes.
Four cases showed equivocal changes in CMT over time postoperatively (Figure 3). One case did not receive a postoperative OCT, and one case demonstrated a significant increase in CMT by 3 months postoperatively. Median final CMT improved to 10 µm compared to baseline by 12 months postoperatively. IOP in all cases initially decreased on postoperative day 1, increased by 1-week postoperatively, and normalized to near preoperative values by 3 months. Transient ocular hypotony was seen in two cases with sutureless PPV; no cases reached IOP above 25 mmHg. The percentage of patients with ELM and IS/OS disruptions decreased by 12 months postoperatively, from 66.7% to 33.3% (Table 3). While looking at percentage of cases with disrupted bands over time, a similar trend as with our other variables is seen: an initial increase postoperatively, but improvement by 12 months after index operation.
Postoperative complications were limited with only one report of mild ERM recurrence, with no need for further vitreoretinal surgery or IOL exchange, treatment of CME, nor clinically significant ocular hypertension. Four out of the six eyes required YAG capsulotomy for symptomatic PCO, and one of those additionally had LASEK to enhance the uncorrected refractive result. Based on the history and assessment recorded during postoperative visits, five of six patients expressed satisfaction with visual results. One case had early dissatisfaction with vision related to anisometropic symptoms, cataract symptoms in the fellow eye, and an unintended residual astigmatism of the index eye of −0.50+0.75×160. This patient was ultimately satisfied following eventual laser vision correction of residual refractive error and eventual cataract surgery in the fellow eye.
Discussion
Combined phacovitrectomy has demonstrated efficacy, and discussion of premium lens options (eg, mfIOLs) is considered standard of care for all patients undergoing cataract extraction. We report a series of six eyes from five patients who underwent combined phacoemulsification with mfIOL implantation and PPV with membrane peeling for coexisting cataract and idiopathic ERM. Patients in the series predominantly had good corrected high-contrasted distance Snellen visual acuities, but suffered from visual deficits from glare, metamorphopsia, and contrast reduction that improved with combined surgery. While five of the six eyes had only corrected NVAs recorded preoperatively, all patients presented with presbyopic complaints. Therefore, this was not a limitation in appreciating the benefit of multifocality with improved uncorrected distance and excellent (J2 or better) NVAs.
Visual recovery was prolonged in some patients, attributable to the time required for macular healing, seen as improvements in disrupted ELM and IS/OS bands on OCT, and the development of symptomatic PCO requiring laser capsulotomy. Disruption of these bands of cells indicates photoreceptor dysfunction and correlates with poorer visual function. Moreover, the recovery of these disruptions has been associated with improvement in visual acuity.23,24 Incidence of PCO in eyes that have had vitrectomy is known to be higher than that in eyes that have not undergone PPV.25,26 The mean refractive outcome of −0.50±0.27 D is similar to that reported by Kim et al, who compared refractive outcomes of combined phacovitrectomy for ERM with cataract surgery alone and found a similar myopic shift.27
Hadayer et al and others have raised concerns about the potential difficulty of performing PPV through an mfIOL due to impaired fundus visualization and intraoperative difficulties such as additional effort required to focus on the peripheral retina and retinal vessels, reduced stereopsis, and impaired view after fluid–air exchange.28,29 The operative view through the apodized diffractive multifocal lens in this case series was not limiting, and there were no intraoperative complications. Our positive experience with visualization through this particular mfIOL may not extrapolate to different diffractive IOL platforms.
Our study has inherent limitations. This was a qualitative analysis with potential case selection bias. Given the small sample size, it was not powered for safety analysis. Due to the retrospective nature, we did not have a standardized refraction paradigm, nor did we collect data on distance-corrected NVA or accommodative amplitudes to better grade degree of presbyopia. A prospective study could improve standardization of follow up, yield comparative analysis, and provide improved monitoring of subjective outcomes. Unfortunately, randomized clinical trials for this indication would be challenging, as this study examines a very small subset of patients undergoing combined surgery with controversial indications biased by strong patient motivations for mfIOL selection. Our data are not necessarily generalizable to other types of mfIOLs. Future investigations warrant a review of surgical outcomes in patients who have undergone PPV prior to, as well as distantly following cataract surgery with mfIOL implantation.
Ultimately our pilot study suggests combined phacovitectomy with mfIOL implantation in a carefully selected group of patients with ERM yielded good visual acuity, multifocality, a decrease in symptomatic metamorphopsia, and results which most patients found satisfactory. Patient selection is very important while considering multifocal lens for combined macular and phacoemulsifaction surgery, and patient expectations should be tempered to account for delayed achievement of maximal visual acuity and possible incomplete resolution of macular abnormalities and dysfunction postoperatively.
Summary statement
This retrospective, interventional, non-comparative case series evaluated functional and anatomical outcomes of eyes that underwent same-day combined phacoemulsification surgery for cataract with mfIOL implantation and PPV with membrane peeling for idiopathic ERM.
Acknowledgments
This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc, New York, NY, to the Department of Ophthalmology, University of Cincinnati (Karl C Golnik, MD, Chairman).
An abstract of the study was previously presented at the University of Cincinnati 22nd Annual Ophthalmology Conference and Research Symposium on April 22, 2017, in Cincinnati, OH.
Footnotes
Disclosure
Dr. Michael Snyder reports disclosures outside the submitted work from: Alcon, Johnson and Johnson Vision, Bausch and Lomb, Glaukos, Gore, Epsilon, VEO Ophthalmics, and Humanoptics. Dr Christopher D Riemann reports personal fees from Alcon, Alimera, Allergan, B&L Valeant, BMC/Eyetube, CSTILII, Gore, Humanoptics, NotalVision, Orbit, AGTC, Arepio, Chengdu KangHong, Clearside, Genentech/Roche, Neurotech, Novartis, Ophthotec, Regeneron, and Lowry-MacTel Registry; other disclosures from Chruman, Iamc2, iVeena, Macor, Northmark Pharmacy, VEO, and Vortex; and personal fees and IP from Haag Streit, Janssen/J&J, Kaleidoscope, MedOne, Reliance, outside the submitted work and personal fees and stock options from TrueVision, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Alio J, Plaza-Puche AB, Fernandez-Buenaga R, Pikkel J, Maldonado M. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017;62:611–634. doi: 10.1016/j.survophthal.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Altaie R, Ring CP, Morarji J, Patel DV, McGhee CN. Prospective analysis of visual outcomes using apodized, diffractive multifocal intraocular lenses following phacoemulsification for cataract or clear lens extraction. Clin Exp Ophthalmol. 2012;40:148–154. doi: 10.1111/j.1442-9071.2011.02671.x. [DOI] [PubMed] [Google Scholar]
- 3.de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016(12):CD003169. doi: 10.1002/14651858.CD003091.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Vries NE, Webers CA, Montes-Mico R, et al. Long-term followup of a multifocal apodized diffractive intraocular lens after cataract surgery. J Cataract Refract Surg. 2008;34:1476–1482. doi: 10.1016/j.jcrs.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Vega L, Alfonso JF, Rodriguez PP, Montes-Mico R. Clear lens extraction with multifocal apodized diffractive intraocular lens implantation. Ophthalmology. 2007;114:1491–1498. doi: 10.1016/j.ophtha.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 6.Rosen E, Alio JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42:310–328. doi: 10.1016/j.jcrs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Demetriades AM, Gottsch JD, Thomsen R, et al. Combined phacoemulsification, intraocular lens implantation, and vitrectomy for eyes with coexisting cataract and vitreoretinal pathology. Am J Ophthalmol. 2003;135:291–296. doi: 10.1016/s0002-9394(02)01972-4. [DOI] [PubMed] [Google Scholar]
- 8.Foster RE, Lowder CY, Meisler DM, Zakov ZN, Meyers SM, Ambler JS. Combined extracapsular cataract extraction, posterior chamber intraocular lens implantation, and pars plana vitrectomy. Ophthalmic Surg. 1993;24:446–452. [PubMed] [Google Scholar]
- 9.Hohn F, Kretz F, Pavlidis M. Surgical and functional results of hybrid 25-27-gauge vitrectomy combined with coaxial 2.2 mm small incision cataract surgery. J Ophthalmol. 2016;2016:9186351. doi: 10.1155/2016/9186351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jun Z, Pavlovic S, Jacobi KW. Results of combined vitreoretinal surgery and phacoemulsification with intraocular lens implantation. Clin Exp Ophthalmol. 2001;29:307–311. doi: 10.1046/j.1442-9071.2001.00439.x. [DOI] [PubMed] [Google Scholar]
- 11.Savastano A, Savastano MC, Barca F, Petrarchini F, Mariotti C, Rizzo S. Combining cataract surgery with 25-gauge high-speed pars plana vitrectomy: results from a retrospective study. Ophthalmology. 2014;121:299–304. doi: 10.1016/j.ophtha.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Scharwey K, Pavlovic S, Jacobi KW. Combined clear corneal phacoemulsification, vitreoretinal surgery, and intraocular lens implantation. J Cataract Refract Surg. 1999;25:693–698. doi: 10.1016/s0886-3350(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 13.Sisk RA, Murray TG. Combined phacoemulsification and sutureless 23-gauge pars plana vitrectomy for complex vitreoretinal diseases. Br J Ophthalmol. 2010;94:1028–1032. doi: 10.1136/bjo.2009.175984. [DOI] [PubMed] [Google Scholar]
- 14.Toussaint BW, Appenzeller MF, Miller DM, et al. Stability of the acrysof toric intraocular lens in combined cataract surgery and transconjunctival sutureless vitrectomy. Retina. 2015;35:1065–1071. doi: 10.1097/IAE.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 15.de Bustros S, Thompson JT, Michels RG, Enger C, Rice TA, Glaser BM. Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol. 1988;105:160–164. doi: 10.1016/0002-9394(88)90180-8. [DOI] [PubMed] [Google Scholar]
- 16.Yiu G, Marra KV, Wagley S, et al. Surgical outcomes after epiretinal membrane peeling combined with cataract surgery. Br J Ophthalmol. 2013;97:1197–1201. doi: 10.1136/bjophthalmol-2013-303189. [DOI] [PubMed] [Google Scholar]
- 17.Chung TY, Chung H, Lee JH. Combined surgery and sequential surgery comprising phacoemulsification, pars plana vitrectomy, and intraocular lens implantation: comparison of clinical outcomes. J Cataract Refract Surg. 2002;28:2001–2005. doi: 10.1016/s0886-3350(02)01354-8. [DOI] [PubMed] [Google Scholar]
- 18.Braga-Mele R, Chang D, Dewey S, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313–322. doi: 10.1016/j.jcrs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Dick HB, Krummenauer F, Schwenn O, Krist R, Pfeiffer N. Objective and subjective evaluation of photic phenomena after monofocal and multifocal intraocular lens implantation. Ophthalmology. 1999;106:1878–1886. doi: 10.1016/S0161-6420(99)90396-2. [DOI] [PubMed] [Google Scholar]
- 20.Dawson SR, Shunmugam M, Williamson TH. Visual acuity outcomes following surgery for idiopathic epiretinal membrane: an analysis of data from 2001 to 2011. Eye. 2014;28(2):219–224. doi: 10.1038/eye.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly G, Melamud A, Lipscomb P, Toussaint BW. Surgical outcomes in patients with macular pucker and good preoperative visual acuity after vitrectomy with membrane peeling. Retina. 2015;35(9):1817–1821. doi: 10.1097/IAE.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 22.Chinskey ND, Shah GK. Epiretinal membrane peeling in patients with good preoperative vision. J Vitreoretin Dis. 2017;1:52–56. doi: 10.1177/2474126416680669. [DOI] [Google Scholar]
- 23.Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93:171–175. doi: 10.1136/bjo.2008.146381. [DOI] [PubMed] [Google Scholar]
- 24.Shimozono M, Oishi A, Hata M, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012;153:698–704. doi: 10.1016/j.ajo.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Iwase T, Oveson BC, Nishi Y. Posterior capsule opacification following 20- and 23-gauge phacovitrectomy (posterior capsule opacification following phacovitrectomy) Eye (Lond) 2012;26:1459–1464. doi: 10.1038/eye.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jun JH, Kim KS, Chang SD. Nd:YAG capsulotomy after phacoemulsification in vitrectomized eyes: effects of pars plana vitrectomy on posterior capsule opacification. J Ophthalmol. 2014;2014:840958. doi: 10.1155/2014/840958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M, Kim HE, Lee DH, Koh HJ, Lee SC, Kim SS. Intraocular lens power estimation in combined phacoemulsification and pars plana vitrectomy in eyes with epiretinal membranes: A case-control study. Yonsei Med J. 2015;56(3):805–811. doi: 10.3349/ymj.2015.56.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadayer A, Jusufbegovic D, Schaal S. Retinal detachment repair through multifocal intraocular lens-overcoming visualization challenge of the peripheral retina. Int J Ophthalmol. 2017;10(6):1008–1010. doi: 10.18240/ijo.2017.06.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mainster MA, Reichel E, Warren KA, Harrington PC. Ophthalmoscopy and vitreoretinal surgery in patients with an ARRAY refractive multifocal intraocular lens implant. Ophthalmic Surg Lasers. 2002;33:74–76. [PubMed] [Google Scholar]