Abstract
Background
Hypertension is a prominent preventable cause of premature morbidity and mortality. People with hypertension and established cardiovascular disease are at particularly high risk, so reducing blood pressure below standard targets may be beneficial. This strategy could reduce cardiovascular mortality and morbidity but could also increase adverse events. The optimal blood pressure target in people with hypertension and established cardiovascular disease remains unknown.
Objectives
To determine if 'lower' blood pressure targets (≤ 135/85 mmHg) are associated with reduction in mortality and morbidity as compared with 'standard' blood pressure targets (≤ 140 to 160/ 90 to 100 mmHg) in the treatment of people with hypertension and a history of cardiovascular disease (myocardial infarction, angina, stroke, peripheral vascular occlusive disease).
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomized controlled trials up to February 2017: the Cochrane Hypertension Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (from 1946), Embase (from 1974), the World Health Organization International Clinical Trials Registry Platform, and ClinicalTrials.gov. We also searched the Latin American and Caribbean Health Science Literature Database (from 1982) and contacted authors of relevant papers regarding further published and unpublished work. There were no language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) with more than 50 participants per group and at least six months follow‐up. Trial reports needed to present data for at least one primary outcome (total mortality, serious adverse events, total cardiovascular events, cardiovascular mortality). Eligible interventions were lower target for systolic/diastolic blood pressure (≤ 135/85 mmHg) compared with standard target for blood pressure (≤ 140 to 160/90 to 100 mmHg).
Participants were adults with documented hypertension or who were receiving treatment for hypertension and cardiovascular history for myocardial infarction, stroke, chronic peripheral vascular occlusive disease or angina pectoris.
Data collection and analysis
Two review authors independently assessed search results and extracted data using standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included six RCTs that involved a total of 9795 participants. Mean follow‐up was 3.7 years (range 1.0 to 4.7 years). Five RCTs provided individual patient data for 6775 participants.
We found no change in total mortality (RR 1.05, 95% CI 0.90 to 1.22) or cardiovascular mortality (RR 0.96, 95% CI 0.77 to 1.21; moderate‐quality evidence). Similarly, no differences were found in serious adverse events (RR 1.02, 95% CI 0.95 to 1.11; low‐quality evidence). There was a reduction in fatal and non fatal cardiovascular events (including myocardial infarction, stroke, sudden death, hospitalization or death from congestive heart failure) with the lower target (RR 0.87, 95% CI 0.78 to 0.98; ARR 1.6% over 3.7 years; low‐quality evidence). There were more participant withdrawals due to adverse effects in the lower target arm (RR 8.16, 95% CI 2.06 to 32.28; very low‐quality evidence). Blood pressures were lower in the lower' target group by 9.5/4.9 mmHg. More drugs were needed in the lower target group but blood pressure targets were achieved more frequently in the standard target group.
Authors' conclusions
No evidence of a difference in total mortality and serious adverse events was found between treating to a lower or to a standard blood pressure target in people with hypertension and cardiovascular disease. This suggests no net health benefit from a lower systolic blood pressure target despite the small absolute reduction in total cardiovascular serious adverse events. There was very limited evidence on adverse events, which lead to high uncertainty. At present there is insufficient evidence to justify lower blood pressure targets (≤ 135/85 mmHg) in people with hypertension and established cardiovascular disease. More trials are needed to answer this question.
Blood pressure targets in people with cardiovascular disease
Review question
We assessed whether lower blood pressure goals are better than standard blood pressure goals for people with high blood pressure who also have heart or vascular problems.
Background
Many people with heart or vascular problems also have high blood pressure. Some clinical guidelines recommend a lower blood pressure goal (135/85 mmHg or lower) in people with previous heart or vascular problems compared with those without (≤140 to 160 mmHg systolic and ≤ 90 to 100 mmHg diastolic are standard blood pressure goals). It is unclear if the lower goals lead to overall health benefits.
Search date
We searched for evidence up to February 2017.
Study characteristics
We included six trials with 9795 participants who were followed‐up for between a year and 4.7 years. We analyzed data to detect differences between lower and standard blood pressure goals on numbers of deaths and serious adverse events (leading to hospital admission).
Key results
We found no differences in total numbers of deaths, heart or vascular deaths or serious harms between lower and standard blood pressure goal approaches. Based on very little information, we found more dropouts due to drug‐related harms in the lower blood pressure target group. The only significant benefit among people in the lower group in the studies analyzed was a slight decrease in total heart or vascular problems, but there was no overall health benefit.
Quality of the evidence
The best available evidence does not support lower blood pressure goals over standard goals in people with elevated blood pressure and heart or vascular problems. More new trials are needed to answer this question. Overall, quality evidence was assessed as low to moderate according to the GRADE assessment.
Summary of findings
Summary of findings for the main comparison.
Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity
| Lower blood pressure targets compared with standard blood pressure targets for mortality and morbidity | ||||||
| Patient or population: Cardiovascular disease with high blood pressure Setting: Outpatients (average duration of trials 4 years) Intervention: Lower blood pressure targets (≤135/85 mmHg) Comparison: Standard blood pressure targets (≤140 to 160/90 to 100 mmHg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard blood pressure target | Risk with lower blood pressure target | |||||
| Total mortality | Study population | RR 1.05 (0.90 to 1.22) | 9795 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 68 per 1000 | 71 per 1000 (61 to 83) | |||||
| Serious adverse events | Study population | RR 1.02 (0.95 to 1.11) | 9795 (6 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | ||
| 186 per 1000 | 189 per 1000 (177 to 206) | |||||
| Total cardiovascular events | Study population | RR 0.87 (0.78 to 0.98) | 9795 (6 RCTs) | ⊕⊕⊝⊝ LOW 1,3 | ARR = 1.6% over 4 years (0.2% to 2.7%) NNTB = 63 over 4 years (37 to 500) |
|
| 123 per 1000 | 107 per 1000 (96 to 121) | |||||
| Cardiovascular mortality | Study population | RR 0.96 (0.77 to 1.21) |
9795 (6 RCTs) |
⊕⊕⊕⊝ MODERATE 1 | ||
| 32 per 1000 | 30 per 1000 (24 to 38) |
|||||
|
Withdrawals due to adverse effects |
Study population | RR 8.16 (2.06 to 32.28) | 690 (2 RCT) | ⊕⊝⊝⊝ VERY LOW 1,4 | ||
| 7 per 1000 | 60 per 1000 (15 to 239) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; ARR: attributed risk ratio; NNTB: number needed to treat for an additional beneficial outcome | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to serious imprecision (95% CI is wider than the minimal important difference).
2 Downgraded one level due to incomplete available data.
3 Downgraded one level due to high risk of bias.
4 Downgraded two levels because only two of the smaller studies reported this outcome.
Background
Description of the condition
Hypertension (high blood pressure) is one of the most preventable causes of premature morbidity and mortality worldwide. It was described as the second leading risk factor for the global burden of disease in 2013 (Forouzanfar 2015). Hypertension is a major risk factor for ischaemic and haemorrhagic stroke, myocardial infarction, heart failure, chronic kidney disease, peripheral vascular disease (PVD), cognitive decline and premature death (NICE 2016).
Historically more emphasis was placed on diastolic than on systolic blood pressure as a predictor of cardiovascular morbidity and fatal events. However a large number of observational studies have demonstrated both systolic and diastolic blood pressures show a graded independent relationship with mortality and morbidity (ESH‐ESC 2013). Untreated hypertension may be associated with a progressive rise in blood pressure, possibly culminating in a treatment‐resistant state due to associated vascular and kidney damage (NICE 2016).
Epidemiological studies suggest that the risk associated with blood pressure is a continuous relationship and for blood pressures above 115/70 mmHg, the risk of cardiovascular events doubles for every 20/10 mmHg rise in blood pressure. This suggests that for every 20 mmHg lower systolic blood pressure (SBP) or 10 mmHg diastolic blood pressure (DBP), the risk of a cardiovascular event is reduced by about 50% (Lewington 2002).
Blood pressure is normally distributed in a population and there is no natural cut‐off point above which hypertension definitively exists and below which it does not. In any individual person, systolic and/or diastolic blood pressures maybe elevated. Diastolic pressure is more commonly elevated in people younger than 50. With ageing,systolic hypertension becomes a more significant problem, as a result of progressive stiffening and loss of compliance of larger arteries. (NICE 2016).
Cardiovascular disease remains the leading cause of death around the world (Townsend 2016). Cardiovascular disease accounts for more than all communicable, neonatal, maternal and nutritional disorders combined, and double the number of deaths caused by cancers. Globally, cardiovascular disease accounts for approximately 17 million deaths annually, nearly one third of the total number of deaths. Of these, complications of hypertension account for 9.4 million deaths worldwide every year. Despite of that, between 1990 and 2013 age‐standardized death rates fell by 22% for cardiovascular and circulatory diseases, mainly due to trends in high‐ and middle‐income countries (GBD 2013). Ischaemic heart disease (IHD) and cerebrovascular diseases are both considered to be major cardiovascular diseases, resulting in 130 million disability‐adjusted life years lost in 2010 (WHO 2010).
Thus, cardiovascular secondary prevention is considered to be a key issue. People who have had atherosclerotic stroke should be included among those deemed to be at high risk (20% over 10 years) of further atherosclerotic coronary events. A significant percentage of those who have a first myocardial infarction are expected to experience recurrent myocardial infarction, heart failure, stroke or fatal coronary heart disease (CHD). In fact, within five years of a first myocardial infarction around 20% to 30% of the population aged over 65 years will experience recurrent myocardial infarction or fatal CHD (Mozaffarian 2015).
Description of the intervention
Target blood pressures are used in clinical practice by clinicians to make treatment decisions related to the intensity of antihypertensive therapy for each patient.
The standard blood pressure target has generally been an arbitrary threshold blood pressure above which treatment is recommended. Over time the threshold has become lower. The standard systolic blood pressure target declined from a target of ≤ 160 mmHg to a target of ≤ 140 mmHg and the diastolic blood pressure target has decreased from ≤100 mmHg to ≤ 90 mmHg in people aged up to 80 years (ESH‐ESC 2007; NICE 2016). Even lower blood pressure targets have been proposed for people with history of cardiovascular events (AHA 2007; ESH‐ESC 2007; JNC‐7 2003).
Recently, a review of the available evidence has led to a re‐appraisal of some recommendations made by international guidelines, particularly among older people , those with diabetes or previous cardiovascular disease (ESH‐ESC 2013; JNC‐8 2014; Joint ESC 2016).
How the intervention might work
Some evidence suggests that in people at high risk thresholds for antihypertensive treatment should be lower than for those at lower risk. It has also been suggested that to maximize the cost‐effectiveness of hypertension management, the intensity of the therapeutic approach should be graded as a function of total cardiovascular risk (ESH‐ESC 2007). However, there is a trend to homogenize blood pressure goals. For example, the European guidelines on hypertension recommend < 140/90 mmHg in most clinical situations (ESH‐ESC 2013).
People with a history of cardiovascular disease are considered to be in a high‐risk population. The effect of lowering the blood pressure values in these people could have a greater absolute reduction of morbidity and mortality but could also be associated with an absolute increase in adverse events.
Reducing blood pressure below standard targets using drug therapy has been recommended in guidelines as a strategy for those with history of cardiovascular disease. Nevertheless, lower may not always be better. A J‐curve has been described for blood pressure in coronary artery disease (Bangalore 2010; Messerli 2006). In people with coronary artery disease low blood pressure (< 110 to 120/60 to 70 mmHg) was associated with an increased risk of future cardiovascular events (Bangalore 2010).
A recent cohort study (Vidal‐Petiot 2016) explored the association between achieved blood pressure and cardiovascular events in people with hypertension and history of coronary disease. The study concluded that in participants where < 120/70 mmHg was reached, an association with more cardiovascular adverse events was detected, supporting the J‐curve hypothesis (Vidal‐Petiot 2016).
Uncertainty remains on many aspects of this controversial topic leading to differing opinions (Mancia 2014; Verdecchia 2014).
Why it is important to do this review
The arterial pressure threshold above which the benefits of treatment outweigh the harms in people with hypertension and cardiovascular disease is unclear.
Blood pressure targets lower than standard targets have been recommended in some, but not all, clinical guidelines. The following are the recommendations for blood pressure targets in people with hypertension and cardiovascular disease from various guidelines published recently.
The Joint National Committee‐7 Report (JNC‐7 2003) recommended blood pressure targets < 140/90 mmHg for people with uncomplicated hypertension. In people with hypertension and either diabetes or kidney disease the recommended blood pressure target was < 130/80 mmHg. However, the statement was updated in 2014 and made some changes to the goals policy (JNC‐8 2014). According to JNC‐8 2014, in the general population aged 60 years it is suggested to treat to goals of SBP < 150 mmHg and DBP < 90 mmHg. In the general population aged up to 60 years, the guideline maintains the recommendation of treating to goals of SBP < 140 mmHg and DBP < 90 mmHg. In people with diabetes or kidney disease, new targets are similar to those for general population. No direct recommendation was made for those with previous cardiovascular disease, although it is acknowledged as a relevant question to be assessed and answered (JNC‐8 2014).
The 2007 European Society of Hypertension and European Society of Cardiovascular guidelines for the management of arterial hypertension (ESH‐ESC 2007) recommended that blood pressure should be reduced to < 140/90 mmHg (systolic/diastolic) and to lower values, if tolerated, in all people with hypertension. The blood pressure goal was < 130/80 mmHg in people with diabetes and others at high risk, such as those with associated clinical conditions (stroke, myocardial infarction, kidney dysfunction, proteinuria). The reappraisal of European guidelines on hypertension management (ESH 2009) remarks that the recommendation to lower blood pressure ≤ 130/80 mmHg in people with diabetes or a history of cardiovascular disease is not supported by incontrovertible trial evidence. The most recent update (ESH‐ESC 2013) proposed SBP goal < 140 mmHg for those at low to moderate cardiovascular risk, diabetes, previous stroke, CHD or kidney disease. In older people with hypertension, good evidence is considered to recommend reducing SBP to between 150 and 140 mmHg, regardless of age provided they are in good physical and mental health. A DBP target of < 90 mmHg is always recommended, except in those with diabetes, in whom values < 85 mmHg are suggested.
The 2016 European Guidelines on cardiovascular disease prevention in clinical practice (Joint ESC 2016) indicate there was sufficient evidence to recommend a blood pressure target < 140/90 mmHg in all people who are hypertensive (except older people in whom the benefit has not been tested in randomized trials). According to the Joint ESC 2016, the recommendation to aim for a lower systolic blood pressure goal < 130 mmHg in people with diabetes and those at very high cardiovascular risk (previous cardiovascular events) is not consistently supported by trial evidence. Thus, it would be prudent to recommend lowering blood pressure to values within the range 130 to 139/80 to 85 mmHg, and possibly, closer to lower values in this range for all people with hypertension.
In its Recommendations for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension the 2015 Canadian Hypertension Education Program (CHEP 2015) made a proposal to reach blood pressure targets < 140/90 mmHg in most situations, including people with previous cardiovascular disease. Nevertheless, the last update of this guideline (CHEP 2016) is prone to an intensive intervention in some people with high cardiovascular risk, including those with cardiovascular disease. Specifically, the guideline calls to consider a < 120 mmHg target, taking into account the SPRINT results (SPRINT 2015).
A Cochrane Review found that treating hypertension to lower than standard blood pressure target ≤ 140 to 160/90 to 100 mmHg was not proven to reduce mortality or morbidity in the overall population (Arguedas 2009). Another Cochrane Review analysing the same question in people with diabetes found a reduction in the incidence of stroke with the lower goal, but also significant increase in the number of serious adverse events (Arguedas 2013).
Two non‐Cochrane reviews have also been published on this issue (Ettehad 2016; Xie 2016). Ettehad 2016 combined data from all relevant clinical trials published on blood pressure reduction. The effects of blood pressure decrease were estimated in terms of mortality or cardiovascular morbidity and according to different basal characteristics, such as established cardiovascular disease. A decrease in mortality and other cardiovascular events was identified as blood pressure was reduced. The review found inconsistent results on safety issues. Xie 2016 focused on the efficacy and safety of blood pressure decrease intensive strategies, including clinical trials with at least six months follow‐up that randomized participants to more intensive versus less intensive blood pressure targets, different blood pressure targets or different blood pressure changes from baseline. Participants in the intensive group showed a decreased risk in terms of less ictus and relevant cardiovascular events.
Several guidelines have been published that directly focus on the main objective of this Cochrane Review ‐ cardiovascular secondary prevention. The 2007 guidelines for the Treatment of Hypertension in the Prevention and Management of Ischemic Heart Disease from the American Heart Association (AHA 2007) recommended blood pressure targets < 130/80 mmHg for people with demonstrated coronary artery disease or risk equivalents (carotid artery disease, peripheral arterial disease, abdominal aortic aneurysm and for high‐risk people). Subsequently, when performance measures were proposed based on these recommendations, limitations were admitted due to the lack of clinical trials that directly compared clinical outcomes of large populations of people with coronary disease randomized to different blood pressure targets (Drozda 2011). This guideline was updated in 2015 (Rosendorff 2015). The update concluded that < 140/90 mmHg would seem a reasonable target for the secondary prevention of cardiovascular events in people with hypertension and coronary artery disease.
Conversely, with less support of evidence, a lower blood pressure target (< 130/80 mmHg) could be appropriate in some people with coronary artery disease, previous myocardial infarction, stroke, or coronary artery disease equivalents (carotid artery disease, peripheral artery disease, abdominal aortic aneurysm).
There are limited data that specifically assess the optimal blood pressure target in relation to secondary stroke prevention. The American guidelines note that goals for target blood pressure level or reduction from pretreatment baseline are uncertain and should be individualized (Kernan 2014). For people who have had a recent lacunar stroke a systolic blood pressure of < 130 mmHg is accepted as reasonable; for people who have had other types of stroke, < 140/90 mmHg is recommended.
There is also the potential that lowering blood pressure too much may cause adverse cardiovascular events (Filippone 2011). Some observations have suggested that excessive lowering of blood pressure using drug treatment is associated with an increased number of deaths due to coronary heart disease (Farnett 1991), particularly in those with coronary artery disease (Bangalore 2010; Messerli 2006). Taking into account that controversy remains over a potential J‐curve phenomenon (Mancia 2014; Verdecchia 2014), additional studies are expected to clarify the dilemma.
Therefore, at present the optimal blood pressure target to reduce morbidity and mortality in people with hypertension and history of cardiovascular disease is unknown. This Review aimed to establish if a more strict blood pressure target should be recommended for these people.
Objectives
To determine if lower blood pressure targets (≤ 135/85 mmHg) are associated with reduction in mortality and morbidity as compared with standard blood pressure targets (≤ 140 to 160/90 to 100 mmHg) in the treatment of people with hypertension and a history of cardiovascular disease (myocardial infarction, angina, stroke, peripheral vascular occlusive disease).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) with more than 50 participants per group and at least six months follow‐up. We also included a study if ≥ 70% of participants met this criterion, or individual patient data were available, or data from relevant participants were provided separately enabling specific inclusion of this population as defined. Blinding was not possible. Trial reports needed to present data for at least one primary outcome to be eligible for inclusion.
We excluded trials that used anything other than accepted randomized allocation methods such as alternate allocation, week of presentation, or retrospective controls. There were no restrictions on publication language.
Types of participants
Participants had to be at least 18 years of age with hypertension documented in a standard way or to be receiving treatment for hypertension, and with a positive cardiovascular history of myocardial infarction, stroke (not including transient ischaemic attack (TIA)), chronic peripheral vascular occlusive disease or angina pectoris.
Trials were not limited by any other factor or baseline risk.
Types of interventions
Intervention: lower blood pressure treatment target: systolic/diastolic ≤ 135/85 mmHg; mean blood pressure ≤ 102 mmHg.
Control: standard blood pressure treatment target: systolic/diastolic ≤ 140 to 160/90 to 100 mmHg; mean blood pressure ≤ 107 to 120 mmHg.
Mean blood pressure (MBP) was also accepted as a valid way of measuring interventions, taking into account the prespecified targets and according to the following equation: MBP = [(2 x diastolic) + systolic] / 3.
Types of outcome measures
Primary outcomes
Total mortality.
Total serious adverse events.
Total cardiovascular events including myocardial infarction, stroke, sudden death, hospitalization or death from congestive heart failure and other significant vascular events such as ruptured aneurysms (excluding angina, transient ischaemic attack, surgical or other procedures or accelerated hypertension). In practice it was measured as total number of participants with at least one cardiovascular event, including fatal and non fatal cardiovascular events.
Cardiovascular mortality.
We defined serious adverse events according to the International Conference on Harmonisation Guidelines (ICH 1995) as any event that leads to death, that was life threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability, or was a congenital anomaly or birth defect.
If a study used a different definition for serious adverse events, the inclusion of data was resolved by consensus among the review authors.
All four primary outcomes were included in 'Summary of findings' tables.
Secondary outcomes
Participant withdrawals due to adverse effects.
Systolic blood pressure and the difference from baseline at one year, or both.
Diastolic blood pressure and the difference from baseline at one year, or both.
Proportion of participants reaching the target blood pressure level.
Number of antihypertensive drugs that each participant needed at the end of the study.
Participant withdrawals due to adverse effects was considered to be an important outcome and was included in 'Summary of findings' tables.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches in the following databases for randomised controlled trials without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (CRS‐Web) (searched 5 February 2017);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (searched 5 February 2017);
MEDLINE Ovid (from 1946 onwards), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 5 February 2017);
Embase Ovid (searched 5 February 2017);
ClinicalTrials.gov (www.clinicaltrials.gov) searched 5 February 2017);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) searched 5 February 2017).
We also searched the Latin American and Caribbean Health Science Literature Database Bireme (searched 2 March 2017).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for databases are provided in Appendix 1. We did not apply a language restriction to the database searches.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE and Epistemonikos for systematic reviews) to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches of CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses, and Web of Knowledge.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
We searched Trip Database (www.tripdatabase.com/), updated to February 2017.
We attempted to identify additional trials by searching the reference lists of included trials and (systematic) reviews, meta‐analyses, and health technology assessment reports (Appendix 2). Authors of trials reporting incomplete information were contacted to provide the missing information.
Dealing with duplicate publications
When more than one publication of an original trial was identified, we assessed those articles together to maximise data collection. In the case of substantial disagreements between articles, study authors were contacted.
References from published studies
We examined the references of included and excluded studies to identify further references linked to potentially eligible RCTs.
Language
There were no language restrictions. Any study not published in English, French or Spanish was translated.
Correspondence
We contacted trial investigators to request data from subgroups of participants with cardiovascular disease, missing data or to clarify study details.
Data collection and analysis
Search results were independently assessed by review authors working in pairs. One review author (LCS) reviewed all results. We used Early Review Organizing Software version 2.0 (www.eros‐systematic‐review.org) for screening and classifying references.
Selection of studies
Two independent review authors carried out the selection of papers, excluding records when the title, keywords and abstract showed that it was not a RCT, there were fewer than 50 participants per group, the follow‐up was less than six months, no review primary outcomes were addressed, participants did not match prespecified criteria, blood pressure targets were not the only intervention or specific targets were different from those prespecified. We obtained the full text of all remaining articles considered for inclusion, which were excluded if inclusion criteria were not met. We obtained the full text of papers that could not be assessed from information in the abstract. We provisionally included studies that were likely to include subgroups of participants that met our criteria, and contacted study authors to request data for the subgroup.
Discrepancies were resolved by discussion or by a third review author if necessary. When an issue was considered to be a highly significant point, a plenary discussion was scheduled.
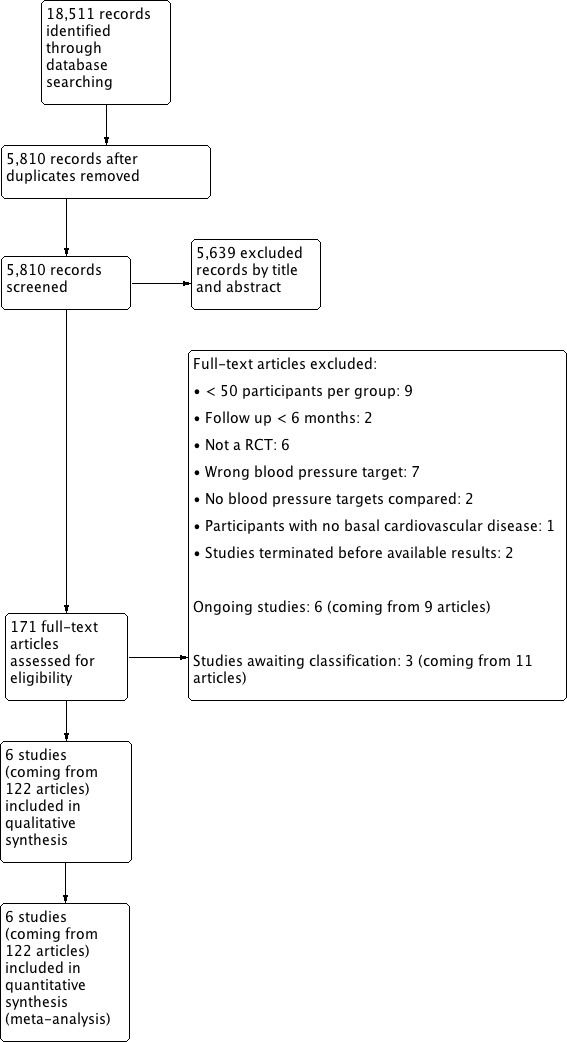
We constructed a PRISMA flow diagram depicting the study selection process (Figure 1).
Data extraction and management
Two review authors independently extracted data from included trials using a previously prepared data extraction form, including basic information, verification of study eligibility, assessment of risk of bias, baseline study characteristics, results in outcomes and subgroup analysis. Extracted data were cross‐checked by another review author.
Differences between review authors were resolved by discussion and involvement of a third author, when necessary. We used Review Manager 2014 for data analyses. Quantitative analyses of outcomes were based on intention‐to‐treat principle.
We used Microsoft Access and Microsoft Excel for organizing and analyzing individual patient data.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the six domains of the Cochrane Risk of Bias Tool, according to the method described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any difference in opinion was resolved by discussion among all review authors.
We tried to find study protocols for comparison with published study reports.
Review authors reported the overall risk of bias for all included studies according to the following:
low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met;
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
We performed sensitivity analyses excluding trials with high or high and unclear risk of bias.
Measures of treatment effect
We used Review Manager 2014 for analyses. Quantitative analyses of outcomes were based on intention‐to‐treat results. We used risk ratio (RR) and a fixed‐effect model, if appropriate, to combine dichotomous outcomes across trials. We calculated absolute risk reduction (ARR), or absolute risk increase (ARI) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) for total mortality, total serious adverse events and total cardiovascular events. We estimated the 95% confidence intervals (CI). Combined outcomes were recorded and analyzed as participants with at least one event in the outcome.
We combined data for the blood pressure reached and the difference from baseline using a weighted mean difference (WMD) method. This combines a weight based on the number of participants in the trial and the within‐study variance. If the trial did not report the within‐study variance for decrease in blood pressure, the standard deviation (SD) was imputed from the average standard deviation from the other trials. This imputation is a limitation and to overcome it, the 99% CI was reported instead of the standard 95% CI reported for all other data. Sensitivity analyses were carried out to assess the impact of changing the assumptions made.
Unit of analysis issues
The analysis of outcomes was based on the randomized participants, but if cluster‐randomized trials were included, we planned to conduct appropriate analysis. We have taken special care to identify if data presented were the total number of events or the total number of participants with a first event. We contacted study authors for clarification when necessary.
We selected data for the longest follow‐up of the trial.
Dealing with missing data
We contacted study authors to obtain additional information not provided in published articles.
Assessment of heterogeneity
We used Chi² and I² statistics to test for heterogeneity of treatment effect among trials. We consider a Chi² value P < 0.05 or I² value > 50% indicative of heterogeneity. We used a random‐effects model to test for statistical significance when significant heterogeneity existed where ‘random’ distribution of intervention effects could be justified.
We planned to investigate possible causes for data showing more than moderate heterogeneity (I² > 60%). If sources of heterogeneity could not be identified, studies were excluded from meta‐analysis.
Assessment of reporting biases
We planned to construct a funnel plot if 10 or more studies were included in the meta‐analysis to test for asymmetry.
Data synthesis
Two review authors analyzed data using RevMan (Review Manager 2014) and reported data in accordance with Cochrane Handbook guidance (Higgins 2011). If meta‐analysis was not appropriate, we planned to provide a narrative description of the results.
Subgroup analysis and investigation of heterogeneity
If possible, subgroup analysis were planned for:
Participants with diabetes.
Male and female participants.
People aged ≥ 75 years.
We aimed to investigate clinical heterogeneity by examining differences in achieved blood pressure among trials, trial duration, the different interventions for hypertension used and history of stroke or coronary heart disease as inclusion criteria.
Sensitivity analysis
We tested the robustness of the results using several sensitivity analysis including:
The risk of bias of the trials.
Trials which were industry sponsored versus non industry sponsored.
We also tested the robustness of the results by repeating the analysis using different measures of effects size (odds ratio) and different statistical models.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The search identified 18,511 records. After removal of duplicates and partial screening, 5810 records remained and were assessed on the basis of title and abstract and 5639 records excluded. We obtained the full text of 171 study reports; following exclusions, 13 reports remained. We contacted the authors of these 13 studies for further information and subsequently excluded seven studies based on information obtained.
We included six studies in the review that met inclusion criteria (Figure 1).
Figure 1.
Results of the search.
Included studies
We included six studies (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015; SPS3 2013).
Four trials (SPS3 2013, and subgroups of participants with basal cardiovascular disease in ACCORD BP 2010; Past BP 2016, SPRINT 2015) compared two different systolic blood pressure targets that met our inclusion criteria. One trial (HOT 1998) compared two different diastolic blood pressure targets within our criteria for lower and standard targets in a subgroup of participants with secondary cardiovascular prevention. One trial (AASK 2002) compared two mean blood pressure targets in a subgroup of participants who met our predefined inclusion criteria. Comparative basal characteristics of these six studies are described in Table 3.
Table 1.
Baseline characteristics of included study participants
| Mean (SD) unless otherwise stated | AASK 2002 | ACCORD BP 2010 | HOT 1998 | Past BP 2016 | SPRINT 2015 | SPS3 2013 |
| Number of participants | 155 | 1531 | 3232 | 295 | 1562 | 3020 |
| Sex (% male) | 68% | 63% | 53% | 64% | 76% | 63% |
| Age in years | 57 (9) | 62 (8) | 62 (‐) | 71 (9) | 70 (9) | 63 (‐) |
| Ethnic group (% Caucasian) | 0% | 62% | 92% | 98% | 71% | 51% |
| Diabetes | 0% | 100% | 12% | 10% | 0% | 37% |
| Current smoker | 31% | 13% | 16% | 13% | 14% | 20% |
| Systolic blood pressure | 149 (28) | 138 (16) | 174 (15) | 143 (14) | 138 (16) | 143 (19) |
| Diastolic blood pressure | 93 (16) | 74 (11) | 106 (3) | 80 (10) | 74 (12) | 79 (11) |
| Ischaemic heart disease (IHD) | 25% | 86% | 95% | 22% | ‐‐‐ | 10% |
| Stroke | 69% | 20% | 7% | 85% | 0% | 99% |
| Peripheral vascular disease | 23% | ‐‐‐ | ‐‐‐ | 7% | ‐‐‐ | ‐‐‐ |
| Thiazides | ‐‐‐ | 51% | ‐‐‐ | 35% | ‐‐‐ | 45% |
| ACEI/ARB | ‐‐‐ | 84% | ‐‐‐ | 65% | ‐‐‐ | 63% |
| Calcium channel blockers | ‐‐‐ | 26% | ‐‐‐ | 43% | ‐‐‐ | 32% |
| Beta‐blockers | ‐‐‐ | 57% | ‐‐‐ | 20% | ‐‐‐ | 25% |
| Other antihypertensive drugs | ‐‐‐ | 28% | ‐‐‐ | 11% | ‐‐‐ | 12% |
| Number of antihypertensive drugs | ‐‐‐ | 3.0 (1.4) | 1.0 (‐‐) | 1.1 (0.8) | 2.1 (1.0) | 1.7 (1.2) |
(‐‐) no information is available. Ischaemic heart disease, stroke and peripheral vascular disease percentages are totally independent of each other because participants can have more than one cardiovascular event at the same time. A similar explanation can be offered with respect to percentages in the different classes of antihypertensive drugs.
Abbreviations: ACEI ‐ angiotensin converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker.
Methods
All included trials were randomized and open with blinded end point design. In AASK 2002, participants were also randomly assigned (in a 3 x 2 factorial design) to either metoprolol, ramipril or amlodipine treatment. In ACCORD BP 2010, participants were also randomized to either intensive or standard glycaemic control according to a 2 x 2 factorial design. HOT 1998 also used a 3 x 2 factorial design and participants were also randomized to receive either acetylsalicylic acid (aspirin) or placebo. SPS3 2013 had a 2 x 2 factorial design with additional randomization to aspirin + placebo or aspirin + clopidogrel.
The mean follow‐up duration was 3.7 years (range 1.0 to 4.7 years).
Participants
The total number of participants included in the review was 9795 (lower target, 5456; standard target, 4339). AASK 2002 included 155 participants (14% of total AASK study); ACCORD BP 2010 included 1531 participants (32% of total ACCORD study); HOT 1998 included 3232 participants (17% of total HOT study); Past BP 2016 included 295 participants (56% of total Past BP trial); SPRINT 2015 included 1562 participants (17% of total SPRINT study); and SPS3 2013 included 3020 participants (100% of total SPS3 study).
AASK 2002 and SPRINT 2015 were conducted in the USA: ACCORD BP 2010 in the USA and Canada: Past BP 2016 in the UK; SPS3 2013 in eight countries in the Americas and Europe, and HOT 1998 in over 20 countries in Asia, the Americas and Europe.
Basal participant characteristics differed among trials (Table 3).
For participants' basal cardiovascular condition, we accepted the following participant profiles as valid secondary prevention:
AASK 2002: participants with ischaemic heart disease (IHD), stroke or peripheral vascular disease (PVD);
ACCORD BP 2010: participants with myocardial infarction, stroke or angina;
HOT 1998: participants with myocardial infarction, stroke or angina;
Past BP 2016: included participants who had stroke or, less frequently, IHD;
SPRINT 2015: all included participants had IHD or PVD;
SPS3 2013: some participants had IHD but all had recent lacunar stroke.
Myocardial infarction and angina identified by electrocardiogram (ECG) or coronary revascularization, and silent events, were considered to meet the inclusion criteria. In general, stroke was the prevalent condition in AASK 2002, Past BP 2016 and SPS3 2013, whereas ischaemic heart attack was the most prevalent condition in ACCORD BP 2010, HOT 1998 and SPRINT 2015.
AASK 2002 and SPRINT 2015 excluded people with history of diabetes, but HOT 1998, Past BP 2016 and SPS3 2013 included some people with diabetes; all ACCORD BP 2010 participants had this cardiovascular risk factor.
All studies included more men than women with a mean age from 57 to 71 years.
Ethnicity varied from all or mostly Caucasian (HOT 1998; Past BP 2016), to mixed populations (ACCORD BP 2010, SPRINT 2015, SPS3 2013) and Afro‐American participants (AASK 2002).
Trials included participants with reduced kidney function (AASK 2002), additional cardiovascular risk factors (ACCORD BP 2010; SPRINT 2015), previous stroke (Past BP 2016; SPS3 2013) or people who were generally hypertensive (HOT 1998).
The baseline blood pressure required for inclusion also varied. AASK 2002 and HOT 1998 required diastolic blood pressure (DBP) ≥ 95 mmHg or DBP 100 to 115 mmHg respectively, whereas ACCORD BP 2010 and SPRINT 2015 required systolic blood pressure (SBP) 130 to 180 mmHg, Past BP 2016 sought SBP 125 mmHg and most SPS3 2013 participants (75%) had SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg.
HOT 1998 was fully industry funded and AASK 2002 was partially industry funded. ACCORD BP 2010, Past BP 2016, SPRINT 2015 and SPS3 2013 were fully publicly funded. ACCORD BP 2010, SPRINT 2015 and SPS3 2013 were supported by the National Institutes of Health in the USA. Past BP 2016 was funded by the National Institute for Health Research (NIHR) in the UK.
Interventions
Participants in AASK 2002 were randomized to MBP 102 to 107 mmHg (standard target) or MBP < 92 mmHg (lower target). ACCORD BP 2010 and SPRINT 2015 randomized participants to SBP < 140 mmHg (standard target) or SBP < 120 mmHg (lower target). The participants in Past BP 2016 were randomized to SBP < 140 mmHg (standard target) or < 130 mmHg (lower target). Participants in SPS3 2013 were randomized to SBP 130 to 149 mmHg (standard target) or SBP < 130 mmHg (lower target). Participants in HOT 1998 were randomized to DBP ≤ 90 mmHg (standard target) or to DBP ≤ 85 mmHg or ≤ 80 mmHg (lower target).
In AASK 2002, if the blood pressure goal could not be achieved by the drug initially randomized (metoprolol, ramipril or amlodipine), additional open‐labelled antihypertensives (furosemide, doxazosin, clonidine, hydralazine or minoxidil) were added sequentially. Felodipine was proposed as basal therapy in HOT 1998, adding other drugs following a five‐step regimen. In SPRINT 2015, the protocol encouraged the use of drug classes with the strongest evidence for reduction in cardiovascular outcomes, including thiazide‐type diuretics (chlorthalidone encouraged as the first‐line agent), loop diuretics (for participants with advanced chronic kidney disease), and beta‐adrenergic blockers (for those with coronary artery disease). No specific drug instructions were provided in ACCORD BP 2010, Past BP 2016 or SPS3 2013.
Outcomes
The primary analysis in AASK 2002 focused on change in glomerular filtration rate, measuring relevant cardiovascular events as secondary outcomes. In ACCORD BP 2010, HOT 1998 and SPRINT 2015 the main outcome was the occurrence of several types of cardiovascular events. The primary outcome in Past BP 2016 was change in systolic blood pressure between baseline and one year.Time to recurrent stroke was the main analysis in SPS3 2013.
Additional notes
AASK 2002 was conducted between February 1995 and September 2001; ACCORD BP 2010 was carried out between January 2001 and June 2009; HOT 1998 was conducted between October 1992 and August 1997; Past BP 2016 was carried out between July 2008 and July 2012: SPRINT 2015 was conducted between November 2010 and March 2013; SPS3 2013 was carried out between February 2003 and April 2012.
Excluded studies
We excluded twenty‐nine records following assessment of full text reports (Figure 1). Among them, it was considered useful to provide a more detailed information about five excluded studies (MDRD 1994; NCT01230216; PODCAST 2013; REIN‐2 2005; RESTART‐AP 2013).
MDRD 1994 mainly focused on the effects of dietary protein restriction and blood pressure control on the progression of chronic kidney disease. Individual patient data were provided by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK, USA). However, after a first analysis this study was excluded because there were fewer than 50 participants per group (an inclusion criterion) (lower target (N = 56), 8 total deaths; standard target (N = 47), 3 total deaths).
NCT01230216 was designed to assess whether an intensive blood pressure target could reduce the percent of atheroma volume measured by intravascular ultrasound in hypertensive patients with coronary artery disease. The study has been terminated early due to slow patients enrolment.
The primary outcome for PODCAST 2013 was Addenbrooke’s Cognitive Examination. Secondary outcomes included vascular events, quality of life, functional outcome, depression and death. The trial recruited 83 participants in the pilot phase . Low recruitment meant the trial did not proceed, and did not meet the 50 participants per arm inclusion criterion for this review.
REIN‐2 2005 was designed to establish whether further blood pressure lowering therapy in addition to angiotensin converting enzyme inhibitors (ACE‐I) could benefit people with chronic kidney disease. Accordingly, the primary objective assessed the effect of intensified versus conventional blood pressure control on progression to end‐stage kidney disease. Individual patient data were provided by the Istituto di Ricerche Farmacologiche Mario Negri (Bergamo, Italy). It was confirmed there were fewer than 50 participants per arm, so this study did not meet this inclusion criterion. (Lower target (N = 34), 2 deaths; standard target (N = 39), 2 deaths).
RESTART‐AP 2013 was designed to determine whether restarting antithrombotic agents had an impact on the number of new onset cerebral microbleeds and if intensive blood pressure lowering reduced their numbers. The study authors confirmed that insufficient funding was available, and the study was terminated early.
Studies awaiting classification
Three studies await classification (ABCD‐H 1998; BBB 1994; Cardio‐Sis 2014). These studies did not report data for participants with cardiovascular disease at baseline. We have requested these data from study authors, but had not received data before publication of this review.
Ongoing studies
We identified six ongoing studies (ESH‐CHL‐SHOT 2014; HOSP 2006; INFINITY 2013; ISRCTN37694103; NCT01198496; NCT03015311). We will evaluate these studies when complete for possible inclusion in future updates of this review.
Risk of bias in included studies
The summary of the risk of bias assessment of each trial is shown in Figure 2. The assessment of risk of bias was based on both published and unpublished data.
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Except for SPRINT 2015, where no specific information on allocation method was provided, all other included studies used a computerized system for randomization. Methods for allocation were judged as low risk of bias for five studies (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPS3 2013). SPRINT 2015 was judged as unclear risk of bias.
Blinding
None of the included studies was blinded to participants or clinicians because of the need to titrate antihypertensives to reach a specific blood pressure goal. However, clinical events were assessed by an independent committee blinded to the group allocation in all trials. Hence, all trials were assessed at low risk of performance and detection bias.
Incomplete outcome data
The available information (both published and unpublished) for four trials (AASK 2002; ACCORD BP 2010; Past BP 2016; SPRINT 2015) did not suggest a significant imbalance between arms for withdrawals or dropouts, and were assessed at low risk of attrition bias.
In HOT 1998 14% of total ECG could not be obtained, leading to some uncertainty on silent myocardial infarctions. We decided to assume a conservative perspective and consider unclear risk of bias for this trial.
There was no information about how many participants lost to follow‐up were allocated to standard or lower blood pressure target groups in SPS3 2013, which was assessed at unclear risk of attrition bias.
Selective reporting
Protocols and published articles were assessed for AASK 2002, ACCORD BP 2010, HOT 1998, Past BP 2016 and SPRINT 2015 with no sign of reporting bias confirmed. These trials were assessed at low risk of reporting bias.
Serious adverse effects reporting in SPS3 2013 related to hypotension and blood pressure management only. We contacted study authors for clarification but no response was received. This study was assessed at high risk of selective reporting bias.
Other potential sources of bias
With the exception of SPS3 2013, all data used in this Cochrane Review came from subgroups of participants not predefined in the original study protocols and this constitutes a potential source of bias.
Some studies were partially (AASK 2002) or fully (HOT 1998) funded by pharmaceutical industry sources, which constitutes another potential source of bias.
Early termination of SPRINT 2015 was also considered a potential source of bias.
Effects of interventions
See: Table 1
Lower versus standard blood pressure targets
We included six randomized controlled trials (RCTs) (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015; SPS3 2013) that met inclusion criteria. Data were obtained from published and unpublished sources. We assumed that silent myocardial infarction complied with the definition of cardiovascular event when provided.
Primary outcomes
Total mortality
There was no difference in total mortality between lower and standard blood pressure target groups (RR 1.05, 95% CI 0.90 to 1.22, P = 0.53; 6 studies; Analysis 1.1). When the absolute effect was measured, three additional total deaths per 1000 participants were identified in the lower target (95% CI 7 fewer to 15 more total deaths per 1000 participants). There was a total of 373 deaths (of 5456 participants) in the lower target group and 294 (of 4339 participants) in the standard target group (Figure 3).
Analysis 1.1.
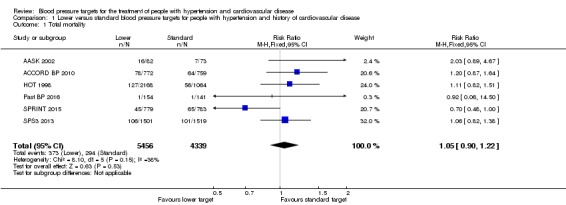
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 1 Total mortality.
Figure 3.
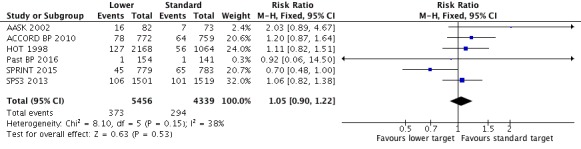
Forest plot of comparison: 1 Lower versus Standard, outcome: 1.1 Total mortality.
Serious adverse events
All included studies provided data for analysis of serious adverse events. A broad definition of serious adverse event was adopted, according to the ICH 1995 definition. We included in participants with any cause of death, any cardiovascular event (as predefined in our protocol) or any other serious adverse event as defined by trial authors, avoiding double‐counting of participants. When all data were pooled, there was no difference in serious adverse events between the lower and standard blood pressure target groups (RR 1.02, 95% CI 0.95 to 1.11, P = 0.48; Analysis 1.2). When the absolute effect was measured, three additional serious adverse events per 1000 participants were identified in the lower target group (95% CI 9 fewer to 20 more serious adverse events per 1000 participants). There were 966 participants with at least one serious adverse events (of 5456 participants) in the lower target group and 805 (of 4339 participants) in the standard target group (Figure 4). SPRINT 2015 was considered to report the full range of serious adverse events (Analysis 1.2.1) and five studies reported subsets of events (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPS3 2013; Analysis 1.2.2).
Analysis 1.2.
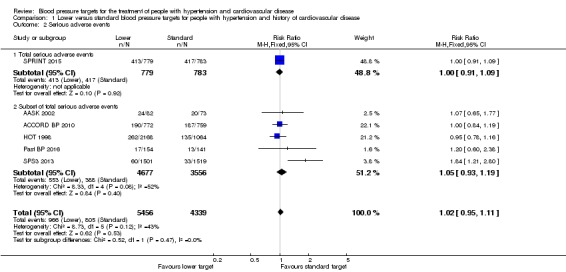
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 2 Serious adverse events.
Figure 4.
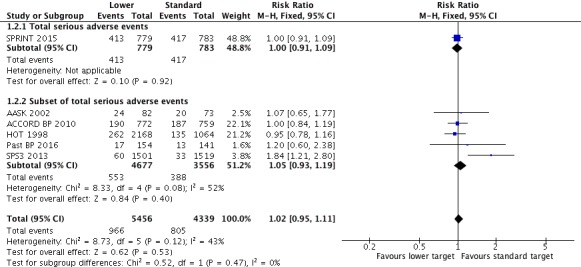
Forest plot of comparison: 1 Lower versus Standard, outcome: 1.2 Serious adverse events.
Cardiovascular events
Data from 27 participants in AASK 2002 were analyzed in relation to individual cardiovascular events for myocardial infarction, stroke and heart failure hospitalization; data from seven further participants were analyzed from a direct cardiovascular mortality diagnosis.
Five included studies provided data by means of well‐defined categories. The total number of cardiovascular events was significantly reduced in the lower blood pressure target group compared with the standard group (RR 0.87, 95% CI 0.78 to 0.98, P = 0.02; 6 studies, Analysis 1.3). When the absolute effect was measured, 16 fewer cardiovascular events per 1000 participants were identified in the lower blood pressure target group (95% CI 2 to 27 fewer cardiovascular events per 1000 participants). There were 555 participants with cardiovascular events (of 5456 participants) in the lower target group and 535 (of 4339 participants) in the standard target group (Figure 5).
Analysis 1.3.
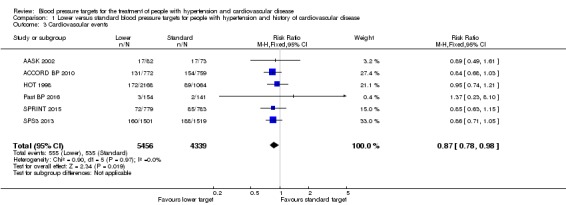
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 3 Cardiovascular events.
Figure 5.
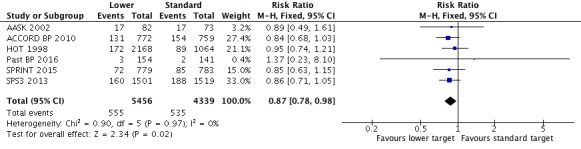
Forest plot of comparison: 1 Lower versus Standard, outcome: 1.4 Cardiovascular events.
Cardiovascular mortality
Some comments relating to AASK 2002 need to be made before reporting analysis results. AASK 2002 researchers used two different documents to register causes of death (CARDIO_REVW Form #38 and CC_DEATH Form #48). There was no complete overlap between forms. After discussion, we considered there to be valid cardiovascular mortality when the researcher answered 'yes' to question 4 in Form #38: "Was there a cardiovascular death?" This indicated 11 deaths. Data from Form #48 were also analyzed case‐by‐case by two clinicians (a cardiologist and a general practitioner) and two additional deaths were identified after a careful validation process.
Five trials provided data by means of well‐defined categories. There was no difference in cardiovascular mortality between the lower and standard blood pressure target groups (RR 0.96, 95% CI 0.77 to 1.21, P = 0.75; Analysis 1.4). There were 169 cardiovascular deaths (among 5456 participants) in the lower target group and 137 (among 4339 participants) in the standard target group (Figure 6).
Analysis 1.4.
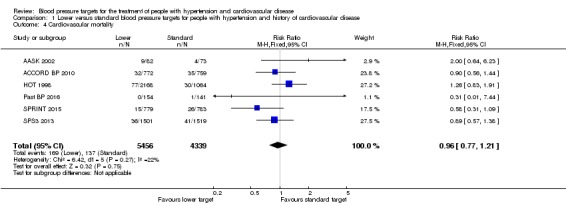
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 4 Cardiovascular mortality.
Figure 6.
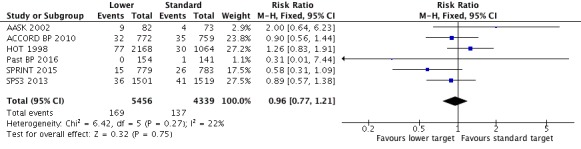
Forest plot of comparison: 1 Lower versus Standard, outcome: 1.3 Cardiovascular mortality.
Secondary outcomes
Withdrawals due to adverse effects
Four trials (AASK 2002; ACCORD BP 2010; SPRINT 2015; SPS3 2013) did not provide any information about withdrawals due to adverse effects in participant with basal cardiovascular disease.
Data were extracted from free text notes only in HOT 1998; Past BP 2016 provided better quality data. Despite limited information, a difference in withdrawals due to adverse effects was found between the lower and standard blood pressure target groups (RR 8.16, 95% CI 2.06 to 32.28, P = 0.003; Analysis 1.5). There were 22 withdrawals due to adverse effects (among 420 participants) in the lower target group and only two (among 270 participants) in the standard target group.
Analysis 1.5.
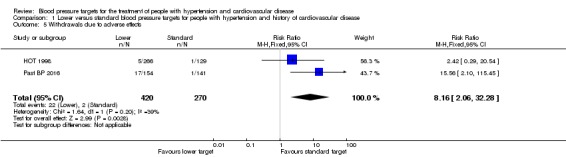
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 5 Withdrawals due to adverse effects.
Blood pressure target achieved at 1 year
There were 3391/5143 (66%) participants who reached the target in the lower target group and 3042/4098 (74%) participants in the standard target group (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015; SPS3 2013). Therefore, more people in the standard group achieved particular blood pressure targets.
Systolic blood pressure change from baseline at end of 1 year
After the first year of therapy, the average systolic blood pressure achieved was significantly lower in the lower blood pressure target group (MD ‐9.52 mmHg, 95% CI ‐4.93 mmHg to ‐14.11 mmHg, P < 0.0001; 6 trials; Analysis 1.7). Heterogeneity among trials was high, so a random‐effects model was preferred for this analysis. The different targets and the specific basal characteristics for each trial were considered the most likely causes for this heterogeneity.
Analysis 1.7.
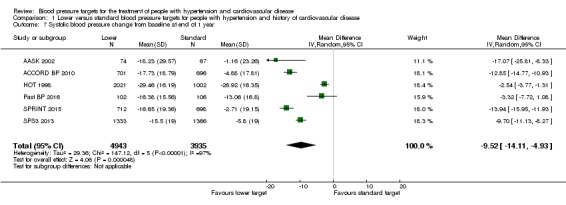
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 7 Systolic blood pressure change from baseline at end of 1 year.
Diastolic blood pressure change from baseline at end of 1 year
After the first year of therapy, the average diastolic blood pressure achieved was significantly lower in the lower blood pressure target group (MD ‐4.93 mmHg, 95% CI ‐2.61 mmHg to ‐7.26 mmHg, P < 0.0001; 5 trials; Analysis 1.8). Heterogeneity between trials for this outcome was high, so a random‐effects model was chosen for this analysis. The different targets and the specific basal characteristics for each trial were considered the most likely causes for this heterogeneity.
Analysis 1.8.
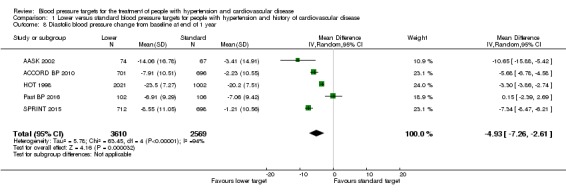
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 8 Diastolic blood pressure change from baseline at end of 1 year.
Number of antihypertensive drugs needed at the end of study
At the end of study, the number of antihypertensive drugs needed was significantly lower in the standard blood pressure target group (average 1.9 drugs) than the lower blood pressure target group (average 2.4 drugs) (MD 0.56, 95% CI 0.16 to 0.95, P = 0.006; 5 trials; Analysis 1.9). Heterogeneity between trials for this outcome was high, so a random‐effects model was chosen for this analysis. The different targets and the specific basal characteristics for each trial were considered the most likely causes for this heterogeneity.
Analysis 1.9.
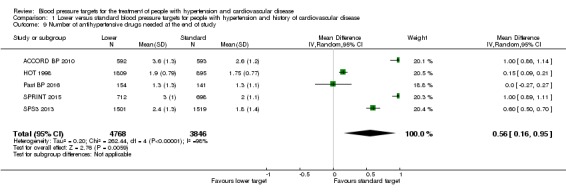
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 9 Number of antihypertensive drugs needed at the end of study.
Discussion
Pharmacological treatment of high blood pressure is aimed to reduce morbidity and mortality. Specific blood pressure targets have been proposed in guidelines for people with hypertension who have established cardiovascular disease, but optimal thresholds remain uncertain because the benefit to harm ratio of more intensive treatment has not been established.
This Cochrane Review explored the current evidence from randomized control trials (RCTs) and assessed relevant outcomes linked to two alternative strategies: standard blood pressure target (≤ 140 to 160/90 to 100 mmHg) and lower blood pressure target (≤ 135/85 mmHg).
We included six RCTs with a total of 9795 participants and a mean follow‐up of 3.7 years (range 1.0 to 4.7 years). Four studies compared systolic blood pressure targets, one compared diastolic blood pressure targets and one compared mean blood pressure targets. Individual patient data were available for five trials (AASK 2002; ACCORD BP 2010; HOT 1998; Past BP 2016; SPRINT 2015).
Two previous Cochrane Reviews adopted different strategies for analysis. Arguedas 2009 pooled trial data for the main analysis, but Arguedas 2013 considered each target (systolic or diastolic) separately. Both approaches are suitable and relevant and our Cochrane Protocol (Gorricho 2013) did not specify any particular strategy. In this Cochrane Review we decided to use the pooled data as the main analysis, but we also tested if results were consistent when blood pressure targets were considered separately. To avoid misclassification problems, a third category (mean blood pressure) was added to systolic/diastolic.
Summary of main results
Evidence from the six included trials indicated that blood pressure targets were more frequently achieved in the standard blood pressure target arm 3042/4098 (74%) than the lower target arm 3391/5143 (66%).
More antihypertensive drugs were used in the lower blood pressure target group (average 2.4 drugs) than in the standard arm (average 1.9 drugs).
There were broad differences for systolic (9.5 mmHg) and diastolic (4.9 mmHg) blood pressure changes from baseline in the lower arm.
No benefits in total mortality (RR 1.05, 95% CI 0.90 to 1.22) or cardiovascular mortality (RR 0.96, 95% CI 0.77 to 1.21) were detected. Subsequent analyses separating trials by systolic, diastolic or mean blood pressure targets did not change these results. A slight decrease was found with regard to fatal and non fatal cardiovascular events (including myocardial infarction, stroke, sudden death, hospitalization or death from congestive heart failure) (RR 0.87, 95% CI 0.78 to 0.98) in favour of lower blood pressure target, with no difference in total serious adverse events (RR 1.02, 95% CI 0.95 to 1.11). When systolic target trials were considered separately, a similar decrease in total cardiovascular events was found in favour of the lower target but a lack of a difference in total serious adverse events was also confirmed.
Most withdrawals due to adverse effects occurred in the lower target arm (RR 8.16, 95% CI 2.06 to 32.28). However, little evidence was available, making establishment of a trustworthy global assessment of benefits and harms very challenging.
Importantly, no significant heterogeneity was detected for any primary outcome. Therefore, at present there does not seem to be sufficient sound evidence to justify more strict blood pressure targets (≤ 135/85 mmHg) than the standard range (≤ 140 to 160/90 to 100 mmHg) in people with hypertension and established cardiovascular disease.
Significant heterogeneity was detected for two outcomes, blood pressure difference from baseline at one year and number of hypertensive drugs that each patient needed at the end of study. The different targets and the specific basal characteristics for each trial were considered the most likely causes for this heterogeneity. Subgroup analysis indicated significant heterogeneity in the male subgroup for cardiovascular mortality. The source of heterogeneity could be linked to a decrease in the number of participants and events and differences in trial design between HOT 1998 and ACCORD BP 2010/SPRINT 2015.
The minimum 5 mmHg difference in systolic or diastolic blood pressure targets predefined as clinically significant in our protocol agrees with previous guideline decisions (NICE 2016). Nonetheless, as Arguedas 2009 and Arguedas 2013 reported, it could be argued that this difference is not large enough to detect significant changes in relevant outcomes. To test this hypothesis, an additional sensitivity analysis was conducted in participants with diabetes, excluding the intermediate < 85 mmHg target in HOT 1998 and the results were very similar between the main analysis in participants with cardiovascular disease and the subgroup analysis in participants with diabetes and high differences in targets (Table 4).
Table 2.
Lower versus standard blood pressure target; people with diabetes, difference in targets ≥10 mmHg
| Outcome | Studies | Participants | Statistical Method | Effect Estimate |
| Total mortality | ACCORD BP 2010, HOT 1998 | 1793 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81,1.48] |
| Cardiovascular mortality | ACCORD BP 2010, HOT 1998 | 1793 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51,1.22] |
| Cardiovascular events | ACCORD BP 2010, HOT 1998 | 1793 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66,0.99] |
| Serious adverse events | ACCORD BP 2010, HOT 1998 | 1793 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82,1.15] |
Four subgroup analyses were specified in our Cochrane Protocol (people with diabetes, participant sex and people aged ≥ 75 years) designed to explore potential differences in specific populations. Despite the large amount of information retrieved from individual patient data for this review, few data were available for people aged ≥ 75 years to draw any definite conclusion. When participant data were split according to sex or only participants with diabetes were considered, similar magnitudes of effect were found to those described in the main analysis. People with diabetes and established cardiovascular disease could be seen at first as being in a higher risk category than people who do not have diabetes (Mancia 2011). However, estimates for people with diabetes were quite similar to those in the general population with basal cardiovascular disease: no differences in total mortality, cardiovascular mortality or total cardiovascular events associated with lower target, and no differences for both target strategies in serious adverse events. There was insufficient evidence to detect greater effect from a lower blood pressure target in these subgroups, although the sample sizes were not large enough to exclude a significant effect.
Two sensitivity analyses were planned to test the robustness of the results: risk of bias of the trials; and industry sponsored versus non industry sponsored.
Because overall risk of bias was rated as high, sensitivity analysis could not be performed. A slight difference was found in total cardiovascular events in favour of the lower blood pressure target in non industry sponsored trials (ACCORD BP 2010; Past BP 2016; SPRINT 2015; SPS3 2013), with no parallel better result in serious adverse events.
Overall completeness and applicability of evidence
Cardiovascular diseases are prevalent and high blood pressure is an added risk factor commonly treated in this population. Evidence based guidelines focused on this issue are needed. Unfortunately, data from randomized controlled trials designed to clarify this uncertainty remain insufficient.
This review included six trials but only one (SPS3 2013, 3020 participants) met the inclusion criteria as a whole study. The other five studies contributed individual patient data for subgroups of participants (AASK 2002, 155 participants; ACCORD BP 2010, 1531 participants; HOT 1998, 3232 participants, Past BP 2016, 295 participants; SPRINT 2015, 1562 participants).
Although this review analyzed a significant body of evidence, and results are considered to be robust, results cannot be considered as conclusive. Several ongoing trials (ESH‐CHL‐SHOT 2014; ISRCTN37694103; NCT01198496) have been designed to explicitly answer relevant questions in people with established cardiovascular disease; it is anticipated that these studies will yield additional evidence.
Over 6000 participants provided data on systolic targets and over 3000 on diastolic targets. Neither subanalysis substantially changed overall results in primary outcomes when all target strategies were considered together. From this perspective, the results of this review can be generalized for physicians prescribing antihypertensive drugs, no matter the specific target strategy (systolic, diastolic or both) chosen.
As identified by Arguedas 2009, and probably fuelled by the intention‐to‐treat approach, this review did not find real differences as wide as expected between arms in achieved systolic and diastolic blood pressure, according to the predefined targets for each study. The standard target was achieved in all six included trials but only ACCORD BP 2010, Past BP 2016 and SPS3 2013 achieved the required blood pressure in the lower target (in HOT 1998 the ≤ 80 mmHg target was not achieved). This underlines the difficulty of putting the intervention into practice, as often happens in real life. Accordingly, this aspect could be seen as both a limitation or a strength.
Quality of the evidence
We downgraded the quality of the evidence for total mortality and cardiovascular mortality to moderate due to imprecision and lack of data. In our opinion, other potential limitations (e.g. cardiovascular disease subgroups were not predefined in several studies) are unlikely to lower confidence in the estimate, taking into account large sample sizes, the design of SPS3 2013 (31% of total participants), the sensitivity analysis performed about potential risk of bias, and the strength of the individual patient data analysis.
We also downgraded the quality of evidence for other outcomes: total cardiovascular events and serious adverse events were assessed as providing low‐quality evidence; withdrawals due to adverse effects provided very low‐quality evidence. Total cardiovascular events data were affected by a high risk of bias. Furthermore, insufficient data were available on drug side effects, and as for withdrawals, imprecision was especially marked, leading to further downgrading of evidence quality. (See Table 1).
Potential biases in the review process
Because of study requirements, none of the included studies were blinded to participants or clinical researchers. However, all studies implemented mechanisms to assess outcomes by independent blinded committees. Consequently, potential performance bias was considered high and detection bias was judged as low.
Another potential source of bias came from the fact that most included participants (all but those in SPS3 2013) were part of subgroup studies and some (25% non hypertensive participants in SPS3 2013) did not comply with our inclusion criteria. SPS3 2013 data were included because the participants who complied with our inclusion criteria reached the predefined 70% threshold. However, to adapt the study interventions to those defined in our review, HOT 1998 participants in two different targets (< 85 mmHg and < 80 mmHg) were pooled for only the lower blood pressure target.
Additionally, primary outcomes in AASK 2002 were not aligned with our review's interest. It must also be stressed that most subgroups included a high number of participants and all were analyzed as individual patient data.
The differences between trials in type and definition of outcomes could also be a source of bias (see Outcomes in Characteristics of included studies tables). For example, not all studies provided adequate information about how silent myocardial infarctions were dealt with, revealing differences among studies that included heart failure hospitalization as an outcome.
No homogeneous information among trials was observed for serious adverse events, the most comprehensive outcome on safe. Only SPRINT 2015 was deemed to report the total number of serious adverse events, according to its international standardized definition (ICH 1995). Other included trials provided an unreliably low number of serious adverse events (HOT 1998; SPS3 2013); reported only those events judged by researchers as probably related to the interventions (ACCORD BP 2010); considered serious adverse events from a extremely narrow perspective (Past BP 2016); or did not offer any specific information on this outcome (AASK 2002). Deaths, major cardiovascular events and serious adverse effects reported by trialists were included as serious adverse events in analyses when only partial information was available. Because of these concerns, reporting bias was strongly suspected for certain outcomes such as serious adverse events and withdrawals due to adverse effects, where few data were reported.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review with meta‐analysis that assessed blood pressure targets in people with established cardiovascular disease from randomized controlled trials that directly compared different target strategies.
We found no evidence of additional benefit from a lower blood pressure target compared to a standard blood pressure target in terms of total mortality or cardiovascular mortality. We found a slight decrease in total cardiovascular events in favour of the lower blood pressure target (RR 0.87, 95% CI 0.78 to 0.98), with no difference in total serious adverse events between strategies.
Some prominent hypertension guidelines (JNC‐8 2014; NICE 2016) have not issued direct recommendations on blood pressure targets for people with previous cardiovascular disease. Those reviews or guidelines that include explicit recommendations mainly come from observational data or post hoc analyses of achieved blood pressure in trials designed for various purposes (Bangalore 2013). This perspective could easily lead to selection bias, favouring a lower risk of experiencing a cardiovascular event in participants with lower achieved blood pressure. Only one study (SPS3 2013) directly compared clinical outcomes in people who had stroke treated to different blood pressure targets; no studies have been conducted in people with cardiovascular disease.
Our results do not seem to support the widespread implementation of an intensive target strategy (≤ 135/85 mmHg) for cardiovascular secondary prevention. The conservative approach is also recommended by ESH‐ESC 2013 and CHEP 2015, where a < 140/90 mmHg target has been recommended in most patient situations, including people with previous cardiovascular disease. In addition, a similar systematic review on chronic kidney disease did not show either that a blood pressure target < 125/75 to 130/80 mmHg is more beneficial than a target < 140/90 mmHg (Upadhyay 2011).
However, based on SPRINT 2015 data, CHEP 2016 recommends consideration of lower targets in some high cardiovascular risk patients. Other guidelines, such as Joint ESC 2016, Rosendorff 2015 and Kernan 2014, only partially agree with our view. In Joint ESC 2016 a 130 to 139 mmHg systolic target is recommended, whereas a more intensive effort (80 to 85 mmHg) is supported as the diastolic goal. No specific supportive evidence was provided on this statement. Two American guidelines focusing on coronary and stroke patients are available. Rosendorff 2015 suggests < 140/90 mmHg as a reasonable target for secondary prevention of cardiovascular events in coronary patients, but a lower target (< 130/80 mmHg) is also considered useful for some individuals, but they admitted that this is not supported by evidence and no additional details of potential benefit profiles were offered. Kernan 2014 recommends a < 140/90 mmHg target strategy as a general rule for stroke patients but points out that 130/80 mmHg could be reasonable for patients with a recent lacunar stroke, based mainly on SPS3 2013 results. However, the SPS3 2013 study did not achieve a statistically significant difference between lower and standard targets for any of the primary or secondary outcomes measured. In SPS3 2013 the difference detected in intracerebral haemorrhages (a subtype of intracranial haemorrhages not pre‐planned even as a secondary outcome) could well have been due to chance. Surprisingly, despite there being no substantial benefit confirmed with the lower target, the SPS3 2013 authors concluded that, based on their results, the use of a systolic blood pressure target of < 130 mmHg was likely to be beneficial in patients with recent lacunar stroke.
A recent systematic review (Ettehad 2016) identified large‐scale blood pressure lowering trials to quantify the effects of reducing 10 mmHg (systolic blood pressure) in terms of mortality and cardiovascular outcomes. This analysis was conducted for the main comparison and several subgroups, one of them in patients with established cardiovascular disease. The results showed benefits for this subgroup in mortality and cardiovascular events when blood pressure was reduced, but inconsistent results in safety outcomes were also reported. The authors concluded that lowering current normotensive levels are supported by their review, provided there is a relevant absolute risk. In this regard, relevant limitations must be taken into account. First, heterogeneity was extremely high in Ettehad 2016, including large differences among populations, basal comorbidities and comparisons in treatment groups. In fact, some included studies compared the effect of different blood pressure targets, the effect of different drugs, or even drugs versus placebo. Second, the review did not consider individual patient data, leading to a particularly low accuracy when conclusions are assumed on participants with or without basal cardiovascular disease. Finally, among the included studies comparing different blood pressure targets, too diverse strategies were mixed, from < 120 mmHg to < 150 mmHg systolic blood pressure target. Certainly this review has been able to gather a big amount of information but, at the same time, a careful approach should be demanded in order to not draw misleading conclusions.
Another systematic review (Xie 2016) has paid attention to clinical trials comparing only blood pressure targets. While this design seems to be more appropriate than in the previous case, inclusion criteria were also established with high laxity. Limits are not well defined with regard to what is considered an intensive or standard target. Because of that, two studies can share the same target but, simultaneously, be assigned to different groups, standard or intensive (Brunström 2016). Participants with a wide range of blood pressure targets are mixed leading to little informative results, even if data from a large number of patients were collected. The authors declare that, in high cardiovascular risk, benefits from intensive treatment clearly overcome potential harms, even in patients with < 140 mmHg, claiming for changes in current guidelines.
In contrast, our systematic review does not support that view. We have not identified any advantages after taking into account more appropriate inclusion criteria, substantial amount of individual patient data and despite the benefit found in terms of total cardiovascular events, when a more informative outcome, 'serious adverse events', was considered. Furthermore, even if SPRINT 2015 mortality results show a trend favouring the lower strategy, no overall benefits in these outcomes were detected and adverse events were poorly informed by all the concerned clinical trials. In our opinion, whereas the scientific community is dealing with this key lack of information, recommendations on blood pressure targets in hypertensive patients with cardiovascular disease should give priority to caution.
Authors' conclusions
The best evidence available at this time from randomized controlled trials does not support blood pressure targets < 140 to 160/90 to 100 mmHg in people with hypertension and established cardiovascular disease (myocardial infarction, stroke, chronic peripheral vascular occlusive disease or angina pectoris).
We analysed systolic, diastolic or mean blood pressure goals as a whole and separately, with similar findings. In addition to the lack of benefit in total or cardiovascular mortality for the lower blood pressure target, the slight decrease in total cardiovascular events linked to a lower systolic blood pressure target needs to be considered within the context of the numerical increase in mortality and serious adverse events.
Predefined subgroup analyses in older people, those with diabetes, or based on participant's sex did not suggest any differences in these conclusions.
According to the best available evidence, lower targets for people with hypertension and established cardiovascular disease do not achieve a net health benefit.
Well designed, randomized controlled trials assessing lower blood pressure targets in people with hypertension and established cardiovascular disease are needed to ascertain the benefits and harms from intensive and more conservative strategies.
Six ongoing studies in people with stroke and coronary disease have been identified (ESH‐CHL‐SHOT 2014; HOSP 2006; INFINITY 2013; ISRCTN37694103; NCT01198496; NCT03015311) but additional studies exploring other types of basal cardiovascular diseases are also required (e.g. peripheral vascular disease, haemorrhagic stroke, etc). Future research should aim to report mortality rates and all serious adverse event outcomes.
Having access to individual patient data and other relevant documents (protocols, clinical study reports, raw data) becomes a major strength of systematic reviews with meta‐analysis. Thus, authors of past or future trials are highly encouraged to share their databases.
Acknowledgements
We are grateful to:
James M Wright and the Cochrane Hypertension Group, for their encouragement, support and assistance. Same gratitude to Juan Erviti, the Navarre Cochrane Group and the Drug Prescribing Unit, Navarre Health Service, Navarre, Spain.
The Biomedical Information Center of Navarre provided most of the published documents reviewed in this report. Stephen Adams, Vancouver, Canada, provided assistance with some references that were especially difficult to find.
Miguel Ángel Imízcoz, cardiologist, provided specific advice related to cardiovascular events ascertainment from individual patient data.
Agustín Ciapponi and Demian Glujovsky, Institute of Clinical Effectiveness and Health Policy) (IECS), Buenos Aires, Argentina, provided access to Early Review Organizing Software.
Annalisa Perna, Giuseppe Remuzzi and Piero Ruggenenti, Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy, provided individual patient data for REIN‐2 2005.
Kate Fletcher, University of Birmingham, Birmingham, UK, and Jonathan Mant, University of Cambridge, Cambridge, UK, provided individual patient data for Past BP 2016.
Larrye Loss and Julie Ye, AstraZeneca, Wilmington (DE), USA, provided access to protocol, forms, Clinical Study Report and individual patient data for the HOT 1998 study. This manuscript was not prepared in collaboration with AstraZeneca staff and does not necessarily reflect the opinions or views of the company. According to the Data Transfer Agreement, AstraZeneca was entitled to make comments to the final report but approval was given with no remarks.
The AASK 2002and MDRD 1994 studies were conducted by the AASK/MDRD Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from the AASK 2002 and MDRD 1994 studies reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the AASK 2002 and MDRD 1994 studies and does not necessarily reflect the opinions or views of the AASK/MDRD studies, the NIDDK Central Repositories, or the NIDDK.
This manuscript was prepared using ACCORD and SPRINT_POP Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordination Center and does not necessarily reflect the opinions or views of the ACCORD, SPRINT_POP or the National Heart, Lung and Blood Institute (NHLBI).
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp cardiovascular diseases/ (2106946) 2 ((heart or myocardial) adj5 (attack$ or disease$ or infarc$)).tw. (293806) 3 (coronary adj5 (disease$ or syndrome$)).tw. (137399) 4 ((cardiovascular or peripheral or vascular) adj5 disease$).tw. (178128) 5 atrial fibril$.tw. (45630) 6 ((cardiac or heart) adj failure).tw. (126714) 7 angina$.tw. (47194) 8 exp ischemia/ (54251) 9 (ischaemi$ or ischemi$).tw. (292064) 10 exp stroke/ (104093) 11 (CVA or poststroke or post‐stroke or stroke or strokes).tw. (168922) 12 apoplexy.tw. (2460) 13 cerebrovascul$.tw. (40594) 14 cerebral vascular.tw. (4997) 15 ((brain$ or cerebral$ or lacunar) adj2 (accident$ or infarct$)).tw. (21275) 16 or/1‐15 (2333464) 17 ((goal? or intensive$ or strict$ or target$ or tight$) adj6 (antihypertensive? or anti‐hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat$)).tw. (127273) 18 hypertension/ (214139) 19 hypertens$.tw. (338263) 20 exp blood pressure/ (268607) 21 (blood pressure or bloodpressure).tw. (234315) 22 or/18‐21 (641204) 23 randomised controlled trial.pt. (446708) 24 controlled clinical trial.pt. (91795) 25 randomized.ab. (339791) 26 placebo.ab. (167931) 27 clinical trials as topic/ (181004) 28 randomly.ab. (234418) 29 trial.ti. (151734) 30 or/23‐29 (1003739) 31 animals/ not (humans/ and animals/) (4280946) 32 30 not 31 (919109) 33 16 and 17 and 22 and 32 (2756) 34 remove duplicates from 33 (2592)
Database: Cochrane Hypertension Specialised Register Search date: 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ((intensive* NEAR bp) OR (intensive* NEAR dbp) OR (intensive* NEAR pressure*) OR (intensive* NEAR sbp)) AND INSEGMENT #2 ((strict* NEAR bp) OR (strict* NEAR dbp) OR (strict* NEAR pressure*) OR (strict* NEAR sbp)) AND INSEGMENT #3 ((target* NEAR bp) OR (target* NEAR dbp) OR (target* NEAR pressure*) OR (target* NEAR sbp)) AND INSEGMENT #4 ((tight* NEAR bp) OR (tight* NEAR dbp) OR (tight* NEAR pressure*) OR (tight* NEAR sbp)) AND INSEGMENT #5 #1 OR #2 OR #3 OR #4 AND INSEGMENT #6 ((cardiovascular NEAR disease*) OR (heart NEAR attack*) OR (heart NEAR disease*) OR (heart NEAR infarct*)) AND INSEGMENT #7 ((peripheral NEAR disease*) OR (myocardial NEAR attack*) OR (myocardial NEAR disease*) OR (myocardial NEAR infarct*)) AND INSEGMENT #8 ((coronary NEAR disease*) OR (coronary NEAR syndrome*) OR (vascular NEAR disease*) OR (atrial fibril*)) AND INSEGMENT #9 ((cardiac failure) OR (heart failure) OR (angina*) OR (ischemi*)) AND INSEGMENT #10 (stroke OR (strokes) OR (ischaemi*) OR (CVA)) AND INSEGMENT #11 (apoplexy OR (cerebrovascul*) OR (cerebral vascular) OR (brain accident*)) AND INSEGMENT #12 ((brain infarct*) OR (cerebral NEAR accident*) OR (lacunar NEAR accident*) OR (lacunar NEAR infarct*)) AND INSEGMENT #13 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 AND INSEGMENT #14 RCT:DE AND INSEGMENT #15 (Review OR Meta‐Analysis):MISC2 AND INSEGMENT #16 #14 OR #15 AND INSEGMENT #17 #5 AND #13 AND #16 AND INSEGMENT
Database: Cochrane Central Register of Controlled Trials via Cochrane Register of Studies (CRS‐Web) Search date: 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ((intensive* NEAR bp) OR (intensive* NEAR dbp) OR (intensive* NEAR pressure*) OR (intensive* NEAR sbp)) AND CENTRAL:TARGET #2 ((strict* NEAR bp) OR (strict* NEAR dbp) OR (strict* NEAR pressure*) OR (strict* NEAR sbp)) AND CENTRAL:TARGET #3 ((target* NEAR bp) OR (target* NEAR dbp) OR (target* NEAR pressure*) OR (target* NEAR sbp)) AND CENTRAL:TARGET #4 ((tight* NEAR bp) OR (tight* NEAR dbp) OR (tight* NEAR pressure*) OR (tight* NEAR sbp)) AND CENTRAL:TARGET #5 #1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET #6 ((cardiovascular NEAR disease*) OR (heart NEAR attack*) OR (heart NEAR disease*) OR (heart NEAR infarct*)) AND CENTRAL:TARGET #7 ((peripheral NEAR disease*) OR (myocardial NEAR attack*) OR (myocardial NEAR disease*) OR (myocardial NEAR infarct*)) AND CENTRAL:TARGET #8 ((coronary NEAR disease*) OR (coronary NEAR syndrome*) OR (vascular NEAR disease*) OR (atrial fibril*)) AND CENTRAL:TARGET #9 ((cardiac failure) OR (heart failure) OR (angina*) OR (ischemi*)) AND CENTRAL:TARGET #10 (stroke OR (strokes) OR (ischaemi*) OR (CVA)) AND CENTRAL:TARGET #11 (apoplexy OR (cerebrovascul*) OR (cerebral vascular) OR (brain accident*)) AND CENTRAL:TARGET #12 ((brain infarct*) OR (cerebral NEAR accident*) OR (lacunar NEAR accident*) OR (lacunar NEAR infarct*)) AND CENTRAL:TARGET #13 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 AND CENTRAL:TARGET #14 #5 AND #13 AND CENTRAL:TARGET
Database: Embase 1974 to 3 February 2017 Search Date: 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp cardiovascular disease/ (3645720) 2 ((heart or myocardial) adj5 (attack$ or disease$ or infarc$)).tw. (439356) 3 (coronary adj5 (disease$ or syndrome$)).tw. (220152) 4 ((cardiovascular or peripheral or vascular) adj5 disease$).tw. (284235) 5 atrial fibril$.tw. (88261) 6 ((cardiac or heart) adj failure).tw. (219465) 7 angina$.tw. (67941) 8 exp ischemia/ (711070) 9 (ischaemi$ or ischemi$).tw. (450594) 10 exp stroke/ (166219) 11 (CVA or poststroke or post‐stroke or stroke or strokes).tw. (293951) 12 apoplexy.tw. (2947) 13 cerebrovascul$.tw. (63078) 14 cerebral vascular.tw. (7090) 15 ((brain$ or cerebral$ or lacunar) adj2 (accident$ or infarct$)).tw. (33109) 16 or/1‐15 (3842174) 17 ((goal? or intensive$ or strict$ or target$ or tight$) adj6 (antihypertensive? or anti‐hypertensive? or bp or control or dbp or diastolic or pressure? or sbp or systolic or treat$)).tw. (216542) 18 exp hypertension/ (631881) 19 (antihypertens$ or anti‐hypertens$ or hypertens$).tw. (546500) 20 exp blood pressure/ (513661) 21 (blood pressure or bloodpressure).mp. (528055) 22 or/18‐21 (1157259) 23 randomised controlled trial/ (477823) 24 crossover procedure/ (55043) 25 double‐blind procedure/ (140751) 26 (randomi?ed or randomly).tw. (949122) 27 (crossover$ or cross‐over$).tw. (87124) 28 placebo.ab. (244056) 29 (doubl$ adj blind$).tw. (177675) 30 assign$.ab. (301727) 31 allocat$.ab. (110573) 32 or/23‐31 (1412226) 33 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5886095) 34 32 not 33 (1240641) 35 16 and 17 and 22 and 34 (4697) 36 remove duplicates from 35 (4504)
Database: LILACS Search date: 2 March 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ ((cardiovascular diseases) or (heart attack$) or (myocardial infarct$) or (heart disease$) or (myocardial disease$) or (coronary disease$) or (coronary syndrome$) or (cardiovascular disease$) or (peripheral disease$) or (vascular disease$) or (atrial fibril$) or (cardiac failure) or (heart failure) or (angina$) or (ischaemi$) or (ischemi$) or (stroke$) or (CVA) or (poststroke) or (post‐stroke) or (apoplexy) or (cerebrovascul$) or (cerebral vascular) or (brain$ accident$) or (brain infarct$) or (cerebral$ accident$) or (cerebral$ infarct$) or (lacunar accident$) or (lacunar infarct$)) and ((intensive$ bp) or (intensive$ dbp) or (intensive$ pressure?) or (intensive$ sbp) or (strict$ bp) or (strict$ dbp) or (strict$ pressure?) or (strict$ sbp) or (target$ bp) or (target$ dbp) or (target$ pressure?) or (target$ sbp) or (tight$ bp) or (tight$ dbp) or (tight$ pressure?) or (tight$ sbp)) and ((hypertension) or (hypertens$) or (blood pressure) or (bloodpressure)) and (((PT:”randomised controlled trial”) or (PT:”controlled clinical trial”) or (AB:”randomi?ed”) or (AB:”placebo”) or (clinical trials) or (AB:”randomly”) or (TI:”trial”)) and not ((animals) and not (humans and animals)))
Database: ClinicalTrials.gov (via Cochrane Register of Studies) Search date: 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: (intensive OR strict OR target OR tight) AND (blood pressure) AND (randomised) Study type: Interventional Studies Conditions: (hypertension) AND (angina OR cardiovascular OR myocardial infarction OR peripheral vascular OR stroke) Outcome measure: blood pressure
Database: WHO International Clinical Trials Registry Platform Search Date: 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 intensive AND blood pressure AND randomized #2 strict AND blood pressure AND randomized #3 target* AND blood pressure AND randomized #4 tight AND blood pressure AND randomized #5 #1 OR #2 OR #3 OR #4 OR #5
Database: TRIP Database Search date: 5 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ (blood) AND (pressure) AND (targets) AND (intensive) AND (standard)
Appendix 2. Reviews and guidelines checked
Arguedas 2009; Arguedas 2013; Bangalore 2011; Bangalore 2013; Bangalore 2017; BPLTTC 2013; BPLTTC 2014; Drozda 2011; ESH‐ESC 2013; Ettehad 2016; Feldstein 2014; Lv 2012; Lv 2013; McBrien 2012; NICE 2016; Rosendorff 2009; Rosendorff 2015; Roy 2010; SBU 2007; Verdecchia 2016; Xie 2016.
Data and analyses
Comparison 1.
Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 6 | 9795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.22] |
| 2 Serious adverse events | 6 | 9795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.11] |
| 2.1 Total serious adverse events | 1 | 1562 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.09] |
| 2.2 Subset of total serious adverse events | 5 | 8233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.19] |
| 3 Cardiovascular events | 6 | 9795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.98] |
| 4 Cardiovascular mortality | 6 | 9795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.77, 1.21] |
| 5 Withdrawals due to adverse effects | 2 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.16 [2.06, 32.28] |
| 6 Blood pressure target achieved at 1 year | 6 | 9241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.12, 1.18] |
| 7 Systolic blood pressure change from baseline at end of 1 year | 6 | 8878 | Mean Difference (IV, Random, 95% CI) | ‐9.52 [‐14.11, ‐4.93] |
| 8 Diastolic blood pressure change from baseline at end of 1 year | 5 | 6179 | Mean Difference (IV, Random, 95% CI) | ‐4.93 [‐7.26, ‐2.61] |
| 9 Number of antihypertensive drugs needed at the end of study | 5 | 8614 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.16, 0.95] |
Analysis 1.6.
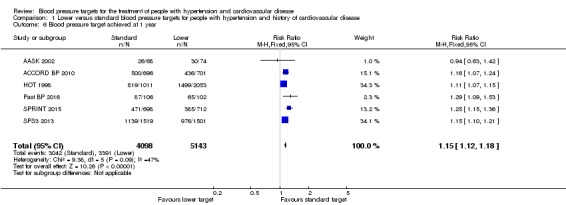
Comparison 1 Lower versus standard blood pressure targets for people with hypertension and history of cardiovascular disease, Outcome 6 Blood pressure target achieved at 1 year.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Multicenter, 3 x 2 factorial design, intention‐to‐treat (ITT) strategy. Participants randomized equally to a usual mean arterial pressure goal of 102 to 107 mmHg or to a lower mean arterial pressure goal of 92 mmHg or lower, and to treatment with metoprolol, ramipril or amlodipine. When the blood pressure goal was not achieved using the randomized drug, other open‐labelled antihypertensive agents were added to participants' treatment. Participants and investigators were not masked to the blood pressure goal. The follow‐up was 3 to 6.4 years (mean 3.8 years). |
|
| Participants | African‐American men and women, aged 18 to 70 years, with hypertension defined as sitting DBP ≥ 95 mmHg and reduced kidney function, defined as glomerular filtration rate (GFR) between 20 and 65 mL/min per 1.73 m². Exclusion criteria included DBP < 95 mmHg, known history of diabetes mellitus, urinary protein to creatinine ratio > 2.5, accelerated or malignant hypertension within 6 months, secondary hypertension, evidence of non blood pressure‐related causes of chronic kidney disease (CKD), serious systemic disease or clinical congestive heart failure (CHF). Baseline characteristics for participants included in the review (%) or mean ± SD): men/women (68/32%); age (57 ± 9 years); SBP (149 ± 28 mmHg); DBP (93 ± 16 mmHg); mean blood pressure (MBP) (112 ± 19 mmHg); current smoker (31%); types of drugs at 1 year: No information available. Previous cardiovascular condition: ischaemic heart disease (IHD) (25%), stroke (69%), peripheral vascular disease (PVD) (23%). Country: USA |
|
| Interventions | Standard (usual) target: MBP 102 to 107 mmHg Lower target: MBP < 92 mmHg |
|
| Outcomes | The primary analysis was based on the rate of change in GFR (GFR slope). As a key secondary analysis, all cardiovascular events including cardiovascular deaths and hospitalizations for myocardial infarctions (MI), strokes, heart failure, revascularization procedures and other hospitalised cardiovascular events were reviewed and classified by a blinded endpoints committee according to a prespecified protocol | |
| Funding sources | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Also partially funded by other National Institutes of Health grants, Office of Research in Minority Health, Pfizer, AstraZeneca and King Pharmaceuticals |
|
| Declarations of interest | "Dr Wright has no stock ownership but has received research grants, honoraria, and consult fees from Astra, Bayer, Bristol‐Myers Squibb, Eli Lilly and Co, Merck & Co, Novartis Pharma AG, Pharmacia, Pfizer, Sankyo Inc, GlaxoSmithKline, and Solvay/Unimed. Dr Appel has received honoraria from Astra and Novartis Pharma AG. Dr Cheek is a speaker for Wyeth, Novartis, and Sanofi‐Synthelabo, and investigator for Abbott Laboratories. Dr Middleton is a speaker for Merck and a consultant for King Pharmaceuticals" | |
| Notes | The amlodipine arm was halted in September 2000. The blood pressure achieved at the end of the trial was: standard target: MBP 104 ± 7; lower target: MBP 95 ± 8 A public repository provided individual patient data from hypertensive participants with established cardiovascular disease to be used in this systematic review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The computer screen displayed the blood pressure group to which the patient had been randomised (usual or low)" (p. S157) |
| Allocation concealment (selection bias) | Low risk | "The computer screen displayed the blood pressure group to which the patient had been randomised (usual or low). Random permuted blocks with randomly varying block sizes were utilized" (p. S157) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All cardiovascular events including cardiovascular deaths and hospitalizations for myocardial infarctions, strokes, heart failure, revascularization procedures, and other hospitalised cardiovascular events were reviewed and classified by a blinded endpoints committee according to a prespecified protocol". (p. S161) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not seem to be a significant imbalance in follow‐up flow diagram, according to Figure 1 (pp. 2421‐31) |
| Selective reporting (reporting bias) | Low risk | Protocol checked to cardiovascular outcomes |
| Other bias | Unclear risk | Subgroup of participants with basal cardiovascular disease not predefined |
| Methods | Multicentre, 2 x 2 factorial design, ITT strategy. Participants and investigators were not masked to blood pressure goal. All participants in the ACCORD BP trial were randomly assigned to either intensive or standard glycaemic control, and were also randomly assigned to either intensive or standard blood pressure control. The follow‐up was 4 to 8 years (mean 4.7 years) |
|
| Participants | Men and women with type 2 diabetes mellitus and a glycated haemoglobin level of 7.5% or more, aged 40 to 79 years with cardiovascular disease or 55 to 79 years with anatomical evidence of a substantial amount of atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease (dyslipidaemia, hypertension, smoking or obesity). Exclusion criteria included body mass index (BMI) > 45, serum creatinine (sCR) level > 1.5 mg/dL and other serious illness. Participants with SBP between 130 and 180 mmHg who were taking three or fewer antihypertensive medications and who had the equivalent of a 24‐hour protein excretion rate < 1.0 g were also eligible for the blood pressure trial. Baseline characteristics for participants included in the review (% or mean ± SD): men/women (63/37%); age (62 ± 8 years); age ≥ 75 years (7%); SBP (138 ± 16 mmHg); DBP (74 ± 11 mmHg); current smoker (13%); ethnic group: White (62%), non White (38%). Types of drugs at 1 year: thiazides (51%), ACEI/ARB (84%), calcium channel blockers (CC)B (26%), beta blocker (57%), other (28%). Previous cardiovascular condition: IHD (86%), stroke (20%). Country: USA, Canada |
|
| Interventions | Standard target: SBP < 140 mmHg Lower (intensive) target: SBP < 120 mmHg |
|
| Outcomes | The primary end point for ACCORD was the first occurrence of a major cardiovascular event, which was defined as the composite of non fatal MI, non fatal stroke, or cardiovascular death. Prespecified secondary outcomes included the combination of the primary outcome plus revascularization or hospitalization for CHF; the combination of a fatal coronary event, non fatal MI, or unstable angina; non fatal MI; fatal or non fatal stroke; non fatal stroke; death from any cause; death from cardiovascular causes; and hospitalization or death due to heart failure | |
| Funding sources | Supported by contracts from the NHLBI. The National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Eye Institute and the Centers for Disease Control and Prevention also contributed funding. General Clinical Research Centers provide support at many sites. Several companies provided study medications | |
| Declarations of interest | Drs Bigger, Buse, Byington, Corson, Cushman, Cutler, Evans, Friedewald, Gerstein, Goff, Grimm, Ismail‐Beigi, Katz, Peterson and Probstfield declared different types of relationships with NIH institutions and pharmaceutical companies (consultancy, grants, honoraria…) | |
| Notes | The glycaemia ACCORD trial was stopped on 6 February 2008. The blood pressures achieved at the end of the trial were: standard target: SBP 133.5 ± 0.4 mmHg; lower target: SBP 119.3 ± 0.4 mmHg. A public repository provided individual patient data from hypertensive participants with established cardiovascular disease to be used in this systematic review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally on the study’s Web site with the use of permuted blocks to maintain concealment of future study‐group assignments" (pp. 1575‐85) |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally on the study’s Web site with the use of permuted blocks to maintain concealment of future study‐group assignments" (pp. 1575‐85) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design is not compatible with blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "ACCORD utilized a centralized adjudication process for all deaths, and hospitalizations for myocardial infarction and strokes. Upon identification of a potential outcome, clinical site staff obtained medical records or details regarding the case. Personal identifiers and information that may have alerted adjudicators to treatment assignment (e.g. A1C values) were masked by the clinical site and the medical records sent to the Coordinating Center" (pp. 1575‐85; Appendix 1) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Consort diagram (section 2) (pp.1575‐85; Appendix 1) |
| Selective reporting (reporting bias) | Low risk | No reporting bias (protocol was checked) |
| Other bias | Unclear risk | Subgroup of participants with basal cardiovascular disease not predefined |
| Methods | Multicentre, 3 x 2 factorial design, ITT strategy, Prospective randomized open with blinded end point (PROBE) trial. All participants in the HOT trial were randomly assigned to three therapeutic goals (DBP ≤ 90 mmHg, ≤ 85 mmHg or ≤ 80 mmHg) and to receive either acetylsalicylic acid (aspirin) 75 mg daily or placebo under double blind conditions. Participants were randomized on the basis of the following baseline variables: age, sex, previous antihypertensive therapy, smoking, previous MI, previous other CHD, previous stroke, and diabetes mellitus. The follow‐up was 3.3 to 4.9 years (mean 3.8 years) |
|
| Participants | Hypertensive men and women aged between 50 and 80 years (mean 62 years) with essential hypertension were eligible for the study. Required DBP ≥100 mmHg and ≤115 mmHg on two occasions, at least one week apart. Exclusion criteria included: malignant or secondary hypertension, stroke or MI within 12 months before randomization, decompensated CHF, serious disease affecting survival during the next 2 to 3 years, requirement for BB, ACEI or diuretic treatments for reasons other than hypertension, requirement for antiplatelet or anticoagulant treatment and those with diabetes who required insulin. Baseline characteristics for participants included in the review (% or mean ± SD): men/women (53/47%); SBP (174 ± 15 mmHg); DBP (106 ± 3 mmHg); diabetes (12%); current smoker (16%); ethnic group: White (92%), non White (8%). Previous cardiovascular condition: IHD (95%), stroke (7%). Countries: Argentina, Austria, Belgium, Canada, Denmark, East Asia, Finland, France, Germany, Great Britain, Greece, Hungary, Israel, Italy, Mexico, Norway, South East Asia, Spain, Sweden, Switzerland, Netherlands and USA. |
|
| Interventions | Standard target: DBP ≤ 90 mmHg Lower target: DBP ≤ 85 mmHg or ≤ 80 mmHg |
|
| Outcomes | The principal aims of the study included to assess the relationship between pooled major cardiovascular events (non fatal MI, non fatal stroke or cardiovascular death) and the target blood pressures or DBP achieved during treatment. Secondary analyses examined the relationship between target DBP and specific outcomes, such as total or cardiovascular mortality, fatal and non fatal CHD or stroke and hospitalization | |
| Funding sources | Astra AB (Sweden), Astra Merck Inc (USA), Teva (Israel), Hoechst (Argentina) | |
| Declarations of interest | Not reported | |
| Notes | Silent MIs were documented by taking an electrocardiogram (ECG) at randomization and at the final visit. The blood pressures achieved at the end of the trial were: standard target: DBP 85 ± 5 mmHg; lower target: DBP 82 ± 5 mmHg A private repository provided individual patient data from hypertensive participants with established cardiovascular disease that were used in this Cochrane Review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was computer‐generated based on communications by fax between investigators and the Study Coordinating Centre" (pp. 1755‐62; and protocol, section 7.3) |
| Allocation concealment (selection bias) | Low risk | "The randomisation was computer‐generated based on communications by fax between investigators and the Study Coordinating Centre" (pp. 1755‐62; and protocol, section 7.3) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design is not compatible with blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An Independent Clinical Event Committee evaluated all events (masked)" (pp. 1755‐62; and protocol, section 7.2) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "14% of the ECG could not be obtained leading to uncertainty on silent myocardial infarctions. On the other hand, no significant differences among targets have been detected" (Clinical Study Report, p. 23, and pp. 1755‐62, Figure 1) |
| Selective reporting (reporting bias) | Low risk | The database shows all required results (study protocol, sections 3.1 and 3.2) |
| Other bias | Unclear risk | Subgroup of participants with basal cardiovascular disease not predefined |
| Methods | Multicentre, primary care‐based pragmatic RCT. The randomization method used minimization to balance the randomized groups on the basis of age (< 80, ≥ 80 years), sex, diabetes mellitus, atrial fibrillation, baseline SBP and practice. Participants were followed up from trial entry for 1 year |
|
| Participants | Men and women with stroke/transient ischaemic attack (TIA) diagnosis through review of medical records and participant interview. Exclusion criteria included SBP < 125 mmHg at baseline, already taking 3 or more antihypertensive agents, orthostatic hypotension, participant already had a treatment target of 130 mmHg SBP specified or insufficient corroborative evidence of stroke/TIA from medical record and participant interview | |
| Interventions | Standard target: SBP ≤ 140 mmHg Intensive target: SBP ≤ 130 mmHg, or 10 mmHg reduction if baseline SBP 125 to 140 mmHg |
|
| Outcomes | The primary outcome measure was change in SBP between baseline and 12 months. Key secondary outcomes included side effects, tolerability and adverse events; clinical outcomes (including major cardiovascular events (composite of fatal and non fatal stroke, MI or fatal CHD and other cardiovascular death), all cause mortality and hospital admissions). Key secondary events (stroke, MI, fatal CHD and other cardiovascular death) were reviewed by independent clinicians blinded to treatment to ensure unbiased coding of these events | |
| Funding sources | Financial support from the National Institute for Health Research (NIHR) Programme Grants for Applied Research funding scheme | |
| Declarations of interest | "JM has received grants from Ferrer and the NIHR; RJMcM has received grants from Ferrer during the conduct of the study and grants and personal fees from Omron, grants from Lloyds Pharmacy, personal fees from the Japanese Society of Hypertension, and personal fees from the American Society of Nephrology outside the submitted work; AR has received grants from the University of Birmingham during the conduct of the study; FDRH has received grants from the NIHR and non‐financial support from Omron and Microlife during the conduct of the study; no other relationships or activities that could appear to have influenced the submitted work" | |
| Notes | The study has been concluded and published. Agreement was made with the study authors to include data from hypertensive participants with established cardiovascular disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The central study team at the University of Birmingham randomised patients, with minimisation based on age, sex, diabetes mellitus, atrial fibrillation, baseline systolic blood pressure, and general practice. The research nurse ascertained treatment allocation either by telephone or online" (p. i708) "If the patient is eligible and willing to take part, the nurse will also gain written informed consent prior to randomisation, and will telephone the randomisation service to obtain treatment group allocation" (p. 37) |
| Allocation concealment (selection bias) | Low risk | "The randomisation will use minimisation to balance the randomised groups on the basis of age (< 80, ≥ 80), sex, diabetes mellitus, atrial fibrillation (because of the difficulties of obtaining accurate BP measurements in this group), baseline systolic BP and practice" (p. 37) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The outcome measure was not blinded, but a nurse not directly involved in the participant’s care obtained it by using an automated sphygmomanometer, so systematic recording bias is unlikely" (p. i708). "Key secondary events (stroke; myocardial infarction; fatal coronary heart disease and other cardiovascular death) will be reviewed by independent clinicians blinded to treatment to ensure unbiased coding of these events" (p. 37) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Primary outcome data were available for 379 participants at one year follow‐up (182 (68%) in the intensive target arm and 197 (75%) in the standard target arm). All patients were followed up for clinical events and deaths" (p. i708, Figure 1) |
| Selective reporting (reporting bias) | Low risk | No reporting bias (the protocol publication was checked) |
| Other bias | Unclear risk | Only half of the total number of study participants met the review inclusion criteria (participants with previous stroke) |
| Methods | Multicentre, randomized, parallel, controlled trial. Blinded to the outcomes assessor. The intervention was stopped early after a median follow‐up of 3.26 years |
|
| Participants | Men and women aged ≥50 years and SBP 130 to 180 mmHg (on 0 or 1 medication), 130 to 170 mmHg (on up to 2 medications), 130 to 160 mmHg (on up to 3 medications) or 130 to 150 mmHg (on up to 4 medications). Participants also had at least one of the following risk factors:
Two major exclusion criteria: diabetes mellitus and stroke. Other exclusion criteria were secondary hypertension, proteinuria, recent cardiovascular event or procedure, and symptomatic heart failure within the past 6 months |
|
| Interventions | Standard target: SBP < 140 mmHg Intensive target: SBP < 120 mmHg |
|
| Outcomes | The primary outcome was a composite of non fatal MI, acute coronary syndrome not resulting in MI, non fatal stroke, non fatal acute decompensated heart failure, and death from cardiovascular disease. Three subgroups were of particular interest: participants with and without CKD, Black or non Black participants, and participants aged < or ≥75 years. SPRINT prespecified secondary outcomes included components of the primary outcome, total mortality, and a composite of the primary outcome (i.e. cardiovascular disease‐free survival). Additional secondary cardiovascular disease outcomes included peripheral arterial disease, coronary revascularization, TIA, left ventricular hypertrophy (LVH) on ECG, and atrial fibrillation or flutter. Peripheral arterial disease included carotid and peripheral revascularization, abdominal aortic aneurysm repair and other objectively defined peripheral arterial disease events | |
| Funding sources | Federal funds from the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging and the National Institute of Neurological Disorders and Stroke | |
| Declarations of interest | The study authors declared no conflicts of interest (In Clinical Trials 2014;11(5):532–46) In NEJM 2015;26;373(22):2103‐16, Dr. Ambrosius, Dr. Johnson, Dr. Rahman, Dr. Reboussin, Dr. Rocco, Dr. Sink, Dr. Williamson and Dr. Wright, Jr. report grant support from NIH/NHLBI and non‐financial support from Takeda Pharmaceuticals International, Inc, and Arbor Pharmaceuticals, LLC, during the conduct of the study. Dr. Cheung and Dr. Goff report grant support from the National Institutes of Health during the conduct of the study. Dr. Cushman reports grant support from the National Institutes of Health (NIH) and non‐financial support from Takeda Pharmaceuticals International, Inc., and Arbor Pharmaceuticals, LLC, during the conduct of the study; and personal fees from Takeda and Novartis outside the submitted work. Dr. Cutler reports non‐financial support from Takeda International Pharmaceuticals Inc. and Arbor Pharmaceuticals, Inc., during the conduct of the study, and personal fees from the National Heart, Lung, and Blood Institute outside the submitted work. Dr. Fine, Ms. Snyder and Dr. Whelton report non‐financial support from Takeda Pharmaceuticals International, Inc., and Arbor Pharmaceuticals, LLC, during the conduct of the study. Dr. Kimmel reports personal fees from Academic Press outside the submitted work. Dr. Lewis reports grant support from the NIH and non‐financial support from Takeda Pharmaceuticals International and Arbor Pharmaceuticals during the conduct of the study; and grant support from Novo Nordisk outside the submitted work. Dr. Oparil reports grant support from the NIH/NHLBI during the conduct of the study; grant support from Merck and Co., the NIH/NHLBI, Novartis, and Arbor Pharmaceuticals, LLC, grant support and personal fees from AstraZeneca and Bayer, grant support, personal fees and non‐financial support from Medtronic, and personal fees from Forest Laboratories, Inc., Amgen (Onyx – Subsidiary), Boehringer Ingelheim, and GlaxoSmithKline outside the submitted work. In addition, Dr. Oparil was co‐chair (JNC 8): "Evidence‐Based Guideline for the Management of High Blood Pressure in Adults: Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8), and Co‐Chair, 2007‐2013 (JAMA 311(5):507‐520, 2014). |
|
| Notes | Four institutes of the National Institutes of Health (NIH) co‐sponsored SPRINT. The authors declared no conflicts of interest. A public repository provided individual patient data for hypertensive participants with established cardiovascular disease for use in this Cochrane Review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participant randomization: SPRINT will use an internet‐based, web browser randomisation procedure. Clinical Sites access the randomisation application through the study web site. Access to this application is password protected and its communications are encrypted. Once security requirements are satisfied, a series of questions identify and verify the eligibility of the participant. When the session is complete, an e‐mail is sent to the Clinic Coordinator, the appropriate CCN, and the CC indicating that the participant has been properly randomised and appended to the database" (pp. 2103‐16) |
| Allocation concealment (selection bias) | Unclear risk | No detailed information was provided on the randomization system used in the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Participants and study personnel were aware of the study‐group assignments, but outcome adjudicators were not" (pp. 2103‐16) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias detected (pp. 2103‐16, Figure 1) |
| Selective reporting (reporting bias) | Low risk | No reporting bias detected (protocol checked) |
| Other bias | High risk | Only about 17% of total participants met review inclusion criteria (participants with established cardiovascular disease). The trial was assessed as biased because it was stopped early for benefit |
| Methods | Multicentre open‐label clinical trial, ITT strategy, 2 x 2 factorial design with randomization to both an antiplatelet intervention and a target level of SBD control. The follow‐up was 0 to 8.6 years (mean 3.7 years) |
|
| Participants | Eligible participants were aged ≥ 30 years, were normotensive or hypertensive, had a recent (within 180 days), symptomatic, MRI‐confirmed lacunar stroke, and were without surgically amenable ipsilateral carotid artery stenosis or high‐risk cardio‐embolic sources. Main exclusion criteria included disabling stroke (modified Rankin score ≥ 4), previous intracranial haemorrhage from non traumatic causes, or cortical ischaemic stroke. Baseline characteristics for participants included in the review (% or mean ± SD): men/women (63/37%); age (63 years); SBP (143 ± 19 mmHg); DBP (79 ± 11 mmHg); diabetes (37%); current smoker (20%); ethnic group: White (51%), non White (49%). Types of drugs at 1 year: thiazides (45%), ACEI/ARB (63%), CCB (32%), BB (25%), other (12%). Previous cardiovascular condition: IHD (10%), stroke (99%). Countries: USA, Canada, Mexico, Ecuador, Peru, Chile, Argentina and Spain |
|
| Interventions | Standard (higher) target: SBP 130 to 149 mmHg Lower target: SBP < 130 mmHg |
|
| Outcomes | The primary outcome was time to recurrent stroke (first of fatal or non fatal ischaemic stroke or central nervous system haemorrhage). All possible clinical stroke events are assessed at the clinical site by both the local neurology investigator and a neurologist blinded to the assigned treatment arms. Secondary outcomes included acute MI and death, classified as vascular or non vascular. Safety events were major cognitive decline, major extracranial (systemic) haemorrhage, serious complication of hypotension and other SPS3‐related serious adverse events. Serious adverse events were major vascular events and severe adverse events related to hypotension. No information about non vascular deaths or severe adverse events other than hypotension‐related was provided |
|
| Funding sources | National Institute of Neurological Disorders and Stroke (USA) | |
| Declarations of interest | The authors declared no conflicts of interest | |
| Notes | The antiplatelet component of the trial was terminated at the recommendation of the data and safety monitoring committee because of lack of efficacy combined with evidence of harm. The blood pressures achieved at the end of the trial were: standard target: SBP 138 ± 1 mmHg; lower target: SBP 127 ± 1 mmHg |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation assignments were generated using a permuted‐block design (variable block size), stored in each clinical centre’s electronic data entry system, and protected from preview" (pp. 164‐75) |
| Allocation concealment (selection bias) | Low risk | "Randomisation assignments were generated using a permuted‐block design (variable block size), stored in each clinical centre’s electronic data entry system, and protected from preview" (pp. 164‐75) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study design was not compatible with blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The Prospective, Randomized, Open‐label, Blinded Endpoint (PROBE) study design, a standard for international blood pressure trials, was utilised" (pp. 164‐75) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 90 (3%) participants were lost to follow‐up and an additional 465 (15%) ended follow‐up early for the following reasons: withdrawn consent (N = 242), site closure (N = 151), physician request (N = 12), and other reasons (N = 60). No information about how these numbers refer to higher‐target and lower‐target groups |
| Selective reporting (reporting bias) | High risk | Only serious adverse events related to hypotension and blood pressure management were reported. Despite repeated attempts to obtain clarification from the study authors, no response was received |
| Other bias | Low risk | All SPS3 participants met the base cardiovascular disease criteria |
Abbreviations: ACEI ‐ angiotensin converting enzyme inhibitors; ARB ‐ angiotensin II receptor blocker; BB ‐ beta blocker; CCB ‐ calcium channel blocker; CHF ‐ congestive heart failure; CKD ‐ chronic kidney disease; CVD ‐ cardiovascular disease; DBP ‐ diastolic blood pressure; ECG ‐ electrocardiography/electrocardiogram; GFR ‐ glomerular filtration rate; IHD ‐ ischaemic heart disease; ITT ‐ intention‐to‐treat; LVH ‐ left ventricular hypertrophy; MBP ‐ mean blood pressure; MI ‐ myocardial infarction; PVD ‐ peripheral vascular disease; RCT ‐ randomized controlled trial; SBP ‐ systolic blood pressure; SD ‐ standard deviation; TIA ‐ transient ischaemic attack.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| MDRD 1994 | Fewer than 50 participants in each group with cardiovascular disease at baseline |
| NCT01230216 | Study not completed due to slow patients enrolment |
| PODCAST 2013 | Fewer than 50 participants in each group with cardiovascular disease at baseline. The study is in progress but the recruitment phase has closed |
| REIN‐2 2005 | Fewer than 50 patients in each group with cardiovascular disease at baseline |
| RESTART‐AP 2013 | Study not completed due to lack of funding, according to information from the authors |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multicentre, controlled, randomized, 2 x 2 factorial design. The ABCD‐H trial included hypertensive (DBP ≥90.0 mmHg) non insulin‐dependent diabetic participants (NIDDM). Participants were randomized to one of four arms: intensive treatment with nisoldipine, intensive treatment with enalapril, moderate treatment with nisoldipine, or moderate treatment with enalapril. Participants and investigators were not masked to blood pressure goal. The follow‐up was 5 years |
| Participants | Adults with NIDDM aged between 40 and 74 years and a minimum DBP ≥ 90.0 mmHg were recruited. Exclusion criteria included MI, unstable angina or CVA within the previous six months, CABG surgery within the previous three months, Class III‐IV NYHA CHF, absolute need for therapy with ACEI or CCBs, haemodialysis or peritoneal dialysis, or serum creatinine concentration > 3 mg/dL (265 mmol/L). Country: USA |
| Interventions | Standard (moderate) target: DBP 80.0 to 89.0 mmHg Intensive target: DBP ≤ 75.0 mmHg |
| Outcomes | The primary endpoint was the effect of intensive or moderate blood pressure control on the change in the 24 hour creatinine clearance, which was assessed every six months. Secondary end points included the effect of intensive as compared with moderate blood pressure control on the incidence of cardiovascular events, retinopathy, clinical neuropathy, urinary albumin excretion, and left ventricular hypertrophy. All cardiovascular events were reviewed by an independent endpoints committee blinded to the patients’ assigned treatment groups. Cardiovascular outcomes were defined as death due to cardiovascular events (sudden death, progressive heart failure, fatal MI, fatal arrhythmias, CVAs, or ruptured aortic aneurysm); non fatal MI; non fatal CVA; heart failure requiring hospital admission; or pulmonary infarction |
| Notes | The trial included a number unspecified participants with basal angina in the published article. Authors were contacted to clarify this issue but no definite answer was received before publication of this review. After 67 months of study, the committee recommended the discontinuation of nisoldipine therapy among the participants with hypertension |
| Methods | Multicentre, prospective, randomized open with blinded end point (PROBE) design |
| Participants | Adults aged 47 to 67 years were included if their treated DBP was in the range 90 to 100 mmHg on at least three consecutive visits. Specific exclusion criteria were: history of IHD, pathological ECG or both, somatic disorders expected to cause a significant deterioration in health within the next few years or inability to participate. Country: Sweden |
| Interventions | Standard (unchanged) target: DBP 90 to 100 mmHg Intensive target: DBP ≤ 80 mmHg |
| Outcomes | Three main questions were asked:
|
| Notes | Study data have been lost. The principal author (Prof Lennart Hansson) is deceased; Dr Bjorn Dahlöf confirmed that data have not been retained. Bayer was also contacted but confirmed the company does not have any data available for the BBB study. The journal Blood Pressure, in which BBB results were published, confirmed the manuscript received was essentially as the published, and the documentation was destroyed about 10 years before (following Prof Hansson's death). The Swedish Council on Heath Tecnology Assessment assessed the study in a report (No. 170/2) but did not have access to the original data. We also approached the Östra Hospital, where Prof Hansson was working at the time the study was conducted. No records were found, and we were told that the legal requirement to keep records safe expired after 15 years |
| Methods | Prospective multicentre, randomized study with two parallel groups, ITT strategy, open‐label design. The follow‐up was 2 years |
| Participants | Adults aged over 55 years with uncontrolled SBP (≥ 150 mmHg) and at least one additional cardiovascular risk factor (cigarette smoking, total cholesterol ≥ 5.2 mmol/L, HDL cholesterol < 1.0 mmol/L, LDL cholesterol ≥ 3.4 mmol/L, family history of premature CVD in a first degree relative (< 65 years in women and < 55 years in men), previous TIA or stroke, or established coronary or peripheral arterial disease). Exclusion criteria included diabetes, kidney failure, chronic atrial fibrillation or flutter, clinically significant hepatic or haematological disorders, alcoholism, drug addiction, causes precluding ECG interpretation for LVH, significant valvular heart disease or any disease causing reduced life expectancy. Baseline characteristics for participants potentially included in the review (in percentages (%) or mean ± SD): men/women (52/48%); age (71 ± 7 years); SBP (159 ± 9 mmHg); DBP (85 ± 9 mmHg); current smoker (7%); ethnic group: white (100%). Country: Italy |
| Interventions | Standard (conventional) target: SBP < 140 mmHg Lower (aggressive) target: SBP < 130 mmHg |
| Outcomes | The primary study outcome was the prevalence of electrocardiographic LV (left ventricular) hypertrophy at the final two year visit. The main prespecified secondary outcome was a composite of all‐cause mortality, non fatal MI, non fatal stroke, TIA, CHF NYHA stages III‐IV requiring hospitalization, angina pectoris with objective evidence of myocardial ischaemia, new‐onset atrial fibrillation, coronary revascularization, aortic dissection, occlusive peripheral arterial disease, and kidney failure requiring dialysis. For participants with more than one event, the survival time up to the first event was used in the analysis. The comparison between the groups in the serial changes in SBP and DBP was another secondary end point of the study |
| Notes | 216 participants (115 standard, 101 lower) met the inclusion criteria for the review but additional information on outcomes is needed to obtain useful data. The authors were contacted and they forwarded our questions to the Steering Committee. An answer had not been received from the committee before review publication. Blood pressures achieved at the end of the trial were: standard target: SBP 139 ± 14 mmHg; lower target: SBP 134 ± 14 mmHg |
Abbreviations: ACEI ‐ angiotensin converting enzyme inhibitors; CABG ‐ coronary artery bypass graft; CHF ‐ coronary heart failure; CCB ‐ calcium channel blockers; CVA ‐ cerebrovascular accident; CVD ‐ cardiovascular disease; DBP ‐ diastolic blood pressure; ECG ‐ electrocardiogram; HDL ‐ high density lipoprotein; IHD ‐ ischaemic heart disease; ITT ‐ intention‐to‐treat; LDL ‐ low density lipoprotein; LVH ‐ left ventricular hypertrophy; MI ‐ myocardial infarction; NIDDM ‐ non insulin dependent diabetes mellitus; SBP ‐ systolic blood pressure; SD ‐ standard deviation.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ESH‐CHL‐SHOT Study |
| Methods | Prospective, multinational, randomized trial, with a 3 x 2 factorial design: three different SBP targets; two different LDL‐C targets. The trial is designed as a Prospective, Randomized, Open‐Blind Endpoint evaluation (PROBE) trial. The expected mean follow‐up is 4 years |
| Participants | Men and women aged ≥ 65 years. Qualifying event is stroke or TIA 1 to 6 months prior to randomization. Untreated people should have SBP ≥ 140 mmHg and those on antihypertensive treatment could be included irrespective of their blood pressure. People not receiving statin treatment with LDL‐C > 2.8 mmol/L, and those on statin treatment with any LDL‐C value could be included. All participants should have antiplatelet therapy (or anticoagulant whenever indicated) unless contraindicated. Exclusion criteria included people with unstable clinical conditions; clinical disturbances caused by non stroke pathology; people with haemodynamically significant carotid stenosis or requiring carotid revascularization, secondary hypertension, SBP > 140 mmHg under three antihypertensive drugs at full doses and orthostatic hypotension, those with LDL‐C > 2.8 mmol/L under full dose of a statin, LDL‐C > 4.5 mmol/L under low dose of a statin or untreated, history of MI if baseline LDL‐C was < 1.8 mmol/L; dementia; severe disability (modified Rankin scale > 4); severe CKD defined as serum creatinine > 250 mmol/L |
| Interventions | Standard target: SBP < 135 to 145 mmHg Intensive target: SBP < 125 to 135 mmHg or < 125 mmHg |
| Outcomes | The primary endpoint is time to occurrence of (recurrent) stroke (fatal and non fatal). Secondary cardiovascular end points are time to occurrence of:
|
| Starting date | April 2013 |
| Contact information | Alberto Zanchetti, Istituto Auxologico Italiano, Via L. Ariosto 13, 20145 Milan, Italy. Tel: +39 02 619112237; e‐mail: alberto.zanchetti@auxologico.it |
| Notes | The published byline includes 53 co‐authors; no reported conflicts of interest. The activity of the General Coordinating Centre in Milan is supported by institutional research funds of Fondazione Istituto Auxologico Italiano. It also collaborates the European Society of Hypertension and the Chinese Hypertension League |
| Trial name or title | HOSP Study |
| Methods | Multicentre, prospective, randomized, open, 2 x 2 factorial design, blinded end point study. Participants were randomly assigned to a modest or strict blood pressure control group, and to an amlodipine or losartan group. The participants were to be followed up for 5 years |
| Participants | Men and women aged 40 to 79 years and clinical diagnosis of hypertension. Exclusion criteria included severe hypertension (treated with ≥3 antihypertensive drugs), people unable to change antihypertensive drugs to a calcium antagonist or an angiotensin antagonist, serious medical conditions and women who may become to be pregnant |
| Interventions | Standard (modest) target: Home SBP < 140 mmHg Intensive (strict) target: Home SBP < 130 mmHg |
| Outcomes | Primary outcome measures: Combined cardiovascular events. Secondary outcome measures: Total mortality, cardiovascular mortality, MI and new‐onset angina, stroke and TIA, kidney failure, aortic and peripheral artery diseases, left ventricular mass and function, urinary albumin and Kidney function |
| Starting date | April 2000 |
| Contact information | Yuhei Kawano, MD, PhD, Division of Hypertension and Nephrology, National Cardiovascular Center, 5‐7‐1 Fujishirodai, Suita, Osaka 565‐8565, Japan. E‐mail: ykawano@hsp.ncvc.go.jp |
| Notes | We have tried to contact Dr Kawano to obtain information on the current situation of the trial |
| Trial name or title | INFINITY Study |
| Methods | Prospective, randomized, open‐label trial with blinded endpoints (PROBE design). The expected mean follow‐up was 4 years |
| Participants | Men and women aged ≥ 75 years with SBP > 150 mmHg (untreated state) and at risk for cerebrovascular disease (history of smoking, dyslipidaemia, type 2 diabetes, long standing hypertension, family history). Participants had visible (≥ 0.5%) white matter hyper intensities lesions on cerebral MRI screening. To be eligible for inclusion, participants needed to maintain 24 hour SBP < 145 mmHg in the standard treatment group or SBP < 130 mmHg in the intensive treatment group if the clinical SBP was 150 to 170 mmHg and taking 0 to 2 antihypertensives, or SBP was > 170 mmHg and taking 0 to 1 antihypertensives. Exclusion criteria included uncontrolled diabetes mellitus (HBA1c > 10%), history of stroke, dementia, or clinically impaired gait, body mass index > 45 kg/m² and/or arm circumference > 44 cm), poor kidney function, active liver disease or serum transaminases > 3 times the upper limit of normal, major cardiovascular event (e.g. MI) or procedure (e.g. CABG surgery) in past 3 months, uncompensated CHF, or chronic atrial fibrillation that disallows ambulatory blood pressure monitoring to be successfully performed |
| Interventions | Standard target: 24 h SBP < 145 mmHg Intensive target: 24 h SBP < 130 mmHg |
| Outcomes | Primary outcome measures: change from baseline in mobility parameters (self‐paced walk and stance times), at 18 months and at 36 months; and change from baseline in cognitive function (executive function, processing speed), at 18 months and at 36 months. Secondary outcome measures: Accrual of white matter hyperintensity over 36 months including degeneration of tissue and tissue perfusion. Adverse events, tolerability, and health‐related quality of life were also to be evaluated |
| Starting date | December 2011 |
| Contact information | William B White, MD, Division of Hypertension and Clinical Pharmacology, Calhoun Cardiology Center, University of Connecticut School of Medicine, 263 Farmington Ave, Farmington, CT 06030‐3940 USA. E‐mail: wwhite@nso1.uchc.edu |
| Notes | Sponsored by the National Institute of Aging |
| Trial name or title | PRESERVE Study |
| Methods | Randomized controlled trial, interventional |
| Participants | Participant inclusion criteria:
Participant exclusion criteria
|
| Interventions | Participants randomized to either intensive or standard blood pressure treatment. The intensive blood pressure lowering arm aims for SBP < 125 mmHg. The standard blood pressure lowering arm aims for SBP 130 to 140 mmHg, as recommended by current guidelines. The trial will compare two strategies for lowering blood pressure not looking at specific blood pressure drugs |
| Outcomes | Primary outcome: Composite cognitive score, which is an overall score for the cognitive tests carried out at baseline, 12 months and 24 months. Secondary outcome measures:
measured at baseline, 12 months and 24 months |
| Starting date | 14 October 2011 |
| Contact information | Ms Eithne Smith. Stroke and Dementia Research Centre. St. George's University of London, Cranmer Terrace. London. SW17 0RE. United Kingdom preserve@sgul.ac.uk |
| Notes | Trial website: http://www.preserve.sgul.ac.uk |
| Trial name or title | RESPECT Study |
| Methods | Multicentre, randomized, parallel, open‐label study. The follow‐up period will be 3 years |
| Participants | Men and women aged 50 to 85 years, hypertensive patients and history of stroke who satisfy the following criteria:
Exclusion criteria include secondary or severe hypertension, MI or angioplasty within 3 months prior to the screening, current or previous heart failure with NYHA classification class III or more, or ejection fraction < 35%, severe bilateral carotid stenosis or major cerebral artery occlusion, severe paralysis due to stroke, current kidney or liver dysfunction |
| Interventions | Standard target: < 140/90 mmHg (or < 130/80 if current diabetes, kidney disease or MI) Intensive target: < 120/80 mmHg |
| Outcomes | Primary outcome measures: prevention of recurrent stroke. Participants on blood pressure treatment achieving their respective blood pressure target will be followed for recurrence of stroke. Secondary outcome measures: Incidence of events other than stroke. Under strict blood pressure control, not only the recurrence of stroke but also occurrence of cardiovascular events (such as MI and heart failure), angioplasty, and death will be reduced |
| Starting date | October 2010 |
| Contact information | Hiroko Usami, PhD. Biomedis International Ltd. Tfn: 81‐1‐3‐6252‐3282. hiroko‐u@biomedis.co.jp |
| Notes | Trial website: http://www.respect‐study.com/ |
| Trial name or title | STEP Study |
| Methods | Multicentre, prospective, randomized, open‐labelled, blinded‐end point trial. The follow‐up period will be 4 years |
| Participants | Men and women aged 60 to 80 years, with SBP between 140 and 190 mm Hg in the three screening visits or currently under antihypertensive treatment and signed the written informed content. Exclusion criteria: SBP ≥190 mm Hg or DBP < 60 mm Hg; known secondary cause of hypertension; history of large atherosclerotic cerebral infarction or haemorrhagic stroke (not lacunar infarction and TIA); hospitalization for MI or unstable angina within the previous 6 months; coronary revascularization (PCI or CABG) within the previous 12 months; planned to perform coronary revascularization (PCI or CABG) in the next 12 months; history of sustained atrial fibrillation or ventricular arrhythmias at entry influencing the measurement of electronic blood pressure; NYHA class III‐IV heart failure at entry or hospitalization for exacerbation of chronic heart failure within the previous 6 months; severe valvular disease or valvular disease likely to require surgery or percutaneous valve replacement during the trial; dilated or hypertrophic cardiomyopathy, rheumatic heart disease, or congenital heart disease; uncontrolled diabetes (serum fasting glucose ≥ 200 mg/dL (11.1 mmol/L, HbA1c > 8%); lab tests indicating abnormal liver or kidney function (ALT > 3 times the upper limit of normal value, or ESKD on dialysis, or eGFR < 30 mL/min, or sCr > 2.5 mg/dL (> 221 µmol/L); severe somatic disease such as cancer; severe cognitive impairment or mental disorders; participating in other clinical trials |
| Interventions | Standard target: 130 to 149 mmHg Intensive target: 110 to 129 mmHg |
| Outcomes | Primary outcome measures: A composite end point comprised of MI, first occurrence of symptomatic stroke (ischaemic or haemorrhagic, fatal or non fatal), hospitalization for unstable angina or acute decompensated heart failure, coronary revascularization (PCI, CABG)), and death from cardiovascular causes. Secondary outcome measures: Major coronary events comprised of MI, hospitalization for unstable angina or acute decompensated heart failure, coronary revascularization (PCI, CABG), and death from cardiovascular causes; first occurrence of symptomatic stroke (ischaemic or haemorrhagic, fatal or non fatal); all‐cause death; cardiovascular death; MI; hospitalization for unstable angina; hospitalization for acute decompensated heart failure; coronary revascularization (PCI, CABG); first occurrence of diabetes mellitus; decline in cognitive function; decline in renal function or development of ESKD; major artery function changes |
| Starting date | December 2016 |
| Contact information | Weili Zhang, MD (zhangweili1747@yahoo.com); Guomei Wu (wuguomei513@163.com) |
| Notes |
Abbreviations: ALT ‐ alanine aminotransferase; CABG ‐ coronary artery bypass graft; CHD ‐ coronary heart disease; CHF ‐ congestive heart failure; CVD ‐ cardiovascular disease; DBP ‐ diastolic blood pressure; eGFR ‐ estimated glomerular filtration rate; ESKD ‐ end‐stage kidney disease; LDL‐C ‐ low density lipoprotein cholesterol; MI ‐ myocardial infarction; MRI ‐ magnetic resonance imaging; MRI DWI ‐ magnetic resonance imaging diffusion weighted imaging; PCI ‐ percutaneous coronary intervention; SBP ‐ systolic blood pressure.
Contributions of authors
LC Saiz is the lead author. He coordinated the review, entered the text of the review into RevMan, conducted the external correspondence, appraised inclusion criteria and quality, extracted study data and analyzed data.
J Gorricho led the protocol, appraised inclusion criteria and quality of studies, extracted study data and drafted the final review.
J Garjón appraised inclusion criteria and quality of studies, extracted study data and drafted the final review.
MC Celaya appraised inclusion criteria and quality of studies, and drafted the final review.
L Muruzábal appraised inclusion criteria and quality of studies, and drafted the final review.
R Montoya appraised inclusion criteria and quality of studies, and interpreted the analysis from a clinical perspective.
A López appraised inclusion criteria and quality of studies, and interpreted the analysis from a policy perspective.
MM Malón appraised inclusion criteria and quality of studies, and interpreted the analysis from a clinical perspective.
All authors participated in writing of discussion and conclusions.
Sources of support
Internal sources
-
Navarre Health Service and Health Department of the Government of Navarre, Spain.
Working time of authors (employees of the Government of Navarre).
Facilities.
External sources
-
European Social Fund Operational Programme 2007‐2013, Other.
50% of the full research project, as salary for the Pharmacotherapy Research Coordinator in the Navarre Health Service (LCS).
-
University of British Columbia, Vancouver, Canada.
Bibliographic searches. Methodological support.
Declarations of interest
LC Saiz: None known.
J Gorricho: None known.
J Garjón: None known.
MC Celaya: None known.
L Muruzábal: None known.
R Montoya: None known.
A López: None known.
MM Malón: None known.
New
References
References to studies included in this review
- Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial—correction. JAMA2006; Vol. 295, issue 23:2726. [DOI: 10.1001/jama.295.23.2726-c] [DOI] [PubMed]; Appel LJ, Middleton J, Miller ER 3rd, Lipkowitz M, Norris K, Agodoa LY, et al. The rationale and design of the AASK cohort study. Journal of the American Society of Nephrology : JASN 2003;14(7 Suppl 2):S166‐72. [PUBMED: 12819323] [DOI] [PubMed] [Google Scholar]; Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). Journal of the American Society of Nephrology : JASN 2003;14(7 Suppl 2):S154‐65. [PUBMED: 12819322] [DOI] [PMC free article] [PubMed] [Google Scholar]; Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. American Journal of Kidney Diseases 2006;48(5):739‐51. [PUBMED: 17059993] [DOI] [PubMed] [Google Scholar]; Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288(19):2421‐31. [PUBMED: 12435255] [DOI] [PubMed] [Google Scholar]
- ACCORD Study Group. Cushman C, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. New England Journal of Medicine 2010;362(17):1575‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. American Journal of Cardiology 2007;99(12A):21i‐33i. [PUBMED: 17599422] [DOI] [PubMed] [Google Scholar]; Cushman WC, Grimm RH Jr, Cutler JA, Evans GW, Capes S, Corson MA, et al. ACCORD Study Group. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. American Journal of Cardiology 2007;99(12A):44i‐55i. [PUBMED: 17599425] [DOI] [PubMed] [Google Scholar]
- Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study ‐ patient characteristics: randomization, risk profiles, and early blood pressure results. Blood Pressure 1994;3(5):322‐7. [PUBMED: 7866597] [DOI] [PubMed] [Google Scholar]; Hansson L, Zanchetti A. The Hypertension Optimal Treatment (HOT) Study: 24‐month data on blood pressure and tolerability. Blood Pressure 1997;6(5):313‐7. [PUBMED: 9360003] [DOI] [PubMed] [Google Scholar]; Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351(9118):1755‐62. [PUBMED: 9635947] [DOI] [PubMed] [Google Scholar]; Hansson L, for the HOT Study Group. The Hypertension Optimal Treatment Study (the HOT Study). Blood Pressure 1993;2(1):62‐8. [PUBMED: 8193735] [DOI] [PubMed] [Google Scholar]; Zanchetti A, Hansson L, Clement D, Elmfeldt D, Julius S, Rosenthal T, et al. Benefits and risks of more intensive blood pressure lowering in hypertensive patients of the HOT study with different risk profiles: does a J‐shaped curve exist in smokers?. Journal of Hypertension 2003;21(4):797‐804. [DOI: 10.1097/01.hjh.0000052482.18130.b2; PUBMED: 12658027] [DOI] [PubMed] [Google Scholar]; Zanchetti A, Hansson L, Dahlöf B, Elmfeldt D, Kjeldsen S, Kolloch R, et al. Effects of individual risk factors on the incidence of cardiovascular events in the treated hypertensive patients of the Hypertension Optimal Treatment Study. HOT Study Group. Journal of Hypertension 2001;19(6):1149‐59. [PUBMED: 11403365] [DOI] [PubMed] [Google Scholar]
- Fletcher K, Mant J, McManus R, Campbell S, Betts J, Taylor C, et al. Protocol for Past BP: a randomised controlled trial of different blood pressure targets for people with a history of stroke of transient ischaemic attack (TIA) in primary care. BMC Cardiovascular Disorders 2010;10:37. [DOI: 10.1186/1471-2261-10-37; PUBMED: 20696047] [DOI] [PMC free article] [PubMed] [Google Scholar]; Mant J, McManus R, Roalfe A, Fletcher K, Taylor C, Martin U, et al. RCT of different systolic blood pressure targets for people with a history or stroke or transient ischaemic attack: the PAST‐BP (Prevention After Stroke ‐ Blood Pressure) study. Journal of Human Hypertension 2014;28:627‐8. [Google Scholar]; Mant J, McManus RJ, Roalfe A, Fletcher K, Taylor CJ, Martin U, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST‐BP (Prevention After Stroke—Blood Pressure) randomised controlled trial. BMJ 2016;352:i708. [DOI: 10.1136/bmj.i708] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clinical Trials (London, England) 2014;11(5):532‐46. [DOI: 10.1177/1740774514537404; PUBMED: 24902920] [DOI] [PMC free article] [PubMed] [Google Scholar]; SPRINT Research Group. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood‐pressure control. New England Journal of Medicine 2015;373(22):2103‐16. [DOI: 10.1056/NEJMoa1511939] [DOI] [PMC free article] [PubMed] [Google Scholar]; Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow G, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016;315(24):2673‐82. [DOI: 10.1001/jama.2016.7050; PUBMED: 27195814] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al. SPS3 Investigators. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. International Journal of Stroke 2011;6(2):164‐75. [DOI: 10.1111/j.1747-4949.2010.00573.x; PUBMED: 21371282] [DOI] [PMC free article] [PubMed] [Google Scholar]; McClure LA, Szychowski JM, Benavente O, Hart RG, Coffey CS. A post hoc evaluation of a sample size re‐estimation in the Secondary Prevention of Small Subcortical Strokes study. Clinical Trials (London, England) 2016;13(5):537‐44. [DOI: 10.1177/1740774516643689; PUBMED: 27094488] [DOI] [PMC free article] [PubMed] [Google Scholar]; SPS3 Study Group. Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, et al. Blood‐pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382(9891):507‐15. [DOI: 10.1016/S0140-6736(13)60852-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. The effects of dietary protein restriction and blood‐pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. New England Journal of Medicine 1994;330(13):877‐84. [PUBMED: 8114857] [DOI] [PubMed] [Google Scholar]
- NCT01230216. Effect of intensive blood pressure control on progression of coronary atherosclerosis: randomized evaluation by intravascular ultrasound. clinicaltrials.gov/ct2/show/NCT01230216 (first received 28 October 2010).
- Blackburn DJ, Krishnan K, Fox L, Ballard C, Burns A, Ford GA, et al. Prevention of Decline in Cognition after Stroke Trial (PODCAST): a study protocol for a factorial randomised controlled trial of intensive versus guideline lowering of blood pressure and lipids. Trials 2013;14:401. [PUBMED: 24266960] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene‐Iordache B, Turturro M, et al. Blood‐pressure control for renoprotection in patients with non‐diabetic chronic renal disease (REIN‐2): multicentre, randomised controlled trial. Lancet 2005;365(9463):939‐46. [PUBMED: 15766995] [DOI] [PubMed] [Google Scholar]
- Arima H, Wang J, Bladin C, Heeley E, Billot L, Barzi F, et al. Rationale of the REstart or STop Antithrombotics Randomised Trial in Asia Pacific (RESTART‐AP). Cerebrovascular Diseases 2013;36(Suppl 1):39‐40. [Google Scholar]
References to studies awaiting assessment
- Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non‐insulin‐dependent diabetes and hypertension. New England Journal of Medicine 1998;338(10):645‐52. [PUBMED: 9486993] [DOI] [PubMed] [Google Scholar]; Estacio RO, Savage S, Nagel NJ, Schrier RW. Baseline characteristics of participants in the Appropriate Blood Pressure Control in Diabetes trial. Controlled Clinical Trials 1996;17(3):242‐57. [PUBMED: 8877260] [DOI] [PubMed] [Google Scholar]; Savage S, Johnson Nagel N, Estacio RO, Feig PU, MacCarthy EP, Lukken NJ, et al. The ABCD (Appropriate Blood Pressure Control in Diabetes) trial. Rationale and design of a trial of hypertension control (moderate or intensive) in type II diabetes. Online Journal of Current Clinical Trials 1993;104(‐):‐. [PUBMED: 8305994] [PubMed] [Google Scholar]
- Hannson L. The BBB Study: the effect of intensified antihypertensive treatment on the level of blood pressure, side‐effects, morbidity and mortality in "well‐treated" hypertensive patients. Behandla Blodtryck Battre. Blood Pressure 1994;3(4):248‐54. [PUBMED: 7994450] [DOI] [PubMed] [Google Scholar]; The BBB study group. The BBB study: a prospective randomized study of intensified antihypertensive treatment.. Journal of Hypertension 1988;6(9):693‐7. [PUBMED: 2903191] [DOI] [PubMed] [Google Scholar]
- Cardio‐Sis Study Group. Verdecchia P, Staessen JA, Achilli A, Simone G, Ganau A, Mureddu G, et al. Randomized study of traditional versus aggressive systolic blood pressure control (Cardio‐Sis): rationale, design and characteristics of the study population. Journal of Human Hypertension 2008;22(4):243‐51. [DOI: 10.1038/sj.jhh.1002313] [DOI] [PubMed] [Google Scholar]; Reboldi G, Angeli F, Simone G, Staessen JA, Verdecchia P. Cardio‐Sis Investigators. Tight versus standard blood pressure control in patients with hypertension with and without cardiovascular disease. Hypertension 2014;63(3):475‐82. [DOI: 10.1161/HYPERTENSIONAHA.113.02089] [DOI] [PubMed] [Google Scholar]; Verdecchia P, Staessen JA, Angeli F, Simone G, Achilli A, Ganau G, et al. Cardio‐Sis investigators. Usual versus tight control of systolic blood pressure in non‐diabetic patients with hypertension (Cardio‐Sis): an open‐label randomised trial. Lancet 2009;374(9689):525‐33. [DOI: 10.1016/S0140-6736(09)61340-4] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba‐Badiale M, et al. Blood pressure and LDL‐cholesterol targets for prevention of recurrent strokes and cognitive decline in the hypertensive patient: design of the European Society of Hypertension‐Chinese Hypertension League Stroke in Hypertension Optimal Treatment randomized trial. Journal of Hypertension 2014;32(9):1888‐97. [DOI: 10.1097/HJH.0000000000000254; PUBMED: 24979303] [DOI] [PubMed] [Google Scholar]
- Kawano Y. HOSP study. Nihon Rinsho. Japanese Journal of Clinical Medicine 2006;64(Suppl 6):465‐9. [PUBMED: 16983778] [PubMed] [Google Scholar]; Kawano Y, Mannami T, Saitoh D, et al. Hypertension control based on home systolic pressure (HOSP) study: Design and interim report of the main study. Journal of Hypertension 2006;20(Suppl 6):329. [Google Scholar]
- White WB, Marfatia R, Schmidt J, Wakefield DB, Kaplan RF, Bohannon RW, et al. INtensive versus standard ambulatory blood pressure lowering to prevent functional DeclINe in the ElderlY (INFINITY). American Heart Journal 2013;165(3):258‐65.e1. [DOI: 10.1016/j.ahj.2012.11.008; PUBMED: 23453090] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN37694103. How intensively should we treat blood PRESsure in established cERebral small VEssel disease?. www.isrctn.com/ISRCTN37694103 (first received 25 January 2012).
- NCT01198496. Recurrent stroke prevention clinical outcome study (RESPECTS) [Phase IV study for effect of intensive blood‐pressure control using anti‐hypertensive agents in essential hypertension with history of stroke]. clinicaltrials.gov/ct2/show/NCT01198496 (first received 28 August 2010).
- NCT03015311. Strategy of blood pressure intervention in the elderly hypertensive patients (STEP) [Strategy of systolic blood pressure intervention in the elderly hypertensive patients: a prospective randomized open‐label blinded‐endpoint trial]. clinicaltrials.gov/ct2/show/NCT03015311 (first received 2 January 2017).
Additional references
- Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, et al. American Heart Association Council for High Blood Pressure Research, American Heart Association Council on Clinical Cardiology, American Heart Association Council on Epidemiology and Prevention. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Council on Clinical Cardiology and Epidemiology and Prevention. Circulation 2007;115(21):2761‐88. [DOI: 10.1161/circulationaha.107.183885] [DOI] [PubMed] [Google Scholar]
- Arguedas JA, Perez MI, Wright JM. Treatment blood pressure targets for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD004349.pub2] [DOI] [PubMed] [Google Scholar]
- Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008277.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore S, Messerli FH, Wun CC, Zuckerman AL, DeMicco D, Kostis JB, et al. J‐curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. European Heart Journal 2010;31(23):2897‐908. [DOI: 10.1093/eurheartj/ehq328] [DOI] [PubMed] [Google Scholar]
- Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random‐effects meta‐analyses of randomized trials. Circulation 2011;123(24):2799‐810. [PUBMED: 21632497] [DOI] [PubMed] [Google Scholar]
- Bangalore S, Kumar S, Volodarskiy A, Messerli FH. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random effects meta‐analysis of randomised trials. Heart (British Cardiac Society) 2013;99(9):601‐13. [PUBMED: 22914531] [DOI] [PubMed] [Google Scholar]
- Bangalore S, Toklu B, Gianos E, Schwartzbard A, Weintraub H, Ogedegbe G, et al. Optimal systolic blood pressure target after SPRINT: insights from a network meta‐analysis of randomized trials. American Journal of Medicine 2017;130(6):707‐19. [DOI: 10.1016/j.amjmed.2017.01.004] [DOI] [PubMed] [Google Scholar]
- Blood Pressure Lowering Treatment Trialists' Collaboration. Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta‐analysis of randomised controlled trials. BMJ 2013;347:f5680. [DOI: 10.1136/bmj.f5680] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure‐lowering treatment based on cardiovascular risk: a meta‐analysis of individual patient data. Lancet 2014;384(9943):591‐8. [DOI: 10.1016/S0140-6736(14)61212-5] [DOI] [PubMed] [Google Scholar]
- Brunström M, Carlberg B. Lower blood pressure targets: to whom do they apply?. Lancet 2016;387(10017):405‐6. [DOI: 10.1016/S0140-6736(15)00816-8] [DOI] [PubMed] [Google Scholar]
- Daskalopoulou SS, Rabi DM, Zarnke KB, Dasgupta K, Nerenberg K, Cloutier L, et al. The 2015 Canadian Hypertension Education Program. Recommendations for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Canadian Journal of Cardiology 2015;31(5):549‐68. [DOI: 10.1016/j.cjca.2015.02.016] [DOI] [PubMed] [Google Scholar]
- Leung AA, Nerenberg K, Daskalopoulou SS, McBrien K, Zarnke KB, Dasgupta K, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Canadian Journal of Cardiology 2016;32(5):569‐88. [DOI: 10.1016/j.cjca.2016.02.066] [DOI] [PubMed] [Google Scholar]
- Drozda J Jr, Messer JV, Spertus J, Abramowitz B, Alexander K, Beam CT, et al. ACCF/AHA/AMA‐PCPI 2011 performance measures for adults with coronary artery disease and hypertension: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association‐Physician Consortium for Performance Improvement. Circulation 2011;124(2):248‐70. [PUBMED: 21670226] [DOI] [PubMed] [Google Scholar]
- Mancia G, Laurent S, Agabiti‐Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Journal of Hypertension 2009;18(6):308‐47. [DOI: 10.1097/hjh.0b013e328333146d] [DOI] [PubMed] [Google Scholar]
- Mancia G, Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2007;28(12):1462‐536. [DOI: 10.1093/eurheartj/ehm236; PUBMED: 17562668] [DOI] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 2013;34(28):2159‐219. [DOI: 10.1093/eurheartj/eht151] [DOI] [PubMed] [Google Scholar]
- Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet 2016;387(10022):957‐67. [DOI] [PubMed] [Google Scholar]
- Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J‐Curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous?. JAMA 1991;265(4):489‐95. [PubMed] [Google Scholar]
- Feldstein CA. Lowering blood pressure to prevent stroke recurrence: a systematic review of long‐term randomized trials. Journal of the American Society of Hypertension : JASH 2014;8(7):503‐13. [DOI: 10.1016/j.jash.2014.05.002; PUBMED: 25064772] [DOI] [PubMed] [Google Scholar]
- Fillippone EJ, Foy A, Newman E. Goal‐directed antihypertensive therapy: Lower may not always be better. Cleveland Clinic Journal of Medicine 2011;78(2):123‐33. [DOI: 10.3949/ccjm.78a.10101] [DOI] [PubMed] [Google Scholar]
- GBD 2013 Risk Factors Collaborators, Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(10010):2287‐323. [DOI: 10.1016/S0140-6736(15)00128-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385(9963):117‐71. [DOI: 10.1016/S0140-6736(14)61682-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
- European Medicines Agency. Clinical Safety Data Management: Definitions and Standards for Expedited Reporting.(CPMP/ICH/377/95). 1995. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002749.pdf (accessed prior to 25 September 2017).
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood PressureEducation Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA 2003;289(19):2560‐72. [DOI: 10.1001/jama.289.19.2560] [DOI] [PubMed] [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507‐20. [PUBMED: 24352797] [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal 2016;37(29):2315‐81. [DOI: 10.1093/eurheartj/ehs092] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(7):2160‐236. [PUBMED: 24788967] [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9362):1903‐13. [DOI] [PubMed] [Google Scholar]
- Lv J, Neal B, Ehteshami P, Ninomiya T, Woodward M, Rodgers A, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta‐analysis. PLoS Medicine 2012;9(8):e1001293. [PUBMED: 22927798] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Ehteshami P, Sarnak MJ, Tighiouart H, Jun M, Ninomiya T, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta‐analysis. CMAJ : Canadian Medical Association Journal 2013;185(11):949‐57. [DOI: 10.1503/cmaj.121468; PUBMED: 23798459] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Grassi G, Zanchetti A. Antihypertensive treatment and blood pressure in diabetic and nondiabetic patients: the lower, the better?. Diabetes Care 2011;34(Suppl 2):S304‐7. [PUBMED: 21525473] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Grassi G. Aggressive blood pressure lowering is dangerous: the J‐curve: pro side of the arguement. Hypertension 2014;63(1):29‐36. [PUBMED: 24336629] [DOI] [PubMed] [Google Scholar]
- McBrien K, Rabi DM, Campbell N, Barnieh L, Clement F, Hemmelgarn BR, et al. Intensive and standard blood pressure targets in patients with type 2 diabetes mellitus: systematic review and meta‐analysis. Archives of Internal Medicine 2012;172(17):1296‐303. [PUBMED: 22868819] [DOI] [PubMed] [Google Scholar]
- Messerli FH, Mancia G, Conti R, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: can aggressively lowering pressure in hypertensive patients with coronary artery disease be dangerous?. Annals of Internal Medicine 2006;144(12):884‐93. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics ‐ 2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29‐322. [PUBMED: 25520374] [DOI] [PubMed] [Google Scholar]
- National Clinical Guideline Centre. Hypertension: clinical management of primary hypertension in adults. Hypertension: the clinical management of primary hypertension in adults.. London (UK): National Clinical Guideline Centre, 2011 Aug (updated 2016 Nov); Vol. Clinical Guidelines:CG127.
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rosendorff C, Black HR. Evidence for a lower target blood pressure for people with heart disease. Current Opinion in Cardiology 2009;24(4):318‐24. [PUBMED: 19395951] [DOI] [PubMed] [Google Scholar]
- Rosendorff C, Lackland DT, Allison M, Aronow WS, Black HR, Blumenthal RS, et al. American Heart Association, American College of Cardiology, and American Society of Hypertension. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension 2015;65(6):1372‐407. [PUBMED: 25828847] [DOI] [PubMed] [Google Scholar]
- Roy M, Mahmood N, Rosendorff C. Evidence for aggressive blood pressure‐lowering goals in patients with coronary artery disease. Current Atherosclerosis Reports 2010;12(2):134‐9. [PUBMED: 20425249] [DOI] [PubMed] [Google Scholar]
- SBU. Moderately elevated blood pressure. A systematic literature review. Swedish Council on Health Technology Assessment in Health Care (SBU); 2007. SBU report no 170. www.sbu.se/en/publications/sbu‐assesses/moderately‐elevated‐blood‐pressure/ (accessed prior to 25 September 2017).
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. European Heart Journal 2016;37(42):3232‐45. [DOI: 10.1093/eurheartj/ehw334] [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Annals of Internal Medicine 2011;154(8):541‐8. [DOI: 10.7326/0003-4819-154-8-201104190-00335; PUBMED: 21403055] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Reboldi G. Aggressive blood pressure lowering is dangerous: the J‐curve: con side of the arguement. Hypertension 2014;63(1):37‐40. [PUBMED: 24336630] [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Angeli F, Gentile G, Reboldi G. More Versus Less Intensive Blood Pressure–Lowering Strategy. Cumulative Evidence and Trial Sequential Analysis. Hypertension 2016;68:642‐53. [DOI: 10.1161/HYPERTENSIONAHA.116.07608] [DOI] [PubMed] [Google Scholar]
- Vidal‐Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388(10056):2142‐52. [DOI: 10.1016/S0140-6736(16)31326-5] [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global status report on noncommunicable diseases 2010. Description of the global burden of NCDs, their risk factors and determinants. www.who.int/nmh/publications/ncd_report2010/en/. Geneva: World Health Organization, (accessed prior to 25 September 2017).
- Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta‐analysis. Lancet 2016;387(10017):435‐43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Gorricho J, Garjón J, Celaya MC, Muruzábal L, Montoya R, López Andrés A, et al. Blood pressure targets for the treatment of patients with hypertension and cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010315] [DOI] [PMC free article] [PubMed] [Google Scholar]


