Abstract
Background
Endometrial cancer is one of the most common gynaecological cancers in the world. Rates of endometrial cancer are rising, in part because of rising obesity rates. Endometrial hyperplasia is a precancerous condition in women that can lead to endometrial cancer if left untreated. Endometrial hyperplasia occurs more commonly than endometrial cancer. Progesterone tablets currently used to treat women with endometrial hyperplasia are associated with adverse effects in up to 84% of women. The levonorgestrel intrauterine device (Mirena Coil, Bayer HealthCare Pharmaceuticals, Inc., Whippany, NJ, USA) may improve compliance, but it is invasive, is not acceptable to all women, and is associated with irregular vaginal bleeding in 82% of cases. Therefore, an alternative treatment for women with endometrial hyperplasia is needed. Metformin, a drug that is often used to treat people with diabetes, has been shown in some human studies to reverse endometrial hyperplasia. However, the effectiveness and safety of metformin for treatment of endometrial hyperplasia remain uncertain.
Objectives
To determine the effectiveness and safety of metformin in treating women with endometrial hyperplasia.
Search methods
We searched the Cochrane Gynaecology and Fertility Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PubMed, Google Scholar, OpenGrey, Latin American Caribbean Health Sciences Literature (LILACS), and two trials registers from inception to 10 January 2017. We searched the bibliographies of all included studies and reviews on this topic. We also handsearched the conference abstracts of the European Society of Human Reproduction and Embryology (ESHRE) 2015 and the American Society for Reproductive Medicine (ASRM) 2015.
Selection criteria
We included randomised controlled trials (RCTs) and cross‐over trials comparing metformin (used alone or in combination with other medical therapies) versus placebo or no treatment, any conventional medical treatment, or any other active intervention for women with histologically confirmed endometrial hyperplasia of any type.
Data collection and analysis
Two review authors independently assessed studies for eligibility, extracted data from included studies, and assessed the risk of bias of included studies. We resolved disagreements by discussion or by deferment to a third review author. When study details were missing, review authors contacted study authors. The primary outcome of this review was regression of endometrial hyperplasia histology (with or without atypia) towards normal histology. Secondary outcome measures included recurrence of endometrial hyperplasia, progression of endometrial hyperplasia to endometrial cancer, hysterectomy rate, abnormal uterine bleeding, health‐related quality of life, and adverse effects during treatment.
Main results
We included three RCTs in which a total of 77 women took part. We rated the quality of the evidence as very low for all outcomes owing to very serious risk of bias (associated with poor reporting, attrition, and limitations in study design) and imprecision.
We performed a meta‐analysis of two trials with 59 participants. When metformin was compared with megestrol acetate in women with endometrial hyperplasia, we found insufficient evidence to determine whether there were differences between groups for the following outcomes: regression of endometrial hyperplasia histology towards normal histology (odds ratio (OR) 3.34, 95% confidence interval (CI) 0.97 to 11.57, two RCTs, n = 59, very low‐quality evidence), hysterectomy rates (OR 0.91, 95% CI 0.05 to 15.52, two RCTs, n = 59, very low‐quality evidence), and rates of abnormal uterine bleeding (OR 0.91, 95% CI 0.05 to 15.52, two RCTs, n = 44 , very low‐quality evidence). We found no data for recurrence of endometrial hyperplasia or health‐related quality of life. Both studies (n = 59) provided data on progression of endometrial hyperplasia to endometrial cancer as well as one (n = 16) reporting some adverse effects in the metformin arm, notably nausea, thrombosis, lactic acidosis, abnormal liver and renal function among others.
Another trial including 16 participants compared metformin plus megestrol acetate versus megestrol acetate alone in women with endometrial hyperplasia. We found insufficient evidence to determine whether there were differences between groups for the following outcomes: regression of endometrial hyperplasia histology towards normal histology (OR 9.00, 95% CI 0.94 to 86.52, one RCT, n = 16, very low‐quality evidence), recurrence of endometrial hyperplasia among women who achieve regression (OR not estimable, no events recorded, one RCT, n = 8, very low‐quality evidence), progression of endometrial hyperplasia to endometrial cancer (OR not estimable, no events recorded, one RCT, n = 13, very low‐quality evidence), or hysterectomy rates (OR 0.29, 95% CI 0.01 to 8.37, one RCT, n = 16, very low‐quality evidence). Investigators provided no data on abnormal uterine bleeding or health‐related quality of life. In terms of adverse effects, three of eight participants (37.5%) in the metformin plus megestrol acetate study arm reported nausea.
Authors' conclusions
At present, evidence is insufficient to support or refute the use of metformin alone or in combination with standard therapy ‐ specifically, megestrol acetate ‐ versus megestrol acetate alone, for treatment of endometrial hyperplasia. Robustly designed and adequately powered randomised controlled trials yielding long‐term outcome data are needed to address this clinical question.
Plain language summary
Metformin for endometrial hyperplasia
Review question
Is metformin an effective and safe treatment for people with endometrial hyperplasia?
Background
Endometrial cancer (cancer of the lining of the womb) is a common cancer that affects the reproductive organs in women worldwide. Endometrial hyperplasia is a precancerous condition in women that can lead to endometrial cancer, if left untreated. Successful treatment of women with endometrial hyperplasia can prevent endometrial cancer. Endometrial hyperplasia is usually treated by providing progesterone hormone tablets, inserting the levonorgestrel intrauterine system (Mirena Coil) into the womb, advising overweight women to lose weight, or performing a hysterectomy for women who do not want any future pregnancy. However, progesterone tablets are associated with side effects in up to 84% of women, and this can prevent women from completing treatment. Also, progesterone tablets do not always work, and endometrial hyperplasia can return in up to 14% to 30% of women after treatment. The Mirena Coil is associated with irregular vaginal bleeding in up to 82% of women, and many women find it painful to use or otherwise unacceptable. Therefore, an alternative treatment for endometrial hyperplasia is required. Metformin, an oral tablet that usually is used to treat diabetes, has been shown to cure endometrial hyperplasia in some human studies. Although people taking metformin may experience side effects, treatment is usually well tolerated. If women experience fewer side effects when taking metformin rather than progesterone tablets, and if metformin effectively treats endometrial hyperplasia, then compliance will be better and the cure rate will improve. This could reduce the number of women who end up with endometrial cancer. However, the effectiveness and safety of metformin used to treat women with endometrial hyperplasia remain uncertain.
Study characteristics
We included three randomised controlled trials in which a total of 77 women took part. Two studies compared metformin versus megestrol acetate (a form of progesterone), and one study compared metformin plus megestrol acetate versus megestrol acetate alone. Women in all studies received treatment for approximately 12 weeks. The evidence is current to 10 January 2017.
Key results
Comparisons of metformin versus megestrol acetate have provided insufficient evidence to show differences in effectiveness for curing endometrial hyperplasia. It remains uncertain whether there is any difference between metformin and megestrol acetate in reducing hysterectomy rates or abnormal uterine bleeding in women with endometrial hyperplasia. Although both studies provided data on progression of endometrial hyperplasia to endometrial cancer, there were no events in either arm, and study authors reported no data on adverse effects.
When metformin plus megestrol acetate is compared with megestrol acetate, differences in effectiveness between groups treating endometrial hyperplasia remain unclear. Three of eight patients in the metformin plus megestrol acetate study arm reported nausea. Occurrence of other adverse events is unclear.
Quality of the evidence
We rated the quality of evidence as very low for all outcomes owing to very serious risk of bias (associated with poor reporting, attrition, and limitations in study design) and imprecision.
Summary of findings
Background
Description of the condition
Endometrial hyperplasia is a precancerous endometrial lesion that commonly presents with abnormal uterine bleeding. It is thought to be due to unopposed, prolonged exposure of the endometrium to oestrogen and, if managed expectantly, can progress to endometrial carcinoma, although the condition may resolve spontaneously. It is diagnosed histologically and subsequently can be categorised into four subtypes: simple, simple with atypia, complex, and complex with atypia (Kurman 1985). Risk of progression to endometrial carcinoma is dependent on the type of endometrial hyperplasia, and progression rates vary widely across the literature. This discrepancy is likely due, in part, to the fact that many cases of endometrial hyperplasia, especially when atypia is present, are managed pre‐emptively with a hysterectomy. However, atypia is thought to be a strong risk factor for progression to adenocarcinoma (Kurman 1985). The latest World Health Organization (WHO) classification for endometrial hyperplasia now differentiates only two categories of endometrial hyperplasia: hyperplasia without atypia and atypical hyperplasia/endometrioid intraepithelial neoplasia (Zaino 2014). Progression rates have been reported as less than 5% for non‐atypical hyperplasia but 28% for atypical hyperplasia cumulatively over 20 years. This difference in progression risk has been seen at interval‐specific time points of four years, nine years, and 20 years post diagnosis (Lacey 2010). Risk factors for endometrial hyperplasia are, predictably, very similar to those for endometrial carcinoma and include obesity, diabetes mellitus, nulliparity, tamoxifen use, oestrogen therapy, and polycystic ovary syndrome (PCOS) (Torres 2012).
Polycystic ovary syndrome is a metabolically driven gynaecological disorder that is thought to affect 10% of women of child‐bearing age (Chang 2002). A diagnosis of PCOS must fulfil the widely accepted Rotterdam criteria for two or more of the following in the absence of another cause of chronic anovulation: hyperandrogenism (clinical or biochemical), chronic oligo/anovulation, and polycystic ovaries apparent on ultrasound (ESHRE/ASRM 2004). Prevalence of endometrial hyperplasia in women with PCOS varies greatly in the literature ‐ between 5% and 10% (Holm 2012; Rudnicka 2009) ‐ but risk of endometrial carcinoma is well founded, as women with PCOS possess a three‐fold increased risk of developing endometrial carcinoma when compared with the non‐PCOS population (Haoula 2012).
The aim of endometrial hyperplasia treatment, whether or not PCOS is a comorbidity, is to control abnormal vaginal bleeding while minimising risk of progression to endometrial carcinoma. Historically, endometrial hyperplasia without atypia has been treated medically with oral progestogens (alone or in combination with oestrogen in PCOS) or intrauterine progestogens, inhibiting oestrogen‐driven cell growth and inducing withdrawal bleeds (Yang 2011). This treatment provides the benefit of preserving fertility but is associated with side effects ‐ in the short term, headaches, mood changes, and acne or breast tenderness, and over the longer term, risk of a thromboembolic event or breast cancer (BNF 2017). The side effects of these medications has had the effect of potentially hindering compliance, consequently producing a relatively high relapse rate. In one study, 30.3% and 13.7% of women treated with oral progestogens and intrauterine levonorgestrel, respectively, had relapse of their endometrial hyperplasia (Gallos 2013). However, longer‐term side effects of progestogens can be mitigated by educating women about the symptoms of thromboembolic events and by ensuring that they attend regular breast cancer screening programmes. For women with atypia and for those who are resistant to progestogens, surgical hysterectomy is the treatment of choice (Shafiee 2014).
Description of the intervention
Metformin, a biguanide that acts as an insulin sensitiser, is the oral hypoglycaemic agent most commonly used for treatment of type 2 diabetes mellitus. It acts to inhibit hepatic gluconeogenesis, decreasing liver glucose production and thereby reducing levels of circulating glucose and insulin.
Metformin is also prescribed for women with PCOS to induce weight loss and improve menstrual regularity, both as monotherapy and in combination with a progestogen. It is frequently used to treat ovulatory dysfunction in women with PCOS who have shown resistance to treatment with clomiphene. Despite widespread use of metformin in women with PCOS, a systematic review comparing metformin with the oral contraceptive pill found no definitive improvement in clinical or biochemical features (Costello 2007). Metformin has an established side effect profile that includes nausea and vomiting, diarrhoea, abdominal pain, and changes in taste, as well as rarer or less‐publicised effects such as lactic acidosis or decreased B12 absorption, possibly leading to anaemia and potentially irreversible neuronal damage if left unmonitored and uncorrected for prolonged periods (de Jager 2010).
How the intervention might work
Hyperinsulinaemia secondary to insulin resistance is thought to exhibit a mitogenic effect, inducing cell division via mitosis ‐ a risk factor for hyperplasia ‐ and, ultimately, carcinoma development. This effect is likely due to its activity at the insulin‐like growth factor‐1 receptor, promoting proliferation and angiogenesis, which can be demonstrated by the positive correlation between diabetes and breast and gynaecological cancers (Vrachnis 2016). Insulin‐mediating effects of metformin, then, show evidence of reducing incidence and improving survival among these malignancies, although the evidence is mixed (Chlebowski 2012; Nevadunsky 2014). The link between insulin resistance and cell proliferation offers an intriguing potential therapeutic target for reversing hyperplasia and preventing endometrial carcinoma. Some early trials have corroborated this link, showing effectiveness of metformin in inducing endometrial atrophy in benign endometrial proliferative disorders; one reported atrophy and therefore reversal of endometrial hyperplasia in 96% of women treated with metformin (Tabrizi 2014).
Other proposed mechanisms of the anticancer properties of metformin include its direct effects on cell signalling pathways, including inhibition of the mammalian target of rapamycin (mTOR) and inhibition of mitogen‐activated protein kinase (MAPK) and Akt activity. These pathways are involved in cell proliferation and therefore play a key role in both hyperplasia and cancerous lesions in any tissue. As metformin inhibits these pathways, cell proliferation will be hindered, reducing the chance of development of cancerous lesions (Alimova 2009; Ben 2011).
Why it is important to do this review
Medical therapy for endometrial hyperplasia currently involves multiple side effects and continued risk of recurrence. Therefore, a systematic review of a novel, alternative therapy is needed to collate the evidence to date and to guide future clinical trials. Risk of progression from endometrial hyperplasia to carcinoma is significant; up to 28% of women with endometrial hyperplasia with atypia go on to develop carcinoma ‐ the most common fatal gynaecological malignancy (Lacey 2010). This rate is expected to increase globally by up to 100% over the next 20 years (Dowling 2011). The biguanide insulin sensitiser metformin has been linked to reversal of endometrial hyperplasia and, if it can be used in this way, may contribute to decreasing the prevalence of endometrial carcinoma without use of hormonal contraceptives or irreversible infertility following a hysterectomy (Tabrizi 2014). Although publications include in vitro studies reporting reversed endometrial hyperplasia in mice and cell lines, as well as some case reports, results of treatment are relatively ambiguous, and the mode of action, effectiveness, and safety of metformin remain unclear (Erdemoglu 2009; Legro 2007; Rosato 2011; Session 2003; Shen 2008). This review may help to clarify the role of metformin in the treatment of women with this disease.
Objectives
To determine the effectiveness and safety of metformin in treating women with endometrial hyperplasia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), both published and unpublished. We also included cross‐over trials, but we planned to use in the analysis only data from the first phase of these trials.
Types of participants
We included women with histologically confirmed endometrial hyperplasia of any type.
Types of interventions
We included trials comparing metformin with placebo or no treatment, conventional medical treatment (typically progestogens, e.g. oral or intrauterine), or any other active intervention. We also included trials that provided co‐interventions (e.g. metformin plus progesterone vs progesterone), but we planned to analyse results of these studies separately.
Types of outcome measures
Primary outcomes
1. Regression of endometrial hyperplasia histology (with or without atypia) towards normal histology
Secondary outcomes
2. Recurrence of endometrial hyperplasia
3. Progression of endometrial hyperplasia to endometrial cancer
4. Hysterectomy rate
5. Abnormal uterine bleeding
6. Health‐related quality of life, as reported in the included studies
7. Adverse effects during treatment, as reported in the included studies
Search methods for identification of studies
We searched for all published and unpublished RCTs of metformin for endometrial hyperplasia without language restriction. Review authors liaised with the Cochrane Gynaecology and Fertility Group Trials Search Co‐ordinator and Information Specialist when conducting the search.
Electronic searches
In accordance with guidance from the Cochrane Gynaecology and Fertility Group, we created search strategies for the following electronic databases to identify all relevant RCTs. We searched the following databases from date of inception to 10 January 2017.
Cochrane Gynaecology and Fertility Specialised Register (Procite platform; inception to 10 January 2017) (Appendix 1).
Cochrane Central Register of Studies Online (CRSO) (Web platform; searched 10 January 2017) (Appendix 2).
Ovid MEDLINE (Ovid platform;1946 to 10 January 2017) (Appendix 3).
Ovid Embase (Ovid platform; 1980 to 10 January 2017) (Appendix 4).
EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL) (Ebsco platform;1961 to 10 January 2017) (Appendix 5).
PubMed (Web platform; searched 10 January 2017) (Appendix 6).
Google Scholar (Web platform; searched 10 January 2017) (Appendix 7).
We also searched the following trials registers and databases to identify ongoing and unpublished trials.
ClinicalTrials.gov (Web platform; searched 10 January 2017) (Appendix 8).
World Health Organization International Trials Registry Platform search portal (Web platform; searched 10 January 2017) (Appendix 9).
OpenGrey (Web platform; searched 10 January 2017) (Appendix 10).
Latin American Caribbean Health Sciences Literature (LILACS) (Web platform; searched 10 January 2017) (Appendix 11).
We have presented a list of search strategies in the appendices. For unpublished trials, we emailed the contact person to obtain further information to aid our assessment as to whether they should be included.
Searching other resources
We handsearched the bibliographies of all included studies and reviews on this topic. We also handsearched conference abstracts of the European Society of Human Reproduction and Embryology (ESHRE) 2015 and the American Society for Reproductive Medicine (ASRM) 2015, as these were not included in CENTRAL at the time of the search. Previous abstracts from these conferences had been incorporated into the Cochrane Gynaecology and Fertility Specialised Register.
Data collection and analysis
Selection of studies
We uploaded the titles and abstracts of all studies retrieved by electronic searches to a reference manager programme (Covidence) and removed duplicates. Two review authors (two of NC, TO, JS, HS) independently assessed titles and abstracts to identify studies for potential inclusion in the review. We sought full‐text reports for potentially relevant studies. Two review authors (two of NC, TO, JS, HS) then independently assessed each full‐text report against the inclusion criteria and documented a justification for exclusion of each study. Review authors resolved disagreements between them regarding trial suitability by discussion or by consultation with a third review author. We screened studies for duplicate publication by comparing study author names and study locations, dates, and durations. When uncertainty about study methods or the possibility of duplicate studies arose, we contacted the authors of relevant papers. We constructed a flow chart (Figure 1) to illustrate selection of studies for inclusion in this review according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) guidelines (Moher 2009).
1.
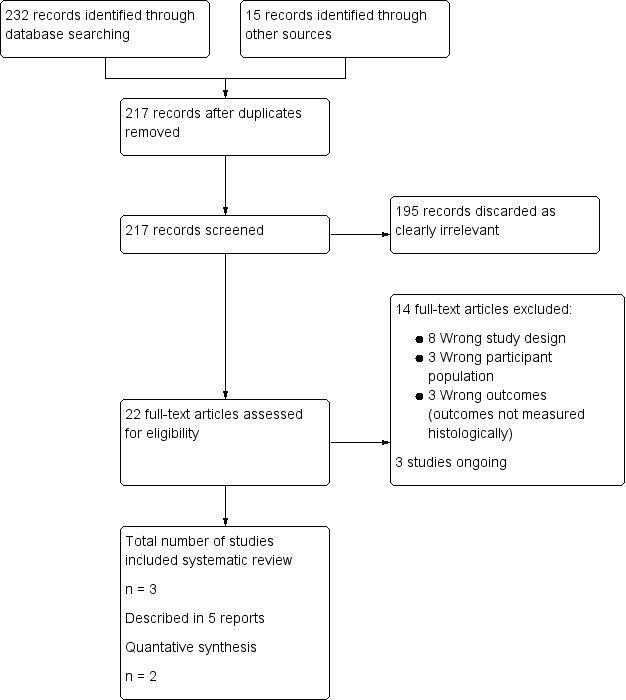
Flow diagram.
Data extraction and management
Two review authors independently extracted data using a data extraction form based on the 'Checklist of items to consider in data collection or data extraction' provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If, during study selection, we found a study that had been published multiple times, we planned to extract and collate study data into a single file. We treated such studies as a single unit of interest for the review and attributed multiple references to the single file. When necessary, we contacted study authors to request additional data on their methods and/or results.
Assessment of risk of bias in included studies
Two review authors (two of NC, TO, HS, JS) independently assessed each included study for risk of bias using the Cochrane 'Risk of bias' assessment tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed bias according to the following domains.
Selection bias (random sequence generation and allocation concealment).
Performance bias (blinding of participants and personnel).
Detection bias (blinding of outcome assessments).
Attrition bias (incomplete outcome data).
Reporting bias (selective reporting).
Other bias (other sources of bias).
We graded risk of bias as 'low', 'high', or 'unclear' for all domains mentioned above by using the 'Criteria for judging risk of bias' in the ‘Risk of bias’ assessment tool (Higgins 2011). We resolved disagreements by discussion and, when necessary, by consultation with a third review author. We have provided a justification for each judgement in the 'Risk of bias' table and, when possible, a quote from the study to support this judgement. We considered our risk of bias assessment when interpreting findings of the review, for example, when performing the sensitivity analysis. To minimise bias in selective reporting of trial outcomes, we planned, when possible, to compare published protocols against methods and outcomes described in the final report.
Measures of treatment effect
For survival outcomes (e.g. regression of endometrial hyperplasia, recurrence of endometrial hyperplasia, progression to endometrial carcinoma), we planned to calculate hazard ratios if data were available. Otherwise, we would calculate rates at a set time point, using the Mantel‐Haenszel odds ratio (OR) and the numbers of events in control and intervention groups.
For continuous data, we planned to use means, standard deviations, and mean differences (MDs). We planned to treat ordinal data, such as side effect severity scoring systems and health‐related quality of life questionnaires, as continuous data for purposes of analysis. If different scales were used to report similar outcomes (e.g. change in endometrial thickness), we planned to calculate the standardised mean difference (SMD). We planned to express the SMD effect as small (0.2 to < 0.5), medium (0.5 to < 0.8), or large (≥ 0.8) (Cohen 1988), and to provide 95% confidence intervals (CIs) for all outcomes.
We planned to report dichotomous and continuous outcomes measured after short‐term treatment (up to six months post treatment), medium‐term treatment (6 to 12 months post treatment), and long‐term treatment (more than 12 months post treatment).
Unit of analysis issues
We performed the primary analysis per woman. If a valid analysis was not possible, we planned to briefly summarise the data but not include them in the meta‐analysis. We planned to include in the analysis only first‐phase data from cross‐over trials.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original trialists. When contacting study authors for missing information, we sent a first reminder email 14 days and a second reminder email 21 days after the initial email. When data could not be obtained, we analysed only available data. We have discussed the potential impact of missing data in the Discussion section of the review.
Assessment of heterogeneity
We planned to consider whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity using the measure of I². We considered an I² greater than 50% to indicate substantial heterogeneity (Higgins 2003; Higgins 2011).
Assessment of reporting biases
Reporting bias is a potential issue for all reviews. We aimed to identify and minimise reporting bias in our analysis by creating a comprehensive search strategy and utilising a multitude of electronic databases, including those that record unpublished work and work prepared in languages other than English. This ensured that we maximised the yield of eligible studies included in the review and were able to identify cases of data duplication.
We planned that if we included 10 or more studies in a single analysis, we would use a funnel plot to explore the possibility of small‐study effects (i.e. the tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If identified studies were sufficiently similar, we aimed to combine the data In Review Manager software (RevMan 2014) using a fixed‐effect model for the following comparisons.
Metformin versus placebo or no treatment.
Metformin versus progestogens.
Metformin versus other active intervention.
Metformin plus co‐intervention versus co‐intervention alone.
We aimed to stratify analyses by dose of metformin (high, moderate, low). We have graphically displayed the results of these meta‐analyses, with increasing odds (regardless of whether the outcome is beneficial) demonstrated by a marker right of the centre‐line, and decreasing odds by a marker left of the centre‐line.
Subgroup analysis and investigation of heterogeneity
When data were available, we aimed to conduct subgroup analyses to obtain separate evidence for the following subgroups.
Women with PCOS.
Women with atypical endometrial hyperplasia.
If pooled data demonstrate substantial heterogeneity (> 50%), we planned to consider additional subgroup analyses (e.g. by dose or route of metformin) and/or sensitivity analyses. We planned to acknowledge the degree of heterogeneity when interpreting the meta‐analysis.
Sensitivity analysis
We aimed to conduct a sensitivity analysis for the primary outcome to determine whether conclusions are robust to our choice of methods with regards to study eligibility and analysis. Through this sensitivity analysis, we planned to explore whether review conclusions would have been different if:
all studies with high risk of bias in one or more domains were excluded from the analysis;
a random‐effects model had been implemented; or
the effect estimate had been expressed as risk ratio (RR) rather than OR.
Overall quality of the body of evidence: 'Summary of findings' tables
Two review authors working independently prepared 'Summary of findings' tables by using GRADEpro Guideline Development Tool software (GRADEpro GDT 2015) and Cochrane methods (Higgins 2011). In this table, we have presented a concise overview of the quality of available evidence for the main comparison (metformin vs megestrol acetate) and for an additional comparison (metformin plus megestrol acetate vs megestrol acetate alone) pertaining to all review outcomes (regression of endometrial hyperplasia towards normal histology, recurrence of endometrial hyperplasia, progression of endometrial hyperplasia to endometrial cancer, hysterectomy rate, abnormal uterine bleeding, health‐related quality of life as reported in included studies, and adverse effects during treatment as reported in included studies). In accordance with GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias), two review authors independently rated the quality of the evidence as 'high', 'moderate', 'low', or 'very low'. We have documented the justification for each grade awarded and have incorporated the overall grade into our final conclusions.
Results
Description of studies
Results of the search
Through the search, we retrieved 217 records; we excluded 195 on the basis of title and abstract review. We sought the full text of the remaining 22 articles and found that eight met our inclusion criteria. We excluded 14 articles. Of the eight full‐text articles reviewed, five report findings of three completed studies, and three describe ongoing studies. See study tables Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies and the PRISMA flow chart in Figure 1.
Included studies
We included in this review three RCTs, which are described in five articles; three articles describe one study.
Study design and setting
All three included studies were single‐centre studies based in hospital outpatient clinics in China or Iran (Shan 2014; Sharifzadeh 2016; Tabrizi 2014).
Participants
In total, investigators randomised 110 women with endometrial hyperplasia across the three RCTs. Critically, histopathological diagnoses reported by each study differed. One included all endometrial hyperplasia, with or without atypia (Tabrizi 2014). One reported only hyperplasia with atypia (Shan 2014). The third described hyperplasia without atypia (Sharifzadeh 2016). Tabrizi included in the randomisation participants with endometrioid endometrial carcinoma and disordered proliferative endometrium, which we excluded from our analysis in this review (Tabrizi 2014). Shan restricted participants to those under 45 years of age (Shan 2014). The other two studies applied no age restriction.
Interventions
Two studies compared metformin against megestrol acetate (Sharifzadeh 2016; Tabrizi 2014). The other included study examined metformin and megestrol acetate dual therapy against megestrol acetate monotherapy (Shan 2014).
Outcomes
Two studies measured outcomes at 12 weeks (Shan 2014; Sharifzadeh 2016). One measured outcomes at three months (Tabrizi 2014). Thus for all eligible studies, analysis could be performed only on "short‐term treatment" outcomes. All studies reported on the review's primary outcome ‐ regression of endometrial hyperplasia towards normal histology. Additionally, study authors reported on progression of endometrial hyperplasia to endometrial cancer, when it occurred.
Excluded studies
We excluded 14 full‐text reports for the following reasons.
Eight were not RCTs.
Three did not include women with endometrial hyperplasia.
Three did not measure outcomes histologically.
Ongoing studies
We identified three ongoing studies, two of which are due to be completed in 2017 (we contacted study authors throughout the review process to request preliminary results) and one in 2019.
Risk of bias in included studies
We have shown risk of bias as judged by review authors for all domains in Figure 2 and have summarised this information in Figure 3.
2.
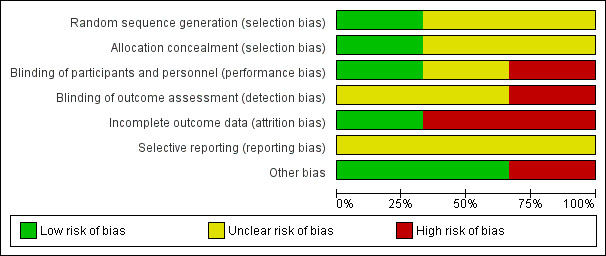
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged that one study was at low risk of bias related to sequence generation, as researchers used a computer to generate a randomised table (Shan 2014). We determined that the remaining two studies were at unclear risk of this bias, as trialists did not describe the method used (Sharifzadeh 2016; Tabrizi 2014).
Allocation concealment
We thought that one study was at low risk of allocation concealment bias, as investigators used sealed envelopes (Sharifzadeh 2016). We determined that two studies were at unclear risk of bias, as study authors did not describe the method used (Shan 2014; Tabrizi 2014).
Blinding
We considered under our primary outcome that lack of blinding may influence symptom reporting by both investigators and patients but not histological results. Therefore, two studies in which neither patients nor investigators were blinded had high risk of bias for this domain (Shan 2014; Sharifzadeh 2016). One study did report blinding of data analysers (Shan 2014). The remaining study described no blinding process, and we therefore judged this trial to be at unclear risk of bias for this domain (Tabrizi 2014).
Incomplete outcome data
Two studies were at low risk related to attrition bias. One of these studies reported that no participants of 43 were lost from the study (Tabrizi 2014). The other reported that only three participants of 45 were lost: One was lost to follow‐up and two discontinued the intervention (Sharifzadeh 2016). We judged the remaining study to be at high risk of bias in this domain, as only 16 of 30 participants completed 12 weeks of therapy and were included in the analysis (Shan 2014).
Selective reporting
We judged all three studies to be at unclear risk of reporting bias owing to lack of publication of protocols before studies were published (Shan 2014; Sharifzadeh 2016; Tabrizi 2014). Two studies excluded participants "lost to follow‐up" from any analysis, but it remains unclear whether available case analysis had been planned within their protocols (Shan 2014; Sharifzadeh 2016).
All three studies reported all outcomes appropriately.
Other potential sources of bias
We judged that one study had potential sampling bias, as participants were not matched between intervention and control groups by histology, age, features of metabolic syndrome, or PCOS diagnosis, and therefore investigators did not control for these known risk factors for development of endometrial hyperplasia (Tabrizi 2014). This study also had potential exclusion bias as, although diagnosis of diabetes was an exclusion criterion, some pre‐intervention blood glucose values appear to show some undiagnosed cases of diabetes but were still included, showing inconsistent exclusion of diabetic patients ‐ again a known effector of endometrial histology.
We identified no potential sources of within‐study bias in the remaining two studies.
Effects of interventions
Summary of findings for the main comparison. Metformin compared with megestrol acetate for endometrial hyperplasia.
| Metformin compared with megestrol acetate for endometrial hyperplasia | ||||||
|
Patient or population: women with endometrial hyperplasia Setting: hospital outpatient clinic Intervention: metformin Comparison: megestrol acetate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with megestrol acetate | Risk with metformin | |||||
| Regression of endometrial hyperplasia (with or without atypia) towards normal histology Assessed by histological examination Follow‐up: 3 months | 615 per 1000 | 842 per 1000 (608 to 949) | OR 3.34 (0.97 to 11.57) | 59 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | Normal histology is defined as atrophic or proliferative endometrium. |
| Recurrence of endometrial hyperplasia | See comment | Not estimable | (0 studies) | ‐ | No data for recurrence of endometrial hyperplasia for this comparison | |
| Progression of endometrial hyperplasia to endometrial cancer Assessed by histological examination Follow‐up: 3 months | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 59 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWc,d | |
| Hysterectomy rate Follow‐up: 3 months | 37 per 1000 | 34 per 1000 (2 to 374) | OR 0.91 (0.05 to 15.52) | 61 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWc,e | |
| Abnormal uterine bleeding Assessed by self‐report Follow‐up: 3 months | 48 per 1000 | 44 per 1000 (2 to 437) | OR 0.91 (0.05 to 15.52) | 44 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,f | |
| Health‐related quality of life (HRQL) | See comment | Not estimable | (0 studies) | ‐ | No data for HRQL for this comparison | |
| Adverse effects during treatment | See comment | Not estimable | (0 studies) | ‐ | No data for adverse effects during treatment for this comparison, with the exception of abnormal uterine bleeding (see above) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for very serious risk of bias: One study was an open‐label study; the other did not detail random sequence generation or allocation concealment or blinding; inadequately applied its inclusion criteria to study participants; and was at high risk of attrition bias.
bDowngraded by two levels for very serious imprecision: Both studies have very small sample sizes, and confidence intervals are compatible with a large effect in either group or with null effect.
cDowngraded by two levels for very serious risk of bias: One study was an open‐label study; the other did not detail random sequence generation or allocation concealment or blinding; inadequately applied its inclusion criteria to study participants; and was at high risk of attrition bias. We had concern about inadequate follow‐up time to detect this outcome.
dDowngraded by two levels for very serious imprecision: Inadequate sample size to capture a relatively uncommon event over too short a period means that confidence intervals could not be generated for this outcome.
eDowngraded by two levels for very serious imprecision: Both studies have an inadequate sample size studied over too short a period, and confidence intervals are compatible with a large effect in either group or with null effect.
fDowngraded by two levels for very serious risk of bias: The included study was an open‐label study that recorded only abnormal uterine bleeding that was self‐reported.
Summary of findings 2. Metformin plus megestrol acetate compared with megestrol acetate for endometrial hyperplasia.
| Metformin plus megestrol acetate compared with megestrol acetate for endometrial hyperplasia | ||||||
| Patient or population: women with endometrial hyperplasia Setting: hospital outpatient clinic Intervention: metformin plus megestrol acetate Comparison: megestrol acetate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with megestrol acetate | Risk with metformin plus megestrol acetate | |||||
| Regression of endometrial hyperplasia (with or without atypia) towards normal histology Assessed by histological examination Follow‐up: 3 months | 250 per 1000 | 750 per 1000 (239 to 966) | OR 9.00 (0.94 to 86.52) | 16 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | Normal histology is defined as proliferative or secretory endometrium. |
| Recurrence of endometrial hyperplasia Follow‐up: median 10.5 months | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 8 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c | Measured in the subset of women who achieved regression |
| Progression of endometrial hyperplasia to endometrial cancer Follow‐up: median 10 months | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 16 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,c | |
| Hysterectomy rate | 125 per 1000 | 40 per 1000 (1 to 545) | OR 0.29 (0.01 to 8.37) | 16 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | |
| Abnormal uterine bleeding | See comment | Not estimable | (0 studies) | ‐ | No data for abnormal uterine bleeding for this comparison | |
| Health‐related quality of life (HRQL) | See comment | Not estimable | (0 studies) | ‐ | No data for HRQL for this comparison | |
| Adverse effects during treatment Assessed self‐report | 3/8 (37.5%) of participants who took metformin had nausea that settled without further treatment. Adverse effects, including "thrombosis, lactic acidosis, abnormal liver and renal function, and other toxicities or complaints" were recorded. | ‐ | (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for very serious risk of bias: The included study provided inadequate detail regarding study design, which was insufficiently clarified when study authors were approached. The random sequence generation was altered, and the allocation concealment was unclear. This was an open‐label study with attrition of 6 of the original 22 randomised participants during the period of the study who were not evaluated by an intention‐to‐treat analysis. For the outcome of regression, only a subset of women were included in the analysis.
bDowngraded by two levels for very serious imprecision: Very low event rate and/or confidence intervals are compatible with a large effect in one or both groups, or with null effect.
cDowngraded by two levels for very serious imprecision: Sample size was tiny, follow‐up was short, and no events were reported.
1. Metformin versus megestrol acetate
We included two studies in this comparison.
Primary outcome
1.1 Regression of endometrial hyperplasia histology (with or without atypia) towards normal histology
Evidence was insufficient to show whether there was any difference in rates of regression of endometrial hyperplasia histology towards normal histology when metformin was compared with megestrol acetate (odds ratio (OR) 3.34, 95% confidence interval (CI) 0.97 to 11.57, two RCTs, n = 59, I² = 0%, very low‐quality evidence; Analysis 1.1). Although one study reported the presence or absence of atypia, data were insufficient to track these participants from initial to final histological results; therefore, we performed no subgroup analysis (Tabrizi 2014).
1.1. Analysis.
Comparison 1 Metformin versus megestrol acetate, Outcome 1 Regression of endometrial hyperplasia (with or without atypia) towards normal histology (defined here as atrophic or proliferative endometrium).
Secondary outcomes
1.2 Progression of endometrial hyperplasia to endometrial cancer
Two studies provided data for this outcome (n = 59) but reported no events in either arm (Analysis 1.2).
1.2. Analysis.
Comparison 1 Metformin versus megestrol acetate, Outcome 2 Progression of endometrial hyperplasia to endometrial cancer.
1.3 Hysterectomy rate
It is uncertain whether metformin and megestrol acetate are different in terms of hysterectomy rates (OR 0.91, 95% CI 0.05 to 15.52, two RCTs, n = 61, very low‐quality evidence; Analysis 1.3).
1.3. Analysis.
Comparison 1 Metformin versus megestrol acetate, Outcome 3 Hysterectomy rate.
1.4 Abnormal uterine bleeding
It is unresolved whether metformin and megestrol acetate showed a difference in rates of abnormal uterine bleeding (OR 0.91, 95% CI 0.05 to 15.52, two RCTs, n = 44, very low‐quality evidence; Analysis 1.4). The only study contributing data to this analysis reported only two events.
1.4. Analysis.
Comparison 1 Metformin versus megestrol acetate, Outcome 4 Abnormal uterine bleeding.
Other secondary outcomes
Study authors provided no data on recurrence of endometrial hyperplasia, health‐related quality of life, or adverse events, with the exception of abnormal uterine bleeding, as described above.
2. Metformin plus megestrol acetate versus megestrol acetate alone
We included only one study in this comparison.
Primary outcome
2.1 Regression of endometrial hyperplasia histology (with or without atypia) towards normal histology
Whether metformin plus megestrol acetate and megestrol acetate alone are different in terms of rates of regression of endometrial hyperplasia histology towards normal histology remains uncertain (OR 9.00, 95% CI 0.94 to 86.52, one RCT, n = 16, very low‐quality evidence; Analysis 2.1). The single study included in this analysis recorded only eight events.
2.1. Analysis.
Comparison 2 Metformin plus megestrol acetate versus megestrol acetate, Outcome 1 Regression of endometrial hyperplasia (with or without atypia) towards normal histology (defined here as proliferative/secretory endometrium).
Secondary outcomes
2.2 Recurrence of endometrial hyperplasia
Whether metformin plus megestrol acetate and megestrol acetate alone are different in terms of rates of recurrence of endometrial hyperplasia among women who achieve regression remains unresolved (OR not estimable, 95% CI not estimable, one RCT, n = 8, very low‐quality evidence; Analysis 2.2). The single study included in this analysis recorded no events.
2.2. Analysis.
Comparison 2 Metformin plus megestrol acetate versus megestrol acetate, Outcome 2 Recurrence of endometrial hyperplasia.
2.3 Progression of endometrial hyperplasia to endometrial cancer
Whether metformin plus megestrol acetate and megestrol acetate alone are different in terms of rates of progression of endometrial hyperplasia to endometrial cancer remains uncertain (OR not estimable, 95% CI not estimable, one RCT, n = 16, I² = not applicable, very low‐quality evidence; Analysis 2.3). The single study included in this analysis recorded no events.
2.3. Analysis.
Comparison 2 Metformin plus megestrol acetate versus megestrol acetate, Outcome 3 Progression of endometrial hyperplasia to endometrial cancer.
2.4 Hysterectomy rate
Whether metformin plus megestrol acetate and megestrol acetate alone are different in terms of hysterectomy rates in women with endometrial hyperplasia remains unresolved (OR 0.29, 95% CI 0.01 to 8.37, one RCT, n = 16, I² = not applicable, very low‐quality evidence; Analysis 2.4). The single study included in this analysis recorded only two events: one in the intervention arm and one in the study control arm.
2.4. Analysis.
Comparison 2 Metformin plus megestrol acetate versus megestrol acetate, Outcome 4 Hysterectomy rate.
2.5 Adverse effects during treatment
Three of eight women (37.5%) in the metformin plus megestrol acetate study arm reported nausea; however, this was resolved without further treatment (Analysis 2.5). Investigators recorded additional adverse effects during the follow‐up period, including thrombosis, lactic acidosis, abnormal liver and renal function, and other toxicities and complaints, but whether these were merely inquired about or occurred within participant populations remains unclear.
2.5. Analysis.
Comparison 2 Metformin plus megestrol acetate versus megestrol acetate, Outcome 5 Adverse effects during treatment.
| Adverse effects during treatment | |
|---|---|
| Study | |
| Shan 2014 | 3 patients in the metformin plus megestrol acetate group reported nausea that settled without medical treatment. |
Other secondary outcomes
Study authors provided no data on abnormal uterine bleeding or health‐related quality of life.
Other analyses
Data were insufficient for performance of subgroup analyses.
Sensitivity analysis by risk of bias was not appropriate because we judged all included studies to have high risk of bias. Sensitivity analyses by effect estimate and choice of statistical model did not substantially change the main findings of this review.
Discussion
Summary of main results
Two of the three studies included in this review compared metformin against megestrol acetate, and one study compared metformin and megestrol acetate dual therapy against megestrol acetate monotherapy.
Trial results provided no evidence to support or refute short‐term use of metformin either alone or in combination with megestrol acetate for treatment of endometrial hyperplasia versus megestrol acetate alone. Whether these treatments are different in terms of rates of hysterectomy, abnormal uterine bleeding, alteration in endometrial histology, or rates of progression to endometrial cancer remains uncertain. The quality of the evidence is very low.
Overall completeness and applicability of evidence
We undertook a comprehensive search of a range of databases and trials registries with no language restrictions to identify published, unpublished, and ongoing studies. Thus we are confident that we have identified all potentially relevant randomised controlled trials (RCTs). However, we identified for inclusion in this review only three studies conducted in only two countries; all had small sample sizes, and consequently overall applicability remains unclear. Completeness of evidence was limited by lack of subgroup data (whether effects were the same in individuals both with and without atypia) and by the relatively short‐term follow‐up explored (12 weeks or 3 months in all three studies).
Quality of the evidence
Using GRADE, review authors determined that the evidence was of very low quality both for the main comparison of metformin versus megestrol acetate and for the additional comparison of metformin plus megestrol acetate versus megestrol acetate alone. For both comparisons, we downgraded the quality of evidence owing to very serious risk of bias (associated with poor reporting, attrition, and limitations in study design) and imprecision as major factors. All studies had very small sample sizes, especially when assessing relatively rare events such as progression of endometrial hyperplasia to endometrial cancer. Confidence intervals were compatible with a large effect in one or both groups, or with a null effect.
Potential biases in the review process
We conducted this review according to guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors independently performed each step of the review process, thus reducing potential bias when subjective decisions were required. When the two review authors disagreed, we sought the opinions of a third review author. Furthermore, this review has undergone peer review; thus we are confident that the findings reported here are a true representation of current evidence pertaining to this question.
Investigators excluded from the study's final analysis 6 of the 22 randomised participants (Shan 2014). Study authors provided no details of participants excluded owing to incomplete data, nor did they provide data previously collected for these participants. Study authors provided no data on participants who dropped out. This was reflected in our rating of attrition bias for the study.
We note that standard therapy doses for metformin and megestrol acetate and megestrol acetate in combination with metformin differed, and we recognise the impact this may have had on the therapeutic response. However, we did not directly compare in this review studies in which these doses differed but included them in two separate comparisons.
We made the decision to include one study even though the authors of this study performed only subgroup analysis (Tabrizi 2014). We made this decision because the subgroup analysis was relevant to the study, and further evidence available in the literature was limited.
Agreements and disagreements with other studies or reviews
To our knowledge, no systematic review has compared the effects of metformin versus megestrol acetate nor the effects of metformin plus megestrol acetate versus megestrol acetate alone.
Authors' conclusions
Implications for practice.
Review authors found insufficient evidence to support or refute the use of metformin given alone or in combination with standard therapy, specifically megestrol acetate, for treatment of women with endometrial hyperplasia.
Implications for research.
Well‐designed randomised controlled clinical trials are required to investigate the effectiveness and safety of metformin for treating women with endometrial hyperplasia in comparison with other interventions, including oral or intrauterine progestogens. These trials should provide long‐term follow‐up of women with endometrial hyperplasia (both with and without atypia) in all arms of the trial, including assessment of progression to endometrial carcinoma and assessment of quality of life.
Acknowledgements
We would like to thank the Cochrane Gynaecology and Fertility Group (formerly the Menstrual Disorders and Subfertility Group) in Auckland for help and support provided, especially Marian Showell for assistance with the search protocols, and Helen Nagels as the Managing Editor. We thank Jane Marjoribanks for invaluable advice on writing up this review.
Appendices
Appendix 1. Gynaecology and Fertility Specialised Register search strategy
From inception to 10 January 2017
Procite platform
Keywords CONTAINS "endometrial hyperplasia" or "endometrial proliferation" or "endometrial thickness" or "proliferation" or "hyperplasia" or Title CONTAINS "endometrial hyperplasia" or "endometrial proliferation" or "endometrial thickness" or "proliferation" or "hyperplasia"
AND
Keywords CONTAINS "metformin" or "glucophage" or Title CONTAINS "metformin" or "glucophage"
(20 hits)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 10 January 2017
Web platform
#1MESH DESCRIPTOR Endometrial Hyperplasia EXPLODE ALL TREES 108 #2(Endometr* adj5 Hyperplas*):TI,AB,KY 373 #3(endometri* adj5 ?proliferat*):TI,AB,KY 109 #4#1 OR #2 OR #3446 #5MESH DESCRIPTOR Metformin EXPLODE ALL TREES 1777 #6metformin:TI,AB,KY 4409 #7glucophage:TI,AB,KY 32 #8(dimethylbiguanidine or dimethylguanylguanidine):TI,AB,KY 1 #9(dimethylbiguanidium or glucovance):TI,AB,KY 6 #10#5 OR #6 OR #7 OR #8 OR #9 4410 #11#4 AND #10 4
Appendix 3. MEDLINE search strategy
From 1946 to 10 January 2017
Ovid platform
1 exp Endometrial Hyperplasia/ (3592) 2 (endometri$ adj5 hyperplas$).tw. (4707) 3 (endometri$ adj3 ?proliferat$).tw. (2820) 4 or/1‐3 (8286) 5 exp Metformin/ (11182) 6 metformin.tw. (15758) 7 glucophage.tw. (110) 8 (dimethylbiguanidine or dimethylguanylguanidine).tw. (4) 9 (dimethylbiguanidium or glucovance).tw. (18) 10 or/5‐9 (17514) 11 4 and 10 (51) 12 randomized controlled trial.pt. (507659) 13 controlled clinical trial.pt. (98156) 14 randomized.ab. (438219) 15 randomised.ab. (87256) 16 placebo.tw. (209885) 17 clinical trials as topic.sh. (197782) 18 randomly.ab. (298723) 19 trial.ti. (201663) 20 (crossover or cross‐over or cross over).tw. (79922) 21 or/12‐20 (1280596) 22 exp animals/ not humans.sh. (4853032) 23 21 not 22 (1182904) 24 11 and 23 (4)
Appendix 4. Embase search strategy
From 1980 to 10 January 2017
Ovid platform
1 exp endometrium hyperplasia/ (6938) 2 (endometri$ adj5 hyperplas$).tw. (5824) 3 (endometri$ adj3 proliferat$).tw. (3376) 4 or/1‐3 (11242) 5 exp metformin/ (47713) 6 metformin.tw. (23994) 7 glucophage.tw. (1571) 8 (dimethylbiguanidine or dimethylguanylguanidine).tw. (4) 9 (dimethylbiguanidium or glucovance).tw. (195) 10 or/5‐9 (48540) 11 4 and 10 (138) 12 Clinical Trial/ (1019530) 13 Randomized Controlled Trial/ (472724) 14 exp randomization/ (84526) 15 Single Blind Procedure/ (28735) 16 Double Blind Procedure/ (138900) 17 Crossover Procedure/ (54650) 18 Placebo/ (326024) 19 Randomi?ed controlled trial$.tw. (153072) 20 Rct.tw. (23004) 21 random allocation.tw. (1649) 22 randomly.tw. (343380) 23 randomly allocated.tw. (26969) 24 allocated randomly.tw. (2221) 25 (allocated adj2 random).tw. (847) 26 Single blind$.tw. (18934) 27 Double blind$.tw. (174826) 28 ((treble or triple) adj blind$).tw. (672) 29 placebo$.tw. (250674) 30 prospective study/ (394570) 31 or/12‐30 (2008326) 32 case study/ (94777) 33 case report.tw. (327043) 34 abstract report/ or letter/ (994732) 35 or/32‐34 (1407253) 36 31 not 35 (1956293) 37 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5761760) 38 36 not 37 (1831795) 39 11 and 38 (41)
Appendix 5. CINAHL search strategy
From 1961 to 10 January 2017
Ebsco platform
1. (MM "Endometrial Diseases+") 3,905
2. TX (endometr* N5 hyperplas*) 329
3. TX (endometr* N3 proliferat*) 111
4. 1 OR 2 OR 3 4,184
5. (MM "Metformin") 1,959
6. TX Metformin 5,076
7. TX glucophage 36
8. TX (dimethylbiguanidium or glucovance) 6
9. 5 OR 6 OR 7 OR 8 5,080
10. 4 AND 9 18
Appendix 6. PubMed search strategy
Searched 10 January 2017
Web platform
1. Endometrial Hyperplasia[mh]
2. (endometri* and hyperplas*)[tw]
3. (endometri* and proliferat*)[tw]
4. or/1‐3
5. Metformin[mh]
6. metformin[tw]
7. glucophage[tw]
8. (dimethylbiguanidine or dimethylguanylguanidine)[tw]
9. (dimethylbiguanidium or glucovance)[tw]
10. or/5‐9
11. 4 and 10
12. randomized controlled trial[ptyp]
13. controlled clinical trial[ptyp]
14. randomized[tw]
15. randomized[tw]
16. placebo[tw]
17. randomly[tw]
18. trial[tw]
19. (crossover or cross‐over or cross over)[tw]
20. or/12‐20
21. animals[mh] not humans[mh]
22. 20 not 21
23. 11 and 22
Appendix 7. Google Scholar search strategy
Searched 10 January 2017
Web platform
Keywords include: "endometrium", "endometrial", "hyperplasia, "proliferation", "metformin"
Appendix 8. ClinicalTrials.gov search strategy
Searched 10 January 2017
Web platform
(endometrial OR endometrium) AND (hyperplasia OR proliferation) AND (metformin OR glucophage OR dimethylbiguanidine OR dimethylguanylguanidine OR glucovance OR dimethylbiguanidium)
Appendix 9. World Health Organization International Trials Registry Platform search strategy
Searched 10 January 2017
Web platform
(endometrial OR endometrium) AND (hyperplasia OR proliferation) AND (metformin OR glucophage OR dimethylbiguanidine OR dimethylguanylguanidine OR glucovance OR dimethylbiguanidium)
Appendix 10. OpenGrey search strategy
Searched 10 January 2017
Web platform
(endometrial OR endometrium) AND (hyperplasia OR proliferation) AND (metformin OR glucophage OR dimethylbiguanidine OR dimethylguanylguanidine OR glucovance OR dimethylbiguanidium)
Appendix 11. LILACS search strategy
Searched 10 January 2017
Web platform
(endometrial OR endometrium) AND (hyperplasia OR proliferation) AND (metformin OR glucophage OR dimethylbiguanidine OR dimethylguanylguanidine OR glucovance OR dimethylbiguanidium)
Data and analyses
Comparison 1. Metformin versus megestrol acetate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regression of endometrial hyperplasia (with or without atypia) towards normal histology (defined here as atrophic or proliferative endometrium) | 2 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.97, 11.57] |
| 2 Progression of endometrial hyperplasia to endometrial cancer | 2 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Hysterectomy rate | 2 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.05, 15.52] |
| 4 Abnormal uterine bleeding | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.05, 15.52] |
Comparison 2. Metformin plus megestrol acetate versus megestrol acetate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regression of endometrial hyperplasia (with or without atypia) towards normal histology (defined here as proliferative/secretory endometrium) | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.94, 86.52] |
| 2 Recurrence of endometrial hyperplasia | 1 | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Progression of endometrial hyperplasia to endometrial cancer | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Hysterectomy rate | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 8.37] |
| 5 Adverse effects during treatment | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Shan 2014.
| Methods | Randomised controlled trial 22 randomised participants, 16 included in the analysis Timing: August 2012 to January 2013 Setting: hospital outpatient clinic, in China |
|
| Participants | Women given a pathological diagnosis of endometrial atypical hyperplasia Aged 45 years or less Participants expressed a desire to preserve fertility. |
|
| Interventions | Metformin plus megestrol acetate (n = 8): received 500 mg of oral metformin 3 times a day and 160 mg of oral megestrol acetate daily Megestrol acetate (n = 8): received 160 mg of oral megestrol acetate daily Both groups: 12 weeks of therapy |
|
| Outcomes | Regression of endometrial hyperplasia histology (with or without atypia) towards normal histology; Recurrence of endometrial hyperplasia among women who had regression; Progression of endometrial hyperplasia to endometrial cancer; Hysterectomy rate; Adverse effects during treatment, as reported in the included studies | |
| Notes | Of 6 not included in the analysis, 3 participants were lost to follow‐up and 3 had incomplete data, leaving 8 participants in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with authors: "Using computer, we got a randomized table with a total sample of 16. Actually, we used function "RAND" in excel to form the table and changed these randomized numbers to 0 or 1. When a subject was enrolled, she would get a number. 0 means MA only and 1 means MA + metformin." |
| Allocation concealment (selection bias) | Unclear risk | Communication with authors: "Using computer, we got a randomized table with a total sample of 16. Actually, we used function "RAND" in excel to form the table and changed these randomized numbers to 0 or 1. When a subject was enrolled, she would get a number. 0 means MA only and 1 means MA + metformin." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Communication with authors: "This study was an open label study. However, when [analyzing] the data, the analyzer kept unknown of the certain meaning of number 0 or 1. And only investigator or sponsor had the right of access to personal data of subjects." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Communication with authors: "This study was an open label study. However, when [analyzing] the data, the analyzer kept unknown of the certain meaning of number 0 or 1." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 30 participants, 16 completed 12 weeks of therapy. 8 women chose to undergo an operation and were excluded before randomisation, 3 were lost during follow‐up, and 3 had incomplete data and were excluded from the end analysis (no blood test results available). It is unclear why the absence of blood test data meant that participants were omitted from histological assessment. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published before publication of the study. Outcomes have been appropriately reported. 3 participants died following completion of the study and were not included in the analysis, but it is unclear whether available case analysis had been planned. |
| Other bias | Low risk | No suggestion of other sources of bias |
Sharifzadeh 2016.
| Methods | Randomised controlled trial 45 randomised participants, 42 included in the analysis Timing: February to May 2016 Setting: hospital outpatient clinic, in Iran |
|
| Participants | Women with histopathologically confirmed simple endometrial hyperplasia without atypia | |
| Interventions | Metformin (n = 22): received 500 mg oral metformin twice daily for 4 weeks, then 500 mg 3 times a day for a further 8 weeks Megestrol acetate (n = 20): 40 mg oral megestrol acetate daily for 12 weeks Both groups: 12 weeks of therapy |
|
| Outcomes | Regression of endometrial hyperplasia histology (with or without atypia) towards normal histology | |
| Notes | 1 participant were lost to follow‐up and 2 discontinued treatment, both in the megestrol acetate arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Communication with authors: "Randomization was performed using sealed, sequentially distributed envelopes to which the letters A and B had been allocated: the letter A to the metformin group and the letter B to the megestrol group. The patients chose one of the envelopes which were opened by the investigator’s colleague and, based on the letters, the groups of patients were determined." |
| Allocation concealment (selection bias) | Low risk | Communication with authors: "Randomization was performed using sealed, sequentially distributed envelopes to which the letters A and B had been allocated: the letter A to the metformin group and the letter B to the megestrol group. The patients chose one of the envelopes which were opened by the investigator’s colleague and, based on the letters, the groups of patients were determined." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Communication from trial authors: "The investigators and patients were not blind to interventions." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Communication from trial authors: "The investigators and patients were not blind to interventions." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was lost to follow‐up and 2 discontinued intervention. |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not published. 1 participant was lost to follow‐up and 2 discontinued intervention. These participants were excluded from the analysis, but it is unclear whether available case analysis had been planned. |
| Other bias | Low risk | No suggestion of other sources of bias |
Tabrizi 2014.
| Methods | Randomised controlled trial (subgroup analysis for the purposes of this review; see below) 43 randomised participants, all 43 analysed by study authors Review authors excluded 26 participants as they had a histological diagnosis of "Disordered proliferative endometrium" or "Endometrioid endometrial carcinoma" at baseline, leaving 17 participants for analysis Timing: May to August 2013 Setting: hospital outpatient clinic, in Iran |
|
| Participants | Study authors state that they included participants with abnormal uterine bleeding and a histological diagnosis of disordered endometrial proliferation or simple hyperplasia; however, the randomised participant cohort also includes individuals with endometrial carcinoma and complex hyperplasia, with and without atypia. | |
| Interventions | Metformin (n = 11): received oral metformin 500 mg daily in the first to fourth week, then 500 mg twice daily from the fourth week onwards Megestrol acetate (n = 6): received oral megestrol acetate 40 mg daily Both groups: 3 months of therapy |
|
| Outcomes | Regression of endometrial hyperplasia histology (with or without atypia) towards normal histology; Progression of endometrial hyperplasia to endometrial cancer | |
| Notes | We contacted study authors to request more information on random sequence generation, allocation concealment, and blinding processes for participants, personnel, and outcomes. They provided no relevant information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information within method regarding assessing risk of bias, even after study authors were contacted. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information within method regarding assessing risk of bias, even after study authors were contacted. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information within method regarding assessing risk of bias, even after study authors were contacted. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information within method regarding assessing risk of bias, even after study authors were contacted. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome data incomplete for participants included in this review relative to the whole cohort randomised |
| Selective reporting (reporting bias) | Unclear risk | No protocol was published before the study report was published, so it is difficult to establish whether selective outcome reporting occurred. No adverse effects were reported. |
| Other bias | High risk | 1. Potential sampling bias: intervention and control groups not matched by histology, age, features of metabolic syndrome, or PCOS. Study reports inclusion criteria to be histology of DPE or SH only, yet participants with CH and EEC are included in the metformin pretreatment group. 2. Exclusion bias as diabetic patients were excluded, yet if pre‐intervention blood glucose values are assumed to be fasting, it is likely that some individuals with undiagnosed diabetes were included. Inconsistent exclusion of diabetic patients may have occurred. |
CH: Complex Hyperplasia
DPE: Disordered Proliferative Endometrium
EEC: Endometrioid Endometrial Cancer
MA: Megestrol Acetate
PCOS: Polycystic Ovary Syndrome
SH: Simple Hyperplasia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Campagnoli 2013 | Wrong study design (review of role of metformin in endometrial pathology; not original study) |
| IRCT201412085563N6, 2015 | Wrong study design (single‐arm study; not blinded; no placebo or control) |
| Legro 2007 | Wrong patient population (patients with PCOS, not EH) |
| Mitsuhashi 2014 | Wrong patient population (patients with preoperative endometrial cancer, not EH) |
| Mitsuhashi 2016 | Wrong study design (patients with endometrial cancer used in second study arm instead of control patients) |
| NCT01685762 | Wrong study design (single‐arm study) |
| NCT02035787, 2014 | Wrong study design (single‐arm, open‐label study) |
| Perez‐Lopez 2014 | Wrong patient population (patients with endometrial cancer instead of patients with EH) |
| Randall 2014 | Wrong study design (review of existing studies; no original data) |
| Shen 2008 | Wrong study design (single‐arm, non‐blinded study) |
| Sivalingam 2015 | Wrong outcomes (outcomes not measured histologically) |
| Sivalingam 2016 | Wrong outcomes (outcomes not measured histologically) |
| Sivalingham 2015b | Wrong outcomes (outcomes not measured histologically) |
| Zhou 2015 | Wrong study design (no control arm) |
EH: Endometrial Hyperplasia
PCOS: Polycystic Ovary Syndrome
Characteristics of ongoing studies [ordered by study ID]
IRCT201410275283N11.
| Trial name or title | Comparison of metformin‐megestrol acetate combination and megestrol acetate alone on endometrial histology in patients with disordered proliferative or hyperplastic endometrium |
| Methods | Randomised clinical trial |
| Participants | Female patients with abnormal uterine bleeding and having disordered proliferative or hyperplastic endometrium with or without atypia |
| Interventions | Metformin 500 mg twice a day with megestrol 40 mg daily vs megestrol acetate 40 mg daily for 3 months |
| Outcomes |
Primary outcome Endometrial pathology |
| Starting date | 21 November 2014 |
| Contact information | Dr. Manizheh Sayyah‐Melli, Tabriz, Iran; wrhrcenter@gmail.com |
| Notes | Due to complete recruitment 30 April 2017 http://www.irct.ir/searchen.php?keyword=IRCT201410275283N11&field=a&lang=en |
NCT01686126.
| Trial name or title | Improving the treatment for women with early stage cancer of the uterus (feMMe) |
| Methods | Randomised parallel‐group open‐label trial |
| Participants |
|
| Interventions | 1. Levonorgestrel (Mirena) 52 mg Intrauterine drug delivery system + Metformin 2. Levonorgestrel (Mirena) 52 mg Intrauterine drug delivery system 3. Levonorgestrel (Mirena) 52 mg Intrauterine drug delivery system + Weight loss |
| Outcomes |
Primary outcome Pathological complete response Secondary outcomes 1. Predicted response to treatment 2. Predicted response to treatment through blood and tissue molecular biomarkers 3. Increased molecular understanding of the biological pathogenesis of "early" EAC |
| Starting date | October 2012 |
| Contact information | Vanessa L Taylor; vanessa.taylor3@health.qld.gov.au |
| Notes | Study author correspondence: expected completion July 2019 https://clinicaltrials.gov/ct2/show/NCT01686126 |
NCT01968317.
| Trial name or title | Megestrol acetate plus metformin to megestrol acetate in patients with endometrial atypical hyperplasia or early stage endometrial adenocarcinoma |
| Methods | Randomized parallel‐group open‐label trial |
| Participants | 1. 18‐ to 45‐year‐old females 2. Primarily with confirmed diagnosis of endometrial atypical hyperplasia based upon D&C or hysteroscopy OR primarily with confirmed diagnosis of endometrial adenocarcinoma (G1, low tumour grade) based upon D&C or hysteroscopy, and 3 MRI parameters showing no myometrial invasion, extension beyond corpus, or enlarged lymph nodes 3. Desire to retain reproductive function or uterus 4. Need to be able to undergo correlative treatment and follow‐up |
| Interventions | Megestrol acetate + Metformin or megestrol acetate alone |
| Outcomes |
Primary outcome Pathological response rate Secondary outcomes 1. Toxicity evaluation 2. Rate of relapse 3. Rate of pregnancy |
| Starting date | October 2013 |
| Contact information | Xiaojun Chen; cxjlhjj@163.com |
| Notes | Study author correspondence: trial recruited over 90 patients; expected completion at end of 2017 https://clinicaltrials.gov/ct2/show/NCT01968317 |
BMI: Body Mass Index
CT: Computed Tomography
D&C: Dilation & Curettage
EAC: Esophageal Adenocarcinoma
MRI: Magnetic Resonance Imaging
Differences between protocol and review
In the protocol, we stated that for the outcomes regression of endometrial hyperplasia and progression to endometrial carcinoma, we would calculate Mantel‐Haenszel odds ratios. In the review, we have added that if data are available, we will (in preference) calculate hazard ratios for the outcomes regression of endometrial hyperplasia, recurrence of endometrial hyperplasia, and progression to endometrial carcinoma, as hazard ratios include participants who dropped out of the study and therefore provide the best way to analyse these outcomes.
In the protocol, our objective was "To determine the efficacy and safety of metformin in treating women with endometrial hyperplasia." Upon editorial recommendation, we have changed this to "To determine the effectiveness and safety of metformin in treating women with endometrial hyperplasia."
Contributions of authors
WA and NC initiated the review; NC, TO, and HS drafted and finalised the background and objectives; JS, TO, and HS drafted and finalised the methods sections with assistance from CM. WA reviewed the final protocol. HS performed all searches. NC, TO, JS, and HS then screened titles and abstracts for potential inclusion. Two of NC, TO, JS, and HS assessed each paper for potential inclusion. JS performed searches of unpublished trials and contacted relevant authors. NC, TO, HS, and JS extracted data from included studies, with WA providing clinical interpretation of data. NC, TO, HS, JS, and CM drafted the review, and WA reviewed the final draft.
Sources of support
Internal sources
No sources of support, UK.
External sources
No sources of support, UK.
Declarations of interest
WA is a Clinical Associate Professor and a Consultant Gynaecologist at Queen's Medical Centre, at Nottingham, in the UK. He previously submitted an application to the Nottingham Clinical Trials Unit (in the process of developing a research grant application to the UK NIHR) to conduct a clinical trial comparing metformin with progesterone for treatment of endometrial hyperplasia. A systematic review was suggested to support the trial.
New
References
References to studies included in this review
Shan 2014 {published and unpublished data}
- Kim MK, Seong SJ. Conservative treatment for atypical endometrial hyperplasia: what is the most effective therapeutic method?. Journal of Gynacologic Oncology 2014;25(3):164‐5. [DOI: 10.3802/jgo.2014.25.3.164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo X, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. Journal of Gynecologic Oncology 2014;25(3):214‐20. [DOI: 10.3802/jgo.2014.25.3.214.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo X, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia: preliminary results of a pilot study. International Journal of Gynecological Cancer. Philadelphia: Lippincott Williams & Wilkins, 2014; Vol. 4:1306‐7.
Sharifzadeh 2016 {published data only}
- Sharifzadeh F, Aminimoghaddam S, Kashanian M, Fazaeli M, Sheikhansari N. A comparison between the effects of metformin and megestrol on simple endometrial hyperplasia. Gynecological Endocrinology 2016:152‐5. [DOI: 10.1080/09513590.2016.1223285] [DOI] [PubMed] [Google Scholar]
Tabrizi 2014 {published and unpublished data}
- Tabrizi AD, Melli MS, Foroughi M, Ghojazadeh M, Bidadi S. Antiproliferative effect of metformin on the endometrium ‐ a clinical trial. Asian Pacific Journal of Cancer Prevention 2014;15(23):10067‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Campagnoli 2013 {published data only}
- Campagnoli C, Abbà C, Ambroggio S, Brucato T, Pasanisi P. Life‐style and metformin for the prevention of endometrial pathology in postmenopausal women. Gynecological Endocrinology 2013;29(2):119‐24. [DOI] [PubMed] [Google Scholar]
IRCT201412085563N6, 2015 {published data only}
- IRCT201412085563N6, Vice Chancellor for Research Tabriz University of Medical Sciences. The effect of letrozole and metformin on endometrial histology in patients with disordered proliferative endometrium and simple hyperplasia uterus cancer. www.irct.ir 2015.
Legro 2007 {published data only}
- Legro RS, Zaino RJ, Demers LM, Kunselman AR, Gnatuk CL, Williams NI, et al. The effects of metformin and rosiglitazone, alone and in combination, on the ovary and endometrium in polycystic ovary syndrome. American Journal of Obstetrics and Gynecology 2007;196(4):402.e1‐402.e11. [DOI] [PubMed] [Google Scholar]
Mitsuhashi 2014 {published data only}
- Mitsuhashi A, Kiyokawa T, Sato Y, Shozu M. Effects of metformin on endometrial cancer cell growth in vivo: a preoperative prospective trial. Cancer 2014;120(19):2986‐95. [DOI] [PubMed] [Google Scholar]
Mitsuhashi 2016 {published data only}
- Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility‐sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Annals of Oncology 2016;27(2):262‐6. [DOI] [PubMed] [Google Scholar]
NCT01685762 {published and unpublished data}
- NCT01685762. UNC Lineberger Comprehensive Cancer Center. Metformin for the Treatment of Endometrial Hyperplasia. clinicaltrials.gov 2017.
NCT02035787, 2014 {published and unpublished data}
- NCT02035787. UNC Lineberger Comprehensive Cancer Center. Metformin With the Levonorgestrel‐Releasing Intrauterine Device for the Treatment of Complex Atypical Hyperplasia (CAH) and Endometrial Cancer (EC) in Non‐surgical Patients. clinicaltrials.gov 2014.
Perez‐Lopez 2014 {published data only}
- Perez‐Lopez FR. Metformin treatment and evolution of endometrial cancer. Climacteric 2014;17(2):207‐9. [PubMed] [Google Scholar]
Randall 2014 {published data only}
- Randall LM, Gibson SJ, Monk BJ. The American Society of Clinical Oncology 50th Annual Meeting 2014: an overview and summary of selected abstracts. Gynecologic Oncology 2014;134(2):222‐7. [DOI] [PubMed] [Google Scholar]
Shen 2008 {published data only}
- Shen ZQ, Zhu HT, Lin JF. Reverse of progestin‐resistant atypical endometrial hyperplasia by metformin and oral contraceptives. Obstetrics & Gynecology 2008;112(2):465‐7. [DOI] [PubMed] [Google Scholar]
Sivalingam 2015 {published data only}
- Sivalingam V, McVey R, Gilmour K, Ali S, Roberts C, Renehan A, et al. A presurgical window‐of‐opportunity study of metformin in obesity‐driven endometrial cancer. The Lancet 2015;385 North American Edition:S90. [DOI] [PubMed] [Google Scholar]
Sivalingam 2016 {published data only}
- Sivalingam VN, Kitson S, McVey R, Roberts C, Pemberton P, Gilmour K, et al. Measuring the biological effect of presurgical metformin treatment in endometrial cancer. British Journal of Cancer 2016;114(3):281‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sivalingham 2015b {published data only}
- Sivalingama VN, McVeyb R, Kitsona S, Gilmourc K, Alid S, Robertsa C, et al. Pre‐surgical window study of metformin in obesity‐driven endometrial cancer. Gynecologic Oncology. 2015; Vol. 137(Suppl 1):18. [DOI: ]
Zhou 2015 {published data only}
- Zhou R, Yang Y, Lu Q, Wang J, Miao Y, Wang S, et al. Prognostic factors of oncological and reproductive outcomes in fertility‐sparing treatment of complex atypical hyperplasia and low‐grade endometrial cancer using oral progestin in Chinese patients. Gynecologic Oncology 2015;139(3):424‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT201410275283N11 {published data only (unpublished sought but not used)}
- IRCT201410275283N11. Comparison of metformin‐megestrol combination and megestrol alone on endometrial histology in patients with disordered proliferative or hyperplastic endometrium.. http://en.search.irct.ir/view/20539 (first received 28 November 2014).
NCT01686126 {published data only (unpublished sought but not used)}
- NCT01686126. Improving the treatment for women with early stage cancer of the uterus (feMMe). https://clinicaltrials.gov/ct2/show/NCT01686126 (first received 12 September 2012).
NCT01968317 {published data only (unpublished sought but not used)}
- NCT01968317. Megestrol acetate plus metformin to megestrol acetate in patients with endometrial atypical hyperplasia or early stage endometrial adenocarcinoma. https://clinicaltrials.gov/ct2/show/NCT01968317 (first received 16 October 2013).
Additional references
Alimova 2009
- Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle (Georgetown, Tex) 2009;8(6):909‐15. [PUBMED: 19221498] [DOI] [PubMed] [Google Scholar]
Ben 2011
- Ben Sahra I, Regazzetti C, Robert G, Laurent K, Marchand‐Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell‐cycle arrest through REDD1. Cancer Research 2011;71(13):4366‐72. [PUBMED: 21540236] [DOI] [PubMed] [Google Scholar]
BNF 2017
- Joint Formulary Committee. British National Formulary. 3rd Edition. London: BMJ Group and Pharmaceutical Press, 2017. [Google Scholar]
Chang 2002
- Chang RJ, Heindel JJ, Dunaif A. Polcystic ovarian syndrome. Polycystic Ovarian Syndrome. New York: Marcel Dekker Inc., 2002. [Google Scholar]
Chlebowski 2012
- Chlebowski RT, McTiernan A, Wactawski‐Wende J, Manson JE, Aragaki AK, Rohan T, et al. Diabetes, metformin, and breast cancer in postmenopausal women. Journal of Clinical Oncology 2012;30(23):2844‐52. [PUBMED: 22689798] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Eribaum, 1988. [Google Scholar]
Costello 2007
- Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin‐sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. The Cochrane Database of Systematic Reviews 2007;1(1):CD005552. [PUBMED: 17253562] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 10 January 2017. Melbourne: Veritas Health Innovation.
de Jager 2010
- Jager J, Kooy A, Lehert P, Wulffele MG, Kolk J, Bets D, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B‐12 deficiency: randomised placebo controlled trial. BMJ (Clinical Research ed) 2010;340:c2181. [PUBMED: 20488910] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dowling 2011
- Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Medicine 2011;9:33. [PUBMED: 21470407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erdemoglu 2009
- Erdemoglu E, Güney M, Giray SG, Take G, Mungan T. Effects of metformin on mammalian target of rapamycin in a mouse model of endometrial hyperplasia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2009;145(2):195‐9. [DOI] [PubMed] [Google Scholar]
ESHRE/ASRM 2004
- Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome. Fertility and Sterility 2004;81(1):19‐25. [PUBMED: 14711538] [DOI] [PubMed] [Google Scholar]
Gallos 2013
- Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. Relapse of endometrial hyperplasia after conservative treatment: a cohort study with long‐term follow‐up. Human Reproduction (Oxford, England) 2013;28(5):1231‐6. [PUBMED: 23466671] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 10 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Haoula 2012
- Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Human Reproduction (Oxford, England) 2012;27(5):1327‐31. [PUBMED: 22367984] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(557):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Holm 2012
- Holm NS, Glintborg D, Andersen MS, Schledermann D, Ravn P. The prevalence of endometrial hyperplasia and endometrial cancer in women with polycystic ovary syndrome or hyperandrogenism. Acta Obstetricia et Gynecologica Scandinavica 2012;91(10):1173‐6. [PUBMED: 22583042] [DOI] [PubMed] [Google Scholar]
Kurman 1985
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long‐term study of "untreated" hyperplasia in 170 patients. Cancer 1985;56(2):403‐12. [PUBMED: 4005805] [DOI] [PubMed] [Google Scholar]
Lacey 2010
- Lacey JV Jr, Sherman ME, Rush BB, Ronnett BM, Ioffe OB, Duggan MA, et al. Absolute risk of endometrial carcinoma during 20‐year follow‐up among women with endometrial hyperplasia. Journal of Clinical Oncology 2010;28:788‐92. [DOI: 10.1200/JCO.2009.24.1315; PUBMED: 20065186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nevadunsky 2014
- Nevadunsky NS, Arsdale A, Strickler HD, Moadel A, Kaur G, Frimer M, et al. Metformin use and endometrial cancer survival. Gynecologic Oncology 2014;132(1):236‐40. [PUBMED: 24189334] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosato 2011
- Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, et al. Metabolic syndrome and endometrial cancer risk. Annals of Oncology 2011;22(4):884‐9. [PUBMED: 20937645] [DOI] [PubMed] [Google Scholar]
Rudnicka 2009
- Rudnicka E, Wierzba W, Radowicki S. Evaluation of endometrial histologic morphology in patients with polycystic ovary syndrome [Ocena obrazow morfologicznych endometrium u pacjentek z zespolem policystycznych jajnikow.]. Ginekologia Polska 2009;80(2):103‐6. [PUBMED: 19338206] [PubMed] [Google Scholar]
Session 2003
- Session DR, Kalli KR, Tummon IS, Damario MA, Dumesic DA. Treatment of atypical endometrial hyperplasia with an insulin‐sensitizing agent. Gynecological Endocrinology 2003;17(5):405‐7. [DOI: 10.1080/09513590312331290298] [DOI] [PubMed] [Google Scholar]
Shafiee 2014
- Shafiee M, Khan G, Ariffin R, Abu J, Chapman C, Deen S, et al. Preventing endometrial caner risk in polycystic ovarian syndrome (PCOS) women: could metformin help?. Gynecologic Oncology 2014;132:248‐53. [DOI] [PubMed] [Google Scholar]
Torres 2012
- Torres ML, Weaver AL, Kumar S, Uccella S, Famuyide AO, Cliby WA, et al. Risk factors for developing endometrial cancer after benign endometrial sampling. Obstetrics and Gynecology 2012;120(5):998‐1004. [PUBMED: 23090515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vrachnis 2016
- Vrachnis N, Lavazzo C, Iliodromiti Z, Sifakis S, Alexandrou A, Siristatidis C, et al. Diabetes mellitus and gynecologic cancer: molecular mechanisms, epidemiological, clinical and prognostic perspectives. Archives of Gynaecology and Obstetrics 2016;293(2):239‐46. [DOI] [PubMed] [Google Scholar]
Yang 2011
- Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends in Endocrinology and Metabolism 2011;22(4):145‐52. [PUBMED: 21353793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaino 2014
- Zaino R, Carinelli SG, Ellenson LH. Tumours of the Uterine Corpus: Epithelial Tumours and Precursors. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH editor(s). WHO Classification of Tumours of Female Reproductive Organs. 4th Edition. Vol. 6, Lyon: WHO Press, 2014:125‐6. [Google Scholar]
References to other published versions of this review
Clement 2016
- Clement NS, Oliver TRW, Shiwani H, Sanner JRF, Mulvaney CA, Atiomo W. Metformin for endometrial hyperplasia. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]


