Abstract
Background
While most guidance recommends the use of insulin in women whose pregnancies are affected by pre‐existing diabetes, oral anti‐diabetic agents may be more acceptable to women. The effects of these oral anti‐diabetic agents on maternal and infant health outcomes need to be established in pregnant women with pre‐existing diabetes or impaired glucose tolerance, as well as in women with previous gestational diabetes mellitus preconceptionally or during a subsequent pregnancy. This review is an update of a review that was first published in 2010.
Objectives
To investigate the effects of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who are planning a pregnancy, or pregnant women with pre‐existing diabetes, on maternal and infant health. The use of oral anti‐diabetic agents for the management of gestational diabetes in a current pregnancy is evaluated in a separate Cochrane Review.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2016) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs assessing the effects of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who were planning a pregnancy, or pregnant women with pre‐existing diabetes. Cluster‐RCTs were eligible for inclusion, but none were identified.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data and assessed the risk of bias of the included RCTs. Review authors checked the data for accuracy, and assessed the quality of the evidence using the GRADE approach.
Main results
We identified six RCTs (707 women), eligible for inclusion in this updated review, however, three RCTs had mixed populations (that is, they included pregnant women with gestational diabetes) and did not report data separately for the relevant subset of women for this review. Therefore we have only included outcome data from three RCTs; data were available for 241 women and their infants. The three RCTs all compared an oral anti‐diabetic agent (metformin) with insulin. The women in the RCTs that contributed data had type 2 diabetes diagnosed before or during their pregnancy. Overall, the RCTs were judged to be at varying risk of bias. We assessed the quality of the evidence for selected important outcomes using GRADE; the evidence was low‐ or very low‐quality, due to downgrading because of design limitations (risk of bias) and imprecise effect estimates (for many outcomes only one or two RCTs contributed data).
For our primary outcomes there was no clear difference between metformin and insulin groups for pre‐eclampsia (risk ratio (RR) 0.63, 95% confidence interval (CI) 0.33 to 1.20; RCTs = 2; participants = 227; very low‐quality evidence) although in one RCT women receiving metformin were less likely to have pregnancy‐induced hypertension (RR 0.58, 95% CI 0.37 to 0.91; RCTs = 1; participants = 206; low‐quality evidence). Women receiving metformin were less likely to have a caesarean section compared with those receiving insulin (RR 0.73, 95% CI 0.61 to 0.88; RCTs = 3; participants = 241; low‐quality evidence). In one RCT there was no clear difference between groups for large‐for‐gestational‐age infants (RR 1.12, 95% CI 0.73 to 1.72; RCTs = 1; participants = 206; very low‐quality evidence). There were no perinatal deaths in two RCTs (very low‐quality evidence). Neonatal mortality or morbidity composite outcome and childhood/adulthood neurosensory disability were not reported.
For other secondary outcomes we assessed using GRADE, there were no clear differences between metformin and insulin groups for induction of labour (RR 1.42, 95% CI 0.62 to 3.28; RCTs = 2; participants = 35; very low‐quality evidence), though infant hypoglycaemia was reduced in the metformin group (RR 0.34, 95% CI 0.18 to 0.62; RCTs = 3; infants = 241; very low‐quality evidence). Perineal trauma, maternal postnatal depression and postnatal weight retention, and childhood/adulthood adiposity and diabetes were not reported.
Authors' conclusions
There are insufficient RCT data to evaluate the use of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who are planning a pregnancy, or in pregnant women with pre‐existing diabetes. Low to very low‐quality evidence suggests possible reductions in pregnancy‐induced hypertension, caesarean section birth and neonatal hypoglycaemia with metformin compared with insulin for women with type 2 diabetes diagnosed before or during their pregnancy, and no clear differences in pre‐eclampsia, induction of labour and babies that are large‐for‐gestational age. Further high‐quality RCTs that compare any combination of oral anti‐diabetic agent, insulin and dietary and lifestyle advice for these women are needed. Future RCTs could be powered to evaluate effects on short‐ and long‐term clinical outcomes; such RCTs could attempt to collect and report on the standard outcomes suggested in this review. We have identified three ongoing studies and four are awaiting classification. We will consider these when this review is updated.
Plain language summary
Oral anti‐diabetic agents for women with diabetes or previous diabetes planning a pregnancy, or pregnant women with pre‐existing diabetes
What is the issue?
Pre‐existing diabetes and gestational diabetes can increase the risks of a number of poor outcomes for both mothers and their babies. For the mother, these include pregnancy‐induced high blood pressure (pre‐eclampsia) with additional fluid retention and protein in the urine; and giving birth by caesarean. For the infant, these can include preterm birth; as well as an increased risk of the presence of physical defects at birth such as heart defects, brain, spine, and spinal cord defects, Down syndrome; and spontaneous abortion. Other complications at birth include babies that are large for their gestational age, and obstructed labour (shoulder dystocia) caused by one of the shoulders becoming stuck in the birth canal once the baby's head has been born.
Why is this important?
Being pregnant can trigger diabetes (gestational diabetes) in women with impaired glucose tolerance. Women who have had gestational diabetes are at risk of developing diabetes later in life. This means that management is important for women with impaired glucose tolerance or previous gestational diabetes, as well as for women with established diabetes. Women with established diabetes need good blood sugar control before they become pregnant. Insulin gives good blood sugar control and does not affect the development of the baby, but women may find oral anti‐diabetic agents more convenient and acceptable than insulin injections. However little is known about the effects of these oral agents.
This review sought to investigate the effects of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who were planning a pregnancy, or pregnant women with pre‐existing diabetes, on maternal and infant health. This review is an update of a review that was first published in 2010.
What evidence did we find?
We searched for evidence from randomised controlled trials (RCTs) on 31 October 2016 and included six RCTs (707 women). Three RCTs included women with current gestational diabetes and did not report data separately for the population of women relevant to this review. Therefore we have only included outcome data from three RCTs, involving 241 pregnant women and their infants. The quality of the evidence was assessed as being low or very low and the overall risk of bias of the RCTs was varied. The three RCTs all compared an oral anti‐diabetic agent (metformin) with insulin in pregnant women with pre‐existing (type 2) diabetes.
There was no clear difference in the development of pre‐eclampsia (high blood pressure and protein in the urine) for women who received metformin compared with insulin (2 RCTs; 227 women; very low‐quality evidence), though women receiving metformin were less likely to have pregnancy‐induced high blood pressure in one RCT (206 women; low‐quality evidence). Women who received metformin were less likely to have a caesarean section birth (3 RCTs; 241 women; low‐quality evidence), though no difference was observed in induction of labour (2 RCTs; 35 women; very low‐quality evidence). There was no clear difference between groups of infants born to mothers who received metformin or insulin for being large‐for‐gestational age (1 RCT; 206 infants; very low‐quality evidence), though infants born to mothers who received metformin were less likely to have low blood sugar (hypoglycaemia) (3 RCTs; 241 infants; very low‐quality evidence). There were no infant deaths (before birth or shortly afterwards) (2 RCTs; very low‐quality evidence). The RCTs did not report on many important short‐ and long‐term outcomes, including perineal trauma and a combined outcome of infant death or morbidity, postnatal depression and weight retention for mothers, and adiposity or disability in childhood or adulthood for infants.
What does this mean?
There is not enough evidence to guide us on the effects of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who are planning a pregnancy, or pregnant women with pre‐existing diabetes. Further large, well‐designed, RCTs are required and could assess and report on the outcomes suggested in this review, including both short‐ and long‐term outcomes for mothers and their infants.
Summary of findings
Summary of findings for the main comparison. Maternal outcomes: oral anti‐diabetic agent (metformin) compared with insulin for women with established type 2 diabetes mellitus.
| Maternal outcomes: oral anti‐diabetic agent (metformin) compared with insulin for women with established type 2 diabetes mellitus | ||||||
| Patient or population: women with type 2 diabetes Setting: USA (2 RCTs), Pakistan (1 RCT) Intervention: oral anti‐diabetic agent (metformin) Comparison: insulin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with insulin | Risk with oral anti‐diabetic (metformin) | |||||
| Hypertensive disorders of pregnancy: pre‐eclampsia | Study population | RR 0.63 (0.33 to 1.20) | 227 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 186 per 1000 | 117 per 1000 (61 to 223) | |||||
| Hypertensive disorders of pregnancy: pregnancy‐induced hypertension | Study population | RR 0.58 (0.37 to 0.91) | 206 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | ||
| 360 per 1000 | 209 per 1000 (133 to 328) | |||||
| Caesarean section | Study population | RR 0.73 (0.61 to 0.88) | 241 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 765 per 1000 | 558 per 1000 (466 to 673) | |||||
| Induction of labour | Study population | RR 1.42 (0.62 to 3.28) | 35 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2,4 | ||
| 316 per 1000 | 448 per 1000 (196 to 1000) | |||||
| Perineal trauma | Study population | ‐ | (0 RCTs) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Postnatal depression | Study population | ‐ | (0 RCTs) | ‐ | None of the included RCTs reported these outcomes | |
| See comment | See comment | |||||
| Postnatal weight retention | Study population | ‐ | (0 RCTs) | ‐ | None of the included RCTs reported these outcomes | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Study limitations (‐2): most of the weight in this analysis was from 1 RCT with very serious design limitations
2 Imprecision (‐2): wide 95% CI crossing the line of no effect and small sample sizes of RCTs
3 Study limitations (‐2): 1 RCT with very serious design limitations contributed data
4 Study limitations (‐1): 2 RCTs with design limitations contributed data
Summary of findings 2. Infant outcomes: oral anti‐diabetic agent (metformin) compared with insulin for women with established type 2 diabetes mellitus.
| Infant outcomes: oral anti‐diabetic (metformin) compared with insulin for women with established diabetes | ||||||
| Patient or population: women with type 2 diabetes mellitus Setting: USA (2 RCTs) and Pakistan (1 RCT) Intervention: oral anti‐diabetic agent (metformin) Comparison: insulin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with insulin | Risk with oral anti‐diabetic (metformin) | |||||
| Large‐for‐gestational age | Study population | RR 1.12 (0.73 to 1.72) | 206 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 270 per 1000 | 302 per 1000 (197 to 464) | |||||
| Perinatal mortality | Study population | ‐ | 220 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3,4 | No perinatal mortality in the 2 RCTs | |
| See comment | See comment | |||||
| Hypoglycaemia | Study population | RR 0.34 (0.18 to 0.62) | 241 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 5,6 | ||
| 277 per 1000 | 94 per 1000 (50 to 172) | |||||
| Neonatal mortality or morbidity composite | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Childhood/adulthood neurosensory disability | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Childhood/adulthood adiposity | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| Childhood/adulthood diabetes | Study population | ‐ | (0 studies) | ‐ | None of the included RCTs reported this outcome | |
| See comment | See comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Study limitations (‐2): 1 RCT with very serious design limitations contributed data
2 Imprecision (‐2): wide 95% CI crossing the line of no effect and small sample size of RCT
3 Study limitations (‐1): 2 RCTs with design limitations contributed data
4 Imprecision (‐2): no events
5 Study limitations (‐2): most of the weight in this analysis was from 1 RCT with very serious design limitations
6 Imprecision (‐1): small sample sizes of RCTs
Background
Description of the condition
Established diabetes prior to pregnancy and pre‐existing diabetes in pregnancy
Pre‐existing (pregestational) diabetes affects pregnant women who have been diagnosed with type 1 or type 2 diabetes ‐ or, in rare cases, other forms of diabetes mellitus ‐ prior to becoming pregnant. While some parts of the world have a significantly higher prevalence of established diabetes than others, in 2015 it was estimated that globally one in 11 (9%) adults aged 20 to 79 years ‐ that is 415 million ‐ had diabetes; this is projected to reach one in 10 (10%) ‐ or 642 million ‐ by 2040 (IDF 2015). An additional 193 million adults are estimated to have undiagnosed diabetes, and a further 318 million, are estimated to have impaired glucose tolerance, which puts them at high risk of developing the disease (IDF 2015). In 2015, it was estimated that 20.9 million, or 16.2% of live births globally were affected by some form of hyperglycaemia in pregnancy ‐ 14.9% (or approximately 3.1 million) of these pregnancies were affected by type 1 or 2 diabetes first detected in pregnancy, or detected prior to pregnancy (established diabetes) (IDF 2015). Prevalence estimates of pre‐existing diabetes in pregnancy have previously been reported as approximately 1% (Bell 2008; Correa 2015; Lawrence 2008), but prevalence is known to be increasing rapidly, with concurrent rises in obesity and type 2 diabetes.
In addition to the impact of diabetes on health, pre‐existing diabetes in pregnancy is commonly associated with a number of adverse health outcomes for mothers and their infants. For pregnant women, pre‐existing diabetes has been associated with caesarean section, pregnancy‐induced hypertension or pre‐eclampsia, and preterm birth (Langer 2000a; Ray 2001; Walkinshaw 2005). Pregnancy in women with pre‐existing diabetes may also exacerbate the effects of diabetes on renal function and retinopathy (Leguizamon 2007; Sheth 2002). Pre‐existing diabetes in pregnancy is associated with an increased risk of fetal congenital anomaly and spontaneous abortion (Kitzmiller 1996). Fetal hyperinsulinaemia associated with pre‐existing diabetes may affect infants by increasing the incidence of: macrosomia (birthweight exceeding 4000 g); large‐for‐gestational age (birthweight greater than 90th centile for age); shoulder dystocia; neonatal hypoglycaemia; preterm birth; hyperbilirubinaemia; hypocalcaemia, and neonatal intensive care admission (Jensen 2004; Macintosh 2006; Ray 2001; Walkinshaw 2005; Weintrob 1996). Furthermore, long‐term follow‐up of infants of diabetic mothers suggests that exposure to maternal diabetes in utero increases the risk of obesity and type 2 diabetes for these children in the future (Dabelea 2000).
Previous gestational diabetes
Gestational diabetes mellitus is defined as "carbohydrate intolerance resulting in hyperglycaemia of variable severity with onset or first recognition during pregnancy" (WHO 1999). The reported incidence of gestational diabetes varies between different populations and the method and criteria by which the diagnosis is made, with studies estimating incidence rates that range between 1% and 28% (Jiwani 2012). Gestational diabetes is associated with an increased risk of a number of adverse perinatal outcomes including macrosomia, shoulder dystocia, perineal trauma, pre‐eclampsia, and neonatal hypoglycaemia (Reece 2010). Although gestational diabetes resolves in 90% of cases, women with a history of gestational diabetes represent a unique group of women who are at significant risk for developing recurrent gestational diabetes and later established diabetes (Kim 2002; Kim 2007). It has been estimated that there is a 2% risk of progression to established diabetes in the subsequent pregnancy for women with gestational diabetes (Khambalia 2013).
Despite the potential need for intervention for these groups of women, there is limited evidence about the use of oral anti‐diabetic agents preconceptionally or during pregnancy.
Description of the intervention
Management of established diabetes before pregnancy, and pre‐existing diabetes during pregnancy
The occurrence of adverse outcomes in women with pre‐existing diabetes and their infants is inversely related to the level of glycaemic control achieved during pregnancy. Therefore, there is a strong focus on the management of maternal glucose concentrations in preconception and antenatal care of women with established diabetes.
Prior to conception, it is recommended that women with established diabetes receive multidisciplinary care including an assessment of diabetes complications, advice on glycaemic control, diet, the importance of family planning, maternal diabetes complications and fetal risks (ADA 2015; ADIPS 2005; CDA 2013; NICE 2015). Oral anti‐diabetic agents are more commonly used by people with type 2 diabetes than people with type 1 diabetes, who commonly will not achieve adequate glycaemic control on oral anti‐diabetics alone. Currently, it is recommended that oral anti‐diabetic agents be substituted for insulin in women planning pregnancy and during pregnancy (ADA 2015; ADIPS 2005; CDA 2013; NICE 2015).
Oral anti‐diabetic agents, also referred to as oral hypoglycaemic agents or oral antihyperglycaemic agents, act in a variety of ways. While widely used in men and women with type 2 diabetes, their use in women with established diabetes who are planning a pregnancy or are pregnant has been controversial, with conflicting reports about their safety. As a result of these concerns, insulin has been the preferred agent for glycaemic management in women with pre‐existing diabetes during pregnancy (ADA 2015; ADIPS 2005; CDA 2013; NICE 2015). Current recommendations suggest that women planning or continuing a pregnancy use insulin, although oral anti‐diabetic agents may be considered on an individual basis, since the harm from uncontrolled diabetes may outweigh any potential harm from the oral agents.
Lack of safety data for the use of oral anti‐diabetic agents in pregnancy has prevented them from being routinely recommended for use during pregnancy. It has also been argued that the use of oral anti‐diabetic agents alone, including glyburide (glibenclamide) and metformin, may be inadequate to manage the post‐prandial glycaemic peaks associated with type 2 diabetes successfully (Jovanovic 2007). However, oral anti‐diabetic agents are convenient, may be preferable to insulin injections, and may not require the intensive education associated with insulin therapy. Where oral agents alone are not sufficient to achieve glycaemic control, they may be used in combination to reduce the frequency or dose of insulin.
A retrospective study of women in South Africa with established diabetes, who remained on oral anti‐diabetic agents, transferred from oral agents to insulin, or transferred from diet alone to insulin, reported no difference in fetal anomaly rates (Ekpebegh 2007). This study, however, did report a significantly higher perinatal mortality rate for women continuing on oral anti‐diabetic agents alone compared with women who received insulin. Meta‐analyses and reviews of observational studies have been unable to provide definitive conclusions about the effects of oral anti‐diabetic agents in pregnancy (Gutzin 2003; Ho 2007).
The Tieu 2017 Cochrane Review found no evidence of benefit for preconception care compared with no preconception care, or any protocol of preconception care over another, for women with established diabetes. Cochrane Reviews have also assessed various management strategies during pregnancy for women with pre‐existing diabetes. The O'Neill 2017 Cochrane Review, which assessed different insulin types and regimens, recently concluded that no firm conclusions could be drawn on the basis of current evidence. The Farrar 2016 Cochrane Review similarly concluded that there is currently no evidence to support the use of one particular form of insulin administration over another. Furthermore, reviews of different intensities of glycaemic control (Middleton 2016), and techniques of monitoring blood glucose for women with pre‐existing diabetes in pregnancy (Moy 2014), have not been able to reach firm conclusions about best practice either.
Management of previous gestational diabetes before and during pregnancy
While the importance of management for women with gestational diabetes has been recognised (Crowther 2005; Landon 2009), the most appropriate form of treatment is uncertain (Alwan 2009). The Cochrane Review 'Treatment for gestational diabetes' concluded that while women with gestational diabetes should be considered for specific treatment in addition to routine antenatal care, the decision about whether to offer dietary advice or more intensive treatment (including insulin or oral agents) was unclear (Alwan 2009), as were the effects on long‐term outcomes for the women and their infants (Alwan 2009). This review assessed a range of management options, including the use of oral anti‐diabetic agents such as metformin and glyburide (glibenclamide). The review found that women who received oral anti‐diabetic agents compared with insulin were less likely to have a caesarean section, and their infants were less likely to develop neonatal hypoglycaemia; there were however, no differences in other important outcomes such as induction of labour and shoulder dystocia (Alwan 2009).
The Alwan 2009 Cochrane Review has now been separated into reviews that address lifestyle interventions (Brown 2017a), dietary supplementation with myo‐inositol (Brown 2016a), different intensities of glycaemic control (Martis 2016), as well as insulin (Brown 2016b), and oral anti‐diabetic agents (Brown 2017b). The recent Brown 2017b review concluded that there are insufficient data from comparisons of oral anti‐diabetic agents versus placebo/standard care (lifestyle advice) in women with gestational diabetes to inform clinical practice, or to draw any meaningful conclusions regarding the benefits or harms of one oral anti‐diabetic agent over another (e.g. metformin versus glibenclamide; and glibenclamide versus acarbose) (Brown 2017b). The Brown 2016b review, which will include comparisons of oral anti‐diabetic agents with insulin is currently being prepared.
The Tieu 2013 Cochrane Review found no evidence to assess the role of interconception care for women with a history of gestational diabetes.
How the intervention might work
Oral anti‐diabetic agents
Common anti‐diabetic agents include sulfonylureas, biguanides, thiazolidinediones, alpha‐glucosidase inhibitors, meglitinides and peptide analogues. Biguanides, including metformin, reduce peripheral insulin resistance, inhibit gluconeogenesis and reduce plasma triglyceride concentrations (DeFronzo 1995; Stumvoll 1995; Yogev 2004). Since metformin does cross the placenta, there have been concerns about its use in pregnancy (Hellmuth 2000; Kovo 2007; Slocum 2002). Since the publication of the Alwan 2009 Cochrane Review, the use of metformin for the treatment of gestational diabetes has been evaluated in a large randomised controlled trial (Rowan 2008a). This trial found that compared with insulin, while metformin was not associated with increased perinatal complications, it was associated with a tendency for less severe neonatal hypoglycaemia, less maternal weight gain and greater maternal acceptability. In this study metformin was commenced in the latter half of pregnancy, between 20 and 34 weeks' gestation.
There is no definitive evidence of the safety of metformin in pregnancies complicated by pre‐existing diabetes (Ho 2007). However, since it has been suggested that metformin increases the incidence of pregnancy and reduces pregnancy loss in women with polycystic ovary syndrome (PCOS), there are data available from babies born to mothers with PCOS who took metformin during pregnancy. Follow‐up at 18 months of age of 126 infants born to 109 mothers with PCOS, who conceived while taking metformin and continued to take it during pregnancy, reported that the metformin‐exposed infants were of similar size and had similar motor‐social development to infants of women not known to have PCOS (Brock 2005; Glueck 2004).
Sulfonylureas, for example glyburide (glibenclamide) and glimepiride, enhance insulin secretion and peripheral tissue sensitivity to insulin while also reducing hepatic clearance of insulin (DeFronzo 1984; Homko 2006; Simonson 1984; Yogev 2004). The main side effect of these agents is hypoglycaemia, and while first generation sulfonylureas cross the placenta, it is unclear whether second generation agents, including glyburide (glibenclamide), do, and what effect this might have on the developing fetus (Jovanovic 2007; Kraemer 2006; Sivan 1995; Slocum 2002). The ability of sulfonylureas to stimulate fetal hyperinsulinaemia is a major concern (Coetzee 2007). However, a randomised controlled trial of treatment of women with gestational diabetes, included in the Alwan 2009 Cochrane Review, which investigated glyburide (glibenclamide; a second generation sulfonylurea), or insulin, found that there were no differences in macrosomic or large‐for‐gestational‐age infants between the two groups (Langer 2000a).
Alpha‐glucosidase inhibitors such as acarbose and miglitol reduce postprandial glucose concentrations by decreasing the breakdown and absorption of carbohydrates in the intestine (Slocum 2002; Yogev 2004). There is little evidence of the use of these agents in pregnancy (Ho 2007). Alpha‐glucosidase inhibitors are typically used in combination with other oral anti‐diabetic agents or insulin (Yogev 2004).
Thiazolidinediones, including rosiglitazone and pioglitazone, result in increased insulin sensitivity and decreased lipid availability (Slocum 2002; Yogev 2004). There is little evidence about the use of thiazolidinediones in pregnancy (Ho 2007), although, placental transfer of thiazolidinediones has been reported (Chan 2005). Furthermore, concerns have been expressed about the use of rosiglitazone in type 2 diabetes due to an increased risk of adverse cardiovascular outcomes (Nissen 2007).
Meglitinides increase pancreatic insulin secretion. There is little evidence of their use in pregnancy (Slocum 2002), and a similar absence of evidence for the use of peptide analogues such as incretin mimetics and dipeptidyl peptidase‐4 inhibitors.
Why it is important to do this review
With the rising prevalence of type 1, type 2 and gestational diabetes, there is an increasing need for evidence‐based management of women with established diabetes or a history of gestational diabetes both preconceptionally and during pregnancy. While most guidelines recommend the use of insulin in place of oral anti‐diabetic agents, oral agents may have benefits, particularly in terms of acceptability and adherence. However, there is little evidence about the effects of these agents on maternal and infant health. It is necessary, therefore, to assess the benefits and harms of anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who are planning a pregnancy, and pregnant women with pre‐existing diabetes mellitus. The use of oral anti‐diabetic agents for the management of gestational diabetes in a current pregnancy was previously evaluated in the Cochrane Review 'Treatment of gestational diabetes' (Alwan 2009); specific assessments of oral anti‐diabetic agents (Brown 2017b), and comparisons of these agents with insulin for women with gestational diabetes (Brown 2016b), are made in two new Cochrane Reviews.
Objectives
To investigate the effect of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes who are planning a pregnancy, or pregnant women with pre‐existing diabetes, on maternal and infant health. The use of oral anti‐diabetic agents for the management of gestational diabetes in a current pregnancy is evaluated in a separate Cochrane Review.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised and quasi‐randomised controlled trials, and cluster‐randomised trials, and to exclude cross‐over trials. We planned to include trials published in abstract form only where there was sufficient information available to assess study eligibility and risk of bias.
Types of participants
Women with established type 1 or 2 diabetes mellitus, impaired glucose tolerance or previous gestational diabetes mellitus planning a pregnancy or pregnant women with pre‐existing type 1 or 2 diabetes mellitus. Trials involving women with gestational diabetes in a current pregnancy were excluded from this review.
Types of interventions
Oral anti‐diabetic agent versus no medication
Oral anti‐diabetic agent versus another oral anti‐diabetic agent
Oral anti‐diabetic agent versus insulin
Oral anti‐diabetic agent versus insulin plus an oral anti‐diabetic agent
Oral anti‐diabetic agent plus insulin versus insulin
Different regimens of any of the above
Types of outcome measures
For this update, we used the standard outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes and pre‐existing diabetes.
Primary outcomes
Mother
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Caesarean section
Infant/child/adult
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Neonatal mortality or morbidity composite (e.g. perinatal mortality, shoulder dystocia, bone fracture, and admission to the neonatal unit)
Childhood/adulthood neurosensory disability
Secondary outcomes
Mother: short‐term
Spontaneous abortion/miscarriage
Gestational diabetes mellitus
Induction of labour
Perineal trauma
Placental abruption
Postpartum haemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention
Sense of well‐being and quality of life
Views of the intervention
Adverse effects of the intervention
Breastfeeding at discharge, six weeks postpartum, six months or longer
Use of additional pharmacotherapy for glycaemic control
Glycaemic control during/end of treatment (e.g. blood glucose or haemoglobin A1c (HbA1c))
Hypoglycaemia
Mortality
Complications of diabetes (retinopathy, neuropathy, nephropathy, ischaemic heart disease, cerebrovascular disease, peripheral vascular disease)
Mother: long‐term
Postnatal depression
Postnatal weight retention or return to prepregnancy weight
Body mass index (BMI)
Gestational diabetes mellitus in a subsequent pregnancy
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
Infant
Congenital anomaly
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia (birthweight greater than 4000 g and birthweight greater than 4500 g)
Small‐for‐gestational age
Birthweight and Z score
Head circumference and Z score
Length and Z score
Ponderal index
Adiposity
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia
Hyperbilirubinaemia
Hypocalcaemia
Polycythaemia
Infection
Relevant biomarker changes associated with the intervention (e.g. cord C peptide, cord insulin)
Child/adult
Weight and Z scores
Height and Z scores
Head circumference and Z scores
Adiposity (e.g. as measured by BMI, skinfold thickness)
Cardiovascular health (as defined by trialists including blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
Type 1 diabetes
Type 2 diabetes
Impaired glucose tolerance
Employment, education and social status/achievement
Health service
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit/nursery admission
Duration of stay in neonatal intensive care unit/nursery
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the management provided
Costs associated with the intervention
Cost of maternal care
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 October 2016).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeTieu 2010.
For this update, the following methods were used for assessing the 29 new reports that were identified as a result of the updated search, and four reports that were reassessed from the previous version of this review.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed all the potential studies identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager 5 software (RevMan 2014), and checked for accuracy.
When information regarding eligibility or data was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update the quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook for the following outcomes.
Mother
Hypertensive disorders of pregnancy (including pre‐eclampsia, pregnancy‐induced hypertension, eclampsia)
Caesarean section
Gestational diabetes mellitus or type 2 diabetes (if applicable)
Induction of labour
Perineal trauma
Postnatal depression
Postnatal weight retention or return to prepregnancy weight
Infant/child/adult
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Neonatal mortality or morbidity composite (e.g. perinatal mortality, shoulder dystocia, bone fracture, and admission to the neonatal unit)
Hypoglycaemia
Childhood/adulthood neurosensory disability
Childhood/adulthood adiposity
Childhood/adulthood diabetes
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach, which uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference where outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome through different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. In future updates of the review, if cluster‐randomised trials are included, we will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs, and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and we will perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are an inappropriate design for this intervention.
Cross over trials are not a suitable design for trials looking at interventions in labour and have been excluded.
Studies with more than two groups
In Ainuddin 2015, results for the group randomised to metformin were reported separately for those women who remained on metformin alone and those women who subsequently received insulin in addition to metformin. We combined the metformin groups to create a single pair‐wise comparison, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16, section.5.4), by considering the metformin alone and the metformin plus insulin groups together.
In Notelovitz 1971, we were unable to report any results from this four‐armed RCT (chlorpropamide, tolbutamide, insulin and diet therapy) as women with type 2 diabetes or gestational diabetes mellitus were not reported separately. However, if we had been able to obtain these data separately, we would have created three single pair‐wise comparisons (by combining both oral anti‐diabetic agents compared with insulin, both oral anti‐diabetic agents compared with diet therapy and one oral anti‐diabetic agent compared with the other anti‐diabetic agent).
Dealing with missing data
We noted levels of attrition for included studies. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
As there were fewer than 10 trials included in the analyses, we were unable to investigate reporting biases (such as publication bias) using funnel plots. In future updates, as more data become available, we will investigate reporting biases using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by visual assessment, we will investigate further.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar.
In future updates, with more included studies, if there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. Where we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses but there were insufficient data to do so.
Type of diabetes (e.g. established type 1 diabetes mellitus versus established type 2 diabetes mellitus versus impaired glucose tolerance versus previous gestational diabetes mellitus).
Gestational age of women at randomisation (e.g. preconception versus first trimester versus second trimester versus third trimester).
Glycaemic control prior to pregnancy/randomisation (e.g. glycaemic targets achieved versus not achieved).
We planned to use only primary outcomes in subgroup analyses. In future updates, we plan to assess differences between subgroups by interaction tests available in RevMan 2014. We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies (rated at unclear or high risk of bias for these components) being excluded from the analyses in order to assess whether this made any difference to the overall result, however there were insufficient data to do this.
Results
Description of studies
Results of the search
In the previous version on this review we identified no trials eligible for inclusion (Tieu 2010).
An updated search was carried out in October 2016; 29 new reports were identified. We reassessed three studies (four reports) which were either excluded (Notelovitz 1971), ongoing (Hague 2010), or awaiting assessment (Hutchinson 2008) in the previous version. Some studies were published in multiple reports. In total, we have now included six studies and excluded 22. Four studies are awaiting further classification, and three studies are ongoing. See Figure 1.
1.
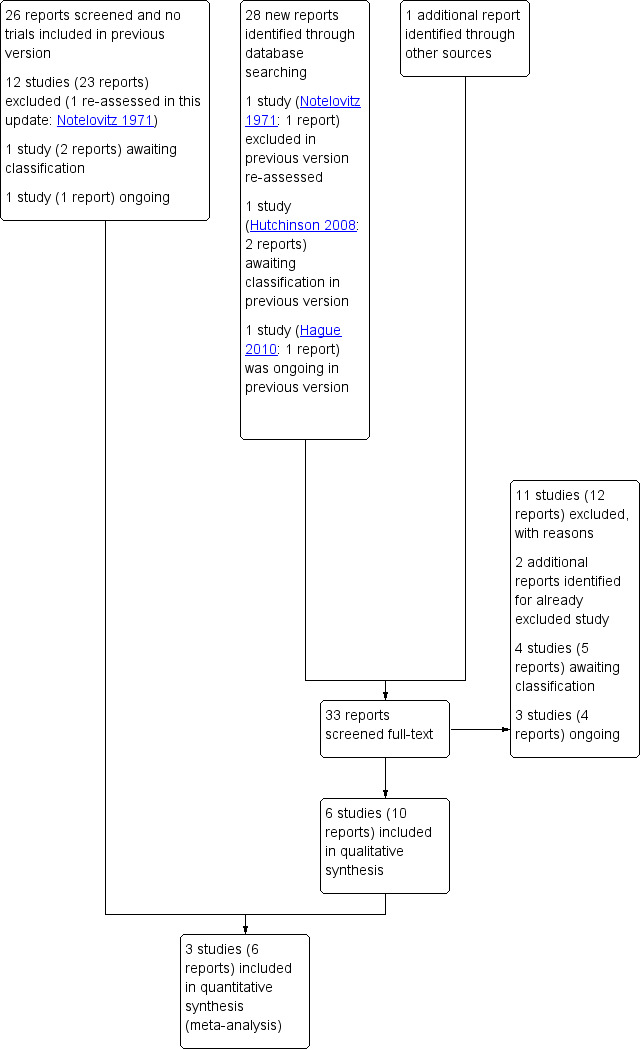
Study flow diagram
To date, three of the four studies awaiting further classification have been published as abstracts only (Coiner 2014; Hutchinson 2008; Reyes‐Munoz 2014); we have contacted the trial authors regarding availability of full reports in order to assess study eligibility and risk of bias further. The fourth study provided insufficient detail for us to determine study eligibility (specifically regarding whether women with established diabetes were included) (Waheed 2013). For further details see Characteristics of studies awaiting classification.
The three ongoing trials are all assessing metformin in pregnant women with type 2 diabetes (Feig 2011), gestational diabetes or type 2 diabetes (Sheizaf 2006), or at high risk for gestational diabetes (Van der Linden 2014). For further details see Characteristics of ongoing studies.
Included studies
We included six trials in this review (Ainuddin 2015; Beyuo 2015; Hickman 2013; Ibrahim 2014; Notelovitz 1971; Refuerzo 2015). However, as three of the trials had mixed populations (women with gestational diabetes and women with established diabetes) (Beyuo 2015; Ibrahim 2014; Notelovitz 1971), and did not report data separately for the relevant subset of women for this review, we have only included outcome data from three of the included trials (Ainuddin 2015; Hickman 2013; Refuerzo 2015). The Hickman 2013 trial also had a mixed population, but the trial authors provided data for the group of women with established diabetes separately for inclusion in this review. We have contacted the authors of the three trials with mixed populations to ask about the availability of data for women with established diabetes, and await responses. We describe the characteristics of the six included trials below.
Settings
The included trials were conducted in Pakistan (Ainuddin 2015), Ghana (Beyuo 2015), Egypt (Ibrahim 2014), South Africa (Notelovitz 1971) and the USA (Hickman 2013; Refuerzo 2015).
Dates when study conducted
Ainuddin 2015 was conducted from January 2009 to January 2014, Beyuo 2015 from January 2013 to October 2015, Hickman 2013 from July 2008 to December 2009, Ibrahim 2014 from August 2011 to April 2012, and Refuerzo 2015 from September 2009 to August 2011. Notelovitz 1971 did not report when the study was conducted.
Funding
Funding sources were reported by four of the trials; Hickman 2013 and Refuerzo 2015 were funded by non‐commercial organisations; Notelovitz 1971 was funded by a commercial organisation (Pfizer Laboratories Ltd); and Ibrahim 2014 identified the trialists as the source of funding. Ainuddin 2015 did not describe source of funding and Beyuo 2015 reported “no source of funding”.
Declarations of interest
Three of the trials reported that there were no conflicts of interests for any of the authors (Ainuddin 2015; Beyuo 2015; Ibrahim 2014). The remaining three trials did not report any information regarding declarations of interest (Hickman 2013; Notelovitz 1971; Refuerzo 2015).
Participants
Overall, the six trials randomised 707 women and their babies, with sample sizes ranging from 25 women in Refuerzo 2015 to 250 women in Ainuddin 2015.
Two trials included only women with established diabetes; both included women with singleton pregnancies, with type 2 diabetes diagnosed prior to pregnancy, and cases of newly diagnosed overt diabetes in pregnancy beyond the first trimester (Ainuddin 2015), or a self‐reported history of type 2 diabetes for less than 10 years prior to 20 weeks' gestation (Refuerzo 2015).
Four trials included mixed populations of women with gestational diabetes and established type 2 diabetes, prior to 20 weeks' gestation (Hickman 2013), from 20 to 30 weeks' gestation (Beyuo 2015), between 20 and 34 weeks' gestation (Ibrahim 2014), or whose duration of pregnancy would allow at least six consecutive weeks of treatment (Notelovitz 1971).
Interventions
While five of the six included trials compared metformin with insulin (Ainuddin 2015; Beyuo 2015; Hickman 2013; Ibrahim 2014; Refuerzo 2015), their intervention and control regimens varied. Four of the trials assessed 500 mg metformin daily (Ainuddin 2015; Beyuo 2015; Hickman 2013; Refuerzo 2015), which was increased, as required, up to a maximum of 2500 mg daily in the intervention group. In these four trials, if glycaemic control (variously defined) was not achieved with the maximum dose of metformin, insulin was added as supplementary therapy. In the fifth trial (Ibrahim 2014), women commenced on 1500 mg metformin daily, which was raised to 2000 mg daily. In this trial, if glycaemic control was not achieved women were switched to a conventional insulin dose‐raising regimen (Ibrahim 2014). Insulin regimens in the comparison groups were broadly similar; it was commonly given in two doses, with the total daily dose titrated to achieve glycaemic control. Total daily doses were calculated as follows: in Ainuddin 2015 0.6 IU/kg body weight in first trimester, 0.7 IU/kg in second trimester, 0.8 IU/kg from 28 to 32 weeks, 0.9 IU/kg from 32 to 36 weeks, 1 IU/kg from 36 weeks onwards; in Beyuo 2015 0.3 IU/kg body weight at initiation (20 to 30 weeks' gestation); in Hickman 2013 0.7 IU/kg bodyweight at initiation (prior to 20 weeks' gestation); and in Refuerzo 2015: 0.7 IU/kg bodyweight in the first trimester, 0.8 IU/kg in the second trimester, and 0.9 to 1.0 IU/kg/day in the third trimester. Ibrahim 2014 treated women with poor glycaemic control with a daily dose of at least 1.12 IU/kg bodyweight.
The Notelovitz 1971 trial had four groups that compared chlorpropamide, tolbutamide, insulin and diet restriction alone; no further details were provided regarding specific regimens.
For detailed descriptions of the included studies, see Characteristics of included studies.
Excluded studies
We excluded a total of 22 studies. Seventeen of the trials evaluated treatments for gestational diabetes (Anjalakshi 2007; Bertini 2005; Casey 2015; Corrado 2011; George 2015; Golladay 2005; Hague 2003; Langer 2000b; Martinez 2010; Moore 2005; Moore 2007; Mukhopadhyay 2012; Niromanesh 2012; Rowan 2008b; Singh 2011Wali 2012; Zanganeh 2010), and one assessed treatments for women with hyperglycaemia that did not meet the criteria for gestational diabetes (Myers 2013); these trials are, or will be, considered in the other relevant Cochrane Reviews. Three trials evaluated treatment specifically for women with polycystic ovary syndrome (Carlsen 2007; Vanky 2004; Vanky 2005). One potentially relevant trial was planned in women with previous gestational diabetes, but subsequently not conducted (Hague 2010). For further details see Characteristics of excluded studies.
Risk of bias in included studies
Overall we judged the trials to be at varying risks of bias; see Figure 2 and Figure 3.
2.
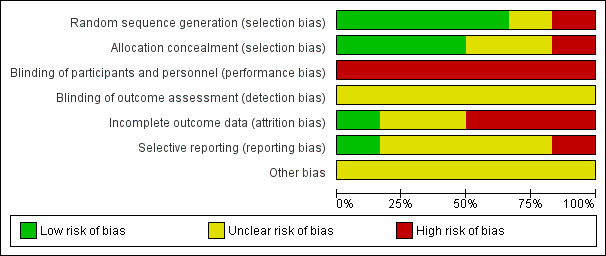
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
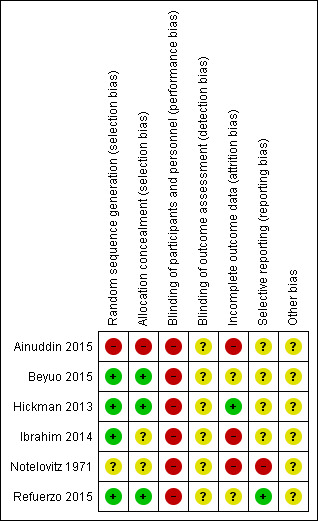
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We judged that three of the six trials used adequate methods for sequence generation and allocation concealment, and thus were at low risk of selection bias (Beyuo 2015; Hickman 2013; Refuerzo 2015). One trial reported an adequate method for sequence generation, but the method of allocation concealment was unclear (Ibrahim 2014). Another trial did not report the methods for sequence generation or allocation concealment (Notelovitz 1971), and so we judged this trial to be at an unclear risk of selection bias. The final trial used quasi‐randomisation, and we judged it to be at high risk of selection bias (Ainuddin 2015).
Blinding
We judged all of the six included trials to be at high risk of performance bias (blinding of women and study personnel was not possible due to the nature of the differing treatments received by the intervention and comparison groups) (Ainuddin 2015; Beyuo 2015; Hickman 2013; Ibrahim 2014; Notelovitz 1971; Refuerzo 2015). We judged all six trials to be at an unclear risk of detection bias; three trials were reported to be 'open label', but none of these trials specifically reported on whether it was possible to blind outcome assessment (Ainuddin 2015; Beyuo 2015; Refuerzo 2015), and the other three trials provided no details (Hickman 2013; Ibrahim 2014; Notelovitz 1971).
Incomplete outcome data
We judged only one trial to be at low risk of attrition bias (Hickman 2013), as over 90% of the women randomised were included in the analyses, and loss to follow‐up and exclusions occurred in similar numbers, and for similar reasons between groups. We judged two trials to be at an unclear risk of attrition bias; in Beyuo 2015 there appeared to be higher attrition in the insulin group, with 75% of women completing the study, compared with 90% in the metformin group; and in Refuerzo 2015 only 84% of the women randomised were analysed (21/25). We judged three trials to be at a high risk of attrition bias (Ainuddin 2015; Ibrahim 2014; Notelovitz 1971); in Ainuddin 2015 almost 20% of women were excluded from the analyses, and notably, in the analyses, the metformin group was separated into metformin alone, and metformin plus insulin; we combined these groups for the purpose of this review. Ibrahim 2014 reported that 'per‐protocol treatment analyses' were performed (with women who chose to switch from their allocated group excluded from the analyses); and for Notelovitz 1971 it also appeared that analyses were not intention‐to‐treat, as it was reported that, "In the final analysis" women were analysed in the group in which they "completed treatment" or "were treated with for the greater part of their pregnancy".
Selective reporting
We judged only one trial to be at a low risk of reporting bias, as the expected outcomes were reported as they were in the trial registration (Refuerzo 2015). We judged four of the trials to be at an unclear risk of reporting bias, due to the provision of limited detail regarding prespecified outcomes in the trial registrations available, or limited reporting of expected outcomes to date, or both (Ainuddin 2015; Beyuo 2015; Hickman 2013; Ibrahim 2014). We judged one trial to be at a high risk of reporting bias, as it did not prespecify outcomes, and provided incomplete reporting of some outcomes (Notelovitz 1971).
Other potential sources of bias
We judged all six trials to be at unclear risk of other bias. Four of the trials appeared to have imbalances in baseline characteristics despite randomisation (Ainuddin 2015; Beyuo 2015; Hickman 2013; Refuerzo 2015), while the other two provided very limited methodological details and information regarding the comparability of groups at baseline (Ibrahim 2014; Notelovitz 1971).
Effects of interventions
Oral anti‐diabetic agent (metformin) versus insulin
Mother: primary outcomes
Hypertensive disorders of pregnancy
Both Ainuddin 2015 and Refuerzo 2015 reported on pre‐eclampsia and overall, there was no clear difference between the metformin and insulin groups (risk ratio (RR) RR 0.63, 95% confidence interval (CI) 0.33 to 1.20; RCTs = 2; participants = 227; very low‐quality evidence; Analysis 1.1). In Ainuddin 2015, however, women receiving metformin, were less likely to have pregnancy‐induced hypertension (RR 0.58, 95% CI 0.37 to 0.91; RCTs = 1; participants = 206; low‐quality evidence; Analysis 1.2).
1.1. Analysis.
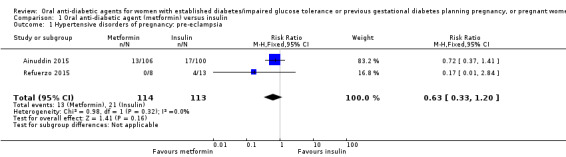
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 1 Hypertensive disorders of pregnancy: pre‐eclampsia.
1.2. Analysis.
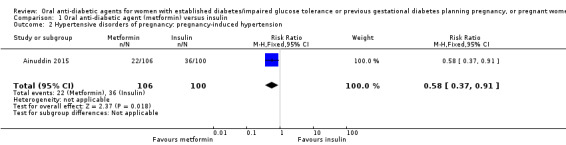
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 2 Hypertensive disorders of pregnancy: pregnancy‐induced hypertension.
Caesarean section
Pooling of data from Ainuddin 2015, Hickman 2013 and Refuerzo 2015 showed that women in the metformin group were less likely to have a caesarean section birth compared with those in the insulin group (RR 0.73, 95% CI 0.61 to 0.88; RCTs = 3; participants = 241; low‐quality evidence; Analysis 1.3).
1.3. Analysis.
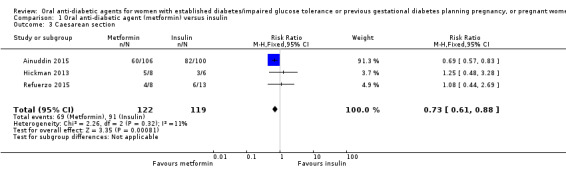
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 3 Caesarean section.
Infant/child/adult
Large‐for‐gestational age
There was no clear difference between the metformin and insulin groups for large‐for‐gestational age infants in Ainuddin 2015 (RR 1.12, 95% CI 0.73 to 1.72; RCTs = 1; participants = 206; very low‐quality evidence; Analysis 1.4).
1.4. Analysis.
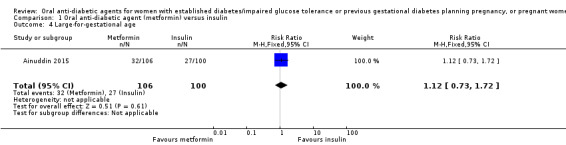
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 4 Large‐for‐gestational age.
Perinatal mortality (stillbirth and neonatal mortality)
There were no perinatal deaths in Ainuddin 2015 or Hickman 2013 (RCTs = 2; participants = 220; very low‐quality evidence; Analysis 1.5).
1.5. Analysis.
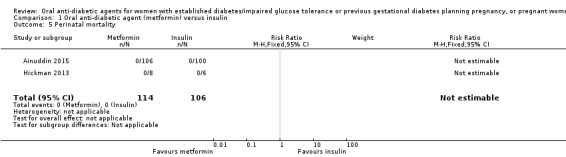
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 5 Perinatal mortality.
Neonatal mortality or morbidity composite
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on this outcome.
Childhood/adulthood neurosensory disability
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on this outcome.
Mother: short‐term secondary outcomes
In the metformin group in Hickman 2013, one woman experienced a 13‐week intrauterine fetal death attributed to a large subchorionic haematoma noted on ultrasound at 12 weeks (Analysis 1.6).
1.6. Analysis.
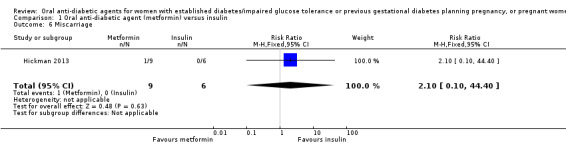
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 6 Miscarriage.
Meta‐analysis of data from Hickman 2013 and Refuerzo 2015 showed no clear difference between the metformin and insulin groups for induction of labour on (RR 1.42, 95% CI 0.62 to 3.28; RCTs = 2; participants = 35; very low‐quality evidence; Analysis 1.7). In Hickman 2013, no women had postpartum haemorrhage that required treatment (Analysis 1.8). In Ainuddin 2015, on average, women in the metformin group gained less weight in pregnancy than those in the insulin group (mean difference (MD) ‐1.30 kg, 95% CI ‐1.57 to ‐1.03; RCTs = 1; participants = 206; Analysis 1.9). Hickman 2013 additionally reported on weight gain during pregnancy (this was presented as groups medians, with interquartile ranges in Analysis 1.10) with results also favouring the metformin group.
1.7. Analysis.
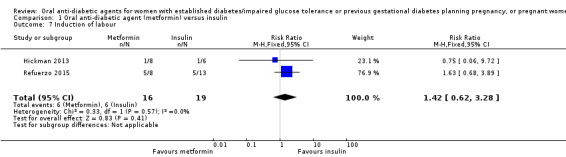
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 7 Induction of labour.
1.8. Analysis.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 8 Postpartum haemorrhage.
1.9. Analysis.
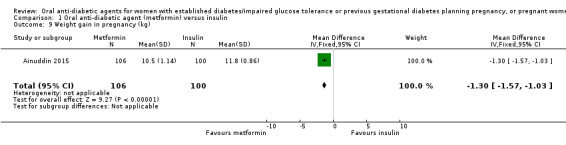
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 9 Weight gain in pregnancy (kg).
1.10. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 10 Weight gain in pregnancy (kg).
| Weight gain in pregnancy (kg) | ||
|---|---|---|
| Study | Metformin (N=9) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 3.16 (2.88, 4.50) | Median (IQR): 10.78 (8.15, 14.42) |
Adherence to the intervention was assessed in the Ainuddin 2015 trial. This showed that women in the metformin group were more likely to report 'never or rarely' forgetting to take their medication (RR 1.37, 95% CI 1.14 to 1.64; RCTs = 1; participants = 206), and were less likely to report forgetting to take their medication two to four times per week (RR 0.45, 95% CI 0.28 to 0.72; RCTs = 1; participants = 206; Analysis 1.11). In Hickman 2013, there was no clear difference between groups for the proportions of women who had missed appointments (RR 2.00, 95% CI 0.59 to 6.79; RCTs = 1; participants = 15), or who completed more than 50% of their log book (RR 1.90, 95% CI 0.89 to 4.04; RCTs = 1; participants = 15; Analysis 1.12).
1.11. Analysis.
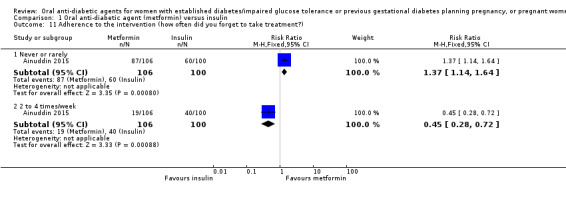
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 11 Adherence to the intervention (how often did you forget to take treatment?).
1.12. Analysis.
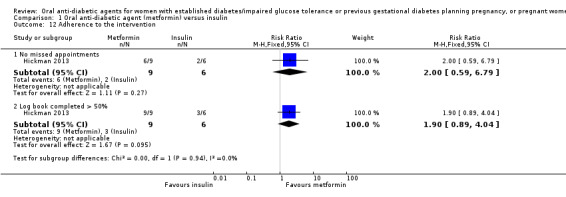
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 12 Adherence to the intervention.
In Ainuddin 2015, women in the metformin group were more likely to report that they would choose metformin in their next pregnancy (RR 7.70, 95% CI 4.52 to 13.14; RCTs = 1; participants = 206), were less likely to report that they would choose insulin (RR 0.07, 95% CI 0.03 to 0.19; RCTs = 1; participants = 206), and were less likely to report that they were 'not sure' which medication they would choose (RR 0.11, 95% CI 0.04 to 0.29; RCTs = 1; participants = 206) compared with women in the insulin group (Analysis 1.13). In the Ainuddin 2015 trial (Analysis 1.14), when asked what part of the diabetes treatment they found easy, no women in either the metformin or insulin groups reported that doing finger pricks was easy; no women in the metformin group, compared with 25/100 in the insulin group reported that dietary control was easy (RR 0.02, 95% CI 0.00 to 0.30; RCTs = 1; participants = 206), though more women in the metformin group reported that drug treatment was easy (RR 1.33, 95% CI 1.19 to 1.49; RCTs = 1; participants = 206; Analysis 1.14). In the Ainuddin 2015 trial (Analysis 1.15), when asked what part of the diabetes treatment they found difficult, women in the metformin group were more likely to report that doing finger pricks was difficult (RR 3.46, 95% CI 2.42 to 4.95; RCTs = 1; participants = 206), and were less likely to report that drug treatment was difficult (RR 0.14, 95% CI 0.08 to 0.26; RCTs = 1; participants = 206) compared with women in the insulin group; there was no clear difference in the proportion of women finding diet control difficult in the metformin and insulin groups (RR 0.75, 95% CI 0.31 to 1.84; RCTs = 1; participants = 206; Analysis 1.15). In Hickman 2013 women in the metformin group were more likely to report that they would choose the same treatment in the future (RR 11.14, 95% CI 0.78 to 159.58; RCTs = 1; participants = 11; P = 0.08; Analysis 1.16).
1.13. Analysis.
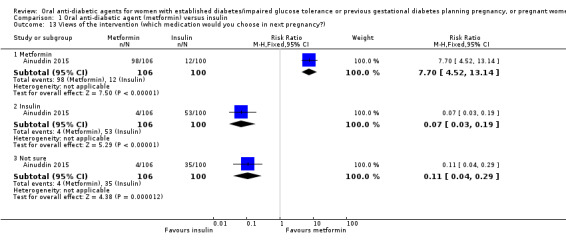
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 13 Views of the intervention (which medication would you choose in next pregnancy?).
1.14. Analysis.
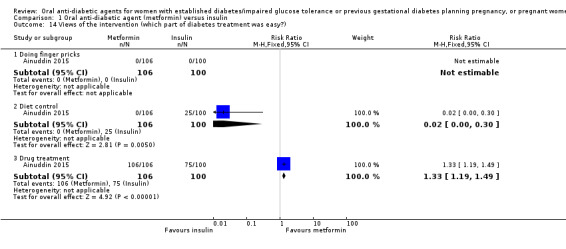
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 14 Views of the intervention (which part of diabetes treatment was easy?).
1.15. Analysis.
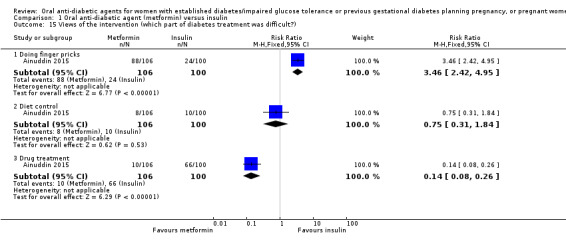
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 15 Views of the intervention (which part of diabetes treatment was difficult?).
1.16. Analysis.
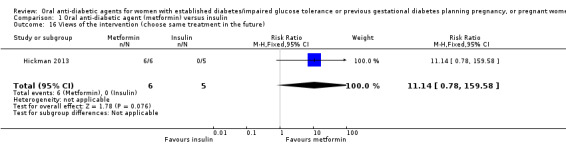
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 16 Views of the intervention (choose same treatment in the future).
Regarding adverse effects of the intervention, in the Ainuddin 2015 trial, six women in the metformin group had gastrointestinal side effects resulting in dose limitation compared with none in the insulin group (RR 12.27, 95% CI 0.70 to 215.04; RCTs = 1; participants = 206). No women in either the metformin or insulin groups had gastrointestinal side effects that resulted in treatment cessation or lactic acidosis (Analysis 1.17).
1.17. Analysis.
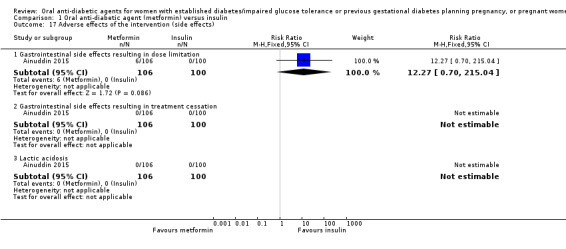
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 17 Adverse effects of the intervention (side effects).
There was no clear difference between groups in Hickman 2013 for exclusive breastfeeding (time point not clear) (RR 1.59, 95% CI 0.79 to 3.23; RCTs = 1; participants = 11; Analysis 1.18).
1.18. Analysis.
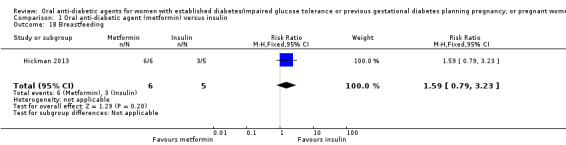
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 18 Breastfeeding.
With regard to the need for additional pharmacotherapy, Ainuddin 2015 reported that 105/125 women in the metformin group required supplemental insulin to maintain glycaemic control, while Refuerzo 2015 reported that none of the 25 women receiving metformin 'failed' with this therapy and needed insulin. Hickman 2013 reported that 3/9 women in the metformin group required supplemental insulin.
In Ainuddin 2015, with regard to glycaemic control, there was no clear difference in average fasting (MD 0.01 mg/dL, 95% CI ‐0.90 to 0.92; RCTs = 1; participants = 206; Analysis 1.19) or random blood glucose throughout pregnancy (MD ‐0.22 mg/dL, 95% CI ‐1.63 to 1.19; RCTs = 1; participants = 206; Analysis 1.20). Similarly, in Refuerzo 2015, there were no clear differences between groups in the average change in HbA1c from enrolment to the third trimester or birth (MD ‐0.48%, 95% CI ‐1.05 to 0.09; RCTs = 1; participants = 21; Analysis 1.21), or in the proportion of women with HbA1c less than 7% in the third trimester or at birth (RR 1.03, 95% CI 0.76 to 1.39; RCTs = 1; participants = 18; Analysis 1.22). Hickman 2013 reported on glycaemic control (HbA1c in the second and third trimester, delivery glucose, and postpartum fasting glucose), which has been presented as group medians, with interquartile ranges in Analysis 1.23.
1.19. Analysis.
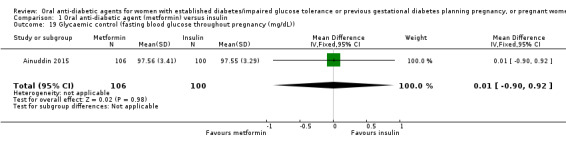
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 19 Glycaemic control (fasting blood glucose throughout pregnancy (mg/dL)).
1.20. Analysis.
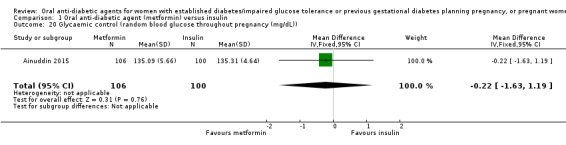
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 20 Glycaemic control (random blood glucose throughout pregnancy (mg/dL)).
1.21. Analysis.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 21 Glycaemic control (change in HbA1c from enrolment to third trimester/birth (%)).
1.22. Analysis.
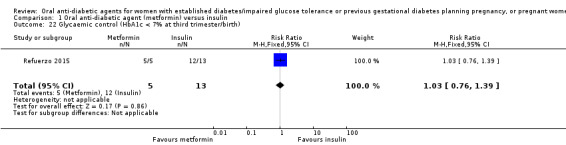
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 22 Glycaemic control (HbA1c < 7% at third trimester/birth).
1.23. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 23 Glycaemic control.
| Glycaemic control | ||
|---|---|---|
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | HbA1c 2nd trimester (%) Median (IQR): 5.55 (5.54, 5.70) |
HbA1c 2nd trimester (%) Median (IQR): 5.70 (5.35, 6.28) |
| Hickman 2013 | HbA1c 3rd trimester (%) Median (IQR): 5.85 (5.73, 6.00) |
HbA1c 3rd trimester (%) Median (IQR): 5.85 (5.53, 6.55) |
| Hickman 2013 | Delivery glucose (mg/dL) Median (IQR): 96.00 (92.00, 113.00) |
Delivery glucose (mg/dL) Median (IQR): 127.50 (109.25, 122.00) |
| Hickman 2013 | Postpartum fasting glucose (mg/dL) Median (IQR): 97.50 (78.50, 108.75) |
Postpartum fasting glucose (mg/dL) Median (IQR): 125.50 (109.75, 136.75) |
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on: gestational diabetes mellitus*; perinatal trauma; placental abruption; postpartum infection; sense of well‐being and quality of life; hypoglycaemia; mortality; or complications of diabetes.
*Outcome not relevant to trials involving women with established diabetes prior to pregnancy and pre‐existing diabetes in pregnancy
Mother: long‐term secondary outcomes
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on: postnatal depression; postnatal weight retention or return to prepregnancy weight; body mass index; gestational diabetes in a subsequent pregnancy*; type 1 diabetes*; type 2 diabetes*; impaired glucose tolerance*; and cardiovascular health.
*Outcome not relevant to trials involving women with established diabetes prior to pregnancy and pre‐existing diabetes in pregnancy
Infant: secondary outcomes
There were no congenital anomalies (major malformations) in Hickman 2013 (Analysis 1.24). There were no stillbirths or neonatal deaths in Ainuddin 2015 or Hickman 2013 (Analysis 1.25; Analysis 1.26). Ainuddin 2015 did not show a clear difference in gestational age at birth between the metformin and insulin groups (MD ‐0.30 weeks, 95% CI ‐0.66 to 0.06; RCTs = 1; participants = 206; Analysis 1.27); Hickman 2013 and Refuerzo 2015 also reported on gestational age (presented as group medians, with (interquartile) ranges in Analysis 1.28). There was no clear difference between the metformin and insulin groups for preterm birth in Hickman 2013 and Refuerzo 2015 (RR 0.42, 95% CI 0.08 to 2.30; RCTs = 2; participants = 35; Analysis 1.29). No infants had an Apgar score less than seven at five minutes in Hickman 2013 (Analysis 1.30). There were no clear differences for macrosomia (> 4000 g) (RR 0.37, 95% CI 0.04 to 3.10; RCTs = 2; participants = 35; Analysis 1.31) in Hickman 2013 and Refuerzo 2015.
1.24. Analysis.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 24 Congenital anomaly (major malformations).
1.25. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 25 Stillbirth.
1.26. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 26 Neonatal mortality.
1.27. Analysis.
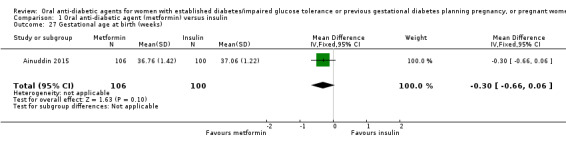
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 27 Gestational age at birth (weeks).
1.28. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 28 Gestational age at birth (weeks).
| Gestational age at birth (weeks) | |||
|---|---|---|---|
| Study | Metformin | Insulin | P value |
| Hickman 2013 | Median (IQR): 38.40 (37.10, 38.86) N=9 |
Median (IQR): 37.50 (35.79, 38.00) N=6 |
|
| Refuerzo 2015 | Median (range): 37 (35‐40) N=8 |
Median (range): 37 (35‐41) N=13 |
0.977 |
1.29. Analysis.
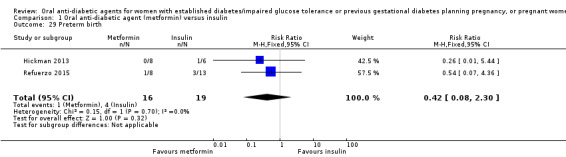
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 29 Preterm birth.
1.30. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 30 Apgar score < 7 at 5 minutes.
1.31. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 31 Macrosomia (> 4000 g).
In Ainuddin 2015 infants born to mothers in the metformin group were more likely to be small‐for‐gestational age compared with those born to mothers in the insulin group (RR 8.49, 95% CI 2.02 to 35.66; RCTs = 1; participants = 206; Analysis 1.32). Meta‐analysis of data from Ainuddin 2015 and Refuerzo 2015 showed no clear difference for birthweight between infants born to mothers in the metformin and insulin groups (MD ‐0.13 kg, 95% CI ‐0.29 to 0.04; RCTs = 2; participants = 227; Analysis 1.33). Hickman 2013 also reported on birthweight, head circumference, length and cord C peptide (these data are presented as group medians, with interquartile ranges in Analysis 1.34; Analysis 1.35; Analysis 1.36; Analysis 1.44).
1.32. Analysis.
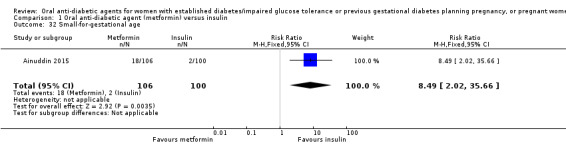
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 32 Small‐for‐gestational age.
1.33. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 33 Birthweight (kg).
1.34. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 34 Birthweight (g).
| Birthweight (g) | ||
|---|---|---|
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 3071.50 (2978.75, 3237.75) | Median (IQR): 3295.50 (2964.25, 3566.75) |
1.35. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 35 Head circumference (cm).
| Head circumference (cm) | ||
|---|---|---|
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 33.50 (32.48, 34.63) | Median (IQR): 33.50 (32.25, 34.75) |
1.36. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 36 Length (cm).
| Length (cm) | ||
|---|---|---|
| Study | Metformin (N=8) | Insulin (N=6) |
| Hickman 2013 | Median (IQR): 49.00 (48.07, 50.53) | Median (IQR): 49.50 (48.45, 52.25) |
1.44. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 44 Relevant biomarker (cord C peptide).
| Relevant biomarker (cord C peptide) | ||
|---|---|---|
| Study | Metformin (N=6) | Insulin (N=4) |
| Hickman 2013 | Median (IQR): 1.25 (0.92, 1.65) | Median (IQR): 3.95 (2.78, 5.13) |
There were no clear differences between the metformin and insulin groups for shoulder dystocia (RR 4.67, 95% CI 0.21 to 102.47; RCTs = 2; participants = 35; Analysis 1.37) in Hickman 2013 and Refuerzo 2015, or across Ainuddin 2015 and Hickman 2013 in the proportion of infants with clavicle fracture or birth injury (RR 0.94, 95% CI 0.14 to 6.57; RCTs = 2; participants = 220; Analysis 1.38). However, infants in the metformin group were less likely to have hyperbilirubinaemia or jaundice (RR 0.44, 95% CI 0.24 to 0.81; RCTs = 2; participants = 220; Analysis 1.41), or sepsis (RR 0.26, 95% CI 0.08 to 0.81; RCTs = 2; participants = 220; Analysis 1.42).
1.37. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 37 Shoulder dystocia.
1.38. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 38 Bone fracture (birth injury/birth trauma with clavicle fracture).
1.41. Analysis.
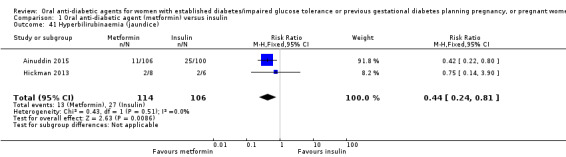
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 41 Hyperbilirubinaemia (jaundice).
1.42. Analysis.
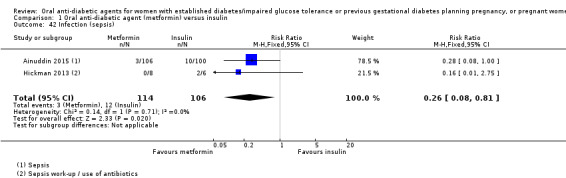
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 42 Infection (sepsis).
Meta‐analysis of data from Ainuddin 2015; Hickman 2013 and Refuerzo 2015 showed no clear difference between groups for respiratory distress syndrome (RR 0.52, 95% CI 0.24 to 1.13; RCTs = 3; participants = 241; Analysis 1.39). Infants born to mothers in the metformin group were less likely to have hypoglycaemia (RR 0.34, 95% CI 0.18 to 0.62; RCTs = 3; participants = 241; very low‐quality evidence; Analysis 1.40). However, in Ainuddin 2015, infants born to women in the metformin group had, on average, higher blood glucose at birth (MD 3.57 mg/dL, 95% CI 0.26 to 6.88; RCTs = 1; participants = 206; Analysis 1.43).
1.39. Analysis.

Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 39 Respiratory distress syndrome.
1.40. Analysis.
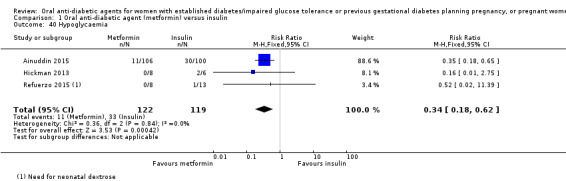
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 40 Hypoglycaemia.
1.43. Analysis.
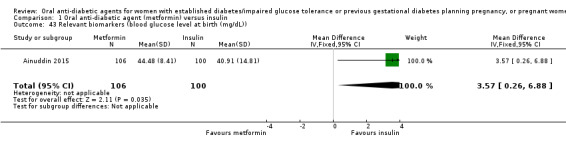
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 43 Relevant biomarkers (blood glucose level at birth (mg/dL)).
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on: ponderal index; adiposity; nerve palsy; hypocalcaemia; or polycythaemia.
Child/adult: secondary outcomes
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on: weight; height; head circumference; adiposity; cardiovascular health; type 1 diabetes; type 2 diabetes; impaired glucose tolerance; employment, education and social status/achievement.
Health service: secondary outcomes
In Hickman 2013 there was no clear difference in antenatal hospital admissions between groups (RR 0.22, 95% CI 0.03 to 1.66; RCTs = 1; participants = 15; Analysis 1.45). Meta‐analysis of data from Ainuddin 2015, Hickman 2013 and Refuerzo 2015 showed that infants born to mothers who received metformin compared with insulin were less likely to be admitted to the neonatal intensive care unit (RR 0.37, 95% CI 0.27 to 0.52; RCTs = 3; participants = 241; Analysis 1.46). In Refuerzo 2015 there was no clear difference reported in length of postnatal stay for the infants (reported as median and range) (Analysis 1.47). Hickman 2013 reported that the three neonates who were admitted to the neonatal intensive care unit in the insulin group had lengths of stay of: five days, three to four weeks and 46 days.
1.45. Analysis.
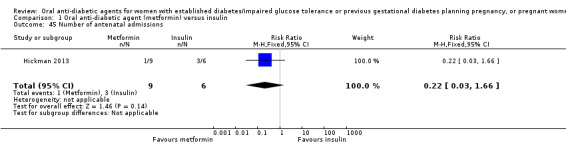
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 45 Number of antenatal admissions.
1.46. Analysis.
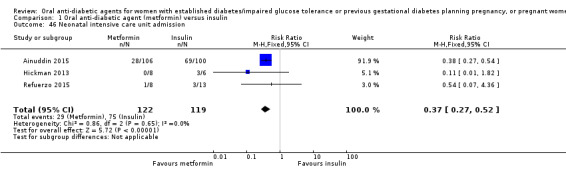
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 46 Neonatal intensive care unit admission.
1.47. Analysis.
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 47 Length of postnatal stay (baby) (days).
| Length of postnatal stay (baby) (days) | |||
|---|---|---|---|
| Study | Metformin | Insulin | P value |
| Refuerzo 2015 | Median (range): 3 (1‐8) N=8 |
Median (range): 2 (1‐12) N=13 |
0.697 |
In Ainuddin 2015, total costs of treatment throughout pregnancy were, on average, lower for women in the metformin group compared with those in the insulin group (MD ‐65.30 USD, 95% CI ‐77.92 to ‐52.68; RCTs = 1; participants = 206; Analysis 1.48).
1.48. Analysis.
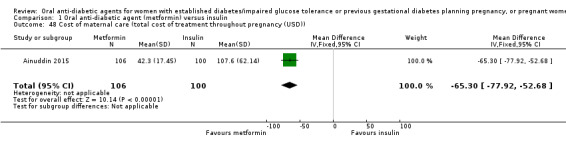
Comparison 1 Oral anti‐diabetic agent (metformin) versus insulin, Outcome 48 Cost of maternal care (total cost of treatment throughout pregnancy (USD)).
Ainuddin 2015, Hickman 2013 and Refuerzo 2015 did not report on: number of hospital or health professional visits; length of antenatal stay; length of postnatal stay (mother); costs to families associated with the management provided; costs associated with the intervention; or cost of offspring care.
Subgroup and sensitivity analyses
We were not able to conduct the planned subgroup analyses or sensitivity analyses due to a paucity of data.
Discussion
Summary of main results
The increasing prevalence and effects of established diabetes on maternal and infant health outcomes highlight the importance of evaluating evidence‐based management for women with established diabetes, impaired glucose tolerance or previous gestational diabetes both preconceptionally and during pregnancy. Additionally, women with impaired glucose tolerance or gestational diabetes in a previous pregnancy represent a group of women with relatively increased insulin resistance, and are at risk of developing type 2 diabetes. Appropriate management of these women is unclear.
Six trials were eligible for inclusion in the review (Ainuddin 2015; Beyuo 2015; Hickman 2013; Ibrahim 2014; Notelovitz 1971; Refuerzo 2015), but only three trials contributed data and are included in the analyses (Ainuddin 2015; Hickman 2013; Refuerzo 2015). These three trials provided data for 241 women who had type 2 diabetes diagnosed before, or during, pregnancy, and their infants.
For our primary outcomes there was no clear difference between the metformin and insulin groups across two trials for pre‐eclampsia (very low‐quality evidence) (Ainuddin 2015; Refuerzo 2015), although in the Ainuddin 2015 trial women receiving metformin were less likely to have pregnancy‐induced hypertension (low‐quality evidence). Women taking metformin were less likely to have a caesarean section birth compared with those taking insulin across the three trials (low‐quality evidence) (Ainuddin 2015; Hickman 2013; Refuerzo 2015). In Ainuddin 2015 there was no clear difference between the metformin groups for large‐for‐gestational‐age infants (very low‐quality evidence). There were no perinatal deaths reported in two trials (very low‐quality evidence) (Ainuddin 2015; Hickman 2013).The trials did not report on neonatal mortality, a morbidity composite outcome, or childhood or adulthood neurosensory disability.
For important secondary outcomes, assessed using the GRADE approach, there were no clear differences between groups in the number of women undergoing induction of labour across two trials (very low‐quality evidence) (Hickman 2013; Refuerzo 2015), though neonatal hypoglycaemia was reduced in the metformin group across the three trials (very low‐quality evidence) (Ainuddin 2015; Hickman 2013; Refuerzo 2015). For mothers, perineal trauma, postnatal depression, and postnatal weight retention were not reported, while for infant outcomes, childhood or adulthood adiposity, and childhood or adulthood diabetes, were not reported.
Few other of this review's secondary outcomes were reported, however additional benefits were observed for metformin, with less weight gain in pregnancy reported in two trials (Ainuddin 2015; Hickman 2013), improved adherence in one trial (Ainuddin 2015), more favourable views of treatment in two trials (Ainuddin 2015; Hickman 2013), less neonatal hyperbilirubinaemia and infection in two trials (Ainuddin 2015; Hickman 2013), less neonatal intensive care unit admission in three trials (Ainuddin 2015; Hickman 2013; Refuerzo 2015), and lower costs of maternal care in one trial (Ainuddin 2015); however there were more small‐for‐gestational‐age infants with metformin in one trial (Ainuddin 2015).
Overall completeness and applicability of evidence
Though we included six trials in this review, we were not able to include outcome data from three of them (Beyuo 2015; Ibrahim 2014; Notelovitz 1971), as they had mixed populations (women with gestational diabetes and established diabetes) and did not report data separately for the subset of women relevant to this review. Two of the three trials that were able to provide data were from the USA (Hickman 2013; Refuerzo 2015), and one was from Pakistan (Ainuddin 2015). These three trials all compared an oral anti‐diabetic agent (metformin) with insulin, therefore, currently no data are available regarding the comparative effects of oral anti‐diabetic agents versus no treatment, or different oral anti‐diabetic agents. Similarly, no data are available regarding the effects of oral anti‐diabetic agents for women with established type 1 or 2 diabetes mellitus, impaired glucose tolerance or previous gestational diabetes mellitus planning a pregnancy, as the three trials that contributed data all recruited pregnant women with type 2 diabetes diagnosed prior to pregnancy (or cases of newly diagnosed overt diabetes in pregnancy, or both).
We selected a number of outcomes for quality assessment using the GRADE approach: while three trials reported on caesarean section, perinatal mortality and neonatal hypoglycaemia, only two reported on pre‐eclampsia and induction of labour, and only one on large‐for‐gestational age infants. There were no data for: perineal trauma, postnatal depression, postnatal weight retention, neonatal mortality or morbidity composite; childhood or adulthood neurosensory disability, or childhood or adulthood diabetes.
Quality of the evidence
Risk of bias in the included studies was mixed. One of the trials contributing data was quasi‐randomised and thus at high risk of selection bias. None of the trials that contributed data were able to blind women or the staff providing care and this may have had an impact on some outcomes. It was not clear in the trials whether there were attempts to blind outcome assessors. In one of the trials that contributed data there was high loss to follow‐up (> 20%) (Ainuddin 2015).
We determined the evidence for outcomes assessed using the GRADE approach to be low quality or very low quality. Evidence was downgraded due to design limitations (risk of bias) and/or imprecision due to small sample sizes, low event rates, and/or uncertain effect estimates.
Potential biases in the review process
We took steps to minimise bias in the review process. Data extraction was carried out by two researchers independently and all data were checked. The GRADE approach assessments were made by two people independently and discrepancies resolved by discussion. We acknowledge that we may have missed relevant trials because we did not formerly search trials registers such as ClinicalTrials.gov or the WHO International Clinical Trials Registry Platform (ICTRP), which became mandatory after October 2016, but we will search these registers in the next update.
Agreements and disagreements with other studies or reviews
There is a dearth of evidence on the use of oral anti‐diabetic agents for women with established diabetes, impaired glucose tolerance or previous gestational diabetes who are planning a pregnancy, or pregnant women with pre‐existing diabetes. Current evidence focuses on women with gestational diabetes, and we refer readers to the Cochrane Reviews Brown 2017b and Brown 2016b for assessments of oral anti‐diabetic agents versus no treatment or alternative oral anti‐diabetic agents, and oral anti‐diabetic agents versus insulin, respectively, in women with gestational diabetes.
One recent systematic review assessed short‐ and long‐term outcomes of metformin compared with insulin in pregnancy, and included trials in women with gestational diabetes or type 2 diabetes (Butalia 2017). The review included data from two of the trials that provided outcome data in our review (Hickman 2013; Refuerzo 2015), one trial included in our review from which we did not include data (Ibrahim 2014), and an additional 13 trials (all in women with gestational diabetes). In agreement with the results of our review, Butalia 2017 reported reductions in pregnancy‐induced hypertension, maternal weight gain in pregnancy, neonatal hypoglycaemia, and neonatal intensive care unit admission with metformin versus insulin, and no clear differences between treatments for pre‐eclampsia, glycaemic control, preterm birth and macrosomia. Conversely, Butalia 2017 reported no clear difference between metformin and insulin for caesarean birth (a reduction with metformin was observed in our review) or small‐for‐gestational age infants (an increase with metformin was observed in our review), and a reduction in large‐for‐gestational age infants (no clear difference was observed in our review).The Butalia 2017 review concluded that while metformin appeared to have short‐term benefits, with no observed harms, information on long‐term benefits and harms is limited, and thus it recommended further follow‐up studies prior to use becoming routine. One of the ongoing trials identified in this review (Feig 2011), the Metformin in Women with Type 2 Diabetes in Pregnancy Trial (MiTy Trial), which compares the effectiveness of the addition of metformin to insulin with standard care (insulin plus placebo) in pregnant women with type 2 diabetes, will provide these longer‐term data from its planned MiTy Kids two‐year follow‐up (NCT01832181).
A systematic review of guidelines for the preconception care of women with type 2 diabetes in pregnancy identified that, while all the included guidelines recommended insulin prior to pregnancy to achieve target concentrations of blood glucose, the more recent ones suggested use of metformin as an adjunct or alternative, for example in situations when insulin is refused or a woman develops resistance (Mahmud 2010). This guideline review noted that while "the safety of currently available oral antidiabetic agents (metformin and glyburide) during pregnancy looks promising, the complete safety and efficacy profile during the full term of pregnancy has not yet been established" (Mahmud 2010).
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to evaluate the use of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance or previous gestational diabetes planning a pregnancy or pregnant women with pre‐existing diabetes. Low‐ to very‐low quality evidence suggests possible reductions in pregnancy‐induced hypertension, caesarean section birth and neonatal hypoglycaemia with metformin (an oral anti‐diabetic agent) compared with insulin in pregnant women with type 2 diabetes, and no clear differences in pre‐eclampsia, induction of labour or babies who are large‐for‐gestational age. Therefore, decisions about the use of oral anti‐diabetic agents for these women will probably depend on factors such as women's preferences, available resources, and local or national clinical practice guidelines.
Implications for research.
Despite limited evidence of the effects of oral anti‐diabetic agents for women included in this review, the potential benefits relating to women's acceptability and adherence with oral anti‐diabetic agents suggest that further evidence is required. Large, high‐quality randomised controlled trials are required to evaluate the effects of oral anti‐diabetic agents in women with established diabetes, impaired glucose tolerance, or previous gestational diabetes who are planning a pregnancy and pregnant women with pre‐existing diabetes. In particular, trials could compare oral anti‐diabetic agents with insulin or dietary and lifestyle control, and compare different oral anti‐diabetic agents. Trials could attempt to collect and report on the standard outcomes suggested in this review, such as short‐term maternal and infant outcomes including glycaemic control parameters and women's views, long‐term maternal and infant outcomes, and outcomes relating to the use and costs of health services. We have identified three ongoing studies, and four are awaiting classification. We will consider these in the next update of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 19 October 2017 | Amended | Minor edits to clarify that this review is an update of a review that was first published in 2010 (Tieu 2010). |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 10, 2010
| Date | Event | Description |
|---|---|---|
| 31 October 2016 | New citation required and conclusions have changed | Oral anti‐diabetic agent (metformin) versus insulin (six new trials): all conclusions are new. |
| 31 October 2016 | New search has been performed | Search updated. Six trials have been included (Ainuddin 2015; Beyuo 2015; Hickman 2013; Ibrahim 2014; Notelovitz 1971; Refuerzo 2015). The 'Oral anti‐diabetic agent (metformin) versus insulin' comparison has been added, outcomes revised and methods updated, including two 'Summary of findings' tables (see Differences between protocol and review). One new author (Emily Shepherd) was involved in this update. |
| 10 January 2011 | Amended | Contact details updated. |
| 2 December 2010 | Amended | Corrected affiliation for Suzette Coat and William Hague. |
Acknowledgements
We acknowledge the support from the Cochrane Pregnancy and Childbirth editorial team in Liverpool, and the Australia and New Zealand Satellite of Cochrane Pregnancy and Childbirth (funded by the Australian National Health and Medical Research Council (NHMRC)).
We thank Therese Dowswell from Cochrane Pregnancy and Childbirth who provided support for this update (including duplicate study screening, data extraction and quality assessment; and generation of the 'Summary of findings' tables). Therese Dowswell's contribution to this project was supported by the National Institute for Health Research (NIHR), via Cochrane programme grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
As part of the prepublication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth's international panel of consumers and the Statistical Adviser.
Data and analyses
Comparison 1. Oral anti‐diabetic agent (metformin) versus insulin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypertensive disorders of pregnancy: pre‐eclampsia | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.33, 1.20] |
| 2 Hypertensive disorders of pregnancy: pregnancy‐induced hypertension | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.91] |
| 3 Caesarean section | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.88] |
| 4 Large‐for‐gestational age | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 5 Perinatal mortality | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Miscarriage | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.1 [0.10, 44.40] |
| 7 Induction of labour | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.62, 3.28] |
| 8 Postpartum haemorrhage | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Weight gain in pregnancy (kg) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.57, ‐1.03] |
| 10 Weight gain in pregnancy (kg) | Other data | No numeric data | ||
| 11 Adherence to the intervention (how often did you forget to take treatment?) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Never or rarely | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.14, 1.64] |
| 11.2 2 to 4 times/week | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.28, 0.72] |
| 12 Adherence to the intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 No missed appointments | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] |
| 12.2 Log book completed > 50% | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [0.89, 4.04] |
| 13 Views of the intervention (which medication would you choose in next pregnancy?) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Metformin | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.70 [4.52, 13.14] |
| 13.2 Insulin | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.03, 0.19] |
| 13.3 Not sure | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.29] |
| 14 Views of the intervention (which part of diabetes treatment was easy?) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Doing finger pricks | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Diet control | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.30] |
| 14.3 Drug treatment | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.19, 1.49] |
| 15 Views of the intervention (which part of diabetes treatment was difficult?) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Doing finger pricks | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [2.42, 4.95] |
| 15.2 Diet control | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.84] |
| 15.3 Drug treatment | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.08, 0.26] |
| 16 Views of the intervention (choose same treatment in the future) | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.14 [0.78, 159.58] |
| 17 Adverse effects of the intervention (side effects) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Gastrointestinal side effects resulting in dose limitation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.27 [0.70, 215.04] |
| 17.2 Gastrointestinal side effects resulting in treatment cessation | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Lactic acidosis | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Breastfeeding | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.79, 3.23] |
| 19 Glycaemic control (fasting blood glucose throughout pregnancy (mg/dL)) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.90, 0.92] |
| 20 Glycaemic control (random blood glucose throughout pregnancy (mg/dL)) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐1.63, 1.19] |
| 21 Glycaemic control (change in HbA1c from enrolment to third trimester/birth (%)) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐1.05, 0.09] |
| 22 Glycaemic control (HbA1c < 7% at third trimester/birth) | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.39] |
| 23 Glycaemic control | Other data | No numeric data | ||
| 24 Congenital anomaly (major malformations) | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Stillbirth | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Neonatal mortality | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Gestational age at birth (weeks) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.66, 0.06] |
| 28 Gestational age at birth (weeks) | Other data | No numeric data | ||
| 29 Preterm birth | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.08, 2.30] |
| 30 Apgar score < 7 at 5 minutes | 1 | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Macrosomia (> 4000 g) | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.10] |
| 32 Small‐for‐gestational age | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.49 [2.02, 35.66] |
| 33 Birthweight (kg) | 2 | 227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.29, 0.04] |
| 34 Birthweight (g) | Other data | No numeric data | ||
| 35 Head circumference (cm) | Other data | No numeric data | ||
| 36 Length (cm) | Other data | No numeric data | ||
| 37 Shoulder dystocia | 2 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [0.21, 102.47] |
| 38 Bone fracture (birth injury/birth trauma with clavicle fracture) | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.14, 6.57] |
| 39 Respiratory distress syndrome | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.24, 1.13] |
| 40 Hypoglycaemia | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.18, 0.62] |
| 41 Hyperbilirubinaemia (jaundice) | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.24, 0.81] |
| 42 Infection (sepsis) | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.81] |
| 43 Relevant biomarkers (blood glucose level at birth (mg/dL)) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | 3.57 [0.26, 6.88] |
| 44 Relevant biomarker (cord C peptide) | Other data | No numeric data | ||
| 45 Number of antenatal admissions | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.66] |
| 46 Neonatal intensive care unit admission | 3 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.27, 0.52] |
| 47 Length of postnatal stay (baby) (days) | Other data | No numeric data | ||
| 48 Cost of maternal care (total cost of treatment throughout pregnancy (USD)) | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐65.3 [‐77.92, ‐52.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ainuddin 2015.
| Methods | Quasi‐randomised controlled trial | |
| Participants | 250 women randomised Setting: hospitals affiliated with Dow University of Health Sciences, Karachi, Pakistan from January 2009 to January 2014 Inclusion criteria: women with type 2 diabetes diagnosed prior to pregnancy, and cases of newly diagnosed overt diabetes in pregnancy (IADPSG criteria: fasting blood glucose ≥ 7.0 mmol/L, random blood glucose ≥ 11.1 mmol/L and HbA1c ≥ 6.5%), between 20 and 48 years, with singleton pregnancies beyond the first trimester Exclusion criteria: women with contraindications or intolerance to metformin intake, women diagnosed with GDM, or with type 1 or 2 diabetes already on insulin treatment, fetal anomaly on ultrasound, ruptured membranes in second trimester, any other medical disorder, or diabetes related complications |
|
| Interventions |
Experimental intervention (N = 125 randomised, 106 analysed): metformin Metformin was started at 500 mg daily orally and increased up to 2500 mg in 3 doses as tolerated by the women until glycaemic control was achieved. Target blood glucose concentrations were: fasting blood glucose ≤ 5.5 mmol/L (100 mg/dL), and postprandial blood glucose (1.5 hours post meal) ≤ 7 mmol/L (126 mg/dL). If target blood glucose concentrations were not maintained, even after maximum dose of metformin, insulin was added as supplementary treatment. Control/comparison (N = 100 randomised, 100 analysed): insulin Insulin was prescribed either as a combination of short‐acting and intermediate‐acting human insulin administered as two daily injections given in the morning and in the evening before meals; or as a combination of multiple injections of short‐acting insulin before meals and intermediate‐acting insulin at bed‐time; depending on individuals' requirements to achieve glycaemic targets. Dose was calculated according to body weight – 24‐hour dose calculated using 0.6 units/kg body weight in first trimester, 0.7 units/kg in second trimester, 0.8 units/kg from 28‐32 weeks, 0.9 units/kg from 32‐36 weeks, 1 unit/kg from 36 weeks onwards. All women: were advised of dietary modifications and instructed to eat 3 meals and 3 snacks daily, with diets based on body weight. Women were followed up in antenatal clinics and received iron, calcium, vitamin B12 and folic acid supplements. Women were taught to self‐monitor blood glucose using home monitors, and were advised to maintain a written/electronic record; women who could not self‐monitor had their concentrations tested at each visit, or were admitted to day‐care ward when required. Fasting and 3 postprandial blood glucose concentrations were recorded. Adjustment of drug doses was made at each weekly/fortnightly antenatal visit until 36 weeks, then weekly until term/birth. |
|
| Outcomes | Review outcomes reported in manuscript: pregnancy‐induced hypertension; pre‐eclampsia; caesarean section; large‐for‐gestational age*; perinatal mortality; use of pharmacotherapy (need for supplementary insulin); stillbirth; neonatal mortality; small‐for‐gestational age*; bone fracture (birth trauma with clavicle fracture); respiratory distress syndrome; infection; hypoglycaemia; hyperbilirubinaemia (jaundice); NICU admission; weight gain in pregnancy; glycaemic control during/at end of intervention (mean fasting and random blood glucose); adherence to the intervention (measures of treatment compliance); views of intervention (measures of treatment acceptability reported); gestational age at birth; birthweight; neonatal biomarker changes associated with the intervention (mean blood glucose at birth); costs of maternal care (total cost of treatment throughout pregnancy). *Unclear whether customised birthweight charts were used. |
|
| Notes |
NCT01855763 Funding: not reported Conflicts of interest: authors reported that there were no conflicts. Results for the group randomised to metformin were reported separately for those women who remained on metformin alone and those women who subsequently received insulin in addition to metformin. Overall results according to randomisation group (intention‐to‐treat) were not reported. In our data analyses we have reported results for groups as randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised, with odd number assignment to metformin treatment and even number assignment for insulin treatment. |
| Allocation concealment (selection bias) | High risk | See above. Allocation could be anticipated at the point of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: “open labelled” and “Blinding was no possible because of different routes of administration of drugs”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above; though blinding of outcome assessment not specifically detailed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 106/125 in the metformin group were analysed (13 lost to follow‐up/delivered elsewhere; 6 discontinued treatment due to side effects). 100/125 in the insulin group were analysed (20 lost to follow‐up/delivered elsewhere; 5 non‐compliant). Overall, almost 20% of women were excluded from analyses. In their analyses, the metformin group was separated into metformin alone, and metformin plus insulin; these groups have been combined for the purpose of this review. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes reported did not match those specified at trial registration. For some outcomes, data not provided, e.g. quote: “Fasting, postprandial blood glucose levels and HbA1C levels were statistically comparable”. |
| Other bias | Unclear risk | Women in the metformin alone group were younger, and had lower parity. |
Beyuo 2015.
| Methods | Randomised controlled trial | |
| Participants | 104 women randomised Setting: Maternity Unit and the Diabetes Centre of the Korle Bu Teaching Hospital, Ghana, from January 2013 to October 2013 Inclusion criteria: women aged 18 to 45 years who were pregnant with a singleton fetus at gestational age 20 to 30 weeks, diagnosed with type 2 diabetes mellitus or GDM, who met the hospital's criteria for starting insulin, with unsatisfactory glycaemic control despite diet and exercise management Exclusion criteria: women with type 1 or 2 diabetes mellitus who previously failed to achieve glycaemic control on metformin monotherapy, women with allergies to metformin |
|
| Interventions |
Experimental intervention (N = 52 randomised, 43 analysed (11 with type 2 diabetes)): metformin Women received metformin at a starting dose of 500 mg once a day, which was increased gradually over 2 weeks; the maximum daily dose was 2500 mg per day. Insulin was added if targets could not be reached on metformin alone. Control/comparison (N = 52, 40 analysed (17 with type 2 diabetes)): insulin Women were prescribed both soluble insulin and premixed insulin (no brand restriction) administered subcutaneously in the deltoid region. Total daily dose at initiation was calculated for most women as 0.3 IU/kg body weight; women admitted with high blood glucose and managed on a sliding scale with soluble insulin had their starting doses based on total daily requirement. The daily dose was divided into 2, with 2/3 of the dose being given in the morning 30 minutes before breakfast, and 1/3 given in the evening 30 minutes before supper. The total dose was titrated for each woman to achieve the glycaemic targets. Few women combined both soluble insulin administered 3 times/day before meals with premixed insulin on a regular basis to achieve glycaemic control targets. Women who did not achieve glycaemic targets on their outpatient doses after 2 attempts at titration were admitted and treated with soluble insulin to determine their new requirements. All women: treatment targets were: fasting blood sugar < 5.5 mmol/L and 2‐hour postprandial glucose < 7.0 mmol/L (as recommended by Australian Diabetes in Pregnancy Society) |
|
| Outcomes | Review outcomes reported in manuscript: use of pharmacotherapy (need for supplemental insulin); glycaemic control during/at end of intervention (fasting blood glucose; 1‐hour postprandial blood glucose; 2‐hour postprandial blood glucose) | |
| Notes | ACTRN12614000942651. Trial was registered retrospectively "to due financial constraints". Funding: the trialists reported that there was no source of funding. Conflicts of interest: the authors declared that there were no conflicts. Also included in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. 11/43 women in the metformin group and 17/40 in the insulin group had type 2 diabetes mellitus; results were not reported separately for these women, and thus no data from the trial could be included in this review. The review authors contacted the trialists on 8 November 2016 regarding the availability of data for the subset of women with type 2 diabetes. We received a reply on 9 November 2016; the trialists have agreed to provided these data in due course. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For a set of four patients seen at the clinic for the first time, they were made to ballot by picking randomly one paper with an inscription each from an opaque envelope. This assigned participants to one of the two treatment group [sic]... The sequence of picking was in the order in which they reported to the clinic; "first to report, first to pick"." |
| Allocation concealment (selection bias) | Low risk | As above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "open‐label" and "The lack of blinding is a limitation of this study... This could have led to over estimation of the effect of metformin". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above; trial reported as "open‐label" and no specific mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the metformin group: 1 woman delivered outside the hospital; 3 were lost to follow‐up; 1 withdrew consent. In the insulin group: 6 delivered outside the hospital; 5 were lost to follow‐up; 1 discharged herself from the clinic against medical advice. 47/52 women allocated to metformin (90%) and 40/52 in the insulin group (76%) completed the study and were analysed; attrition was higher in the insulin group. |
| Selective reporting (reporting bias) | Unclear risk | The publication only reported on glycaemic control, though the measurement of additional outcomes was reported. Quotes: "Secondary outcome measures included GBF, 1HPG, maternal weight gain, pregnant outcome and feto‐neonatal outcomes. Only the glycaemic control is discussed in this publication" and "The dose of metformin or insulin required .... and peri‐partum events like gestational age at delivery, type of delivery, fetal birth weight, and Neonatal Intensive Care Unit (NICU) admissions were retrieved from patients notes and analysed. All patients were weighed". |
| Other bias | Unclear risk | Groups were comparable for most baseline characteristics; women in the metformin group were recruited at a higher gestational age (quote: "There was, however, a significant difference in the gestational age at enrolment with the metformin group being recruited at a higher gestational age, p = 0.017"). |
Hickman 2013.
| Methods | Randomised controlled trial | |
| Participants | 31 women randomised Setting: University of North Carolina Women's Hospital, North Carolina, USA from July 2008 to March 2010; and at WakeMed Hospital, Raleigh, North Carolina, USA, from January 2009 to December 2009 Inclusion criteria: pregnant women who presented for prenatal care prior to 20 weeks' gestation who had a diagnosis of type 2 diabetes controlled on an oral hypoglycaemic agent prior to pregnancy; women with a diagnosis of A2 GDM prior to 20 weeks' gestation (2 or more abnormal values on 100 g 3‐hour OGTT using National Diabetes Data Group criteria) with failure to achieve adequate glycaemic control with dietary modification Exclusion criteria: women on insulin prior to pregnancy, under 18 years of age, who did not speak English or Spanish, carrying a triplet or higher‐order multiple pregnancy or known fetal anomaly, with evidence of end organ damage, or a major medical comorbidity in addition to diabetes, or contraindication to metformin (hepatic or renal compromise, allergy, prior adverse reaction, history of diabetes ketoacidosis) |
|
| Interventions |
Experimental intervention (N = 15 randomised; 14 analysed): metformin Women received instructions on proper administration of metformin, with morning dose taken with breakfast, and evening dose taken with dinner; women were started on 500 mg once or twice a day; those taking metformin prior to pregnancy continued on the same dose, while those taking another agent were converted to metformin. Women who failed to achieve adequate glycaemic control with maximal daily dose (2500 mg) had regular or NPH insulin added as needed. Control/comparison (N = 16 randomised; 14 analysed): insulin Women received conventional weight‐based insulin regimen (twice‐daily regular and NPH insulin). Women were taught insulin administration; a total starting dose of 0.7 U/kg/day was divided, with 2/3 taken in the morning (2/3 NPH and 1/3 regular) and 1/3 in the evening (1/2 NPH and 1/2 regular). Any women on oral agents prior to pregnancy discontinued those agents. All women: received nutrition counselling regarding a proper diet for people with diabetes and attended an education class where they were instructed on identifying, preventing and treating hypoglycaemia; all women received a glucose meter, were taught the methods of capillary blood glucose monitoring, and were instructed to perform and document fasting and 1‐hour postprandial concentrations; women received a glucagon kit for hypoglycaemia treatment. Women who did not achieve optimal glycaemic control (> 50% of the 1‐hour postprandial values > 130 mg/dL) had their insulin or metformin dose titrated. |
|
| Outcomes | Review outcomes reported for women: caesarean section, perinatal mortality, miscarriage, induction of labour, postpartum haemorrhage, weight gain in pregnancy, adherence to the intervention, views of the intervention, breastfeeding, glycaemic control, antenatal admissions and cost of care. Review outcomes for infants reported: congenital anomaly, stillbirth, neonatal mortality, gestational age at birth, preterm birth, Apgar score < 7 at 5 minutes, macrosomia, birthweight, head circumference, length, shoulder dystocia, birth trauma, respiratory distress syndrome, hypoglycaemia, jaundice, infection, cord c peptide, NICU admission and length of postnatal stay | |
| Notes |
NCT00835861 Funding: the Bowes‐Cefalo Young Researcher Award Grant Conflicts of interest: not reported Trial sample size was originally 230 women; "Three months into recruitment, it became apparent we would not reach our target enrolment within the time period our funding allowed". Recuited for a fixed 2‐year period. Also included in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. 9/14 women in the metformin group and 6/14 in the insulin group had known pre‐existing diabetes; in the trial reports results were not reported separately for these women. The review authors contacted the trialists on 10 November 2016 regarding availability of data for the subset of women with pre‐existing diabetes. We received a reply on 17 November 2016; the trialists provided additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme" |
| Allocation concealment (selection bias) | Low risk | Quote: "a nurse not involved in the study prepared opaque, sequentially numbered envelopes containing group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Given the nature of the two treatments, neither the patients or providers were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above and quote: "Maternal and neonatal information was abstracted from the medical record by the principal investigator...or a trained study nurse (WakeMed)". No specific mention of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/16 women in the insulin group did not receive the intervention (1 was judged to have inadequate mental capacity; 1 was diagnosed with fetal death at 7 weeks); 1/15 women in the metformin group did not receive the intervention (withdrew consent); therefore 28/31 (90%) women were included in analyses (with 15 women included in the analyses in this review). |
| Selective reporting (reporting bias) | Unclear risk | Though a trial registration number was provided, the outcomes (as reported in trial report) were only detailed in full in the 2014 update of the registration; unclear whether outcomes, outcome definitions, time points for measurement etc. were all prespecified. |
| Other bias | Unclear risk | Most baseline characteristics were reported to be comparable between groups; "however, women in the metformin group were older (p < 0.01)". With such a small sample size, it is difficult to assess comparability of groups. |
Ibrahim 2014.
| Methods | Randomised controlled trial | |
| Participants | 90 women randomised Setting: Ain Shams University Maternity Hospital, Egypt, from August 2011 to April 2012 Inclusion criteria: pregnant women with GDM or pre‐existing diabetes mellitus, between 20 and 34 weeks' gestation, who showed insulin resistance (defined as poor glycaemic control at a daily dose of ≥ 1.12 units/kg; with poor glycaemic control defined as fasting blood glucose > 95 mg/dL and/or 2‐hour postprandial blood glucose > 120 mg/dL) Exclusion criteria: women with type 1 diabetes mellitus, with secondary diabetes, or with liver or renal impairment |
|
| Interventions |
All women: for women with newly diagnosed diabetes, or who had not started on insulin therapy at the time of admission, insulin was started at a daily dose of 0.7 IU/kg in the second trimester, or 0.8 IU/kg at the third trimester. Insulin was increased in women admitted for poor glycaemic control, and raised at a rate of 1 IU for every 10 mg/dL higher than the target blood glucose concentration (target blood glucose: fasting blood glucose 60 mg/dL to 95 mg/dL; 2‐hour postprandial blood glucose < 120 mg/dL). The total dose of insulin was given in 2 doses of a mixture of regular insulin and neutral protamine Hagedorn insulin (ratio 3:7; 100 IU/mL; 2/3 in the morning and 1/3 in the evening). Only women who were admitted to hospital for poor glycaemic control after reaching a daily dose equivalent to or exceeding the threshold (1.12 IU/kg) were recruited. Experimental intervention (N = 46): metformin and insulin Women received oral metformin without increasing the insulin dose. Women received 1500 mg, divided into 3 doses, taken with meals, in addition to insulin at the last dose reached. If the target blood glucose values were not attained (after 5 days) the metformin dose was raised to 2000 mg per day for 5 days. If after 10 days women had not reached target blood glucose concentrations, they were switched to the conventional insulin dose‐raising regimen. Control/comparison (N = 44): insulin Women had their insulin dose increased (rate as above). All women: women who showed proper glycaemic control were discharged from hospital and followed until birth, with glycaemic control checked fortnightly until birth; if at any time women showed poor control, they were admitted and had their insulin dose increased. |
|
| Outcomes | Review outcomes reported: caesarean section; maternal hypoglycaemia; congential anomaly; stillbirth; macrosomia; respiratory distress syndrome; neonatal hypoglycaemia; NICU admission; glycaemic control ('proper' glycaemic control); gestational age at birth; birthweight; readmission for poor glycaemic control | |
| Notes |
NCT01915550 Funding: funded by the authors Conflicts of interest: authors reported that there were no conflicts This trial is awaiting classification in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. 39/90 women had GDM and 51/90 had pre‐existing diabetes mellitus; results not reported separately for these women, and thus no data from the trial included in this review. Review authors contacted the trialists on 8 November 2016 regarding data availability for subset of women with type 2 diabetes. Awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer‐generated randomization system". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "To minimize the risk of selection bias, the allocation table was checked after applying eligibility criteria on recruited women". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No detail provided; considered unfeasible/unlikely in view of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Women who chose to switch from the allocated group to the other one were cancelled and not included in the final statistical analysis. Per‐protocol treatment analysis was performed". 8/90 women were lost to follow‐up (3/46 and 15/44). 43/46 women in the metformin group and 39/44 in the insulin group were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration only prespecified primary outcome; no trial protocol available to assess further for selective reporting. Though a number of relevant outcomes reported, many expected outcomes were not (e.g. pre‐eclampsia; large‐for‐gestational age). |
| Other bias | Unclear risk | Baseline characteristics not presented by group; it was reported that there were "no significant differences between women of both groups" regarding a range of characteristics. |
Notelovitz 1971.
| Methods | Randomised parallel trial | |
| Participants | 207 women were included (unclear if this was the total number randomised) Setting: Department of Obstetrics and Gynaecology, Addington Hospital, Durban, South Africa (study dates not reported) Inclusion criteria: known diabetics and women with glycosuria or family or obstetrical histories suggestive of diabetes were screened (100 g OGTT, with 2‐hour blood glucose ≥ 140 mg/100 mL), whose duration of pregnancy would allow at least 6 consecutive weeks of treatment Exclusion criteria: "The patients who qualified for the series had their treatment selected on a random‐sample basis, with the exception of established diabetics already on specific therapy". (Somewhat unclear whether this was an exclusion criterion, or whether these women were included in the trial, but not randomised.) |
|
| Interventions |
Experimental intervention 1 (N = 58 analysed): chlorpropamide Experimental intervention 2 (N = 46): tolbutamide Control/comparison 1 (N = 47 analysed): insulin Control/comparison 2 (N = 56 analysed): diet restriction alone |
|
| Outcomes | Review outcomes reported: perinatal mortality; congenital anomaly; stillbirth; neonatal mortality; Apgar score (< 5 or 5 to 7); neonatal hypoglycaemia; glycaemic control (good, fair, poor) | |
| Notes | Funding: "thank Pfizer Laboratories Ltd for financial support" Conflicts of interest: not reported Trial included in Cochrane Reviews 'Insulin for the treatment of women with gestational diabetes' and 'Insulin for the treatment of women with gestational diabetes'. It is unclear what proportion of women in the trial had GDM or type 2 diabetes mellitus; results not reported separately for these women, and thus no data from the trial included in this review. Review authors contacted the trialists on 8 November 2016 regarding whether data were available for the subset of women with type 2 diabetes. Awaiting response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients who qualified for the series had their treatment selected on a random‐sample basis". |
| Allocation concealment (selection bias) | Unclear risk | No further details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details provided; considered unfeasible/unlikely in view of the interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It appears that the analyses were not intention‐to‐treat, and rather that "In the final analysis" women were analysed in the group in which they "completed treatment" or "were treated with for the greater part of their pregnancy". Later it was reported that the insulin group was 'loaded' with women who did not respond to other forms of therapy and were subsequently "treated with insulin for the greater part of the pregnancy". |
| Selective reporting (reporting bias) | High risk | It appears that outcomes were not (clearly) prespecified; some outcomes appear to be under‐reported (such as neonatal hypoglycaemia "routine blood‐sugar estimations ... were not always obtained ... and the incidence of neonatal hypoglycaemia could therefore not be accurately assessed"... "Sympomatic hypoglycaemia ... was not found to be any more common."). |
| Other bias | Unclear risk | Limited methodological detail provided; limited detail provided regarding baseline characteristics of the women |
Refuerzo 2015.
| Methods | Randomised controlled trial | |
| Participants | 25 women randomised Setting: University of Texas Health Science Centers at Houston and Brownsville, USA, from September 2009 to August 2011 Inclusion criteria: pregnant women at < 20 weeks' gestation, with a self‐reported history of type 2 diabetes mellitus with treatment of either diet control or oral hypoglycaemic agents before pregnancy, with type 2 diabetes for < 10 years Exclusion criteria: women who were on insulin before pregnancy, had multiple gestations, type 1 diabetes mellitus, known fetal chromosomal or structural defects or contraindications to the use of metformin including renal disease, liver disease, recent myocardial infarction or sepsis, or HbA1c > 9% |
|
| Interventions |
Experimental intervention (N = 11 randomised; 8 analysed): metformin 500 mg metformin daily was initiated, and women returned for routine prenatal visits weekly; if > 50% of glucose values were abnormal, metformin was increased to 500 mg twice a day; the metformin was increased by 500 mg as needed for a maximum dose of 2500 mg a day. Once glycaemic control was achieved, the women were followed up every 2 weeks. Women who required > 2500 mg without achieving glycaemic control were considered to have failed metformin therapy and were started on insulin, but continued on metformin. Women receiving metformin before pregnancy resumed the dose they were on at the start of pregnancy, and increased as above. Control/comparison (N = 14; 13 analysed): insulin Insulin regimen was based on maternal weight and gestational age: first trimester: 0.7 units/kg/day; second trimester: 0.8 units/kg/day; third trimester 0.9‐1.0 units/kg/day. The total insulin dose was divided into morning dose (2/3 NPH and 1/3 regular insulin), and evening dose (1/2 NPH and 1/2 regular insulin). Insulin was increased or decreased 10% to 20% according to self‐monitored blood glucose values. Women receiving insulin before pregnancy resumed or switched to an equivalent regimen as above. All women: all women received prenatal care through a high‐risk diabetic clinic; at their initial prenatal visit, an American Diabetes Association diet was recommended based on weight, and instructions were provided on self‐monitoring of blood glucose (> 95 mg/dL fasting, and 120 mg/dL postprandial considered abnormal); instructions for exercise were also provided. |
|
| Outcomes | Review outcomes reported: pre‐eclampsia; caesarean section; induction of labour; use of additional pharmacotherapy; preterm birth; macrosomia; shoulder dystocia; respiratory distress syndrome; hypoglycaemia (reported need for dextrose); NICU admission; glycaemic control during/at end of intervention (HbA1c < 7%); gestational age at birth; birthweight; length of stay (baby) | |
| Notes |
NCT00678080 Funding: supported by Center for Clinical and Translational Sciences, funded by National Institutes of Health Clinical and Translational Award UL1000371. Conflicts of interest: not reported Due to strict inclusion and exclusion criteria there were limitations in enrolment, and the trial did not reach the anticipated sample size (N = 50 women per group planned); thus the authors employed a Bayesian analysis “typically used to determine the effects of treatment in comparison studies with small sample sizes”. The low recruitment rate and small sample size meant that the study was insufficiently powered to detect differences between groups for most outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Women were randomly assigned to either metformin or insulin by the central investigational drug pharmacy at UT Health in Houston, TX”. |
| Allocation concealment (selection bias) | Low risk | As above, and quote: “This randomization was conducted independent of the medication the participant was receiving before the onset of pregnancy”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “open‐label” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | As above; though blinding of outcome assessment was not specifically detailed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/11 women in the metformin group were withdrawn/excluded (1 was lost; for 2 the physician started insulin) and 1/14 in the insulin group was withdrawn (changed her mind); 84% were analysed overall, with an already small sample size. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as per trial registration; no evidence of selecting outcome reporting. |
| Other bias | Unclear risk | Baseline characteristics reported to be 'similar'; though difficult to determine with small numbers (e.g. more morbidly obese women in the insulin group: 38.5% versus 12.5%). |
Abbreviations
1HPG: 1‐hour plasma glucose GBF: gastric blood flow GDM: gestational diabetes mellitus HbA1c: glycated haemoglobin IADPSG: International Association of the Diabetes and Pregnancy Study Groups NICU: neonatal intensive care unit NPH: neutral protamine Hagedorn OGTT: oral glucose tolerance test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anjalakshi 2007 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Bertini 2005 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Carlsen 2007 | Study evaluated treatment for women with PCOS. |
| Casey 2015 | Study evaluated treatment for women with GDM (may be eligible for inclusion in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Corrado 2011 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Dietary supplementation with myo‐inositol in women during pregnancy for treating gestational diabetes'). |
| George 2015 | Study evaluated treatment for women with GDM (may be eligible for inclusion in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Golladay 2005 | Study is evaluating treatment for women with GDM (ongoing study in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Hague 2003 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Hague 2010 | Planned trial assessing metformin for women with previous GDM. Personal communication: trial was not undertaken. |
| Langer 2000b | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Martinez 2010 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Moore 2005 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Moore 2007 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Mukhopadhyay 2012 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Myers 2013 | Study evaluated treatment for women with 'mild' GDM (may be eligible for inclusion in Cochrane Review 'Interventions for pregnant women with hyperglycaemia not meeting gestational diabetes and type 2 diabetes diagnostic criteria'). |
| Niromanesh 2012 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Rowan 2008b | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Singh 2011 | Study is evaluating treatment for women with GDM (ongoing study in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Vanky 2004 | Study evaluated treatment for pregnant women with PCOS. |
| Vanky 2005 | Study evaluated treatment for pregnant women with PCOS. |
| Wali 2012 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
| Zanganeh 2010 | Study evaluated treatment for women with GDM (included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'). |
Abbreviations
GDM: gestational diabetes mellitus PCOS: polycystic ovary syndrome
Characteristics of studies awaiting assessment [ordered by study ID]
Coiner 2014.
| Methods | Randomised controlled trial |
| Participants | 32 women with diabetes in pregnancy (who had undergone amniocentesis for fetal lung maturity studies) |
| Interventions | 4 groups: metformin versus glyburide versus insulin versus metformin and insulin. Additional control group: non‐diabetic mothers |
| Outcomes | Reported in abstract: concentrations of insulin, glucose, and adiponectin |
| Notes | Published to date as abstract only. Review authors contacted the trialists on 8 November 2016. Awaiting response. |
Hutchinson 2008.
| Methods | Randomised controlled trial |
| Participants | 172 women with GDM or type 2 diabetes not previously requiring insulin |
| Interventions | Combined glyburide and metformin versus insulin. Women monitored their glucose concentrations at home and reported weekly; medication changes were made for optimal glycaemic control; women were seen twice weekly after 28 weeks, in addition to routine obstetric care. |
| Outcomes | Reported in abstract: gestational age at birth; birthweight; cord glucose, fructosamine, HbA1c; neonatal 1‐hour glucose; NICU admissions for hypoglycaemia; infant length of stay |
| Notes |
NCT00371306 Published to date as abstract only. Review authors contacted the trialists on 8 November 2016. Received a response 9 November 2016 noting that there was never a full manuscript for this trial. Included in the Cochrane Review 'Insulin for the treatment of women with gestational diabetes' (no outcome data incorporated in review). |
Reyes‐Munoz 2014.
| Methods | Randomised controlled trial |
| Participants | 58 pregnant women with singleton pregnancies at high risk of developing GDM (3 or more of the following criteria: > 25 years, BMI > 27 kg/m2, history of infertility, polycystic ovary syndrome, medical history of GDM, history of macrosomic, history of diabetes in first degree, or known impaired glucose metabolism). |
| Interventions | Medical nutrition therapy plus metformin versus medical nutrition therapy without metformin |
| Outcomes | Reported in abstract: GDM |
| Notes |
NCT01675310 Published to date as abstract only. Review authors contacted the trialists on 8 November 2016. Awaiting response. |
Waheed 2013.
| Methods | Randomised controlled trial |
| Participants | 68 women were randomised Setting: Department of Obstetrics and Gynaecology, Maternal and Child Health Centre, Pakistan Institute of Medical Sciences, Islamabad, Pakistan, from May 2010 to January 2011 Inclusion criteria: pregnant women with diabetes, with blood sugar > 100 mg/dl and random blood sugar > 140 mg/dl, beyond 14 weeks' gestation Exclusion criteria: women with renal and hepatic impairment or type 1 diabetes |
| Interventions |
Experimental intervention (N = 34 randomised): metformin Women received a starting dose of 500 mg metformin once daily, increased up to 1500 mg, if needed to achieve glycaemic control. Glycaemic profile was repeated after 1 month and at term to check control of blood sugar. Control/comparison (N = 34 randomised): insulin No details provided All women: efficacy was measured in terms of glycaemic control: term fasting blood sugar between 63 mg/dL and 100 mg/dL, random blood sugar < 140 mg/dL and HbA1c < 6.1%. |
| Outcomes | Review outcomes reported: glycaemic control during/at end of intervention (fasting blood glucose, random blood glucose, HbA1c) |
| Notes | Funding: not reported Conflicts of interest: not reported Included in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. Manuscript Introduction focuses on GDM, however it is not entirely clear in the Methodology section which women were recruited, "All pregnant women with diabetes ...". Review authors contacted the trialists on 8 November 2016 regarding whether the women randomised included a subset of women with pre‐existing diabetes. Awaiting response. |
Abbreviations
BMI: body mass index GDM: gestational diabetes mellitus HbA1c: glycated haemoglobin NICU: neonatal intensive care unit
Characteristics of ongoing studies [ordered by study ID]
Feig 2011.
| Trial name or title | Metformin in women with type 2 diabetes in pregnancy trial (MiTy) |
| Methods | Randomised controlled trial Funding: the trial is funded by the Canadian Institute of Health Research MOP 106678. The metformin and placebo tablets have been donated by Apotex Inc. |
| Participants | Location: 21 centres in Canada and 1 centre in Australia Inclusion criteria: pregnant women with a singleton fetus, with type 2 diabetes, between 18 to 45 years of age, currently on insulin, with a gestational age of 6 + 0 to 22 + 6 weeks. Women are eligible if they had undiagnosed type 2 diabetes prior to 20 weeks (fasting glucose concentrations ≥ 7.0 mmol/L, HbA1c values of ≥ 0.065 (48 mmol/mol) or a 2‐hour ≥ 11.1 mmol/L on a 75 g OGTT). Exclusion criteria: women diagnosed with type 2 diabetes after 20 weeks' gestation; women with type 1 diabetes; a known intolerance to metformin; current, significant gastrointestinal problems; active Crohn's or colitis; acute or chronic metabolic acidosis; a history of diabetic ketoacidosis or lactic acidosis; with excessive alcohol intake; congestive heart failure; contraindications to metformin (renal insufficiency, shock or sepsis, previous hypersensitivity); with a fetus with a known potentially lethal anomaly; with higher order pregnancies; or with prior trial participation. |
| Interventions | Eligible women will be randomised to receive either metformin (provided in 500 mg tablets) or placebo (identical appearance, taste, labelling and expiry dates, dispensed and administered in the same manner), to be added to their usual insulin regimen, from the morning after randomisation until birth. |
| Outcomes | Primary outcome: composite defined as the occurrence of 1 or more of the following: pregnancy loss, preterm birth, birth injury, moderate/severe respiratory distress, neonatal hypoglycaemia, and NICU admission > 24 hours Secondary outcomes: individual components of the composite; large‐for‐gestational‐age infants; congenital anomalies; cord blood gas pH < 7.0; hyperinsulinaemia as measured by elevated cord blood C peptide > 1.7 μg/L; sepsis; hyperbilirubinaemia; shoulder dystocia; fetal fat mass as measured by neonatal anthropometric analysis; maternal weight gain; maternal insulin doses; maternal glycaemic control (HbA1c and capillary glucose measurements); maternal hypoglycaemia defined as mild (< 3.6 mmol/L (65 mg/dL), symptomatic and asymptomatic or requiring treatment), or severe (loss of consciousness or confusion requiring assistance); pre‐eclampsia, or gestational hypertension, or both; number of hospitalisations prior to admission for birth; duration of hospital stays for the mother prior to admission for birth and associated with birth; caesarean birth; duration of hospital stay for the infant |
| Starting date | May 2011. Sample size of 500 is planned. Estimated completion date: June 2018 |
| Contact information | Denice Feig, MD, Mount Sinai Hospital, Canada d.feig@utoronto.ca |
| Notes | NCT01353391 |
Sheizaf 2006.
| Trial name or title | Metformin for the treatment of diabetes in pregnancy |
| Methods | Randomised controlled trial Funding: not stated |
| Participants | Location: Israel Inclusion criteria: pregnant, diagnosed with GDM or type 2 diabetes, singleton pregnancy Exclusion criteria: women with diabetic nephropathy or proliferative retinopathy, or unable to swallow tablets |
| Interventions | Metformin, comparison not stated |
| Outcomes | Primary outcomes: glycaemic control, pregnancy complications Secondary outcomes: not stated |
| Starting date | January 2007. Sample size of 200 is planned. |
| Contact information | Boaz Sheizaf, MD, Division of Obstetrics and Gynecology, Soroka University Medical Center, Israel bsheizaf@bgu.ac.il |
| Notes | NCT00414245 Listed as 'ongoing study' in Cochrane Review 'Insulin for the treatment of women with gestational diabetes'. |
Van der Linden 2014.
| Trial name or title | Metformine to prevent gestational diabetes mellitus (Medico‐GDM trial) |
| Methods | Randomised controlled trial Funding: not stated |
| Participants | Location: Netherlands Inclusion criteria: women with high risk (according to Dutch national criteria) for GDM, aged between 18 to 40 years, at 8 to 12 weeks' gestation, able to communicate and read in Dutch. Exclusion criteria: multiple pregnancy, diabetes mellitus diagnosed before the current pregnancy, high fasting glucose at first trimester (> 5.3 mmol/L), cardiac insufficiency, renal insufficiency, liver disease, use of medication other than paracetamol or vitamins |
| Interventions | 500 mg metformin twice daily for the first week, after that 1000 mg twice daily, versus no intervention. All women will receive a diet that contains a 2000 calories/day, with an adequate distribution of carbohydrates during the day. |
| Outcomes | Primary outcome: GDM Secondary outcomes: pregnancy‐induced hypertension; weight gain during pregnancy; abnormal daily glucose curve after pregnancy; insulin therapy required; head circumference; birthweight; height; pH of umbilical‐cord; serious neonatal complications (including: severe birth defects, stillbirth, birth trauma, respiratory distress, admission to neonatal intensive care unit, low 5 minute Apgar score (< 7) and premature birth); neonatal hypoglycaemia that requires therapy; need for phototherapy; small‐for‐gestational age; birthweight > 90th percentile, birthweight < 10th percentile |
| Starting date | September 2014. Sample size of 400 is planned. Estimated completion date: September 2017 |
| Contact information | Joke van der Linden, Dr, Maasstad Hospital wetenschapsbureau@maasstadziekenhuis.nl |
| Notes | NCT02275845 |
Abbreviations
GDM: gestational diabetes mellitus HbA1c: glycated haemoglobin NICU: neonatal intensive care unit OGTT: oral glucose tolerance test
Differences between protocol and review
For this update, we have revised the outcomes, using the standard outcome set agreed by consensus between review authors of Cochrane Pregnancy and Childbirth systematic reviews for prevention and treatment of gestational diabetes mellitus and pre‐existing diabetes (which we adapted, as appropriate for this review question).
We updated the methods so that they met those outlined in the standard template used by Cochrane Pregnancy and Childbirth (including use of the GRADE approach to assess the quality of the body of evidence and the use of 'Summary of findings' tables).
Contributions of authors
Joanna Tieu wrote the protocol and the first version of this review with help from Philippa Middleton. Philippa Middleton, Suzette Coat and William Hague were involved in editing.
For this 2017 update, Emily Shepherd and Joanna Tieu screened studies for inclusion, extracted data and assessed quality for included trials. Emily Shepherd wrote the text with input and feedback from Joanna Tieu, Philippa Middleton, Suzette Coat and William Hague.
Sources of support
Internal sources
ARCH: Australian Research Centre for Health of Women and Babies, Robinson Research Institute, The University of Adelaide, Australia.
External sources
-
NHMRC: National Health and Medical Research Council, Australia.
Funding for the Pregnancy and Childbirth Australian and New Zealand Satellite
-
NIHR: National Institute for Health Research, UK.
Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
Joanna Tieu is supported by an NHMRC postgraduate scholarship and Arthritis Australia Ken Muirden fellowship (jointly funded by the Australian Rheumatology Association and Roche).
Suzette Coat is involved in the conduct of the Treatment of Booking Gestational diabetes Mellitus Study (TOBoGM), Bishop 2017. This study seeks to determine whether diagnosis and treatment of gestational diabetes mellitus in the first trimester in women with risk factors for overt diabetes is beneficial to the woman and her fetus/baby.
William Hague: I am a chief investigator (CIB) for the Treatment of Booking GDM trial (TOBoGM), Bishop 2017, currently funded by a NHMRC project grant. I was an AI in the Mothers After Gestational Diabetes in Australia (MAGDA) study (O'Reilly 2016), and also a co‐author on some of the work by Van Ryswyk 2016, which impinge on this review. This was taken into account when writing the review.
Philippa Middleton: none known.
Emily Shepherd: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Ainuddin 2015 {published data only}
- Ainuddin JA, Karim N, Zaheer S, Ali SS, Hasan AA. Metformin treatment in type 2 diabetes in pregnancy: an active controlled, parallel‐group, randomized, open label study in patients with type 2 diabetes in pregnancy. Journal of Diabetes Research 2015;2015:325851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01855763. Metformin in gestational diabetes and type 2 diabetes in pregnancy in a developing country. clinicaltrials.gov/ct2/show/NCT01855763 (first received 9 May 2013).
Beyuo 2015 {published data only}
- Beyuo T, Obed SA, Adjepong‐Yamoah KK, Bugyei KA, Oppong SA, Marfoh K. Metformin versus insulin in the management of pre‐gestational diabetes mellitus in pregnancy and gestational diabetes mellitus at the Korle Bu Teaching Hospital: a randomized clinical trial. PLOS ONE 2015;10(5):e0125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickman 2013 {published data only}
- Hickman MA, McBride R, Boggess KA, Strauss R. Metformin compared with insulin in the treatment of pregnant women with overt diabetes: a randomized controlled trial. American Journal of Perinatology 2013;30(6):483‐90. [DOI] [PubMed] [Google Scholar]
- NCT00835861. Effectiveness of metformin compared to insulin in pregnant women with mild preexisting or early gestational diabetes (MIPOD). clinicaltrials.gov/ct2/show/results/NCT00835861 (first received 2 February 2009).
Ibrahim 2014 {published data only}
- Ibrahim MI, Hamdy A, Shafik A, Taha S, Anwar M, Faris M. The role of adding metformin in insulin‐resistant diabetic pregnant women: a randomized controlled trial. Archives of Gynecology and Obstetrics 2014;289(5):959‐65. [DOI] [PubMed] [Google Scholar]
- NCT01915550. The role of adding metformin in insulin‐resistant diabetic pregnant women ‐ a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01915550 (first received 2 August 2013).
Notelovitz 1971 {published data only}
- Notelovitz M. Sulphonylurea therapy in the treatment of the pregnant diabetic. South African Medical Journal 1971;45:226‐9. [PubMed] [Google Scholar]
Refuerzo 2015 {published data only}
- NCT00678080. Metformin versus insulin in pregnant women with type 2 diabetes. clinicaltrials.gov/ct2/show/NCT00678080 (first received 8 May 2008).
- Refuerzo JS, Gowen R, Pedroza C, Hutchinson M, Blackwell SC, Ramin S. A pilot randomized, controlled trial of metformin versus insulin in women with type 2 diabetes mellitus during pregnancy. American Journal of Perinatology 2015;32(2):163‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anjalakshi 2007 {published data only}
- Anjalakshi C, Balaji V, Balaji MS, Seshiah V. A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Research and Clinical Practice 2007;76(3):474‐5. [DOI] [PubMed] [Google Scholar]
Bertini 2005 {published data only}
- Bertini AM, Silva JC, Taborda W, Becker F, Bebber FR, Viesi JM, et al. Perinatal outcomes and the use of oral hypoglycemic agents. Journal of Perinatal Medicine 2005;33(6):519‐23. [DOI] [PubMed] [Google Scholar]
Carlsen 2007 {published data only}
- Carlsen SM, Kjotrod S, Vanky E, Romundstad P. Homocysteine levels are unaffected by metformin treatment in both nonpregnant and pregnant women with polycystic ovary syndrome. Acta Obstetricia et Gynecologica Scandinavica 2007;86(2):145‐50. [DOI] [PubMed] [Google Scholar]
Casey 2015 {published data only}
- Casey BM, Duryea EL, Abbassi‐Ghanavati M, Tudela CM, Shivvers SA, McIntire DD, et al. Glyburide in women with mild gestational diabetes: a randomized controlled trial. Obstetrics and Gynecology 2015;126(2):303‐9. [DOI] [PubMed] [Google Scholar]
Corrado 2011 {published data only}
- Corrado F, D'Anna R, di G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabetic Medicine 2011;28(8):972‐5. [DOI] [PubMed] [Google Scholar]
George 2015 {published data only}
- George A, Mathews JE, Sam D, Beck M, Benjamin SJ, Abraham A, et al. Comparison of neonatal outcomes in women with gestational diabetes with moderate hyperglycaemia on metformin or glibenclamide ‐ a randomised controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(1):47‐52. [DOI] [PubMed] [Google Scholar]
Golladay 2005 {published data only}
- NCT00160485. Glyburide compared to insulin in the management of White's classification A2 gestational diabetes. clinicaltrials.gov/ct2/show/NCT00160485 (first received 8 September 2005).
Hague 2003 {published data only}
- Hague WM, Davoren PM, Oliver J, Rowan J. Contraindications to use of metformin. Metformin may be useful in gestational diabetes [comment]. BMJ 2003; Vol. 326:762. [PubMed]
Hague 2010 {published data only}
- ACTRN12610000157077. Metformin in the prevention of gestational diabetes: the MPG trial. anzctr.org.au/trial_view.aspx?ID=320961 (first received 16 February 2010).
Langer 2000b {published data only}
- Langer O, Conway D, Berkus M, Xenakis EM. Oral hypoglycaemic agent is comparable to insulin in GDM management. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S6. [Google Scholar]
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. New England Journal of Medicine 2000;343(16):1134‐8. [DOI] [PubMed] [Google Scholar]
- Langer O, Yogev Y, Brustman L, Rosenn B. Is there a relationship between severity of gestational diabetes (GDM) and pregnancy outcome in insulin‐ and glyburide‐treated patients?. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S105. [Google Scholar]
- Langer O, Yogev Y, Rosenn B, Brustman L. Glyburide therapy: the relationship between dosage and level of gestational diabetes (GDM) severity. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S105. [DOI] [PubMed] [Google Scholar]
- Langer O, Yogev Y, Xenakis EM, Rosenn B. Insulin and glyburide therapy: dosage, severity level of gestational diabetes, and pregnancy outcome. American Journal of Obstetrics and Gynecology 2005;192(1):134‐9. [DOI] [PubMed] [Google Scholar]
- Letonturier P. Gestational diabetes: an alternative to insulin therapy?. Presse Medicale 2001;30:169‐70. [PubMed] [Google Scholar]
Martinez 2010 {published data only}
- Martinez P, Abdulhaj Martinez M, Andres Nunez P, Garcia Leon P, Lopez Sanchez EJ, Gonzalez Ramirez AR. A randomized study comparing metformin and insulin in the treatment of gestational diabetes mellitus. Interim results. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):381. [Google Scholar]
Moore 2005 {published data only}
- Moore L, Clokey D, Robinson A. A randomized trial of metformin compared to glyburide in the treatment of gestational diabetes. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S92. [Google Scholar]
Moore 2007 {published data only}
- Moore L, Briery C, Martin R, Hood E, Bofill J, Morrison J. Metformin (M) vs. insulin (I) in A2 diabetes: a randomized clinical trial [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S8. [Google Scholar]
- Moore LE, Briery CM, Clokey D, Martin RW, Williford NJ, Bofill JA, et al. Metformin and insulin in the management of gestational diabetes mellitus: preliminary results of a comparison. Journal of Reproductive Medicine 2007;52(11):1011‐5. [PubMed] [Google Scholar]
Mukhopadhyay 2012 {published data only}
- Mukhopadhyay P, Bag TS, Kyal A, Saha DP, Khalid N. Oral hypoglycemic glibenclamide: can it be a substitute to insulin in the management of gestational diabetes mellitus? A comparative study. Journal of South Asian Federation of Obstetrics and Gynaecology (SAFOG) 2012;4(1):28‐31. [Google Scholar]
Myers 2013 {published data only}
- ISRCTN86503951. Management of mild gestational diabetes mellitus (GDM). isrctn.com/ISRCTN86503951 (first received 23 December 2013).
Niromanesh 2012 {published data only}
- IRCT201105075591N2. Metformin compared with insulin in control of blood sugar in gestational diabetes mellitus. en.search.irct.ir/view/5930 (first received 31 March 2011).
- Niromanesh S, Alavi A, Sharbaf FR, Amjadi N, Moosavi S, Akbari S. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Research and Clinical Practice 2012;98(3):422‐9. [DOI] [PubMed] [Google Scholar]
Rowan 2008b {published data only}
- Rowan J. A trial in progress: gestational diabetes. Diabetes Care 2007;30:S214‐S219. [DOI] [PubMed] [Google Scholar]
- Rowan J. Metformin in gestational diabetes: follow up of offspring of mothers treated with insulin compared with metformin. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335 (first received 6 September 2005).
- Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care 2010;33(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, for the MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine 2008;358(19):2003‐15. [DOI] [PubMed] [Google Scholar]
- Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow‐up (MiG TOFU): body composition at 2 years of age. Diabetes Care 2011;34(10):2279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2011 {published data only}
- CTRI/2011/08/001956. Can oral medication replace insulin injections in pregnant women with diabetes. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3378 (first received 19 August 2011).
Vanky 2004 {published data only}
- Salvesen KA, Vanky E, Carlsen SM. Metformin treatment in pregnant women with polycystic ovary syndrome ‐ is reduced complication rate mediated by changes in the uteroplacental circulation?. Ultrasound in Obstetrics and Gynecology 2007;29(4):433‐7. [DOI] [PubMed] [Google Scholar]
- Vanky E, Salvesen KA, Bjerve K, Carlsen SM. C‐Reactive protein in metformin treated pregnant PCOS women. XXXIV Congress of Nordic Federation of Societies of Obstetrics and Gynecology; 2004 June 12‐15;Helsinki, Finland. 2004:76.
- Vanky E, Salvesen KA, Heimstad R, Fougner KJ, Romundstad P, Carlsen SM. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Human Reproduction 2004;19(8):1734‐40. [DOI] [PubMed] [Google Scholar]
- Vanky E, Salvesen KA, Hjorth‐Hansen H, Bjerve K, Carlsen SM. Beneficial effect of metformin on pregnancy outcome in women with polycystic ovary syndrome is not associated with major changes in C‐reactive protein levels or indices of coagulation. Fertility & Sterility 2006;85(3):770‐4. [DOI] [PubMed] [Google Scholar]
Vanky 2005 {published data only}
- NCT00159536. Metformin in pregnant PCOS women. clinicaltrials.gov/ct2/show/NCT00159536 (first received 8 September 2005).
Wali 2012 {published data only}
- ACTRN12612001272886. A phase three open label randomized controlled trial to compare the efficacy of oral hypoglycemic agents (OHA) with insulin in the treatment of gestational diabetes mellitus (GDM). anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363218 (first received 7 December 2012).
Zanganeh 2010 {published data only}
- Zangeneh M. The comparative study of therapeutic effects of insulin and glibenclamide in the gestational diabetes mellitus. http://en.search.irct.ir/view/4374 (first received 1 December 2010).
References to studies awaiting assessment
Coiner 2014 {published data only}
- Coiner J, Rowe M, DeVente J. The treatment of diabetes in pregnancy; metformin vs glyburide and insulin biomedical evidence of fetopathy. American Journal of Obstetrics and Gynecology 2014; Vol. 210, issue 1 Suppl 1:S148.
Hutchinson 2008 {published data only (unpublished sought but not used)}
- Hutchinson A, Haugabrook C, Long L, Mason L, Kipikasa J, Adair D. A comparison of glyburide/metformin and insulin for gestational diabetes. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S200. [Google Scholar]
- NCT00371306. Comparison of glucovance to insulin for diabetes during pregnancy. clinicaltrials.gov/ct2/show/NCT00371306 (first received 1 September 2006).
Reyes‐Munoz 2014 {published data only}
- Reyes‐Munoz E, Ortega‐Gonzalez C, Mier‐Cabrera J, Avila‐Carrasco A, Espino S, Ayala‐Yanez R. Medical nutrition therapy plus metformin for preventing gestational diabetes among high‐risk women. Obstetrics and Gynecology 2014;123 Suppl 1:168S. [Google Scholar]
Waheed 2013 {published data only}
- Waheed S, Malik FP, Mazhar SB. Efficacy of metformin versus insulin in the management of pregnancy with diabetes. Journal of the College of Physicians and Surgeons‐‐Pakistan 2013;23(12):866‐9. [PubMed] [Google Scholar]
References to ongoing studies
Feig 2011 {published data only}
- Feig DS, Murphy K, Asztalos E, Tomlinson G, Sanchez J, Zinman B, et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): a multi‐center randomized controlled trial. BMC Pregnancy and Childbirth 2016;16(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01353391. Metformin in women with type 2 diabetes in pregnancy a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01353391 (first received 2 May 2011).
Sheizaf 2006 {published data only}
- NCT00414245. Metformin for the treatment of diabetes in pregnancy. clinicaltrials.gov/ct2/show/record/NCT00414245 (first received 20 December 2006).
Van der Linden 2014 {published data only}
- NCT02275845. Metformin vs control to prevent gestational diabetes mellitus (GDM) in women with a high risk for GDM, an open label randomized controlled trial. The Medico‐GDM trial. clinicaltrials.gov/ct2/show/NCT02275845 (first received 23 October 2014).
Additional references
ADA 2015
- American Diabetes Association. Management of diabetes in pregnancy. Diabetes Care 2015;38(Suppl 1):S77‐S79. [DOI] [PubMed] [Google Scholar]
ADIPS 2005
- McElduff A, Cheung NW, McIntyre HD, Lagstrom JA, Oats JJ, Ross GP, et al. The Australasian Diabetes in Pregnancy Society consensus guidelines for the management of type 1 and type 2 diabetes in relation to pregnancy. Medical Journal of Australia 2005;183(7):373‐7. [PubMed] [Google Scholar]
Alwan 2009
- Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2008
- Bell R, Bailey K, Cresswell T, Hawthorne G, Critchley J, Lewis‐Barned N, et al. Trends in prevalence and outcomes of pregnancy in women with pre‐existing type I and type II diabetes. BJOG: an international journal of obstetrics and gynaecology 2008;115(4):445‐52. [DOI] [PubMed] [Google Scholar]
Bishop 2017
- Bishop C, Simmons D. The Treatment Of BOoking Gestational diabetes Mellitus Study: Evaluating the impact on obstetric outcomes of immediate versus delayed care for gestational diabetes diagnosed at booking. http://www.anzctr.org.au/ACTRN12616000924459.aspx 2017. [ACTRN12616000924459]
Brock 2005
- Brock B, Smidt K, Ovesen P, Schmitz O, Rungby J. Is metformin therapy for polycystic ovary syndrome safe during pregnancy?. Basic & Clinical Pharmacology & Toxicology 2005;96:410‐2. [DOI] [PubMed] [Google Scholar]
Brown 2016a
- Brown J, Crawford TJ, Alsweiler J, Crowther CA. Dietary supplementation with myo‐inositol in women during pregnancy for treating gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012048.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2016b
- Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD012037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017a
- Brown J, Alwan NA, West J, Brown S, McKinlay CJ, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD011970.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2017b
- Brown J, Martis R, Hughes B, Rowan J, Crowther CA. Oral anti‐diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD011967.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Butalia 2017
- Butalia S, Gutierrez L, Lodha A, Aitken E, Zakariasen A, Donovan L. Short‐ and long‐term outcomes of metformin compared with insulin alone in pregnancy: a systematic review and meta‐analysis. Diabetic Medicine 2017;34(1):27‐36. [DOI] [PubMed] [Google Scholar]
CDA 2013
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee, Canadian Diabetes Association. 2013 Clinical Practice Guidelines for the prevention and management of diabetes in Canada. Canadian Journal of Diabetes 2013;37(Suppl 1):S1‐S212. [DOI] [PubMed] [Google Scholar]
Chan 2005
- Chan LYS, Yeung JH, Lau TK. Placental transfer of rosiglitazone in the first trimester of human pregnancy. Fertility and Sterility 2005;83(4):955‐8. [DOI] [PubMed] [Google Scholar]
Coetzee 2007
- Coetzee EJ. Counterpoint: oral hypoglycemic agents should be used to treat diabetic pregnant women. Diabetes Care 2007;30:2980‐2. [DOI] [PubMed] [Google Scholar]
Correa 2015
- Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Maternal and Child Health Journal 2015;19:635‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2005
- Crowther C, Hiller J, Moss J, McPhee A, Jeffries W, Robinson J, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352:2477‐86. [DOI] [PubMed] [Google Scholar]
Dabelea 2000
- Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow‐up research in the Pima Indians. Journal of Maternal‐Fetal Medicine 2000;9:83‐8. [DOI] [PubMed] [Google Scholar]
DeFronzo 1984
- DeFronzo RA, Simonson DC. Oral sulfonylurea agents suppress hepatic glucose production in non‐insulin‐dependent diabetic individuals. Diabetes Care 1984;7(Suppl 1):72‐80. [PubMed] [Google Scholar]
DeFronzo 1995
- DeFronzo RA, Goodman AM, the Multicenter Metformin Study Group. Efficacy of metformin in patients with non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1995;333(9):541‐9. [DOI] [PubMed] [Google Scholar]
Ekpebegh 2007
- Ekpebegh CO, Coetzee EJ, Merwe L, Levitt NS. A 10‐year retrospective analysis of pregnancy outcome in pregestational type 2 diabetes: comparison of insulin and oral glucose‐lowering agents. Diabetic Medicine 2007;24:253‐8. [DOI] [PubMed] [Google Scholar]
Farrar 2016
- Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD005542.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glueck 2004
- Glueck CJ, Goldenberg N, Pranikoff J, Lotspring M, Sieve L, Wang P. Height, weight, and motor‐social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Human Reproduction 2004;19(6):1323‐30. [DOI] [PubMed] [Google Scholar]
Gutzin 2003
- Gutzin SJ, Kozer E, Magee LA, Feig DS, Koren G. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta‐analysis. Canadian Journal of Clinical Pharmacology 2003;10(4):179‐83. [PubMed] [Google Scholar]
Hellmuth 2000
- Hellmuth E, Damm P, Molsted‐Pedersen L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabetic Medicine 2000;17(7):507‐11. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ho 2007
- Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy: an appraisal of the current evidence for oral anti‐diabetic drug use in pregnancy. Annals of the Academy of Medicine, Singapore 2007;36:672‐8. [PubMed] [Google Scholar]
Homko 2006
- Homko CJ, Reece EA. Insulins and oral hypoglycemic agents in pregnancy. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(11):679‐86. [DOI] [PubMed] [Google Scholar]
IDF 2015
- International Diabetes Federation (IDF). IDF Diabetes Atlas. 7th Edition. Brussels: International Diabetes Federation, 2015. [Google Scholar]
Jensen 2004
- Jensen DM, Dam P, Moelsted‐Pedersen L, Ovesen P, Westergaard JG, Moeller M, et al. Outcomes in type 1 diabetic pregnancies. Diabetes Care 2004;27(12):2819‐23. [DOI] [PubMed] [Google Scholar]
Jiwani 2012
- Jiwani A, Marseille E, Lohse N, Damm P, Hod M, Kahn JG. Gestational diabetes mellitus: results from a survey of country prevalence and practices. Journal of Maternal Fetal and Neonatal Medicine 2012;25(6):600‐10. [DOI] [PubMed] [Google Scholar]
Jovanovic 2007
- Jovanovic L. Point: oral hypoglycemic agents should not be used to treat diabetic pregnant women. Diabetes Care 2007;30:2976‐9. [DOI] [PubMed] [Google Scholar]
Khambalia 2013
- Khambalia AZ, Ford JB, Nassar N, Shand AW, McElduff A, Roberts CL. Occurrence and recurrence of diabetes in pregnancy. Diabetic Medicine 2013;30:452‐6. [DOI] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862‐8. [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim C, Berger DK, Chamany S. Recurrence of gestational diabetes mellitus: a systematic review. Diabetes Care 2007;30:1314‐9. [DOI] [PubMed] [Google Scholar]
Kitzmiller 1996
- Kitzmiller JL, Buchanan TA, Kjos S, Combs CA, Ratner RE. Pre‐conception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care 1996;19(5):514‐41. [DOI] [PubMed] [Google Scholar]
Kovo 2007
- Kovo M, Haroutinunian S, Feldman N, Hoffman A, Glezerman M. Determination of metformin transfer across the human placenta using a dually perfused ex vivo placental cotelydon model. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;136(1):29‐33. [DOI] [PubMed] [Google Scholar]
Kraemer 2006
- Kraemer J, Klein J, Lubetsky A, Koren G. Perfusion studies of glyburide transfer across the human placenta: implications for fetal safety. American Journal of Obstetrics and Gynecology 2006;195(1):270‐4. [DOI] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thorn E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361(14):1339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langer 2000a
- Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. New England Journal of Medicine 2000;343(16):1134‐8. [DOI] [PubMed] [Google Scholar]
Lawrence 2008
- Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999‐2005. Diabetes Care 2008;31(5):899‐904. [DOI] [PubMed] [Google Scholar]
Leguizamon 2007
- Leguizamon G, Igarzabal ML, Reece EA. Preconceptional care of women with diabetes mellitus. Obstetrics and Gynecology Clinics of North America 2007;34(2):225‐39. [DOI] [PubMed] [Google Scholar]
Macintosh 2006
- Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ 2006;333(7560):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahmud 2010
- Mahmud M, Mazza D. Preconception care of women with diabetes: a review of current guideline recommendations. BMC Women's Health 2010;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martis 2016
- Martis R, Brown J, Alsweiler J, Crawford TJ, Crowther CA. Different intensities of glycaemic control for women with gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011624.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2016
- Middleton P, Crowther CA, Simmonds L. Different intensities of glycaemic control for pregnant women with pre‐existing diabetes. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD008540.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moy 2014
- Moy FM, Ray A, Buckley BS. Techniques of monitoring blood glucose during pregnancy for women with pre‐existing diabetes. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD009613.pub2] [DOI] [PubMed] [Google Scholar]
NCT01832181
- NCT01832181. Metformin in women with type 2 diabetes in pregnancy kids trial (MiTy Kids). clinicaltrials.gov/ct2/show/NCT01832181 (first received 8 April 2013).
NICE 2015
- National Institute for Health and Clinical Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. London: NICE, 2015. [PubMed] [Google Scholar]
Nissen 2007
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New England Journal of Medicine 2007;356:2457‐71. [DOI] [PubMed] [Google Scholar]
O'Neill 2017
- O'Neill SM, Kenny LC, Khashan AS, West HM, Smyth RM, Kearney PM. Different insulin types and regimens for pregnant women with pre‐existing diabetes. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD011880.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Reilly 2016
- O'Reilly SL, Dunbar JA, Versace V, Janus E, Best JD, et al. MAGDA Study Group. Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program. PLoS Med 2016;13(7):e1002092. [DOI: 10.1371/journal.pmed.1002092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 2001
- Ray JG, Vermeulen MJ, Shapiro JL, Kenshole AB. Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. Quarterly Journal of Medicine 2001;94:347‐56. [DOI] [PubMed] [Google Scholar]
Reece 2010
- Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(3):199‐203. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowan 2008a
- Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. New England Journal of Medicine 2008;358(19):2003‐15. [DOI] [PubMed] [Google Scholar]
Sheth 2002
- Sheth B. Does pregnancy accelerate the rate of progression of diabetic retinopathy?. Current Diabetes Report 2002;23(8):327‐30. [DOI] [PubMed] [Google Scholar]
Simonson 1984
- Simonson DC, Ferrannini E, Bevilacqua S, Smith D, Barrett E, Carlson R, et al. Mechanism of improvement of glucose metabolism after chronic glyburide therapy. Diabetes 1984;33(9):838‐45. [DOI] [PubMed] [Google Scholar]
Sivan 1995
- Sivan E, Feldman B, Dolitzki M, Nevo N, Dekel N, Karasik A. Glyburide crosses the placenta in vivo in pregnant rats. Diabetologia 1995;38(7):753‐6. [DOI] [PubMed] [Google Scholar]
Slocum 2002
- Slocum JM, Sosa MEB. Use of antidiabetes agents in pregnancy: current practice and controversy. Journal of Perinatal and Neonatal Nursing 2002;16(2):40‐53. [DOI] [PubMed] [Google Scholar]
Stumvoll 1995
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1995;333(9):588‐9. [DOI] [PubMed] [Google Scholar]
Tieu 2013
- Tieu J, Bain E, Middleton P, Crowther CA. Interconception care for women with a history of gestational diabetes for improving maternal and infant outcomes. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD010211.pub2] [DOI] [PubMed] [Google Scholar]
Tieu 2017
- Tieu J, Middleton P, Crowther CA, Shepherd E. Preconception care for diabetic women for improving maternal and infant health. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD007776.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Ryswyk 2016
- Ryswyk EM, Middleton PF, Hague WM, Crowther CA. Women's views on postpartum testing for type 2 diabetes after gestational diabetes: Six month follow‐up to the DIAMIND randomised controlled trial. Primary Care Diabetes 2016;10(2):91‐102. [DOI: 10.1016/j.pcd.2015.07.003] [DOI] [PubMed] [Google Scholar]
Walkinshaw 2005
- Walkinshaw SA. Pregnancy in women with pre‐existing diabetes: management issues. Seminars in Fetal & Neonatal Medicine 2005;10:307‐15. [DOI] [PubMed] [Google Scholar]
Weintrob 1996
- Weintrob N, Karp M, Hod M. Short‐ and long‐ range complications in offspring of diabetic mothers. Journal of Diabetes and its Complications 1996;10:294‐301. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Report of a WHO consultation: definition, diagnosis and classification of diabetes mellitus. Geneva: WHO, 1999. [Google Scholar]
Yogev 2004
- Yogev Y, Langer O. The use of anti‐hyperglycaemic and hypoglycaemic agents in pregnancy. Fetal and Maternal Medicine Review 2004;15(2):133‐43. [Google Scholar]
References to other published versions of this review
Tieu 2010
- Tieu J, Coat S, Hague W, Middleton P. Oral anti‐diabetic agents for women with pre‐existing diabetes mellitus/impaired glucose tolerance or previous gestational diabetes mellitus. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007724.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


