Abstract
Metabolic reprogramming has become a key focus for both immunologists and cancer biologists, with exciting advances providing new insights into underlying mechanisms of disease. Metabolites traditionally associated with bioenergetics or biosynthesis have been implicated in immunity and malignancy in transformed cells, with a particular focus on intermediates of the mitochondrial pathway known as the Krebs cycle. Among these, the intermediates succinate, fumarate, itaconate, 2-hydroxyglutarate isomers (D-2-hydroxyglutarate and L-2-hydroxyglutarate) and acetyl-CoA now have extensive evidence for “non-metabolic” signalling functions in both physiological immune contexts and in disease contexts, such as the initiation of carcinogenesis. This review will describe how metabolic reprogramming, with emphasis placed on these metabolites, leads to altered immune cell and transformed cell function. The latest findings are informative for new therapeutic approaches which could be transformative for a range of diseases.
1. Introduction
The past 5 years has seen a remarkable increase in our knowledge of how intracellular metabolic changes in both tumours and especially immune cells are not only linked to energy demand or biosynthesis, but to discrete effector mechanisms that alter cell behaviour in specific ways. An area of particular focus has been on the Krebs cycle, (also known as the tricarboxylic acid (TCA) cycle or the citric acid cycle (CAC)), the primary oxidative pathway for acetyl-CoA and for the generation of the reducing agents NADH and FADH2 in aerobic organisms. Importantly, NADH and FADH2 are required to transfer electrons to the mitochondrial respiratory chain, also known as the electron transport chain (ETC), a series of enzyme and coenzyme complexes found along the inner mitochondrial membrane (IMM). Transfer of electrons along the ETC occurs via several redox reactions to facilitate the generation of an electrochemical proton (H+) gradient, which subsequently drives the synthesis of energy rich adenosine triphosphate (ATP) by ATP synthase. This process, referred to as oxidative phosphorylation (OXPHOS), requires oxygen (O2) and results in the formation of carbon dioxide (CO2) as a by-product.
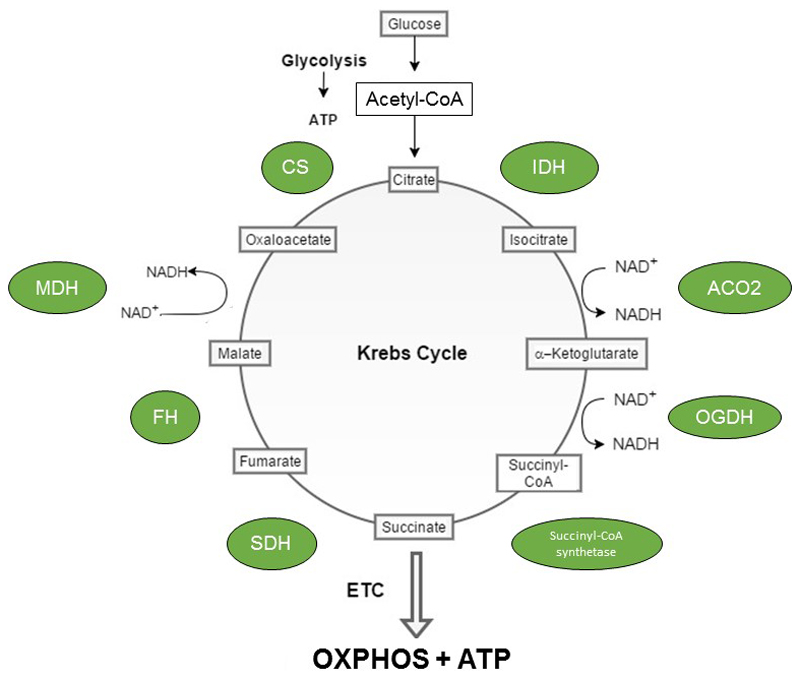
The TCA cycle itself operates in the mitochondrial matrix and is an amphibolic pathway that acts as an important nexus for the integration of multiple catabolic and anabolic pathways, such as glycolysis and gluconeogenesis. As depicted in Figure 1, the pathway consists of eight enzymes namely citrate synthase (CS), aconitase (ACO2), isocitrate dehydrogenase (IDH), α-ketoglutarate dehydrogenase (OGDH), succinyl-CoA synthetase, succinate dehydrogenase (SDH), fumarase (FH) and malate dehydrogenase (MDH). The first reaction, an irreversible aldol condensation, is catalysed by CS and extends the 4-carbon oxaloacetate to 6-carbon citrate, with the additional 2 carbons derived from acetyl-CoA. In the second step, ACO2 catalyses the reversible stereo-specific isomerisation of citrate to isocitrate, via cis-aconitate, in a two-step reaction. To achieve this, ACO2 employs a dehydration-hydration mechanism. Firstly, citrate is protonated at position C3 prior to deprotonation at position C2 to form cis-aconitate (dehydration). Secondly, cis-aconitate flips 180° to ensure the correct stereochemistry (2R, 3S) of the reaction by allowing the dehydration and hydration steps to occur on opposite faces of the intermediate. Subsequently, cis-aconitate is converted to isocitrate (rehydration). The third step, catalysed by IDH, requires NAD+ and results in the oxidation of isocitrate to oxalosuccinate generating NADH. Oxalosuccinate is subsequently decarboxylated to form 5-carbon -ketoglutarate (α-KG) and CO2. In the fourth step, OGDH catalyses the oxidative decarboxylation of α-KG to form succinyl-CoA, NADH and CO2. Succinyl-CoA synthetase then catalyses the hydrolysis of succinyl-CoA, coupled to the condensation of GDP and inorganic phosphate (Pi), or ADP and Pi, to form succinate and either GTP or ATP. As such, the reaction catalysed by OGDH is referred to as substrate level phosphorylation and constitutes the fifth step in the Krebs cycle. In the sixth step, SDH, also complex II of the ETC, catalyses the oxidation of succinate to fumarate. SDH, which uses FAD as a prosthetic group, generates FADH2 from the oxidation of succinate. The two electrons carried by FADH2 are subsequently used to reduce ubiquinone (Q) to ubiquinol (QH2). In the seventh step, FH catalyses the hydration of the α,β-unsaturated carbonyl compound fumarate to form L-malate. The final step in the cycle is catalysed by MDH and results in the oxidation of malate to generate NADH. This step also regenerates oxaloacetate, which can then be re-used by CS to enable continuation of the cycle.
Figure 1. The Krebs cycle.
The Krebs cycle is a metabolic pathway operating in the mitochondrial matrix of all aerobic organisms. Breakdown of nutrients, such as glucose, generates acetyl-CoA which can then be funnelled into this pathway. For a full cycle to be completed, a series of 10 enzymatic reactions are required. These reactions are catalysed by the mitochondrial enzymes citrate synthase (CS), isocitrate dehydrogenase (IDH), aconitase (ACO2), α-ketoglutarate dehydrogenase (OGDH), succinyl-CoA synthetase, succinate dehydrogenase (SDH), fumarase (FH) and malate dehydrogenase (MDH). The primary function of the TCA cycle is to generate reducing equivalents, such as NADH and FADH2 (produced by SDH). NADH and FADH2 can then transfer electrons to the ETC to drive oxidative phosphorylation (OXPHOS) and the production of the high energy nucleoside triphosphate, ATP, via ATP synthase.
Intriguingly, Krebs cycle–derived metabolites have also been attributed signalling functions and impact on multiple processes critical for immune cell activation and cellular transformation. The biological targets of succinate, itaconate, fumarate 2-HG and acetyl-CoA are of direct relevance to immunity and/or tumorigenesis, inhibiting specific enzymes or driving covalent modifications of proteins to alter their functions. A common outcome is altered expression of specific sets of genes, and alterations in the epigenome. The complexities of metabolic rewiring may well ultimately lead to new therapeutic modalities that could have a major impact on the pathogenesis of multiple diseases previously linked to metabolic reprogramming. We are on the cusp of a major shift in our understanding of how intracellular metabolic changes lead to disease, presenting us with the exciting prospect of new approaches to complement or even replace current therapeutic approaches for several diseases where an unmet medical need remains. This review will discuss the extensive evidence for the “non-metabolic” functions of succinate, itaconate, fumarate, 2-HG and acetyl-CoA in turn, focusing on immune cell signalling and carcinogenesis. Finally, the therapeutic opportunities that have, and may arise, from understanding these signalling pathways will also be examined.
2. Succinate as a signal in macrophages and tumour cells
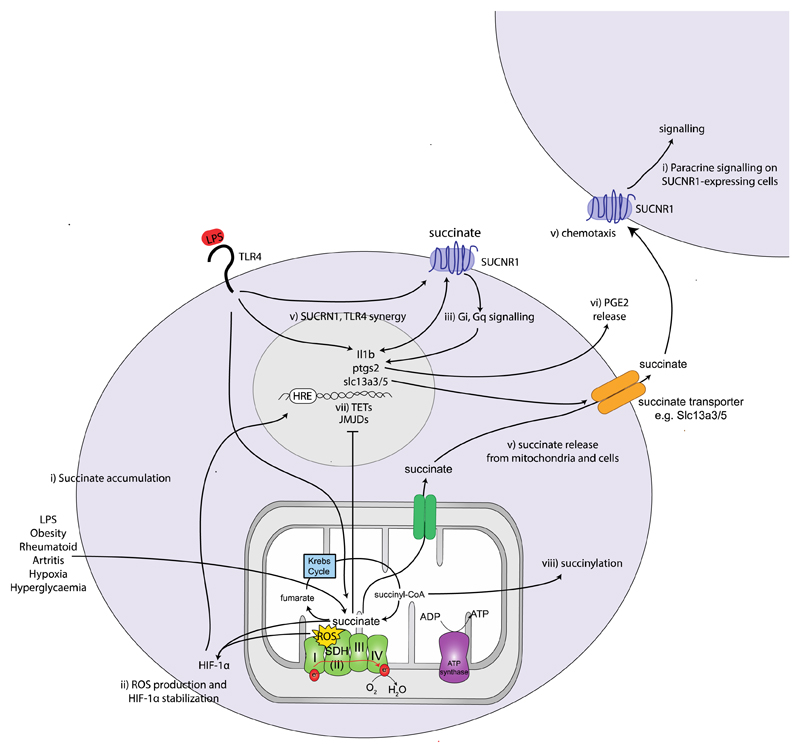
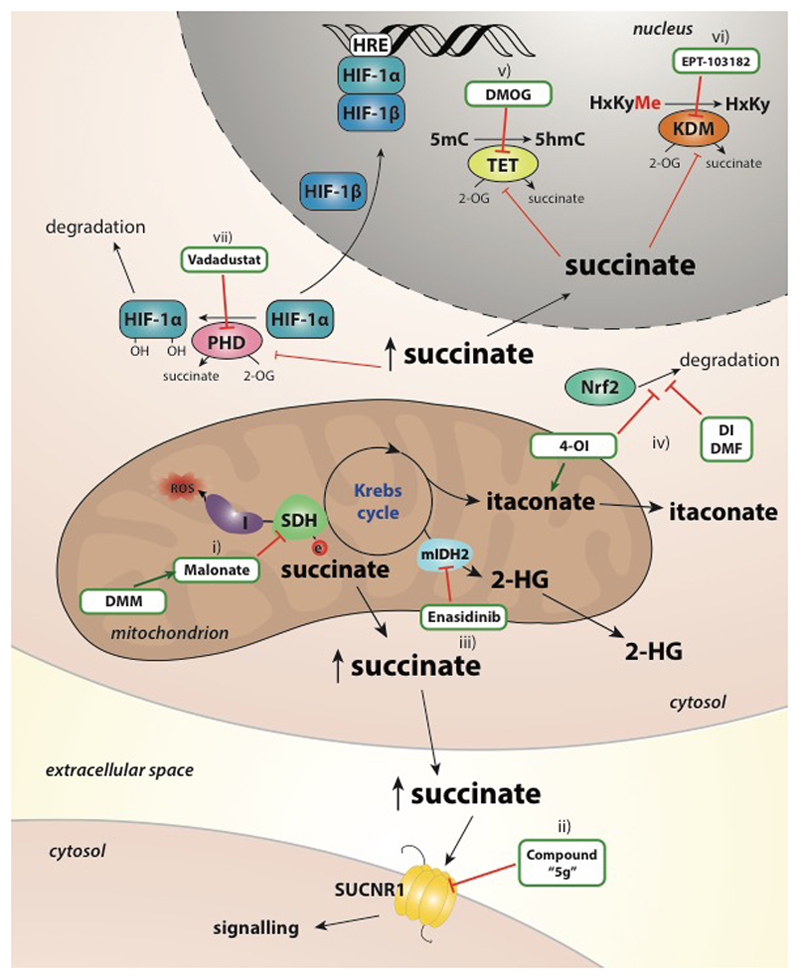
As mentioned, succinate is generated in the mitochondrial matrix in a reversible three-step reaction catalysed by the TCA cycle enzyme succinyl-CoA synthetase, with concomitant production of a high energy nucleoside triphosphate. Recent data indicate that succinate is a pro-inflammatory metabolite that accumulates in LPS/IFN-γ-treated macrophages1,2. As depicted in Figure 2, succinate has been shown to act through several pathways to exert its inflammatory effects: through direct generation of mitochondrial ROS (mtROS), by activation of hypoxia-inducible factor-1alpha (HIF-1α; discussed below), and by through ligation of the G-protein coupled receptor succinate receptor 1 (SUCNR1).
Figure 2. The diverse signalling roles of succinate.
Succinate levels are elevated in response to LPS stimulation, in synovial fluids from rheumatoid arthritis (RA) patients, in the circulation in models of diet-induced obesity and in adipose tissue in response to hypoxia and hyperglycaemia. ii) Succinate oxidation (as well as direct product inhibition) and reactive oxygen species (ROS) stabilizes hypoxia-inducible factor-1alpha (HIF-1α) which binds to the hypoxia-response element (HRE) in the IL-1β promoter iii) Succinate is the ligand for the G-protein coupled receptor succinate receptor 1 (SUCNR1). Ligand binding induces Gi and Gq signalling cascades. iv) SUCNR1 ligation on dendritic cells induces cell migration v) and acts in synergy with TLR ligands to increase interleukin-1 beta (IL-1β) expression and IL-1β and LPS further enhances SUCNR1 expression. v) Endogenously generated succinate is released, e.g. via Slc13a3/5 in neural stem cells (NSCs) and binds to SUCNR1 on the same or nearby SUCNR1-expressing cells. vi) Activation of SUCNR1 on NSCs induces the secretion of anti-inflammatory prostaglandin E2 which is anti-inflammatory. vii) Succinate regulates the activity the Jumonji C-domain containing histone demethylases (JMJDs) and the Ten eleven translocation (TET) family of 5mC hydroxylases, which play a role in histone and DNA demethylation, respectively and can thereby remodel the epigenome. viii) Another consequence of succinate accumulation is the modification of proteins by lysine succinylation.
2.1. Succinate and SUCNR1-mediated signalling in inflammation and immunity
In 2004, He and colleagues made an important discovery when they found that the Krebs cycle-derived metabolites, succinate and α-KG, acted as ligands for the orphan G-protein coupled receptors GPR91 (now known as SUCNR1) and GPR99 (now known as OXRG1), respectively3. The authors demonstrated that succinate acted as an important hypertensive agent, modulating the renin-angiotensin system via SUCNR1 signalling, independent of its role in bioenergetics3. Since this seminal discovery, an important role for SUCNR1 in regulating numerous physiological processes implicated in health and disease have emerged, notably in the prevention of age-related macular degeneration (AMD)175 and immune cell function4. SUCNR1 is highly expressed in mouse kidney, liver, spleen, small intestine3, and on dendritic cells (DCs) 4 and ligand binding induces Gi and Gq signalling cascades3. SUCNR1 ligation on MoDC or the U937 macrophage cell line induces cell migration, suggesting that succinate can act as a chemokine4. Extracellular succinate addition also acts in synergy with TLR ligands to increase TNFα and IL-1β expression4 and increases the capacity of DCs to act as antigen presenting cells. Priming of DCs with succinate and antigen simultaneously, elevates antigen-specific T cell activation, increasing TNFα and IFN-γproduction from these cells4. SUCNR1 is also expressed on tuft cells in the intestine where its signalling promotes type 2 immunity to certain infectious agents, such as tritrichomonad protists, and drives small intestinal remodelling via a tuft cell-ILC2 circuit168-170.
Littlewood-Evans and colleagues (2016) have demonstrated that succinate can act in an autocrine and paracrine manner to increase IL-1β production in macrophages5. Following LPS treatment both intracellular succinate levels and the cell surface expression of SUCNR1 are increased. Interestingly, endogenously generated succinate is released, through an undefined mechanism, and binds to SUCNR1 on the same or nearby SUCNR1-expressing cells to amplify IL-1β production. IL-1β itself further enhances SUCNR1 expression fuelling this cycle of cytokine production. Macrophage activation and IL-1β production are decreased in the absence of SUCR1 both following LPS treatment and in a model of antigen-induced arthritis. This correlates with an observed increase in succinate in synovial fluids from rheumatoid arthritis (RA) patients suggesting that chronically elevated succinate is pathological in this setting6. Intriguingly, HIF-1α expression is also increased in synovial joints of RA patients7 suggesting that succinate may act in two ways to increase inflammation in RA: via SUCNR1 and HIF-1α.
Tissue and circulating succinate is also elevated in other chronic inflammatory settings such as models of diet-induced obesity8. It has been suggested that exposure of adipocytes to hypoxia and hyperglycaemia (for example during obesity) induces succinate release from adipose tissue in mice9 and this is associated with macrophage infiltration into the adipose tissue. The intracellular pathway driving succinate accumulation in these cells or its release from these cells is unclear. In the absence of SUCNR1 a reduction in absolute numbers of macrophages, but not inflammatory signalling, was observed and this resulted in an improvement in adipose tissue inflammation and improved glucose tolerance in mice following high-fat diet feeding. In contrast to previous reports, the authors observed no effect of succinate alone on macrophage infiltration suggestion that additional signals present in the hypoxic environment may also be required for succinate-induced chemotaxis. More recently, succinate has been implicated as a systemic signal for thermogenesis. It was demonstrated that brown adipocytes possess a unique capacity to sequester extracellular succinate, which serves to increase UCP1 activity as a consequence of elevated mtROS160. Interestingly, the authors demonstrate a role for shivering muscle in the generation of succinate following cold exposure. This enters the circulation and is sequestered by the brown adipose tissue. Muscle has been demonstrated to produce succinate in other contexts too175. Specifically, succinate is elevated in plasma after acute exercise and in marathon runners175.
Succinate has also been implicated in the pathogenesis of neuroinflammation. In a mouse model of multiple sclerosis (MS) transplantation of neural stem cells (NSCs) reduced succinate levels in the cerebrospinal fluid and thereby decreased infiltration of damaging macrophages and microglial cells10. Mechanistically, ligation of SUCNR1 on the surface of NSCs upregulates the expression of the dicarboxylate co-transporter Slc13a3/5, which correlates with increased uptake by the NSCs thereby scavenging succinate away from pro-inflammatory immune cells. Activation of SUCNR1 on NSCs also induces the secretion of anti-inflammatory prostaglandin E2 further contributing to their anti-inflammatory phenotype.
Based on these data it might be predicated that SUCNR1 antagonists will be protective in settings where inflammation is exacerbated such as graft rejection or autoimmunity. A selective antagonist for human and rat has been shown to be protective in hypertension, a disease in which succinate is elevated11. Similarly, potent agonists have recently been synthesized which will deepen our understanding of the signalling pathways affected by this important receptor12. Whether these compounds with have therapeutic potential remains to be explored.
2.2. The interplay between hypoxia-inducible factor 1 and succinate in inflammatory signalling and tumorigenesis
Another mechanism by which succinate exerts its signalling properties involves the transcription factor HIF-1, as shown in Figure 2. HIF-1 is a transcriptional regulator central to the response to hypoxia13. HIF-1 was first discovered by Semenza and colleagues in 1992, and it was found to promote erythropoiesis by binding the erythropoietin gene enhancer14. It transcriptionally directs a switch in metabolism from oxidative phosphorylation to glycolysis, which allows rapid ATP production without the need for oxygen15. HIF is best understood for its role in tumour formation. Tumour environments are often oxygen-deprived and such hypoxic conditions lead to activation of HIF-1. HIF-1 binds to hypoxia response elements (HREs) in target genes and increases the expression of numerous glycolytic enzymes and the glucose transporters (GLUT)1 and GLUT3. It also targets genes involved in erythropoiesis, angiogenesis and proliferation.
HIF-1 is composed of a β-subunit, that is constitutively active, and an oxygen-sensitive α subunit. HIF-1α is tightly regulated by prolyl hydroxylases (PHD), a class of α-ketoglutarate (α-KG)-dependent dioxygenases (α-KGDDs) that convert α-KG to succinate and use molecular oxygen to hydroxylate HIF-1α. In the presence of oxygen, conserved proline residues in the HIF-1α subunit are hydroxylated by PHD 1, 2 and 3 16. Hydroxylation provides a recognition site for the binding of Von Hippel-Lindau (VHL) E3 ubiquitin ligase, which ubiquitinates HIF-1α and subsequently targets it for proteasomal degradation17. This degradation is prevented in conditions of limited oxygen, allowing for HIF-1α translocation to the nucleus, dimerization with HIF-1β and binding of target genes13.
HIF-1α is vital for the switch to glycolysis observed in M1 macrophages and DCs following stimulation. It upregulates the expression of several glycolytic genes and aids in the switch to glycolysis by maintaining levels of nicotinamide adenine dinucleotide (NAD+), a vital co-factor in the glycolytic pathway. It does so by increasing the expression of lactate dehydrogenase (LDH), which reduces pyruvate to lactate and consequently generates NAD+, which will be reduced to NADH by an active glycolytic pathway. It also increases pyruvate dehydrogenase kinase (PDK) expression which phosphorylates and inhibits pyruvate dehydrogenase (PDH), thereby limiting acetyl CoA production which enters the Krebs cycle18.
While the primary role of HIF-1α is to induce a switch to glycolysis, which is likely to be important at sites of inflammation where oxygen levels are low13, hypoxia is not essential for HIF-1α activation in immune cells. Ligation of TLR4 by LPS can transcriptionally induce HIF-1α and the induction of a range of HIF-1α target genes under hypoxic and also normoxic conditions2, in an NF-κB-dependent manner19.
LPS can also stabilize HIF-1α indirectly, by increasing succinate levels. Evidence for the role of succinate in HIF-1α stabilization first came from cancer studies. An elevation in succinate had been reported in certain tumours that possess mutations in the gene encoding SDH, the enzyme responsible for the conversion of succinate to fumarate20. The PHDs generate succinate from α-KG, and importantly succinate (acting via product inhibition) will inhibit the PHDs and stabilize HIF-1α21. An additional mechanism by which succinate accumulates tumors involves the mitochondrial chaperone, tumor necrosis factor receptor-associated protein 1 (TRAP1) which is highly expressed in many tumors22. TRAP1 decreases SDH activity and succinate accumulates as a result. More recently, however, it has been demonstrated that succinate accumulation in tumours promotes angiogenesis via upregulation of vascular endothelial growth factor (VEGF) in a HIF-1α-independent manner. Succinate instead activates extracellular regulated kinase (ERK) 1/2 and signal transducer and activator of transcription 3 (STAT3) in a SUCNR1-dependent manner23.
Analogous to tumours, LPS-induced succinate was shown to both directly and indirectly (via ROS) inhibit PHD activity in macrophages resulting in stabilization of HIF-1α2. Like succinate, reactive oxygen species (ROS) are critical regulators of HIF-1α activity. ROS-can stabilize HIF-1α by inducing non-enzymatic decarboxylation of α-KG24 and by oxidizing iron (Fe2+) to Fe3+, two required PHD co-factors25. ROS also impair the activity of Factor Inhibiting HIF (FIH), another member of the α-KGDD family25, which hydroxylates an asparagine residue in the carboxy terminal of HIF-1α resulting in a decrease in transcriptional activity. It should be noted that hypoxia and ischemia, as well as decreased oxidative phosphorylation, drive succinate accumulation in the mitochondrial matrix26,27. As such, following ischemia/hypoxia, or when SDH operation is affected by genetic aberrations (such a tumors harboring SDH mutations) elevated succinate can potentiate the hypoxic phenotype by leading to further HIF-1α stabilization.
LPS-induced HIF-1α not only promotes glycolysis but is also directly pro-inflammatory. The finding that hypoxia was capable of inducing IL-1β production in astrocytes in an NF-κB-independent, HIF-1α-dependent, mechanism 28 led to the discovery of a HRE in the IL-1β promoter 2. The sustained induction of IL-1β in response to LPS was shown to require HIF-1α and to occur under normoxia. The loss of HIF-1α in macrophages decreased TNF-α, IL-1β and IL-1α production, but did not affect production of the anti-inflammatory cytokines IL-4 or IL-10 29. A critical consequence of succinate elevation in response to LPS is the potentiation of inflammatory signalling and in particular IL-1β, in a HIF-1α-dependent manner2. This requires both succinate oxidation by the enzyme succinate dehydrogenase (SDH) and an increase in mitochondrial membrane potential. These factors combine to drive pro-inflammatory ROS production, HIF-1α stabilization and increased IL-1β following LPS treatment2,30. Succinate reciprocally decreases anti-inflammatory cytokines, such as IL-10 and IL-1RA, but whether this is HIF-1α-dependent remains to be explored30. Limiting succinate oxidation with the pro-drug dimethylmalonate (DMM), which releases malonate that inhibits SDH function, profoundly represses LPS-induced HIF-1α, IL-1β and ROS and boosts IL-10 and IL-1RA. ROS production in this setting is believed to be as a result of reverse electron transport (RET) at complex I of the electron transport chain. In support of this the complex I inhibitor rotenone significantly decreased LPS-induced ROS. It should be noted that if the ETC was operating in the forward direction rotenone would boost ROS production. To further investigate this the authors examined macrophages from mice expressing an alternative oxidase (AOX) from Ciona intestinalis31. AOX provides a pathway to oxidize excess electrons that build up in the ubiquinone (CoQ) pool (as a result of succinate accumulation, for example) that can contribute to mtROS production. AOX expression in macrophages also impaired LPS-induced ROS production. These data suggest that complex I of the ETC is an important site of ROS production in macrophages and such ROS production may depend on RET. In other settings complex I can generate significant ROS when operating in the forward direction. Deletion of a critical subunit of complex I, Ndufs4, is sufficient to generate substantial ROS in macrophages, most likely as a result of impaired electron shuttling down the ETC and accumulation at ROS-producing sites in CI, which in turn will promote inflammation32. This is unlikely to be RET-dependent ROS production as the membrane potential, which much be significantly elevated to drive ROS via RET, is decreased in Ndufs4-deficient macrophages. Furthermore, these studies were performed in the absence of a stimulus like LPS which is know to drive Q pool reduction (another phenotype required for RET). These data suggest that complex I can generate ROS through a variety of mechanisms in macrophages to promote inflammation.
The relationship between HIF-1α and succinate in macrophages is quite well understood however this is less well explored in other cell types, particularly adaptive immune cells such as CD4+ T cell subsets. The demonstrating that LPS was capable of driving profound succinate accumulation in macrophages may be of relevance. Whether inflammatory stimuli also boost succinate levels in other immune cells remains to be explored.
2.3. The interplay between succinate, the epigenome and innate immune memory
α-KG and succinate can regulate the activity of other members of the α-KGDD family of enzymes, in particular those involved in the regulation of histone and DNA demethylation, the α-KG-dependent Jumonji C-domain containing histone demethylases (JMJDs) and the Ten eleven translocation (TET) family of 5mC hydroxylases, which play a role in DNA demethylation. Like the PHDs, these enzymes are subject to product inhibition by succinate and therefore their activity is dependent on the ratio of α-KG to succinate 33. In this way succinate, and α-KG, can remodel the epigenome. Methylation marks on histones can both positively and negatively influence gene expression while DNA methylation is typically associated with an open chromatin state and active transcription, therefore epigenetic changes can alter gene function, acting as on/off switches. Importantly, these changes in gene expression are heritable and appear to be intimately linked to the metabolic state of cells34. Succinate, fumarate and α-KG may also indirectly regulate the activity of histone demethylases through their effects on HIF-1α which can bind to and induce the expression of certain histone demethylases including JMJD1A, JMJD2C and JMJD2B, histone 3 lysine 9 (H3K9) demethylases 34.
Another critical consequence of altering the epigenome is the newly emerging concept of innate immune training. It has been recently revealed that innate immune cells demonstrate a form of immunological memory and have the ability to respond more robustly to a second stimulation that is not necessarily related to the first35. At a molecular level this involves epigenetic modifications, leading to stronger gene transcription upon re-stimulation, as opposed to gene recombination that occurs in the adaptive memory response. Epigenetic marks can persist after the stimulus that induced them has resolved and are therefore stable signals that can be sustained for days or even longer. Such innate immune training has been demonstrated to occur when human monocytes were trained in vitro with β-glucan, a component of Candida albicans, and subsequently treated with LPS, with elevated cytokine production observed upon re-stimulation with LPS 36. Interestingly, in addition to potentiating cytokine production in response to LPS, training with β-glucan induced epigenetic upregulation of genes involved in glycolysis such as HIF-1α, Hexokinase (HK) and pyruvate kinase and increased succinate levels37. HIF-1α reciprocally enhanced trained immunity in response to β-glucan. Inhibition of HIF-1α with ascorbate impaired the training effect induced by β-glucan and decreased TNF-α production in these cells, demonstrating a requirement for HIF-1α in the training effect induced by β-glucan. Training with β-glucan was shown to be protective against Staphylococcus aureus infection and this effect was abrogated in HIF-1α-deficient mice. As shown in Figure 2, succinate and other metabolites may therefore be capable of influencing the epigenome through its effects on HIF-1α and perhaps subsequently on IL-1β, which has also been demonstrated to induce trained immunity in monocytes37. Whether other stimuli other than β-glucan are capable driving a similar training phenotype warrants further investigation.
2.4. Succinylation as a covalent modification to regulate multiple targets
Another consequence of dysregulated succinate metabolism is the recently identified post-translational modification (PTM), lysine succinylation. This modification is caused by the accumulation of succinyl-CoA, which can result from SDH inhibition and succinate accumulation38. Treatment of mouse fibroblasts with the SDH inhibitor 3-nitropropionic acid increases succinylation38. This modification induces a 100 Da change in mass, comparable to that of two well-established lysine modifications: acetylation and dimethylation. Importantly, it will mask the positive charge on lysine likely resulting in a significant conformational change. Western blot analysis of whole cell lysates revealed that this modification is evolutionarily conserved and that substrates are numerous39 and include proteins involved in cellular metabolism38. Succinyl-proteome profiling in bacteria40, plants41,42, and HeLa cells all point towards metabolic pathways as key targets for this PTM. A study in yeast identifies histones as targets of this PTM with mutation of succinylation sites having a variety of effects: reducing cell viability, loss of silencing at telomeres and rDNA, and changes in temperature sensitivity43.
While the enzyme responsible for succinylation is yet to be identified, and indeed it is likely to be non-enzymatic by direct reaction between succinyl CoA and the modified protein47, a potent desuccinylase (and demalonylase) has been uncovered44. SirT5, which was previously thought to function primarily as a deacetylase has been shown to have potent desuccinylase activity 44. Interestingly, SDHA is a target of lysine succinylation. SirT5-deficient mice had significantly increased SDH activity suggesting that succinylation positively regulates its activity38. This PTM appears to be LPS-inducible. LPS decreases sirT5 expression in macrophages and increases protein succinylation2.
The α-ketoglutarate dehydrogenase complex (KGDHC) has also been suggested to mediate succinylation in an α-ketoglutarate-dependent manner. Inhibition of KGDHC reduces succinylation of proteins in neuronal cells. The authors identify the PDHC (pyruvate dehydrogenase complex) isocitrate dehydrogenase (ICSD) and fumarase as targets of succinylation with succinylation decreasing ICSD activity and increasing fumarase activity45.
Succinylation can also modulate macrophage function. Succinylation of Lys311 of pyruvate kinase M2 (PKM2), a key glycolytic enzyme required for the shift to glycolysis in activated macrophages, was shown to limit its activity by promoting its tetramer-to-dimer transition46. The authors demonstrate that SIRT5 desuccinylates and activates PKM2 and this limits IL-1β production. Conversely, SIRT5-deficient mice exhibit hypersuccinylation and increased IL-1β. There are many aspects of this PTM that require further investigation. The breath of the targets of succinylation and indeed the enzyme responsible for this PTM remain to be determined as well as precisely how this PTM alters protein function.
These data demonstrate that succinate can have profound impacts on cellular function acting both intracellularly via ROS and HIF-1α, succinylation and histone and DNA modification and extracellularly via SUCNR1.
3. Itaconate as a key anti-inflammatory metabolite and novel player in tumor biology
One of the most striking examples of a Krebs cycle-derived metabolite acting as a signal in immunity is itaconate1,47–50. This previously unidentified carboxylic acid was first discovered in 1836 as a product of citric acid distillation by the Swiss chemist Samuel Baup51. In 1840 it was independently synthesised by the decarboxylation of cis-aconitate, resulting in the introduction of its current name, itaconic acid, an anagram of cis-aconitic acid51. While itaconic acid has long been used in the industrial arena, owing to its reactive methylene group which enables self-polymerisation to polyitaconic acid (a valuable precursor for the synthesis of various polymers), the first (unwittingly) relevant discovery regarding the role of itaconate in mammalian biology came in 1995 when Lee and colleagues cloned immunoresponsive gene 1 (Irg1), a gene found to be potently upregulated in LPS-activated peritoneal macrophages51,52. It was not until 2011 however, that the production of itaconic acid in a mammalian system, and the first suggestion it may play a role in cellular immunity, was uncovered53,54. Intriguingly, it was described in two separate immune contexts, both in the lungs of Mycobacterium tuberculosis (Mtb)-infected mice53 and secreted into the supernatant of LPS-activated RAW264.7 cells (a macrophage cell line)54. A key discovery in the field of itaconate biology followed when Michelucci and colleagues (2013) identified IRG1 as the enzyme responsible for itaconate synthesis in both mouse and human macrophages55. This subsequently led to the renaming of IRG1 to cis-aconitate decarboxylase (CAD). Although a role for itaconate as an anti-bactericidal agent has previously been suggested [see refs 55–58], more recently, itaconate has been found to be an important immunomodulatory metabolite50,59–61.
3.1. Itaconate is an endogenous inhibitor of SDH and an anti-inflammatory metabolite
Despite the known anti-bacterial function of itaconate, its role in regulating macrophage function remained virtually unexplored until 2016. Since 2016, several key studies have uncovered a role for itaconate as a crucial anti-inflammatory metabolite that negatively regulates the inflammatory response and cytokine production50,59–61, as shown in Figure 3. Importantly, Lampropoulou and colleagues (2016) first demonstrated that bone-marrow derived macrophages (BMDMs) activated by LPS and pre-treated with a cell permeable methyl ester derivative of itaconate, dimethyl itaconate (DI), exhibited potent inhibition of pro-inflammatory mediators including nitic oxide (NO), ROS and the cytokines IL-6, IL-12p70 and IL-1β13,50. Furthermore, Irg1-deficient BMDMs, whereby genetic deletion of Irg1 completely abolished itaconate synthesis, exhibited a significant increase in the production of IL-12, IL-6, NO, and under conditions that activate the NLRP3 inflammasome, increased IL-1β and IL-1850. An increase in HIF-1α, a critical regulator of aerobic glycolysis and IL-1β in macrophages, was also observed in Irg1-deficient BMDMs stimulated with LPS13,50. As such, this study was the first to highlight a role for itaconate as an anti-inflammatory metabolite.
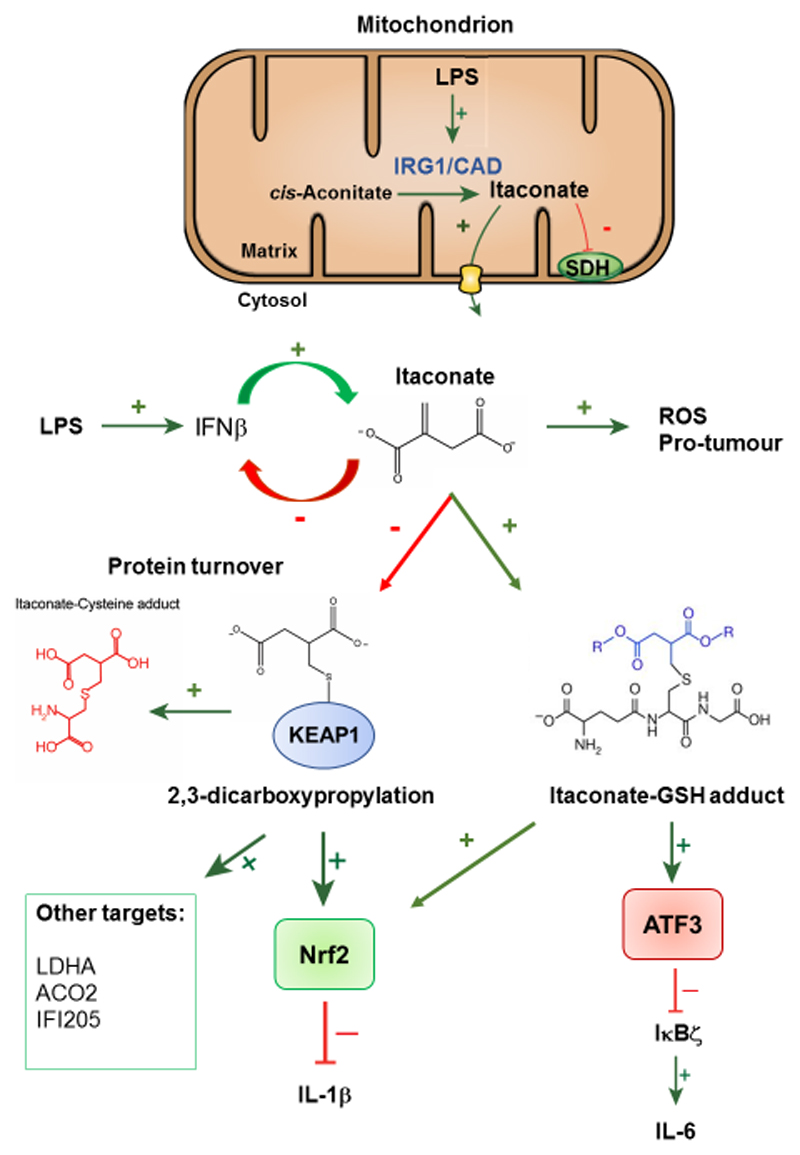
Figure 3. Itaconate is a thiol reactive anti-inflammatory metabolite.
In LPS-activated macrophages, mitochondrial IRG1/CAD synthesises itaconate from cis-aconitate, whereby it can inhibit SDH or is exported out of the mitochondria via the oxoglutarate carrier (OGC). In the cytosol, itaconate alkylates KEAP1, a novel PTM termed 2,3-dicarboxypropylation, and GSH to form an itaconate-GSH adduct, or 2,3-dicarboxypropyl-GSH. This in turn activates the anti-inflammatory and anti-oxidant transcription factor Nrf2 and ATF3. Levels of the metabolite 2,3-dicarboxypropyl cysteine (Itaconate-cysteine adduct) increase, which is indicative of the turnover of 2,3-dicarboxypropylated targets. Activation of Nrf2 acts to negatively regulate the pro-inflammatory cytokine IL-1β, whilst activation of ATF3 inhibits IκBζ and IL-6.
Lampropoulou and colleagues (2016) suggested that mechanistically the ability of itaconate to modulate inflammation arose from its ability to competitively inhibit SDH and succinate oxidation50. Intriguingly, itaconate was first shown to act as a competitive SDH inhibitor more than 60 years ago62, which prompted speculation that it was an endogenous SDH inhibitor, akin to malonate, in macrophages48–50. Supporting this, exogenous treatment of macrophages with DI, or RAW264.7 cells and A549 cells (a lung adenocarcinoma cell line) with itaconic acid, was found to induce succinate accumulation, indicative of SDH inhibition48,49. Likewise, itaconic acid was found to competitively inhibit purified SDH50. Crucially, LPS-stimulated Irg1-deficient BMDMs displayed significantly attenuated succinate accumulation and increased oxygen consumption rates (OCR) further supporting its role in SDH inhibition and metabolic rewiring in macrophages49,50. As such, itaconate inhibition of SDH was proposed to limit inflammation by blocking the generation of ROS derived from RET at complex I50, as previously discussed. Together these papers offered a molecular explanation for succinate accumulation1 and the breakpoint observed in the TCA cycle by Jha and colleagues (2015)1, whereby isotopic tracing using U-13C-glutamine showed that approximately 35% of the pool of succinate, but only 22% of malate, could be attributed to glutamine anaplerosis in BMDMs treated with LPS, suggesting inefficient succinate-to-fumarate transition at SDH.
However, it must also be noted that, compared with malonate, itaconate is a relatively weak competitive inhibitor of SDH47,49, which raised the question as to whether any additional mechanisms could contribute to the anti-inflammatory effects of itaconate. Furthermore, DI, the itaconate derivative used in these studies, is not metabolised to itaconate intracellularly. It has also been shown that exogenous addition of [13C] itaconate to RAW264.7 cells was sufficient to increase intracellular levels of unlabelled succinate (suggestive of SDH inhibition), with no evidence of cellular uptake63. It is possible that the observed effect of itaconic acid on succinate levels could be receptor-mediated, as posited by ElAzzouny and colleagues (2017), especially given the discovery of citric acid cycle intermediate signalling via GPCRs, such as that observed for succinate and SUCNR13,63. It is also important to note that itaconate is an α,β-unsaturated carbonyl compound, making it a potential Michael acceptor that could undergo nucleophilic attack by the thiolate ion of protein cysteine residues, as such the methyl esterification of the proximal carboxyl group conjugated to the alkene would be predicted to increase the electrophilicity of itaconate63. In this way, the effects of DI could possibly be attributed to electrophilic inactivation of metabolic enzymes, as opposed to an increase in intracellular itaconate63. Supporting this, Lampropoulou and colleagues (2016) reported an upregulation of genes involved in Phase II conjugation, glutathione conjugation and biological oxidations from an RNA-seq screen performed in DI-treated BMDMs50.
3.2. Itaconate is an electrophilic metabolite that alkylates redox sensitive cysteines
Independently, Mills and colleagues (2018) also noted that itaconate could potentially alkylate cysteine residues to form a 2,3-dicarboxypropyl adduct59, as shown in Figure 3. As such, it was hypothesised that the anti-inflammatory effects of itaconate could be due to alkylation of the key redox sensing protein KEAP1 and activation of the anti-inflammatory and anti-oxidant transcription factor Nrf259,64. DI was first employed as an experimental tool and was shown to potently induce Nrf2 and several Nrf2 target genes in control and LPS-activated BMDMs59. This strongly suggested that DI was indeed a thiol reactive metabolite, however, esterification on the 1-position would render it an activated Michael acceptor that could result in rapid Nrf2 activation, along with many other reactive intracellular thiols, akin to dimethyl fumarate (DMF)59,65. To overcome the limitations of DI, a new itaconate derivative, 4-octyl itaconate (4-OI), was synthesised whereby the octyl ester group was located on the carboxyl group distal to the alkene59. Importantly, itaconate and 4-OI were shown to have a similar thiol reactivity profile, whereas DI was shown to significantly and acutely deplete GSH levels, confirming it was indeed an activated Michael acceptor59. Furthermore, the ability of DI to induce NQO1 activity (a prototypical Nrf2 target gene) was diminished when pre-incubated with GSH, however, the ability of 4-OI to induce NQO1 activity remained unaffected59. These findings suggested that 4-OI represented a suitable cell-permeable itaconate surrogate with which to probe the physiological function of itaconate59. Importantly, pre-treatment of control and LPS-activated macrophages with 4-OI still resulted in a significant increase in Nrf2 and Nrf2 target genes, namely Heme oxygenase 1 (HMOX1), suggesting itaconate was an endogenous signal governing Nrf2 activation59. This finding was corroborated by Bambouskova and colleagues (2018) who demonstrated a decrease in LPS-induced Nrf2 stabilisation in Irg1-deficient BMDMs, confirming that endogenous itaconate stabilizes Nrf260.
Further supporting the hypothesis that itaconate was a thiol reactive metabolite, a significant increase in the levels of the metabolite 2,3-dicarboxypropyl cysteine (itaconate-cysteine adduct), akin to (S)-2-succinocysteine (2SC) a breakdown product of fumarate-mediated protein succination, was observed in LPS-activated BMDMs59. Mechanistically, 4-OI was shown to alkylate KEAP1 on several cysteine residues, namely cysteine 151 (C151), a principal redox sensing cysteine important for activation of Nrf259,66. Furthermore, using a quantitative unbiased proteomic screen performed in both LPS (which will drive intracellular itaconate accumulation) and 4-OI-treated macrophages, several metabolic and inflammatory proteins were found to be alkylated representing the first demonstration of this novel post-translational modification (PTM), termed 2,3-dicarboxypropylation59. Pre-treatment of LPS-activated BMDMs with 4-OI also resulted in a significant attenuation of IL-1β levels, an effect that was largely abrogated in Nrf2-deficent macrophages59. It should also be noted that Nrf2 can also limit LPS-induced inflammatory gene transcription, in a redox-independent manner, through direct ligation of these genes and inhibition of RNA polymerse II recruitment47. Whether itaconate can mediate cytokine inhibition via this process remains to be explored. 4-OI was also shown to be protective in an LPS lethality model in vivo, prolonging survival, improving body temperature regulation and decreasing pro-inflammatory cytokine production59. In addition, a novel negative feedback loop between IRG1, itaconate and interferon (IFN)-β was elucidated, whereby the induction of Irg1 and itaconate was largely dependent on autocrine/paracrine type I interferon signalling59. Intriguingly, pre-treatment of LPS-activated macrophages with 4-OI resulted in a significant attenuation of IFN-β levels and interferon-stimulated genes (ISGs), namely ISG20, an effect that was at least partially dependent on Nrf259. As such, this study confirmed itaconate as an anti-inflammatory metabolite and provided a unique insight into its mechanism of action with the discovery of a novel PTM. Further work is required to understand the scope of this PTM and its effect on protein function.
In addition to Nrf2 regulation, an NF-kappa-B inhibitor zeta-Activating Transcription Factor 3 (IκBξ-ATF3) inflammatory axis has recently emerged as a target of itaconate and its more electrophilic derivatives60. Bambouskova and colleagues (2018) demonstrated that DI could induce electrophilic stress and Nrf2 in macrophages via depletion of intracellular GSH to form a DI-GSH adduct, akin to 2,3-dicarboxypropylation60, as previously discussed. Interestingly, DI is reported to selectively inhibit LPS-induced IL-6 while TNF-α levels remain unaffected, an effect shown to be mediated through inhibition of IκBξ the major transcription factor governing secondary transcriptional responses to TLR activation60. Mechanistically, inhibition of IκBξ by DI was shown to be mediated by activation of the transcription factor ATF3 and occur at the level of Nfkbiz translation, as assessed by eIF2α phosphorylation, in a Nrf2-independent manner60.
The use of itaconate ester derivatives as endogenous itaconate mimics poses limitations due to varying electrophilicities, depending on whether the ester group lies on the carboxyl group proximal or distal to the methylene. To assess this, Bambouskova and colleagues (2018) synthesised 1-ethyl itaconate (1EI) and 4-ethyl itaconate (4EI) and tested their effect on IκBξ. They demonstrate that 1EI (an activated Michael acceptor) could also inhibit IκBξ however, 4EI (which has similar reactivity to endogenous itaconate) could not inhibit IκBξ unless GSH synthesis was inhibited by buthionine sulfoximine (BSO)60. This highlighted the importance of acute electrophilic stress required to induce this novel pathway. To determine the endogenous relevance of itaconate to the IκBξ-ATF3 inflammatory axis, the authors utilised Irg1-deficient macrophages, however, no differences were observed in IκBξ levels between Irg1-/- and wildtype macrophages stimulated with LPS60. As it takes time for itaconate to accumulate in response to stimulus and due to its much weaker electrophilicity (reactivity with high pKa thiol groups, such as that found on GSH, are likely to take much longer than reactivity with low pKa thiols, such as C151 on KEAP1), it is unlikely to have any physiological relevance to this canonical pathway. However, the authors posited that elevated itaconate levels may affect induction of IκBξ in LPS-tolerized macrophages60. Although induction of IκBξ was much lower in LPS-tolerized macrophages, there was an increase in Irg1-deficient macrophages, suggesting that at later timepoints when itaconate has built up to sufficient levels (> 18 h) it may induce low levels of electrophilic stress by modifying GSH, which subsequently regulates the tolerization process60. As such, a unique link between electrophilic stress and the IκBξ-ATF3 inflammatory axis was uncovered that may be exploited for therapeutic gain.
A role for IRG1 and itaconate in the tolerization process, and as a negative regulator of inflammation, is supported by the findings that IRG1 suppresses the production of the pro-inflammatory cytokines TNF-α, IL-6 and IFN-β in LPS-tolerised BMDMs67. Yingke and colleagues (2013) attributed the effect of IRG1 on cytokine production to ROS-mediated induction of A20, a negative regulator of TLR signalling. Supplementation of ROS in Irg1-deficient BMDMs increased A20 expression and abolished the break of endotoxin tolerance67. More recently, IRG1 was also found to be significantly increased in A20-deficient macrophages, which suggests an interesting feedback loop may exist between IRG1 and A20 expression68. Intriguingly, Yingke and colleagues (2013) also observed increased IFN-β and IRF3 signaling in LPS-tolerized Irg1-silenced macrophages, supporting the existence of a negative feedback loop between IRG1, itaconate and IFN-β, as previously mentioned59. IRG1 has also been shown to mediate the immunomodulatory effects of CO releasing molecule 2 (CORM2)-induced HMOX1 on TNF-αproduction in both LPS-activated macrophages and in a murine endotoxin shock model69, and it also appears to play a major role in embryo implantation in the womb, a process thought to require immunosuppression70–72. More recently, an important role for myeloid-derived IRG1 (and presumably itaconate) in Mtb-infection was uncovered73. Nair and colleagues (2018) convincingly demonstrated an increase in pro-inflammatory cytokine production, including IL-6 and IL-1β, in Mtb-infected Irg1-deficent mice; this effect was independent of its ability to act as an anti-bactericidal agent73. Interestingly, Irg1 was shown to specifically impair neutrophil recruitment to the lungs to curtail excessive lung inflammation and pathology, an effect thought to be a consequence of itaconate-mediated transcriptional regulation of the inflammatory response73. These data suggest that itaconate may dampen the immune response of Mtb-infected mice to limit immunopathology.
The role for itaconate in immune cell activation is still in its infancy and the profound levels to which it is produced in macrophages suggest that it is likely to be central to macrophage function. Further work to decipher its potentially diverse roles and whether it has important signalling roles outside macrophage biology are on-going
3.3. Itaconate in macrophage-tumor crosstalk
Most recently, an intriguing study in peritoneal tumours (B16 melanoma and ID8 ovarian carcinoma) has demonstrated that tumour-infiltrating macrophages release itaconate which potentiates tumour growth74. Itaconate was shown to boost OXPHOS and mtROS generation and subsequent MAPK activation in tumour-infiltrating macrophages (Figure 3). The effect of itaconate on ROS production is consistent with a previous study in zebrafish macrophages, which demonstrated that IRG1 was essential for OXPHOS-driven mtROS production and bactericidal killing172. Furthermore, Weiss and colleagues (2018) demonstrated that ROS production in response to itaconate regulates Nrf2, suggesting there are two mechanisms for itaconate-induced Nrf2 activation; directly via KEAP1 2,3-dicarboxypropylation and indirectly via ROS production (which would then modify KEAP1)75. Nrf2 therefore appears to be an important target for itaconate. Furthermore, IRG1 (and presumably itaconate) levels were also shown to be markedly increased in glioma tissue75. This was associated with a decrease in the microRNA miR-378, which targets Irg1 as well as poorer overall survival and clinicopathological parameters. Overexpression of miR-378 suppressed glioma tumour growth both in vitro and in vivo, the epithelial-mesenchymal transition (EMT) and metastasis75. These data are consistent with previous reports demonstrating that Irg1 acts as an oncogene, driving glioma pathogenesis171. As such, itaconate serves as another example of crosstalk between macrophages and tumours in the context of metabolic reprogramming.
4. Fumarate, a mitochondrial messenger of the immune system and tumor microenvironment
Fumarate is another Krebs cycle intermediate that is generated through the oxidation of succinate by succinate dehydrogenase and is also produced as a breakdown product of tyrosine metabolism and is produced from the urea and purine nucleotide cycles48. Fumarate is then broken down to malate by the enzyme fumarate hydratase (FH), which catalyses the reversible hydration of fumarate to malate both within the Krebs cycle and in the cytosol, as shown in Figure 4.
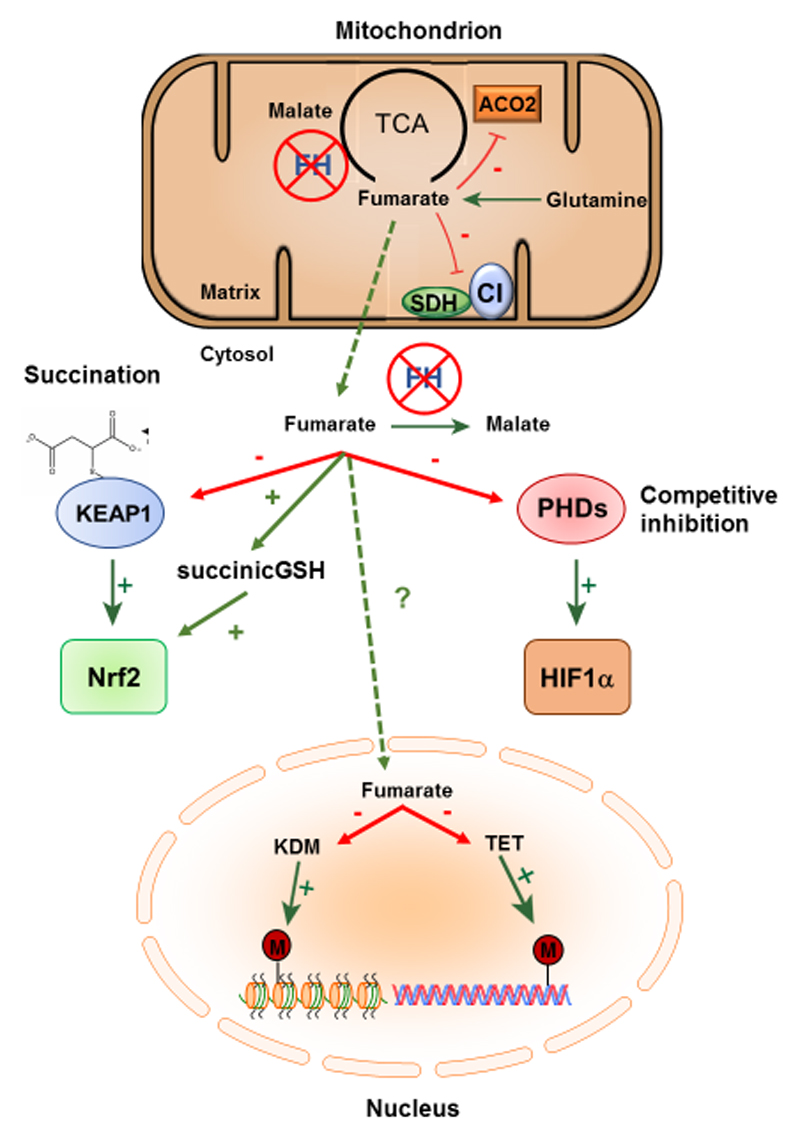
Figure 4. Fumarate is an oncometabolite and epigenetic modifier.
Under conditions of FH-deficiency, fumarate levels markedly increase leading to perturbations in mitochondrial OXPHOS. Elevated fumarate acts as a competitive inhibitor of SDH and inhibits Complex 1 via succination of key [Fe-S] cluster biogenesis proteins. In the cytosol, increased fumarate can succinate KEAP1 and GSH to activate Nrf2, whilst fumarate also acts to competitively inhibit PHDs and stabilise HIF1α. In the nucleus, fumarate acts as an epigenetic modifier, whereby it can inhibit the KDM family of histone demethylases and the TET family of DNA demethylases, which acts as a signal to induce EMT.
4.1. Fumarate as a regulator of the epigenome, innate immune memory and immune cell function
Fumarate has recently emerged as an important inflammatory signal in innate immune training, as outlined above37,97. Innate immune training, also known as trained immunity, is a property of macrophages, monocytes and natural killer cells that enables enhanced responsiveness to re-invading pathogens independently of classical immunological memory carried out by T and B lymphocytes37,97. Importantly, Arts and colleagues (2016) demonstrated that the accumulation of fumarate in β-glucan-treated monocytes was essential for trained immunity by enhancing cytokine production upon re-stimulation with LPS97. Furthermore, increased fumarate levels were shown to be driven by enhanced shunting of glutamine into the TCA cycle, otherwise referred to as glutamine anaplerosis, as ascertained by a combination of biochemical techniques and the use of the glutaminase inhibitor, BPTES97. As previously discussed, fumarate is a known epigenetic regulator exerting its modulatory effects primarily through inhibition of α-KGDDs91,97. To determine the role of increased fumarate levels on the epigenetic landscape, Arts and colleagues (2016) used the cell permeable fumaric acid ester, monomethyl fumarate (MMF), which was shown to alter histone methylation with similarities to β-glucan induced training97. Furthermore, treatment with MMF augmented TNF and IL-6 secretion in β-glucan-trained macrophages stimulated with LPS97. The authors proposed that fumarate accumulation acted to inhibit the KDM5 family of histone demethylases, which subsequently increased the levels of H3K4me3, a marker of active gene transcription, at the promoters of both Tnf and Il6, thus providing the first link between Krebs cycle rewiring and epigenetic regulation in an inflammatory context97.
Outside of trained immunity, fumarate has previously been reported to accumulate in LPS-activated macrophages1,13. Using an integrated high-throughput transcriptional metabolic profiling and analysis pipeline (CoMBI-T), Jha and colleagues (2015) described the induction of an inflammatory argininosuccinate shunt, a metabolic pathway that links the Krebs cycle to the Urea cycle1. Induction of this pathway occurred via an increase in the expression of argininosuccinate synthase (Ass1), which catalyses the formation of argininosuccinate from aspartate, citrulline and ATP98. Together with the argininosuccinate lyase (Asl), Ass1 is responsible for the synthesis of the semi-essential amino acid arginine in most bodily tissues98. Importantly, Ass1 also constitutes the rate-limiting step in the aspartate-argininosuccinate shunt and L-arginine biosynthesis98. As Asl cleaves argininosuccinate into arginine and fumarate, the authors proposed that this metabolic pathway accounted for the elevated fumarate levels observed in macrophages1.
Fumarate can also regulate T cell function. Blewett and colleagues (2016) demonstrated that dimethyl fumarate (DMF), a potent electrophile which is currently used to treat psoriasis and relapse-remitting MS, but not MMF inhibited the activation of human and mouse T cells 49. By employing a proteomic approach the authors discovered a range of proteins which regulate T cell function that are sensitive to covalent modification by DMF including protein kinase C θ (PKCθ). Mechanistically, DMF blocked the association of PKCθ with the costimulatory receptor CD28 and T cell activation.
More recently, Kornberg and colleagues (2018) suggested that a potential consequence of elevated fumarate levels is to negatively regulate glycolysis via succination of the active site cysteine residue (C152) in GAPDH99,100. This suggestion arose from the observation that the cell-permeable fumaric esters, DMF and MMF, modify and irreversibly inhibit GAPDH enzymatic activity and aerobic glycolysis in peripheral blood mononuclear cells (PBMCs) from mice and MS patients treated with DMF99. Induction of aerobic glycolysis is a key marker of activated immune cells and represents an important phenotypic switch to facilitate proliferation and immune cell effector functions99. Two pro-inflammatory T cell subsets implicated in the pathogenesis of MS, namely TH1 and TH17 cells, rely heavily on aerobic glycolysis and treatment with DMF or MMF inhibited their development in vitro, as previously reported99,101,102, 49. Importantly, inhibition of aerobic glycolysis by the specific GAPDH inhibitor heptelidic acid (HA) and 2-deoxyglucose (2-DG) recapitulated the immunomodulatory effects of DMF in vitro, while in vivo administration of HA inhibited the development of experimental autoimmune encephalomyelitis (EAE), a murine model of MS99. As such, this represents an important proof-of-principle study supporting the concept that metabolically targeted therapies may be used successfully to treat inflammatory-driven diseases, while also providing mechanistic insight into the anti-inflammatory action of DMF and MMF99. Intriguingly, endogenous fumarate was also found to succinate GAPDH in both mouse and human macrophages, suggesting that elevated fumarate levels may act as an endogenous anti-inflammatory signal to limit inflammation, however, the precise role of fumarate accumulation remains an open question to be explored. It is important to note that while HA and DMF have proven beneficially in the treatment of debilitating decreases such as MS prolonged/systemic inhibition of glycolysis is likely to be detrimental to the host and will impair immune (both innate and adaptive) cell function which in the context of infection would be unfavourable. Further work is required to fully understand the effect of prolonged glycolytic inhibition.
4.2. Fumarate and cellular transformation
In addition to its role as an inflammatory regulator, fumarate can also be viewed as an oncometabolite Given its central role in energy metabolism, FH was long considered a housekeeping enzyme76. Consistent with this view, homozygous FH loss in fumaric aciduria, an autosomal recessive metabolic disorder, leads to very severe neurological disorders and is fatal in early childhood77. However, in 2002 it was shown that mutations of FH are the cause of Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC), a cancer predisposition syndrome characterised by benign tumours of the smooth muscle of skin and uterus, and a very aggressive form of renal cancer78. These findings indicated not only that cells survive the loss of FH, but in specific tissues, they can also undergo transformation to tumour cells. How FH loss leads to cancer has been a long standing question in the field.
The defining biochemical feature of FH loss is the accumulation of fumarate, which has been implicated in tumorigenesis, acting as an oncometabolite79. To date, several biological functions have been ascribed to fumarate. For instance, accumulated fumarate can bind and inactivate reactive thiol residues of proteins and peptides in a process called succination, a protein modification originally described in diabetes80. This function of fumarate dervives from its unsaturated dicarboxylic acid structure, which makes it a mildly electrophilic molecule that can be involved in a Michael addition with nucleophiles, such as reactive thiol residues of proteins. The first insights into the relevance of succination in HRLCC patients was proposed in 2011, when it was shown that fumarate could bind reactive thiol residues and inactivate KEAP1, the negative regulator of the master antioxidant transcription factor Nrf2, leading to a powerful antioxidant response81,82, as shown in Figure 4. Later, it was also shown that fumarate binds glutathione (forming succinic-glutathione (succinicGSH)), and thereby depletes this antioxidant molecule leading to unscheduled oxidative stress83,84. Of note, fumarate-dependent oxidative stress was shown to drive renal cells to senescence in an FH-deficient mouse model, and its bypass by the co-ablation of p21 increased malignant transformation in the kidneys503. Consistent with an important role of senescence bypass for the development of tumorigenesis, recent data in HLRCC patients showed that CDKN2A (also known as P16), a key player in senescence, is hypermethylated and suppressed in tumours85. Fumarate was shown to cause succination of the mitochondrial aconitase, ACO2, leading to an additional truncation of the TCA cycle86, and also iron-responsive element binding protein (IRP)2, promting the transcription of transferrin87. Finally, it was shown that fumarate can drive succination of several members of the family of Iron Sulfur (Fe-S) cluster biogenesis proteins, causing a depletion of Fe-S clusters required for the activity of mitochondrial enzymes, including complex I88. All these reactions appear to be independent of enzymatic catalysis, driven by the high level of fumarate accumulation and are irreversible. Succination is a hallmark of FH-deficient tumours and an antibody against succinate protein has been proposed as a diagnostic marker for HLRCC89.
As mentioned, fumarate also inhibits a variety of α-KGDDs, enzymes involved in multiple cellular processes, from metabolism to signalling and epigenetics90-92, as shown in Figure 4 Examples of α-KGDDs inhibited by fumarate are the PHDs, negative regulators of HIFs93. Accordingly, fumarate causes HIF stabilisation, even under normoxic conditions, i.e. pseudohypoxia, a hallmark of FH-deficient tumours93. α-KGDDs are also involved in de-methylation of DNA, RNA, and histones. Recent data demonstrated that fumarate acts as a powerful inhibitor of the TET family of DNA demethylases and of a series of histone demethylases91,92. In this context, fumarate is considered an important epigenetic modifier (reviewed in 94). It has also been shown that fumarate accumulation leads to the inhibition of TET-dependent DNA demethylation of a class of antimetastatic miRNAs, the miR200 family, promoting an EMT95, a process involved in cancer initiation and metastasis96. Of note, FH-deficient renal cancers are characterised by a distinct DNA hypermethylation phenotype, which also affects the tumour suppressor CDK2NA85, indicating that this epigenetic activity of fumarate may have a critical role in tumour aetiology, by allowing senescence escape.
Finally, fumarate and FH were recently proposed as key players in the DNA damage response. Originally described in yeast176, FH has a “moonlighting” role in the nucleus that capitalises on the epigenetic-modifying function of fumarate. Upon DNA damage, FH localises in the nucleus, where it is phosphorylated by the catalytic subunit of DNA-PK (DNA-PKcs)177. Here, via its reverse activity, it generates fumarate that inhibits histone lysine demethylase KDM2B, which in turn facilitates the binding of proteins involved in DNA repair177. In support of this unexpected function of fumarate, it was recently shown that the aberrant accumulation of this metabolite in HLRCC correlates with increased endogenous damage, lower DNA repair efficiency and increased sensitivity to poly-ADP ribose polymerase (PARP) inhibitors178. Of note, this biological function of fumarate is also shared with succinate178 and 2HG (see below). It has also recently been shown that FH loss in renal cancer cells leads to increased DNA damage and resistance to ionising radiation (IR), due to a fumarate-dependent inhibition of mitotic entry after IR, even in the presence of unrepaired damage179. Overall, these lines of evidence provide a potential model for how fumarate accumulation promotes genomic alterations that could give rise to cancer formation in HLRCC patients, cooperating with the other above-described oncogenic signals it elicits.
5. 2-hydroxyglutarate in immunity and cancer
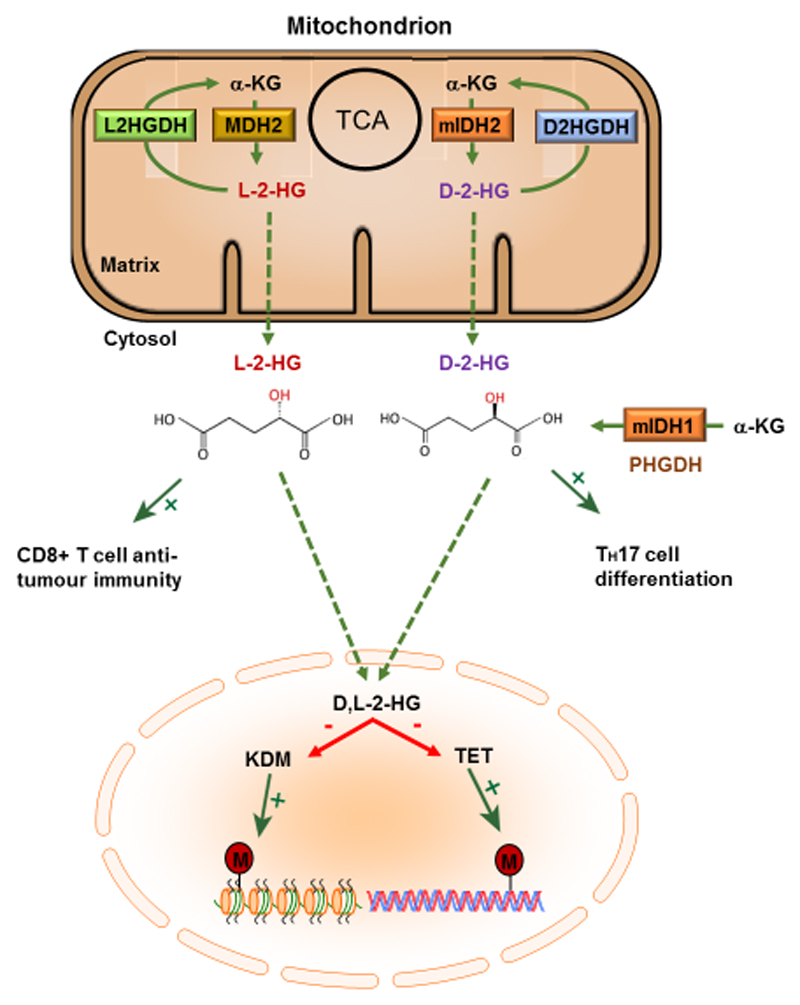
The next intermediate we will consider is 2-HG, which exists in two enantiomeric forms, L-2-HG and D-2-HG103, as shown in Figure 5. Like itaconate, 2-HG was first discovered in the 1800s (in this instance by the German biochemist Karl Heinrich Ritthausen), however, it did not attract much attention until recently when its physiological function was discerned103. Interest in these enantiomers heightened in the 1980s when they were linked to two rare but clinically related diseases, now termed L-2-hydroxyglutaric aciduria (L2HGA) and D-2-hydroxyglutaric aciduria (D2HGA), which manifest early in childhood and usually lead to severe disability and mental retardation in adulthood104-106. The cause of these so-called 2-hydroxyglutaric acidurias (2HGAs) was attributed to germline mutations in L-2-HG dehydrogenase (L2HGDH) and D-2-HG dehydrogenase (D2HGDH), the mitochondrial enzymes responsible for the conversion of 2-HG to α-KG and thus its breakdown107-109. In the case of D2HGA, about 50% of patients present with D2HGDH mutations, while the remaining half possess mutations in the Krebs cycle enzyme isocitrate dehydrogenase 2 (IDH2), which converts isocitrate to α-KG110. A third 2HGA that arises from germline mutations in the mitochondrial citrate carrier SLC25A1, in which both enantiomers accumulate, has also been discovered. This provides an intriguing link between mitochondrial citrate metabolism and 2-HG production111 that remains to be explored. In recent years, two landmark genomic studies carried out in human glioma and acute myeloid leukaemia (AML) found mutations in IDH1/2 and it is now appreciated that these enzymes (which drive elevated production of D-2-HG) are the most frequently mutated metabolic genes in human cancer112-114.
Figure 5. 2-hydroxyglutarate is an oncometabolite and epigenetic modifier.
2-hydroxyglutarate exists as two enantiomers L-2-HG and D-2-HG. Under conditions whereby IDH1/2 are mutated (mIDH1/2), this mutant enzyme preferentially acts on α-KG, as opposed to isocitrate, to generate D-2-HG. In this context, D-2-HG accumulates to high levels, however, under normal conditions D-2HG levels are maintained at low concentrations via D2HGDH. In response to hypoxia or acidic pH, MDH2 can promiscuously generate L-2-HG from α-KG, whose levels are usually maintained at low concentrations via L2HGDH. Accumulation of D-2-HG and/or L-2-HG in the nucleus results in the competitive inhibition of the KDM family of histone demethylases and the TET family of DNA demethylases, thus acting as an important epigenetic modifier driving tumorigenesis and as a regulator of T cell immunity.
5.1. 2-HG as an epigenetic modifier that governs T cell differentiation
While the role of 2-HG in tumorigenesis has been a heavy focus of research over the last several years, more recently, an unexpected role for this unusual metabolite has begun to emerge as a regulator of T cell function and inflammation. It has become increasingly appreciated that different T cell subtypes undergo dramatic metabolic remodelling that governs their proliferation, differentiation and effector functions142. T-helper 17 (TH17) cells, derived from naïve CD4+ T cells, are one such subset that rely heavily on a switch from OXPHOS to glycolysis, a process that requires the activation of HIF-1α and induction of RORγt143,146. On the other hand, induced regulatory T (iTreg) cells, also derived from naïve CD4+ T cells, do not require HIF-1α143,146. Interestingly, Xu and colleagues (2017) observed increased levels in of D-2-HG in TH17 cells, when compared to iTreg cells143. The elevated levels of D-2-HG was associated with increased DNA methylation levels at the Foxp3 locus, the master transcription factor driving iTreg differentiation, resulting in its repression143. Furthermore, TH17 cell differentiation could be initiated by exogenous addition of D-2-HG to naïve CD4+ T cells or by genetic silencing of TET1 and TET2, established targets of 2-HG143-145. Mechanistically, D-2-HG production was driven by the conversion of glutamate to α-KG by the aspartate aminotransferase GOT1. Inhibition of GOT1 by aminooxyacetic acid (AOAA), which decreases D-2-HG levels, promoted expression Foxp3 and the differentiation of TH17 cells to iTregs143. Lastly, AOAA ameliorated disease progression in EAE, a disease in which TH17 cells mediate pathology143. This study identified a crucial role for 2-HG as an epigenetic modifier governing T cell differentiation and suggests that therapeutic strategies aimed at lowering its levels may represent a promising approach to treat TH17-mediated autoimmune disorders.
In addition to CD4+ T cells, an unexpected role for L-2-HG in the promotion of tumour killing acting via CD8+ T cells, cytotoxic T lymphocytes important for anti-tumour immunity, has emerged149. Like TH17 cells, CD8+ T cells are highly dependent on the activation of HIF-1α and glycolysis, which is responsible for mediating trafficking into hypoxic tumour environments and inflamed tissue147,148. Interestingly, Tyrakis and colleagues (2016) observed a dramatic increase in L-2-HG levels, reaching up to 1.5 mM, in response to T-cell receptor triggering in mouse CD8+ T cells149. This increase occurred under normoxic conditions and was dependent on HIF-1α stabilisation149. Mechanistically, HIF-1α stabilisation induced LDHA expression and activity, which promiscuously converted glutamine-derived α-KG to L-2-HG149. Accumulation of L-2-HG altered CD8+ T cell differentiation through modulation of both the histone and DNA methylation landscape of the cell, and also via HIF stability149. Furthermore, exogenous treatment of CD8+ T cells with L-2-HG greatly enhanced the in vivo proliferation, persistence and anti-tumour capacity of adoptively transferred CD8+ T cells149. 2-HG enantiomers are therefore beginning to emerge as important signaling moieties linking metabolic reprogramming, epigenetic alterations and effector functions of immune cells.
5.2. D-2-HG-inhibition of αKGDD and tumorigenesis as a consequence of IDH mutations
Genomic studies have established that somatic mutations in IDH1 and IDH2 are perhaps the first genetic events to occur during tumorigenesis in human glioma and AML, and appear to initiate pathogenesis by a common mechanism103,115-117. The most commonly occurring cancers thought to arise from mutant IDH1/2 (mIDH1/2) include glioma, cartilaginous tumours, AML, angioimmunoblastic T cell lymphoma (AITL) and intrahepatic cholangiocarcinoma (ICC). Additionally, mutations in these genes have also been reported to sporadically arise in prostate cancer, melanoma, medulloblastoma and hepatocellular carcinoma (HCC)103. Remarkably, almost all IDH1/2 mutations occur in a few unique locations in the active site of the enzymes, which results in the acquirement of a neomorphic enzymatic activity converting α-KG to the oncometabolite D-2-HG118-120. As with succinate and fumarate, D-2-HG acts as a competitive inhibitor of α-KGDDs including, but not limited to, the TET family and JMJD family of DNA and histone demethylases, respectively121,22. D-2-HG produced by mIDH1/2 has been shown to drive a CpG island methylator phenotype (G-CIMP) in both glioma and ICC tumours to promote tumorigenesis, and has also been found to occupy the active site of histone demethylases thereby increasing histone methylation in primary gliomas121,123-125. Furthermore, Flavahan and colleagues (2016) demonstrated that the IDH-driven G-CIMP phenotype occurs at cohesion and CCCTC-binding factor (CTCF)-binding sites, compromising this methylation-sensitive insulator protein and enables a constitutive enhancer to interact aberrantly with, and activate, the glioma oncogene PDGFRA173.
Emphasis has often been placed on epigenetic alterations elicited by elevated D-2-HG as a driver of tumorigenesis in IDH mutant cancers, however, it is also apparent that it can antagonise other α-KGDDs. Although the IC50 of D-2-HG toward KDM family members is in the low micromolar range (24-106 μM), it can also inhibit FIH and PHD2, with IC50 values of 1.5 and 7.3 mM, respectively121. In IDH1/2-mutant gliomas, D-2-HG has been reported to accumulate to levels up to 35 mM, as such, D-2-HG could inhibit α-KG-dependent dioxygenases to affect alternative cellular signaling pathways120. Indeed, D-2-HG accumulation in IDH-mutant glioma has been found to inhibit PHD hydroxylase and stabilise HIF-1α126 and HIF-1αstabilisation has been shown to occur in IDH1-mutant brain tissue127. L-2HG is also a potent inhibitor of α-KG-dependent enzymes. It has been reported that under conditions of hypoxia L-2HG is selectively produced by mammalian cells 51. The authors demonstrate that this is independent of IDH1 and 2 and primarily mediated by LDHA and malate dehydrogenase (MDH) via “promiscuous” reduction of αKG and this is enhanced by acidic pH52. Under acidic conditions αKG is protonated and this promotes binding to LDHA and L-2HG production. Functionally, L-2HG is both sufficient to try histone methylation, in particular histone 3 lysine 9 (H3K9me3), and can also promote HIF-1α stabilization in normoxia and therefore may assist with hypoxic adaptation. These data suggest that L-2HG might represent a metabolic response to certain environmental stimuli including hypoxia and acidosis to drive changes in cellular signaling and function. Although it has been reported that D-2-HG is a less potent inhibitor of α-KGDDs than L-2-HG, the concentrations at which it accumulates under pathogenic conditions suggests HIF-1α stabilisation and impaired collagen biogenesis may contribute to tumorigenicity126-130. Intriguingly, another possible mechanism by which D-2-HG drives tumorigenesis is through the promotion of genetic instability, predisposing cells to oncogenic transformation103. D-2-HG has been shown to inhibit α-KG-dependent alkylation repair homologs, ALKBH2 and ALKBH3, critical DNA damage repair enzymes that detoxify 1-methyladenine (1 mA) and 3-methlycytosine (3mC) lesions in DNA derived from alkylating agents121,131. Indeed, IDH-mutant cells display reduced DNA repair kinetics, accumulate DNA double stranded breaks (DSBs) and are sensitized to alkylating agents131,174. Furthermore, D-2-HG has been proposed to promote DNA damage by altering the expression of genes involved in DNA repair, namely the DNA damage sensor ATM (ataxia-telangiectasia mutated), whereby an accumulation of repressive histone methylation markers, H3K9 and H3K27, were found at the ATM promoter in IDH-mutant mouse hematopoietic stem cells and human AML samples132. As such, these findings directly link mIDH-derived D-2-HG accumulation to DNA damage, genetic instability and carcinogenesis.
5.3. The role of alternative D-2-HG targets in cancer initiation and progression
In addition to the role of αKGDD inhibition by D-2-HG is the regulation of histone and DNA methylation and genomic instability, D-2-HG has been found to affect several other cellular pathways that may also contribute to cancer initiation and progression103. Of note, D-2-HG produced by mIDH1 in low grade glioma was found to activate mechanistic target of rapamycin (mTOR) signaling133. Mechanistically, it was proposed that D-2-HG destabilised DEPTOR, a negative regulator of mTORC1/2 partly through inhibition of the histone demethylase KDM4A133. Furthermore, metabolomic profiling of mIDH1/2 gliomas revealed significant alterations in cellular metabolism, an effect recapitulated with the exogenous addition of D-2-HG134. This metabolic rewiring resulted in a decrease in a common dipeptide found in the brain N-acetyl-aspartyl-glutamate (NAAG), and NAAG levels were found to be significantly lower in IDH-mutant human glioma tissue134. Likewise, mutations in IDH were also found to reprogram pyruvate and TCA metabolism, lower dependence on oxidative mitochondrial metabolism and silence LDHA expression, all suggesting that cellular reprogramming elicited by D-2-HG may play a prominent role in the pathogenesis of IDH-mutant tumours135-137. However, the mechanism by which D-2-HG orchestrates these events remains to be explored. Furthermore, D-2-HG activates NF-κB in bone marrow stromal cells in an IκB kinase-independent fashion, thus generating a stromal niche for IDH mutant AML development138. Intriguingly, D-2-HG has also been found to be a competitive inhibitor of SDH, a key metabolic enzyme and tumour suppressor as previously discussed, resulting in the accumulation of succinate, impaired mitochondrial oxygen consumption and increased protein succinylation139. As such, the initiation and pathogenesis of IDH-mutant tumours may share parallels with SDH-mutant tumours. D-2-HG has also been found to bind the DNA methyltransferase DNMT1, which subsequently represses receptor-interacting protein kinase 3 (RIP3). Finally, D-2HG also impairs necroptosis and the small GTPase Cdc42, leading to suppression of mixed lineage kinase 3 (MLK3)-mediated apoptosis140,141. These findings therefore suggest that both suppression of RIP3-mediated necroptosis and MLK3-mediated apoptosis by D-2-HG may contribute to IDH-mutant carcinogenesis140,141.
6. Citrate and ATP-citrate lyase as a source of acetyl-CoA for histone acetylation
One final metabolite to consider in the context of cellular signalling is acetyl-CoA derived from citrate. Acetyl-coenzyme A (acetyl-CoA) is an energy rich intermediate that provides acetyl groups to the Krebs cycle through the generation of citrate which is further oxidized for energy production. Acetyl-CoA is generated from the breakdown of carbohydrates, fatty acids and proteins though glycolysis,β-oxidation, and the degradation of the amino acids leucine, isoleucine and tryptophan respectively48. Acetyl-CoA serves as a building block for the synthesis of lipids, ketone bodies and amino acids. As fatty acid synthesis occurs in the cytosol acetyl-CoA must exit the mitochondria to fulfil this function. To do so, following its conversion to citrate it exits the mitochondria via the mitochondrial citrate carrier and is then converted back to acetyl-CoA (and oxaloacetate) by the enzyme ATP-citrate lyase (ACL). It is this enzyme that endows acetyl-CoA with much of its signalling capacity in the context of tumor and immune cell biology.
Citrate, a component of the TCA cycle has been shown to act as a source of acetyl-CoA to drive histone acetylation and this process can have profound impacts on both tumor and immune cell function. Chromatin structure is regulated in part through modification of histones including histone acetylation53. It has been demonstrated in mammalian cells that histone acetylation is dependent on ACL, the enzyme that converts citrate into acetyl-CoA, a required co-factor for acetylation,54 with ACL silencing decreasing the degree of histone acetylation. One of the earliest studies to identify a role for ACL in histone acetylation demonstrated a requirement for this gene in response to growth factor stimulation and during differentiation, for example the differentiation of murine 3T3- L1 pre-adipocytes into adipocytes. Cells depleted of ACL contained visibly less lipid than control cells. This was linked to decreased expression of genes involved in glucose uptake and glycolysis which are required for adipocytes to engage in fat storage. It was shown that during adipocyte differentiation, global histone acetylation is determined by glucose availability through an ACL-dependent pathway 54. These seminal finding highlighted a clear link between nutrient sensing, metabolism and histone acetylation which has now been extended to immune and tumor cell function.
6.1. Acetyl-CoA and histone acetylation regulate of immune cell function
Acetyl-CoA levels and histone acetylation have both been implicated in the regulation of immune cells. It is well-established that glycolysis is required for effector T cell function and a recent study from Peng and colleagues (2016) provides mechanistic insight into this requirement. The authors demonstrate that LDA, an enzyme that catalyzes the reversible conversion of pyruvate and NADH to lactate and NAD+, is induced in activated T cells and enhances histone acetylation of interferon gamma (Ifng) via the maintenance of acetyl-CoA levels to promote its expression 55. Mice with an T cell-specific LDHA deficiency produced less IFN-γ and were protected from immunopathology. Mechanistically LDHA-deficient T cells showed decreased acetyl-CoA levels and decreased histone H3 acetylation at the lysine 9 residue (H3K9Ac), a histone mark associated with active transcription, on the Ifng promoter. Furthermore, artificial acetyl-CoA reduction, through inhibition of ACLY, decreased IFN-γ expression and acetate supplementation, which augmented acetyl-CoA production, corrected H3K9Ac marks and IFN-γ expression in LDHA-deficient T cells.
The mechanism of IFN-γ regulation proposed by Peng and colleagues (2016) differs to that proposed by Chang and colleagues (2013) who suggest that aerobic glycolysis boosted IFN-γ production by engaging GAPDH and preventing its binding to the Ifng 3′UTR thereby enhancing IFN-γ translation 56. These assays are technically demanding to perform and this discrepancy remains to be clarified. But it should be noted that the study from Peng and colleagues (2016) employed the use of an artificial construct—the bovine growth hormone 3′ UTR—rather than direct manipulation of the Ifng 3′ UTR itself. Multiple mechanisms involving acetyl-CoA may therefore exist to regulate Ifng expression. Activated T cells, which display elevated glycolysis, may remodel their chromatin, thereby generating Ifng mRNA that can be robustly translated, as GAPDH is engaged in glycolysis and no longer binds the Ifng mRNA. These data demonstrate that complex relationship between metabolism and gene regulation. It is interesting to speculate if acetyl-CoA can boost histone acetylation and transcription of other cytokines, or in indeed other immune or tumor cells.
ACLY and histone acetylation have also been implicated in T cell growth57. An unbiased mass spectrometry-based study of the nuclear phosphoproteome of resting and IL-2-treated CD4+ T-lymphocytes revealed that ACLY is phosphorylated in response to IL-2 treatment 57. Pharmacological or genetic ablation of ACLY function impaired IL-2-promoted T-cell growth. The authors demonstrate that ACLY is required to enhance histone acetylation levels and induce the expression of cell cycle regulating genes in response to IL-2. They speculate that this is as a result of acetyl-CoA generation but do not demonstrate this experimentally.
6.2. Acetyl-CoA alters the epigenome of tumor cells
In 2014 Lee and colleagues demonstrated that acetyl-CoA levels and histone acetylation in tumors are regulated by oncogenic activation of Akt and subsequent ACLY phosphorylation58. This was one of the first reports to implicate metabolic reprogramming mediated by oncogenic activation and the regulation of the cancer cell epigenome. Importantly, histone acetylation and phosphorylated Akt levels correlate significantly in human glioma and human prostate cancer and levels of histone acetylation have been shown to be predictive of which patients would later exhibit biochemical failure, with lower levels predictive of worse outcome. These data suggest that histone acetylation levels might be a valuable biomarker to predict tumor relapse. Further study is needed to understand how global histone acetylation levels impact tumor growth and progression, as well as the response to treatment.
ACLY, acetyl-CoA levels and histone acetylation have also been shown to play an important role in the DNA damage response in tumor cells 59. ACLY is phosphorylated downstream of AKT activation, following DNA damage and this facilitates histone acetylation at double strand break (DSB) sites, enabling breast cancer early onset 1 (BRCA1) recruitment and DNA repair by homologous recombination. ACLY deficiency results in impaired BRCA1 recruitment to sites of DSBs and suppression of homologous recombination. The mechanisms of AKT activation by DNA damage signaling and the mechanisms that regulate ACLY nuclear levels, which is critical for its role in regulating BRCA1 recruitment, are less clear.
7. Therapeutic opportunities
The emerging roles of Krebs cycle intermediates in the pathophysiology of biomedically important processes raises the possibility of the generation of a new class of therapies focussed on these metabolites, as shown in Figure 6. This concept has already warranted investigation and was first illustrated following the discovery that succinate accumulated during ischaemia and is then oxidised upon reperfusion to drive the mitochondrial superoxide formation that contributes to ischaemia-reperfusion (IR) injury150,151. Inhibiting the mitochondrial enzyme SDH during ischaemia by preloading tissues with the SDH inhibitor DMM prevented succinate accumulation during ischaemia and protected against tissue damage upon reperfusion150. Malonate itself was also protective when added at reperfusion152,153. This work indicated that simple inhibitors of Krebs cycle enzymes could be used to prevent pathological damage. The production of succinate is also enhanced during inflammation13, acting as a signal from mitochondria to the cytosol to activate pro-inflammatory gene expression while also generating ROS as a further pro-inflammatory redox signal28. As the mechanism of mitochondrial superoxide generation by succinate oxidation during inflammation seems to be the same as that during IR injury (RET)28, it was perhaps unsurprising that DMM could also decrease inflammation in a mouse model of sepsis28. Hence, simple molecules designed to inhibit SDH are a novel class of anti-inflammatory and anti-IR injury compounds.
Figure 6. Therapeutic opportunities targeting metabolic signalling pathways.
i) The SDH inhibitor malonate and the malonate prodrug, DMM (hydrolysed to release malonate), ameliorate damage associated with succinate accumulation during ischaemia. ii) SUCNR1 receptor inhibitors, such as compound “5g” are in development. iii) Enasidinib, which targets mutated IDH2 to reduce 2-HG production, is the leading to the treatment of AML. iv) 4-OI, DI and DMF are anti-inflammatory are may offer therapeutic potential. v) Inhibitors of α-KGDDs are an emerging target, but selectivity is an issue. Dimethyloxaloylglycine (DMOG) acts on TETs but also PHDs. vi) Specific inhibition of histone lysine demethylases (KDMs) has also been challenging but EPT-103182 has shown promise in early pre-clinical work. vii) The specific PHD inhibitor, Vadadustat, has shown promise in chronic kidney disease.
As well as acting within the cell, a portion of the succinate that accumulates during ischemia and inflammation is released from the cell into the extracellular environment through poorly defined processes. This occurs following IR injury158,159, inflammation10, or cold exposure160. The diverse consequence of released succinate remains to be explored, but one likely role is as an inflammatory signal, acting via SUCNR14,5,161,162. There is considerable interest in developing drugs that can counteract the effects of succinate on this receptor162. Although, SUCNR1 receptor inhibitors, such as compound “5g”, are in their infancy they may provide further understanding to the signalling role of succinate and a method of blunting a cascade response during inflammation [5]. Circulating Krebs cycle intermediates are particularly interesting potential drug targets.
As previously discussed, mitochondrial metabolites impact on metabolism following their export from the mitochondrial matrix to the cytosol and nucleus and α-KGDDs are major target for the action of mitochondrial metabolites such as succinate and 2-HG60–62. High levels of succinate or 2-HG inhibit α-KGDDs. These include the PHDs, the TET DNA demethylases, and the JMJDs154-156 Inhibitors of α-KGDDs are often non-specific, either hitting multiple isoforms or a-KGDD family members, such as dimethyloxaloylglycine (DMOG) acting on TETs but also PHDs [7]. Specific inhibition of histone lysine demethylases (KDMs) has also been challenging [8]. EPT-103182 has shown promise in early pre-clinical work, in an osteosarcoma cell line, however is yet to be investigated in clinical trials [9]. However, specific PHD inhibitors have been developed, such as Vadadustat, and have shown promise in chronic kidney disease, increasing erythropoiesis and providing a means of attenuating HIF in other pathologies [10]. The development of more effective drugs that affect the interactions of Krebs cycle intermediates with α-KGDDs is an area of considerable current interest157.
A further therapeutic angle has opened following the discovery of mutations in certain Krebs cycle enzymes that result in the generation of novel metabolites, the best example being mIDH2. Interestingly, a drug Enasidenib has been developed for AML, which binds to the mutated, but not the wild-type, form of IDH2 only to prevent D-2-HG production163.
The generation of itaconate from the Krebs Cycle intermediate cis-aconitate contrasts with most other metabolite signals emanating from mitochondria, in that it primarily acts as a protective, anti-inflammatory metabolite55,59,60. As itaconate acts as an endogenous protective and anti-inflammatory agent, there has been interest in developing drugs that enhance its levels within cells. 4-OI is an itaconate mimic that was protective in a mouse model of sepsis, decreasing cytokine production and increasing survival59. The therapeutic potential of DI was also demonstrated as in vivo administration of DI resulted in an amelioration of skin pathology in a mouse model of psoriasis, a disease also currently treated by the related electrophile DMF60,65. Thus, the development of new therapies designed to fine-tune the cell delivery and activity of Nrf2 activators is a promising therapeutic strategy.
In summary, the emerging role of Krebs cycle intermediates in many central biomedical pathways opens up the possibility of extending mitochondrial pharmacology164-167 to manipulating the levels and impacts of mitochondrial metabolites both within the matrix, the rest of the cell, or following their release from the cell into the circulation.
8. Outstanding questions and concluding remarks
Our understanding of the role of metabolites as signalling molecules has come a long way but is still in its infancy. The clear role for succinate and HIF as pro-inflammatory signals, L-2-HG driving anti-tumour immunity, fumarate as an anti-inflammatory but also pro-tumour signal and acetyl-CoA as an epigenetic regulator reveal some of the consequences of this burgeoning field yet many unanswered questions remain. One critical point to consider is the limited human and in vivo data existing to date. These studies prove especially challenging due to the rapid rate at which steady state metabolism changes. Appropriate methods of euthanasia and tissue extrusion are paramount to get an accurate snap shot of the in vivo metabolic status of cells/tissues making these sorts of analyses extremely challenging. Studies in human immune cells are also currently very limited. Understanding the cellular location of metabolite accumulation as well as how these cells may signal in a paracrine manner remains to be explored. For example, the relevant ratios of the different metabolites, which may vary over time, and in different compartments of the cell, will be determining. It is likely that unidentified GPCRs capable of recognizing metabolites exist and indeed transporters that allow metabolites to enter cells from the extracellular milieu. Whether metabolites such as succinate, itaconate and acetyl-CoA serve as biomarkers or drivers of disease or tumour progression remains to be further explored. Of note, some of the major findings on the function of oncometabolites were obtained from rare cancer syndromes and it will be crucial to understand whether some of the processes elicited by oncometabolites occur in other sporadic cancers, in the absence of inactivating mutations of FH, IDH, or SDH. Is it possible that the often-opposing roles of metabolites in the context of tumor progression and immune cell activation may be detrimental to the host. For example, succinate, which promotes a potentially tumour-fighting, pro-inflammatory state also drives HIF-1α activation in tumours which is associated with cancer progression. On the flip side, it would be interesting to explore if pathogens exploit or manipulate immunometabolism of the host for their own need or indeed if they produce metabolites that can either acts as PAMP/DAMPs or impair immune cell function. It is likely that we are only at the tip of the iceberg in terms of the breath of metabolites with signalling capacity and indeed the breath of the entire metabolome. RThis field still remains in its infancy in comparison to proteomic and transcriptomic analysis but LC/MS approaches to detect metabolites are constantly evolving and this will allow for the detection of a broader range of metabolites and more unbiased analyses which are likely to uncover novel metabolic signals. We can look forward to new insights into how Krebs cycle connects with signalling and perhaps being the ultimate determinant of cell function in health and disease.
References
- 1.Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He W, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 4.Rubic T, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat Immunol. 2008;9:1261–1269. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- 5.Littlewood-Evans A, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213:1655–1662. doi: 10.1084/jem.20160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, et al. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PloS one. 2014;9:e97501. doi: 10.1371/journal.pone.0097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollander AP, Corke KP, Freemont AJ, Lewis CE. Expression of hypoxia-inducible factor 1alpha by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis and rheumatism. 2001;44:1540–1544. doi: 10.1002/1529-0131(200107)44:7<1540::AID-ART277>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Sadagopan N, et al. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens. 2007;20:1209–1215. doi: 10.1016/j.amjhyper.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Van Diepen JA, et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia. 2017;60:1304–1313. doi: 10.1007/s00125-017-4261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peruzzotti-Jametti L, et al. Macrophage-Derived Extracellular Succinate Licenses Neural Stem Cells to Suppress Chronic Neuroinflammation. Cell Stem Cell. 2018;22:355–368 e313. doi: 10.1016/j.stem.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhuniya D, et al. Discovery of a potent and selective small molecule hGPR91 antagonist. Bioorganic & medicinal chemistry letters. 2011;21:3596–3602. doi: 10.1016/j.bmcl.2011.04.091. [DOI] [PubMed] [Google Scholar]
- 12.Geubelle P, et al. Identification and pharmacological characterization of succinate receptor agonists. Br J Pharmacol. 2017;174:796–808. doi: 10.1111/bph.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannahill GM, O'Neill LA. The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS letters. 2011;585:1568–1572. doi: 10.1016/j.febslet.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 16.Epstein ACR, et al. C-elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/S0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 18.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Molecular and cellular biology. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagen T. Oxygen versus Reactive Oxygen in the Regulation of HIF-1alpha: The Balance Tips. Biochemistry research international. 2012;2012 doi: 10.1155/2012/436981. 436981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. The Biochemical journal. 2006;396:517–527. doi: 10.1042/BJ20051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu W, Zhao S. Metabolic changes in cancer: beyond the Warburg effect. Acta biochimica et biophysica Sinica. 2013;45:18–26. doi: 10.1093/abbs/gms104. [DOI] [PubMed] [Google Scholar]
- 23.Selak MA, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Rasola A, Neckers L, Picard D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014;24:455–463. doi: 10.1016/j.tcb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu X, et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget. 2017;8:13174–13185. doi: 10.18632/oncotarget.14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, et al. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. Journal of neuroimmunology. 2006;174:63–73. doi: 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Peyssonnaux C, et al. Cutting edge: Essential role of hypoxia inducible factor-1alpha in development of lipopolysaccharide-induced sepsis. Journal of immunology. 2007;178:7516–7519. doi: 10.4049/jimmunol.178.12.7516. [DOI] [PubMed] [Google Scholar]
- 28.Mills EL, et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457–470 e413. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyssonnaux C, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. The Journal of clinical investigation. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wobben R, et al. Role of hypoxia inducible factor-1alpha for interferon synthesis in mouse dendritic cells. Biological chemistry. 2013;394:495–505. doi: 10.1515/hsz-2012-0320. [DOI] [PubMed] [Google Scholar]
- 32.Jantsch J, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. Journal of immunology. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 33.Benit P, et al. Unsuspected task for an old team: succinate, fumarate and other Krebs cycle acids in metabolic remodeling. Biochimica et biophysica acta. 2014;1837:1330–1337. doi: 10.1016/j.bbabio.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Brigati C, et al. Inflammation, HIF-1, and the epigenetics that follows. Mediators of inflammation. 2010;2010 doi: 10.1155/2010/263914. 263914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends in immunology. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Saeed S, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345 doi: 10.1126/science.1251086. 1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng SC, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345 doi: 10.1126/science.1250684. 1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, et al. SIRT5-Mediated Lysine Desuccinylation Impacts Diverse Metabolic Pathways. Molecular cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, et al. Identification of lysine succinylation as a new post-translational modification. Nature chemical biology. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie L, et al. First succinyl-proteome profiling of extensively drug-resistant Mycobacterium tuberculosis revealed involvement of succinylation in cellular physiology. J Proteome Res. 2015;14:107–119. doi: 10.1021/pr500859a. [DOI] [PubMed] [Google Scholar]
- 41.Feng S, et al. Succinyl-proteome profiling of Dendrobium officinale, an important traditional Chinese orchid herb, revealed involvement of succinylation in the glycolysis pathway. BMC Genomics. 2017;18:598. doi: 10.1186/s12864-017-3978-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He D, et al. Global Proteome Analyses of Lysine Acetylation and Succinylation Reveal the Widespread Involvement of both Modification in Metabolism in the Embryo of Germinating Rice Seed. J Proteome Res. 2016;15:879–890. doi: 10.1021/acs.jproteome.5b00805. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, et al. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du J, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson GE, et al. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015;134:86–96. doi: 10.1111/jnc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F, et al. SIRT5 Desuccinylates and Activates Pyruvate Kinase M2 to Block Macrophage IL-1beta Production and to Prevent DSS-Induced Colitis in Mice. Cell Rep. 2017;19:2331–2344. doi: 10.1016/j.celrep.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 47.Meiser J, Krämer L, Sapcariu S, et al. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. Journal of Biological Chemistry. 2015;291(8):3932–3946. doi: 10.1074/jbc.m115.676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemeth B, Doczi J, Csete D, et al. Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage. The FASEB Journal. 2015;30(1):286–300. doi: 10.1096/fj.15-279398. [DOI] [PubMed] [Google Scholar]
- 49.Cordes T, Wallace M, Michelucci A, et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. Journal of Biological Chemistry. 2016;291(27):14274–14284. doi: 10.1074/jbc.m115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lampropoulou V, Sergushichev A, Bambouskova M, et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metabolism. 2016;24(1):158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okabe M, Lies D, Kanamasa S, Park E. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Applied Microbiology and Biotechnology. 2009;84(4):597–606. doi: 10.1007/s00253-009-2132-3. [DOI] [PubMed] [Google Scholar]
- 52.Lee C, Jenkins N, Gilbert D, Copeland N, O'Brien W. Cloning and analysis of gene regulation of a novel LPS-inducible cDNA. Immunogenetics. 1995;41(5) doi: 10.1007/bf00172150. [DOI] [PubMed] [Google Scholar]
- 53.Shin J, Yang J, Jeon B, et al. 1H NMR-based Metabolomic Profiling in Mice Infected withMycobacterium tuberculosis. Journal of Proteome Research. 2011;10(5):2238–2247. doi: 10.1021/pr101054m. [DOI] [PubMed] [Google Scholar]
- 54.Sugimoto M, Sakagami H, Yokote Y, et al. Non-targeted metabolite profiling in activated macrophage secretion. Metabolomics. 2011;8(4):624–633. doi: 10.1007/s11306-011-0353-9. [DOI] [Google Scholar]
- 55.Michelucci A, Cordes T, Ghelfi J, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proceedings of the National Academy of Sciences. 2013;110(19):7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams JO, Roche TE, McFadden BA. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. 1971;10(8):1384–90. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]
- 57.McFadden BA, Purohit S. Itaconate, an isocitrate lyase-directed inhibitor in Pseudomonas indigofera. Journal of Bacteriology. 1977;131(1):136–144. doi: 10.1128/jb.131.1.136-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naujoks J, Tabeling C, Dill B, et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLOS Pathogens. 2016;12(2):e1005408. doi: 10.1371/journal.ppat.1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills E, Ryan D, Prag H, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–117. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bambouskova M, Gorvel L, Lampropoulou V, et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature. 2018;556(7702):501–504. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nair S, Huynh J, Lampropoulou V, et al. Irg1expression in myeloid cells prevents immunopathology duringM. tuberculosisinfection. J Exp Med. 2018;215(4):1035–1045. doi: 10.1084/jem.20180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ackermann W, Potter V. Enzyme Inhibition in Relation to Chemotherapy. Experimental Biology and Medicine. 1949;72(1):1–9. doi: 10.3181/00379727-72-17313. [DOI] [PubMed] [Google Scholar]
- 63.ElAzzouny M, Tom CT, Evans CR, et al. Dimethyl Itaconate is Not Metabolized into Itaconate Intracellularly. Journal of Biological Chemistry. 2017;292(12):4766–4769. doi: 10.1074/jbc.C117.775270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi E, Suzuki T, Funayama R, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature Communications. 2016;7 doi: 10.1038/ncomms11624. 11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brennan M, Matos M, Li B, et al. Dimethyl Fumarate and Monoethyl Fumarate Exhibit Differential Effects on KEAP1, NRF2 Activation, and Glutathione Depletion In Vitro. PLoS ONE. 2015;10(3):e0120254. doi: 10.1371/journal.pone.0120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito R, Suzuki T, Hiramoto K, et al. Characterizations of Three Major Cysteine Sensors of Keap1 in Stress Response. Mol Cell Biol. 2015 doi: 10.1128/mcb.00868-15. MCB.00868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Zhang P, Wang C, et al. Immune Responsive Gene 1 (IRG1) Promotes Endotoxin Tolerance by Increasing A20 Expression in Macrophages through Reactive Oxygen Species. Journal of Biological Chemistry. 2013;288(23):16225–16234. doi: 10.1074/jbc.m113.454538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Quickelberghe E, Martens A, Goeminne L, Clement L, van Loo G, Gevaert K. Identification of Immune-Responsive Gene 1 (IRG1) as a Target of A20. J Proteome Res. 2018;17(6):2182–2191. doi: 10.1021/acs.jproteome.8b00139. [DOI] [PubMed] [Google Scholar]
- 69.Jamal Uddin M, Joe Y, Kim S, et al. IRG1 induced by heme oxygenase-1/carbon monoxide inhibits LPS-mediated sepsis and pro-inflammatory cytokine production. Cellular and Molecular Immunology. 2015;13(2):170–179. doi: 10.1038/cmi.2015.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luan H, Medzhitov R. Food Fight: Role of Itaconate and Other Metabolites in Antimicrobial Defense. Cell Metabolism. 2016;24(3):379–387. doi: 10.1016/j.cmet.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheon Y, Xu X, Bagchi M, Bagchi I. Immune-Responsive Gene 1 Is a Novel Target of Progesterone Receptor and Plays a Critical Role during Implantation in the Mouse. Endocrinology. 2003;144(12):5623–5630. doi: 10.1210/en.2003-0585. [DOI] [PubMed] [Google Scholar]
- 72.Sherwin J, Smith S, Sharkey A. Identification of genes regulated by leukaemia inhibitory factor in the mouse uterus at the time of implantation. Fertility and Sterility. 2004;82:S271. doi: 10.1016/j.fertnstert.2004.07.723. [DOI] [PubMed] [Google Scholar]
- 73.Nair S, Huynh J, Lampropoulou V, et al. Irg1expression in myeloid cells prevents immunopathology duringM. tuberculosisinfection. J Exp Med. 2018;215(4):1035–1045. doi: 10.1084/jem.20180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiss J, Davies L, Karwan M, et al. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. Journal of Clinical Investigation. 2018 doi: 10.1172/jci99169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi H, Wang D, Sun X, S L. MicroRNA-378 acts as a prognosis marker and inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting IRG1. Eur Rev Med Pharmacol Sci. 2018 Jun;22(12):3837–3846. doi: 10.26355/eurrev_201806_15268. 2018. [DOI] [PubMed] [Google Scholar]