Abstract
Introduction
Drug conjugates are trend topics in Chemical Biology. These entities are an emerging class of highly potent biopharmaceutical drugs, best known in the field of oncology, that have been also designed as a targeted therapy/diagnosis for the treatment/prevention of several bacterial diseases. Antibiotic resistance is now a major threat to public health, and targeted strategies can reduce resistance. The following review aims at giving an overview of the patented therapeutic innovations covering these areas. Particular attention has been given to antibacterial drug conjugates in the last 30 years.
Areas covered
The authors provide an overview of the scientific reports describing the research and development of new drug conjugates for bacterial diseases. The review emphasizes the rationale behind synthesis, biological activities and improvement of the new drug conjugates. New technologies applied for the research in this field have also been discussed. The article is based on the most relevant literature related to the development of new therapeutic solutions. The patents presented in this review have been collected from multiple electronic databases including SciFinder, Pubmed, Espacenet and Mendeley.
Expert opinion
The new drug conjugates described in the current review proved to display improved delivery, efficacy, targeting abilities and fewer side effects. Versatile approaches were invented to achieve these goals.
Keywords: Bacterial diseases, drug conjugates, antibiotic improvement, antibiotic targeting
1. Introduction
Bacteria are exceptionally adaptable organisms and have repeatedly proven their ability to resist novel chemotherapeutic agents [1]. Less than 20 years after the discovery of penicillin, the β-lactamases were able to inactivate the drug [2]. Therefore, the development of antibiotics for control of pathogenic bacteria is of pressing need in this era of drug-resistant infections [3]. Current antibiotics available present undesirable properties as systemic toxicity, short half-life, and increased susceptibility to bacterial resistance [4]. Although most antibiotic classes are administered systemically through oral or intravenous routes, a more efficient delivery system to achieve antibactericide efficacy is needed [5]. In this regard, the scientific community has extensively discussed the chemical conjugation of antibiotics to polymers, nanocarriers, proteins, or antibodies [6]. Through the conjugation of antibiotics, unique properties can be achieved [7]. These conjugated drugs display controlled, sustained release and vary in antibiotic class type, synthetic method, carrier composition, bond liability, and antibacterial activity [8].
Another important area of research is antibacterial vaccination. The need for new preventive solutions is also related to the intrinsic and acquired antibiotic resistance. With the increasingly rapid appearance and spread of antibiotic-resistant bacteria, prevention of infections with appropriately targeted vaccines assumes greater importance and urgency. However, some of the precedents observed with antibiotics may also be applied to vaccines.
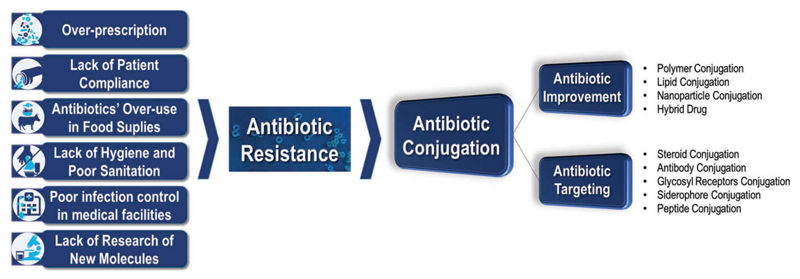
Among the new and more innovative therapeutic strategies, drug conjugates are on the top of the list. The conjugates synthesis, characterization, drug release, and antibacterial activities, if applicable, will be presented and discussed to offer a detailed overview of the currently available tools in this field. To a better comprehension of the data, the analysis of the patents was organized as antibiotic improvement and targeting through bioconjugation applications. Then, subgroups regarding the molecules were used to either improve the properties of the antibiotic (polymers, lipids, nanoparticles, or other therapeutic molecules – hybrids) or to target antibiotics to bacteria (steroids, glycosyl receptors, siderophores, peptides, or antibodies). Each of these topics will be discussed in detail herein (Figure 1).
Figure 1.
Introductory image reflecting the aim of this work. Causes for antibiotic resistance and one of the possible solutions for this concerning health issue. Images adapted from WHO website (www.who.int/drugresistance).
2. Antibiotic improvement
As mentioned in the introduction, clinical application of antibiotics has revealed limitations associated with the bioavailability, toxicity, and biodistribution of the antibiotic, as well as efficacy issues. Moreover, often, the clinical failure of antibiotic therapy is related to lack of targeting moieties and to poor pharmacokinetic properties, among other aspects [5].
Due to these limitations, and since free antibiotics have a fast and short-acting effect, several daily high doses are required to maintain therapeutic concentrations at specific locations. This, in turn, leads to increased side effects, frequently related with the concomitant damage to commensal human microbiota. Hence, among the most common side effects are the ones that affect the gastrointestinal tract, namely, vomiting, abdominal pain, diarrhea, and decreased appetite [5].
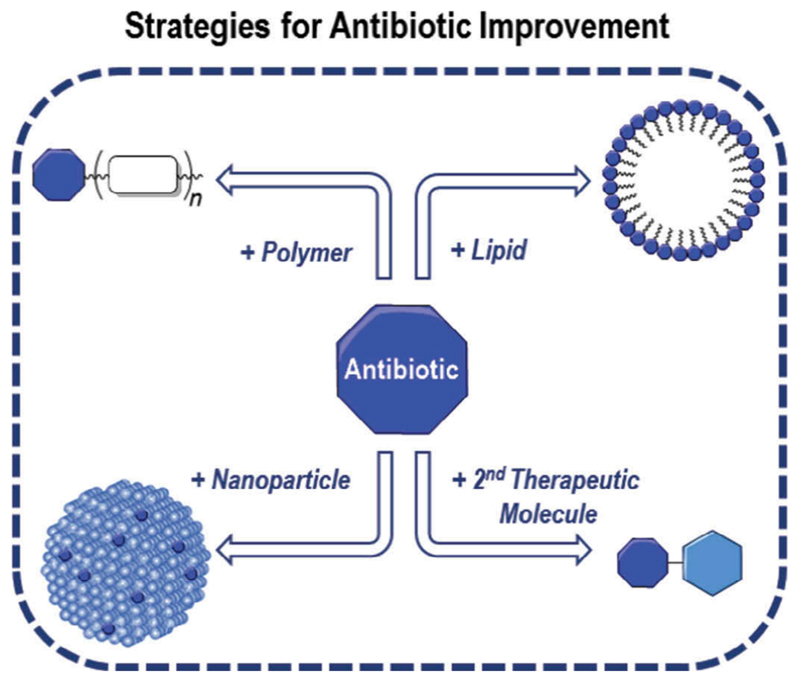
Accordingly, strategies to overcome these impairments that limit the dosage and improve the efficacy of antibiotics are of great interest. Antibiotic conjugation opened a new set of tools to improve the properties of antimicrobials, either by improving their pharmacokinetics, as for conferring properties to these drugs that they did not possess before. Several patents covering these improvements were developed in the last 30 years covering their attachment to polymers, lipids, nanoparticles, or even the conjugation of an antibiotic with another therapeutic molecule (Figure 2).
Figure 2.
Illustration to expose the different approaches for Antibiotic Improvement.
A common characteristic of a drug to achieve a therapeutic effect is lipophilicity due to its relation with membrane permeation in biological systems. Antibiotics are not an exception and the control of this property is often tuned to optimize biodistribution, metabolization, and excretion [9]. However, a hydrophilic balance is required to overcome solubility issues, poor absorption (by the oral route) as well as drug aggregation in systemic circulation. Polymer conjugation is a method to circumvent the lipophilicity of the drug with an additional advantage, the possibility to generate a protective barrier against environmental degradation or deactivation. Therefore, polymeric antibiotic conjugates improve biodistribution and plasma pharmacokinetics, as well as bioavailability of the drug [5].
In the early 1990s, an invention reported the attempt to improve the stability, therapeutic index, circulating half-life, and reduce adverse side effects of an antibiotic by conjugating it to a polymer. Polyethylene glycol (PEG) was then linked via a urethane, ester, or amide bond to several different antibiotics, namely peptides and polyenes like polymyxin and amphotericin B (AmB). The development of polymer-antibiotic conjugates tolerates the increase of therapeutic dose levels and still retains antimicrobial activity, when compared with its unconjugated free form [10]. Later, another patented report of a hydrosolubility improvement of a polyene antibiotic showed antibiotic conjugation with polysaccharide molecules under an aqueous alkaline medium. Starch, inulin, dextran, xylan, levan, or amylose were oxidized to polyaldehydes and reacted with amine functions of piramicin, nystatin, trichomycin, and AmB to generate water-soluble polyene antibiotics [11].
A few years later, once again, polyene compounds were targets of conjugation in order to improve the pharmacological properties of these macrolides. This time around, the conjugation was achieved with different lengths of oligo(ethylene glycol) aiming to raise the critical micelle concentration of the compound, which is an indication of molecular aggregation. With AmB conjugates, the ability to disrupt the membrane integrity of representative microorganism and host cells was evaluated. These amphiphilic antibiotic conjugates showed improved disruptive characteristics toward the cellular membrane of the organism that it is targeted for [12].
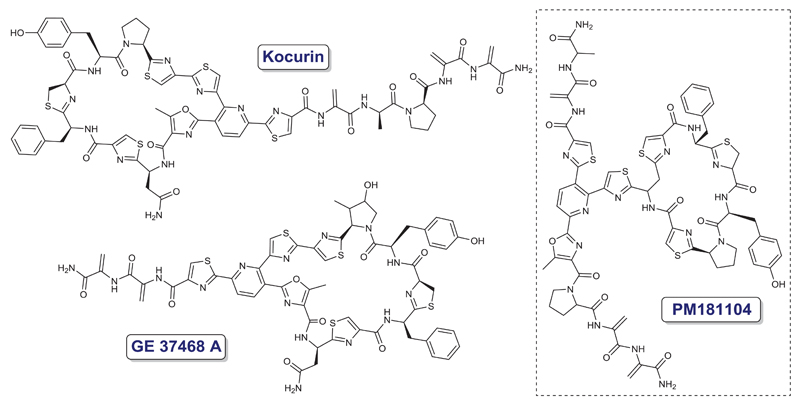
In 1993, a patent covering new antimicrobial quinolonyl lactam esters was presented showing a large range of possible chemical modifications of the pharmacophore [13]. A novel thiazolyl peptide antibiotic (GE37468A) was patented as an inhibitor of bacterial protein synthesis that was active in vitro against gram-positive bacteria [14]. In addition, a follow-up peptide (PM181104) was patented as a possible active substance to use in a pharmaceutical composition able to tackle bacterial infections caused by microorganisms belonging to Staphylococcus, Streptococcus, Enterococcus, or Bacillus species (Figure 3) [13–17].
Figure 3.
Chemical structures of new quinolonyl peptidic antibiotics – kocurin, GE37468A and PM181104.
However, only in 2012, due to reported studies of poor pharmacokinetics of the mentioned thiazolyl cyclic peptides, two approaches were followed to overcome these limitations that hampered optimal therapeutic application [2]. One was based on the evaluation of excipients to improve the pharmacological properties of PM181104 and the other was based on the covalent conjugation of PM181104 with PEG polymers which can effectively be applied in the prevention or treatment of bacterial infections, namely methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) [18,19].
In the late 1990s, there were two other patented antibiotic-polymer conjugations, namely, with activated polyoxyalkylenes or natural polysaccharide adducts. The first strategy was a general patent to cover polymer conjugation with therapeutic molecules, more specifically with small molecule or peptidic antibiotics through the activation of polyoxyalkylene by UV irradiation or with ammonium persulfate treatment. The second approach, unlike the others discussed this far, was based on non-covalent and nonionic bonds between natural polysaccharide polymers and antibiotics. This example showed improvements regarding antibiotic limitations, such as low bioavailability or limited duration of the antibiotic effect, therefore leading to higher therapeutic effectiveness and reduced molar doses of the antibiotic (consequently, less toxic) [20,21].
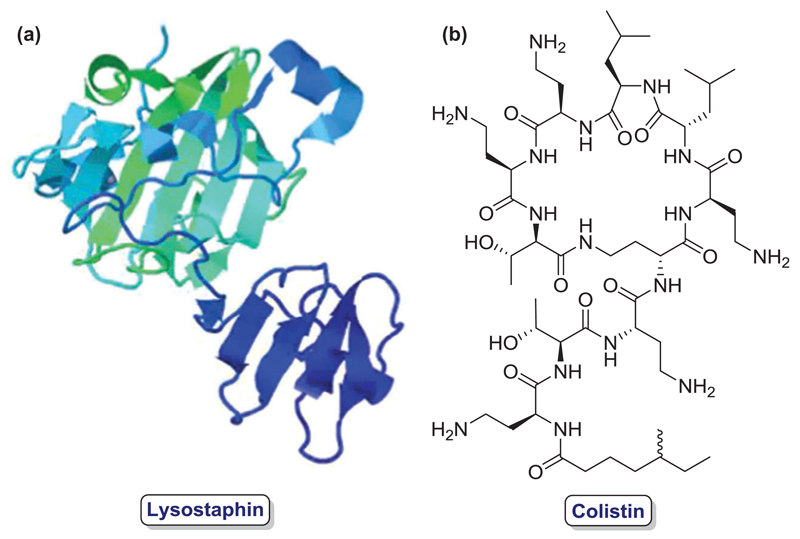
In 2003, an additional advance on conjugation of water-soluble polymers and antibiotics was patented, where bacterial endopeptidase (lysostaphin, Figure 4(a)) was conjugated to poly(alkylene oxides) in order to increase its circulating half-life and thus, treat or prevent staphylococcal infection at less frequent dosages than the unmodified compound. The inventors study thoroughly the effects of this PEGylation on decreased antibody binding and increased bacterial killing, decreased immunogenicity and reduced binding to circulatory system surfaces [22]. Later, in 2006, another invention described the conjugation of polysaccharides to antibiotics, more specifically oxidized regenerated cellulose with gentamicin and its use with or without medical devices, demonstrating significant enhancement of in vitro antimicrobial efficacy [23].
Figure 4.
Different antibiotic biomolecules for polymer conjugation: (a) Lysostaphin (PDB 1QWY) [24]; (b) Colistin.
Three years later, another example of antibiotic-polymer conjugation was described, which was based on a biodegradable polymer conjugated to any therapeutic molecule containing an amino, hydroxyl, or carboxyl functions in their structure, among which a list of antibiotics was also declared [25]. A more specific invention was claimed in 2011 where the conjugation of an antimicrobial peptide (AMP) and a biodegradable polymer was reported as an attempt to overcome bacterial resistance to antibiotic therapy. In this case, colistin (Figure 4(b)), which is a cationic AMP highly active against multidrug-resistant gram-negative bacteria, was conjugated with a dextrin polymer to overcome the poor pharmacokinetics and toxicity of its free form and the reduced in vitro antibacterial potency of the sulfonated colistin derivative. This bioresponsive dextrin-AMP conjugate that increases bioactivity and bioavailability, also binds to lipopolysaccharides, which is a key component of the outer membrane of gram-negative bacteria and, thus, localizes the polymer-antibiotic conjugate at infection sites. Moreover, its macromolecular size can shield the peptide from the immune system and decrease its renal clearance [26].
More recently, another water-soluble polymer conjugate was patented, which was based on the PEGylation (MW ranging from 500 Da to 100 kDa) of antimicrobial peptidic agents, namely, preprolysostaphin, prolysostaphin, mature lysostaphin, and mature active lysostaphin. According to the inventors, these conjugates, either as multiarmed water-soluble polymers or even as hydrogels comprising the antimicrobial agent, were able to inhibit the growth of staphylococcal organisms [27].
Apart from polymer-antibiotic conjugates, another strategy used to circumvent some of the limitations discussed in the beginning of this chapter is based on the conjugation of antibiotics with lipid structures.
An example of conjugation between lipid carriers and antimicrobial drugs was patented to achieve delivery and accumulation of the therapeutic molecule at the site of infection. This polar lipid carrier can be attached directly to the biologically active compound via a stable linker or directly conjugated through a covalent bond (example in Figure 5(a)). Different polar lipid carriers and antimicrobial drugs are mentioned as pharmacologically acceptable for this invention [28].
Figure 5.
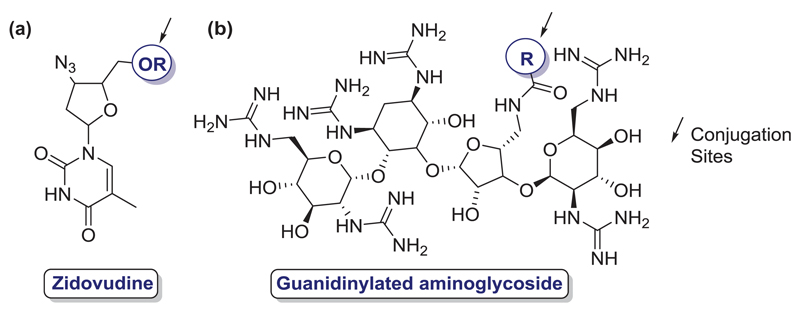
Different antibiotic molecules for Lipid Conjugation: (a) Zidovudine; (b) Guanidinylated aminoglycoside. Sites of conjugation marked in blue.
More recently, another example of the same strategy was given by a guanidinylated aminoglycoside-lipid conjugation. In this case, an aminoglycoside attached to a guanidine group (at least) was conjugated with at least another lipid group (example in Figure 5(b)). With these conjugates, the inventors reported significant improvements comparing with traditional aminoglycoside antibiotics, such as neomycin or kanamycin [29].
With the development of nanoparticles in the last few decades, it is not surprising that, recently, this technology has also been applied to antibiotic improvement in order to evade some of the limitations of these drugs.
In such regard, one of the first patented applications described ‘surfactant-free nanoparticles’ with drug delivery purposes. On this invention, the authors describe a surfactant covalently linked to the polymer to generate conjugates that were envisioned to have a controlled release profile. Theoretically, this would increase bioavailability, patient compliance, and minimize side effects. This process involved a copolymerization of the acrylate or acrylamide monomer, the antibiotic molecule, like an N-thiolated β-lactam, and a surfactant (anionic, cationic, nonionic or Zwitterionic). Like so, the inventors aimed to avoid all the adverse effects on stability and effectiveness through a drug delivery process to be applied when concerning life-threatening bacterial infections, such as the ones cause by MRSA or Bacillus anthracis [30].
Later, another antimicrobial nanoparticle conjugation for controlled-release formulation was patented, which described the methods to covalently immobilize AmB, gentamicin, vancomycin, or ampicillin to silica nanoparticles (SNP), through an activated-linker moiety. SNPs are especially indicated for the formation of stable antimicrobial conjugates, since these particles do not cause cytotoxic responses in patients and also present high stability, durability, and efficient functionalization. The oxidized form of dextran is the common linker among the described embodiments to react with the primary amines, which then are reduced to yield stable secondary amines [31].
In the following year, a similar patent was filed based on the conjugation of an antibacterial agent with a linker or carrier and a nanoparticle to generate conjugated prodrugs. Moreover, the inventors describe its applicability as a method to treat or prevent dandruff or acne situations, using a patented personal care composition [32].
Furthermore, despite the fact that fullerene moiety is not considered as nanoparticle per se, these structures were also used to comprise an antibiotic, thus generating a fullerene (C60)-antibiotic conjugate. This invention was able to be applied to different antibiotics, which led to conjugates able to target bacterial cell walls or bacterial spores. In the specific case of vancomycin being used as the antibiotic moiety, this conjugate was further functionalized with diphosphonate groups to target bone structures, which was then used to treat osteomyelitis [33].
More than highlighting the innate characteristics of antibiotics, another strategy used to improve antibiotic efficacy is based on the conjugation of two different therapeutic molecules envisioning a synergetic effect between them.
In this sense, two inventions were already reported for these hybrid drugs, which were based in conjugations of different antibiotic agents or in the conjugation of an antibiotic with a nitric oxide (NO) donor. The first discovery, based on the conjugation of aminoglycosides with non-ribosomal active antibiotics via ‘click’ chemistry (copper-catalyzed azide-alkyne [3 + 2] cycloaddition), was applied in therapy against different pathogenic microorganisms and compared with each of the agents separately or their mixture. With this strategy, the inventors reported the development of nonresistance inducing antimicrobial conjugates in clinically relevant gram-positive pathogens [34].
More recently, the conjugation between an NO donor and an acyl homoserine lactone, fimbrolide, dihydropyrrolone, or indole to develop antimicrobial conjugates active against biofilm formation and/or development was also patented. These biofilms are particularly difficult to eradicate, since these biological structures confer extra protection to the microorganisms, which in turn lead to an increase of antibiotic resistance when compared to planktonic cells. As an example, a common bacterium that generates biofilms on the surface of the lungs (in the case of cystic fibrosis) is Pseudomonas aeruginosa, which increases the level of antibiotic resistance and protection from the host’s immune system and therefore aggravates the prospects of an effective therapy. In this case, exogenous NO, at low concentrations, is able to induce the transformation of bacteria in biofilms to planktonic bacteria, therefore its association with an antibiotic is of great interest when (difficult to treat) biofilm-associated infections are concerned [35].
Hence, several strategies have been pursued to improve the efficacy and decrease the toxicity of these therapeutic drugs. These enhancements, have led some antibiotic conjugates to clinical trials, which upgraded the properties of the unconjugated form. The polymer-antibiotic conjugation has been one of the most attempted strategies; however, recently, the efforts on diversifying the available tools to overcome antibiotic resistance have been rising and are expected to grow even further in the next years [36]. Combined with these antibiotic developments, the targeting capabilities that the conjugation of these molecules also offer, expands the possibility of overcoming the battle against resistant pathogens. Due to the potential of these conjugates, next, several attempts on targeting antibiotics will also be discussed in detail.
3. Antibiotic targeting
Few biological/clinical applications have been explored outside the field of oncology utilizing noncytotoxic drugs for the drug conjugates therapy [37]. One of the main areas of research, out of cancer research, is focus on infectious diseases, in particular, bacterial-caused diseases. This area has been emerging specially in consequence to the bacterial resistance to traditionally drugs that were originally effective for treatment of infections and/or to the toxicity related to accumulative high doses of drugs. The possibility of therapeutic agents being attached to a specific carrier by chemical modification has provided also a novel drug delivery option. Most of the described compounds could be used in medicine, and are related to antibody-drug conjugate approaches.
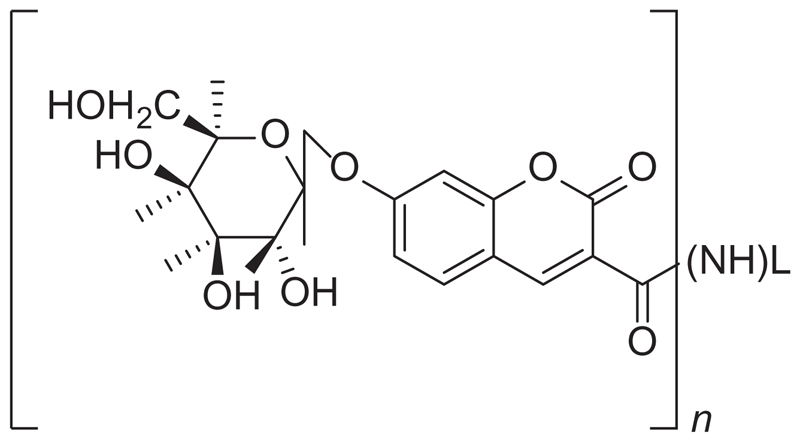
In 1978, a patent presented β-galactosyl-umbelliferone-labeled aminoglycoside antibiotics (Figure 6), assay methods, and reagent means for use therein, of the homogeneous and heterogeneous-specific binding type for determining qualitatively or quantitatively a ligand in a liquid medium [38]. Several aminoglycoside antibiotics were studied, such as gentamicin, sisomicin, nerilmicin, tobramycin, kanamycin, amikacin, and diphenylhydantoin. The conjugate was described to be cleavable by an enzyme to produce a detectable indicator product. The resulting bound species and/or the free species could then be contacted with the cleaving enzyme and the resulting indicator product measured as a function of the presence or amount of the ligand to be determined in the liquid medium assayed. This detection methodology was based on a cleaving enzyme employed to monitor the label in the bound species or free species.
Figure 6.
Structure of β-galactosyl-umbelliferone-labeled aminoglycoside antibiotics. Wherein – (NH)L is an aminoglycoside antibiotic selected from gentamicin, tobramycin, amikacin, kanamycin, sisomicin, or netilmicin bound through an amino group therefore by an amide bond and n equals 1 to a total number of primary amino groups in the selected antibiotic.
Bifunctional antibiotics for targeting bacterial rRNA and to inhibit resistance-causing enzymes were described [39]. A novel group of aminoglycosides was used as the antibiotic moiety of the conjugates, displaying two main functions: effective antibiotic activity and reduction of antibiotic resistance. In addition, the aminoglycosides of the presented invention proved to be suitable for inhibition of the anthrax lethal factor.
One of the first patents describing antibiotic conjugates was based on receptor conjugates for targeting penicillin antibiotics to bacteria [40]. This invention related conjugates comprising an anti-infective agent coupled to a receptor, which binds to a microorganism, and the methods to prepare and uses of these new conjugates. The selectivity of the receptor permitted increased targeting and specificity for the pathogen. This approach was combined to minimization of the dosage and adverse side effects. Glycoproteins, proteins, and glycolipids were described as potential receptors for recognition and attachment of microorganisms to the host cell.
Antibiotic-starch conjugates of both cytostatic agents daunorubicin and doxorubicin (anthracyclines) were studied. Both antibiotics are capable of inhibiting DNA and RNA synthesis, forming highly stable DNA intercalation complexes. In addition, they interact with cytochrome P-450 systems forming semiquinone radicals. As they are not absorbed by oral administration, the traditional way is via short infusions. The high doses necessary for the activity are related to high toxicity (maximum cumulative dose in adults is 550 mg/m3 body surface area). The cost of production is still high, but the effectiveness of this approach has been proved in the mentioned invention.
A recent invention described the uses of ble proteins (specific binding characteristics) for conjugation with antibiotics from the bleomycin family [41]. In particular, bleomycin derivatives are DNA-cleaving glycopeptides, which were found to have new uses in the mentioned invention. These new conjugates are capable of reversibly binding the antibiotic, with applications in several immobilization methods. It was highlighted in this patent the utility of this approach to produce markers for protein expression and/or folding, or for affinity tagging.
Synthetic analogs of bacterial quorum sensors (QS), which are analogs of an autoinducer of Pseudomonas aeruginosa quorum system, butane acyl-homoserine lactone (AHL-2), were described [42]. The invention is directed to these new systems that can afford perturbation of critical survival mechanisms by interface with QS-signaling and/or by precise delivery of an antibiotic to its target. These effects attenuate bacterial virulence and induce the premature expression of immunogenic molecules, exposing the bacteria to the host immune system at an early state of infection (downregulating the transcription and expression of flagellin, a highly immunogenic molecule).
Conjugates assimilated by an induced bacterial iron transport process as their activities, inversely related with iron concentration, were described [43]. This new approach for obtaining antibacterial siderophore-aminopenicillin (ampicillin and amoxicillin) conjugates was described. The activity was specially improved against Pseudomonas aeruginosa, when compared to the nonconjugated drugs.
A generic combination of a transportophore, a bond linker, and an antibiotic covalently bonded was described [44]. The transporter can be a metabolite, a natural product, a metabolite mimic, a metabolite derivative, a fatty acid, a bile acid, a vitamin, a nucleobase, an alcohol, or an organic acid or base. In fact, several examples were studied. The efficacy of the conjugates proved to be higher than the isolated drugs.
Covalent polar lipid conjugates with antimicrobial and antineoplastic drugs for targeting to biological protected sites formed part of an interesting invention [45]. This approach aimed to achieve the delivery to and accumulation of biologically active compounds into organisms, tissues, and cells (specially targeting to biological protected sites). The patent claimed the use of these conjugates for several applications, not only related to infectious diseases. Crossing of the blood– brain barrier was one of the interesting advantages of this methodology. Therefore, efficacy, efficiency, and specificity of the therapeutic agents were achieved.
In 2006, another innovative strategy used to improve antibiotic delivery was patented based on the conjugation of antibacterial agents and bacteriophages, which selectively infect (and kill) a host bacterium. The reported antibacterial application of the invention mentions bacteriophage-drug conjugates through labile or non-labile linker to one or more aminoglycoside molecules. Examples of conjugations reported by the inventors include amine conjugation methods with heterobifuntional crosslinking reagents or by means of an avidin/biotin complex [46].
3.1. Polysaccharide conjugation
Polysaccharide protein conjugate vaccines are primarily used for the prevention of bacterial infections (Figure 7). However, one single patent was claimed regarding capsular gram-positive bacteria bioconjugate vaccines, in 2011 [47]. The invention encompassed gram-positive and other bioconjugate vaccines containing a protein carrier, at least one polysaccharide such as a gram-positive capsular polysaccharide (CP), and, optionally, an adjuvant or pharmaceutically acceptable carrier. Production of the described vaccines was also included in the patent. In this patent, N-acetyl mannosaminuronic acid, N-acetyl l-fucosamine, and N-acetyl d-fucosamine were described to be used in the preparation of Staphylococcus aureus CP vaccines.
Figure 7.
Polysaccharide protein conjugates.
Another patent, previous to the above mentioned, included the study of a water-soluble antibiotic comprising an amino sugar, in the form of a polysaccharide conjugate [48]. An amino sugar was used to prepare the antibiotic conjugates that have been described as more effective, with reduced side effects. Antibiotics combined with polysaccharides were then liberated in aqueous alkaline solutions, and the polysaccharide part can be decomposed by serum-α-amylase.
A patent described the applicability of bioconjugates for imaging purposes to detect infections before they become systemic. Oligosaccharide conjugates for targeting bacteria were described [49]. One of the main advantages is that the conjugate comprising an oligosaccharide and a molecule of interest, under certain conditions, can be transported across the bacterial cell wall.
Antibiotics improved by conjugation with stereospecific carbohydrates and methods for carbohydrate stereoselectivity were studied at the Yale University [50]. Novel anthracyclines with carbohydrate domains proved to display improved antibiotic properties. The role of these domains was associated to the modulation of cytotoxic activity of the antibiotics.
3.2. Peptide conjugation
Triazole-based aminoglycoside-peptide conjugates and methods of use were described in 2009 [51]. Application of click chemistry and antibacterial agents was included in this invention. Modified triazole-linked aminoglycoside-amino acid and -peptide conjugates were synthetized to avoid the multidrug resistance of some antibacterial drugs and also dose-related nephrotoxicity and ototoxicity. The aminoglycoside antibiotic portion may enhance the electrostatic interactions with the lipid bilayer of bacteria, while a peptide moiety may facilitate absorption and uptake or prevent efflux or covalent modification of the aminoglycoside.
Novel nanomedicine-based delivery system to target the delivery of colistin dextrin polymer was invented [52]. This patent claimed targeted antibiotic and antimicrobial treatments for personalized administration, based on novel conjugates comprising an AMP and biodegradable polymer. Colistin, a cationic peptide highly active against multidrug-resistant gram-negative bacteria, was used. Its oral absorption and nephro- and neurotoxicity were limiting its generalizable use. In fact, it was used as a prodrug (colistin methanesulfonate). These new nanodelivery systems are capable to target colistin to infection sites, increasing bioactivity and bioavailability while decreasing systemic toxicity and improving the global clinical effectiveness of the free drug (instead of the prodrug). Due to the increased blood flow and vascular permeability at disease sites, the accumulation of drug in damaged tissues is efficient. This effect was described to be interesting for chronic diseases, such as cystic fibrosis. The main advantages outlined in this patent are the size of the conjugates, that prevents access to toxicity organs (i.e. kidney and brain), the capacity of the dextrins to mask colistin from immune system and enzymes, potentially higher ‘unmasking’ rates in chronic infection sites due to higher amylase concentrations, controlled degradation rates, unmasked colistin exerts its effect on gram-negative bacteria and safeguards against sepsis by binding LPS.
Finally, the inventions of Genentech Inc. were additionally directed to therapeutics [53]. Additional efforts were made to perform assays to better understand the delivery and targeting processes of the new conjugates. Pharmaceutical compositions were presented, as well as new therapeutic alternatives. The studies were extended to humans. Also, multi-therapies were also considered. A kit for treating a bacterial infection comprising a pharmaceutical composition of the invention and instructions for use was developed. In this case, a peptidic linker attached to the antibiotic was also considered.
Other peptide antibiotic conjugates were described and claimed in patents. Formyl-methionyl chemotactic peptide antibiotic conjugates useful in treating infections were described for the first time in 1982 [54]. These peptide-antibiotics conjugates proved to display chemotactic activity, inhibiting also the growth of microorganisms. A particular application of this invention was the potential of peptide-silver sulfadiazine conjugates to treat burns.
Peptide therapeutic conjugates and their uses were described decades later [55]. This invention was directed to compounds formed by three different parts: a peptide vector, capable of enhancing transport of the compound across the blood–brain barrier or into particular cells, a linker, and a therapeutic peptide. The described approach is versatile and the conjugates can be used to treat any disease for which the peptide therapy is useful. In addition, these formulations proved to have increased stability, improved pharmacokinetics or reduced degradation in vivo, as compared to the unconjugated peptides.
Bioconjugates useful for diagnosis and therapeutics were presented by the National Institute of Immunology [56]. These novel bioconjugates and their synthesis were claimed. The compounds comprised an LPXTG peptide motif capable of recognizing sortase. Formulations of the bioconjugates to be used were also described.
3.3. Steroid conjugation
Allergan, Inc. presented three inventions directed to antibiotic conjugates and antibiotic conjugates linked with steroid drugs, between 2012 and 2014 [57–60]. In general, they are related to hybrids of two antibiotics, cleaved enzymatically or hydrolytically in vivo to release active drugs. The pharmaceutical preparations were for topical application to the eyes. A conjugate drug, also referred to as a co-drug, a prodrug, or a hybrid drug, comprised two or more (different or equal) drugs. Each drug contained an appropriate chemical functionality to enable them to be connected together, either directly or by means of a cleavable and biologically labile covalent linker. Hybrid drugs incorporated at least two drugs joined together by a linker moiety such as an ester, a carboxylate, a carbonyl, a carbonate, an amido, a carbamate, a ketone, an amino, an oxo, an ethylene glycol, an alkylene, a PEG, which was cleaved enzymatically or hydrolytically in vivo to release the active drugs.
The hybrid drugs of the invention provide a unique delivery of two antibiotics for the treatment and prevention of ophthalmic bacterial infections. A single-drug entity is advantageous to individual dosing of each drug because of the ability for simultaneous dosing and elimination of washout concerns when applying each drug separately [57]. This approach was applied to fluoroquinolones, cephalosporins, chloramphenicol, aminoglycosides, penicillins, erythromycin, macrolide antibiotics, and oxazolidinones.
In the same year, antibiotic conjugates linked with steroid drugs were also presented by Allergan [58]. The application claimed novel single-drug entities, formed by linkage of an antibiotic with steroidal drug via a linker. Upon topical application to the eye, the conjugate had the same cleavage process as described before. The linkers were similar to those related before. The hybrid drugs of this invention combined antibacterial and anti-inflammatory effects. The use of antibiotic/steroid hybrid drugs was useful when the risk of dangerous infection is high. The anti-inflammatory component is useful in treating the trauma of the ophthalmic tissues associated with that. The hybrids proved to suffer degradation in vivo into the antibiotic and the steroidal moieties. Another invention was centered in the same approaches [59]. In this last case, only amide or ester bonds were used as linkers to connect the antibiotic with the steroid moiety. The tested antibiotics were gatifloxacin, moxifloxacin, chloramphenicol, tobramycin, and amikacin. The studied steroid moieties were dexamethasone, betamethasone, triamcinolone acetonide, prednisolone, and hydrocortisone.
Lehigh University invented methods for the manufacture and use of antimicrobial sterol conjugates [61]. Pharmaceutical compositions and their applications were studied. The synthetic methodologies were also described. Several examples proved to be effective, comparing to the nonconjugated drugs. This invention showed for the first time that the placement of a pendant polyamine and sulfate groups on A and D rings of the sterol may be reserved with retention of antimicrobial activity.
3.4. Antibody conjugation
Two patents, one from 1993 [62] and another one from 2003 [63] described new approaches for the prevention and treatment of sepsis. The first one is focused on the prevention of blood-borne and toxin-mediated diseases. It has been described the use of antibody-antibiotic conjugates, covalently attached, preferably to a nonspecific response (use of nonspecific immunoglobulins). The conjugate is then capable of binding to bacteria via the antibiotic. The conjugate could be bacteriostatic, bactericidal, or both. Polymyxins were the most studied antibiotics, but cephalosporins and penicillins were also included. The synthetic methodologies to prepare the conjugates have been described, as well as the mechanism of action. The second invention further included new approaches for preventing and treating sepsis, which was also based on antibody-antibiotic conjugates. In fact, it is only an update of the concepts presented in the previous patent.
This concept of ‘antibodiotics,’ expression used for antibody-antibiotic conjugates, was explored in several inventions. The first reference of inventions based on antibiotic-antibody conjugates was in 1989 [64]. This invention was directed to a method for the production of antigenic protein-hapten conjugates, and antibodies corresponding thereto. The studied antibiotics were penicillin and other β-lactams. These antipenicillin antibodies were described to be particularly useful in immunoassays of penicillin and other β-lactam antibodies in milk. In the same year, a pharmaceutical preparation and a conjugate of a bactericide and an antibody directed against plaque forming or caries-inducing bacteria (in particular Streptococcus mutans) were described [65]. One of the most explored antibiotics was netilmicin, further conjugated with the antibody in the presence of glutaraldehyde. Pharmaceutical preparations of the conjugate together with a diluent or other additives, in the form of a paste, suspension, solution, or mouthwash, have been described.
Antibacterial antibodies were proposed in different inventions from Kim Stanley A. and Strox Biopharmaceuticals, LLC [66–69]. All of them are directed to the composition of purified antibody conjugates, presenting an antigen-binding portion that binds one antigen derived from Staphylococcus or Streptococcus, with at least one antibiotic.
In 1999, antibacterial antibodies and methods of use were presented [66]. This invention related to different antibodies that are capable of binding to a bacterial antigen, but lack the ability to be bound by bacterial Fc-binding proteins. Therefore, these preparations proved to be effective for treating infections caused by bacteria that express Fc-binding proteins. Several applications have been studied; in particular an example of Staphylococcus aureus CP vaccines preparation has been described. In 2001, this patent was amplified [67].
In 2009, a new invention was related to the extension of the developed technology to more streptococcal infections [68]. This invention is still related to antibacterial antibodies that does not react with bacterial Fc-binding proteins via the antigen-binding portion of their Fab regions. Examples of potentially useful human adjuvants include BCG and Corynebacterium parvum. Antigens can be also incorporated into lipid vesicles to enhance their antigenicity. The arsenal of studied antibiotics changed as the therapeutic alternatives increased.
In 2010, a new invention was based on the same principles, but the purification systems were actualized [69]. The invention is additionally directed to a huge number of bacteria. Upgrades of these inventions were presented from the same company between 2002 and 2012 [70–72].
In 2014 and 2015, Genentech Inc. developed three inventions related to the anti-wall teichoic antibodies and conjugates [73–75].
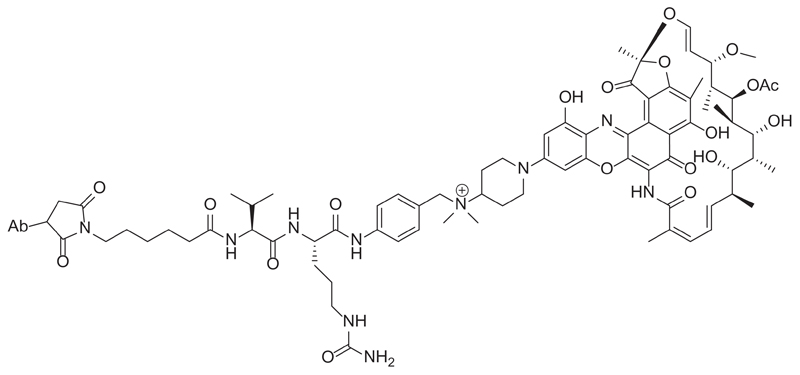
These patents provided a deep study about the anti-wall teichoic acid antibodies and antibiotic conjugates and the methods of using them. In particular, rifamycin-type antibiotics and their antibody conjugates were described (Figure 8) [73].
Figure 8.
Example of an anti-wall teichoic acid antibody-rifamycin conjugate.
Different groups of antibodies were tested and proved to be useful for the described approach. The applicability of the same strategies to antibiotic moieties selected from clindamycin, novobiocin, retapamulin, daptomycin, GSK-2140944, CG-400549, sitafloxacin, teicoplanin, triclosan, napthyridone, radezolid, doxorubicin, ampicillin, vancomycin, imipenem, doripenem, gemcitabine, dalbavancin and azithromycin were further claimed [74]. Once again, different isolated anti-wall teichoic monoclonal antibodies were studied. Another aspect of the invention is the description of an antibody-antibiotic conjugate compound comprising an anti-wall teichoic acid antibody covalently attached by a peptide linker to the antibiotic moiety. This last remarkable conjugate has been reported in literature and it is being studied as an alternative therapy to overcome infection caused by MRSA [76]. In this recent paper, authors described an antibody-antibiotic conjugate consisting of an anti-Staphylococcus aureus antibody conjugated to a highly efficacious antibiotic that was activated only after its releasing in the proteolytic environment of the phagolysosome. The antibody-antibiotic conjugate proved to be superior to vancomycin for treatment of bacteremia and provided direct evidence that intracellular Staphylococcus aureus represents an important component of invasive infections. Rifamycin proved to ablate nonreplicating bacteria and antibiotic-resistant persister cells, and it has been also described to accumulate within the intracellular milieu.
4. Conclusion
In the current analysis, we provide an overview of scientific reports describing the research and development of new conjugates related to bacterial diseases. The review emphasizes the rationale behind synthesis and biological activities (delivery and targeting) related to the new drug conjugates. New technologies applied for the research in this field have also been discussed.
5. Expert opinion
Few biological/clinical applications of drug conjugates have been explored outside the field of oncology. One of the main areas of research, nonrelated to cancer research, is focused on infectious diseases. This is an emerging area especially as a result of increased bacterial resistance to conventional drugs that were originally effective for treatment of infections and/or to the toxicity related to accumulative high doses of drugs. The concern regarding this subject and the urge for new antibiotics is so significant that led FDA, in 2014, to generate a qualified infectious disease product (QIDP) framework based on the Generating Antibiotic Incentives Now (GAIN) project [77]. This program, among other incentives, allows the antibiotics under QIDP status to give a drug an extra 5 years of market exclusivity [77], which have led the pharmaceutical industry to reinvest in this area, netting 5 new FDA antibiotic approvals between 2014 and 2015 [78,79]. Several other compounds are currently in clinical trials [80], and promising molecules are often reported in the literature, as well as new and inventive ways of identifying and generating antibiotics that show great promise. An example of such developments is teixobactin which is the first novel antibiotic isolated from bacteria in decades, raising hope for new isolation techniques that can lead to improved resistance-free antibacterial compounds [81,82].
Apart from these new antibiotic molecules, academics and industry have also produced many new promising approaches, despite requiring further understanding in order to be applied in current therapeutics [36]. However, some examples of conjugated antibiotics can already be found under advanced clinical trials or approved for therapy by pharmaceutical regulatory agencies. Namely, TD-1792 which is a glycopeptide-cephalosporin heterodimer antibiotic currently under phase III clinical trials [83,84] and several vaccines that conjugate carrier proteins and glycosyl receptors of bacteria cell capsule, mainly through reductive amination (such as Menactra®, MenHibrix®, Menveo®, Prevnar®, Synflorix®, Prevnar13®), that are presently used in prophylactic therapy [85–87].
The possibility of conjugating therapeutic agents to a specific carrier by chemical modification has provided a novel drug delivery option. As one of the most significant examples that as a great potential of becoming commercially relevant, one can mention the work developed by Genentech, which reports the development of the first antibiotic-antibody conjugate that is effective against MRSA strands [76]. The new approaches presented were combined to minimization of the dosage and reduction of adverse side effects. In addition, the novel formulations proved to have increased stability, improved pharmacokinetics, or reduced degradation in vivo, as compared to the unconjugated molecules formulations. The short-term clinical outlook for these compounds is the interest of the new conjugates in improving efficacy, delivery, and targeting, with fewer side effects. Moreover, the possibility of such conjugates to avoid antibiotic resistant pathways is one of the most exciting driving forces of this rekindled area. The main limitation to these new approaches is the application to traditional, ‘old-fashion’ drugs. However, the development of these techniques is not fruitless, since in combination with the novel antibiotics that are being developed/isolated, further derivatization of these drugs can deliver therapeutic molecules with better bioavailabilities and pharmacokinetics. Thus, this parallel research can converge in the future, speeding the introduction in the market of such drugs and, therefore, leading to less frightening eras than the ones we are facing at the moment with a very low number of weapons to fight back antibiotic-resistant microorganisms.
Article highlights.
We provide an analysis of therapeutics and discuss commercial aspects regarding drug conjugates for bacterial diseases.
We comment on emerging technologies for antibiotic improvement and targeting.
We discuss the enormous growth potential as future diagnosis, therapeutics and preventive (vaccines).
We discuss the improvements in targeting, efficacy and delivering of the new therapeutic alternatives (drug conjugate vs isolated drug).
We analyzed several strategies pursued to improve the efficacy and decrease the toxicity of these therapeutic drugs.
This box summarizes key points contained in the article.
Funding
We thank FCT Portugal (Postdoctoral Fellowship, SFRH/BPD/103172/2014 to P.M.S.D.C.; FCT Investigator to G.J.L.B.), the EU (Marie-Curie CIG and Marie-Sklodowska Curie ITN Protein Conjugates to G.J.L.B.), Xunta de Galicia (Galician Plan for Research, Innovation and Growth 2011–2015 - Plan I2C, ED481B 2014/086-0 to M.J.M.) and the EPSRC for funding. G.J.L.B. is a Royal Society University Research Fellow and the recipient of an European Research Council Starting Grant (TagIt).
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Stebbins N, Ouimet M, Uhrich K. Antibiotic-containing polymers for localized, sustained drug delivery. Adv Drug Deliv Rev. 2014;78:77–87. doi: 10.1016/j.addr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan G, Thomas B, Parab R, et al. In vitro and in vivo activities of antibiotic PM181104. Antimicrob Agents Chemother. 2013;57(11):5315–5319. doi: 10.1128/AAC.01059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leeb M. Antibiotics: A shot in the arm. Nature. 2004;431:892. doi: 10.1038/431892a. [•• An excellent overview regarding antibiotics.] [DOI] [PubMed] [Google Scholar]
- 4.Butler M, Blaskovich M, Cooper M. Antibiotics in the clinical pipeline in 2013. J Antibiot. 2013;66:571–591. doi: 10.1038/ja.2013.86. [DOI] [PubMed] [Google Scholar]
- 5.Xiong M-H, Bao Y, Yang X-Z, et al. Delivery of antibiotics with polymeric particles. Adv Drug Deliv Rev. 2014;78:63–76. doi: 10.1016/j.addr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Abed N, Couvreur P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int J Antimicrob Agents. 2014;43:485–496. doi: 10.1016/j.ijantimicag.2014.02.009. [• A report of a breakthrough antibiotic carries.] [DOI] [PubMed] [Google Scholar]
- 7.Bera S, Zhanel G, Schweizer F. Design, synthesis, and antibacterial activities of neomycin–lipid conjugates: polycationic lipids with potent gram-positive activity. J Med Chem. 2008;51:6160–6164. doi: 10.1021/jm800345u. [DOI] [PubMed] [Google Scholar]
- 8.Kalhapure R, Suleman N, Mocktar C, et al. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J Pharm Sci. 2015;104:872–905. doi: 10.1002/jps.24298. [•• Some interesting aspects regarding drug delivery systems.] [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim M, Panda S, Birs A, et al. Synthesis and antibacterial evaluation of amino acid–antibiotic conjugates. Bioorg Med Chem Lett. 2014;24:1856–1861. doi: 10.1016/j.bmcl.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 10.Cytogen Corporation. Polymer/antibiotic conjugate. WO1990/015628
- 11.Helfgott Karas PC, Yissum Res Dev C. Water-soluble polyene conjugate. WO1996/005212
- 12.Competitive Technologies of PA, Inc. Amphiphilic polyene macrolide antibiotic compounds. WO1996/032404
- 13.Proctor & Gamble Pharmaceuticals Inc. Antimicrobial quinolonyl lactam esters. US5434147. 1993
- 14.Gruppo Lepetit SPA. Antibiotics ge 37468 a, b and c. WO1994/014838
- 15.Piramal Life Sciences Ltd. Novel antibacterial compounds. WO2007/119201
- 16.Stella S, Montanini N, Le Monnier F, et al. Antibiotic GE37468 A: a new inhibitor of bacterial protein synthesis. I. Isolation and characterization. J Antibiot. 1995;48(8):780–786. doi: 10.7164/antibiotics.48.780. [DOI] [PubMed] [Google Scholar]
- 17.Martín J, Sousa TS, Crespo G, et al. Kocurin, the true structure of PM181104, an anti-methicillin-resistant staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium kocuria palustris. Mar Drugs. 2013;11:387–398. doi: 10.3390/md11020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piramal Enterprises Limited. Polymeric prodrug conjugates. WO2014/053873
- 19.Yemparala V, Damre A, Manohar V, et al. Effect of the excipient concentration on the pharmacokinetics of PM181104, a novel antimicrobial thiazolyl cyclic peptide antibiotic, following intravenous administration to mice. Pharma Sci. 2014;4:34–41. doi: 10.1016/j.rinphs.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micrologix Biotech Inc. Indolicidin and cationic peptides conjugated with polymers. WO1999/043357
- 21.Instituto Biochimico Pavese Pharma SPA. Antibiotic-natural polysaccharide polymer adducts. WO2000/078287
- 22.Biosynexus Incorporated. Antimicrobial polymer conjugates. WO2003/082926
- 23.Ethicon Inc. Antimicrobial composition. WO2007/014087
- 24.Odintsov S, Sabala I, Marcyjaniak M, et al. Latent LytM at 1.3 Å resolution. J Mol Biol. 2004;335(3):775–785. doi: 10.1016/j.jmb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 25.The Bionic Ear Institute. Biodegradable polymer-bioactive moiety conjugates. WO2010/040188
- 26.University College Cardiff Consultants Limited. Therapeutic conjugates. WO2012/035310
- 27.Nektar Therapeutics. Polymer-based compositions and conjugates of antimicrobial agents. US8420068. 2013
- 28.Oregon Health Sciences University. Covalent polar lipid conjugates with antimicrobial and antineoplastic drugs for targeting to biological protected sites. WO2000/033883
- 29.University of Manitoba. Guanidinylated aminoglycoside-lipid conjugates. WO2012/004684
- 30.University of South Florida. Surfactant-free nanoparticles for drug delivery. WO2008/085556
- 31.Matera Lda. Antimicrobial nanoparticle conjugates. WO2012/025514
- 32.Vyome Biosciences. Conjugate-based antifungal and antibacterial prodrugs. WO2012/177986
- 33.William Marsh Rice University. Fullerene (C60) vancomycin conjugates as improved antibiotics. US2004/0241173
- 34.University of Manitoba. Triazole-based aminoglycoside-peptide conjugates and methods of use. WO2009/037592
- 35.Newsouth Innovations PTY Ltd. Dual action nitric oxide donors and their use as antimicrobial agents. WO2014/071457
- 36.Czaplewski L, Bax R, Clokie M, et al. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect Dis. 2016;16(2):239–251. doi: 10.1016/S1473-3099(15)00466-1. [DOI] [PubMed] [Google Scholar]
- 37.Liu R, Wang R, Wang F. Antibody-drug conjugates for non-oncological indications. Exp Opin Biol Ther. 2016;16(5):591–593. doi: 10.1517/14712598.2016.1161753. [•• A recent overview of antibody-drug conjugates for non-oncological indications.] [DOI] [PubMed] [Google Scholar]
- 38.Miles Laboratories Inc. β-Galactosyl-umbelliferone-labeled aminoglycoside antibiotics and intermediates in their preparation. US4226978. 1978
- 39.Technion Research & Development Foundation Ltd. Bifunctional antibiotics for targeting rRNA and resistance-causing enzymes and for inhibition of anthrax lethal factor. US7635685. 2009
- 40.MicroCarb Inc. Receptor conjugates for targeting penicillin antibiotics to bacteria. US5466681. 1994
- 41.Sense Therapeutic Ltd. Uses of ble proteins and antibiotics from the bleomycin family. WO2004/046730
- 42.Los Alamos National Security, LLC. Synthetic analogs of bacterial quorum sensors. US8350061. 2011
- 43.University of Notre Dame Du Lac. Anti-bacterial siderophore-aminopenicillin conjugates. US2013/0281424
- 44.Burnet M, Guse J-H, Kim G. Antibiotic conjugates. US2006/0069047
- 45.Oregon Health Sciences University. Covalent polar lipid conjugates with antimicrobial and antineoplastic drugs for targeting to biological protected sites. WO2000/033883
- 46.Ramot U, Yacoby I, Ron EZ, et al. Targeted drug-carrying viruses. WO/2006/095345
- 47.Glycovaxyn Ag. Capsular Gram-positive bacteria bioconjugate vaccines. WO2011/138361
- 48.Gmbh FKD. Water-soluble antibiotic comprising an amino sugar, in the form of a polysaccharide conjugate. WO2003/000738
- 49.Emory University. Oligosaccharide conjugates for targeting bacteria and uses related thereto. WO2012/097223
- 50.Yale University. Antibiotics improved by conjugation with stereo-specific carbohydrtes and methods for carbohydrate stereoselectivity. WO1992/007862
- 51.University of Manitoba. Triazole-based aminoglycoside-peptide conjugates and methods of use. WO2009/037592
- 52.Wang H. Targeted antibiotic and antimicrobial treatments for personalized administration. US2013/0183323
- 53.Genentech Inc. Anti-wall teichoic antibodies and conjugates. US2015/0366985
- 54.Research Corporation NY. Formyl-methionyl chemotatic peptide antibiotic conjugates useful in treating infections. US4427660. 1982
- 55.Allergan Inc. Peptide therapeutic conjugates and uses thereof. WO2010/063124
- 56.National Institute of Immunology. Novel bioconjugates as therapeutic agent and synthesis thereof. WO2007/108013
- 57.Allergan Inc. Antibiotic Conjugates. US2014/0256658
- 58.Allergan Inc. Antibiotic conjugates directly linked with steroid drugs. WO2014/138350
- 59.Allergan Inc. Steroid antibiotic conjugates. WO2014/138359
- 60.Allergan Inc. Antibiotic conjugates linked with steroid drugs. WO2014/138375
- 61.Lehigh University. Methods for the manufacture and use of antimicrobial sterol conjugates. US5834453. 1997
- 62.Ophidian Pharmaceuticals Inc. Prevention and treatment of sepsis. WO1994/014437
- 63.Pono Corporation. Prevention and treatment of sepsis. US6660267. 1994
- 64.Plc CLS. Method for the production of antigenic protein-hapten conjugates, and antibodies corresponding thereto. WO1989/003040
- 65.Aktiebolag RBL. A pharmaceutical preparation and a conjugate of a bactericide and an antibody directed against plaque forming or caries-inducing bacteria. WO1989/011866
- 66.Kim S. Anti-bacterial antibodies and methods of use. US6322788. 1999
- 67.Kim S. Antibody-conjugated antibodies. US2002/0168368
- 68.Strox Biopharmaceuticals LCC. Anti-staphylococcus aureus antibodies. US2009/0274704
- 69.Strox Biopharmaceuticals LLC. Anti-bacterial antibodies. US2010/0322944
- 70.Strox Biopharmaceuticals LLC. Antibiotic-conjugated antibodies. US7,569,677. 2002
- 71.Strox Biopharmaceuticals LLC. Anti-Staphylococcus aureus antibodies. US7795402. 2010
- 72.Strox Biopharmaceuticals LLC. Anti-bacterial antibodies. US8142780. 2010
- 73.Genentech Inc. Anti-wall teichoic antibodies and conjugates. WO2014/194247
- 74.Genentech Inc. Anti-wall teichoic antibodies and conjugates. WO2014/193722
- 75.Genentech Inc. Anti-wall teichoic antibodies and conjugates. US2015/0366985
- 76.Lehar S, Pillow T, Xu M, et al. Novel antibody-antibiotic conjugate eliminates intracellular S aureus. Nature. 2015;527(7578):323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 77.Keener A. First QIDP drug approved, but designation may fail urgent needs. Nature Med. 2014;20:690–691. doi: 10.1038/nm0714-690. [DOI] [PubMed] [Google Scholar]
- 78.Mullard A. FDA drug approvals. Nature Rev Drug Discov. 2014;2015(14):77–81. doi: 10.1038/nrd4545. [DOI] [PubMed] [Google Scholar]
- 79.Mullard A. FDA drug approvals. Nature Rev Drug Discov. 2015;2016(15):73–76. doi: 10.1038/nrd.2016.15. [DOI] [PubMed] [Google Scholar]
- 80.Coates A, Halls G, Hu Y. Novel classes of antibiotics or more of the same. Brit J Pharmacol. 2011;163:184–194. doi: 10.1111/j.1476-5381.2011.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nichols DCN, Trakhtenberg E, Pham L, et al. Use of Ichip for high-throughput in situ cultivation of “uncultivable” microbial species. App Env Microb. 2010;76(8):2445–2450. doi: 10.1128/AEM.01754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ling LL, Schneider T, Peoples A, et al. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hegde SS, Okusanya OO, Skinner R, et al. Pharmacodynamics of TD-1792, a novel glycopeptide-cephalosporin heterodimer antibiotic used against Gram-positive bacteria, in a neutropenic murine thigh model. Antimicrob Agents Chemother. 2012;56(3):1578–1583. doi: 10.1128/AAC.05382-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stryjewski ME, Potgieter PD, Li Y-P, et al. TD-1792 versus vancomycin for treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother. 2012;56(11):5476–5483. doi: 10.1128/AAC.00712-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poolman J, Frasch C, Nurkka A, et al. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin Vaccine Immunol. 2011;18:327–336. doi: 10.1128/CVI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adamo R, Nilo A, Castagner B, et al. Synthetically defined glycoprotein vaccines: current status and future directions. Chem Sci. 2013;4:2995–3008. doi: 10.1039/c3sc50862e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hedari CP, Khinkarly RW, Dbaibo GS. Meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine: a new conjugate vaccine against invasive meningococcal disease. Infect Drug Resist. 2014;7:85–99. doi: 10.2147/IDR.S36243. [DOI] [PMC free article] [PubMed] [Google Scholar]