Abstract
The sodium (Na+)/hydrogen (H+) exchanger 3 (NHE3) and sodium-potassium adenosine triphosphatase (Na+/K+-ATPase) are two of the most important Na+ transporters in the proximal tubules of the kidney. On the apical membrane side, NHE3 primarily mediates the entry of Na+ into and the exit of H+ from the proximal tubules, directly and indirectly being responsible for reabsorbing ~50% of filtered Na+ in the proximal tubules of the kidney. On the basolateral membrane side, Na+/K+-ATPase serves as a powerful engine driving Na+ out of, while pumping K+ into the proximal tubules against their concentration gradients. While the roles of NHE3 and Na+/K+-ATPase in proximal tubular Na+ transport under in vitro conditions are well recognized, their respective contributions to the basal blood pressure regulation and angiotensin II (ANG II)-induced hypertension remain poorly understood. Recently, we have been fortunate to be able to use genetically modified mouse models with global, kidney- or proximal tubule-specific deletion of NHE3 to directly determine the cause and effect relationship between NHE3, basal blood pressure homeostasis, and ANG II-induced hypertension at the whole body, kidney and/or proximal tubule levels. The purpose of this article is to review the genetic and genomic evidence for an important role of NHE3 with a focus in the regulation of basal blood pressure and ANG II-induced hypertension, as we learned from studies using global, kidney- or proximal tubule-specific NHE3 knockout mice. We hypothesize that NHE3 in the proximal tubules is necessary for maintaining basal blood pressure homeostasis and the development of ANG II-induced hypertension.
Keywords: angiotensin II, hypertension, intestines, kidney, NHE3, proximal tubule
INTRODUCTION
Hypertension, i.e., high blood pressure, is now well recognized to play an important causative role in the pathogenesis of target organ injuries, such as ischemic heart diseases, atherosclerosis, stroke, and renal diseases. The impact and significance of hypertension in public health are best illustrated by the facts that about half of the U.S. adult population will develop hypertension and take antihypertensive drugs in their life time and that the direct and indirect costs of preventing and treating hypertension are estimated at hundreds of billion dollars a year (77, 87). Although hypertension has been extensively studied for decades, the precise mechanisms of hypertension remain incompletely understood. Genetic predisposition, endocrine disorders, increased sympathetic nerve activity, cardiovascular dysfunction, unhealthy dietary styles, and environmental factors all have been shown to contribute independently to the development of hypertension. Indeed, regardless of etiologies, clinical practice has firmly established that diuretics to inhibit sodium (Na+) and fluid reabsorption in the kidney and blockers of the renin-angiotensin system (RAS), such as renin inhibitors, angiotensin-converting enzyme inhibitors, angiotensin II (ANG II) type 1 receptor blockers, and aldosterone receptor antagonists, all significantly lower blood pressure in human essential hypertension (3, 87). Furthermore, long-term restriction of dietary Na+ intake appears to be clinically beneficial in reducing blood pressure in prehypertensive and hypertensive subjects (36, 51, 102). This strongly suggests that the activation of the RAS, or ANG II specifically, and increased Na+ reabsorption by the kidney are two key factors in the maintenance of basal blood pressure and the development of hypertension in humans. However, clinical studies also demonstrated that only half of patients with hypertension have adequate control of their blood pressure with current antihypertensive therapy (77, 87). It is therefore imperative to continue to uncover additional mechanisms of hypertension and devise novel antihypertensive strategies to prevent and treat hypertensive diseases.
The late great renal physiologist Dr. Arthur C. Guyton from the University of Mississippi Medical Center long proposed that the kidney plays a central role in maintaining basal arterial blood pressure and the development of hypertension through a key regulating mechanism, i.e., the pressure-natriuresis response (43, 44). The pressure-natriuresis response is an important feedback mechanism for long-term blood pressure control in which an acute increase in blood pressure will lead to a decrease in Na+ reabsorption primarily in the proximal tubule and restore blood pressure to normal (43, 44). In the kidney, the proximal tubules are responsible for reabsorbing ~70% of filtered Na+ and H2O from the glomeruli; thus sustained increases or decreases in Na+ reabsorption in the proximal tubules will ultimately alter overall body salt and fluid balance and therefore blood pressure (116, 121, 131). The Na+ reabsorption in the proximal tubules is mediated primarily by the Na+ and proton (H+) exchanger subtype 3 (NHE3) from apical membranes (78, 96, 103, 110, 113), and the Na+/K+-ATPase from the basolateral membranes (59, 94, 118, 120, 126, 131). NHE3 is the most important apical (AP) membrane Na+ transporter in the proximal tubules (6, 14, 78, 103, 115). Indeed, the key function of NHE3 is to release H+ into the lumen in exchange for luminal Na+ entering the cells; NHE3 is directly and indirectly responsible for reabsorbing more than 50% of filtered Na+ and 80% of filtered bicarbonate () in the kidney. By contrast, Na+/K+-ATPase is well recognized as the most important active Na+ transporter in the basolateral membranes, playing an indispensable role in driving active Na+ transport across the proximal tubules (35). While we do not discount the relatively minor roles of the Na+ and glucose cotransporter (sglt2), Na+ and bicarbonate cotransporters (Na+/), and aquaporin 1 (AQP1), NHE3, and Na+/K+-ATPase clearly contribute most to basal blood pressure homeostasis and the development of ANG II-induced hypertension.
The purpose of this article is to review and discuss the important role of NHE3 in the regulation of basal blood pressure homeostasis and in the development of ANG II-induced hypertension, as we learned from studies using genetically modified mouse models with global, kidney-, or proximal tubule-specific knockout of NHE3 (68–70). To ensure some relevance to the potential role of Na+/K+-ATPase in the proximal tubules, we also discuss whether the expression of Na+/K+-ATPase is upregulated in the proximal tubules of the kidney to compensate for the loss of NHE3 in global, kidney-selective, and proximal tubule-specific NHE3 knockout mice (68–70). Based on the genetic and genomic evidence obtained from studying genetically modified NHE3 mouse models, we hypothesize that NHE3 in the proximal tubules of the kidney may be a novel mechanism and therapeutic target of ANG II-induced hypertension. The new insights to be gained from future studies in this field will help us better understand renal mechanisms of hypertension and develop proximal tubule-targeting diuretics to treat hypertension in the future.
NHE3 IS PREDOMINANTLY EXPRESSED AND LOCALIZED IN THE PROXIMAL TUBULES OF THE KIDNEY
NHE3 is considered to be the most important Na+ transporter in the apical membranes of the proximal tubules in the kidney, because its action directly and indirectly contributes to the reabsorption of nearly 50% of filtered Na+ and fluid loads (26, 116, 121, 131). NHE3 belongs to the mammalian NHE gene family, which has nine isoforms (26, 127), but NHE3 is most relevant in blood pressure regulation due to its dominant expression in the proximal tubules of the kidney and small intestines and its role in mediating Na+ reabsorption (14). Localization of NHE3 on the apical membranes promotes, whereas internalization of NHE3 from the apical membranes to subapical regions inhibits, Na+ reabsorption in the proximal tubules (25, 50, 60, 64, 129). Apart from the proximal tubules, the loop of Henle [thick ascending limb (TAL)] also express low levels of NHE3 (26). With respect to the expression and localization of other NHE isoforms in the kidney, NHE1 is primarily found in the basolateral membranes of proximal and distal convoluted tubules, TAL, and collecting duct, where it mediates vectorial Na+ transport (15, 62). NHE2 and NHE4 are primarily localized in basolateral membranes of medullary collecting duct (19, 109). It has been shown that NHE3 and NHE5 may traffic between the apical membranes and recycling endosomes under physiological conditions (9, 11, 89, 111). However, NHE 1, 2, and 4 appear to mainly stay on the membranes (26). Like NHE3, NHE2 and NHE8 are also expressed in the apical membranes of the proximal tubules, but their functional roles in mediating Na+ transport remain poorly understood. Furthermore, NHE6, 7, 8, and 9 are reportedly localized in other cell types to regulate intraorganellar pH (41, 42, 82, 86, 124). Overall, NHE3 in the proximal tubules of the kidney plays a key role in the regulation of proximal tubule Na+ and fluid reabsorption and therefore has an impact on blood pressure homeostasis.
EXPRESSION OF NHE3 IN THE PROXIMAL TUBULES OF THE KIDNEY IS REGULATED BY VASOACTIVE, ANTINATRIURETIC, AND NATRIURETIC FACTORS
As the major function of NHE3 in the proximal tubules of the kidney involves stimulation of Na+ reabsorption, which alters blood pressure, its expression in the proximal tubules is regulated by a complex of vasoactive antinatriuretic and natriuretic factors (17, 48, 54, 72, 80, 83). One of these factors is the Na+/H+ exchanger regulatory factor-1, NHERF-1 (113, 117). NHERF-1 has been shown to bind to NHE3 and the Na+-dependent phosphate transporter 2a (Npt2a) and induce cAMP-mediated phosphorylation and inhibition of NHE3 activity in the apical membranes of the proximal tubules (85, 111, 117). cAMP (EPAC)- and protein kinase A (PKA)-dependent mechanisms may be involved in the role of NHERF-1 in the regulation of NHE3 activity in the proximal tubules, as suggested in NHERF1−/− mice (85). However, other factors also play important roles in the regulation of NHE3 expression and activity in the kidney. Dopamine, a natriuretic peptide hormone, plays a significant inhibitory role in NHE3 expression and activity in the kidney (10, 18, 30, 50, 116). Conversely, some antinatriuretic peptide hormones or humoral factors, such as ANG II (16, 29, 72, 99), glucocorticoid (92, 114), glucagon, insulin (37), and oxidative stress, upregulate the NHE3 expression and activity and promote Na+ and fluid reabsorption in proximal tubules of the kidney. ANG II, via activation of AT1 receptor-mediated protein kinase Cα (PKCα) and inositol 1,4,5-triphosphate, and Ca2+/calmodulin-dependent protein kinase II (48), induces NHE3 expression and activity in the proximal tubules (21, 28, 49, 55, 57, 58, 72, 75). Inositol 1,4,5-triphosphate receptor-binding protein interacted with inositol 1,4,5-triphosphate, which is expressed in the proximal tubules, appears to mediate ANG II-induced activation of NHE3 in a Ca2+/calmodulin-dependent protein kinase II-dependent pathway (48). Finally, the circadian clock genes and the circadian clock protein period 1 (Per1) have been shown to play an important role in regulating NHE3 in the kidney (102a, 110). Rohman et al. (102a) demonstrated that NHE3 mRNA and protein expression in the kidney display significant circadian patterns with a decrease in expression when the lights are on and an increase in expression when lights are off. Furthermore, the expression of NHE3 and Per2 appeared to be colocalized in the proximal tubules of the kidney. In contrast, Solocinski et al. (110) showed that pharmacological blockade of nuclear Per1 entry or siRNA knockdown of Per1 expression markedly decreased NHE3 expression in the renal cortex or human proximal tubule cells, suggesting that Per1 rather than Per2 regulates NHE3 in the proximal tubules of the kidney. Whether the circadian clock genes directly and independently regulate NHE3 expression in the proximal tubules of the kidney remains poorly understood (102a, 110). Indeed, the expression and activity of nearly all neural, endocrine, paracrine, vasoactive hormones or factors are theoretically under the regulation of the circadian clock genes. Thus, the expression and activity of NHE3 in the proximal tubules of the kidney may directly respond to the circadian rhythms of the expression and activity of various vasoactive antinatriuretic, neural, and natriuretic hormones or factors.
NHE3 IS NECESSARY FOR MAINTAINING NORMAL BLOOD PRESSURE HOMEOSTASIS, AS REVEALED IN GLOBAL NHE3−/−, KIDNEY-SELECTIVE, AND PROXIMAL TUBULE-SPECIFIC NHE3 KNOCKOUT MICE
In the proximal tubules of the kidney, the key function of NHE3 is to secret H+ ions into the tubular lumen in exchange for luminal Na+, thereby mediating proximal tubular Na+ reabsorption and contributing to body salt, fluid, and acid-base balance and basal blood pressure homeostasis (5, 7, 8, 20, 94, 112). Previous studies in cultured proximal tubule cells or isolated perfused proximal tubules with NHE3 inhibitors have firmly established that NHE3 plays a key role in proximal tubule Na+ and transport. However, these studies were unable to determine directly the role of NHE3 in the blood pressure regulation. Schultheis et al. (78, 103, 115) were the first to generate genetically modified NHE3-deficient mice (Nhe3−/−) to determine the physiological relevance of NHE3 in body salt and fluid balance and blood pressure. These authors showed that deletion of NHE3 globally caused slight to moderate salt wasting from the digestive system with diarrhea and mild acidosis, but most of the Nhe3−/− mice survived to adulthood (103). In the kidney, and fluid absorption were reduced by ~50% in proximal convoluted tubules. Although renal renin, aldosterone in the plasma, the AE1 (Slc4a1) Cl−/ exchanger mRNA expression in the kidney, and epithelial sodium channel (ENaC) and H+, K+-ATPase mRNA expression in the colon were all significantly upregulated, basal blood pressure was still significantly reduced in Nhe3−/− mice. These landmark studies confirm for the first time that NHE3 is the major absorptive Na+/H+ exchanger in the kidney and small intestines and that lack of NHE3 impairs body acid and base and Na+ and fluid balance and basal blood pressure homeostasis.
Our group has also recently studied the roles of NHE3 in the regulation of body salt and fluid balance and blood pressure using Nhe3−/− mice with or without transgenic rescue of the NHE3 gene in small intestines of the digestive system (68, 69). Nhe3−/− mice represent the global NHE3 knockout mouse model (68, 103), whereas tgNhe3−/− mice with transgenic rescue of the NHE3 gene in small intestines of the digestive system may be considered to be a “kidney-selective” NHE3 knockout model (69, 88). We also found that Nhe3−/− and tgNhe3−/− mice grew normally but showed significantly abnormal structural and absorptive phenotypes of the small intestines, as demonstrated by the markedly enlarged cecum segment of the small intestines with accumulation of a large volume of fluid content inside and a marked increase in 24 h fecal Na+ excretion (Fig. 1) (68–70). In our study, however, we found that the transgenic rescue of the NHE3 gene in small intestines in tgNhe3−/− mice was inadequate to fully rescue the abnormal structural and absorptive phenotypes of Nhe3−/− mice (69, 70). The moderate to severe salt wasting from the digestive system of Nhe3−/− and tgNhe3−/− mice caused marked compensatory Na+-retaining responses in the kidney with significantly decreases in 24 h urine output and urinary Na+ excretion. Although the RAS was markedly activated as demonstrated by increased basal plasma ANG II and aldosterone levels, basal systolic, diastolic, and mean arterial blood pressures were still ~12–15 mmHg lower in Nhe3−/− and tgNhe3−/− mice, respectively (Fig. 2) (P < 0.01). Recently, basal arterial blood pressure was also reportedly lower in a new whole kidney nephron segment, panepithelial cell-specific NHE3 knockout mice using the Pax8-Cre/NHE3loxlox approach (34). Our data are consistent with the results of Schultheis et al. (103), Noonan et al. (88), and Fenton et al. (34) and strongly support the scientific premise that NHE3 plays a critical role in maintaining basal blood pressure homeostasis by mediating Na+ reabsorption in the digestive system and the kidney.
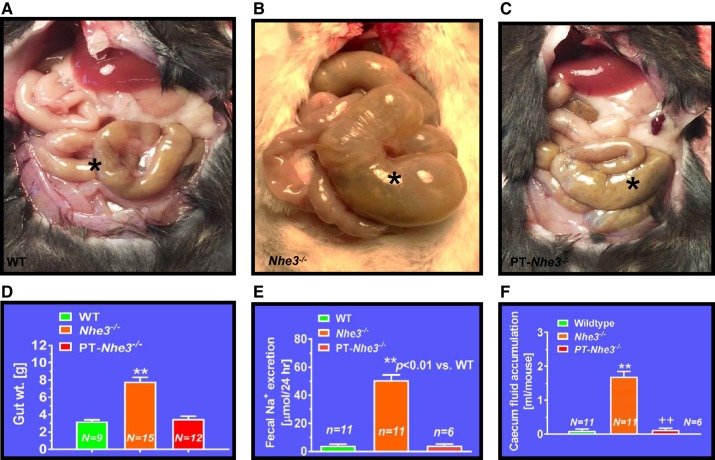
Fig. 1.
Structural and absorptive phenotypes of small intestines of the digestive system in mutant mice with global (Nhe3−/−), kidney- (tgNhe3−/−), and proximal tubule-specific deletion of NHE3 (PT-Nhe3−/−). A: a representative normal cecum segment between small and large intestines in a wild-type mouse (*). B: a representative cecum segment between small and large intestines in a global Nhe3−/− mouse, showing the extremely enlarged cecum segment accumulated with a large volume of fluid content inside (*). C: a representative cecum segment between small and large intestines in a PT-Nhe3−/− mouse, showing the lack of enlarged cecum segment and no accumulation of a large volume of fluid content in the cecum segment (*). Please note that the overall gut weight more than double in global Nhe3−/− mice than wild-type (WT) and PT-Nhe3−/− mice (D), whereas 24 h fecal Na+ excretion (E) and accumulation of fluid content (F) are ~10 times higher in global Nhe3−/− mice than WT and PT-Nhe3−/− mice. These results strongly suggest that there is no off-side NHE3 deletion in small intestines in PT-Nhe3−/− mice. Reproduced from reference (70) with permission.
Fig. 2.
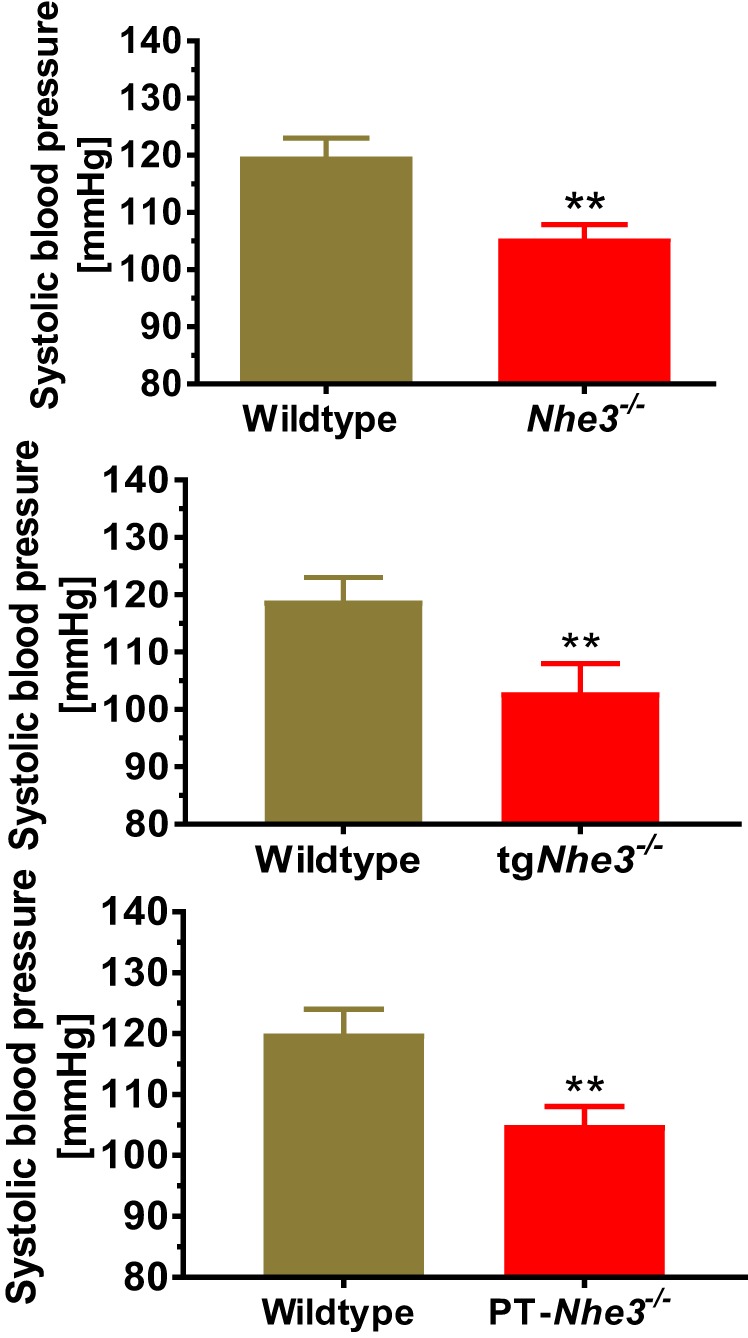
The basal blood pressure phenotype in genetically modified mice with global (Nhe3−/−), kidney- (tgNhe3−/−), and proximal tubule-specific deletion of NHE3 (PT-Nhe3−/−). Basal systolic and intra-arterial blood pressure is significantly lower in adult male Nhe3−/−, tgNhe3−/− and PT-Nhe3−/−mice. Blood pressure was measured continuously for 7 days by the direct implanted telemetry technique. Modified from references (68–70) with permission.
To further determine the role of NHE3 in the proximal tubules of the kidney in body salt and fluid balance and blood pressure regulation, Soleimani at the University of Cincinnati and our group at the University of Mississippi Medical Center generated a novel mouse model with deletion of NHE3 selectively in the proximal tubules of the kidney, PT-Nhe3−/− mice, using the sglt2-Cre/LoxP approach (65, 70). The effectiveness of this approach to genetically delete NHE3 only in the proximal tubules is based on the scientific premise that sglt2 is primarily expressed in the early S1 and S2 segments of the proximal tubules, and thus the sglt2 promoter is expected to drive the Cre expression exclusively in the S1 and S2 segments (65, 70, 101). Using PT-Nhe3−/− mice, we directly tested the hypothesis that deletion of NHE3 selectively in the proximal tubules of the kidney lowers basal blood pressure by increasing the pressure-natriuresis response in mice. To test the hypothesis, adult male and female, age-matched wild-type and PT-Nhe3−/− mice were studied for basal body electrolytes and pH, blood pressure, and kidney function, the pressure-natriuresis response, and the natriuretic responses to acute saline expansion (0.9% NaCl at 10% body wt ip) or to 2 wk of 2% NaCl diet. Under physiological conditions, PT-Nhe3−/− mice showed significantly lower systolic, diastolic, and mean arterial blood pressure than wild-type mice (Fig. 2) (P < 0.01), at the levels basically similar to those of global Nhe3−/− and tgNhe3−/− mice (70). However, unlike Nhe3−/− and tgNhe3−/− mice, which show significant compensatory Na+-retaining responses (68, 69), PT-Nhe3−/− mice showed significantly greater diuretic and natriuretic responses than wild-type mice (P < 0.01). Twenty-four hour fecal Na+ excretion, plasma pH, Na+, and bicarbonate levels were not significantly different from wild-type mice. Furthermore, the pressure-natriuresis response (Fig. 3) and natriuretic responses to acute saline expansion or to 2 wk 2% NaCl diet were all significantly augmented compared with wild-type mice (70).
Fig. 3.
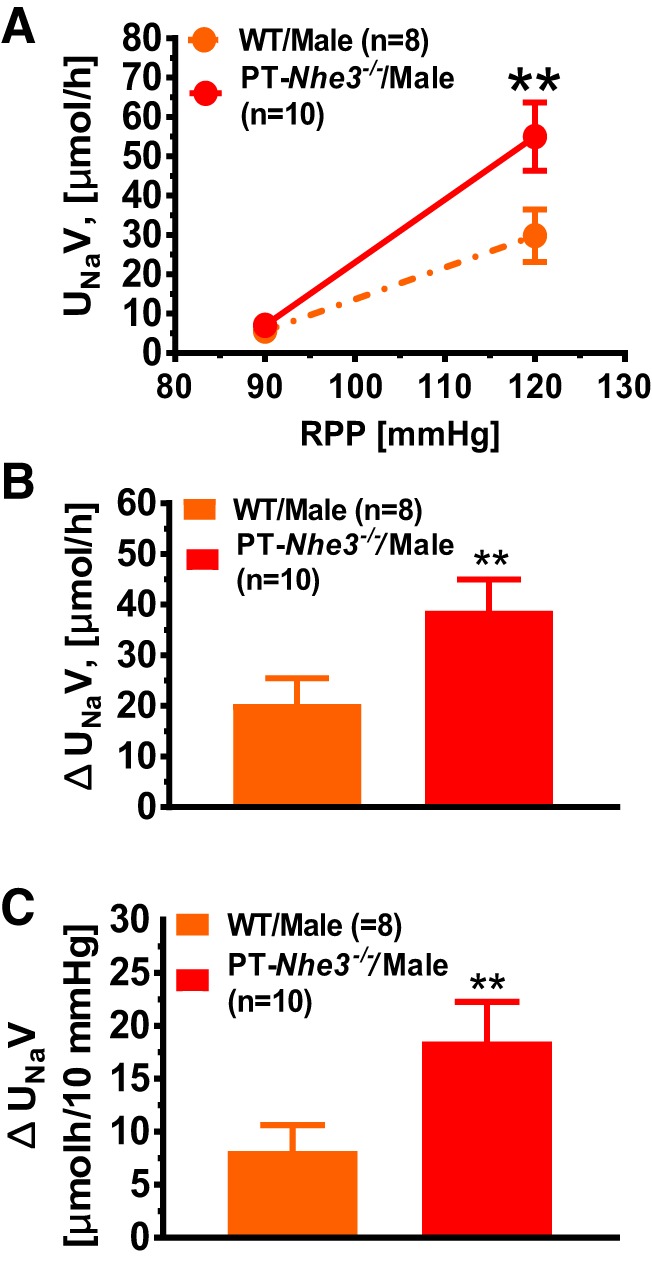
The pressure-natriuresis response in male wild-type and PT-Nhe3−/− mice. In response to an increase of ~30 mmHg in renal perfusion pressure, the pressure-natriuresis response increased ~5-fold in male wild-type mice, whereas the response increased ~8-fold in male PT-Nhe3−/− mice (**P < 0.01). Both net urinary Na+ excretion and as a response to 10 mmHg blood pressure increase were significantly higher in male PT-Nhe3−/− mice (P < 0.01; n = 10) than in wild-type mice (P < 0.01; n = 8). Reproduced from reference (70) with permission.
Taken together, the direct evidence for an important role of NHE3 in maintaining normal basal blood pressure has been confirmed in global Nhe3−/−, kidney-selective tgNhe3−/− or Pax8-Cre/NHE3loxlox, and proximal tubule-specific Nhe3−/− mice (34, 68–70, 88, 103). However, it should be pointed out that the levels of basal blood pressure are not remarkably different in global, kidney-, and proximal tubule-specific NHE3 knockout models. Since the hypotensive phenotype is accompanied by marked absorptive defects in small intestines of Nhe3−/− and tgNhe3−/−, but not in those of PT-Nhe3−/− mice, our data support the physiological importance of NHE3 and the hypothesis that NHE3 in the proximal tubules of the kidney plays an essential role in maintaining basal blood pressure homeostasis by regulating proximal tubule Na+ reabsorption and the pressure and natriuretic response (70).
NHE3 IS NECESSARY FOR THE FULL DEVELOPMENT OF ANG II-INDUCED HYPERTENSION, AS REVEALED IN GLOBAL NHE3−/− AND KIDNEY-SELECTIVE NHE3 KNOCKOUT MICE
We have previously shown that ANG II, at nonpressor concentrations, acts on the AT1 (AT1a) receptor to increase NHE3 expression and activity in rabbit and mouse proximal tubule cells in vitro (66, 67, 71). In vivo, however, the effects of ANG II on the expression or activity of NHE3 in the proximal tubules of the kidney appear to be dose and blood pressure dependent (64, 72, 80, 97). Acute infusion of highly pressor doses of ANG II induces rapid internalization of NHE3 in the proximal tubules and pressure natriuresis, whereas long-term infusion of subpressor doses of ANG II increases the expression and activity of proximal tubule NHE3 and slowly elevates blood pressure (72, 73). Indeed, we have recently demonstrated that NHE3 proteins were significantly increased in the apical membranes of the proximal tubules after 2 wk infusion of a subpressor dose of ANG II (0.5 mg/kg/day ip) but are decreased by 2 wk infusion of a highly pressor dose of ANG II (1.5 mg/kg/day ip) (72). These results suggest that NHE3 may play an important role in the regulation of blood pressure by ANG II through its actions on proximal tubule Na+ transport.
We have recently tested the hypothesis that NHE3 is necessary for the full development of ANG II-induced hypertension in global Nhe3−/− and kidney-selective tgNhe3−/− mouse models (68, 69). Our argument is that NHE3, especially in the proximal tubules of the kidney, may play an important role in the renal mechanisms of ANG II-induced hypertension. To test the hypothesis, age- and body weight-matched adult male wild-type, Nhe3−/− and tgNhe3−/− mice were infused with or without ANG II (1.5 mg/kg/day ip, via osmotic minipump), or ANG II plus treatment with the AT1 receptor blocker losartan (20 mg/kg/day po) for 2 wk. In response to ANG II infusion for 2 wk, blood pressure markedly increased in wild-type mice in a time-dependent manner (P < 0.01). However, the hypertensive response to ANG II was attenuated by more than 50% in Nhe3−/− and tgNhe3−/− mice although plasma ANG II and aldosterone levels were significantly elevated during ANG II infusion (Fig. 4) (68, 69). Similar attenuation of the pressor response to ANG II was observed in anesthetized Nhe3−/− and tgNhe3−/− mice (68, 69). In further preliminary studies, we infused the same dose of ANG II in PT-Nhe3−/− mice and confirmed that ANG II-induced hypertension was also attenuated in PT-Nhe3−/− mice to a similar magnitude of Nhe3−/− and tgNhe3−/− mice (68, 69). Our studies therefore provide unequivocal evidence to support the hypothesis that NHE3 in the proximal tubules of the kidney, along with NHE3 in small intestines, is necessary for the development of ANG II-induced hypertension.
Fig. 4.
The systolic blood pressure responses to ANG II are attenuated in adult male conscious global Nhe3−/− and “kidney-selective” tgNhe3−/−mice, compared with wild-type mice (A, **P < 0.01 vs. the same groups' respective control or baseline levels; ++P < 0.01 vs. the corresponding wild-type groups with the same treatment), and the compensatory upregulation of basal water (aquaporin 1, AQP1) and major Na+ cotransporter proteins, including Na+/ and Na+/K+-ATPase α1, in the proximal tubules of global Nhe3−/− mice (B, ++P < 0.01 vs. wild type). Reproduced from references (68, 69).
EXPRESSION, LOCALIZATION, AND ACTION OF Na+/K+-ATPase IN THE PROXIMAL TUBULES
In the basolateral membranes of the proximal tubules of the kidney, Na+/K+-ATPase, sodium-potassium adenosine triphosphatase, plays a critical role in driving active Na+ transport across the proximal tubular epithelium against a sodium concentration gradient (35, 119–121). Na+/K+-ATPase was discovered in 1957 by the Danish scientist Jens Christian Skou (108), who isolated an enzyme from the cell membranes of nerves that was activated by Mg2+, Na+, and K+ and mediated the active transport of Na+ and K+ across the cell membrane. Skou received the Nobel Prize in Physiology and Medicine in 1997 for discovering Na+/K+-ATPase (24). Na+/K+-ATPase was cloned in the 1980s (104–106) and was found to belong to the P-type family of ATPase with two major subunits, i.e., the catalytic α- and glycosylated β-subunits (27, 35, 56). The α-subunit consists of four isoforms, α1–α4, with a molecular weight of 110 kDa (27, 35), 10 transmembrane domains (M1–M10), and binding sites for ouabain and K+ at the extracellular domain (27, 35). By comparison, the β-subunit includes three isoforms, β1–β3, with a single transmembrane domain and a large extracellular domain (27, 35, 56). The α-subunit plays a key role in the Na+ transport activity of the Na+/K+-ATPase, while the β-subunit provides a supportive role. Additionally, a γ-subunit was cloned with 53 amino acids, but it does not have an effect on the formation and expression of functional Na+/K+-ATPase α/β-subunit complexes (13, 35, 81).
In the kidney, the Na+/K+-ATPase is predominantly localized on the basolateral plasma membranes of the proximal convoluted and proximal straight tubules (33, 59, 100). However, the basolateral membranes of the loop of Henle, distal tubules, and collecting duct also express Na+/K+-ATPase. The role of Na+/K+-ATPase in mediating active Na+ transport in the proximal tubules of the kidney has been extensively investigated since the 1970s. Ouabain, a natural inhibitor of Na+/K+-ATPase, has been widely used in in vitro epithelial Na+ transport studies to determine the role of this enzyme (35). In vitro microperfusion and in vivo micropuncture studies have shown that inhibition of Na+/K+-ATPase by ouabain markedly decreased proximal tubular Na+ reabsorption (38, 40, 45). These early studies have established that Na+/K+-ATPase drives active Na+ extrusion from basolateral plasma membranes and helps promote Na+ entry into proximal tubular cells via the actions of NHE3 and sodium and glucose cotransporters (sglt2), sodium bicarbonate transporter etc. from apical membranes (35, 38, 40, 45).
In addition to mediating active Na+ transport, Na+/K+-ATPase may act as a signal transducer in the proximal tubules of the kidney (1). Vasoactive peptide hormones, such as ANG II, atrial natriuretic peptide, dopamine, and endothelin 1, high-salt diet, increased oxidative stress, regulate proximal tubular Na+ transport in part by regulating the expression or activity of Na+/K+-ATPase, or the endocytosis of Na+/K+-ATPase into proximal tubule cells (23, 35, 61, 76). Indeed, as the most relevant to the subject of this review article, ANG II was shown to directly stimulate the activity and/or the phosphorylation of Na+/K+-ATPase in the proximal tubules of the rat kidney in vitro or in vivo (31, 39, 122, 123, 125, 126). The stimulatory effect of ANG II on Na+/K+-ATPase activity is primarily mediated by AT1 receptors (46). By contrast, ANG II and/or ANG III, via activation of AT2 receptors (90, 129) and nitric oxide-cGMP-dependent signaling (107), may induce the internalization of apical membrane NHE3 and basolateral Na+/K+-ATPase, leading to the inhibition of proximal tubular Na+ reabsorption and natriuresis, and consequently lower blood pressure in rats (60, 91).
LACK OF GLOBAL, KIDNEY-, OR PROXIMAL TUBULE-SPECIFIC OVEREXPRESSION OR DELETION OF Na+/K+-ATPase ANIMAL MODELS
In contrast to NHE3 in the proximal tubules of the kidney, the role of Na+/K+-ATPase or its α- or β-subunits in the regulation of active Na+ transport or reabsorption in the proximal tubules and basal blood pressure homeostasis has not been studied in genetically modified animal models. To date no transgenic mice with either global or tissue-specific deletion or overexpression of Na+/K+-ATPase in the proximal tubules of the kidney have been reported. The difficulty in generating and breeding these mice for further studies is due to the fact that mice with global deletion of the α1-subunit gene die during embryogenesis, and mice with global deletion of α2-subunit gene also die, immediately after birth (12, 27, 52, 53, 84). It would be interesting to determine the roles of Na+/K+-ATPase in the proximal tubules or other tubular segments of the kidney using mutant mouse models with proximal tubule-specific or other nephron segment- or cell-specific deletion or overexpression of α- or β-subunits of Na+/K+-ATPase.
Na+/K+-ATPase IN THE PROXIMAL TUBULES OF THE KIDNEY IS UPREGULATED IN GLOBAL, KIDNEY-SELECTIVE, AND PROXIMAL TUBULE-SPECIFIC NHE3-KNOCKOUT MICE
In the proximal tubules of the kidney, Na+ and fluid are reabsorbed in proportion to the glomerular filtered load through a unique mechanism, i.e., glomerulotubular balance through the regulation of physical and humoral factors (29, 30, 130, 131). This physiological response involves the actions of NHE3, the sglt2, Na+ and amino acid cotransporter, Na+ and phosphate cotransporter 2, and AQP1 on the apical membranes, and Na+/K+-ATPase and Na+ and bicarbonate cotransporter on the basolateral membranes. However, NHE3 and Na+/K+-ATPase play the most important or a predominant role in mediating Na+ reabsorption in the proximal tubules of the kidney (131). We have recently bred global (Nhe3−/−) (68), kidney-selective (tgNhe3−/−) (69), and proximal tubule-specific NHE3-knockout mice (PT-Nhe3−/−) (70) to determine the roles of NHE3 in the regulation of overall body Na+ and fluid balance, proximal tubule Na+ reabsorption, and basal blood pressure homeostasis. We next determined whether Na+/K+-ATPase and other Na+ cotransporters in the proximal tubules of the kidney are upregulated to compensate for the loss of global, kidney, and proximal tubule NHE3 in global, kidney-selective, and proximal tubule-specific NHE3-knockout mice. Finally, we investigated whether ANG II-induced hypertension is attenuated in global, kidney-selective, and proximal tubule-specific NHE3-knockout mice (68, 69).
We focused on three key Na+ transporter and three key signaling proteins in the proximal tubules of the superficial renal cortex of the kidney in global Nhe3−/− (68), kidney-selective tgNhe3−/− (69), and proximal tubule-specific PT-Nhe3−/− mice (70). In the absence of apical membrane NHE3 in these three different strains of NHE3 genetically modified mice, the expression of sglt2 and the water channel protein AQP 1 in apical membranes and the α1-subunit of Na+/K+-ATPase, and Na+ bicarbonate cotransporter (Na+/) proteins in basolateral membranes was significantly increased in the superficial cortex of the kidney (Fig. 4, P < 0.01) (68–70). Furthermore, the expression of ENaC and AQP2 proteins in the renal medulla was also significantly upregulated (70). The upregulation of sglt2, Na+/K+-ATPase, Na+/-, ENaC, AQP1, and AQP2 in the kidney was associated with significant increases in signaling responses, including phosphorylated PKCα, phosphorylated MAP kinases ERK1/2, and phosphorylated glycogen synthase kinase-3α/β (GSK-3α/β) in the proximal tubules of Nhe3−/− or tgNhe3−/− mice (68–70). In wild-type mice, ANG II infusion for 2 wk significantly increased the expression of the α1-subunit of Na+/K+-ATPase, Na+/, and AQP1 in the proximal tubules of the kidney, but this response was not observed in Nhe3−/− or tgNhe3−/− mice (68, 69). These data suggest that the deletion of NHE3 globally, in the whole kidney, or in the proximal tubules of the kidney leads to the compensatory upregulation of the expression of sglt2, Na+/K+-ATPase, Na+/, ENaC, AQP1, and AQP2 or activation of PKCα, MAP kinases ERK1/2, and GSK-3α/β in the proximal tubules of the kidney. This in turn increases Na+ and fluid reabsorption through the actions of these Na+ cotransporters or AQP1 and AQP2 proteins in the proximal tubules or distal nephron segments of the kidney to minimize salt wasting due to the loss of NHE3 in these tissues. However, it should be recognized that the upregulation of the expression of sglt2, Na+/K+-ATPase, Na+/, ENaC, AQP1, and AQP2 in Nhe3−/−, tgNhe3−/−, and PT-Nhe3−/− mice is inadequate to compensate for the loss of NHE3 in small intestines or the proximal tubules of the kidney, with the consequence of lower systolic, diastolic, and mean arterial blood pressure in these animals (68–70). Taken together, NHE3 in the small intestines of the digestive system and the proximal tubules of the kidney plays a key role in maintaining basal blood pressure homeostasis and may be considered to be a potential therapeutic target of hypertension.
CONCLUSIONS AND FUTURE PERSPECTIVES
In summary, we and others have used global, kidney-, and proximal tubule-specific NHE3 knockout mouse models to determine the roles of NHE3 in maintaining body salt and fluid balance, basal blood pressure homeostasis, and the development of ANG II-induced hypertension (34, 65, 68–70, 103, 115). The data derived from these studies using these global and tissue-specific, genetically modified NHE3 knockout mice provide unequivocal evidence to support the scientific hypothesis that NHE3 is necessary, or required, for the maintenance of body salt and fluid balance, basal blood pressure homeostasis, and the development of ANG II-induced hypertension (Fig. 5). Other Na+ and glucose cotransporters, Na+/K+-ATPase, Na+ and bicarbonate cotransporters, Na+ and amino acid cotransporters, ENaC, and AQP1 and 2 in the kidney may also contribute to the blood pressure regulation, but their roles likely pale in comparison to that of NHE3. This conclusion is supported by our observations that most, if not all, of these Na+ cotransporters are markedly upregulated in the proximal tubules, or the medulla, of the kidney in global, kidney-, and proximal tubule-specific Nhe3−/− mice. However, activation or upregulation of these transporters is still inadequate to compensate for the loss of the action of NHE3 and restore blood pressure to normal. These findings have significant and potentially translational or clinical implications for hypertension in humans.
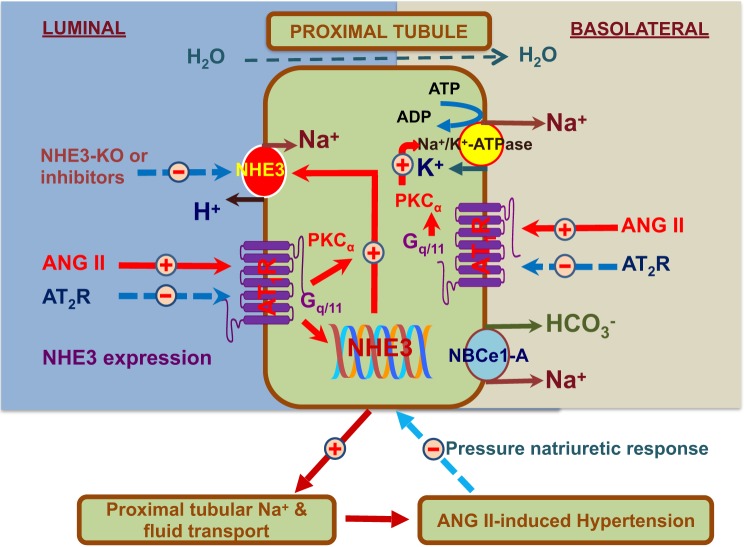
Fig. 5.
A schematic hypothesis that systemic and tissue ANG II activates AT1 receptors on the apical and basolateral membranes of the proximal tubules of the kidney and downstream signaling pathways to increase the expression and activity of NHE3 and Na+/K+-ATPase, which stimulates proximal tubular Na+ reabsorption, causes body Na+ and fluid retention, and induces ANG II-dependent hypertension. Increased arterial pressure will induce the pressure-natriuresis response to prevent further increase in blood pressure at the very early stage of hypertension. However, the initially increased pressure-natriuresis response is insufficient to restore blood pressure to normal in established ANG II-induced hypertension due to the resetting of the response to higher pressures.
Hypertension is currently treated with loop- or distal tubule-selective diuretics, RAS inhibitors, Ca2+ channel blockers, adrenergic receptor antagonists, or aldosterone receptor antagonists (22, 32, 79). Three types of diuretics have been used as antihypertensive drugs: thiazides, which inhibit Na+ and Cl− reabsorption from the distal convoluted tubules by blocking the thiazide-sensitive Na+-Cl− symporter; loop diuretics, which inhibit the Na+-K+-2 Cl− symporter (cotransporter) in the TAL of the loop of Henle; and K+-sparing diuretics, which either block ENaC or act as aldosterone receptor antagonists (32, 79). However, only 50% of patients with hypertension have their blood pressure under adequate control with these classes of antihypertensive drugs, and some patients still develop resistant hypertension. Against this background, no proximal tubule-selective diuretics, with the exception of carbonic anhydrase inhibitors that are mild diuretics, have been developed for antihypertensive treatment. Proximal tubules are responsible for reabsorbing 70% of filtered Na+ load, whereas NHE3 in the proximal tubules directly and indirectly accounts for reabsorbing >50% of filtered Na+ and ~80% of filtered in the kidney (4, 7, 83, 103). It is not surprising to hypothesize that the upregulation of the intratubular RAS and NHE3 expression and activity in the proximal tubules will stimulate Na+ and reabsorption, induce body salt and fluid retention, and contribute to blood pressure control in health and hypertension. Indeed, increased NHE3 expression and activity have been reported in the proximal tubules of spontaneously hypertensive rats (SHR), an animal model of human essential hypertension (47, 63, 128). Even in 5 wk old SHR before blood pressure begins to rise, NHE3 activity is increased by 48% (2) and remains substantially elevated after hypertension is firmly established (63). Furthermore, all known prohypertensive factors, including ANG II, glucocorticoid, hyperinsulinema, hyperglucagonemia, increased oxidative stress, and renal nerve stimulation (16, 37, 48, 72, 92, 93, 116), induce NHE3 expression and activity and promote Na+ reabsorption in the proximal tubules. Thus this raises the possibility to target NHE3 pharmacologically in the proximal tubules of the kidney for additional antihypertensive therapy for those patients with poorly controlled hypertension. A recent proof of concept study by Linz et al. (74) showed that an orally active, nonabsorbable NHE3 inhibitor, SAR218034, significantly inhibited intestinal Na+ reabsorption and reduced blood pressure in 78 wk old SHR-lean rats, which supports this hypothesis. Perhaps, the development of orally active, absorbable, and potent NHE3 inhibitors as proximal tubule-selective diuretics to inhibit NHE3 in the proximal tubules of the kidney may have significant translational impact on future antihypertensive treatment in humans.
GRANTS
This work was supported in part by National Institutes of Health Grants 2RO1DK-067299-06A2, 2R01DK067299-10A1, 1R01DK-102429-01, 2R01DK-102429-03A1, and 1R56HL-130988-01 to J. L. Zhuo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.C.L., X.Z., and J.L.Z. conceived and designed research; X.C.L., X.Z., X.C., C.Z., D.Z., J.Z., and J.L.Z. performed experiments; X.C.L., X.Z., X.C., C.Z., D.Z., J.Z., and J.L.Z. analyzed data; X.C.L., X.Z., X.C., C.Z., D.Z., J.Z., and J.L.Z. interpreted results of experiments; X.C.L., X.Z., and J.L.Z. prepared figures; X.C.L., X.Z., and J.L.Z. drafted manuscript; X.C.L., X.Z., and J.L.Z. edited and revised manuscript; X.C.L., X.Z., X.C., C.Z., D.Z., J.Z., and J.L.Z. approved final version of manuscript.
Supplemental Data
Chemogenetic activation of orexin neurons does not affect water consumption and body composition. Young 5-mo-old and middle-aged (12 mo old) female mice received intracranial injections of control DREADD virus (cDREADD, AAV2-hSyn-DIO-mCherry). Female mice were treated with either water or water with clozapine-N-oxide (CNO) for 2 days. Young and middle-aged mice consumed similar amounts of water and water with CNO (0.25 mg/ml) (n = 10/group) (A). Middle-aged mice have increased body (B) and fat mass (C), as well as the ratio of fat to lean mass (D) relative to young mice. However, no within-age differences in body mass, fat mass, and fat-to-lean ratio were observed between water and water with CNO-treated female mice (n = 10/group; *P < 0.05, **P < 0.01, ***P < 0.005) - .docx (226 KB)
Clozapine-N-oxide (CNO) effects on anxiety, locomotion and memory in 5-mo-old female mice. Young 5-mo-old female mice received intracranial injections of control DREADD virus (cDREADD, AAV2-hSyn-DIO-mCherry). CNO treatment did not affect distance traveled or time spent in open arms in the elevated plus maze (EPM, A and B). The time spent in open arms and the time spent in center of the open field test (OFT) arena was not affected by CNO treatment (C and D). CNO did not affect performance in the novel object recognition test in 5-mo-old female mice (E and F) - .docx (202 KB)
ACKNOWLEDGMENTS
We thank Dr. Gary Shull of the University of Cincinnati College of Medicine for generously providing us with breeding pairs of global Nhe3−/− and tgNhe3−/− mice, Dr. Manoocher Soleimani of the University of Cincinnati School of Medicine for generously providing us with breeding pairs of NHE3-floxed mice, and Dr. Isabelle Rubera from the Laboratoire CNRS 3472 LP2M, Université de Nice Sophia Antipolis, France, and Dr. Rong Li of Johns Hopkin University for generously providing us with breeding pairs of iL1-sglt2-Cre mouse strain, and Elisa Miguel-Qin and Hoang Nguyen for excellent technical assistance over many years.
Current addresses: D. Zhu, Department of Outpatient Medicine, Guangxi Science and Technology University 1st Affiliate Hospital, Wuzhou, Guangxi; X. Zheng, C. Zhao, and J. Zhang, Department of Emergency Medicine, Guangxi Medical University 2nd Affiliated Hospital, Nanning, Guangxi, China.
REFERENCES
- 1.Aizman O, Aperia A. Na,K-ATPase as a signal transducer. Ann N Y Acad Sci 986: 489–496, 2003. doi: 10.1111/j.1749-6632.2003.tb07233.x. [DOI] [PubMed] [Google Scholar]
- 2.Aldred KL, Harris PJ, Eitle E. Increased proximal tubule NHE-3 and H+-ATPase activities in spontaneously hypertensive rats. J Hypertens 18: 623–628, 2000. doi: 10.1097/00004872-200018050-00016. [DOI] [PubMed] [Google Scholar]
- 3.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288: 2981–2997, 2002. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 4.Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev 70: 79–114, 1990. doi: 10.1152/physrev.1990.70.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Alpern RJ, Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest 78: 502–510, 1986. doi: 10.1172/JCI112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 7.Aronson PS. Mechanisms of active H+ secretion in the proximal tubule. Am J Physiol Renal Physiol 245: F647–F659, 1983. doi: 10.1152/ajprenal.1983.245.6.F647. [DOI] [PubMed] [Google Scholar]
- 8.Aronson PS, Nee J, Suhm MA. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature 299: 161–163, 1982. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- 9.Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. J Biol Chem 274: 4383–4388, 1999. doi: 10.1074/jbc.274.7.4383. [DOI] [PubMed] [Google Scholar]
- 10.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int 64: 2133–2141, 2003. doi: 10.1046/j.1523-1755.2003.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird NR, Orlowski J, Szabó EZ, Zaun HC, Schultheis PJ, Menon AG, Shull GE. Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. J Biol Chem 274: 4377–4382, 1999. doi: 10.1074/jbc.274.7.4377. [DOI] [PubMed] [Google Scholar]
- 12.Barcroft LC, Moseley AE, Lingrel JB, Watson AJ. Deletion of the Na/K-ATPase alpha1-subunit gene (Atp1a1) does not prevent cavitation of the preimplantation mouse embryo. Mech Dev 121: 417–426, 2004. doi: 10.1016/j.mod.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Béguin P, Wang X, Firsov D, Puoti A, Claeys D, Horisberger JD, Geering K. The gamma subunit is a specific component of the Na,K-ATPase and modulates its transport function. EMBO J 16: 4250–4260, 1997. doi: 10.1093/emboj/16.14.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol Renal Physiol 265: F736–F742, 1993. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- 15.Biemesderfer D, Reilly RF, Exner M, Igarashi P, Aronson PS. Immunocytochemical characterization of Na(+)-H+ exchanger isoform NHE-1 in rabbit kidney. Am J Physiol Renal Physiol 263: F833–F840, 1992. doi: 10.1152/ajprenal.1992.263.5.F833. [DOI] [PubMed] [Google Scholar]
- 16.Bloch RD, Zikos D, Fisher KA, Schleicher L, Oyama M, Cheng JC, Skopicki HA, Sukowski EJ, Cragoe EJ Jr, Peterson DR. Activation of proximal tubular Na(+)-H+ exchange by angiotensin II. Am J Physiol Renal Physiol 263: F135–F143, 1992. doi: 10.1152/ajprenal.1992.263.1.F135. [DOI] [PubMed] [Google Scholar]
- 17.Bobulescu IA, Dwarakanath V, Zou L, Zhang J, Baum M, Moe OW. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol Renal Physiol 289: F685–F691, 2005. doi: 10.1152/ajprenal.00447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bobulescu IA, Quiñones H, Gisler SM, Di Sole F, Hu MC, Shi M, Zhang J, Fuster DG, Wright N, Mumby M, Moe OW. Acute regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A. Am J Physiol Renal Physiol 298: F1205–F1213, 2010. doi: 10.1152/ajprenal.00708.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bookstein C, Musch MW, DePaoli A, Xie Y, Villereal M, Rao MC, Chang EB. A unique sodium-hydrogen exchange isoform (NHE-4) of the inner medulla of the rat kidney is induced by hyperosmolarity. J Biol Chem 269: 29704–29709, 1994. [PubMed] [Google Scholar]
- 20.Boron WF, Boulpaep EL. The electrogenic Na/HCO3 cotransporter. Kidney Int 36: 392–402, 1989. doi: 10.1038/ki.1989.208. [DOI] [PubMed] [Google Scholar]
- 21.Chan YL, Wang T. The role of protein kinase C in mediating the stimulatory effect of angiotensin II on renal tubular transport. Contrib Nephrol 95: 216–221, 1991. doi: 10.1159/000420662. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 23.Cinelli AR, Efendiev R, Pedemonte CH. Trafficking of Na-K-ATPase and dopamine receptor molecules induced by changes in intracellular sodium concentration of renal epithelial cells. Am J Physiol Renal Physiol 295: F1117–F1125, 2008. doi: 10.1152/ajprenal.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clausen T, Persson AE. Jens Christian Skou awarded the Nobel prize in chemistry for the identification of the Na+, K(+)-pump. Acta Physiol Scand 163: 1–2, 1998. doi: 10.1046/j.1365-201x.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem 273: 2035–2043, 1998. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 26.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol Rev 87: 825–872, 2007. doi: 10.1152/physrev.00030.2006. [DOI] [PubMed] [Google Scholar]
- 27.Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol 290: R524–R528, 2006. doi: 10.1152/ajpregu.00838.2005. [DOI] [PubMed] [Google Scholar]
- 28.Du Z, Ferguson W, Wang T. Role of PKC and calcium in modulation of effects of angiotensin II on sodium transport in proximal tubule. Am J Physiol Renal Physiol 284: F688–F692, 2003. doi: 10.1152/ajprenal.00261.2002. [DOI] [PubMed] [Google Scholar]
- 29.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance: II: impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol 303: F1507–F1516, 2012. doi: 10.1152/ajprenal.00277.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Z, Yan Q, Wan L, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. I. Impact of dopamine on flow-dependent transport. Am J Physiol Renal Physiol 303: F386–F395, 2012. doi: 10.1152/ajprenal.00531.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 278: 28719–28726, 2003. doi: 10.1074/jbc.M303741200. [DOI] [PubMed] [Google Scholar]
- 32.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med 361: 2153–2164, 2009. doi: 10.1056/NEJMra0907219. [DOI] [PubMed] [Google Scholar]
- 33.Ernst SA. Transport ATPase cytochemistry: ultrastructural localization of potassium-dependent and potassium-independent phosphatase activities in rat kidney cortex. J Cell Biol 66: 586–608, 1975. doi: 10.1083/jcb.66.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenton RA, Poulsen SB, de la Mora Chavez S, Soleimani M, Dominguez Rieg JA, Rieg T. Renal tubular NHE3 is required in the maintenance of water and sodium chloride homeostasis. Kidney Int 92: 397–414, 2017. doi: 10.1016/j.kint.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- 36.Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 125: 433–439, 2012. doi: 10.1016/j.amjmed.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Fuster DG, Bobulescu IA, Zhang J, Wade J, Moe OW. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol 292: F577–F585, 2007. doi: 10.1152/ajprenal.00240.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg LC, Knepper MA, Burg MB. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol Renal Physiol 240: F536–F544, 1981. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- 39.Garvin JL. Angiotensin stimulates bicarbonate transport and Na+/K+ ATPase in rat proximal straight tubules. J Am Soc Nephrol 1: 1146–1152, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Goldman ID, Fyfe JM, Bowen D, Loftfield S, Schafer JA. The effect of microtubular inhibitors on transport of alpha-aminoisobutyric acid. Inhibition of uphill transport without changes in transmembrane gradients of Na+, K+, or H+. Biochim Biophys Acta 467: 185–191, 1977. doi: 10.1016/0005-2736(77)90195-X. [DOI] [PubMed] [Google Scholar]
- 41.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530–F538, 2005. doi: 10.1152/ajprenal.00229.2004. [DOI] [PubMed] [Google Scholar]
- 42.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284: F467–F473, 2003. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 43.Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 44.Guyton AC, Hall JE, Coleman TG, Manning RD Jr, Norman RAJ. The dominant role of the kidneys in long-term arterial pressure regulation in normal and hypertensive states, in Hypertension: Pathophysiology, Diagnosis, and Management (Laragh JH, Brenner BM, editors). New York: Raven Press, 1995. [Google Scholar]
- 45.Györy AZ, Kinne R, Moewes B. Energy source for transepithelial sodium transport in rat renal proximal tubules. Pflugers Arch 327: 234–260, 1971. doi: 10.1007/BF00586861. [DOI] [PubMed] [Google Scholar]
- 46.Hakam AC, Hussain T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension 47: 1117–1124, 2006. doi: 10.1161/01.HYP.0000220112.91724.fc. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi M, Yoshida T, Monkawa T, Yamaji Y, Sato S, Saruta T. Na+/H+-exchanger 3 activity and its gene in the spontaneously hypertensive rat kidney. J Hypertens 15: 43–48, 1997. [PubMed] [Google Scholar]
- 48.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 285: 27869–27878, 2010. doi: 10.1074/jbc.M110.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Houillier P, Chambrey R, Achard JM, Froissart M, Poggioli J, Paillard M. Signaling pathways in the biphasic effect of angiotensin II on apical Na/H antiport activity in proximal tubule. Kidney Int 50: 1496–1505, 1996. doi: 10.1038/ki.1996.464. [DOI] [PubMed] [Google Scholar]
- 50.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 51.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension 60: 1200–1206, 2012. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda K, Onimaru H, Yamada J, Inoue K, Ueno S, Onaka T, Toyoda H, Arata A, Ishikawa TO, Taketo MM, Fukuda A, Kawakami K. Malfunction of respiratory-related neuronal activity in Na+, K+-ATPase alpha2 subunit-deficient mice is attributable to abnormal Cl- homeostasis in brainstem neurons. J Neurosci 24: 10693–10701, 2004. doi: 10.1523/JNEUROSCI.2909-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999. doi: 10.1016/S1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 54.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 1802: 1259–1267, 2010. doi: 10.1016/j.bbadis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jourdain M, Amiel C, Friedlander G. Modulation of Na-H exchange activity by angiotensin II in opossum kidney cells. Am J Physiol Cell Physiol 263: C1141–C1146, 1992. doi: 10.1152/ajpcell.1992.263.6.C1141. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem 71: 511–535, 2002. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 57.Karim Z, Defontaine N, Paillard M, Poggioli J. Protein kinase C isoforms in rat kidney proximal tubule: acute effect of angiotensin II. Am J Physiol Cell Physiol 269: C134–C140, 1995. doi: 10.1152/ajpcell.1995.269.1.C134. [DOI] [PubMed] [Google Scholar]
- 58.Karim ZG, Chambrey R, Chalumeau C, Defontaine N, Warnock DG, Paillard M, Poggioli J. Regulation by PKC isoforms of Na(+)/H(+) exchanger in luminal membrane vesicles isolated from cortical tubules. Am J Physiol Renal Physiol 277: F773–F778, 1999. doi: 10.1152/ajprenal.1999.277.5.F773. [DOI] [PubMed] [Google Scholar]
- 59.Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol Renal Physiol 237: F114–F120, 1979. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- 60.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. Response to letter regarding article, “AT2 receptor activation induces natriuresis and lowers blood pressure”. Circ Res 115: e26–e27, 2014. 10.1161/CIRCRESAHA.114.304975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273: 15249–15256, 1998. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 62.Krapf R, Solioz M. Na/H antiporter mRNA expression in single nephron segments of rat kidney cortex. J Clin Invest 88: 783–788, 1991. doi: 10.1172/JCI115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LaPointe MS, Sodhi C, Sahai A, Batlle D. Na+/H+ exchange activity and NHE-3 expression in renal tubules from the spontaneously hypertensive rat. Kidney Int 62: 157–165, 2002. doi: 10.1046/j.1523-1755.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 64.Leong PK, Yang LE, Holstein-Rathlou NH, McDonough AA. Angiotensin II clamp prevents the second step in renal apical NHE3 internalization during acute hypertension. Am J Physiol Renal Physiol 283: F1142–F1150, 2002. doi: 10.1152/ajprenal.00178.2002. [DOI] [PubMed] [Google Scholar]
- 65.Li HC, Du Z, Barone S, Rubera I, McDonough AA, Tauc M, Zahedi K, Wang T, Soleimani M. Proximal tubule specific knockout of the Na+/H+ exchanger NHE3: effects on bicarbonate absorption and ammonium excretion. J Mol Med (Berl) 91: 951–963, 2013. doi: 10.1007/s00109-013-1015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through a microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol 297: F1342–F1352, 2009. doi: 10.1152/ajprenal.90734.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol 303: F1617–F1628, 2012. doi: 10.1152/ajprenal.00219.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li XC, Shull GE, Miguel-Qin E, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol Genomics 47: 479–487, 2015. doi: 10.1152/physiolgenomics.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li XC, Shull GE, Miguel-Qin E, Chen F, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension in NHE3-deficient mice with transgenic rescue of NHE3 in small intestines. Physiol Rep 3: e12605, 2015. doi: 10.14814/phy2.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li XC, Soleimani M, Zhu D, Rubera I, Tauc M, Zheng X, Zhang J, Chen X, Zhuo JL. Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) promotes the pressure-natriuresis response and lowers blood pressure in mice. Hypertension 72: 1328–1336, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol 293: C367–C378, 2007. doi: 10.1152/ajpcell.00463.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li XC, Zhuo JL. Phosphoproteomic analysis of AT1 receptor-mediated signaling responses in proximal tubules of angiotensin II-induced hypertensive rats. Kidney Int 80: 620–632, 2011. doi: 10.1038/ki.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT(1a) receptors induces blood pressure responses to intracellular angiotensin II in AT(1a) receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 304: R588–R598, 2013. doi: 10.1152/ajpregu.00338.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linz D, Wirth K, Linz W, Heuer HO, Frick W, Hofmeister A, Heinelt U, Arndt P, Schwahn U, Böhm M, Ruetten H. Antihypertensive and laxative effects by pharmacological inhibition of sodium-proton-exchanger subtype 3-mediated sodium absorption in the gut. Hypertension 60: 1560–1567, 2012. doi: 10.1161/HYPERTENSIONAHA.112.201590. [DOI] [PubMed] [Google Scholar]
- 75.Liu FY, Cogan MG. Role of protein kinase C in proximal bicarbonate absorption and angiotensin signaling. Am J Physiol Renal Physiol 258: F927–F933, 1990. doi: 10.1152/ajprenal.1990.258.4.F927. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Yan Y, Liu L, Xie Z, Malhotra D, Joe B, Shapiro JI. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. J Biol Chem 286: 22806–22813, 2011. doi: 10.1074/jbc.M111.246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; WRITING GROUP MEMBERS; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 78.Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol Renal Physiol 277: F447–F453, 1999. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- 79.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology . 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 25: 1105–1187, 2007. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 80.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010. doi: 10.1152/ajpregu.00002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mercer RW, Biemesderfer D, Bliss DP Jr, Collins JH, Forbush B III. Molecular cloning and immunological characterization of the gamma polypeptide, a small protein associated with the Na,K-ATPase. J Cell Biol 121: 579–586, 1993. doi: 10.1083/jcb.121.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyazaki E, Sakaguchi M, Wakabayashi S, Shigekawa M, Mihara K. NHE6 protein possesses a signal peptide destined for endoplasmic reticulum membrane and localizes in secretory organelles of the cell. J Biol Chem 276: 49221–49227, 2001. doi: 10.1074/jbc.M106267200. [DOI] [PubMed] [Google Scholar]
- 83.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol 10: 2412–2425, 1999. [DOI] [PubMed] [Google Scholar]
- 84.Moseley AE, Lieske SP, Wetzel RK, James PF, He S, Shelly DA, Paul RJ, Boivin GP, Witte DP, Ramirez JM, Sweadner KJ, Lingrel JB. The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J Biol Chem 278: 5317–5324, 2003. doi: 10.1074/jbc.M211315200. [DOI] [PubMed] [Google Scholar]
- 85.Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007. doi: 10.1074/jbc.M701910200. [DOI] [PubMed] [Google Scholar]
- 86.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 87.National Heart, Lung and Blood Institute The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: NIH Publication, 2003. [PubMed] [Google Scholar]
- 88.Noonan WT, Woo AL, Nieman ML, Prasad V, Schultheis PJ, Shull GE, Lorenz JN. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol 288: R685–R691, 2005. doi: 10.1152/ajpregu.00209.2004. [DOI] [PubMed] [Google Scholar]
- 89.Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331–9339, 1992. [PubMed] [Google Scholar]
- 90.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 30: 1238–1246, 1997. doi: 10.1161/01.HYP.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 91.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension 53: 338–343, 2009. doi: 10.1161/HYPERTENSIONAHA.108.124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pao AC, Bhargava A, Di Sole F, Quigley R, Shao X, Wang J, Thomas S, Zhang J, Shi M, Funder JW, Moe OW, Pearce D. Expression and role of serum and glucocorticoid-regulated kinase 2 in the regulation of Na+/H+ exchanger 3 in the mammalian kidney. Am J Physiol Renal Physiol 299: F1496–F1506, 2010. doi: 10.1152/ajprenal.00075.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pontes RB, Crajoinas RO, Nishi EE, Oliveira-Sales EB, Girardi AC, Campos RR, Bergamaschi CT. Renal nerve stimulation leads to the activation of the Na+/H+ exchanger isoform 3 via angiotensin II type I receptor. Am J Physiol Renal Physiol 308: F848–F856, 2015. doi: 10.1152/ajprenal.00515.2014. [DOI] [PubMed] [Google Scholar]
- 94.Rector FC., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol Renal Physiol 244: F461–F471, 1983. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- 96.Reilly AM, Harris PJ, Williams DA. Biphasic effect of angiotensin II on intracellular sodium concentration in rat proximal tubules. Am J Physiol Renal Physiol 269: F374–F380, 1995. doi: 10.1152/ajprenal.1995.269.3.F374. [DOI] [PubMed] [Google Scholar]
- 97.Riquier-Brison AD, Leong PK, Pihakaski-Maunsbach K, McDonough AA. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am J Physiol Renal Physiol 298: F177–F186, 2010. doi: 10.1152/ajprenal.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosenberg ME, Hostetter TH, Kren S, Chmielewski D. Effect of angiotensin II and norepinephrine on early growth response genes in the rat kidney. Kidney Int 43: 601–609, 1993. doi: 10.1038/ki.1993.88. [DOI] [PubMed] [Google Scholar]
- 100.Rostgaard J, Møller O. Localization of Na+, K+ -ATPase to the inside of the basolateral cell membranes of epithelial cells of proximal and distal tubules in rabbit kidney. Cell Tissue Res 212: 17–28, 1980. doi: 10.1007/BF00234029. [DOI] [PubMed] [Google Scholar]
- 101.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol 15: 2050–2056, 2004. doi: 10.1097/01.ASN.0000133023.89251.01. [DOI] [PubMed] [Google Scholar]
- 102.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DG, Karanja N, Lin PH, Aickin M, Most-Windhauser MM, Moore TJ, Proschan MA, Cutler JA; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344: 3–10, 2001. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 98.Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M. Circadian clock genes directly regulate expression of the Na(+)/H(+) exchanger NHE3 in the kidney. Kidney Int 67: 1410–1419, 2005. doi: 10.1111/j.1523-1755.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 103.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 104.Shull GE, Greeb J, Lingrel JB. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry 25: 8125–8132, 1986. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- 105.Shull GE, Lingrel JB. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem 261: 16788–16791, 1986. [PubMed] [Google Scholar]
- 106.Shull GE, Schwartz A, Lingrel JB. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature 316: 691–695, 1985. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- 107.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest 97: 1978–1982, 1996. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta 23: 394–401, 1957. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- 109.Soleimani M, Singh G, Bizal GL, Gullans SR, McAteer JA. Na+/H+ exchanger isoforms NHE-2 and NHE-1 in inner medullary collecting duct cells. Expression, functional localization, and differential regulation. J Biol Chem 269: 27973–27978, 1994. [PubMed] [Google Scholar]
- 110.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Renal Physiol 309: F933–F942, 2015. doi: 10.1152/ajprenal.00197.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tse CM, Brant SR, Walker MS, Pouyssegur J, Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem 267: 9340–9346, 1992. [PubMed] [Google Scholar]
- 112.Vallon V, Schwark JR, Richter K, Hropot M. Role of Na(+)/H(+) exchanger NHE3 in nephron function: micropuncture studies with S3226, an inhibitor of NHE3. Am J Physiol Renal Physiol 278: F375–F379, 2000. doi: 10.1152/ajprenal.2000.278.3.F375. [DOI] [PubMed] [Google Scholar]
- 113.Wade JB, Liu J, Coleman RA, Cunningham R, Steplock DA, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285: C1494–C1503, 2003. doi: 10.1152/ajpcell.00092.2003. [DOI] [PubMed] [Google Scholar]
- 114.Wang D, Zhang H, Lang F, Yun CC. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am J Physiol Cell Physiol 292: C396–C404, 2007. doi: 10.1152/ajpcell.00345.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang T, Yang CL, Abbiati T, Schultheis PJ, Shull GE, Giebisch G, Aronson PS. Mechanism of proximal tubule bicarbonate absorption in NHE3 null mice. Am J Physiol Renal Physiol 277: F298–F302, 1999. doi: 10.1152/ajprenal.1999.277.2.F298. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Armando I, Upadhyay K, Pascua A, Jose PA. The regulation of proximal tubular salt transport in hypertension: an update. Curr Opin Nephrol Hypertens 18: 412–420, 2009. doi: 10.1097/MNH.0b013e32832f5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weinman EJ, Cunningham R, Shenolikar S. NHERF and regulation of the renal sodium-hydrogen exchanger NHE3. Pflugers Arch 450: 137–144, 2005. doi: 10.1007/s00424-005-1384-8. [DOI] [PubMed] [Google Scholar]
- 118.Wesson LG Jr, Anslow WP Jr, Smith HW. The excretion of strong electrolytes. Bull N Y Acad Med 24: 586–606, 1948. [PMC free article] [PubMed] [Google Scholar]
- 119.Wesson LG, Anslow WP Jr, Smith HW. The renal excretion of strong electrolytes. Fed Proc 7: 132, 1948. [PubMed] [Google Scholar]
- 120.Wetzel RK, Sweadner KJ. Immunocytochemical localization of Na-K-ATPase alpha- and gamma-subunits in rat kidney. Am J Physiol Renal Physiol 281: F531–F545, 2001. doi: 10.1152/ajprenal.2001.281.3.F531. [DOI] [PubMed] [Google Scholar]
- 121.Wilcox CS, Baylis C, Wingo CS. Glomerular-tubular balance and proximal regulation, in The Kidney: Physiology and Pathophysiology (Seldin DW, Giebisch G, editors). New York: Raven Press, 1992. [Google Scholar]
- 122.Xie Z, Jack-Hays M, Wang Y, Periyasamy SM, Blanco G, Huang WH, Askari A. Different oxidant sensitivities of the alpha 1 and alpha 2 isoforms of Na+/K(+)-ATPase expressed in baculovirus-infected insect cells. Biochem Biophys Res Commun 207: 155–159, 1995. doi: 10.1006/bbrc.1995.1166. [DOI] [PubMed] [Google Scholar]
- 123.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem 274: 19323–19328, 1999. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 124.Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium-hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41, 2005. doi: 10.1152/ajpgi.00552.2004. [DOI] [PubMed] [Google Scholar]
- 125.Yingst DR, Araghi A, Doci TM, Mattingly R, Beierwaltes WH. Decreased renal perfusion rapidly increases plasma membrane Na-K-ATPase in rat cortex by an angiotensin II-dependent mechanism. Am J Physiol Renal Physiol 297: F1324–F1329, 2009. doi: 10.1152/ajprenal.90363.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yingst DR, Massey KJ, Rossi NF, Mohanty MJ, Mattingly RR. Angiotensin II directly stimulates activity and alters the phosphorylation of Na-K-ATPase in rat proximal tubule with a rapid time course. Am J Physiol Renal Physiol 287: F713–F721, 2004. doi: 10.1152/ajprenal.00065.2004. [DOI] [PubMed] [Google Scholar]
- 127.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995. doi: 10.1152/ajpgi.1995.269.1.G1. [DOI] [PubMed] [Google Scholar]
- 128.Zhu H, Sagnella GA, Dong Y, Miller MA, Onipinla A, Markandu ND, MacGregor GA. Molecular variants of the sodium/hydrogen exchanger type 3 gene and essential hypertension. J Hypertens 22: 1269–1275, 2004. doi: 10.1097/01.hjh.0000125428.28861.11. [DOI] [PubMed] [Google Scholar]
- 129.Zhuo J, Song K, Harris PJ, Mendelsohn FA. In vitro autoradiography reveals predominantly AT1 angiotensin II receptors in rat kidney. Ren Physiol Biochem 15: 231–239, 1992. [DOI] [PubMed] [Google Scholar]
- 130.Zhuo JL, Harris PJ, Skinner SL. Atrial natriuretic factor modulates proximal glomerulotubular balance in anesthetized rats. Hypertension 14: 666–673, 1989. doi: 10.1161/01.HYP.14.6.666. [DOI] [PubMed] [Google Scholar]
- 131.Zhuo JL, Li XC. Proximal nephron. Compr Physiol 3: 1079–1123, 2013. doi: 10.1002/cphy.c110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemogenetic activation of orexin neurons does not affect water consumption and body composition. Young 5-mo-old and middle-aged (12 mo old) female mice received intracranial injections of control DREADD virus (cDREADD, AAV2-hSyn-DIO-mCherry). Female mice were treated with either water or water with clozapine-N-oxide (CNO) for 2 days. Young and middle-aged mice consumed similar amounts of water and water with CNO (0.25 mg/ml) (n = 10/group) (A). Middle-aged mice have increased body (B) and fat mass (C), as well as the ratio of fat to lean mass (D) relative to young mice. However, no within-age differences in body mass, fat mass, and fat-to-lean ratio were observed between water and water with CNO-treated female mice (n = 10/group; *P < 0.05, **P < 0.01, ***P < 0.005) - .docx (226 KB)
Clozapine-N-oxide (CNO) effects on anxiety, locomotion and memory in 5-mo-old female mice. Young 5-mo-old female mice received intracranial injections of control DREADD virus (cDREADD, AAV2-hSyn-DIO-mCherry). CNO treatment did not affect distance traveled or time spent in open arms in the elevated plus maze (EPM, A and B). The time spent in open arms and the time spent in center of the open field test (OFT) arena was not affected by CNO treatment (C and D). CNO did not affect performance in the novel object recognition test in 5-mo-old female mice (E and F) - .docx (202 KB)