Abstract
Apathy and impulsivity are common and often coexistent consequences of frontotemporal lobar degeneration (FTLD). They increase patient morbidity and carer distress, but remain under-estimated and poorly treated. Recent trans-diagnostic approaches that span the spectrum of clinical presentations of FTLD and parkinsonism, indicate that apathy and impulsivity can be fractionated into multiple neuroanatomical and pharmacological systems. These include ventral/dorsal fronto-striatal circuits for reward-sensitivity, response-inhibition, and decision-making; moderated by noradrenaline, dopamine, and serotonin. Improved assessment tools, formal models of cognition and behavior, combined with brain imaging and psycho-pharmacology, are creating new therapeutic targets and establishing principles for stratification in future clinical trials.
Introduction
Apathy and impulsivity are two constructs that coexist in frontotemporal lobar degeneration syndromes (FTLD), including the behavioral variant of frontotemporal dementia (bvFTD), primary progressive aphasia, progressive supranuclear palsy (PSP), and corticobasal syndrome [1–4]. Epidemiological data indicate that apathy and impulsivity are common in FTLD syndromes [5], and cause significant patient morbidity and carer distress. Despite progress in understanding apathy and impulsivity in other diseases [6], there is a limited evidence base for clinical management in FTLD.
Apathy and impulsivity have been conceived as belonging to opposite ends of a behavioral spectrum of dopamine-dependent abnormal motivation [7]. Although relevant to some aspects of apathy and impulsivity in certain neuropsychiatric disorders, this approach cannot explain their frequent co-occurrence in FTLD, or the fact that FTLD patients with more apathy also manifest more impulsivity (Fig.1)[8]. As a concrete illustration of their co-existence, we commonly observe apathetic patients (e.g., sitting in a chair for hours) whose first action in the day is an uncontrolled and impulsive movement that put them at risk of falling and reporting injuries. This ‘alliance’ of apathy and impulsivity is also acknowledged in the clinical diagnostic criteria for bvFTD [4] and PSP [3].
Figure 1.
Correlation between the self-rated Apathy Evaluation Scale (minimum score 18) and Barratt Impulsiveness Scale (minimum score 30) in 73 patients with frontotemporal lobar degeneration syndromes (PSP 25, CBS 17, PPA 17, bvFTD 14; Pearson’s Correlation r=0.495, p<0.001). PSP, progressive supranuclear palsy; CBS, corticobasal syndrome; PPA, primary progressive aphasia; bvFTD, behavioral variant of frontotemporal dementia.
We propose that apathy and impulsivity are behavioral constructs with multiple components, and that these components are positively correlated due to commonalities in neuroanatomical and pharmacological consequences of pathology, leading to dysregulation of decision-making, response-inhibition, and motivation. Alternatively, apathy and impulsivity may originate from separate brain structures and pharmacological mechanisms which are difficult to fractionate empirically due to the widespread nature of the FTLD-related pathological changes. However, the co-existence of apathy and impulsivity in other, non-degenerative, conditions (e.g., drug addiction) suggests that this latter hypothesis is less likely [9,10].
In parallel with correlative investigations of the neuroanatomical correlates of apathy and impulsivity, we present a computational approach embedded in the decision theory to describe and characterize the co-existence of apathy and impulsivity in FTLD syndromes in terms of latent neurocognitive mechanisms [11,12].
Finally, we highlight the role played by neurotransmitters other than dopamine, in part because apathy and impulsivity in FTLD are clinically unresponsive to standard dopaminergic therapies and in part because of emerging evidence of serotonergic and noradrenergic contributions to both apathy and impulsivity [13–16].
Neurocognitive mechanisms of apathy and impulsivity
The examination of behavioral profiles (latencies, accuracy, choice preferences) in terms of an accumulation-to-threshold decision model [17]; or effort allocation models [18] are key examples of model-based approaches to study apathy and impulsivity. Such models can parameterize effort, fatigue, reward expectations and behavioral biases, and other latent variables related to apathy and impulsivity [19–22]. Differences in the accumulation of “evidence” for effort, or the variation in decision thresholds according to reward, can be mapped to differences in brain structure and function [23].
This powerful modelling approach is beginning to elucidate the etiology of behavioral changes in FTLD, such as the similarly deleterious effect of PSP and Parkinson’s disease (PD) on response inhibition (Fig.2A). A ‘drift-diffusion’ model describes the binary-choice between action and inhibition in a Go/No-Go paradigm, with neuronal ‘accumulators’ integrating the momentary evidence over-time [20–22]. When this evidence reaches a threshold, the agent is committed to response, or inhibition of a response. Despite their profound akinesia, PSP patients, relative to PD patients and controls, had a markedly increased bias towards making a Go response. However, they were severely impaired at accumulating the necessary additional evidence to commit to a response [17]. Through the computational model of patient behavior, one can see how PSP patients are simultaneously prone to impulsivity (i.e., bias towards a responding, plus noise) and apathy (severe difficulty to reach threshold)(Fig.2B)[17]. In contrast to model parameters, the mean reaction-times and errors did not reveal the cognitive deficits that distinguished PSP patients from PD patients and controls[17]. Latent cognitive variables for effort and reward are similarly derived from saccadic responses [24], and although only applied thus far to PD, this approach has potential advantages to study FTLD, where akinesia or rigidity may interfere with responding over and above the cognitive disorders underlying apathy and impulsivity.
Figure 2.
A. Examples of trajectories of the “drift-diffusion” model. The two boundaries (a & 0) represent the Go and No-Go decisions. The drift-rate (velocity) represents the rate of accumulation of evidence. The diffusion process starts at a starting point between the two boundaries (z*a) until the accumulated evidence reaches one of the two boundaries. The predicted movement latency is the sum of the duration of the diffusion process and the non-decision time (Ter). B. Progressive supranuclear palsy (PSP) leads to exaggerated response bias towards the Go decision boundary, reduced non-decision time (Ter) and slow accumulation rate. This combination renders PSP patients both impulsive and severely slow, in a parsimonious and biologically plausible decision-model. The pictures in panel A and B have been adapted from reference [17]. PD, Parkinson’s disease.
Apathy
The composite nature of goal-directed behavior supported the theoretical decomposition of apathy into emotional/motivational, cognitive, and behavioral (‘auto-activation’) subtypes. The first variant of apathy relates to blunted affect, while the cognitive apathy closely resembles the typical executive deficits observed in FTLD syndromes. However, the relationship between apathy and cognition remains unclear; apathy has been linked to rapid cognitive/functional decline [25], while others have reported no correlation between the apathy and cognition [26]. The ‘auto-activation’ apathy reflects a reduced ability to self-generate motor patterns without external prompting. This distinction is clinically heuristic but a clear operationalization of such subtypes is needed to link clinical observations to modern cognitive neuroscience ontologies and their neuroanatomical substrates.
Although direct evidence linking brain structural deficits to different modalities of apathy in FTLD syndromes remains limited, the motivational deficit in apathy has been hypothesized to arise from deficits from orbital/ventro-medial prefrontal cortex (PFC)/ventral striatum circuits; the cognitive apathy from dorsolateral-PFC/caudate networks; and the ‘auto-activation’ apathy from premotor/motor circuits including the supplementary motor area (SMA) and pre-SMA [27]. Dysfunction of the latter circuit in FTLD can cause the failure to self-generate motor patterns, over and above blunted affect or cognitive dysfunction, in keeping with evidence for this circuit in voluntary action selection in health [20,28] and poor signal-to-noise in motor plans arising from the medial frontal cortex [29]. This ‘auto-activation’ deficit can also be formulated as a failure to reach a necessary activation threshold, by leakage, decay or refractoriness in the fronto-parietal neuronal ensembles that represent actions [17].
Nevertheless, there is lack of consistency across studies examining the neuroanatomical substrate of apathy in FTLD, due to limited numbers of patients, lenient statistical thresholds, and the inclusion of single diagnostic entities which reduces the generalisation of previous studies. To overcome these limitations, we recommend multiple modes of assessment of apathy (e.g., behavioural tests, questionnaires from multiple sources, wearables technologies) as well as trans-diagnostic approaches that emphasize the commonality of the manifestation of apathy across the broad clinical spectrum of FTLD diagnoses. This enables a data-driven approach to interrogate the phenomenology and etiology of apathy and impulsivity [8,30]. For example, Lansdall et al. used a principal components analysis of multiple questionnaires and laboratory tests, combined with structural magnetic resonance imaging [8,30]. They found a positive correlation between measures of apathy and impulsivity (Fig.3) and a dissociation between patient ratings, carer ratings, and dissociable neural correlates of the different modes of apathy and impulsivity, depending on the rater (Fig.3)[8]. Carers’ observations of apathetic changes in behavior correlated with diffuse atrophy in fronto-striatal and fronto-temporal regions, while patients’ reports related to deficits in motor networks, suggesting that patients retain insight in some aspects of their disability. These findings imply that the aspects of FTLD which distress carers and patients differ: future studies targeting patient- or carer-reported symptoms should choose outcome measures accordingly.
Figure 3.
Voxel-based-morphometry analyses revealed distinct white-matter or grey-matter correlates for patient related, carer related and task-related principal components (after [8]). Patient self-ratings correlated with white-matter atrophy in cortico-spinal circuits while carer ratings correlated with diffuse grey-matter deficits in fronto-striatal and fronto-temporal regions.
Response-inhibition impairments on behavioral paradigms assessing impulsivity (i.e., stop signal task) correlated with focal cortical atrophy in prefrontal cortex regions involved in cognitive control. The color bar rapresents t-statistics.
Impulsivity
Impulsivity is a multi-faceted construct, which reflects the tendency to act prematurely, with adverse consequences, or with insufficient evidence to make a decision [31]. Such definitions imply the distinction of impulsivity into separate neurocognitive systems, with identifiable neuroanatomical and neurochemical components. For example, aberrant processing of reward-expectation and delay-discounting measures (“risky decision-making” and “waiting impulsivity”), differs from response-inhibition deficits and cognitive dysregulation (“stopping” and “reflection” impulsivity)[31].
The neural determinants of impulsivity in FTLD syndromes include: (1) subcortical FTLD-related pathological changes within striatal, thalamic, and sub-thalamic neurons which affect reward processing and dis-inhibition of thalamo-cortical loops, with consequent biases towards contextually inappropriate actions [21,22,32]; and (2) neocortical pathology, especially in PFC networks, which impair decision-making and action selection processes [33]. Lesions at different points across the functional gradients of interlocking PFC-striato-thalamo-cortical circuits affect different modes of impulsivity [31].
For example, degeneration of “limbic” ventral PFC-striatal circuits leads to risky decision-making and delay intolerance while neurodegeneration in dorsal “motor” and “cognitive” circuits impairs the ability to refrain from or cancel inappropriate actions. These effects span animal models of impulsive disorders [34], neuroimaging data from individuals with impulsive neurodevelopmental disorders [35] and adult patients (e.g., obsessive-compulsive disorders and PD)[21,22,36]. The prevalence of impulsivity in these diverse conditions highlights the value of translational and trans-diagnostic approaches to elucidate the neural underpinnings of impulsivity [8,30]. In the study by Lansdall et al. [12], the response-inhibition deficits observed during laboratory-based behavioral paradigms (e.g., the stop-signal task of response cancellation) correlated with focal atrophy in the inferior frontal gyrus and pre-SMA. These are two critical ‘hubs’ in cognitive and motor control, and the target of therapeutic strategies which we consider in the next section[7,13–15].
Neuropharmacology of apathy and impulsivity
The emotional/motivational contributors to apathy have been linked to the dopaminergic reward system[37], but the pharmacology of ‘auto-activation’ deficits is unclear. A link between dopamine, reward, and motivation is well established in health and PD[38], but the motor and affective components of incentive motivation are dissociated and the principal determinants of apathy in PD may be distinct from apathy in FTLD[39]. In clinical practice, apathy in FTLD syndromes is frequently unresponsive to anti-parkinsonian dopaminergic medications, although dopamine deficiency is common in FTLD, not only the overtly parkinsonian disorders like PSP, but also the bvFTD. For example, half cases of FTD-linked C9orf72 mutation develop parkinsonism, and this common mutation is associated with striatal dopamine deficiency. The extent to which this causes apathy and impulsivity, as opposed to atrophy on frontostriatal circuits, remains unclear. It is possible that dopamine deficiency in some circuits and the relative preservation in other circuits is accompanied by dopaminergic ‘overdose’, as in PD[40], contributing to impulsivity in FTLD syndromes.
We propose that dysfunction of the noradrenergic systems may play a key role in the pathogenesis of apathy, especially in FTLD syndromes[16]. There are early pathological changes in the locus coeruleus (LC) in post mortem tissue from FTLD patients (Fig.4)[33]. The LC is the principal source of noradrenaline in the forebrain, which regulates the neuronal signal-to-noise ratio in the neocortex, gating information processing and modulating arousal[41]. It is possible that the dopaminergic and noradrenergic systems influence different components of goal-directed behavior (e.g., motivation and energization)[41,42], but such a dichotomy is over-simplistic, and there is counter evidence for strong interactions between the dopaminergic and noradrenergic neurotransmission[42].
Figure 4.
Left panel. At the macroscopic examination, a patient with progressive supranuclear palsy (PSP) shows, relative to a healthy control, paler locus coeruleus (red arrows) reflecting reduced intracellular neuromelanin. Right panel. There is also evidence that tau pathology (red arrows) is present in the locus coeruleus in PSP.
Impulsivity in FTLD syndromes may reflect dysfunctions in multiple monoaminergic systems, including serotonin, noradrenaline, and dopamine[43]. The reduction of serotonin in FTLD reported by Bowen and Proctor through post mortem studies, led Hughes and colleagues to test whether the serotonin reuptake inhibitor citalopram could restore the functional systems for response inhibition[44]. As predicted, bvFTD patients had a functional deficit in the PFC when required to inhibit actions, but this deficit was restored by citalopram. Clinical trials are necessary before this approach could be introduced therapeutically, but the study indicates the value of a translational approach, across species and across disorders[13,44].
Noradrenaline is necessary to effectively cancel ongoing behaviors when the context changes, in animal models and healthy humans[45]. There is growing evidence for the role of noradrenaline deficiency in impulsivity in FTLD syndromes[14,15,46]. The early and severe pathology in the LC in FTLD[33,47] suggests that restoring noradrenergic neurotransmission might be a therapeutic target for impulsivity. One candidate is the noradrenergic reuptake inhibitor atomoxetine, which restores activity and connectivity in inhibitory control networks in another disorder with noradrenergic deficiency, PD[14]. Together, these results suggest that targeting noradrenergic transmission may be a useful treatment for apathy and impulsivity in FTLD syndromes.
Concluding remarks
We propose that apathy and impulsivity are not opponent manifestations of a unidimensional behavioral spectrum, but instead are multi-dimensional behavioral constructs resulting from common neuroanatomical and neurochemical deficits (Fig.5). To improve effective therapeutic strategies in FTLD, we recommend targeting apathy and impulsivity jointly, ensuring that chosen assessment tools capture each of their principal dimensions. There is a pressing need to develop improved assessment tools for apathy and impulsivity, to empower clinical trials in terms of stratification and outcome markers. These are especially relevant to trans-diagnostic therapies, which would maximize the impact of effective new treatments to a larger population of patients and carers alike.
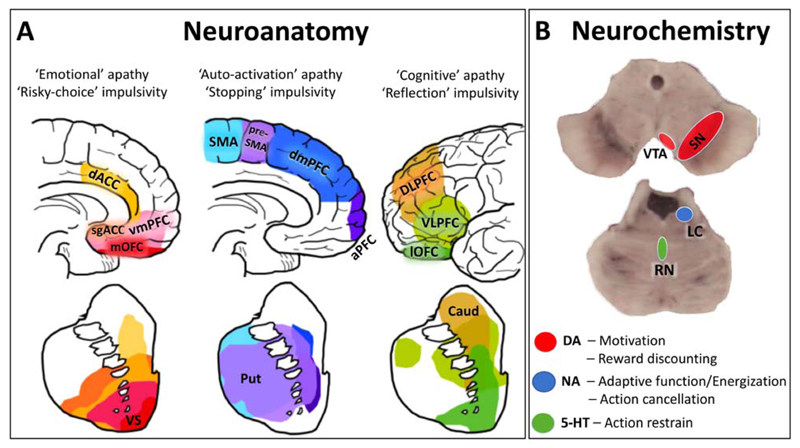
Figure 5.
Shared neuroanatomical and neurochemical mechanisms underlying apathy and impulsivity in frontotemporal lobar degeneration syndromes. A. Different modes of apathy and impulsivity are mediated by relatively segregated fronto-striatal circuits (the frontal and striatal areas sharing the same coloring show direct anatomical and functional connectivity) [48,49]. B. The dopaminergic, noradrenergic, and serotoninergic systems are involved in regulating different aspects of apathy and impulsivity. Abbreviations: dACC, dorsal anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex; mOFC, medial orbitofrontal cortex; VS, ventral striatum; SMA, supplementary motor area; dmPFC, dorsomedial prefrontal cortex; aPFC, anterior prefrontal cortex; Put, putamen; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; lOFC, lateral orbitofrontal cortex; Caud, caudate; VTA, ventral tegmental area; SN, substantia nigra; LC, locus coeruleus; RN, raphe nuclei. The pictures in panel A have been adapted from reference [50].
Highlights.
Apathy and impulsivity are common consequences of frontotemporal lobar degeneration syndromes
Common fronto-striatal loops mediate different modes of apathy and impulsivity across diagnoses
The noradrenergic system is a promising therapeutic target for apathy and impulsivity
Funding
This research was funded by a Wellcome Trust grant to James Rowe (JBR103838), the Medical Research Council (MRC) of Cognition and Brain Sciences Unit (CBSU), Cambridge (MC-A060-5PQ30), and an MRC grant to James Rowe and Luca Passamonti (MR/P01271X/1).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading*
- 1.Alexander SK, Rittman T, Xuereb JH, Bak TH, Hodges JR, Rowe JB. Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry. 2014;85:925–929. doi: 10.1136/jnnp-2013-307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [*This landmark paper revises the diagnostic criteria for progressive supranuclear palsy and eight sub-types based on clinical features. Of particular relevance to this review is the “PSP-F” variant with early frontal cognitive/behavioural signs, including apathy and impulsivity. Cognitive/behavioural changes are widely reported in PSP, but may often be masked by or followed by motor features, and these new criteria provide a critical step towards formal recognition of non-motor consequences of PSP. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyle-Gilchrist IT, Dick KM, Patterson K, Vazquez Rodriquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86:1736–1743. doi: 10.1212/WNL.0000000000002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 7.Sinha N, Manohar S, Husain M. Impulsivity and apathy in Parkinson's disease. J Neuropsychol. 2013;7:255–283. doi: 10.1111/jnp.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, Vazquez Rodriguez P, Wilcox A, Wehmann E, Dick KM, Robbins TW, Rowe JB. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain. 2017;140:1792–1807. doi: 10.1093/brain/awx101. [*This paper reports the structural brain changes associated with different types of apathy and impulsivity, across the spectrum of disorders caused by frontotemporal lobar degeneration (frontotemporal dementia, progressive supranuclear palsy, and corticobasal syndrome). The positive correlation between apathy and impulsivity (as observed by carers) indicates the need for a unified therapeutic strategy. The lack of correlation between patient and carer ratings, and objective behavioural measures, highlights the need of better assessment tools and revised outcome measures for clinical trials. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattie JE, Woods SP, Iudicello JE, Posada C, Grant I, Group T. Elevated neurobehavioral symptoms are associated with everyday functioning problems in chronic methamphetamine users. J Neuropsychiatry Clin Neurosci. 2012;24:331–339. doi: 10.1176/appi.neuropsych.11080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winhusen TM, Somoza EC, Lewis DF, Kropp FB, Horigian VE, Adinoff B. Frontal systems deficits in stimulant-dependent patients: evidence of pre-illness dysfunction and relationship to treatment response. Drug Alcohol Depend. 2013;127:94–100. doi: 10.1016/j.drugalcdep.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pessiglione M, Vinckier F, Bouret S, Daunizeau J, Le Bouc R. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain. 2017 doi: 10.1093/brain/awx278. [DOI] [PubMed] [Google Scholar]
- 12.Teufel C, Fletcher PC. The promises and pitfalls of applying computational models to neurological and psychiatric disorders. Brain. 2016;139:2600–2608. doi: 10.1093/brain/aww209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, Huddleston C, Rae CL, Regenthal R, Sahakian BJ, et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease. Brain. 2014;137:1145–1155. doi: 10.1093/brain/awu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, Huddleston C, Rae CL, Regenthal R, Sahakian BJ, et al. Improving response inhibition in Parkinson's disease with atomoxetine. Biol Psychiatry. 2015;77:740–748. doi: 10.1016/j.biopsych.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z, Rae CL, Nombela C, Ham T, Rittman T, Jones PS, Rodriguez PV, Coyle-Gilchrist I, Regenthal R, Altena E, et al. Predicting beneficial effects of atomoxetine and citalopram on response inhibition in Parkinson's disease with clinical and neuroimaging measures. Hum Brain Mapp. 2016;37:1026–1037. doi: 10.1002/hbm.23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loued-Khenissi L, Preuschoff K. Apathy and noradrenaline: silent partners to mild cognitive impairment in Parkinson's disease? Curr Opin Neurol. 2015;28:344–350. doi: 10.1097/WCO.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Rittman T, Nombela C, Fois A, Coyle-Gilchrist I, Barker RA, Hughes LE, Rowe JB. Different decision deficits impair response inhibition in progressive supranuclear palsy and Parkinson's disease. Brain. 2016;139:161–173. doi: 10.1093/brain/awv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bouc R, Rigoux L, Schmidt L, Degos B, Welter ML, Vidailhet M, Daunizeau J, Pessiglione M. Computational Dissection of Dopamine Motor and Motivational Functions in Humans. J Neurosci. 2016;36:6623–6633. doi: 10.1523/JNEUROSCI.3078-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forstmann BU, Ratcliff R, Wagenmakers EJ. Sequential Sampling Models in Cognitive Neuroscience: Advantages, Applications, and Extensions. Annu Rev Psychol. 2016;67:641–666. doi: 10.1146/annurev-psych-122414-033645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Rowe JB. Dissociable mechanisms of speed-accuracy tradeoff during visual perceptual learning are revealed by a hierarchical drift-diffusion model. Front Neurosci. 2014;8:69. doi: 10.3389/fnins.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [*The effect of subthalamic nucleus (‘deep brain’) stimulation on the cortical electrophysiological basis of decision-making epitomizes the use of diffusion-drift computational models to examine clinical disorders and their treatment. The degree of medial prefrontal cortex activity positively correlated with the decision boundary threshold. Deep brain stimulation of the subthalamic nucleus reduced the threshold and made patient choices more impulsive. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pote I, Torkamani M, Kefalopoulou ZM, Zrinzo L, Limousin-Dowsey P, Foltynie T, Speekenbrink M, Jahanshahi M. Subthalamic nucleus deep brain stimulation induces impulsive action when patients with Parkinson's disease act under speed pressure. Exp Brain Res. 2016;234:1837–1848. doi: 10.1007/s00221-016-4577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyniel F, Goodwin GM, Deakin JW, Klinge C, MacFadyen C, Milligan H, Mullings E, Pessiglione M, Gaillard R. A specific role for serotonin in overcoming effort cost. Elife. 2016;5 doi: 10.7554/eLife.17282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manohar SG, Chong TT, Apps MA, Batla A, Stamelou M, Jarman PR, Bhatia KP, Husain M. Reward Pays the Cost of Noise Reduction in Motor and Cognitive Control. Curr Biol. 2015;25:1707–1716. doi: 10.1016/j.cub.2015.05.038. [*A novel saccadic distraction task was developed to quantify the impact of Parkinson’s disease on the speed and accuracy of the eye movements under varying levels of reward. Critically, a computational model was used to test the hypothesis that dopamine deficits alter reward sensitivity in Parkinson’s disease. Specifically, the model revealed the cost of producing precise responses, balancing effort and reward. This model provides a mechanistic and quantifiable approach to study apathy and its treatment. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dalen JW, Van Wanrooij LL, Moll van Charante EP, Richard E, van Gool WA. Apathy is associated with incident dementia in community-dwelling older people. Neurology. 2017 doi: 10.1212/WNL.0000000000004767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirogovsky-Turk E, Moore RC, Filoteo JV, Litvan I, Song DD, Lessig SL, Schiehser DM. Neuropsychiatric Predictors of Cognitive Decline in Parkinson Disease: A Longitudinal Study. Am J Geriatr Psychiatry. 2017;25:279–289. doi: 10.1016/j.jagp.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Ducharme S, Price BH, Dickerson BC. Apathy: a neurocircuitry model based on frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2017 doi: 10.1136/jnnp-2017-316277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rae CL, Nombela C, Rodriguez PV, Ye Z, Hughes LE, Jones PS, Ham T, Rittman T, Coyle-Gilchrist I, Regenthal R, et al. Atomoxetine restores the response inhibition network in Parkinson's disease. Brain. 2016;139:2235–2248. doi: 10.1093/brain/aww138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolpe N, Wolpert DM, Rowe JB. Seeing what you want to see: priors for one's own actions represent exaggerated expectations of success. Front Behav Neurosci. 2014;8:232. doi: 10.3389/fnbeh.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, Vázquez Rodríguez P, Wilcox A, Wehmann E, Dick KM, Robbins TW, Rowe JB. White matter change with apathy and impulsivity in frontotemporal lobar degeneration syndromes. Neurology. doi: 10.1212/WNL.0000000000005175. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci. 2017;18:158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- 32.Passamonti L, Vazquez Rodriguez P, Hong YT, Allinson KS, Williamson D, Borchert RJ, Sami S, Cope TE, Bevan-Jones WR, Jones PS, et al. 18F-AV-1451 positron emission tomography in Alzheimer's disease and progressive supranuclear palsy. Brain. 2017;140:781–791. doi: 10.1093/brain/aww340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, Van Deerlin VM, Seeley WW, Miller BL, Lee EB, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol. 2016;79:272–287. doi: 10.1002/ana.24559. [*This detailed neuropathological examination of patients with Pick’s disease revealed the spatiotemporial evolution of pathological tau deposition: originating in limbic/paralimbic cortex (Phase I), followed by subcortical structures, including basal ganglia, locus coeruleus and raphe nuclei (Phase II), then the primary motor cortex and pre-cerebellar nuclei (Phase III) and finally the visual cortex (Phase IV). These sequential phases are shown to reflect the evolution of clinical symptoms, degeneration on imaging metrics and disease duration. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16:719–732. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- 37.Chong TT, Husain M. The role of dopamine in the pathophysiology and treatment of apathy. Prog Brain Res. 2016;229:389–426. doi: 10.1016/bs.pbr.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM. Parkinson's disease and dopaminergic therapy--differential effects on movement, reward and cognition. Brain. 2008;131:2094–2105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt L, d'Arc BF, Lafargue G, Galanaud D, Czernecki V, Grabli D, Schupbach M, Hartmann A, Levy R, Dubois B, et al. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- 40.Robbins TW, Cools R. Cognitive deficits in Parkinson's disease: a cognitive neuroscience perspective. Mov Disord. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- 41.Aston-Jones G, Waterhouse B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 2016;1645:75–78. doi: 10.1016/j.brainres.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varazzani C, San-Galli A, Gilardeau S, Bouret S. Noradrenaline and dopamine neurons in the reward/effort trade-off: a direct electrophysiological comparison in behaving monkeys. J Neurosci. 2015;35:7866–7877. doi: 10.1523/JNEUROSCI.0454-15.2015. [*Neurophysiological recording in monkeys demonstrated that the firing of dopaminergic neurons in the substantia nigra encode the reward-value associated with decision-making. Conversely, the firing of noradrenergic neurons in the locus coeruleus was implicated in ‘energizing’ behaviour so as to face the challenges associated with a particular task, especially in terms of the effort required to produce an action. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murley A, Rowe JB. Neurotransmitter deficits from frontotemporal lobar degeneration: a critical review. Brain. doi: 10.1093/brain/awx327. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes LE, Rittman T, Regenthal R, Robbins TW, Rowe JB. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain. 2015;138:1961–1975. doi: 10.1093/brain/awv133. [*This double-blind placebo-controlled pharmaco-magnetoencephalography study demonstrated the impact of serotonergic augmentation on response-inhibition systems in patients with frontotemporal dementia. The NoGo-N2 and NoGo-P3 components in the frontal and temporal lobes were significantly decreased in patients, relative to controls, but restored by drug treatment even in the presence of irreversible focal atrophy. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dela Pena I, Dela Pena IJ, de la Pena JB, Kim HJ, Shin CY, Han DH, Kim BN, Ryu JH, Cheong JH. Methylphenidate and Atomoxetine-Responsive Prefrontal Cortical Genetic Overlaps in "Impulsive" SHR/NCrl and Wistar Rats. Behav Genet. 2017 doi: 10.1007/s10519-017-9861-3. [DOI] [PubMed] [Google Scholar]
- 46.Borchert RJ, Rittman T, Passamonti L, Ye Z, Sami S, Jones SP, Nombela C, Vazquez Rodriguez P, Vatansever D, Rae CL, et al. Atomoxetine Enhances Connectivity of Prefrontal Networks in Parkinson's Disease. Neuropsychopharmacology. 2016;41:2171–2177. doi: 10.1038/npp.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haglund M, Friberg N, Danielsson EJ, Norrman J, Englund E. A methodological study of locus coeruleus degeneration in dementing disorders. Clin Neuropathol. 2016;35:287–294. doi: 10.5414/NP300930. [DOI] [PubMed] [Google Scholar]
- 48.Voon V, Dalley JW. Translatable and Back-Translatable Measurement of Impulsivity and Compulsivity: Convergent and Divergent Processes. Curr Top Behav Neurosci. 2016;28:53–91. doi: 10.1007/7854_2015_5013. [DOI] [PubMed] [Google Scholar]
- 49.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris LS, Kundu P, Dowell N, Mechelmans DJ, Favre P, Irvine MA, Robbins TW, Daw N, Bullmore ET, Harrison NA, et al. Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex. 2016;74:118–133. doi: 10.1016/j.cortex.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]