Abstract
Background
Breathlessness is a common and disabling symptom which affects many people with advanced cardiorespiratory disease and cancer. The most effective treatments are aimed at treating the underlying disease. However, this may not always be possible, and symptomatic treatment is often required in addition to maximal disease‐directed therapy. Opioids are increasingly being used to treat breathlessness, although their mechanism of action is still not completely known. A few good sized, high quality trials have been conducted in this area.
Objectives
To determine the effectiveness of opioid drugs in relieving the symptom of breathlessness in people with advanced disease due to malignancy, respiratory or cardiovascular disease, or receiving palliative care for any other disease.
Search methods
We performed searches on CENTRAL, MEDLINE, EMBASE, CINAHL, and Web of Science up to 19 October 2015. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles.
Selection criteria
We included randomised double‐blind controlled trials that compared the use of any opioid drug against placebo or any other intervention for the relief of breathlessness. The intervention was any opioid, given by any route, in any dose.
Data collection and analysis
We imported studies identified by the search into a reference manager database. We retrieved the full‐text version of relevant studies, and two review authors independently extracted data. The primary outcome measure was breathlessness and secondary outcome measures included exercise tolerance, oxygen saturations, adverse events, and mortality. We analysed all studies together and also performed subgroup analyses, by route of administration, type of opioid administered, and cause of breathlessness. Where appropriate, we performed meta‐analysis. We assessed the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and created three 'Summary of findings' tables.
Main results
We included 26 studies with 526 participants. We assessed the studies as being at high or unclear risk of bias overall. We only included randomised controlled trials (RCTs), although the description of randomisation was incomplete in some included studies. We aimed to include double blind RCTs, but two studies were only single blinded. There was inconsistency in the reporting of outcome measures. We analysed the data using a fixed‐effect model, and for some outcomes heterogeneity was high. There was a risk of imprecise results due to the low numbers of participants in the included studies. For these reasons we downgraded the quality of the evidence from high to either low or very low.
For the primary outcome of breathlessness, the standardised mean post‐treatment dyspnoea score was 0.32 points better in the opioid group compared to the placebo group (ranging from a 0.53 point reduction to a 0.10 point reduction) (12 RCTs, 338 participants, low quality evidence). The standardised mean change from baseline dyspnoea score was 0.11 points better in the opioids group compared to the placebo group (ranging from a 0.40 point reduction to a 0.19 increase) (six RCTs, 194 participants, very low quality evidence). A lower score indicates an improvement in breathlessness.
The evidence for the six‐minute walk test (6MWT) was conflicting. The total distance in 6MWT was 28 metres (m) better in the opioids group compared to placebo (ranging from 113 m to 58 m) (one RCT, 11 participants, very low quality evidence). However, the change in baseline was 48 m worse in the opioids group (ranging from 36 m to 60 m) (two RCTs, 26 participants, very low quality evidence).
The adverse effects reported included drowsiness, nausea and vomiting, and constipation. In those studies, participants were 4.73 times more likely to experience nausea and vomiting compared to placebo, three times more likely to experience constipation, and 2.86 times more likely to experience drowsiness (nine studies, 162 participants, very low quality evidence).
Only four studies assessed quality of life, and none demonstrated any significant change.
Authors' conclusions
There is some low quality evidence that shows benefit for the use of oral or parenteral opioids to palliate breathlessness, although the number of included participants was small. We found no evidence to support the use of nebulised opioids. Further research with larger numbers of participants, using standardised protocols and with quality of life measures included, is needed.
Plain language summary
Opioids for treating breathlessness at the end of life
Background
People with lung disease may experience breathlessness. Initial treatments should focus on the underlying causes of breathlessness. However, as the disease progresses, it may be better to focus on treating the symptoms. As well as standard care, opioids (e.g. morphine, given either by mouth, by nebuliser, or injected) may help relieve these symptoms. However, opioids also have side effects, such as drowsiness, constipation, nausea (feeling sick), and vomiting.
Review question
We wanted to know if opioid drugs reduced breathlessness in people with lung disease. We also looked at whether opioids improved their ability to exercise, and what side effects people had. We also wanted to know if opioid drugs improved their quality of life.
Study characteristics
We searched for studies up to 19 October 2015, and we included 26 studies with 526 people. These people had breathlessness from different types of lung disease. Some were given opioid drugs and some were given other drugs or a placebo, and studies compared the reporting of breathlessness to see if there was any difference. Some studies also looked at the amount of time people could exercise to see if there were any differences. Some people came from home, and some came from the hospital setting.
Key findings
There was some low quality evidence that showed a benefit of using oral or injectable opioid drugs for the treatment of the symptoms of breathlessness. There was no evidence for opioids by nebuliser. Some people experienced drowsiness, nausea, and vomiting. More research is needed using more people, and looking at effects on quality of life.
Quality of the evidence
We rated the quality of the evidence using one of the following grades: very low, low, moderate, or high. Very low quality evidence means we are uncertain about the results. High quality evidence means we are very certain about the results. For this Cochrane review, we found that the evidence was of low to very low quality. We included randomised controlled trials which were blinded, which means that participants and those people that assessed the results did not know whether the participants had received the opioid drug or a placebo. However, the trials were of small size, and some studies did not give enough information to allow us to assess whether they were of good quality.
Summary of findings
Background
Description of the condition
Breathlessness may be described as "a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity" (ATS 1999). Breathlessness, also termed dyspnoea, shortness of breath, air hunger, awareness of respiratory distress, or laboured breathing, may be variably perceived by different patients, depending on multiple physiological, psychological, social, environmental, and cultural factors (Guz 1997). It is a common symptom at the advanced stages of illness, and may be as disabling to the patient and their families as pain, nausea and vomiting, delirium, and other end of life symptoms (Neuman 2006).
Respiratory motor activity is regulated by automatic centres in the brainstem and voluntary signals from the cortex, and controls chest wall expansion, lung inflation, and ventilation. Feedback is provided by chemoreceptors, mechanoreceptors, and sensory receptors. Breathlessness may be explained by a mismatch between afferent sensory information processed at the cortex and respiratory motor command from the cortex and brainstem. Alterations in arterial blood pH (acidity), partial pressure of carbon dioxide (pCO2), and partial pressure of oxygen (pO2) stimulate central chemoreceptors in the medulla and peripheral chemoreceptors in the carotid and aortic bodies, which transmit impulses to the brainstem respiratory centres, and adjust breathing based on acid base homeostasis (Nattie 1995; Fitzgerald 1986). Mechanoreceptors and stretch receptors located in the lung parenchyma and bronchioles sense changes in the expansion of the lung and become irritated by certain mechanical and chemical stimuli, and affect subsequent levels and patterns of breathing (Nishino 2011). Changes in air flow, smooth muscle tone, and impulses from C fibres located adjacent to the alveoli and pulmonary capillaries respond to changes in pulmonary interstitial and capillary pressures (Widdicombe 1982). Sensory receptors in respiratory muscle and the diaphragm involved in spinal and supraspinal reflexes influences central respiratory activity (Bolsher 1987; Bolsher 1988). Each of these mechanisms may contribute to the mismatch of neural activity and consequent mechanical and ventilatory outputs, and create sensations of dyspnoea, air hunger, and increased desire to breathe, which may cause distress.
Recent neuroimaging studies also suggest that neural structures that involve pain and dyspnoea may be shared, further contributing to the affected person's discomfort and distress associated with an increased sensation of ventilation (Brannan 2001; Liotti 2001; Parsons 2001; Peiffer 2001; Evans 2002; von Leupoldt 2009).
There are many currently incurable and progressive cardiopulmonary, neuromuscular, and malignant conditions in which dyspnoea is a common symptom in the advanced stages of disease. The dominant mechanism that leads to dyspnoea may vary between conditions and in many conditions more than one mechanism may be responsible. Illnesses such as interstitial lung disease, pulmonary hypertension, and congestive heart failure stimulate pulmonary receptors (irritant, mechanical, and vascular) leading to an increased respiratory drive and increased afferent input to the respiratory centre. Chronic conditions that are severe enough might also lead to gas exchange abnormalities through mechanisms such as ventilation‐perfusion (V/Q) mismatching (e.g. pulmonary vascular disease) or diffusion impairment (e.g. interstitial lung disease) leading to stimulation of chemoreceptors and increased respiratory drive. Conditions that reduce the oxygen‐carrying capacity of the blood (e.g. anaemia) or reduce cardiac output (e.g. cardiac failure) also stimulate chemoreceptors. Respiratory muscle weakness in conditions such as motor neurone disease or myopathy, and decreased compliance of the chest wall in conditions such as severe kyphoscoliosis and pleural effusion, impair ventilatory mechanics which reduces the afferent feedback for a given efferent input (Manning 1995). There are multiple potential aetiological factors related to breathlessness in chronic obstructive pulmonary disease (COPD). There is an increased resistive load from narrowing of the airways and increased elastic load from hyperinflation resulting in impaired ventilator mechanics. In addition, hypoxia and or hypercapnia may be present, leading to stimulation of chemoreceptors, and finally dynamic airway compression may stimulate receptors within the airway (Parshall 2012).
Multiple mechanisms for breathlessness have also been described in individuals with advanced cancer (Mazzocato 1999). Cancers that involve the lungs may obstruct airways leading to ventilation perfusion mismatch, and pleural effusions are common. Many people with lung cancer also have COPD. Dudgeon 1998 showed that people with terminal cancer often have abnormal spirometry (most commonly a mixed obstructive/restrictive pattern or a restrictive pattern). They also found that respiratory muscle weakness may be an important contributor to dyspnoea and that co‐morbidities such as anaemia and cardiac disease are common.
Initial approaches should aim to treat the underlying causes of breathlessness. However, as the disease progresses, such treatments may be less appropriate due to decreased effectiveness and discomfort caused to the person, and a more symptom‐based approach may be required.
Many pharmacological and non‐pharmacological interventions have been recommended to help alleviate symptoms of breathlessness in advanced disease. Management of symptoms is often multimodal, with varying treatments utilised depending on the person's co‐morbidities, and psychosocial, environmental, and cultural factors.
A Cochrane systematic review on non‐pharmacological interventions demonstrated efficacy for neuro‐electrical muscle stimulation, chest wall vibration, walking aids, breathing training, and use of hand‐held fans (Bausewein 2008). Another Cochrane review demonstrated effectiveness of exertional oxygen therapy in non‐hypoxaemic COPD patients (Uronis 2011), and suggested a slight, but not statistically significant, improvement in adults with heart failure, cancer (not end‐stage disease), and kyphoscoliosis (Cranston 2008).
Some guidelines recommend opioids as the first‐line pharmacological treatment for breathlessness (ATS 1999; Mahler 2010; Parshall 2012; Mahler 2013; Wiseman 2013). A Cochrane review published in 2001 concluded that there was some evidence to support the use of oral and parenteral opioids to palliate breathlessness, but the number of participants studied was small and they recommended that larger trials were needed using standard protocols and incorporating quality of life measures (Jennings 2001).
A Cochrane review (Simon 2010), found no evidence for a beneficial effect of benzodiazepines for the relief of breathlessness in people with advanced cancer and COPD. However, the overall effect size was small and further research is required.
Description of the intervention
Opioids are chemical substances derived from the opium poppy. In the human body they bind to the μ, κ, and δ receptors located in the cerebral cortex, limbic system, midbrain, brainstem, and outside the central nervous system in the bronchioles, alveolar walls, myocardial cells, peripheral sensory nerve fibres, and primary afferent neurons.
How the intervention might work
Exogenous and endogenous opioids specifically bind to the μ receptors to reduce transmission of pain signals (Chahl 1996). Opioids also depress respiratory drive by directly blunting the responsiveness of the brainstem centres, which are affected by hypoxia and hypercapnia. Decreased respiratory output results in a decrease in corollary discharge from the brainstem to perceptual areas in the cerebral cortex and thus reduced the sensation of breathlessness. Corollary discharge describes the hypothesis that a sensory 'copy' of the motor output is sent from the motor cortex to the sensory cortex and imparts a conscious awareness of respiratory effort (Beach 2006).
Opioids may also cause blunting of perceptual sensitivity to sensations of breathlessness. Neuroimaging studies demonstrate that μ opioid receptor agonists can modulate the central processing of breathlessness similar to that of pain relief. Administration of opioids stimulate activity in the anterior cingulate cortex, thalamus, frontal cortex, and brainstem, the same areas which are activated when breathlessness occurs (Banzett 2000; Peiffer 2001; Petrovic 2002; Pattinson 2009).
Peripheral opioid receptors are located in bronchioles and alveolar walls of the respiratory tract (Zebraski 2000). Opioid administration may modulate breathlessness by binding to these opioid receptors. It is theorised that opioid administration could modulate breathlessness by binding to these peripheral opioid receptors. However, to date, studies of nebulised opioids have lacked efficacy compared with systemically administered opioids, and there is a lack of efficacy when nebulised opioids are compared with systemically administered opioids (Polosa 2002; Mahler 2013).
Other effects of opioids include drowsiness, euphoria, confusion, peripheral vasodilation, constipation, nausea and vomiting, and cough suppression.
The choice of preparation and pharmacokinetics of opioids may vary depending on individual needs. Small doses of short‐acting opioids may be commenced in opioid‐naïve people, and once a stable dose has been achieved, may be switched to long acting preparations. Currow 2011 found that 70% of participants derived benefit from 10 mg sustained‐release once‐daily preparations. Transmucosal, transdermal, subcutaneous, or intravenous modes may be more appropriate for people whose swallowing is impaired or who are approaching the final stages of end of life. It is unclear if all opioids and all routes are equal in their ability to relieve breathlessness. Opioids differ significantly in their pharmacodynamic properties, from differences in their absorption, to metabolism and affinity for receptors.
Why it is important to do this review
The use of opioids to treat breathlessness in advanced illness is variably accepted in medical practice, and some health professionals and patients have concerns regarding efficacy and side effects (Oxberry 2012; Rocker 2012). Much of the literature around opioids for breathlessness are narrative reviews and opinion pieces, and a systematic review is required to specifically examine the quality of evidence from randomised controlled trials (RCTs), to evaluate efficacy in terms of symptom control and quality of life, and to assess adverse effects.
This review will build on a previous Cochrane systematic review (Jennings 2001). In more recent years, additional RCTs have been published (Mazzocato 1999; Johnson 2002; Abernethy 2003), mechanisms of action have been further elucidated, and guidelines that examine the risk of bias and assessment of heterogeneity in Cochrane reviews have been updated (Higgins 2011).
Objectives
To determine the effectiveness of opioid drugs in relieving the symptom of breathlessness in patients with advanced disease due to malignancy, respiratory or cardiovascular disease, or receiving palliative care for any other disease.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomised controlled trials (RCTs) compared to either placebo or other treatment, as well as crossover studies in which participants were randomised to order of treatment. We defined 'randomised' as studies that were described by the study author as 'randomised'. There was no language restriction. All identified trials, published and unpublished, were eligible for inclusion.
Types of participants
We considered adults with any type of advanced progressive illness with persistent breathlessness despite optimal or appropriate treatment of reversible factors.
We also included participants suffering from breathlessness due to any type of illness, who were considered to be at an advanced stage of illness, or palliative stage, as defined by the study authors.
Types of interventions
Any opioid drug, given by any route in any dose, for the treatment of breathlessness compared to placebo, or any other pharmacological or non‐pharmacological interventions that were directly compared with the opioid treatment.
Types of outcome measures
Primary outcomes
Subjective measurement of breathlessness intensity or severity, including but not limited to Borg and the modified Borg scale, verbal categorical scales of breathlessness, and visual analogue scales (VAS) of breathlessness (O'Donnell 1998).
Secondary outcomes
Quality of life measure by any scale.
Any physiological and functional assessments of breathlessness including but not limited to six‐minute walk tests (6MWT), shuttle tests, and actigraphy.
Performance status.
Pulse oximetry.
Arterial blood gas analysis.
Adverse events including constipation, delirium, and others.
Mortality.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases up to 19 October 2015.
The Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library) Issue 10 of 12, 2015.
MEDLINE (OVID) 1946 to October week 2 2015.
EMBASE (OVID) 1974 2015 October 16.
CINAHL(EBSCO) 1982 to Octpber 2015.
Web of Science (ISI) to October 2015.
We have presented the search strategies we used in Appendix 1.
Searching other resources
For ongoing studies we searched the following up to 19 October 2015.
The metaRegister of Controlled Trials (mRCT) (http://www.controlled‐trials.com/mrct/).
ClinicalTrials.gov (http://clinicaltrials.gov/).
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/).
We handsearched reference lists of included studies, relevant chapters, and review articles. We used Google to search for conference abstracts.
We attempted to contact the trial investigators of two studies to determine the potential for inclusion. However, we did not receive a reply by the time we completed this review.
Data collection and analysis
Selection of studies
Two review authors (RM and HB) independently screened all abstracts to determine whether they met the inclusion criteria. We sought the full‐text publications of articles that definitely met or may have met the inclusion criteria. Two review authors (RM and HB) then reviewed these full‐text articles to determine eligibility. We resolved any disagreement with discussion and consensus. We included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) study flow diagram in the review to document the screening process (Liberati 2009), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Data extraction and management
Two review authors (RM and HB) independently extracted data from the included studies. Where appropriate, we imported data and pooled them in Cochrane's statistical software, Review Manager (RevMan) (Review Manager 2014), for further analysis.
We used a data collection form for study characteristics and outcome data, which we piloted on one study included in the review.
We extracted the following data.
Methods: study design, duration of the study, study setting, and date of study.
Participants: number, mean age and age range, gender, inclusion and exclusion criteria.
Intervention: intervention, dose, mode of administration, concomitant medications, and exclusions.
Outcomes: primary and secondary outcomes as specified, type of scale used, and time points collected.
Notes: funding for trial and any conflicts of interest for trial authors.
'Risk of bias' summary.
We extracted the mean and standard deviation (SD) values from each study. Where the included studies reported standard error or confidence intervals (CIs) were reported, we converted these to SD values according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in included studies
Two independent authors (HB and RM) assessed the included studies for risk of bias using the Cochrane's 'Risk of bias' assessment tool (Higgins 2011). We assessed the following: allocation (random sequence generation and allocation concealment); blinding of participants and personnel, blinding of outcome assessors; incomplete outcome data; and other bias. We scored each of these domains separately as either low risk of bias, unclear risk of bias (insufficient information to make a judgement), or high risk of bias as outlined below.
-
Generation of allocation sequence:
for each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups;
we assessed the method used to generate the allocation sequence as either: low risk of bias (any truly random process such as random number table or computer random number generator); or unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies that used a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment:
for each included study we described the method used to conceal the allocation sequence in sufficient detail and to determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment;
we assessed the methods as either: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); or unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
-
Blinding or masking (checking for possible performance bias):
for each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes;
we assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as either: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); or unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We intended to exclude studies that were at high risk of bias and were not double‐blinded.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). We assessed the methods used to deal with incomplete data as either: low risk (information from all participants were included in the main results, any dropouts are reported, any systematic differences between the two treatment arms are reported); unclear risk of bias (used 'last observation carried forward' analysis); or high risk of bias (used 'completer' analysis).
Selective reporting bias (checking for within study reporting bias, checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods as either low risk of bias (whether the study fully reported all prespecified outcomes); unclear risk of bias (it appeared not all pre‐outcomes were fully reported); or high risk of bias (the study highlighted not all prespecified outcomes were reported).
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised (Kjaergard 2001; Nüesch 2010; Dechartres 2013). We considered studies to be at low risk of bias if they had 200 participants or more in each treatment arm; at unclear risk of bias if they had 50 to 200 participants per treatment arm; or at high risk of bias if they had fewer than 50 participants per treatment arm.
-
Free of other bias (bias due to problems not covered elsewhere in the table):
for each included study we described any important concerns we had about other possible sources of bias (e.g. baseline imbalance, bias of the presentation data, representation of gender, etc.).
We resolved any disagreement by discussion and consensus.
We performed funnel plot analysis and compared fixed‐effect versus random‐effects magnitude of effect to determine if there was any suggestion of bias.
Measures of treatment effect
We presented results from continuous variables, such as the breathlessness scales, using a fixed‐effect model and calculated standardised mean differences (SMDs) where scales were combined, such as when pooling VAS and Borg scale, with the corresponding 95% CIs. Where scales were not combined, and to assess effect across subgroups, we used the mean difference (MD). Where studies reported results based on a variable range of doses, we used the higher dose.
For dichotomous data, including adverse events, we reported relative risk ratios (RRs) where we could pool the data. Where we were unable to pool these data, we included these results in a descriptive analysis.
Unit of analysis issues
Our unit of analysis was the participant. We did not identify any cluster RCTs. We took measurements from the intervention and control group, and analysed the data as if it was a parallel trial, due to lack of paired data available. We attempted to contact the study authors to obtain paired data. However, due to no response we were unable to obtain original data. Most included studies were crossover trials, and therefore we included the data available in data reports, and acknowledged the limitations of this approach.
Dealing with missing data
Where possible we attempted to contact the principal investigator of the included studies to obtain missing data.
Assessment of heterogeneity
For pooled analyses, we quantified statistical heterogeneity using the I² statistic, which describes the percentage of the total variation across trials due to heterogeneity rather than sampling error. We considered significant statistical heterogeneity to be present if the I² statistic value was greater than 50%.
Where we identified significant heterogeneity, we further assessed this using predetermined subgroups.
Assessment of reporting biases
We attempted to contact the principal investigator of the included study for missing data where reporting bias appeared possible.
Data synthesis
A priori, we decided to analyse continuous data according to a fixed‐effect model, due to the concerns around the small‐study effects on the results of the meta‐analysis for all continuous outcomes (Higgins 2011). However, we provided random‐effects model data in the sensitivity analysis to compare the sensitivity of the results to different statistical methods. We calculated SMDs where we combined scales, such as when we pooled the VAS and Borg scale data, with the corresponding 95% CIs. Where scales were not combined, and to assess effect across subgroups, we used the MD. Where studies reported results based on a variable range of doses, we used the higher dose.
We used RevMan (Review Manager 2014) to perform meta‐analyses and presented our primary outcomes in a 'Summary of findings' table, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using the GRADEPro Guideline Development Tool (GDT) software (GradePro 2015).
We assessed the overall quality of the evidence for each outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (GradePro 2015) and presented the main findings of the review in a transparent and simple tabular format in the 'Summary of findings' tables. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. We chose to present the outcomes stipulated a priori and that which would be clinically meaningful.
The GRADE system uses the following criteria for assigning the grade of evidence based on RCTs.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
We decreased the grade of evidence if the following occurred.
Serious (−1) or very serious (−2) limitation to study quality.
Important inconsistency (−1).
Some (−1) or major (−2) uncertainty about directness.
Imprecise or sparse data (−1).
High probability of reporting bias (−1).
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses.
Type of illness (e.g. chronic obstructive pulmonary disease (COPD), heart failure, malignancy, and neuromuscular disorders).
Mode of delivery of opioid drug (e.g. oral, subcutaneous, intravenous, nebulised, intra‐nasal, sublingual, buccal, transdermal, and other modes).
Dose.
Type of opioid (e.g. morphine, dihydrocodeine, fentanyl).
In the protocol we indicated that we would perform meta‐analyses according to the subgroups of dose and 'Risk of bias' assessment. Due to the wide variation and heterogeneity of reported doses we chose to analyse this in a descriptive analysis. We compared the 'Risk of bias' difference in a sensitivity analysis.
Post‐hoc we chose to include the type of opioid as a subgroup analysis as we felt this would be an important assessment for clinicians and policy makers.
Sensitivity analysis
We performed sensitivity analyses by systematically excluding studies from the overall analysis based on the potential sources of heterogeneity outlined above, and if homogeneous subgroups have not already been identified and analysed separately. We also compared data from fixed‐effect and random‐effects models to assess for heterogeneity.
Results
Description of studies
Results of the search
We identified 376 citations by using the search strategy, and selected 50 articles for full‐text review after screening the abstracts of the initial search results. See Figure 1 for further details.
1.
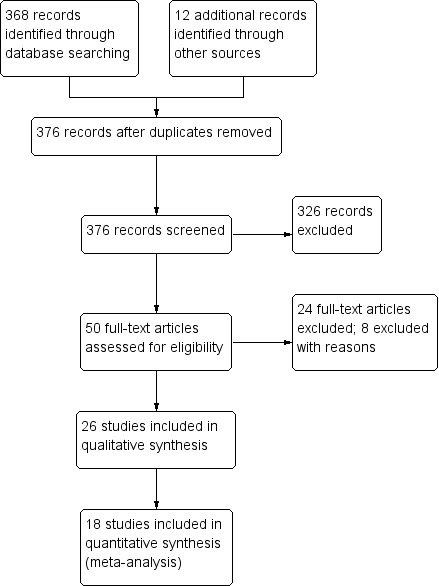
Study flow diagram.
We included 26 studies with 526 participants in the review.
Included studies
See the 'Characteristics of included studies' table.
Study characteristics
Eighteen studies with 276 participants provided data for the primary outcome of breathlessness and were included in the meta‐analysis (Abernethy 2003; Bar‐Or 1982; Bruera 1993; Charles 2008; Chua 1997; Eiser 1991; Harris‐Eze 1995; Hui 2014; Jankleson 1997; Jensen 2012; Johnson 1983; Leung 1996; Light 1996; Mazzocato 1999; Noseda 1997; Oxberry 2011; Poole 1998; Woodcock 1981). Four additional studies examined the primary outcome of breathlessness but we were unable to extrapolate data for meta‐analysis (Davis 1996; Grimbert 2004; Johnson 2002; Masood 1995). Two additional studies did not report the primary outcome, but reported secondary outcomes (Williams 2003; Young 1989). Two additional studies compared opioids to an intervention other than placebo; Navigante 2010 compared morphine to midazolam, Rice 1987 compared codeine to promethazine. One study (Oxberry 2011) compared morphine with oxycodone and placebo.
Twenty‐four included studies were crossover trials. Hui 2014 was a parallel group RCT that compared subcutaneous fentanyl with placebo, and Navigante 2010 was a parallel RCT that compared morphine to midazolam.
Most included studies were performed over a fixed period during one day or on two consecutive days, with a washout period of only on day. Six studies involved more chronic administration of the drug or placebo, continuing for study periods between four days and six weeks, with a washout period of between three days and two weeks (Woodcock 1981; Johnson 1983; Eiser 1991; Poole 1998; Abernethy 2003; Navigante 2010).
Participants
All included studies were small, with fewer than 50 participants per treatment arm. The number ranged from six to 25 participants, with an average of 19 participants per study. Fourteen studies recruited ambulatory care participants (Abernethy 2003; Eiser 1991; Hui 2014; Masood 1995; Harris‐Eze 1995; Rice 1987; Poole 1998; Oxberry 2011; Navigante 2010; Leung 1996; Johnson 1983; Johnson 2002; Young 1989; Woodcock 1981), two studies recruited inpatients (Mazzocato 1999; Noseda 1997), one study had a mix of inpatients and outpatients (Charles 2008), and nine studies did not specify the participant setting (Light 1996; Williams 2003; Jensen 2012; Jankleson 1997; Grimbert 2004; Chua 1997; Bruera 1993; Bar‐Or 1982; Davis 1996).
Fourteen studies involved primarily or exclusively participants with chronic obstructive pulmonary disease (COPD) (Abernethy 2003; Bar‐Or 1982; Light 1996; Poole 1998; Eiser 1991; Jankleson 1997; Jensen 2012; Johnson 1983; Leung 1996; Masood 1995; Noseda 1997; Rice 1987; Woodcock 1981; Young 1989). Six studies included only participants with malignant disease (Bruera 1993; Charles 2008; Davis 1996; Grimbert 2004; Hui 2014; Mazzocato 1999), four studies were comprised primarily of cardiac failure participants (Chua 1997; Johnson 2002; Oxberry 2011; Williams 2003), and one study was comprised of participants with interstitial lung disease (Harris‐Eze 1995). One study involved end stage disease from all causes (Navigante 2010).
Intervention
Eight studies specifically recruited participants not currently on opioids (Abernethy 2003; Harris‐Eze 1995; Jensen 2012; Johnson 1983; Masood 1995; Navigante 2010; Poole 1998; Rice 1987), who were thus opioid naive. Twelve studies did not specify whether opioid use was part of the exclusion criteria, or whether it formed part of the co‐interventions (Bar‐Or 1982; Light 1996; Eiser 1991; Jankleson 1997; Noseda 1997; Woodcock 1981; Young 1989; Davis 1996; Chua 1997; Oxberry 2011; Leung 1996; Williams 2003). Three studies examined participants already on opioids (Bruera 1993; Grimbert 2004; Hui 2014). Bruera 1993 used 50% more of the participant's usual dose in a PRN (pro re nata, or as required) manner. Hui 2014 used a sliding scale of 30 mcg to 350 mcg fentanyl for all interventional participants, and included regular opioids in both the interventional and control arm. Three studies (Charles 2008; Mazzocato 1999; Grimbert 2004) used a predefined dose of opioids, regardless of the participant's current opioid use.
The included studies used the following opioids: oral dihydrocodeine (Bar‐Or 1982; Chua 1997; Johnson 1983; Rice 1987), oral diamorphine (Eiser 1991), intravenous diamorphine (Williams 2003), oral morphine (Light 1996; Mazzocato 1999; Poole 1998; Abernethy 2003; Woodcock 1981), nebulised morphine (Davis 1996; Charles 2008; Grimbert 2004; Harris‐Eze 1995; Leung 1996; Jankleson 1997; Masood 1995; Noseda 1997; Young 1989), subcutaneous fentanyl (Navigante 2010; Hui 2014), subcutaneous morphine (Bruera 1993), nebulised fentanyl (Jensen 2012), oral oxycodone (Oxberry 2011), and hydromorphone (Charles 2008).
The doses of dihydrocodeine ranged from 15 mg three times a day to 60 mg three times a day in 1 mg/1 kg doses. The diamorphine dose ranged from 2.5 to 5 mg four times a day. Sustained release morphine was used in 10 to 20 mg doses. Oxycodone was administered in 2.5 mg doses four times a day. Subcutaneous morphine doses ranged from 2.5 to 10 mg. There was a wide range of nebulised morphine doses used, from 1 mg to 50 mg.
Nine studies delivered the opioids by the oral route (Bar‐Or 1982; Eiser 1991; Johnson 1983; Woodcock 1981; Abernethy 2003; Chua 1997; Light 1996; Oxberry 2011; Poole 1998), two studies used parenteral opioids (Bruera 1993; Hui 2014), and ten studies gave the drugs via nebulisation (Davis 1996; Harris‐Eze 1995; Masood 1995; Young 1989; Grimbert 2004; Jankleson 1997; Leung 1996; Noseda 1997; Charles 2008; Jensen 2012). Some studies compared different routes of administration.
Eight studies continued regular use of co‐interventions including steroids and bronchodilators (Bruera 1993; Masood 1995; Woodcock 1981; Young 1989; Charles 2008; Hui 2014; Mazzocato 1999; Rice 1987). Two studies involved the use of oxygen inhalation (Leung 1996; Noseda 1997). In both cases the measures were applied to the use of the drug and placebo arm and we felt this did not bias the study results.
Outcomes
Twelve studies performed some form of exercise testing (Bar‐Or 1982; Hui 2014; Poole 1998; Chua 1997; Harris‐Eze 1995; Leung 1996; Light 1996; Eiser 1991; Jensen 2012; Johnson 1983; Williams 2003; Woodcock 1981). They used a variety of different exercise tests, including incremental treadmill tests, incremental cycle ergometer tests, non incremental treadmill or endurance treadmill tests, and six‐minute walk tests (6MWT).
There was significant variety in the reporting of breathlessness outcome measure, but all studies used well‐validated scales, including the visual analogue scale (VAS), Borg Scale, and oxygen cost diagram (McGavin 1978; O'Donnell 1998). Several studies did not report breathlessness at a fixed point during exercise (Beauford 1993; Masood 1995). Some studies did not report the primary outcome of breathlessness, did not include sufficient data, did not report standard deviations (SDs) or error, or reported data in such a way that the relevant numbers could not be extrapolated (Young 1989; Davis 1996; Jankleson 1997; Masood 1995; Williams 2003; Grimbert 2004).
In most cases, the studies asked their participants to assess their own levels of breathlessness, by VAS or Borg scale. Some studies asked participants to guess which substance contained the opioid or placebo drug, and other studies offered participants the opportunity to continue on opioid therapy. One study, Poole 1998, used the Chronic Respiratory Disease Questionnaire (CRQ) dyspnoea scale.
Excluded studies
See the 'Characteristics of excluded studies' section.
We excluded eight studies for the following reasons: participants were not randomised (Beauford 1993; Peterson 1996; Shorati 2012; Smith 2009), there was no comparison to a placebo or other intervention (Allard 1999; Bruera 2005; Navigante 2003), or it was a review (Thomas 2010).
Ongoing studies
We identified two ongoing studies (Cuervo Pinna 2012; Daubert 2014).
Risk of bias in included studies
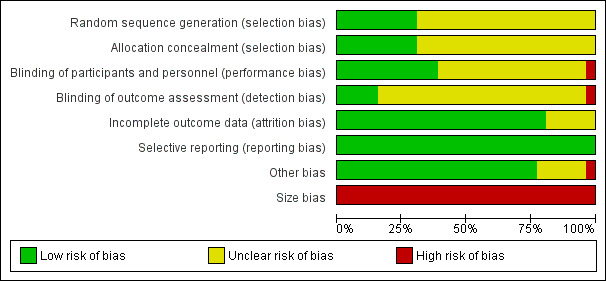
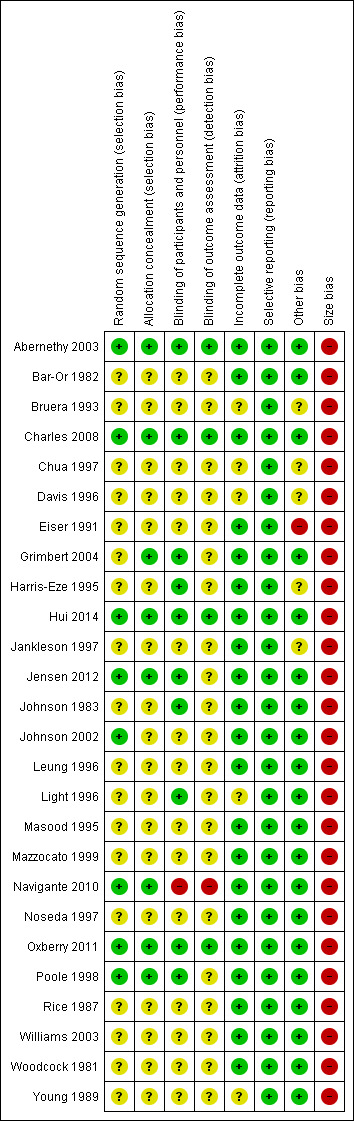
We assessed the risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011), and included the domains of allocation, blinding, incomplete outcome data, and other bias. We judged eight studies to be at an overall low risk of bias. We considered 18 studies to be at an overall unclear risk of bias, that is we had insufficient information to make a judgement, usually due to inadequate descriptions of the methods of randomisation or blinding.
Please see Figure 2 and Figure 3 for a summary of the 'Risk of bias' findings.
2.
3.
We assessed the overall quality of the evidence for each outcome using the GRADE system (GradePro 2015) and presented these results in the 'Summary of findings' tables, which shows the main findings of the review in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
Allocation
We assessed random sequence generation as adequate (low risk) in eight out of 26 studies (Poole 1998; Johnson 2002; Abernethy 2003; Charles 2008; Navigante 2010; Oxberry 2011; Jensen 2012; Hui 2014). Most studies (18 studies) did not describe the methods of sequence generation (unclear risk of bias).
We judged allocation concealment as adequate (low risk) in eight out of 26 studies (Poole 1998; Abernethy 2003; Grimbert 2004; Charles 2008; Navigante 2010; Oxberry 2011; Jensen 2012; Hui 2014), which suggests that information from most of the studies presented an unclear risk of bias. Many studies did not state the method of allocation concealment, though many of the studies reported their design as randomised.
We did not judge any studies as at high risk of allocation or random sequence generation bias.
Blinding
Blinding of participants and personnel with respect to the intervention was adequate, though blinding of the outcome assessment was overall poor.
We judged the blinding of participants and personnel to be adequate in 10 out of 26 studies, indicating low risk of bias (Johnson 1983; Harris‐Eze 1995; Light 1996; Poole 1998; Abernethy 2003;Grimbert 2004; Charles 2008; Oxberry 2011; Jensen 2012; Hui 2014). These studies used placebo interventions, which were reported to have been designed to appear the same as the opioid intervention. We judged blinding of participants and personnel to be at high risk of bias in Navigante 2010 because only the participants were blinded, not the investigators or those that performed the outcome assessment. Of the 15 studies that we assessed as being at an unclear risk of bias for this domain, the studies did not specifically or adequately describe the details to which the intervention and control were blinded, though many studies reported themselves as blinded.
Overall, blinding of the outcome assessment was poor. We assessed only four out of 26 studies as at low risk of bias (Abernethy 2003; Charles 2008; Oxberry 2011; Hui 2014). We judged Navigante 2010 to be at high risk as those performing the outcome assessment were not blinded, and we judged the remaining 21 studies to be at an unclear risk of bias. Most studies did not clearly describe the methods by which the outcome assessment was blinded, though some described themselves as double blinded. This may be in part due to the primary outcome of breathlessness requiring the participant to score their own symptoms.
Incomplete outcome data
The included studies generally reported data completely, with 21 out of 26 studies adequately described. We therefore judged them to be at low risk of bias. We judged the remaining five studies to be at an unclear risk of bias (Bruera 1993; Chua 1997; Davis 1996; Light 1996; Young 1989). The included studies usually recorded adverse events, but generally these did not cause participants to drop out of the study. Most studies were conducted on consecutive days, so loss to follow‐up was less likely to occur.
Selective reporting
We judged the risk of selective reporting to be low in all studies. We did not detect any evidence of selective reporting bias.
Other potential sources of bias
We judged 20 studies to be at low risk for this domain (Woodcock 1981; Bar‐Or 1982; Rice 1987; Young 1989; Masood 1995; Leung 1996; Light 1996; Noseda 1997; Poole 1998; Mazzocato 1999; Johnson 2002; Abernethy 2003; Williams 2003; Grimbert 2004; Charles 2008; Navigante 2010; Oxberry 2011; Jensen 2012; Hui 2014), and one at high risk of bias because it did not state that it systematically studied adverse events (Eiser 1991). We judged the remaining five studies to be at unclear risk of other bias because insufficient information was available.
Size
The studies were of small sample size, with a mean of 19 participants per study, and with fewer than 50 participants per treatment arm. Thus we judged all 26 studies to be at overall high risk of bias for this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
for the main comparison.
| Opioids compared with placebo in people with breathlessness in advanced disease or terminal illness | ||||||
|
Patient or population: adults with refractory breathlessness Setting: inpatient and outpatient setting Intervention: opioids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Opioids | |||||
| Breathlessness: change from baseline1 | The standardised mean change from baseline ranged from −2 to 0.60 in the control group | The standardised mean change from baseline was 0.11 points better in the opioids group compared to the placebo group (ranging from a 0.40 point reduction to a 0.19 point increase in breathlessness) | — | 194 (6 RCTs) | ⊕⊝⊝⊝ very low2,3,4 |
A lower score indicates an improvement in breathlessness |
| Breathlessness: post‐treatment score1 | The standardised mean post‐treatment score ranged from −43 to 49 in the control group | The standardised mean post‐treatment score was 0.32 points better in the opioid group compared to the placebo group (ranging from a 0.53 point reduction to a 0.10 point reduction in breathlessness) | — | 338 (12 RCTs) | ⊕⊕⊝⊝ low2,3 |
A lower score indicates an improvement in breathlessness |
| Exercise tolerance: 6MWT5 ‐ total distance | The total distance in 6MWT was 368m in the placebo group | The total distance in 6MWT was 28 m better in the opioids group compared to placebo (ranging from 113 m to 58 m) | — | 11 participants (1 RCT) | ⊕⊝⊝⊝ very low2,3,4,6 |
— |
| Exercise tolerance: 6MWT5‐ change from baseline | The change from baseline was from ‐21m to 37m in the placebo group | The change in baseline was 48 m worse in the opioids group (ranging from 36 m to 60 m) | — | 26 (2 RCTs) | ⊕⊝⊝⊝ very low1,2,3 |
— |
|
Adverse events: constipation |
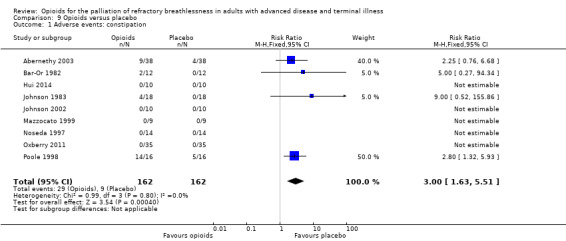
55 per 1000 | 179 per 1000 | RR 3 (95% CI 1.63 to 5.51) | 162 (9 RCTs) |
⊕⊝⊝⊝ very low2,3 |
— |
|
Adverse events: nausea and vomiting |
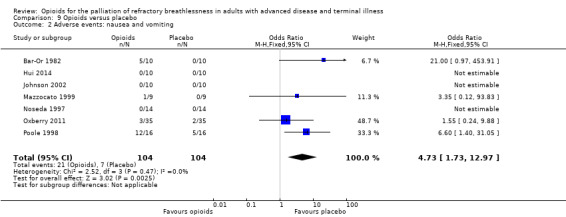
67 per 1000 | 201 per 1000 | RR 4.73 (95% CI 1.73 to 12.97) | 104 (7 RCTs) |
⊕⊝⊝⊝ very low2,3 |
— |
|
Adverse events: drowsiness |
58 per 1000 | 128 per 1000 | RR 2.86 (95% CI 1.17 to 7.02) | 156 (9 RCTs) |
⊕⊝⊝⊝ very low2,3 |
— |
| Quality of life7 | The change from baseline score in the control group was 2.94 | The quality of life change from baseline score in the opioid group was 0.86 points lower (ranging from 9.90 points lower to 8.18 points higher) | — | 16 (1 RCT) | ⊕⊝⊝⊝ very low2,3,4 |
— |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1The study authors assessed breathlessness at variable time points (one hour to six weeks) during the study according to the VAS, Borg scale, and oxygen cost diagram. 2There were limitations in the design and implementation of available studies, which suggested a high risk of bias. 3There were small study sizes. 4There was significant heterogeneity. 5The study authors assessed six minute walk test (6MWT) at variable time points (one hour to six weeks). 6There were large CIs. 7The study authors measured this outcome using the Chronic Respiratory Disease Questionnaire. Only one study included quality of life data that we were able to be include.
Abbreviations: RCT: randomised controlled trial; CI: confidence interval; RR: risk ratio.
2.
| Morphine compared with midazolam in people with breathlessness in advanced disease or terminal illness | ||||||
|
Patient or population: adults with refractory breathlessness Setting: outpatient setting Intervention: morphine Comparison: midazolam | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Midazolam | Morphine | |||||
| Breathlessness: post‐treatment score1 | The mean dyspnoea score in the midazolam group was 4 | The mean post‐treatment score was 2 points higher in the opioids group (ranging from 1.07 to 2.93) | — | 63 (1 RCTs) | ⊕⊝⊝⊝ very low2,3,4 | A lower score indicates an improvement in breathlessness |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1The study authors assessed breathlessness according to the numeric rating scale (NRS) for dyspnoea at 5 days. 2Limitations in the design and implementation of available studies suggest a high risk of bias. 3There was only one study. 4There was evidence of significant heterogeneity.
Abbreviations: RCT: randomised controlled trial; CI: confidence interval; RR: risk ratio.
3.
| Codeine compared with promethazine in people with breathlessness in advanced disease or terminal illness | ||||||
|
Patient or population: adults with refractory breathlessness Setting: outpatient setting Intervention: codeine Comparison: promethazine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Promethazine | Codeine | |||||
| Breathlessness: post‐treatment score1 | The mean dyspnoea score in the promethazine group was 6 | The mean post‐treatment score was 0.30 points lower in the codeine group (ranging from 0.83 points lower to 0.23 points higher) | — | 7 (1 RCT) | ⊕⊝⊝⊝ very low2,3,4 | A lower score indicates an improvement in breathlessness |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Breathlessness was assessed according to the oxygen cost diagram at 1 month. 2Limitations in the design and implementation of available studies suggesting a high risk of bias. 3Only one study. 4Significant heterogeneity.
Abbreviations: RCT: randomised controlled trial; CI: confidence interval; RR: risk ratio.
Primary outcome: breathlessness
Opioids versus placebo
All studies
The primary outcome of breathlessness was reported in 24 out of 26 included studies. We performed meta‐analysis for the main outcome of breathlessness for 18 studies. We analysed change from before and after administration and post‐administration measurement. We used standardised mean differences (SMDs) since the studies measured comparable outcomes on different scales (the SMD can be converted to units in a VAS or Borg score by multiplying the SD for a particular study). Where studies presented standard errors of the mean, we correlated them to SD. We took measurements from the intervention and control group, and analysed the data as if it was a parallel trial, due to lack of paired data available. This may increase the unit of analysis error.
Individually, nine studies reported a statistical benefit using opioids for breathlessness, and 10 studies reported no difference comparing opioids with placebo. Three studies found a significant difference in exercise tolerance, and one found no difference. When we excluded nebulised morphine studies, 10 individual studies found a significant effect on breathlessness, compared to three studies that found no benefit.
We have presented meta‐analyses using SMDs in the figures below. We have included fixed‐effect meta‐analyses for opioids compared with placebo for breathlessness outcome for the following.
All studies.
By type of opioid.
By mode of administration.
By condition.
Please see Figure 4 for more details.
4.
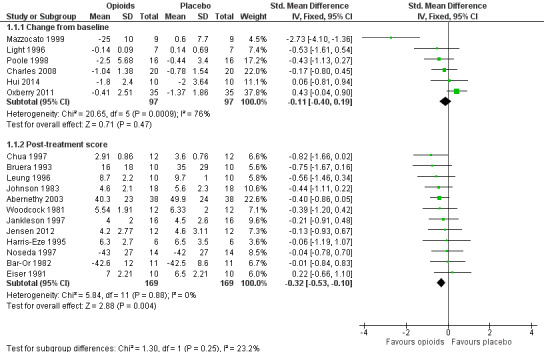
The meta‐analysis demonstrates a small treatment effect for breathlessness (change from baseline, six studies, 194 participants; SMD −0.11, 95% confidence interval (CI) −0.40 to 0.19; P = 0.47 (Analysis 1.1); post‐treatment score, 12 studies, 338 participants, SMD −0.32, 95% CI −0.53 to −0.10; P = 0.004 (Analysis 1.1)). There was statistically significant heterogeneity between the results of the trials for breathlessness change from baseline (I² statistic = 76%, P = 0.0009), but the direction of effect was consistent, and the sample size of the studies was small. We considered the evidence to be of low quality for post‐treatment scores and of very low quality for change from baseline breathlessness.
1.1. Analysis.
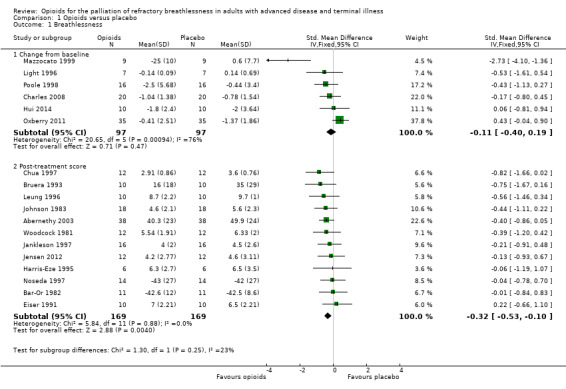
Comparison 1 Opioids versus placebo, Outcome 1 Breathlessness.
Johnson 2002 presented data using the interquartile range and thus we could not pool these data in the meta‐analysis. The study demonstrated a statistically significant improvement in breathlessness from baseline using oral morphine compared to placebo. Masood 1995 presented data using CIs with a very small sample size (12 subjects), so we could not pool its data in the meta‐analysis. The study did not demonstrate any statistically significant difference in terms of breathlessness comparing nebulised or intravenous morphine with placebo. Davis 1996 reported a post‐treatment score expressed as a percentage of pre‐treatment score change from baseline. The ratio for opioids was 0.64 compared to normal saline of 0.84 was statistically significant at P = 0.001, however the difference in ratios between the two groups was not significant (P = 0.17).
Additional sensitivity analyses
We planned a priori to compare data from fixed‐effects and random‐effects models to assess for heterogeneity. There was no difference in effect size or heterogeneity when comparing fixed‐ and random‐effects (Table 4). We systematically excluded studies with multiple domains of high risk of bias, and unclear risk of bias (see Figure 3). When studies with unclear risk of bias were excluded, there was a reduction in effect size and loss of significance.
1. Sensitivity analysis: breathlessness: impact of fixed versus random effects model and unclear bias.
| Meta‐analysis | Number of studies | Pooled SMD | Confidence interval | P value for SMD | Heterogeneity test |
| All studies, fixed‐effect Change from baseline |
6 | ‐0.11 | −0.40 to 0.19 | 0.47 | I² statistic = 76%, P = 0.0009 |
| All studies, random‐effects Change from baseline |
6 | ‐0.40 | −1.04 to 0.24 | 0.22 | I² statistic = 76%, P = 0.0009 |
| All studies, fixed‐effect Post‐treatment score |
12 | −0.32 | −0.53 to −0.10 | 0.004 | I² statistic = 0%, P = 0.88 |
| All studies, random‐effects Post‐treatment score |
12 | −0.32 | −0.53 to −0.10 | 0.004 | I² statistic = 0%, P = 0.88 |
| Studies excluded with unclear bias Change from baseline |
5 | 0.01 | −0.29 to 0.31 | 0.95 | I² statistic = 80%, P = 0.0006 |
| Studies excluded with unclear bias Post‐treatment score |
5 | 0.20 | −0.50 to 0.10 | 0.20 | I² statistic = 0%, P = 0.76 |
Abbreviations: SMD: standardised mean difference.
We have also included an additional post hoc sensitivity analysis to address some of the methodological challenges relating to the quantitative synthesis of the results of cross‐over trials. Cross‐over trials can be an appropriate way to assess short term interventions. The Cochrane Handbook outlines several methods to incorporate crossover data into meta‐analyses (Higgins 2011). It states that using the data as if it was a parallel study is a legitimate method, so long as the limitations are acknowledged. In particular this approach can give rise to a unit of analysis error whereby confidence intervals may be wide and the overall effect is under‐estimated.
An alternative method is to calculate correlation co‐efficients (which describe the ratio of within patient variation to between patient variation) to impute a corrected standard error. Some included studies provide appropriate data to calculate this (standard error of the differences), or a corrected standard error can be imputed using “borrowed” correlation co‐efficients from other studies.
In a subsequent sensitivity analysis we included an alternative meta‐analysis using correlation co‐efficients and corrected standard errors. The data is presented using standardised mean differences. The sensitivity analysis presented, accounting for appropriate use of crossover data, demonstrates a SMD −0.42 (95% CI −0.58 to −0.26); see Figure 5). This is not dissimilar to our previous SMD −0.32 (95% CI −0.53 to −0.10) for post treatment dyspnoea scores and SMD −0.11 (−0.40 to −0.19) for change from baselines scores; there is a significant but small effect size for the use of opioids for breathlessness.
5.
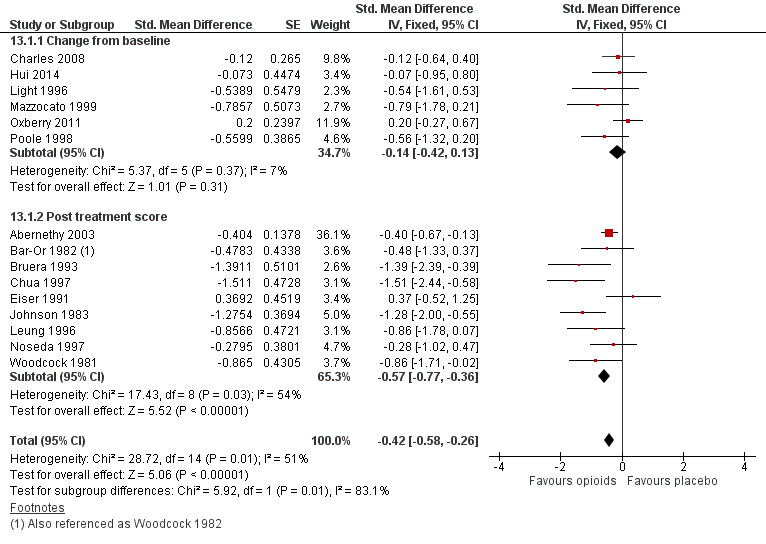
Forest plot of comparison: 13 Opioids versus placebo ‐ sensitivity analysis, outcome: 13.1 Breathlessness.
In order to interpret this in a more meaningful context, we converted this standard mean difference to a mean difference on a commonly used dyspnoea scale, that being the VAS 100 mm scale. Using the post treatment standard deviation from a large study (Abernethy 2003), we calculated an effect size of 9.6 mm (95% CI −13.44 to −5.52) on a 100 mm VAS scale. The point estimate appears to meet the clinically important difference threshold but the confidence intervals still include values which would not be considered clinically significant and therefore there is still some uncertainty about the effectiveness of the intervention.
Type of opioid
There was a strong treatment effect for morphine (post‐treatment scores: six studies, 188 participants; SMD −0.33, 95% CI −0.62 to −0.04; P = 0.02 (Analysis 2.1); change from baseline: four studies, 134 participants, SMD −0.12, 95% CI −0.48 to 0.24; P = 0.50 (Analysis 2.1)), and for dihydrocodeine (post‐treatment score: four studies, 107 participants; SMD −0.41, 95% CI −0.80 to −0.03; P = 0.04 (Analysis 3.1)).
2.1. Analysis.
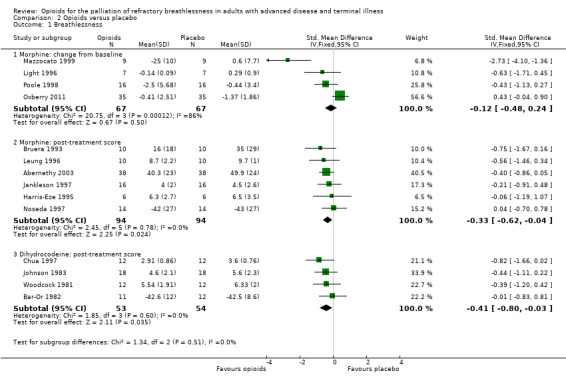
Comparison 2 Opioids versus placebo, Outcome 1 Breathlessness.
3.1. Analysis.
Comparison 3 Opioids versus placebo, Outcome 1 Breathlessness.
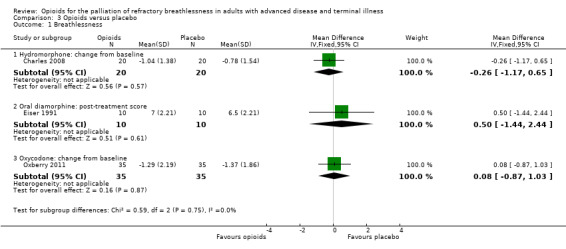
There was no effect for hydromorphone (change from baseline: one study, 20 participants, MD −0.26; 95% CI −1.17 to 0.65; P = 0.57), oral diamorphine (post treatment: one study, 10 participants; MD 0.50, 95% CI −1.44 to 2.44; P = 0.61), oxycodone (change from baseline: one study, 35 participants; MD 0.08, 95% CI −0.87 to 1.03; P = 0.16 (Analysis 3.1)), or fentanyl (change from baseline: one study, 10 participants, MD 0.20, 95% CI −2.50 to 2.90; P = 0.88 (Analysis 4.1); post‐treatment score: one study, 12 participants; MD −0.40, 95% CI −2.76 to 1.96; P = 0.74 (Analysis 4.1)).
4.1. Analysis.
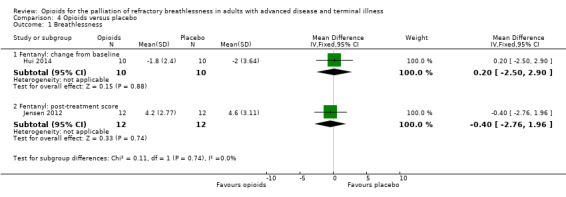
Comparison 4 Opioids versus placebo, Outcome 1 Breathlessness.
Condition
There were insufficient data to suggest opioids would be more beneficial in any specific condition. The effect for COPD was as follows: change from baseline: two studies, 23 participants, SMD −0.49, 95% CI −1.08 to 0.10; P = 0.1; post‐treatment scores: eight studies, 131 participants; SMD −0.24; 95% CI −0.48 to 0.01; P = 0.1, (Analysis 5.1). For cancer‐related dyspnoea it was: change from baseline: three studies, 39 participants, SMD −0.41; 95% CI −0.89 to 0.06; P = 0.21; post‐treatment score: one study, 10 participants, SMD −0.75; 95% CI −1.67 to 0.16; P = 0.11 (Analysis 5.1). There was no significant difference overall for heart failure (change from baseline: one study, 35 participants; SMD 0.43, 95% CI −0.04 to 0.90, P = 0.08 favouring placebo; post‐treatment score: one study, 12 participants, SMD −0.82, 95% CI −1.66 to 0.02; P = 0.06 favouring opioids), and for interstitial lung disease (one study, six participants; SMD −0.06; 95% CI −1.19 to 1.07; P = 0.92 (Analysis 5.1)).
5.1. Analysis.
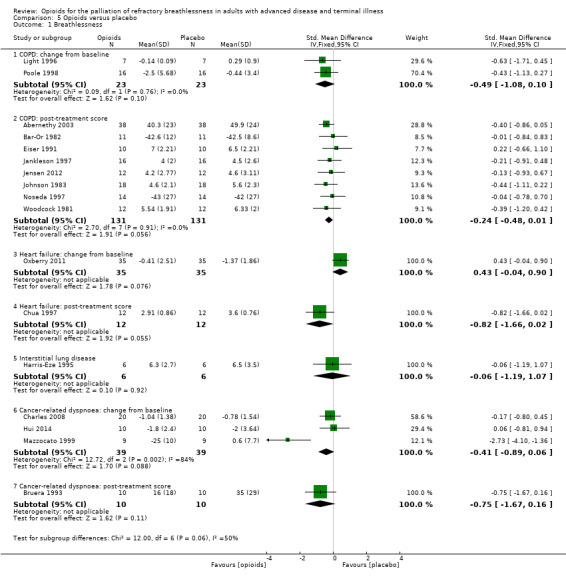
Comparison 5 Opioids versus placebo, Outcome 1 Breathlessness.
Mode of administration
The effect for oral opioids was as follows: change from baseline: three studies, 58 participants; SMD 0.07, 95% CI −0.30 to 0.44; P = 0.72; post‐treatment score: six studies, 95 participants; SMD −0.27, 95% CI −0.56 to 0.02, P = 0.07 (Analysis 6.1)). For the subcutaneous route it was as follows: change from baseline: two studies, 38 participants; MD −2.30, 95% CI −4.87 to 0.27; P = 0.08, post‐treatment score: one study, 10 participants; MD −19.00, 95% CI −40.15 to 2.15; P = 0.08 (Analysis 6.2)).
6.1. Analysis.
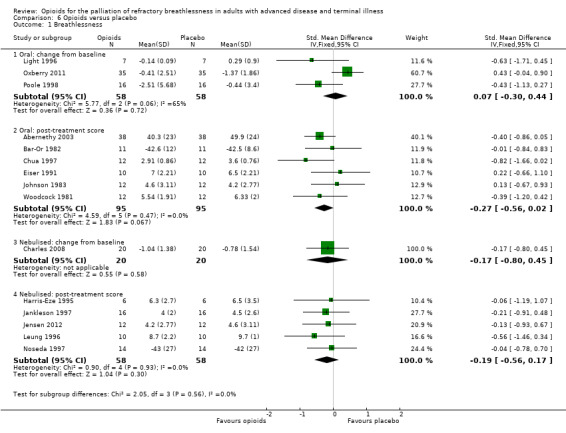
Comparison 6 Opioids versus placebo, Outcome 1 Breathlessness.
6.2. Analysis.
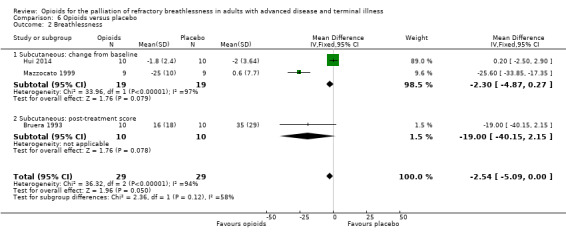
Comparison 6 Opioids versus placebo, Outcome 2 Breathlessness.
There was no difference in breathlessness for nebulised opioids compared to placebo (change from baseline: one study, 40 participants; SMD −0.17; 95% CI −0.80 to 0.45; P = 0.58; post‐treatment score: five studies, 116 participants; SMD −0.19; 95% CI −0.56 to 0.17; P = 0.30 (Analysis 6.1)).
Opioids versus other interventions
Navigante 2010 included 63 participants and examined morphine versus midazolam. The study found a statistically significant treatment effect that favoured midazolam for the outcome of breathlessness (MD 2.00, 95% CI 1.07 to 2.93; P < 0.0001; Analysis 10.1).
10.1. Analysis.
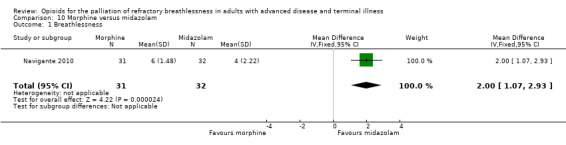
Comparison 10 Morphine versus midazolam, Outcome 1 Breathlessness.
Rice 1987 included seven participants and examined codeine versus promethazine. The effect favoured codeine for breathlessness (MD −0.30; 95% CI −0.83 to 0.23; P = 0.27; Analysis 11.1).
11.1. Analysis.
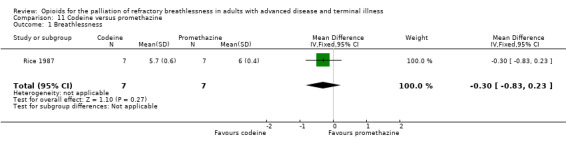
Comparison 11 Codeine versus promethazine, Outcome 1 Breathlessness.
Dose
We attempted to calculate the morphine dose equivalent that would confer relief from breathlessness, but due to significant heterogeneity between trials there was no clear dose threshold. We calculated oral morphine equivalent doses and these are represented in the 'Characteristics of included studies' tables. Light 1996, Oxberry 2011, Bar‐Or 1982, and Abernethy 2003 administered 20 mg to 30 mg oral morphine equivalent daily, and three out of four studies found a benefit (Abernethy 2003; Bar‐Or 1982; Light 1996). Mazzocato 1999, Eiser 1991, and Johnson 1983 administered 13 mg to 15 mg oral morphine equivalent daily, and two out of three studies found a benefit (Johnson 1983; Mazzocato 1999). Woodcock 1981, Johnson 2002, and Chua 1997 administered 5 mg oral morphine equivalent, and all studies found benefit. Poole 1998, Hui 2014, and Bruera 1993 had a wide range of doses and so we could not include these studies in the analysis.
There were few studies that calculated the bioequivalence of nebulised morphine to subcutaneous route. It may be as low as 5% (Masood 1996). Due to a potentially different mode of action, we did not use nebulised opioid doses to calculate bioequivalence.
It is difficult to ascertain the appropriate dose for the relief for breathlessness. It is possible that 5 mg oral morphine daily may confer benefit, but further research is required in this area.
We performed a sensitivity analysis that compared fixed‐effect versus random‐effects data, and excluded those with an unclear risk of bias. However, it made very little difference to the overall result (Table 4). We also excluded those studies with an unclear risk of bias, which reduced the effect size.
We performed funnel plot analysis to estimate the risk of bias by comparing the effect of the intervention effect with each study’s size or precision. In the setting of an intervention effect, and symmetry of the funnel plot, a low risk of publication bias was suggested.
Please see Figure 6 for more details.
6.
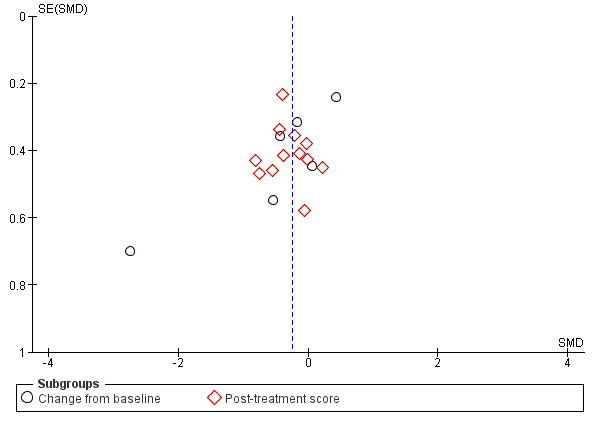
Funnel plot of comparison: 1. Opioids versus placebo, outcome: 1.1 Breathlessness.
Secondary outcomes
Quality of life
Four studies examined the effects on quality of life. Poole 1998 compared morphine to placebo and used the Chronic Respiratory Disease Questionnaire. The study found no difference in the total score. However, there was a statistically significant difference in the mastery domain scores that favoured placebo, and the study authors suggested that participants may feel less in control when using morphine. This was the only study that presented data that we were able to use for meta‐analysis (Analysis 8.1). Eiser 1991 compared morphine to placebo and found no statistically significant difference in well being. Abernethy 2003 compared oral morphine to placebo and reported that there was no significant difference in overall sense of well being, although these data were not reported. Oxberry 2011 comparing oral morphine, oral oxycodone, and placebo in heart failure participants and reported no difference in the SF‐12, a well‐validated 12 question survey for quality of life, although we did not present these data.
8.1. Analysis.
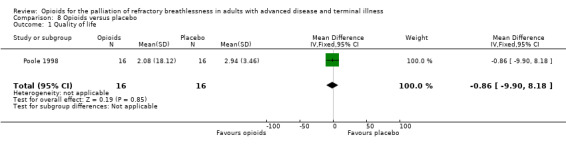
Comparison 8 Opioids versus placebo, Outcome 1 Quality of life.
Exercise tolerance
Fourteen studies examined exercise tolerance, including 12 studies that compared opioids versus placebo.
Please see Figure 7 for more details.
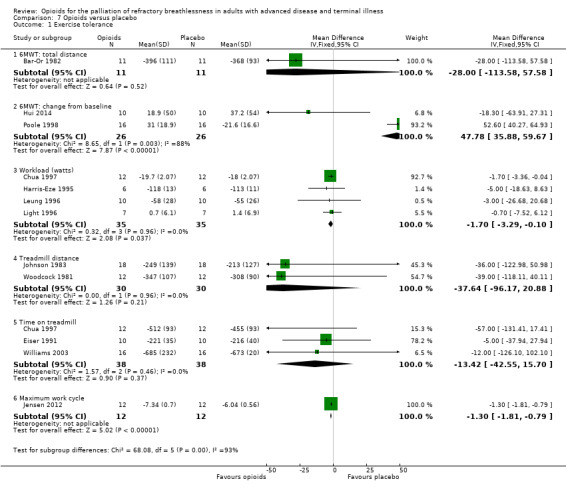
7.
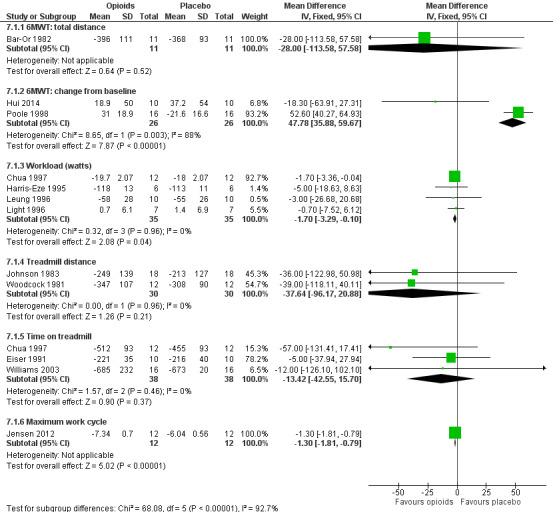
Meta‐analysis demonstrated a significant improvement in maximal workload achieved (four studies, 35 participants; MD −1.70; 95% CI −3.29 to −0.10; P = 0.04), and in maximum work cycle (one study, 12 participants; MD −1.30; 95% CI −1.81 to −0.79; P < 0.00001) (Analysis 7.1).
7.1. Analysis.
Comparison 7 Opioids versus placebo, Outcome 1 Exercise tolerance.
There was an improvement of change in treadmill distance of 37.64 m that favoured opioids (two studies, 30 participants; MD −37.64, 95% CI −96.17 to 20.88; P = 0.21), and an improvement in time on treadmill of 13.42 seconds that favoured opioids (three studies, 38 participants; MD −13.42, 95% CI −42.55 to 15.70; P = 0.37).
There were conflicting results for the effect of opioids on the effects of the 6MWT. The change from baseline distance demonstrated a benefit that favoured placebo (two studies, 26 participants; MD 47.78, 95% CI 35.88 to 59.67; P < 0.00001, with significant heterogeneity, I² statistic = 88%, P = 0.003), which was largely due to one study (Poole 1998). The effect for the 6MWT total distance was as follows; one study, 11 participants; MD −28.00, 95% CI −113.58 to 57.58; P = 0.52) (Analysis 7.1).
Light 1996 assessed minute ventilation in morphine compared with promethazine, and found no difference in workload or minute ventilation.
Rice 1987 assessed a 12‐minute walk test in codeine compared to promethazine, and found no statistical significance.
There were no long‐term data presented for exercise tolerance.
We graded the evidence as of low methodological quality due to the small size of the included trials, significant heterogeneity across trials, and inconsistency of outcome measurements.
Performance status
No studies examined performance status.
Pulse oximetry
Twelve studies measured pulse oximetry, but all found no difference between opioid and placebo treatment.
Arterial blood gas analysis and end tidal carbon dioxide measurement
Only three studies performed arterial blood gas analysis (Bar‐Or 1982; Eiser 1991; Chua 1997). All found no significant difference in arterial oxygen or carbon dioxide levels. Four studies performed end tidal carbon dioxide analysis (Bar‐Or 1982; Harris‐Eze 1995; Light 1996; Chua 1997). Three studies found no significant difference, and one study found a statistically significant increase in end tidal carbon dioxide levels in the dihydrocodeine group compared to placebo (Chua 1997).
Adverse events
Adverse events from opioids are well recognised, and may be part of the practitioner's reluctance to prescribe in the setting of breathlessness. Only 14 studies reported any adverse events, and only nine studies reported data that we were able to use in meta‐analyses (Analysis 9.1; Analysis 9.2; Analysis 9.3). The adverse effects reported included drowsiness, nausea and vomiting, and constipation. In those studies, participants who were 4.73 times more likely to experience nausea and vomiting compared to placebo, three times more likely to experience constipation, and 2.86 times more likely to experience drowsiness. Twelve participants across all studies stopped the trial early due to adverse events in the treatment arm (one participant due to drowsiness and five due to nausea and vomiting (Bar‐Or 1982); three participant s withdrew due to morphine related side effects (Abernethy 2003); two participants withdrew from Oxberry 2011 due to bowel and bladder symptoms; and one participant withdrew from Poole 1998 due to severe constipation).
9.1. Analysis.
Comparison 9 Opioids versus placebo, Outcome 1 Adverse events: constipation.
9.2. Analysis.
Comparison 9 Opioids versus placebo, Outcome 2 Adverse events: nausea and vomiting.
9.3. Analysis.
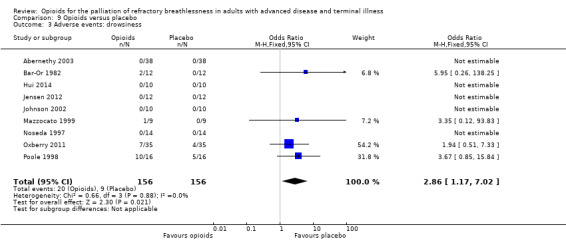
Comparison 9 Opioids versus placebo, Outcome 3 Adverse events: drowsiness.
Mortality
Three participants died during the Noseda 1997 study. However, the study authors did not believe that this was related in any way to the study interventions. All of these participants had advanced disease and the deaths were likely to be expected.
Quality of the evidence
We assessed the quality of evidence from the included studies as of low to very low quality. We only included RCTs, although some studies provided an incomplete description of randomisation. We aimed to included double blind RCTs, however two studies were only single blinded. There was inconsistency in the reporting of outcome measures. We analysed the data according to a fixed‐effect model due to small study bias, and for some outcomes heterogeneity was high. There was a risk of imprecise results due to the low numbers of included participants. For these reasons we downgraded the quality of the evidence to low for breathlessness post‐treatment score, and very low for breathlessness change from baseline.
Please see the 'Summary of findings' tables for more information (Table 1; Table 2; Table 3).
Discussion
Summary of main results
This Cochrane review demonstrates low quality evidence for a small clinically significant effect for oral and subcutaneous opioids compared to placebo in the relief of breathlessness. There is insufficient evidence at this level to suggest that nebulised opioids are more effective than placebo in relieving breathlessness. This may be explained by the difference in pharmacodynamics of opioids. Not all opioids can be administered via inhaled or intranasal modes. In order to be absorbed by the intranasal or intraoral mucosa, opioids need to be lipophilic. Fentanyl fulfils this criterion as it is highly lipid soluble, whereas morphine is hydrophilic. Therefore, morphine is poorly absorbed via this route (Bausewein 2008).
We found that opioids are inferior for the relief of breathlessness when compared to midazolam, based on one study (Navigante 2010). This is consistent with the Cochrane Review by Simon 2010, which demonstrated a non‐significant beneficial effect that favoured benzodiazepines compared to opioids.
Overall completeness and applicability of evidence
The strength of the evidence available is limited by the small sample size of the studies, which involved six to 63 participants with a mean of 19 participants per study, and by the variability of outcome measures utilised, which limits meta‐analysis.
Quality and applicability of evidence is also limited in that studies measured the response to intervention shortly after administration, in a crossover study design, often conducted on two consecutive days with the intervention on one day and control the next. Few studies involved multiple doses or titration according to the participants' individual response.
We analysed the data as if all the included studies were parallel group trials, due to lack of paired data available. This may introduce a unit‐of‐analysis error, the confidence intervals (CIs) may be too wide, and the data may be under weighted, thus disguising clinically important heterogeneity (Elbourne 2002). We analysed the data using a fixed‐effect model due to concerns regarding small‐study bias, and this may underestimate clinically important differences.
The lack of evidence for nebulised studies may be influenced by the lack of consistency between studies, as nebuliser devices between different studies were not randomised, and particle size and distance from device to mouth varied. Therefore the total amount of opioid reaching the lungs may have varied.
The conclusions we can draw from this review are limited to the dosages used in the included studies. The included studies used a wide range of doses, thus an enhanced effect may be seen with higher doses. However, the risk of adverse events, including drowsiness, may also increase.
The studies on breathlessness used a variety of different outcome measures, including the Borg and visual analogue scale (VAS). The point at which studies measured the data also varied, and may or may not have included an exercise test. The studies reported data variably as either a change from baseline or post‐treatment change. This variability in data reporting causes difficulty in interpretation, therefore it is recommended that future studies standardise outcome measures.
Less than half of the studies included assessed pulse oximetry, and only three studies assessed arterial oxygen and carbon dioxide levels. Only one study found a difference in end tidal carbon dioxide levels (Chua 1997). It is unlikely that opioids have a significant impact on oxygen levels in the management of breathlessness.
Not all studies reported adverse outcomes. The most common symptom was drowsiness, followed by nausea and vomiting, and constipation. Adverse effects caused some participants to withdraw from the trial. These trials used high doses of morphine at 20 mg oral morphine daily or more. Further research is required to determine if the same improvement of breathlessness can be achieved at lower doses with a reduction in adverse events.
Very few studies included data on quality of life. This is an important omission as the participants in these studies were all symptomatic, thus quality of life data are particularly relevant.
Quality of the evidence
We assessed the quality of the evidence presented in this Cochrane review using GRADEpro Guideline Development Tool (GDT) software (GradePro 2015) and presented it in the 'Summary of findings' tables (Table 1; Table 2; Table 3). We rated the quality of the evidence using the following grades: very low, low, moderate, or high. Very low quality evidence means we are uncertain about the results. High quality evidence means we are very certain about the results. For this review, we found the evidence to be of low quality.
We only included randomised controlled trials (RCTs), however 17 out of 26 studies had an unclear risk of bias overall, mostly due to inadequate reporting of randomisation and allocation sequence. We aimed to included double blind RCTs, however two studies were only single blinded.
There was significant heterogeneity between studies for the main outcome of breathlessness (I² statistic =74%, P = 0.0009), which may be explained by the small sample size and inconsistency with outcome measures. Therefore, these results should be interpreted with caution.
There was a risk of imprecise results due to the low numbers of included participants.
For these reasons we downgraded the quality of the evidence to low for breathlessness post‐treatment score, and very low for breathlessness change from baseline. Further research using larger studies for longer duration, with consistent outcome measures, and adequate randomisation and blinding, is likely to have an important impact on our confidence in the estimate of effect and likely to change the estimate.
Potential biases in the review process
We conducted this review in accordance with established Cochrane standards. Two review authors independently screened search results and resolved discrepancies by discussion and consensus. We did not restrict the literature search by language and we translated two studies into English to determine suitability for inclusion. Also we contacted the study authors where it was unclear if a study met the inclusion criteria, though none of the study authors responded.
Publication bias is possible, whereby a failure to identify unpublished negative trials could have lead to an overestimation of the effect of opioids for breathlessness.
We analysed the data as if all the included studies were parallel group trials, due to lack of paired data available. This may introduce a unit‐of‐analysis error, the CIs may be too wide, and the data may be under‐weighted, thus disguising clinically important heterogeneity (Elbourne 2002).
Agreements and disagreements with other studies or reviews
This review builds on the review by Jennings 2001 "Opioids for the palliation of breathlessness in advanced disease and terminal illness", which concluded that there is evidence in favour of use for oral or parenteral opioid drugs to treat breathlessness, and there is no supporting evidence to support the use of nebulised opioids for the treatment of breathlessness.
This Cochrane review included a further 11 studies, although we chose not to include all studies previously included by Jennings 2001 due to concerns regarding lack of randomisation.
We also undertook further subgroup analyses, and found a particular benefit using morphine.
This Cochrane review also examined the use of opioids compared to other interventions. Our review included one study, Navigante 2010, which found that opioids were inferior when compared to intravenous midazolam for the relief of breathlessness. This is consistent with the Cochrane review by Simon 2010, which compared benzodiazepines to any other intervention, and found a small effect that favoured benzodiazepines over opioids in one study, with an overall small effect size.
Authors' conclusions
Implications for practice.
For people with breathlessness in advanced disease or terminal illness
There is low quality evidence showing benefit for the use of oral opioids for the relief of breathlessness in adults with advanced disease and terminal illness.
Based on this evidence, it is possible that opioids lead to a short‐term increase in exercise capacity.
There is no evidence to support the use of nebulised opioids for the treatment of breathlessness.
For clinicians
There is low quality evidence showing benefit for the use of oral opioids for the relief of breathlessness in some adults with advanced disease and terminal illness.
Based on this evidence, it is possible that opioids lead to a short‐term increase in exercise capacity.
It is difficult to draw firm conclusions about the clinical significance of the pooled estimate of treatment effect in our meta‐analysis as we used standardised mean difference (SMD) values to combine studies due to the lack of standardised outcome measures but the magnitude of the treatment effect appears small.
There is no evidence to support the use of nebulised opioids for the treatment of breathlessness.
For policy makers
There is low quality evidence showing benefit for the use of oral opioids for the relief of breathlessness in adults with advanced disease and terminal illness.
Based on this evidence, it is possible that opioids lead to a short‐term increase in exercise capacity.
It is difficult to draw firm conclusions about the clinical significance of the pooled estimate of treatment effect in our meta‐analysis as we used the SMD to combine studies due to the lack of standardised outcome measures. However, the magnitude of the treatment effect appears small.
There is no evidence to support the use of nebulised opioids for the treatment of breathlessness.
For funders
There is low quality evidence showing benefit for the use of oral opioids for the relief of breathlessness in adults with advanced disease and terminal illness.
Based on this evidence, it is possible that opioids lead to a short‐term increase in exercise capacity.
It is difficult to draw firm conclusions about the clinical significance of the pooled estimate of treatment effect in our meta‐analysis as we used the SMD to combine studies due to the lack of standardised outcome measures but the magnitude of the treatment effect appears small.
There is no evidence to support the use of nebulised opioids for the treatment of breathlessness.
Given the small sample sizes of these studies, larger trials may assist in providing more robust evidence for opioids for breathlessness.
Implications for research.
Given the small sample sizes of these studies, larger trials including more than 50 participants per treatment arm may assist in providing more robust evidence for opioids for breathlessness.
Randomised, parallel group trials of longer duration (i.e. greater than one day; for several weeks) are likely to be more clinically appropriate.
Effective dosing schedules should be elucidated to determine maximum effect with minimum side effects.
Standardised outcome measures should be used including consistent fixed‐point outcome measures of breathlessness, and quality of life measures.
Feedback
Feedback, 20 April 2017
Summary
Date of Submission: 20‐Apr‐2017
Name: David Currow
Email Address: david.currow@uts.edu.au
Affiliation: ImPACCT Improving Palliative, Aged and Chronic Care through Clinical and Translational Research University of Technology
Role: Professor of Palliative Medicine
Comment: We are writing to raise concerns about significant methodological shortcomings in this Cochrane Review. Given the authoritative nature of Cochrane and the attention paid to it by clinicians and policy makers, we think these concerns need to be addressed urgently. The main limitation is that the cross‐over design of eleven of the twelve studies included in the meta‐analysis was not taken into account in accordance with the published recommended approaches outlined in the Cochrane Handbook. Further, despite acknowledging significant differences between studies, a fixed rather than a random effects model was used which does not account for variations in the true effect between studies. When re‐analysed [1], the precision is greater and the findings of the meta‐analysis are both clinically and statistically significant. The systematic review introduced an additional criterion to judge the quality of the evidence based on the sample size alone. Although “other” criteria can be added, a seemingly arbitrary sample size level at 50 participants was determined to pose a high risk of bias. No rationale for this was provided, and whether or not the studies were adequately powered was not considered. Again, no account was taken as to whether this was a cross over study when making this arbitrary decision. We suggest that this review should be urgently reconsidered and updated. As it stands, this review will mislead clinicians about the role of regular, low dose opioids in the safe, symptomatic relief of chronic breathlessness, a major cause of avoidable suffering globally.
Authors: Assoc Professor Magnus Ekström, Dr Sabrina Bajwah, Professor Martin Bland, Professor David Currow, Dr Jamilla Hussain, Professor Miriam Johnson.
1. Ekstrom M, Bajwah S, Bland M, et al. One evidence base; three stories: do opioids relieve chronic breathlessness? Thorax Online First. Published April 4 2017: http://thorax.bmj.com/content/early/2017/04/04/thoraxjnl‐2016‐209868.
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
Reply
Thank you for your feedback letter for the Cochrane Review: Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness, and we also note your research letter published in Thorax entitled: One evidence base; three stories: do opioids relieve chronic breathlessness?1
The opioids for breathlessness data is complex, and fraught with statistical difficulties in the interpretation and summation of the data.
One of the limitations includes the use of crossover studies. This can be an appropriate way to assess short term interventions. The Cochrane Handbook (Higgins 2011) outlines several methods to incorporate crossover data into meta‐analyses. In using the data as if it was a parallel study, the limitations should be acknowledged, in that it can give rise to a unit of analysis error whereby confidence intervals may be wide, and the overall effect is under‐estimated.
An alternative method is to calculate correlation co‐efficients (which describe the ratio of between‐patient standard deviation with the within patient variation) to impute a corrected standard error. Some included studies provide appropriate data to calculate this (standard error of the differences), or a corrected standard error can be imputed using “borrowed” correlation co‐efficients from other studies.
In this Cochrane Review we used the former method. Using the internal report Meta‐analysis of crossover trials in a systematic review of opioids for breathlessness: Report to the Cochrane Pain, Palliative Care and Supportive Care Group: 2001 by Julian Higgins (Cochrane statistician), we present in the sensitivity analysis an alternative meta‐analysis using correlation co‐efficients and corrected standard errors. The data are presented using standardised mean differences, as discussed below.
The sensitivity analysis presented in Effects of interventions, accounting for appropriate use of crossover data, demonstrates a change from baseline SMD ‐0.14 (95% CI ‐0.40 to 0.13) and a post treatment score SMD ‐0.55 (95% CI ‐0.76 to ‐0.35); see Figure 5. This is not too dissimilar to our previous SMD ‐0.32 (‐0.53 to ‐0.10) for post treatment dyspnoea scores and SMD ‐0.11 (‐0.40 to 0.19) change from baseline scores, and is consistent with our previous conclusion there is a significant but small effect size for the use of opioids for breathlessness.
Ekstrom et al1 raise concerns regarding the use of a fixed effects versus a random effects model. Based on the assumption that studies would have a small sample size we chose a priori to use a fixed effects model. As Higgins 2011 describes: a random effects model will award relatively more weight to smaller studies because smaller studies are more informative for learning about the distribution of the effects across studies than for learning about an assumed common intervention effect. Therefore, if a random effects model is inappropriately applied, in particular, if the results of small studies are systematically different to the results of larger ones, the random effects model can inappropriately exacerbate the effects of any bias (Kjaergard 2001).
The choice and rationale for a fixed effects model was outlined in advance in our protocol. This protocol was peer reviewed prior to publication. Consistent with Higgins 2011, we presented both a fixed effects and random effects model in the sensitivity analysis, and found no differences in effect. Following additional sensitivity analysis as described above, there remains very little difference between the fixed effects model in the change from baseline scores (SMD ‐0.14 (95% CI ‐0.40 to 0.13)) and the random effects model (SMD ‐0.21 (95% CI 0.21 (95% CI ‐0.55 to 0.12)), and in the post treatment score fixed effects model (SMD ‐0.55 (95% CI ‐0.76 to ‐0.35)) and random effects model (SMD ‐0.69 (95% CI ‐1.08 to ‐0.29)).
A second limitation from the opioids for breathlessness data is the use of different scales to measure the same outcome (eg VAS, NRS, Borg), but measure different lengths, different extremes, and different gradations of intensity. In order to combine data on different scales, standardised mean differences are required, which is calculated by dividing a pooled estimate of between‐patient standard deviation. However, combining this between‐patient standard deviation with the within patient variation imputed from the corrected standard error described as above to incorporate crossover trials is not always possible from the available data. It is difficult to interpret the resulting standardised mean differences from cross‐over trials.
Transforming the data as described above works if the data is reported as either change from baseline or post treatment scores, however it is unclear if it is also appropriate to combine them in a single meta‐analysis and to combine them in a single meta‐analysis using standardised mean difference (SMD).
Higgins 2011 states that post treatment scores can be combined with change from baseline scores when using an unstandardised mean difference, however, they should not be combined as a standardised mean difference using the standard deviation of the change scores (as these are not the same units as the standard deviation of the final scores). Therefore, it makes it difficult to combine data from different scales as outlined above, as well as combining post treatment and change from baseline scores in one single meta‐analysis. Originally, we separated post treatment and change from baseline scores. In subsequent sensitivity analyses performed in response to the feedback, we combined these but separated by scale, in Analysis 12.1.
12.1. Analysis.
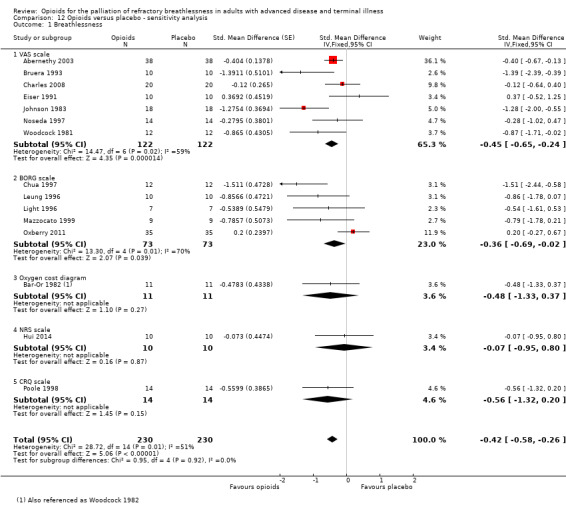
Comparison 12 Opioids versus placebo ‐ sensitivity analysis, Outcome 1 Breathlessness.
Ekstrom et al1 discussed at length the primary outcome of breathlessness, but they did not take into account adverse events or multidimensional assessment of the use of opioids. We noted increased adverse events including drowsiness, nausea, and constipation, as well as a significant difference in the mastery domain scores in one included trial, suggesting that participants may feel less in control when using morphine. We believe it is important to consider the evidence in its entirety, rather than focusing on only one effect size score.
Ekstrom et al1 have suggested that we downgraded the quality of evidence based on concerns about study size alone. We used GRADE methodology to rate the quality of the evidence and our decision to downgrade the quality of the evidence was based on the fact that more than 50% of included trials did not report on allocation concealment, blinding of participants or personnel, or blinding of outcome assessment. This is potentially a serious limitation when the primary outcome is subjective as in this review. We acknowledge that study size per se does not influence the internal validity of trial results and that some of the trials included in the review were designed with sufficient statistical power.
The ‘size bias’ criterion was suggested during the editorial review of our manuscript as there is empiric evidence that study size may be a surrogate marker of trial quality when the reporting on aspects of trial quality is poor (Kjaergard 2001). In other fields, small study effects have been shown to distort the results of meta‐analyses (Nüesch 2010). Many of the papers included in the review did not provide sufficient information to adequately assess trial quality, and because all the studies included were small in relative terms (< 50 participants per trial) we believe that it is important to highlight that the quantitative data synthesis is based on the pooling of relatively small studies.
Ekstrom et al1 have asserted in their re‐analysis that opioids are associated with both a statistically significant and clinically significant reduction in breathlessness. In their meta‐analysis the point estimate was 0.8 on a NRS which is just at the threshold for clinical significance based on work by Johnson et al2, however they did not present the 95% confidence interval for the point estimate using the NRS and it likely that this interval includes values that are not clinically significant, potentially reducing the level certainty in the estimate of effectiveness.
In order to interpret our results in a more meaningful context, we converted this standard mean difference to a mean difference on a commonly used dyspnoea scale; the VAS 100 mm scale. For chronic refractory breathlessness, the MCID is in the order of ‐9 mm on a 100 mm VAS (validated in COPD and heart failure patients)3, indicating that in the chronic setting, relatively small reductions in breathlessness may be perceived as beneficial by patients. Using the post treatment standard deviation from a large study (Abernethy 2003), we calculated an effect size of change from baseline as ‐3.36 (95% CI ‐9.60 to 3.12) and a post treatment score as ‐13.2 (95% CI ‐18.24 to ‐8.4) on a 100 mm VAS scale. The post treatment score meets the clinically important difference threshold but the lower limit of the confidence interval falls just below this threshold.
We included the study by Woodcock 1982, but this is more correctly referenced in our review as Bar‐Or 1982 (Bar‐Or 1982). We included Johnson 2002 in the review, but excluded it from the meta‐analyses as the data was not normally distributed and medians and interquartile ranges cannot be imputed into a meta‐analysis, consistent with the Cochrane Handbook. Although Ekstrom et al1 commented that study selection should align to predefined eligibility criteria with reasons for exclusion stated to minimise selection bias, our studies were selected according to a published protocol with study types, inclusion and exclusion criteria, which Ekstrom et al1 did not do in their commentary.
While we value the opinion provided by Ekstrom et al1, the additional sensitivity analyses reported here do not change our review conclusions. There is some small, low quality evidence that shows benefit for the use of parental or oral opioids to palliate breathlessness in the short term. The magnitude of this benefit is at best modest and given the potential adverse events and the lack of any evidence suggesting an improvement in overall quality of life, longer‐term studies with multi‐dimensional scales are required to ascertain whether any benefits outweigh the potential long term risks4, particularly where opioids are being used in those with chronic stable disease in the outpatient setting.
Acknowledgements: We thank Christopher Cates for his extensive input in this sensitivity analysis and comments on this letter, Kerry Dwan, Toby Lasserson and the Statistical Methods Group, and Julian Higgins for his report on the interpretation of this data.
References:
1Ekström M, Bajwah S, Bland JM, Currow D, Hussain J, Johnson M. One evidence base; three stories: do opioids relieve chronic breathlessness? Thorax 2017
2 Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Clinically important differences in the intensity of chronic refractory breathlessness. Journal of Pain and Symptom Management. 2013 Dec;46(6):957‐63.
3 Johnson M, Hui D, Currow D. Opioids, Exertion, and Dyspnea: A Review of the Evidence. American Journal of Hospice and Palliative Medicine 2016;33(2):194‐200.
4 Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop.Annals of Internal Medicine. 2015 Feb 17;162(4):276‐86. doi: 10.7326/M14‐2559.
Contributors
As Hayley Barnes (review Contact Person) is the PaPaS Feedback Editor, the feedback and author response was co‐ordinated by PaPaS Managing Editor Anna Erskine and Co‐ordinating Editor Christopher Eccleston.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2014 Review first published: Issue 3, 2016
| Date | Event | Description |
|---|---|---|
| 14 May 2019 | Amended | Final amendments to analyses. |
| 1 May 2019 | Amended | We addressed errors in data extraction and updated our meta‐analyses accordingly. |
| 8 February 2018 | Review declared as stable | See Published notes |
| 23 October 2017 | Amended | Minor change to Feedback |
| 21 August 2017 | Feedback has been incorporated | See Feedback. |
| 1 August 2016 | Amended | Minor changes to text, e.g. in Abstract, Results, and Summary of findings table. |
| 8 June 2016 | Amended | Abstract results amended |
Notes
2018
A restricted search in January 2018 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in early 2019. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
2019
A restricted search in May 2019 identified five potentially relevant studies: four small published studies (Abdallah 2017 (20 participants); Hui 2016 (20 participants); Hui 2017 (20 participants); and Janowiak 2017 (10 participants)), one larger study (Minchom 2016 (173 participants)), and two large ongoing studies (Currow 2017; Verberkt 2016). However, we judged that the published studies were unlikely to change the conclusions. We have now stabilised this review pending the publication of the two ongoing studies, following discussion with the authors and editors. The review will be re‐assessed for updating in 2021. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
References
Abdallah SJ, Wilkinson‐Maitland C, Saad N, Li PZ, Smith BM, Bourbeau J, Jensen D. Effect of morphine on breathlessness and exercise endurance in advanced COPD: a randomised crossover trial. Eur Respir J. 2017 Oct 19;50(4) (20 participants)
Hui D, Kilgore K, Park M, Williams J, Liu D, Bruera E. Impact of Prophylactic Fentanyl Pectin Nasal Spray on Exercise‐Induced Episodic Dyspnea in Cancer Patients: A Double‐Blind, Randomized Controlled Trial. J Pain Symptom Manage. 2016 Oct;52(4):459‐68 (26 participants)
Hui D, Kilgore K, Frisbee‐Hume S, Park M, Liu D, Balachandran DD, Bruera E. Effect of Prophylactic Fentanyl Buccal Tablet on Episodic Exertional Dyspnea: A Pilot Double‐Blind Randomized Controlled Trial. J Pain Symptom Manage. 2017 Dec;54(6):798‐805. doi: 10.1016/j.jpainsymman. 2017 (20 participants)
Janowiak P, Krajnik M, Podolec Z, Bandurski T, Damps‐Konstańska I, Sobański P, Currow DC, Jassem E. Dosimetrically administered nebulized morphine for breathlessness in very severe chronic obstructive pulmonary disease: a randomized, controlled trial. BMC Pulm Med. 2017 Dec 11;17(1):186 (10 participants)
Minchom A, Punwani R, Filshie J, Bhosle J, Nimako K, Myerson J, Gunapala R, Popat S, O'Brien ME. A randomised study comparing the effectiveness of acupuncture or morphine versus the combination for the relief of dyspnoea in patients with advanced non‐small cell lung cancer and mesothelioma. Eur J Cancer. 2016 (173 participants)
Ongoing studies
Currow D, Watts GJ, Johnson M, McDonald CF, Miners JO, Somogyi AA, Denehy L, McCaffrey N, Eckert DJ, McCloud P, Louw S, Lam L, Greene A, Fazekas B, Clark KC, Fong K, Agar MR, Joshi R, Kilbreath S, Ferreira D, Ekström M; Australian national Palliative Care Clinical Studies Collaborative (PaCCSC). A pragmatic, phase III, multisite, double‐blind, placebo‐controlled, parallel‐arm, dose increment randomised trial of regular, low‐dose extended‐release morphine for chronic breathlessness: Breathlessness, Exertion And Morphine Sulfate (BEAMS) study protocol. BMJ Open. 2017 Jul 17;7(7):e018100 (aim: 171 participants)
Verberkt CA, van den Beuken‐van Everdingen MH, Franssen FM, Dirksen CD, Schols JM, Wouters EF, Janssen DJ. A randomized controlled trial on the benefits and respiratory adverse effects of morphine for refractory dyspnea in patients with COPD: Protocol of the MORDYC study. Contemp Clin Trials. 2016 Mar;47:228‐34 (aim: 124 participants)
Acknowledgements
We acknowledge the support of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Group, in particular Anna Erskine, and Dr Adrian Tookman, Ann O’Mara, Chris Eccleston, Newton Opiyo, and Toby Lasserson who provided peer review comments.
We thank Christopher Cates for his extensive input in this sensitivity analysis and Julian Higgins for his report in the interpretation of this data.
This project was supported by the National Institute for Health Research, via Cochrane Incentive Award funding to the Cochrane PaPaS Group, Award Reference number 14/175/05. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: the views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (the Cochrane Library)
#1 MeSH descriptor: [Analgesics, Opioid] this term only
#2 (papaveretum or morphine or fentanyl or hydromorphone or oxycodone or pentazocine or methadone or opioid* or opiate* or codeine or dextromoramide or OTFC or diamorphine or dihydrocodeine or dextropropoxyphene or meptazinol or sufentanil or alfentanil or remifentanil or nalbuphine or meptazinol or dipipanone or pethidine or tramadol or buprenorphine):ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Dyspnea] this term only
#5 (dyspnoea* or breathless* or (short* near/2 breath*)):ti,ab,kw (Word variations have been searched)
#6 #4 or #5
#7 #3 and #6
MEDLINE (OVID)
1 Analgesics, Opioid/
2 (papaveretum or morphine or fentanyl or hydromorphone or oxycodone or pentazocine or methadone or opioid* or opiate* or codeine or dextromoramide or OTFC or diamorphine or dihydrocodeine or dextropropoxyphene or meptazinol or sufentanil or alfentanil or remifentanil or nalbuphine or meptazinol or dipipanone or pethidine or tramadol or buprenorphine).tw.
3 Dyspnea/
4 (dyspnoea* or breathless* or (short* adj2 breath*)).tw.
5 or/1‐2
6 or/3‐4
7 5 and 6
8 randomised controlled trial.pt.
9 controlled clinical trial.pt.
10 randomized.ab.
11 placebo.ab.
12 drug therapy.fs.
13 randomly.ab.
14 trial.ab.
15 or/8‐14
16 exp animals/ not humans.sh.
17 15 not 16
18 7 and 17
EMBASE (OVID)
1. Analgesics, Opioid/
2. (papaveretum or morphine or fentanyl or hydromorphone or oxycodone or pentazocine or methadone or opioid* or opiate* or codeine or dextromoramide or OTFC or diamorphine or dihydrocodeine or dextropropoxyphene or meptazinol or sufentanil or alfentanil or remifentanil or nalbuphine or meptazinol or dipipanone or pethidine or tramadol or buprenorphine).tw.
3. Dyspnea/
4. (dyspnoea* or breathless* or (short* adj2 breath*)).tw.
5. or/1‐2
6. or/3‐4
7. 5 and 6
8. random$.tw.
9. factorial$.tw.
10. crossover$.tw.
11. cross over$.tw.
12. cross‐over$.tw.
13. placebo$.tw.
14. (doubl$ adj blind$).tw.
15. (singl$ adj blind$).tw.
16. assign$.tw.
17. allocat$.tw.
18. volunteer$.tw.
19. Crossover Procedure/
20. double‐blind procedure.tw.
21. Randomized Controlled Trial/
22. Single Blind Procedure/
23. or/8‐22
24. (animal/ or nonhuman/) not human/
25. 23 not 24
26. 7 and 25
Web of Science (ISI)
#8 #7 AND #3
Indexes=SCI‐EXPANDED, A&HCI, SSCI, CPCI‐SSH, CPCI‐S Timespan=All years
#7 #6 OR #5 OR #4
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
#6 TOPIC: ((((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*))))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
#5 TOPIC: (((controlled clinical trial OR controlled trial OR clinical trial OR placebo)))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
#4 TOPIC: (((randomised OR randomised OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomised controlled trial)))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
#3 #2 AND #1
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
#2 TS=((dyspnoea* or breathless* or (short* NEAR/2 breath*)))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
#1 TOPIC: ((papaveretum or morphine or fentanyl or hydromorphone or oxycodone or pentazocine or methadone or opioid* or opiate* or codeine or dextromoramide or OTFC or diamorphine or dihydrocodeine or dextropropoxyphene or meptazinol or sufentanil or alfentanil or remifentanil or nalbuphine or meptazinol or dipipanone or pethidine or tramadol or buprenorphine))
Indexes=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan=All years
CINAHL (EBSCO)
S17 S7 AND S16
S16 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
S15 (allocat* random*)
S14 (MH "Quantitative Studies")
S13 (MH "Placebos")
S12 placebo*
S11 (random* allocat*)
S10 (MH "Random Assignment")
S9 (Randomi?ed control* trial*)
S8 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S7 (S3 AND S6)
S6 S4 OR S5
S5 (dyspnoea* or breathless* or (short* N2 breath*))
S4 (MH "Dyspnea")
S3 S1 OR S2
S2 (papaveretum or morphine or fentanyl or hydromorphone or oxycodone or pentazocine or methadone or opioid* or opiate* or
codeine or dextromoramide or OTFC or diamorphine or dihydrocodeine or dextropropoxyphene or meptazinol or sufentanil or alfentanil
or remifentanil or nalbuphine or meptazinol or dipipanone or pethidine or tramadol or buprenorphine)
S1 (MH "Analgesics, Opioid")#1 MeSH descriptor: [Analgesics, Opioid] this term only
#2 (papaveretum or morphine or fentanyl or hydromorphone or oxycodone or pentazocine or methadone or opioid* or opiate* or codeine or dextromoramide or OTFC or diamorphine or dihydrocodeine or dextropropoxyphene or meptazinol or sufentanil or alfentanil or remifentanil or nalbuphine or meptazinol or dipipanone or pethidine or tramadol or buprenorphine):ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Dyspnea] this term only
#5 (dyspnoea* or breathless* or (short* near/2 breath*)):ti,ab,kw (Word variations have been searched)
#6 #4 or #5
#7 #3 and #6
Data and analyses
Comparison 1. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 18 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Change from baseline | 6 | 194 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.40, 0.19] |
| 1.2 Post‐treatment score | 12 | 338 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.53, ‐0.10] |
Comparison 2. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Morphine: change from baseline | 4 | 134 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.48, 0.24] |
| 1.2 Morphine: post‐treatment score | 6 | 188 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.62, ‐0.04] |
| 1.3 Dihydrocodeine: post‐treatment score | 4 | 107 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.80, ‐0.03] |
Comparison 3. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Hydromorphone: change from baseline | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.17, 0.65] |
| 1.2 Oral diamorphine: post‐treatment score | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.44, 2.44] |
| 1.3 Oxycodone: change from baseline | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.87, 1.03] |
Comparison 4. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fentanyl: change from baseline | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐2.50, 2.90] |
| 1.2 Fentanyl: post‐treatment score | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.76, 1.96] |
Comparison 5. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 COPD: change from baseline | 2 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐1.08, 0.10] |
| 1.2 COPD: post‐treatment score | 8 | 262 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.48, 0.01] |
| 1.3 Heart failure: change from baseline | 1 | 70 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.04, 0.90] |
| 1.4 Heart failure: post‐treatment score | 1 | 24 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.66, 0.02] |
| 1.5 Interstitial lung disease | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐1.19, 1.07] |
| 1.6 Cancer‐related dyspnoea: change from baseline | 3 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.89, 0.06] |
| 1.7 Cancer‐related dyspnoea: post‐treatment score | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.67, 0.16] |
Comparison 6. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 15 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oral: change from baseline | 3 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.30, 0.44] |
| 1.2 Oral: post‐treatment score | 6 | 190 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐0.56, 0.02] |
| 1.3 Nebulised: change from baseline | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.80, 0.45] |
| 1.4 Nebulised: post‐treatment score | 5 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.56, 0.17] |
| 2 Breathlessness | 3 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐2.54 [‐5.09, 0.00] |
| 2.1 Subcutaneous: change from baseline | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐4.87, 0.27] |
| 2.2 Subcutaneous: post‐treatment score | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐19.0 [‐40.15, 2.15] |
Comparison 7. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exercise tolerance | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 6MWT: total distance | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐28.0 [‐113.58, 57.58] |
| 1.2 6MWT: change from baseline | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 47.78 [35.88, 59.67] |
| 1.3 Workload (watts) | 4 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.29, ‐0.10] |
| 1.4 Treadmill distance | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐37.64 [‐96.17, 20.88] |
| 1.5 Time on treadmill | 3 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐13.42 [‐42.55, 15.70] |
| 1.6 Maximum work cycle | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐1.81, ‐0.79] |
Comparison 8. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐9.90, 8.18] |
Comparison 9. Opioids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events: constipation | 9 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.63, 5.51] |
| 2 Adverse events: nausea and vomiting | 7 | 208 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.73 [1.73, 12.97] |
| 3 Adverse events: drowsiness | 9 | 312 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.86 [1.17, 7.02] |
Comparison 10. Morphine versus midazolam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [1.07, 2.93] |
Comparison 11. Codeine versus promethazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.83, 0.23] |
Comparison 12. Opioids versus placebo ‐ sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 15 | 460 | Std. Mean Difference (Fixed, 95% CI) | ‐0.42 [‐0.58, ‐0.26] |
| 1.1 VAS scale | 7 | 244 | Std. Mean Difference (Fixed, 95% CI) | ‐0.45 [‐0.65, ‐0.24] |
| 1.2 BORG scale | 5 | 146 | Std. Mean Difference (Fixed, 95% CI) | ‐0.36 [‐0.69, ‐0.02] |
| 1.3 Oxygen cost diagram | 1 | 22 | Std. Mean Difference (Fixed, 95% CI) | ‐0.48 [‐1.33, 0.37] |
| 1.4 NRS scale | 1 | 20 | Std. Mean Difference (Fixed, 95% CI) | ‐0.07 [‐0.95, 0.80] |
| 1.5 CRQ scale | 1 | 28 | Std. Mean Difference (Fixed, 95% CI) | ‐0.56 [‐1.32, 0.20] |
Comparison 13. Opioids versus placebo ‐ sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breathlessness | 15 | Std. Mean Difference (Fixed, 95% CI) | ‐0.42 [‐0.58, ‐0.26] | |
| 1.1 Change from baseline | 6 | Std. Mean Difference (Fixed, 95% CI) | ‐0.14 [‐0.42, 0.13] | |
| 1.2 Post treatment score | 9 | Std. Mean Difference (Fixed, 95% CI) | ‐0.57 [‐0.77, ‐0.36] |
13.1. Analysis.
Comparison 13 Opioids versus placebo ‐ sensitivity analysis, Outcome 1 Breathlessness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abernethy 2003.
| Methods | Randomised, double blind crossover study | |
| Participants | Opioid naive adults with dyspnoea despite treatment for reversible factors for end stage respiratory, cardiac or palliative conditions 48 randomised participants Mean age: 76 years 35/48 male Exclusion criteria were recent use of opioids, confusion, obtundation, adverse reactions to opioids, and history of substance misuse |
|
| Interventions | 20 mg oral sustained release morphine sulphate or placebo tablet Co‐interventions included coloxyl and senna |
|
| Outcomes | VAS dyspnoea scale Respiratory rate Sedation/obtundation Conspitation |
|
| Notes | Outcome measurement at day 4 and day 8 The study authors concluded that sustained release oral morphine at low dosage provides significant symptomatic improvement in refractory dyspnoea in the community setting Dose is equivalent to 20 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and blinding were co‐ordinated through the hospital pharmacy’s centralised service. This included computerised generation of the allocation sequence in random permuted blocks and blinded disbursement of medication. |
| Allocation concealment (selection bias) | Low risk | Randomisation and blinding were coordinated through the hospital pharmacy’s centralised service. This included computerised generation of the allocation sequence in random permuted blocks and blinded disbursement of medication. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. The placebo medication was identical in appearance and taste to the active medication; the bottle indicated which medication to take each day. Participants were unblinded to the investigators for serious adverse events only. There was no blinding for constipation. To accommodate this, the only investigator aware of the constipation was the study nurse (AM), who was not involved in the analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The placebo medication was identical in appearance and taste to the active medication; the bottle indicated which medication to take each day. Participants were unblinded to the investigators for serious adverse events only. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study assessed the primary outcome dyspnoea at the end of 4th day of the treatment period only. There were 10 withdrawals during treatment, with some due to adverse events. |
| Selective reporting (reporting bias) | Low risk | The study collected statistical details about some adverse events and some outcomes, such as overall well being and MRC Exercise tolerance scores, but did not report these in detail in the study report except to say there were no significant differences. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There were < 50 participants per treatment arm. |
Bar‐Or 1982.
| Methods | Randomised crossover study | |
| Participants | Participants with chronic obstructive pulmonary disease (COPD), otherwise not specifically stated 11 participants |
|
| Interventions | 30 mg three times daily (TDS) or 60 mg TDS oral dihydrocodeine Compared to oral placebo |
|
| Outcomes | Used oxygen cost diagram to measure breathlessness Six‐minute walking test (6MWT) Oxygen consumption Adverse events |
|
| Notes | Abstract, so limited information provided They concluded that there was a marked improvement in subjective disability when 30 mg TDS was given, but not 60 mg TDS Dose is equivalent to 18 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly state the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 16 randomised participants and 5 withdrawals due to adverse effects, all discussed but not likely related to the primary outcome measure. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There was a small sample size, and this study is likely to be at high risk of bias; there were < 50 participants per arm. |
Bruera 1993.
| Methods | Randomised crossover study | |
| Participants | Terminally ill participants with lung cancer or lung metastases or lymphangitis On supplemental oxygen 10 inpatient participants Normal cognitive function On regular subcutaneous morphine for pain Stable morphine dose for 5 days |
|
| Interventions | Morphine subcutaneous: mean dose 32 mg, standard deviation (SD) 12 mg: 50% higher than regular dose versus placebo 24 hour wash‐out period |
|
| Outcomes | VAS dyspnoea scale Oxygen saturations Respiratory rate |
|
| Notes | VAS at 60 minutes following injection used in results The study authors concluded that treatment is safe and effective |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not state the methods of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not state the methods of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not state the withdrawals, dropouts and protocol deviations. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting. |
| Other bias | Unclear risk | It is unclear whether or not there was other risk due to lack of detail in methods. |
| Size bias | High risk | There is size bias as there were only 10 participants in total, and < 50 per treatment arm. |
Charles 2008.
| Methods | Double blind, randomised crossover study | |
| Participants | Those with cancer experiencing dyspnoea English speaking, over 18 years, expected prognosis of 7 days, Minimental state exam (MMSE) > 24/30, experiencing dyspnoea with no reversible cause 20 participants Mean age: 69 years 11 males, 9 females |
|
| Interventions | 5 mg nebulised hydromorphone compared to 3 mL nebulised saline Co‐interventions were recorded but not stated |
|
| Outcomes | VAS dyspnoea scale Respiratory rate Pulse rate Oxygen saturation |
|
| Notes | The study measured outcomes at 10, 20, 30 and 60 minutes post‐dose The study authors concluded that there were no significant differences between treatments |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study randomised treatment order using a random number generator. To ensure double blinding, a non‐clinical research doctor prepared treatments composed of medications (commercially obtained) and blinding agents and checked with a non‐clinical nurse, neither of whom was involved with the care of the participant. A research nurse, who was unaware of the order sequence and who was not involved with the clinical care of the participant, subsequently administered pre‐prepared and randomised treatments. The non‐clinical research doctor held a master plan of the randomizations so that treatments could be unblinded in an emergency. There were no such emergencies over the course of the study. |
| Allocation concealment (selection bias) | Low risk | This was probably ok as the doctor responsible for randomisation was not involved in the care of the participants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. Oral medications were administered in orange juice. Blinding as above seems reasonable. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. The VAS was self administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 5 withdrawals. The study authors did not describe the reasons per initial group allocation but for the whole group. They stated that the reasons were not systematically related to treatments or order of treatments. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There was size bias as there were only 20 participants in total, and < 50 per treatment arm. |
Chua 1997.
| Methods | Randomised crossover study Exercise study Incremental treadmill test (modified Bruce protocol) |
|
| Participants | Stable chronic heart failure 12 male participants Mean age 65.5 years, (range 58 to 75 years) Average left ventricular ejection fraction (LVEF) 21.3% (range 8% to 39%) New York Heart Association (NYHA) functional class 2 and 3 No chest pain or inducible ischaemia during previous exercise testing No history pulmonary disease All patients on diuretics and angiotensin‐converting‐enzyme (ACE) inhibitors |
|
| Interventions | Dihydrocodeine 1 mg/kg vs placebo Tests took place on separate days |
|
| Outcomes | Modified Borg score (dyspnoea) Pulse Systolic blood pressure (BP) End‐tidal CO2 concentration % Partial pressure of oxygen (PaO2) Modified Borg score (fatigue) Hypoxic chemosensitivity Hypercapnic chemosensitivity Peak O2 consumption Ventilation to carbon dioxide output (VE‐VCO2 slope) Exercise duration |
|
| Notes | The study measured outcomes at 3 minutes, 6 minutes, and at peak exercise The study authors concluded that dihydrocodeine was associated with a reduction of exercise ventilation, an improvement in exercise tolerance and a decrease in breathlessness The dose is equivalent to an average of 6 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study assessed hypoxic and hypercapnic chemosensitivities in these participants 1 hour after the participants received placebo or dihydrocodeine (1 mg/kg body weight) in a randomised, double‐blind design on 2 separate days, followed by treadmill exercise testing on each occasion. The study gave placebo and dihydrocodeine in the form of a drink made up to 200 mL with bitter lemon, which was prepared by the Department of Pharmacy, Royal Brompton Hospital. |
| Allocation concealment (selection bias) | Unclear risk | There were no details other than the above. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was unclear whether or not the participants could tell the difference between the 2 drinks from description above. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not state the number of withdrawals, dropouts, or protocol deviations. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting. |
| Other bias | Unclear risk | It is unclear whether or not there was other risk due to the lack of detail in the methods. |
| Size bias | High risk | There was size bias as there were only 12 participants in total, and < 50 per treatment arm. |
Davis 1996.
| Methods | Randomised crossover study | |
| Participants | People with cancer 79 participants 34 men, 45 women Median age 60 years (range 20 to 81 years) |
|
| Interventions | Morphine single nebulised dose, range 5 mg to 50 mg or placebo Interventions made on separate days |
|
| Outcomes | VAS score for breathlessness Modified Borg score VAS scores for nausea and drowsiness |
|
| Notes | The study measured outcomes at 5, 30, 60, and 90 minutes and at 2, 3, 4, 6, 8, 12, and 24 hours post‐treatment The study authors concluded that there was no significant difference in response to nebulised morphine and normal saline Did not provide sufficient information or standard deviations to be included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was an abstract only and did not provide any details regarding randomisation. It is likely that a computer stratified randomisation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not report the methods of random sequence generation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not report the methods of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not report the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was crossover data for 66 participants only, and the study authors did not state the reason for this. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting. |
| Other bias | Unclear risk | It is unclear whether or not there was other risk due to lack of detail in methods. |
| Size bias | High risk | There were < 50 participants per treatment arm. |
Eiser 1991.
| Methods | Randomised crossover study Three 2‐week periods followed by exercise tests with no wash‐out interval Exercise testing (6MWT and treadmill test) at end of each study period 4 withdrawals (1 due to chest infection, 1 because of itching on diamorphine, 1 due to constipation on diamorphine, and 1 due to headache due to cerebral metastases) |
|
| Participants | Those diagnosed with severe, stable COPD 14 participants 8 men; 6 women Mean age: 65 years Mean forced expiratory volume in one second (FEV1): 32% predicted Mean partial pressure of oxygen (paO2) 9.0 range 7.1 to 10.9 kPa Mean partial pressure of carbon dioxide (paCO2) 5.1 range 3.4 to 6.5 kPa |
|
| Interventions | Diamorphine 2.5 mg four times daily (QDS), or diamorphine 5 mg QDS or placebo | |
| Outcomes | Daily diary cards with 10 cm VAS for dyspnoea, feeling of well‐being, drowsiness, number of bronchodilator puffs At the end of each 2 week period: FEV1 PaO2 PaCO2 A‐aPO2 (alveolar‐arterial oxygen tension difference) 6MWT (six minute walk test) VAS dyspnoea for 6MWT Time on treadmill VAS dyspnoea for treadmill O2 saturation End‐tidal PCO2 Morphine levels |
|
| Notes | Dyspnoea assessed "at completion of each type of exercise" The study authors detected no significant effect of opioid compared with placebo The dose is equivalent to 15 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was double‐blind, randomised, and cross‐over with no wash‐out intervals. The study authors did not provide any further details for the methods. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not state the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the first study there were 4 drop outs, 2 due to unrelated reasons and 2 due to side effects in the diamorphine group. However, this is unlikely to influence the results of the assessment of breathlessness. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting. |
| Other bias | High risk | The study authors did not state that adverse events were systematically studied (apart from drowsiness). |
| Size bias | High risk | There were only 14 participants in total, and < 50 per treatment arm. |
Grimbert 2004.
| Methods | Randomised crossover study | |
| Participants | Participants receiving palliative care for lung cancer, experiencing dyspnoea 12 participants Mean age 63 years 11 men, 1 woman Exclusion criteria: heart failure, asthma, allergy to morphine, previous addiction to morphine |
|
| Interventions | Nebulised morphine sulphate 20 mg, compared to nebulised saline 48 hours of treatment with 24 hours washout period |
|
| Outcomes | VAS dyspnoea scale ‐ no interpretable data Respiratory rate Oxygen saturation |
|
| Notes | The study authors concluded that both nebulised morphine and nebulised saline produced the same improvement in dyspnoea We translated this study into English The primary outcome data was unable to be extrapolated from the figures so this was not included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | The study concealed allocation, collected from pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy prepared the intervention as a clear, colourless solution. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not clearly state the blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study authors reported all follow‐up of participants. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There was a small sample size of only 12 participants and a high size bias. There were< 50 participants per treatment arm. |
Harris‐Eze 1995.
| Methods | Randomised crossover study Exercise study Incremental cycle ergometer |
|
| Participants | Interstitial lung disease (ILD) 6 participants: 5 male, 1 female Mean age: 49 years FEV1 2.54, SD 0.69 Stable, no change in medication over 2 months No history of opioid abuse No opioid drugs for 1 month |
|
| Interventions | Morphine 2.5 mg, morphine 5 mg, or placebo 15 minutes before exercise test 3‐day washout period |
|
| Outcomes | Modified Borg Scale‐mean value at end exercise Exercise duration Heart rate Maximal workload ECG Oxygen saturations (SaO2) O2 uptake (VO2) CO2 output (VCO2) End‐tidal CO2 minute ventilation (VI) Respiratory frequency Tidal volume |
|
| Notes | The study authors measured outcomes at 15 minutes post‐dose The study authors reported no significant effect of nebulised morphine compared with placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly describe the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly describe the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study participants were given the nebulized study medication (saline (control), morphine 2.5 mg, or morphine 5.0 mg) in randomised, double‐blinded fashion. An attendant who was not involved in the remainder of the protocol prepared the study medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The adequacy of blinding was unclear, and we were unable to tell whether unblinding was possible from the description (see above), could influence measurement of Borg scale but not some of the exercise test parameters. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting. |
| Other bias | Unclear risk | It is unclear whether or not there was other bias. |
| Size bias | High risk | There were only 6 participants in total, which is a small sample size; there were < 50 participants per treatment arm. |
Hui 2014.
| Methods | Double blinded, randomised parallel placebo controlled study 6MWT |
|
| Participants | People diagnosed with cancer, aged ≥ 18 years, with breakthrough dyspnoea > 3/10, ambulatory, Karnovsky score > 50%, on a stable opioid dose 20 participants Mean age 50 years (range 30 to 75 years) 11 female 70% Caucasian, 15% black, 15% Hispanic Exclusion criteria: dyspnoea > 7/10, supplemental oxygen > 6L, delirium, allergy to fentanyl, substance abuse, recent coronary disease |
|
| Interventions | Subcutaneous fentanyl, dosed on a sliding scale, dose ranging from 30 mcg to 350 mcg compared to subcutaneous saline Co‐interventions included regular opioids, bronchodilators, steroids |
|
| Outcomes | NRS dyspnoea scale Borg fatigue scale Heart rate Respiratory rate Oxygen saturation Blood pressure Adverse events |
|
| Notes | The study measured outcomes before and after the 6MWT The study authors concluded that prophylactic fentanyl was safe and improved dyspnoea, fatigue, walk distance, and respiratory rate, and that there was also a large placebo effect The dose is equivalent to 1.5 mg to 9 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomised sequence generation. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by using a secured Web site that was only accessible to the study pharmacist after participant enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded. Both the participants and research staff conducting the study assessments were blinded to the study intervention and the randomisation sequence. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both the participants and research staff conducting the study assessments were blinded to the study intervention and the randomisation sequence. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study with no loss of follow up |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This was a parallel trial with 20 participants in each arm (< 50 participants per treatment arm). |
Jankleson 1997.
| Methods | Double blind, randomised crossover study Exercise study 6MWT |
|
| Participants | Exercise tolerance limited by dyspnoea in the setting of stable COPD 16 participants 11 male, 5 female Mean age 69 years (range 61 to 85 years) Mean FEV1 0.93 Mean forced vital capacity (FVC) 2.21 Mean PaO2 9.6 kPa Mean PaCO2 5.4 kPa |
|
| Interventions | Nebulised morphine 20 mg or morphine 40 mg or placebo immediately before and 1 hour before exercise test Tests separated by 1 or 2 days |
|
| Outcomes | Modified Borg score 6MWT SaO2 Heart rate Plasma morphine levels |
|
| Notes | The study authors reported no significant effect of opioid compared with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study followed a double‐blind cross‐over, placebo‐controlled design. The hospital pharmacist prepared and allocated 3 test solutions, morphine sulphate (20 and 40 mg in 5 mL solutions with 0.9% saline) and placebo (5 mL 0.9% saline), in a double‐blind, random order on each of 3 test days, which were no more than 2 days apart. |
| Allocation concealment (selection bias) | Unclear risk | The study followed a double‐blind cross‐over, placebo‐controlled design. The hospital pharmacist prepared and allocation the 3 test solutions, morphine sulphate (20 and 40 mg in 5 mL solutions with 0.9% saline) and placebo (5 mL 0.9% saline), in a double‐blind, random order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See above – it may be sufficient but we cannot tell how similar the placebo and morphine nebs were. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop outs. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting. |
| Other bias | Unclear risk | It was unclear if there was any other bias. |
| Size bias | High risk | There was a small sample size, and this study was at high risk of bias with < 50 participants per study arm. |
Jensen 2012.
| Methods | Randomised, double blind, placebo controlled crossover study | |
| Participants | Diagnosed with COPD, aged 40 years or older, cigarette smokers FEV1 < 0.7 12 participants Mean age 70 years (range ± 2.3 years) 7 males, 5 females Exclusion criteria: those with significant diseases other than COPD, those with sleep disordered breathing, those who has used opioids in the last 2 days |
|
| Interventions | Nebulised 50 mcg fentanyl citrate compared to nebulised saline Participants withdrew from beta‐agonists, anticholinergics, caffeine, and theophylline prior to the trial |
|
| Outcomes | Dyspnoea on the Borg scale Exercise time Dyspnoea unpleasantness Leg discomfort Heart rate Oxygen saturation VO2 VCO2 VE VT FR |
|
| Notes | The study authors measured outcomes at pre‐exercise, isotime (highest equivalent of exercise time achieved), and peak exercise They concluded that single‐dose inhalation of fentanyl citrate was associated with significant and potentially clinically important improvements in exercise tolerance in people with COPD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospital pharmacist performed randomisation, blinding, and dispensing of study medications. This person was an unblinded third party who was not affiliated with either subject recruitment or data collection and analysis. |
| Allocation concealment (selection bias) | Low risk | Double blinded. The hospital pharmacist performed randomisation, blinding, and dispensing of study medications. This person was an unblinded third party who was not affiliated with either subject recruitment or data collection and analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This is likely to have been adequate given both placebo in fentanyl solutions were the same volume and dispensing was independent of those involved in conducting the study. However, it is not entirely clear whether the placebo and drug had the same appearance/taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clearly reported. There were 3 drop outs in the placebo group: 2 were due to side effects (before exercise test) and 1 due to protocol violation. There was 1 drop out in intervention group due to side effects prior to exercise test. Balanced and unlikely to affect the results |
| Selective reporting (reporting bias) | Low risk | We did not find any evidence of selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There was a small sample size, and high risk of bias; there were < 50 participants per study arm. |
Johnson 1983.
| Methods | Randomised, double blind, crossover study 2 consecutive 1 week periods followed by exercise test Incremental treadmill test 1 drop‐out: developed chest infection and right heart failure on dihydrocodeine |
|
| Participants | Those with stable COPD with severe breathlessness, and severe airflow obstruction 19 participants 15 men, 3 women Mean age 64.9 years, SD 9.1 years FEV1 830, SD 260 mL PaO2 9.3 SD 0.8 kPa; PaCO2 4.8 SD 0.5 kPa At least Grade 3 breathlessness (MRC scale) No recent hospital admissions No sedative drugs Continued usual bronchodilators and steroids |
|
| Interventions | Dihydrocodeine 15 mg or placebo over 2 consecutive 1 week periods. Drug to be taken 30 minutes before exercise up to 3 times daily Tests at end of each week period |
|
| Outcomes | Pedometer distance for 1 week Daily VAS for breathlessness PEFR FEV1 FVC Incremental treadmill test Distance walked VAS for breathlessness at 75% distance walked on placebo day |
|
| Notes | The study authors concluded that dihydrocodeine 15 mg 30 minutes before exercise offers appreciable benefit to participants with severe breathlessness due to chronic airflow obstruction Dose is equivalent to 4.5 oral morphine, up to 3 times daily |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors reported allocation concealment but did not clearly state the methods. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were given similar solutions of tablets with opioid or placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study authors clearly stated the methods of withdrawals, drop outs, and reasons, which did not appear to change the study outcomes. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting bias. |
| Other bias | Low risk | We did not detect any other bias. |
| Size bias | High risk | There was a small sample size, and high risk of bias; there were < 50 participants per arm. Measured interquartile range so could not be included in the meta‐analysis |
Johnson 2002.
| Methods | Randomised, double blind, placebo controlled crossover study | |
| Participants | NYHA Class III or IV heart failure, clinically stable, medically optimised 10 participants Mean age 67 years (range 45 to 85 years) All male Exclusion criteria: renal impairment, malignant disease |
|
| Interventions | 5 mg oral morphine solution unless creatinine > 200ml/dL, then 2.5 mg was administered Compared to oral placebo solution |
|
| Outcomes | VAS breathlessness score Sedation score Constipation Nausea Quality of life Blood pressure Pulse Respiratory rate Catecholamines |
|
| Notes | Outcomes measured at 1 hour, day 2, 3, and 4 The study authors concluded that morphine relieves breathlessness due to chronic heart failure The dose is equivalent to 5 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study authors used a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Hospital pharmacy but not clear if allocation concealed as no further details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors stated that the study was to be double blind but gave no further details about blinding or the nature of the placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study authors accounted for all participants. They recruited 10 participants and reported on 10 participants. |
| Selective reporting (reporting bias) | Low risk | The study authors reported all primary and secondary outcomes, including withdrawals. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were fewer than 50 participants per study arm. |
Leung 1996.
| Methods | Randomised, double blind, crossover study Exercise study Incremental cycle ergometer |
|
| Participants | COPD (1 pt ILD) 10 participants 6 male, 4 female Mean age 62 years, (range 51 to 71 years) Mean FEV1 = 1.12 Exclusion criteria: CO2 retention and ischaemic heart disease |
|
| Interventions | Morphine 5 mg in 5mL or placebo 15 minutes before exercise test 100% O2 inhaled during exercise test Tests on separate days |
|
| Outcomes | Modified Borg score Maximum power output VE max Heart rate |
|
| Notes | The study authors reported no significant effect of opioid compared with placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recruited participants remained in the study. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were fewer than 50 participants per study arm. |
Light 1996.
| Methods | Randomised, crossover study Exercise study Incremental cycle ergometer |
|
| Participants | COPD 7 male participants Mean age 66.4 years SD 3.25 years FEV1 0.99 SD 0.3 FEV1/FVC 0.35 SD 0.07 Exercise limited by breathlessness Stable disease Exclusion criteria: PaCO2 > 45 mmHg, FEV1 > 1.39 L, long‐term oxygen supplementation, cardiac disease, history of narcotic abuse, other significant disease affecting exercise performance, use of tranquillisers, hypnotics, mood altering drugs or opioids in week prior to study, alcoholism in past 5 years |
|
| Interventions | Morphine 30 mg or placebo once orally 60 minutes before exercise test, compared to 30 mg morphine plus 10 mg prochlorperazine, or compared to 30 mg morphine plus 25 mg promethazine Tests on separate days |
|
| Outcomes | Modified Borg score each minute of exercise Workload Exercise duration VO2 VCO2 VE PETO2 PETCO2 Heart rate SaO2 |
|
| Notes | The study authors concluded that the administration of 30 mg morphine plus promethazine significantly improved the exercise tolerance of participants with COPD, without significantly impairing the mental capabilities of the participants The dose is equivalent to 30 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebos were identical in appearance; the participants were blinded to the intervention and the placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not state the number of participants enrolled or recruited, and only provided a statement about the number that completed the study. It is unclear whether or not this had impact on the trial outcomes. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size; there were < 50 participants per arm. |
Masood 1995.
| Methods | Double blind, randomised, crossover study Exercise study Incremental cycle ergometer |
|
| Participants | Stable severe COPD with disabling breathlessness 12 men ADLs limited by breathlessness FEV1 < 1.51 Exclusion criteria: exacerbations needing antibiotics, change in oral steroid dose or hospital admission within 2 months, overt cardiac disease, contra‐indication to exercise testing, pCO2 > 7.0, use of opioids, benzodiazepines, or other sedative agent within 1 month |
|
| Interventions | Morphine 10 mg nebulised or morphine 25 mg nebulised or morphine 1 mg intravenous or morphine 2.5 mg intravenous or placebo nebulised or placebo intravenous 15 minutes before exercise tests Each test was separated by at least 48 hours |
|
| Outcomes | Heart rate Respiratory rate VO2 RER SaO2 VAS for breathlessness Exercise duration Plasma morphine levels Ventilation |
|
| Notes | Nebulised Beta 2 agonist given before exercise tests Authors conclude no significant effect of opioid compared with placebo on exercise tolerance or breathlessness The primary outcome did not include standard deviation therefore could not be included in the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not state whether or not they blinded participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recruited participants remained in the study. |
| Selective reporting (reporting bias) | Low risk | The study authors presented all primary and secondary outcomes described in methods. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were < 50 participants per arm |
Mazzocato 1999.
| Methods | Randomised crossover study | |
| Participants | Those with dyspnoea due to advanced cancer Normal limits on MMSE, absence of brain tumour, acute incapacitating respiratory decompensation 9 participants Mean age 73 years (range 66 to 83 years) 4 female, 5 male |
|
| Interventions | Subcutaneous morphine 5 mg, compared to subcutaneous saline Continued usual co‐interventions |
|
| Outcomes | VAS dyspnoea scale Borg dyspnoea scale Pain Somnolence Anxiety Respiratory effort score (respiratory frequency, presence of cyanosis, and utilisation of accessory respiratory muscles) Oxygen saturations |
|
| Notes | Outcomes measured at 45 minutes on day 1, and crossover on day 2 The study authors concluded that morphine appears effective for cancer dyspnoea, and it does not compromise respiratory function at the dose level used The dose was equivalent to 15 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Nine participants gave their informed consent. Seven opioid‐naive participants were randomised to 5 mg subcutaneous morphine or placebo on day 1. The study authors described this study as a double blind cross over study. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors gave the methods of blinding of participants as “our study was done under double‐blind conditions” but did not provide any further details to assess the adequacy of this. Note that some participants in the morphine group received different doses of morphine if they were already taking oral morphine and it is unclear how this was concealed or blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state whether or not they performed blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The graph (figure 1) with data for primary outcome shows data collected on all participants enrolled. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were < 50 participants per arm. |
Navigante 2010.
| Methods | Randomised parallel study | |
| Participants | Ambulatory participants with moderate to severe dyspnoea at rest Mean age 55 years 31 participants in the morphine arm and 32 participants in the midazolam arm |
|
| Interventions | Oral morphine: 3 mg then incremental steps at 30 minutes until 50% reduction in dyspnoea Compared to oral midazolam: 2 mg up titrated 25% until 50% reduction in dyspnoea |
|
| Outcomes | Dyspnea intensity Adverse events |
|
| Notes | Compared morphine to midazolam | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (using a random number generator in 1:1 ratio in blocks of 6) to 1 of the 2 treatment groups. Numbered envelopes that were used to implement the randomisation were concealed until interventions were assigned. |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned (using a random number generator in 1:1 ratio in blocks of 6) to 1 of the 2 treatment groups. Numbered envelopes that were used to implement the randomisation were concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study was single blinded, that is only participants were blinded. However, adequacy was unclear – similarity of preparation – morphine group given laxatives. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This study was single blinded, and only participants were blinded. The investigators were aware of the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was only 1 withdrawal in each group, which was unrelated to the intervention. |
| Selective reporting (reporting bias) | Low risk | The study authors appear to have reported all outcome data. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There were 30 participants per treatment arm and the study was designed as a parallel trial; there were < 50 participants per treatment arm. |
Noseda 1997.
| Methods | Placebo‐controlled, double blind, randomised crossover study There were 3 drop outs: 3 participants died over the study period, during the night: the study authors did not consider their deaths to be related to the treatment |
|
| Participants | Hospital inpatients with severe lung disease, experiencing distressing dyspnoea not relieved by medical therapy Mean age 69 years (± 11 years) Mean FEV1 0.92 SD 0.18 Normal cognitive function 17 participants |
|
| Interventions | Nebulised morphine 10 mg + O2 or morphine 20 mg + O2, or morphine 10 mg or placebo + O2 at 2L/min Tests took place over consecutive days |
|
| Outcomes | VAS for breathlessness SaO2% Respiratory rate |
|
| Notes | Results using morphine alone were not analysed The study authors reported no benefit of morphine compared with placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly describe the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly describe the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All morphine and saline solutions were prepared and coded independently in the hospital pharmacy. However, the study authors did not provide any further description. For the 10 mg/no oxygen group, prongs were applied but no oxygen and it is not possible to determine whether or not the participants or investigators were truly blinded to the intervention based on the description above. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not state the methods of blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | This study had incomplete data due to deaths during the trial, which were clearly reported and would be expected in this study type. The deaths occurred overnight and were unlikely to be related to the intervention, which took place during the day. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were < 50 participants per arm. |
Oxberry 2011.
| Methods | Controlled double blind crossover study | |
| Participants | Those with heart failure LVEF < 45%, on standard medical therapy 35 participants Mean age 70 years (± 11 years) Male 8% Exclusion criteria: co‐existing respiratory illness, peak expiratory flow (PEF) < 150 L/min, opioid sensitivities, renal impairment i.e. glomerular filtration rate (eGFR) < 30 mL/min |
|
| Interventions | Oral morphine 5 mg four times daily (QID) compared to oral oxycodone 2.5 mg four times daily (QID) compared to oral placebo Treatment arm 4 days with 3 day washout period |
|
| Outcomes | NRS dyspnoea scale NRS coping NRS satisfaction |
|
| Notes | Outcomes assessed daily The study authors concluded that there was no benefit over placebo for the relief of breathlessness with short‐term low‐dose oral opioids for congestive heart failure The dose is equivalent to 20 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study drug manufacturer (Calderdale and Huddersfield NHS Pharmacy Manufacturing Unit, Huddersfield, UK) randomised the order of interventions for each participant using a random number generation programme. It did not use block design and there were 6 possible sequence combinations. The pharmacy dispensed all 3 medications for use in the required sequence with identical labels except for the treatment order. Hence the investigators and participants remained blinded to the treatment sequence and allocation was conducted distant to the research team. |
| Allocation concealment (selection bias) | Low risk | The placebo was designed to have very similar characteristics to the active medications (a clear, colourless liquid with the same viscosity and similar taste). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy dispensed all 3 medications for use in the required sequence with identical labels except for the treatment order. Hence the investigators and participants remained blinded to the treatment sequence and allocation was conducted distant to the research team. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded to outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a small number of drop outs, which were all completely reported and unlikely to influence results and reasons stated. |
| Selective reporting (reporting bias) | Low risk | The study was registered prior to recruitment and audited study. The study reported all prespecified outcomes. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | There were < 50 participants per treatment arm. |
Poole 1998.
| Methods | Randomised, double blind, crossover study Two 6‐week treatment periods followed by exercise tests 2‐week wash‐out period 6MWT 2 drop‐outs |
|
| Participants | Those with breathlessness caused by COPD, following pulmonary rehabilitation 16 participants 11 men, 5 women Mean age 70.7 years, SD 1.6 years FEV1 0.6, SD 0.4 Mean pO2 9.8 mean pCO2 5.3 Exclusion criteria: CCF, paCO2 > 5.4, FEV1 > 1.49, alcoholism, psychiatric disorder, on opiates, change in drugs in past month, or hospitalised in past 2 months |
|
| Interventions | Oral morphine sulphate sustained release 10 mg to 20 mg daily‐ twice daily (BD) or placebo Average dose 25 mg over 24 hours Tests at end of treatment periods |
|
| Outcomes | Chronic Respiratory Disease Questionnaire 6MWT SaO2 Spirometry Breathlessness scores on Likert scale before and at the end of six minutes walk Side effects |
|
| Notes | The study authors concluded that there was no significant difference after opioid administration The dose is equivalent to 15 mg morphine, uptitrated to BD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The Auckland Hospital Pharmacy performed randomisation in blocks of 4. They maintained the randomisation schedule, stored the study medicines in a controlled drug safe, and dispensed them at study visits according to the regulations governing the use of controlled medicines. |
| Allocation concealment (selection bias) | Low risk | The placebo capsules were identical in appearance but were filled only with lactose. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The pharmacy supplied identical tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Staff involved in administering questionnaires and 6MWTs were not blinded to adverse events and therefore may have guessed the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 2 dropouts from the morphine group, but this is unlikely to affect the results. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were < 50 participants per arm. |
Rice 1987.
| Methods | Double blind crossover study | |
| Participants | Clinically stable males with COPD FEV < 60% 7 participants 3 withdrawals (1 due to side effects of codeine, 1 due to worsening respiratory function) Age range 59 to 79 years All male Exclusion criteria: PCO2 > 55, history of chemical dependence |
|
| Interventions | 30 mg codeine four times daily (QID) compared to 25 mg promethazine QID Co‐interventions: beta agonists, theophylline, prednisolone Outcomes measured at baseline and at 1 month |
|
| Outcomes | VAS dyspnoea scale Breathlessness rating |
|
| Notes | The study authors concluded that the benefits of codeine or promethazine are uncertain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects were randomised in sets of 4 in a double‐blind fashion to begin oral treatment with identical pills that contained either 30 mg codeine or 25 mg promethazine, both taken 4 times daily. There were limited details in the study report. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly report the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not clearly state whether they blinded participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state whether there was blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant dropped out on the third day of the first study arm after developing acute urinary retention while taking codeine. Three participants had marked worsening of their respiratory symptoms and required hospitalisation. Two participants were receiving codeine, 1 on the first study arm and 1 on the second arm. The third subject was receiving promethazine during the first arm. There was a small number of drop outs but also a small total number of participants. There were roughly similar reasons per group. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We did not detect any other bias. |
| Size bias | High risk | This study had a small sample size despite it being a parallel study; there were < 50 participants per arm |
Williams 2003.
| Methods | Randomised crossover study | |
| Participants | Stable heart failure Age range 38 to 75 years Mostly male (N = 15) |
|
| Interventions | Diamorphine 1 or 2 mg given intravenously Compared to intravenous saline |
|
| Outcomes | Primary outcome of breathlessness was not measured Oxygen consumption Exercise duration Respiratory rate |
|
| Notes | Naloxone 0.4 mg was given at the end of the test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly state the methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not clearly state whether they blinded participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state whether or not they performed blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study authors reported all follow‐up and completion data. |
| Selective reporting (reporting bias) | Low risk | We did not detect any selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size; there were < 50 participants per treatment arm. |
Woodcock 1981.
| Methods | Randomised, crossover study Exercise study Incremental treadmill test |
|
| Participants | Those with severe airway obstruction with breathlessness on exertion 12 participants 10 men, 2 women Mean age 62 years No recent hospital admissions At least Grade 3 breathlessness (MRC scale) Normal or low pCO2 FEV1 0.73, SD 0.31 paO2 72.6, SD 6.86 paCO2 35.3, SD 2.4 All ex‐smokers stopped smoking at least 6 months before the study |
|
| Interventions | Dihydrocodeine 1 mg/kg given once, orally, 45 minutes before treadmill test (incremental speed to exhaustion) or alcohol or caffeine Placebo Tests on consecutive days |
|
| Outcomes | VAS for breathlessness during treadmill test (measured at 75% of distance walked on day of placebo) Exercise tolerance (distance walked to exhaustion on treadmill) Ventilation O2 consumption FVC FEV1 |
|
| Notes | Salbutamol 200 mcg, inhaled, 30 minutes before study The study authors concluded that opioids may be valuable in the treatment of breathlessness The dose is equivalent to an average of 6 mg oral morphine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not clearly state whether they performed random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The study authors did not clearly state whether they performed allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not clearly state whether they performed blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not clearly state whether they performed blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study, although the study authors did not specifically state the number of participants enrolled. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were < 50 participants per arm |
Young 1989.
| Methods | Cross‐over study Exercise study Cycle endurance test Nebulised opioid |
|
| Participants | COPD (9) or idiopathic pulmonary fibrosis (2) Mean age 58.4 years (39 to 74) Exercise tolerance limited by dyspnoea FEV1 0.4 to 1 |
|
| Interventions | Morphine 5 mg or placebo neb 15 mins before exercise test Tests on separate days 100% O2 inhaled during exercise test |
|
| Outcomes | Cycle ergometer exercise test at 80% of pre‐determined Emax Endurance time Ventilation during last minute of exercise FEV1 |
|
| Notes | The study authors did not use dyspnoea as an outcome measure, although they selected participants because exercise was limited by dyspnoea. They initially studied 18 participants and 7 excluded on run‐in day because exercise was limited by other factors: the participants were excluded from the study authors’ and from this review analysis Mean endurance time increased by 64 seconds: 1 participant had an increase of 400 seconds. If the study authors had excluded that participant from their analysis, the mean increase would have been approximately 25 seconds and not statistically significant. The study authors concluded that morphine had a significant effect on exercise endurance time vs placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study authors did not state the methods of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The hospital pharmacist performed allocation, but the study authors did not further describe the method used to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study authors did not state whether they performed blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study authors did not state whether they performed blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not clearly report complete participant data and dropouts. |
| Selective reporting (reporting bias) | Low risk | We did not detect any evidence of selective reporting bias. |
| Other bias | Low risk | We judged that this trial appeared to be free of other sources of bias. |
| Size bias | High risk | This study had a small sample size, and was at high risk of bias; there were < 50 participants per arm. |
Chronic obstructive pulmonary disease (COPD), three times daily (TDS), six‐minute walking test (6MWT), standard deviation (SD), minimental state exam (MMSE), left ventricular ejection fraction (LVEF), New York Heart Association (NYHA), angiotensin‐converting‐enzyme (ACE), blood pressure (BP), partial pressure of oxygen (PaO2), ventilation to carbon dioxide output (VE‐VCO2 slope), mean forced expiratory volume in one second (FEV1), mean partial pressure of carbon dioxide (paCO2), A‐aPO2 (alveolar‐arterial oxygen tension difference), interstitial lung disease (ILD), oxygen saturations (SaO2), O2 uptake (VO2), CO2 output (VCO2), end‐tidal CO2 minute ventilation (VI), visual analogue scale (VAS), numeric rating scale (NRS), glomerular filtration rate (eGFR), four times a day (QID or QDS), twice daily (BD).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allard 1999 | This study did not compare the intervention to a placebo or any other intervention. |
| Beauford 1993 | This study doesn't appear to be randomised, and we were unable to contact the study authors. |
| Bruera 2005 | This study did not compare the intervention to a placebo. |
| Navigante 2003 | This study compared morphine plus midazolam to oxygen therapy. |
| Peterson 1996 | This study doesn't appear to be randomised. |
| Shorati 2012 | This study doesn't appear to be randomised. We were unable to contact the study authors. |
| Smith 2009 | This was a pilot trial of 2 participants only, and it doesn't appear to have randomised participants to treatment. |
| Thomas 2010 | This was a review of 2 randomised controlled trials. |
Characteristics of ongoing studies [ordered by study ID]
Cuervo Pinna 2012.
| Trial name or title | Cuervo Pinna 2012 |
| Methods | This is a crossover clinical trial in which the study population will do the 6MWT with the study drug and placebo. |
| Participants | Patients with advanced cancer. Patients must have dyspnoea at rest or dyspnoea moderate effort with an intensity of at least 3 on a scale from 0 to 10. |
| Interventions | Oral Transmucosal Fentanyl Citrate versus placebo |
| Outcomes | Primary variable: VAS scale change from baseline. Improvement of the severity of dyspnoea after completion of the 6MWT in patients with advanced cancer. Response to treatment was considered an improvement greater than or equal to two points on the previous level of dyspnea.The evaluation and determination of changes in the level of severity of dyspnoea is done through Visual Analogue Scale (VAS) included in the Edmonton Symptom Assessment System (ESAS). Secondary variables: |
| Starting date | 2012 |
| Contact information | Cuervo Pinna M. Extremadura, Palliative Care Support Team, Badajoz, Spain |
| Notes |
Daubert 2014.
| Trial name or title | Daubert 2014 |
| Methods | Prospective, randomised, double blind controlled trial, randomly assigned to either receive lorazepam or morphine. Symptom relief will be evaluated using the Edmonton Symptom Assessment Scale. Patients will receive treatment for 14 days. |
| Participants | People greater than 18 years of age, enrolled in a hospice service, diagnosed with dyspnoea, able to take oral medications |
| Interventions | opioids compared to benzodiazepines |
| Outcomes | The primary outcome will be the change in patients' perception of their quality of life. This will be assessed using the Functional Assessment of Chronic Illness Therapy‐Palliative Care scale, which patients will complete prior to initiation of therapy and after 7 and 14 days of treatment. An intention to‐ treat analysis will be performed and the change in quality of life observed will be compared between groups using a multivariable logistic regression analysis to adjust for confounding variables. |
| Starting date | Research is in progress from 2014 |
| Contact information | Daubert E, Bolesta S, Wilkes University, E‐mail: eliza.daubert@wilkes.edu |
| Notes |
Differences between protocol and review
In the protocol we indicated that we would perform meta‐analyses according to the subgroups of dose and 'Risk of bias' assessments. Due to the wide variation and heterogeneity of reported doses we chose to analyse this in a descriptive analysis. We compared the 'Risk of bias' difference in a sensitivity analysis.
Post‐hoc we chose to include the type of opioid as a subgroup analysis as we felt this would be an important assessment for clinicians and policy makers.
Contributions of authors
HB and RM drafted the protocol. HB and RM screened the abstracts, and full‐text articles for inclusion, extracted the data, and drafted the review. JM and NS provided comments and revisions of the review. HB will be responsible for updating this Cochrane review.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research Cochrane Review Incentive Scheme (14‐175‐05), UK.
Declarations of interest
HB has no relevant conflicts of interest to declare. JM has no relevant conflicts of interest to declare. NS has no relevant conflicts of interest to declare. RM has no relevant conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abernethy 2003 {published data only}
- Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003;327(7414):523‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currow DC, Plummer J, Frith P, Abernethy AP. Can we predict which patients with refractory dyspnea will respond to opioids?. Journal of Palliative Medicine 2007;10(5):1031‐6. [DOI] [PubMed] [Google Scholar]
Bar‐Or 1982 {published data only}
- Bar‐Or D, Marx JA, Good J. Breathlessness, alcohol and opiates. The New England Journal of Medicine 1982;306(22):1363–4. [DOI] [PubMed] [Google Scholar]
Bruera 1993 {published data only}
- Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Annals of Internal Medicine 1993;119(9):906‐7. [DOI] [PubMed] [Google Scholar]
Charles 2008 {published data only}
- Charles MA, Reymond L, Israel F. Relief of incident dyspnea in palliative cancer patients: a pilot, randomized, controlled trial comparing nebulized hydromorphone, systemic hydromorphone, and nebulized saline. Journal of Pain and Symptom Management 2008;36(1):29‐38. [DOI] [PubMed] [Google Scholar]
Chua 1997 {published data only}
- Chua T, Harrington D, Ponikowski P, Webb‐Peploe K, Poole‐Wilson P, Coats A. Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failure. Journal of the American College of Cardiology 1997;29(1):147‐52. [DOI] [PubMed] [Google Scholar]
Davis 1996 {published data only}
- Davis C, Penn K, A’Hern R, Daniels J, Slevin M. Single dose randomised controlled trial of nebulised morphine in patients with cancer related breathlessness. Palliative Medicine 1996;10:64–5. [Google Scholar]
Eiser 1991 {published data only}
- Eiser N, Denman WT, West, C Luce P. Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the "pink puffer" syndrome. European Respiratory Journal 1991;4(8):926‐31. [PubMed] [Google Scholar]
Grimbert 2004 {published data only}
- Grimbert D, Lubin O, Monte M, Vecellio None L, Perrier M, Carré P, et al. Dyspnea and morphine aerosols in the palliative care of lung cancer [Dyspnée et aérosols de morphine dans les soins palliatifs du cancer broncho‐pulmonaire]. Revue des Maladies Respiratoires 2004;21(6 Pt 1):1091‐7. [DOI] [PubMed] [Google Scholar]
Harris‐Eze 1995 {published data only}
- Harris‐Eze AO, Sridhar G, Clemens RE, Zintel TA, Gallagher CG, Marciniuk DD. Low‐dose nebulized morphine does not improve exercise in interstitial lung disease. American Journal of Respiratory and Critical Care Medicine 1995;152(6):1940‐5. [DOI] [PubMed] [Google Scholar]
Hui 2014 {published data only}
- Hui D, Xu A, Frisbee‐Hume S, Chisholm G, Bruera, E. Prophylactic subcutaneous fentanyl for exercise‐induced breakthrough dyspnea: a preliminary double‐blind, randomized controlled trial. Supportive Care in Cancer 2013;21:S191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D, Xu A, Frisbee‐Hume S, Chisholm G, Morgado M, Reddy S, et al. Effects of prophylactic subcutaneous fentanyl on exercise‐induced breakthrough dyspnea in cancer patients: a preliminary double‐blind, randomized, controlled trial. Journal of Pain and Symptom Management 2014;47(2):209‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jankleson 1997 {published data only}
- Jankelson D, Hosseini K, Mather LE, Seale JP, Young IH. Lack of effect of high doses of inhaled morphine on exercise endurance in chronic obstructive pulmonary disease. European Respiratory Journal 1997;10(10):2270–4. [DOI] [PubMed] [Google Scholar]
Jensen 2012 {published data only}
- Jensen D, Alsuhail A, Viola R, Dudgeon DJ, Webb KA, O’Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high‐intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. Journal of Pain and Symptom Management 2012;43(4):706‐19. [DOI] [PubMed] [Google Scholar]
Johnson 1983 {published data only}
- Johnson MA, Woodcock AA, Geddes DM. Dihydrocodeine for breathlessness in “pink puffers”. British Medical Journal (Clinical Research Edition) 1983;286(6366):675–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2002 {published data only}
- Johnson MJ, McDonagh TA, Harkness A, McKayd SE, Dargie HJ. Morphine for the relief of breathlessness in patients with chronic heart failure‐‐a pilot study. European Journal of Heart Failure 2002;4(6):753–6. [DOI] [PubMed] [Google Scholar]
Leung 1996 {published data only}
- Leung R, Hill P, Burdon J. Effect of inhaled morphine on the development of breathlessness during exercise in patients with chronic lung disease. Thorax 1996;51(6):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Light 1996 {published data only}
- Light RW, Stansbury DW, Webster JS. Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPD. Chest 1996;109(4):975–81. [DOI] [PubMed] [Google Scholar]
Masood 1995 {published data only}
- Masood AR, Reed JW, Thomas SH. Lack of effect of inhaled morphine on exercise‐induced breathlessness in chronic obstructive pulmonary disease. Thorax 1995;50(6):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzocato 1999 {published data only}
- Mazzocato C, Buclin T, Rapin CH. The effects of morphine on dyspnea and ventilatory function in elderly patients with advanced cancer: A randomized double‐blind controlled trial. Annals of Oncology 1999;10(12):1511‐4. [DOI] [PubMed] [Google Scholar]
Navigante 2010 {published data only}
- Cerchietti LCA, Navigante, AH. Midazolam as adjunct therapy to morphine to relieve dyspnea? Authors' reply. Journal of Pain and Symptom Management 2007;33(3):234‐6. [DOI] [PubMed] [Google Scholar]
- Navigante AH, Castro MA, Cerchietti LC. Morphine versus midazolam as upfront therapy to control dyspnea perception in cancer patients while its underlying cause is sought or treated. Journal of Pain and Symptom Management 2010;39(5):820‐30. [DOI] [PubMed] [Google Scholar]
Noseda 1997 {published data only}
- Noseda A, Carpiaux JP, Markstein C, Meyvaert A, Maertelaer V. Disabling dyspnoea in patients with advanced disease: lack of effect of nebulized morphine. European Respiratory Journal 1997;10(5):1079–83. [DOI] [PubMed] [Google Scholar]
Oxberry 2011 {published data only}
- Oxberry SG, Bland JM, Clark AL, Cleland JG, Johnson MJ. Minimally clinically important difference in chronic breathlessness: every little helps. American Heart Journal 2012;164(2):229‐35. [DOI] [PubMed] [Google Scholar]
- Oxberry SG, Torgerson DJ, Bland JM, Clark AL, Cleland JG, Johnson MJ. Short‐term opioids for breathlessness in stable chronic heart failure: a randomized controlled trial. European Journal of Heart Failure 2011;13(9):1006–12. [DOI] [PubMed] [Google Scholar]
Poole 1998 {published data only}
- Poole PJ, Veale AG, Black PN. The effect of sustained‐release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(6):1877–80. [DOI] [PubMed] [Google Scholar]
Rice 1987 {published data only}
- Rice KL, Kronenberg RS, Hedemark LL, Niewoehner DE. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. British Journal of Diseases of the Chest 1987;81(3):287–92. [DOI] [PubMed] [Google Scholar]
Williams 2003 {published data only}
- Williams SG, Wright DJ, Marshall P, Reese A, Tzeng BH, Coats AJS, et al. Safety and potential benefits of low dose diamorphine during exercise in patients with chronic heart failure. Heart 2003;89(9):1085‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodcock 1981 {published data only}
- Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. The New England Journal of Medicine 1981;305(27):1611–6. [DOI] [PubMed] [Google Scholar]
Young 1989 {published data only}
- Young IH, Daviskas E, Keena VA. Effect of low dose nebulised morphine on exercise endurance in patients with chronic lung disease. Thorax 1989;44(5):387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Allard 1999 {published data only}
- Allard P, Lamontagne C, Bernard P, Tremblay CJ. How effective are supplementary doses of opioids for dyspnea in terminally ill cancer patients? A randomized continuous sequential clinical trial. Journal of Pain and Symptom Management 1999;17(4):256–65. [DOI] [PubMed] [Google Scholar]
Beauford 1993 {published data only}
- Beauford W, Saylor TT, Stansbury DW, Avalos K, Light RW. Effects of nebulized morphine sulfate on the exercise tolerance of the ventilatory limited COPD patient. Chest 1993;104(1):175–8. [DOI] [PubMed] [Google Scholar]
Bruera 2005 {published data only}
- Bruera E, Sala R, Spruyt O, Palmer L, Zhang T, Willey J. Nebulized versus subcutaneous morphine for patients with cancer dyspnea: a preliminary study. Journal of Pain and Symptom Management 2005;29(6):613‐8. [DOI] [PubMed] [Google Scholar]
Navigante 2003 {published data only}
- Navigante AH, Castro M, Cerchietti L. Morphine plus midazolam versus oxygen therapy on severe dyspnea management in the last week of life in hipoxemic advanced cancer patients [Morfina más midazolan versus oxigenoterapia en el control de la disnea severa durante la última semana de vida en pacientes hipoxémicos con cáncer avanzado]. Medicina Paliativa 2003;10(1):14‐9. [Google Scholar]
Peterson 1996 {published data only}
- Peterson GM, Young RS, Dunne PF, Galloway JG, Parks TE. Pilot study of nebulised morphine for dyspnoea in palliative care patients. Australian Journal of Hospital Pharmacy 1996;26(5):545–7. [Google Scholar]
Shorati 2012 {published data only}
- Shohrati M, Ghanei M, Harandi AA, Foroghi S, Harandi, AA. Effect of nebulized morphine on dyspnea of mustard gas‐exposed patients: a double‐blind randomized clinical trial study. Pulmonary Medicine 2012;2012:610921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Smith TJ, Coyne P, French W, Ramakrishnan V, Corrigan P. Failure to accrue to a study of nebulized fentanyl for dyspnea: lessons learned. Journal of Palliative Medicine 2009;12(9):771‐2. [DOI] [PubMed] [Google Scholar]
Thomas 2010 {published data only}
- Thomas J, Stambler N, Israel RJ. Methylnaltrexone, a peripheral opioid antagonist for opioid‐induced constipation in advanced illness: an analysis of dyspnea in patients with chronic obstructive pulmonary disease (COPD) in two double‐blind randomized trials. 18th International Congress on Palliative Care; Oct 5‐8, 2010; Montreal. Journal of Palliative Care 2010;26(3):242. [Google Scholar]
References to ongoing studies
Cuervo Pinna 2012 {published data only}
- Cuervo Pinna MA. A randomized cross‐over clinical trial to evaluate the efficacy of oral transmucosal fentanyl citrate in the treatment of dyspnea on exertion in patients with advanced cancer. Palliative Medicine 2012;26(4):569‐70. [DOI] [PubMed] [Google Scholar]
Daubert 2014 {published data only}
- Daubert E, Bolesta S. Effect of lorazepam versus morphine on quality of life in hospice patients with dyspnea and anxiety. Journal of the American Pharmacists Association 2014;54(2):e193. [Google Scholar]
Additional references
ATS 1999
- American Thoracic Society. Dyspnea. Mechanisms, assessment, and management: a consensus statement. Amercan Journal of Respiratory and Critical Care Medicine 1999;159(1):321‐40. [DOI] [PubMed] [Google Scholar]
Banzett 2000
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activate the insular cortex. NeuroReport 2000;11(10):2117‐20. [DOI] [PubMed] [Google Scholar]
Bausewein 2008
- Bausewein C, Booth S, Gysels M, Higginson IJ. Non‐pharmacological interventions for breathlessness in advanced stages of malignant and non‐malignant diseases. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005623.pub2] [DOI] [PubMed] [Google Scholar]
Beach 2006
- Beach D, Schwartzstein RM. The genesis of breathlessness ‐ what do we understand?. In: Booth S, Dudgeon D editor(s). Dyspnoea in Advanced Disease ‐ A Guide to Clinical Management. 1st Edition. Oxford: Oxford University Press, 2006:1‐18. [Google Scholar]
Bolsher 1987
- Bolser DC, Lindsey BG, Shannon R. Medullary inspiratory activity: influence of intercostal tendon organs and muscle spindle endings. Journal of Applied Physiology 1987;62(3):1046‐56. [DOI] [PubMed] [Google Scholar]
Bolsher 1988
- Bolser DC, Lindsey BG, Shannon R. Respiratory pattern changes produced by intercostal muscle/rib vibration. Journal of Applied Physiology 1988;64(6):2458‐62. [DOI] [PubMed] [Google Scholar]
Brannan 2001
- Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, et al. Neuroimaging of cerebral activations and deactivations associated with hypercapnia and hunger for air. Proceedings of the National Academy of Sciences of the United States of America 2001;98(4):2029‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chahl 1996
- Chahl LA. Opioids ‐ mechanisms of action. Australian Prescriber 1996;19:63‐5. [Google Scholar]
Cranston 2008
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004769.pub2] [DOI] [PubMed] [Google Scholar]
Currow 2011
- Currow DC, McDonald C, Oaten S, Kenny B, Allcroft P, Frith P, et al. Once‐daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. Journal of Pain and Symptom Management 2011;42(3):388‐99. [DOI] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dudgeon 1998
- Dudgeon J, Lertzman M. Dyspnea in the advanced cancer patient. Journal of Pain and Symptom Management 1998;16(4):212‐9. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Evans 2002
- Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. Journal of Neurophysiology 2002;88(3):1500‐11. [DOI] [PubMed] [Google Scholar]
Fitzgerald 1986
- Fitzgerald RS, Lahiri S. Reflex response to chemoreceptor stimulation. In: Cherniack NS, Widdicombe JG editor(s). Handbook of Physiology, Section 3: The Respiratory System, Vol. 2. Control of Breathing. Bethesda: American Physiological Society, 1986:313‐62. [Google Scholar]
GradePro 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org. GRADEpro Guideline Development Tool [Software]. McMaster University (developed by Evidence Prime, Inc.). Available from www.gradepro.org, 2015.
Guz 1997
- Guz A. Brain, breathing and breathlessness. Respiration Physiology 1997;109(3):197‐204. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):W65‐94. [DOI] [PubMed] [Google Scholar]
Liotti 2001
- Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanalp B, et al. Brain responses associated with consciousness of breathlessness (air hunger). Proceedings of the National Academy of Sciences of the United States of America 2001;98(4):2035‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahler 2010
- Mahler DA, Selecky PA, Harrod CG, Benditt JO, Carrieri‐Kohlman V, Curtis JR, et al. American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest 2010;137(3):674‐91. [DOI] [PubMed] [Google Scholar]
Mahler 2013
- Mahler DA. Opioids for refractory dyspnoea. Expert Review of Respiratory Medicine 2013;7(2):123‐35. [DOI] [PubMed] [Google Scholar]
Manning 1995
- Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. The New England Journal of Medicine 1995;333(23):1547‐53. [DOI] [PubMed] [Google Scholar]
Masood 1996
- Masood AR, Thomas SH. Systemic absorption of nebulized morphine compared with oral morphine in healthy subjects. British Journal of Clinical Pharmacology 1996;41(3):250‐2. [DOI] [PubMed] [Google Scholar]
McGavin 1978
- McGavin CR, Artvinli M, Naoe H, McHardy GJR. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. British Medical Journal 1978;2(6132):241–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nattie 1995
- Nattie E. Central chemoreceptors. In: Dempsey JA, Pack AL editor(s). Regulation of Breathing. 2nd Edition. New York: Marcel Dekker, 1995:473‐510. [Google Scholar]
Neuman 2006
- Neuman A, Gunnbjörnsdottir M, Tunsäter A, Nyström L, Franklin KA, Norrman E, et al. Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respiratory Medicine 2006;100(10):1843‐9. [DOI] [PubMed] [Google Scholar]
Nishino 2011
- Nishino T. Dyspnoea: underlying mechanisms and treatment. British Journal of Anaesthesia 2011;106(4):463‐74. [DOI] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 1998
- O’Donnell DE, L.M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158(5):1557–65. [DOI] [PubMed] [Google Scholar]
Oxberry 2012
- Oxberry S, Jones L, Clark AL, Johnson MJ. Attitudes to morphine in chronic heart failure patients. Postgraduate Medical Journal 2012;88(1043):515‐21. [DOI] [PubMed] [Google Scholar]
Parshall 2012
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. American Journal of Respiratory and Critical Care Medicine 2012;185(4):435‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsons 2001
- Parsons LM, Egan G, Liotti M, Brannan S, Denton D, Shade R, et al. Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proceedings of the National Academy of Sciences of the United States of America 2001;98(4):2041‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pattinson 2009
- Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, et al. Opioids depress cortical centers responsible for the volitional control of respiration. Journal of Neuroscience 2009;29(25):8177‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peiffer 2001
- Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. American Journal of Respiratory and Critical Care Medicine 2001;163(4):951‐7. [DOI] [PubMed] [Google Scholar]
Petrovic 2002
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia‐‐ imaging a shared neuronal network. Science 2002;295(5560):1737‐40. [DOI] [PubMed] [Google Scholar]
Polosa 2002
- Polosa R, Simidchiev A, Walters EH. Nebulised morphine for severe interstitial lung disease. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rocker 2012
- Rocker G, Young J, Donahue M, Farquar M, Simpson C. Perspectives of patients, family caregivers and physicians about the use of opioids for refractory dyspnea in advanced chronic obstructive pulmonary disease. Canadian Medical Association Journal 2012;184(9):E497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2010
- Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007354.pub2] [DOI] [PubMed] [Google Scholar]
Uronis 2011
- Uronis H, McCrory DC, Samsa G, Currow D, Abernethy A. Symptomatic oxygen for non‐hypoxaemic chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006429.pub2] [DOI] [PubMed] [Google Scholar]
von Leupoldt 2009
- Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, et al. Dyspnoea and pain share emotion‐related brain network. NeuroImage 2009;48(1):200‐6. [DOI] [PubMed] [Google Scholar]
Widdicombe 1982
- Widdicombe JG. Pulmonary and respiratory tract receptors. Journal of Experimental Biology 1982;100:42‐57. [DOI] [PubMed] [Google Scholar]
Wiseman 2013
- Wiseman R, Rowett D, Allcroft P, Abernethy A, Currow DC. Chronic refractory dyspnoea‐‐evidence based management. Australian Family Physician 2013;42(3):137‐40. [PubMed] [Google Scholar]
Zebraski 2000
- Zebraski SE, Kochenash SM, Raffa RB. Lung opioid receptors: pharmacology and possible target for nebulized morphine in dyspnea. Life Sciences 2000;66(23):2221‐31. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jennings 2001
- Jennings AL, Davies AN, Higgins JPT, Anzures‐Cabrera J, Broadley KE. Opioids for the palliation of breathlessness in advanced disease and terminal illness. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002066] [DOI] [PubMed] [Google Scholar]


