Abstract
Background
Acute respiratory infections (ARIs) comprise of a large and heterogeneous group of infections including bacterial, viral, and other aetiologies. In recent years, procalcitonin (PCT), a blood marker for bacterial infections, has emerged as a promising tool to improve decisions about antibiotic therapy (PCT‐guided antibiotic therapy). Several randomised controlled trials (RCTs) have demonstrated the feasibility of using procalcitonin for starting and stopping antibiotics in different patient populations with ARIs and different settings ranging from primary care settings to emergency departments, hospital wards, and intensive care units. However, the effect of using procalcitonin on clinical outcomes is unclear. This is an update of a Cochrane review and individual participant data meta‐analysis first published in 2012 designed to look at the safety of PCT‐guided antibiotic stewardship.
Objectives
The aim of this systematic review based on individual participant data was to assess the safety and efficacy of using procalcitonin for starting or stopping antibiotics over a large range of patients with varying severity of ARIs and from different clinical settings.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE, and Embase, in February 2017, to identify suitable trials. We also searched ClinicalTrials.gov to identify ongoing trials in April 2017.
Selection criteria
We included RCTs of adult participants with ARIs who received an antibiotic treatment either based on a procalcitonin algorithm (PCT‐guided antibiotic stewardship algorithm) or usual care. We excluded trials if they focused exclusively on children or used procalcitonin for a purpose other than to guide initiation and duration of antibiotic treatment.
Data collection and analysis
Two teams of review authors independently evaluated the methodology and extracted data from primary studies. The primary endpoints were all‐cause mortality and treatment failure at 30 days, for which definitions were harmonised among trials. Secondary endpoints were antibiotic use, antibiotic‐related side effects, and length of hospital stay. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable hierarchical logistic regression adjusted for age, gender, and clinical diagnosis using a fixed‐effect model. The different trials were added as random‐effects into the model. We conducted sensitivity analyses stratified by clinical setting and type of ARI. We also performed an aggregate data meta‐analysis.
Main results
From 32 eligible RCTs including 18 new trials for this 2017 update, we obtained individual participant data from 26 trials including 6708 participants, which we included in the main individual participant data meta‐analysis. We did not obtain individual participant data for four trials, and two trials did not include people with confirmed ARIs. According to GRADE, the quality of the evidence was high for the outcomes mortality and antibiotic exposure, and quality was moderate for the outcomes treatment failure and antibiotic‐related side effects.
Primary endpoints: there were 286 deaths in 3336 procalcitonin‐guided participants (8.6%) compared to 336 in 3372 controls (10.0%), resulting in a significantly lower mortality associated with procalcitonin‐guided therapy (adjusted OR 0.83, 95% CI 0.70 to 0.99, P = 0.037). We could not estimate mortality in primary care trials because only one death was reported in a control group participant. Treatment failure was not significantly lower in procalcitonin‐guided participants (23.0% versus 24.9% in the control group, adjusted OR 0.90, 95% CI 0.80 to 1.01, P = 0.068). Results were similar among subgroups by clinical setting and type of respiratory infection, with no evidence for effect modification (P for interaction > 0.05). Secondary endpoints: procalcitonin guidance was associated with a 2.4‐day reduction in antibiotic exposure (5.7 versus 8.1 days, 95% CI ‐2.71 to ‐2.15, P < 0.001) and lower risk of antibiotic‐related side effects (16.3% versus 22.1%, adjusted OR 0.68, 95% CI 0.57 to 0.82, P < 0.001). Length of hospital stay and intensive care unit stay were similar in both groups. A sensitivity aggregate‐data analysis based on all 32 eligible trials showed similar results.
Authors' conclusions
This updated meta‐analysis of individual participant data from 12 countries shows that the use of procalcitonin to guide initiation and duration of antibiotic treatment results in lower risks of mortality, lower antibiotic consumption, and lower risk for antibiotic‐related side effects. Results were similar for different clinical settings and types of ARIs, thus supporting the use of procalcitonin in the context of antibiotic stewardship in people with ARIs. Future high‐quality research is needed to confirm the results in immunosuppressed patients and patients with non‐respiratory infections.
Plain language summary
Testing blood procalcitonin levels to decide when to start and stop antibiotics in adults with acute respiratory tract infections
Review question
What are the effects of using procalcitonin to start or discontinue antibiotics in people with acute respiratory infections compared to routine care on mortality and treatment failure?
Background
In people with acute respiratory infections, unnecessary antibiotic use significantly contributes to increasing bacterial resistance, medical costs, and the risk of drug‐related adverse events. The blood marker procalcitonin increases in bacterial infections and decreases when patients recover from the infection. Procalcitonin can be measured in the blood of patients by different commercially available assays with a turnaround time of around one to two hours and support clinical decision making about initiation and discontinuation of antibiotic therapy.
Search date
We conducted electronic searches on 10 February 2017. We conducted searches for ongoing trials on 12 April 2017.
Study characteristics
All included trials randomised participants with acute respiratory infections to receive antibiotics based on procalcitonin levels ('procalcitonin‐guided' group) or a control group. The trials were performed in primary care, the emergency department and medical wards, and the intensive care unit. Included participants had acute upper or lower respiratory infections, including pneumonia, bronchitis, exacerbation of chronic obstructive pulmonary disease, and others.
Study funding sources
All studies were investigator‐initiated trials. Half of the trials were funded by national agencies or did not report funding, and half of the trials received funding from the biomarker industry (e.g. Thermo Fisher Scientific).
Key results
We studied 6708 participants from 26 trials in 12 countries. Mortality at 30 days was significantly lower in procalcitonin‐guided participants compared to control participants (286 deaths in 3336 procalcitonin‐guided participants (8.6%) versus 336 deaths in 3372 controls (10.0%)). There was no significant difference with regard to treatment failures. Results were similar for different clinical settings (primary care, emergency department, intensive care unit) and types of respiratory infection. Regarding antibiotic exposure, participants in the procalcitonin‐guided group had a 2.4‐day reduction in antibiotic exposure and a reduction in antibiotic‐related side effects (16.3% versus 22.1%).
Quality of the evidence
The quality of the evidence was high for mortality and antibiotic exposure. Most of the trials did not use blinding, however we did not expect that mortality would be biased by this limitation. The quality of the evidence was moderate for treatment failure and antibiotic‐related side effects because the definitions for these endpoints among trials were not identical.
Summary of findings
Summary of findings for the main comparison. Procalcitonin algorithm compared to standard care for guiding antibiotic therapy in acute respiratory tract infections.
| Procalcitonin algorithm compared to standard care for guiding antibiotic therapy in acute respiratory tract infections | ||||||
| Patient or population: people with acute respiratory tract infections Settings: primary care, emergency department, intensive care unit Intervention: PCT‐guided care Comparison: standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | PCTalgorithm | |||||
| Mortality Follow‐up: 30 days | Study population | OR 0.83 (0.70 to 0.99) | 6708 (26 studies) | ⊕⊕⊕⊕ High1 | ||
| 100 per 1000 |
86 per 1000 (76 to 95) |
|||||
| Treatment failure Clinical assessment3 Follow‐up: 30 days | Study population | OR 0.90 (0.80 to 1.01) | 6708 (26 studies) | ⊕⊕⊕⊝ Moderate2 3 | ||
| 249 per 1000 |
230 per 1000 (216 to 245) |
|||||
|
Antibiotic‐related side effects Follow‐up: 30 days |
Study population 221 per 1000 |
163 per 1000 (145 to 182) |
OR 0.68 (0.57 to 0.82) | 3034 (6 studies) | ⊕⊕⊕⊝ Moderate4 | |
| Antibiotic exposure Total days of antibiotic therapy in all randomised participants | The mean antibiotic exposure in the control groups was 8.1 days. | The mean antibiotic exposure in the intervention groups was 2.43 dayslower (2.15 to 2.71) | ‐ | 6708 (26 studies) | ⊕⊕⊕⊕ High1 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio: PCT: procalcitonin | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1No downgrading for serious concerns. Still, there is some concern about unconcealed allocation in several trials in the emergency department and intensive care settings. There is also some concern about low adherence with the PCT algorithm in the intervention group. We consider unblinded outcome assessment as not relevant for the outcome of death. 2Downgraded one level for serious inconsistency: trials used differing definition of treatment failure and some rare events were not systematically assessed among trials. 3For the primary care setting, treatment failure was defined as death, hospitalisation, acute respiratory infection (ARI)‐specific complications (e.g. empyema for lower ARI, meningitis for upper ARI), recurrent or worsening infection, and participants reporting any symptoms of an ongoing respiratory infection (e.g. fever, cough, dyspnoea) at follow‐up. For the emergency department setting, treatment failure was defined as death, intensive care unit (ICU) admission, rehospitalisation after index hospital discharge, ARI‐associated complications (e.g. empyema or acute respiratory distress syndrome for lower ARI), and recurrent or worsening infection within 30 days of follow‐up. For the ICU setting, treatment failure was defined as death within 30 days of follow‐up. 4Downgraded one level for incomplete reporting: only 6 trials reported side effects from antibiotics, and none of these trials were conducted in the ICU setting.
Background
Acute respiratory infections (ARIs) account for over 10% of global disease burden and are the most common reason for antibiotic therapy in primary care and hospital settings (Evans 2002; Gonzales 1997; Zaas 2014).
Description of the condition
Acute respiratory infections comprise a heterogeneous group of infections including bacterial, viral, and other aetiologies. As many as 75% of all antibiotic doses are prescribed for ARIs, despite their mainly viral cause (Doan 2014; Evans 2002). Early initiation of adequate antibiotic therapy is the cornerstone in the treatment of bacterial ARIs and is associated with improved clinical outcomes (Hoare 2006; Kumar 2006; Kumar 2009; Liberati 2009b; Spurling 2010). However, overuse of antibiotics by overprescription in outpatients with bronchitis (Arnold 2005), for instance, and prolonged duration of antibiotic therapy in people with bacterial ARIs in the hospital and intensive care unit (ICU) settings is associated with increased resistance to common bacteria, high costs, and adverse drug reactions (Gonzales 1997; Goossens 2005; Lawrence 2009; Zaas 2014).
Description of the intervention
The presence of a diagnostic 'gold standard' or reference standard represents the best available method for establishing the presence or absence of a disease. Optimally, a morphological verification such as histopathology or, in the case of ARIs, growth of typical pathogens in blood cultures or sputum cultures can be obtained to establish the 'correct' diagnosis. Regrettably, the use of blood cultures as the assumed gold standard in ARIs lacks sensitivity, specificity, or both, with only around 10% of people with pneumonia having positive cultures and some of them being false positives (Muller 2010). In this diagnostic uncertainty, surrogate biomarker to estimate the likelihood for the presence of a bacterial infection and to grade disease severity are of great interest (Schuetz 2015). In such a circumstance, two fundamentally different concepts are employed. One concept tends to ignore potential dilemmas in the accuracy of the alleged gold standard but assumes a well‐defined illness, which is represented by the assumption drawn following a diagnostic test or a clinical diagnosis. The second concept discards alleged gold standards and focuses on patient outcomes. In the case of ARIs, the clinical benefit of a diagnostic biomarker, such as procalcitonin (PCT), can be measured by clinical outcomes of randomised intervention studies, assuming that if the person recovered without antibiotics then there was no relevant bacterial illness.
In recent years, PCT has emerged as a promising marker for the diagnosis of bacterial infections because higher levels are found in severe bacterial infections but remain fairly low in viral infections and non‐specific inflammatory diseases (Muller 2000; Muller 2001; Muller 2010). Procalcitonin is released in multiple tissues in response to bacterial infections via a direct stimulation of cytokines, such as interleukin (IL)‐1β, tumour necrosis factor (TNF)‐ɑ, and IL‐6. Conversely, PCT production is blocked by interferon gamma, a cytokine released in response to viral infections (Muller 2000). Hence, PCT may be used to support clinical decision making for the initiation and discontinuation of antibiotic therapy in different types of infections and indications (Sager 2017; Schuetz 2016). Randomised controlled trials (RCTs) have demonstrated the feasibility of such a strategy in different ARI patient populations and different settings ranging from primary care to emergency departments and hospital wards to medical and surgical ICUs (Bloos 2016; Branche 2015; Corti 2016; De Jong 2016; Deliberato 2013; Layios 2012; Long 2014; Maravić‐Stojković 2011; Oliveira 2013; Shehabi 2014; Verduri 2015; Wang 2016).
How the intervention might work
Procalcitonin levels correlate with the risk of relevant bacterial infections and decrease upon recovery. Procalcitonin testing may therefore help physicians decide in which patients antibiotics are needed and when it is safe to stop treatment (Kutz 2015). The use of PCT in clinical protocols may thus decrease antibiotic consumption in two ways: by preventing unnecessary antibiotic prescriptions and by limiting durations of antibiotic treatment (Sager 2017; Schuetz 2011a).
Why it is important to do this review
While several RCTs have evaluated PCT‐guided antibiotic treatment, most individual trials included participants with different types of respiratory and non‐respiratory infections and lacked the statistical power to assess the risk for mortality and severe infectious disease complications associated with PCT‐guided decision making. Previous meta‐analyses of RCTs investigating the effect of PCT algorithms on antibiotic use focused on the critical care setting, people with suspicion of bacterial infections, and people with sepsis and respiratory infections (Heyland 2011; Hoeboer 2015; Tang 2009; Wacker 2013). However, these meta‐analyses used aggregated data and were not able to investigate the effects of PCT on different ARI diagnoses and on outcomes other than mortality. A previous meta‐analysis based on individual participant data published in the Cochrane Library did not find a significant difference in clinical outcomes, but confidence intervals remained relatively wide (Schuetz 2012). Safety of using PCT for antibiotic decision making remained thus unproven.
Objectives
The aim of this systematic review based on individual participant data was to assess the safety and efficacy of using procalcitonin for starting or stopping antibiotics over a large range of patients with varying severity of ARIs and from different clinical settings.
Methods
Criteria for considering studies for this review
Types of studies
Prospective RCTs comparing a strategy to initiate or discontinue antibiotic therapy based on PCT levels with a control arm without PCT measurements were eligible for inclusion. Participants were randomised to receive antibiotics either based on PCT levels ('PCT‐guided' group) or a control group without knowledge of PCT levels, including antibiotic management based on usual care or guidelines. We did not include non‐randomised studies.
Types of participants
We included adult participants with clinical diagnoses of ARIs: either a lower ARI including community‐acquired pneumonia (CAP), hospital‐acquired pneumonia (HAP), ventilator‐associated pneumonia (VAP), acute bronchitis, exacerbation of asthma, or exacerbation of chronic obstructive pulmonary disease (COPD); or an upper ARI including common cold, rhino‐sinusitis, pharyngitis, tonsillitis, or otitis media. We also included people with sepsis and suspected ARIs in the analyses. We excluded trials if they focused exclusively on children or used PCT to escalate antibiotic therapy. We made no exclusions based on language of reports or clinical setting. We included trials from primary care, emergency departments, and medical and surgical ICUs.
Types of interventions
Strategies to initiate or discontinue antibiotic therapy based on PCT levels compared with usual care were eligible.
Types of outcome measures
We defined primary and secondary outcomes to a follow‐up time of 30 days. For trials with shorter follow‐up periods, we used the available information (i.e. until hospital discharge). We excluded all trials with different follow‐up times for mortality in a sensitivity analysis.
Primary outcomes
All‐cause mortality following randomisation up to a follow‐up time of 30 days.
Setting‐specific treatment failure within 30 days of inclusion.
For the primary care setting, we defined treatment failure as death, hospitalisation, ARI‐specific complications (e.g. empyema for lower ARIs, meningitis for upper ARIs), recurrent or worsening infection, and still having ARI‐associated discomfort at 30 days. For the emergency department setting, we defined treatment failure as death, ICU admission, rehospitalisation after index hospital discharge, ARI‐associated complications (e.g. empyema or acute respiratory distress syndrome for lower ARIs), and recurrent or worsening infection within 30 days of follow‐up. For the medical and surgical ICU setting, we defined treatment failure as death within 30 days of follow‐up and recurrent or worsening infection.
Secondary outcomes
Antibiotic use (initiation of antibiotics, duration of antibiotics, and total exposure to antibiotics (total amount of antibiotic days divided by total number of participants)).
Length of hospital stay for hospitalised participants.
Length of ICU stay for critically ill participants.
Number of days with restricted activities within 14 days after randomisation for primary care participants.
Antibiotic‐related side effects.
Search methods for identification of studies
We updated the search strategy for this review in February 2017 in collaboration with the Cochrane Acute Respiratory Infections Group's Information Specialist. We performed data collection based on the protocol of a previous meta‐analysis of individual participant data published in the Cochrane Library (Schuetz 2008).
Electronic searches
We updated the searches for this review in February 2017, running the search across all databases from the date of inception to 10 February 2017. We screened all new references identified by the search. We searched the following databases for published studies:
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 1), part of the Cochrane Library, which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, www.cochranelibrary.com/ (accessed 10 February 2017) (Appendix 1);
MEDLINE Ovid (1966 to 10 February 2017) (Appendix 2);
Embase.com (1980 to 10 February 2017) (Appendix 3).
We used the search strategy in Appendix 4 to conduct searches for the 2012 version of this review (Schuetz 2012).
We also searched for ongoing and completed trials in the following trial register:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/; searched 12 April 2017).
We did not apply any language or publication restrictions.
Searching other resources
We contacted experts for further eligible trials.
Data collection and analysis
We requested individual participant data from the investigators of all included trials. We checked all provided data against published reports, and if needed, corrected any discrepancies.
We prepared this review update according to PRISMA guidelines and the PRISMA‐IPD guideline (Liberati 2009a; Stewart 2015).
Selection of studies
At least two review authors (RS, YW, PS) independently assessed trial eligibility based on titles, abstracts, full‐text reports, and further information from investigators as needed.
Data extraction and management
We checked data from each trial against reported results and resolved any queries with the principal investigator, trial data manager, or statistician. The mortality and adverse outcome rates from trials included in this review may differ slightly from previous reports because we treated data in a consistent manner across all trials.
Assessment of risk of bias in included studies
Two review authors (RS, YW) assessed the methodological quality of each included study using the Cochrane 'Risk of bias' tool and resolved any disagreements by discussion (Higgins 2011). Methodological criteria included: adequate sequence generation and concealment of treatment allocation; blinding of participants, physicians and clinical outcome assessment; whether the study was free of selective reporting; and the proportion of participants lost to follow‐up. We documented the proportion of participants in the PCT group that adhered to the PCT algorithm used in each study, defining adherence to the PCT algorithm of lower than 70% as high risk of bias, and, if not reported, as unclear risk. Due to the study design of the included studies, physicians were aware of the participants' study group because in the intervention group physicians used the PCT result for decision making about antibiotic treatment, while in the control group no PCT result was communicated to the physicians. Blinding of physicians was therefore not feasible, resulting in an unclear risk for performance bias in all studies.
We assessed the quality of evidence at the outcome level using the GRADE approach (GRADEpro GDT 2014).
Measures of treatment effect
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using multivariable hierarchical logistic regression for the co‐primary endpoints of mortality from any cause and treatment failure (Thompson 2001; Turner 2000). We fitted corresponding linear and logistic regression models for continuous and binary secondary endpoints, respectively. We calculated Kaplan‐Meier curves for time to death for graphical display.
We used Stata version 12.1 (College Station, TX) for statistical analyses (Stata 12.1).
Unit of analysis issues
The unit of our primary analysis was the individual study participant. We analysed all participants in the study group to which they were randomised. We calculated summary estimates using aggregated data from individual trials as a sensitivity analysis.
Dealing with missing data
We received the full data sets from all trials included in the individual participant data analysis (n = 26) with all available follow‐up information (if recorded in the trials).
We assumed in our main analysis that participants lost to follow‐up did not experience an event. We explored if a complete‐case analysis (excluding participants lost to follow‐up) or an analysis assuming that participants lost to follow‐up experienced an event would change the results for the primary outcomes of mortality and treatment failure in sensitivity analyses. We checked all individual participant data against the published results but did not find significant differences that warranted further exploration.
Assessment of heterogeneity
We performed prespecified analyses stratified by clinical setting (i.e. primary care, emergency department, ICU) and ARI diagnosis (CAP, COPD, bronchitis, VAP) to investigate the consistency of results across our heterogeneous patient populations in terms of disease severity. We formally tested for potential subgroup effects by adding the clinical setting and ARI diagnosis in turn to the regression model together with the corresponding interaction term with the PCT group as a fixed‐effect model. We assessed heterogeneity by estimating the I2 statistic (the percentage of total variance across trials that is due to heterogeneity rather than chance) in meta‐analyses using aggregated data and by testing for heterogeneity using the Cochran Q test (Higgins 2003).
Assessment of reporting biases
We assessed reporting bias by attempting to identify if the study was included in a trial registry, a protocol was available, and if the methods section provided a list of outcomes. We compared listed outcomes from those sources to outcomes reported in the published papers.
Data synthesis
We used multivariable hierarchical logistic regression to combine participant data from the trials (Thompson 2001; Turner 2000). Apart from the group variable indicating the use of a PCT algorithm, we included important prognostic factors such as participant age and ARI diagnosis as an additional fixed effect; to account for within‐ and between‐trial variability, we added a categorical trial variable to the model as a random effect. In meta‐analyses with aggregated trial data we calculated summary ORs using a random‐effects model and the Mantel‐Haenszel facility of Review Manager 5 (RevMan 2014).
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: all‐cause mortality at 30 days, setting‐specific treatment failure at 30 days, total exposure to antibiotics, and antibiotic‐related side effects (Table 1). The results reported in this table correspond to the main IPD analysis and are slightly different from the aggregate data analysis. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed prespecified analyses stratified by clinical setting and ARI diagnosis and formally tested for potential subgroup effects by adding an interaction term into the statistical model.
Sensitivity analysis
We performed prespecified sensitivity analyses based on the main quality indicators: allocation concealment, blinded outcome assessment, adherence to the PCT algorithm (we defined low adherence to PCT algorithms as < 70%), and follow‐up time for mortality other than one month. We also performed an aggregate data meta‐analysis using all trials with potentially eligible participants.
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
After removal of duplicates, we identified 998 records that we further assessed based on title and abstracts, excluding 919 records. We obtained 79 full‐text study reports, and following assessment excluded 39 that did not meet our inclusion criteria. Eight studies were ongoing trials. From 32 eligible RCTs (9909 participants) including 18 new trials for this 2017 update, we obtained individual participant data from 26 trials including 6708 participants, which were included in the main individual participant data meta‐analysis (see Figure 1). We did not obtain individual participant data for four trials, and two trials did not include participants with confirmed ARIs. The sensitivity aggregate analysis includes all 32 trials.
1.
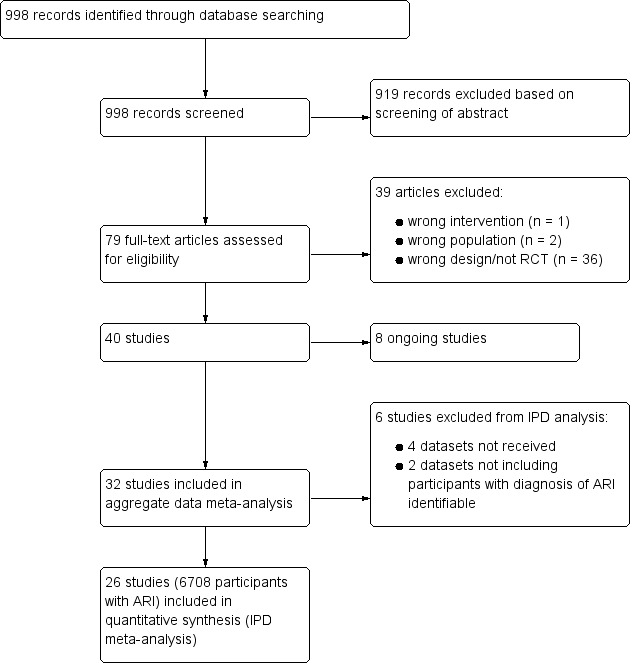
Study flow diagram.
Abbreviations: ARI: acute respiratory infection; IPD: individual participant data; RCT: randomised controlled trial
Included studies
We included a total of 26 studies involving 6708 participants in the main individual participant data meta‐analysis. Study characteristics are presented in Table 2.
1. Characteristics of included trials.
| Study ID | Country | Setting, type of trial | Clinical diagnosis | Type of PCTalgorithm and PCTcut‐offs used (µg/L) | N: ARI participants (study total) | Primary endpoint | Follow‐up time | Reasons for exclusion of patients |
| Annane 2013 | France | ICU, multicentre | Severe sepsis without overt source of infection and negative blood culture | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 5.0) | 0 (62) | Participants on AB on day 5 post randomisation | Hospital stay | 62 non‐ARI patients (4 of them with post randomisation consent withdrawal) |
| Bloos 2016 | Germany | ICU, multicentre | Severe sepsis or septic shock | Discontinuation at day 4, 7, and 10; R against AB: < 1.0 or > 50% drop to previous value | 219 (1180) | 28‐day mortality | 3 months | 91 post randomisation exclusions (informed consent not obtainable), 870 not ARI patients |
| Bouadma 2010 | France | ICU, multicentre | Suspected bacterial infections during ICU stay without prior AB (> 24 h) | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 394 (630) | All‐cause mortality | 2 months | 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice); 227 non‐ARI patients |
| Branche 2015 | USA | ED, medical ward, single centre | Lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 265 (300) | Antibiotic exposure and safety | 3 months | 35 non‐ARI patients |
| Briel 2008 | Switzerland | Primary care, multicentre | Upper and lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 458 (458) | Days with restricted activities | 1 month | No exclusions |
| Burkhardt 2010 | Germany | Primary care, multicentre | Upper and lower ARI | Initiation; R against AB: < 0.25; R for AB: > 0.25 | 550 (571) | Days with restricted activities | 1 month | 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) |
| Christ‐Crain 2004 | Switzerland | ED, single centre | Lower ARI with X‐ray confirmation | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 219 (243) | AB use | 2 weeks | 24 non‐ARI patients |
| Christ‐Crain 2006 | Switzerland | ED, medical ward, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 286 (302) | AB use | 6 weeks | 16 non‐ARI patients |
| Corti 2016 | Denmark | ED, single centre | AECOPD | Initiation and duration; R against AB < 0.25 (0.15)/80% decrease; R for AB > 0.25 | 120 (120) | AB use | 28 days | No exclusions |
| De Jong 2016 | Netherlands | ICU, multicentre | Critically ill patients with presumed infection | Duration; R against AB: < 0.5 or > 80% drop | 994 (1575) | AB use | 1 year | 29 post randomisation exclusions (25 protocol violations, 4 withdrew informed consent), 552 non‐ARI patients |
| Deliberato 2013 | Brazil | ICU, single centre | Septic patients with proven bacterial infection | Duration; R against AB: < 0.5 or > 90% drop | 66 (81) | AB use | ICU discharge or 14 days' post randomisation | 15 non‐ARI patients |
| Ding 2013 | China | ICU, single centre | Acute exacerbation of pulmonary fibrosis | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 0 (78) | AB use | 1 month | 10 post randomisation exclusions (7 lost to follow‐up, 3 withdrew informed consent), 68 data not shared |
| Hochreiter 2009 | Germany | Surgical ICU, single centre | Suspected bacterial infections and > 1 SIRS criteria | Duration; R against AB: < 1 or > 65% drop over 3 d | 43 (110) | AB use | Hospital stay | 67 non‐ARI patients |
| Kristoffersen 2009 | Denmark | ED, medical ward, multicentre | Lower ARI without X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 (> 0.5) | 210 (223) | AB use | Hospital stay | 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) |
| Layios 2012 | Belgium | ICU, single centre | Suspected infection | Initiation; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 160 (509) | AB use | 1 month | 120 no PCT measurements, 10 missing data, 219 non‐ARI patients |
| Lima 2016 | Brazil | ED, medical ward, single centre | Febrile neutropenia | Duration; R against AB: < 0.5 for 2 days or > 90% drop than highest measured concentration | 0 (62) | AB use | 28 days | 1 post randomisation exclusion (withdrew informed consent), 62 non‐ARI patients |
| Long 2009 | China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 127 (149) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent |
| Long 2011 | China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 156 (172) | AB use | 1 month | 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) |
| Long 2014 | China | ED, single centre | Severe acute exacerbation of asthma | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 180 (180) | AB use | 1 year | No exclusions |
| Maravić‐Stojković 2011 | Serbia | ICU surgical, single centre | Infection after open heart surgery | Initiation; R for AB: > 0.5 | 5 (205) | AB use, AB cost | Hospital stay | 200 non‐ARI patients |
| Najafi 2015 | Iran | ICU, single centre | SIRS without apparent source of infection | Initiation; R for AB: > 2 | 0 (60) | AB use | Hospital stay | 60 patient data not shared |
| Nobre 2008 | Switzerland | ICU, single centre | Suspected severe sepsis or septic shock | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 52 (79) | AB use | 1 month | 27 non‐ARI patients |
| Ogasawara 2014 | Japan | Medical ward, single centre | Aspiration pneumonia | Predefined duration; AB for 3 d: < 0.5; AB for 5 d: 0.5 to 1.0; AB for 7 d: > 1 | 0 (105) | Relapse and 30‐day mortality | 1 month | 9 post randomisation exclusions (2 withdrew consent, 7 others), 96 data not shared |
| Oliveira 2013 | Brazil | ICU, multicentre | Severe sepsis or septic shock | Discontinuation; initial < 1.0: R against AB: 0.1 at day 4; initial > 1.0: R against: > 90% drop | 58 (97) | AB use | 28 days or hospital discharge | 3 post randomisation exclusions (2 withdrew consent, 1 technical problems), 36 patients with a final diagnosis other than ARI |
| Schroeder 2009 | Germany | Surgical ICU, single centre | Severe sepsis following abdominal surgery | Duration; R against AB: < 1 or > 65% drop over 3 d | 8 (27) | AB use | Hospital stay | 19 non‐ARI patients |
| Schuetz 2009 | Switzerland | ED, medical ward, multicentre | Lower ARI with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 1304 (1381) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent, 55 non‐ARI patients |
| Shehabi 2014 | Australia | ICU, multicentre | Suspected sepsis, undifferentiated infections | Duration; R against AB: < 0.25 (< 0.1) or > 90% drop | 156 (400) | AB use | 3 months | 6 post randomisation exclusions (6 withdrew consent), 238 non‐ARI patients |
| Stolz 2007 | Switzerland | ED, medical ward, single centre | Exacerbated COPD | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 208 (226) | AB use | 2 to 3 weeks | 18 post randomisation exclusions (absence of COPD) |
| Stolz 2009 | Switzerland, USA | ICU, multicentre | VAP when intubated > 48 h | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 101 (101) | AB‐free days alive | 1 month | No exclusions |
| Tang 2013 | China | ED, single centre | Exacerbation of asthma | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 0 (265) | AB use | 6 weeks | 10 post randomisation exclusions (5 lost to follow‐up, 3 died, 2 withdrew consent), 255 data not shared |
| Verduri 2015 | Italy | ED, medical ward, multicentre | AECOPD | Initiation; R against AB:< 0.1; R for AB: > 0.25 | 178 (183) | Number of exacerbations | 6 months | 5 post randomisation exclusions (5 lost to follow‐up because they did not meet the inclusion criteria) |
| Wang 2016 | China | ICU, single centre | AECOPD | All participants had initial PCT < 0.1; AB group treated with AB for at least 3 days, control group no AB in the first 10 days | 191 (194) | Treatment success within 10 days | 30 days | 3 post randomisation exclusions (3 with pneumonia according to CT scan) |
AB: antibiotic AECOPD: acute exacerbation of chronic obstructive pulmonary disease ARI: acute respiratory infection CAP: community‐acquired pneumonia COPD: chronic obstructive pulmonary disease CT: computed tomography d: days ED: emergency department h: hours ICU: intensive care unit PCT: procalcitonin R: recommendation for or against antibiotics SIRS: systemic inflammatory response syndrome VAP: ventilator‐associated pneumonia
Participants
Baseline characteristics of included participants were similar in the PCT and control groups with respect to important prognostic features (Table 3). Most participants were recruited either in the emergency department or ICU setting, and CAP was the most frequent ARI diagnosis, reported in more than 40% of participants.
2. Baseline characteristics of included participants.
| Parameter | Control (n = 3372) | PCT group (n = 3336) |
| Demographics | ||
| Age (year), mean (SD) | 61.2 ± 18.4 | 60.7 ± 18.8 |
| Male gender, n (%) | 1910 (56.6%) | 1898 (56.9%) |
| Clinical setting, no (%) | ||
| Primary care | 501 (14.9%) | 507 (15.2%) |
| Emergency department | 1638 (48.6%) | 1615 (48.4%) |
| Intensive care unit | 1233 (36.6%) | 1214 (36.4%) |
| Primary diagnosis | ||
| Total upper ARI, n (%) | 280 (8.3%) | 292 (8.8%) |
| Common cold | 156 (4.6%) | 149 (4.5%) |
| Rhino‐sinusitis, otitis | 67 (2.0%) | 73 (2.2%) |
| Pharyngitis, tonsillitis | 46 (1.4%) | 61 (1.8%) |
| Total lower ARI, n (%) | 3092 (91.7%) | 3044 (91.2%) |
| Community‐acquired pneumonia | 1468 (43.5%) | 1442 (43.2%) |
| Hospital‐acquired pneumonia | 262 (7.8%) | 243 (7.3%) |
| Ventilator‐associated pneumonia | 186 (5.5%) | 194 (5.8%) |
| Acute bronchitis | 287 (8.5%) | 257 (7.7%) |
| Exacerbation of COPD | 631 (18.7%) | 621 (18.6%) |
| Exacerbation of asthma | 127 (3.8%) | 143 (4.3%) |
| Other lower ARI | 131 (3.9%) | 144 (4.3%) |
| Procalcitonin upon enrolment | ||
| PCT< 0.1 ug/L | 921 (35.6%) | 981 (30.9%) |
| PCT 0.1 to 0.25 ug/L | 521 (20.1%) | 608 (19.2%) |
| PCT > 0.25 to 0.5 ug/L | 308 (11.9%) | 383 (12.1%) |
| PCT > 0.5 to 2.0 ug/L | 358 (13.8%) | 520 (16.4%) |
| PCT > 2.0 ug/L | 482 (18.6%) | 679 (21.4%) |
ARI: acute respiratory infection COPD: chronic obstructive pulmonary disease PCT: procalcitonin SD: standard deviation
Settings
Trials were conducted in 12 countries: Switzerland, Germany, France, Italy, USA, China, Denmark, Netherlands, Brazil, Belgium, Australia, and Serbia. Trials were conducted in different clinical settings including primary care, emergency departments and medical wards, and ICU. There were two primary care trials with upper and lower respiratory infection patients; 11 emergency department and medical ward trials with lower ARI patients; and 13 ICU trials with mostly septic patients due to infections of the lower respiratory tract.
Interventions
Procalcitonin algorithms used in the different trials were similar in concept and recommended initiation and/or continuation of antibiotic therapy based on similar PCT cut‐off levels (reviewed in Schuetz 2011a). However, there were differences: some trials in primary care and the emergency department used only a single PCT measurement on admission to guide initiation of antibiotics, while the other trials (predominantly in hospitalised patients with severe infections) used repeated measurements for guiding the duration of treatment. One trial used a point‐of‐care device (Corti 2016). Adherence to algorithms varied, ranging from 44% to 100% (Table 4).
3. Quality assessment of trials.
| Study ID | Allocation concealment | Blinded outcome assessment | Follow‐up for mortality | Adherence to PCT algorithm in PCT group | Follow‐up for mortality |
| Annane 2013 | Yes (central randomisation) | No | 58/58 (100%) | 63% adherence | LOS |
| Bloos 2016 | Yes (central randomisation) | No | 1045/1089 (96%) | 49.6% adherence | 28 days and 90 days |
| Bouadma 2010 | Yes (central randomisation) | Yes | 393/394 (100%) | 47% adherence | 28 days and 60 days |
| Branche 2015 | Yes (central randomisation using blocks of 4) | No | 250/300 (83.3%) | 64% adherence | 1 month and 3 months |
| Briel 2008 | Yes (central randomisation) | Yes | 454/458 (99%) | 85% adherence | 28 days |
| Burkhardt 2010 | Yes (central randomisation) | Yes | 546/550 (99%) | 87% adherence | 28 days |
| Christ‐Crain 2004 | No (alternating weeks) | No | 230/243 (95%) | 83% adherence | 10 to 14 days |
| Christ‐Crain 2006 | Yes (sequentially numbered, opaque, sealed envelopes) | No | 300/302 (99%) | 87% adherence | 56 days |
| Corti 2016 | Yes (randomisation algorithm was concealed to treating clinicians and participants) | No | 120/120 (100%) | 61.1% adherence | 28 days |
| De Jong 2016 | Yes (central randomisation) | No | 1546/1546 (100%) | 44% adherence | 28 days and 1 year |
| Deliberato 2013 | Yes (opaque, sealed envelopes) | No | 81/81 (100%) | 52% adherence | LOS |
| Ding 2013 | Yes (central randomisation) | No | 68/78 (87.2%) | Not reported | 30 days |
| Hochreiter 2009 | No (unconcealed drawing of lots) | No | 43/43 (100% until discharge) | Not reported | LOS |
| Kristoffersen 2009 | Yes (central randomisation) | No | 210/210 (100% until discharge) | 59% adherence | LOS |
| Layios 2012 | Not reported | No | 509/509 (100%) | Not reported | Intensive care unit LOS |
| Lima 2016 | Yes (sequentially numbered, opaque, sealed envelopes) | No | 61/62 (98.4%) | 73.3% adherence | 28 days and 90 days |
| Long 2009 | No (odd and even patient ID numbers) | No | 127/127 (100%) | Not reported | Not reported |
| Long 2011 | No (odd and even patient ID numbers) | No | 156/156 (100%) | Not reported | 28 days |
| Long 2014 | Yes (central randomisation) | No | 169/180 (93.9%) | 96.6% adherence | LOS and 1 year |
| Maravić‐Stojković 2011 | Yes (central randomisation) | No | 205/205 (100%) | Not reported | 30 days and LOS |
| Najafi 2015 | Yes (central randomisation) | No | 30/30 (100%) | Not reported | LOS |
| Nobre 2008 | Yes (sequentially numbered, opaque, sealed envelopes) | No | 52/52 (100%) | 81% adherence | 28 days and LOS |
| Ogasawara 2014 | Not reported | No | 96/96 (100%) | Not reported | 30 days |
| Oliveira 2013 | Yes (central randomisation) | No | 94/94 (100%) | 86.2% adherence | 28 days |
| Schroeder 2009 | No (unconcealed drawing of lots) | No | 8/8 (100% until discharge) | Not reported | LOS |
| Schuetz 2009 | Yes (central randomisation) | Yes | 1358/1359 (100%) | 91% adherence | 28 days |
| Shehabi 2014 | Yes (central randomisation) | Yes | 394/394 (100%) | Not reported | LOS and 90 days |
| Stolz 2007 | Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 208/208 (100%) | Not reported | 6 months |
| Stolz 2009 | Yes (sequentially numbered, opaque, sealed envelopes) | No | 101/101 (100%) | Not reported | 28 days |
| Tang 2013 | Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 258/265 (97.4%) | Not reported | 6 weeks |
| Verduri 2015 | Yes (central randomisation) | No | 178/178 (100%) | Not reported | 6 months |
| Wang 2016 | Yes (those responsible for allocation concealment were not involved in the measurement of results) | No | 191/191 (100%) | 82.3% adherence (17 participants in the control group received AB) | 30 days |
LOS: length of stay PCT: procalcitonin
Comparators
In control group participants, PCT was not used to guide treatment decisions, but this decision was up to the treating physician team. In some trials, physicians were asked to follow antibiotic guidelines for control group participants (Briel 2008; Schuetz 2009). In one trial, the control group was guided with C‐reactive protein levels (Oliveira 2013).
Funding sources
All studies were investigator‐initiated trials. Half of the trials were funded by national agencies or did not report funding; the other half of the trials received funding from the biomarker industry (e.g. Thermo Fisher Scientific).
Excluded studies
We excluded a total of 39 studies due to wrong intervention (n = 1), wrong population (n = 2), and wrong design (not RCT) (n = 36). A total of nine studies reported as ongoing in the 2012 review were now available for assessment; we included four of these studies in this current update (Annane 2013; Bloos 2016; De Jong 2016; Lima 2016), and did not include five studies due to wrong population (paediatrics).
Ongoing studies
Our searches of the trial register identified seven ongoing studies that we will assess for inclusion for the next review update (Ongoing studies). These studies focus on the utility of PCT in people with pneumonitis (NCT02862314), pulmonary embolism (NCT02261610), lower respiratory infection (NCT02130986), heart failure (NCT02787603), and intraoperative positive‐end expiratory pressure optimisation (NCT02931409). Two trials are antibiotic efficacy trials (NCT02332577; NCT02440828).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and Figure 3. The risk of bias was mostly low for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting; unclear for blinding of personnel in all studies; and mostly high for blinding of outcome assessment.
2.
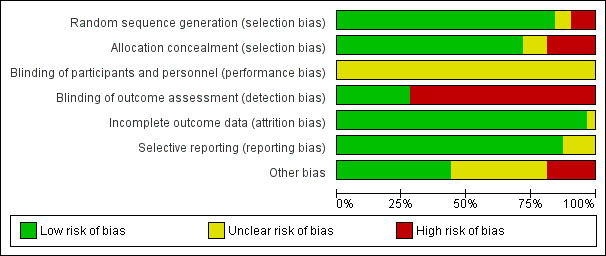
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies randomised participants to intervention (PCT testing) or control groups. A total of 25 trials with mainly computer‐generated lists and centralised randomisation were at low risk of selection bias. Seven trials were at high or unclear risk of selection bias. Risk for selection bias with regard to random sequence generation was due to weekly allocation (Christ‐Crain 2004), unnumbered envelopes (Christ‐Crain 2006; Stolz 2007), use of odd and even patient identification numbers (Long 2009; Long 2011), and unconcealed drawing of lots (Hochreiter 2009; Schroeder 2009).
Blinding
None of the included trials blinded physicians to group allocation because PCT was used for decision making in the intervention group, thus all trials had unclear risk for blinding of participants and personnel.
All trials used blinded outcome assessment (Briel 2008; Bouadma 2010; Branche 2015; Layios 2012; Schuetz 2009; Shehabi 2014; Stolz 2007; Tang 2013), employing blinded telephone interviews to assess vital status and other outcomes.
Incomplete outcome data
The included trials had a high follow‐up for mortality with few participants lost to follow‐up (Table 4). In seven trials, outcome assessment was done after hospital or ICU discharge (Deliberato 2013; Hochreiter 2009; Kristoffersen 2009; Layios 2012; Long 2009; Schroeder 2009; Shehabi 2014). One trial had a high number of post randomisation exclusions (six in the intervention arm versus four in the control group) and thus had an unclear risk of bias (Ding 2013).
Selective reporting
No reporting bias was found when study protocols and final results were compared. However, we did not find registration numbers for four trials (Ding 2013; Layios 2012; Maravić‐Stojković 2011; Najafi 2015), which we considered to be at unclear risk of bias. We found no evidence of reporting bias by visual inspection of funnel plots (Figure 4).
4.

Funnel plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.
Other potential sources of bias
Another potential source of bias relates to low adherence to the PCT algorithms, particularly for safety endpoints. Overall, adherence varied, ranging from 44% to 100% (Table 4).
With regard to funding, 16 trials reported no industry funding (six did not report any funding, 10 reported public funding), and in 16 trials Thermo Fisher, the producer of the PCT assay, funded or co funded the studies by providing free‐of‐charge PCT kits or additional research funds, or both.
Effects of interventions
See: Table 1
Primary outcomes
1. All‐cause mortality following randomisation up to a follow‐up time of 30 days
There were 286 deaths in 3336 PCT‐guided participants (8.6%) compared to 336 in 3372 controls (10.0%) resulting in a significantly lower mortality associated with PCT‐guided therapy (adjusted odds ratio (OR) 0.83, 95% confidence interval (CI) 0.70 to 0.99, P = 0.037) (Table 5). This effect was consistent across clinical settings (P for interaction > 0.05), although mortality could not be estimated in primary care trials because only one death was reported in a control group participant. The effect on mortality was also consistent among different ARI diagnoses (CAP, COPD, bronchitis, VAP) (P for interaction > 0.05). As a further sensitivity analysis and to investigate heterogeneity among trials, we also calculated an aggregate data meta‐analysis based on the aggregate results of all 32 potentially eligible trials (thus not limited to ARI participants only). In this analysis, the results proved robust, although the mortality estimate did not reach statistical significance (OR 0.89, 95% CI 0.78 to 1.01; Analysis 1.1; Figure 5). There was no evidence of heterogeneity (I2 = 0%).
4. Clinical endpoints overall and stratified by setting and ARI diagnosis.
| Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction | |
| Overall | 3372 | 3336 | ||
| 30 days mortality, n (%) | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Treatment failure, n (%) | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Length of ICU stay, mean (±SD) | 13.3 ± 16.0 | 13.7 ± 17.2 | 0.39 (‐0.81 to 1.58), P = 0.524 | NA |
| Length of hospital stay, mean (±SD) | 13.7 ± 20.6 | 13.4 ± 18.4 | ‐0.19 (‐0.96 to 0.58), P = 0.626 | NA |
| Antibiotic‐related side effects, n (%) | 336 (22.1%) | 247 (16.3%) | 0.68 (0.57 to 0.82), P < 0.001 | NA |
| According to setting | ||||
| Primary care | 501 | 507 | ||
| 30 days mortality, n (%) | 1 (0.2%) | 0 (0.0%) | NA | NA |
| Treatment failure, n (%) | 164 (32.7%) | 159 (31.4%) | 0.96 (0.73 to 1.25), P = 0.751 | 0.715 |
| Days with restricted activities, mean (±SD) | 8.9 ± 4.2 | 8.9 ± 4.1 | 0.07 (‐0.44 to 0.59), P = 0.777 | NA |
| Antibiotic‐related side effects, n (%) | 128 (25.7%) | 102 (20.2%) | 0.65 (0.46 to 0.91), P = 0.012 | 0.596 |
| Emergency department | 1638 | 1615 | ||
| 30 days mortality, n (%) | 62 (3.8%) | 57 (3.5%) | 0.91 (0.63 to 1.33), P = 0.635 | 0.546 |
| Treatment failure, n (%) | 292 (17.8%) | 259 (16.0%) | 0.87 (0.72 to 1.05), P = 0.141 | 0.807 |
| Length of hospital stay, mean (±SD) | 8.2 ± 10.5 | 8.1 ± 7.5 | ‐0.14 (‐0.73 to 0.44), P = 0.631 | 0.684 |
| Antibiotic‐related side effects, n (%) | 208 (20.3%) | 145 (14.4%) | 0.66 (0.52 to 0.83), P = 0.001 | 0.596 |
| Intensive care unit | 1233 | 1214 | ||
| 30 days mortality, n (%) | 273 (22.3%) | 229 (19.0%) | 0.84 (0.69 to 1.02), P = 0.081 | 0.619 |
| Length of ICU stay, mean (±SD) | 14.8 ± 16.2 | 15.3 ± 17.5 | 0.56 (‐0.82 to 1.93), P = 0.427 | 0.849 |
| Length of hospital stay, mean (±SD) | 26.3 ± 26.9 | 25.8 ± 23.9 | ‐0.33 (‐2.28 to 1.62), P = 0.739 | 0.641 |
| According to diagnosis | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| 30 days mortality, n (%) | 206 (14.1%) | 175 (12.2%) | 0.82 (0.66 to 1.03), P = 0.083 | 0.958 |
| Treatment failure, n (%) | 385 (26.2%) | 317 (22.0%) | 0.78 (0.66 to 0.93), P = 0.005 | 0.052 |
| Length of ICU stay, mean (±SD) | 10.5 ± 10.3 | 11.9 ± 13.3 | 1.45 (0.15 to 2.75), P = 0.029 | 0.119 |
| Length of hospital stay, mean (±SD) | 13.3 ± 15.7 | 13.9 ± 16.1 | 0.74 (‐0.25 to 1.73), P = 0.143 | 0.094 |
| Antibiotic‐related side effects, n (%) | 186 (27.7%) | 127 (19.1%) | 0.62 (0.48 to 0.80), P < 0.001 | 0.227 |
| Exacerbation of COPD | 631 | 621 | ||
| 30 days mortality, n (%) | 24 (3.8%) | 19 (3.1%) | 0.8 (0.43 to 1.48), P = 0.472 | 0.847 |
| Treatment failure, n (%) | 110 (17.4%) | 104 (16.7%) | 0.94 (0.70 to 1.27), P = 0.704 | 0.676 |
| Length of hospital stay, mean (±SD) | 9.3 ± 13.9 | 8.4 ± 7.2 | ‐0.60 (‐1.84 to 0.64), P = 0.342 | 0.658 |
| Antibiotic‐related side effects, n (%) | 30 (10.9%) | 29 (10.5%) | 0.93 (0.53 to 1.63), P = 0.805 | 0.198 |
| Acute bronchitis | 287 | 257 | ||
| 30 days mortality, n (%) | 0 (0.0%) | 2 (0.8%) | NA | NA |
| Treatment failure, n (%) | 55 (19.2%) | 52 (20.2%) | 1.11 (0.72 to 1.70), P = 0.643 | 0.4 |
| Length of hospital stay, mean (±SD) | 2.6 ± 5.7 | 2.2 ± 4.7 | ‐0.21 (‐0.90 to 0.48), P = 0.556 | 0.97 |
| Antibiotic‐related side effects, n (%) | 54 (21.6%) | 39 (17.3%) | 0.77 (0.49 to 1.22), P = 0.263 | 0.657 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| 30 days mortality, n (%) | 29 (15.6%) | 23 (12.0%) | 0.75 (0.41 to 1.39), P = 0.366 | 0.644 |
| Treatment failure, n (%) | 51 (27.4%) | 44 (22.7%) | 0.78 (0.48 to 1.28), P = 0.332 | 0.522 |
| Length of ICU stay, mean (±SD) | 23.5 ± 20.5 | 21.8 ± 19.1 | ‐1.74 (‐5.64 to 2.17), P = 0.383 | 0.441 |
| Length of hospital stay, mean (±SD) | 33.8 ± 27.6 | 32.0 ± 23.1 | ‐2.14 (‐7.04 to 2.75), P = 0.391 | 0.448 |
Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals
ARI: acute respiratory infection CI: confidence interval COPD: chronic obstructive pulmonary disease ICU: intensive care unit NA: not applicable OR: odds ratio PCT: procalcitonin SD: standard deviation
1.1. Analysis.

Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 1 Mortality at 30 days.
5.

Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.
2. Setting‐specific treatment failure within 30 days of inclusion
Treatment failure was not significantly lower in PCT‐guided participants (23.0% versus 24.9%, adjusted OR 0.90, 95% CI 0.80 to 1.01, P = 0.068). These results were similar among subgroups by clinical setting and type of respiratory infection (P for interaction > 0.05). With an OR of 0.90 (95% CI 0.81 to 0.99), treatment failure was significantly lower in PCT group participants in an aggregate data meta‐analysis based on all 32 potentially eligible trials (thus relying on the original definition of treatment failure as used in the trials). There was no evidence of heterogeneity (I2 = 0%) (Figure 6). We also performed several predefined sensitivity analyses, which showed no evidence for interactions (see summary in Table 6).
6.
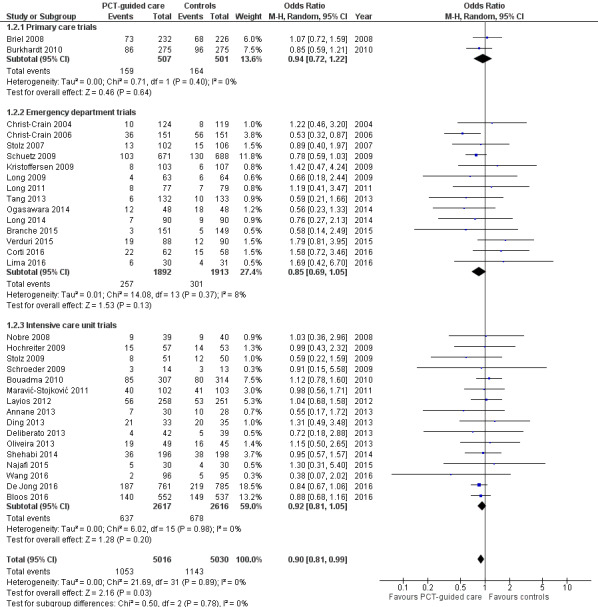
Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.2 Treatment failure at 30 days.
5. Sensitivity analysis.
| Mortality | ||||
| Main analysis | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Adherence | ||||
| High adherence | 82 (4.5%) | 75 (4.1%) | 0.88 (0.63 to 1.22), P = 0.434 | 0.617 |
| Low adherence (< 70% or not reporting) | 254 (16.4%) | 211 (14.0%) | 0.83 (0.67 to 1.02), P = 0.073 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 305 (10.6%) | 250 (8.8%) | 0.80 (0.67 to 0.97), P = 0.021 | 0.229 |
| High risk for allocation concealment bias (or not reporting) | 31 (6.5%) | 36 (7.3%) | 1.12 (0.66 to 1.91), P = 0.672 | |
| Blinding | ||||
| Blinded outcome assessment | 113 (6.5%) | 102 (5.9%) | 0.85 (0.64 to 1.13), P = 0.259 | 0.537 |
| No blinded outcome assessment | 223 (13.8%) | 184 (11.5%) | 0.81 (0.65 to 1.01), P = 0.062 | |
| Follow‐up | ||||
| Follow up for mortality at 1 month | 275 (10.7%) | 224 (8.9%) | 0.81 (0.67 to 0.99), P = 0.039 | 0.325 |
| Follow up for mortality < 1 month | 61 (7.6%) | 62 (7.6%) | 0.94 (0.64 to 1.38), P = 0.756 | |
| Treatment failure | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Adherence | ||||
| High adherence | 419 (23.1%) | 381 (21.0%) | 0.89 (0.76 to 1.04), P = 0.148 | 0.752 |
| Low adherence (< 70% or not reporting) | 422 (27.1%) | 387 (25.5%) | 0.92 (0.77 to 1.08), P = 0.301 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 776 (26.8%) | 699 (24.6%) | 0.89 (0.79 to 1.01), P = 0.069 | 0.486 |
| High risk for allocation concealment bias (or not reporting) | 65 (13.6%) | 69 (13.8%) | 0.99 (0.68 to 1.44), P = 0.939 | |
| Blinding | ||||
| Blinded outcome assessment | 412 (23.5%) | 367 (21.1%) | 0.88 (0.75 to 1.03), P = 0.117 | 0.574 |
| No blinded outcome assessment | 429 (26.5%) | 401 (25.1%) | 0.94 (0.79 to 1.11), P = 0.438 | |
CI: confidence interval NA: not applicable OR: odds ratio PCT: procalcitonin
Secondary outcomes
1. Antibiotic use (initiation of antibiotics, duration of antibiotics, and total exposure to antibiotics (total amount of antibiotic days divided by total number of participants))
Procalcitonin guidance was associated with a reduction in total antibiotic exposure (mean 8.1 days compared to 5.7 days, regression coefficient ‐2.43 days (95% CI ‐2.71 to ‐2.15), P < 0.001). Also, duration of antibiotic treatment in treated participants was shorter (mean 9.4 days compared to 8.0 days, adjusted coefficient ‐1.83 days (95% CI ‐2.15 to ‐1.5), P < 0.001) (Table 7).
6. Antibiotic treatment overall and stratified by setting and ARI diagnosis.
| Parameter | Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction |
| Overall | 3372 | 3336 | ||
| Initiation of antibiotics, n (%) | 2894 (86.3%) | 2351 (71.5%) | 0.27 (0.24 to 0.32), P < 0.001 | |
| Duration of antibiotics (days), mean (±SD) | 9.4 ± 6.2 | 8.0 ± 6.5 | ‐1.83 (‐2.15 to ‐1.50), P < 0.001 | |
| Total exposure of antibiotics (days), mean (±SD) | 8.1 ± 6.6 | 5.7 ± 6.6 | ‐2.43 (‐2.71 to ‐2.15), P < 0.001 | |
| Setting‐specific outcomes | ||||
| Primary care | 501 | 507 | ||
| Initiation of antibiotics, n (%) | 316 (63.1%) | 116 (22.9%) | 0.13 (0.09 to 0.18), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.3 ± 2.5 | 7.0 ± 2.8 | ‐0.52 (‐1.07 to 0.04), P = 0.068 | 0.064 |
| Total exposure of antibiotics (days), mean (±SD) | 4.6 ± 4.1 | 1.6 ± 3.2 | ‐3.02 (‐3.45 to ‐2.58), P < 0.001 | 0.101 |
| Emergency department | 1638 | 1615 | ||
| Initiation of antibiotics, n (%) | 1354 (83.2%) | 1119 (71.3%) | 0.49 (0.41 to 0.58), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.8 ± 5.4 | 7.3 ± 5.1 | ‐2.45 (‐2.86 to ‐2.05), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 8.2 ± 6.2 | 5.2 ± 5.4 | ‐3.02 (‐3.41 to ‐2.62), P < 0.001 | < 0.001 |
| Intensive care unit | 1233 | 1214 | ||
| Initiation of antibiotics, n (%) | 1224 (99.8%) | 1116 (91.9%) | 0.02 (0.01 to 0.05), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.8 ± 7.8 | ‐1.23 (‐1.82 to ‐0.65), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.1 ± 7.9 | ‐1.44 (‐1.99 to ‐0.88), P < 0.001 | < 0.001 |
| Disease‐specific outcomes | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| Initiation of antibiotics, n (%) | 1455 (99.4%) | 1340 (92.9%) | 0.08 (0.04 to 0.15), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 10.5 ± 6.2 | 8.0 ± 5.7 | ‐2.45 (‐2.87 to ‐2.02), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 10.4 ± 6.2 | 7.5 ± 5.9 | ‐2.94 (‐3.38 to ‐2.50), P < 0.001 | 0.004 |
| Exacerbation of COPD | 631 | 621 | ||
| Initiation of antibiotics, n (%) | 453 (71.8%) | 266 (42.8%) | 0.29 (0.23 to 0.36), P < 0.001 | 0.017 |
| Duration of antibiotics (days), mean (±SD) | 7.4 ± 5.3 | 7.2 ± 6.7 | ‐1.15 (‐2.00 to ‐0.31), P = 0.007 | 0.003 |
| Total exposure of antibiotics (days), mean (±SD) | 5.3 ± 5.6 | 3.1 ± 5.6 | ‐2.22 (‐2.83 to ‐1.60), P < 0.001 | 0.506 |
| Acute bronchitis | 287 | 257 | ||
| Initiation of antibiotics, n (%) | 189 (65.9%) | 68 (26.5%) | 0.18 (0.12 to 0.26), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.1 ± 3.0 | 6.4 ± 3.5 | ‐0.35 (‐1.15 to 0.45), P = 0.393 | 0.359 |
| Total exposure of antibiotics (days), mean (±SD) | 4.7 ± 4.2 | 1.7 ± 3.3 | ‐2.95 (‐3.59 to ‐2.31), P < 0.001 | 0.33 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| Initiation of antibiotics, n (%) | 186 (100.0%) | 193 (99.5%) | NA | NA |
| Duration of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.22 (‐3.80 to ‐0.65), P = 0.006 | 0.253 |
| Total exposure of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.45 (‐4.09 to ‐0.82), P = 0.003 | 0.786 |
Note: Duration refers to the total days of antibiotic therapy in participants in whom antibiotics were initiated. Total exposure refers to the total days of antibiotic therapy in all randomised participants.
Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals
ARI: acute respiratory infection CI: confidence interval COPD: chronic obstructive pulmonary disease NA: not applicable OR: odds ratio PCT: procalcitonin SD: standard deviation
2. Length of hospital stay for hospitalised participants
However, the effect on antibiotic consumption differed according to clinical setting. In the primary care setting, lower antibiotic exposure was mainly due to lower initial prescription rates (P < 0.001 for interaction between primary care setting and PCT group on antibiotic prescriptions). Similarly, lower antibiotic exposure due to lower prescription rates was found in selected infections such as acute bronchitis (adjusted OR 0.18, 95% CI 0.12 to 0.26; P for interaction < 0.001). Lower antibiotic prescription rates (adjusted OR 0.49, 95% CI 0.41 to 0.58) and shorter duration of antibiotic therapy in participants with initiation of antibiotic (adjusted coefficient ‐2.45 days, 95% CI ‐2.86 to ‐2.05) contributed to the lower overall exposure in the emergency department setting.
Length of hospital stay and ICU stay were similar in both groups with no evidence for different effects in subgroups (P for interaction > 0.05).
3. Length of ICU stay for critically ill participants
For the ICU setting, the lower exposure was mainly explained by shorter treatment durations (adjusted difference in days ‐1.23, 95% CI ‐1.82 to ‐0.65). Similarly, for CAP, the lower exposure was mainly explained by shorter durations (adjusted difference in days ‐2.45, 95% CI ‐2.87 to ‐2.02).
4. Number of days with restricted activities within 14 days after randomisation for primary care participants
For studies conducted in the primary care setting, there was no difference in days with restricted activities of daily living between PCT and control group participants (days, 8.9 ± 4.2 versus 8.9 ± 4.1, regression coefficient 0.07 (95% CI ‐0.44 to 0.59), P = 0.777).
5. Antibiotic‐related side effects
There was also a significant reduction in antibiotic‐related side effects (16.3% versus 22.1%, adjusted OR 0.68, 95% CI 0.57 to 0.82, P < 0.001). This outcome was only assessed in some of the primary care and emergency department trials (n = 6), and not in ICU trials. There was no evidence for subgroup effects (P for interaction > 0.05).
Discussion
Summary of main results
This updated systematic review and meta‐analysis included 32 trials, of which 26 trials were used for the main individual participant data analysis. Trials were conducted in 12 countries and included different clinical settings and types of respiratory infections. There was mostly low risk for random sequence generation, allocation concealment, incomplete outcome data, and selective reporting. There was unclear risk in all studies for blinding of personnel, and mostly high risk for blinding of outcome assessment. The results indicate a significant reduction in mortality (high‐quality evidence according to GRADE, Table 1) and non‐significant result for treatment failure (moderate‐quality evidence according to GRADE, Table 1) when PCT was used to guide initiation and duration of antibiotic treatment in ARI participants compared to control participants. Additionally, antibiotic consumption and side effects from antibiotics were significantly reduced across different clinical settings and types of ARIs. There was no effect on length of hospital stay and ICU length of stay. Results were similar in subgroup and sensitivity analyses including an aggregate data analysis with all 32 potentially eligible trials. Limitations include incomplete individual participant data, with four research groups not agreeing to the sharing of individual participant data; incomplete follow‐up information in some of the trials where no outcome assessment was done after 30 days of enrolment; differences in definitions of treatment failure among trials; and exclusion of some patient populations such as immunosuppressed people. Still, results from this updated individual participant data meta‐analysis support the use of PCT in the context of antibiotic stewardship in people with ARIs.
Overall completeness and applicability of evidence
The strengths of our review include an explicit study protocol, a comprehensive search to retrieve all relevant trials, access to individual participant‐level data from all but four of the included trials, and standardised outcome definitions across trials, thereby overcoming limitations of meta‐analyses using aggregated data. To minimise the risk of data‐driven associations, we prespecified a limited number of prognostic factors and subgroup variables for our statistical model. We allowed for potential clustering effects by using random‐effects models for included trials. Our results proved robust in sensitivity analyses focusing on high‐quality trials and on participants with complete follow‐up data.
The accuracy of PCT for diagnosing bacterial infections has been called into question by previous meta‐analyses of observational studies, which demonstrated mixed results (Jones 2007; Simmonds 2005; Tang 2007; Uzzan 2006). However, a more recent meta‐analysis using positive culture as the reference method found moderate to high discrimination of systematic inflammatory response syndrome and sepsis (Wacker 2013). Since there are no available gold standards for the diagnosis of the clinical conditions included in our analysis, most studies used clinical consensus criteria, which may differ among studies. Rather than relying on these imperfect diagnostic criteria, we were able to assess the value of PCT algorithms by means of RCTs measuring clinically relevant, participant‐level outcomes.
Despite these merits, this review has several limitations. We limited our analysis to adults with ARIs who were mostly immunocompetent, and excluded some pathogens (i.e. Legionella or Pseudomonas infections). The results of these trials may therefore not be generalised to people who are immunocompromised, with specific pathogens or infections other than ARIs, or children. Previous RCTs have shown that PCT guidance also reduces antibiotic exposure in a neonatal sepsis population but not in children with fever without a source (Manzano 2010). We found several ongoing RCTs in children evaluating PCT algorithms that should shed further light on the benefits and harms of PCT use for children. The included trials compared the PCT strategy to a control group where antibiotic therapy was guided based on 'usual practice' or based on current guideline recommendations. The magnitude of antibiotic reduction obviously correlates strongly with antibiotic prescription patterns, and in regions of low antibiotic prescription the PCT strategy may have smaller effects.
Quality of the evidence
Characteristics of the individual trials are presented in Table 2. Most trials had a follow‐up of one month, with two trials assessing outcome after 14 to 21 days and several trials following participants until hospital discharge (or ICU discharge) only. Procalcitonin algorithms used in the different trials were similar in concept and recommended initiation and/or continuation of antibiotic therapy based on similar PCT cut‐off levels (Table 2). However, there were differences: some trials in primary care and the emergency department used only a single PCT measurement on admission to guide initiation of antibiotics (Burkhardt 2010; Christ‐Crain 2004), while the other trials (predominantly in hospitalised participants with severe infections) used repeated measurements for guiding the duration of treatment. Adherence to algorithms was variable. In terms of methodological quality, trials had concealed allocation, but in several trials blinded outcome assessment was not done. All trials achieved complete or near‐complete follow‐up for mortality. None of the trials blinded participants or physicians to group allocation. The overall quality of the evidence according to GRADE was moderate to high (Table 1).
Potential biases in the review process
Due to the differences in patient populations included in this analysis, which ranged from primary care to the ICU, we adapted the definition of treatment failure to clinical settings by including setting‐specific components in this composite outcome. This may challenge the clinical interpretation in the overall analysis.
Agreements and disagreements with other studies or reviews
While mortality did not differ significantly in our initial meta‐analysis (adjusted OR 0.94, 95% CI 0.71 to 1.23) (Schuetz 2012), we found a significantly lower mortality rate in PCT‐guided participants in this update. This result was robust in subgroup analyses and in our sensitivity analysis. Also, when considering all trials in the aggregate data analysis, mortality tended to be reduced, although not significantly (OR 0.89, 95% CI 0.78 to 1.01). Importantly, the largest‐yet ICU trial from the Netherlands has reported a significantly lower mortality in PCT‐guided participants (De Jong 2016).
Two of the included individual trials reported reduced length of stay, particularly within the ICU. Yet, despite a marked reduction in the duration of antibiotic therapy across trials and settings, there was no difference in length of ICU and hospital stay between the two groups in our comprehensive analysis. One might expect that clinically stable patients with discontinued intravenous antibiotics could be safely discharged unless there are extenuating circumstances. Perceived needs by physicians to further monitor these patients in the unit or inability to transfer patients to other inpatient or aftercare locations may partly explain this finding.
There is ongoing controversy about the diagnostic performance of PCT and other blood markers to correctly identify patients with a bacterial infection. In fact, several observational studies have questioned the added value of PCT in addition to clinical signs, such as a primary care study authored by van Vugt and colleagues reporting no additional benefit of PCT to a clinical assessment (van Vugt 2013). Importantly, in the context of respiratory infections, diagnostic studies are limited by the lack of a reference standard, with blood cultures only detecting a minority of cases (e.g. only 10% to 20% of patients with clinically and radiologically confirmed CAP have positive blood cultures) (Muller 2010; Wacker 2013). Interventional research, such as the trials included in the current analysis, do not rely on a reference standard but compare resource use (e.g. antibiotics) and clinical outcomes in people with and without use of the diagnostic marker. For the primary care setting, PCT had a very strong effect on antibiotic consumption (reduction of antibiotic exposure by 70%, from 4.6 to 1.6 days) without compromising disease resolution and patient safety. Of note, we were not able to assess the effect of PCT on mortality due to the very low risk situation with only one non‐survivor (control group) among the 1008 included participants.
The available evidence from RCTs, as summarised in this report, supports the use of PCT for de‐escalation of antibiotic therapy for people with ARIs. The same may not be true for escalation of antibiotic therapy when PCT levels increase as demonstrated in a recent large sepsis trial (Jensen 2011), where PCT‐guided escalation of diagnostic procedures and antimicrobial therapy in the ICU did not improve survival and led to organ‐related harm and prolonged ICU stays.
Authors' conclusions
Implications for practice.
Emerging bacterial resistance to multiple antibiotic agents calls for more stringent efforts to reduce the empiric use of antimicrobial agents in self limited and non‐bacterial diseases and to shorten the duration of antibiotic treatment in bacterial infection with clinical resolution. The results of our study suggest that procalcitonin (PCT) is a safe and effective tool to guide clinical decisions for antibiotic initiation and duration of treatment. In all trials, PCT was used to inform physicians about the need for initiation or discontinuation of antibiotic therapy, or both. However, there were differences in PCT protocols among trials depending on the clinical setting (see Table 2 for details about PCT recommendations used in the individual trials) (Schuetz 2011a; Schuetz 2015). In brief, PCT was mainly used to inform about initiation of antibiotic treatment in primary care trials, and re‐measurement of PCT was recommended in participants not being treated with antibiotics and not showing a resolution of illness at follow‐up. In the emergency room and hospital ward setting, PCT was used to inform about initiation of antibiotic treatment (mainly in low‐risk patients with bronchitis or chronic obstructive pulmonary disease exacerbation), and also about discontinuation of treatment in community‐acquired pneumonia patients. In intensive care unit patients, PCT was mainly used to monitor treatment and discontinue antibiotics in participants with clinical improvement and a drop in PCT levels. Thus for clinical practice, PCT should also be adapted to clinical settings and the risk of patients ‐ similar to patients with suspicion of pulmonary embolism where D‐dimer levels are used differently depending on the pre‐test probability (Konstantinides 2008).
The use of PCT to guide initiation and duration of antibiotic treatment in people with acute respiratory infections (ARIs) was associated with lower mortality rates and significantly reduced antibiotic consumption and associated side effects across different clinical settings and ARI diagnoses. Of note, mortality was very low in primary care patients, and we were thus not able to assess the effect of PCT on mortality. The use of PCT embedded in clinical algorithms has the potential to improve the antibiotic management of ARI patients and has substantial clinical and public health implications to reduce antibiotic exposure and the associated risk of antibiotic resistance. Several assays for the measurement of PCT are currently available (Schuetz 2017), and the US Food and Drug Administration recently cleared the Vidas assay, among others, for antibiotic stewardship and prognostication of patients using a PCT kinetics algorithm (Schuetz 2016). Importantly, all trials have used highly sensitive assays to measure PCT in order to have optimal sensitivity and thus test performance. Factors such as accessibility and time taken to get reports of the tests are equally important in whether PCT will be used in the clinical decision‐making process for antibiotic therapy in ARIs. In this regard, a point‐of‐care test would be important, especially for the primary care setting (Kutz 2016).
Importantly, all trials included PCT into clinical algorithms, and physicians could deviate from the PCT algorithm if needed. Poststudy surveys have been published in order to better understand the effects and challenges of PCT testing in clinical practice (Albrich 2012; Balk 2017).
Implications for research.
Future studies should establish cost‐effectiveness by considering country‐specific costs of PCT measurement (around USD 20 to USD 30 per sample) and potential savings in consumption of antibiotics and other healthcare resources (Stojanovic 2017).
In addition, it would be interesting to conduct a head‐to‐head trial comparing a PCT strategy to a strategy based on another biomarker, such as C‐reactive protein (CRP) or interleukin‐6 (Meili 2015; Meili 2016). A similar randomised controlled trial was recently conducted in primary care in the Netherlands with a treatment algorithm based on either CRP levels, communication training, or both, compared to a control group (Cals 2009). The trial authors reported a 42% relative reduction in antibiotic use with CRP guidance, which was similar to the effect of communication training in this setting. However, the usefulness of CRP for antibiotic guidance outside the primary care setting is not yet supported by controlled intervention trials.
While there is strong evidence for the use of PCT in respiratory infections, its role in other infections remains unclear. Several studies have investigated PCT as a diagnostic and antibiotic stewardship marker in different types of infections (Albrich 2012; Drozdov 2015; Sager 2017). However, larger trials powered for safety are needed to understand the effect of PCT outside respiratory infections.
Feedback
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections, 22 October 2017
Summary
In the 2012 update of this review (Schuetz, 2012), (Maravic‐Stojkovic, 2011) was excluded due to “[no] evidence of respiratory infection”. In this 2017 update, (Maravic‐Stojkovic, 2011) has now been included. The reasons for this change do not seem to be explicitly provided in the review. The “What’s new” section does say “new trials” were included but (Maravic‐Stojkovic, 2011) had already been published, identified and excluded in the previous review.
Schuetz, P., Müller, B., Christ‐Crain, M., Stolz, D., Tamm, M., Bouadma, L., … Briel, M. (2012). Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.cd007498.pub2
Maravic‐Stojkovic, V., Lausevic‐Vuk, L., Jovic, M., Rankovic, A., Borzanovic, M., & Marinkovic, J. (2011). Procalcitonin‐based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: A randomized controlled trial. Srpski Arhiv Za Celokupno Lekarstvo, 139(11‐12), 736–742. doi:10.2298/sarh1112736m
Best,
Martin Vuillème Affiliation: Volunteer translator
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
Reply
Based on the 2012 review of abstracts we did not believe that the Maravic dataset would have “patients with acute respiratory tract infection included” (our main inclusion criterion) based on the description in the abstract “The prospective study included 205 patients who underwent open heart surgery. The patients were randomly assigned for procalcitonin‐guided antibiotic treatment (PCT‐group; n=102) or standard care (standard group; n=103). On the basis of serum procalcitonin concentrations, usage of antibiotics was encouraged (PCT≥0.5 ng/mL) or discouraged.”
For the 2017 update we decided to check again all trials with investigators and indeed found that 5 out of the 205 patients had hospital‐acquired pneumonia (HAP) – these 5 patients were then included in the final analysis.
Thus – the 5 patients from the Maravic study should have been included in the 2012 review already but slipped our attention due to the focus of the study on cardiac surgery patients.
Contributors
Philipp Scheutz
Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections, 15 March 2019
Summary
This review reports findings in the summary of findings table for four outcomes (mortality, treatment failure, anitbiotic‐related side effects, anitbiotic exposure) but data and meta‐analyses are only included for the first two. Can the study level findings and meta‐analyses please be made available for the third and fourth outcomes? This review is being considered by the WHO Essential Diagnostics List and seeing this data is very important.
Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment?: No
Jon Deeks, Professor of Biostatistics, University of Birmingham
Reply
Thank you for your comments. Currently, we only have these results for our individual patient data analysis, but will include analyses for the third and fourth outcomes using random‐effects in our next update.
Contributors
Philipp Scheutz
What's new
| Date | Event | Description |
|---|---|---|
| 17 May 2019 | Feedback has been incorporated | Feedback comment and reply added to the review. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 26 October 2017 | Amended | Correction to Sumary of findings table GRADE icon. |
| 26 October 2017 | Feedback has been incorporated | Feedback comment and reply added to the review. |
| 10 February 2017 | New citation required and conclusions have changed | Procalcitonin‐guided antibiotic treatment improves clinical outcomes and reduces antibiotic consumption. |
| 10 February 2017 | New search has been performed | We updated our searches and analyses. We included 12 new trials in individual participant analysis (Bloos 2016; Branche 2015; Corti 2016; De Jong 2016; Deliberato 2013; Layios 2012; Long 2014; Maravić‐Stojković 2011; Oliveira 2013; Shehabi 2014; Verduri 2015; Wang 2016). We identified six new trials that were excluded from individual participant analysis (Annane 2013; Ding 2013; Lima 2016; Najafi 2015; Ogasawara 2014; Tang 2013). We identified seven new ongoing trials (NCT02130986; NCT02261610; NCT02332577; NCT02440828; NCT02787603; NCT02862314; NCT02931409). Our new results show that mortality is significantly lower in the procalcitonin group. |
Acknowledgements
We thank all participating patients and staff of the clinics of emergency medicine, internal medicine, and departments of clinical chemistry from all participating hospitals for their most helpful support during the individual studies. We also wish to thank the following people for commenting on the draft protocol: Hayley Edmonds, Anette Holm, Renato Seligman, Richard Shoemaker, and Roger Damoiseaux; and for reviewing the draft review we wish to thank Anne Lyddiatt, Noorin Bhimani, Anette Holm, Renato Seligman, Mark Jones, and Roger Damoiseaux. We also thank Qing Wang (for translating Chinese articles) and Benjamin Kasenda (for helping with quality assessment of trials in which the primary investigators were involved).
Appendices
Appendix 1. CENTRAL (Cochrane Library via Wiley) search strategy
[mh Calcitonin] OR Procalcitonin:ti,ab OR ProCT:ti,ab OR (calcitonin:ti,ab AND (precursor:ti,ab OR precursors:ti,ab))
AND
[mh "Anti‐Bacterial Agents"] OR (antibiotic OR Antibiotics OR antibacterial OR anti‐bacterial OR amoxicillin OR amoxycillin OR penicillin OR ampicillin OR cotrimoxazole OR chloramphenicol OR trimethoprim OR sulphamethoxazole OR "tmp smx" OR tmp‐smx):ti,ab
AND
[mh Biomarkers] OR (Biomarker OR Biomarkers OR Marker OR Level OR Levels OR Guide OR Guidance):ti,ab
Appendix 2. MEDLINE (Ovid) search strategy
exp Calcitonin/ OR Procalcitonin.tw. OR ProCT.tw. OR (calcitonin.tw. AND (precursor.tw. OR precursors.tw.))
AND
exp Anti‐Bacterial Agents/ OR (antibiotic OR Antibiotics OR antibacterial OR anti‐bacterial OR amoxicillin or amoxycillin OR penicillin OR ampicillin OR cotrimoxazole OR chloramphenicol OR trimethoprim OR sulphamethoxazole OR tmp smx OR tmp‐smx).tw,nm.
AND
exp Biomarkers/ OR (Biomarker OR Biomarkers OR Marker OR Level OR Levels OR Guide OR Guidance).tw.
AND
((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or randomised.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) NOT (exp animals/ not humans.sh.)
Appendix 3. Embase.com (Elsevier) search strategy
'Calcitonin'/exp OR Procalcitonin:ti,ab OR ProCT:ti,ab OR (calcitonin:ti,ab AND (precursor:ti,ab OR precursors:ti,ab))
AND
'antiinfective agent'/exp OR (antibiotic OR Antibiotics OR antibacterial OR anti‐bacterial OR amoxicillin OR amoxycillin OR penicillin OR ampicillin OR cotrimoxazole OR chloramphenicol OR trimethoprim OR sulphamethoxazole OR "tmp smx" OR tmp‐smx):ti,ab
AND
'biological marker'/exp OR (Biomarker OR Biomarkers OR Marker OR Level OR Levels OR Guide OR Guidance):ti,ab
AND
random* OR factorial OR crossover OR placebo OR blind OR blinded OR assign OR assigned OR allocate OR allocated OR 'crossover procedure'/exp OR 'double‐blind procedure'/exp OR 'randomised controlled trial'/exp OR 'single‐blind procedure'/exp NOT ('animal'/exp NOT ('animal'/exp AND 'human'/exp))
Appendix 4. Previous MEDLINE (Ovid) search strategy
Search used for previous versions of this Cochrane Review
1 procalcitonin.tw.
2 calcitonin precursor*.tw.
3 exp Anti‐Bacterial Agents/
4 antibiotic.tw.
5 1 or 2
6 3 or 4
7 5 and 6
Data and analyses
Comparison 1. Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 30 days | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.98] |
| 1.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.36] |
| 1.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.00] |
| 2 Treatment failure at 30 days | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 2.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 2.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
1.2. Analysis.
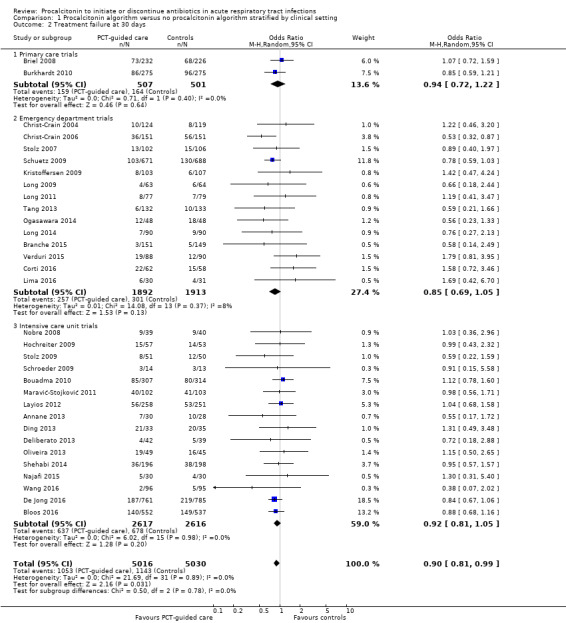
Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 2 Treatment failure at 30 days.
Comparison 2. Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 30 days stratified by adherence | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.37] |
| 1.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.97] |
| 2 Treatment failure at 30 days stratified by adherence | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.02] |
| 2.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 3 Mortality at 30 days stratified by allocation concealment | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 3.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.01] |
| 3.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 4 Treatment failure at 30 days stratified by allocation concealment | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 4.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 4.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.04] |
| 5 Mortality at 30 days stratified by blinded outcome assessment | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 5.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.32] |
| 5.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.95] |
| 6 Treatment failure at 30 days stratified by blinded outcome assessment | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 6.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 6.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 7 Mortality at 30 days stratified by follow up | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 7.1 Trials with 1 month follow up for mortality | 18 | 7337 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 7.2 Trials with different follow up for mortality | 14 | 2709 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.30] |
2.1. Analysis.
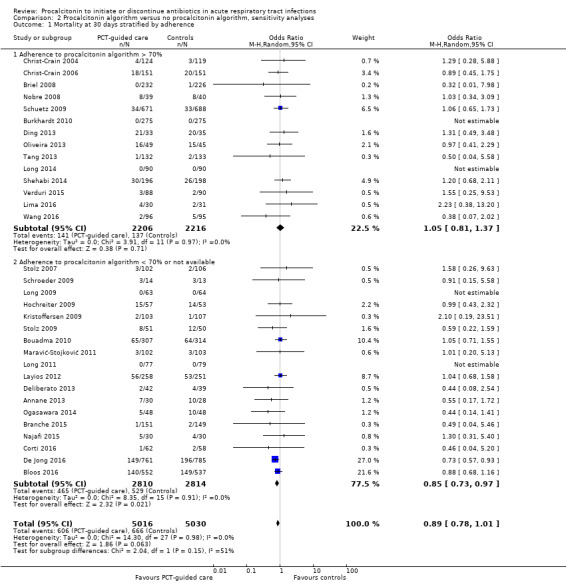
Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 1 Mortality at 30 days stratified by adherence.
2.2. Analysis.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 2 Treatment failure at 30 days stratified by adherence.
2.3. Analysis.
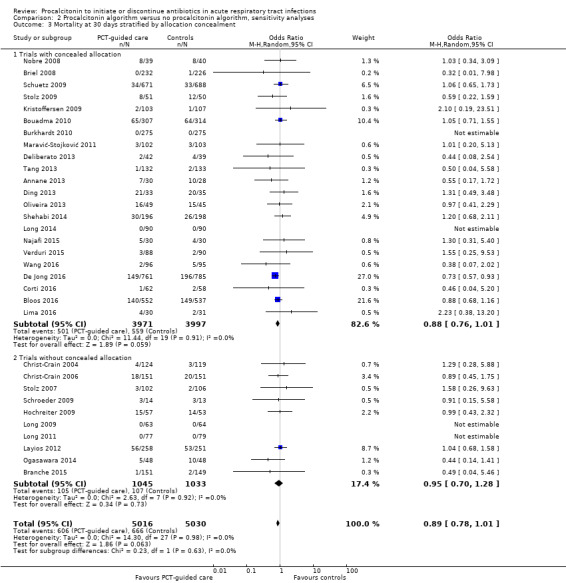
Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 3 Mortality at 30 days stratified by allocation concealment.
2.4. Analysis.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 4 Treatment failure at 30 days stratified by allocation concealment.
2.5. Analysis.
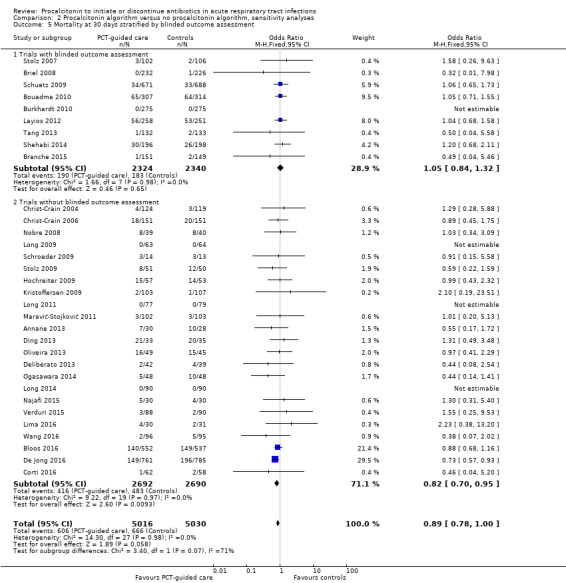
Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 5 Mortality at 30 days stratified by blinded outcome assessment.
2.6. Analysis.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 6 Treatment failure at 30 days stratified by blinded outcome assessment.
2.7. Analysis.
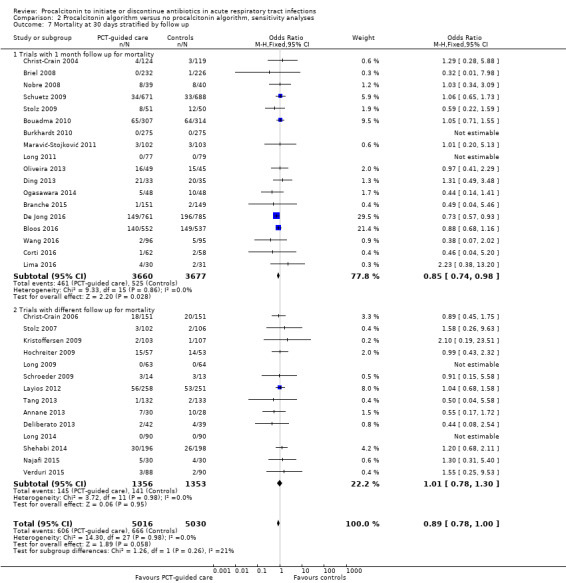
Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 7 Mortality at 30 days stratified by follow up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Annane 2013.
| Methods | Randomised, multicentre, single‐blind clinical trial in 8 French ICUs | |
| Participants | Inclusion criteria: Adults admitted to a participating ICU were eligible if they, < 48 h, had SIRS, acute dysfunction of at least 1 organ, absence of indisputable clinical infection, and negative microbial cultures Exclusion criteria: Pregnancy, burns over ≥ 15% of body surface area, trauma, outpatient or inpatient cardiac arrest, post‐orthopaedic surgery status, drug‐related neutropenia, withdrawal of life‐supportive therapies or a decision to withhold them, indisputable clinical infection or antibiotic exposure ≥ 48 h during the time shortly before ICU admission Included in this study: 62/1250 screened patients were eligible for the study, of whom 31 were randomised to each arm. 4 post randomisation exclusion (4 withdrew their consent) | |
| Interventions | Guiding antibiotic decisions in ICU patients with non‐microbiologically proven apparent severe sepsis Algorithm used in this study: In the experimental arm, both initiation and discontinuation of antibiotics were guided by a PCT‐based algorithm, applied at 6 h and on day 3 and day 5 post randomisation. Briefly, antibiotic therapy was not to be started or was to be halted when PCT was < 0.25 μg/L, was strongly discouraged when PCT was ≥ 0.25 to < 0.5 μg/L, was recommended when PCT was ≥ 0.5 to < 5 μg/L, and was strongly recommended when PCT was ≥ 5 μg/L. Owing to the fact that surgery can increase PCT levels, for 12 participants enrolled in the 48‐hour postoperative period, the respective PCT cut‐offs were < 4 μg/L, ≥ 4 to < 9 μg/L, and ≥ 9 μg/L. Investigators were strongly advised not to overrule the algorithm every day up to the study day 5. In the control arm, the decision to start or stop antibiotic therapy was at the discretion of the participant’s physician, without knowledge of the participant’s PCT concentrations. | |
| Outcomes |
|
|
| Notes | Funding: Research grant partly by Thermo Fisher B·R·A·H·M·S France. The sponsor had no input in study design, conduct, or reporting. Follow‐up time: Until hospital discharge or 30 days' post randomisation, whichever came first Registration: NCT01025180 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1 ratio according to a computer‐generated list. Randomisation was centralised through a secured web site and performed by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using permutation blocks |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The control arm remained blinded to PCT levels. Masking of antibiotic therapy was not feasible in this study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators remained blinded to PCT levels in the control arm, but no blinding of overall outcome assessment was mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for participants on antibiotic on day 5 was: 58/62. 4 participants withdrew their consent. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01025180). |
| Other bias | Unclear risk | Moderate adherence in the PCT arm: physicians were non‐compliant with the PCT‐based algorithm in 19% of participants at 6 h, 17% on day 3, and 37% on day 5. The study was stopped prematurely owing to the low incidence of eligible patients. As a consequence, the study population is small with low statistical power. |
Bloos 2016.
| Methods | Randomised, investigator‐initiated, multicentre, partially blinded clinical trial, in 33 multidisciplinary ICUs across Germany | |
| Participants | Inclusion criteria: Adults with severe sepsis or septic shock (severe sepsis was defined as SIRS caused by infection combined with acute organ dysfunction. Septic shock was defined as sepsis in combination with arterial hypotension or need for vasopressor therapy despite adequate fluid resuscitation) Exclusion criteria: Pregnant or lactating women, patients with selenium intoxication, individuals with infections for which guidelines recommend a longer duration of antimicrobial therapy, immunocompromised patients, and those without commitment to full therapy or where death was imminent owing to coexisting diseases were excluded from the trial. Included in this study: 1180 participants were randomised; 91 participants were excluded from the final analysis because informed consent was not obtainable in the deferred consent process, resulting in 1089 participants with valid data. | |
| Interventions | Guiding antibiotic decisions and effect of sodium selenite administration in people with severe sepsis or septic shock Algorithm used in this study: Using a 2 × 2 factorial design, participants were randomly assigned to receive intravenous sodium selenite or placebo as well as antimicrobial therapy guided by a PCT algorithm or conventional antimicrobial therapy. In participants randomised to the PCT guidance arm, PCT was measured locally on days 0, 1, 4, 7, 10, and 14 after randomisation if the participant was still in the ICU. Procalcitonin concentration on day 0 or day 1 served as the baseline value. Depending on the PCT results, an algorithm provided recommendations to change or discontinue antimicrobial therapy or trigger diagnostic procedures to optimise source control. On day 4, no change in antimicrobial therapy was recommended if the PCT level dropped by at least 50% compared with the baseline value. Otherwise, change or optimisation of antimicrobial therapy or interventions regarding source control were recommended. On the other days, stopping antimicrobial therapy was recommended if the PCT level was 1 ng/mL or lower or if the PCT level dropped by at least 50% compared with the previous value. Otherwise, change or optimisation of antimicrobial therapy or interventions regarding source control were recommended. The treating physician was allowed to overrule the algorithm recommendation. In the group without PCT guidance, no PCT measurements were obtained until day 14; changes in antimicrobial therapy were made at the discretion of the treating physician. Investigators agreed to treat all participants according to the Guidelines of the Germany Sepsis Society, which included recommendations to re‐evaluate antimicrobial therapy after 48 to 72 hours and to restrict duration of antimicrobial therapy to no more than 10 days. |
|
| Outcomes |
|
|
| Notes | Funding: The study infrastructure was partially funded by grant 01 KI 0106 from the German Federal Ministry of Education and Research. Biosyn (Germany) and Thermo Fisher (Germany) provided study medication and financial support via unrestricted grants. Follow‐up: 90 days Trial registration: NCT00832039 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based, centralised randomisation |
| Allocation concealment (selection bias) | Low risk | quote "Using a 2 × 2 factorial design, we randomly assigned patients to receive intravenous sodium selenite or placebo as well as antimicrobial therapy guided by a PCT algorithm or conventional antimicrobial therapy without PCT guidance with an allocation ratio of 1:1:1:1 by use of a central randomisation web server. Randomisation was stratified by study centre, sex, and sepsis severity" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Due to the study design, blinding of physicians was not feasible. Procalcitonin values were only available in participants with study intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment was mentioned in study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Postrandomisation exclusions were about even among intervention arms with a very low dropout rate for mortality |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to trial registration (NCT00832039) |
| Other bias | Unclear risk | Overruling of the PCT algorithm was very high (adherence was lower than 50%) |
Bouadma 2010.
| Methods | Randomised, multicentre clinical trial in 9 French ICUs | |
| Participants |
Inclusion criteria: Patients with suspected bacterial infections during ICU stay without prior AB (> 24 h) Exclusion criteria: Aged under 18 years; known pregnancy; expected stay in the ICU of less than 3 days; bone marrow transplant or chemotherapy‐induced neutropenia (< 500 neutrophils per mL); infections for which long‐term antibiotic treatment is strongly recommended (i.e. infective endocarditis, osteoarticular infections, anterior mediastinitis after cardiac surgery, hepatic or cerebral abscesses, chronic prostatitis, or infection with Mycobacterium tuberculosis, Pneumocystis jirovecii, or Toxoplasma gondii); poor chance of survival, defined as a simplified acute physiology score (SAPS II) of more than 65 points at screening; and do‐not‐resuscitate orders. Included in this analysis: 394 participants with CAP and VAP out of 630 randomised participants; 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice), and 227 not considered for this analysis due to diagnosis other than ARI |
|
| Interventions | Guiding antibiotic decisions in ICU patients with repeated PCT measurements Algorithm used in this study: Investigators were encouraged to discontinue ABs when PCT concentration was less than 80% of the peak concentration or an absolute concentration of less than 0.5 μg/L was reached. |
|
| Outcomes |
|
|
| Notes |
Funding: Research grant from the Départment à la Recherche Clinique et au Développement, Assistance Publique‐Hopitaux de Paris (PHRC AOR06019), France. B·R·A·H·M·S, Germany (manufacturer of PCT assay) provided all assay‐related materials free of charge for the study (Kryptor machines if not already available on‐site and kits and maintenance required for study‐related measurements). Follow‐up: Fixed period of 60 days for mortality Registration: NCT00472667 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent, centralised, computer‐generated randomisation sequence (CleanWEB, Telemedicine Technologies, Boulogne, France) |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Although treatment assignments were not masked, all investigators were unaware of aggregate outcomes during the study and primary endpoints were strictly defined and not patient‐reported." Quote: "An adjudication committee comprised of 4 specialists in infectious diseases and critical care medicine who were masked to the randomisation assignment reviewed and validated all infectious episode classifications by consensus." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 393/394 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT00472667). |
| Other bias | Unclear risk | Low adherence to PCT algorithm in PCT group (47%) |
Branche 2015.
| Methods | Single‐centre, randomised, open‐label clinical trial in Rochester, NY, USA | |
| Participants | Inclusion criteria: Adults ≥ 21 years of age with symptoms compatible with LRTI (i.e. admission diagnosis of pneumonia, acute exacerbations of COPD, bronchitis, asthma, influenza, viral syndrome, respiratory failure, and congestive heart failure CHF) were identified by reviewing the daily admission census. Exclusion criteria: Patients with characteristics indicative of a high risk for bacterial infection (i.e. ICU requirement, active chemotherapy or radiation, immunosuppression, definitive infiltrate on chest radiograph, enrolment systolic blood pressure of < 90 mmHg, and ≥ 15% band forms in peripheral blood). Patients who had conditions known to increase PCT levels (i.e. trauma, renal failure, and pancreatitis) or who had received antibiotics prior to admission were also excluded. Included in this study: 300/685 screened patients were eligible for the study, of whom 151 were randomised to the intervention group and 149 to the non‐intervention group | |
| Interventions | Guiding antibiotic decisions in hospitalised patients with respiratory infections Algorithm used in this study: In the intervention group serum PCT and viral/atypical pathogen PCR testing were performed on admission (in addition to all standard‐of‐care diagnostic tests). Antibiotic decisions were made based on a PCT algorithm (for PCT values of ≤ 0.1 ng/mL, initiation of antibiotic treatment is strongly discouraged; for values of 0.11 to 0.24 ng/mL, initiation is discouraged; for values of 0.25 to 0.49 ng/mL, initiation is encouraged; and for values of ≥ 0.5 ng/ mL, initiation is strongly encouraged). In the standard care group standard‐of‐care testing (bacterial and viral cultures of respiratory samples, hospital influenza/RSV duplex PCR, and urine Legionella antigen analysis) were obtained on admission and antibiotic decisions were made by the attending physician. Participants in the standard care group had PCT and viral testing samples frozen and tested at study termination. |
|
| Outcomes |
|
|
| Notes | Funding: This work was supported by Rochester General Hospital (KIDD Fund); the National Institutes of Health, National Institute of Allergy and Infectious Diseases (contract HHSN27220120005C); and BioFire (FilmArray respiratory panel instrument). Follow up time: Study personnel reviewed the EMR daily until hospital discharge. Participants were contacted by phone at 30 days and 3 months by personnel blinded to randomisation, who collected information about healthcare utilisation, antibiotic use and complications, and return to baseline health. Registration: NCT01907659 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by the presence of COPD and were randomly assigned at a ratio of 1:1, using blocks of 4, to receive standard care or the intervention. |
| Allocation concealment (selection bias) | Low risk | Small block size (4) for randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not feasible due to the study design. Although the control arm remained blinded, there was a risk for spillover care resulting from providers caring for participants in both arms. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants in the standard care group had blood samples frozen and tested at study termination, making the data only available at the end of the study. Phone follow‐up by personnel blinded to randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 3 months of follow‐up 36 participants were lost to follow‐up, 13 died, and 4 withdrew their consent (237/300 completed 3‐month follow‐up). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01907659). |
| Other bias | High risk | Low overall adherence to the PCT algorithm (64%) Small sample size |
Briel 2008.
| Methods | Randomised clinical trial, multicentre in 53 primary care practices in northwest Switzerland | |
| Participants |
Inclusion criteria: People with upper or lower ARIs in primary care and the physician's intention to prescribe antibiotics on the basis of evidence‐based guidelines Exclusion criteria: Antibiotic use within the previous 28 days, psychiatric disorders or inability to give written informed consent, not being available for follow‐up, not fluent in German, severe immunosuppression, cystic fibrosis, active tuberculosis, and the need for immediate hospitalisation Included in this analysis: 458 out of 458 randomised participants |
|
| Interventions | Guiding antibiotic decisions in primary care with repeated measurements Algorithm used in this study: In participants with PCT levels lower than 0.1 μg/L, a bacterial infection was considered highly unlikely and the use of ABs was discouraged. In participants with a PCT level higher than 0.25 μg/L, a bacterial infection was considered likely and the use of ABs was recommended. For PCT concentrations of 0.1 to 0.25 μg/L, a bacterial infection was considered unlikely and the use of ABs was not recommended. When ABs were withheld from participants, a second measurement of the PCT level was mandatory within 6 to 24 hours for safety reasons. The use of ABs was recommended if this second measurement was higher than 0.25 μg/L or if the PCT level had increased from the first measurement by more than 50% and the participant showed no clinical improvement. All participants given ABs based on PCT level were reassessed after 3 days. Discontinuation of AB treatment was then recommended in participants with a PCT level of 0.25 μg/L or lower. |
|
| Outcomes |
|
|
| Notes |
Funding: Swiss National Science Foundation (Grant 3300C0‐107772) and Association for the Promotion of Science and Postgraduate Training of the University Hospital Basel, Switzerland. B·R·A·H·M·S, Germany provided assay and kit material related to the study. Follow‐up: Fixed period of 28 days Registration: ISRCTN73182671 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician generated the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation communicated by phone to physician. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded medical students performed interviews with participants at 14 and 28 days. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 454/458 (99%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 85% adherence to PCT algorithm in PCT group |
Burkhardt 2010.
| Methods | Randomised clinical trial, multicentre in 15 primary care practices in the Hanover, Germany area | |
| Participants |
Inclusion criteria: Adults with upper or lower ARIs in primary care Exclusion criteria: Treatment with antibiotics during the previous 2 weeks, chronic liver disease, major surgery that had required hospitalisation during the last 4 weeks, autoimmune or systemic disorders, dialysis, medullary C‐cell carcinoma and other inflammatory diseases Included in this analysis: 550 out of 571 randomised participants; 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) |
|
| Interventions | Guiding antibiotic decisions in primary care with initial measurement only Algorithm used in this study: PCT value < 0.25 µg/L indicated that a relevant bacterial infection of the respiratory tract is unlikely. |
|
| Outcomes |
|
|
| Notes |
Funding: B·R·A·H·M·S AG, Germany Follow‐up: Fixed period of 28 days Registration: NCT00827060 and NCT00688610 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated Quote: "Baseline adaptive randomisation was realised through a web‐based randomisation data bank (IOMTech GmbH, Berlin, Germany), which had been programmed specifically for that purpose." |
| Allocation concealment (selection bias) | Low risk | Central randomisation Quote: "In the central laboratory, the web‐based randomisation of the patient into the PCT group or the control group took place." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Structured interviews by blinded personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 546/550 (99%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 87% adherence to PCT algorithm in PCT group |
Christ‐Crain 2004.
| Methods | Randomised clinical trial, single‐centre, emergency department at the University Hospital Basel, Switzerland | |
| Participants |
Inclusion criteria: People with lower ARIs presenting at a medical emergency department Exclusion criteria: Severely immunocompromised people, i.e. with HIV infection and a CD4 count less than 200 cells per mL, neutropenic patients, and stem cell transplant recipients; those with cystic fibrosis or active tuberculosis; and individuals with nosocomial pneumonia Included in this analysis: 243 participants out of 243 randomised participants |
|
| Interventions | Guiding antibiotic decisions in emergency department patients with different ARIs with initial PCT values only Algorithm used in this study: A PCT value of 0.1 to 0.25 µg/L was regarded as an indication that bacterial infection was unlikely and use of ABs was discouraged. A serum PCT between 0.25 and 0.5 g/L was deemed indicative of a possible bacterial infection, and the treating doctor was advised to initiate antimicrobial treatment. A PCT value of 0.5 µg/L or greater was judged suggestive of the presence of bacterial infection and AB treatment was strongly recommended. For participants on antimicrobial therapy at the time of admission, discontinuation of ABs was recommended if PCT concentrations were less than 0.25 µg/L. |
|
| Outcomes |
|
|
| Notes |
Funding: Freiwillige Akademische Gesellschaft Basel, Switzerland; Department of Internal Medicine and the Divisions of Endocrinology and Pneumology, University Hospital Basel; B·R·A·H·M·S AG, Germany and Orgenium Laboratories, Finland, provided assay material and partial support of the investigator‐initiated study. Follow‐up: Fixed period of 10 to 14 days; in participants with acute exacerbations of COPD the follow‐up period comprised 4 to 6 months Registration: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned eligible patients either standard antimicrobial therapy (standard group) or PCT‐guided antimicrobial treatment (PCT group) according to a computer‐generated week wise‐randomisation scheme." |
| Allocation concealment (selection bias) | High risk | Recruiting physicians were aware of group allocation based on week‐wise randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 230/243 (95%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 83% adherence to PCT algorithm in PCT group |
Christ‐Crain 2006.
| Methods | Randomised clinical trial, single‐centre, emergency department at the University Hospital Basel, Switzerland | |
| Participants |
Inclusion criteria: CAP with X‐ray confirmation in the emergency department Exclusion criteria: People with cystic fibrosis or active pulmonary tuberculosis, people with hospital‐acquired pneumonia, and severely immunocompromised individuals Included in this analysis: 302 out of 302 randomised participants |
|
| Interventions | Guiding antibiotic decisions in emergency department patients with CAP with repeated PCT measurements Algorithm used in this study: a PCT level of less than 0.1 µg/L suggested the absence of bacterial infection, and the initiation or continuation of ABs was strongly discouraged. A PCT level between 0.1 and 0.25 µg/L indicated that bacterial infection was unlikely, and the initiation or continuation of ABs was discouraged. A PCT level from 0.25 to 0.5 µg/L was considered indicative of a possible bacterial infection, and the initiation or continuation of AB therapy was encouraged. A PCT level greater than 0.5 µg/L strongly suggested the presence of bacterial infection, and AB treatment and continuation was strongly encouraged. Re‐evaluation of the clinical status and measurement of serum PCT levels were recommended after 6 to 24 h in all participants from whom ABs were withheld. PCT levels were reassessed after 4, 6, and 8 d. Antibiotics were discontinued on the basis of the PCT cut‐offs defined above. In participants with very high PCT values on admission (e.g. greater than 10 µg/L), discontinuation of ABs was encouraged if levels decreased to less than 10% of the initial value (e.g. 1 µg/L, instead of less than 0.25 µg/L). |
|
| Outcomes |
|
|
| Notes |
Funding: Funding obtained from B·R·A·H·M·S (Hennigsdorf, Germany), Pfizer (Schweiz AG), and Mepha (Schweiz AG) was used for assay material and salaries of technical personnel involved in laboratory work and for shipping and handling of data and specimens and presentation of data at scientific meetings. Additional support, which provided more than two‐thirds of the total study costs, was granted by funds from the Departments of Internal Medicine and Emergency Medicine, the Stiftung Forschung Infektionskrankheiten (SFI), and mainly from the Departments of Endocrinology and Pulmonary Medicine, University Hospital Basel, Switzerland. Follow‐up: Fixed period of 6 weeks Registration: ISRCTN04176397 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "On admission, patients were randomly assigned to one of the two groups by sealed, opaque envelopes." Envelopes were not numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 300/302 (99%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 87% adherence to PCT algorithm in PCT group |
Corti 2016.
| Methods | Randomised, single‐centre clinical trial in an ED of a university hospital in Denmark | |
| Participants | Inclusion criteria: 1) 18 years old and 2) admitted with an AECOPD (clinician’s diagnosis at admission), defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Exclusion criteria: 1) person unable to understand or respond to oral or written information; 2) previously been enrolled in the study; and 3) do‐not‐resuscitate order in place Included in this analysis: 120/630 screened people with AECOPD were randomised and used for the ITT analysis (62 in the PCT group, 58 in the control group). | |
| Interventions | The aim was to assess whether PCT‐guided antibiotic treatment could reduce the overall use of antibiotics among people hospitalised for AECOPD. Algorithm used in this study: In the control group, antibiotic therapy followed treatment strategies for AECOPD according to GOLD guidelines. In the PCT group, initiation or continuation of antibiotics was strongly discouraged if PCT was 0.15 ng/mL or lower and discouraged if levels were between 0.15 ng/mL and 0.25 ng/mL. Initiation or continuation of antibiotics was encouraged if PCT was > 0.25 ng/mL. In participants with PCT over 5 ng/mL on admission, the algorithm recommended stopping antibiotics when PCT levels decreased by 80% of the peak value. | |
| Outcomes |
|
|
| Notes | Funding: Thermo Fisher Scientific, MA, USA, and bioMérieux Denmark ApS supported the study non‐financially. Follow‐up: 28 days Registration: NCT01950936 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated according to the random part of the civil registration number in Denmark |
| Allocation concealment (selection bias) | Low risk | The randomisation algorithm was concealed to treating clinicians and participants |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not feasible, but PCT was only measured in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mentioning of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 120/120 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01950936) |
| Other bias | High risk | Moderate adherence to the PCT algorithm in PCT group (61.1%) |
De Jong 2016.
| Methods | Randomised, multicentre, controlled, open‐label intervention trial in 15 hospitals in the Netherlands | |
| Participants | Inclusion criteria: Eligible patients had to be at least 18 years of age, be admitted to the ICU, and have received their first dose of antibiotics no longer than 24 h before inclusion to the trial for an assumed or proven infection. Exclusion criteria: Patients were excluded in cases of systemic antibiotics as prophylaxis only, antibiotics solely as part of selective decontamination of the digestive tract, prolonged therapy (e.g. endocarditis), expected ICU stay of less than 24 h, severe immunosuppression, severe infections (due to viruses, parasites, or Mycobacterium tuberculosis), and moribund patients. Patients who received corticosteroids were not excluded. Included in this study: 1575/4507 screened patients were enrolled in the study (776 in the PCT group, 799 in the control group) for an intention‐to‐treat analysis. | |
| Interventions | Guiding antibiotic decisions in critically ill ICU patients Algorithm used in this study: Antibiotics in the standard‐of care group were stopped according to local or national guidelines and according to the discretion of attending physicians. Procalcitonin concentration was not measured in the standard‐of‐care group. For participants randomly assigned to the PCT‐guided group, once‐a‐day measurements of PCT concentrations were taken and made available to the attending physicians, including a baseline measurement as close to initiation of antibiotics as possible, at least within 24 h. The study protocol advised stopping the prescribed antibiotics if PCT concentration had decreased by 80% or more of its peak value (relative stopping threshold) or when it reached a value of 0.5 μg/L or lower (absolute stopping threshold). The attending physician was free to decide whether to continue antibiotic treatment in participants who had reached these thresholds. |
|
| Outcomes |
|
|
| Notes | Funding: Thermo Fisher Scientific Follow‐up: 1‐year follow‐up Trial Registration: NCT01139489 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done centrally by use of a computer‐generated list produced by an independent research organisation (the Julius Centre for Human Research, Utrecht, Netherlands). |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by an independent organisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and investigators were aware of treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1546 of 1575 participants included in the intention‐to‐treat population analysis |
| Selective reporting (reporting bias) | Low risk | The outcomes correspond to the trial registration (NCT01139489). |
| Other bias | High risk | Physicians did not adhere to the stopping advice in more than half of the participants. About 30% of participants randomly assigned to the PCT group were discharged from ICU before the algorithm recommended stopping antibiotic treatment. |
Deliberato 2013.
| Methods | Randomised, single‐centre, controlled clinical trial at an ICU of a tertiary care, private hospital in São Paulo, Brazil | |
| Participants | Inclusion criteria: People with microbiologically confirmed infections (blood, urine, tracheal aspirate, or bronchoalveolar lavage fluid cultures) with sepsis, severe sepsis, and septic shock Exclusion criteria were: Onset of antibiotic therapy more than 48 hours before the date when the cultures were performed; people under 18 years old; known pregnancy; infections requiring prolonged antibiotic therapy, such as bacterial endocarditis, hepatic or brain abscess, deep abscess, mediastinitis, and osteomyelitis; severe infection caused by viruses, parasites, fungi, or mycobacteria; chronic localised infections, such as chronic osteomyelitis or chronic prostatitis; people without indication for ICU admission, as determined by the attending physician; and negative cultures (blood, urine, tracheal aspirate, or bronchoalveolar lavage fluid) in people with suspected sepsis, severe sepsis, or septic shock Included in this study: 81/265 eligible patients randomised for a intention‐to‐treat analysis; after further exclusions, 51/265 patients remained for per‐protocol analysis. | |
| Interventions | Guiding antibiotic decisions in ICU patients with proven bacterial infection Algorithm used in this study: All participants received antibiotic therapy. For the control group, stopping antibiotic therapy was at the discretion of the attending physician. For the intervention group, physicians were guided by the PCT protocol to stop antibiotic treatment. The protocol stated 2 conditions: 1) PCT dropped more than 90% from the peak level, or 2) an absolute value < 0.5 ng/mL was reached. |
|
| Outcomes |
|
|
| Notes | Funding: No funding declared in the main article. Follow‐up time: Data were recorded from 2 days before the bacteraemia (when applicable), with day 0 defined as the day sepsis was diagnosed, until 14 days after or at ICU discharge, whichever came first. Registration: NCT01494675 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A blind randomisation scheme was used where a black box contained 100 folders, and 2 authors randomly drew 1 folder as soon as an informed consent was present. |
| Allocation concealment (selection bias) | Low risk | Folders were randomly and blindly assigned as “PCT group” or “standard group”. 2 of the authors would randomly draw 1 folder from a black box containing 100 folders (50 “PCT group” and 50 “control group”). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of the treating physicians was not feasible in this study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of the outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the intention‐to‐treat analysis the follow‐up for mortality was 81/81 (100%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01494675). |
| Other bias | High risk | Low adherence to the PCT algorithm (47.6%) Single‐centre study where not all attending physicians agreed to participate (also a reason for exclusions in the PCT arm in the per‐protocol analysis and low adherence) |
Ding 2013.
| Methods | Randomised, single‐centre, open‐label, controlled clinical trial in HeNan Hospital, China | |
| Participants | Inclusion criteria: All patients with suspected AE‐IPF admitted to the respiratory department were assessed for eligibility from January 2009 to December 2011. Acute exacerbation of idiopathic pulmonary fibrosis was defined according to the criteria established by the Idiopathic Pulmonary Fibrosis Clinical Research Network: (1) previous or concurrent diagnosis of idiopathic pulmonary fibrosis; (2) unexplained worsening or development of dyspnoea within 30 days; (3) high‐resolution computed tomography with new bilateral ground‐glass; (4) abnormality and/or consolidation superimposed on a background reticular or honeycomb pattern consistent with usual interstitial pneumonia pattern; (5) no evidence of pulmonary infection by endotracheal aspirate or bronchoalveolar lavage; (6) exclusion of left heart failure, pulmonary embolism, and identifiable cause of acute lung injury. Exclusion criteria: Patients treated with antibiotics during the previous 2 weeks were excluded. Included in this study: 68 of 78 randomised participants finished at follow‐up. | |
| Interventions | Guiding antibiotic decisions in people with acute exacerbation of idiopathic pulmonary fibrosis Algorithm used in this study: Serum PCT level was measured every 3 days. The first PCT measurement was available before the clinical decision to start antibiotics treatment. Participants whose serum PCT value exceeded the threshold of 0.25 ng/mL were administered antibiotics and were treated until PCT value fell to ≤ 0.25 ng/mL. In the routine treatment group, the decision to administer antibiotics was guided by the clinical experience of the clinician, typically by conventional laboratory tests such as sputum bacteriology and white blood cell count. |
|
| Outcomes |
|
|
| Notes | Funding: No funding declared in the main paper. Follow‐up: 30 days Trial registration: No trial registration found for this clinical trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to either PCT‐guided antibiotic treatment or a control group receiving routine antibiotic therapy by the statistician using computer‐generated random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding was performed. "In the routine treatment group, patients were treated by antibiotics according to the clinical experience of clinicians typically guided by conventional laboratory tests, such as sputum bacteriology and white blood cell count." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment was mentioned in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Postrandomisation exclusion rate was relatively high but similar in both study arms (6 in the intervention arm versus 4 in the control group). |
| Selective reporting (reporting bias) | Unclear risk | No trial registration was found for this trial. |
| Other bias | Low risk | 100% adherence to PCT protocol in the per‐protocol analysed participants (all protocol violations were defined as "withdrawn"). |
Hochreiter 2009.
| Methods | Randomised clinical trial, single‐centre, ICU in Germany | |
| Participants |
Inclusion criteria: Patients in the surgical ICU with suspected bacterial infections and > 1 SIRS criteria Exclusion criteria: Patients who refused study consent, whose antibiotic treatment had been initiated before intensive care admission, or who had therapy limitations Included in this analysis: 43 (110); 67 not considered for this analysis due to diagnosis other than ARI |
|
| Interventions | Guiding antibiotic decisions in postoperative patients in a surgical ICU Algorithm used in this study: Antibiotic therapy in the PCT‐guided group was discontinued if clinical signs and symptoms of infection improved and PCT decreased to less than 1 µg/L, or if the PCT value was more than 1 µg/L, but had dropped to 25% to 35% of the initial value over 3 days. |
|
| Outcomes |
|
|
| Notes |
Funding: B·R·A·H·M·S AG Follow‐up: Until hospital discharge Registration: ISRCTN10288268 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unconcealed drawing of lots |
| Allocation concealment (selection bias) | High risk | Unconcealed drawing of lots |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 393/394 (100%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed |
Kristoffersen 2009.
| Methods | Randomised clinical trial, multicentre, 3 hospitals in Denmark | |
| Participants |
Inclusion criteria: Hospitalised patients with suspected pneumonia (no X‐ray confirmation); quote: "The assessment of eligibility (i.e. the clinical diagnosis) was made by the admitting physician and was based on medical history and physical examination." Exclusion criteria: Not meeting the diagnostic criteria Included in this analysis: 210 out of 223 randomised participants; 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) |
|
| Interventions | Guiding antibiotic decisions in CAP patients with initial values only Algorithm used in this study: Physicians were not asked to wait for PCT results before initiating antimicrobial therapy, therefore PCT values were, in most cases, used to motivate either cessation or continuation of already initiated treatments. Discontinuation of AB treatment was recommended if PCT at admission was below 0.25 µg/L, despite delays in test results. |
|
| Outcomes |
|
|
| Notes |
Funding: The Danish Medical Research Council and the Danish Lung Association provided financial support. Follow‐up: Until hospital discharge Registration: NCT00415753 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 210/210 (100% until discharge) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | High risk | 59% adherence to PCT algorithm in PCT group |
Layios 2012.
| Methods | Randomised, single‐centre, prospective, controlled clinical study in 5 ICUs in Belgium | |
| Participants | Inclusion criteria: Patients older than 18 yrs of age and hospitalised for > 2 days in 1 of the 5 ICUs Included in this study: Of 509 randomised participants, 441 developed infection, and PCT was obtained and analysed in 389 of these participants. | |
| Interventions | Guiding antibiotic decisions in ICU patients Algorithm used in this study: According to the proposal by Mueller and colleagues, for participants in the PCT group, the use of antibiotics was more or less strongly discouraged if PCT level was < 0.25 μg/L or 0.50 μg/L, respectively, and more or less recommended if PCT level was above 1 μg/L or 0.50 μg/L, respectively. This strategy was applied to all infectious episodes encountered during participants' ICU stay. |
|
| Outcomes |
|
|
| Notes | Funding: No information provided. Follow‐up: During ICU stay Trial registration: No trial registration found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of randomisation provided. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Procalcitonin levels in the control arm were blinded for treating physicians, but the study arm to which participants had been assigned was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the end of ICU stay, participants’ charts were reviewed by infectious disease specialists blinded to PCT results, who classified them as confirmed, probable, possible, or no infection using all the clinical data and biological results including microbiological cultures and results from investigational procedures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality 509/509 (100%) |
| Selective reporting (reporting bias) | Unclear risk | No trial registration found for this research article. |
| Other bias | High risk | Low adherence to PCT algorithm (46.3%) |
Lima 2016.
| Methods | Randomised, single‐centre, prospective, controlled clinical trial in Minas Gerais, Brazil | |
| Participants | Inclusion criteria: Presence of febrile neutropenia (axillary temperature ˜37.8°C, neutrophils count < 500 cells/mm3) in people with diagnosis of haematological disease (except people undergoing allogeneic stem cell transplantation); with expected duration of neutropenia of more than 3 days; ongoing broad‐spectrum antibiotic therapy according to institutional guideline, based on Infectious Diseases Society of America; and no current use of therapeutic antibiotics or antifungals with the last day of use was > 14 days before inclusion in the study Exclusion criteria: Severe organ dysfunction (e.g. hypotension, ICU admission, trans‐retinoic acid syndrome, respiratory insufficiency, and disseminated intravascular coagulation); previous proven or probable invasive fungal infection according to the Springer Ann Hematol (2016) European Organisation for Research and Treatment of Cancer‐Mycosis Study Group (EORTC‐MSG) criteria; infections due to Pseudomonas spp, Acinetobacter spp, Staphylococcus aureus, Mycobacterium tuberculosis, Pneumocystis jirovecii, Toxoplasma gondii, or HIV; infections requiring antibodies for a long time (e.g. infectious endocarditis, osteomyelitis); grade 3 or 4 oral mucositis, since this condition increases the risk of S aureus bacteraemia; and pregnancy Included in this study: 62 randomised participants, 1 post randomisation exclusion due to withdrawal of informed consent | |
| Interventions | Guiding antibiotic decisions in people with febrile neutropenia Algorithm used in this study: Attending physicians were encouraged to discontinue antibiotics in participants when both of the following criteria were met: (i) no febrile episodes for 2 consecutive days (if no Ionger neutropenia) or 3 consecutive days (if still neutropenia), and (ii) PCT concentration at least 90% lower than highest baseline Ievels or lower than 0.5 ng/mL for 2 consecutive days, regardless of the initial Ievels. The final decision to discontinue antibiotics was left at the attending physician's discretion. Procalcitonin Ievels were measured for 2 additional days following antibiotics interruption to monitor a possible relapse of infection. For safety reasons, at least 5 days of antibiotic therapy were ensured for all included participants. Participants with bacteraemia were treated for at least 7 days. In the control group, duration of antibiotic therapy was based on institutional protocol, according to Infectious Diseases Society of America recommendations. |
|
| Outcomes |
|
|
| Notes | Funding: This work was supported by Funda of the de Amparo a Pesquisa do Eatado dc MiDaa Ocrais (.APQ‐01956‐10). Follow‐up: 3 months Trial registration: NCT00928291 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised on the third day of follow‐up, in a 1:1 ratio for the PCT group and the control group. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using a table of random, computer‐generated numbers, and sealed, opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | For participants randomised into the control group, results of PCT serum Ievels were kept concealed during the study and were only revealed for the final analysis. However, physicians were aware of the study group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment was mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most randomised participants finished follow‐up. Only 1 post randomisation exclusion |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to trial registration. |
| Other bias | Low risk | High adherence to the PCT protocol (73.3%) |
Long 2009.
| Methods | Randomised clinical trial, single‐centre, emergency department outpatients in China | |
| Participants |
Inclusion criteria: CAP with X‐ray confirmation Exclusion criteria: Use of antibiotic therapy in 2 weeks before enrolment, systemic immune deficiency, organ dysfunction, tumour, mental illness, CAP onset ≥ 5 days, coexisting extrapulmonary infection requiring antibiotic therapy Included in this analysis: 127 out of 127 randomised participants |
|
| Interventions | Guiding antibiotic decisions in CAP patients with repeated levels Algorithm used in this study: A PCT level of less than 0.1 µg/L suggested the absence of bacterial infection, and the initiation or continuation of ABs was strongly discouraged. A PCT level between 0.1 and 0.25 µg/L indicated that bacterial infection was unlikely, and the initiation or continuation of ABs was discouraged. A PCT level of 0.25 µg/L or greater was considered indicative of a possible bacterial infection, and the initiation or continuation of AB therapy was encouraged. Re‐evaluation of the clinical status and measurement of PCT levels was recommended after 6 to 12 h in all participants from whom ABs were withheld. |
|
| Outcomes |
|
|
| Notes |
Funding: Training fund of Shanghai No. 5 Hospital Follow‐up: 28 days Registration: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd and even patient ID numbers |
| Allocation concealment (selection bias) | High risk | Odd and even patient ID numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 210/210 (100% until discharge) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
Long 2011.
| Methods | Randomised clinical trial, single‐centre, emergency department outpatients in China | |
| Participants |
Inclusion criteria: CAP with X‐ray confirmation in an outpatient setting Exclusion criteria: Pregnancy, commencement of antibiotic therapy ≥ 48 h before enrolment, systemic immune deficiency, withholding of life‐support, and active tuberculosis Included in this analysis: 156 out of 172 randomised participants; 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) |
|
| Interventions | Guiding antibiotic decisions in CAP patients with repeated levels Algorithm used in this study: A PCT level of less than 0.1 µg/L suggested the absence of bacterial infection, and the initiation or continuation of ABs was strongly discouraged. A PCT level between 0.1 and 0.25 µg/L indicated that bacterial infection was unlikely, and the initiation or continuation of ABs was discouraged. A PCT level of 0.25 µg/L or greater was considered indicative of a possible bacterial infection, and the initiation or continuation of AB therapy was encouraged. Re‐evaluation of the clinical status and measurement of PCT levels was recommended after 6 to 12 h in all participants from whom ABs were withheld. |
|
| Outcomes |
|
|
| Notes |
Funding: The study was sponsored by a grant from the Shanghai Fifth People's Hospital Science Foundation (09YRCPY11). Follow‐up: Fixed period of 4 weeks Registration: NA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd and even patient ID numbers |
| Allocation concealment (selection bias) | High risk | Odd and even patient ID numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 156 (156) (100%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
Long 2014.
| Methods | Randomised, single‐centre, open‐label, controlled clinical trial in Shanghai, China | |
| Participants | Inclusion criteria: People aged 18 to 65 years with severe acute exacerbations of asthma. A severe asthma exacerbation was defined as at least 1 of the following: need for systemic corticosteroids, or an increase from a stable maintenance dose, for at least 3 days and/or hospitalisation or ED visit because of asthma requiring systemic corticosteroids. Exclusion criteria: People with antibiotic use within the previous 14 days, psychiatric disorders or other inability to give written informed consent, not being available for follow‐up, severe immunosuppression, heart failure, cystic fibrosis, active tuberculosis, pregnancy, and chest radiography–confirmed pneumonia Included in this study: 180/216 screened individuals were eligible for the study (90 intervention group, 90 non‐intervention group); 169 finished the follow‐up. | |
| Interventions | Guiding antibiotic decisions in people with acute severe exacerbation of asthma Algorithm used in this study: Antibiotic treatment was strongly discouraged when serum PCT level was less than 0.1 μg/L; antibiotic treatment was discouraged when serum PCT level was less than 0.25 μg/L; and antibiotic treatment was encouraged when serum PCT level was higher than 0.25 μg/L. When antibiotics were withheld from participants, a second measurement of the PCT level was mandatory within 6 to 24 hours for safety reasons. The use of antibiotics was recommended if this second measurement was higher than 0.25 μg/L. Physicians were permitted to overrule the algorithm, but they had to indicate the reasons for overruling. The control group received antibiotic according to the discretion of the treating physician, who was unaware of the participant’s PCT levels. |
|
| Outcomes |
|
|
| Notes | Funding: The study was sponsored by a grant from the Shanghai Fifth People’s Hospital Science Foundation and Minhang District Natural Science Foundation of Shanghai. The funding bodies had no involvement in the design, collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. Follow‐up time: 12 months Registration: ChiCTR‐TRC‐12002534 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to either intervention was conducted according to computer‐generated random numbers produced by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | After randomisation, an opaque, sealed, sequentially numbered envelope containing the PCT or control protocol was prepared for each participant according to group assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study with blinding of PCT level in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 169/180 participants completed 1‐year follow‐up visit (11 participants lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (ChiCTR‐TRC‐12002534). |
| Other bias | Low risk | High adherence to PCT algorithm (93.3%) |
Maravić‐Stojković 2011.
| Methods | Randomised, single‐centre, open‐label, controlled clinical trial at a 200‐bed academic tertiary care hospital in Belgrade, Serbia | |
| Participants | Inclusion criteria: People scheduled to undergo open heart surgery on cardio‐pulmonal bypass. We assessed people who were selected for elective cardiac surgery at the 200‐bed academic tertiary care hospital. The criterion for inclusion in the study was the type of operation: coronary artery bypass grafting (CABG) surgery, valve reconstruction, combined CABG and valve procedures. Entry criteria included stable and unstable angina pectoris, valve insufficiency, left ventricle ejection fraction (LVEF) above 30%, and epidemiological status with saprophyte bacteria. Exclusion criteria: People selected for redo cardiac operations, thoracic aortic surgery, as well as people having active endocarditis and people with LVEF < 30%. People with preoperative signs of infection (leukocyte count > 12,000/L; body temperature > 38°C) were also excluded. Included in this study: 205/205 included participants finished for follow‐up (102 PCT group/103 standard group). | |
| Interventions | Guiding antibiotic decisions in patients after cardiac surgery Algorithm used in this study: Antibiotic prophylaxis was performed in all participants. The participants were divided at the time of surgery into the standard group and the PCT group. In the standard group, the antibiotic use was applied according to the criteria based on the laboratory and clinical signs; no antibiotic therapy was administrated routinely in the absence of clinical signs of infection or a bacteriologic positive sample. In the PCT group, the use of antibiotics was encouraged or discouraged on the basis of serum PCT concentrations. A serum PCT concentration of 0.5 ng/mL or less indicated the absence of bacterial infection, at which point the use of antibiotics was discouraged. |
|
| Outcomes |
|
|
| Notes | Funding: No funding is mentioned in the main article. Follow‐up: 1 year Trial registration: No trial registration found. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | The participants were divided at the time of surgery into the standard group and the PCT group by centralised randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of physicians due to the study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 205/205 (100%) |
| Selective reporting (reporting bias) | Unclear risk | No trial registration found. |
| Other bias | Unclear risk | No information about adherence |
Najafi 2015.
| Methods | Randomised, single‐centre, single‐blinded clinical trial in a 30‐bed ICU in Tehran, Iran | |
| Participants | Inclusion criteria: Patients with at least 2 of 4 criteria including body temperature above 38°C or below 36°C; tachycardia > 90/min; tachypnoea > 20/min; and leukocytosis > 12 × 109/L or a leftward shift with more than 10% band cells or leukopenia < 4 × 109/L were defined as patient with SIRS. Exclusion criteria: Documented infection, pus from wound or abscess, empyema, thrombophlebitis, infection due to viral or parasites, hypoxaemia (PO2 < 60 mmHg), oliguria (urine output < 30 mL/h), Glasgow Coma Scale 3 without sedation, parenteral antibiotic usage 24 hours before admission to ICU, hospitalisation 48 hours before enrolment, conditions requiring prolonged antibiotic therapy such as endocarditis, chronic localised infection such as osteomyelitis, and severely immunocompromised patients Included in this study: 60 participants were randomised (30 in the intervention group and 30 in the control group). | |
| Interventions | Guiding antibiotic decisions in critically ill ICU patients Algorithm used in this study: In case group, according to serum level of PCT, participants were divided into 3 groups as: PCT level 0.5 ng/mL or less (group A), PCT value of 0.5 to 2 ng/mL (group B), and PCT level 2 ng/mL or more (group C). Group A indicated a low probability of bacterial infection; use of antibiotics was discouraged, and PCT level was rechecked after 12 hours. In group B, with a medium probability of infection, antibiotic therapy was not administered, and PCT level was rechecked after 8 hours. In group C, with a high probability of bacterial infection, participants underwent antibiotic treatment. If the PCT level was higher than 2 ng/mL after recheck in group A and B, antibiotics therapy was administered; if the PCT level was lower than 2 ng/mL, participants underwent close observation, and PCT was rechecked until culture results were obtained. |
|
| Outcomes |
|
|
| Notes | Funding: No funding declared in the original research article. Follow‐up: No precise follow‐up found in the original research article. Trial registration: No trial registration found in the original research article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants were randomly divided into 2 groups by computer‐based random number generation. |
| Allocation concealment (selection bias) | Low risk | All participants were randomly divided into 2 groups by computer‐based random number generation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Procalcitonin was only measured in the intervention arm, but blinding was not feasible due to the study design. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment was mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants finished the study and were assessed for the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration was found for this study. |
| Other bias | Unclear risk | Adherence to the PCT algorithm was not known. |
Nobre 2008.
| Methods | Randomised clinical trial, single‐centre, medical ICU in Switzerland | |
| Participants |
Inclusion criteria: Suspected severe sepsis or septic shock in the ICU Exclusion criteria:
Included in this analysis: 52 out of 79 randomised participants; 27 not considered for this analysis due to a diagnosis other than RTI |
|
| Interventions | Guiding antibiotic decisions in ICU patients with repeated measurements Algorithm used in this study: Procalcitonin levels measured at baseline and daily. For participants presenting a favourable clinical course, investigators used predefined “stopping rules” based on circulating PCT levels to encourage physicians to discontinue ABs. Participants with baseline PCT level ≥ 1 µg/L were re‐evaluated at day 5. Investigators encouraged treating physicians to discontinue ABs when:
Participants with PCT level below 1 µg/L at baseline were re‐evaluated at day 3; treating physicians were encouraged to discontinue ABs when PCT level was below 0.1 µg/L and careful clinical evaluation ruled out severe infection. |
|
| Outcomes |
|
|
| Notes |
Funding: B·R·A·H·M·S AG Follow‐up: Fixed follow‐up period of 28 days Registration: NCT00250666 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 52/52 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 81% adherence to PCT algorithm in PCT group |
Ogasawara 2014.
| Methods | Randomised, single‐centre, prospective, open‐label, non‐inferiority clinical trial in Shizuoka, Japan | |
| Participants | Inclusion criteria: Patients at risk for aspiration, who had been hospitalised after developing pneumonia, were enrolled. Aspiration pneumonia was clinically diagnosed on the basis of the findings on computed tomography (e.g. bronchopneumonia in the dorsal lower lobes), combined with a history of aspiration pneumonia, stroke or dementia, poor systemic condition, or any combination of these (e.g. bedridden patients or patients fed by a nasogastric tube or percutaneous endoscopic gastrostomy). Selection criteria included the following: at least 1 month had elapsed since the last treatment for relapsed pneumonia, and ventilator use was not scheduled for the pneumonia treatment Exclusion criteria: Patients with a known severe allergy to any drugs; patients with sepsis or a severe infectious disease; patients with severe underlying diseases (e.g. malignancy, COPD, heart failure) that affected the prognosis; and patients who could not safely have cessation of oral intake or hydration as a treatment for aspiration pneumonia because of dementia Included in this study: The study enrolled 105 participants; 2 participants withdrew their informed consent, 5 were excluded because of other final diagnoses, and 1 was excluded because of a defect in the PCT data. The ITT population thus comprised 96 participants: 48 in the PCT group and 48 in the control group | |
| Interventions | Guiding antibiotic decisions in patients with aspiration pneumonia and assessment of the continuation of oral intake Algorithm used in this study: Procalcitonin levels were measured via outsourcing to SRL (Tokyo, Japan); the results were obtained 2 or 3 days after admission. In the PCT group, if the PCT levels upon admission were < 0.5 ng/mL, 0.5 to 1.0 ng/mL, or > 1.0 ng/mL, the duration of antibiotic therapy was determined to be 3, 5, or 7 days, respectively. If the PCT level upon admission was 45.0 ng/mL, antibiotic treatment was continued until it was less than 10% of the peak PCT level reached. In the control group, antibiotic therapy followed the recommendations of the Japanese Respiratory Society guideline for management of community‐acquired pneumonia in adults. Antibiotic therapy was discontinued if 3 of the following 4 criteria were met: fever declined (body temperature < 37.0°C), normalisation of leukocyte count, decrease in the CRP level to 30% of the maximum, and an obvious improvement as observed by chest radiography. In both groups, the choice of antibiotic regimen was left to the discretion of the treating physician. |
|
| Outcomes |
|
|
| Notes | Funding: No funding declared in the main research paper. Follow‐up: 30 days Trial registration: UMIN000004800 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated in 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No clear mention of allocation concealment is made in the main article. Citation "Following enrolment, the patients were randomly allocated in a 1:1 ratio to groups assigned different durations of antibiotic therapy…" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Procalcitonin was only measured in the intervention arm, but study design was open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most participants were followed for 30 days. Reasons for post randomisation exclusions are clearly reported in the article. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to trial registration. |
| Other bias | Unclear risk | As cited by authors under limitations, time until analysis of PCT concentrations was available was relatively long (3 days). Adherence to PCT algorithm not known |
Oliveira 2013.
| Methods | Randomised, multicentre, open‐label, controlled clinical trial in the ICUs of 2 university hospitals in Brazil | |
| Participants | Inclusion criteria: All adult patients 18 years of age or older with suspected severe sepsis or septic shock were assessed for potential inclusion. Exclusion criteria: Confirmed microbiological infection by Pseudomonas aeruginosa,Acinetobacter baumannii, Listeria spp,Mycobacterium tuberculosis, or fungi; Staphylococcus aureus bacteraemia; suspected or confirmed severe infections caused by viruses or parasites; infections that required long‐term treatment, regardless of the aetiologic agent (e.g. bacterial endocarditis); localised chronic infections (e.g. chronic osteomyelitis); > 48 hours of antibiotic treatment; immunosuppressed patients (such as those diagnosed with HIV), patients with neutropenia (< 500 neutrophils/mm3), patients post‐solid organ transplant, patients under immunosuppressive therapy, and patients who received more than 1 mg/kg of prednisone or equivalent; patients under palliative care; patients who suffered multiple trauma, burns, or major surgery in the previous 5 days; patients diagnosed with pulmonary neoplasias, carcinoid tumours, or medullary tumours of the thyroid; and patients who remained in the ICU for 24 hours or less Included in this study: 94/355 patients assessed for eligibility were included in the final analysis (49 in the PCT group and 45 in the CRP group). | |
| Interventions | Guiding antibiotic decisions with CRP versus PCT in septic patients Algorithm used in this study: Antibiotic therapy was discontinued following a protocol based on serum levels of these markers, according to the allocation group. For both groups, at least 7 full days of antibiotic therapy were ensured in participants with SOFA score greater than 10 and/or bacteraemia at inclusion, and participants with evident resolution of the infectious process had antibiotics stopped after 7 days, despite biomarker levels. |
|
| Outcomes |
|
|
| Notes | Funding: Supported in part by the Minas Gerais Research Foundation (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG) Follow‐up: 28 days or until death or hospital discharge Trial registration: NCT00934011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes were used for the randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding due to the study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT00934011). |
| Other bias | Low risk | High adherence to the PCT protocol (87.8%) |
Schroeder 2009.
| Methods | Randomised clinical trial, single‐centre, surgical ICU in Germany | |
| Participants |
Inclusion criteria: Patients after abdominal surgery with antibiotic treatment because of severe sepsis in the surgical ICU Exclusion criteria: Patients were excluded if they did not meet the respective inclusion criteria, refused informed consent, or had already received antibiotic treatment prior to admission to the ICU. Included in this analysis: 8 out of 27 randomised participants; 19 not considered for this analysis due to diagnosis other than RTI |
|
| Interventions | Guiding antibiotic decisions in postoperative patients in a surgical ICU Algorithm used in this study: In the PCT‐guided group, antibiotic therapy was discontinued if clinical signs and symptoms of sepsis improved and PCT values had either decreased to 1 µg/L or less or had dropped to 25% to 35% of the initial PCT concentration over 3 consecutive days. |
|
| Outcomes |
|
|
| Notes |
Funding: NA Follow‐up: Until hospital discharge Registration: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unconcealed drawing of lots |
| Allocation concealment (selection bias) | High risk | Unconcealed drawing of lots |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 8/8 (100% until discharge) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
Schuetz 2009.
| Methods | Randomised clinical trial, multicentre, 6 sites in Switzerland | |
| Participants |
Inclusion criteria: Clinical diagnosis of CAP, ECOPD, bronchitis with X‐ray confirmation Exclusion criteria: People with active intravenous drug use, severe immunosuppression other than corticosteroid use, life‐threatening medical comorbidity leading to possible imminent death, hospital‐acquired pneumonia (development of pneumonia 48 hours after hospital admission or if they were hospitalised within 14 days before presentation), and chronic infection necessitating antibiotic treatment Included in this analysis: 1359 out of 1381 randomised participants; 22 post randomisation exclusions due to withdrawal of consent |
|
| Interventions | Guiding antibiotic decisions in emergency department patients with different ARIs with repeated measurements Algorithm used in this study: Initiation or continuation of ABs was strongly discouraged if PCT was less than 0.1 µg/L and discouraged if levels were 0.25 µg/L or lower. Initiation or continuation of ABs was strongly encouraged if PCT was higher than 0.5 µg/L and encouraged if levels were higher than 0.25 µg/L. If ABs were withheld, hospitalised patients were clinically re‐evaluated and PCT measurement was repeated after 6 to 24 hours. |
|
| Outcomes |
|
|
| Notes |
Funding: This work was supported in part by grant SNF 3200BO‐116177/1 from the Swiss National Science Foundation and contributions from santésuisse and the Gottfried and Julia Bangerter‐Rhyner‐Foundation, the University Hospital Basel, the Medical University Clinic Liestal, the Medical Clinic Buergerspital Solothurn, the Cantonal Hospitals Muensterlingen, Aarau and Lucerne, respectively, the Swiss Society for Internal Medicine, and the Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Basel. B·R·A·H·M·S Inc, the major manufacturer of the PCT assay, provided all assay‐related material, Kryptor machines if not already available onsite, and kits and maintenance required for 10,000 measurements related to the study. Follow‐up: Fixed follow‐up period after 30 days and 180 days Registration: ISRCTN95122877 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Central randomisation using a study web site |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviews by blinded medical students, data safety monitoring board |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 1358/1359 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match previously published protocol. |
| Other bias | Low risk | 91% adherence to PCT algorithm in PCT group |
Shehabi 2014.
| Methods | Randomised, multicentre, single‐blind, controlled clinical trial in 11 ICUs in Australia | |
| Participants | Inclusion criteria: Patients older than 18 years of age, admitted to ICU within the previous 72 hours, receiving parenteral and/or enteral antibiotics for a suspected bacterial infection (with 2 or more SIRS criteria) and expected to remain in the ICU for longer than 24 hours were eligible. Exclusion criteria: Patients receiving antibiotics for surgical prophylaxis or with proven bacterial infection requiring more than 3 weeks’ antibiotic therapy, isolated systemic fungal or systemic viral infection in the absence of bacterial infection, neutropenia with a neutrophil count less than 1000 cells/mL, receiving immunosuppressive agents, cardiac surgery or trauma or heat stroke within 48 hours, medullary thyroid or small cell lung cancer, patient not expected to survive to hospital discharge, or known pregnancy Included in this study: Of 400 randomised participants, 394 finished 90 days' follow‐up for survival. 6 withdrew their consent. | |
| Interventions | Guiding antibiotic decisions in critically ill patients in the ICU with undifferentiated infection or suspected sepsis Algorithm used in this study: Cessation of antibiotics was recommended if initial or any subsequent PCT was negative (<0.10 ng/mL) or if initial or any subsequent PCT was between 0.10 to 0.25 ng/mL, and infection was highly unlikely; Subsequent PCT level declined more than 90% from baseline, and 2. Assess antibiotic appropriateness and/or adequacy of source control if PCT level at 48 hours is 70% of baseline value. Daily PCT results were made available to the treating clinician for participants randomised to the PCT group. Antibiotic prescription in both the standard care and PCT groups was according to the Australian Antibiotics Therapeutic Guidelines and the antimicrobial stewardship (implemented by infectious diseases twice‐weekly rounds and on‐need consultations). The algorithm was implemented only in the ICU. |
|
| Outcomes |
|
|
| Notes | Funding: Funded by a competitive grant from the Intensive Care Foundation of Australia and New Zealand. Material support was provided by Roche Diagnostics, Thermo Fisher Scientific, and bioMérieux. Roche Diagnostics and Thermo Fisher Scientific provided additional unrestricted grant funding. Follow‐up: 90 days' post randomisation for survival Trial registration: ACTRN12610000809033 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were variable block randomised 1:1 via a secured central study web site into either a PCT‐guided or clinician‐guided group. |
| Allocation concealment (selection bias) | Low risk | Central randomisation with variable blocks |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | For the standard care group, clinicians were blinded to the PCT levels, but the physicians were aware of the participants' study group due to the study design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were collected by professional research personnel at each site and entered into a central secured database at the Clinical Informatics and Data Management Unit, Department of Epidemiology and Preventive Medicine, Monash University and analysed by a blinded biostatistician at Monash University, Melbourne, Australia. The study was monitored by an independent data safety and monitoring committee, with no interim analysis performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The follow‐up for mortality was 394/394 (100%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (ACTRN12610000809033). |
| Other bias | Low risk | High adherence to the PCT algorithm (97%). "The proportion of study days where the PCT algorithm was not followed was less than 3%, the majority of which was due to missed PCT sampling." |
Stolz 2007.
| Methods | Randomised clinical trial, single‐centre, University Hospital Basel, Switzerland | |
| Participants |
Inclusion criteria: Clinical diagnosis of COPD exacerbation Exclusion criteria: People who were considered to be vulnerable study participants (i.e. those with psychiatric comorbidity) were excluded from the study. Other exclusion criteria were immunosuppression, asthma, cystic fibrosis, and the presence of infiltrates on chest radiographs on hospital admission. Included in this analysis: 208 out of 226 randomised participants; 18 post randomisation exclusions due to absence of COPD according to GOLD criteria |
|
| Interventions | Guiding antibiotic decisions in COPD patients with repeated measurements Algorithm used in this study: Procalcitonin level of 0.1 µg/L was considered indicative of the absence of bacterial infection, and the use of ABs was discouraged. A level of 0.1 to 0.25 µg/L indicated possible bacterial infection, and the use of ABs was discouraged or encouraged, respectively, based on the stability of the participant’s clinical condition. A PCT level of 0.25 µg/L was considered suggestive of the presence of bacterial infection, and AB treatment was encouraged. |
|
| Outcomes |
|
|
| Notes |
Funding: This study was funded by the Clinic of Pulmonary Medicine; the Clinic of Endocrinology, Diabetes and Clinical Nutrition; and the Emergency Department of the University Hospital Basel. B·R·A·H·M·S provided PCT assays for this investigator‐driven study. Follow‐up: Short‐term follow‐up visit after 14 to 21 days; long‐term follow‐up visit at 6 months Registration: ISRCTN77261143 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation list. |
| Allocation concealment (selection bias) | High risk | Sealed envelopes, not numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 208/208 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
Stolz 2009.
| Methods | Randomised clinical trial, multicentre with 7 European and US intensive care units | |
| Participants |
Inclusion criteria: VAP when intubated for > 48 h Exclusion criteria: Patients were excluded it they 1) were pregnant; 2) were enrolled in another trial; 3) had received immunosuppressants or long‐term corticosteroid therapy (> 0.5 mg/kg per day for > 1 month); 4) were severely immunosuppressed, including AIDS; or 5) had a coexisting extrapulmonary infection diagnosed between day 1 and 3 requiring antibiotic therapy for > 3 days. Included in this analysis: 101 (101) (100%) |
|
| Interventions | Guiding antibiotic decisions in VAP patients with repeated measurements Algorithm used in this study: A PCT level of < 0.25 µg/L suggested the absence of VAP, and discontinuation of ABs was strongly encouraged. A PCT level between 0.25 µg/L and 0.5 µg/L or a decrease by ≥ 80% as compared to day 0 indicated that bacterial infection was unlikely, and reduction or discontinuation of ABs was encouraged. A PCT level ≥ 0.5 µg/L or decrease by < 80% as compared to day 0 was considered indicative of unresolved bacterial infection, and reduction or discontinuation of AB was discouraged. A PCT level of > 1 µg/L strongly suggested unresolved bacterial infection, and AB discontinuation was strongly discouraged. |
|
| Outcomes |
|
|
| Notes |
Funding: Funding was granted by the Clinic of Pulmonary Medicine, University Hospital Basel. Funding obtained from B·R·A·H·M·S AG (Hennigsdorf, Germany) Follow‐up: Fixed follow‐up period of 28 days Registration: ISRCTN61015974 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation list. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was through arbitrary allocation to one of the two treatment assignments based on sealed, opaque envelopes. Block size was 20 envelopes. Treating physicians were not aware of envelope contents before randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded study member |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up for mortality: 101/101 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
Tang 2013.
| Methods | Randomised, single‐centre, single‐blinded, controlled clinical trial in the emergency department of the Fifth People’s Hospital of Shanghai, China | |
| Participants | Inclusion criteria: 1) ≥ 18 years old; 2) has any, or all, of the following clinical features as defined by the Global Initiative for National Asthma (GINA) Guidelines: dyspnoea, wheeze, acute cough, increased work of breathing, increased requirement for beta2‐agonist from baseline use, O2 saturation < 95%, a peak expiratory flow (PEF) at randomisation ≤ 80% of their known best (within the last 12 months) or, in the absence of this information, of their predicted PEF Exclusion criteria: 1) treatment with antibiotics within 2 weeks prior to recruitment; 2) bacterial infection in other parts of body than the respiratory system; 3) chest X‐ray‐confirmed pneumonia; 4) suffering from other chronic respiratory diseases; 5) suffering from severe organ dysfunction Included in this study: 265 people were eligible, and 255 participants completed the study. | |
| Interventions | Guiding antibiotic decisions in patients with acute exacerbation of asthma Algorithm used in this study: Participants in the PCT group were treated with antibiotics based on their PCT serum level according to the following guidelines: antibiotics treatment was strongly discouraged when serum PCT level was < 0.1 μg/L; antibiotics treatment was discouraged when serum PCT level was < 0.25 μg/L; antibiotics treatment was encouraged when serum PCT level was > 0.25 μg/L. |
|
| Outcomes |
|
|
| Notes | Funding: The study was sponsored by a grant from the Shanghai Fifth People’s Hospital Science Foundation and Minhang District Natural Science Foundation of Shanghai. Follow‐up: 6 weeks Trial registration: ICTRP ChiCTR‐TRC‐12002534 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to either intervention was conducted according to computer‐generated random numbers produced by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | After randomisation, an opaque, sealed, and sequentially numbered envelope containing the PCT or control protocol was prepared for each participant according to the group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Attending physicians responsible for participants in the control group remained unaware of the participants’ PCT concentrations throughout the study, but blinding was not feasible due to the study design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants, laboratory technicians, investigators, and research designers were blinded to participant assignments until the data analysis was completed. There were no protocol violations during the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 265/265 participants completed follow‐up for mortality. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol: ICTRP ChiCTR‐TRC‐12002534. |
| Other bias | Low risk | 100% adherence to the PCT algorithm. "There were no protocol violations during the study." |
Verduri 2015.
| Methods | Randomised, multicentre, open, controlled, parallel‐group, non‐inferiority trial involving 18 university/city hospital pulmonary departments in Italy | |
| Participants | Inclusion criteria: Study participants were male or female, 18 years of age, current or former smokers, and diagnosed with COPD stages I‐IV as defined by GOLD guidelines available at the time the study was designed, with protocol deviation. Participants were hospitalised for severe ECOPD requiring antibiotic treatment, i.e. type 1 exacerbation (increased dyspnoea, sputum volume, and sputum purulence verified by the attending clinician) according to Anthonisen, and/or characterised by respiratory failure. Exacerbation of chronic obstructive pulmonary disease was defined as “an acute event characterised by a worsening of the patient’s respiratory symptoms that is beyond normal day‐to‐day variations and leads to a change in medication”. Exclusion criteria: Bronchial asthma, unstable concomitant disease (cardiovascular, renal, hepatic, gastrointestinal, neurological, metabolic, musculoskeletal, neoplastic, respiratory, or other disease), pregnancy and breastfeeding, clinically significant laboratory abnormalities suggestive of unstable concomitant disease, survival for 1 year unlikely, and inability to give written consent. Antibiotic pretreatment before hospital admission and radiographic signs of pneumonia did not preclude eligibility for the study. Included in this study: 183 participants were randomised, of which 178 participants, 88 in the PCT group and 90 in the standard care group, were analysed. | |
| Interventions | Guiding antibiotic decisions in people with severe exacerbations of COPD Algorithm used in this study: On admission, all patients received a 3‐day course of antibiotics (either amoxicillin plus clavulanate or quinolones) according to 2005 international guidelines. Procalcitonin levels were measured on hospital admission, on day 1, and on day 2. On day 2 each eligible patient was randomly assigned to 1 of the 2 treatment plans. quote "Participants randomised to the standard group continued antibiotic therapy for 10 days, whereas participants randomised to the PCT group either continued treatment for 10 days or stopped on day 3, depending on their PCT levels, according to previously recommended cut‐off values. Specifically, participants continued antibiotic treatment for 10 days if 1 or more of the PCT values on the first 3 days of hospitalisation were 0.25 μg/L. When PCT values were < 0.25 μg/L but > 0.1 μg/L on any occasion, antibiotic treatment was continued for 10 days if participants were clinically unstable or had acute respiratory failure; otherwise, treatment was stopped on day 3. If all PCT values were consistently < 0.1 μg/L, treatment was stopped on day 3." All participants were also treated with systemic corticosteroids for 14 days, plus regular inhaled short‐acting or long‐acting bronchodilators. |
|
| Outcomes |
|
|
| Notes | Funding: The trial was approved and funded by the Agenzia Italiana del Farmaco (AIFA), the Italian agency for drugs, which is an official body of the Italian Ministry of Health. Follow‐up: Follow‐up visits were scheduled on day 1, day 3, and 6 months after discharge; telephone interviews were conducted at 2, 4, and 5 months after discharge. Trial registration: NCT01125098 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was web based, and only statisticians and the web site administrator knew the randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Eligible patients were randomly assigned to receive standard antibiotic therapy (standard group) or PCT‐guided antibiotic treatment (PCT group) according to a 1:1 permuted block computer‐generated scheme, stratified according to hospital |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not feasible due to the study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment mentioned in the study. The authors state that: "... because we anticipated that the primary outcome (exacerbations of COPD) would be strong and easy to identify, and thus unlikely to be biased by investigator influence, we did not adopt any procedure to reduce bias during the follow‐up part of the trial." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 187 of 192 randomised participants were included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Results correspond to the trial registration. |
| Other bias | Low risk | High adherence to the PCT protocol (95.5%) The study planned to enrol 400 participants to have enough statistical power for the primary endpoint, but randomised only 183. |
Wang 2016.
| Methods | Randomised, single‐centre, single‐blinded, controlled clinical trial in the Beijing Luhe Hospital, China | |
| Participants | Inclusion criteria: Patients with AECOPD who were 40 years of age, had sound understanding and language abilities, and who had a PCT level < 0.1 ng/mL were included. Exclusion criteria: Fever (38°C), tracheal intubation within 24 h after hospital admission, a PCT level of 0.1 ng/mL on admission, pneumonia, chronic renal failure, history of malignant disease, immunosuppressive therapy, and refusal to participate Included in this study: 194 randomised participants. 191 finished the 30‐day follow‐up. | |
| Interventions | Guiding antibiotic decisions in patients with acute exacerbation of chronic obstructive pulmonary disease Algorithm used in this study: Patients with a PCT concentration < 0.1 ng/mL were randomised. Antibiotics were withheld from participants in the control group. However, antibiotics could be administered later for participants whose clinical condition was unstable or who had a worsening of symptoms and signs, and for those with positive evidence of bacteria as assessed by the attending physicians. In the antibiotic group, antibiotics were administered routinely. |
|
| Outcomes |
|
|
| Notes | Funding: The study was sponsored by the National Science Fund for Distinguished Young Scholars (81425001/H0104) for Dr Bin Cao. Follow‐up: 30 days after hospital discharge Trial registration: ChiCTR‐TRC‐14004726 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer digital table method was used to generate randomisation numbers. |
| Allocation concealment (selection bias) | Low risk | Researchers in this study had 24‐hour access to randomisation numbers, allowing immediate and concealed allocation to the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding feasible due to the study protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Individuals responsible for allocation concealment were not allowed to take part in the measurement of results, but no overall blinding of the outcome assessment was mentioned in this study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 participants were excluded during the 30‐day follow‐up, due to a diagnosis of pneumonia according to chest X‐ray. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to the trial registration (ChiCTR‐TRC‐14004726). |
| Other bias | Low risk | High adherence to the PCT protocol (82.3%) |
AB: antibiotic AECOPD: acute exacerbation of chronic obstructive pulmonary disease AE‐IPF: acute exacerbation of idiopathic pulmonary fibrosis ARIs: acute respiratory infections CAP: community‐acquired pneumonia COPD: chronic obstructive pulmonary disease CPIS: Clinical Pulmonary Infection Score CRP: C‐reactive protein d: day ED: emergency department ECOPD: exacerbation of chronic obstructive pulmonary disease EMR: electronic medical record FEV1%: forced expiratory volume for 1 second expressed as a percentage of the forced vital capacity GOLD: Global Initiative for Chronic Obstructive Lung Disease h: hour ICU: intensive care unit ID: identification ITT: intention‐to‐treat LRTI: lower respiratory tract infection NA: not available ODIN: Organ Dysfunction and/or Infection PaO2/FiO2: relationship between arterial oxygen tension (PaO2) and inspiratory oxygen fraction (FiO2) Pa: arterial PCR: polymerase chain reaction PCT: procalcitonin RSV: respiratory Syncytial virus RTI: respiratory tract infection SaO2: oxygen saturation SIRS: systemic inflammatory response syndrome SOFA: Sequential Organ Failure Assessment VAP: ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dharaniyadewi 2013 | Poster presentation only |
| Esposito 2012 | Not adult participants (paediatrics) |
| Heyland 2011 | Meta‐analysis of previous RCTs |
| Jensen 2011 | Not using procalcitonin to de‐escalate antibiotic therapy but for improving mortality by escalation of therapy |
| Jones 2007 | Meta‐analysis of observational studies |
| Kook 2012 | Not an RCT; before‐after study design |
| Liew 2011 | Not an RCT |
| Liu 2013 | Not an RCT |
| Qu 2012 | Not a respiratory infection (pancreatitis) |
| Saeed 2011 | Not an RCT |
| Schuetz 2010 | Not an RCT; before‐after study design (post study survey) |
| Simmonds 2005 | Meta‐analysis of observational studies |
| Simon 2004 | Meta‐analysis of observational studies |
| Stocker 2010 | Included a paediatric population only |
| Tang 2007 | Meta‐analysis of observational studies |
| Tang 2009 | Meta‐analysis of RCTs |
| Uzzan 2006 | Meta‐analysis of observational studies |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02130986.
| Trial name or title | PCT Antibiotic Consensus Trial (ProACT) |
| Methods | Randomised, single‐blind, multicentre study |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Algorithm used in this study: Procalcitonin versus usual care group in patients with LRTI in the ED. Procalcitonin cut‐offs: < 0.1 ng/L. Antibiotics strongly discouraged 0.1 to 0.25 ng/L. Antibiotics discouraged > 0.25 to 0.5 ng/L. Antibiotics recommended > 0.5 ng/L. Antibiotics strongly recommended |
| Outcomes |
|
| Starting date | November 2014 |
| Contact information | Elizabeth A Gimbel, BS; gimbele@upmc.edu Kourtney A Wofford, BA; woffordka@upmc.edu |
| Notes |
Collaborators: University of Pittsburgh National Institute of General Medical Sciences (NIGMS), bioMérieux Registration: NCT02130986 |
NCT02261610.
| Trial name or title | Pulmonary embolism and PCT. PE‐PCT study |
| Methods | Single‐centre, prospective, randomised trial in France |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Procalcitonin algorithm to guide antibiotic therapy. In the control group, the use of antibiotics will be guided by clinical criteria |
| Outcomes |
|
| Starting date | November 2014 |
| Contact information | Patrick Lacarin; placarin@chu‐clermontferrand.fr |
| Notes |
Collaborators: Thermo Fisher Scientific Registration: NCT02261610 |
NCT02332577.
| Trial name or title | Study to compare the efficacy of pristinamycin (Pyostacine) versus amoxicillin in the treatment of acute community acquired pneumonia |
| Methods | A multicentre, non‐inferiority, randomised, double‐blind, phase IV study in France and Tunisia |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: pristinamycin + placebo versus amoxicillin + placebo. To evaluate the clinical efficacy of pristinamycin at a dose of 2 g x 2/day for 2 days then 1 g x 3/day for 5 to 7 days versus amoxicillin 1 g x 3/day for 7 to 9 days, 5 to 9 days after the end of treatment |
| Outcomes |
|
| Starting date | April 2015 |
| Contact information | Contact‐Us@sanofi.com |
| Notes |
Collaborators: Clinical Sciences & Operations Registration: NCT02332577 |
NCT02440828.
| Trial name or title | Addition of tobramycin inhalation in the treatment of ventilator associated pneumonia (VAPORISE) |
| Methods | Randomised trial, parallel assignment, double‐blind (participant, investigator) in the Netherlands and Spain |
| Participants |
Inclusion criteria: 1) mechanical ventilation 48 hours or more; and 2) new or progressive radiologic pulmonary infiltrate; together with at least 2 of the following 3 criteria (< 24 h):
Exclusion criteria:
|
| Interventions |
Algorithm used in this study: Experimental arm: tobramycin inhalation twice daily; tobramycin inhalation (Bramitob) 300 mg and standard intravenous antibiotics treatment. Intervention: drug: tobramycin inhalation Placebo comparator: placebo twice daily; placebo inhalation and standard intravenous antibiotics treatment. Intervention: drug: placebo |
| Outcomes |
|
| Starting date | March 2015 |
| Contact information | Rogier Hoek, MD; r.hoek@erasmusmc.nl Menno Van der Eerden, MD, PhD; m.vandereerden@erasmusmc.nl |
| Notes |
Collaborators: Chiesi Farmaceutici S.p.A. Registration: NCT02440828 |
NCT02787603.
| Trial name or title | PCT in Early Antibiotic Interruption in Patient With Bacterial Pulmonary infeCtion and Acute Heart Failure (EPICAD) |
| Methods | Randomised trial with parallel assignment, open label in Brazil |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Algorithm used in this study: Experimental: group A: interruption of antibiotic treatment based on PCT measurement No intervention: group B: antibiotic therapy period determined by the physician without knowledge of PCT levels |
| Outcomes |
|
| Starting date | January 2015 |
| Contact information | Mucio Tavares, PhD, MD; mucio@incor.usp.br Aline Bossa, MSc; aline.bossa@incor.usp.br |
| Notes |
Collaborators: University of Sao Paulo General Hospital, bioMérieux Registration: NCT02787603 |
NCT02862314.
| Trial name or title | PCT Pneumonia/Pneumonitis Associated With ASPIration (PROPASPI) |
| Methods | Randomised trial, parallel assignment, open label in France |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Algorithm used in this study: Experimental: PCT group. The PCT concentration is measured at inclusion. No intervention: control group. Concentrations of PCT are not measured. |
| Outcomes |
|
| Starting date | February 2015 |
| Contact information | Gilles Capellier, MDPH; gilles.capellier@univ‐fcomte.fr Sophie Depierre; sdepierre@chu‐besancon.fr |
| Notes |
Collaborators: Centre Hospitalier Universitaire de Besancon Registration: NCT02862314 |
NCT02931409.
| Trial name or title | Intra‐operative PEEP optimisation: effects on postoperative pulmonary complications and inflammatory response |
| Methods | Randomised trial, parallel assignment, single‐blind in Hungary |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Algorithm used in this study: Experimental: Optimal PEEP patients submitted to general anaesthesia and open radical cystectomy and urinary diversion (20 participants) will be submitted an alveolar recruitment maneuver using the sustained airway pressure by the CPAP method, applying 30 cmH2O PEEP for 30 seconds followed by a decremental PEEP titration procedure directed by static pulmonary compliance (Cstat). During PEEP titration procedure, PEEP will be decreased from 14 cmH2O by 2 cmH2O every 4 minutes, until a final PEEP of 6 cmH2O. Optimal PEEP is considered to be a PEEP value resulting the highest possible Cstat measured by ventilator. After PEEP titration procedure, a lung protective mechanical ventilation will be performed using optimal PEEP and low tidal volumes. Active comparator: Standard PEEP. Patients submitted to general anaesthesia and open radical cystectomy and urinary diversion (20 participants) will be submitted an alveolar recruitment manoeuvre using the sustained airway pressure by the CPAP method, applying 30 cmH2O PEEP for 30 seconds followed by a standard lung protective mechanical ventilation using a PEEP value of 6 cmH2O and low tidal volumes (6 mL/kg) |
| Outcomes |
|
| Starting date | October 2016 |
| Contact information | Zoltán Ruszkai, MD; ruszkai.zoltan@peterfykh.hu |
| Notes |
Collaborator: Péterfy Sándor Hospital, Szeged University Registration: NCT02931409 |
AB: antibiotic ACCP: American College of Chest Physicians APACHE II: Acute Physiology and Chronic Health Evaluation II BMI: body mass index BNP: B‐type natriuretic peptide COPD: chronic obstructive pulmonary disease CPAP: continuous positive airway pressure CRP: C‐reactive protein CT: computed tomography d: day ED: emergency department ESBL: extended‐spectrum beta‐lactamase h: hour GOLD: Global Initiative for Chronic Obstructive Lung Disease ICU: intensive care unit LRTI: lower respiratory tract infection NT‐proBNP: N‐terminal pro‐B‐type natriuretic peptide NYHA: New York Heart Association Pa: arterial PaO2/FiO2: relationship between arterial oxygen tension (PaO2) and inspiratory oxygen fraction (FiO2) PCT: procalcitonin PEEP: positive end‐expiratory pressure PORT: Pneumonia Patient Outcomes Research Team RCT: randomised controlled trial SIRS: systemic inflammatory response syndrome SOFA: Sequential Organ Failure Assessment score
Differences between protocol and review
We changed the co‐primary endpoint of combined disease‐specific failure at 30 days (mentioned in the protocol) to setting‐specific treatment failure at 30 days as defined above for reasons of standardisation across trials. We limited the analysis of the secondary outcome number of 'sick days' (days with restricted activities from the ARI within 14 days following randomisation) to the primary care trials because other trials did not assess this outcome.
In addition, based on referee comments during the initial editorial process of the initial review (Schuetz 2012), we added further sensitivity analyses to investigate the robustness of our results. Specifically, we performed sensitivity analyses excluding trials with low adherence to PCT algorithms (< 70%) or not reporting adherence. We also performed sensitivity analyses with respect to methodological quality criteria (allocation concealment and blinded outcome assessment). We further performed a sensitivity analysis excluding trials with a follow‐up time for mortality different than one month. We conducted meta‐analyses with aggregated data of included trials to further investigate heterogeneity (inconsistency measure I2 statistic and Cochran Q test) of intervention effects and trial subgroups based on adherence to PCT algorithms.
Contributions of authors
Philipp Schuetz, Beat Mueller, Heiner Bucher, and Matthias Briel conceived the study and wrote the initial protocol.
Philipp Schuetz and Matthias Briel performed the analysis of the initial review, and Philipp Schuetz, Yannick Wirz, and Ramon Sager performed the analysis of the 2017 update including the writing of the manuscript. Philipp Schuetz, Mirjam Christ‐Crain, Daiana Stolz, Michael Tamm, Lila Bouadma, Charles E Luyt, Michel Wolff, Jean Chastre, Florence Tubach, Kristina B Kristoffersen, Olaf Burkhardt, Tobias Welte, Stefan Schroeder, Vandack Nobre, Long Wei, Heiner C Bucher, Djillali Annane, Konrad Reinhart, Angela Branche, Pierre Damas, Maarten Nijsten, Dylan W de Lange, Rodrigo O Deliberato, Stella SS Lima, Vera Maravić‐Stojković, Alessia Verduri, Bin Cao, Yahya Shehabi,, Albertus Beishuizen, Jens‐Ulrik S Jensen, Caspar Corti, Matthias Briel, Beat Mueller, Jos A Van Oers, Ann R Falsey, and Evelien de Jong are investigators of included trials, provided data from their respective trials, and resolved queries about their trial data.
All authors amended and commented on the manuscript and approved the final version. Philipp Schuetz, Beat Mueller, and Matthias Briel oversaw the study and act as guarantors.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Gottfried and Julia Bangerter‐Rhyner‐Foundation, the Swiss Foundation for Grants in Biology and Medicine and santésuisse, Switzerland.
Unrestricted research grant to cover salary time related to this review
-
National Institute for Health Research, UK.
This update was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding (NIHR Cochrane Programme Grant 16‐72‐15) to the Acute Respiratory Infections Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
Philipp Schuetz received support (paid to his employer) from Thermo Fisher, Roche Diagnostics, Abbott and bioMerieux to attend meetings and fulfil speaking engagements. These conflicts breach Cochrane's Commercial Sponsorship Policy (Clause 3), therefore Philipp Schuetz will step down as lead author at the next update of the review. Dr Schuetz's declared conflicts were referred to the Funding Arbiter Panel and Cochrane’s Deputy Editor‐in‐Chief who have agreed this course of action but as an exception which does not set a precedent for similar situations in the future. Beat Mueller reports that within the last 3 years he was part of the speaker bureau of B·R·A·H·M·S and bioMérieux to give educational talks. His institution received compensation for flight and travel expenses, and to cover his absence from work. Yannick Wirz: None known. Ramon Sager: None known. Mirjam Christ‐Crain received support from B·R·A·H·M·S and bioMérieux to attend meetings and fulfilled speaking engagements. Daiana Stolz received fees for lectures or occasional advisory committees from Boehringer Ingelheim, Almirall, Novartis, Glaxo, AstraZeneca, and Roche. Dr Stolz's institution received unrestricted research grants from ResMed, Weinmann AG, AstraZeneca, Boston Scientific, and Curetis AG. Michael Tamm: None known. Lila Bouadma: None known. Charles E Luyt received lecture fees from B·R·A·H·M·S and Merck Sharp & Dohme‐Chibret. Michel Wolff received consulting and lectures fees from Merck Sharp & Dohme‐Chibret, Janssen‐Cilag, Gilead, and Astellas Pharma. Jean Chastre received consulting and lecture fees from Pfizer, B·R·A·H·M·S, Wyeth, Johnson & Johnson, Nektar‐Bayer, and Arpida. Florence Tubach: My institution received some funds from B·R·A·H·M·S for implementing the PRORATA trial, whose data are involved in the review. Kristina B Kristoffersen: None known. Olaf Burkhardt received research support from B·R·A·H·M·S. Tobias Welte received lecture fees and research support from B·R·A·H·M·S. Stefan Schroeder received lecture fees and research support from B·R·A·H·M·S. Vandack Nobre: None known. Long Wei: None known. Heiner C Bucher: None known. Neera Bhatnagar: None known. Djillali Annane: None known. Konrad Reinhart received fees for consultancy from Adrenomed, Henningsdorf Berlin, Germany; holds equity in InflaRx, Jena, Germany; and is unpaid chair of the Global Sepsis Alliance, which receives funds from several companies with interest in sepsis diagnostics. Angela Branche: This work was partially supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (contract HHSN27220120005C). This author has nothing else to declare. Pierre Damas: None known. Maarten Nijsten: None known. Dylan W de Lange: The department where Dylan de Lange worked received financial compensation for a randomisation tool during the SAPS trial. Rodrigo O Deliberato: None known. Stella SS Lima: None known. Vera Maravić‐Stojković: None known. Alessia Verduri: None known. Bin Cao: None known. Yahya Shehabi received unrestricted research and educational grants from Thermo Fisher, bioMérieux, Pfizer, and Orion Pharma. Albertus Beishuizen: None known. Jens‐Ulrik S Jensen declares that he was invited to the European Respiratory Society meeting 2016 by Roche Pharmaceuticals. Otherwise, he has no disclosures. Caspar Corti received an unrestricted grant of USD 2000 from Thermo Fisher Scientific, MA, USA; bioMérieux Denmark ApS supported the study non‐financially. Jos A Van Oers: None known. Ann R Falsey: The relationships with noted industry partners had no role or influence in the study. Evelien de Jong received lecturing fees from Thermo Fisher. Carolina F Oliveira: None known. Bianca Beghe: None known. Matthias Briel: Unrestricted grant from B·R·A·H·M·S AG (now Thermo Fisher) that partially covered working hours to a previous version of this Cochrane Review. B·R·A·H·M·S had no role in the design, conduct, analysis, or writing of our manuscript.
Funding: The initial review was partly funded by unrestricted research grants from B·R·A·H·M·S/Thermo Fisher Scientific, the Gottfried and Julia Bangerter‐Rhyner‐Foundation, the Swiss Foundation for Grants in Biology and Medicine (SSMBS, PASMP3‐127684/1), and santésuisse to cover salary time related to this review. The sponsors had no role in the study design, data collection, data analysis or data interpretation, or writing of the report. No funding was received for this update.
No commercial sponsor had any involvement in the design and conduct of this review, namely collection, management, analysis, and interpretation of the data; and preparation, decision to submit, review, or approval of the manuscript.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Annane 2013 {published data only}
- Annane D, Maxime V, Faller JP, Mezher C, Clec'h C, Martel P, et al. Procalcitonin levels to guide antibiotic therapy in adults with non‐microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open 2013;3(2):pii: e002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloos 2016 {published data only}
- Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin‐guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Internal Medicine 2016;176(9):1266‐76. [DOI] [PubMed] [Google Scholar]
Bouadma 2010 {published data only}
- Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010;375(9713):463‐74. [DOI] [PubMed] [Google Scholar]
Branche 2015 {published data only}
- Branche AR, Walsh EE, Vargas R, Hulbert B, Formica MA, Baran A, et al. Serum procalcitonin measurement and viral testing to guide antibiotic use for respiratory infections in hospitalized adults: a randomized controlled trial. Journal of Infectious Diseases 2015;212(11):1692‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briel 2008 {published data only}
- Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin‐guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Archives of Internal Medicine 2008;168(18):2000‐7. [DOI] [PubMed] [Google Scholar]
Burkhardt 2010 {published data only}
- Burkhardt O, Ewig S, Haagen U, Giersdorf S, Hartmann O, Wegscheider K, et al. Procalcitonin guidance and reduction of antibiotic use in acute respiratory tract infection. European Respiratory Journal 2010;36(3):601‐7. [DOI] [PubMed] [Google Scholar]
Christ‐Crain 2004 {published data only}
- Christ‐Crain M, Jaccard‐Stolz D, Bingisser R, Gencay M, Huber P, Tamm M, et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet 2004;1363(9409):600‐7. [DOI] [PubMed] [Google Scholar]
Christ‐Crain 2006 {published data only}
- Christ‐Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community‐acquired pneumonia: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2006;174(1):84‐93. [DOI] [PubMed] [Google Scholar]
Corti 2016 {published data only}
- Corti C, Fally M, Fabricius‐Bjerre A, Mortensen K, Jensen BN, Andreassen HF, et al. Point‐of‐care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. International Journal of Chronic Obstructructive Pulmonary Disease 2016;11:1381‐9. [DOI: 10.2147/COPD.S104051] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jong 2016 {published data only}
- Jong E, Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open‐label trial. Lancet Infectious Diseases 2016;16(7):819‐27. [DOI] [PubMed] [Google Scholar]
Deliberato 2013 {published data only}
- Deliberato RO, Marra AR, Sanches PR, Martino MD, Ferreira CE, Pasternak J, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagnostic Microbiology and Infectious Disease 2013;76(3):266‐71. [DOI] [PubMed] [Google Scholar]
Ding 2013 {published data only}
- Ding J, Chen Z, Feng K. Procalcitonin‐guided antibiotic use in acute exacerbations of idiopathic pulmonary fibrosis. International Journal of Medical Sciences 2013;10(7):903‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hochreiter 2009 {published data only}
- Hochreiter M, Kohler T, Schweiger AM, Keck FS, Bein B, Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Critical Care 2009;13(3):R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristoffersen 2009 {published data only}
- Kristoffersen KB, Sogaard OS, Wejse C, Black FT, Greve T, Tarp B, et al. Antibiotic treatment interruption of suspected lower respiratory tract infections based on a single procalcitonin measurement at hospital admission ‐ a randomized trial. Clinical Microbiology and Infection 2009;15(5):481‐7. [DOI] [PubMed] [Google Scholar]
Layios 2012 {published data only}
- Layios N, Lambermont B, Canivet JL, Morimont P, Preiser JC, Garweg C, et al. Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Critical Care Medicine 2012;40(8):2304‐9. [DOI] [PubMed] [Google Scholar]
Lima 2016 {published data only}
- Lima SS, Nobre V, Castro Romanelli RM, Clemente WT, Silva Bittencourt HN, Melo AC, et al. Procalcitonin‐guided protocol is not useful to manage antibiotic therapy in febrile neutropenia: a randomized controlled trial. Annals of Hematology 2016;95(7):1169‐76. [DOI] [PubMed] [Google Scholar]
Long 2009 {published data only}
- Long W, Deng XQ, Tang JG, Xie J, Zhang YC, Zhang Y, et al. Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community acquired pneumonia. Zhonghua Nei Ke Za Zhi 2009;48(3):216‐9. [PubMed] [Google Scholar]
Long 2011 {published data only}
- Long W, Deng X, Zhang Y, Lu G, Xie J, Tang J. Procalcitonin guidance for reduction of antibiotic use in low‐risk outpatients with community acquired pneumonia. Respirology 2011;76(1):266‐9. [DOI] [PubMed] [Google Scholar]
Long 2014 {published data only}
- Long W, Li LJ, Huang GZ, Zhang XM, Zhang YC, Tang JG, et al. Procalcitonin guidance for reduction of antibiotic use in patients hospitalized with severe acute exacerbations of asthma: a randomized controlled study with 12‐month follow‐up. Critical Care 2014;18(5):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maravić‐Stojković 2011 {published data only}
- Maravić‐Stojković V, Laušević‐Vuk L, Jović M, Ranković A, Borzanović M, Marinković J. Procalcitonin‐based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srpski Arhiv Celokupno Lekarstvo 2011;139(11‐12):736‐42. [PubMed] [Google Scholar]
Najafi 2015 {published data only}
- Najafi A, Khodadadian A, Sanatkar M, Shariat Moharari R, Etezadi F, Ahmadi A, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Medica Iranica 2015;53(9):562‐7. [PubMed] [Google Scholar]
Nobre 2008 {published data only}
- Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. American Journal of Respiratory and Critical Care Medicine 2009;177(5):498‐505. [DOI] [PubMed] [Google Scholar]
Ogasawara 2014 {published data only}
- Ogasawara T, Umezawa H, Naito Y, Takeuchi T, Kato S, Yano T, et al. Procalcitonin‐guided antibiotic therapy in aspiration pneumonia and an assessment of the continuation of oral intake. Respiratory Investigation 2014;52(2):107‐13. [DOI] [PubMed] [Google Scholar]
Oliveira 2013 {published data only}
- Oliveira CF, Botoni FA, Oliveira CR, Silva CB, Pereira HA, Serufo JC, et al. Procalcitonin versus C‐reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Critical Care Medicine 2013;41(10):2336‐43. [DOI] [PubMed] [Google Scholar]
Schroeder 2009 {published data only}
- Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, et al. Procalcitonin (PCT)‐guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Archives of Surgery 2009;394(2):221‐6. [DOI] [PubMed] [Google Scholar]
Schuetz 2009 {published data only}
- Schuetz P, Christ‐Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009;302(10):1059‐66. [DOI] [PubMed] [Google Scholar]
Shehabi 2014 {published data only}
- Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. ProGUARD Study Investigators, ANZICS Clinical Trials Group. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2014;190(10):1102‐10. [DOI] [PubMed] [Google Scholar]
Stolz 2007 {published data only}
- Stolz D, Christ‐Crain M, Bingisser R, Leuppi J, Miedinger D, Muller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin‐guidance with standard therapy. Chest 2007;131(1):9‐19. [DOI] [PubMed] [Google Scholar]
Stolz 2009 {published data only}
- Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, et al. Procalcitonin for reduced antibiotic exposure in ventilator‐associated pneumonia: a randomised study. European Respiratory Journal 2009;34(6):1364‐75. [DOI] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang J, Long W, Yan L, Zhang Y, Xie J, Lu G, et al. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: a randomized controlled trial. BMC Infectious Diseases 2013;13:596. [DOI: 10.1186/1471-2334-13-596] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verduri 2015 {published data only}
- Verduri A, Luppi F, D'Amico R, Balduzzi S, Vicini R, Liverani A, et al. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial. PLoS ONE 2015;10(3):e0118241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang JX, Zhang SM, Li XH, Zhang Y, Xu ZY, Cao B. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. International Journal of Infectious Diseases 2016;48:40‐5. [DOI: 10.1016/j.ijid.2016.04.024] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dharaniyadewi 2013 {published data only}
- Dharaniyadewi D, Lie KC, Sukmana N, Rumende CM. Effect of semi‐quantitative procalcitonin assay on the adequacy of empirical antibiotics and mortality in septic patients. Citical Care 2013;17(Suppl 4):P15. [Google Scholar]
Esposito 2012 {published data only}
- Esposito S, Tagliabue C, Picciolli I, Semino M, Sabatini C, Consolo S, et al. Procalcitonin measurements for guiding antibiotic treatment in pediatric pneumonia. Respiratory Medicine 2011;105(12):1939‐45. [DOI] [PubMed] [Google Scholar]
Heyland 2011 {published data only}
- Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Critical Care Medicine 2011;39(7):1792‐9. [DOI] [PubMed] [Google Scholar]
Jensen 2011 {published data only}
- Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin‐guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Critical Care Medicine 2011;39(9):2048‐58. [DOI] [PubMed] [Google Scholar]
Jones 2007 {published data only}
- Jones AE, Fiechtl JF, Brown MD, Ballew JJ, Kline JA. Procalcitonin test in the diagnosis of bacteremia: a meta‐analysis. Annals of Emergency Medicine 2007;50(1):34‐41. [DOI] [PubMed] [Google Scholar]
Kook 2012 {published data only}
- Kook JL, Chao SR, Le J, Robinson PA. Impact of the use of procalcitonin assay in hospitalised patients with pneumonia at a community care hospital. Infection Control and Hospital Epidemiology 2012;33(4):424‐6. [DOI] [PubMed] [Google Scholar]
Liew 2011 {published data only}
- Liew YX, Chlebicki MP, Lee W, Hsu LY, Kwa AL. Use of procalcitonin (PCT) to guide discontinuation of antibiotic use in an unspecified sepsis is an antimicrobial stewardship program (ASP). European Journal of Clinical Microbiology and Infectious Diseases 2011;30:853‐5. [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu BH, Li HF, Lei Y, Zhao SX, Sun ML. Clinical significance of dynamic monitoring of procalcitonin in guiding the use of antibiotics in patients with sepsis in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2013;25(11):690‐3. [DOI] [PubMed] [Google Scholar]
Qu 2012 {published data only}
- Qu R, Ji Y, Ling Y, Ye CY, Yang SM, Liu YY, et al. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single‐center controlled trial. Saudi Medical Journal 2012;33(4):382‐7. [PubMed] [Google Scholar]
Saeed 2011 {published data only}
- Saeed K, Dryden M, Bourne S, Paget C, Proud A. Reduction in antibiotic use through procalcitonin testing in patients in the medical admission unit or intensive care unit with suspicion of infection. Journal of Hospital Infection 2011;78(4):289‐92. [DOI] [PubMed] [Google Scholar]
Schuetz 2010 {published data only}
- Schuetz P, Batschwaroff M, Dusemund F, Albrich W, Burgi U, Maurer M, et al. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post‐study survey. European Journal of Clinical Microbiology and Infectious Diseases 2010;29(3):269‐77. [DOI] [PubMed] [Google Scholar]
Simmonds 2005 {published data only}
- Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta‐analysis of individual patient data from randomized trials: a review of methods used in practice. Clinical Trials 2005;2(3):209‐17. [DOI] [PubMed] [Google Scholar]
Simon 2004 {published data only}
- Simon L, Gauvin F, Amre DK, Saint‐Louis P, Lacroix J. Serum procalcitonin and C‐reactive protein levels as markers of bacterial infection: a systematic review and meta‐analysis. Clinical Infectious Diseases 2004;39(2):206‐17. [DOI] [PubMed] [Google Scholar]
Stocker 2010 {published data only}
- Stocker M, Fontana M, Helou S, Wegscheider K, Berger TM. Use of procalcitonin‐guided decision‐making to shorten antibiotic therapy in suspected neonatal early‐onset sepsis: prospective randomized intervention trial. Neonatology 2010;97(2):165‐74. [DOI] [PubMed] [Google Scholar]
Tang 2007 {published data only}
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta‐analysis. Lancet Infectious Diseases 2007;7(3):210‐7. [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang H, Huang T, Jing J, Shen H, Cui W. Effect of procalcitonin‐guided treatment in patients with infections: a systematic review and meta‐analysis. Infection 2009;37(6):497‐507. [DOI] [PubMed] [Google Scholar]
Uzzan 2006 {published data only}
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta‐analysis. Critical Care Medicine 2006;34(7):1996‐2003. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02130986 {unpublished data only}
- NCT02130986. Procalcitonin Antibiotic Consensus Trial (ProACT). clinicaltrials.gov/ct2/show/study/NCT02130986 First received: May 1, 2014.
NCT02261610 {unpublished data only}
- NCT02261610. Pulmonary Embolism and PCT. PE‐PCT Study. clinicaltrials.gov/ct2/show/NCT02261610 First received: September 5, 2014.
NCT02332577 {unpublished data only}
- NCT02332577. Study to compare the efficacy of pristinamycin (Pyostacine) versus amoxicillin in the treatment of acute community acquired pneumonia. clinicaltrials.gov/ct2/show/NCT02332577 First received: January 5, 2015.
NCT02440828 {unpublished data only}
- NCT02440828. Addition of tobramycin inhalation in the treatment of ventilator associated pneumonia (VAPORISE). clinicaltrials.gov/ct2/show/NCT02440828 First received: March 13, 2015.
NCT02787603 {unpublished data only}
- NCT02787603. Procalcitonin in Early Antibiotic Interruption in Patient With Bacterial Pulmonary infeCtion and Acute Heart Failure (EPICAD). clinicaltrials.gov/ct2/show/NCT02787603 First received: May 25, 2016.
NCT02862314 {unpublished data only}
- NCT02862314. PROcalcitonin Pneumonia/Pneumonitis Associated With ASPIration (PROPASPI). clinicaltrials.gov/ct2/show/NCT02862314 First received: July 29, 2016.
NCT02931409 {unpublished data only}
- NCT02931409. Intraoperative PEEP optimization: effects on postoperative pulmonary complications and inflammatory response. clinicaltrials.gov/ct2/show/NCT02931409 First received: October 5, 2016.
Additional references
Albrich 2012
- Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kuhn F, et al. Effectiveness and safety of procalcitonin‐guided antibiotic therapy in lower respiratory tract infections in "real life": an international, multicenter post‐study survey (ProREAL). Archives of Internal Medicine 2012;172(9):715‐22. [DOI] [PubMed] [Google Scholar]
Arnold 2005
- Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003539.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balk 2017
- Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health‐care utilization and costs in critically ill patients in the United States. Chest 2017;151(1):23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cals 2009
- Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ 2009;338:b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doan 2014
- Doan Q, Enarson P, Kissoon N, Klassen TP, Johnson DW. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006452.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drozdov 2015
- Drozdov D, Schwarz S, Kutz A, Grolimund E, Rast AC, Steiner D, et al. Procalcitonin and pyuria‐based algorithm reduces antibiotic use in urinary tract infections: a randomized controlled trial. BMC Medicine 2015;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2002
- Evans AT, Husain S, Durairaj L, Sadowski LS, Charles‐Damte M, Wang Y. Azithromycin for acute bronchitis: a randomised, double‐blind, controlled trial. Lancet 2002;359(9318):1648‐54. [DOI] [PubMed] [Google Scholar]
Gonzales 1997
- Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278(11):901‐4. [PubMed] [Google Scholar]
Goossens 2005
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross‐national database study. Lancet 2005;365(9435):579‐87. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed April 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoare 2006
- Hoare Z, Lim WS. Pneumonia: update on diagnosis and management. BMJ 2006;332(7549):1077‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoeboer 2015
- Hoeboer SH, Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta‐analysis. Clinical Microbiology and Infection 2015;21(5):474‐81. [DOI] [PubMed] [Google Scholar]
Konstantinides 2008
- Konstantinides S. Clinical practice. Acute pulmonary embolism. New England Journal of Medicine 2008;359(26):2804‐13. [DOI] [PubMed] [Google Scholar]
Kumar 2006
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Medicine 2006;34(6):1589‐96. [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009;136(5):1237‐48. [DOI] [PubMed] [Google Scholar]
Kutz 2015
- Kutz A, Briel M, Christ‐Crain M, Stolz D, Bouadma L, Wolff M, et al. Prognostic value of procalcitonin in respiratory tract infections across clinical settings. Critical Care (London, England) 2015;19(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kutz 2016
- Kutz K, Hausfater P, Oppert M, Alan M, Grolimund E, Gast C, et al. Comparison between B·R·A·H·M·S PCT direct, a new sensitive point‐of‐care testing device for rapid quantification of procalcitonin in emergency department patients and established reference methods ‐ a prospective multinational trial. Clinical Chemistry and Laboratory Medicine 2016;54(4):577‐84. [DOI] [PubMed] [Google Scholar]
Lawrence 2009
- Lawrence KL, Kollef MH. Antimicrobial stewardship in the intensive care unit: advances and obstacles. American Journal of Respiratory and Critical Care Medicine 2009;179(6):434‐8. [DOI] [PubMed] [Google Scholar]
Liberati 2009a
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [DOI] [PubMed] [Google Scholar]
Liberati 2009b
- Liberati A, D'Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD000022.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzano 2010
- Manzano S, Bailey B, Girodias JB, Galetto‐Lacour A, Cousineau J, Delvin E. Impact of procalcitonin on the management of children aged 1 to 36 months presenting with fever without source: a randomized controlled trial. American Journal of Emergency Medicine 2010;28(6):647‐53. [DOI] [PubMed] [Google Scholar]
Meili 2015
- Meili M, Muller B, Kulkarni P, Schutz P. Management of patients with respiratory infections in primary care: procalcitonin, C‐reactive protein or both?. Expert Review of Respiratory Medicine 2015;9(5):587‐601. [DOI] [PubMed] [Google Scholar]
Meili 2016
- Meili M, Kutz A, Briel M, Christ‐Crain M, Bucher HC, Mueller B, et al. Infection biomarkers in primary care patients with acute respiratory tract infections ‐ comparison of procalcitonin and C‐reactive protein. BMC Pulmonary Medicine 2016;16(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2000
- Muller B, Becker KL, Schachinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Critical Care Medicine 2000;28(4):977‐83. [DOI] [PubMed] [Google Scholar]
Muller 2001
- Muller B, Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Medical Weekly 2001;131(41‐2):595‐602. [DOI] [PubMed] [Google Scholar]
Muller 2010
- Muller F, Christ‐Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, et al. Procalcitonin levels predict bacteremia in patients with community‐acquired pneumonia: a prospective cohort trial. Chest 2010;138(1):121‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sager 2017
- Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin‐guided diagnosis and antibiotic stewardship revisited. BMC Medicine 2017;15(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuetz 2011a
- Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Archives of Internal Medicine 2011;171(15):1322‐31. [DOI] [PubMed] [Google Scholar]
Schuetz 2015
- Schuetz P, Aujesky D, Muller C, Muller B. Biomarker‐guided personalised emergency medicine for all ‐ hope for another hype?. Swiss Medical Weekly 2015;145:w14079. [DOI] [PubMed] [Google Scholar]
Schuetz 2016
- Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. International Journal of Cardiology 2016;223:390‐7. [DOI: 10.1016/j.ijcard.2016.08.204] [DOI] [PubMed] [Google Scholar]
Schuetz 2017
- Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin monitoring sepsis (MOSES) study. Critical Care Medicine 2017;45(5):781‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spurling 2010
- Spurling GKP, Mar CB, Dooley L, Foxlee R. Delayed antibiotics for respiratory infections. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD004417.pub3] [DOI] [PubMed] [Google Scholar]
Stata 12.1 [Computer program]
- StataCorp LP. Stata Statistical Software: Release 12.1.. College Station (TX): StataCorp LP, 2005.
Stewart 2015
- Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred reporting items for systematic review and meta‐analyses of individual participant data: the PRISMA‐IPD Statement. JAMA 2015;313(16):1657‐65. [DOI] [PubMed] [Google Scholar]
Stojanovic 2017
- Stojanovic I, Schneider JE, Wei L, Hong Z, Keane C, Schuetz P. Economic evaluation of procalcitonin‐guided antibiotic therapy in acute respiratory infections: a Chinese hospital system perspective. Clinical Chemistry and Laboratory Medicine 2017;55(4):561‐70. [DOI] [PubMed] [Google Scholar]
Thompson 2001
- Thompson SG, Turner RM, Warn DE. Multilevel models for meta‐analysis, and their application to absolute risk differences. Statistical Methods in Medical Research 2001;10(6):375‐92. [DOI] [PubMed] [Google Scholar]
Turner 2000
- Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2000;19(24):3417‐32. [DOI] [PubMed] [Google Scholar]
van Vugt 2013
- Vugt SF, Broekhuizen BD, Lammens C, Zuithoff NP, Jong PA, Coenen S, et al. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ 2013;346:f2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wacker 2013
- Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta‐analysis. Lancet Infectious Diseases 2013;13(5):426‐35. [DOI] [PubMed] [Google Scholar]
Zaas 2014
- Zaas AK, Garner BH, Tsalik EL, Burke T, Woods CW, Ginsburg GS. The current epidemiology and clinical decisions surrounding acute respiratory infections. Trends in Molecular Medicine 2014;20(10):579‐88. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schuetz 2008
- Schuetz P, Briel M, Christ‐Crain M, Wolbers M, Stolz D, Tamm M, et al. Procalcitonin to initiate or withhold antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews 2008, Issue 10. [DOI: 10.1002/14651858.CD007498] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuetz 2010a
- Schuetz P, Albrich W, Christ‐Crain M, Chastre J, Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Review of Anti‐Infective Therapy 2010;8(5):575‐87. [DOI] [PubMed] [Google Scholar]
Schuetz 2011
- Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Archives of Internal Medicine 2011;171(15):1322‐31. [DOI] [PubMed] [Google Scholar]
Schuetz 2012
- Schuetz P, Muller B, Christ‐Crain M, Stolz D, Tamm M, Bouadma L, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007498.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


