Abstract
Background
Diverticular disease is a common condition that increases in prevalence with age. Recent theories on the pathogenesis of diverticular inflammation have implicated chronic inflammation similar to that seen in ulcerative colitis. Mesalamine, or 5‐aminosalicylic acid (5‐ASA), is a mainstay of therapy for individuals with ulcerative colitis. Accordingly, 5‐ASA has been studied for prevention of recurrent diverticulitis.
Objectives
To evaluate the efficacy of mesalamine (5‐ASA) for prevention of recurrent diverticulitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8), in the Cochrane Library; Ovid MEDLINE (from 1950 to 9 September 2017); Ovid Embase (from 1974 to 9 September 2017); and two clinical trials registries for ongoing trials ‐ Clinicaltrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform database (9 September 2017). We also searched proceedings from major gastrointestinal conferences ‐ Digestive Disease Week (DDW), United European Gastroenterology Week (UEGW), and the American College of Gastroenterology (ACG) Annual Scientific Meeting ‐ from 2010 to September 2017. In addition, we scanned reference lists from eligible publications, and we contacted corresponding authors to ask about additional trials.
Selection criteria
We included randomised controlled clinical trials comparing the efficacy of 5‐ASA versus placebo or another active drug for prevention of recurrent diverticulitis.
Data collection and analysis
We used standard methodological procedures as defined by Cochrane. Three review authors assessed eligibility for inclusion. Two review authors selected studies, extracted data, and assessed methodological quality independently. We calculated risk ratios (RRs) for prevention of diverticulitis recurrence using an intention‐to‐treat principle and random‐effects models. We assessed heterogeneity using criteria for Chi2 (P < 0.10) and I2 tests (> 50%). To explore sources of heterogeneity, we conducted a priori subgroup analyses. To assess the robustness of our results, we carried out sensitivity analyses using different summary statistics (RR vs odds ratio (OR)) and meta‐analytical models (fixed‐effect vs random‐effects).
Main results
We included in this review seven studies with a total of 1805 participants. We judged all seven studies to have unclear or high risk of bias. Investigators found no evidence of an effect when comparing 5‐ASA versus control for prevention of recurrent diverticulitis (31.3% vs 29.8%; RR 0.69, 95% confidence interval (CI) 0.43 to 1.09); very low quality of evidence).
Five of the seven studies provided data on adverse events of 5‐ASA therapy. The most commonly reported side effects were gastrointestinal symptoms (epigastric pain, nausea, and diarrhoea). No significant difference was seen between 5‐ASA and control (67.8% vs 64.6%; RR 0.98, 95% CI 0.91 to 1.06; P = 0.63; moderate quality of evidence), nor was significant heterogeneity observed (I2 = 0%; P = 0.50).
Authors' conclusions
The effects of 5‐ASA on recurrence of diverticulitis are uncertain owing to the small number of heterogenous trials included in this review. Rates of recurrent diverticulitis were similar among participants using 5‐ASA and control participants. Effective medical strategies for prevention of recurrent diverticulitis are needed, and further randomised, double‐blinded, placebo‐controlled trials of rigorous design are warranted to specify the effects of 5‐ASA (mesalamine) in the management of diverticulitis.
Plain language summary
Does 5‐ASA prevent the recurrence of diverticulitis?
Background
Diverticula are small bulging pouches that can form in the lining of the digestive system, particularly in the colon. Diverticulitis is inflammation of these pouches, and it is an important complication of diverticular disease. Approximately one‐third to one‐quarter of patients who recover from one episode of diverticulitis will experience recurrence. The inflammation underlying diverticulitis may be similar to that seen in inflammatory bowel disease. 5‐Aminosalicylic acid (5‐ASA) is an anti‐inflammatory drug that has proved effective as treatment for ulcerative colitis and therefore may be useful for prevention of recurrent diverticulitis
Objectives
We aimed to evaluate whether 5‐ASA prevented recurrence of diverticulitis.
Study characteristics
A review of the literature identified seven studies with a total of 1805 participants for analysis. A search of the literature was conducted on 9 September 2017. These trials assigned participants with a diagnosis of diverticulitis to receive 5‐ASA or an alternative therapy. Four trials compared 5‐ASA versus placebo; one compared 5‐ASA plus probiotic versus probiotic; one compared 5‐ASA plus antibiotic versus antibiotic; and one compared 5‐ASA versus no therapy. Participants were followed to compare the recurrence rate of diverticulitis and side effects among treatment arms.
Key findings
Our analysis determined that approximately one‐third of participants receiving 5‐ASA had recurrence of diverticulitis (31.3%). Participants receiving non‐5‐ASA therapy experienced a similar rate of recurrence (29.8%). Adverse event rates were similar among 5‐ASA therapy and comparison therapies. The most commonly reported side effects of 5‐ASA therapy were gastrointestinal symptoms (epigastric pain, nausea, and diarrhoea).
Quality of the evidence
Overall, the quality of evidence available for analysis of recurrence of diverticulitis is considered to be very low. None of the included studies was considered to have low risk of bias for all criteria. These trials were designed differently. For example, some studies required a CT scan for diagnosis of diverticulitis, and others relied on less reliable clinical and laboratory criteria. Comparison therapies varied, with some studies using placebo, and others using antibiotics and probiotics. Although we combined the findings of these studies in our analysis, these different comparison arms made direct comparisons problematic. The confidence interval does not exclude an appreciable benefit or no difference.
Overall, the quality of evidence available for analysis of adverse effects was moderate. Two of the included studies provided no usable data.
Summary of findings
Summary of findings for the main comparison. 5‐ASA compared with control (all trials) for prevention of recurrent diverticulitis.
| 5‐ASA compared with control (all trials) for prevention of recurrent diverticulitis | ||||||
| Patient or population: patients with the need for management of diverticulitis Setting: hospital Intervention: 5‐ASA Comparison: placebo, probiotic, antibiotic | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control (all trials) | Risk with 5‐ASA | |||||
| Recurrence of diverticulitis Follow‐up: range 1 to 2 years | Study population | RR 0.69 (0.43 to 1.09) | 1805 (7 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 30 per 100 | 21 per 100 (13 to 33) | |||||
| Number of participants with adverse events (epigastric pain, nausea, diarrhoea) Follow‐up: range 1 to 2 years | Study population | RR 0.98 (0.91 to 1.06) | 1421 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa,d | ||
| 65 per 100 | 63 per 100 (59 to 68) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 5‐ASA: 5‐aminosalicylic acid; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for serious risk of bias (four of seven studies were assessed to be at high risk of bias).
bThe confidence interval does not exclude an appreciable benefit or no difference.
cDowngraded one level for inconsistency (significant heterogeneity of results might be explained by control regimens and methods of diagnosis).
dThe confidence interval excludes appreciable benefit or harm.
Background
Description of the condition
Diverticular disease is common in developed countries, and its prevalence increases with age. More than 50% of people over the age of 80 have colonic diverticula (Parks 1975; Barroso 2015). Diverticulitis is the most common clinical manifestation of diverticulosis. Diverticulitis is defined as inflammation, infection, or both, associated with colonic diverticula (Painter 1971; Parks 1975; Buchs 2015). Common clinical manifestations include abdominal pain, altered bowel habits, fever. and leukocytosis, which have a considerable impact on the well‐being of patients (Humes 2008; Strate 2012). Recurrent diverticulitis is an important clinical outcome, with reported rates ranging from as low as 7% to as high as 62% (Peery 2013). However, the best accepted estimate of recurrence risk is one‐third to one‐quarter of patients (Parks 1970; Stollman 1999; Chautems 2002; Morris 2014).
No clinical classification of diverticular disease has been universally accepted. Literature is replete with terms of unclear significance. We present our definition of terms to avoid confusion. 'Diverticulosis' is merely the presence of diverticula. 'Diverticular disease' is clinically significant and symptomatic diverticulosis. DIverticular disease may occur in the form of 'diverticulitis' or 'symptomatic uncomplicated diverticular disease' (SUDD) (Strate 2012). The term 'complicated diverticulitis', in this review, refers to the presence of abscess, obstruction, or purulent or faecal peritonitis (Szojda 2007). Some investigators have described an acute and chronic diverticulitis, with chronic referring to recurrent diverticulitis or the presence of segmental colitis associated with diverticulosis syndrome (SCAD), which is an inflammatory process that affects colonic luminal mucosa in segments that are also affected by diverticulosis (Strate 2012). However, this classification is not universally accepted and is not used in this review.
Description of the intervention
Mesalamine, or 5‐ASA, is used mainly for treatment of individuals with ulcerative colitis, and is believed to control inflammation through several mechanisms including inhibition of nuclear factor‐kappa B (NF‐KB). Study authors have observed that colonic diverticular disease is often associated with chronic low‐grade inflammation (Horgan 2001; Floch 2006; Tursi 2008). These observations have led to the hypothesis that 5‐ASA may also be beneficial in the management of diverticulitis.
Treatment with 5‐ASA is generally well tolerated but can be associated with mild to serious adverse effects. The most common side effects include headache, malaise, abdominal cramping, diarrhoea, and gas. Less common effects include hair loss, skin rash, diarrhoea, and worsening of inflammation of the colon (colitis). However, to the review authors' knowledge, no reports have described worsening inflammation when 5‐ASA was used in cases of diverticulitis. Rare but serious adverse effects include pancreatitis, pneumonitis, pericarditis, and interstitial nephritis (Ransford 2002; Karagozian 2007).
How the intervention might work
The pathogenesis of diverticulitis remains uncertain. A prevailing hypothesis is that obstruction of the neck of the diverticulum by inspissated stool or a fecalith leads to low‐grade inflammation and stasis within a diverticulum (Berman 1968; Williams 1995). The resulting micro‐environment favours bacterial overgrowth and leads to diminished venous outflow and local ischaemia (Kohler 1999). Obstruction, infection, and ischaemia promote active mucosal inflammation that manifests as diverticulitis. Microperforation of an inflamed diverticulum can occur, and can remain localised (as a phlegmon or pericolic or pelvic abscess) or lead to purulent or faecal peritonitis.
It has been suggested that some of the mechanisms that underlie inflammation in diverticulitis are similar to those seen in inflammatory bowel disease (IBD) (Peppercorn 2004; Floch 2006; Sheth 2008). These entities appear to converge in a condition known as segmental colitis associated with diverticulosis syndrome (SCAD) (Strate 2012). Individuals with SCAD have a chronic inflammation that resembles IBD and has no known cause (Goldstein 2000; Freeman 2008).
5‐ASA has anti‐inflammatory and immunosuppressive effects (Nielsen 2007). It has been hypothesised that 5‐ASA may also modulate inflammation in diverticulitis and reduce the frequency of recurrent attacks (Tursi 2002; Di Mario 2006).
Why it is important to do this review
It is estimated that between 15% and 25% of patients with diverticulosis will experience an episode of diverticulitis (Parks 1975; Stollman 1999). Between 15% and 30% of those admitted with diverticulitis will require surgery during that admission (Parks 1975), and approximately one‐third will experience recurrent episodes of diverticulitis (Stollman 1999; Chautems 2002). Recent studies have found lower recurrence rates of around 16% (Buchs 2015). Diverticulitis and disease‐related complications can be associated with significant morbidity and a negative impact on quality of life. We have performed a systematic review of the literature to assess the efficacy of 5‐ASA in the management of diverticulitis relative to other active therapies and placebo.
Objectives
To evaluate the efficacy of 5‐ASA (mesalamine) for prevention of recurrent diverticulitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials (including cluster and cross‐over randomised controlled trials (RCTs)) comparing the efficacy of 5‐ASA versus control (placebo, no treatment, or another active drug) for prevention of recurrent diverticulitis.
Types of participants
Participants older than 18 years of age, with diverticulitis diagnosed by endoscopy, radiology, and/or clinical symptoms.
Types of interventions
Administration of 5‐ASA, orally or rectally and at any dose, to at least one treatment arm. Comparators could include placebo or another active medical therapy.
Types of outcome measures
Primary outcomes
Rate of recurrence of diverticulitis
Recurrent diverticulitis is diagnosed mainly on clinical grounds with support of laboratory, endoscopic, and radiological investigations. A computerised tomography (CT) scan is preferred for diagnosis but is not required by our protocol for selection. We accepted the diagnostic criteria of the investigators for this outcome.
Secondary outcomes
Adverse effects of therapy
Any reported adverse events are eligible. Because of anticipated heterogeneity in the definition of these outcomes, we accepted those of the original study authors.
Search methods for identification of studies
We designed search strategies by using a combination of subject headings and text words related to 5‐ASA and diverticulitis.
Electronic searches
We conducted a comprehensive literature search to identify all published and unpublished RCTs with no language restriction. We searched the following electronic databases to identify potential studies.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8), in the Cochrane Library (Appendix 1).
Ovid MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations (Ovid) 1946 to 9 September 2017 (Appendix 2).
Ovid Embase 1974 to 9 September 2017 (Appendix 3).
We did not apply the standard Cochrane search strategy filter for RCTs because we identified a relatively small number of hits during searches of MEDLINE and Embase.
Searching other resources
We searched the following clinical trials registries on 9 September 2017, for protocols for ongoing trials.
Clinical trials.gov.
World Health Organization (WHO) International Clinical Trials Registry Platform search portal (http://www.who.int/ictrp/en/).
We handsearched abstracts in conference proceedings from Digestive Disease Week (published in Gastroenterology andGastrointestinal Endoscopy), United European Gastroenterology Week (published in Gut), and the American College of Gastroenterology Annual Scientific Meeting (published in American Journal of Gastroenterology) (2010 to 2017). We scanned reference lists from retrieved articles to identify additional citations that may have been overlooked during the database search.
Data collection and analysis
Selection of studies
Two review authors (FC, MA) independently screened citations for peer‐reviewed papers identified by the above search strategies for potential relevance. We obtained full texts for potentially relevant citations. Two review authors (of FC, MA, and YY) then independently reviewed these citations for inclusion in the review by applying four criteria.
Confirmed diverticulitis diagnosed by endoscopy, radiology, and/or clinical symptoms.
5‐ASA administered to at least one treatment arm (at randomisation).
Treatment allocation randomised or quasi‐randomised.
Symptomatic recurrence of diverticulitis as a measured outcome.
Each investigator rated each criterion on a three‐point scale: yes, no, or not stated. Studies with a 'yes' for all four criteria were eligible for inclusion. A third review author resolved disagreements.
Data extraction and management
Two independent review authors (of FC, MA, and YY) used a standardised form to extract prespecified data from eligible studies. When necessary, we contacted authors of the original studies for clarification of data, additional information, or both. Extracted data included the following.
Numbers of participants enrolled and allocated to each treatment arm.
Participant characteristics (age, gender).
Type of intervention administered in each treatment arm (dose, formulation, frequency, duration).
Numbers of participants in each treatment arm in symptomatic remission and with recurrent diverticulitis.
Numbers, nature, and severity of reported adverse events in each treatment arm.
Number of participants in each arm lost to follow‐up or dropout.
Study definitions of recurrent diverticulitis and symptom remission.
Assessment of risk of bias in included studies
Review authors assessed risk of bias using the Cochrane tool for risk of bias assessment (Higgins 2011). This tool measures factors that impact the quality of a trial, including the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data.
Selective reporting.
Other risk of bias (such as baseline imbalance, blocked randomisation in unblinded trials, conduct of the study affected by interim results, etc.).
We assessed risk of bias domains as having high, unclear, or low risk of bias using the 'Risk of bias' tool (see Appendix 4; Higgins 2011). We summarised risk of bias for the primary outcome within a study across all domains. We considered a study to have “high risk of bias” when we judged risk of bias as high for one or more domains; “low risk of bias” only when we judged risk of bias as low for all domains; and "unclear risk of bias" when reporting did not permit judgement of high or low risk. We also reported any other important concerns that we had about bias identified in the studies.
Measures of treatment effect
We performed statistical analysis according to the study classification presented above. We calculated the pooled risk ratio (RR) and corresponding 95% confidence intervals (CIs) for recurrent diverticulitis, and we calculated the number of participants with adverse events. We did not calculate a pooled RR for individual adverse events because data were insufficient.
Unit of analysis issues
We included in this review trials that randomised participants to 5‐ASA versus control. We did not identify any cluster‐randomised or cross‐over trials for inclusion in this review. Thus, the unit of analysis is the individual participant.
Dealing with missing data
We attempted to contact study authors to ask for missing data. If no outcome data were available, we used the intention‐to‐treat (ITT) approach to analyse all randomised participants according to their treatment assignments. We used a conservative approach and presumed that missing participants had failed treatment (worst‐case scenario).
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test (P < 0.10 indicates significant heterogeneity) and the I2 statistic (> 50% indicates substantial heterogeneity) and a random‐effects model, along with visual inspection of forest plots. When we found significant or substantial heterogeneity, we investigated possible explanations by performing subgroup analyses. Potential sources of heterogeneity hypothesised a priori include the following.
Control regimens (5‐ASA + probiotics vs probiotics, 5‐ASA + antibiotics vs antibiotics, 5‐ASA vs placebo, 5‐ASA vs no treatment, and 5‐ASA + probiotics vs placebo).
Method of diagnosis (diverticulitis confirmed by CT scan/ultrasonography vs others).
Risk of bias (low vs unclear and high).
Publication type (abstract vs full text).
We did not exclude studies or abstracts at high risk of bias; instead, we reported pooled estimates for both subgroups along with tests of subgroup differences. We reported data for two risk of bias subgroups and planned to explore whether risk of bias explains the heterogeneity (e.g. sometimes studies at high risk of bias can explain heterogeneity).
Assessment of reporting biases
We planned to assess small‐study effects or publication bias by examining the relationship between treatment effects and standard error of the estimate using a funnel plot (Sterne 2011). The general recommendation is that a funnel plot should be included only if the meta‐analysis includes 10 or more studies. Because only seven studies were eligible, we did not assess publication bias in this systematic review.
Data synthesis
We performed and presented meta‐analysis of outcomes for the comparison of 5‐ASA versus control. We performed meta‐analysis only if we identified two or more trials with similar comparisons and outcome measures. We calculated a pooled risk ratio (RR) using a random‐effects model (Mantel‐Haenszel), as both outcomes were dichotomous. When studies reported repeated observations, we analysed data measured at the last follow‐up time point. When studies allocated participants to more than one 5‐ASA treatment arm, we combined these studies for the primary analysis.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for subgroups for primary outcomes defined by the following study‐level covariates, when reported.
Control regimens (5‐ASA + probiotics vs probiotics, 5‐ASA + antibiotics vs antibiotics, 5‐ASA vs placebo, 5‐ASA vs no treatment, and 5‐ASA + probiotics vs placebo).
5‐ASA dose.
Participant age and gender.
Number of prior episodes of diverticulitis.
Method of diagnosis (diverticulitis confirmed by CT scan/ultrasonography vs other methods).
Risk of bias (low vs unclear and high).
Publication type (abstract vs full text).
All studies were full papers; therefore insufficient study‐level data were available for 5‐ASA dose, participant age and gender, and number of prior episodes. Subgroup analyses were possible only for control regimens, methods of diagnosis, and risk of bias.
Sensitivity analysis
We performed the following sensitivity analyses.
Summary statistic (risk ratios vs odds ratios).
Meta‐analysis modelling (fixed‐effect vs random‐effects).
GRADE and 'Summary of findings' table
We evaluated the overall quality of evidence for primary (rate of recurrence of diverticulitis) and secondary (adverse effects of therapy) outcomes using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Schünemann 2009). We presented this evaluation in a 'Summary of findings' table.
We could downgrade evidence from high quality by one level (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, or outcomes), imprecision (wide confidence interval), and publication bias.
We applied the following definitions in grading the quality of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: Any estimate of effect is very uncertain.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 714 citations through the electronic database search. We identified one additional study, published only in abstract form, by searching conference proceedings (Gaman 2011). After removing duplicates, we screened 528 studies for eligibility. Of these, we excluded 505 studies because they did not meet the inclusion criteria. We found that 23 studies were eligible for inclusion. We obtained full text for 18 studies; the remaining five studies were available only in abstract form. In summary, seven studies (reported in eight references) fulfilled our inclusion criteria. For details on study selection, see PRISMA flow chart Figure 1.
1.
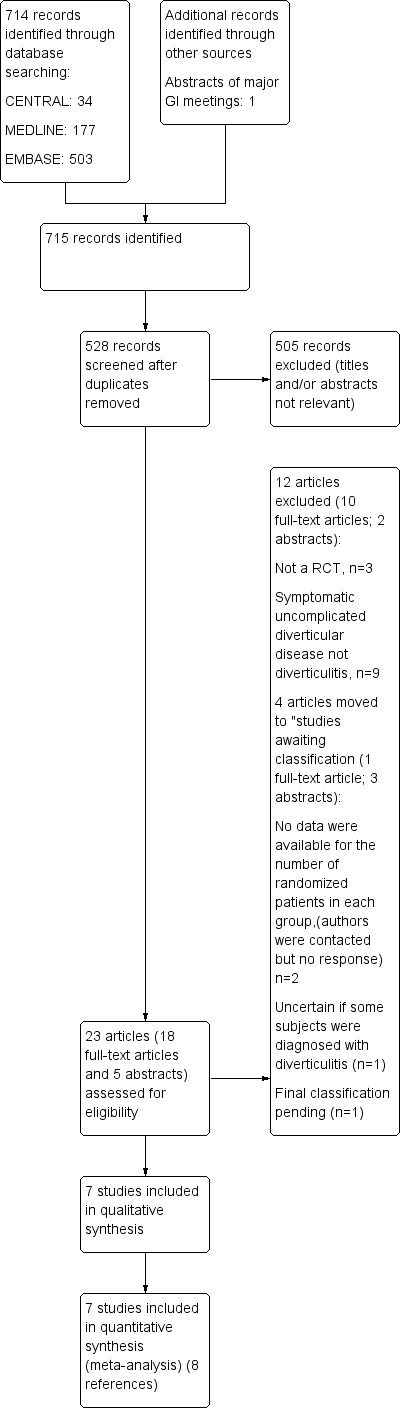
Study flow diagram.
Included studies
Trespi reported 12‐month follow‐up results from a single‐centre randomised trial that compared 5‐ASA versus no therapy for prevention of complications of diverticulitis (Trespi 1999). Diagnosis was confirmed by radiological imaging in the presence of fever, abdominal distension, or major change in bowel habit. Investigators randomised 166 participants after initial treatment with ampicillin/sulbactam 1.5 g IM twice daily plus rifaximin 400 mg PO twice daily for seven days. They randomised participants to 5‐ASA 400 mg twice daily or to no further therapy for eight weeks. After 12 months, the dropout rate was higher in the 5‐ASA group than in the control group (n = 15, 19%; vs n = 12, 14%); most cases of dropout were due to poor compliance or withdrawal of consent. After four years, 44 participants dropped out (27%). Recurrence rates were 12/81 (15%) vs 39/85 (46%) (Trespi 1999). Study authors did not state definitive criteria for recurrent diverticulitis. However, they followed participants for relapse of symptoms and repeated blood tests at intervals to detect relapse of inflammation. Risk of bias was high because outcome data were incomplete.
Tursi published a 12‐month RCT that compared 5‐ASA 800 mg three times daily plus rifaximin 400 mg twice daily for seven days followed by 5‐ASA 800 mg twice daily plus rifaximin 400 mg twice daily for seven days every month (n = 109) versus rifaximin 400 mg twice daily alone for seven days every month (n = 109) among 218 participants with recurrent attacks of acute colonic diverticulitis (Tursi 2002). Researchers defined diverticulitis as inflammation and/or infection associated with diverticula of the colon and confirmed diagnosis by colonoscopy or double contrast radiography. Clinical criteria included abdominal pain, fever, and leukocytosis with elevated inflammatory markers. A total of 193 participants were fully compliant with therapy. Three participants in the 5‐ASA arm (2.8%) and 16 in the rifaximin monotherapy arm (18.0%) experienced recurrence of acute diverticulitis (P < 0.005). Investigators diagnosed recurrence on the basis of clinical symptoms (abdominal pain, altered bowel habits, fever) and endoscopic examination (inflamed mucosa at endoscopy). The severity of symptoms was lower with 5‐ASA (85.6% vs 49.4%; P < 0.0005 at 12 months). One participant in the 5‐ASA group developed transient urticaria (0.9%) and nine reported epigastric pain (8.3%), but treatment allocation was not reported. Therefore, we did not include this study in the analysis of adverse events. Study authors concluded that rifaximin plus 5‐ASA was more effective than rifaximin alone for prevention of recurrence of diverticulitis.
Tursi reported a randomised single‐centre trial that compared the efficacy of 5‐ASA (balsalazide) 2.25 g/d for 10 days per month plus a probiotic mixture VSL#3 450 billion/d for 15 days per month versus the probiotic alone for 12 months for prevention of recurrent diverticulitis in 30 participants with uncomplicated diverticulitis of the colon (Tursi 2007a). Investigators defined diagnosis by the presence of symptomatic diverticula at colonoscopy with signs of inflammation but without complications. They induced remission with rifaximin 800 mg/d and 5‐ASA 2.25 g/d for 10 days. As no blinding of participants or physicians was reported, we considered this study to be at high risk of bias for "allocation concealment" and "blinding of participants and personnel". One participant in each group was lost to follow‐up (one in the 5‐ASA arm with poor compliance, and one lost to follow‐up in the control arm). In the 5‐ASA arm, one participant had recurrent diverticulitis and two experienced recurrent symptoms without diverticulitis. In the control arm, two participants experienced recurrent diverticulitis and four had recurrent symptoms without diverticulitis. Results showed no statistical significant differences between the two groups for remission rates or overall symptom scores. Symptom scores for constipation, abdominal pain, and bloating were significantly lower in the 5‐ASA group. Investigators evaluated recurrent diverticulitis on the basis of clinical symptoms (abdominal pain, altered bowel habit, fever) and/or endoscopic examination findings. They reported no adverse events in either group throughout the study. Study authors concluded that the combination of 5‐ASA and probiotic was better than probiotic monotherapy in preventing relapse of uncomplicated diverticulitis of the colon, although their findings did not reach statistical significance.
Parente reported a randomised, multi‐centre, double‐blind, placebo‐controlled trial comparing 5‐ASA 800 mg twice daily for 10 days per month versus placebo for 24 months for preventing recurrence of diverticulitis in 96 participants (Parente 2013). Diagnosis of diverticulitis was based on clinical symptoms (abdominal pain, fever, leukocytosis, and increased erythrocyte sedimentation (ESR)/C‐reactive protein (CRP)) and was confirmed by abdominal ultrasonography or CT scanning. The primary endpoint recurrence of diverticulitis was diagnosed clinically in the presence of abdominal pain, leukocytosis, and/or fever. Recurrence was confirmed by cross‐sectional imaging (CT or ultrasonography). Secondary endpoints included time to relapse, physical condition, and quality of life as evaluated by the Therapeutic Impact Questionnaire (TIQ); use of additional drugs; and treatment tolerability. Four participants did not receive study drug after randomisation (treatment allocation not stated), and study authors' modified ITT analysis included only 92 participants. Sixteen participants were lost to follow‐up because of dropouts (n = 4) and serious side effects (n = 8 with 5‐ASA vs n = 4 with placebo). Therefore, we assessed this study as having high risk of bias for incomplete outcome data. After 24 months, the incidence of diverticulitis recurrence was 13.3% (n = 6) in the 5‐ASA arm and 27.7% (n = 13) in the placebo arm (difference not significant). TIQ scores for physical condition were significantly better with 5‐ASA than with placebo (P = 0.02). Global additional drug consumption was less among participants taking 5‐ASA (P < 0.03). Adverse events were more common with placebo (48.9% vs 35.6%). Study authors concluded that intermittent 5‐ASA did not reduce risk of relapse but improved participants' physical condition and reduced requirements for other gastrointestinal drugs.
Stollman reported a multi‐centre randomised, double‐blind, double‐dummy, placebo‐controlled trial conducted to assess the efficacy of 5‐ASA in reducing gastrointestinal symptoms after an acute attack of diverticulitis (Stollman 2013 (DIVA)). Participants received 5‐ASA (2.4 g once daily), 5‐ASA plus probiotic(Bifidobacterium infantis 35624), or placebo for 12 weeks, and were followed for an additional nine months. Investigators randomised 117 participants within seven days after CT imaging confirmed acute diverticulitis. They assessed efficacy using a global gastrointestinal symptomatic score (GSS) wherein the maximum severity of 10 symptoms was reported on a seven‐point Likert scale. The primary outcome was GSS at 12 weeks. Secondary outcome measures included percentage of GSS responders and change in GSS at 12 weeks and 52 weeks, withdrawal due to surgery for diverticulitis, and recurrent diverticulitis. Study authors noted that recurrent diverticulitis was diagnosed on clinical grounds, and that a CT scan was not required by the protocol. They reported a trend towards improved symptoms with 5‐ASA but reported statistical significance only for complete response rate and specific rectosigmoid symptoms. Investigators noted no statistical significant differences in rate of recurrence of diverticulitis. Subgroup analysis by regimens and controls (Analysis 2.1) split the shared placebo group into two groups with smaller sample size and included data for two subgroups, according to the Cochrane guideline (Chapter 16.5.4). In all, 9 (25%) vs 8 (20%) vs 12 (30%) participants in 5‐ASA + probiotic, 5‐ASA, and placebo groups, respectively, did not complete treatment after 12 weeks, and 12 (33%), 13 (33%), and 19 (46%) did not complete the 52‐week study. Although study authors provided reasons for withdrawal, the overall number of participants without outcome data was greater than 20%, with more withdrawals in the placebo group. Therefore, we considered this study to be at high risk of bias for incomplete outcome data.
2.1. Analysis.
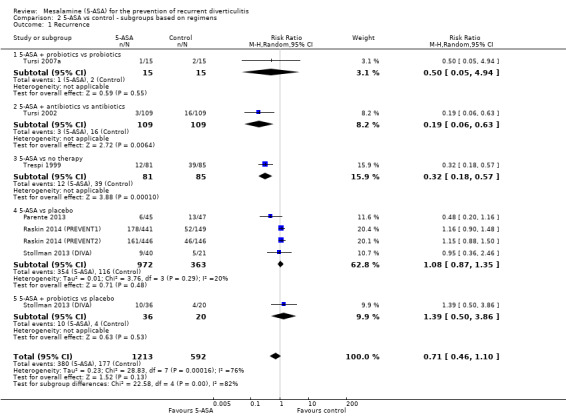
Comparison 2 5‐ASA vs control ‐ subgroups based on regimens, Outcome 1 Recurrence.
Raskin conducted identical multi‐centre, multi‐national, dose‐response trials (Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2). PREVENT1 enrolled 590 participants and PREVENT2 enrolled 592 participants with one or more episodes of acute diverticulitis in the previous 24 months that resolved without surgery. Investigators confirmed diverticulitis by CT, magnetic resonance imaging (MRI), ultrasonography, colonoscopy, sigmoidoscopy, or barium enema. They randomised participants to one of three doses of 5‐ASA (1.2 g, 2.4 g, or 4.8 g) or to placebo once daily for 104 weeks. The primary endpoint was absence of recurrence by week 104. Secondary endpoints included time to recurrence of diverticulitis and proportion of participants requiring surgical intervention. This study defined recurrent diverticulitis as surgical intervention at any time for diverticular disease or a positive CT scan result. In PREVENT1, only the 5‐ASA dose of 4.8 g was associated with a lower recurrence rate than placebo (52.7% vs 64.6%; P = 0.047). PREVENT2 reported no significant difference in recurrence between any 5‐ASA dose and placebo. Study authors concluded that 5‐ASA was not superior to placebo for preventing recurrent diverticulitis.
Of note, four of the seven included studies were conducted in Italy (Parente 2013; Trespi 1999; Tursi 2002; Tursi 2007a). One was conducted in the United States (Stollman 2013 (DIVA)). Two were multi‐national studies (Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)).
Excluded studies
We excluded twelve studies (see table of Excluded studies). Nine studies because they included participants with symptomatic uncomplicated diverticular disease but not diverticulitis (Brandimarte 2004; Mario 2005; Tursi 2006; Comparato 2007a; Comparato 2007b; Tursi 2007b; Gaman 2011; Kruis 2013a; Tursi 2013b), and furthermore three studies because they were not randomised controlled trials (Gatta 2012; Tursi 2013; Festa 2015).
Studies awaiting assessment
We could not classify four remaining studies from our searches (Kruis 2013b; Kruis 2014; Bassi 2015; Kruis 2017). Two were randomised, double‐blind, placebo‐controlled phase 3 studies that were available only in conference abstract form and came from the same study group. We did not include these studies because they provided insufficient data, and, unfortunately, even after three years, they had produced no full publications (Kruis 2013b; Kruis 2014). One study randomised 330 participants who had been treated successfully for uncomplicated left‐sided diverticulitis to receive 5‐ASA 1.5 g or 3 g once daily or placebo for 96 weeks. Among 164 participants assessed at 96 weeks, recurrence‐free proportions were 6.9%, 9.8%, and 21.8%, respectively (P > 0.05) (Kruis 2014). A third trial randomised 345 participants with uncomplicated left‐sided diverticulitis to 5‐ASA 3 g once daily or placebo. After 48 weeks, 67.9% of participants given 5‐ASA and 74.4% of those given placebo were recurrence free (P > 0.05) (Kruis 2013b). None of these trials found 5‐ASA to be significantly superior to placebo for prevention of recurrence of uncomplicated diverticulitis. We contacted study authors for further information, but they did not respond. The fourth study, which reported two randomised, double‐blind, placebo‐controlled, multi‐centre trials, is awaiting final classification (Kruis 2017). The final excluded study was a randomised, open‐label study that also was available only in conference abstract form. We did not include this study because information was insufficient for review authors to determine whether patients with diverticulitis were included in the study. Investigators randomised 34 participants after induction of remission with metronidazole and mesalazine for 14 days. Participants received mesalazine 1.6 g once daily or mesalazine 1.6 g plus probiotic (L. casei DG 16 billion/d for 10 days per month). After 12 months of treatment, four participants (11.8%) were symptom free. Study authors provided no other results and concluded that both mesalazine and probiotics were effective in preventing recurrence in uncomplicated symptomatic diverticular disease of the colon (Bassi 2015).
We have presented these trials in the Characteristics of studies awaiting classification section.
Risk of bias in included studies
We have presented results of risk of bias analysis in Figure 2 and Figure 3. Two review authors (of FC, YY, and MA) independently assessed risk of bias of eligible trials using the Cochrane 'Risk of bias' tool (Chapter 8, Higgins 2011; Appendix 4). We considered four studies as having high risk of bias, and the remaining three as having unclear risk of bias.
2.
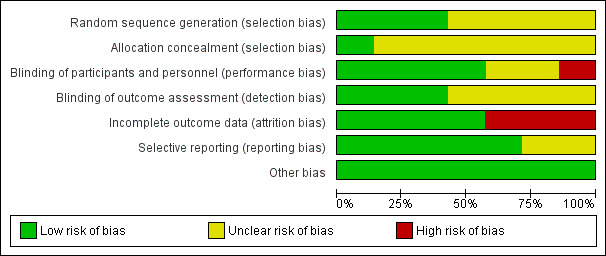
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
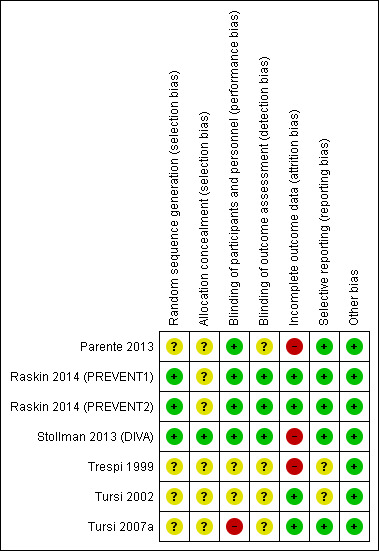
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered three studies to have low risk of bias for random sequence generation (Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). We considered the other four studies to have unclear risk of bias because study authors provided insufficient information. We considered only one study to have low risk of bias for allocation concealment (Stollman 2013 (DIVA)). The remaining studies did not provide sufficient information.
Blinding
Four studies were double‐blinded (Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). One study was an open‐label study; therefore we considered it to have high risk of bias for participants and personnel (Tursi 2007a). The other studies did not report methods of blinding. Only three studies clearly stated that outcome assessors were blinded (Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). We considered the other studies to have unclear risk of bias for outcome assessment.
Incomplete outcome data
Three studies had high risk of bias for the domain "incomplete outcome data" (Trespi 1999; Parente 2013; Stollman 2013 (DIVA)). We have discussed details under Characteristics of included studies.
Selective reporting
Five studies reported all important outcomes; we therefore considered them to have low risk of bias for selective reporting. We considered two studies to have unclear risk for selective reporting because study authors did not clearly report data on adverse events (Trespi 1999; Tursi 2002).
Other potential sources of bias
We considered all studies to have low risk of other biasg.
Effects of interventions
See: Table 1
Recurrence of diverticulitis
We included seven studies with a total of 1805 participants in the analysis for the primary outcome of recurrence of diverticulitis. We saw no statistically significant reduction in recurrence of diverticulitis with 5‐ASA versus control (31.3% vs 29.8%) with RR of 0.69 (95% CI 0.43 to 1.09; P = 0.11). We noted significant heterogeneity between studies (I2 = 0.79; P < 0.0001) (Analysis 1.1; Figure 4). We rated the overall quality of evidence for the outcome recurrence of diverticulitis as very low (Table 1).
1.1. Analysis.
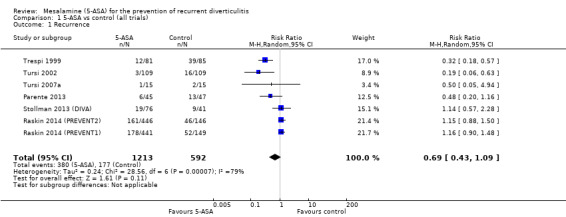
Comparison 1 5‐ASA vs control (all trials), Outcome 1 Recurrence.
4.
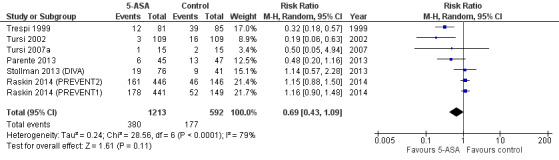
Forest plot of comparison: 5‐ASA vs all 'control' interventions, outcome: 6.1 Recurrence.
Number of participants with adverse events
Only five studies provided sufficient data for analysis of the frequency of adverse events (Tursi 2007a; Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Among these, one trial reported no adverse event in either group (Tursi 2007a). The others reported no significant differences in the frequency of adverse events between 5‐ASA and control groups (67.8% vs 64.6%; RR 0.98, 95% CI 0.91 to 1.06; P = 0.63) and showed no significant heterogeneity (I2 = 0; P = 0.50). We performed no further subgroup analyses for adverse events. See Figure 5 (Analysis 1.2). We rated the overall quality of evidence for this outcome as moderate (Table 1).
5.
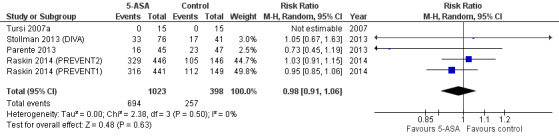
Forest plot of comparison: 1 5‐ASA vs control (all trials), outcome: 1.2 Participants with adverse events.
1.2. Analysis.
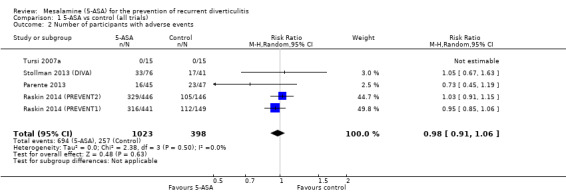
Comparison 1 5‐ASA vs control (all trials), Outcome 2 Number of participants with adverse events.
Subgroup analyses
Subgroup analysis of study regimens revealed a significant difference in comparisons of 5‐ASA + antibiotics versus antibiotic monotherapy (RR 0.19, 95% CI 0.06 to 0.63) and 5‐ASA versus no therapy (RR 0.32, 95% CI 0.18 to 0.57) (Analysis 2.1; Figure 6). However, we included only one small study in each of these subgroups (Trespi 1999; Tursi 2002). Analysis showed no significant differences for 5‐ASA + probiotics versus probiotic monotherapy (RR 0.50, 95% CI 0.05 to 4.94) in the Tursi study; for 5‐ASA versus placebo (RR 1.08, 95% CI 0.87 to 1.35) in the Parente, Stollman, and Raskin studies; and for 5‐ASA + probiotics versus placebo (RR 1.39, 95% CI 0.46 to 1.10) in the Stollman study (Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2); Tursi 2007a). These subgroups showed significant heterogeneity (test for subgroup differences, I2 = 0.82; P = 0.0002).
6.
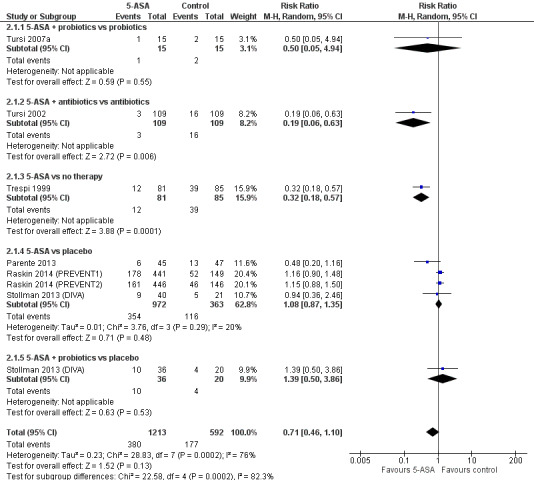
Forest plot of comparison: 2 5‐ASA vs control ‐ subgroups by regimens and controls, outcome: 2.1 Recurrence.
Methods used for confirmation of a diverticulitis before enrolment were not consistent across included trials. Four trials utilised CT scan or ultrasonography for primary diagnosis of diverticulitis (Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Subgroup analysis of studies that used CT or ultrasonography for primary diagnosis revealed no significant treatment effect (36.1% vs 31.3%; RR 1.11, 95% CI 0.80 to 1.35) (Analysis 3.1; Figure 7). These studies showed no significant heterogeneity (I2 = 0.18; P = 0.30). The remaining three trials used clinical assessment, laboratory indices, and colonoscopy to confirm the diagnosis (Trespi 1999; Tursi 2002; Tursi 2007a). Their pooled analysis favoured 5‐ASA for prevention of diverticulitis (7.8% vs 27.3%; RR 0.24, 95% CI 0.18 to 0.50) with no significant heterogeneity (I2 = 0; P = 0.62). Differences between these two subgroups were statistically significant (I2 = 0.95; P < 0.000001). Furthermore, only three trials utilised CT scan for primary diagnosis of both diverticulitis and recurrence (Parente 2013; Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Pooled analysis revealed no significant treatment effect (RR 1.07, 95% CI 0.81 to 1.40) (Analysis 3.2). Heterogeneity was not significant (I2 = 45%; P = 0.16).
3.1. Analysis.
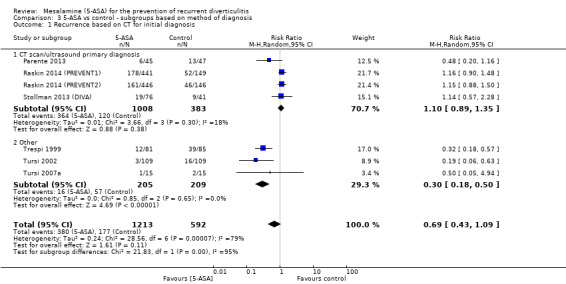
Comparison 3 5‐ASA vs control ‐ subgroups based on method of diagnosis, Outcome 1 Recurrence based on CT for initial diagnosis.
7.
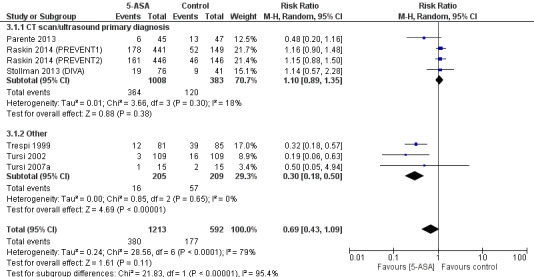
Forest plot of comparison: 3 5‐ASA vs control ‐ subgroups based on method of diagnosis, outcome: 3.1 Recurrence based on CT for initial diagnosis.
3.2. Analysis.
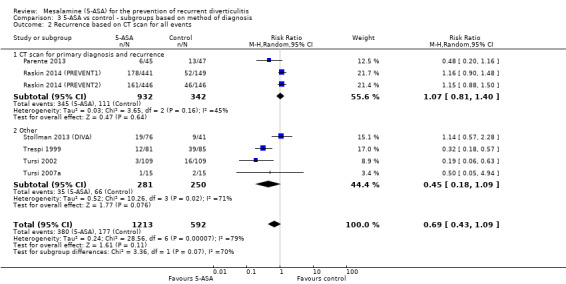
Comparison 3 5‐ASA vs control ‐ subgroups based on method of diagnosis, Outcome 2 Recurrence based on CT scan for all events.
Subgroup analysis based on risk of bias revealed no significant differences between 5‐ASA and control groups among studies with unclear risk of bias (34.3% vs 28.2%; RR 0.93, 95% CI 0.59 to 1.49; test for heterogeneity I2 = 77%; P = 0.001) or among studies with high risk of bias (17.5% vs 3.5%; RR 0.55, 95% CI 0.27 to 1.10; test for heterogeneity I2 = 61%; P = 0.06) (Figure 8). Results showed no statistical significant difference between the two subgroups (I2 = 35%; P = 0.22).
8.
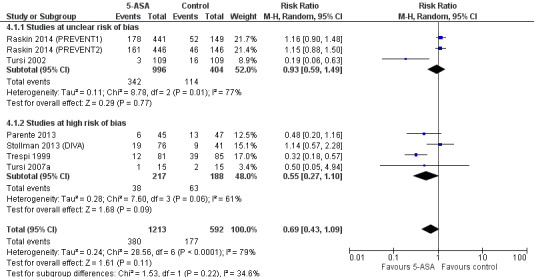
Forest plot of comparison: 5‐ASA vs control (double‐blind, placebo‐controlled trials), outcome: 7.1 Recurrence.
Sensitivity analysis
Results of this analysis were robust to the method of analysis. We used the random‐effects model for the overall analysis, for a pooled RR of 0.69 (95% CI 0.43 to 1.09). When we used a fixed‐effect model, the pooled RR was 0.90 (95% CI 0.77 to 1.06). Results remained non‐significant when we used the odds ratio (OR); the pooled OR was 0.60 (95% CI 0.32 to 1.13).
Discussion
Recurrence of diverticulitis after an initial episode is a common and important clinical problem. At present, no pharmacological agents have been approved for prevention of recurrent diverticulitis. Prophylactic surgery was previously recommended after two confirmed significant episodes of diverticulitis, but more recent guidelines advocate a more conservative approach (Rafferty 2006). This shift has been driven by the lack of evidence supporting routine elective colectomy among patients with recurrent diverticulitis (Chapman 2006; Collins 2008). Recently, even the use of antibiotics for diverticulitis has been questioned (Chabok 2012). 5‐Aminosalicylic acid (ASA) offers the promise of modifying the underlying inflammation in patients with diverticular disease. This review evaluated available evidence for 5‐ASA for prevention of recurrence of diverticulitis.
Summary of main results
We included seven randomised controlled trials (RCTs) in this review (Trespi 1999; Tursi 2002; Tursi 2007a; Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Four compared 5‐ASA versus placebo (Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). One compared 5‐ASA in combination with probiotics versus probiotic monotherapy (Tursi 2007a). One compared 5‐ASA with an antibiotic (rifaximin) versus antibiotic monotherapy (Tursi 2002). One compared 5‐ASA versus no therapy (Trespi 1999). Stollman also reported a comparison of 5‐ASA plus probiotics versus placebo (Stollman 2013 (DIVA)).
Recurrent diverticulitis
The primary outcome of this review was prevention of recurrent diverticulitis. Overall, 5‐ASA was not superior to control interventions for prevention of diverticulitis. This result was robust to all sensitivity analyses. As expected, heterogeneity among the included studies was significant (I2 = 0.79; P < 0.0001). Control interventions used in trials that informed this review were varied (placebo, probiotic, antibiotic, and no therapy). Subgroup analysis based on treatment comparators revealed significant treatment effects with 5‐ASA + antibiotics versus antibiotic monotherapy (risk ratio (RR) 0.19, 95% confidence interval (CI) 0.06 to 0.63) and with 5‐ASA versus no therapy (RR 0.32, 95% CI 0.18 to 0.57), but each of these comparisons was based on only one small study. Both were open‐label trials with high or unclear risk of bias.
Our review demonstrates the challenges associated with clinical research in diverticular disease. The pathogenesis of diverticulitis, a relatively common diagnosis, remains uncertain. This uncertainty focuses on whether infection or inflammation is the primary disturbance, and it affects the control therapies utilised in clinical trials. It is worth noting that the efficacy of both probiotics and antibiotics for preventing recurrence of diverticulitis remains unproven.
Treatment effects can best be assessed in clinical trials that enrol a well‐defined, homogeneous, and responsive patient population. Among trials included in this review, methods used to diagnose index and recurrent episodes of diverticulitis were not consistent. The definition or diagnosis of a diverticulitis episode or recurrence was shown to be important in our review, as trials that required cross‐sectional imaging were less likely to demonstrate a treatment effect than trials that did not require cross‐sectional imaging. Four trials required use of computerised tomography (CT) scanning for primary diagnosis of diverticulitis (Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Pooled analysis of these trial results showed no significant treatment effect. Three trials required a CT scan for diagnosis of primary diverticulitis and recurrent events (Parente 2013; Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). These three trials best explored the objective of this review. Pooled analysis of results of these three trials revealed no significant treatment effect (RR 1.07, 95% CI 0.81 to 1.40). In sensitivity analyses, this result remained robust with use of odds ratios (ORs) and a fixed‐effect model. The three trials that did not use a CT scan for diagnosis of diverticulitis and relied mostly on clinical symptoms favoured 5‐ASA therapy (RR 0.30, 95% CI 0.18 to 0.50) (Trespi 1999; Tursi 2002; Tursi 2007a). It is possible to overestimate and misdiagnose cases of recurrence if only symptoms and laboratory indices are used (Sarma 2008). We recommend that diagnoses of diverticulitis be confirmed by CT or ultrasonography in future clinical trials.
Pooled analysis of results of clinical trials of diverticular disease is further challenged by the heterogeneous study design. Open‐label trials are common, and even the controlled trials included in this review were at risk of significant bias. Our overall results were consistent even after we removed studies identified as having high risk of bias. However, we assessed none of the included studies as having low risk of bias across all categories. Four of the included trials used a placebo‐controlled, double‐blind design (Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). These studies constitute the best evidence regarding 5‐ASA therapy for diverticular disease, and each failed to demonstrate efficacy for prevention of recurrent diverticulitis.
Many of the trials included in this review were small and had limited power to detect a treatment effect. PREVENT1 and PREVENT2 enrolled the largest patient cohorts and provided power calculations. Stollman reported that enrolment of 216 participants was required for adequate power but enrolled only 117 participants (Stollman 2013 (DIVA)). Therefore, this study was underpowered. The remaining studies reported no sample size calculation.
Dosing regimens for 5‐ASA also varied across studies. Four studies administered 5‐ASA daily (Trespi 1999; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). The other three trials used cyclical regimens (Tursi 2002; Tursi 2007a; Parente 2013). The total daily dose also varied across trials: Trepsi 1997 used 400 mg twice daily; Tursi 2002, 0.8 g three times daily for 7 days per month; Tursi 2007a, 2.25 g once daily for 10 days per month; Parente 2013, 0.8 g twice daily for 10 days per month; Stollman 2013, 2.4 g once daily; and PREVENT1 and PREVENT2, 1.2 g, 2.4 g, or 4.8 g once daily (Trespi 1999; Tursi 2002; Tursi 2007a; Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Available data were insufficient for review authors to explore a dose effect for treatment of diverticular disease.
In summary, two individual studies have reported significant reductions in recurrence of diverticulitis with 5‐ASA therapy (Trespi evaluated 5‐ASA vs no therapy in 1999, and Tursi compared 5‐ASA plus antibiotic vs antibiotic alone in 2002), but the other five eligible studies have demonstrated no effect (Trespi 1999; Tursi 2002; Tursi 2007a; Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Pooled analysis revealed no overall statistically significant benefit. We have concluded that available trials included in this review do not support the use of 5‐ASA therapy for prevention of recurrent acute diverticulitis.
Adverse events
We found 5‐ASA to be well tolerated. Five studies reported adverse event rates that could be analysed (Tursi 2007a; Parente 2013; Stollman 2013 (DIVA); Raskin 2014 (PREVENT1); Raskin 2014 (PREVENT2)). Total adverse events were no more common in the 5‐ASA arms than in the control arms. Results showed no significant heterogeneity (I2 = 0; P = 0.50), but this finding should be interpreted with caution, as the I2 test provides little power to reject the null hypothesis of homogeneity (I2 = 0) when few studies are available, even if substantial heterogeneity is present (Loannidis 2007).
Overall completeness and applicability of evidence
This review sought to evaluate the efficacy of 5‐ASA in preventing recurrence of diverticulitis. We were prepared to include studies from any source, regardless of publication status or language. Of the originally 23 eligible studies for inclusion, we were able to retrieve full manuscripts for all but two studies. These two were published only in abstract form and provided insufficient data for analysis (Figure 1). Pooled results of included studies could not establish a role for 5‐ASA for this indication, and it is unlikely that these two abstracts would change our overall results. We believe that this review accurately reflects available evidence for 5‐ASA for prevention of recurrent diverticulitis.
We were not able to analyse adverse effects in all included trials. In addition, we could analyse only total adverse effects. as the data reported were insufficient to permit any substantive evaluation of individual adverse events. We concluded that in trials included in this review, 5‐ASA was well tolerated and performed similarly to placebo. However, we must concede that two of the included trials did not provide analysable data regarding adverse events. What effect this had, if any, on our final results remains uncertain.
The present standard of medical care for diverticulitis involves antibiotics in the acute setting but no subsequent therapy to prevent recurrence and/or complications. The evidence presented here does not call for any change in this treatment paradigm.
Quality of the evidence
Overall, the quality of evidence for the outcome recurrence of diverticulitis is very low owing to study limitations and significant heterogeneity (Table 1). We included in this review only seven randomised controlled trials that enrolled a total of 1805 participants with diverticulitis. We considered four of seven studies to be at high risk of bias and noted that heterogeneity among studies was significant. The confidence interval does not exclude appreciable benefit or no difference. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change this estimate.
The overall quality of evidence for the outcome number of participants with adverse events is moderate. Only five studies with 1421 participants contributed data to this outcome. Three of five studies were at high risk of bias. Results showed no significant heterogeneity for the outcome adverse events but should be interpreted with caution because included studies were few.
Potential biases in the review process
A potential limitation of this review is that included studies used various methods to diagnose a diverticulitis event. This may make direct comparison of these trial results difficult.
We did not explore publication bias in this review because we included only seven studies for the primary outcome and five for the secondary outcome. Application of funnel plot asymmetry tests to detect publication bias is inappropriate or is not meaningful for this review, as we included only a few studies for the outcomes reported in this review (Loannidis 2007).
Agreements and disagreements with other studies or reviews
This review is consistent with other published systematic reviews in this area. In 2011, Maconi published a review of medical therapy for both treatment of symptoms of diverticular disease and prevention of recurrent diverticulitis (Maconi 2011). These review authors concluded that medical therapy (including 5‐ASA) did not improve symptoms, but that its role in preventing recurrent diverticulitis needed to be further defined. In 2012, Unlu published a review of medical therapy for prevention of recurrent diverticulitis (Unlu 2012). These review authors identified three trials, two of which evaluated 5‐ASA (Tursi 2002; Tursi 2007a). They concluded that the evidence supporting medical therapy was of low quality, but that 5‐ASA was the most promising of the available medical therapies. Tursi published a review of recent advances in the management of colonic diverticulitis, wherein he concluded that the evidence for 5‐ASA for preventing recurrence was promising, but that dosing and schedules remained unclear (Tursi 2012). This same review author later concluded, in a review on new medical strategies for management of acute diverticulitis, that evidence on effective strategies for prevention of recurrence is lacking (Tursi 2015).
Authors' conclusions
Implications for practice.
The efficacy of 5‐aminosalicylic acid (5‐ASA) for prevention of recurrence of diverticulitis is uncertain owing to the very low quality of available evidence. Accordingly, we recommend no change in practice.
Implications for research.
Additional trials of rigorous design are needed to explore whether 5‐ASA is effective for prevention of recurrence of diverticulitis. Such trials should use standardised criteria to diagnosis diverticulitis, and should follow a randomised, double‐blind, placebo‐controlled design with adequate statistical power. The research design should also allow for comparison of significant complications of diverticulitis including but not exclusive to surgery for diverticulitis, colonic stenosis, abscess, and diverticular bleeding. The research design should allow for comparison of common and rare adverse events and should compare dosages and schedules.
Acknowledgements
The review authors would like to thank the Cochrane Colorectal Cancer Group for continuous support in developing and carrying out the search strategies applied for this review (Sys Johnsen) and for substantial editorial support (Henning Keinke Andersen and Sara Hallum) and thorough evaluation provided by appointed editors and peer referees.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor: [Diverticulum] explode all trees
MeSH descriptor: [Diverticulitis] explode all trees
MeSH descriptor: [Diverticulosis, Colonic] explode all trees
diverticul*:ti,ab,kw (Word variations have been searched)
#1 or #2 or #3 or #4
MeSH descriptor: [Mesalamine] explode all trees
MeSH descriptor: [Sulfasalazine] explode all trees
5‐aminosalicylic acid or 5‐ASA or aminosalicylate* or mesalamine or mesalazine:ti,ab,kw (Word variations have been searched)
Mesacol or Mezavant or Mesacron or Mesalazina or Mesasal or Mesaneo:ti,ab,kw (Word variations have been searched)
Asacol or Apriso or Asacolon or Asalit or Azodisal or Canasa or Claversal:ti,ab,kw (Word variations have been searched)
Delzicol or Dipentum or Ipocal or Lialda or Lixacol or Octasa or olsalazine or Pentasa:ti,ab,kw (Word variations have been searched)
Rowasa or Salofalk or balsalazide or Giazo or Colazal or Colazide:ti,ab,kw (Word variations have been searched)
salicylazosulfapyridine or sulfasalazine or sulphasalazine:ti,ab,kw (Word variations have been searched)
#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#5 and #14
Appendix 2. MEDLINE search strategy
exp Diverticulum/
exp Diverticulitis/
exp diverticulosis, colonic/
diverticul*.tw.
or/1‐4
exp Mesalamine/
exp Sulfasalazine/
(5‐aminosalicylic acid or 5‐ASA or aminosalicylate* or mesalamine or mesalazine).tw.
(Mesacol or Mezavant or Mesacron or Mesalazina or Mesasal or Mesaneo).tw.
(Asacol or Apriso or Asacolon or Asalit or Azodisal or Canasa or Claversal).tw.
(Delzicol or Dipentum or Ipocal or Lialda or Lixacol or Octasa or olsalazine or Pentasa).tw.
(Rowasa or Salofalk or balsalazide or Giazo or Colazal or Colazide).tw.
(salicylazosulfapyridine or sulfasalazine or sulphasalazine).tw.
or/6‐13
5 and 14
Appendix 3. Embase search strategy
exp diverticulosis/
exp diverticulitis/
diverticul*.tw.
1 or 2 or 3
exp mesalazine/
exp salazosulfapyridine/
exp balsalazide/
exp olsalazine/
(5‐aminosalicylic acid or 5‐ASA or aminosalicylate* or mesalamine or mesalazine).tw.
(Mesacol or Mezavant or Mesacron or Mesalazina or Mesasal or Mesaneo).tw.
(Asacol or Apriso or Asacolon or Asalit or Azodisal or Canasa or Claversal).tw.
(Delzicol or Dipentum or Ipocal or Lialda or Lixacol or Octasa or olsalazine or Pentasa).tw.
(Rowasa or Salofalk or balsalazide or Giazo or Colazal or Colazide).tw.
(salicylazosulfapyridine or sulfasalazine or sulphasalazine).tw.
or/5‐14
4 and 15
Appendix 4. Criteria for judging risk of bias in the 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence | |
| Criteria for judgement of ‘Low risk’ of bias | Investigators describe a random component in the sequence generation process such as: · Referring to a random number table; · Using a computer random number generator; · Tossing a coin; · Shuffling cards or envelopes; · Throwing dice; · Drawing lots; or · Using minimisation*. *Minimisation may be implemented without a random element, and this is considered equivalent to being random. |
| Criteria for judgement of ‘High risk’ of bias | Investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · Sequence generated by odd or even date of birth; · Sequence generated by some rule based on date (or day) of admission; or · Sequence generated by some rule based on hospital or clinic record number. Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example: · Allocation by judgement of the clinician; · Allocation by preference of the participant; · Allocation based on results of a laboratory test or series of tests; or · Allocation by availability of the intervention. |
| Criteria for judgement of ‘Unclear risk’ of bias | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment | |
| Criteria for judgement of ‘Low risk’ of bias | Participants and investigators enrolling participants could not foresee assignment because 1 of the following, or an equivalent method, was used to conceal allocation. · Central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation). · Sequentially numbered drug containers of identical appearance. · Sequentially numbered, opaque, sealed envelopes. |
| Criteria for judgement of ‘High risk’ of bias | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · Using an open random allocation schedule (e.g. a list of random numbers); · Using assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or were nonopaque or not sequentially numbered); · Alternation or rotation; · Date of birth; · Case record number; or · Any other explicitly unconcealed procedure. |
| Criteria for judgement of ‘Unclear risk’ of bias | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or is not described in sufficient detail to allow a definitive judgement – for example, if use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, or opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study | |
| Criteria for judgement of ‘Low risk’ of bias | Any 1 of the following: · No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding; or · Blinding of participants and key study personnel ensured, and unlikely that blinding could have been broken. |
| Criteria for judgement of ‘High risk’ of bias | Any 1 of the following: · No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or · Blinding of key study participants and personnel attempted, but likely that blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for judgement of ‘Unclear risk’ of bias | Any 1 of the following: · Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; or · The study did not address this outcome. |
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors | |
| Criteria for judgement of ‘Low risk’ of bias | Any 1 of the following: · No blinding of outcome assessment, but review authors judge that outcome measurement is not likely to be influenced by lack of blinding; or · Blinding of outcome assessment ensured, and unlikely that blinding could have been broken. |
| Criteria for judgement of ‘High risk’ of bias | Any 1 of the following: · No blinding of outcome assessment, and outcome measurement is likely to be influenced by lack of blinding; or · Blinding of outcome assessment, but likely that blinding could have been broken, and outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for judgement of ‘Unclear risk’ of bias | Any 1 of the following: · Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; or · The study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Attrition bias due to quantity, nature, or handling of incomplete outcome data | |
| Criteria for judgement of ‘Low risk’ of bias | Any 1 of the following: · No missing outcome data; · Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · For dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; or · Missing data imputed using appropriate methods. |
| Criteria for judgement of ‘High risk’ of bias | Any 1 of the following: · Reason for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups; · For dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; or · Potentially inappropriate application of simple imputation. |
| Criteria for judgement of ‘Unclear risk’ of bias | Any 1 of the following: · Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomised not stated, no reasons for missing data provided); or · The study did not address this outcome. |
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting | |
| Criteria for judgement of ‘Low risk’ of bias | Any of the following: · The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes of interest in the review have been reported in the prespecified way; or · The study protocol is not available but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| Criteria for judgement of ‘High risk’ of bias | Any 1 of the following: · Not all of the study’s prespecified primary outcomes have been reported; · One or more primary outcomes are reported using measurements, analysis methods, or subsets of data (e.g. subscales) that were not prespecified; · One or more reported primary outcomes are not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · One or more outcomes of interest in the review are reported incompletely, so that they cannot be entered into a meta‐analysis; or · The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that most studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table | |
| Criteria for judgement of ‘Low risk’ of bias | The study appears to be free of other sources of bias. |
| Criteria for judgement of ‘High risk’ of bias | There is at least one important risk of bias. For example, the study: · Had a potential source of bias related to the specific study design used; · Has been claimed to have been fraudulent; or · Had some other problem. |
| Criteria for judgement of ‘Unclear risk’ of bias | There may be a risk of bias, but either: · Information is insufficient to assess whether an important risk of bias exists; or · Rationale or evidence is insufficient to show that an identified problem will introduce bias. |
Data and analyses
Comparison 1. 5‐ASA vs control (all trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence | 7 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 2 Number of participants with adverse events | 5 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.06] |
Comparison 2. 5‐ASA vs control ‐ subgroups based on regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence | 7 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.46, 1.10] |
| 1.1 5‐ASA + probiotics vs probiotics | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 4.94] |
| 1.2 5‐ASA + antibiotics vs antibiotics | 1 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.63] |
| 1.3 5‐ASA vs no therapy | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.18, 0.57] |
| 1.4 5‐ASA vs placebo | 4 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.87, 1.35] |
| 1.5 5‐ASA + probiotics vs placebo | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.50, 3.86] |
Comparison 3. 5‐ASA vs control ‐ subgroups based on method of diagnosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence based on CT for initial diagnosis | 7 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 1.1 CT scan/ultrasound primary diagnosis | 4 | 1391 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.89, 1.35] |
| 1.2 Other | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.50] |
| 2 Recurrence based on CT scan for all events | 7 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 2.1 CT scan for primary diagnosis and recurrence | 3 | 1274 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.81, 1.40] |
| 2.2 Other | 4 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.18, 1.09] |
Comparison 4. 5‐ASA vs control ‐ subgroup analyses based on risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrence | 7 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.43, 1.09] |
| 1.1 Studies at unclear risk of bias | 3 | 1400 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.59, 1.49] |
| 1.2 Studies at high risk of bias | 4 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.27, 1.10] |
4.1. Analysis.
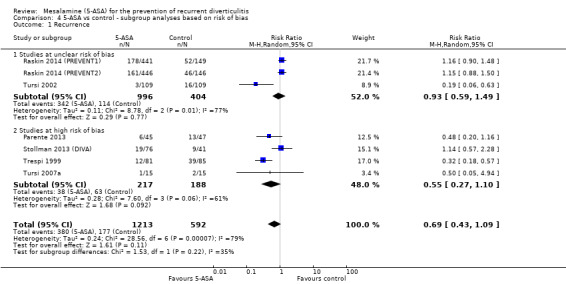
Comparison 4 5‐ASA vs control ‐ subgroup analyses based on risk of bias, Outcome 1 Recurrence.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Parente 2013.
| Methods | RCT, multi‐centre, Italy | |
| Participants | 96 patients with recent first episode of uncomplicated diverticulitis | |
| Interventions | 5‐ASA mesalazine (Pentacol) 800 mg twice daily for 10 days every month vs placebo 1 tablet twice daily for 10 days every month for 24 months | |
| Outcomes | Diverticulitis recurrence, followed for 24 months Secondary endpoints: time to relapse; impact of prophylactic treatment on physical condition and quality of life assessed by means of the Therapy Impact Questionnaire (TIQ); additional gastrointestinal drug savings, as pharmacoeconomic objective; treatment tolerability |
|
| Notes | This study was defined by study authors as a pilot study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled; placebos were identical in appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21.7% of participants had no outcome data. Of these, 4 did not receive any study drug after randomisation. It is not clear which groups they belonged to. Therefore, study authors modified ITT analysis (n = 92) and did not include all randomised participants. 16 were lost to follow‐up: 4 participants dropped out (all in placebo group), 12 with serious side effects (8 on 5‐ASA, 4 on placebo). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None detected |
Raskin 2014 (PREVENT1).
| Methods | RCT, multi‐centre, global, dose‐response phase 3 placebo‐controlled study | |
| Participants | 590 patients with ≥ 1 episode of acute diverticulitis in the previous 24 months that resolved without surgery | |
| Interventions | 5‐ASA (multi‐matrix mesalamine ) 1.2 g, 2.4 g, 4.8 g, or placebo once daily for 104 weeks | |
| Outcomes | Diverticulitis recurrence free at 104 weeks Secondary endpoints: time to recurrence of diverticulitis and proportion of participants requiring surgical intervention, adverse events | |
| Notes | Data for mesalamine were combined in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated fixed‐block randomisation schedule. Randomisation was stratified by country and by number of previous episodes of diverticulitis. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study, matching placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded, based on ClinicalTrial.gov information (NCT00545740). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Al outcomes were reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported for 583 participants (99%). 75 (13%) participants withdrew from the study. |
| Other bias | Low risk | None detected |
Raskin 2014 (PREVENT2).
| Methods | RCT, multi‐centre, global, dose‐response phase 3 placebo‐controlled study | |
| Participants | 592 patients with ≥ 1 episode of acute diverticulitis in the previous 24 months that resolved without surgery | |
| Interventions | 5‐ASA (multi‐matrix mesalamine) 1.2 g, 2.4 g, 4.8 g, or placebo once daily for 104 weeks | |
| Outcomes | Diverticulitis recurrence free at 104 weeks Secondary endpoints: time to recurrence of diverticulitis and proportion of participants requiring surgical intervention, adverse events | |
| Notes | Data for mesalamine were combined in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated fixed‐block randomisation schedule. Randomisation was stratified by country and by number of previous episodes of diverticulitis. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study, matching placebo tablets were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded, based on ClinicalTrial.gov information (NCT00545103). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Al outcomes were reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported for 586 participants (99%). 56 (10%) withdrew from the study. |
| Other bias | Low risk | None detected |
Stollman 2013 (DIVA).
| Methods | RCT, multi‐centre, USA | |
| Participants | 117 patients with acute diverticulitis, mean age 57.6, male 56, female 61 | |
| Interventions | 5‐ASA (mesalamine 2.4 g/d) (n = 40) vs mesalamine 2.4 g/d plus probiotic (Bifidobacterium infantis 35624, 1 billion units) (n = 36) vs placebo (n = 41) | |
| Outcomes | Global symptomatic score (GSS) at 12 weeks. Percentage of responders/Change in GSS at weeks 12 and 52. Recurrent diverticulitis. Withdrawal due to surgery for diverticulitis, recurrent diverticulitis, adverse events Treatment period 12 weeks followed by 9‐month treatment‐free observation |
|
| Notes | Relapse data were extracted from the website https://clinicaltrials.gov/ct2/show/NCT00554099, NCT00554099. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Once the patient was determined to be eligible for the study, the investigator or a designated representative called an interactive voice response system for participant randomisation and allocation of study medication. Participants were stratified on the basis of the number of prior episodes of diverticulitis (1 attack vs > 1 attack). |
| Allocation concealment (selection bias) | Low risk | Central randomisation system; personnel called centre for randomisation and allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, caregivers, and investigators blinded. "The treatment codes were controlled by the Sponsor’s Clinical Supplies group. The double‐blind packaging of mesalamine and matching placebo were identical, labelled bottles. The double‐blind packaging for the probiotic and matching placebo were identical, labelled blister card kits." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported. Likely outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24.8% dropped out during the treatment phase. 9 (25%) vs 8 (20%) vs 12 (30%) participants did not complete treatment after 12 weeks of treatment, and 12 (33%), 13 (33%), and 19 (46%) did not complete the 52‐week study, in mesalamine + probiotic, mesalamine and placebo groups, respectively. Although reasons for withdrawal were provided, the overall number of participants without outcome data > 20% and the number of withdrawals were higher in the placebo group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No clear evidence of other bias |
Trespi 1999.
| Methods | RCT, single‐centre, open‐label , Italy | |
| Participants | 187 patients with acute diverticulitis, mean age 61.1, 101 male, 86 female | |
| Interventions | 166 participants were randomised after initial treatment with ampicillin/sulbactam 1.5 g IM twice daily plus rifaximin 400 mg PO twice daily for 7 days. Mesalazine (Pentacol tablets‐‐SOFAR S.p.A.) 400 mg twice daily per os for 8 weeks (n = 81) vs no supplementary treatment (n = 85) for 8 weeks | |
| Outcomes | Treatment for acute diverticulitis, prevention of complications of symptomatic diverticular disease, incidence of diverticular disease complications Follow‐up 12 months and 4 years |
|
| Notes | Study designed for 3‐year follow up. Preliminary results for first 12 months were reported in 1997 (Trespi 1997); we used the 4‐year follow‐up results (Trespi 1999). Did not report data for adverse events. Study authors excluded from the analysis 21 participants for the following reasons: major inflammatory complications (n = 9), liver cirrhosis (n = 2), chronic renal failure (n = 1), peptic ulcer (n = 3), allergy to salicylates (n = 1), lack of consensus to the study (n = 5) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After 12 months, 27 (16%) dropouts. In total, 15 5‐ASA (19%) and 12 controls (14%); dropouts (9 major complications, 3 massive haemorrhage, 32 dropouts). Dropout rate was higher in 5‐ASA group than in control group, most due to higher poor compliance or withdrawal of consent. After 4 years, dropouts were 44 (27%) (24 vs 20) for the following reasons: major complications (e.g. abdominal abscess or bleeding, n = 9: 4 M group + 5 C group), death due to stroke or heart attack (n = 3, 1 M + 2 C), lack of compliance or interruption of consensus to treatment (n = 32, 19 M + 13 C). |
| Selective reporting (reporting bias) | Unclear risk | Data for adverse events are not clearly reported. It is not clear whether study authors have excluded data. |
| Other bias | Low risk | None found |
Tursi 2002.
| Methods | RCT, single‐centre, Italy | |
| Participants | 218 patients with history of recurrent diverticulitis (2 attacks of acute diverticulitis in the previous year), mean age 64.3, 131 male, 87 female | |
| Interventions | 800 mg three times daily plus rifaximin 400 mg twice daily for 7 days, then followed by mesalazine 800 mg twice daily plus rifaximin 400 mg twice daily for 7 days every month (n = 109) to rifaximin 400 mg twice daily alone for 7 days every month (n = 109), for 12 months | |
| Outcomes | Prevention of diverticulitis recurrence Rapidity of symptom improvement Regulation of bowel habits Side effects All participants underwent colonoscopy after 3, 6, and 12 months. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described; probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessor was blinded but likely not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants did not complete the study: 2 died (1 in mesalazine with stroke and 1 in rifaximin with myocardial infarction), and 4 participants were lost to follow‐up (1 vs 3). 19 participants were not fully compliant. Additional information was not provided. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. However, when reporting adverse events, study authors reported 1 participant with transient urticaria (in the group rifaximin + mesalazine, 0.91%) and 9 with epigastric pain (8.25%), "related to rifaximin and mesalazine, respectively", without clearly stating which group they belong to. As mesalazine was used only in the combination group, it seems all adverse events were noted in this group (9 or 10 vs 0 participants). |
| Other bias | Low risk | No other risk of bias is noted. |
Tursi 2007a.
| Methods | RCT, single‐centre, Italy | |
| Participants | 30 patients with uncomplicated acute diverticulitis after remission, mean 60.1 years, 19 males, 11 female | |
| Interventions | 5‐ASA balsalazide 2.25 g once daily for 10 days every month plus probiotic VSL#3 450 billions/d for 15 days every month vs probiotic alone (VSL#3) 450 billions/d for 15 days every month. Remission was induced with rifaximin 800 mg/d and 5‐ASA 2.25 g/d for 10 days. Follow‐up: 12 months |
|
| Outcomes | Maintainence of remission after an attack, overall scores at end of follow‐up, single symptom assessment, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | "the patients who met inclusion criteria were enrolled in the study and randomised, in an unblinded fashion, one to one into the two groups of treatments, after giving their informed consent" It is likely allocation was not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Randomised in an "unblinded" fashion. Treatment regimens were different, no placebo was used, likely participants and physicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described but probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant from each group lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None found |
5‐ASA: 5‐aminosalicylic acid. ITT: intention‐to‐treat. RCT: randomised controlled trial. TIQ: Therapeutic Impact Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brandimarte 2004 | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Comparato 2007a | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Comparato 2007b | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Festa 2015 | Not a randomised controlled trial |
| Gaman 2011 | Conference abstract. Included participants with diverticulosis, not diverticulitis. Reported the effect of mesalazine in preventing recurrence of disease and occurrence of diverticulosis |
| Gatta 2012 | Not a randomised controlled trial |
| Kruis 2013a | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Mario 2005 | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Tursi 2006 | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Tursi 2007b | Symptomatic uncomplicated diverticular disease, not diverticulitis |
| Tursi 2013 | Not a randomised controlled trial |
| Tursi 2013b | Symptomatic uncomplicated diverticular disease, not diverticulitis |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Bassi 2015.
| Methods | RCT, prospective, randomised, open‐label |
| Participants | 34 patients with symptomatic uncomplicated diverticular disease |
| Interventions | 5‐ASA mesalazine 1.6 g/d or mesalazine 1.6 g/d + L. casei DG 16 billion/d for 10 days/month for 18 months |
| Outcomes | 4 participants (11.8%) were symptom free after the 12th month of treatment. |
| Notes | Both mesalazine and L. casei seem to be effective in preventing recurrence of uncomplicated symptomatic diverticular disease of the colon. |
Kruis 2013b.
| Methods | RCT, double‐blind, placebo‐controlled |
| Participants | 345 patients with uncomplicated left‐sided diverticulitis |
| Interventions | 5‐ASA mesalamine 3 g granules once daily or placebo (3 g placebo granules) once daily for 48 weeks |
| Outcomes | Proportions of participants who were recurrence‐free: 67.9% on 5‐ASA vs 74.4% on placebo (P > 0.05) |
| Notes | 5‐ASA not significantly superior over placebo for prevention of recurrence of uncomplicated diverticulitis |
Kruis 2014.
| Methods | RCT, double‐blind, placebo‐controlled |
| Participants | 330 participants treated for uncomplicated left‐sided diverticulitis |
| Interventions | 5‐ASA 1.5 g or 3 g daily or placebo for 96 weeks |
| Outcomes | Recurrence‐free rates were 6.9%, 9.8%, and 21.8%, respectively (P > 0.05). |
| Notes | The study was prematurely terminated for lack of observed efficacy after an interim analysis. |
Kruis 2017.
| Methods | Two RCTs, double‐blind, placebo‐controlled, multi‐centre |
| Participants | Patients with prior episodes of uncomplicated left‐sided diverticulitis |
| Interventions | First trial: 5‐ASA 3 g vs placebo; second trial: 5‐ASA 1.5 g, 5‐ASA 3 g, or placebo |
| Outcomes | First trial: 67.9% in the 5‐ASA group vs 74.4% in the placebo group were recurrence free (P = 0.226). Second trial: 6.9% in the 5‐ASA 1.5 g group, 9.8% in the 5‐ASA 3 g group, 23.1% in the placebo group were recurrence free at 96 weeks |
| Notes | First trial prematurely terminated owing to futility at planned interim analysis |
5‐ASA: 5‐aminosalicylic acid. RCT: randomised controlled trial.
Differences between protocol and review
None.
Contributions of authors
Drafted the protocol: JM, FC. Developed a search strategy: YY, JM, FC. Searched for trials: FC, MA. Selected which trials should be included: FC, MA. Assessed risk of bias in included studies: FC, YY, MA. Extracted data from trial publications: FC, YY, MA. Entered data into RevMan: FC, YY. Carried out the analysis: FC, YY. Interpreted the analysis: FC, YY, JM. Drafted the final review: FC, YY, JM.
Declarations of interest
None to report.
New
References
References to studies included in this review
Parente 2013 {published data only}
- Parente F, Bargiggia S, Prada A, Bortoli A, Giacosa A, Monti C, et al. Intermittent treatment with mesalazine in the prevention of diverticulitis recurrence: a randomised multicentre pilot double‐blind placebo‐controlled study of 24‐month duration. International Journal of Colorectal Disease 2013;28:1423‐31. [DOI] [PubMed] [Google Scholar]
Raskin 2014 (PREVENT1) {published data only}
- Raskin J, Kamm M, Jamal M, Marquez J, Meizer E, Schoen R, et al. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology 2014;147:793‐802. [DOI] [PubMed] [Google Scholar]
Raskin 2014 (PREVENT2) {published data only}
- Raskin J, Kamm M, Jamal M, Marquez J, Meizer E, Schoen R, et al. Mesalamine did not prevent recurrent diverticulitis in phase 3 controlled trials. Gastroenterology 2014;147:793‐802. [DOI] [PubMed] [Google Scholar]
Stollman 2013 (DIVA) {published data only}
- Stollman N, Magowan S, Shanahan F, Quigley E. A randomized controlled study of mesalamine after acute diverticulitis: results of the DIVA trial. Journal of Clinical Gastroenterology 2013;47(7):621‐9. [DOI] [PubMed] [Google Scholar]
Trespi 1999 {published data only}
- Trespi E, Colla C, Panizza P, Polino MG, Venturini A, Bottani G, et al. Therapeutic and prophylactic role of mesalazine (5‐ASA) in symptomatic diverticular disease of the large intestine. 4 Year follow‐up results [<Ruolo terapeutico e profilattico della mesalazina (5‐ASA) nella malattia diverticolare sintomatica del crasso. Risultati a 4 anni di follow‐up.>]. Minerva Gastroenterologica e Dietologica 1999;45(4):245‐52. [PubMed] [Google Scholar]
- Trespi E, Panizza P, Colla C, Bottani G, Vecchi P, Matti C. Efficacy of low dose mesalazine (5‐ASA) in the treatment of acute inflammation and prevention of complications inpatients with symptomatic diverticular disease [Efficacia della mesalazina (5‐ASA) a basse dosi nel trattamento della flogosi acuta e nella prevenzione delle complicanze in pazienti con malattia diverticolare sintomatica. Risultati preliminari]. Minerva Gastroenterologica e Dietologica 1997;43(3):157‐62. [PubMed] [Google Scholar]
Tursi 2002 {published data only}
- Tursi A, Brandimarte G, Daffina R. Long‐term treatment with mesalamine and rifaximin versus rifaximin alone for patients with recurrent attacks of acute diverticulitis of the colon. Digestive and Liver Disease 2002;34:510‐5. [DOI] [PubMed] [Google Scholar]
Tursi 2007a {published data only}
- Tursi A, Giovanni B, Marco Giorgetti G, Elisei W, Aiello F. Balsalazide and/or high potency probiotic mixture (VSL#3) in maintaining remission after attack of acute, uncomplicated diverticulitis of the colon. International Journal of Colorectal Disease 2007;22:1103‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brandimarte 2004 {published data only}
- Brandimarte G, Tursi A. Rifaximin plus mesalazine followed by mesalazine alone is highly effective in obtaining remission of symptomatic uncomplicated diverticular disease. Medical Science Monitor 2004;10(5):170‐3. [PubMed] [Google Scholar]
Comparato 2007a {published data only}
- Comparato G, Fanigliulo L, Aragona G, Cavestro GM, Cavallaro LG, Leandro G, et al. Quality of life in uncomplicated symptomatic diverticular disease: is it another good reason for treatment?. Digestive Diseases 2007;25(3):252‐9. [DOI] [PubMed] [Google Scholar]
Comparato 2007b {published data only}
- Comparato G, Fanigliulo L, Cavallaro LG, Aragona G, Cavestro GM, Iori V, et al. Prevention of complications and symptomatic recurrences in diverticular disease with mesalazine: a 12‐month follow‐up. Digestive Diseases & Sciences 2007;52(11):2934‐41. [DOI] [PubMed] [Google Scholar]
Festa 2015 {published data only}
- Festa V, Papi C, Chiesara F, Moretti A, Dezi A, Bianchi M, et al. Prevention of recurrent diverticulitis: a pragmatic trial with mesalazine versus rifaximin. Digestive and Liver Disease. 21st National Congress of Digestive Diseases, Italian Federation of Societies of Digestive Diseases 2015;47:e169. [Google Scholar]
Gaman 2011 {published data only}
- Gaman A, Teodorescu R, Georhescu EF, Abagiu MT. Prophylactic effects of mesalamine in diverticular disease. Falk Symposium "Diverticular disease: a fresh approach to a neglected disease". Guerzenich Congress Center, Cologne, Germany, 2011; Vol. 178:Abstract 13.
Gatta 2012 {published data only}
- Gatta L, Mario F, Curlo M, Vaira D, Pilotto A, Lucarini P, et al. Long‐term treatment with mesalazine in patients with symptomatic uncomplicated diverticular disease. Internal and Emergency Medicine 2012;7(2):133‐7. [DOI] [PubMed] [Google Scholar]
Kruis 2013a {published data only}
- Kruis W, Meier E, Schumacher M, Mickisch O, Greinwald R, Mueller R. Randomised clinical trial: mesalazine (salofalk granules) for uncomplicated diverticular disease of the colon ‐ a placebo‐controlled study. Alimentary Pharmacology and Therapeutics 2013;37(7):680‐90. [DOI] [PubMed] [Google Scholar]
Mario 2005 {published data only}
- Mario FD, Aragona G, Leandro G, Comparato G, Fanigliulo L, Cavallaro LG, et al. Efficacy of mesalazine in the treatment of symptomatic diverticular disease. Digestive Diseases and Sciences 2005;50(3):581‐6. [DOI] [PubMed] [Google Scholar]
Tursi 2006 {published data only}
- Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Mesalazine and/or Lactobacillus casei in preventing recurrence of symptomatic uncomplicated diverticular disease of the colon: a prospective, randomized, open‐label study. Journal of Clinical Gastroenterology 2006;40(4):312‐6. [DOI] [PubMed] [Google Scholar]
Tursi 2007b {published data only}
- Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Continuous versus cyclic mesalazine therapy for patients affected by recurrent symptomatic uncomplicated diverticular disease of the colon. Digestive Diseases & Sciences 2007;52(3):671‐4. [DOI] [PubMed] [Google Scholar]
Tursi 2013 {published data only}
- Tursi A, Mario F, Brandimarte G, Elisei W, Picchio M, Loperfido S, et al. Intermittent versus every‐day mesalazine therapy in preventing complications of diverticular disease: a long‐term follow‐up study. European Review for Medical and Pharmacological Sciences 2013;17(23):3244‐8. [DOI] [PubMed] [Google Scholar]
Tursi 2013b {published data only}
- Tursi A, Brandimarte G, Elisei W, Picchio M, Forti G, Pianese G, et al. Randomised clinical trial: mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease – a double‐blind, randomised, placebo‐controlled study. Alimentary Pharmacology & Therapeutics 2013;38(7):741‐51. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bassi 2015 {published data only}
- Bassi R. Preventing recurrence of symptomatic diverticular disease of the colon: mesalazine with or without Lactobacillus casei: a prospective randomized, open label study. 10th Scientific and Annual Meeting of the European Society of Coloproctology, Dublin. Ireland. 2015; Vol. 17:49‐50.
Kruis 2013b {published data only}
- Kruis W, Eisenbach T, Lohr H, Muser M, Tan GT, Lukas M, et al. Double‐blind, randomized, placebo‐controlled, multicenter trial of mesalamine for the prevention of recurrence of diverticulitis. Gastroenterology. 2013; Vol. 144 (5 Suppl 1):S139.
Kruis 2014 {published data only}
- Kruis W, Kardalinos V, Curtin A, Dorofeyev AE, Zakko SF, Wolkner J, et al. Daily mesalamine fails to prevent recurrent diverticulitis in a large placebo controlled multicenter trial. Digestive Disease Week. 2014; Vol. 146, issue 5 Suppl 1:S187.
Kruis 2017 {published data only}
- Kruis W, Kardalinos V, Eisenbach T, Lukas M, Vich T, Buganic I, et al. Randomised clinical trial: mesalazine versus placebo in the prevention of diverticulitis recurrence. Alimentary Pharmacology &Therapeutics 2017;46:282‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Barroso 2015
- Barroso AO, Quigley EM. Diverticula and diverticulitis: time for a reappraisal. Journal of Gastroenterology and Hepatology 2015;11(10):680‐8. [PMC free article] [PubMed] [Google Scholar]
Berman 1968
- Berman LG, Burdick D, Heitzman ER, Prior JT. A critical reappraisal of sigmoid peridiverticulitis. Surgery, Gynecology & Obstetrics 1968;127:481‐91. [PubMed] [Google Scholar]
Buchs 2015
- Buchs NC, Mortensen NJ, Ris F, Morel P, Gervaz P. Natural history of uncomplicated sigmoid diverticulitis. World Journal of Gastrointestinal Surgery 2015;7(11):313‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chabok 2012
- Chabok A, Pahlman L, Hjern F, Haapaniemi S, Smedh K, AVOD Study Group. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. British Journal of Surgery 2012;99:532‐9. [DOI] [PubMed] [Google Scholar]
Chapman 2006
- Chapman JR, Dozois EJ, Wolff BG. Diverticulitis: a progressive disease? Do multiple recurrences predict less favourable outcomes?. Annals of Surgery 2006;243:876‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chautems 2002
- Chautems RC, Ambrosetti P, Ludwig A, Mermillod B, Morrel P, Soravia C. Long term follow up after first acute episode of sigmoid diverticulitis: is surgery mandatory? A prospective study of 188 patients. Diseases of the Colon and Rectum 2002;45:962‐6. [DOI] [PubMed] [Google Scholar]
Collins 2008
- Collins D, Winters DC. Elective resection for diverticular disease: an evidence based review. World Journal of Surgery 2008;32:2429‐33. [DOI] [PubMed] [Google Scholar]
Di Mario 2006
- Mario F, Comparato G, Fanigliulo L. Cavallaro LG, Cavestro GM, et al. Use of mesalazine in diverticular disease. Journal of Clinical Gastroenterology 2006;40(Suppl 3):S155‐9. [DOI] [PubMed] [Google Scholar]
Floch 2006
- Floch MH. A hypothesis: is diverticulitis a type of inflammatory bowel disease?. Journal of Clinical Gastroenterology 2006;40(Suppl 3):121‐5. [DOI] [PubMed] [Google Scholar]
Freeman 2008
- Freeman NJ. Natural history and long‐term clinical behaviour of segmental colitis associated with diverticulosis (SCAD syndrome). Digestive Disease Science 2008;53:2452‐7. [DOI] [PubMed] [Google Scholar]
Goldstein 2000
- Goldstein NS, Leon‐Armin C, Mani A. Crohn's colitis‐like change in sigmoid diverticulitis specimens is usually an idiosyncratic inflammatory response to the diverticulosis rather than Crohn's colitis. Americal Journal of Surgical Pathology 2000;24:668. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Horgan 2001
- Horgan AF, McConnell EJ, Wolff BG. Atypical diverticular disease: surgical results. Diseases of the Colon & Rectum 2001;44:1315‐6. [DOI] [PubMed] [Google Scholar]
Humes 2008
- Humes DJ, Simpson J, Neal KR, Scholefield JH, Spiller RC. Psychological and colonic factors in painful diverticulosis. British Journal of Surgery 2008;95:195‐8. [DOI] [PubMed] [Google Scholar]
Karagozian 2007
- Karagozian R, Burakoff R. The role of mesalamine in the treatment of ulcerative colitis. Therapeutics and Clinical Risk Management 2007;3(5):893‐903. [PMC free article] [PubMed] [Google Scholar]
Kohler 1999
- Kohler L, Sauerland S, Neugubauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. Surgical Endoscopy 1999;13:430‐6. [DOI] [PubMed] [Google Scholar]
Loannidis 2007
- Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta‐analyses. British Medical Journal 2007;335(7626):914‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maconi 2011
- Maconi G, Barbara G, Bosetti C, Cuomo R, Annibale B. Treatment of diverticular disease of the colon and prevention of acute diverticulitis: a systematic review. Diseases of Colon and Rectum 2011;54(10):1326‐38. [DOI] [PubMed] [Google Scholar]
Morris 2014
- Morris AM, Regenboden SE, Hardiman KM, Hendren S. Sigmoid diverticulitis: a systematic review. Journal of the American Medical Association 2014;311(3):287‐97. [DOI] [PubMed] [Google Scholar]
Nielsen 2007
- Nielsen OH, Munck LK. Drug Insight: aminosalicylates for the treatment of IBD. Nature Clinical Practice Gastroenterology and Hepatology 2007;4:160‐70. [DOI] [PubMed] [Google Scholar]
Painter 1971
- Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of western civilization. British Medical Journal 1971;2:450‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parks 1970
- Parks TG, Connell AM. The outcome in 455 patients admitted for treatment of diverticular disease of the colon. British Journal of Surgery 1970;57:775‐8. [DOI] [PubMed] [Google Scholar]
Parks 1975
- Parks TG. Natural history of diverticular disease of the colon. Clinical Gastroenterology 1975;4:53‐69. [PubMed] [Google Scholar]
Peery 2013
- Peery A, Sandler R. Diverticular disease. Clinical Gastroenterology and Hepatology 2013;11(12):1532‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peppercorn 2004
- Peppercorn MA. The overlap of inflammatory bowel disease and diverticular disease. Journal of Clinical Gastroenterology 2004;38(Suppl 5):8‐10. [DOI] [PubMed] [Google Scholar]
Rafferty 2006
- Rafferty J, Shellito P, Hyman NH: Standards Committee of the American Society of Colon and Rectal Surgeons. Practice parameters for sigmoid diverticulitis. DIseases of the Colon and Rectum 2006;49:939‐44. [DOI] [PubMed] [Google Scholar]
Ransford 2002
- Ransford RA, Langman MJ. Sulphasalazine and mesalazine: serious adverse reactions re‐evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut 2002;51(4):536‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarma 2008
- Sarma D, Longo WE, NDSG. Diagnostic imaging for diverticulitis. Journal of Clinical Gastroenterology 2008;42:1139‐41. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brozek J, Oxman A, editors. The GRADE Working Group, 2009. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendation. Available from http://www.cc‐ims.net/gradepro. Version 3.2 (updated March 2009).
Sheth 2008
- Sheth AA, Longo W, Floch MH. Diverticular disease and diverticulitis. American Journal of Gastroenterology 2008;103:1550‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Egger M, Moher D, on behalf of the Cochrane Bias Methods Group. Chapter 10: Addressing reporting bias. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stollman 1999
- Stollman N, Raskin J. Practice guidelines: diagnosis and management of diverticular disease of the colon in adults. American Jounal of Gastroenterology 1999;94(11):3110‐21. [DOI] [PubMed] [Google Scholar]
Strate 2012
- Strate L, Modi R, Cohen E, Spiegel B. Diverticular disease as a chronic illness: evolving epidemiologic and clinical insights. American Journal of Gastroenterology 2012;107:1486‐93. [DOI] [PubMed] [Google Scholar]
Szojda 2007
- Szojda MM, Cuesta MA, Mulder CM, Felt‐Bersma RJ. Review article: management of diverticulitis. Alimentary, Pharmacology & Therapeutics 2007;26(Suppl 2):67‐76. [DOI] [PubMed] [Google Scholar]
Trespi 1997
- Trespi E, Panizza P, Colla C, Bottani G, Vecchi P, Matti C. Efficacy of low dose mesalazine (5‐ASA) in the treatment of acute inflammation and prevention of complications inpatients with symptomatic diverticular disease [Efficacia della mesalazina (5‐ASA) a basse dosi nel trattamento della flogosi acuta e nella prevenzione delle complicanze in pazienti con malattia diverticolare sintomatica. Risultati preliminari]. Minerva Gastroenterologica e Dietologica 1997;43(3):157‐62. [PubMed] [Google Scholar]
Tursi 2008
- Tursi A, Brandimarte G, Elisei W, Giorgetti GM, Inchingolo CD, Danese S, et al. Assessment and grading of mucosal inflammation in colonic diverticular disease. Journal of Clinical Gastroenterology 2008;42(6):699‐703. [DOI] [PubMed] [Google Scholar]
Tursi 2012
- Tursi A. Advances in the management of colonic diverticulitis. Canadian Medical Association Journal 2012;184(13):1470‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tursi 2015
- Tursi A. New medical strategies for the management of acute diverticulitis. Expert Review of Gastroenterology & Hepatology 2015;9(10):1293‐304. [DOI] [PubMed] [Google Scholar]
Unlu 2012
- Unlu C, Daniels L, Vrouenraets BC, Boermeester MA. Systematic review of medical therapy to prevent recurrent diverticulitis. International Journal of Colorectal Disease 2012;27(9):1131‐6. [DOI] [PubMed] [Google Scholar]
Williams 1995
- Williams RA, Davis IP. DIverticular disease of the colon. In: Haubrich WS, Schaffner F editor(s). Brockus Gastroenterology. 5th Edition. Philadelphia: WB Saunders, 1995:1637‐56. [Google Scholar]


